Abstract
Older compared to younger adults show greater amygdala activity to positive emotions, and are more likely to interpret emotionally ambiguous stimuli (e.g., surprised faces) as positive. While some evidence suggests this positivity effect results from a top-down, effortful mechanism, others suggest it may emerge as the default or initial response. The amygdala is a key node in rapid, bottom-up processing and patterns of amygdala activity over time (e.g., habituation) can shed light on the mechanisms underlying the positivity effect. Younger and older adults passively viewed neutral and surprised faces in an MRI. Only in older adults, amygdala habituation was associated with the tendency to interpret surprised faces as positive or negative (valence bias), where a more positive bias was associated with greater habituation. Interestingly, although a positive bias in younger adults was associated with slower responses, consistent with an initial negativity hypothesis in younger adults, older adults showed faster categorizations of positivity. Together, we propose that there may be a switch to a primacy of positivity in aging.
Keywords: Emotion, Aging, Positivity Effect, Valence Bias, Amygdala
1. Introduction
During normative adulthood, the transition into older age is accompanied by a decrease in the extent to which arousing, negative information impacts attention and memory (Mather, 2016). For instance, whereas in younger adults the processing of negative information is facilitated (Öhman et al., 2001) and interferes with attention toward competing neutral information (Müller et al., 2008), older adults show a reduction in this attention interference effect (Mather and Carstensen, 2003). Further, older compared to younger adults show less accurate memory recall of negative, but not positive, events (Charles et al., 2003). These age-related shifts away from negativity, customarily termed the “positivity effect” (Mather and Knight, 2005), are consistent with a general increase in reported emotional well-being among older adults (Charles, 2010).
Extensive neuroimaging work has examined the neural mechanisms underlying this positivity effect and has, for example, highlighted age-related differences in amygdala function. When viewing negative, but not positive, information, older compared to younger adults show less amygdala activation (e.g., Leclerc and Kensinger, 2011; Mather et al., 2004; but see Moriguchi et al. 2011). Related work in older adults has linked the positivity effect to increased activation in the medial prefrontal cortex (mPFC; Dolcos et al., 2014; Leclerc and Kensinger, 2011; Williams et al., 2006), suggesting that greater positivity may arise from a downregulation of amygdala activity via frontal cortical signals (Hariri et al., 2003). These findings support the notion that the positivity effect emerges via a relatively effortful process (i.e., top-down regulatory signals; Reed and Carstensen, 2012) which selectively control amygdala activity (Mather, 2016) and down-regulate potentially negative information (Carstensen, 2006; Mather and Knight, 2005).
Having said that, in the broader literature, the evidence supporting the notion that the positivity effect is a result of effortful regulation is mixed, highlighting the need for new approaches to explore this question. For instance, recent behavioral studies have shown a positivity bias in attention in older adults may be the result of a relatively effortless process (Allard et al., 2010; Gronchi et al., 2018). However, this positivity bias in older adults was eliminated with the addition of a concurrent working memory task, suggesting the rapid bias toward positivity in older adults may indeed require effortful processes related to cognitive control (Kennedy et al., 2019). Further, although some studies have demonstrated that the P1 component measured in electroencephalography (EEG), which reflects early (70–130 ms) visual attention processes (Hillyard et al., 1998), is amplified for positive relative to negative images in older, but not younger, adults (Hilimire et al., 2014; Houston et al., 2018), others found that the P1 shows no such age-by-emotion interaction (Meng et al., 2015). Moreover, the late positive potential (LPP), a relatively late component (400–1000 ms) which is reliably amplified by emotionally arousing content (Hajcak et al., 2011), is enhanced for positive relative to negative images for older, but not younger, adults during an active task (Langeslag and van Strien, 2009). However, this effect was not evident during free viewing of images (Renfroe et al., 2016) or when the images were task irrelevant (Pehlivanoglu and Verhaeghen, 2019), which suggests that the influence of task instruction on the LPP may differ in older versus younger adults. Taken together, this mixed evidence showing a positivity bias during early time-windows challenges, but does not rule out, the prediction that the positivity effect depends on effortful cognitive mechanisms.
The majority of work on the positivity effect relies on stimuli conveying clear positive or negative valence, and measures age-related differences in a) attention shifts toward or away from competing positive and negative information, or b) brain responses evoked by positive and negative information. More recently, work with dual-valence ambiguity (i.e., stimuli that could be validly interpreted as either positive or negative) have extended these findings by demonstrating the older adults have more positive interpretations of these stimuli than younger adults (Bucks et al., 2008; Neta and Tong, 2016; Shuster et al., 2017). Indeed, dual-valence ambiguity enables a measure of bias toward or away from positivity/negativity within a single item. For instance, whereas happy and angry expressions convey relatively clear positive and negative information, respectively, surprised expressions are ambiguous in that they signal both positive (e.g., an unexpected gift) and negative outcomes (e.g., witnessing a car crash). The increased positive categorizations of surprised faces in older compared to younger adults suggests that, when the information within a single stimulus may convey multiple valid interpretations, older adults are more likely to ascribe positivity (i.e., positive valence bias, or the tendency to interpret emotional ambiguity as having a positive meaning).
