Abstract
Objective:
Therapeutic management of ductal carcinoma in situ (DCIS) is heterogeneous among countries worldwide, and some treatment indications are still controversial. To investigate DCIS management in different countries; identify both consensual practices and controversial topics; and survey opinions about the future management of DCIS.
Materials and Methods:
The Senologic International Society network members participated to an online survey using a questionnaire, between November 2021 and February 2022.
Results:
Twenty-two responses from 20 different countries showed that organized breast cancer screening programs were present for 87% participants, and DCIS cases represented 13.7% of all breast cancers. Most participants used the grade classification (100%), the morphological classification (78%) and performed immunohistochemistry assays (73%). In case of conservative treatment, the mean re-excision rate was 10.3% and clear margins of mean 2.5 mm were considered healthy. Radical mastectomy rate was 35.5% with a breast reconstruction rate of 53%. Tumor bed boost indications were heterogeneous, and 73% of participants indicated hormone therapy for hormone-positive DCIS. Surgery and radiotherapy omission for some low-risk DCIS were considered by 73% of participants. Multigene assays were used by 43% of participants. Concerning future changes in DCIS management, participants mostly answered surgical de-escalation (48%), radiotherapy de-escalation (35) and/or active surveillance for some cases (22%).
Conclusion:
This survey provided an overview of the current practices of DCIS management worldwide. It showed that some areas are rather consensual: incidence increases over time, treatment in young women, pathological classifications, definition of healthy margins, the skin-sparing mastectomy and immediate breast reconstruction. However, some topics are still debated and result in heterogeneous practices, such as evolution in the age of diagnosis, the benefit of de-escalation in low-risk DCIS among elderly women, indications for hormone therapy, radiotherapy omission, or multigene assays. Further evidence is needed to reach consensus on these points, and innovative approaches are still under evaluation in clinical trials. The International Senologic Society, by its members, encourages precision medicine and personalized treatments for DCIS, to avoid overtreatment and overdiagnosis, and provide better healthcare to women with DCIS.
Keywords: Ductal carcinoma in situ, clinical practices, survey, precision medicine, treatment de-escalation, innovative approaches
Key Points
• Ductal carcinoma in situ (DCIS) is defined as a proliferation of malignant cells in the breast ducts without crossing of the basal membrane.
• Differences in DCIS characteristics, diagnosis and management exist between countries worldwide.
• The Senologic International Society (SIS) is dedicated to promoting breast health and improving the care of breast cancer patients, taking into consideration, medical, social, economic and ethical constraints. The objective of this survey was to investigate the management of DCIS though members of the SIS.
• As active members of the SIS and breast specialists, participants were invited if they wished to participate to be co-authors to the pending publication.
Introduction
Ductal carcinoma in situ (DCIS) of the breast is defined as a proliferation of malignant cells in the lumen of mammary ducts without visible rupture of the basement membrane on optic microscopy. This term encompasses a highly heterogeneous group of lesions that differ in their clinical presentation, histologic and biologic characteristics, and outcomes (1). DCIS is considered as an early form of breast cancer [Tis(DCIS) according to the 2018 Tumor-node-metastasis classification and stage 0 (TisN0M0) according to the Union for International Cancer Control (UICC) classification] (2). Breast cancer screening, whether individual or organized, has increased the diagnosis of DCIS as this pathology is mostly asymptomatic (it can nonetheless be the cause of a nipple discharge or a palpable mass). Thus, the frequency of DCIS has increased over the last 30 years (3, 4, 5, 6).
The therapeutic management of a DCIS is aimed at preventing the development of an invasive breast cancer (IBC). Different treatments are available for DCIS: surgery; radiotherapy; and hormonal therapies. Several factors are involved in the choice of appropriate treatment plan: the age of the patient; her comorbidities and risk factors; the size of the DCIS and its prognostic factors; the clinical presentation (nipple discharge, mass); and the patient choice. Treatment indications are different among countries worldwide, and they evolved over time. This shows that some are still controversial.
Without treatment, it is estimated that about 8 to 17.6% of DCIS will progress to invasive cancer at 10 years, and this proportion has been reported to be up to 20-30% in some studies (7, 8). It therefore brings up the issue of overtreatment because more than 70% of patients diagnosed with DCIS will not develop an IBC. Current areas of concern include the need for better patient selection to identify those who will develop IBC and those who will not. Indeed, possibilities of therapeutic optimization for some cases of DCIS may be abstention from radiotherapy, or even abstention from all treatment and “active” surveillance.
The Senologic International Society (SIS), founded in 1976, affiliated to the UICC since 2019, is a unique worldwide federation of scientific societies, breast cancer patients associations and groups, located across five continents, with a priority mission: to improve breast health by constantly putting the patient in the center of its concerns. It is a society turned towards the future with a particular focus on innovation, transdiscipline inclusivity and contribution to optimization of breast cancer care (www.sisbreast.org).
