Abstract
Many patients evaluated in the emergency department (ED) for acute coronary syndrome (ACS) develop post-traumatic stress symptoms (PTSS), but little is known about symptom trajectories over time. We estimated longitudinal trajectories of PTSS from ED to 1 year after evaluation for suspected ACS (N = 1000), and the effect of threat perceptions and discharge diagnosis. Participants reported on threat perceptions in the ED, ongoing cardiac threat at 1 month, and PTSS at 1, 6, and 12 months. Latent growth mixture modeling identified 3 PTSS trajectories over 1 year: Resilient (81.75%), Chronic-Worsening (13.69%), and Acute-Recovering (4.56%). Chronic-Worsening and Acute-Recovering classes reported significantly higher ED and cardiac threat perceptions than Resilient class. Discharge diagnosis did not differ (χ2(2) = 2.93, p = .231). PTSS are common following evaluation for suspected ACS, and trajectories vary, but targeting threat perceptions may reduce PTSS and improve clinical course, whether or not patients are ultimately diagnosed with ACS.
Keywords: Trauma, Posttraumatic stress disorder, Acute coronary syndrome, Threat perceptions, Cardiovascular disease
1. Introduction
Acute coronary syndrome (ACS) is life-threatening, highly distressing, and for many traumatic; 1 in 8 ACS patients screen positive for posttraumatic stress disorder (PTSD) [16,17,22]. However, little is known about variations in ACS patients’ posttraumatic stress symptom (PTS) onset or clinical course. We estimated longitudinal trajectories of PTS following hospitalization for suspected ACS. We also compared patients with confirmed ACS to those who ultimately ruled out, and estimated the influence of threat during hospitalization and subsequent cardiac threat perceptions on longitudinal symptom trajectories.
Cardiovascular disease (CVD) remains the leading cause of death in the United States, accounting for over 900,000 deaths and 2.2 million hospitalizations annually [1,21,42]. After years of steady decline, CVD mortality rates have begun to plateau, and, in some subgroups, increase after nearly 40 years [39]. Patients with ACS (1.1 million hospitalizations per year) are at high risk for recurrent cardiac events and mortality [3,29,37].
Because most ACS patients survive the event, secondary risk reduction and quality of life after hospital discharge are critical. Psychological disorders, such as depression, anxiety, and PTSD, are common after ACS, and negatively impact quality of life [10,16,17,25,40]. Crucially, psychopathology is also an independent risk factor for mortality and cardiac event recurrence [7,8,18,35,36].
For the 12–15% of individuals who screen positive for ACS-induced PTSD [16,17,20], ACS-induced PTSD is associated with cardiac event recurrence and mortality [15,36]. Thus, whereas all ACS survivors are at high risk for CVD recurrence and mortality, ACS-induced PTS confer yet greater risk.
Millions of adults are evaluated for ACS in emergency departments each year, and those who ultimately rule out are at similar risk for PTSD as those who receive a diagnosis of ACS [24]. This is not surprising, given findings from a recent large study of Medicare claims data that patients evaluated for ACS were only slightly more accurate than chance at reporting whether they were ultimately diagnosed with ACS [43], as the physiological symptoms of ACS can have other etiologies in patients who have enough cardiovascular risk factors to be evaluated for ACS in the first place.
Little is known about variations in psychological stress response in the year after evaluation for ACS. Longitudinal studies of adjustment following other types of trauma have documented a number of clinically relevant symptom trajectories over time [4,5]. These trajectories include chronic difficulties, acute symptom elevations followed by gradual recovery, delayed-onset symptoms, and stable psychological and physical health or resilience (Galatzer-Levy, Huang, & Bonanno, 2018). In cardiac patients, only depression [7,8] and anxiety [19,27] symptom trajectories have been explored.
In the present study, we estimated longitudinal trajectories of PTS following emergency department (ED) evaluation for suspected ACS in the REactions to Acute Care and Hospitalizations (REACH) study. Participants in the REACH study are approached in the ED while they are being evaluated for suspected ACS. Thus, upon discharge, some participants are given a discharge diagnosis of confirmed ACS while others ultimately rule-out for ACS and may receive a non-cardiovascular diagnosis. Prior research has shown that there are no differences in rates of PTS at 1-month after evaluation, whether or not the event is diagnosed ACS, as patients who meet our inclusion criteria are high risk for ACS [25]. Thus, an additional aim of the present study was to compare PTS trajectories of participants diagnosed with “confirmed ACS” vs “rule-out ACS.”
A final aim of the study was to examine threat perceptions as predictors of PTS trajectories. Perceived threat during the peri-traumatic period predicts the development of subsequent PTSD [22,38]. One source of perceived threat, unique to life-threatening medical events, is the ED environment; ED crowding in the ED and nearby patient acuity have been associated with heightened threat perception in the ED [16,23]. A second source of threat perception stems specifically from the cardiac event. In line with the enduring somatic threat (EST) model [14], patients with heightened cardiac threat perceptions in the month after the ACS have the most severe PTS at 1-month post-ACS [28]. The EST model suggests that an underlying fear of mortality contributes to PTSD symptoms, leading to ongoing, interoceptive monitoring and catastrophic interpretation of interoceptive signals. In the present study, we examined variations in PTS trajectories in relation to ED threat perceptions measured during the ED visit and ongoing cardiac threat perceptions measured 1-month after hospital discharge.
