SUMMARY
Human-mediated changes to natural ecosystems have consequences for both ecosystem and human health. Historically, efforts to preserve or restore ‘biodiversity’ can seem to be in opposition to human interests. However, the integration of biodiversity conservation and public health has gained significant traction in recent years, and new efforts to identify solutions that benefit both environmental and human health are ongoing. At the forefront of these efforts is an attempt to clarify ways in which biodiversity conservation can help reduce the risk of zoonotic spillover of pathogens from wild animals, sparking epidemics and pandemics in humans and livestock. However, our understanding of the mechanisms by which biodiversity change influences the spillover process is incomplete, limiting the application of integrated strategies aimed at achieving positive outcomes for both conservation and disease management. Here, we review the literature, considering a broad scope of biodiversity dimensions, to identify cases where zoonotic pathogen spillover is mechanistically linked to changes in biodiversity. By reframing the discussion around biodiversity and disease using mechanistic evidence — while encompassing multiple aspects of biodiversity including functional diversity, landscape diversity, phenological diversity, and interaction diversity — we work toward general principles that can guide future research and more effectively integrate the related goals of biodiversity conservation and spillover prevention. We conclude by summarizing how these principles could be used to integrate the goal of spillover prevention into ongoing biodiversity conservation initiatives.
Introduction
The COVID-19 pandemic has brought the threat of zoonoses into the public spotlight, creating widespread demand for better management of the ecological sources of disease spillover and emergence. However, even prior to this pandemic, there has been an increasing recognition amongst experts of the ties between healthy ecosystems and human health. This has led to broader support for global conservation initiatives and spurred the United Nations’ adoption of sustainable development goals (the 2030 Agenda). The prevention of zoonotic spillovers is a biosecurity imperative with a patent connection to the human–wildlife interface; thus, efforts are underway to identify solutions that both promote biodiversity conservation and facilitate zoonotic disease management1. However, given our incomplete understanding of the mechanisms linking biodiversity to infectious disease spillover, a clear vision of how to effect positive solutions for both human health and the environment is needed. Increased attention to, and resources for, zoonotic disease prevention make it an opportune time to study the mechanisms connecting changes in biodiversity with zoonotic disease spillover, and to identify (potentially synergistic) solutions for biodiversity conservation and global health.
There has been a contentious debate about the existence and generality of the relationship between biodiversity and disease: in particular, the extent to which maintaining biodiversity protects against disease via a dilution effect versus the alternative possibility that biodiversity can increase infectious disease transmission via an amplification effect (see for example references2-9). With a few notable exceptions10-16, this debate has largely focused on correlations between host-species richness and the prevalence of pathogens in host reservoir populations. However, this narrow way of framing the impacts of species richness on host prevalence in most of the empirical literature provides limited insight into the range of mechanisms by which biodiversity affects disease, rendering it difficult to integrate into public health interventions. Here, we expand the focus to the broader mechanistic relationships among a variety of biodiversity components and the zoonotic spillover process. We then follow with a review of general principles with applied relevance. Finally, we highlight opportunities where ongoing conservation initiatives could consider and possibly integrate these mechanisms further in order to reduce disease spillover risks (Figure 1, Table 1, and Table 2).
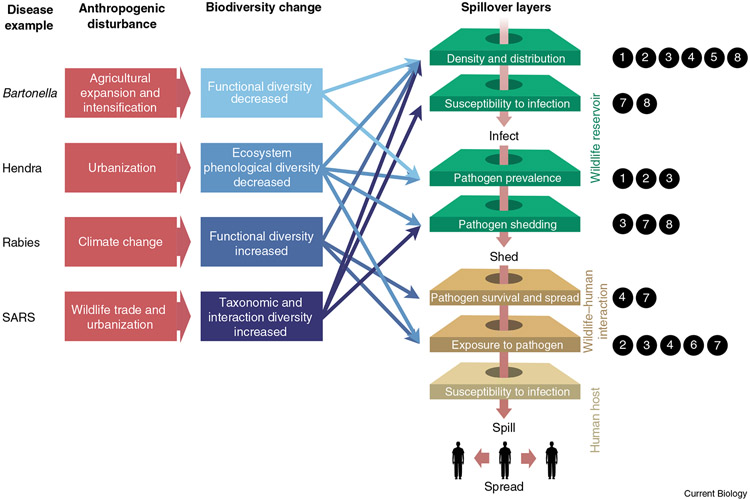
Figure 1. The anthropogenic disturbance, biodiversity change, and spillover cascade.
To understand mechanisms connecting anthropogenic disturbance with spillover via biodiversity change, it is imperative to investigate how anthropogenic disturbance impacts biodiversity, and how those effects drive the perforation of the layers (intermediate processes) leading to spillover (shown using four case studies from Table 1 as examples). Zoonotic spillover arises from the alignment of multiple processes (depicted as layers). Apart from human susceptibility to infection, we found that each layer can be affected by biodiversity change, especially when considering biodiversity along multiple axes (Box 1). Connecting biodiversity change to explicit processes helps us to better understand how, when, and why biodiversity change impacts zoonotic disease risk. Numbers next to each layer correspond to the eight case studies highlighted in Table 1. All references for these case studies are included in Table 1.
Table 1.
Case studies of mechanisms connecting anthropogenic disturbance with biodiversity change and the subsequent effects on infectious disease spillover.
| Infectious disease case studies |
||||||
|---|---|---|---|---|---|---|
| Anthropogenic disturbance |
Biodiversity change (type and direction) |
Mechanisms of biodiversity change |
Spillover layers affected | Disease impacts | No. in Figure 1 |
References |
| Agricultural expansion and intensification | Functional diversity (decreased) | Loss of large consumers increases rodent richness and abundance | Wildlife host density and distribution, and pathogen prevalence | Increased prevalence of Bartonella in rodents in Kenya | 1 | 33 |
| Landscape diversity (decreased) | Resources become limited, pushing animals into human-modified landscapes | Wildlife host density and distribution, and pathogen prevalence; human exposure to pathogen | Increased prevalence and spillover (zoonotic transmission) of P. knowlesi in Borneo | 2 | 63 | |
| Urbanization | Ecosystem phenological diversity (decreased) | Resources become limited, pushing migrating animals to form resident populations in human-modified landscapes | Wildlife host density and distribution, pathogen prevalence, and pathogen shedding; human exposure to pathogen | Increased prevalence, shedding, and spillover of Hendra virus | 3 | 21 |
| Climate change | Functional diversity (increased) | Polar species replaced by migrating nonpolar species (via predation and resource competition) | Wildlife host density and distribution; pathogen survival and spread; human exposure to pathogen | Increased spillover risk of rabies in Alaska as a polar reservoir of rabies (Arctic fox) is being replaced by a more human-landscape adaptable reservoir species (red fox) | 4 | 120,125 |
| Taxonomic and interaction diversity (increased) | Drought and reduction in water resources lead to increased density and diversity of hosts around shared water resources | Wildlife host density and distribution | Increased spillover risk of E. coli in Botswana | 5 | 130,131 | |
| Invasive species | Taxonomic, functional, and interaction diversity (decreased) | Introduction of Burmese python reduces abundance of large- and medium-sized mammals | Human exposure to pathogen | Increased spillover risk of Everglade virus in Florida as mosquito disease vectors feed on rodent reservoirs more frequently | 6 | 136,137 |
| Wildlife trade | Taxonomic, genetic, functional, interaction, and landscape diversity (decreased) | Removal of wild, mostly large-bodied animals (via hunting, trapping, transfer, killing) or overfishing directly reduces abundance and diversity of terrestrial and marine wildlife species | Wildlife host susceptibility to infection, and pathogen shedding; pathogen survival and spread; human exposure to pathogen | Increased spillover risk of Ebola in the Congo Basin as demand for wild meat from small-bodied mammals such as bats (Ebola reservoirs) increases (hunters and preparers of the bushmeat are exposed to bat bites, scratches, or blood) | 7 | 169,171,173,174 |
| Wildlife trade and urbanization | Taxonomic and interaction diversity (increased) | Wildlife markets aggregate novel assemblages of hosts, increasing host richness that is unique to markets and the food supply chain | Wildlife host density and distribution, susceptibility to infection, and pathogen shedding | Increased wildlife susceptibility to infection, reservoir density, pathogen shedding and spread of SARS viruses | 8 | 162,166,167 |
Figure 1 illustrates the overall framework for linking anthropogenic disturbance to biodiversity change to disease spillover via the spillover layers being affected in each case study.
Table 2.
Examples of ongoing biodiversity and sustainability initiatives that could potentially incorporate spillover prevention.
| Initiative | Year founded |
Description | Biodiversity goals | Potential health goals? | Potential extensions for preventing spillover |
Generality | References |
|---|---|---|---|---|---|---|---|
| The Bonn Challenge | 2011 | Launched by the Government of Germany and the International Union for Conservation of Nature to reduce deforestation and promote ecosystem restoration | Obtain pledges for 150 million hectares of degraded and deforested landscapes globally on which to begin restoration by 2020 (which was successfully reached in 2017) and 350 million hectares by 2030 | Improve human health, wellbeing, and livelihood by conserving and restoring degraded or deforested landscapes (no mention of infectious disease burden or spillover per se) | Landscape restoration of wildlife habitat, especially for large-bodied predators and consumers, could potentially help reduce spillover risk driven by increase in rodent abundance due to competitor and predator release related to agriculture and deforestation | 1–3 | 175 |
| Convention on Biological Diversity | 1992 | A list of goals (2020–2050) for sustainable nature-based solutions for improving planetary health and human well-being, set by the United Nations | Address mitigation of biodiversity loss and anthropogenic disturbances | Improve human health and well-being (no mention of infectious disease burden or spillover per se) | The Convention on Biological Diversity handbooks, including in 2020, do not mention actionable next steps for implementing nature-based solutions. How nature-based solutions may target spillover prevention merits further investigation | 1–3 | 195,221 |
| Convention on International Trade in Endangered Species (CITES) of Wild Fauna and Flora | 1973 | A global agreement (182 countries) to regulate the international wildlife trade and ban trade of endangered species | Support surveillance efforts to track species under threat in the international wildlife trade and control illegal wildlife trade activity | Mission statement does not include the prevention of spillover (or improving human health or well-being) | CITES could adopt a pathogen screening regulation scheme to be implemented by all of its member countries to prevent the global spread of emerging diseases that may also hurt endangered wild populations | 2,4 | 189,158 |
| Thirty-By-Thirty Resolution to Save Nature | 2020 | Part of a global effort, spearheaded by the Wyss Campaign for Nature, National Geographic Society, and over 100 organizations | The Natural Resources Defense Council proposed a ‘commitment to protect nature and life on Earth’ urging the US federal government to conserve at least 30% of US lands and 30% of ocean regions by the year 2030 | Mission statement does not recognize the additional human health benefits of reduced spillover risk via the proposed conservation efforts (e.g. conservation of wildlife habitat and corridors for safe passage of wildlife between intact habitats) | Wildlife corridors would aid conservation of natural predators and large consumers, which could help reduce spillover risk of zoonotic disease where predators keep reservoir populations in check (e.g. rodents) or where corridors help migrations of large herbivores (e.g. caribou) reducing brucellosis risk | 1–3 | 176,177,222,223 |
| Payments for Ecosystem Services (PES) Program in Costa Rica | 1997 | PES requires those who benefit from ecosystem services to compensate stewards of these services (e.g. landowners keeping forests intact should be compensated for the services their forests provide, such as carbon sequestration, clean air, and clean rivers) | Forest conservation and restoration aimed to improve biodiversity conservation and other recognized ecosystem services (e.g. watershed services, carbon sequestration, and landscape beauty) | PES programs do not explicitly include infectious disease or spillover prevention | Spillover prevention could be embedded in existing efforts (or be introduced as its own ecosystem service). PES schemes that conserve contiguous and diverse forests could potentially benefit spillover prevention by reducing density of small-bodied mammal reservoir hosts, and intact forests serve as carbon sinks (thereby mitigating climate change effects on spillover) | 1–3 | 178,179 |
| Project Finance for Permanence (PFP) | 2010 | A model that includes restoring and conserving contiguous intact ecosystems. PFP programs, e.g. Amazon Region Protected Areas (ARPA), are funded by foundations, NGOs (e.g. WWF), and government agencies | Aims to improve the abundance and management of intact ecosystems. ARPA intends to create, consolidate, and maintain a 60-million-hectare network of protected areas in the Brazilian Amazon | Although not a specific PFP objective, ARPA has likely reduced cases of malaria transmission in the Inner Amazon by slowing the rate of deforestation. This example highlights the potential joint benefits of the PFP model for conservation and public health | Spillover prevention is not yet incorporated in PFP programs, although they could be extended to zoonotic spillover prevention via similar mechanisms to PES programs | 1–3 | 182,183,224 |
Several initiatives are listed along with the four generalities discussed in the main text section ‘Incorporating concepts of ecological diversity to mitigate spillover risk’ that may be considered applicable. Generality numbers in the tables refer to: 1) Large, intact habitat reduces overlap among host species and promotes wildlife health; 2) Loss of predators and competitors reduces regulation of reservoir host and vector populations; 3) Reservoir hosts are better adapted to human-modified systems; and 4) Human activity may increase opportunities for novel interspecies contacts.
