Summary
Background
In human papillomavirus (HPV)-vaccinated women, reductions in cervical disease and related procedures results in more women having intact transformation zones, potentially increasing the risk of cervical lesions caused by non-vaccine-preventable types, a phenomenon termed “clinical unmasking”. We aimed to evaluate HPV vaccine efficacy against cervical intraepithelial neoplasia grades 2+ (CIN2+) and cervical intraepithelial neoplasia grades 3+ (CIN3+) attributed to non-preventable types, through 11 years post-vaccination in the long-term follow-up phase of the Costa Rica HPV Vaccine Trial (CVT).
Methods
CVT is a randomised (1:1), double-blinded trial which vaccinated women in Costa Rica aged 18-25 years with Cervarix®, the bivalent AS04-adjuvanted HPV vaccine (N=3727), or control hepatitis A vaccine (N=3739), administered intramuscularly with 0·5 mL doses at 0, 1, and 6 months. The allocation sequence was generated using a blocked randomisation method, with permuted block sizes of 14, 16, and 18. Blinding of vaccine allocation was maintained throughout the 4-year randomised trial phase, after which controls were provided the HPV vaccine and exited the study; a screening-only, unvaccinated control group (UCG) was enrolled. The UCG and HPV-vaccine arm were then followed for seven years, during which treatment allocation was not masked. One of the prespecified primary endpoints for the long-term follow-up phase of CVT was precancers associated with HPV types not prevented by the vaccine (primary outcome 1b), which we defined as histologically-confirmed incident CIN2+/CIN3+ attributed to non-preventable types (any type except HPV16/18/31/33/45). Because clinical unmasking may occur later when less aggressive genotypes cause disease, our primary analytical period was years 7-11, the observational follow-up phase. We also examined years 1-4 and both periods combined. Eligibility criteria for each analytical period included all women with at least one follow-up visit in the respective period and excluded women with a prior endpoint (i.e., modified intention-to-treat cohort). For each outcome, women were followed until the endpoint was reached or a cervical procedure was performed. We computed relative and absolute reductions in endpoints, and 95% confidence intervals (95%CIs). The randomised, blinded trial phase has been completed while an unblinded subset of women in the HPV-vaccinated arm is still under active investigation (Clinicaltrials.gov, NCT00128661, NCT00867464).
Findings
Between June 28, 2004, and December 21, 2005, 7466 women enrolled in CVT (HPV-vaccine arm=3727; control arm=3739). Between March 30, 2009, and July 5, 2012, 2836 women enrolled in the new UCG. The primary analytical cohort (years 7-11) included 2767 women in the HPV-vaccine arm and 2563 in the UCG for the CIN2+ endpoint assessment and 2826 women in the HPV-vaccine arm and 2592 in the UCG for the CIN3+ endpoint assessment. Median follow-up time during years 7-11 for women included for the CIN2+ analysis was 52·8 months (interquartile range 44·0-60·7) for the HPV-vaccine arm and 49·8 months (interquartile range 42·0-56·9) for the UCG. During years 7-11, clinical unmasking was observed with a significant negative VE against CIN2+ attributed to non-preventable types [−71·2% (95%CI −164%, −12.5%)]. Through 11 years, we observed 9·2 (95%CI 0.8, 17.8) additional CIN2+ events attributed to non-preventable types per 1000 vaccinated women versus unvaccinated women; despite this increase, we observed 27·0 (95%CI 14·2, 39·9) fewer CIN2+ events irrespective of type per 1000 vaccinated women. Similarly, VE against CIN3+ attributed to non-preventable types during years 7-11 was −135% (95%CI −330%, −33·5%).
Interpretation
Higher CIN2+/CIN3+ rates due to non-preventable types in vaccinated versus unvaccinated women suggests unmasking could attenuate long-term reductions in high-grade disease following successful implementation of HPV vaccination programs in screened populations. Importantly, the net benefit of vaccination remains considerable therefore, HPV vaccination should still be prioritized as primary prevention for cervical cancer.
Funding
CVT is funded by the National Cancer Institute (N01-CP-11005) and National Institutes of Health Office of Research on Women's Health.
Introduction
The International Agency for Research on Cancer has identified 12 main carcinogenic HPV genotypes (HPV16/18/31/33/35/39/45/51/56/52/58/59).1 Persistent carcinogenic human papillomavirus (HPV) infection is associated with the development of cervical cancer, of which 70% are attributable to HPV16/18, and 20% are attributable to HPV31/33/45/52/58.2 Commercially available HPV vaccines have high efficacy against vaccine-targeted HPV infections and high-grade cervical precancers in HPV-naïve women.3 The bivalent AS04-adjuvanted HPV vaccine, Cervarix®, covers HPV16/18, and provides partial cross-protection against phylogenetically-related carcinogenic types, including HPV31/33/45.4,5 Further, antibody quantity and quality are generally stable over 11 years following bivalent AS04-adjuvanted vaccination, and levels well exceed those of natural immunity.6,7 Together, vaccine-preventable types contribute to the largest proportion of cervical cancer cases globally, with ranked cumulative proportions as follows: HPV16 (61%), HPV18 (+10%, 71%), HPV45 (+6%, 77%), HPV33 (+4%, 81%), and HPV31 (+3%, 84%).8
Two distinct phenomena following vaccination that have been of interest are virological replacement (a biological, viral niche phenomenon in which non-vaccine-preventable types preferentially replace vaccine-preventable types following the elimination of preventable types through vaccination)9 and clinical unmasking (a result of fewer clinical treatments for vaccine-preventable associated disease in the vaccinated population). Elimination of vaccine-targeted HPV types is unlikely to lead to virological replacement with non-targeted types because multiple infections are common, and different HPV genotypes do not appear to compete for a common niche, as shown in most studies investigating replacement.9,10 However, the possibility remains that vaccination could lead to increases in disease caused by non-vaccine-preventable types through unmasking, as possibly shown in the pneumococcal conjugate vaccine literature.11,12 Applied to HPV, unmasking is hypothesized to occur when reductions in cervical procedures in vaccinated women result in more at-risk women having intact cervical transformation zones. This leads to an increase, or “unmasking”, of high-grade lesions caused by non-vaccine-preventable HPV types that would have been removed by excisional/ablative treatment after the development of disease from vaccine-preventable type infections or would be acquired after treatment but failed to develop because the tissue in which most CIN2+ lesions arise had been removed.
