Abstract
Acquired thrombotic thrombocytopenic purpura (aTTP) is a rare and life‐threatening autoimmune thrombotic microangiopathy. Caplacizumab, evaluated in phase II and III studies in adults, shortens the time to platelet count response and reduces aTTP exacerbations, has a favorable safety profile, and can potentially reduce refractoriness and mortality associated with aTTP. Since no children with aTTP were enrolled in these clinical trials, caplacizumab has been initially approved for use only in adult patients with aTTP (10 mg). Pediatric dosing recommendations were developed using model‐based simulations. A semimechanistic pharmacokinetic/pharmacodynamic population model has been developed describing the interaction between caplacizumab and von Willebrand factor antigen (vWF:Ag) following intravenous and subcutaneous administration of caplacizumab in different adult populations, at various dose levels, using nonlinear mixed‐effects modeling. Based on the allometrically scaled pharmacokinetic/pharmacodynamic model, different dosing regimens were simulated in 8000 children (aged 2‐18 years). Simulated caplacizumab exposures and vWF:Ag levels across different age categories were compared to an adult reference group. A simulated daily dose of 5 mg in children weighing <40 kg and of 10 mg in children weighing ≥40 kg resulted in similar exposures and vWF:Ag suppression across age and weight groups. Despite the lack of pediatric clinical data, the results of this modeling and simulation analysis constituted the basis for the European extension of indication for caplacizumab (10 mg) to adolescents aged >12 years and with a body weight ≥40 kg. This represents a rare case in which regulatory authorities have deemed a modeling and simulation study robust enough to approve a variation of indication.
Keywords: aTTP, caplacizumab, model‐informed drug development, pediatric dosing, pharmacokinetics, pharmacodynamics, von Willebrand factor
Acquired thrombotic thrombocytopenic purpura (aTTP) is an acute, immune‐mediated hematologic disorder with a mortality rate of up to 90%, if left untreated. Patients with aTTP produce autoantibodies that inhibit the von Willebrand factor (vWF) cleaving enzyme ADAMTS‐13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13). As a result, hyperadhesive, ultra‐large multimers of vWF (UL‐vWF) released from the endothelium are not cleaved appropriately, leading to spontaneous platelet aggregation. The consumption of platelets in these microthrombi results in thrombocytopenia, the main clinical hallmark of aTTP, microangiopathic hemolytic anemia, and may lead to ischemia and end‐organ damage. 1
The annual incidence of aTTP is ≈6 cases per 1 million people in the United Kingdom 2 and 3 to 11 cases per 1 million people in the United States. 3 , 4 The condition is more common in women than in men (2:1), 1 and while it can occur at any age, it is more frequent in people aged 20 to 50 years. In children (aged <18 years) the incidence of aTTP is about 3% of that in adults. 3 Demographic and clinical features of children with aTTP are similar to those of adults with aTTP, and the pathophysiology of the disease is similar across ages. 3
Until the recent approval of caplacizumab (Cablivi, Sanofi Genzyme), the mainstay of therapy for aTTP has been plasma exchange (PE) and immunosuppressive therapies. 5 PE is aimed at removing UL‐vWF and autoantibodies directed against ADAMTS13, as well as at restoring ADAMTS‐13 activity. Immunosuppressive therapies, typically with corticosteroids and rituximab, are used to inhibit the formation of autoantibodies involved in the aTTP disease process. Despite this historical treatment, the disease still presented several challenges: about 40% of patients experienced a decrease of ADAMTS‐13 activity after remission, potentially translating into relapsing episodes of aTTP 6 , 7 , 8 , 9 ; up to 50% of patients suffered from disease exacerbations shortly after stopping PE 10 ; finally, mortality rate was still up to 20% in spite of treatment, with most deaths occurring within 30 days of diagnosis. 9 , 11 Besides, up to 46% of treated patients did not respond to PE and corticosteroids, thus requiring additional therapy. 5 Altogether, these considerations indicated the need for a therapy that could rapidly inhibit the formation of microvascular thrombi (thus reducing the risk of organ damage) and maintain the platelet‐protective effect until the underlying autoimmune activity has been resolved. To this aim, caplacizumab has recently been developed.
Caplacizumab is a humanized, bivalent, anti‐vWF Nanobody with a size of ≈28 kilodaltons. Caplacizumab binds to the A1 domain of vWF and specifically interferes with the interaction of vWF with platelets. The drug can interact with vWF in both its active and inactive forms, thereby preventing vWF‐mediated platelet aggregation. The levels of circulating vWF antigen (vWF:Ag), measured in peripheral blood, are higher in patients with aTTP than in healthy subjects. 4 Upon treatment with caplacizumab, a transient reduction of vWF:Ag can be detected in the circulation, and this reduction is considered a good indicator of the attainment of the desired pharmacological effect. Additionally, vWF:Ag is considered a good biomarker to characterize the exposure‐response relationship of caplacizumab. 4 , 12
Caplacizumab has been approved in Europe (September 2018), 13 the United States (February 2019), 14 and several other countries, for use in the treatment of adult patients with aTTP, in combination with PE and immunosuppression. Marketing authorization was based on 2 multicenter randomized placebo‐controlled trials. 15 , 16 , 17 Besides the results of these clinical studies, both applications for marketing authorizations included modeling and simulation studies to support the selected dosing regimen. 18 , 19 The model‐based analysis was informed by pooled data from 7 phase I to III studies of caplacizumab in different adult populations (healthy volunteers, patients undergoing percutaneous coronary intervention [PCI], and patients with aTTP) with a wide range of intravenous (IV) and subcutaneous (SC) doses. A model was developed to describe the interplay between caplacizumab and its target vWF:Ag. The model's appropriateness and value in justifying the proposed dosing of caplacizumab was acknowledged in the assessment reports by both the European Medicines Agency (EMA) 18 and the US Food and Drug Administration (FDA). 19 Among other aspects, the modeling was used to support the lack of need for weight‐based dose adjustments in the relevant adult body weight range.