The brain mechanisms underlying a positive valence bias have been explored, albeit primarily in younger populations (i.e., children and younger adults, but see Sakaki et al. (2013) for related work). For instance, individuals with a more negative valence bias show increased amygdala and decreased vmPFC activity evoked by valence-ambiguous expressions of surprise (Kim, Somerville, Johnstone, et al., 2003; Petro et al., 2021). More recent evidence has shown that the same lateral frontal regions that are recruited during explicit emotion regulation are also recruited in response to surprised faces, but more so in individuals with a positive valence bias (Petro et al., 2018). These findings are consistent with other work that suggests that the initial response to dual-valence ambiguity (in young adults) is more negative, and that positivity may rely on a regulatory process that overrides the initial negativity (Kim, Somerville, Johnstone, et al., 2003; Neta et al., 2020; Neta and Tong, 2016). These results might suggest that the positivity effect in older adults is the product of top-down, effortful processing that overrides the initial negativity in order to produce a more positive bias. However, the evidence that the positivity effect may emerge in early perceptual stages (Gronchi et al., 2018; Hilimire et al., 2014; Houston et al., 2018) raises the possibility of a shift in older adulthood such that the initial response to dual-valence ambiguity is positive rather than negative.
The goal of the present work is to explore the behavioral and neural mechanisms of the positive valence bias in older adults (compared to younger adults). Although some behavioral work in young adults has lent support for an initial negativity (e.g., slower responses for positive than negative trials, an initial attraction to the competing – negative – response when categorizing as positive; Neta et al., 2009; Neta et al., 2020), these approaches may be less compelling in aging. For example, behavioral responses such as response time and other motor responses show a general slowing in older age (Proctor et al., 2005). As such, we complemented our behavioral findings with neuroimaging measures that could provide a more complete description of the mechanism supporting a positive valence bias in aging.
Specifically, the amygdala is considered a key node in the rapid, bottom-up processing of stimuli conveying biological relevance (LaBar and LeDoux, 1996). With respect to facial expressions, the amygdala shows a robust response to negative (Johnstone et al., 2005), positive (Costafreda et al., 2008), and even ambiguous (Kim, Somerville, Johnstone, et al., 2003; Neta et al., 2013) expressions. Of note, although there is a robust response initially, the amygdala response habituates across repeated exposures (Geissberger et al., 2020; Plichta et al., 2014), particularly when no further learning is required (Bordi et al., 1993; Breiter et al., 1996). Alternatively, amygdala activity shows weaker or no habituation in response to uncertainty (e.g., when stimuli are presented in unpredictable patterns or when individuals show a high intolerance for uncertainty; Herry et al., 2007; Tanovic et al., 2018), suggestive of a sustained enhancement of vigilance (Herry et al., 2007). Interestingly, in the case of dual-valence ambiguity, young adults show greater habituation to fear faces (relatively clear negativity) than to surprised faces (Whalen et al., 2009). The authors suggest that this lack of amygdala habituation for surprise may resemble slower extinction patterns because the ambiguity conveys uncertain outcomes which require sustained vigilance to promote further learning. On the other hand, expressions conveying clear valence (e.g., fear) convey a relatively predictable outcome and thus do not promote sustained vigilance (i.e., the amygdala response habituates).
In the current study, we examined behavioral and neural (e.g., amygdala habituation) responses to surprised faces in older compared to younger adults. We predicted that, consistent with previous studies (Neta and Tong, 2016; Shuster et al., 2017), older adults would show a more positive valence bias than younger adults. Further, if the positive bias in older (but not younger) adults is not a result of effortful regulation but rather a perception of these expressions as more clearly positive, then these individuals would show greater amygdala habituation (i.e., less in need of further learning). In other words, if a positive response arises early on to signal safety and the negative response alternative – or potential threat – is not considered (as previous work suggests it is in younger adults; see Neta et al., 2020), then no further learning would be required. In this case, we expected also that positivity will not be a result of frontal cortical activity in older adults. In contrast, if the positive bias is preceded by an initial negativity, then the amygdala would putatively show sustained activity in response to an uncertain but potential threat, as evidenced in younger adults. Further, in this case, a more positive valence bias in older adults would be associated with greater activity in the frontal cortex than a more negative bias, also as evidenced in young adults. In sum, we predicted that, in contrast to what is seen in younger adults, older adults with a positive bias would show greater amygdala habituation (and no relationship between valence bias and frontal cortical activity) in response to surprised faces, suggesting a primacy for positivity in response to dual-valence ambiguity.
2. Methods
2.1. Participants
Data were collected from 57 young (28 female, ages 17–30 years, mean(SD) age = 20.75(2.93)) and 52 older adults (36 female, ages 60–88 years, mean(SD) age = 69.92(6.83)) who reported having no history of neurological or psychiatric disorders, nor taking any psychotropic medication. The data from the sample of younger adults have been analyzed previously in Petro, Tong, Henley, and Neta (2018), but this previous analysis did not investigate effects related to amygdala habituation. During recruitment, older adults were administered the Modified Telephone Interview for Cognitive Status (Welsh et al., 1993); those with a score of 9/20 or higher on the recall portion of the interview and a total score of 24/39 or higher were invited to participate in the study. All recruitment and experiment protocols were approved by the University of Nebraska Committee for the Protection of Human Subjects in accordance with the Declaration of Helsinki. Each participant was given monetary compensation.