Biodiversity encompasses all forms of variability among living organisms and the ecological complexes of which they are a part; these different forms of variability have long been studied and summarized into related but alternative definitions of biodiversity by other ecological fields17 (Box 1). Change in taxonomic diversity, including species richness, is often an observable outcome of changes in other types of biodiversity, which more explicitly guide conservation efforts such as the loss of functional groups, changes in interaction networks, and heterogeneity in habitat composition. Identifying how these underlying axes drive proximate changes in ecosystem processes like disease transmission is critical for responding to human-mediated (that is, anthropogenic) change10-16. Zoonotic spillover is influenced by many ecological processes before a pathogen actually spills over into a human host. Therefore, changes in biodiversity can mechanistically affect spillover through several pathways including effects on the density, distribution, and susceptibility of reservoir hosts, as well as pathogen prevalence, infectiousness, survival, dissemination, and reservoir host–human contact18,19 (Figure 1). Once in the recipient (human) host, a series of biological and epidemiological factors determine whether onward transmission is possible18-21 (Figure 1). To harmonize spillover prevention and biodiversity conservation, a clear mechanistic understanding is needed of how increases and decreases in multiple aspects of biodiversity, from individuals to populations to communities to ecosystems, influence spillover processes (Figure 1).
Box 1. Dimensions of biodiversity.
There are a number of dimensions that comprise ‘biodiversity’, each with multiple axes affecting zoonotic spillover risk. Below are a handful of examples described by Naeem et al.22, with suggestions for how to measure and track each aspect using the universally developed Group on Earth Observations Biodiversity Observation Network’s essential biodiversity variables (EBVs)225.
Genetic diversity includes aspects of genomic variability, including nucleotide, allelic, chromosomal, and genotypic variation. Genetic diversity has yet to be studied in the context of biodiversity change and zoonotic disease risk; however, multiple reviews14,15 have described how observable patterns in taxonomic diversity are likely, at least in part, the result of genotypic variation governing phenotypic variation in host physiology and behavior (that is, host resistance, tolerance, and competence) and thus can influence zoonotic disease risk. EBVs: Intraspecific genetic diversity, Genetic differentiation.
Taxonomic diversity refers to the number and relative abundance of taxa (for example, species, genera, and higher levels of taxonomic organization). Disease–diversity relationships are typically described within the context of species richness. One example relevant to spillover is an increase in diversity of host species, so that vectors take ‘wasted bites’ on non-competent hosts. In many cases, change in taxonomic diversity per se does not influence zoonotic disease spillover; however, change in the other dimensions of biodiversity are evident through changes in taxonomic diversity. EBVs: Species distributions, Species abundances, Community abundance, Taxonomic/phylogenetic diversity.
Functional diversity refers to the variation in the degree of expression of multiple functional traits: that is, the different types of processes in a community that are important to its structure and dynamic stability. Examples relevant to spillover include loss of predators and competitors and increase in abundance of generalist, synanthropic animals. EBV: Trait diversity.
Interaction diversity refers to the number and relative abundance of interactions among species in a community226. These biotic interactions include contact, competition, facilitation, and predation. Examples relevant to spillover include a loss of interactions regulating reservoir host species or an increased number of novel cross-species interactions via crowding. EBV: Interaction diversity.
Ecosystem phenological diversity is the diversity in the phenological dates of life within an ecosystem (for example, flowering time). Phenological diversity is a subset of temporal diversity, which is broadly thought of as change in biodiversity over time. An example relevant to spillover is the reduction in the seasonal availability of resources, which in turn affects sedentary movement and eating habits. EBV: Phenology.
Landscape diversity* is composed of compositional and configuration diversity. Landscape compositional diversity includes diversity of habitat patches, and configuration diversity includes the number, size, and arrangement of habitat patches. An example relevant to spillover is an increase in the number of reservoir habitat patches while decreasing their size, thereby providing increased opportunity for reservoir host–human or host–vector contact. *Note that landscape ecologists commonly refer to ‘landscape diversity’ as ‘heterogeneity’. EBVs: Live cover fraction, Ecosystem distribution.
This review focuses on how infectious-disease systems change with shifts in biodiversity, highlighting case studies that suggest causal mechanisms (Figure 1 and Table 1). We group case studies based on the leading International Union for Conservation of Nature-classified threats to biodiversity. Although examples that mechanistically link environmental change to zoonotic spillover via at least one metric of biodiversity change are scarce, our review identifies emerging generalities across disease systems and anthropogenic disturbances. We find the best support for an influence of functional, interaction, ecosystem phenological, and landscape diversity on spillover risk but recognize that there are additional dimensions of biodiversity not explicitly studied that are likely to influence spillover (for example, genetic diversity22). Within our description of the generalities, we identify ongoing sustainability initiatives that could incorporate spillover prevention, emphasizing how reframing the discussion about biodiversity and disease may facilitate win–win outcomes for health and the environment.
Anthropogenic disturbance, biodiversity change, and disease spillover
Land conversion, agricultural intensification, and urbanization
As of 2019, agricultural expansion and intensification were the leading causes of biodiversity loss17. Agricultural development both clears and fragments previously intact ecosystems, creating edge habitats that increase human encroachment on wildlife, homogenizing landscapes to reduce availability of natural resources for wildlife, and releasing pesticides, fertilizers, and antimicrobial compounds into the environment. Urbanization, characterized by the presence of built environments, similarly clears intact ecosystems while increasing air, water, light, and land pollution23. Moreover, urbanization significantly increases human density: 70% of the world’s population is expected to live in urban areas by 205024. All of these factors contribute to population declines or even local extinctions of species25-27 and may influence the dynamics of infectious diseases that have an important environmental component in their transmission cycle28.
Clearing intact ecosystems for agriculture, urbanization, and other land modifications (including development of forestry) drives the loss of large- and medium-bodied animals (that is, defaunation) while supporting the persistence or growth of populations of small-bodied animals29-32. Recent research has made it clear that loss of functional diversity (defined in Box 1) due to non-random patterns of defaunation has significant effects on zoonotic spillover risk10,11,16,33-39. Increase in disease spillover risk due to changes in functional diversity of animal communities may occur through the expansion or invasion of opportunistic zoonotic hosts that thrive in human-modified landscapes or through the cascading effect of human-induced extirpation of predators and competitors of zoonotic species, as described below.
Small-bodied mammals are common pathogen reservoirs, with the rodent and bat orders containing the highest number of known zoonotic hosts40-43. Certain taxa of small-bodied animals are likely to predominate in human-modified landscapes due to traits that make them adaptable to living in proximity to humans44,45. These traits, including diet and habitat generalism, fast-paced life history, high population density, and proximity with human settlements are positively correlated with zoonotic reservoir status12,34,41. On a global scale, the richness and abundance of zoonotic hosts (especially birds, bats, and rodents) positively correlate with the degree of human-mediated land modification34,46. Local studies in Kenya, Tanzania, and Madagascar found that this change in functional diversity, such that communities are dominated by animals with traits conducive to adaptation to human environments, increases zoonotic disease risk: rodent communities in croplands had a higher proportion of competent zoonotic reservoir hosts and higher prevalence of zoonotic pathogens than in unmanaged areas16,35,47.
Loss of functional diversity in ecological communities may also be driven by the loss of predators and competitors that help regulate populations of reservoir hosts and vectors. Land conversion can drive the replacement of large herbivores with small herbivores, altering the overall effect of herbivores on the plant community and ecosystem as a whole33,48. In savanna ecosystems in central Kenya, exclusion of large herbivores through fencing, an experimental simulation of what often occurs with agricultural intensification, resulted in changes in the plant community and competitive release of small herbivores, leading to the increase in abundance of competent rodent hosts (Saccostomus mearnsei) and prevalence of Bartonella and its vectors33,49 (Figure 1, Table 1, and Figure 2A). Predators of reservoir hosts and vectors might also exert a crucial role in modulating the risk of disease spillover for humans10,11. In Senegal, the construction of the Diama dam in 1986 to prevent saltwater intrusion and support agricultural intensification blocked the migration of a native predator (the giant river prawn, Macrobrachium vollenhoveni) that consumes snail vectors and free-living Schistosoma spp., resulting in increased transmission of vector-borne parasites to humans36. These findings have been linked to construction of other large dams as well, and the subsequent increases in schistosomiasis transmission throughout Africa38. In terrestrial zoonotic disease systems, the presence of leopards may decrease risk of rabies transmission to humans by preying on stray dogs in Mumbai, India37. Further, predator loss can trigger significantly more complex trophic cascades. The loss of wolves in the northeastern United States was followed by an increase in coyotes. This resulted in increased predation by coyotes on mesopredators (such as foxes), leading to a dramatic reduction of predators of small mammals that control the abundance of rodents that carry Lyme disease11. This release of competent rodent reservoir hosts from predation has been linked to expansions in Lyme disease in the last two decades10,11.
Figure 2. Taxa and habitats affected by agricultural intensification, urbanization, and species invasion.
(A) The competent rodent host species (Saccostomus mearnsei) of Bartonella in Kenya (image courtesy of Hillary Young). Reduced functional diversity, due to loss of large consumers and driven by agricultural expansion and intensification, increases rodent richness and abundance and thus Bartonella spillover risk. (B) The natural habitat of the flying fox (a fruit bat of the genus Pteropus) is threatened by land conversion and urbanization (reducing ecosystem phenological diversity), which in turn aggregates flying foxes at higher densities in urban areas and brings humans into closer proximity with these natural reservoirs of Hendra virus (photo by Elizabeth Shanahan). (C) Supplemental feeding of elk (Cervus canadensis) during winter months in Yellowstone National Park (image courtesy of United States Geological Survey). Agricultural conversion of land in North America has limited the availability of natural winter-feeding grounds for elk (reduced ecosystem phenological diversity). Large populations are dependent on supplemental feeding, reducing migration and promoting high density aggregations, thus increasing the risk of brucellosis spillover to livestock and humans. (D) A Burmese python (Python bivittatus) in the Everglades in Florida, USA (photo by Anne Devan-Song). This invasive species has reduced biodiversity in the Everglades (taxonomic, functional, and interaction diversity), thereby increasing the rate at which vectors feed on competent hosts of Everglade virus and thus spillover risk in this region.
In general, land conversion for agriculture can affect landscape diversity (Box 1), thereby altering species distributions and changing contact patterns between wildlife and humans50-52. Landscape diversity can be described as compositional diversity (including patch-type diversity, defined as richness of habitat types among patches) and configuration diversity (including number, size, and arrangement of habitat patches). These aspects of landscape diversity have nonlinear and complex responses to anthropogenic change53. As many existing biodiversity initiatives center around land conservation and restoration, including landscape diversity in the biodiversity–disease discussion is crucial for identifying synergistic solutions for biodiversity conservation and preventing zoonotic spillover. Within monocultures, all metrics of landscape diversity are reduced. However, in relation to intact ecosystems, moderate agricultural conversion has various effects on patch-type diversity, decreases patch size and thus variation in patch size, and increases the distance among intact habitat patches54-56. Fragmenting of habitat into small patches can shift the distribution of reservoir species, causing them to aggregate at high densities near humans and increasing their contacts — with humans, previously unencountered mammals, and vectors — thereby increasing potential for transmission57. For example, Plasmodium knowlesi malaria is expanding in Malaysia and across Southeast Asia, partially due to forest loss and agricultural land conversion58-63. These disturbances drive the primary reservoir hosts, long-tailed macaques (Macaca fascicularis) and pig-tailed macaques (Macaca nemestrina), to occupy small forest fragments within or next to agricultural areas where they overlap with anthropophilic mosquito vectors and people63-65. This shift in distribution not only increases the density of reservoirs, potentially increasing transmission among reservoir hosts, but also increases potential for macaque–vector–human transmission63 (Table 1). High profile zoonotic pathogens, such as Ebola virus, may similarly spill over in forest fragments66,67, highlighting the links between changes in landscape configuration and diversity on zoonotic spillover risk.
Shifts in landscape diversity that skew functional diversity towards favoring reservoir hosts may also increase the risk of zoonotic spillover by antimicrobial-resistant organisms. Runoff from antibiotic-fed livestock forms wastewater lagoons where diverse bacteria mix. There they face strong selective pressures to evolve and share (via horizontal gene transfer) genes conferring resistances to those antibiotics68,69. This also occurs in aquaculture waters70, wastewater from antibiotic-treated crops71, and effluent from wastewater treatment plants72. Wildlife that come in contact with polluted waters or soils can pick up these antimicrobial-resistant bacteria and transport them to both neighboring and distant croplands or livestock operations, where they can spill over to people73-77. Global rates of antimicrobial resistance are on the rise, driven by the misuse of antibiotics in both clinical settings and agriculture, with an estimated 700,000 deaths worldwide caused by antimicrobial-resistant bacterial infections78. Although existing research on wild animal reservoirs of antimicrobial-resistant bacteria is severely limited79, initial studies show that animal populations proximate or adaptable to human-modified habitats have higher prevalence of antimicrobial-resistant bacteria than animals with little to no contact with humans80, perhaps due to higher host competency and/or exposure rates to these potentially infectious agents. Smith et al.80 found that the prevalence of antimicrobial-resistant bacteria in agricultural areas decreased as the amount of native habitat increased, possibly due to reduced contact between birds and livestock runoff. As a result, landscape composition and configuration may reduce the likelihood of birds becoming inoculated with and transmitting antimicrobial-resistant bacteria. Landscape diversity may decrease antimicrobial-resistance risk both by protecting croplands from livestock wastewater runoff and by providing vegetation that acts as a natural ecosystem filter81. The effect of biodiversity changes on antimicrobial-resistance spillover is severely understudied but warrants significant attention79,80 given the threat of antimicrobial-resistant bacteria to global public health82.