Unmasking of non-targeted/non-cross-protected HPV types (non-preventable types) is concerning because it may attenuate the anticipated reduction of the cervical cancer burden in populations where HPV vaccination is widely available and cervical cancer screening is regularly conducted. However, prior studies have been unable to investigate unmasking because non-preventable types are less aggressive13 and require long-term follow-up post-vaccination. Given the Costa Rica HPV Vaccine Trial’s (CVT) long-term follow-up, we can now evaluate unmasking by quantifying relative and absolute rate differences in disease for a range of HPV types between HPV-vaccinated and unvaccinated groups. If unmasking is a true phenomenon, we would expect to observe an excess of high-grade cervical lesions attributed to non-preventable types in screened, vaccinated women compared to screened, unvaccinated women. Quantifying the relative reduction between groups would result in a negative vaccine efficacy (VE). Estimating the absolute difference between groups informs the potential public health impact of an intervention in which unmasking can occur. For example, are the net reductions in endpoints due to vaccine-preventable types among HPV-vaccinated women offset by the net increase in endpoints due to non-preventable types? Here, we evaluated the bivalent AS04-adjuvanted HPV vaccine’s protection against cervical intraepithelial neoplasia grades 2 or worse (CIN2+) and CIN grades 3 or worse (CIN3+) attributed to vaccine-preventable and non-preventable HPV types on relative and absolute scales, in the long-term follow-up phase of CVT.
Methods
Study design and participants
CVT (NCT00128661) is a randomised, double-blinded community-based trial which examined the efficacy of the bivalent HPV vaccine against HPV16/18-associated infections and precancerous cervical lesions. Women in Costa Rica were enrolled between June 28, 2004, and December 21, 2005, if they were aged 18-25 years and in general good health. All women provided written, informed consent. Research activity for CVT was approved by Institutional Review Boards of Instituto Costarricense de Investigación y Enseñanza en Nutritión y Salud in Costa Rica and the United States National Cancer Institute (Bethesda, MD, USA). Detailed information on study design and methodology are previously described.14 After 4 years, women in the HPV-vaccine arm were invited to enroll in the long-term follow-up study between March 30, 2009, and July 5, 2012, to be seen biennially for seven additional years over 11 total years of follow-up. At this time, women in the control arm were offered the HPV vaccine and attended one final visit two years later before exiting the study. A new, observational unvaccinated control group (UCG) from the same birth cohort and geographic regions as the HPV-vaccine arm was enrolled and followed biennially for seven years. Additional information on the long-term follow-up study are reported elsewhere.15
Randomisation and masking
Women enrolled in CVT were randomly assigned (1:1) to receive an HPV16/18 AS04-adjuvanted vaccine or control hepatitis A vaccine, using a blocked randomisation method, with permuted block sizes of 14, 16, and 18. The allocation sequence was generated using SAS (version 8.2) by staff at the National Cancer Institute. Allocation was concealed to participants, study personnel, and investigators, and was maintained throughout the 4-year randomised phase of the trial, after which participants were informed of their vaccination status and women in the control arm were offered the HPV vaccine. Detailed randomization and masking procedures are published elsewhere.14 Participants in the long-term follow-up study were not masked because a screening-only, observational unvaccinated group was enrolled by design.
Procedures
Participants were vaccinated intramuscularly with either Cervarix, the bivalent AS04-adjuvanted HPV vaccine, or control hepatitis A vaccine in the deltoid muscle with 0·5 mL doses at 0, 1, and 6 months. Because HPV is a sexually transmitted infection, cytologic assessment and HPV DNA testing were conducted at enrollment and at subsequent follow-up visits among sexually active women, by obtaining exfoliated cervical cells using a Cervex-Brush® (Rovers Medical Devices BV, Oss, The Netherlands) rinsed in PreservCyt solution (Hologic, Marlborough, MA, USA). During the randomised phase, women in the HPV-vaccine and control arms received annual cervical screening. Clinical management was determined by cytology alone, which was the standard of care for cervical cancer screening at the time of the study. Cervical samples were collected and banked for HPV testing for research purposes, as this was a primary endpoint of the trial.
Because UCG was enrolled after the randomised phase, routine cervical screening was unknown prior to enrollment; therefore, a cervical screening and clinical management protocol was implemented at enrollment (year 4) to identify and treat prevalent disease (through year 6). This ensured comparability between the HPV vaccine-arm and UCG for the long-term analytical period (years 7-11). During the long-term follow-up study, women in both groups received biennial cytological screening. As clinical practices changed over time, the clinical management algorithm was modified to include HPV co-testing in the final biennial study visit. Screened HPV-positive patients who did not require immediate treatment attended an additional visit to conclusively define their clinical status.
Disease endpoints were determined by examining histological slides from cervical biopsies or specimens collected from loop electrosurgical excisional procedures (LEEPs), interpreted by one pathologist in Costa Rica for clinical management. One pathologist in the United States blinded to the Costa Rica pathology diagnoses additionally reviewed all slides. A second pathologist in the United States reviewed any discrepant diagnoses (43% of all samples); the final diagnosis was assigned based on majority rule.
During the randomised phase of CVT, HPV DNA testing was performed on cervical samples using the SPF10 PCR Primer System and a DNA enzyme immunoassay with a line probe assay. HPV genotyping was performed on positive samples using reverse hybridisation. During the extended follow-up phase, HPV DNA testing was replaced with TypeSeq and HPV genotyping was performed using the Ion S5 next-generation sequencing and a custom Torrent Suite plugin analysis (Thermo Fisher Scientific, Waltham, MA, USA). More information on HPV DNA testing and genotyping are presented in the appendix p1.
Outcomes
One of the prespecified primary outcomes of CVT’s long-term follow-up study was the long-term safety and impact of HPV 16/18 vaccination, including vaccine efficacy against precancers associated with non-preventable HPV types (primary outcome 1b, appendix p188). Serious adverse events during the long-term follow-up (i.e., safety of the vaccine) have been previously reported.16 Here, the primary outcomes were histologically-confirmed incident CIN2+ and CIN3+ attributed to non-preventable types defined as any detected HPV type except HPV16/18/31/33/45 (see appendix p1 for the list of genotypes detected by our assay). To evaluate clinical unmasking, we additionally investigated histologically-confirmed incident CIN2+ and CIN3+: 1) irrespective of HPV type (primary outcome 1ei, appendix p188); 2) attributed to vaccine types (HPV16/18) (primary outcome 1ai, appendix p188); and 3) attributed to cross-protected types (HPV31/33/45, excluding HPV16/18 coinfection) (primary outcome 1aii, appendix p188). HPV type attribution was based on HPV genotypes detected in the cervical sample immediately preceding the histological diagnosis. Women with multiple HPV infections were assigned based on hierarchical carcinogenicity.8 We also examined a post-hoc virological endpoint, long-term incident HPV persistence, defined as two or more consecutive type-specific positive HPV tests at least 2 years apart with no intervening negatives (appendix p1). Short-term virological endpoints were previously analyzed in virological replacement studies.9,17
Statistical analyses
We described characteristics of women in the HPV-vaccine arm and the UCG at the year 7 visit, including age, marital status, lifetime sexual partners, HPV positivity, median follow-up time per woman during years 7-11, and median clinic visits per woman. Data on race/ethnicity were not collected. In prior publications, we reported baseline characteristics of the HPV-vaccine and control arms,14 and characteristics of the control arm and UCG at year 4.15 Sample size and power calculations for the randomised, blinded phase of the trial are reported elsewhere.14 For the observational long-term follow-up phase, the target enrollment for unvaccinated women was 3000 to provide a comparable sample size to the original control arm in CVT.15 The analytic approach for our primary outcomes was prespecified in the long-term follow-up study protocol (appendix p233-235). Because unmasking may occur later in follow-up when less aggressive HPV genotypes cause disease, our primary analytical period was years 7-11, the observational phase of the trial. Women in the control arm who received HPV vaccination after year 4 were not included during years 7-11. We also examined an early analytical period (years 1-4) and both periods combined to estimate the total impact of cervical cancer screening in vaccinated and unvaccinated young adult women. Eligibility criteria for each analytical period included all women with at least one follow-up visit in the respective period and excluded women with a prior endpoint (i.e., modified intention-to-treat cohort). For each outcome, women were followed until the endpoint was reached or a LEEP was performed.
