Abstract
OBJECTIVES
This study sought to evaluate atrial fibrillation (AF) ablation outcomes based on scar patterns and contiguous area available for AF wavefronts to propagate.
BACKGROUND
The relevance of ablation scar pattern acting as a barrier for electrical propagation in recurrence after catheter ablation for persistent AF is unknown.
METHODS
Three-month post-ablation atrial cardiac magnetic resonance was used to determine post-ablation scar. The left atrium (LA) was divided into 5 areas based on anatomical landmarks and scar patterns. The length of gaps in scar on the area boundaries was used to calculate fibrillatory areas (FAs) by adding the weighted contribution of adjacent areas. Cylindrical as well as patient-specific computational models were used to further confirm findings.
RESULTS
A total of 75 patients that underwent an initial ablation for AF with 2 years of follow-up were included. The average maximum FA was 7,896 ± 1,988 mm2 in patients with recurrence (n = 40) and 6,559 ± 1,784 mm2 in patients without recurrence (n = 35) (p < 0.008). After redo ablation in 19 patients with recurrence, average maximum FA was 7,807 ± 1,392 mm2 in 9 patients with recurrence and 5,030 ± 1,765 mm2 in 10 without recurrence (p < 0.007). LA volume and total scar were not significant predictors of recurrence after the first ablation. In the cylindrical model, AF self-terminated after reducing the FAs. In the patient-specific models, simulation matched the clinical outcomes with larger FAs associated with post-ablation arrhythmia recurrences.
CONCLUSIONS
This data provides mechanistic insights into AF recurrence, suggesting that post-ablation scar pattern dividing the atria into smaller regions is an important and better predictor than LA volume and total scar, with improved long-term outcomes in persistent AF. (J Am Coll Cardiol EP 2020;■:■–■) © 2020 by the American College of Cardiology Foundation.
Keywords: atrial fibrillation, atrial mapping, ablation, fibrillation, magnetic resonance imaging, mechanisms of atrial fibrillation
The outcomes of pulmonary vein isolation ablation alone, which is the cornerstone of ablation for paroxysmal atrial fibrillation (AF) (1), are worse for persistent AF (2). This is likely due to the wider influence of non–pulmonary vein triggers and atrial substrates such as localized sources or foci, intra-atrial re-entrant circuits, and multiple atrial wavelets that contribute to the maintenance of AF in persistent cases (3,4). Empiric ablation beyond the pulmonary veins is commonly performed in patients with persistent AF including targeting the posterior wall, coronary sinus, ligament or vein of Marshall, fractionated areas in the atrium, or other nonpulmonary drivers, but despite these additional ablation approaches, long-term results have been inconsistent (5–7).
West and Landa (8) did an experimental arrhythmia study an atrial segments and proved that a critical mass is required for the initiation and maintenance of arrhythmia, which is dependent on the mean conduction time and refractory period to make the reentry excitation possible. The surgical Cox maze III procedure based on a similar concept has a high success rate of >96% after 10 years post-surgery and was founded on compartmentalization of the atrium (9). Currently, modified versions of the original surgical Cox maze are performed as surgical treatments (10). These incisional approaches create robust scar lines without any gaps interrupting macro–re-entrant circuits and rotors that sustain AF. Contemporary Cox maze IV procedures use a combination of cryothermal or radiofrequency (RF) ablations to replace the surgical incisions of the previous maze procedures (11,12). Percutaneous catheter ablation, despite the relatively worse outcomes, continues to be performed in much larger numbers as the procedure is less invasive and has a faster recovery time (13).
Catheter ablation can be used with the goal of replicating the Cox maze procedures. Transmural, complete linear ablation lines create barriers to electrical propagation and divide the atrial surface into separate regions in which the fibrillatory waves propagate and sustain AF. However, not all the ablated sites result in permanent scar, and there are generally gaps in the ablation lines that need to be accounted for calculations of the critical mass required to trigger and maintain AF (14,15). Procedural patient outcomes based on ablation patterns, and as a result, the compartmentalized areas available for fibrillatory waves to propagate, are unknown. The objective of this study was to evaluate the outcome of RF ablation based on the way it isolates and compartmentalizes different regions in the left atrial (LA) wall. We hypothesized that larger contiguous regions available for AF to trigger and the waves to propagate will result in worse outcomes. We have accounted for gaps in ablation lines in a novel way to determine the area available for waves to propagate and sustain AF, and we refer to this area as fibrillatory area (FA).
With the development of “virtual heart” methodology it is now possible to study the effects of pathological defects at the tissue, cell and protein levels on the conduction of the heart, and associated cardiac arrhythmias (16,17). We have used computational models in this study to help better understand the importance of the size of FAs and gaps in ablation lines in both 2-dimensional (2D) cylindrical geometric models as well as 3-dimensional (3D) patient-specific human geometric atrial models.
METHODS
STUDY POPULATION.
A total of 102 persistent AF patients (61 with and 41 without arrhythmia recurrence post-ablation) were selected who underwent initial AF RF ablation at the University of Utah. LA RF ablation was done as previously described (18) with wide antral pulmonary vein isolation done in all patients, with additional posterior wall debulking or anterior wall ablation lesions done based on operator preference. These selected consecutive patients had a 3 month post-ablation late gadolinium enhancement cardiac magnetic resonance (LGE-CMR) and at least 2 years of subsequent follow-up data. The recurrence data post–90-day blanking was obtained based on chart review from electrocardiography and Holter or monitored cardiac telemetry data as well clinic notes from follow-up visits to the cardiologist or electrophysiologist or to the emergency room. This retrospective study was approved by the Institutional Review Board at the University of Utah.
CMR ACQUISITION.
LGE-CMR scans were done on clinical scanners (Siemens Healthcare, Erlangen, Germany) 15 min after injection of contrast agent (0.1 mmol/kg; MultiHance; Bracco Diagnostics, Princeton, New Jersey) using an institutional standard inversion recovery prepared, 3D gradient echo pulse sequence as described (19). LGE-CMR images were obtained with acquired voxel size of 1.25 mm × 1.25 mm × 2.5 mm and reconstructed to voxel spacing of 0.625 mm × 0.625 mm × 1.25 mm.
ATRIAL SEGMENTATION AND SCAR DETECTION.
In order to reduce the effect of artifact, we used a median image filter with the radius of 1 pixel on the acquired LGE-CMRs. Segmentation of LA wall was performed as McGann et al. (20) described, and ablation scar was detected on segmented LA wall of post-ablation LGE-CMR using Corview image processing software (University of Utah, Salt Lake City, Utah) and automatic thresholding with the k-means clustering method (21).
QUANTIFICATION OF FIBRILLATORY AREAS.