Caplacizumab had initially been approved only for adults (≥18 years) 18 since, due to the rarity of the disease in children, 3 no clinical data on pediatric patients were available (as attempts to enroll such patients in the phase II and III studies [ALX0681‐2.1/10, TITAN, 2010‐019375‐30/NCT01151423 and ALX0681‐C301, HERCULES, 2015‐001098‐42/NCT02553317] had proven unsuccessful). Therefore, in 2018 the EMA agreed that clinical studies of caplacizumab in the pediatric population are not feasible and thus deleted this obligation from the pediatric investigation plan. 21 Furthermore, the EMA requested the results of the remaining pediatric investigation plan measure–a modeling and simulation study (ALX‐0681‐MS‐01) that explored an appropriate dosing strategy in children to be incorporated in the European label for Cablivi and to expand the indication to include adolescents weighing at least 40 kg. 21 In Europe, this pediatric label extension was approved in 2020. 20 Adding to the current knowledge on aTTP therapy for children, a few cases of pediatric patients with aTTP effectively treated with caplacizumab have been reported in the literature so far. 22 , 23 , 24 , 25 , 26 Altogether, these reports suggest that caplacizumab is effective in the treatment of children and adolescents with aTTP as well as in pediatric patients with relapsing aTTP. 24
This article presents the results of the pediatric modeling and simulation study that allowed inclusion of adolescents in the label for caplacizumab in Europe without support of clinical trial data. 20 The objective of this modeling and simulation study was to establish a suitable dosing regimen of caplacizumab in adolescents and children aged >2 years. The basis for this study was the pharmacokinetic/pharmacodynamic (PK/PD) model previously developed for the same drug in adults, 12 , 18 , 19 using allometric scaling principles. To achieve this goal, the expected caplacizumab and vWF:Ag concentrations in pediatric patients with aTTP were initially simulated using a flat dosing regimen of 10 mg SC daily caplacizumab. These simulations were then compared with those performed for a weight‐based dosing of 5 mg for children with body weight <40 kg and 10 mg for children weighing at least 40 kg. Finally, suitability of the weight‐based dosing regimen was confirmed by comparing the caplacizumab exposures and vWF:Ag suppression levels simulated in children with those observed in adult patients with aTTP. 27
Methods
Simulations were performed to establish a suitable dosing regimen in adolescents and children aged >2 years, and were based on the PK/PD model previously developed in adults 4 , 12 as well as on data from the literature for demographic factors in a pediatric population.
Adult Model
Data Used for the Development of the PK/PD Model in Adults
Data from 7 studies (of which some included substudies) was used to develop the PK/PD model already described in adults 4 , 12 :
Two phase I studies in healthy subjects and one phase I study in patients with stable angina undergoing PCI, conducted to characterize the pharmacokinetics, pharmacodynamics, safety, and tolerability of caplacizumab. In these studies, caplacizumab was administered in single and multiple doses by IV infusion (ALX‐0081‐01/06, 2006‐006502‐28; ALX‐0081‐1.2/08a/08b/08c OLE, 2007‐007263‐24), 28 or in single and multiple doses by SC injection (ALX0681‐1.1/08 first and second part, 2008‐006624‐60). 29
One phase I study in healthy subjects to evaluate the bioequivalence of a new reconstituted lyophilized formulation with the liquid formulation of caplacizumab used in previous studies (ALX‐0681‐C102, 2014‐001294‐13/NCT02189733). 30
One phase II study (ALX‐0081‐2.1/09, 2009‐012206‐39/NCT01020383) 31 in high‐risk patients undergoing PCI, conducted to evaluate the safety, tolerability, and efficacy of caplacizumab compared with that of the glycoprotein IIb/IIIa inhibitor ReoPro.
Two studies in patients with aTTP: 1 phase II study (ALX0681‐2.1/10, TITAN, 2010‐019375‐30/NCT01151423) 15 and 1 phase III study (ALX0681‐C301, HERCULES, 2015‐001098‐42/NCT02553317), 16 both performed to evaluate the safety, tolerability, and efficacy of caplacizumab as an adjunct to PE treatment.
Pooling data from these trials generated a study population of 541 subjects: 317 males and 224 females with ages ranging from 18 to 85 years, body weight ranging from 46.5 to 150 kg, and a creatinine clearance ranging from 11.9 to 244 mL/min. Of the 541 subjects, 100 were healthy volunteers, 225 patients with stable angina (PCI group), and 216 patients with aTTP. The pooled data generated a total of 3629 PK and 6295 PD observations (vWF:Ag), which were used to inform the modeling. Pooling of these observations was justified by in vitro and in vivo data showing that caplacizumab interacts similarly with normal multimers of vWF (observed in healthy volunteers and patients undergoing PCI) and with UL‐vWF multimers (observed in patients with aTTP). The large, pooled data set offered the best possibility to characterize the caplacizumab‐vWF:Ag interaction and the associated covariates. At the same time, during model development, differences among the 3 subject groups, for example, disease progression, baseline characteristics, and effects of standard of care, were explored and characterized. When applying the model for the simulations, only the characteristics of patients with aTTP were considered (as specified below).
Development of the PK/PD Model in Adults
The population PK/PD analysis was conducted by nonlinear mixed‐effects modeling using NONMEM, version 7.3.0 (ICON Development Solutions, Hanover, Maryland). 32 The model was developed stepwise. Initially, a subset of the data set, including only data from healthy volunteers and PCI patients, was used for the model development. In a second step, the model was updated to describe the specific characteristics related to the aTTP disease status and standard of care (ie, PE). For this purpose, only the subset of the data set including patients with aTTP was used. Finally, the effects of the covariates age, sex, race, blood group, body weight, creatinine clearance (CrCl), antidrug antibodies, and concomitant treatment were evaluated.
During model development, discrimination between models was mainly based on the inspection of graphical and numerical diagnostics, including standard goodness‐of‐fit plots and prediction‐corrected visual predictive checks, as well as changes in the objective function value provided by NONMEM.
Final Population PK/PD Model Used for Simulation in Children
The final population PK/PD model characterizing the interaction between caplacizumab and vWF:Ag in different adult populations following IV and SC administration was based on a full target‐mediated drug disposition model structure (Figure 1). 12 The model included a 2‐compartment drug disposition model with first‐order linear elimination of free drug and two parallel first‐order absorption processes following SC dosing of caplacizumab. The model described the formation of drug‐vWF complexes with the ability to form both dimers and trimers. The production, maturation, and release of vWF:Ag were described by transit compartments and a vWF:Ag pool with feedback effects stimulating the production and release of vWF:Ag when vWF:Ag decreased below the subject's baseline level. For patients with aTTP, disease progression, and the effects of PE treatment were adequately captured by (1) a time‐dependent disease progression model and (2) removal of free vWF:Ag, free drug, and drug‐vWF complexes by PE.
Figure 1.