2. Methods
2.1. Participants
The present study includes the first 1000 English- and Spanish-speaking patients enrolled from November 2013 to February 2016 in the REACH study. REACH is an ongoing prospective observational cohort of a consecutive sample of patients presenting to an urban ED in New York City with symptoms of suspected ACS. All participants had pre-existing risk factors for ACS, reported symptoms consistent with ACS, and were initially considered by ED physicians to (more likely than not) be diagnosed with ACS. We excluded patients with ST elevation myocardial infarction (STEMI) due to hospital emergency department fast track procedures for catheterization. Additional exclusion criteria included inability to follow the protocol (due to dementia or substance abuse), need for immediate psychiatric intervention, and lack of availability for follow-up (e.g. due to terminal non-cardiovascular illness). The study was completed in accordance with the latest version of the Declaration of Helsinki, approved by the Institutional Review Board of Columbia University Irving Medical Center (CUIMC), and all participants gave informed consent before completing study procedures.
2.2. Procedure
We first approached participants in the ED during evaluation for suspected ACS and administered informed consent and demographic questionnaires. At this time, participants reported on the ACS symptoms that brought them to the hospital and on current threat perceptions (in the ED). Upon transfer to an inpatient bed (or by phone, if discharged), we completed the baseline interview, where participants recall prior ED threat perceptions and complete a measure of Acute Stress Disorder symptoms keyed to the ACS event. One month, 6 months, and 12 months after discharge, participants reported on PTSD symptoms specific to the cardiac event (i.e., ACS-induced PTSD) via telephone interview. Participants received a $30 payment for completing assessments.
We extracted discharge diagnosis and baseline medical comorbidities from patient medical records. Patients determined not to have met criteria for “confirmed ACS,” were designated “ruled out” for ACS. The majority of rule-out ACS patients remain at higher than normal risk for cardiovascular events, as most had pre-existing coronary artery disease, or other chronic diseases that caused ED physicians to initiate ACS evaluation.
2.3. Measures
2.3.1. Demographic variables
We collected demographic data in the ED. Our participant population is highly diverse, with ~50% of participants identifying as Dominican or Hispanic. A large number of participants identified “Dominican” or “Hispanic” as both their race and ethnicity rather than reporting two distinct racial and ethnic identities. Therefore, for the purposes of this study, we created a combined race/ethnicity variable with categories Black, White, Hispanic, and Other.
2.3.2. Index ACS status and medical covariates
Using medical records, we recorded participants’ discharge diagnosis (confirmed ACS or rule-out). Detailed cardiovascular and non-cardiovascular diagnoses (i.e. gastrointestinal distress, musculoskeletal pain, anxiety/panic attack) in the rule-out ACS participants have been previously reported [25]. We also recorded whether or not participants had experienced a cardiovascular event in the past. Covariates from the medical record included the Global Registry of Acute Coronary Events (GRACE) risk score [13] and Charlson Comorbidity Index scores [9]. GRACE risk scores can range from 1 to 263, with higher scores representing greater risk for mortality. Charlson Comorbidity Index scores can range from 0 to 37, with higher scores indicating more severe medical comorbidity.
2.3.3. ACS-induced posttraumatic stress (PTS) symptoms
We measured ACS-induced Acute Stress Disorder symptoms after ED discharge (median 3 days) using the Acute Stress Disorder Scale (ASDS) [6]. Participants reported on acute stress symptoms in relation to the “heart problem that brought you to the hospital” (e.g., “did you ever feel numb or distant from your emotions?”). Participants scored responses on a 5-point Likert scale ranging from 1, “Not at all,” to 5, “Very much,” and we summed item responses to compute a total symptom score. As in our prior studies, we did not include 4 dissociation items. We measured ACS-induced symptoms of PTSD (i.e., PTSD with respect to the “heart problem, ED visit, and hospitalization”) at 1, 6, and 12 months using the PTSD Checklist (PCL-S) [41]. Partway through the study, APA released the DSM-V and the corresponding PCL-5 was published. We adjusted the assessment used in our study using common items across these two instruments, by creating a 17-item summary score of PTS symptoms (common to both scales; i.e., DSM-IV criteria) experienced within the past month. Participants rated items on a 5-point Likert scale scored from 1, “Not at all,” to 5, “Extremely,” and we obtained total PTS symptom severity by summing the 17 items.
2.3.4. Psychosocial covariates
2.3.4.1. ED threat perceptions.
We assessed threat perceptions in response to evaluation for suspected ACS in the ED during ED evaluation and after transfer to an inpatient bed using a 7-item measure of ED Threat Perceptions [11,33]. Participants rated the extent to which certain statements (e.g., “I am afraid,” “I feel helpless”) reflected their ED experiences on a 4-point Likert scale ranging from 1, “Not at all,” to 4, “Extremely.” The internal consistency of the ED Threat Perceptions scale was high (Cronbach’s = 0.81).
2.3.4.2. Cardiac threat perceptions.
We assessed ongoing perceptions of cardiac threat at 1 month via telephone interview using cardiac threat related items from the Anxiety Sensitivity Index-Revised (ASI) [34]. This measure was assessed 1-month after the participants’ index cardiac events as we were interested in understanding the sequalae specific to cardiac-related interoception that participants experience in an ongoing fashion in the time after hospitalization and discharge. We chose not to include this measure in the ED because, in line with our inclusion criteria, participants were suspected to have had a cardiac event; therefore, participants were likely to experience cardiac-related threat perceptions acutely in the ED. Items included “It scares me when my heart beats rapidly”, “When my chest feels tight, I get scared that I won’t be able to breathe properly”, “When I notice my heart skipping a beat, I worry that there is something seriously wrong with me”, and “When I feel pain in my chest, I worry that I’m going to have a heart attack.” Participants responded on a Likert scale ranging from 0, “Very little” to 4, “Very much” and we then summed items to create a total cardiac threat score. Higher scores reflect higher levels of perceived cardiac threat. This variable was introduced after the study had already begun, and therefore data on this measure were only available for N = 526. Internal consistency was high (α = 0.87).