We reported the incidence proportion (attack rate) and corresponding 95% confidence intervals (95%CIs) of all outcomes in the vaccinated and unvaccinated groups during years 1-4, 7-11, and all years combined. We also reported VEs, absolute rate differences (ARDs), and corresponding 95%CIs for each outcome. Analyses for persistence were conducted at the infection level; therefore, VEs and ARDs were calculated using generalized estimating equation corrections to account for correlations of different HPV types within the same woman. All calculations are described in the appendix p1-2.
Several post-hoc analyses were conducted. To provide supporting evidence for the proposed mechanism of unmasking, we examined the association between 1) HPV vaccination and LEEPs prior to year 7, and 2) LEEPs prior to year 7 among HPV-vaccinated women and CIN2+ attributed to non-preventable types in years 7-11, using Fisher’s exact tests. We further quantified HPV genotype distribution among the CIN2+ and CIN3+ events caused by non-preventable types in the HPV-vaccine arm in years 7-11 (i.e., unmasked events). Because multivalent HPV vaccines are available, we assigned type attribution according to the protection afforded by Gardasil®9, the currently approved nonavalent vaccine, which covers non-carcinogenic types (HPV6/11) and carcinogenic types (HPV16/18/31/33/45/52/58). Among events caused by non-preventable types by the bivalent AS04-adjuvanted vaccine (any type except HPV16/18/31/33/45), we categorized type attribution into mutually exclusive groups: 1) other carcinogenic types protected by the nonavalent vaccine (HPV52/58 only, without any other types); 2) other carcinogenic types, as classified by the International Agency for Research on Cancer, not protected by the nonavalent vaccine (any HPV35/39/51/56/59); 3) any non-carcinogenic type only (including types that are phylogenetically related to known carcinogenic types, which have been shown in rare cases of cervical cancer8); and 4) unknown (HPV type could not be determined due to genotyping failure).
The primary analytical period and full study follow-up over 11 years are not randomised analyses because of the observational UCG recruited for the long-term follow-up. Therefore, in our post-hoc analyses, we evaluated two biases that may have occurred. First, because women in UCG were not as actively screened prior to enrollment, more disease could have accumulated in UCG by the year 7 analysis compared to the control and HPV-vaccine arms, in which regular screening follow-up could detect disease earlier. To evaluate detection bias, we compared cumulative proportions of CIN2+ and CIN3+ events between the control arm (years 0-6) and UCG (years 4-6) prior to year 7, using a Fisher’s exact test. Because the control arm is intended to represent the counterfactual of the HPV-vaccine arm, the cumulative disease proportions for UCG should also represent the control arm to ensure comparability between the HPV-vaccine arm and UCG in years 7-11. Second, the cervical screening/clinical management protocol applied to the UCG at study entry was deliberately stringent but could have resulted in excess LEEPs, thus preventing some CIN2+ from developing, resulting in artificially low VEs. To evaluate this treatment bias, we compared proportions of treatment with LEEPs in women prior to year 7 between the HPV-vaccine arm and both control groups. We also evaluated the proportion of women who were truncated (removed from potentially reaching an endpoint), calculated by dividing the total number of LEEPs among women without CIN2+ by the total number of at-risk women at enrollment conclusion. Differential truncation proportions would be concerning because LEEPs in women without CIN2+ would remove the potential for disease in these at-risk women who could have reached an endpoint had they not received a LEEP (“truncation”). We focused our evaluation of truncation between the HPV-vaccine arm and UCG because our primary analytical period was years 7-11. If screening procedures were similar between groups, the proportion of truncated women should be similar. P-values<0·05 were considered statistically significant. Statistical analyses were conducted in SAS (version 9.4). The CVT and long-term follow-up study are registered with Clinicaltrials.gov [NCT00128661, NCT00867464].
Role of the funding source
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the US National Cancer Institute (NCI). The trial is sponsored and funded by the NCI (contract N01-CP-11005) with funding support from the National Institutes of Health Office of Research on Women’s Health. GlaxoSmithKline Biologicals provided vaccine and support for aspects of the trial associated with regulatory submission needs of the company under a Clinical Trials Agreement (FDA BB-IND 7920) during the four-year, randomised blinded phase of the study, but had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The NCI and Costa Rica investigators are responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. All authors have access to the raw data.
Results
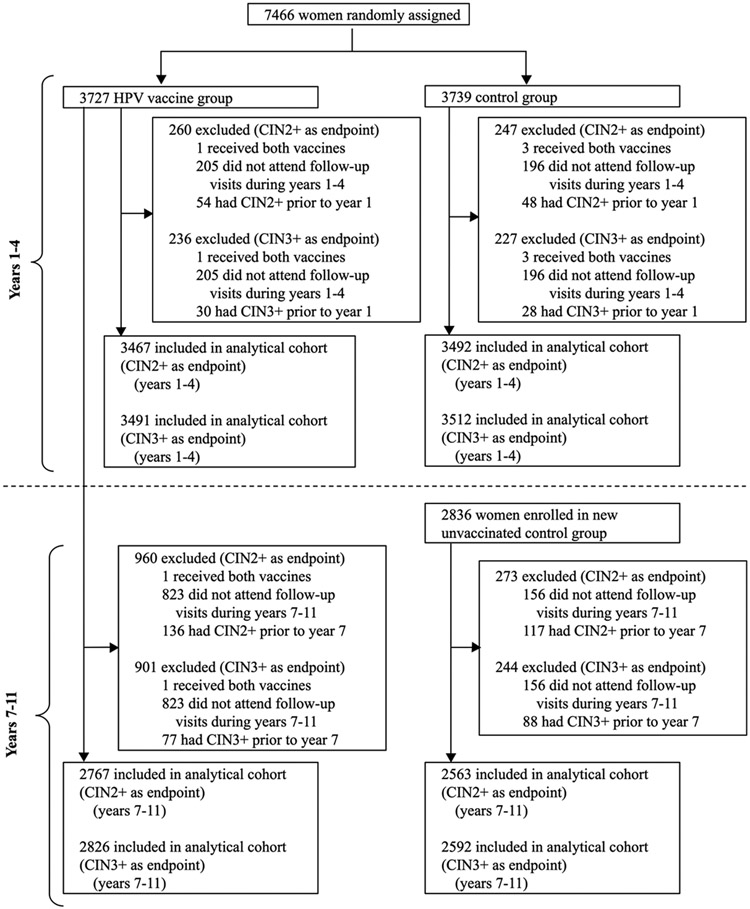
A total of 7466 women were enrolled in CVT (HPV-vaccine arm=3727; control arm=3739) from June 28, 2004, through December 21, 2005 (figure 1). Between March 30, 2009, and July 5, 2012, 2836 women were enrolled in the new UCG. After excluding women who received both vaccines, did not attend follow-up visits, and already had a prior endpoint, 3467 women from the HPV-vaccine arm and 3492 from the control arm were included in the analytical cohort for the CIN2+ endpoint in years 1-4. For the CIN3+ endpoint, the analytical cohort for years 1-4 included 3491 women from the HPV-vaccine arm and 3512 from the control arm. For the long-term follow-up phase, 2767 women in the HPV-vaccine arm and 2563 in UCG were included in the analytical cohort for the CIN2+ endpoint in years 7-11. For the CIN3+ endpoint, the analytical cohort for years 7-11 included 2826 women from the HPV-vaccine arm and 2592 from UCG.