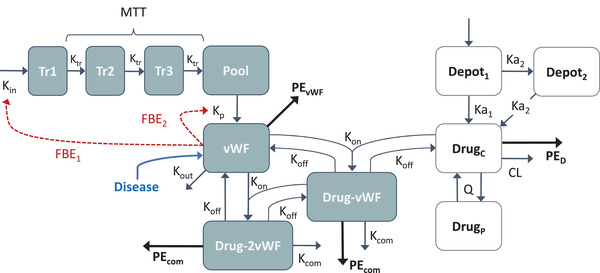
Schematic overview of the final population PK/PD model developed in different adult populations and describing the interaction between caplacizumab and vWF:Ag following IV and SC caplacizumab administration. Disease: disease effect on baseline free vWF. Total vWF = free vWF + vWF bound in dimer complex + 2 x vWF bound in trimer complex. Total drug = free drug/Vc + drug bound in dimer complex + drug bound in trimer complex. CL, drug non–target‐mediated clearance; Depot1, depot compartment SC dosing; Depot2, delayed absorption compartment following SC dosing; DrugC, free drug compartment, DrugP, peripheral drug compartment; Drug‐vWF: dimer (drug‐von Willebrand factor) complex; Drug‐2vWF, trimer (drug‐vWF‐drug) complex; FBE1, feedback effect parameter 1; FBE2, feedback effect parameter 2; Ka1, first‐order (fast) absorption rate for SC dosing; Ka2, first‐order (slow) absorption rate for SC dosing; Kcom, complex elimination rate; Kin: production rate for vWF; Koff, dissociation rate constant; Kon, association rate constant; Kout, elimination rate of free vWF; Kp, pool transfer rate; Ktr, transit rate constant; MTT, mean transit time; PEcom, PE‐mediated complex elimination rate; PED, PE‐mediated drug elimination rate; PEvWF, PE‐mediated free vWF elimination rate; Pool, vWF precursor pool; Q, drug intercompartmental clearance; Tr1, Tr2, Tr3, vWF precursor transit compartment; vWF, free vWF.
The final population PK/PD model was used to support the approval of caplacizumab in the adult population. Reviewers from both the EMA 18 and the FDA 19 deemed the model appropriate for the intended use. Simulations were performed using the final model only for patients with aTTP, to evaluate the effect of, among others, change in doses and patient body weight.
Model‐Based Simulations in Children
Generation of Simulated Populations
A simulated adult population of 1000 patients with aTTP was generated by assuming the same covariate distribution as that observed in the 2 clinical studies in patients with aTTP included in the PK/PD analysis (TITAN 15 and HERCULES 16 ). From these studies, complete covariate vectors (ie, removing all individuals with missing covariates) were extracted for the following covariates: body weight, body mass index, CrCl, age, and sex. From these unique covariate vectors (n = 197), 1000 samples were taken with replacement. The 1000 sampled vectors had median and/or mean values similar to those of the original covariates. The original and resampled means (with standard deviation) for body weight were, respectively, 83.62 (21.35) kg and 82.54 (20.83) kg; for CrCl they were 107.02 (54.56) mL/min and 102.54 (48.97) mL/min; and for age they were 44.42 (13.04) years and 44.56 (13.21) years.
A simulated population of 8000 children was generated from data sampled from the National Health and Nutrition Examination Survey III database. 33 Overall, 1000 children were randomly sampled for each age category (ie, 2‐year slots from the age of 2 years up to and including 17 years of age). Demographics of the simulated pediatric population are reported in the Results section.
Simulated Treatment Regimen
The treatment regimen simulated in the pediatric population is schematically represented in Figure 2 and consisted of:
IV loading dose (with the same dose level as for the maintenance dose)
One‐hour daily PE treatment for 7 days (PE starting 3 hours after the IV loading dose)
SC once‐daily dosing of caplacizumab for 40 days (injection starting 4 hours after the IV loading dose, that is, at the end of PE treatment)
Follow‐up for 60 additional days
Figure 2.
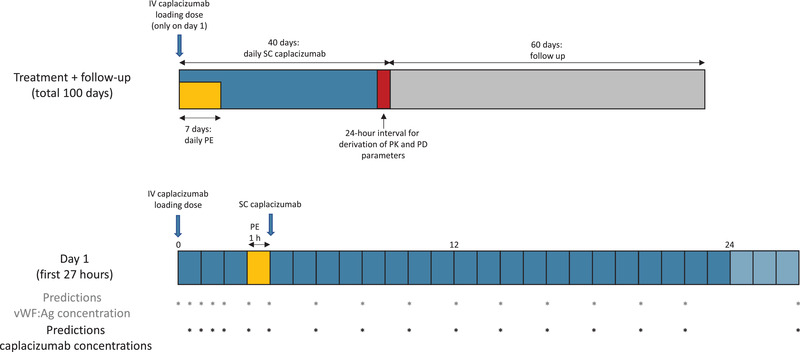
Schematic overview of the simulated treatment regimen for the whole treatment period (upper bar) and the first day of treatment (lower bar). Asterisks indicate the time points at which predictions were made for the concentration of caplacizumab or vWF:Ag. IV, intravenous; SC, subcutaneous; vWF:Ag, von Willebrand factor antigen.
Predictions of caplacizumab concentrations were simulated at 0.5, 1, 1.5, 2, 3, and 4 hours after the initial IV dose (Figure 2). Predictions of vWF:Ag concentrations were simulated at the same time points listed above, with an additional baseline measurement (Figure 2). Special care was taken to always predict concentrations immediately before and after PE treatment, that is, just before the SC dose. After SC caplacizumab administration (days 1‐40), both types of predictions were simulated at the following postdose hours: 2, 4, 6, 8, 10, 12, 14, 16, 18, and 23 (Figure 2). A prediction was then made 24 hours after caplacizumab dosing was halted, and then every 48 hours for the remaining follow‐up period (29 predictions over 58 days). All simulations were performed for a total of 100 days.
Pediatric Population: Simulation Settings and Assumptions
Based on data from the literature, the following parameters in children with aTTP were assumed to be similar to those in adults with aTTP: expression of vWF 34 (baseline vWF:Ag levels) (with a median of 65.6 nM [164 IU/dL], range of 9‐224 nM [22.5‐560 IU/dL], in agreement with Ablynx 15 , 16 ), affinity of caplacizumab to the vWF target, 20 and distribution of patients with aTTP between sexes. 3
With regard to body weight, the simulated pediatric population had a similar body composition as that of US children in the National Health and Nutrition Examination Survey database, 33 and the body weight distribution in the simulated adult population was assumed to be similar to the studied aTTP adult population. 20
In addition, CrCl, as well as the typical clearance, intercompartmental clearance, central volume of distribution, and peripheral volume of distribution values in children were allometrically scaled to adults with an assumed normal renal function for a 70‐kg subject of 120 mL/min.
Software Used for the Analysis
The simulations were performed using NONMEM version 7.3.0 32 installed on a Xeon‐based server (Intel, Santa Clara, California) running Scientific Linux 6.3. NONMEM runs were performed using the gfortran compiler, version 4.4.6. Data management and further processing of NONMEM output were performed using R version 3.3.3 (2017‐03‐06; R Foundation for Statistical Computing, Vienna, Austria). 35
Results
Subject Demographics and Characteristics
The demographic characteristics for each age category of the simulated pediatric population are reported in Table 1. The pediatric population included a total of 8000 children divided into 8 age categories. In addition, a simulated adult population of 1000 patients with aTTP was generated by assuming the same covariate distribution as that observed in the 2 clinical studies in patients with aTTP included in the previous PK/PD analysis (see Methods section for details).