2.4. Data analysis
We performed latent growth mixture modeling using Mplus 8.0 [32] to identify distinct trajectories of PTS symptoms over the one-year period after evaluation for suspected ACS (baseline and 1, 6, and 12 months). Because ASDS (T1) and PTSD symptoms (T2–T4) were scored on different scales, we standardized scores prior to model estimation following [12]. For all models, we weighted time intervals between measures to account for nonequivalence. We examined unconditional models with no covariates, comparing only the intercept (no growth), followed by intercept and slope parameters (linear growth), and finally by intercept, slope, and quadratic parameters (nonlinear growth). The linear model provided improved fit over the intercept only model, whereas the nonlinear growth model failed to converge. All subsequent models used linear growth parameters with the variance of the intercept allowed to be freely estimated while the slope variance was fixed. To determine the best fitting trajectory solution, we compared progressive models of 1 to 4 classes using fit statistics, including Akaike (AIC), Bayesian (BIC), sample-size adjusted Bayesian information criterion (SSBIC) indices, entropy values, LoMendell-Rubin (LRT) and bootstrap likelihood ratio tests (BLRT). We selected the final model based on overall model fit and interpretability [5,31].
Little is known about the trajectories of PTS after an ACS event; therefore, after determining the best fitting unconditional model, a number of potentially relevant demographic and medical covariates were tested in addition to our two initial psychosocial covariates of interest to examine their effect on trajectory class membership. We conducted these analyses initially outside of the model using one-way ANOVA and Chi-Square analyses because of marked reductions in sample size with the ASI cardiac threat perceptions variable. Demographic covariates included age, gender, and race/ethnicity, to assess the extent to which demographic variables predicted class trajectory. Medical covariates were examined to determine whether or not pre-existing cardiovascular risk factors or comorbid illnesses impacted trajectory class. Medical covariates included GRACE risk scores and Charlson Comorbidity Index scores, cardiovascular event history, and discharge ACS status. When examining the effect of discharge ACS status on trajectory class, we used both a Chi-Square analysis outside of the model and an omnibus Wald test as part of a known-class analysis.
In line with Edmondson’s EST model [14,28] and other relevant literature, we examined the association of ED and 1-month cardiac threat perceptions on trajectory class. Based on the results of covariate analyses outside of the model, variables that significantly predicted trajectory class membership were then included in a final conditional model using Least Squares Regression analyses within the LGMM framework.
3. Results
3.1. Unconditional model
We present descriptive characteristics of the sample, stratified by discharge ACS status, in Table 1. Information indices (AIC, BIC, SSBIC) for one- to four-class mixture models were progressively smaller as class size increased, suggesting incrementally improved fit (see Supplemental Online Materials 1 and 2). Entropy remained high and the LRT and BRT indicated significant improvement in fit up to the three-class model solution. However, for a four-class model solution, entropy decreased and LRT was no longer significant. Based on these considerations, we selected the three-class model as the optimal solution (see Fig. 1).
Table 1.
Participant characteristics as a function of ACS status at discharge.
| Characteristic | M (SD) or % (n) | M (SD) or % (n) | M (SD) or % (n) | p-Value | Range |
|---|---|---|---|---|---|
| Confirmed ACS diagnosis (n = 318) | Non-cardiovascular diagnosis (n = 682) | Total (N = 1000) | |||
| Demographics | |||||
| Age, years | 62.71 (12.54) | 59.91 (13.35) | 60.80 (13.15) | .002 | 22–100 |
| Female | 11.50 (115) | 34.10 (341) | 45.60 (456) | .000 | – |
| Race | .009 | – | |||
| White | 21.07 (67) | 14.37 (98) | 16.50 (165) | – | |
| Black | 16.04 (51) | 21.70 (148) | 19.90 (199) | – | |
| Other | 8.81 (28) | 5.57 (38) | 6.60 (66) | – | |
| Hispanic | 51.57 (164) | 56.01 (380) | 54.40 (544) | – | |
| Medical record covariates | |||||
| GRACE score | 97.30 (30.37) | 91.22 (29.98) | 93.19 (29.56) | .003 | 18–200 |
| Charlson Comorbidity Index | 2.16 (1.86) | 1.65 (1.99) | 1.82 (1.97) | .000 | 0–11 |
| Prior cardiovascular event | 54.40 (173) | 20.97 (143) | 31.60 (316) | .000 | – |
| In-hospital assessment | |||||
| Perceived threat in the ED | 11.00 (4.13) | 10.93 (4.50) | 10.95 (4.38) | .814 | 6–24 |
| Depressive symptoms at baselinea | 6.16 (5.67) | 6.68 (6.03) | 6.51 (5.91) | .207 | 0–24 |
| PTSD total severity score at baseline for prior traumab | 25.26 (12.69) | 26.08 (13.59) | 25.81 (13.30) | .381 | 17–85 |
| Confirmed ACS diagnosis (n = 182) | Non-cardiovascular diagnosis (n = 344) | Total (N = 526) | |||
| 1-month follow-up | |||||
| Perceived cardiac threat | 6.85 (5.10) | 7.17 (5.20) | 7.06 (5.16) | .504 | 0–16 |
| ACS-induced PTSD symptoms | 25.29 (11.46) | 25.06 (11.76) | 25.14 (11.64) | .834 | 17–74 |
| 6-month follow-up | |||||
| ACS-induced PTSD symptoms | 23.39 (11.03) | 26.36 (13.41) | 25.33 (12.70) | .023 | 17–83 |
| 12-month follow-up | |||||
| ACS-induced PTSD symptoms | 23.27 (10.45) | 24.51 (11.41) | 24.10 (11.11) | .291 | 17–84 |
Note. GRACE = Global Registry of Acute Coronary Events. ED = emergency department. PTSD = posttraumatic stress disorder. ACS = acute coronary syndrome.