Land conversion can also reduce the phenological diversity of natural ecosystems and food sources (that is, diversity of temporal or cyclical biological cycles; see ‘Ecosystem phenological diversity’, defined in Box 1), which can cause nomadic and migrating species to forgo migration in favor of occupying the same habitat year-round. In some cases, formation of resident populations may shift reservoir-host dynamics and alter zoonotic spillover risk, particularly when loss of seasonal, high-quality natural resources is paired with provisioning of non-seasonal, subpar food83. For example, the reservoir hosts of Hendra virus, the Pteropus spp. fruit bats, form large nomadic groups that track seasonally abundant nectar sources. Loss of optimal winter resources, at least in part due to habitat loss, drives these animals into small resident groups feeding on permanent, suboptimal food within and around cities21,84,85 (Figure 1, Figure 2B, and Table 1). Not only does this bring these bats into closer proximity to humans but also food stress associated with these suboptimal resources may promote viral shedding, increasing the likelihood of the virus spilling into amplifying hosts (that is, hosts in which a pathogen can rapidly replicate to high concentrations, for example horses in this case) and humans86. Similarly, agricultural conversion has limited the availability of high-quality winter resources for elk, which serve as reservoir hosts of Brucella abortus (Figure 2C). Large elk populations are now supported by lower-quality supplemental feeding, which reduces migration and promotes high-density aggregations, thereby increasing the spread of Brucella among these animals and potentially spillover to livestock87-90. Climate change may further exacerbate loss of phenological diversity and interrelated shifts in animal movement; however, this has not been explicitly linked to zoonotic spillover91.
Finally, the rural to urban transition that has occurred over time has released local economies from dependence on local agriculture and opened up trade of goods, services, and ideas with more distant places92. Through trade with rural areas, urbanization interacts with other biodiversity threats to drive changes in zoonotic spillover; for example, via introduction of pathogens through the wildlife trade and introduction of invasive species93. Drastic reduction of non-human-adapted animals in completely converted land (that is, cities) may reduce the frequency of spillover of novel zoonotic pathogens94. At the same time, interactions between urbanization and other anthropogenic disturbances creates circumstances for pathogen introduction, especially if pathogens can be sustained via human–human transmission. For example, urban centers serve as hubs for long-distance shipping, with urban wildlife markets often containing higher densities and diversity of wildlife. Thus, urban wildlife markets create unique assemblages of species, subsequently increasing the likelihood of novel cross-species transmission95. Then, in the rare case where the biology of the pathogen allows frequent human–human transmission (for example, high infectivity to humans, asymptomatic transmission, aerosol transmission19), the large and dense human populations found in cities can facilitate rapid pathogen spread, resulting in large epidemics94 or even pandemics. Spread of novel zoonotic pathogens may be mitigated by increased health and subsequent reduced susceptibility in affluent urban areas96. However, the opposite may be true in urban areas that are unplanned or designed to oppress groups of people (that is, without centralized infrastructure and equitable distribution of resources). In these areas, human health might be compromised by increased pollution, lack of affordable healthcare, and limited access to healthy food and clean water93,97.
Climate change
Species may respond to climate change through phenotypic plasticity98, rapid adaptive evolution99, and altitudinal and latitudinal range shifts to the edge of their geographic ranges100-102. Alternatively, species may undergo local population extinctions, range shifts, or even global extinction103-107. Further, the velocity of rising temperatures varies across different regions of the world, affecting species and populations differently108. Together these responses can drive biodiversity change in complex, nonlinear, and interdependent ways. Here, we focus on case studies of range shifts in response to rapid anthropogenic climate change, as it is the most immediately observable impact of climate change on wildlife hosts that harbor zoonotic pathogens109,110. Plastic, adaptive, and local declines or extirpation responses are currently well researched111-113, with the amphibian decline being perhaps the most emblematic case114, but they are rarely connected to pathogen spillover.
The abundance of different species with certain traits or ecosystem functions (for example, diet, habitat, activity patterns, etc.), and thus functional diversity, may decline with range shifts, especially at high latitudes, although taxonomic diversity (Box 1) of some systems may increase with range shifts115-117. This is largely attributed to generalists outnumbering specialists in systems impacted by global change, as generalists are able to thrive in a variety of ecological conditions, including human-modified landscapes, whereas specialists need specific resources and/or habitats to survive. At the same time, correlative analyses suggest that zoonotic reservoirs are more likely to be generalist species34,39,118, as they are more likely to live in closer proximity to people and contact a wider range of other host species. Further, climate-induced loss of forest habitat may lead to an increase in abundance of extreme generalists with zoonotic reservoir potential, as in the case of the highly adaptable deer mice harboring Sin Nombre virus119.
The Alaskan Arctic is currently exhibiting climate-induced shifts in host species, with an increase in the abundance of zoonotic hosts more likely to contact humans. Before contemporary climate change, the ranges of Arctic and red foxes (Figure 3A,B), both of which serve as reservoir hosts for rabies, were separated120. However, with climate change, the home range of the generalist red fox has expanded northward, encroaching on the territory of the Arctic fox, which is more of a habitat specialist121. Arctic fox numbers were already in decline due to other effects of climate change, such as the loss of sea ice and tundra habitat as well as loss of lemming prey, but red foxes are expediting this decline through intraguild predation and competition for resources122-124. As Arctic fox populations are replaced by red fox populations, the red fox will become the primary reservoir for rabies spillover. As immigrant red foxes increasingly interact with resident Arctic foxes, this shift in the reservoir community will likely increase epizootic peaks of rabies, increasing both the transmission rate and the overall density of susceptible individuals125. Further, because the larger-bodied red fox displays more aggressive behavior than the Arctic fox120, and because it is more adaptable to human-dominated landscapes, contact rates between wild rabies reservoirs and dogs or humans might increase, thus increasing rabies spillover risk (Figure 1, Table 1, and Figure 3A,B).
Figure 3. Taxa and habitats affected by climate change and wildlife trade.
(A) An Arctic fox (Vulpes lagopus) in Alaska (image courtesy of Alaska Department of Fish and Game). Climate change may increase functional diversity in polar and temperate regions as native fauna, such as the Arctic fox, is being replaced by northwardly range-shifting species, such as the red fox (B; Vulpes vulpes) (photo by Peter Hudson). This could potentially increase rabies spillover to humans in Alaska as the red fox is generally a more human-landscape adaptable reservoir species. (C) Several species aggregating around a small water hole in southern Africa (photo by Nick Fox). In the tropics and sub-tropics, climate change is reducing water availability, which may increase taxonomic and interaction diversity. This in turn could increase spillover risk of E. coli as more hosts start to share common water resources. (D) Elephants in Tarangire National Park, Tanzania, protected from poaching (photo by Peter Hudson). The wildlife trade is reducing wild elephant populations and other large-bodied animals, thereby decreasing biodiversity (taxonomic, genetic, functional, interaction, and landscape diversity) and leading to a higher demand for meat from small-bodied mammals such as bats, potentially increasing spillover risk of Ebola and other disease borne by small mammals.
Climate change may reduce other dimensions of biodiversity beyond functional diversity. For instance, climate change may reduce landscape diversity by reducing patch diversity and subsequently increase the likelihood of cross-species transmission through increased habitat overlap and taxonomic diversity in confined areas126. For instance, the melting of sea ice alters, disrupts, or even prevents migration patterns of animals such as wild caribou127, increasing the chance of intermingling among caribou and with other wild or domestic ungulates. Thus, people who rely on caribou and/or other livestock might be at higher risk of brucellosis spillover under a warming climate in temperate regions128. Similarly, in water-stressed parts of Africa, extreme droughts can force many animals — that may previously have used different water bodies and had little to no contact with one another, such as humans, wildlife, and livestock — to congregate at common water sources129,130 (Figure 3C), increasing traffic and reducing water quality due to elevated fecal loads. In Chobe National Park, Botswana, these patterns and processes are associated with increased loads of Escherichia coli, the leading cause of diarrheal outbreaks130. Following drought events in and around Chobe National Park, heavy seasonal rainfall and flooding mobilize pathogen-containing feces, subsequently leading to human diarrheal outbreaks in neighboring communities131 (Table 1). Further, these water sources have the potential to serve as melting pots of antimicrobial resistant bacteria and sources of novel pathogen emergence132.
Invasive species
Invasive species (that is, organisms that establish and spread outside their native range) present a significant threat to ecosystems and human well-being by negatively impacting native biodiversity and ecosystem services133. Through processes such as predation, competition, and environmental modification, invasive species can drastically decrease the biodiversity of an ecosystem; an estimated 30 species of invasive predators alone are responsible for at least 58% of all bird, mammal, and reptile extinctions globally134. Invasive species can indirectly impact infectious disease by altering the structure and composition of the native community in ways that either increase or decrease pathogen transmission.
Altering a native community in a way that increases zoonotic spillover risk has been empirically demonstrated for the Everglade virus, a mosquito-borne zoonotic virus. The introduction of the Burmese python (Python bivittatus; Figure 2D) to the Florida Everglades has led to large-scale declines in functional and taxonomic mammalian diversity due to predation and subsequent precipitous loss of large and small-bodied mammals135,136. With the loss of deer, racoons, and opossums as food sources for blood-sucking arthropods, mosquito vectors of Everglades virus turned increasingly to the primary reservoir host of the virus, the hispid cotton rat (Sigmodon hispidus). This has resulted in higher rates of Everglade virus infection in mosquitoes, potentially increasing the risk of virus exposure to humans136,137. The Burmese python–Everglade virus case study is a clear example of the dilution effect: higher taxonomic diversity of hosts reduces disease risk because the vector takes ‘wasted bites’ (from a pathogen-transmission perspective) on non-competent hosts. The loss of taxonomic diversity therefore increases disease spillover risk, with the dilution effect most commonly occurring for vector-borne, zoonotic pathogens, as is the case here9.
In contrast, introduction of invasive species can sometimes reduce transmission of infectious disease from vectors to people through predation on various vector life stages: for example, larvivorous fish preying on malaria vectors138 and crayfish consuming schistosome intermediate hosts139. However, despite crayfish lowering the risk of schistosomiasis by voraciously consuming snail intermediate hosts and free-living parasites, invasive crayfish compromised other dimensions of human health by consuming rice and degrading canal banks with their burrows140. Consequently, in scenarios where invasive species reduce disease risk there can still be a tension between biodiversity impacts of invasive species and their specific ecological roles in infectious-disease dynamics.
Invasive species may also affect infectious-disease dynamics by acting as vectors or reservoir hosts40,47,141-143, sharing pathogens with native species144-146, or providing resources for reservoirs and/or vectors143,147. In these cases, biodiversity conservation via invasive species control may simultaneously reduce zoonotic spillover risk143. The same processes that drive species introductions, including global trade and travel, may also drive disease emergence, suggesting that win–win solutions for protecting ecosystems from species invasion and humans from pathogen spillover might be possible, albeit potentially challenging from a technical or political perspective148.
Wildlife hunting, trade, and consumption
One in five vertebrate species is impacted by trade149, with some wildlife facing population declines and/or species extinction due, mainly or in part, to the impacts of wildlife trade — some legal but primarily illegal (for example, tigers, rhinoceroses, elephants, sharks, and pangolins)150,151 (Figure 3D). The illegal wildlife trade is estimated to be the world’s second largest underground business (hypothesized to be a 5–20 billion-dollar industry) after narcotics152. The legal wildlife trade, estimated to be an even larger business (300 billion-dollar industry), also poses a threat to biodiversity as the majority of legal wildlife trade (78%) is composed of wild-caught animals, as opposed to those reared in captivity153. The local increase or decrease of biodiversity, as well as novel contacts made during translocation and trade between species that do not co-occur naturally in the wild, may drive spillover and disease emergence, as explained below.
Epidemiological and genetic analyses have linked wildlife hunting, trade, and consumption to spillover and spread of many high-profile zoonotic pathogens: rabies virus, Crimean-Congo hemorrhagic fever virus, the plague-causing bacteria Yersinia pestis, monkeypox virus, coronaviruses, HIV, Marburg, and Ebola viruses150,151,153-156. However, in order to stop or mitigate the spillover process, we need to better understand the mechanisms linking the wildlife trade to the eco-epidemiological process of spillover (Figure 1).
The wildlife trade highlights how anthropogenic pressures can increase spillover risk via a direct increase in both taxonomic diversity and the number of interactions across taxa on very small spatial scales (see ‘Interaction diversity’ defined in Box 1). Throughout the supply chain, the wildlife trade brings together high densities of species that typically would not contact each other in natural habitats. These unique assemblages and interactions can promote cross-species transmission, increasing the likelihood that a pathogen may be transmitted to amplifying hosts and/or humans154,157-163. Trade may also impact the spillover process by promoting pathogen shedding from animals because of stress during transportation and unsanitary conditions at markets154,157-163. For example, the ancestor to SARS-CoV-1 is suspected to have been transmitted from horseshoe bats (most likely Rhinolophus sinicus) to palm civets, two species that do not interact in wild settings. However, palm civets served as amplifying or intermediate hosts within wildlife markets, bringing the virus in closer proximity to humans164-166. Seroprevalence and virological testing surveys of civets on farms versus those brought to markets in Guangdong, China suggest that palm civets were exposed to the virus at the end of the supply chain165-167. In a study in Vietnam, the prevalence of coronaviruses in field rats caught or reared for human consumption and sold in markets was more than double that of field rats in the wild162. Further, coronavirus prevalence was ten times higher in field rats sold or served in restaurants compared with field rats in the wild162. Thus, the wildlife trade creates opportunities for increased transmission among multiple wild animal species and puts humans in closer proximity to stressed and infected wildlife, fueling the potential for spillover of pathogens (Figure 1 and Table 1).