Table 1.
Relevant Demographic Characteristics of the Simulated Pediatric Population and Proportion of Subjects in Each Age Category Receiving the 5‐mg or 10‐mg Dose in the Weight‐Dependent Dosing Simulation
| Age Category | Age Range | Male Body Weight (kg) Median (5th‐95th Percentile) | Female Body Weight (kg) Median (5th‐95th Percentile) | Females (%) | <40 kg and 5‐mg Dose (%) | ≥40 kg and 10‐mg Dose (%) |
|---|---|---|---|---|---|---|
| 1 | ≥2 to <4 | 14.8 (12.2–18.6) | 14.2 (11.4‐18.1) | 66.0 | 100.0 | ‐ |
| 2 | ≥4 to <6 | 19.4 (15.2‐26.2) | 18.7 (15.2‐24.8) | 68.6 | 100.0 | … |
| 3 | ≥6 to <8 | 25.4 (19.7‐36.8) | 23.9 (18.8‐36.9) | 65.9 | 97.4 | 2.6 |
| 4 | ≥8 to <10 | 31.7 (23.9‐52.1) | 31.3 (23.3‐50.3) | 67.1 | 79.7 | 20.3 |
| 5 | ≥10 to <12 | 40.1 (30.2‐64.2) | 43.9 (30.2‐69.0) | 67.1 | 41.0 | 59.0 |
| 6 | ≥12 to <14 | 53.3 (35.1‐83.8) | 53.7 (40.2‐83.8) | 67.8 | 8.0 | 92.0 |
| 7 | ≥14 to <16 | 64.6 (48.2‐98.4) | 58.2 (44.7‐85.9) | 64.4 | … | 100.0 |
| 8 | ≥16 to <18 | 69.8 (54.9‐105.6) | 60.3 (46.7‐89.7) | 63.8 | … | 100.0 |
| 9 | ≥18 (adults) | 85.0 (63.0‐120.0) | 79.7 (52.1‐122.6) | 67.0 | … | 100.0 |
Simulations of Different Dosing Strategies: Flat vs Weight‐Based Dosing
All individual steady‐state area under the plasma concentration–time curve (AUCss) values were derived at day 40 for each age category and are displayed using box plots (Figure 3). Emphasis on outliers was toned down in these plots as outliers are generally dependent on the number of subjects/simulations per age category and do not contribute to the comparison of the general tendencies in the different groups.
Figure 3.
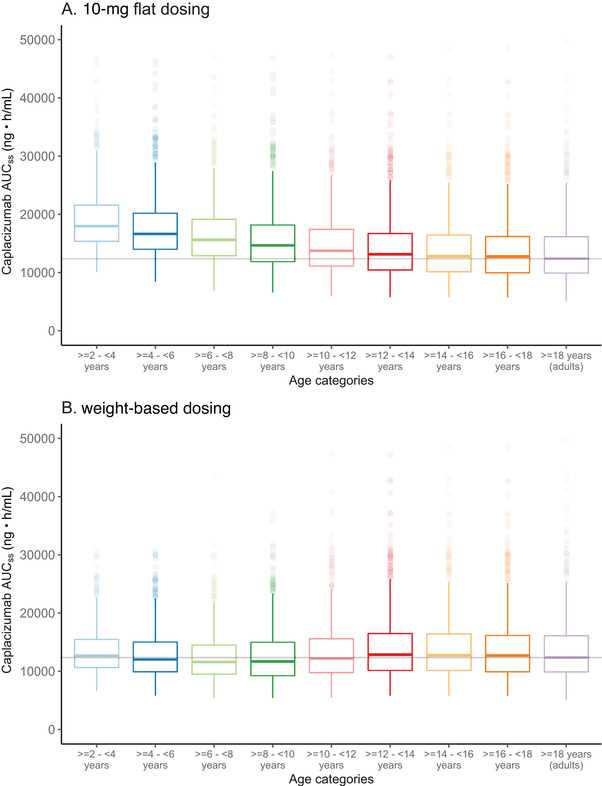
Box plots of caplacizumab AUCss for the different age categories, following (A) daily SC flat dosing of 10 mg or (B) weight‐based dosing of caplacizumab for 40 days. Treatment included an IV loading dose on day 1 and 1‐hour PE treatment during the first 7 days. Horizontal, colored lines indicate the median AUCss, boxes indicate the 25th and 75th percentiles, and the upper/lower whiskers extend from the box to the largest/smallest value no further than 1.5*IQR from the box. For ease of comparison, the horizontal gray line indicates the median AUCss in the adult population. Emphasis on outlying values (ie, higher or lower than the whisker) were toned down. AUCss, steady‐state area under the plasma concentration–time curve; IQR, interquartile range; PE, plasma exchange; SC, subcutaneous.
When simulating a flat dosing strategy of 10 mg of caplacizumab, given daily SC for 40 days, including an IV loading dose and a 1‐hour PE treatment during the first 7 days (Figure 2), caplacizumab concentrations indicated a higher exposure for lower age categories (Figure 3A). This trend of increased AUCss for the lower age categories was further confirmed when comparing other parameters. For lower age categories, also maximum concentration at steady state (Css,max) and minimum concentration at steady state (Css,min) were higher than those in the reference adult population (data not shown), thus confirming the need for a dose reduction in low‐weight pediatric patients.
Simulations of the weight‐based dosing strategy followed the same treatment schedule as the flat dosing (Figure 2) but differed in that pediatric patients with body weight <40 kg were treated with 5 mg daily caplacizumab and those with body weight of at least 40 kg (or adults) received 10 mg daily. The results show that with the proposed weight‐based dosing approach, similar AUCss could be obtained across the different pediatric age groups (Figure 3B). Furthermore, these AUCss predictions did not show clear deviations from the AUCss predicted in the adult population.
When considering the median AUCss (Figure 3B), Css,max, and Css,min (data not shown) for each age group, we noticed a trend indicating a slight decrease in the median values of these parameters in the first 3 age categories. However, this downward trend was not evident in the subsequent age groups (group 4, ≥8 to <10 years; and group 5, ≥10 to <12 years), in which an increasing number of children received the 10‐mg caplacizumab dose (Table 1). These observations were confirmed when the parameters AUCss, Css,max, and Css,min were summarized by 2 larger weight categories (ie, <40 or ≥40 kg) (Table 2). When these 2 groups of children were compared to the adult group, all parameters showed slightly lower median levels in children weighing <40 kg and slightly higher levels in children weighing at least 40 kg (Table 2).
Table 2.