In view of the current concerns, the objective of this survey was to investigate, through members of the SIS, a wide range of questions about DCIS management and national guidelines. Each participant was asked to collate the DCIS data and recommendations of their own country to answer the questionnaire, leading to the identification of both consensual practices and controversial topics, which would require further investigation and, finally, opinions about the future management of DCIS.
Materials and Methods
Members of the SIS network were invited to participate in an online survey with a Microsoft Forms questionnaire. Between the 17th of November 2021 and the 15th of February 2022, participants were invited to answer the questionnaire via email. The answers were directly recorded into an online database and only one response per participant was allowed, but more than one response was authorized from the same countries, because of regional disparities in any single country.
The online survey consisted of 27 questions. Section 1 (6 questions) was about the respondents’ information, such as affiliation and medical specialty, and the number of cases of DCIS managed per year. Then, in Section 2 (2 questions) the respondents were asked about discovery mode, such as presence of a breast cancer screening program and its modalities. Section 3 was about epidemiology (4 questions) and asked about the incidence of DCIS and its evolution. After that, respondents were asked about pathology in Section 4 (3 questions) concerning the use of different classifications (grade, morphology and immunohistochemistry assays). In Section 5 (7 questions) respondents’ actual practices concerning treatment (surgery, radiotherapy, and hormonal therapy) were investigated. Finally, in Section 6 (5 questions) respondents were asked about future perspectives concerning topics such as treatment de-escalation and molecular/genetic signatures. The questionnaire is available as Supplementary Material S1 (Appendix 1).
Results
Twenty-two completed questionnaires were returned. Participants came from 20 different countries on five continents (Figure 1), with 2.7 billion inhabitants. Participants were mostly surgeons (77%, n = 17), radiologists (14%, n = 3), or radiation (5%, n = 1) or medical (5%, n = 1) oncologists. Results of the survey are shown in Table 1.
Figure 1.
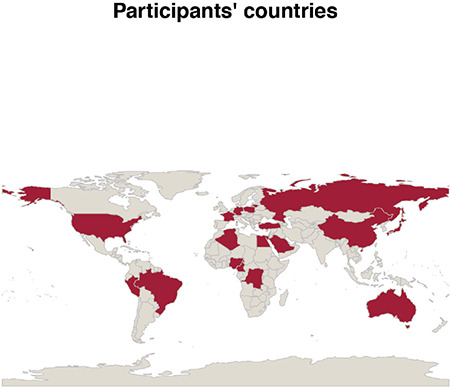
Participants’ countries
Table 1. Survey results.
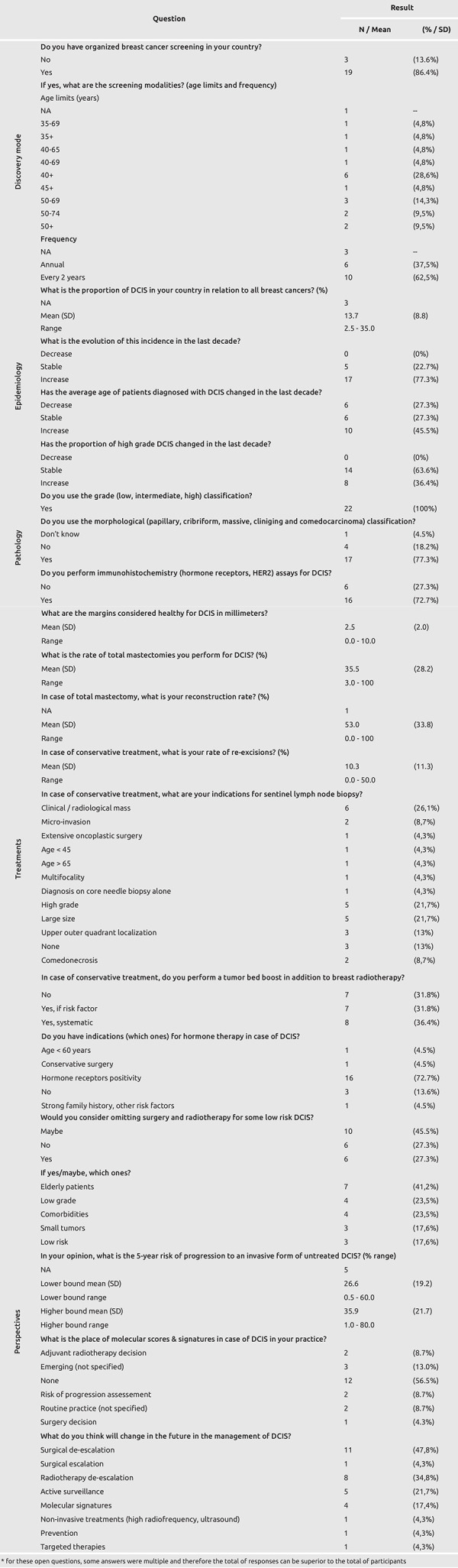
Most participants’ countries had organized breast cancer screening (87%, n = 19). All of these screening programs included women between 50 and 65 years-old and most reported a recall interval of every two years (63%, n = 10).