The wildlife trade for human consumption can take on various forms, including commercial harvesting of wild animals on land and at sea. Together, these interact to amplify the effects of overharvesting, leading to a decrease of many types of biodiversity, such as taxonomic, genetic, functional, interaction, and landscape diversity (Box 1). For example, the wild meat trade in Ghana, which has driven population declines of some mammalian species in the last few decades, correlates with local declines in fish supply, probably due to commercial overfishing off the coast168,169. Conceivably, during periods when the demand for wild meat is high, hunters and people involved with butchering and preparation are at a higher risk of disease spillover from bites, scratches, and contacts with bodily fluids of animals that serve as pathogen reservoirs. In the Congo basin and other regions where pathogens have recently emerged, wild meat serves as an important protein source in impoverished households. This makes the banning of wild meat a controversial topic170 even though genetic and epidemiological evidence suggest that wild meat consumption has contributed to the rise of emerging diseases and recent outbreaks via spillover from wildlife to humans of pathogens like Ebola (Table 1), HIV, Marburg, and monkeypox viruses154,171,172. In Cameroon, simian foamy viruses regularly spill over and infect wild meat hunters, but no human–human transmission has yet been established154. Conversely, although HIV has adapted to undergo human–human transmission, phylogenetic analyses suggest that approximately ten spillover events occurred over the past century before it eventually evolved to cause a pandemic, suggesting that frequent spillover during bushmeat hunting was critical for its emergence151.
Overexploitation of wild meat and other anthropogenic pressures have also been correlated with a decrease in the proportion of large-bodied mammals and an increase in the proportion of small-bodied mammals brought to market173,174. As a result, preliminary research suggests that overharvesting of wildlife may influence the types of wild animals that hunters and consumers are contacting, potentially presenting new zoonotic spillover risks. However, mechanistic links between change in composition of wildlife markets and zoonotic disease risk have not yet been established.
Incorporating concepts of ecological diversity to mitigate spillover risk
Although mechanistic research linking changes in biodiversity to zoonotic spillover risk is limited due to expense and logistical challenges, by considering more mechanism-based changes in biodiversity, we collect enough empirical examples to propose four general concepts that have potential to inform biodiversity conservation. These generalities may motivate further integration of biodiversity and zoonotic pathogen spillover research, potentially opening more avenues of funding and facilitating the incorporation of multi-disciplinary methods for collecting and analyzing data. To illustrate this application of our synthesis, we identify ongoing biodiversity and sustainability initiatives that could use these generalities to incorporate spillover prevention. Using the framework we propose may, for example, help to avoid unintended harms from biodiversity conservation or broaden the benefits of biodiversity conservation. Echoing Halsey8, we distinguish between generality, that which is mostly considered true, and universality, that which is considered true in all possible contexts. These four generalities (described below) may be more or less applicable for different ecosystems and disease threats.
Generality number 1: Large, intact habitat reduces overlap among host species and promotes wildlife health
Loss of spatially and phenologically diverse habitat alters the spatiotemporal distributions of reservoirs, leading to increased overlap with other vertebrate hosts, vectors, and humans. This generality suggests an opportunity: preserving and restoring large, contiguous, and heterogeneous habitats could minimize harmful contact between humans and wildlife and between host species that do not commonly interact (for example, a reservoir and an amplifying host). Such an approach might additionally reduce the density of reservoir hosts and subsequent intraspecific contact and transmission. The Bonn Challenge175, Thirty-by-Thirty Resolution to Save Nature176,177, Payments for Ecosystem Services178-180, and Project Finance for Permanence projects181-183 all include conservation and/or restoration of natural ecosystems but do not incorporate spillover prevention in project design and implementation (Table 2). Intact and diverse contiguous landscapes may also promote landscape immunity, defined as ecological conditions that maintain and strengthen immunity in resident fauna so as to reduce pathogen susceptibility and shedding, particularly for potential reservoir species including bats and rodents184,185. Further, targeted habitat conservation and restoration could encourage previous migration patterns by re-creating or maintaining phenological diversity of high-quality food sources, such as nectar resources for bats21,143. However, in some cases, resource provisioning — through invasive species, crops, and even waste disposal practices — may reduce migration even when endemic, phenologically diverse habitats are available186,187. More research differentiating the impact of habitat restoration versus limiting human provisions is needed. Importantly, some biodiversity conservation initiatives, such as Payment for Ecosystem Services in Costa Rica179, include agroforestry, which could hypothetically increase human exposure risk to zoonotic disease188. In these cases, the effect of landscape diversity and specific agroforestry practices on spillover should be considered so as not to put biodiversity conservation and public health at odds. Overall, studying the mechanistic effect of landscape diversity and ecosystem phenological diversity on each spillover process (Figure 1) should lead to new insights that can guide evidence-based policy for both conserving natural ecosystems and reducing spillover risk.
Generality number 2: Loss of predators and competitors reduces regulation of reservoir host and vector populations
Loss of large consumers and predators (changes in functional diversity) can result in increased abundance of animals with fast growth rates and relatively small ranges, such as rodent reservoirs and arthropod vectors. Regulation of poaching (for example, via the Convention on International Trade in Endangered Species189 initiative) and habitat conservation, preservation, and restoration of contiguous, intact ecosystems could support populations of large predators and herbivores174,190,191. In turn, predators and large consumers may be important in ecotones between intact and anthropogenic landscapes, where they can regulate populations of small-bodied reservoirs that thrive in human-modified areas. The initiatives aimed to restore and conserve habitat in Table 2 could be adapted to support populations of wildlife that help regulate rodent populations. For example, the Thirty-by-Thirty Resolution to Save Nature176,177 proposes conservation of wildlife habitat and corridors for safe passage of wildlife between intact habitats. This plan could be improved by configuring habitats and corridors to best support populations of keystone predators and large consumers in areas of zoonotic disease risk. More research is needed to understand the impacts that large herbivores and predators have on zoonotic disease regulation, especially within and around ecotones. If more evidence supports a beneficial effect of conserving predators and large herbivores for reducing spillover risk without increasing human–wildlife conflict, conservation of predators and large consumers may offer another promising solution.
Generality number 3: Reservoir hosts are better adapted to human-modified systems
Human modification further affects functional diversity by changing habitats and shifting communities toward dominance by species that are resilient to anthropogenic disturbance or thrive in human-dominated landscapes. Change in functional diversity towards such ‘synanthropic’ species has been observed across taxonomic groups of vertebrates including rodents, birds, and carnivores. Similar effects have been observed for disease vectors: generalists thrive in urban areas and have high capacity to transmit pathogens to humans38,192,193. Integrative approaches, such as direct management of invasive rodents and vectors or indirect management through preserving intact habitat and mitigating impacts of climate change to reduce range shifts of reservoirs and vectors, are likely necessary143,194. Initiatives that guide policy and coordinate action to protect biodiversity from multiple anthropogenic threats, such as the Convention on Biological Diversity195, may be particularly well suited to prevent spillover from these human-adapted reservoirs and vectors. For example, the Convention on Biological Diversity sets global priorities and coordinates global action on invasive species and climate change, providing a platform to jointly manage invasive reservoir hosts and vectors while advocating for climate resilient ecosystems on a global scale.
Generality number 4: Human activity may increase opportunities for novel interspecies contacts
Commercial wildlife trade, introduction of invasive species, and transportation of livestock and companion animals are activities that increase interaction diversity, introduce more opportunities for cross-species transmission, and facilitate the emergence of new pathogens with zoonotic spillover potential. The Convention on International Trade in Endangered Species189 aims to control the illegal wildlife trade but does not include objectives that prevent spillover. Adopting global regulations on pathogen screening and ethical and sanitary animal husbandry standards in the international wildlife trade could be a natural next step in advancing management of zoonotic spillover. Overall, regulations and initiatives that reduce diversity of novel interspecies interactions should be adjusted to incorporate spillover prevention.
Other international initiatives are currently working towards sustainable solutions for promoting both public health and conservation, such as the UN Sustainable Development Goals196, Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) Nature’s Contributions to People197, International Union for Conservation of Nature’s Global Standards for Nature-Based Solutions198, Bridge Collaborative199, Pan American and World Health Organizations (PAHO/WHO) Climate Change and Health200, Global Health Security Agenda201, and the collaboration among Food and Agriculture Organization (FAO), World Organisation for Animal Health (OIE), and WHO (FAO-OIE-WHO Collaboration)202. The initiatives included in Table 2 have not yet incorporated spillover prevention.
We emphasize that the initiatives described here must only be implemented based upon local context, centered around the needs, demands, and culture of the local people. A number of global restoration and conservation efforts have been criticized as colonialist and thus detrimental to vulnerable and marginalized groups of people. For example, the Bonn Challenge has been criticized for foresting historically savannah ecosystems, thereby impacting ecosystem function and rangeland livelihoods203. The Payment for Ecosystem Services in Costa Rica has been rebuked as not adequately compensating people for the service they provide204. Further, Thirty by Thirty has been challenged for disproportionately, negatively impacting Indigenous communities via exclusion from land ownership and management, despite the outsized, positive effect that some Indigenous practices have on biodiversity205. These initiatives may be improved by creating context-dependent management plans that are designed around and implemented by local communities and Indigenous groups. One way to achieve this is through conservation of land via Indigenous Protected Areas: although defined differently depending on the country, Indigenous Protected Areas are generally large areas of intact ecosystems managed or co-managed by Indigenous groups. More than 46% of national reserves within Australia are Indigenous Protected Areas206, and a small but increasing proportion of protected land in Canada is comprised of Indigenous Protected Areas (for example, Thaidene Nëné Indigenous Protected Area, the homeland of the Łutsël K’é Dene First Nation)207. The United States and countries with similar Thirty by Thirty goals can and should create similar protected areas. Another successful model is Health in Harmony’s programs in Borneo, Madagascar, and Brazil, which start with ‘radical listening’ within rainforest communities to co-develop community-based conservation and health programs that reduce deforestation and provide affordable healthcare access208.
We additionally emphasize that biodiversity conservation is not a panacea for zoonotic spillover prevention, and many systems are too complex or understudied to define clear links between biodiversity change and spillover risk. For example, highly diverse multi-host, multi-vector systems such as West Nile Virus, Ross River virus209,210, leishmaniasis211, and Chagas disease212 require more studies to document ecological drivers of reservoir and vector abundances and capacities to transmit disease. Further, reservoir host species that contribute most to transmission may be variable along geographic and land-use gradients213-218. Even when conservation-related levers for spillover prevention exist, their impacts should be compared to those of other approaches (including economic and biomedical) and implemented from a community-based, environmental-justice perspective. Thus, sustainable solutions for alleviating zoonotic disease burden while conserving biodiversity should be evaluated based on specific knowledge of the socio-ecological context1.
Conclusions and future directions
We identified mechanistic evidence in the literature that anthropogenically driven biodiversity change may increase zoonotic spillover risk. Several common themes emerged. First, the loss of intact habitat increases overlap between reservoirs and other vertebrate hosts, vectors, and humans. Second, loss of large-bodied consumers and predators (defaunation) can result in increased abundance of rodent reservoirs. Third, human-modified landscapes change the functional diversity of species assemblages, increasing the proportion of species that are able to adapt and thrive in anthropogenic environments, and thereby increasing human exposure to zoonotic pathogens. Fourth, other forms of anthropogenic disturbance generated by agriculture and trade of domestic animals and wildlife lead to the introduction of invasive species and increase interaction diversity, facilitating opportunities for cross-species transmission and thus the potential for emergence of novel pathogens with zoonotic spillover potential. Hence, anthropogenic drivers of biodiversity change interact in complex ways, including synergies and both direct and indirect effects. The combined impacts of many different anthropogenic disturbances may exacerbate the effects of biodiversity change on spillover risk.
Certain disease systems are either understudied or too complex to elucidate the effects of biodiversity changes on spillover risk. In addition, some components of the spillover process (Figure 1) are better studied than others in this context. Based on our review, the effects of biodiversity changes on wildlife-host susceptibility, pathogen shedding, and pathogen prevalence in the reservoir are three important steps of spillover that are understudied and warrant further investigation. These aspects are difficult to observe219, but another possible reason that they have been understudied could be a lack of appreciation for how wildlife health — and not just presence or absence of disease agents — may affect zoonotic spillover risk. When exposed to stress from anthropogenic activities, wildlife hosts may experience suppressed immunity, rendering them more susceptible to opportunistic infections, more pathogen shedding, and altered behavior that further increases their exposure to pathogens185,220. Thus, increased pathogen surveillance and health assessments of wildlife may be useful for understanding mechanisms by which environmental stressors affect wild animal health and lead to changes in the process of disease spillover to people and domestic animals. Finally, there is an urgent need for spatially and temporally replicated field studies incorporating biodiversity change, pathogen dynamics, and wildlife host immunology184,185, in addition to human health outcomes.
The world is undergoing rapid anthropogenic change with detrimental effects on biodiversity and the health of organisms, including humans. Efforts are underway to combat the impact of anthropogenic disturbances on biodiversity. However, since biodiversity change may affect zoonotic disease spillover through multiple mechanisms, it is imperative that biodiversity conservation efforts also incorporate actions to prevent spillover. Spillover is an issue not only for public health, but also for conservation of threatened wildlife. Here, we argue that reframing discussions of biodiversity and disease around a more inclusive definition of biodiversity and considering the context of each of the complex social-ecological systems in which the spillover process occurs (Figure 1 and Box 1) are essential to highlight mechanistic links between biodiversity and zoonotic spillover. This approach sheds light on how to develop sustainable interventions that prevent zoonotic spillover while protecting biodiversity—to the benefit of both humans and the environment.