Past 2-week depressive symptoms assessed with the Patient Health Questionnaire depression scale (PHQ-8).
PTSD total severity score at baseline calculated by summing responses on the PTSD Checklist-Civilian version (PCL-C) with respect to the most stressful event identified on the LEC.
Fig. 1.
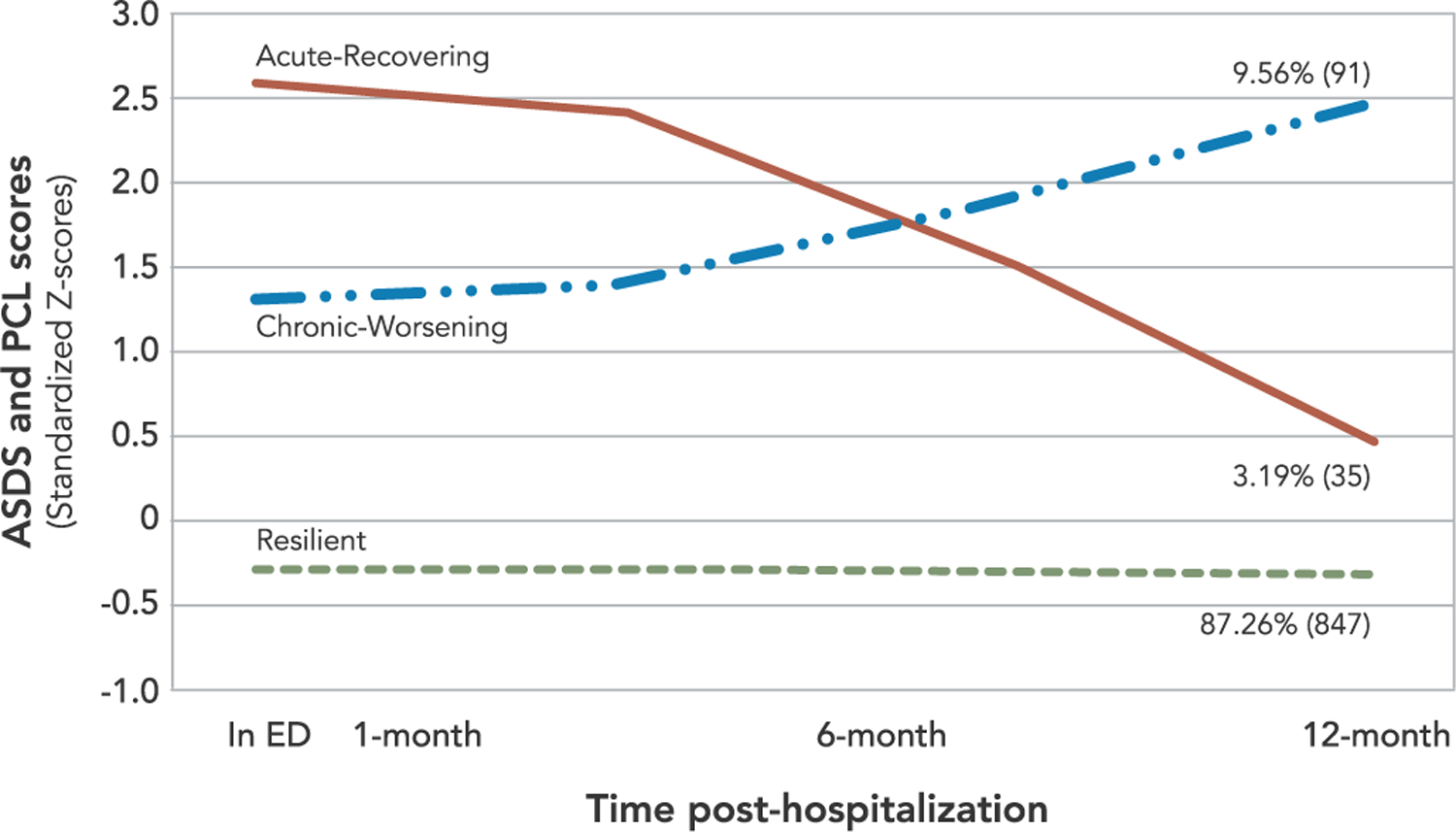
Three-class unconditional model of Posttraumatic Stress Symptoms (N=973)
The largest of the three classes, labeled Resilient (87.26%), was characterized by low PTS symptoms across all time points with a low intercept (b = −0.26, SE = 0.02, p < .001) and flat, nonsignificant slope (b = −0.03, SE = 0.03, p = .28). The second largest class, labeled Chronic-Worsening (9.56%), described individuals who showed clinically elevated PTS at T1 and T2 and worsening at T3 and T4. This group had a high intercept (b = 1.31, SE = 0.17, p < .001) and a significant positive linear slope (b = 1.17, SE = 0.17, p < .001). The third and smallest class, labeled Acute-Recovering (3.19%), was characterized by individuals who had acute initial PTS at T1 and T2, followed by marked reductions across T3 and T4. This group had the highest intercept (b = 2.55, SE = 0.33, p < .001) and significant negative linear slope (b = −2.11, SE = 0.48, p < .001).