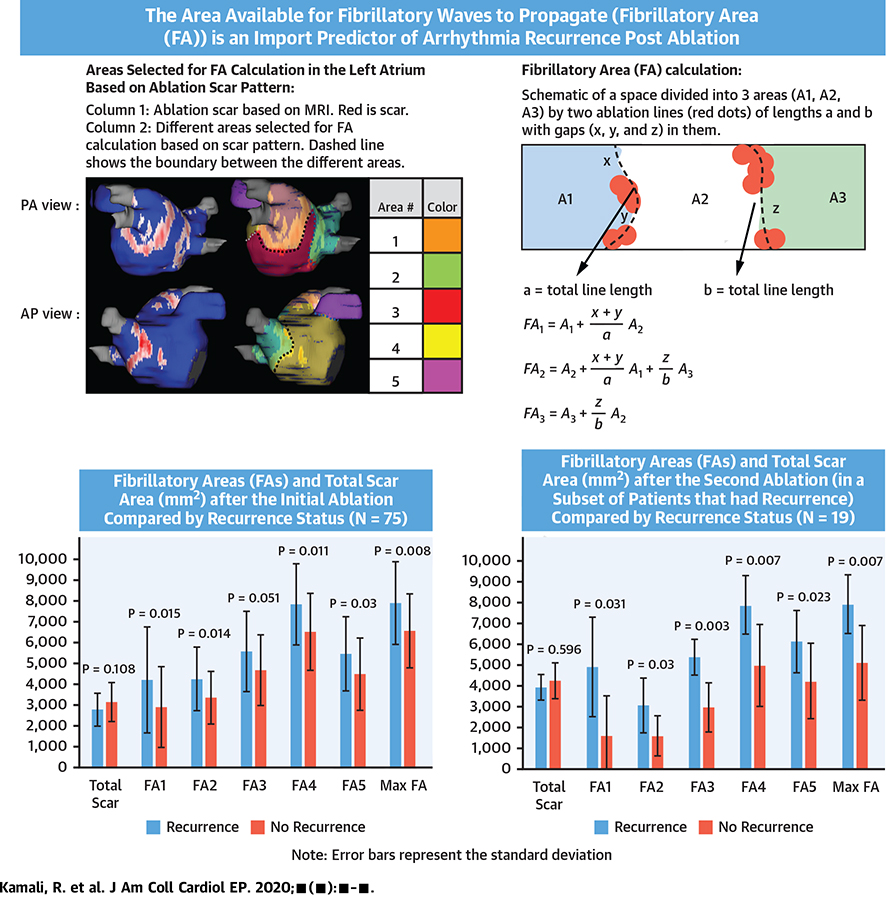
After the detection of ablation scar on the LA geometry, we divided the atrium into 5 regions based on LA landmarks and the pattern of scar around the pulmonary vein ostium after wide antral ablation attempts for all the ablations. Region 1 was the area around the left-sided pulmonary veins, region 2 was the area surrounding the right-sided pulmonary veins, region 3 was below the pulmonary veins in the lower part of the posterior wall, region 4 covered the anterior wall, and region 5 was the appendage. This arrangement of region selection was based on large contiguous areas separated by standard ablation lines around the pulmonary veins. The borders of the regions were drawn in a way that they included the maximum scar that separated 2 adjacent regions (Figure 1). The length of the shared border between the adjacent regions as well as the gap lengths in the scar on the border lines were measured, as was the surface area covered by each of the 5 regions.
FIGURE 1. Region Assignment for Calculating Fibrillatory Areas in a Typical Patient.
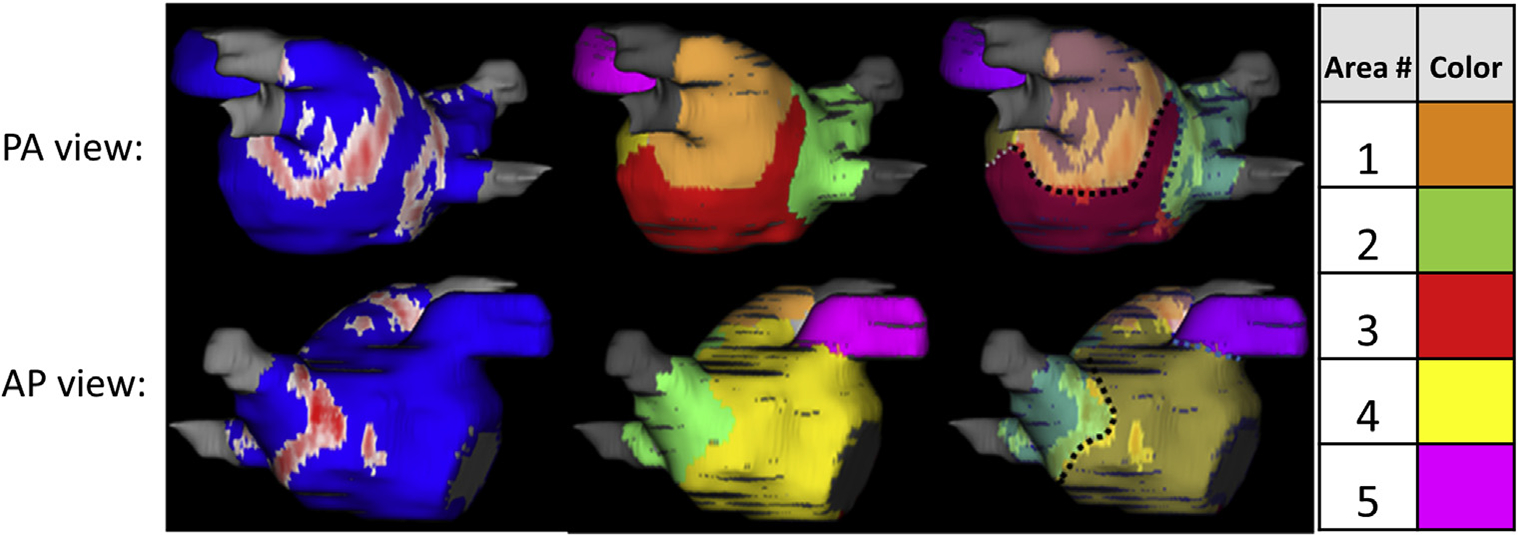
(Left column) Different views of the segmented and processed post-ablation cardiac magnetic resonance with scar region in red and normal tissue in blue. (Middle column) The different regions marked on it in different colors with their assigned numbers. (Right column) The assigned boundary lines (with dashed lines) between the different areas overlaid on the scar distribution. AP = anterior-posterior; PA = posterior-anterior.
We then calculated the scar-free area in each of the regions (called effective area [EA]).
| (1) |
where Ai is the surface area of region i.
The relative gap length to border length factor between regions was used to add a weighted portion of adjacent EAs when calculating FAs. If there was no scar between adjacent EAs, FA would simply be the sum of the EAs.
The following equation was used in calculation of the 5 (i=1:5) FAs in the atrium.
| (2) |
A schematic in the Supplemental Appendix details the calculation of the FA.
We started with an initial cohort of patients and calculated FAs for them. We correlated the FAs with arrhythmia recurrence. A subset of the patients with arrhythmia recurrences were reablated. Based on CMR done after the second ablation, scars were reassessed, and FAs were recalculated and correlated with arrhythmia recurrence. Figure 2 shows the study design.
FIGURE 2. Schematic Showing the Study Design.