ACKNOWLEDGEMENTS
We thank Gretchen Daily, Elizabeth Hadly, and members of the Mordecai Lab (Alexander Becker, Devin Kirk, Marissa Childs, Lisa Couper, Johanna Farner, Mallory Harris, Isabel Dewel, Gowri Vadmal) for thoughtful feedback on early drafts of the manuscript. We are grateful to Hillary Young, Elizabeth Shanahan, Anne Devan-Song, Peter Hudson, Nick Fox, Paul Cross from the United States Geological Survey, and Kimberlee Beckmen and Riley Woodford from the Alaska Department of Fish and Game for providing photographs. We thank the anonymous reviewers for feedback that significantly improved this manuscript. C.K.G., E.A.M. and L.M. were supported by the National Science Foundation (NSF; DEB-2011147, with the Fogarty International Center). N.N. was supported by the Philanthropic Educational Organization (PEO) Scholar Award from the International Chapter of the PEO Sisterhood, and the Stanford Data Science Scholars program. M.P.K. and L.M. were supported by the Natural Capital Project. S.H.S. and G.A.D.L. were partially supported by the NSF (DEB-2011179), the Belmont Forum of Climate Environment and Health and NSF initiative (ICER-2024383), and a Lyme disease seed grant from Stanford University, Department of Psychiatry and Behavioral Sciences. R.K.P. was funded by the DARPA PREEMPT program (Cooperative Agreement: D18AC00031), the NSF (DEB-1716698), and the USDA National Institute of Food and Agriculture (Hatch project 1015891). E.A.M. was also supported by the NSF (DEB-1518681), the National Institute of General Medical Sciences (R35GM133439), the Terman Award, the Stanford King Center for Global Development, the Stanford Woods Institute for the Environment, and the Stanford Center for Innovation in Global Health.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Hopkins SR, Sokolow SH, Buck JC, De Leo GA, Jones IJ, Kwong LH, LeBoa C, Lund AJ, MacDonald AJ, Nova N, et al. (2020). How to identify win–win interventions that benefit human health and conservation. Nat. Sustain 4, 298–304. [Google Scholar]
- 2.Randolph SE, and Dobson ADM (2012). Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology 139, 847–863. [DOI] [PubMed] [Google Scholar]
- 3.Lafferty KD, and Wood CL (2013). It’s a myth that protection against disease is a strong and general service of biodiversity conservation: Response to Ostfeld and Keesing. Trends Ecol. Evol 28, 503–504. [DOI] [PubMed] [Google Scholar]
- 4.Ostfeld RS, and Keesing F (2013). Straw men don’t get Lyme disease: response to Wood and Lafferty. Trends Ecol. Evol 28, 502–503. [DOI] [PubMed] [Google Scholar]
- 5.Salkeld DJ, Padgett KA, and Jones JH (2013). A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol. Lett 16, 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood CL, Lafferty KD, DeLeo G, Young HS, Hudson PJ, and Kuris AM (2014). Does biodiversity protect humans against infectious disease? Ecology 95, 817–832. [DOI] [PubMed] [Google Scholar]
- 7.Civitello DJ, Cohen J, Fatima H, Halstead NT, Liriano J, McMahon TA, Ortega CN, Sauer EL, Sehgal T, Young S, et al. (2015). Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc. Natl. Acad. Sci. USA 112, 8667–8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halsey S (2019). Defuse the dilution effect debate. Nat. Ecol. Evol 3, 145–146. [DOI] [PubMed] [Google Scholar]
- 9.Rohr JR, Civitello DJ, Halliday FW, Hudson PJ, Lafferty KD, Wood CL, and Mordecai EA (2020). Towards common ground in the biodiversity–disease debate. Nat. Ecol. Evol 4, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostfeld RS, and Holt RD (2004). Are predators good for your health? Evaluating evidence for top-down regulation of zoonotic disease reservoirs. Front. Ecol. Environ 2, 13–20. [Google Scholar]
- 11.Levi T, Kilpatrick AM, Mangel M, and Wilmers CC (2012). Deer, predators, and the emergence of Lyme disease. Proc. Natl. Acad. Sci. USA 109, 10942–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson PTJ, de Roode JC, and Fenton A (2015). Why infectious disease research needs community ecology. Science 349, 1259504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levi T, Massey AL, Holt RD, Keesing F, Ostfeld RS, and Peres CA (2016). Does biodiversity protect humans against infectious disease? Comment. Ecology 97, 536–542. [DOI] [PubMed] [Google Scholar]
- 14.Ostfeld RS, and Keesing F (2012). Effects of host diversity on infectious disease. Annu. Rev. Ecol. Evol. Syst 43, 157–182. [Google Scholar]
- 15.Johnson PTJ, Ostfeld RS, and Keesing F (2015). Frontiers in research on biodiversity and disease. Ecol. Lett 18, 1119–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young HS, McCauley DJ, Dirzo R, Nunn CL, Campana MG, Agwanda B, Otarola-Castillo ER, Castillo ER, Pringle RM, Veblen KE, et al. (2017). Interacting effects of land use and climate on rodent-borne pathogens in central Kenya. Philos. Trans. R. Soc. Lond. B Biol. Sci 372, 20160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaz S, Settele E, Brondizio E, Ngo H, Gueze J, Agard A, Balvanera K, Brauman S, Buthchart K, Chan L, et al. (2019). IPBES (2019): Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (Bonn Germany: IPBES secretariat; ), p. 1148, 10.5281/zenodo.3831673. [DOI] [Google Scholar]
- 18.Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, and Lloyd-Smith JO (2017). Pathways to zoonotic spillover. Nat. Rev. Microbiol 15, 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wasik BR, de Wit E, Munster V, Lloyd-Smith JO, Martinez-Sobrido L, and Parrish CR (2019). Onward transmission of viruses: how do viruses emerge to cause epidemics after spillover? Philos. Trans. R. Soc. Lond. B Biol. Sci 374, 20190017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd-Smith JO, George D, Pepin KM, Pitzer VE, Pulliam JRC, Dobson AP, Hudson PJ, and Grenfell BT (2009). Epidemic dynamics at the human-animal interface. Science 326, 1362–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plowright RK, Eby P, Hudson PJ, Smith IL, Westcott D, Bryden WL, Middleton D, Reid PA, McFarlane RA, Martin G, et al. (2015). Ecological dynamics of emerging bat virus spillover. Proc. R. Soc. B Biol. Sci 282, 20142124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naeem S, Duffy JE, and Zavaleta E (2012). The functions of biological diversity in an age of extinction. Science 336, 1401–1406. [DOI] [PubMed] [Google Scholar]
- 23.Bai X, McPhearson T, Cleugh H, Nagendra H, Tong X, Zhu T, and Zhu Y-G (2017). Linking urbanization and the environment: Conceptual and empirical advances. Annu. Rev. Environ. Resour 42, 215–240. [Google Scholar]
- 24.United Nations, Department of Economic and Social Affairs, Population Division (2019). World Urbanization Prospects 2018. Highlights (New York: United Nations; ), https://population.un.org/wup/Publications/Files/WUP2018-Highlights.pdf. [Google Scholar]
- 25.Krebs JR, Wilson JD, Bradbury RB, and Siriwardena GM (1999). The second Silent Spring? Nature 400, 611–612. [Google Scholar]
- 26.Laurance WF, Sayer J, and Cassman KG (2014). Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol 29, 107–116. [DOI] [PubMed] [Google Scholar]
- 27.Estrada A, Garber PA, Rylands AB, Roos C, Fernandez-Duque E, Fiore AD, Nekaris KA-I, Nijman V, Heymann EW, Lambert JE, et al. (2017). Impending extinction crisis of the world’s primates: Why primates matter. Sci. Adv 3, e1600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohr JR, Barrett CB, Civitello DJ, Craft ME, Delius B, DeLeo GA, Hudson PJ, Jouanard N, Nguyen KH, Ostfeld RS, et al. (2019). Emerging human infectious diseases and the links to global food production. Nat. Sustain 2, 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, et al. (2011). Trophic downgrading of planet Earth. Science 333, 301–306. [DOI] [PubMed] [Google Scholar]
- 30.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, and Collen B (2014). Defaunation in the Anthropocene. Science 345, 401–406. [DOI] [PubMed] [Google Scholar]
- 31.Graham SI, Kinnaird MF, O’Brien TG, Vågen T-G, Winowiecki LA, Young TP, and Young HS (2019). Effects of land-use change on community diversity and composition are highly variable among functional groups. Ecol. Appl 29, e01973. [DOI] [PubMed] [Google Scholar]
- 32.Gutiérrez-Granados G, and Dirzo R (2021). Logging drives contrasting animal body-size effects on tropical forest mammal communities. For. Ecol. Manag 481, 118700. [Google Scholar]
- 33.Young HS, Dirzo R, Helgen KM, McCauley DJ, Billeter SA, Kosoy MY, Osikowicz LM, Salkeld DJ, Young TP, and Dittmar K (2014). Declines in large wildlife increase landscape-level prevalence of rodent-borne disease in Africa. Proc. Natl. Acad. Sci. USA 111, 7036–7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibb R, Redding DW, Chin KQ, Donnelly CA, Blackburn TM, Newbold T, and Jones KE (2020). Zoonotic host diversity increases in human-dominated ecosystems. Nature 584, 398–402. [DOI] [PubMed] [Google Scholar]
- 35.McCauley DJ, Salkeld DJ, Young HS, Makundi R, Dirzo R, Eckerlin RP, Lambin EF, Gaffikin L, Barry M, and Helgen KM (2015). Effects of land use on plague (Yersinia pestis) activity in rodents in Tanzania. Am. J. Trop. Med. Hyg 92, 776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sokolow SH, Huttinger E, Jouanard N, Hsieh MH, Lafferty KD, Kuris AM, Riveau G, Senghor S, Thiam C, N’Diaye A, et al. (2015). Reduced transmission of human schistosomiasis after restoration of a native river prawn that preys on the snail intermediate host. Proc. Natl. Acad. Sci. USA 112, 9650–9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braczkowski AR, O’Bryan CJ, Stringer MJ, Watson JE, Possingham HP, and Beyer HL (2018). Leopards provide public health benefits in Mumbai, India. Front. Ecol. Environ 16, 176–182. [Google Scholar]
- 38.Sokolow SH, Jones IJ, Jocque M, La D, Cords O, Knight A, Lund A, Wood CL, Lafferty KD, Hoover CM, et al. (2017). Nearly 400 million people are at higher risk of schistosomiasis because dams block the migration of snail-eating river prawns. Philos. Trans. R. Soc. B Biol. Sci 372, 20160127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson CK, Hitchens PL, Pandit PS, Rushmore J, Evans TS, Young CCW, and Doyle MM (2020). Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proc. Biol. Sci 287, 20192736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Faria MT, Calderwood MS, Athanazio DA, McBride AJA, Hartskeerl RA, Pereira MM, Ko AI, and Reis MG (2008). Carriage of Leptospira interrogans among domestic rats from an urban setting highly endemic for leptospirosis in Brazil. Acta Trop. 108, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han BA, Schmidt JP, Bowden SE, and Drake JM (2015). Rodent reservoirs of future zoonotic diseases. Proc. Natl. Acad. Sci. USA 112, 7039–7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luis AD, O’Shea TJ, Hayman DTS, Wood JLN, Cunningham AA, Gilbert AT, Mills JN, and Webb CT (2015). Network analysis of host-virus communities in bats and rodents reveals determinants of cross-species transmission. Ecol. Lett 18, 1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han BA, Kramer AM, and Drake JM (2016). Global patterns of zoonotic disease in mammals. Trends Parasitol. 32, 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purvis A, Gittleman JL, Cowlishaw G, and Mace GM (2000). Predicting extinction risk in declining species. Proc. R. Soc. Lond. B Biol. Sci 267, 1947–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cusack J (2011). Characterising small mammal responses to tropical forest loss and degradation in Northern Borneo using capture-mark-recapture methods. Unpublished masters dissertation (Imperial College London: ), https://www.iccs.org.uk/wp-content/thesis/consci/2011/Cusack.pdf. [Google Scholar]
- 46.Mendoza H, Rubio AV, García-Perña GE, Suzán G, and Simonetti JA (2019). Does land-use change increase the abundance of zoonotic reservoirs? Rodents say yes. Eur. J. Wildl. Res 66, 6. [Google Scholar]
- 47.Herrera JP, Wickenkamp NR, Turpin M, Baudino F, Tortosa P, Goodman SM, Soarimalala V, Ranaivoson TN, and Nunn CL (2020). Effects of land use, habitat characteristics, and small mammal community composition on Leptospira prevalence in northeast Madagascar. PLoS Negl. Trop. Dis 14, e0008946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young HS, McCauley DJ, Helgen KM, Goheen JR, Otárola-Castillo E, Palmer TM, Pringle RM, Young TP, and Dirzo R (2013). Effects of mammalian herbivore declines on plant communities: observations and experiments in an African savanna. J. Ecol 101, 1030–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Titcomb G, Allan BF, Ainsworth T, Henson L, Hedlund T, Pringle RM, Palmer TM, Njoroge L, Campana MG, Fleischer RC, et al. (2017). Interacting effects of wildlife loss and climate on ticks and tick-borne disease. Proc. R. Soc. B Biol. Sci 284, 20170475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benton TG, Vickery JA, and Wilson JD (2003). Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol. Evol 18, 182–188. [Google Scholar]
- 51.Chace JF, and Walsh JJ (2006). Urban effects on native avifauna: a review. Landscape Urban Plan. 74, 46–69. [Google Scholar]
- 52.Durán AP, Green JMH, West CD, Visconti P, Burgess ND, Virah-Sawmy M, and Balmford A (2020). A practical approach to measuring the biodiversity impacts of land conversion. Methods Ecol. Evol 11, 910–921. [Google Scholar]