Incidence showed that DCIS cases represented 13.7% of all breast cancers [standard deviation (SD) = 8.8%, range 2.5 – 35%]. Most participants noted that DCIS incidence had increased in the last decade (77%, n = 17), while the age of diagnosis was stable (27%, n = 6), increased (46%, n = 10) or decreased (27%, n = 6). Moreover, 64% of participants reported that the proportion of high-grade DCIS was stable over time in the last decade (n = 14) or had increased (36%, n = 8) but no respondent reported a decrease.
Concerning histopathology, all participants used the grade classification (n = 22), the majority used the morphological classification (78%, n = 17) and performed immunohistochemistry assays (73%, n = 16).
Answers in the DCIS treatment section showed that healthy margins ranged from 0 to 10 mm, with a mean of 2.5 mm (SD = 2 mm). Mean radical mastectomy rates were 35.5% (SD = 28.2%, range 3 – 100%). In case of radical mastectomy, mean breast reconstruction rate was 53% (SD = 33.8%, range 0 – 100%). Conversely, in case of conservative treatment the mean re-excision rate was 10.3% (SD = 11.3%, range 0 – 50%). Then, in case of conservative treatment, participants were asked about indications for sentinel lymph node biopsy. The most frequently reported indications were: clinical/radiological mass (26%, n = 6); high grade (22%, n = 5); and larger size (22%, n = 5). Seven (32%) participants reported that tumor bed boost in case of conservative treatment was not performed, while 7 (32%) indicated that tumor bed boost would be performed if risk factors were present and 8 (36%) performed it systematically. Finally, most participants indicated hormone therapy if hormone receptors were expressed by the tumor (73%, n = 16) and 3 (14%) reported no indication for this treatment.
Concerning future perspectives of DCIS management, 16 (73%) participants considered (yes or maybe) surgery and radiotherapy omission for some low-risk DCIS. The most frequent situations reported were elderly patients (41%, n = 7), low grade (24%, n = 4) and presence of comorbidities (24%, n = 4). When asked their estimate of 5-year risk of progression to an invasive form in untreated DCIS, participants mean response was 26.6% – 35.9% (mean upper and lower bounds, respectively). Concerning molecular scores and signatures, 12 participants (57%) did not use them in routine practice. When asked about future changes in DCIS management, participants mostly answered surgical de-escalation (48%, n = 11), radiotherapy de-escalation (35%, n = 8) and/or active surveillance for some cases (22%, n = 5).
Discussion and Conclusion
With its international survey, the SIS wanted to investigate the current trends and therapeutic management of DCIS worldwide. As reported in Table 2, this survey highlighted that while some points are rather consensual, other are still controversial.
Table 2. DCIS management consensual and debated topics.
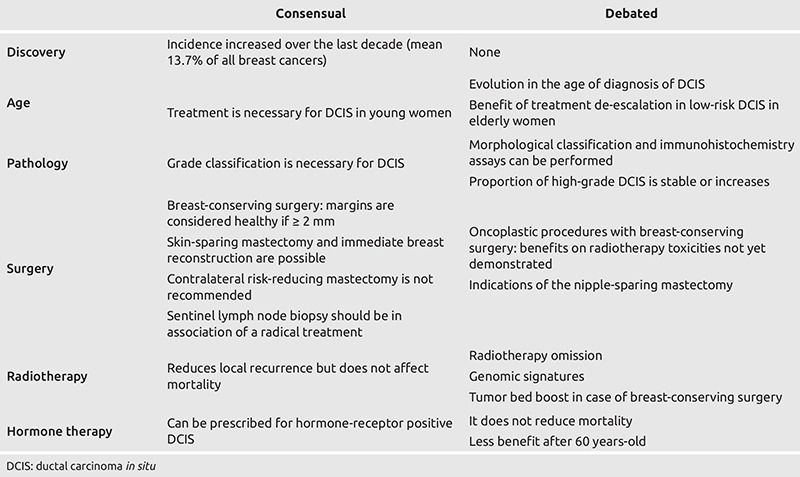
Diagnosis
While some cases of DCIS are diagnosed because of a nipple discharge (typically with blood or serous liquid) or a palpable mass, most are discovered by screening which may be either individual or as part of an organized program. Most of the respondents’ countries have an organized screening program, thus explaining the rate of 13.6% of DCIS among all breast cancers. Interestingly, participants’ responses about evolution of the age at diagnosis were heterogeneous. This could be explained by differences in organized screening programs. Participants also reported that the proportion of high-grade DCIS had a tendency to either be stable or to increase.