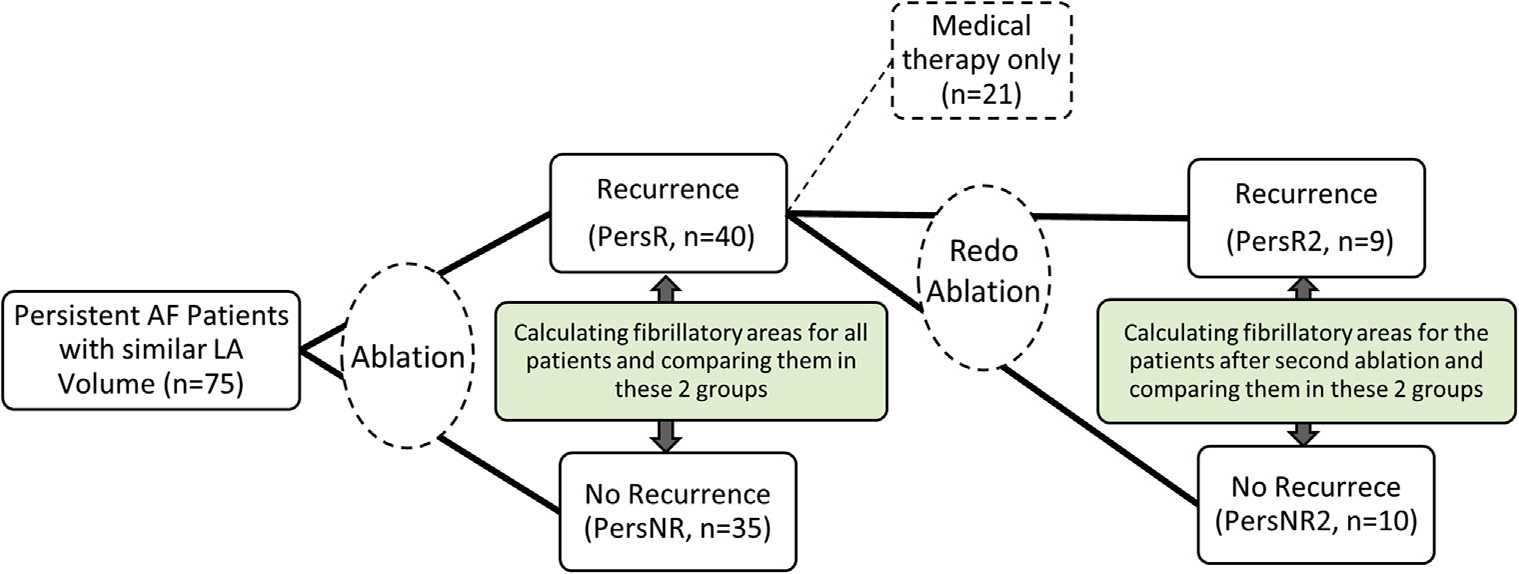
●●●. AF = atrial fibrillation; LA = left atrial; PersNR = patients with no recurrence; PersR = patients with atrial fibrillation recurrence.
COMPUTATIONAL AF MODELS.
We used CARPentry software package (NumeriCor GmbH, Graz, Austria) (22) for creating 2D cylindrical and 3D patient-specific computational AF models to further investigate the importance of the size of FAs and gaps in ablation lines in the ability of the model to sustain AF.
2D cylindrical geometric models.
A 2D cylindrical model with a surface area similar to the average adult human atrium and a random fibrosis pattern was generated, as described in the Supplemental Appendix. We implemented 4 different ablation patterns with varying gap lengths in the ablation lines, such that the calculated FA would be different (as shown in Figure 3). FAs were then calculated for each region. The goal of this simulation was to investigate the relationship between the FAs measured in different models and the ability of the model to sustain AF.
FIGURE 3. Two-Dimensional Cylindrical Simulation Results for Different Scar and Fibrosis Patterns, Paced From the Same Sample Point Along With a Simple Demonstration of the Selected Areas for Fibrillatory Area Calculations.
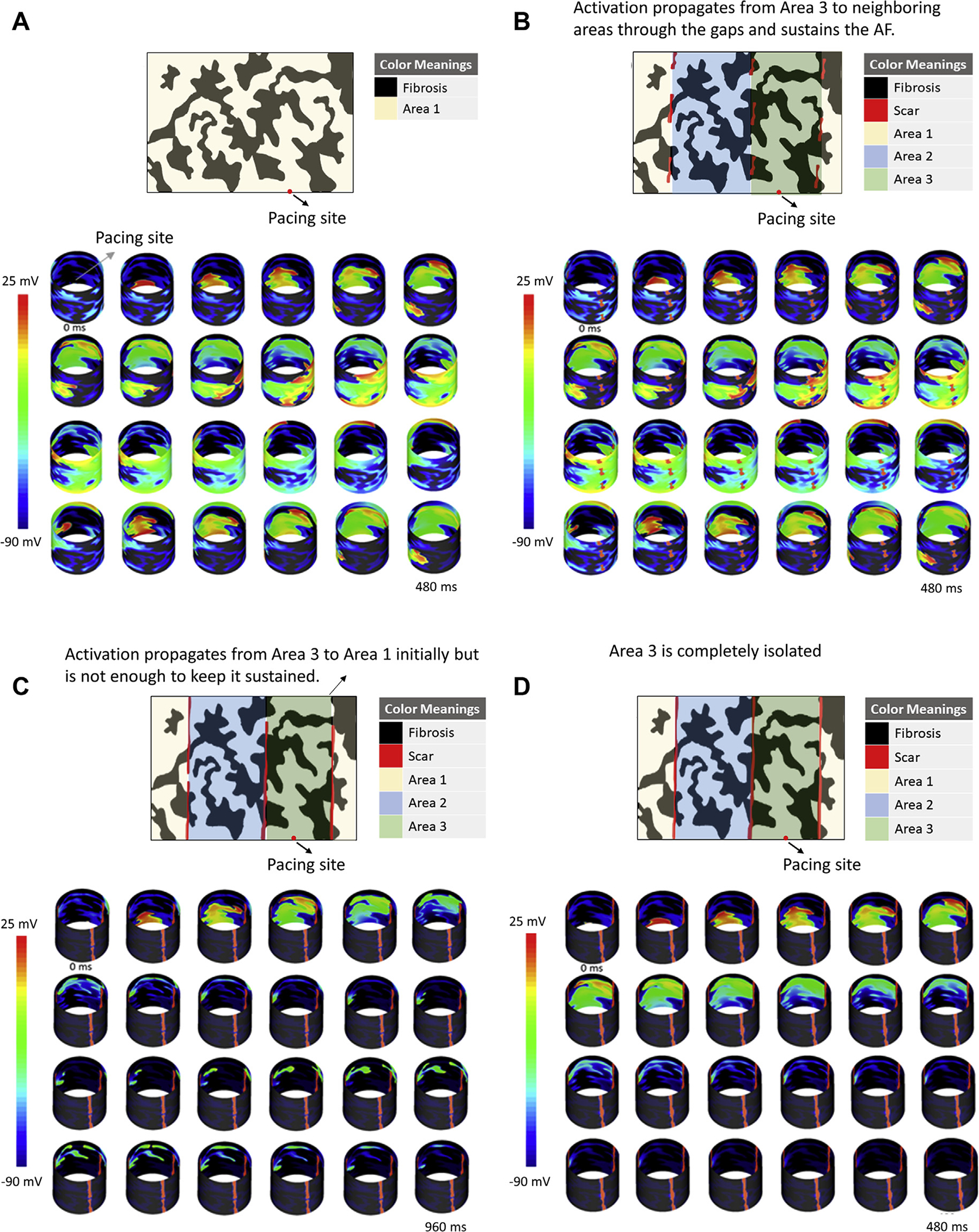
(A) No scar. (B) Leaving large gaps in the ablation lines. (C) Leaving less gaps between the regions. (D) Leaving no gaps. The top panel of shows the scar lines (red) with the gaps. The bottom panel shows snap shots of the transmembrane potential at different time points. AF = atrial fibrillation.
3D patient-specific human geometric atrial models.
We picked 2 patients with similar atrial volume and total scarred area for these simulations. One patient was from the arrhythmia recurrence group and 1 patient was from the no recurrence after the initial ablation group. The patients had both pre- and post-ablation LGE-CMR images that were used for detecting fibrosis and scar patterns, using the k-means clustering method (21), respectively. The mesh generation process is explained in detail in the Supplemental Appendix. Fiber orientation was estimated for our patient-specific models by registering our geometries to an atlas heart model with known fiber orientations (23).