3.2. Preliminary analyses of possible predictors of class membership
Analyses of covariates in a conditional model tend to produce better specified solutions relative to an unconditional model [26,30]. We evaluated demographic, medical, and psychosocial variables as predictors of class membership independent of the model using Chi-Square and one-way ANOVAs. No demographic or medical variables showed significant effects, except gender (χ2(1) = 16.74, p < .001). However, both psychosocial variables of interest, ED threat perceptions (F [2, 971] = 24.38, p < .001) and cardiac threat perceptions (F [2, 525] = 43.70, p < .001), significantly differentiated trajectory membership.
3.3. Conditional model
To more fully examine how covariates predicted class membership and whether they influence the shape and prevalence of the trajectories, we tested a conditional model that included variables identified in the previous analyses: gender, ED threat perceptions, and cardiac threat perceptions. As previously noted, a considerable amount of data was missing for the cardiac threat perceptions measure because this measure was added after data collection for the study had begun. Of the N = 1000, only 526 completed the cardiac threat perceptions measure, lowering the sample size for our conditional model by 45% (n = 526).
The conditional model successfully converged. Class membership proportions and shape did not change substantially when compared to the unconditional model (see Supplemental Online Material 1. The Resilient class was, again, the largest (81.74%), followed by Chronic-Worsening (13.69%), and the smallest group, Acute-Recovering (4.56%) (Fig. 2).
Fig. 2.
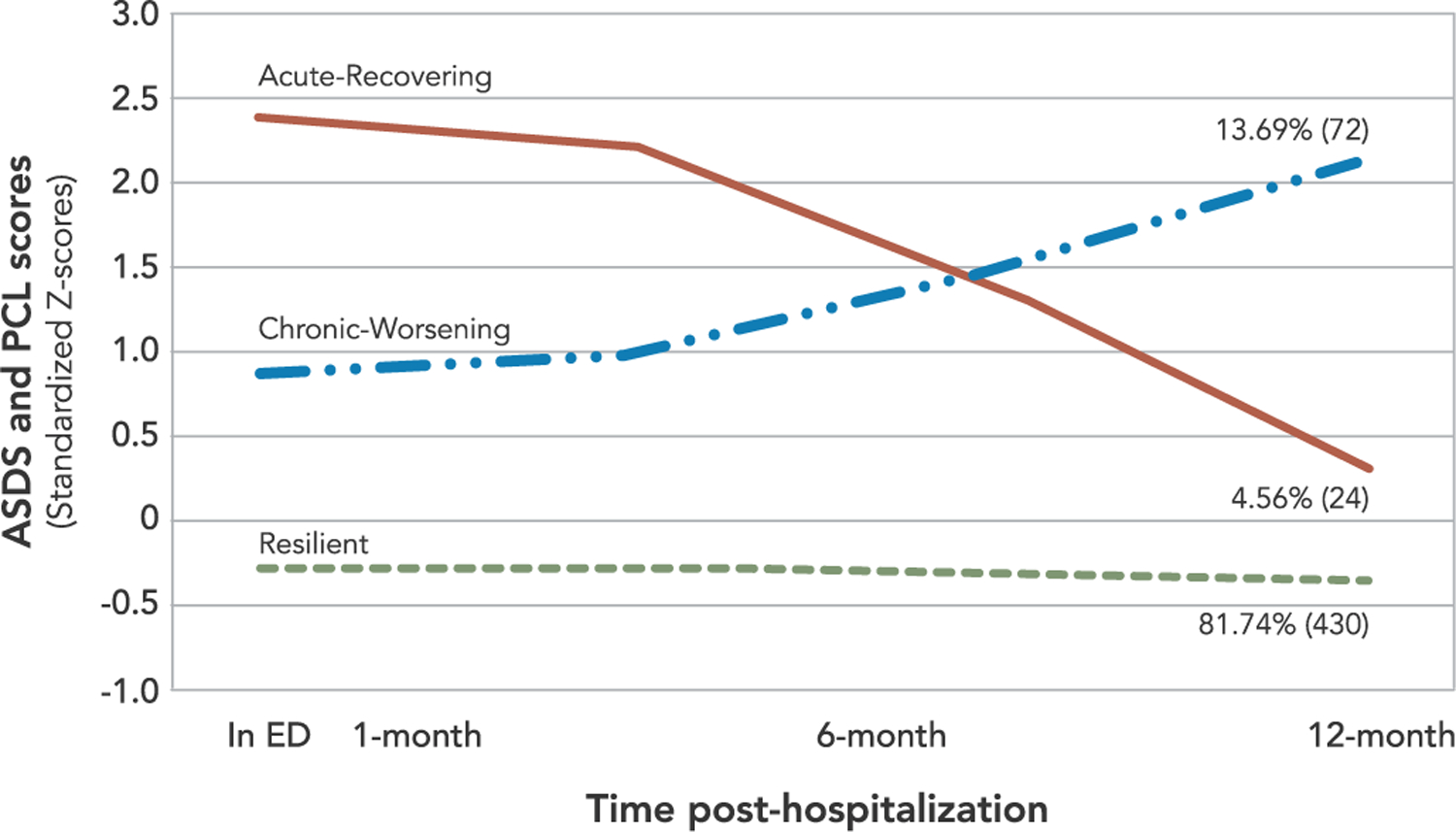
Three-class conditional model of Posttraumatic Stress Symptoms (N=526)
To examine the covariates as predictors of class membership, we first used the Resilient class as the reference group. Compared to the Resilient class, ED threat perceptions were significantly higher for individuals in the Chronic-Worsening class (b = 0.13, SE = 0.03, p < .001) and Acute-Recovering class (b = 0.29, SE = 0.06, p < .001). Cardiac threat perceptions were also significantly higher in the Chronic-Worsening class (b = 0.24, SE = 0.04, p < .001) and Acute-Recovering class (b = 0.35, SE = 0.08, p < .001). In a second set of analyses we used the Chronic-Worsening class as the reference group. Compared to the Chronic-Worsening class, the Acute-Recovering class reported significantly higher ED threat perceptions (b = 0.15, SE = 0.06, p = .008) but no significant difference in cardiac threat perceptions (b = 0.11, SE = 0.08, p = .213). Gender did not significantly differentiate any of the trajectory classes.