Concerning pathology, the 2019 World Health Organization’s (WHO) classification of breast tumors recommends the grade classification (9), which is based on cytonuclear morphology. Morphological classification was described in previous versions but not in the latest one. It has different subtypes: Comedo (comedocarcinoma) and non-comedo, further divided into cribriform, micropapillary, papillary and solid (10). Still, the latest classification considers solid papillary carcinoma in situ as a separate entity (9). Finally, WHO states that there is no universal agreement on the benefits of hormone receptor testing in DCIS (9) as hormone therapy is still controversial.
Surgery
Breast-Conserving Surgery
Breast-conserving surgery (BCS) is now widely performed for DCIS, and its rate increased along with the progressive discovery of small infraclinical DCIS through implementation of mammographic screening. Scientific data has demonstrated that BCS is a safe technique compared to modified radical mastectomy (MRM) (11). However, BCS raised two additional issues regarding local recurrence risk: Negative margins and adjuvant radiotherapy. Radiotherapy is discussed in the dedicated section below. Today, a margin of more than 2 mm has been found to minimize the local recurrence risk for BCS with radiotherapy (12, 13). Moreover, wider negative margins do not improve local control for DCIS (12, 14) and are not recommended by the American Society of Clinical Oncology (15).
Subsequently, new surgical techniques have been developed in conjunction with the widespread adoption of BCS and can be applied to patients with DCIS. Oncoplastic procedures include different techniques ranging from ipsilateral glandular rearrangement to contralateral reduction mammoplasties and symmetry procedures. However, data on radiotherapy toxicity in patients (with invasive or in situ breast cancer) undergoing oncoplastic procedures is still limited and studies are contradictory (16, 17). Thus, although these techniques may reduce radiotherapy complications associated with larger-breasted patients, they need additional procedures with their specific complications and in some case may even increase radiotherapy toxicity (18). Therefore, oncoplastic techniques can be applied in DCIS according to specific indications to obtain a better aesthetic result. However, oncoplastic techniques should not be applied with the main aim of reducing radiotherapy toxicity as insufficient data is available.
Mastectomy
Ipsilateral MRM was once the standard treatment for DCIS. Nowadays, this technique has been replaced by BCS and, in case of diffuse DCIS, replaced by conservative mastectomies, such as the nipple-sparing mastectomy (NSM) or the skin-sparing mastectomy (SSM). MRM remains appropriate for patients who refuse or are contraindicated for reconstructive surgery.
Concerning SSM, several studies evaluated the oncologic safety of this technique. One study conducted on 199 patients (102 with SSM and 97 with MRM) found a higher 5-year recurrence rate in the SSM group (5.9% versus 0%, p = 0.012) (19). In contrast, a study on 399 patients (192 with SSM and 207 with MRM found no difference in 10-year recurrence rates (1.04% versus 0.97%, p>0.05) (19). Similarly, two cohort studies were conducted in DCIS patients with SSM only. The first included 223 and found a recurrence rate of 5.1% with a mean follow up of 82 months (20). The second included 44 DCIS patients with SSM with a follow up of at least 6 years, and found no recurrences (21).
For NSM, there was another issue to assess - the nipple recurrence rate. In a retrospective cohort of 199 NSM (22) with a median follow up of 97 months, the authors found a local recurrence rate of 4.5%, and a nipple recurrence rate of 3%. Multivariate analysis showed that negative progesterone receptor status was an independent risk factor for local recurrence rate. Surprisingly, margin status and tumor-to-nipple distance were not associated with increased risk for either local or nipple recurrence. In another retrospective cohort of 69 DCIS patients with NSM (23) and a mean follow-up 143 months, local recurrence rate was 11.6%, of which 1.4% was nipple recurrence. The disease-free survival rate was 88.4% and the overall survival rate was 98.6%. Finally, in a retrospective cohort of 41 NSM (24) the authors reported the long-term follow-up data for the remaining 19 patients (46%). In this group, they observed one local recurrence (5.3%).
Conservative mastectomies seem therefore to be oncologically safe, except for the cases in which there may already be DCIS involvement of the nipple (i.e., in the cases of nipple discharge and/or a short tumor-to-nipple distance). Immediate breast reconstruction is therefore feasible for some DCIS.
For women with DCIS, there is also a trend toward increased contralateral risk-reducing mastectomy (RRM) (25). However, benefits of contralateral RRM are not yet demonstrated and may be non-existent. Indeed, from a prospective database of 2,759 patients who had unilateral conservative surgery for DCIS between 1978 and 2011, Miller et al. (26) found a contralateral cancer rate 2.5 times lower than the ipsilateral recurrence rate, estimated at 5.8% at 10 years (compared with 14.5% ipsilateral recurrence). Therefore, the benefit of contralateral RRM would be less compared to unilateral mastectomy for DCIS, which is decreasing in the current context of surgical de-escalation for DCIS. In another retrospective study of 24,766 bilateral RRM for unilateral DCIS, the authors showed that the financial cost of this procedure is significant and has been steadily increasing since 2005 (27). For these reasons, and especially because of insufficient benefit from reduced mortality, performing contralateral RRM cannot be recommended for DCIS.