Figure 1. Study profile.
CIN = cervical intraepithelial neoplasia.
At the year 7 visit, more women in the HPV-vaccine arm were single (not married, widowed, or divorced) compared to UCG (table 1). There were small differences in age distribution and lifetime number of sexual partners. However, women in the HPV-vaccine arm and UCG at year 7 were similar in their HPV positivity (except for HPV 16/18 because of vaccination and other carcinogenic HPV, suggesting an early signal of unmasking). Median follow-up time during years 7-11 was 52·8 months (interquartile range 44·0-60·7) for the HPV-vaccine arm and 49·8 months (interquartile range 42·0-56·9) for the UCG.
Table 1.
Descriptive characteristics of the HPV vaccine arm and new unvaccinated control group at the year 7 visit (long-term follow-up study month 24 visit).
| Characteristic | HPV Vaccine (N = 2767) |
UCG (N = 2563) |
|---|---|---|
| Age, years, n (%) | ||
| <25 | 103 (4·0) | 74 (3·1) |
| 25-26 | 629 (24·2) | 493 (20·8) |
| 27-28 | 635 (24·4) | 616 (26·0) |
| 29+ | 1233 (47·4) | 1189 (50·1) |
| Median, IQR (range) | 28, 26-30 (24-36) | 29, 27-31 (23-35) |
| Did not attend visit | 167 | 191 |
| Marital status, n (%) | ||
| Single | 721 (27·7) | 434 (18·3) |
| Married | 1730 (66·5) | 1747 (73·7) |
| Widowed/divorced | 149 (5·7) | 190 (8·0) |
| No answer/did not attend visit | 167 | 192 |
| Lifetime sexual partners, n (%) | ||
| 0 | 85 (3·3) | 46 (1·9) |
| 1 | 600 (23·1) | 574 (24·2) |
| 2 | 449 (17·3) | 459 (19·4) |
| 3+ | 1466 (56·4) | 1292 (54·5) |
| Did not attend visit | 167 | 192 |
| HPV positivity, n (%) a | ||
| HPV 16/18 | 26 (1·0) | 151 (6·4) |
| HPV 31/33/45 | 62 (2.4) | 123 (5.2) |
| Other carcinogenic HPV (non-HPV 16/18/31/33/45) | 395 (15·2) | 302 (12·7) |
| Non-carcinogenic HPV | 559 (21·5) | 467 (19·7) |
| No HPV | 1682 (64·8) | 1532 (64·6) |
| No result/did not attend visit | 172 | 192 |
| Follow-up time during years 7-11 | ||
| Median total follow-up time per woman, months, IQR (range) | 52·8, 44·0-60·7 (0·0-110·3) | 49.8, 42.0-56.9 (0·0-104·5) |
| Median total clinic visits per woman, IQR (range) | 3, 3-4 (1-14) | 3, 3-4 (1-14) |
IQR = interquartile range; HPV = human papillomavirus; UCG = unvaccinated control group.
The sum of the percentages for HPV positivity does not equal 100 because women who had multiple type infections were counted in multiple rows.
During years 7-11, we observed significantly higher rates of CIN2+ attributed to non-preventable types (without HPV16/18/31/33/45) in the HPV-vaccine arm (61 events) compared to UCG (33 events), resulting in negative VE for these types [VE=−71·2% (95%CI −164%, −12·5%)] (table 2). Similarly, VE against CIN3+ attributed to non-preventable types during years 7-11 was −135% (95%CI −330%, −33·5%) (41 versus 16 events) (table 3). Unmasking was not observed during earlier years of follow-up, with similar rates of CIN2+/CIN3+ attributed to non-preventable types between the HPV-vaccine and control arms in years 1-4 (tables 2 and 3). For the virological endpoint, we observed a non-significant negative VE against ≥2-year persistent HPV infection with non-preventable types in years 7-11 [VE=−24·4% (95%CI −61·2%, 4·0%)] (151 versus 117 events) (appendix p3).
Table 2.
Bivalent HPV vaccine efficacy against incident CIN2+ by HPV types among women with at least 1 follow-up visit during each analytical period and no endpoint prior to each analytical period.
| Years 1-4 |
Years 7-11 |
Combined |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV Vaccine (N = 3467) |
Control (N = 3492) |
Vaccine Efficacy |
Absolute Rate Difference |
HPV Vaccine (N = 2767) |
UCG (N = 2563) | Vaccine Efficacy | Absolute Rate Difference |
HPV Vaccine |
Control | Vaccine Efficacy |
Absolute Rate Difference |
|||||
| HPV Type | # | Attack Rate (95% CI) |
# | Attack Rate (95% CI) |
% (95% CI) | Δ (95% CI) | # | Attack Rate (95% CI) |
# | Attack Rate (95% CI) |
% (95% CI) | Δ (95% CI) | Attack Rate (95% CI) |
Attack Rate (95% CI) |
% (95% CI) | Δ (95% CI) |
| Irrespective of HPV type | 102 | 29·4 (24·2, 35·5) | 153 | 43·8 (37·4, 51·0) | 32·9% (13·8%, 47·8%) | −14·4 (−23·0, −5·4) | 80 | 28·9 (23·1, 35·7) | 109 | 42·5 (35·2, 50·9) | 32·0% (9·3%, 49·2%) | −13·6 (−23·4, −3·5) | 57·5 (49·3, 65·7) | 84·5 (74·5, 94·5) | 32·0% (18·1%, 43·7%) | −27·0 (−39·9, −14·2) |
| HPV 16/18 | 38 | 11·0 (7·9, 14·9) | 83 | 23·8 (19·1, 29·2) | 53·9% (32·6%, 68·9%) | −12·8 (−18·2, −6·8) | 5 | 1·8 (0·7, 4·0) | 47 | 18·3 (13·7, 24·1) | 90·1% (76·8%, 96·5%) | −16·5 (−18·9, −12·5) | 12·7 (8·9, 16·5) | 41·3 (34·3, 48·4) | 69·2% (57·3%, 78·9%) | −28·6 (−36·7, −20·6) |
| HPV 31/33/45 (excluding HPV 16/18 coinfection) | 21 | 6·1 (3·9, 9·1) | 27 | 7·7 (5·2, 11·1) | 21·7% (−38·8%, 56·2%) | −1·7 (−5·4, 2·2) | 14 | 5·1 (2·9, 8·3) | 29 | 11·3 (7·7, 16·0) | 55·3% (16·1%, 77·0%) | −6·3 (−10·4, −1·4) | 11·0 (7·3, 14·6) | 18·6 (13·7, 23·4) | 40·9% (9·9%, 62·8%) | −7·6 (−13·9, −1·5) |
| Without HPV 16/18/31/33/45 | 43 | 12·4 (9·1, 16·5) | 43 | 12·3 (9·0, 16·4) | −0·7% (−54·1%, 34·2%) | 0·1 (−5·1, 5·3) | 61 | 22·0 (17·1, 28·0) | 33 | 12·9 (9·0, 17·8) | −71·2% (−164%, −12·5%) | 9·2 (2·1, 15·6) | 33·8 (27·4, 40·2) | 24·6 (19·1, 30·2) | −37·3% (−86·0%, −2·7%) | 9·2 (0·8, 17·8) |
CI = confidence interval; CIN = cervical intraepithelial neoplasia; HPV = human papillomavirus; UCG = unvaccinated control group.