Electrophysiological model parameterization.
We used the Courtemanche cellular model for atrial action potential (24) and modified the membrane parameters to reproduce realistic human action potential curves as measured by Kim et al. (25) during chronic AF. The Ito, ICaL, and IKur currents were reduced by 80%, 30%, and 90%, respectively, and IKr was increased by 50% and modified for the fibrotic regions by incorporating reduced inward rectifier potassium current (50%), L-type calcium current (50%), and sodium current (65%) (26). The Courtemanche model was also used for scarred areas with reduced conductivity values by a factor of 100 when compared with normal tissue. The electrophysiological parameters are listed in Supplemental Table 1. Electrical propagation was simulated with monodomain formulation.
Protocol for checking the AF inducibility of the models.
For each model, we chose 5 different pacing sites that were evenly distributed in the cylindrical model and were based on anatomical landmarks in atrial models. We paced the models with a 2-beat pacing train with a cycle length of 400 ms followed by a third extra stimuli with a decreasing coupling interval down to 200 ms in 20-ms decrements. Simulations were run for 10 s from the last pacing stimulus. We considered AF to be self-sustaining in a model if we had rotor activity until the end of the simulation (10 s).
STATISTICS AND ANALYSIS.
Continuous variables were expressed as mean ± SD. Cox proportional hazards ratio models were used to assess the association between each of the exposures (total area, FA areas, total EA, maximum FA [FAmax], volume, and scar area) and the hazard of recurrence with and without adjusting for CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, age 65–74 years, sex category) score and body mass index (BMI). CHA2DS2-VASc score was used as a combined surrogate for all risk factors included in its calculation. Because BMI is not included in the CHA2DS2-VASc calculation, we accounted for it by keeping it as a separate variable. For adjustments, in a regression we separated both BMI and CHA2DS2-VASc effects from each of the measured and calculated parameters. Model selection for the most significant predictor among the 5 FAs was done with a stepwise selection method with a significance level for removal from the model (terms with p ≥ pr [0.1] were eligible for removal) and a significance level for addition to the model (terms with p ≤ pe [0.05] were eligible for addition). Results were reported by hazard ratios and their 95% confidence intervals. We have interpreted the results as hazard ratio for a 1-unit (different based on the predictor: mm2 or ml) difference in the predictor variable of the first arrhythmia recurrence; p values <0.05 indicate statistically significant results. Stata software version 13 (StataCorp, College Station, Texas) was used to perform all statistical analyses.
RESULTS
After the segmentation of their LA, 75 patients with comparable LA volumes (94 ± 27 ml) were selected for further analysis. We excluded patients with very large or very small LA sizes, as size would be a factor in the calculated FA. During a 2-year follow-up period, 40 patients (26 men, 14 women; mean 69.33 ± 7.26 years of age) had atrial arrhythmia recurrences with a median of 203.5 (interquartile range: 130 to 297) days post-ablation, and 35 patients (26 men, 9 women; mean 67.77 ± 9.96 years of age) stayed in sinus rhythm in the median of 1,007 (interquartile range: 687 to 1,610) days of follow-up post-ablation. The baseline characteristics of all the patients are shown in Supplemental Table 2 along with the type of medications that they were taking post-ablation. Based on this table, drugs did not have an impact on controlling the rhythm in the patients who did not have recurrence.
ANALYSIS OF DATA AFTER THE FIRST ABLATION.
Based on CMR images after first ablations the values for average FAs, total atrial area, total scarred area, EAs and atrial volumes for patients with AF recurrence post-ablation and patients with no recurrence along with the unadjusted p values, confidence intervals, and hazard ratios are shown in Table 1. Adjusted values with CHA2DS2-VASc score and BMI for different models are also reported in Table 2.
TABLE 1.
Calculated Parameters After Initial Ablation for PersR and PersNR: Unadjusted Model
| PersR and PersNR | ||||
|---|---|---|---|---|
|
|
||||
| PersR (n = 40) | PersNR (n = 35) | Hazard Ratio per mm2 or ml (95% CI) | p Value | |
|
| ||||
| Total area | 13,092 ± 2,161 | 12,782 ± 2,096 | 1.005 (0.990–1.019) | 0.545 |
| Total EA | 10,314 ± 1,883 | 9,635 ± 1,886 | 1.011 (0.995–1.026) | 0.173 |
| Volume, ml | 98 ± 28 | 91 ± 25 | 1.010 (0.998–1.022) | 0.082 |
| Total scar | 2,778 ± 794 | 3,146 ± 938 | 0.967 (0.929–1.007) | 0.108 |
| FA1 | 4,219 ± 2,545 | 2,910 ± 1,948 | 1.016 (1.003–1.029) | 0.015 |
| FA2 | 4,245 ± 1,516 | 3,357 ± 1,259 | 1.026 (1.005–1.047) | 0.014 |
| FA3 | 5,568 ± 1,931 | 4,680 ± 1,695 | 1.017 (0.999–1.034) | 0.050 |
| FA4 | 7,834 ± 1,946 | 6,512 ± 1,848 | 1.021 (1.004–1.038) | 0.011 |
| FA5 | 5,459 ± 1,783 | 4,485 ± 1,732 | 1.019 (1.002–1.037) | 0.03 |
| FAmax | 7,896 ± 1,988 | 6,559 ± 1,784 | 1.022 (1.006–1.039) | 0.008 |
BMI = body mass index; CHA2DS2-VASc = congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, age 65–74 years, sex category; CI = confidence interval; EA = effective area; FA = fibrillatory area; FAmax = maximum fibrillatory area; PersNR = patients with no recurrence; PersR = patients with atrial fibrillation recurrence.
TABLE 2.
Calculated Parameters After Initial Ablation for PersR and PersNR: Adjusted Model
| Hazard Ratio (95% CI) | p Value | |
|---|---|---|
|
| ||
| Total area | 1.005 (0.990–1.021) | 0.477 |
| CHA2DS2-VASc | 0.926 (0.774–1.107) | 0.401 |
| BMI | 0.998 (0.939–1.059) | 0.95 |
| Total EA | 1.012 (0.996–1.028) | 0.142 |
| CHA2DS2-VASc | 0.919 (0.767–1.102) | 0.366 |
| BMI | 0.992 (0.935–1.052) | 0.802 |
| FA2 | 1.031 (1.007–1.055) | 0.01 |
| CHA2DS2-VASc | 0.847 (0.704–1.019) | 0.08 |
| BMI | 0.988 (0.931–1.048) | 0.694 |
| FAmax | 1.026 (1.008–1.045) | 0.004 |
| CHA2DS2-VASc | 0.878 (0.727–1.060) | 0.177 |
| BMI | 0.988 (0.931–1.049) | 0.712 |
| Volume | 1.010 (0.998–1.022) | 0.074 |
| CHA2DS2-VASc | 0.926 (0.776–1.106) | 0.399 |
| BMI | 0.997 (0.940–1.057) | 0.922 |
| Total scar | 0.969 (0.931–1.009) | 0.133 |
| CHA2DS2-VASc | 0.953 (0.792–1.147) | 0.613 |
| BMI | 1.005 (0.947–1.066) | 0.86 |
Abbreviations as in Table 1.
Total area, LA volume, EAs, and total scar area were not significantly associated with the hazard of recurrence of AF in both adjusted and unadjusted models.