Among the recruited participants, 3 younger adults and 1 older adult failed to accurately categorize clearly valenced facial expressions on at least 60% of the trials and so were excluded from further analysis, consistent with prior work (Neta et al., 2009; Neta and Tong, 2016). Indeed, this accuracy threshold is a particularly important exclusionary criteria given the difficulty in discerning the specific interpretations of dual-valence ambiguity (i.e., valence bias) if stimuli with clear valence are not accurately categorized. As such, 54 young (26 female, ages 17–30 years, mean(SD) age = 20.83(2.98)) and 51 older adults (36 female, ages 60–88 years, mean(SD) age = 69.94(6.90)) were included in the analysis of behavioral data.
In addition, 3 younger adults and 7 older adults did not complete the neuroimaging portion of the task (session 2, see below). The imaging data from 1 additional older adult were excluded due to technical failure during the session 2 task. Thus, 51 younger (25 female, ages 17–30 years, mean(SD) age = 20.73(2.93)) and 43 older adults (31 female, ages 60–88 years, mean(SD) age = 70.21(6.81)) were included in the analysis of MRI data.
2.2. Procedures
2.1.1. Session 1: Valence Bias Assessment.
See Figure 1 for an illustration of the experimental tasks. Session 1 comprised a behavioral testing session. All stimuli were presented on E-Prime software (Psychology Software Tools, Pittsburgh, PA, USA). To measure baseline valence bias, participants viewed images of happy, angry, and surprised facial expressions and categorized (via keyboard press) each image as either positive or negative. The experimental design was taken from previous work (Neta et al., 2009). Stimuli included 34 discrete identities, 14 of which (7 females, ages 21–30 years) were drawn from the NimStim Set of Facial Expressions (Tottenham et al., 2009), and 20 (10 females, ages 20–30 years) from the Karolinska Directed Emotional Faces database (Goeleven et al., 2008). Each image was presented for 500 ms and separated by an interstimulus interval of 1500 ms. The images were presented across 2 blocks, each of which consisted of 24 images (6 angry, 6 happy, 12 surprise, per block) presented in a pseudorandom order in which no expression was presented in more than 2 subsequent trials, and blocks were counterbalanced between participants. Participants were given a short break between blocks, and resumed the experiment via key-press at their convenience. Note that we intermixed expression conditions here to encourage participants to categorize each face, rather than provide repeat categorization decisions across a series (i.e., a block) of subsequent identical expressions.
Figure 1: Depiction of procedure.
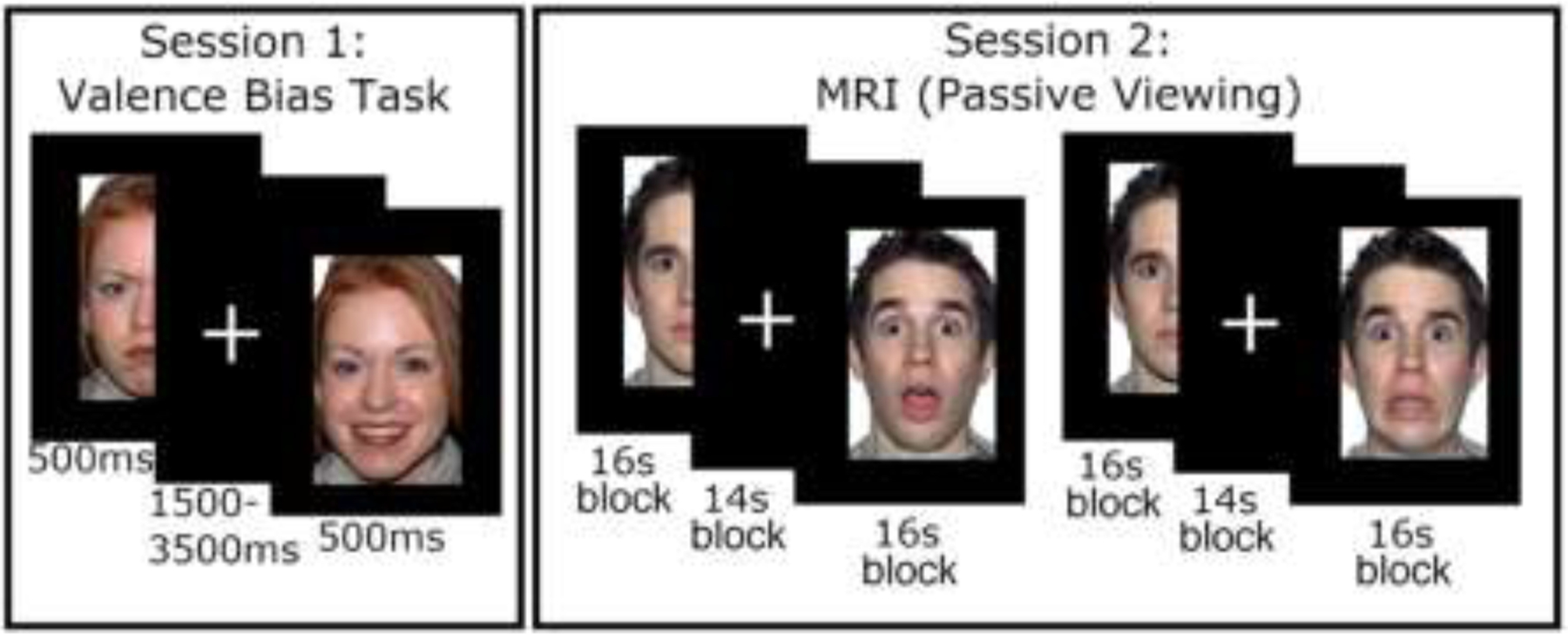
In the valence bias task (left panel), participants viewed happy, angry, and surprised faces, and categorized each image as either positive or negative. In the MRI session (right panel), participants passively viewed a new set of faces (i.e., not seen in session 1) during two runs with blocks of surprised and neutral faces followed by two runs with blocks of fearful and neutral faces.