Overview of the Simulated AUCss, Css,max, and Css,min by Weight Category, for the Corresponding Caplacizumab Dose, on Day 40, Following Once‐Daily Subcutaneous Weight‐Based Dosing of Caplacizumab
| AUCss (ng • h/mL) | Css,max (ng/mL) | Css,min (ng/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weight Range (kg) | Number of Subjects | Median | 5th Percentile | 95th Percentile | Median | 5th Percentile | 95th Percentile | Median | 5th Percentile | 95th Percentile |
| <40 | 4261 | 11820 | 7438 | 20 507 | 600.0 | 379.6 | 1005.9 | 376.0 | 230.6 | 661.9 |
| ≥40 | 3739 | 12970 | 7745 | 24 126 | 640.0 | 398.7 | 1150.2 | 442.0 | 249.2 | 872.4 |
| Adults | 1000 | 12381 | 7295 | 23 794 | 609.0 | 371.8 | 1123.6 | 435.0 | 242.1 | 873.4 |
AUCss, steady‐state area under the the plasma concentration–time curve; Css,max, maximum concentration at steady state; Css,min, minimum concentration at steady state.
We then analyzed the relationship between the simulated AUCss of each subject and their body weight (Figure 4A) or age (Figure 4B). When simulations were plotted against body weight, the AUCss showed a gradually decreasing trend in young pediatric patients with body weight <40 kg (Figure 4A). The AUCss values became closer to those in adults as pediatric patients received the 10‐mg dose when they reached a weight ≥40 kg (Figure 4A and Table 1). When simulated exposures were plotted against children's specific age (Figure 4B), rather than age categories (Figure 3B), the AUCss were similar and relatively stable across ages. Similar trends, related to both weight and age, were also observed for Css,max (data not shown).
Figure 4.
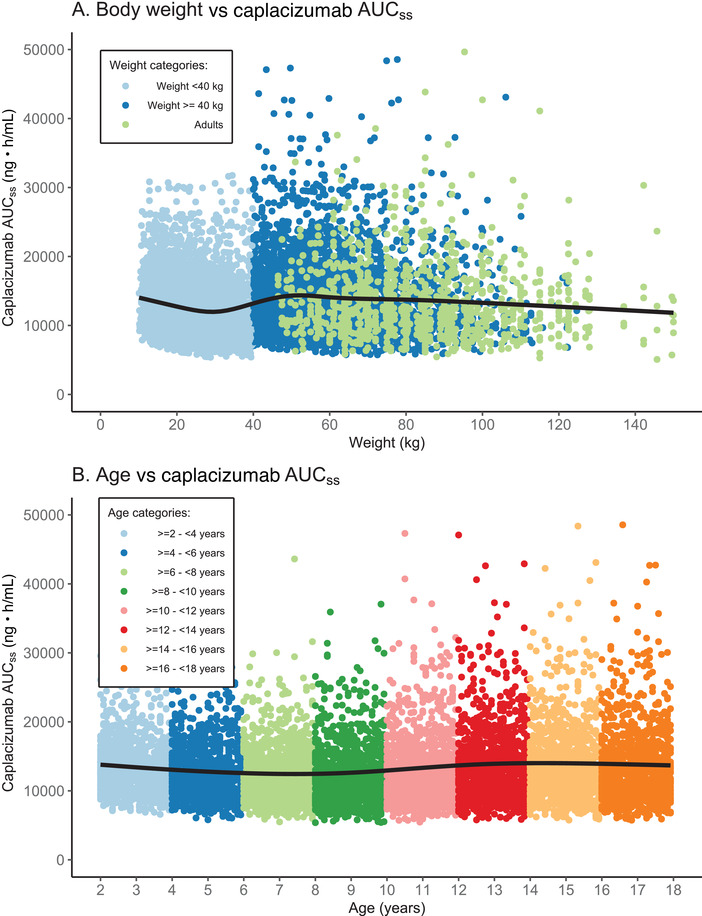
Relationship between simulated caplacizumab AUCss and (A) body weight or (B) age following daily SC weight‐based dosing for 40 days. Treatment included an IV loading dose on day 1 and 1‐hour PE treatment during the first 7 days. In A, the simulated AUCss of the 8000 pediatric patients are shown (colored by weight category, as indicated in the legend), together with the simulated AUCss of the 1000 adults. In B, the simulated AUCss of the 8000 pediatric patients are shown (colored by age category, as indicated in the legend). For both plots, the black line is a smooth line indicating the trend of the data. AUCss, steady‐state area under the plasma concentration–time curve; IV, intravenous; PE, plasma exchange; SC, subcutaneous.
Simulation of the Effects of Weight‐Based Dosing on Disease Progression (vWF:Ag Time Course)
In the first part of this study, we established that a weight‐based dosing is needed for pediatric patients with aTTP to ensure caplacizumab exposures similar to those observed in adult patients with aTTP receiving the recommended daily SC 10‐mg dosing regimen. We then proceeded by using the previously developed PK/PD model to, first, determine the model‐predicted total plasma concentrations of caplacizumab in children, and then assess the PD response, quantified in terms of total vWF:Ag levels.
When plotting the simulated median caplacizumab concentration time‐course for each age category (Figure 5A), we confirmed that similar plasma concentration levels are predicted across the different age groups. In addition, when plotting the vWF:Ag levels as change from baseline (Figure 5B), our predictions suggested that similar caplacizumab exposures lead to similar levels of vWF:Ag suppression for the different age categories. Also, a faster return to baseline vWF:Ag concentrations was generally predicted for younger age groups.
Figure 5.
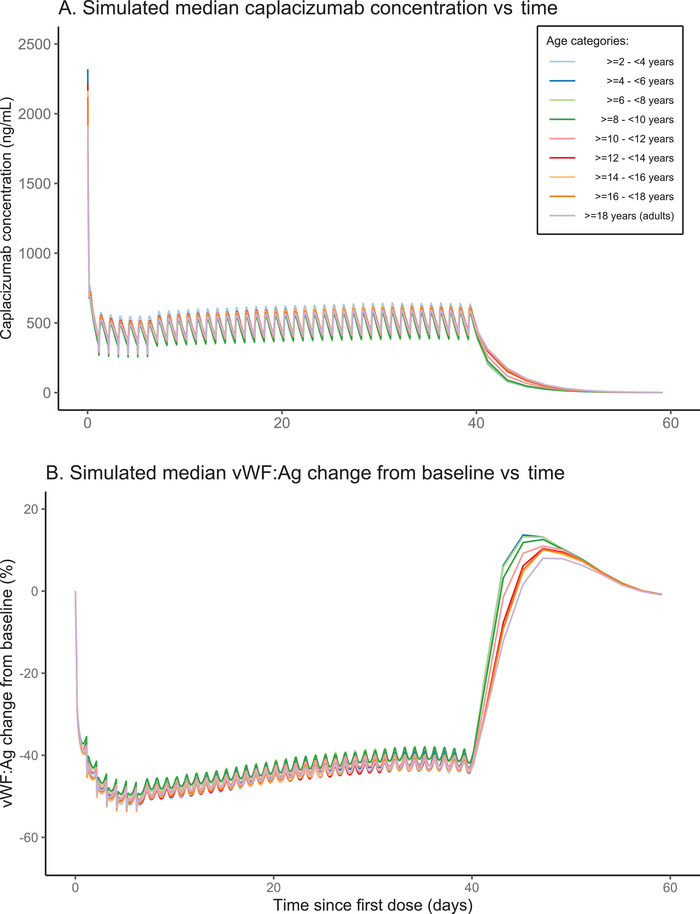
(A) Simulated median caplacizumab concentration time‐course and (B) simulated median vWF:Ag change from baseline (%) following daily SC weight‐based dosing of caplacizumab for 40 days. Treatment included an IV loading dose on day 1 and 1‐hour PE treatment during the first 7 days. Both plots show data for different age categories (colored as indicated in the legend). Simulations were performed for 100 days. For ease of visualization, only simulations up to day 60 are shown. IV, intravenous; PE, plasma exchange; SC, subcutaneous; vWF:Ag, von Willebrand factor antigen.