Rates are expressed as per 1,000 women.
Table 3.
Bivalent HPV vaccine efficacy against incident CIN3+ by HPV types among women with at least 1 follow-up visit during each analytical period and no endpoint prior to each analytical period.
| Years 1-4 |
Years 7-11 |
Combined |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV Vaccine (N = 3491) |
Control (N = 3512) |
Vaccine Efficacy | Absolute Rate Difference |
HPV Vaccine (N = 2826) |
UCG (N = 2592) | Vaccine Efficacy | Absolute Rate Difference |
HPV Vaccine |
Control | Vaccine Efficacy | Absolute Rate Difference |
|||||
| HPV Type | # | Attack Rate (95% CI) |
# | % (95% CI) |
% (95% CI) | · (95% CI) | # | Attack Rate (95% CI) |
# | Attack Rate (95% CI) |
% (95% CI) |
· (95% CI) | Attack Rate (95% CI) |
Attack Rate (95% CI) |
% (95% CI) | · (95% CI) |
| Irrespective of HPV type | 58 | 16·6 (12·8, 21·3) | 78 | 22·2 (17·7, 27·5) | 25·2% (−5·0%, 46·9%) | −5·6 (−11·9, 0·9) | 56 | 19·8 (15·1, 25·5) | 60 | 23·1 (17·9, 29·5) | 14·4% (−23·4%, 40·7%) | −3·3 (−11·1, 4·5) | 36·1 (29·5, 42·7) | 44·8 (37·4, 52·3) | 19·5% (−3·3%, 37·5%) | −8·7 (−18·6, 1·3) |
| HPV 16/18 | 22 | 6·3 (4·1, 9·4) | 47 | 13·4 (10·0, 17·6) | 52·9% (22·4%, 72·1%) | −7·1 (−11·1, −2·5) | 4 | 1·4 (0·4, 3·4) | 28 | 10·8 (7·3, 15·4) | 86·9% (65·3%, 96·1%) | −9·4 (−11·4, −5·8) | 7·7 (4·7, 10·7) | 23·9 (18·5, 29·4) | 67·9% (51·1%, 80·4%) | −16·3 (−22·5, −10·2) |
| HPV 31/33/45 (excluding HPV 16/18 coinfection) | 15 | 4·3 (2·5, 6·9) | 13 | 3·7 (2·1, 6·2) | −16·1% (−149%, 45·3%) | 0·6 (−2·3, 3·4) | 11 | 3·9 (2·0, 6·8) | 16 | 6·2 (3·7, 9·8) | 36·9% (−36·2%, 71·6%) | −2·3 (−5·7, 1·5) | 8·1 (5·0, 11·3) | 9·7 (6·2, 13·3) | 16·6% (−40·6%, 52·4%) | −1·6 (−6·4, 2·9) |
| Without HPV 16/18/31/33/45 | 21 | 6·0 (3·8, 9·0) | 18 | 5·1 (3·1, 7·9) | −17·4% (−123%, 37·8%) | 0·9 (−2·6, 4·3) | 41 | 14·5 (10·6, 19·4) | 16 | 6·2 (3·7, 9·8) | −135% (−330%, −33·5%) | 8·3 (3·0, 12·8) | 20·3 (15·3, 25·3) | 11·2 (7·4, 14·9) | −81·7% (−191%, −19·9%) | 9·1 (2·8, 15·5) |
CI = confidence interval; CIN = cervical intraepithelial neoplasia; HPV = human papillomavirus; UCG = unvaccinated control group.
Rates are expressed as per 1,000 women.
To address the possible public health impact, we examined ARDs by HPV type through 11 years. When combining the two follow-up periods, we estimated that, per 1000 women, there were 9·2 (95%CI 0·8, 17·8) additional CIN2+ events attributable to non-preventable HPV types in vaccinated versus unvaccinated women (table 2). In comparison, per 1000 women, we observed 28·6 (95%CI 20·6, 36·7) fewer HPV16/18-associated CIN2+ and 7·6 (95%CI 1·5, 13·9) fewer HPV31/33/45-associated CIN2+ in vaccinated versus unvaccinated women. When considering the observed reductions in CIN2+ attributed to vaccine-targeted types and cross-protected types, the expected overall reduction in CIN2+ is approximately −36·2 per 1000 women (sum of reductions in both groups). However, the unmasking effect attenuated the observed overall reduction to 27·0 (95%CI 14·2, 39·9) fewer CIN2+ events per 1000 women irrespective of HPV type. For CIN3+, over 11 years post-vaccination, the unmasking effect resulted in 9·1 (95%CI 2·8, 15·5) additional CIN3+ events attributed to non-preventable HPV types per 1000 vaccinated women compared to unvaccinated (table 3). ARDs for CIN3+ irrespective of type and HPV31/33/45-associated CIN3+ through 11 years were not statistically significant, and qualitatively, these point estimates were not as pronounced for CIN3+ compared to CIN2+.
We observed supporting evidence for the proposed mechanism for unmasking to occur for histological endpoints: 1) fewer cumulative LEEPs were performed in HPV-vaccinated (221 of 3736, 5·9%) versus unvaccinated (277 of 3736, 7·4%) women prior to year 7 (Fisher’s exact test p=0.01, appendix p4); and 2) among HPV-vaccinated women, 0 (0%) CIN2+ events attributed to non-preventable types during years 7-11 were among 170 women who received LEEPs prior to year 7, compared to 63 (2·3%) events among 2713 women without LEEPs prior to year 7 (Fisher’s exact test p=0.03, appendix p5).