All regional FAs, except FA3, were significantly associated with the recurrence of AF in unadjusted models. FA3 was marginally significant. FAmax was significantly associated with the hazard of recurrence in both adjusted and unadjusted models. When CHA2DS2-VASc score and BMI were adjusted, a 1-mm2 increase in FAmax was associated with 2.66% increase in the hazard of recurrence.
MODEL SELECTION FOR THE MOST SIGNIFICANT PREDICTOR AMONG THE 5 FAs.
FA2 was the most significant factor associated with recurrence of AF after the first ablation. When adjusting for CHA2DS2-VASc score and BMI, the hazard of recurrence increased 3.14% with a 1-mm2 increase in FA2 (Tables 1 and 2).
ANALYSIS OF DATA AFTER SECOND ABLATION.
Twenty patients with AF recurrences after the first ablation had a redo ablation, and among them 9 had recurrences. The same criteria with and without adjustments for CHA2DS2-VASc score and BMI for patients with AF recurrence post redo ablation and patients with no recurrence are reported in Tables 3 and 4.
TABLE 3.
Calculated Parameters After Redo Ablation for PersR and PersNR: Unadjusted Model
| PersR2 and PersNR2 | ||||
|---|---|---|---|---|
|
|
||||
| PersR2 (n = 9) | PersNR2 (n = 11) | Hazard Ratio per mm2 or ml (95% CI) | p Value | |
|
| ||||
| Total area, mm2 | 13,346 ± 1,637 | 12,050 ± 1,874 | 1.023 (0.993–1.053) | 0.133 |
| Total EA, mm2 | 9,476 ± 9,476 | 7,867 ± 1,826 | 1.027 (0.995–1.058) | 0.094 |
| Volume, ml | 104 ± 20 | 88 ± 22 | 1.040 (1.001–1.081) | 0.043 |
| Total scar, mm2 | 3,870 ± 612 | 4,183 ± 860 | 0.975 (0.886–1.071) | 0.596 |
| FA1, mm2 | 4,837 ± 2,356 | 1,618 ± 1,837 | 1.033 (1.004–1.062) | 0.023 |
| FA2, mm2 | 3,009 ± 1,291 | 1,566 ± 956 | 1.059 (1.005–1.115) | 0.030 |
| FA3, mm2 | 5,299 ± 851 | 2,919 ± 1,158 | 1.153 (1.048–1.267) | 0.003 |
| FA4, mm2 | 7,782 ± 1,402 | 4,917 ± 1,935 | 1.045 (1.011–1.078) | 0.007 |
| FA5, mm2 | 6,038 ± 1,465 | 4,174 ± 1,785 | 1.037 (1.003–1.070) | 0.031 |
| FAmax, mm | 7,807 ± 1,392 | 5,030 ± 1,765 | 1.046 (1.012–1.079) | 0.007 |
Abbreviations as in Table 1.
TABLE 4.
Calculated Parameters After Redo Ablation for PersR and PersNR: Adjusted Model
| Hazard Ratio (95% CI) | p Value | |
|---|---|---|
|
| ||
| Total area | 1.019 (0.988–1.049) | 0.235 |
| CHA2DS2-VASc | 0.935 (0.531–1.645) | 0.816 |
| BMI | 1.056 (0.910–1.226) | 0.471 |
| Total EA | 1.023 (0.992–1.054) | 0.147 |
| CHA2DS2-VASc | 0.918 (0.510–1.649) | 0.774 |
| BMI | 1.061 (0.917–1.226) | 0.427 |
| FA3 | 1.149 (1.029–1.282) | 0.013 |
| CHA2DS2-VASc | 0.883 (0.442–1.758) | 0.723 |
| BMI | 1.001 (0.825–1.214) | 0.989 |
| FAmax | 1.042 (1.004–1.082) | 0.03 |
| CHA2DS2-VASc | 1.006 (0.511–1.975) | 0.987 |
| BMI | 1.028 (0.868–1.216) | 0.748 |
| Volume | 1.043 (1.002–1.084) | 0.037 |
| CHA2DS2-VASc | 0.777 (0.443–1.363) | 0.379 |
| BMI | 1.074 (0.929–1.240) | 0.334 |
| Total scar | 0.958 (0.874–1.045) | 0.362 |
| CHA2DS2-VASc | 0.861 (0.520–1.424) | 0.559 |
| BMI | 1.101 (0.950–1.274) | 0.2 |
Abbreviations as in Table 1.
Total area, EA, and total scar area were not statistically significant when associated with the hazard of AF recurrence in both the adjusted and unadjusted models. LA volume was significantly associated with the hazard of recurrence after a redo ablation in both adjusted and unadjusted models. When CHA2DS2-VASc score and BMI were adjusted, a 1-ml increase in volume was associated with a 4.29% increase in the hazard of recurrence.
All FAs and FAmax were significantly associated with recurrence after the redo ablation in the unadjusted model. With adjustment for CHA2DS2-VASc score and BMI, a 1-mm2 increase in FAmax was associated with a 4.22% increase in the hazard of recurrence.
MODEL SELECTION FOR THE MOST SIGNIFICANT PREDICTOR AMONG THE 5 FAs.
FA3 was the most significant predictor for recurrence after the redo ablation, and after adjustment for CHA2DS2-VASc score and BMI, a 1-mm2 increase in FA was associated with a 14.91% increase in hazard of recurrence.
2D CYLINDRICAL SIMULATIONS.
The 2D cylindrical model was implemented with 3 ablation lines with varying gap lengths. AF with self-sustaining rotor activities was induced in the model without any scar and with ablation lines having large gaps and large FAs (Figures 3A and 3B). In models with smaller gaps in ablation lines resulting in smaller FAs, AF spontaneously terminated (Figures 3C and 3D). The calculated FAs for each corresponding scar pattern are shown in Table 5, with FAmax of 4,694 and 3,204 mm2 in models that sustained AF versus FAmax of 2,412 and 2,356 mm2 in models that did not sustain AF. The videos for each simulation are in the Supplemental Appendix.
TABLE 5.
Calculated FAs for the 2-Dimensional Cylindrical Models
| FA1 (mm2) | FA2 (mm2) | FA3 (mm2) | Simulated Rotor Activity | |
|---|---|---|---|---|
|
| ||||
| a | 4,694 | Continues until the end of simulation | ||
| b | 2,947 | 2,921 | 3,204 | Continues until the end of simulation |
| c | 2,260 | 2,412 | 1,551 | Terminated at 965 ms |
| d | 2,203 | 2,356 | 1,551 | Terminated at 365 ms |
Abbreviations as in Table 1.
3D PATIENT-SPECIFIC LA SIMULATIONS.
We implemented patient-specific models by incorporating fibrosis and scar patterns based on pre- and post-ablation CMRs. Rotor activity was observed and sustained in the AF model of the patient with post-ablation recurrence (Figure 4), and no sustained rotor activity was seen in the AF model of the patient that did not have arrhythmia recurrences post-ablation (Figure 5). The videos for each simulation are in the Supplemental Appendix. For the patient with recurrence, the LA volume was 113 ml, total area was 15,640 mm2, total scar area was 2,880 mm2, and FA1 to FA5 were 8,910 mm2, 4,585 mm2, 6,054 mm2, 7,912 mm2, and 4,542 mm2, respectively, with the FAmax being 8,910 mm2.
FIGURE 4. Three-Dimensional Left Atrial Simulation Showing Sustained Rotor Activity Until the End of Simulation (10 s) in a Patient With Arrhythmia Recurrence Post-Ablation.