2.2.2. Session 2: Magnetic resonance imaging (MRI).
Session 2 followed session 1 by approximately 7 days (Younger Adults: mean(SD) days = 7.84(2.09), range = 6–20 days; Older Adults: mean(SD) days = 7.09(0.87), range = 6–11 days). During the MRI scanning, participants freely viewed blocks of faces across four runs of blood-oxygen-level dependent (BOLD) imaging. The procedural changes implemented in session 2 (e.g., free viewing as opposed to a categorization task) were chosen because block designs have been shown to evoke a robust BOLD signal (Maus et al., 2012), and because tasks requiring explicit judgments may attenuate amygdala activity (Costafreda et al., 2008; Neta et al., 2013). Although we had to separate behavioral and brain data to different sessions, and use different paradigms for each session (i.e., trial-wise versus blocked), previous work has demonstrated that the valence bias is stable across the period of one year (Neta et al., 2009), so we expect it would generalize across sessions.
For the younger adults, all stimuli during session 2 were presented using E-Prime software, whereas Experiment Builder (SR Research Ltd., 2015) was used to present stimuli to the older adult participants. We ensured that stimulus properties and procedures were identical across platforms. The first two runs each consisted of 3 blocks of surprised faces and 3 blocks of neutral faces; the ordering of these blocks was pseudo-random such that no expression was repeated in more than 2 subsequent blocks. After these two runs, an additional two runs were completed in which fearful instead of surprised faces were presented, but the BOLD analysis of these runs is largely outside the scope of the current report (except for defining an amygdala region of interest; see below). Each block consisted of 32 faces (4 presentations of 8 unique identities – 4 females and 4 males, ages 20–29 years – taken from the Umeå University Database of Facial Expressions; Samuelsson et al., 2012), each presented for 200 ms and separated by a fixation cross for 300 ms, as in prior work (Kim, Somerville, Johnstone, et al., 2003; Petro et al., 2018). The identities also repeated across blocks for each expression. Thus, the duration of each block was 16 seconds, separated by 14 seconds during which a fixation cross was presented. Following these four runs, there were two additional runs during which participants completed an emotion regulation task, which is also outside the scope of the current report (but see supplemental section S5).
2.3. MRI acquisition and processing
2.3.1. Scan parameters.
The MRI images were collected in a Siemens 3T Skyra scanner using a 32-channel head coil at the University of Nebraska-Lincoln, Center for Brain, Biology & Behavior. The structural images were acquired using a T1-weighted MPRAGE sequence with the following parameters: TR = 2.2s, TE = 3.37 ms, slices = 192 interleaved, voxel size = 1.0 × 1.0 × 1.0 mm, matrix = 256 × 256, FOV = 256 mm, flip angle = 7 (degrees), total acquisition time = 5:07. BOLD images were collected while participants freely viewed the faces using an echo planar imaging (EPI) sequence with the following parameters: TR = 2.5 s, TE = 30 ms, slices 42 interleaved, voxel size = 2.5 × 2.5 × 3.0 mm, matrix = 88 × 88 mm, FOV = 220 mm, flip angle = 80 (degrees), total acquisition time = 3:24. The image slices were acquired parallel with the inter-commissural plane, and the volume positioned to cover the entire brain.
2.3.2. MRI Preprocessing.
Preprocessing of the imaging data was conducted using the Analysis of Functional Neuroimages (AFNI) suite of programs (Cox, 1996), and subsequent analysis of preprocessed imaging data was conducted in using both AFNI and MATLAB. The first four volumes of each run were discarded to allow for scanner stabilization. The BOLD times-eries, separately for each voxel, were first de-spiked by removing values with outlying data. Then, slice timing correction was accomplished by re-referencing each scan to the first slice. The slice time corrected volumes were then realigned to the minimum outlying image. All volumes were then aligned with the anatomical image, and then warped to the Talairach template atlas (Talairach and Tournoux, 1988) provided by AFNI. This step, which accounts for potential anatomical differences between age groups, was conducted using a non-linear transformation as implemented by AFNI (i.e., tlrc_NL_warp option in afni_proc.py). All functional volumes were then spatially smoothed using a 6mm3 full-width at half maximum kernel. The BOLD time-series, separately for each voxel, was normalized by dividing each time point by the average BOLD value across all time points and then multiplying all time points by 100. Any images containing movement exceeding 0.9 mm3, as calculated during spatial realignment, were censored frame-wise from further analysis.
2.4. Data Analysis
All data are available on the Open Science Framework (link: https://osf.io/47n6b/).
2.4.1. Behavior.
The valence bias score was calculated as the percent of negative categorizations made for surprised faces out of the total number of categorizations of surprise (i.e., excluding omissions). For example, a participant that categorized all surprised faces as negative would be assigned a valence bias of 100%, but one that categorized all surprised faces as positive would be assigned a valence bias of 0%. Thus, a low score on this valence bias measure reflects not just low negativity, but also high positivity. Response times were also recorded for analysis.