3.4. Discharge ACS status
We examined whether PTS symptom trajectories differed by ACS diagnosis. We found no significant difference in proportion of participants in each trajectory in a 2 × 3 contingency analysis comparing confirmed versus rule-out ACS across PTS symptom trajectories in the unconditional model, χ2(2) = 1.43, p = .489, or in the conditional model, χ2(2) = 2.93, p = .231. Results are given in Table 2. We also tested discharge ACS status as a variable within the LGMM using a known-class analysis and omnibus Wald test in both models. Results of the omnibus Wald test were not significant, indicating that stratification of models based on ACS status (confirmed versus rule-out ACS) did not meaningfully improve model fit (unconditional: χ2(1) = 0.170, p = .681; conditional: χ2(1) = 0.128, p = .720).
Table 2.
Chi-square analyses of trajectory class membership stratified by ACS status in unconditional (N = 973) and conditional (N = 526) growth mixture models.
| Resilient | Acute-Recovering | Chronic | χ2(2) | p-Value | |
|---|---|---|---|---|---|
|
|
|||||
| % (n) | % (n) | % (n) | |||
| Unconditional model | |||||
| Confirmed ACS | 88.9% (279) | 2.9% (9) | 8.3% (26) | 1.43 | .489 |
| Rule-out ACS | 86.2% (568) | 3.9% (26) | 9.9% (65) | ||
| Conditional model | |||||
| Confirmed ACS | 85.2% (155) | 2.7% (5) | 12.1% (22) | 2.93 | .231 |
| Rule-out ACS | 79.9% (275) | 5.5% (19) | 14.5% (50) | ||
4. Discussion
Each year, millions of individuals are hospitalized for suspected ACS. Many experience these events as traumatic, and some go on to develop PTS symptoms. We identified 3 unique trajectories of PTS symptoms over the course of 1-year post-hospitalization in a large, ethnically diverse sample. A clear majority of ACS patients in the current study (87%) were classified in a Resilient trajectory denoted by low PTS symptoms at all time points. A smaller group (10%) was classified in a Chronic-Worsening trajectory characterized by high initial PTS symptoms that worsened over the course of the year. Finally, a third, small group (3%) was classified in an Acute-Recovering trajectory characterized by considerably elevated initial PTS symptoms that steadily resolved over the course of the year.
We were particularly interested in whether the PTS trajectories were impacted by threat perceptions during ED evaluation, ongoing interoceptive distress concerning cardiac signals, and by discharge diagnosis (ACS or rule-out). We found that patients who were highly distressed during the ED visit were significantly more likely to show either an acute-recovering or chronic-worsening trajectory (12.7% of the sample combined). In light of current diagnostic criteria for PTSD, this finding is intuitive. According to the DSM-5, peritraumatic factors, including severity of the trauma and perceived threat to life, increase the likelihood of developing PTSD [2]. Although our ED threat measure reflected peritraumatic fear of dying and vulnerability, the hectic hospital environment may have exacerbated participants’ distress [27].
Interestingly, however, ED threat perceptions were significantly more strongly associated with acute PTS symptoms that resolved over the ensuing year (i.e., Acute-Recovering pattern) than with the Chronic-Worsening pattern. Thus, although individuals with heightened peritraumatic threat in the ED suffer acute PTS symptoms, they do not necessarily develop chronic PTSD. It will be crucial in future studies to further tease out how threat ED perceptions may inform these different clinical sequelae.
In contrast to threat perceptions during ED evaluation, the perception of ongoing cardiac threat predicted both the Acute-Recovering and the Chronic-Worsening trajectory with equal likelihood. This finding supports the EST model, which theorizes that ongoing somatic threat perceptions are of particular importance for PTS after an acute, life-threatening cardiac event. Heighted cardiac threat is reminiscent of the archetypal hypervigilance and arousal behaviors in response to triggering traumatic reminders characteristic of PTSD. However, unique to our study and this sample, the trigger is a physiological signal both of relevance to the index ACS event and important in identifying a potential future event. Patients are in a unique role of experiencing present cardiac sensations as simultaneously triggering memories of the initial trauma and as potentially signifying a current/future traumatic cardiac event. Whereas PTSD is generally considered a disorder of fear memory processing, the EST model suggests that the present and future temporal focus of cardiac threat perceptions may be an important clinical target.
We also examined other potentially relevant predictor variables. Neither demographic nor medical status variables differed meaningfully across trajectories. Notably, the PTS symptom patterns were also relatively invariant in relation to discharge diagnosis. Despite 68% of patients in our study receiving a rule-out ACS discharge diagnosis, the trajectories for this group and those with a confirmed ACS event were essentially identical, which agrees with prior cross-sectional findings [25]. While perhaps surprising, our findings suggest that pathophysiology and ultimate diagnosis do not differentially impact PTS reactions following an acute cardiac event. Instead, initial perceived threat, subjective trauma experience, and ongoing concerns about cardiac risk are more powerful and predictive of clinical course and psychological sequalae than clinical diagnosis or severity (Edmondson & Cohen, 2013). Prior findings suggest that patients evaluated for ACS may be uncertain of their discharge diagnosis [43]. Future studies of PTS in patients evaluated for ACS should determine the influence of patient understanding of discharge diagnosis.