Radiotherapy
Historically, whole breast irradiation was performed in case of BCS for DCIS based on the data from studies of IBC (18). Since then, several randomized controlled trials have been published on the benefit of radiotherapy after BCS for DCIS. Two meta-analyses published in 2007 (28) and 2010 (29) showed that radiotherapy reduced 10-year local recurrence rates by 15% (even for low-risk patients) and the odds ratio (OR) of local recurrence was 0.4 [95% confidence interval (95% CI) =0.31–0.53, p<0.001]. However, these meta-analyses failed to demonstrate a significant benefit in distant recurrence rates and in survival. Conversely, one of them showed a significant increase in contralateral breast events [3.85% versus 2.5%, OR = 1.53 [95% CI 1.05–2.24], p = 0.03]. A more recent meta-analysis, published in 2018 (30), showed a decreased risk of local [relative risk (RR) = 0.53 (95% CI = 0.45–0.62)] and regional [RR = 0.54 (95% CI = 0.32–0.91)] recurrence, but still no benefit in distant recurrence nor mortality.
Moreover, practices are heterogeneous regarding additional tumor bed boost during radiotherapy. Participants reported different practices as some of them always did additional tumor bed boost while other never did, and some only in the presence of risk factors. A review showed that tumor bed boost was more often performed when risk factors, such as young age (<40 or 50 years), presence of clinical symptoms, tumor size >20 mm, high nuclear grade, presence of necrosis, insufficient surgical margins, associated atypical lesions, and lobular carcinoma in situ, were present (31).
Therefore, indications for post-operative radiotherapy have been questioned. It has been suggested that radiotherapy could be omitted for low-risk patients, such as those with low grade, small tumor size and elderly patients. Moreover, multigene assays have been developed for this purpose and are discussed below. Today, trials are currently underway to evaluate the omission of radiotherapy for some patients. For instance, the ROMANCE trial (Radiotherapy Omission in Low Risk Ductal in Situ Carcinoma Breast) is currently underway and includes patients aged 55 and older to better define the benefits/disadvantages and indications for radiotherapy. This tendency suggests that future management of DCIS would omit radiotherapy for low-risk patients. However, it is still necessary to identify those patients, whether by clinical characteristics (age), pathological features of DCIS (low grade), or multigene assays.
Hormone Therapy
The place of hormone therapy in the treatment of DCIS is still debated. Two major trials have evaluated the impact of tamoxifen after conservative surgery and radiation therapy for the adjuvant treatment of DCIS. The long-term analysis of the NSABP B-24 randomized controlled trial, which compared tamoxifen (n = 899) versus placebo (n = 900) in patients with DCIS treated by conservative surgery and radiotherapy, found a reduced risk of ipsilateral invasive recurrence (6.6% versus 9.0% at 5 years) with tamoxifen (32). Participants were both pre-menopausal (35.9%) and post-menopausal (64.1%). Moreover, addition of tamoxifen did not result in a statistically significant reduction in mortality risk [hazard ratio (HR)=0.86, 95% CI 0.66–1.11].
The UK/ANZ DCIS randomized controlled trial, which compared three groups: radiotherapy versus tamoxifen versus radiotherapy + tamoxifen in 1701 patients who had undergone surgery for DCIS, found a reduction in the risk of in situ recurrence (HR=0.70, 95% CI 0.51-0.86), with no change in invasive recurrence (33). Similarly, mortality was not affected by tamoxifen.
The randomized controlled trial NSABP B-35, which included 3,104 postmenopausal patients with hormone receptor-positive DCIS treated with conservative surgery and radiotherapy, compared anastrozole versus tamoxifen. The results showed a significant reduction in contralateral breast cancers with anastrozole (HR=0.64; 95% CI, 0.43–0.96; p = 0.032) compared to tamoxifen but there was no benefit of one therapy compared to the other in patients over the age of 60 (34).
Due to the uncertain benefit and the presence of toxicities (e.g., venous thromboembolic events or higher risk of endometrial cancer described with tamoxifen (34), hormone therapy has no place in the management of DCIS in many European countries. Conversely, in the United States, hormone therapy is more widely prescribed. Indeed, the National Comprehensive Cancer Network describes the option of prescribing hormone therapy for 5 years in women with hormone-receptor-expressing DCIS treated with surgery alone or conservative surgery and radiation therapy, without mentioning age limits (35). However, evidence reported above suggests that hormone therapy may have less benefit among older women. Therefore, this treatment could be considered for younger women with some DCIS subtypes, although it remains controversial. Efforts should be made to assess which women will benefit the most (i.e., improve survival) with DCIS hormone therapy.