- 53.Mladenoff DJ, White MA, Pastor J, and Crow TR (1993). Comparing spatial pattern in unaltered old-growth and disturbed forest landscapes. Ecol. Appl 3, 294–306. [DOI] [PubMed] [Google Scholar]
- 54.Watson JEM, Evans T, Venter O, Williams B, Tulloch A, Stewart C, Thompson I, Ray JC, Murray K, Salazar A, et al. (2018). The exceptional value of intact forest ecosystems. Nat. Ecol. Evol 2, 599–610. [DOI] [PubMed] [Google Scholar]
- 55.Gibbons P, and Boak M (2002).The value of paddock trees for regional conservation in an agricultural landscape. Ecol. Manag. Restor 3, 205–210. [Google Scholar]
- 56.Urrutia AL, González-Gónzalez C, Van Cauwelaert EM, Rosell JA, García Barrios L, and Benítez M (2020). Landscape heterogeneity of peasant-managed agricultural matrices. Agric. Ecosyst. Environ 292, 106797. [Google Scholar]
- 57.Bloomfield LSP, McIntosh TL, and Lambin EF (2020). Habitat fragmentation, livelihood behaviors, and contact between people and nonhuman primates in Africa. Landscape Ecol. 35, 985–1000. [Google Scholar]
- 58.Chang MS, Hii J, Buttner P, and Mansoor F (1997). Changes in abundance and behaviour of vector mosquitoes induced by land use during the development of an oil palm plantation in Sarawak. Trans. R. Soc. Trop. Med. Hyg 91, 382–386. [DOI] [PubMed] [Google Scholar]
- 59.Cox-Singh J, Davis TME, Lee K-S, Shamsul SSG, Matusop A, Ratnam S, Rahman HA, Conway DJ, and Singh B (2008). Plasmodium knowlesi malaria in humans is widely distributed and potentially life-threatening. Clin. Infect. Dis 46, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brock PM, Fornace KM, Parmiter M, Cox J, Drakeley CJ, Ferguson HM, and Kao RR (2016). Plasmodium knowlesi transmission: integrating quantitative approaches from epidemiology and ecology to understand malaria as a zoonosis. Parasitology 143, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fornace KM, Abidin TR, Alexander N, Brock P, Grigg MJ, Murphy A, William T, Menon J, Drakeley CJ, and Cox J (2016). Association between landscape factors and spatial patterns of Plasmodium knowlesi infections in Sabah, Malaysia. Emerg. Infect. Dis 22, 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manin BO, Ferguson HM, Vythilingam I, Fornace K, William T, Torr SJ, Drakeley C, and Chua TH (2016). Investigating the contribution of peri-domestic transmission to risk of zoonotic malaria infection in humans. PLoS Negl. Trop. Dis 10, e0005064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davidson G, Chua TH, Cook A, Speldewinde P, and Weinstein P (2019). Defining the ecological and evolutionary drivers of Plasmodium knowlesi transmission within a multi-scale framework. Malar. J 18, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brant HL, Ewers RM, Vythilingam I, Drakeley C, Benedick S, and Mumford JD (2016). Vertical stratification of adult mosquitoes (Diptera: Culicidae) within a tropical rainforest in Sabah, Malaysia. Malar. J 15, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vythilingam I, Wong ML, and Wan-Yussof WS (2018). Current status of Plasmodium knowlesi vectors: a public health concern? Parasitology 145, 32–40. [DOI] [PubMed] [Google Scholar]
- 66.Rulli MC, Santini M, Hayman DTS, and D’Odorico P (2017). The nexus between forest fragmentation in Africa and Ebola virus disease outbreaks. Sci. Rep 7, 41613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olivero J, Fa JE, Farfán MÁ, Márquez AL, Real R, Juste FJ, Leendertz SA, and Nasi R (2020). Human activities link fruit bat presence to Ebola virus disease outbreaks. Mammal. Rev 50, 1–10. [Google Scholar]
- 68.Tymensen L, Zaheer R, Cook SR, Amoako KK, Goji N, Read R, Booker CW, Hannon SJ, Neumann N, and McAllister TA (2018). Clonal expansion of environmentally adapted Escherichia coli contributes to propagation of antibiotic resistance genes in beef cattle feedlots. Sci. Total Environ 637–638, 657–664. [DOI] [PubMed] [Google Scholar]
- 69.Zhang M, Liu Y-S, Zhao J-L, Liu W-R, He L-Y, Zhang J-N, Chen J, He L-K, Zhang Q-Q, and Ying G-G (2018). Occurrence, fate and mass loadings of antibiotics in two swine wastewater treatment systems. Sci. Total Environ 639, 1421–1431. [DOI] [PubMed] [Google Scholar]
- 70.Lulijwa R, Rupia EJ, and Alfaro AC (2020). Antibiotic use in aquaculture, policies and regulation, health and environmental risks: a review of the top 15 major producers. Rev. Aquac 12, 640–663. [Google Scholar]
- 71.Taylor P, and Reeder R (2020). Antibiotic use on crops in low and middle-income countries based on recommendations made by agricultural advisors. CABI Agric. Biosci 1, 1. [Google Scholar]
- 72.Sabri NA, Schmitt H, Van der Zaan B, Gerritsen HW, Zuidema T, Rijnaarts HHM, and Langenhoff AAM (2020). Prevalence of antibiotics and antibiotic resistance genes in a wastewater effluent-receiving river in the Netherlands. J. Environ. Chem. Eng 8, 102245. [Google Scholar]
- 73.Bonnedahl J, and Järhult JD (2014). Antibiotic resistance in wild birds. Ups. J. Med. Sci 119, 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rivadeneira P, Hilson C, Justice-Allen A, and Jay-Russell M (2016). Pathogen risks related to the movement of birds frequenting livestock and fresh produce growing areas in the southwestern U.S. Proc. Vertebr. Pest Conf 27, 258–263. [Google Scholar]
- 75.Borges CA, Cardozo MV, Beraldo LG, Oliveira ES, Maluta RP, Barboza KB, Werther K, and Ávila FA (2017). Wild birds and urban pigeons as reservoirs for diarrheagenic Escherichia coli with zoonotic potential. J. Microbiol 55, 344–348. [DOI] [PubMed] [Google Scholar]
- 76.Ishibashi S, Sumiyama D, Kanazawa T, and Murata K (2019). Prevalence of antimicrobial-resistant Escherichia coli in endangered Okinawa rail (Gallirallus okinawae) inhabiting areas around a livestock farm. Vet. Med. Sci 5, 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Navarro-Gonzalez N, Castillo-Contreras R, Casas-Díaz E, Morellet N, Concepción Porrero M, Molina-Vacas G, Torres RT, Fonseca C, Mentaberre G, Domínguez L, et al. (2018). Carriage of antibiotic-resistant bacteria in urban versus rural wild boars. Eur. J. Wildl. Res 64, 60. [Google Scholar]
- 78.O’Neill J (2014). Antimicrobial resistance: Tackling a crisis for the health and wealth of nations (London, UK: The Review on Antimicrobial Resistance; ), https://wellcomecollection.org/works/rdpck35v. [Google Scholar]
- 79.Lagerstrom KM, and Hadly EA (2021). The under-investigated wild side of Escherichia coli: genetic diversity, pathogenicity and antimicrobial resistance in wild animals. Proc. R. Soc. B Biol. Sci 288, 20210399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith OM, Edworthy AB, Taylor JM, Jones MS, Tormanen AP, Kennedy CM, Fu Z, Latimer CE, Cornell KA, Michelotti LA, et al. (2020). Agricultural intensification heightens food safety risks posed by wild birds. J. Appl. Ecol 57, 2246–2257. [Google Scholar]
- 81.Karp DS, Gennet S, Kilonzo C, Partyka M, Chaumont N, Atwill ER, and Kremen C (2015). Comanaging fresh produce for nature conservation and food safety. Proc. Natl. Acad. Sci. USA 112, 11126–11131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dhingra S, Rahman NAA, Peile E, Rahman M, Sartelli M, Hassali MA, Islam T, Islam S, and Haque M (2020). Microbial resistance movements: an overview of global public health threats posed by antimicrobial resistance, and how best to counter. Front. Public Health 8, 535668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Becker DJ, Streicker DG, and Altizer S (2018). Using host species traits to understand the consequences of resource provisioning for host–parasite interactions. J. Anim. Ecol 87, 511–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Plowright RK, Foley P, Field HE, Dobson AP, Foley JE, Eby P, and Daszak P (2011). Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.). Proc. Biol. Sci 278, 3703–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kessler MK, Becker DJ, Peel AJ, Justice NV, Lunn T, Crowley DE, Jones DN, Eby P, Sánchez CA, and Plowright RK (2018). Changing resource landscapes and spillover of henipaviruses. Ann. N. Y. Acad. Sci 1429, 78–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Plowright RK, Peel AJ, Streicker DG, Gilbert AT, McCallum H, Wood J, Baker ML, and Restif O (2016). Transmission or within-host dynamics driving pulses of zoonotic viruses in reservoir–host populations. PLoS Negl. Trop. Dis 10, e0004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith BL (2001). Winter feeding of elk in western North America. J. Wildl. Manag 65, 173–190. [Google Scholar]
- 88.Jones JD, Kauffman MJ, Monteith KL, Scurlock BM, Albeke SE, and Cross PC (2014). Supplemental feeding alters migration of a temperate ungulate. Ecol. Appl 24, 1769–1779. [DOI] [PubMed] [Google Scholar]
- 89.Brennan A, Cross PC, Portacci K, Scurlock BM, and Edwards WH (2017). Shifting brucellosis risk in livestock coincides with spreading seroprevalence in elk. PLoS One 12, e0178780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Couch CE, Wise BL, Scurlock BM, Rogerson JD, Fuda RK, Cole EK, Szcodronski KE, Sepulveda AJ, Hutchins PR, and Cross PC (2021). Effects of supplemental feeding on the fecal bacterial communities of Rocky Mountain elk in the Greater Yellowstone Ecosystem. PLoS One 16, e0249521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin Y, and Hyyppä J (2019). Characterizing ecosystem phenological diversity and its macroecology with snow cover phenology. Sci. Rep 9, 15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berdegué JA, Rosada T, and Bebbington AJ (2014). The rural transformation. In International Development: Ideas, Experience, and Prospects, Currie-Alder B, Kanbur R, Malone DM, and Medhora R, eds. (Oxford: Oxford University Press; ), pp. 463–478. [Google Scholar]
- 93.Santiago-Alarcon D, and MacGregor-Fors I (2020). Cities and pandemics: urban areas are ground zero for the transmission of emerging human infectious diseases. J. Urban Ecol 6, juaa012. [Google Scholar]
- 94.Faust CL, McCallum HI, Bloomfield LSP, Gottdenker NL, Gillespie TR, Torney CJ, Dobson AP, and Plowright RK (2018). Pathogen spillover during land conversion. Ecol. Lett 21, 471–483. [DOI] [PubMed] [Google Scholar]
- 95.Naguib MM, Li R, Ling J, Grace D, Nguyen-Viet H, and Lindahl JF (2021). Live and wet markets: food access versus the risk of disease emergence. Trends Microbiol. 29, 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wood CL, McInturff A, Young HS, Kim D, and Lafferty KD (2017). Human infectious disease burdens decrease with urbanization but not with biodiversity. Philos. Trans. R. Soc. Lond. B Biol. Sci 372, 20160122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moore M, Gould P, and Keary BS (2003). Global urbanization and impact on health. Int. J. Hyg. Environ. Health 206, 269–278. [DOI] [PubMed] [Google Scholar]
- 98.Bonamour S, Chevin L-M, Charmantier A, and Teplitsky C (2019). Phenotypic plasticity in response to climate change: the importance of cue variation. Philos. Trans. R. Soc. Lond. B Biol. Sci 374, 20180178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Catullo RA, Llewelyn J, Phillips BL, and Moritz CC (2019). The potential for rapid evolution under anthropogenic climate change. Curr. Biol 29, R996–R1007. [DOI] [PubMed] [Google Scholar]
- 100.Chen I-C, Hill JK, Ohlemüller R, Roy DB, and Thomas CD (2011). Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026. [DOI] [PubMed] [Google Scholar]
- 101.Nogués-Bravo D, Rodríguez-Sánchez F, Orsini L, de Boer E, Jansson R, Morlon H, Fordham DA, and Jackson ST (2018). Cracking the code of biodiversity responses to past climate change. Trends Ecol. Evol 33, 765–776. [DOI] [PubMed] [Google Scholar]
- 102.Parmesan C, and Yohe G (2003). A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. [DOI] [PubMed] [Google Scholar]
- 103.Ceballos G, Ehrlich PR, and Dirzo R (2017). Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 114, E6089–E6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Román-Palacios C, and Wiens JJ (2020). Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 117, 4211–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, and Courchamp F (2012). Impacts of climate change on the future of biodiversity. Ecol. Lett 15, 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hetem RS, Fuller A, Maloney SK, and Mitchell D (2014). Responses of large mammals to climate change. Temperature 1, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Waller N, Gynther I, Freeman A, Lavery TH, and Leung L (2017). The Bramble Cay melomys Melomys rubicola (Rodentia: Muridae): a first mammalian extinction caused by human-induced climate change? Wildl. Res 44, 9–21. [Google Scholar]
- 108.Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, and Ackerly DD (2009). The velocity of climate change. Nature 462,1052–1055. [DOI] [PubMed] [Google Scholar]
- 109.Waits A, Emelyanova A, Oksanen A, Abass K, and Rautio A (2018). Human infectious diseases and the changing climate in the Arctic. Environ. Int 121, 703–713. [DOI] [PubMed] [Google Scholar]
- 110.Carlson CJ, Albery GF, Merow C, Trisos CH, Zipfel CM, Eskew EA, Olival KJ, Ross N, and Bansal S (2020). Climate change will drive novel cross-species viral transmission. bioRxiv. 10.1101/2020.01.24.918755. [DOI] [PubMed] [Google Scholar]