Discussion
This article describes a population PK/PD model‐based simulation aimed at establishing a suitable dosing regimen for caplacizumab in children with aTTP aged >2 years. According to our results, a suitable dose in children is −5 mg if body weight is <40 kg and 10 mg if body weight is at least 40 kg. 27 The simulation for this dosing, given with the same regimen as that recommended for adults, resulted in similar caplacizumab exposures and vWF:Ag suppression as those observed in the adult aTTP patient population. The results of this analysis were submitted to the EMA with the application for a type II variation to support a pediatric label change. Based on this analysis, and without the request of pediatric clinical trial data, the EMA extended the indication and corresponding dose recommendations of caplacizumab to adolescents aged >12 years and with body weight of at least 40 kg. Additionally, Section 5.2 of the prescribing information (reporting the PK properties) was updated with the results of the present modeling and simulation study.
In children, 2 forms of TTP have been described: a congenital form (accounting for about one‐third of the cases, usually manifesting already in neonates, and often chronic) and an acquired form (accounting for the remaining two‐thirds of the cases). 8 Both forms are characterized by severe ADAMTS‐13 deficiency and are life threatening. The course of aTTP is similar across ages, from childhood to adulthood. Demographic characteristics of adults and children with aTTP are also similar, 3 , 36 with more cases occurring in female patients and the first episode of aTTP occurring between ages 4 months and 17 years (median, 13 years). 8 In a French pediatric cohort, the mortality rate of aTTP was reported to be 9% and the clinical relapse rate ≈25%. 3 , 36 As in adults, the onset of aTTP in children also needs rapid diagnosis and therapeutic management. The first‐line treatment in children is based on daily plasma replacement therapy (plasma infusion or PE) and usually steroid treatment. Some children, especially those refractory to treatment, may need immunomodulation with rituximab. However, the effects of rituximab are normally detectable 2 weeks after the first infusion and thus may not be able to prevent early death; furthermore, daily PE needs to be continued during the course of rituximab administration. 8 Published studies discussing the management of aTTP in children report that current aTTP treatment needs improvement and that children would clearly benefit from the introduction of innovative therapeutic drugs, such as caplacizumab. 8
A few case reports of children with aTTP using caplacizumab have been published, and all showed that pediatric patients responded well to therapy, with few or no side effects reported and no new safety findings. 22 , 23 , 24 , 25 , 26 However, despite the efforts, clinical studies involving a sufficient number of children have proven not feasible, leaving modeling and simulation studies the only option to explore pediatric dosing. Caplacizumab is an orphan drug for which a PK/PD model has already been developed for a population of adult patients with aTTP. 12 This PK/PD model was used to support the approval of caplacizumab in adults, and reviewers from both the EMA 18 and the FDA 19 deemed the model appropriate for the intended use. For the current study, the following parameters in children with aTTP were assumed to be similar to those in adults with aTTP, based on data from the literature: expression of vWF 34 (baseline vWF:Ag levels), affinity of caplacizumab to the vWF target, distribution of patients with aTTP between sexes. 3 In addition, CrCl, as well as the typical clearance, intercompartmental clearance, central volume of distribution, and peripheral volume of distribution values in children were allometrically scaled to adults with an assumed normal renal function for a 70‐kg subject of 120 mL/min. These considerations allowed using the previously developed population PK/PD model to perform simulations and, finally, suggest an appropriate dosing regimen in children. A recent publication by Gill et al 37 indicates that the expression of vWF may on average be somewhat lower in young healthy children undergoing tonsillectomy (being ≈20% lower in children under 3 years compared to teenagers). This difference is small in relation to the wide variability observed for baseline vWF:Ag levels in patients with aTTP. 4 The simulations here performed assumed a wide range of variability for baseline vWF:Ag levels: median of 63 nM (157 IU/dL) and a 90% prediction interval of 36.9 to 116 nM (92‐290 IU/dL). In general, baseline vWF:Ag levels have been shown to be higher in patients with aTTP than in healthy subjects, and currently no arguments suggest that this should not be the case for children. 4 The importance of baseline vWF:Ag levels was explored while creating the original PK/PD model that supported the adult indication. In that modeling analysis, lower baseline vWF:Ag levels were found to be associated with lower caplacizumab plasma concentrations and, consequently, with a less pronounced reduction in vWF (% reduction). However, these effects were not considered to be of a magnitude that warranted any dose adaptations.
Based on our simulations, we concluded that the weight‐based dosing regimen in children results in similar caplacizumab exposures and similar vWF:Ag suppression as those observed in adult subjects with aTTP receiving caplacizumab 10 mg daily. The specific treatment regimen that was used for the simulations is described in the Methods section and reflects the actual posology of caplacizumab in adults (Figure 2). 38 During an initial exploratory stage, alternative dosing strategies have been investigated, until the 2 more adequate strategies were found: the 10‐mg flat dosing and the weight‐based one, implying a 50% dose reduction in children weighing <40 kg. As expected, the simulations of exposure following a flat dosing (10 mg for all subjects) clearly indicated that this regimen determines a higher exposure in children with a low body weight, that is, primarily children aged <10 years and thereby average body weight <40 kg (Figure 3A, Table 1). On the other hand, a dose adjustment to 5 mg in children with a body weight <40 kg resulted in similar predicted exposures across age and weight groups (Figures 3B and 4; and Table 2). With this dose adjustment, the predicted median for AUCss and Css,max in each 2‐year age category is expected to be within ±8% of the median value for the adult population. The predicted median Css,min for children aged 6 to 8 years is 14% lower than the median in adults, but the predicted lower 5th percentile is only 4% lower than the equivalent adult percentile. Our results show that similar caplacizumab exposures lead to similar vWF:Ag suppression levels for the different age categories (Figure 5B). Noticeably, a faster return to baseline vWF:Ag concentrations is predicted for younger age groups.