Multigene Assays
Similarly, with what has happened in invasive breast cancer, multi-gene assays have been developed for DCIS that provide prognostic and predictive scores. Two are currently used: Oncotype DX DCIS and DCISionRT. These multigene assays stratify different groups according to their risk of local recurrence.
A study evaluating Oncotype DX DCIS in women treated by BCS alone (n = 571) versus BCS and radiotherapy (n = 689) showed that low-risk patients treated by BCS alone had a small benefit from radiotherapy by reducing the 10-years local recurrence, while those with a high risk had a greater benefit (36).
Similarly, in another study assessing DCISionRT in the SweDCIS randomized clinical trial cohort (504 women with DCIS) the authors showed that in the high risk group, radiotherapy significantly decreased relative 10-year local recurrence (both for in situ and invasive tumors) while in the low risk group there were no significant risk differences observed with radiotherapy (37). In another study of 526 women with DCIS, the authors showed that among low-risk DCIS defined with classical clinical and pathological characteristics, this signature reclassified 42% of patients into the high-risk group and showed that these patients had significant benefit from radiotherapy (38).
These findings suggest that multigene assays are a promising tool to distinguish high and low-risk DCIS. However, to date there is no data about benefits in terms of mortality. Multigene assays are emerging in routine clinical practice among the survey participants, and future insights could improve radiotherapy or hormone therapy decisions to better select patients who will benefit from it.
In conclusion, this survey provided an overview of the current practices of DCIS management worldwide. While some points are rather consensual (such as healthy margins or pathological classifications), others are still widely debated and result in heterogeneous practices. DCIS treatments have significantly evolved over the last few decades, resulting in different surgical techniques or radiotherapy and hormone therapy indications. Further investigations are needed to reach consensus on these points. Moreover, while several clinical trials and observational studies are available, to our knowledge this is the first international survey published about DCIS management. This kind of initiative provides valuable insights about this topic as they could not be investigated otherwise. Finally, the SIS, through its members, encourages precision medicine and personalized treatments for DCIS, to avoid overtreatment and overdiagnosis, and provide better healthcare to women with DCIS.
Acknowledgments
We are thankful to Gérard Hrodej for the help for contacting the SIS network members
Footnotes
Ethics Committee Approval: This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments.
Informed Consent: Written consent was obtained from all individual participants included in the study. The samples as well as the associated medical data were made anonymous, allowing their automated processing within the framework of research.
Peer-review: Externally peer-reviewed.
Authorship Contributions
Concept: C.M., M.L., K.A., B.A.O., E.A., S.A., M.B., M.M.C., E.E., T.E., L.G., X.H., S.I., E.M., M.A.M., A.M., V.O., S.Ö., T.Ö., V.Ö., T.P., G.S., A.S., V.S., S.S.; Design: C.M., M.L., K.A., B.A.O., E.A., S.A., M.B., M.M.C., E.E., T.E., L.G., X.H., S.I., E.M., M.A.M., A.M., V.O., S.Ö., T.Ö., V.Ö., T.P., G.S., A.S., V.S., S.S.; Data Collection and/or Processing: C.M., M.L., K.A., B.A.O., E.A., S.A., M.B., M.M.C., E.E., T.E., L.G., X.H., S.I., E.M., M.A.M., A.M., V.O., S.Ö., T.Ö., V.Ö., T.P., G.S., A.S., V.S., S.S.; Analysis and/or Interpretation: C.M., M.L., K.A., B.A.O., E.A., S.A., M.B., M.M.C., E.E., T.E., L.G., X.H., S.I., E.M., M.A.M., A.M., V.O., S.Ö., T.Ö., V.Ö., T.P., G.S., A.S., V.S., S.S.; Literature Search: C.M., M.L., K.A., B.A.O., E.A., S.A., M.B., M.M.C., E.E., T.E., L.G., X.H., S.I., E.M., M.A.M., A.M., V.O., S.Ö., T.Ö., V.Ö., T.P., G.S., A.S., V.S., S.S.; Writing: C.M., M.L., K.A., B.A.O., E.A., S.A., M.B., M.M.C., E.E., T.E., L.G., X.H., S.I., E.M., M.A.M., A.M., V.O., S.Ö., T.Ö., V.Ö., T.P., G.S., A.S., V.S., S.S.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Punglia RS, Bifolck K, Golshan M, Lehman C, Collins L, Polyak K, et al. Epidemiology, biology, treatment, and prevention of ductal carcinoma in situ (DCIS) JNCI Cancer Spectr. 2018;2:pky063. doi: 10.1093/jncics/pky063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cserni G, Chmielik E, Cserni B, Tot T. The new TNM-based staging of breast cancer. Virchows Arch. 2018;472:697–703. doi: 10.1007/s00428-018-2301-9. [DOI] [PubMed] [Google Scholar]