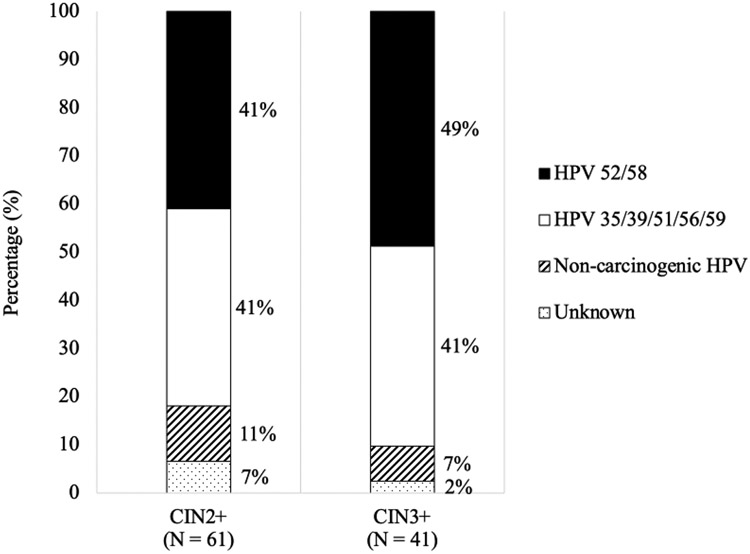
During years 7-11, 61 total CIN2+ events were caused by non-preventable types in HPV-vaccinated women during the long-term follow up period (i.e., unmasked lesions). Of these 61 events, 25 (41%) were attributed to HPV52/58 (types covered by the nonavalent vaccine), 25 (41%) to carcinogenic types not covered by the nonavalent vaccine, 7 (11%) could not be attributed to a carcinogenic type, and 4 (7%) were unknown (figure 2). For CIN3+, 41 total events were unmasked, of which 20 (49%) were attributed to HPV52/58, 17 (41%) to carcinogenic types not covered by the nonavalent vaccine, 3 (7%) could not be attributed to a carcinogenic type, and 1 (2%) was unknown. More granular distributions of HPV types in CIN2+/CIN3+ attributed to non-preventable types by period and study arm are in the appendix p6 and p10.
Figure 2. Distribution of HPV types in “unmasked” CIN2+ and CIN3+ events caused by non-vaccine-preventable types in the HPV vaccine group in years 7-11.
CIN = cervical intraepithelial neoplasia.
In evaluating detection bias, we observed 203 (5·4%) of 3736 women were diagnosed with CIN2+ in the control arm compared to 120 (4·2%) of 2836 in UCG prior to year 7 (Fisher’s exact test p=0.03, appendix p7). The cumulative proportion of CIN3+ diagnoses prior to year 7 was 2·9% (108 of 3736) in the control arm compared to 3·2% (90 of 2836) in UCG (Fisher’s exact test p=0.51). In evaluating treatment bias, we observed 153 (69·2%) of 221 total LEEPs in the HPV-vaccine arm had CIN2+ compared to 194 (70·0%) of 277 LEEPs in the control arm and 119 (75·3%) of 158 LEEPs in UCG prior to year 7 (appendix p8). Of the 3726 women in the HPV-vaccine arm, 68 received a LEEP that resulted in no CIN2+ diagnosis, indicating that 1·8% of women in the HPV-vaccine arm were potentially truncated from the year 7-11 analysis, compared to 1·4% for UCG (Fisher’s exact test p=0·17). We further investigated patterns of LEEPs prior to year 7 and found that LEEPs were not preferentially performed by HPV type and study arm (appendix p9).
Discussion
To our knowledge, this is the first study to suggest that unmasking of high-grade cervical neoplasia caused by non-targeted/non-cross-protected HPV types may occur following HPV vaccination. Specifically, HPV16/18-vaccinated women had significantly higher rates of CIN2+ and CIN3+ attributed to non-preventable HPV types compared to unvaccinated women in years 7-11, resulting in negative VE. Despite observing a large negative VE, ARDs between vaccinated and unvaccinated women only showed moderate increases (relative to the large negative VE) in events caused by non-preventable types. The absence of unmasking in earlier years of follow-up supports previous observations that non-preventable types are less aggressive.13
While most studies showed a lack of evidence for type replacement,9 particularly in clinical trials, some studies had inconclusive findings.18 We postulate that our results are more likely an observation of unmasking and not viral replacement, as the findings corroborate the proposed mechanism for unmasking to occur such that HPV vaccination can lead to decreases in cervical procedures, which allows non-preventable HPV types to manifest as lesions many years following vaccination. We reported that HPV vaccination was associated with subsequently fewer LEEPs, and LEEPs among HPV-vaccinated women were significantly associated with decreased incidence of non-preventable type CIN2+.
Real-world effectiveness studies have shown a nearly 90% reduction in invasive cervical cancer in women vaccinated at young ages,19-21 including among bivalent AS04-adjuvanted HPV vaccinees, which exceeds the expected protection based on attribution of HPV genotypes in cervical cancers. These effectiveness estimates will likely shift over time due to the differential timing of the progression of non-preventable HPV types to cervical cancer. Thus, we hypothesize the full impact of unmasking on invasive cervical cancer will not be observed until much longer follow-up time has accrued. Further, we note that the unmasking effect may differ based on vaccine product. For instance, we speculate unmasking may be reduced among nonavalent vaccinees, as we observed that among bivalent HPV vaccinees, nearly half of the unmasked CIN2+ and CIN3+ events were attributed to HPV52/58, the two additional carcinogenic types targeted by the currently licensed nonavalent vaccine.
Our findings suggest that in populations where cervical cancer screening is routinely and effectively conducted, the vaccine impact against cervical disease irrespective of HPV type may decline over time due to increases in disease attributed to non-preventable types following vaccination. Yet, we reiterate that the high net benefit to cervical cancer prevention will persist. Specifically, the vaccine has high efficacy against vaccine-targeted types HPV16/18, the most carcinogenic types due to their disproportionally high prevalence and attributable fraction in cancer cases.2,8,22 Non-preventable types are less likely to progress to cancer, even relative to their rate of development to CIN2 or CIN3.22 As HPV vaccination continues to become more widespread and follow-up lengthens, potential shifts in the type distribution in pre-cancers and cancers may be partially explained by unmasking, as suggested here and in a study that used modeling to estimate the apparent increase in cervical precancers and cancers attributed to non-preventable-types due to unmasking.23 Future effectiveness studies with longer follow-up and cervical cancer as the endpoint will definitively address whether unmasking occurs. Studies modeling the net effect of unmasking on the reduction of relevant endpoints will inform expectations of the impact on relevant endpoints.