Note that the categorizations across expression conditions and across the two age samples were not normally distributed (all Shapiro-Wilkes ps < .05), thus non-parametric statistics are used for analyses of valence bias. For the moderation analyses, robust statistics were used in the regression. These robust regressions were implemented using Matlab’s fitlm command, and used a bi-square weight function with a tuning constant of 4.685.
To test age-related differences in valence bias, the bias scores for each age group were submitted to a Yuen’s t-test of trimmed means as described by Wilcox (2016). A Yuen’s t-test was used for all group comparisons between younger and older adults throughout the manuscript. Further, given that previous work in younger adults has demonstrated that slower responses are associated with more positive categorizations (Neta et al., 2009; Neta and Tong, 2016), we explored this relationship by submitting individual categorizations of surprised faces to a regression with three predictors: 1) response time of the categorization, 2) age group, and 3) the interaction between response time and age group. The interaction term coefficient represented the moderating effect of age group on the relationship between valence bias and response time.
2.4.2. Functional MRI - Amygdala BOLD Activation and Habituation.
Given that previous research has shown that a dorsal region amygdala, not typically captured by structural amygdala definitions, is particularly sensitive to the ambiguity conveyed by surprised faces (Kim, Somerville, McLean, et al., 2003), a functional amygdala region was defined. To identify amygdala voxels that were not biased to a particular expression, the BOLD signal was submitted to a general linear model (GLM) containing regressors which modeled the stimulus onset and duration for each facial expression condition (surprised, fearful, neutral) separately. The regression matrix also contained 6 motion regressors (calculated during spatial realignment) and 2 regressors modeling polynomial trends to control for BOLD signal drifts. The beta values calculated from each task regressor were averaged together, separately at each voxel, and submitted to a one-sample t-test, yielding an estimate of the BOLD activation during the blocks of faces. To identify amygdala activation, these t-values were passed through a cluster-forming (p < 0.001) and -extent (k = 23) threshold, which were calculated according to Gaussian Random Field guidelines for multiple comparison correction (Friston et al., 1994). This process revealed a cluster in both the right (k = 99, Talaraich (x, y, z) = 21, −6, −9) and left (k = 50, Talaraich (x, y, z) = −21, −6, −9) amygdala (Figure 2A). Notably, while this amygdala region of interest (ROI) was defined across all participants, older adults showed greater faces > baseline activation here than younger adults (Yuen’s t-test; Mdifference = 0.08 [95% CI, .04, .12], t48.32 = 4.34, p < .001, d = 1.25).
Figure 2: Relationship between amygdala habituation and valence bias in younger and older adults.
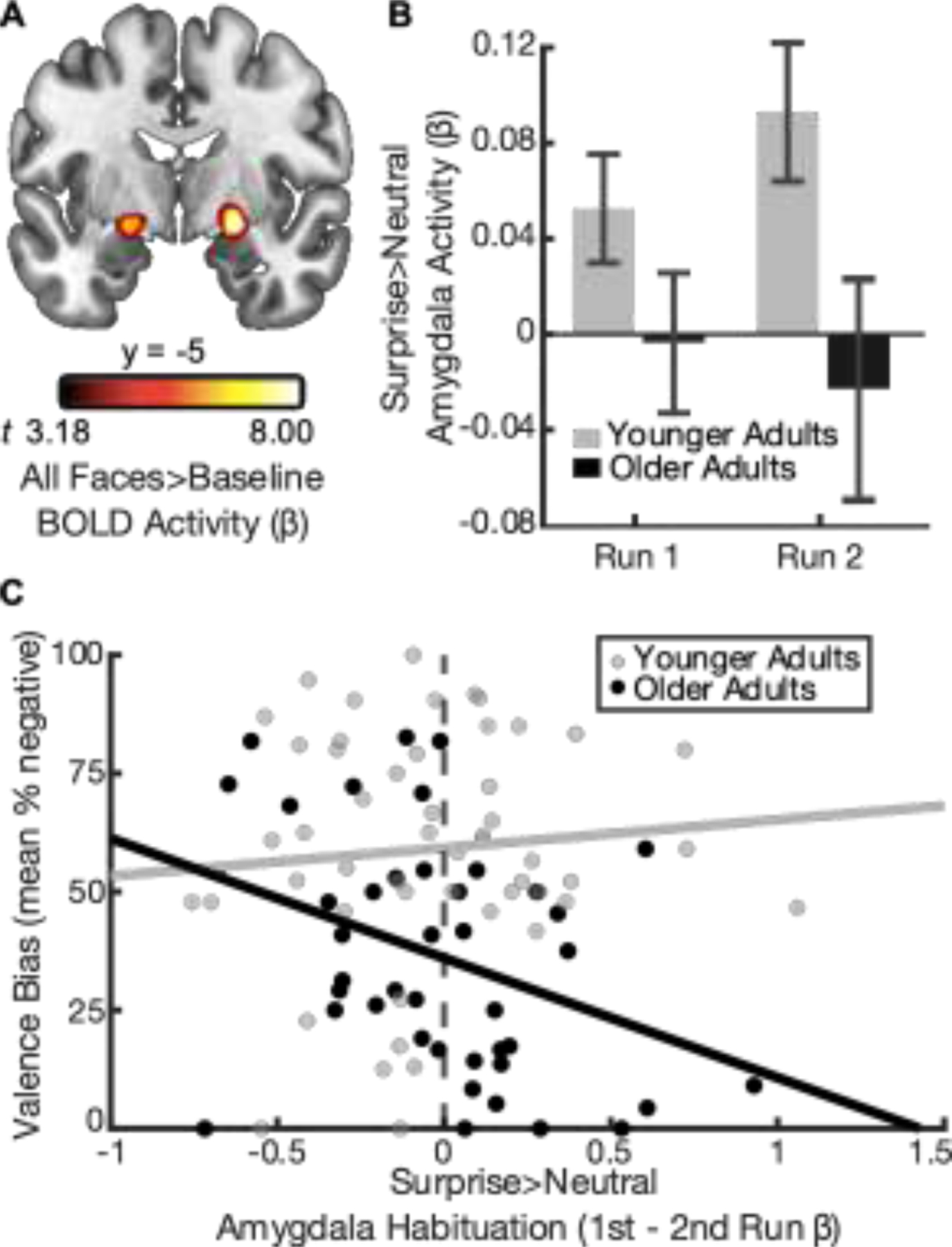
(A) A seed region in the bilateral amygdalae was defined using the contrast of all facial expressions (surprise, neutral, fear) versus baseline (p < .001). (B) Surprise > neutral amygdala activity decreased from run 1 to run 2 for older (black bars) but not younger adults (gray bars). Error bars illustrate the between-subjects standard error. (C) Age group moderated the relationship between valence bias and amygdala habituation (B = −30.42 [95% CI, −56.35, −4.49], t90 = −2.33, p = .02, d = 0.49).