- 3.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 4.Sørum R, Hofvind S, Skaane P, Haldorsen T. Trends in incidence of ductal carcinoma in situ: the effect of a population-based screening programme. Breast. 2010;19:499–505. doi: 10.1016/j.breast.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Puig-Vives M, Pollan M, Rue M, Osca-Gelis G, Saez M, Izquierdo A, et al. Rapid increase in incidence of breast ductal carcinoma in situ in Girona, Spain 1983-2007. Breast. 2012;21:646–651. doi: 10.1016/j.breast.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Molinié F, Vanier A, Woronoff AS, Guizard AV, Delafosse P, Velten M, et al. Trends in breast cancer incidence and mortality in France 1990-2008. Breast Cancer Res Treat. 2014;147:167–175. doi: 10.1007/s10549-014-3073-9. [DOI] [PubMed] [Google Scholar]
- 7.Ryser MD, Weaver DL, Zhao F, Worni M, Grimm LJ, Gulati R, et al. Cancer Outcomes in DCIS Patients Without Locoregional Treatment. J Natl Cancer Inst. 2019;111:952–960. doi: 10.1093/jnci/djy220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders ME, Schuyler PA, Simpson JF, Page DL, Dupont WD. Continued observation of the natural history of low-grade ductal carcinoma in situ reaffirms proclivity for local recurrence even after more than 30 years of follow-up. Mod Pathol. 2015;28:662–669. doi: 10.1038/modpathol.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer. World Health Organization Classification of Tumors: Breast tumors. 5th Ed. 2019. [Internet]
- 10.International Agency for Research on Cancer. World Health Organization Classification of Tumors: Breast tumors . 4th Ed. 2012. [Internet]
- 11.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 12.Pilewskie M, Morrow M. Margins in breast cancer: How much is enough? Cancer. 2018;124:1335–1341. doi: 10.1002/cncr.31221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tadros AB, Smith BD, Shen Y, Lin H, Krishnamurthy S, Lucci A, et al. Ductal carcinoma in situ and margins <2 mm: contemporary outcomes with breast conservation. Ann Surg. 2019;269:150–157. doi: 10.1097/SLA.0000000000002439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong JS, Kaelin CM, Troyan SL, Gadd MA, Gelman R, Lester SC, et al. Prospective study of wide excision alone for ductal carcinoma in situ of the breast. J Clin Oncol. 2006;24:1031–1036. doi: 10.1200/JCO.2005.02.9975. [DOI] [PubMed] [Google Scholar]
- 15.Morrow M, Van Zee KJ, Solin LJ, Houssami N, Chavez-MacGregor M, Harris JR, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Ductal Carcinoma In Situ. J Clin Oncol. 2016;34:4040–4046. doi: 10.1200/JCO.2016.68.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emiroglu M, Salimoglu S, Karaali C, Sert I, Gungor O, Sert F, et al. Oncoplastic reduction mammoplasty for breast cancer in women with macromastia: Oncological long-term outcomes. Asian J Surg. 2017;40:41–47. doi: 10.1016/j.asjsur.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Peled AW, Sbitany H, Foster RD, Esserman LJ. Oncoplastic mammoplasty as a strategy for reducing reconstructive complications associated with postmastectomy radiation therapy. Breast J. 2014;20:302–307. doi: 10.1111/tbj.12257. [DOI] [PubMed] [Google Scholar]
- 18.Shah C, Wobb J, Manyam B, Kundu N, Arthur D, Wazer D, et al. Management of Ductal Carcinoma In Situ of the Breast: A Review. JAMA Oncol. 2016;2:1083–1088. doi: 10.1001/jamaoncol.2016.0525. [DOI] [PubMed] [Google Scholar]
- 19.Timbrell S, Al-Himdani S, Shaw O, Tan K, Morris J, Bundred N. Comparison of Local Recurrence After Simple and Skin-Sparing Mastectomy Performed in Patients with Ductal Carcinoma In Situ. Ann Surg Oncol. 2017;24:1071–1076. doi: 10.1245/s10434-016-5673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlson GW, Page A, Johnson E, Nicholson K, Styblo TM, Wood WC. Local recurrence of ductal carcinoma in situ after skin-sparing mastectomy. J Am Coll Surg. 2007;204:1074-1078; discussion 1078–1080. doi: 10.1016/j.jamcollsurg.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 21.Spiegel AJ, Butler CE. Recurrence following treatment of ductal carcinoma in situ with skin-sparing mastectomy and immediate breast reconstruction. Plast Reconstr Surg. 2003;111:706–711. doi: 10.1097/01.PRS.0000041440.12442.05. [DOI] [PubMed] [Google Scholar]