Our bias assessment confirmed that replacing the control arm with an observational control did not induce our observation of unmasking. As previously reported, the control arm and UCG varied in education, marital status, and number of pregnancies.15 However, the predicted future risk of HPV infection was similar between groups, indicating that the observed differences likely did not impact HPV exposure.15 Further, the cumulative CIN3+ detection prior to year 7 was similar between the control arm and UCG, suggesting that the intensive screening and management protocol in years 4-6 appropriately identified prevalent disease. The cumulative proportion of CIN2+ events prior to year 7 was slightly lower in UCG compared to the control arm though, we do not believe this caused a meaningful difference in the results. Additionally, the proportion of LEEPs in the UCG and HPV arm were qualitatively similar, suggesting that the screening and management protocol in years 4-6 did not excessively remove disease in the UCG to induce large negative VEs in years 7-11. Lastly, the truncation proportion (removal of at-risk women without CIN2+ who could have had an endpoint had they not received a LEEP) between groups was generally non-differential.
Our study has limitations. Because endpoints in CVT were truncated at high-grade cervical lesions for treatment and management, we were unable to evaluate the impact of unmasking on cancer. We were also unable to test tissue DNA due to financial constraints therefore, attributing HPV causation of the lesions based on the HPV genotype in the proceeding cervical sample could have resulted in misclassification beyond what testing the tissue could have achieved. Further, our study used standard of care for cervical cancer screening during the time in which our study was conducted. However, presently, screening more often utilizes HPV co-testing or HPV testing alone as a measure of primary screening. Because HPV testing is more sensitive than cytology testing,24 our study may have yielded a stronger unmasking effect than in populations that incorporate HPV testing into their screening procedures, which could mitigate unmasking by prompting more clinical intervention in vaccinated women. Our study had notable strengths, including a robust study design with long-term follow-up of vaccinated and unvaccinated women, high retention rates, compliance of specimen collection procedures (>90%), and active follow-up.
In conclusion, our study provides proof-of-concept that unmasking may attenuate the anticipated long-term reductions in cervical precancer following successful implementation of HPV vaccination programs in highly screened populations. Additional follow-up will better elucidate the impact of unmasking on cervical precancer and cancer. Importantly, despite the observation of unmasking, the long-term net benefit of vaccination against precancerous cervical lesions irrespective of type remains considerable through 11 years. Therefore, HPV vaccination should still be prioritized as primary prevention for cervical cancer.
Supplementary Material
Research in Context.
The bivalent AS04-adjuvanted human papillomavirus (HPV) vaccine is highly effective against HPV16/18-associated cervical intraepithelial neoplasia grade 2 or worse (CIN2+) in HPV-naïve women and provides protection against phylogenetically-related oncogenic types, including HPV31, 33, and 45. The elimination of vaccine-targeted HPV types is unlikely to lead to virological replacement with non-targeted types because multiple infections are common, and HPV genotypes do not appear to compete for a common niche. However, the possibility remains that vaccination could lead to increases in disease caused by non-vaccine-preventable types through a distinct phenomenon termed “unmasking”, a result of fewer prior treatments of the cervical transformation zone for vaccine-preventable HPV-associated precancers in the vaccinated population. We searched PubMed from June 8, 2006, to August 1, 2021, for studies on the clinical unmasking of cervical precancers attributed to non-vaccine-preventable types. We included any publications containing the following search terms in the abstract or title: “(HPV AND unmasking); (HPV AND non-preventable types); (HPV AND vaccine efficacy AND non-preventable types)”. To our knowledge, only one study has directly examined unmasking in the context of HPV, which used mathematical modeling to estimate increases in cervical precancers attributed to non-preventable types due to unmasking. Because carcinogenic HPV genotypes that are not prevented by HPV vaccination are less aggressive and require long-term follow-up postvaccination to be able to observe high-grade disease endpoints, no studies that we are aware of have been able to directly investigate unmasking in an observational study within a larger clinical trial setting.
Added value of this study.
We report the long-term efficacy of the bivalent AS04-adjuvanted HPV vaccine against cervical intraepithelial neoplasia (CIN) grade 2 or worse (CIN2+) and CIN grade 3 or worse (CIN3+) attributed to non-vaccine-targeted/non-cross-protected HPV types (any type except HPV16/18/31/33/45, i.e., non-preventable types) through 11 years post-initial-vaccination in the Costa Rica HPV Vaccine Trial (CVT). In the long-term follow-up of CVT (years 7-11), we observed significantly higher rates of CIN2+ and CIN3+ attributed to non-preventable HPV types in vaccinated versus unvaccinated women, resulting in negative vaccine efficacy for these types. Importantly, despite the observation of unmasking, vaccinated women still had long-term absolute reductions in high-grade lesions irrespective of type women through 11 years. Given CVT’s 11 years of active follow-up, this is the first study, to our knowledge, to be able to evaluate clinical unmasking of precancerous cervical lesions attributed to non-vaccine-preventable HPV types in vaccinated women.
Implications of all the available evidence.
This long-term follow-up analysis of the Costa Rica HPV Vaccine Trial provides proof-of-concept that the clinical unmasking of cervical precancer caused by non-vaccine-preventable HPV types could attenuate long-term reductions in high-grade cervical precancers following successful implementation of HPV vaccination programs in screened populations. However, the increase in high-grade cervical neoplasia due to non-preventable types have a lower potential for progression to cancer given their lower carcinogenicity than the vaccine-preventable types. Importantly, the net protection of the HPV vaccine against high-grade cervical precancers irrespective of type remained considerable, emphasizing the importance of vaccination for cervical cancer prevention.
Acknowledgements
The Costa Rica HPV Vaccine Trial is a long-standing collaboration between investigators in Costa Rica and the NCI. The trial is sponsored and funded by the NCI (contract N01-CP-11005), with funding support from the National Institutes of Health (NIH) Office of Research on Women's Health. GlaxoSmithKline Biologicals (GSK) provided vaccine and support for aspects of the trial associated with regulatory submission needs of the company under a Clinical Trials Agreement (FDA BB-IND 7920) during the four-year, randomised blinded phase of our study. Jaimie Shing, Allan Hildesheim, Joshua Sampson, John Schiller, Douglas Lowy, Mónica Sierra, and Aimée Kreimer were employees of the NIH when the majority of this work was done. Rolando Herrero was an employee of the International Agency for Research on Cancer (IARC) when most of his contributions to this study were done. Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization. We extend a special thanks to the women of Guanacaste and Puntarenas, Costa Rica, who gave of themselves in participating in this effort. In Costa Rica, we acknowledge the tremendous effort and dedication of the staff involved in this project; we would like to specifically acknowledge the meaningful contributions by Carlos Avila, Loretto Carvajal, Rebeca Ocampo, Cristian Montero, Diego Guillen, and Mario Alfaro. In the United States, we extend our appreciation to the team from Information Management Services (IMS) responsible for the development and maintenance of the data system used in the trial and who serve as the data management center for this effort, especially Jean Cyr, Julie Buckland, John Schussler, and Brian Befano. We thank Dr. Diane Solomon (CVT: medical monitor & QC pathologist) for her invaluable contributions during the randomised blinded phase of the trial and the design of the LTFU and Nora Macklin (CVT) and Kate Torres (LTFU) for the expertise in coordinating the study. We thank the members of the Data and Safety Monitoring Board charged with protecting the safety and interest of participants during the randomised, blinded phase of our study (Steve Self, Chair, Adriana Benavides, Ruth Karron, and Ritu Nayar) and members of the external Scientific HPV Working Group who have contributed to the success of our efforts over the years (Henriette Raventós, Chair, Joanna Cain, Diane Davey, Gypsyamber D’Souza, Wasima Rida, Richard Roden, Maria del Rocío Sáenz Madrigal, and Margaret Stanley).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
JTS and DRL report that they are named inventors on US Government-owned HPV vaccine patents with expired licenses to GlaxoSmithKline and Merck. The other authors declare that they have no conflicts of interest.