- 111.Parmesan C (2006). Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst 37, 637–669. [Google Scholar]
- 112.Couper L, Farner J, Caldwell J, Childs M, Harris M, Kirk D, Nova N, Shocket M, Skinner E, Uricchio L, et al. (2021). How will mosquitoes adapt to climate warming? eLife 10, e69630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Réale D, McAdam AG, Boutin S, and Berteaux D (2003). Genetic and plastic responses of a northern mammal to climate change. Proc. R. Soc. B Biol. Sci 270, 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pounds JA, Bustamante MR, Coloma LA, Consuegra JA, Fogden MPL, Foster PN, La Marca E, Masters KL, Merino-Viteri A, Puschendorf R, et al. (2006). Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439, 161–167. [DOI] [PubMed] [Google Scholar]
- 115.Davey CM, Chamberlain DE, Newson SE, Noble DG, and Johnston A (2012). Rise of the generalists: evidence for climate driven homogenization in avian communities. Glob. Ecol. Biogeogr 21, 568–578. [Google Scholar]
- 116.Hof AR, Jansson R, and Nilsson C (2012). Future climate change will favour non-specialist mammals in the (sub)Arctics. PLoS One 7, e52574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brustolin MC, Nagelkerken I, Ferreira CM, Goldenberg SU, Ullah H, and Fonseca G (2019). Future ocean climate homogenizes communities across habitats through diversity loss and rise of generalist species. Glob. Chang. Biol 25, 3539–3548. [DOI] [PubMed] [Google Scholar]
- 118.Walsh MG, and Hossain S (2020). Population structure and diet generalism define a preliminary ecological profile of zoonotic virus hosts in the Western Ghats, India. Epidemics 33, 100416. [DOI] [PubMed] [Google Scholar]
- 119.Lehmer EM, Korb J, Bombaci S, McLean N, Ghachu J, Hart L, Kelly A, Jara-Molinar E, O’Brien C, and Wright K (2012). The interplay of plant and animal disease in a changing landscape: the role of sudden aspen decline in moderating Sin Nombre virus prevalence in natural deer mouse populations. EcoHealth 9, 205–216. [DOI] [PubMed] [Google Scholar]
- 120.Hersteinsson P, and MacDonald DW (1992). Interspecific competition and the geographical distribution of red and arctic foxes Vulpes vulpes and Alopex lagopus. Oikos 64, 505–515. [Google Scholar]
- 121.Elmhagen B, Tannerfeldt M, Verucci P, and Angerbjörn A (2000). The arctic fox (Alopex lagopus): an opportunistic specialist. J. Zool 251, 139–149. [Google Scholar]
- 122.Rodnikova A, Ims RA, Sokolov A, Skogstad G, Sokolov V, Shtro V, and Fuglei E (2011). Red fox takeover of arctic fox breeding den: an observation from Yamal Peninsula, Russia. Polar Biol 34, 1609. [Google Scholar]
- 123.Berteaux D, Thierry A-M, Alisauskas R, Angerbjörn A, Buchel E, Doronina L, Ehrich D, Eide NE, Erlandsson R, Flagstad Ø, et al. (2017). Harmonizing circumpolar monitoring of Arctic fox: benefits, opportunities, challenges and recommendations. Polar Res. 36, 10.1080/17518369.2017.1319602. [DOI] [Google Scholar]
- 124.Elmhagen B, Berteaux D, Burgess RM, Ehrich D, Gallant D, Henttonen H, Ims RA, Killengreend ST, Niemimaa J, Norén K, et al. (2017). Homage to Hersteinsson and Macdonald: climate warming and resource subsidies cause red fox range expansion and Arcticfox decline. Polar Res. 36, 10.1080/17518369.2017.1319109. [DOI] [Google Scholar]
- 125.Simon A, Tardy O, Hurford A, Lecomte N, Bélanger D, and Leighton P (2019). Dynamics and persistence of rabies in the Arctic. Polar Res. 38, 3366. [Google Scholar]
- 126.Plowright RK, Cross PC, Tabor GM, Almberg E, and Hudson PJ (2012). Climate change and infectious disease dynamics. In New Directions in Conservation Medicine: Applied Cases of Ecological Health, Aguirre AA, Ostfield RS, and Daszak P, eds. (Oxford: Oxford University Press; ), pp. 111–121. [Google Scholar]
- 127.Post E, Bhatt US, Bitz CM, Brodie JF, Fulton TL, Hebblewhite M, Kerby J, Kutz SJ, Stirling I, and Walker DA (2013). Ecological consequences of sea-ice decline. Science 341, 519–524. [DOI] [PubMed] [Google Scholar]
- 128.Hueffer K, Parkinson AJ, Gerlach R, and Berner J (2013). Zoonotic infections in Alaska: disease prevalence, potential impact of climate change and recommended actions for earlier disease detection, research, prevention and control. Int. J. Circumpolar Health 72, 19562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Clifford D, Kazwala DR, and Coppolillo P (2008). Evaluating and managing zoonotic disease risk in rural Tanzania. figshare. Journal contribution, 10.6084/m9.figshare.156276.v1. [DOI] [Google Scholar]
- 130.Fox JT, and Alexander KA (2015). Spatiotemporal variation and the role of wildlife in seasonal water quality declines in the Chobe River, Botswana. PLoS One 10, e0139936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alexander KA, Heaney AK, and Shaman J (2018). Hydrometeorology and flood pulse dynamics drive diarrheal disease outbreaks and increase vulnerability to climate change in surface-water-dependent populations: A retrospective analysis. PLoS Med. 15, e1002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sanderson CE, Fox JT, Dougherty ER, Cameron ADS, and Alexander KA (2018). The changing face of water: a dynamic reflection of antibiotic resistance across landscapes. Front. Microbiol 9, 1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dasgupta P (2021). Economics of Biodiversity: The Dasgupta Review (London, UK: HM Treasury; ). [Google Scholar]
- 134.Doherty TS, Glen AS, Nimmo DG, Ritchie EG, and Dickman CR (2016). Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. USA 113, 11261–11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dorcas ME, Willson JD, Reed RN, Snow RW, Rochford MR, Miller MA, Meshaka WE, Andreadis PT, Mazzotti FJ, Romagosa CM, et al. (2012). Severe mammal declines coincide with proliferation of invasive Burmese pythons in Everglades National Park. Proc. Natl. Acad. Sci. USA 109, 2418–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hoyer IJ, Blosser EM, Acevedo C, Thompson AC, Reeves LE, and Burkett-Cadena ND (2017). Mammal decline, linked to invasive Burmese python, shifts host use of vector mosquito towards reservoir hosts of a zoonotic disease. Biol. Lett 13, 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Burkett-Cadena ND, Blosser EM, Loggins AA, Valente MC, Long MT, Campbell LP, Reeves LE, Bargielowski I, and McCleery RA (2021). Invasive Burmese pythons alter host use and virus infection in the vector of a zoonotic virus. Commun. Biol 4, 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Walshe DP, Garner P, Adeel AA, Pyke GH, and Burkot TR (2017). Larvivorous fish for preventing malaria transmission. Cochrane Database Syst. Rev 12, CD008090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mkoji GM, Hofkin BV, Kuris AM, Stewart-Oaten A, Mungai BN, Kihara JH, Mungai F, Yundu J, Mbui J, Rashid JR, et al. (1999). Impact of the crayfish Procambarus clarkii on Schistosoma haematobium transmission in Kenya. Am. J. Trop. Med. Hyg 61, 751–759. [DOI] [PubMed] [Google Scholar]
- 140.Angeler DG, Sánchez-Carrillo S, García G, and Alvarez-Cobelas M (2001). The influence of Procambarus clarkii (Cambaridae, Decapoda) on water quality and sediment characteristics in a Spanish floodplain wetland. Hydrobiologia 464, 89–98. [Google Scholar]
- 141.De Lima H, De Guglielmo Z, Rodríguez A, Convit J, and Rodriguez N (2002). Cotton rats (Sigmodon hispidus) and black rats (Rattus rattus) as possible reservoirs of Leishmania spp. in Lara State, Venezuela. Mem. Inst. Oswaldo Cruz 97, 169–174. [DOI] [PubMed] [Google Scholar]
- 142.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. (2013). The global distribution and burden of dengue. Nature 496, 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reaser JK, Witt A, Tabor GM, Hudson PJ, and Plowright RK (2021). Ecological countermeasures for preventing zoonotic disease outbreaks: when ecological restoration is a human health imperative. Restor. Ecol 29, e13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tompkins DM, White AR, and Boots M (2003). Ecological replacement of native red squirrels by invasive greys driven by disease. Ecol. Lett 6, 189–196. [Google Scholar]
- 145.Colla SR, Otterstatter MC, Gegear RJ, and Thomson JD (2006). Plight of the bumble bee: Pathogen spillover from commercial to wild populations. Biol. Conserv 129, 461–467. [Google Scholar]
- 146.Mordecai EA (2013). Consequences of pathogen spillover for cheatgrass-invaded grasslands: coexistence, competitive exclusion, or priority effects. Am. Nat 181, 737–747. [DOI] [PubMed] [Google Scholar]
- 147.Allan BF, Dutra HP, Goessling LS, Barnett K, Chase JM, Marquis RJ, Pang G, Storch GA, Thach RE, and Orrock JL (2010). Invasive honeysuckle eradication reduces tick-borne disease risk by altering host dynamics. Proc. Natl. Acad. Sci. USA 107, 18523–18527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lodge DM, Williams S, MacIsaac HJ, Hayes KR, Leung B, Reichard S, Mack RN, Moyle PB, Smith M, Andow DA, et al. (2006). Biological invasions: Recommendations for U.S. policy and management. Ecol. Appl 16, 2035–2054. [DOI] [PubMed] [Google Scholar]
- 149.Scheffers BR, Oliveira BF, Lamb I, and Edwards DP (2019). Global wildlife trade across the tree of life. Science 366, 71–76. [DOI] [PubMed] [Google Scholar]
- 150.Zhang L, Hua N, and Sun S (2008). Wildlife trade, consumption and conservation awareness in southwest China. Biodivers. Conserv 17, 1493–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rosen GE, and Smith KF (2010). Summarizing the evidence on the international trade in illegal wildlife. EcoHealth 7, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wyler LS, and Sheikh PA (2013). International Illegal Trade in Wildlife: Threats and U.S. Policy (Washington, DC: Congressional Research Service; ), https://fas.org/sgp/crs/misc/RL34395.pdf. [Google Scholar]
- 153.Smith KM, Zambrana-Torrelio C, White A, Asmussen M, Machalaba C, Kennedy S, Lopez K, Wolf TM, Daszak P, Travis DA, et al. (2017). Summarizing US wildlife trade with an eye toward assessing the risk of infectious disease introduction. EcoHealth 14, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wolfe ND, Switzer WM, Carr JK, Bhullar VB, Shanmugam V, Ta-moufe U, Prosser AT, Torimiro JN, Wright A, Mpoudi-Ngole E, et al. (2004). Naturally acquired simian retrovirus infections in central African hunters. Lancet 363, 932–937. [DOI] [PubMed] [Google Scholar]
- 155.Gómez A, and Aguirre AA (2008). Infectious diseases and the illegal wildlife trade. Ann. N. Y. Acad. Sci 1149, 16–19. [DOI] [PubMed] [Google Scholar]
- 156.Pavlin BI, Schloegel LM, and Daszak P (2009). Risk of importing zoonotic diseases through wildlife trade, United States. Emerg. Infect. Dis 15, 1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bell D, Roberton S, and Hunter PR (2004). Animal origins of SARS coronavirus: possible links with the international trade in small carnivores. Philos. Trans. R. Soc. Lond. B Biol. Sci 359, 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Travis DA, Watson RP, and Tauer A (2011). The spread of pathogens through trade in wildlife. Rev. Sci. Tech 30, 219–239. [DOI] [PubMed] [Google Scholar]
- 159.Greatorex ZF, Olson SH, Singhalath S, Silithammavong S, Khammavong K, Fine AE, Weisman W, Douangngeun B, Theppangna W, Keatts L, et al. (2016). Wildlife trade and human health in Lao PDR: An assessment of the zoonotic disease risk in markets. PLoS One 11, e0150666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Judson SD, Fischer R, Judson A, and Munster VJ (2016). Ecological contexts of index cases and spillover events of different Ebolaviruses. PLoS Pathog. 12, e1005780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cantlay JC, Ingram DJ, and Meredith AL (2017). A review of zoonotic infection risks associated with the wild meat trade in Malaysia. EcoHealth 14, 361–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huong NQ, Nga NTT, Long NV, Luu BD, Latinne A, Pruvot M, Phuong NT, Quang LTV, Hung VV, Lan NT, et al. (2020). Coronavirus testing indicates transmission risk increases along wildlife supply chains for human consumption in Viet Nam, 2013–2014. PLoS One 15, e0237129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kasper K, Schweikhard J, Lehmann M, Ebert CL, Erbe P, Wayakone S, Nguyen TQ, Le MD, and Ziegler T (2020). The extent of the illegal trade with terrestrial vertebrates in markets and households in Khammouane Province, Lao PDR. Nat. Conserv 41, 25–45. [Google Scholar]
- 164.Hu B, Zeng L-P, Yang X-L, Ge X-Y, Zhang W, Li B, Xie J-Z, Shen X-R, Zhang Y-Z, Wang N, et al. (2017). Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 13, e1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ye Z-W, Yuan S, Yuen K-S, Fung S-Y, Chan C-P, and Jin D-Y (2020). Zoonotic origins of human coronaviruses. Int. J. Biol. Sci 16, 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nova N (2021). Cross-species transmission of coronaviruses in humans and domestic mammals, what are the ecological mechanisms driving transmission, spillover, and disease emergence? Front. Public Health 9,717941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kan B, Wang M, Jing H, Xu H, Jiang X, Yan M, Liang W, Zheng H, Wan K, Liu Q, et al. (2005). Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. J. Virol 79, 11892–11900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Africanews (2019). Ghana running out of sea food due to overfishing, https://www.africanews.com/2019/07/30/overfishing. [Google Scholar]