One goal of the present study was to analyze amygdala activity changes across runs of the experiment (i.e., habituation). To accomplish this goal, the BOLD time-series at each voxel was submitted to a GLM which contained regressors modeling the onset and duration of each block separately. Thus, a separate beta value was calculated for each stimulus block. The betas within the bilateral amygdalae ROI were extracted and averaged across all voxels separately for each block. The block-by-block betas were first averaged together across all blocks for either facial expression condition (surprised and neutral), yielding an index of amygdala activity across the duration of the experiment for surprised and neutral faces. In addition, to investigate the changes in amygdala activity between the two experimental runs, the beta values were averaged together for the 3 blocks within each experimental run (i.e., a single value per run), separately for each condition. Averaging across blocks per run ensured a reliable measure of amygdala activity per run (Kim, Somerville, Johnstone, et al., 2003). The change in amygdala activity was computed by subtracting each participant’s average run 2 beta from the average for run 1.
To determine whether surprise-related amygdala activity was related to valence bias, and if this relationship differed across age group, the surprise > neutral amygdala betas, averaged across the entire experiment, were submitted as a predictor in a regression with the outcome of valence bias. The full set of predictors consisted of: 1) surprise > neutral amygdala betas, 2) age group, and 3) the interaction between surprise > neutral betas and age group. The interaction term represented the moderating effect of age group on the relationship between valence bias and amygdala activity. Further, in order to explore effects related to amygdala habituation, we also ran an additional regression replacing surprise > neutral amygdala beta values with surprise > neutral habituation values (run 1 > run 2 betas). In other words, these habituation betas were submitted to the same regression with predictors of valence bias, age group, and their interaction.
2.4.3. Functional MRI – Frontal cortical activation.
In our previous study, we found that more positive younger adults showed greater surprise > neutral activity in the left middle frontal gyrus, identified in a set of regions showing reappraise > maintain activation. From this region, we tested if younger and older adults showed different levels of surprise > neutral activation using an independent samples t-test, and if this activation correlated with valence bias in older adults using a Spearman’s rank correlation. Lastly, we compared the correlation coefficient for older versus young adults using a z-test.
2.4.4. Age differences in amygdala functional connectivity during surprise vs neutral trials.
To determine if the surprise > neutral amygdala activity was functionally connected to any mPFC region, we conducted a context-dependent connectivity analysis (i.e., psychophysiological interaction or PPI; see section S5.1.5 for a full description of this method). Here, the brain-wide t-values associated with surprise > neutral amygdala connectivity were passed through a cluster-forming (p < .01) and -extent threshold (k > 75) according to Gaussian Random Field theory guidelines for multiple comparison correction (Friston et al., 1994).
3. Results
3.1. Behavior
Both younger and older adults categorized angry faces as negative (younger adults: mean(SD) % negative = 94.21(8.82); older adults: mean(SD) % negative = 90.21(10.10)) and happy faces as positive (younger adults: mean(SD) % negative = 6.43(8.91); older adults: mean(SD) % negative = 5.34(7.82)), whereas categorizations of surprised faces showed more inter-subject variability (younger adults: mean(SD) % negative = 58.69(23.89); older adults: mean(SD) % negative = 36.62(24.75); see distribution of categorizations in Figure S1). For the purpose of the current study, the categorizations of angry and happy faces, which convey relatively clear valence, were used only as criteria for accurate performance. Only the categorizations of surprised faces were used to assess individual differences in valence bias. Consistent with previous work (Neta and Tong, 2016; Shuster et al., 2017), we found age-related differences in valence bias such that older adults categorized surprised faces as more positive than younger adults (Mdifference = −24.28 [95% CI, −34.57, −13.99], t57.18 = −4.72, p < .001, d = −1.25).