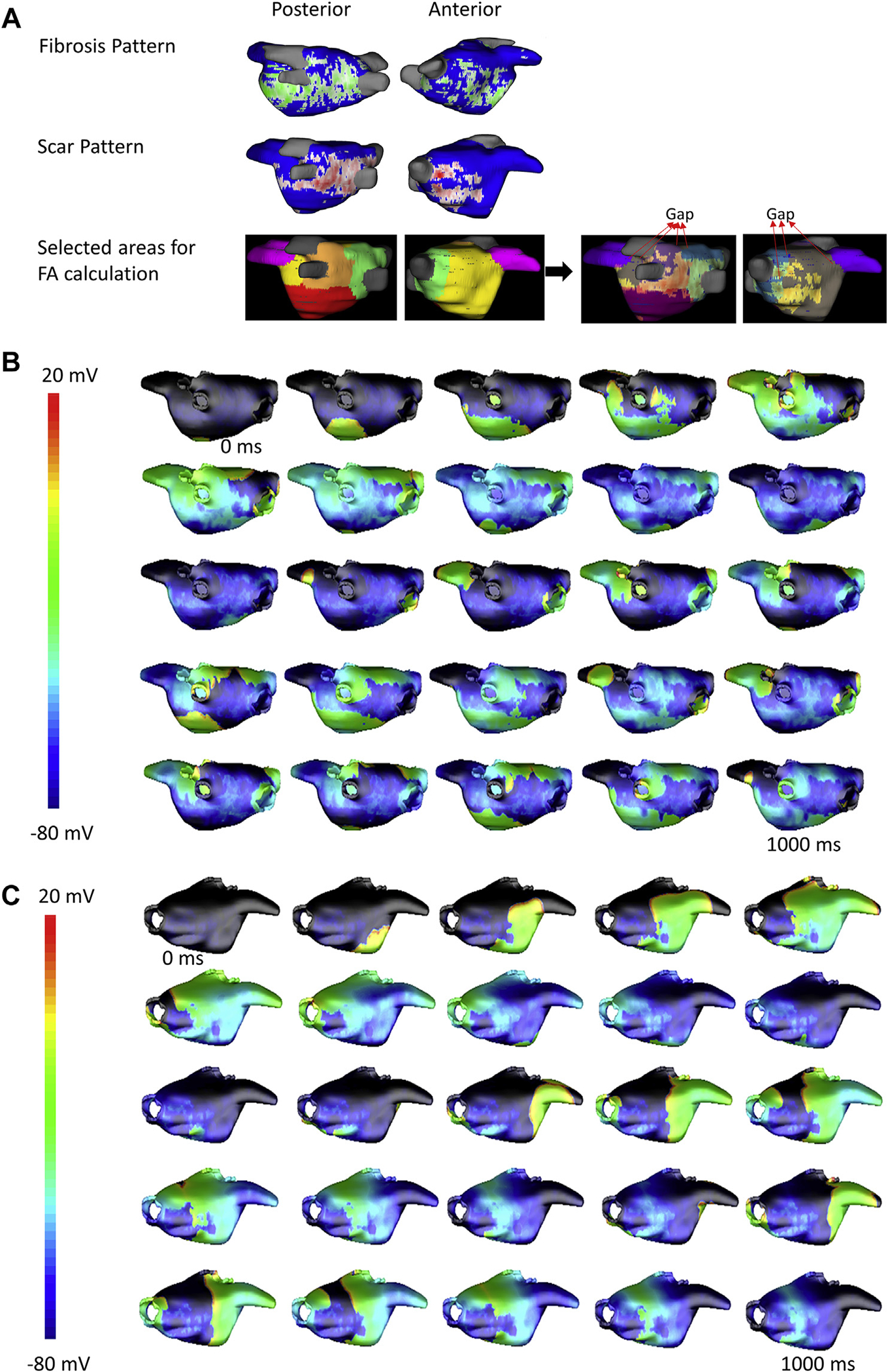
(A) Fibrosis (green, top row) and scar (red, middle row) in the left atrium based on pre- and post-ablation cardiac magnetic resonance, respectively, along with the different regions used for fibrillatory area (FA) calculation (bottom row) are shown. Gaps in the scar through which activation spreads to adjacent regions is marked on the right panels. Snapshots of the transmembrane potential in the left atrial wall at different time points are shown in (B) posterior view and (C) anterior view.
FIGURE 5. Three-Dimensional Left Atrial Simulation Showing Rotor Activity That Terminates Before the End of Simulation (10 s) in a Patient With No Arrhythmia Recurrence Post-Ablation.
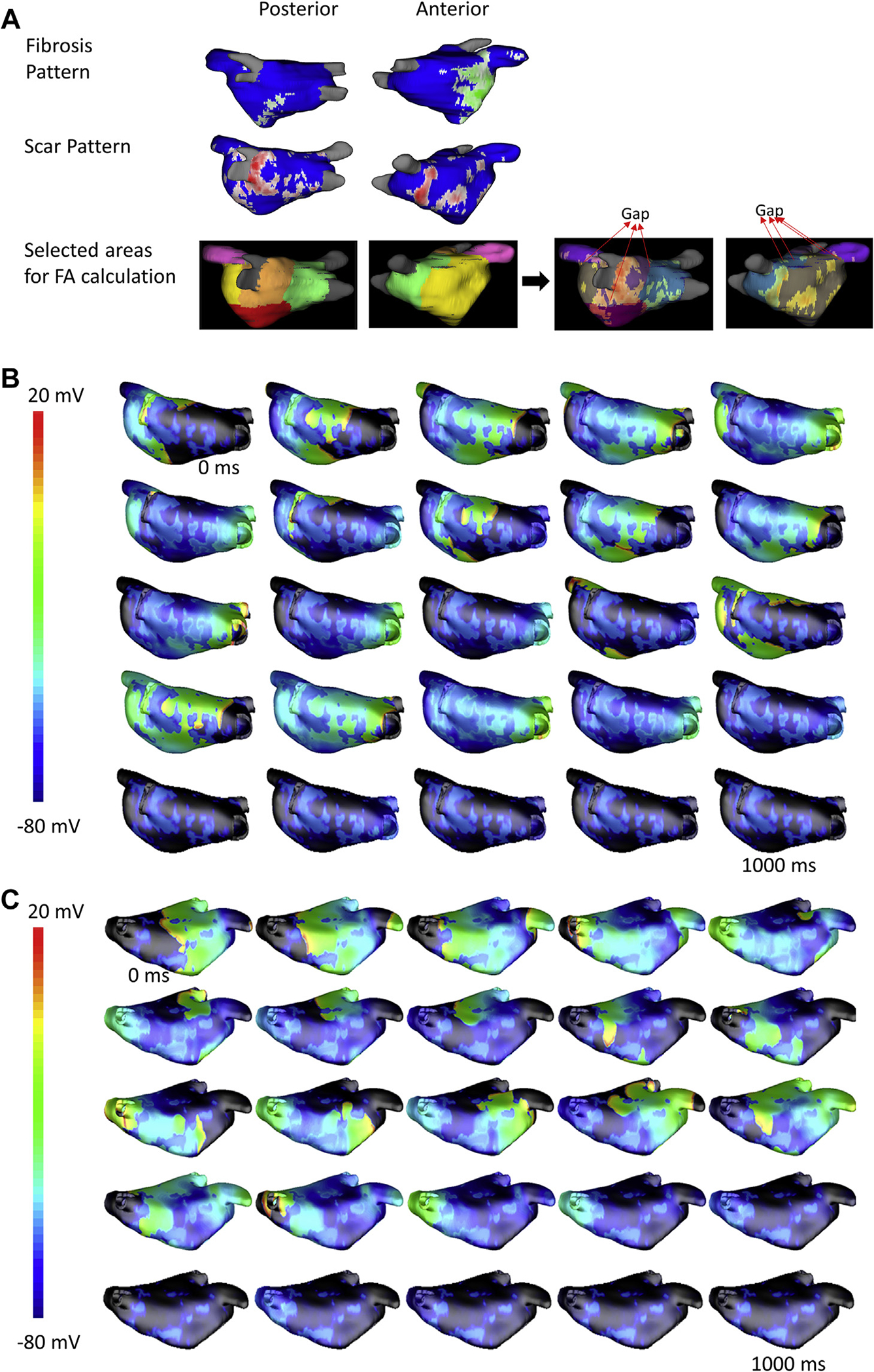
(A) Fibrosis (green, top row) and scar (red, middle row) in the left atrium based on pre- and post-ablation cardiac magnetic resonance, respectively, along with the different regions used for FA calculation (bottom row) are shown. Gaps in the scar through which activation spreads to adjacent regions are marked on the right panels. Snapshots of the transmembrane potential in the left atrial wall at different time points are shown in (B) posterior view and (C) anterior view.