Given the mechanism of action of caplacizumab and the current knowledge of aTTP in the pediatric population, efficacy and safety of caplacizumab in children are likely to be similar to those in adults. 20 Furthermore, similar caplacizumab exposures in children and adults are assumed to elicit comparable effects in both populations. While the modeling and simulation study reported here refers to children between ages 2 and 18 years, pediatric label change for caplacizumab has been approved by the EMA only for adolescents aged >12 years and with body weight of at least 40 kg. For the remaining pediatric population, the PK properties of caplacizumab have been reported in Section 5.2 of the prescribing information and can be used as guidance for clinicians that need to treat younger patients. Regarding the specific population of infants and children from birth up to 2 years, the EMA issued a waiver based on the consideration that “the specific medicinal product does not represent a significant therapeutic benefit as clinical studies are not feasible.” 21 Besides, the clinical characteristics of the condition presenting at this early age may require specific management, for example, in the rare, congenital form of TTP, prophylactic plasma therapy is the only therapeutic option currently available. 8
To the best of our knowledge, this represents a rare case in which regulatory authorities have deemed simulation studies sufficiently robust to support a pediatric indication and posology, in absence of clinical study data. 39 , 40
Conclusion
The PK/PD model previously developed for caplacizumab in adults was used to perform a simulation analysis in a population of 8000 children with aTTP. According to the results, the recommended dose in children (aged 2‐18 years) was 5‐mg caplacizumab if body weight is <40 kg, and 10 mg if body weight is at least 40 kg. The modeling and simulation data that were presented here have been submitted to the EMA via a type II variation. 20 Despite the absence of clinical study data, the EMA deemed the results sufficiently robust to approve an extension of the indication for caplacizumab, which now includes the recommended posology of 10 mg for pediatric patients aged >12 years and weighing over 40 kg.
Conflicts of Interest
M.B. and E.H. are employees of Pharmetheus AB, contracted as external consultants by Ablynx/Sanofi‐Aventis Group. B.D., F.C., R.D.P.S., M.L.S.‐M. are all employees of Sanofi‐Aventis Group and may hold shares and/or stock options in the company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Funding
This work was funded by Sanofi‐Aventis Group.
Acknowledgments
The authors thank Viviana Moroso, PhD, of Pharmetheus AB (Uppsala, Sweden), for providing medical writing support, in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
Data availability statement
Qualified researchers may request access to model control files used for the presented simulations, individual level simulated data and related documents. Potential patient‐level data will be anonymized, and documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
References
- 1. Shatzel JJ, Taylor JA. Syndromes of thrombotic microangiopathy. Med Clin North Am. 2017;101(2):395‐415. [DOI] [PubMed] [Google Scholar]
- 2. Scully M, Yarranton H, Liesner R, et al. Regional UK TTP Registry: correlation with laboratory ADAMTS 13 analysis and clinical features. Br J Haematol. 2008;142(5):819‐826. [DOI] [PubMed] [Google Scholar]
- 3. Reese JA, Muthurajah DS, Hovinga JAK, Vesely SK, Terrell DR, George JN. Children and adults with thrombotic thrombocytopenic purpura associated with severe, acquired Adamts13 deficiency: Comparison of incidence, demographic and clinical features: TTP in children and adults. Pediatr Blood Cancer. 2013;60(10):1676‐1682. [DOI] [PubMed] [Google Scholar]
- 4. Sargentini‐Maier ML, De Decker P, Tersteeg C, Canvin J, Callewaert F, De Winter H. Clinical pharmacology of caplacizumab for the treatment of patients with acquired thrombotic thrombocytopenic purpura. Expert Rev Clin Pharmacol. 2019;12(6):537‐545. [DOI] [PubMed] [Google Scholar]
- 5. Hollifield AL, Arnall JR, Moore DC. Caplacizumab: an anti–von Willebrand factor antibody for the treatment of thrombotic thrombocytopenic purpura. Am J Health Syst Pharm. 2020;77(15):1201‐1207. [DOI] [PubMed] [Google Scholar]
- 6. Hie M, Gay J, Galicier L, et al. Preemptive rituximab infusions after remission efficiently prevent relapses in acquired thrombotic thrombocytopenic purpura. Blood. 2014;124(2):204‐210. [DOI] [PubMed] [Google Scholar]
- 7. Coppo P, Cuker A, George JN. Thrombotic thrombocytopenic purpura: toward targeted therapy and precision medicine. Res Pract Thromb Haemost. 2019;3(1):26‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joly BS, Coppo P, Veyradier A. Pediatric thrombotic thrombocytopenic purpura. Eur J Haematol. 2018;101(4):425‐434. [DOI] [PubMed] [Google Scholar]
- 9. Hanlon A, Metjian A. Caplacizumab in adult patients with acquired thrombotic thrombocytopenic purpura. Ther Adv Hematol. 2020;11:204062072090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coppo P, Veyradier A. Current management and therapeutical perspectives in thrombotic thrombocytopenic purpura. Presse Médicale. 2012;41(3):e163‐e176. [DOI] [PubMed] [Google Scholar]
- 11. Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836‐2846. [DOI] [PubMed] [Google Scholar]
- 12. Sargentini‐Maier B. Caplacizumab dosing rational in aTTP patients supported by mechanism based PKPD modelling. PAGE 28 (2019) Abstr 8800 [www.page‐meeting.org/?abstract=8800]. https://www.page‐meeting.org/?abstract=8800. Accessed January 19, 2021.
- 13. Sanofi: Cablivi(TM) (caplacizumab) approved in Europe for adults with acquired thrombotic thrombocytopenic purpura (aTTP) ‐ Sanofi. https://www.sanofi.com/en/media‐room/press‐releases/2018/2018‐09‐03‐07‐00‐00. Accessed January 12, 2021.
- 14. Sanofi: FDA approves Cablivi® (caplacizumab‐yhdp), the first Nanobody®‐based medicine, for adults with acquired thrombotic thrombocytopenic purpura (aTTP) ‐ Sanofi. https://www.sanofi.com/en/media‐room/press‐releases/2019/2019‐02‐06‐17‐43‐21. Accessed January 12, 2021.
- 15. Ablynx . A Phase II, Single‐Blind, Randomized, Placebo‐Controlled Trial to Study the Efficacy and Safety of Anti‐von Willebrand Factor Nanobody® Administered as Adjunctive Treatment to Patients With Acquired Thrombotic Thrombocytopenic Purpura. https://clinicaltrials.gov/ct2/show/NCT01151423. Published 2019. Accessed January 14, 2021.