For both age groups, the responses for categorizing the valence of surprised faces in session 1 (younger adults: mean(SD) ms = 821.26(205.62); older adults: mean(SD) ms = 871.94(204.39)) were slower than for angry (younger adults: mean(SD) ms = 715.12(160.73); older adults: mean(SD) ms = 716.66(122.58)) and happy faces (younger adults: mean(SD) ms = 681.86(154.18); older adults: mean(SD) ms = 682.70(94.76)). Interestingly, there were no age differences in response times for surprised expressions (Mdifference = 29.65 [95% CI, −56.50, 115.81], t61.44 = 0.69, p = .49, d = 0.18).
Notably, the multiple regression revealed that age group moderated the relationship between valence bias and response time (B = 0.08 [95% CI, 0.03, 0.13], t101 = 3.44, p < .001, d = .69; Figure 3; Table S1). Follow-up tests revealed that valence bias and response time were negatively related within the younger adults (B = −0.04 [95% CI, −0.07, −0.01], t52 = −2.74, p < .01, d = 0.76), consistent with previous work (Neta et al., 2009; Neta and Tong, 2016), but were positively related within the older adults (B = 0.04 [95% CI, 0.01, 0.08], t49 = 2.31, p = .03, d = .66). In other words, younger adults with a more negative bias categorized surprised faces faster than those with a more positive bias, but in older adults, a positive bias was faster.
Figure 3: Relationship between valence bias and response time.
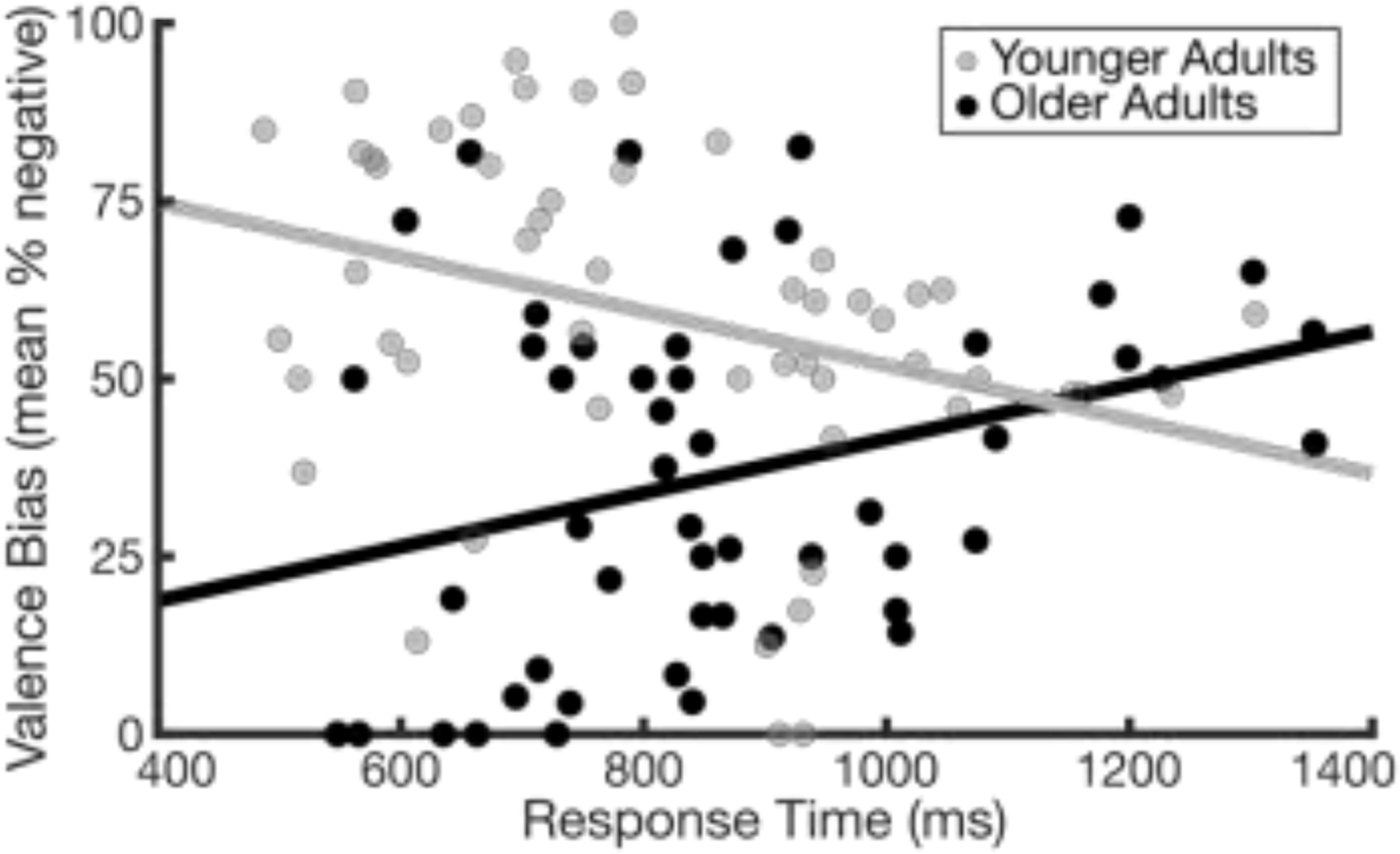
Age group moderated the relationship between valence bias and response time (B = 0.08 [95% CI, 0.03, 0.13], t101 = 3.44, p < .001, d = 0.69). Slower responses were related to a more positive than negative valence bias in younger adults (gray dots; B = −0.04 [95% CI, −0.07, −0.01], t52 = −2.74, p < .01, d = 0.76), whereas a more positive valence bias was related to faster responses in older adults (black dots; B = 0.04 [95% CI, 0.01, 0.08], t49 = 2.31, p = .03, d = 0.66).