For the patient without post-ablation recurrence, the LA volume was 109 ml, the total area was 154.9 cm2, the total scar area was 29.4 cm2, and FA1 to FA5 were 1,738 mm2, 4,119 mm2, 2,472 mm2, 6,049 mm2, and 5,228 mm2, respectively, with the FAmax being 6,049 mm2.
DISCUSSION
In this study, we investigated the relevance of ablation scar pattern to ablation outcomes. To our knowledge this is the first study to investigate the correlation between areas available for fibrillatory waves to propagate and outcomes after routine AF ablation. We used LGE-CMR to detect post-ablation scar. We also factored the extent of the gaps in ablation lines in the calculation of FAs. Based on quantitative calculation of FAs, we found that the area available for fibrillatory waves to propagate has a direct influence on ablation outcomes in patients with persistent AF. Even after adjustment for different clinical variables of arrhythmia recurrence risk (BMI and CHA2DS2-VASc), the FAs stand out as determinants of AF recurrence. This is an important finding that can certainly influence how we ablate patients with persistent AF who have recurrence after pulmonary vein isolation.
Micro–re-entrant rotational activity has previously been demonstrated on isolated animal hearts (27). Some human studies have also shown that AF is sustained by temporally and spatially stable rotors, and phase mapping algorithms might be used for targeting these arrhythmogenic regions (28). Using ablation techniques to target these areas would conceptually be ideal, but studies done using this approach have yielded mixed results (29). Propagating waves without the existence of stable re-entrant activity is another theory used to explain persistent AF (3,30). Limiting physical area available for such waves to propagate and sustain arrhythmia should result in AF termination. This concept has been well tested in the surgical Cox maze procedures founded on atrial compartmentalization that continue to have the best long-term outcomes for persistent AF despite technology advances in ablation tools and mapping systems (31).
Surgical incisional techniques are quite effective in creating robust ablation lines without any gaps, but they are time-consuming, augment risk of the procedure, and require a high level of operator expertise. Percutaneous ablation procedures (cryothermal or RF energy) that use methodology similar to surgical approaches often leave gaps in ablation lines despite acute block (14). In this study, we have outlined the concept of using FA as a predictor of ablation outcomes by incorporating these gaps in ablation lesions. This concept is critical when considering arrhythmia mechanisms beyond macro–re-entry flutter. This concept of FA can be used in pre- and periprocedural planning to create more effective ablation lesion sets in patients with persistent AF. Some efforts to use additional lesion lines to improve outcomes were tested in the STAR AF II trial, but the results indicated additional lines did not improve outcomes (32). Complicating our understanding of the potential value of adding linear ablations to pulmonary vein isolation is that durable continuous linear lesions are difficult to complete (19). The STAR AF II trial was done in the era before contact force catheters were used, and studies have shown that in that era up to 50% of the targeted area did not result in long-term scar (19,33) and that this affected outcomes. LGE-CMR has been used noninvasively to visualize atrial ablation–related scar (18,34). In one analysis that examined the completeness of linear lesions 3 months post-ablation in all patients regardless of arrhythmia recurrence, gaps were found in 61% of the pulmonary vein isolation lesion sets, 28% of the roof lines, and 24% of the mitral isthmus lines (15). In the pre–contact force era, when the STAR AF II trial was done, up to 33% of lesions did not result in scar (19). Based on this analysis and the LGE-CMR data, it is likely that a significant number of ablation lines in the STAR AF II trial had significant gaps. The restoration of conduction across these gaps is considered to be a major risk factor in the recurrence of atrial arrhythmias (35).
An important concept derived from our data is that additional ablation lines placed randomly as part of a stepwise ablation approach or within a planned lesion set are likely not effective strategies. In fact, additional line with gaps in them can actually be proarrhythmic, leading to atrial flutter. The additional lines are required with the goal of minimizing the fibrillatory area available for waves to propagate and sustain AF. In our study, the total scar by itself was not an important determinant of outcome, which is important when considering additional ablation in general and strategies of debulking. Randomized studies with well-planned linear ablations done in a patient-specific manner to reduce FAs depending on the LA size or surface area will be needed to further test the hypothesis proposed here. The surgical Cox maze outcomes are very supportive of this concept (10).
Cardiac simulations are becoming an effective tool to test novel treatment concepts. We have used 3D patient-specific models to test the concept of FAs and their role in sustaining AF. In the cylindrical model, we tested the concept of FA by incorporating different-sized gaps in ablation lines and found that larger FAs were needed for AF to be sustained. Finally, we implemented patient-specific models with similar volume and amount of scar but different scar patterns and showed that FAs can have a direct influence in ablation outcomes. Such models can be used to plan ablation procedures in patients both for an index procedure and a redo ablation in which ablation strategies are often limited when pulmonary vein isolation is verified, and additional ablation is required. Such techniques will need to be tested in future prospective studies.
STUDY LIMITATIONS.
CMR quality needs to be high for the best detection and assessment of ablation lines and potential gaps. Studies have shown post-ablation scar reproducibility, but high-quality imaging is necessary for proper scar delineation. We used patients of similar atrial size in our study to avoid potential bias of having larger FAs in patients with a bigger atrium just on account of the larger atria having a larger surface area. Future studies with larger patients will be needed to test this further by comparing the absolute FA sizes and larger atria, which might need additional lines to keep the maximum fibrillatory areas in the range that will not result in AF sustaining itself.
Modeling also has limitations in not including detailed individual electrophysiological and structural characteristics such as regional differences in action potential shape and subject-specific fiber orientation as these parameters are simply not available for each patient. As a result, we used values used in prior work based on available atlases. The right atrium was excluded in our models, and is often involved in more advanced subtypes of AF. Adding the right atrium will be imperative in future studies.
CONCLUSIONS
The ablation scar pattern is an important determinant of AF ablation outcomes in persistent AF. Our study shows that having smaller areas for fibrillatory waves to propagate will result in better ablation outcomes in patients with persistent AF. Patient-specific computational simulations confirm this finding.
Supplementary Material
CENTRAL ILLUSTRATION ■■■.
PERSPECTIVES.
The ablation pattern resulting in different areas available for fibrillatory waves to propagate has a direct influence on ablation outcomes in patients with persistent AF. AF ablation should be done with the goal of minimizing these areas, both in initial and redo ablation procedures.