- 169.Brashares JS, Arcese P, Sam MK, Coppolillo PB, Sinclair ARE, and Balmford A (2004). Bushmeat hunting, wildlife declines, and fish supply in West Africa. Science 306, 1180–1183. [DOI] [PubMed] [Google Scholar]
- 170.Eskew EA, and Carlson CJ (2020). Overselling wildlife trade bans will not bolster conservation or pandemic preparedness. Lancet Planet. Health 4, e215–e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Leroy EM, Rouquet P, Formenty P, Souquière S, Kilbourne A, Froment J-M, Bermejo M, Smit S, Karesh W, Swanepoel R, et al. (2004). Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science 303, 387–390. [DOI] [PubMed] [Google Scholar]
- 172.Kurpiers LA, Schulte-Herbrüggen B, Ejotre I, and Reeder DM (2016). Bushmeat and emerging infectious diseases: Lessons from Africa. In Problematic Wildlife: A Cross-Disciplinary Approach, Angelici FM, ed. (Cham: Springer International Publishing; ), pp. 507–551. [Google Scholar]
- 173.Fa JE, Olivero J, Farfán MÁ, Márquez AL, Duarte J, Nackoney J, Hall A, Dupain J, Seymour S, Johnson PJ, et al. (2015). Correlates of bushmeat in markets and depletion of wildlife. Conserv. Biol 29, 805–815. [DOI] [PubMed] [Google Scholar]
- 174.Ripple WJ, Newsome TM, Wolf C, Dirzo R, Everatt KT, Galetti M, Hayward MW, Kerley GIH, Levi T, Lindsey PA, et al. (2015). Collapse of the world’s largest herbivores. Sci. Adv 1, e1400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bonn Challenge (2020). About the Challenge, https://www.bonnchallenge.org/about. (Accessed 7 June 2021.) [Google Scholar]
- 176.Chase A, and O’Shea H (2020). Saving nature will take bold action: “Thirty by Thirty”. Natural Resources Defense Council Expert Blog, https://www.nrdc.org/experts/alison-chase/saving-nature-will-take-bold-action-thirty-thirty. [Google Scholar]
- 177.Campaign for Nature, https://www.campaignfornature.org. (Accessed 7 June 2021.) [Google Scholar]
- 178.Schomers S, and Matzdorf B (2013). Payments for ecosystem services: A review and comparison of developing and industrialized countries. Ecosyst. Serv 6, 16–30. [Google Scholar]
- 179.Sánchez-Azofeifa GA, Pfaff A, Robalino JA, and Boomhower JP (2007). Costa Rica’s payment for environmental services program: intention, implementation, and impact. Conserv. Biol 21, 1165–1173. [DOI] [PubMed] [Google Scholar]
- 180.Grima N, Singh SJ, Smetschka B, and Ringhofer L (2016). Payment for ecosystem services (PES) in Latin America: Analysing the performance of 40 case studies. Ecosyst. Serv 17, 24–32. [Google Scholar]
- 181.Fondo Nacional de Financiamiento Forestal (2018). FONAFIFO completed projects, https://www.fonafifo.go.cr/en/conozcanos/proyectos-finalizados/. (Accessed 7 June 2021.) [Google Scholar]
- 182.World Wildlife Fund (2015). Project Finance for Permanence: Key Outcomes and Lessons Learned, https://www.worldwildlife.org/publications/project-finance-for-permanence-key-outcomes-and-lessons-learned. [Google Scholar]
- 183.Redstone Strategy Group (2011). Project Finance for Permanence: Lessons from Landscape-scale Conservation Deals, https://www.redstonestrategy.com/wp-content/uploads/2016/07/2013-01-04-PFP-Paper.pdf. [Google Scholar]
- 184.Plowright RK, Reaser JK, Locke H, Woodley SJ, Patz JA, Becker DJ, Oppler G, Hudson PJ, and Tabor GM (2021). Land use-induced spillover: a call to action to safeguard environmental, animal, and human health. Lancet Planet. Health 5, e237–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Becker DJ, Albery GF, Kessler MK, Lunn TJ, Falvo CA, Czirják GÁ, Martin LB, and Plowright RK (2020). Macroimmunology: The drivers and consequences of spatial patterns in wildlife immune defence. J. Anim. Ecol 89, 972–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tortosa FS, Caballero JM, and Reyes-López J (2002). Effect of rubbish dumps on breeding success in the white stork in southern Spain. Waterbirds 25, 39–43. [Google Scholar]
- 187.Satterfield DA, Maerz JC, and Altizer S (2015). Loss of migratory behaviour increases infection risk for a butterfly host. Proc. R. Soc. B Biol. Sci 282, 20141734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rosenstock TS, Dawson IK, Aynekulu E, Chomba S, Degrande A, Fornace K, Jamnadass R, Kimaro A, Kindt R, Lamanna C, et al. (2019). A planetary health perspective on agroforestry in sub-Saharan Africa. One Earth 1, 330–344. [Google Scholar]
- 189.World Wildlife Fund (2021). Convention on International Trade in Endangered Species of Wild Fauna and Flora, https://www.worldwildlife.org/pages/cites. (Accessed 7 June 2021.) [DOI] [PubMed] [Google Scholar]
- 190.Stoner C, Caro T, Mduma S, Mlingwa C, Sabuni G, and Borner M (2007). Assessment of effectiveness of protection strategies in Tanzania based on a decade of survey data for large herbivores. Conserv. Biol 21, 635–646. [DOI] [PubMed] [Google Scholar]
- 191.McIntosh AR, McHugh PA, Plank MJ, Jellyman PG, Warburton HJ, and Greig HS (2018). Capacity to support predators scales with habitat size. Sci. Adv 4, eaap7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vittor AY, Pan W, Gilman RH, Tielsch J, Glass G, Shields T, Sánchez-Lozano W, Pinedo VV, Salas-Cobos E, Flores S, et al. (2009). Linking deforestation to malaria in the Amazon: Characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am. J. Trop. Med. Hyg. 81, 5–12. [PMC free article] [PubMed] [Google Scholar]
- 193.Xia S, Dweck H, Lutomiah J, Sang R, McBride C, Rose N, Ayala D, and Powell J (2021). Larval breeding sites of the mosquito Aedes aegypti in forest and domestic habitats in Africa and the potential association with oviposition evolution. Authorea, 10.22541/au.161717899.92634794/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sokolow SH, Nova N, Pepin KM, Peel AJ, Pulliam JRC, Manlove K, Cross PC, Becker DJ, Plowright RK, McCallum H, et al. (2019). Ecological interventions to prevent and manage zoonotic pathogen spillover. Philos. Trans. R. Soc. B Biol. Sci 374, 20180342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Convention on Biological Diversity (2019). POST2020 Global Biodiversity Framework: Updated synthesis of the proposals of parties and observers on the structure of the post-2020 global biodiversity framework and its targets, https://www.cbd.int/doc/c/ef28/32d6/883b8de693927c8baa2e4d0a/post2020-prep-01-inf-03-en.pdf. [Google Scholar]
- 196.United Nations Department of Economic and Social Affairs. The 17 Goals, https://sdgs.un.org/goals. (Accessed 7 June 2021.) [Google Scholar]
- 197.Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES). Nature’s contributions to people, http://www.ipbes.net/glossary/natures-contributions-people. (Accessed June 7, 2021.) [Google Scholar]
- 198.International Union for Conservation of Nature (2020). IUCN global standard for NbS, https://www.iucn.org/theme/nature-based-solutions/resources/iucn-global-standard-nbs. (Accessed 7 June 2021.) [Google Scholar]
- 199.Bridge Collaborative, https://bridgecollaborativeglobal.org/. (Accessed 7 June 2021.) [Google Scholar]
- 200.Pan American Health Organization. Climate Change and Health, https://www.paho.org/en/topics/climate-change-and-health. (Accessed 7 June 2021.) [Google Scholar]
- 201.Global Health Security Agenda, https://ghsagenda.org/. (Accessed 7 June 2021.) [Google Scholar]
- 202.FAO, OIE, and WHO (2010). The FAO-OIE-WHO Collaboration. A tripartite concept note, https://www.who.int/foodsafety/zoonoses/final_concept_note_Hanoi.pdf. [Google Scholar]
- 203.Vetter S (2020). With power comes responsibility — a rangelands perspective on forest landscape restoration. Front. Sustain. Food Syst 4, 549483. [Google Scholar]
- 204.Arriagada RA, Sills EO, Ferraro PJ, and Pattanayak SK (2015). Do payments pay off? Evidence from participation in Costa Rica’s PES Program. PLoS One 10, e0131544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Agrawal A, Bawa K, Brockington D, Brosius P, D’Souza R, DeFries R, Dove MR, Duffy R, Kabra A, Kothari A, et al. An open letter to the lead authors of ‘Protecting 30% of the planet for nature: costs, benefits and implications, https://openlettertowaldronetal.wordpress.com/. (Accessed 14 August 2021.) [Google Scholar]
- 206.Australian Government, Department of Agriculture, Water and the Environment. Indigenous Protected Areas, https://www.environment.gov.au/land/indigenous-protected-areas. (Accessed 14 August 2021.) [Google Scholar]
- 207.Government of Canada, Parks Canada. Thaidene Nene National Park Reserve, https://www.pc.gc.ca/en/pn-np/nt/thaidene-nene/gestion-management/protected. (Accessed 14 August 2021.) [Google Scholar]
- 208.Jones IJ, MacDonald AJ, Hopkins SR, Lund AJ, Liu ZY-C, Fawzi NI, Purba MP, Fankhauser K, Chamberlin AJ, Nirmala M, et al. (2020). Improving rural health care reduces illegal logging and conserves carbon in a tropical forest. Proc. Natl. Acad. Sci. USA 117, 28515–28524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Stephenson EB, Peel AJ, Reid SA, Jansen CC, and McCallum H (2018). The non-human reservoirs of Ross River virus: a systematic review of the evidence. Parasit. Vectors 11, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kain MP, Skinner E, van den Hurk AF, McCallum H, and Mordecai E (2021). Physiology and ecology combine to determine host and vector importance for Ross River virus. eLife 10, e67018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Stephens CR, González-Salazar C, Sánchez-Cordero V, Becker I, Rebollar-Tellez E, Rodríguez-Moreno Á, Berzunza-Cruz M, Balcells CD, Gutiérrez-Granados G, Hidalgo-Mihart M, et al. (2016). Can you judge a disease host by the company it keeps? Predicting disease hosts and their relative importance: a case study for leishmaniasis. PLoS Negl. Trop. Dis 10, e0005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jansen AM, Xavier S.C. das C., and Roque ALR (2018). Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasit. Vectors 11, 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kilpatrick AM, Daszak P, Jones MJ, Marra PP, and Kramer LD (2006). Host heterogeneity dominates West Nile virus transmission. Proc. Biol. Sci 273, 2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Brown HE, Childs JE, Diuk-Wasser MA, and Fish D (2008). Ecological factors associated with West Nile virus transmission, northeastern United States. Emerg. Infect. Dis 14, 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bradley CA, Gibbs SEJ, and Altizer S (2008). Urban land use predicts West Nile virus exposure in songbirds. Ecol. Appl. Publ. Ecol. Soc. Am 18, 1083–1092. [DOI] [PubMed] [Google Scholar]
- 216.Bowden SE, Magori K, and Drake JM (2011). Regional differences in the association between land cover and West Nile virus disease incidence in humans in the United States. Am. J. Trop. Med. Hyg 84, 234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nolan MS, Schuermann J, and Murray KO (2013). West Nile Virus infection among humans, Texas, USA, 2002–2011. Emerg. Infect. Dis 19, 137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kain MP, and Bolker BM (2019). Predicting West Nile virus transmission in North American bird communities using phylogenetic mixed effects models and eBird citizen science data. Parasit. Vectors 12, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Plowright RK, Becker DJ, McCallum H, and Manlove KR (2019). Sampling to elucidate the dynamics of infections in reservoir hosts. Philos. Trans. R. Soc. B Biol. Sci 374, 20180336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Baker SE, Cain R, van Kesteren F, Zommers ZA, D’Cruze N, and Macdonald DW (2013). Rough trade: Animal welfare in the global wildlife trade. BioScience 63, 928–938. [Google Scholar]
- 221.International Union for the Conservation of Nature, Commission on Ecosystem Management. Nature-based Solutions, https://www.iucn.org/commissions/commission-ecosystem-management/our-work/nature-based-solutions. (Accessed June 7, 2021.) [Google Scholar]
- 222.US Department of the Interior, US Department of Agriculture, US Department of Commerce, Council on Environmental Quality (2021). Conserving and Restoring America the Beautiful (Washington, DC: US Department of the Interior; ), https://www.doi.gov/sites/doi.gov/files/report-conserving-and-restoring-america-the-beautiful-2021.pdf. [Google Scholar]
- 223.Natural Resources Defense Council. 30x30: NRDC’s commitment to protect nature and life on Earth, https://www.nrdc.org/30x30-nrdcs-commitment-protect-nature-and-life-earth. (Accessed 7 June 2021.) [Google Scholar]
- 224.MacDonald AJ, and Mordecai EA (2019). Amazon deforestation drives malaria transmission, and malaria burden reduces forest clearing. Proc. Natl. Acad. Sci. USA 116, 22212–22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.The Group on Earth Observations Biodiversity Observation Network. What are EBVs? https://geobon.org/ebvs/what-are-ebvs/. (Accessed 10 August 2021.) [Google Scholar]
- 226.Dyer LA, Walla TR, Greeney HF, Stireman III JO, and Hazen RF (2010). Diversity of interactions: A metric for studies of biodiversity. Biotropica 42, 281–289. [Google Scholar]