Our findings should be interpreted within the context of several limitations. First, the REACH study was conducted at a single site in an urban setting and one of the nation’s largest and busiest hospitals. Therefore, these findings may not be generalizable to patients presenting with ACS in other ED settings. A second consideration concerns our measurements of PTS symptoms and cardiac threat. Our study used a self-report questionnaire to assess PTS symptoms rather than a clinical interview. Therefore, we cannot conclude a clinical diagnosis of PTSD and instead report on symptoms of PTS. Further, our study had missing data for our cardiac threat measure. This measure, comprised of cardiac threat related items from the ASI [34] was introduced after data collection for this study had begun, resulting in only half of our total sample completing the cardiac threat measure.
While our study presents novel findings on the predictors of PTS trajectories in the 12-months following a suspected ACS event, our study lacks data on the clinical effects of these trajectories, including event recurrence, future hospitalizations, and mortality. In addition, our study did not formally assess participants’ psychological treatment for dysfunction and distress associated with PTS nor medical treatment for CVD. However, prior research has reported self-reported treatment-seeking behaviors in this sample [39]. Future research should take into consideration clinical outcomes and treatment for PTS and CVD in order to better understand the impact of trajectory status and membership in this population.
ED treatment for suspected ACS events is a potentially traumatic experience that results in heterogeneous patterns of PTS symptoms. While the majority of individuals are resilient, about 1 in 8 patients report elevated PTS symptoms. Our findings detail the impact of patients’ threat perceptions during ED evaluation, as well as the unique relationship of ongoing cardiac threat perceptions with PTS symptom trajectories. It is important for medical and psychological clinicians to consider that PTS symptoms may be present and highly distressing in patients evaluated for CVD events in the ED, regardless of discharge diagnosis. The subjective experience of presenting to the ED with ACS symptoms can be traumatic, and may result in chronic psychological symptoms. Future research should continue to explore symptom trajectories and determinants of PTSD due to other life-threatening medical events. Such research can inform and improve peritraumatic and clinical intervention efforts. Interventions targeting PTS, threat perceptions in the ED, and/or ongoing cardiac/interoceptive threat perceptions may reduce psychological distress, improve quality of life, and perhaps reduce secondary CVD risk after acute cardiac events.
Supplementary Material
Funding
This work was supported by the National Institutes of Health [HL117832, HL128497, HL128310].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.genhosppsych.2019.11.006.
Declaration of competing interest
None.
References
- [1].American Heart Association. Heart disease and stroke statistics — 2019 update. A report from the American Heart Association. Circulation 2019;139:e56–528. 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- [2].American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- [3].Bertoni A, Bonds D, Thom T, Chen G, Goff D. Acute coronary syndrome national statistics: challenges in definitions. Am Heart J 2005;149(6):1055–61. [DOI] [PubMed] [Google Scholar]
- [4].Bonanno GA, Kennedy P, Galatzer-Levy IR, Lude P, Elfström ML. Trajectories of resilience, depression, and anxiety following spinal cord injury. Rehabil Psychol 2012;57(3):236–47. 10.1037/a0029256. [DOI] [PubMed] [Google Scholar]
- [5].Bonanno GA. Loss, trauma, and human resilience: have we underestimated the human capacity to thrive after extremely aversive events? Am Psychol 2004;59(1):20–8. 10.1037/0003-066x.59.1.20. [DOI] [PubMed] [Google Scholar]
- [6].Bryant RA, Moulds ML, Guthrie RM. Acute Stress Disorder Scale: a self-report measure of acute stress disorder. Psychol Assess 2000;12(1):61–8. [PubMed] [Google Scholar]
- [7].Burton CL, Galatzer-Levy IR, & Bonanno GA (In Press). Treatment type and demographic characteristics as predictors for cancer adjustment: prospective trajectories of depression symptoms in a population sample. Health Psychol [DOI] [PubMed] [Google Scholar]
- [8].Burton CL, Galatzer-Levy IR, Bonanno GA. Treatment type and demographic characteristics as predictors for cancer adjustment: prospective trajectories of depressive symptoms in a population sample. Health Psychol 2015;34(6):602. [DOI] [PubMed] [Google Scholar]
- [9].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Disease 1987;40:373–83. 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- [10].Cohen BE, Marmar CR, Neylan TC, Schiller NB, Ali S, Whooley MA. Posttraumatic stress disorder and health-related quality of life in patients with coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry 2009;66:1214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cornelius T, Agarwal S, Garcia O, Chaplin W, Edmondson D, Chang BP. Development and validation of a measure to assess patients’ threat perceptions in the emergency department. Acad Emerg Med 2018. 10.1111/acem.13513. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].DeRoon-Cassini TA, Mancini AD, Bonanno GA, Rusch MD. Psychopathology and resilience following traumatic injury: a latent growth mixture model analysis. Rehabil Psychol 2010;55(1):1–11. http://doi.org/2010-03250-001 [pii]. https://doi.org/10.1037/a0018601. [DOI] [PubMed] [Google Scholar]
- [13].Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA 2004;291:2727–33. [DOI] [PubMed] [Google Scholar]