Acknowledgments
Dr. Ranjan is currently supported by National Heart, Lung, and Blood Institute grant no. R01 HL142913.
ABBREVIATIONS AND ACRONYMS
- 2D
2-dimensional
- 3D
3-dimensional
- AF
atrial fibrillation
- BMI
body mass index
- CMR
cardiac magnetic resonance
- EA
effective area
- FA
fibrillatory area
- FAmax
maximum fibrillatory area
- LA
left atrial/atrium
- LGE
late gadolinium enhancement
- RF
radiofrequency
Footnotes
AUTHOR DISCLOSURES
Dr. Ranjan has served as a consultant to Abbott, Biosense Webster, and Medtronic; and has served as the principal investigator for research grants from Biosense Webster and Abbott to the University of Utah. Dr. Bunch has received research grant support from Boehringer Ingelheim, Boston Scientific, and Altathera. The remaining authors have nothing to disclose. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For an expanded Methods section and supplemental tables, please see the online version of this paper.
REFERENCES
- 1.Haissaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- 2.Willems S, Klemm H, Rostock T, et al. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: a prospective randomized comparison. Eur Heart J 2006;27:2871–8. [DOI] [PubMed] [Google Scholar]
- 3.Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res 2002;54:204–16. [DOI] [PubMed] [Google Scholar]
- 4.Haïssaguerre M, Hocini M, Sanders P, et al. Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation 2006;113:616–25. [DOI] [PubMed] [Google Scholar]
- 5.Stiles MK, Sanders P, Lau DH. Targeting the substrate in ablation of persistent atrial fibrillation: Recent lessons and future directions. Front Physiol 2018;9:1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nademanee K, Lockwood E, Oketani N, Gidney B. Catheter ablation of atrial fibrillation guided by complex fractionated atrial electrogram mapping of atrial fibrillation substrate. J Cardiol 2010;55:1–12. [DOI] [PubMed] [Google Scholar]
- 7.Cutler MJ, Johnson J, Abozguia K, et al. Impact of voltage mapping to guide whether to perform ablation of the posterior wall in patients with persistent atrial fibrillation. J Cardiovasc Electrophysiol 2016;27:13–21. [DOI] [PubMed] [Google Scholar]
- 8.West TC, Landa JF. Minimal mass required for induction of a sustained arrhythmia in isolated atrial segments. Am J Physiol 1962;202:232–6. [DOI] [PubMed] [Google Scholar]
- 9.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg 2003;126:1822–7. [DOI] [PubMed] [Google Scholar]
- 10.Weimar T, Schena S, Bailey MS, et al. The Cox-Maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhythm Electrophysiol 2012;5:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weimar T, Bailey MS, Watanabe Y, et al. The Cox-Maze IV procedure for lone atrial fibrillation: a single center experience in 100 consecutive patients. J Interv Card Electrophysiol 2011;31:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaynor SL, Diodato MD, Prasad SM, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg 2004;128:535–42. [DOI] [PubMed] [Google Scholar]
- 13.Pappone C, Rosanio S, Oreto G, et al. Circumferential radiofrequency ablation of pulmonary vein ostia. Circulation 2000;102:2619–28. [DOI] [PubMed] [Google Scholar]
- 14.Ranjan R, Kato R, Zviman MM, et al. Gaps in the ablation line as a potential cause of recovery from electrical isolation and their visualization using CMR. Circ Arrhythm Electrophysiol 2011;4:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mujović N, Marinković M, Marković N, et al. Persistency of left atrial linear lesions after radiofrequency catheter ablation for atrial fibrillation: Data from an invasive follow-up electrophysiology study. J Cardiovasc Electrophysiol 2017;28:1403–14. [DOI] [PubMed] [Google Scholar]
- 16.Trayanova NA. Whole-heart modeling: applications to cardiac electrophysiology and electromechanics. Circ Res 2011;108:113–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vigmond E, Vadakkumpadan F, Gurev V, et al. Towards predictive modelling of the electrophysiology of the heart. Exp Physiol 2009;94:563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamashita K, Kamali R, Kwan E, MacLeod RS, Dosdall DJ, Ranjan R. Effective ablation settings that predict chronic scar after left atrial ablation. J Am Coll Cardiol EP 2020;6:143–52. [DOI] [PubMed] [Google Scholar]
- 19.Parmar BR, Jarrett TR, Burgon NS, et al. Comparison of left atrial area marked ablated in electroanatomical maps with scar in CMR. J Cardiovasc Electrophysiol 2014;25:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGann C, Akoum N, Patel A, et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on CMR. Circ Arrhythm Electrophysiol 2014;7:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry D, Morris A, Burgon N, McGann C, MacLeod R, Cates J. Automatic classification of scar tissue in late gadolinium enhancement cardiac CMR for the assessment of left-atrial wall injury after radiofrequency ablation. Proc SPIE Int Soc Opt Eng 2012;8315. 10.1117/12.910833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vigmond EJ, Weber dos Santos R, Prassl AJ, Deo M, Plank G. Solvers for the cardiac bidomain equations. Prog Biophys Mol Biol 2007;96:3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vadakkumpadan F, Arevalo H, Ceritoglu C, Miller M, Trayanova N. Image-based estimation of ventricular fiber orientations for personalized modeling of cardiac electrophysiology. IEEE Trans Med Imaging 2012;31:1051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol 1998;275:H301–21. [DOI] [PubMed] [Google Scholar]
- 25.Kim B-S, Kim Y-H, Hwang G-S, et al. Action potential duration restitution kinetics in human atrial fibrillation. J Am Coll Cardiol 2002;39:1329–36. [DOI] [PubMed] [Google Scholar]
- 26.Ramos-Mondragón R, Galindo CA, Avila G. Role of TGF-β on cardiac structural and electrical remodeling. Vasc Health Risk Manag 2008;4:1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation 2000;101:194–9. [DOI] [PubMed] [Google Scholar]
- 28.Haissaguerre M, Hocini M, Shah AJ, et al. Noninvasive panoramic mapping of human atrial fibrillation mechanisms: a feasibility report. J Cardiovasc Electrophysiol 2013;24:711–7. [DOI] [PubMed] [Google Scholar]
- 29.Lin Y-J, Lo M-T, Chang S-L, et al. Benefits of atrial substrate modification guided by electrogram similarity and phase mapping techniques to eliminate rotors and focal sources versus conventional defragmentation in persistent atrial fibrillation. J Am Coll Cardiol EP 2016;2:667–78. [DOI] [PubMed] [Google Scholar]
- 30.Nattel S New ideas about atrial fibrillation 50 years on. Nature 2002;415:219–26. [DOI] [PubMed] [Google Scholar]
- 31.Cox JL. Cardiac surgery for arrhythmias. J Cardiovasc Electrophysiol 2004;15:250–62. [DOI] [PubMed] [Google Scholar]
- 32.Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 33.Parmar BR, Jarrett TR, Kholmovski EG, et al. Poor scar formation after ablation is associated with atrial fibrillation recurrence. J Interv Card Electrophysiol 2015;44:247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters DC, Wylie JV, Hauser TH, et al. Detection of pulmonary vein and left atrial scar after catheter ablation with 3-dimensional navigator-gated delayed enhancement mr imaging: initial experience 1. Radiology 2007;243:690–5. [DOI] [PubMed] [Google Scholar]
- 35.Ouyang F, Antz M, Ernst S, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins. Circulation 2005;111:127–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.