- 22.Wu ZY, Kim HJ, Lee J, Chung IY, Kim JS, Lee SB, et al. Recurrence outcomes after nipple-sparing mastectomy and immediate breast reconstruction in patients with pure ductal carcinoma in situ. Ann Surg Oncol. 2020;27:1627–1635. doi: 10.1245/s10434-019-08184-z. [DOI] [PubMed] [Google Scholar]
- 23.Lago V, Maisto V, Gimenez-Climent J, Vila J, Vazquez C, Estevan R. Nipple-sparing mastectomy as treatment for patients with ductal carcinoma in situ: A 10-year follow-up study. Breast J. 2018;24:298–303. doi: 10.1111/tbj.12947. [DOI] [PubMed] [Google Scholar]
- 24.Leclère FM, Panet-Spallina J, Kolb F, Garbay JR, Mazouni C, Leduey A, et al. Nipple-sparing mastectomy and immediate reconstruction in ductal carcinoma in situ: a critical assessment with 41 patients. Aesthetic Plast Surg. 2014;38:338–343. doi: 10.1007/s00266-013-0236-8. [DOI] [PubMed] [Google Scholar]
- 25.Yao K, Stewart AK, Winchester DJ, Winchester DP. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998-2007. Ann Surg Oncol. 2010;17:2554–2562. doi: 10.1245/s10434-010-1091-3. [DOI] [PubMed] [Google Scholar]
- 26.Miller ME, Muhsen S, Olcese C, Patil S, Morrow M, Van Zee KJ. Contralateral breast cancer risk in women with ductal carcinoma in situ: is it high enough to justify bilateral mastectomy? Ann Surg Oncol. 2017;24:2889–2897. doi: 10.1245/s10434-017-5931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malapati SJ, Singh SRK, Kumar R, Mouabbi J, Abdalla A, Dul C, et al. Abstract P2-08-04: Bilateral mastectomy in ductal carcinoma in situ: 10-year analysis of national inpatient sample database. Cancer Research. 2020;80(4 Supplement):P2-08–04. [Google Scholar]
- 28.Viani GA, Stefano EJ, Afonso SL, De Fendi LI, Soares FV, Leon PG, et al. Breast-conserving surgery with or without radiotherapy in women with ductal carcinoma in situ: a meta-analysis of randomized trials. Radiat Oncol. 2007;2:28. doi: 10.1186/1748-717X-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Correa C, McGale P, Taylor C, Wang Y, Clarke M, et al; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garg PK, Jakhetiya A, Pandey R, Chishi N, Pandey D. Adjuvant radiotherapy versus observation following lumpectomy in ductal carcinoma in-situ: A meta-analysis of randomized controlled trials. Breast J. 2018;24:233–239. doi: 10.1111/tbj.12889. [DOI] [PubMed] [Google Scholar]
- 31.Kuntz L, Le Fèvre C, Hild C, Keller A, Gharbi M, Mathelin C, et al. Survie globale et sans récidive locale en cas de radiothérapie du lit tumoral des carcinomes canalaires in situ du sein : revue de la littérature [Overall survival and survival without local recurrence in case of radiotherapy of the tumor bed of ductal carcinomas in situ of the breast: Review of the literature] Gynecol Obstet Fertil Senol. 2021;49:255–265. doi: 10.1016/j.gofs.2020.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103:478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuzick J, Sestak I, Pinder SE, Ellis IO, Forsyth S, Bundred NJ, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margolese RG, Cecchini RS, Julian TB, Ganz PA, Costantino JP, Vallow LA, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet. 2016;387:849–856. doi: 10.1016/S0140-6736(15)01168-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Comprehensive Cancer Network. (NCCN) Clinical Practice Guidelines in Oncology, Breast Cancer, Version 5.2020. 2020. [Internet]
- 36.Rakovitch E, Nofech-Mozes S, Hanna W, Sutradhar R, Baehner FL, Miller DP, et al. Multigene Expression Assay and Benefit of Radiotherapy After Breast Conservation in Ductal Carcinoma in Situ. J Natl Cancer Inst. 2017;109:djw256. doi: 10.1093/jnci/djw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wärnberg F, Karlsson P, Holmberg E, Sandelin K, Whitworth PW, Savala J, et al. Prognostic risk assessment and prediction of radiotherapy benefit for women with ductal carcinoma in situ (DCIS) of the breast, in a randomized clinical trial (SweDCIS) Cancers (Basel) 2021;13:6103. doi: 10.3390/cancers13236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bremer T, Whitworth PW, Patel R, Savala J, Barry T, Lyle S, et al. A biological signature for breast ductal carcinoma in situ to predict radiotherapy benefit and assess recurrence risk. Clin Cancer Res. 2018;24:5895–5901. doi: 10.1158/1078-0432.CCR-18-0842. [DOI] [PubMed] [Google Scholar]