- [14].Edmondson D An enduring somatic threat model of posttraumatic stress disorder due to acute life-threatening medical events. Soc Personal Psychol Compass 2014;8(3):118–34. 10.1111/spc3.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, Neria Y. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta-analytic review. PLoS ONE 2012;7(6):e38915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Edmondson DE, Shimbo D, Ye S, Wyer P, Davidson KW. The association of emergency department crowding during treatment for acute coronary syndrome with subsequent posttraumatic stress disorder symptoms. JAMA Intern Med 2013:472–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J 2013;166:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction: impact on 6-month survival. JAMA 1993;270(15):1819–25. 10.1001/jama.1993.03510150053029. [DOI] [PubMed] [Google Scholar]
- [19].Froese A, Hackett TP, Cassem NH, et al. Trajectories of anxiety and depression in denying and nondenying acute myocardial infarction patients during hospitalization. J Psychosom Res 1974;18:413–20. [DOI] [PubMed] [Google Scholar]
- [20].Gander ML, Känel RV. Myocardial infarction and post-traumatic stress disorder: frequency, outcome, and atherosclerotic mechanisms. European Journal of Cardiovascular Prevention & Rehabilitation 2006;13(2):165–72. [DOI] [PubMed] [Google Scholar]
- [21].Global Burden of Cardiovascular Diseases Collaboration. The burden of cardiovascular diseases among US states, 1990–2016. JAMA Cardiol 2018;3(5):375–89. 10.1001/jamacardio.2018.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Holbrook TL, Hoyt DB, Stein MB, Sieber WJ. Perceived threat to life predicts posttraumatic stress disorder after major trauma: risk factors and functional outcome. Journal of Trauma 2001;51:287–92. [DOI] [PubMed] [Google Scholar]
- [23].Konrad B, Hiti D, Chang BP, Retuerto J, Julian J, Edmondson D. Cardiac patients’ perceptions of neighboring patients’ risk: influence on psychological stress in the ED and subsequent posttraumatic stress. BMC Emerg Med 2017;17(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kronish IM, Edmondson D, Moise N, Chang BP, Wei Y, Veneros DL, et al. Posttraumatic stress disorder in patients who rule out versus rule in for acute coronary syndrome. Gen Hosp Psychiatry 2018;53:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lane D, Carroll D, Ring C, Beevers DG, Lip GY. The prevalence and persistence of depression and anxiety following myocardial infarction. Br J Health Psychol 2002;7:11–21. [DOI] [PubMed] [Google Scholar]
- [26].Li L, Hser Y-I. On inclusion of covariates for class enumeration of growth mixture models. Multivar Behav Res 2011;46(2):266–302. 10.1080/00273171.2011.556549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McCarthy ML, Aronsky D, Jones ID, Miner JR, Band RA, Baren JM, et al. The emergency department occupancy rate: a simple measure of emergency department crowding? Ann Emerg Med 2008;51(1):15–24. [DOI] [PubMed] [Google Scholar]
- [28].Meli L, Alcántara C, Sumner JA, Swan B, Chang BP, Edmondson D. Enduring somatic threat perceptions and post-traumatic stress disorder symptoms in survivors of cardiac events. J Health Psychol 2017. 10.1177/1359105317705982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131(4):e29–322. [DOI] [PubMed] [Google Scholar]
- [30].Muthén B Latent variable analysis: growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of quantitative methodology for the social sciences. Newbury Park, CA: Sage Publications; 2004. p. 345–68. [Google Scholar]
- [31].Muthen B. Statistical and substantive checking in growth mixture modeling: comment on Bauer and Curran (2003). Psychol Methods 2003;8(3):369–77. 10.1037/1082-989x.8.3.369. [DOI] [PubMed] [Google Scholar]
- [32].Muthen BO, Muthen LK. Mplus user’s guide. 1998–2010.
- [33].Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull 2003;129(1):52–73. [DOI] [PubMed] [Google Scholar]
- [34].Peterson RA, Reiss S. Anxiety sensitivity index revised test manual. Worthington, OH: International Diagnostic Services; 1993. [Google Scholar]
- [35].Roest AM, Martens EJ, Denollet J, et al. Prognostic association of anxiety post myocardial infarction with mortality and new cardiac events: a meta-analysis. Psychosom Med 2010;72:563–9. [DOI] [PubMed] [Google Scholar]
- [36].Shemesh E, Yehuda R, Milo O, Dinur I, Rudnick A, Vered Z, et al. Posttraumatic stress, nonadherence, and adverse outcome in survivors of a myocardial infarction. Psychosom Med 2004;66(4):521–6. [DOI] [PubMed] [Google Scholar]
- [37].Terkelsen CJ, Lassen JF, Nørgaard BL, Gerdes JC, Jensen T, Gøtzsche LBH, et al. Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur Heart J 2004;26(1):18–26. [DOI] [PubMed] [Google Scholar]
- [38].van Wingen GA, Geuze E, Vermetten E, Fernández G. Perceived threat predicts the neural sequelae of combat stress. Mol Psychiatry 2011;16(6):664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wall HK, Ritchey MD, Gillespie C, Omura JD, Jamal A, George MG. Vital signs: prevalence of key cardiovascular disease risk factors for Million Hearts 2022—United States, 2011–2016. Morb Mortal Wkly Rep 2018;67(35):983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wasson LT, Shaffer J, Alcantara C, Schwartz JE, Edmondson D. The association of posttraumatic stress disorder and quality of life during the first year after acute coronary syndrome. Int J Cardiol 2014;176(3):1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD checklist (PCL): reliability, validity, and diagnostic utility. Paper presented at the annual convention of the International Society for Traumatic Stress Studies, San Antonio, TX. 1993. [Google Scholar]
- [42].Wright JS, Wall HK, Ritchey MD. Million Hearts 2022: small steps are needed for cardiovascular disease prevention. JAMA 2018;320(18):1857–8. 10.1001/jama.2018.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yasaitis LC, Berkman LF, Chandra A. Comparison of self-reported and Medicare claims-identified acute myocardial infarction. Circulation 2015;131:1477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