Contributor Information
Jaimie Z Shing, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
Shangying Hu, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA; Department of Cancer Epidemiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Rolando Herrero, Agencia Costarricense de Investigaciones Biomédicas (ACIB), Fundación INCIENSA, San José, Costa Rica; Early Detection and Prevention Section, International Agency for Research on Cancer, World Health Organization, Lyon, France.
Allan Hildesheim, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
Carolina Porras, Agencia Costarricense de Investigaciones Biomédicas (ACIB), Fundación INCIENSA, San José, Costa Rica.
Joshua N Sampson, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
John Schussler, Information Management Services, Silver Spring, Maryland, USA.
John T. Schiller, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
Douglas R. Lowy, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
Mónica S Sierra, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
Loretto Carvajal, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA; Agencia Costarricense de Investigaciones Biomédicas (ACIB), Fundación INCIENSA, San José, Costa Rica.
Aimée R Kreimer, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA.
Data Sharing
Participant data can be shared with outside collaborators for research to understand more about the performance of the HPV vaccine, immune response to the vaccine, and broader study factors associated with the natural history of HPV infection and risk factors for infection and disease. Outside collaborators can apply to access our data from the blinded phase of the Costa Rica Vaccine Trial (NCT00128661). Outside collaborators can apply for access to the data online. Data for the long-term follow-up phase are not yet available. Study protocols are provided in the appendix. A trial summary, current publications, and contact information are available online at https://dceg.cancer.gov/research/who-we-study/cohorts/costa-rica-vaccine-trial.
References
- 1.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological Agents. International Agency for Research on Cancer, 2012. [Google Scholar]
- 2.de Sanjosé S, Serrano B, Tous S, et al. Burden of human papillomavirus (HPV)-related cancers attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI Cancer Spectr 2019; 2. DOI: 10.1093/jncics/pky045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev 2018; 5: CD009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsang SH, Sampson JN, Schussler J, et al. Durability of cross-protection by different schedules of the bivalent HPV vaccine: the CVT trial. JNCI J Natl Cancer Inst 2020; 112: 1030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheeler CM, Castellsagué X, Garland SM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13: 100–10. [DOI] [PubMed] [Google Scholar]
- 6.Kreimer AR, Sampson JN, Porras C, et al. Evaluation of durability of a single dose of the bivalent HPV vaccine: the CVT trial. J Natl Cancer Inst 2020; 112: 1038–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsang SH, Schiller JT, Porras C, et al. HPV16 infection decreases vaccine-induced HPV16 antibody avidity: the CVT trial. npj Vaccines 2022; 7: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbyn M, Tommasino M, Depuydt C, Dillner J. Are 20 human papillomavirus types causing cervical cancer? J Pathol 2014; 234: 431–5. [DOI] [PubMed] [Google Scholar]
- 9.Tota JE, Struyf F, Merikukka M, et al. Evaluation of type replacement following HPV16/18 vaccination: pooled analysis of two randomized trials. J Natl Cancer Inst 2017; 109. DOI: 10.1093/jnci/djw300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Man I, Vänskä S, Lehtinen M, Bogaards JA. Human papillomavirus genotype replacement: still too early to tell? The Journal of Infectious Diseases 2021; 224: 481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination: a discussion of the evidence. Lancet Lond Engl 2011; 378: 1962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipsitch M Interpreting results from trials of pneumococcal conjugate vaccines: a statistical test for detecting vaccine-induced increases in carriage of nonvaccine serotypes. Am J Epidemiol 2001; 154: 85–92. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto K, Oki A, Furuta R, et al. Predicting the progression of cervical precursor lesions by human papillomavirus genotyping: a prospective cohort study. Int J Cancer 2011; 128: 2898–910. [DOI] [PubMed] [Google Scholar]
- 14.Herrero R, Hildesheim A, Rodríguez AC, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine 2008; 26: 4795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez P, Hildesheim A, Herrero R, et al. Rationale and design of a Long Term Follow-up study of women who did and did not receive HPV 16/18 vaccination in Guanacaste, Costa Rica. Vaccine 2015; 33: 2141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porras C, Tsang SH, Herrero R, et al. Efficacy of the bivalent HPV vaccine against HPV 16/18-associated precancer: long-term follow-up results from the Costa Rica Vaccine Trial. The Lancet Oncology 2020; 21: 1643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tota JE, Ramanakumar AV, Jiang M, et al. Epidemiologic approaches to evaluating the potential for human papillomavirus type replacement postvaccination. Am J Epidemiol 2013; 178: 625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesher D, Soldan K, Lehtinen M, et al. Population-level effects of human papillomavirus vaccination programs on infections with nonvaccine genotypes. Emerg Infect Dis 2016; 22: 1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med 2020; 383: 1340–8. [DOI] [PubMed] [Google Scholar]
- 20.Kjaer SK, Dehlendorff C, Belmonte F, Baandrup L. Real-world effectiveness of human papillomavirus vaccination against cervical cancer. J Natl Cancer Inst 2021; 113: 1329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falcaro M, Castañon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. The Lancet 2021; 398: 2084–92. [DOI] [PubMed] [Google Scholar]
- 22.Bzhalava D, Guan P, Franceschi S, Dillner J, Clifford G. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology 2013; 445: 224–31. [DOI] [PubMed] [Google Scholar]
- 23.Choi YH, Chapman R, Gay N, Jit M. Potential overestimation of HPV vaccine impact due to unmasking of non-vaccine types: Quantification using a multi-type mathematical model. Vaccine 2012; 30: 3383–8. [DOI] [PubMed] [Google Scholar]
- 24.Bruhn LV, Andersen SJ, Hariri J. HPV-testing versus HPV-cytology co-testing to predict the outcome after conization. Acta Obstetricia et Gynecologica Scandinavica 2018; 97: 758–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Participant data can be shared with outside collaborators for research to understand more about the performance of the HPV vaccine, immune response to the vaccine, and broader study factors associated with the natural history of HPV infection and risk factors for infection and disease. Outside collaborators can apply to access our data from the blinded phase of the Costa Rica Vaccine Trial (NCT00128661). Outside collaborators can apply for access to the data online. Data for the long-term follow-up phase are not yet available. Study protocols are provided in the appendix. A trial summary, current publications, and contact information are available online at https://dceg.cancer.gov/research/who-we-study/cohorts/costa-rica-vaccine-trial.