- 16. Ablynx . A Phase III Double‐Blind, Randomized, Parallel Group, Multicenter Placebo‐Controlled Trial to Study the Efficacy and Safety of Caplacizumab in Patients With Acquired Thrombotic Thrombocytopenic Purpura. https://clinicaltrials.gov/ct2/show/NCT02553317. Published 2019. Accessed January 14, 2021.
- 17. Ablynx . Prospective Follow‐up Study for Patients Who Completed Study ALX0681‐C301 (HERCULES) to Evaluate Long‐Term Safety and Efficacy of Caplacizumab (Post‐HERCULES). https://clinicaltrials.gov/ct2/show/NCT02878603 Published 2020. Accessed January 14, 2021.
- 18.EMA assessment report: Cablivi. https://www.ema.europa.eu/en/documents/assessment-report/cablivi-epar-public-assessment-report_en.pdf. Published June 28, 2018. Accessed January 12, 2021.
- 19.FDA NDA/BLA Multi‐disciplinary Review and Evaluation {BLA 761112} {Caplacizumab (Cablivi®)}. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761112Orig1s000MultiR.pdf. Published February 5, 2019. Accessed January 12, 2021.
- 20.EMA assessment report: Cablivi. https://www.ema.europa.eu/en/documents/variation-report/cablivi-h-c-4426-ii-0021-epar-assessment-report-variation_en.pdf. Published April 30, 2020. Accessed January 18, 2021.
- 21. European Medicines Agency decision P/0294/2018 of 12 September 2018 on the acceptance of a modification of an agreed paediatric investigation plan for caplacizumab (EMEA‐001157‐PIP01‐11‐M02). 8.
- 22. Bhoopalan SV, Hankins J, George J, Ryder A, Onder AM, Puri L. Use of caplacizumab in a child with refractory thrombotic thrombocytopenic purpura. Pediatr Blood Cancer. 2019;66(7):e27737. [DOI] [PubMed] [Google Scholar]
- 23. Dutt T, Shaw RJ, Stubbs MJ, et al. Real‐world evidence of caplacizumab use in the management of acute TTP [published online ahead of print November 4, 2020]. Blood. [Google Scholar]
- 24. Kaczmarek V, Holle J, Astudillo R, Kempf C, Bufler P, Müller D. Caplacizumab for relapsing thrombotic thrombocytopenic purpura. Pediatr Nephrol. 2019;34(9):1625‐1628. [DOI] [PubMed] [Google Scholar]
- 25. Nagel MB, Ryder A, Lobbins M, Bhatt N. Refractory acquired thrombotic thrombocytopenic purpura treated with caplacizumab in a pediatric patient with systemic lupus erythematosus [published online ahead of print 2021]. Pediatr Blood Cancer. 10.1002/pbc.28534 [DOI] [PubMed] [Google Scholar]
- 26. Boudali J, Hallak B, Haeck M, et al. Immune‐mediated thrombotic thrombocytopenic purpura in childhood treated by caplacizumab, about 3 cases [published online ahead of print February 22, 2021]. J Nephrol. 10.1007/s40620-021-00992-5 [DOI] [PubMed] [Google Scholar]
- 27. Sargentini L, Hansson E, Bergstrand M, Callewaert F, Sousa R. Caplacizumab model‐based dosing recommendations in pediatric patients with acquired thrombotic thrombocytopenic purpura ‐PB2249. HemaSphere. 2019;3(S1):1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jozef Bartunek, Emanuele Barbato, Josefin‐Beate Holz, et al. Abstract 2009: ALX‐0081 a novel anti‐thrombotic: results of a single‐dose phase 1 study in healthy volunteers and further development in patients with stable angina undergoing PCI. Circulation. 2008;118(suppl_18):S_656‐S_656. [Google Scholar]
- 29. Abd‐Elaziz K, Kamphuisen PW, Lyssens C, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of anti‐Vwf nanobody® ALX‐0681 after single and multiple subcutaneous administrations to healthy volunteers. Blood. 2009;114(22):1063‐1063.19443663 [Google Scholar]
- 30. Ablynx . A Phase I, Single Center, Open‐Label, Randomized, Single Dose Cross‐Over Study in Healthy Male Subjects to Investigate the Bioequivalence and Tolerability of Liquid and Reconstituted Lyophilized Subcutaneous Formulations of Caplacizumab. https://clinicaltrials.gov/ct2/show/NCT02189733. Published 2014. Accessed January 14, 2021.
- 31. Ablynx . A Phase 2 Randomized, Open Label Clinical Trial in High Risk Percutaneous Coronary Intervention (PCI) Patients Receiving Standard Antithrombotic Treatment Plus Either ALX‐0081 or GPIIb/IIIa Inhibitor (ReoPro®) Over a Period of 24 Hours. https://clinicaltrials.gov/ct2/show/NCT01020383. Published 2019. Accessed January 14, 2021.
- 32. Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ. NONMEM User's Guides. (1989‐2014) Ellicott City, MD. Icon Development Solutions; 2014. [Google Scholar]
- 33. NHANES–National health and nutrition examination survey homepage. https://www.cdc.gov/nchs/nhanes/index.htm. Published December 18, 2020. Accessed January 12, 2021.
- 34. Sosothikul D, Seksarn P, Lusher JM. Pediatric reference values for molecular markers in hemostasis. J Pediatr Hematol Oncol. 2007;29(1):19‐22. [DOI] [PubMed] [Google Scholar]
- 35. R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.r-project.org/. Accessed January 18, 2021. [Google Scholar]
- 36. Joly BS, Stepanian A, Leblanc T, et al. Child‐onset and adolescent‐onset acquired thrombotic thrombocytopenic purpura with severe ADAMTS13 deficiency: a cohort study of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016;3(11):e537‐e546. [DOI] [PubMed] [Google Scholar]
- 37. Gill JC, Conley SF, Johnson VP, et al. Low VWF levels in children and lack of association with bleeding in children undergoing tonsillectomy. Blood Adv. 2020;4(1):100‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.EMA summary of product characteristics: Cablivi. https://www.ema.europa.eu/en/documents/product-information/cablivi-epar-product-information_en.pdf. Published October 14, 2020. Accessed January 15, 2021.
- 39. European Medicines Agency . EMA drug approval: Cablivi‐web page. https://www.ema.europa.eu/en/medicines/human/EPAR/cablivi. Published September 17, 2018. Accessed January 12, 2021.
- 40.FDA Drug Approval Package: CABLIVI‐web page. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761112Orig1s000TOC.cfm. Accessed January 12, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to model control files used for the presented simulations, individual level simulated data and related documents. Potential patient‐level data will be anonymized, and documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.