3.2. Functional MRI - Amygdala activity as a function of valence bias
When considering amygdala activity across all blocks, there was no difference between younger and older adults in surprise > neutral activity (Mdifference = −0.05 [95% CI, −0.12, 0.03], t46.17 = −1.32, p = .19, d = −0.39). Further, across all participants, a bivariate robust regression revealed that valence bias was not related to surprise > neutral amygdala activity (B = −0.38 [95% CI, −24.96, 24.21], t92 = −0.03, p = .98, d = −0.01), nor was the relationship between surprise > neutral amygdala activation and valence bias moderated by age group (B = −39.25 [95% CI, −87.65, 9.16], t90 = −1.61, p = .11, d = −0.34; Figure S2; Table S2).
When considering patterns of habituation (run 1 > run 2) in surprise > neutral amygdala activity, the relationship between surprise > neutral habituation and valence bias was moderated by age group (B = −30.42 [95% CI, −56.35, −4.49], t90 = −2.33, p = .02, d = −0.49; Figure 2C; Table S3). Follow-up analyses revealed that older adults showed a negative relationship between amygdala habituation and valence bias (B = −26.81 [95% CI, −44.01, −9.60], t41 = −3.15, p < .01, d = −0.98), such that greater habituation was related to a more positive bias. In contrast, younger adults showed no relationship between these variables (B = 2.35 [95% CI, −17.14, 22.44], t49 = 0.24, p = .82, d = 0.07). These age differences are not related to differences in rate of habituation, since amygdala habituation did not differ by age (Yuen’s t-test; Mdifference = .03 [95% CI, −0.11, 0.18], t54.74 = 0.46, p = .65, d = 0.13).
To further probe the moderation effect, whereby older but not younger adults showed a significant relationship between amygdala habituation and valence bias, we examined the relationship between valence bias and amygdala activation in each run and condition separately for the older adults. Specifically, the older adult amygdala betas for surprise and neutral blocks separately, for run 1 and run 2 separately, were submitted to a bivariate robust regression with valence bias, resulting in a total of 4 regression analyses (Figure S3). In other words, there was a regression for 1) surprise-related activity in run 1 and 2) in run 2, and for 3) neutral-related activity in run 1 and 4) in run 2. During run 1, surprise-related activity was negatively related to valence bias (B = −36.79 [95% CI, −71.12, −2.47], t41 = −2.17, p = .04, d = −0.68), such that greater amygdala activity was associated with a more positive valence bias. In contrast, neutral-related activity was not related to valence bias (B = 20.61 [95% CI, −18.91, 60.13], t41 = 1.05, p = .30, d = 0.33). During run 2, surprise activity was not related to valence bias (B = 0.66 [95% CI, −30.62, 31.94], t41 = 0.04, p = .97, d = 0.01) but for neutral faces trended toward a relationship such that greater amygdala activity was associated with a more positive valence bias (B = −20.43 [95% CI, −44.27, 3.41], t41 = −1.73, p = .09, d = −0.54). Further, an additional analysis compared the effects in the left and right amygdala separately and found a stronger effect in the right (see supplemental materials section S4.2).
Interestingly, there was some variability in rate of habituation in older adults including a subset of individuals showing an increase in surprise > neutral amygdala activity from run 1 to 2 (Figure 4, white filled dots). As illustrated in Figure 4, those with negative values (white filled dots, n = 23; mean(SD) = −0.27(0.22)) showed a repetition enhancement, while those with positive values (black filled dots, n = 20; mean(SD) = 0.35(.40)) showed repetition suppression, or habituation. One potential explanation for these differences in older adults might be related to cognitive function. To test this, we operationalized cognitive function using response times in session 1 categorizations, such that faster responses represented relatively better cognitive function. We then compared response time with changes in amygdala activity, and explored age-related differences in this relationship. Each participant’s average response time for surprised face categorizations was calculated, and then submitted as the outcome in a robust regression with predictors of 1) surprise > neutral amygdala habituation, 2) age group, and 3) the interaction between surprise > neutral amygdala habituation and age group. This test revealed that age group moderated the relationship between response time and amygdala habituation (B = −220.63 [95% CI, −431.84, −9.41], t90 = −2.08, p = .04, d = −0.44; Table S4). Follow-up bivariate robust regressions revealed that response time and amygdala habituation were not related in younger adults (B = 81.80 [95% CI, −90.85, 254.45], t49 = 0.95, p = .35, d = 0.27), but were negatively related in older adults (B = −128.13 [95% CI, −254.80, −1.45], t41 = −2.04, p = .048, d = −0.64) such that greater habituation was associated with faster responses in categorizations of surprised faces.
Figure 4: Variability in amygdala habituation and response time in older adults.
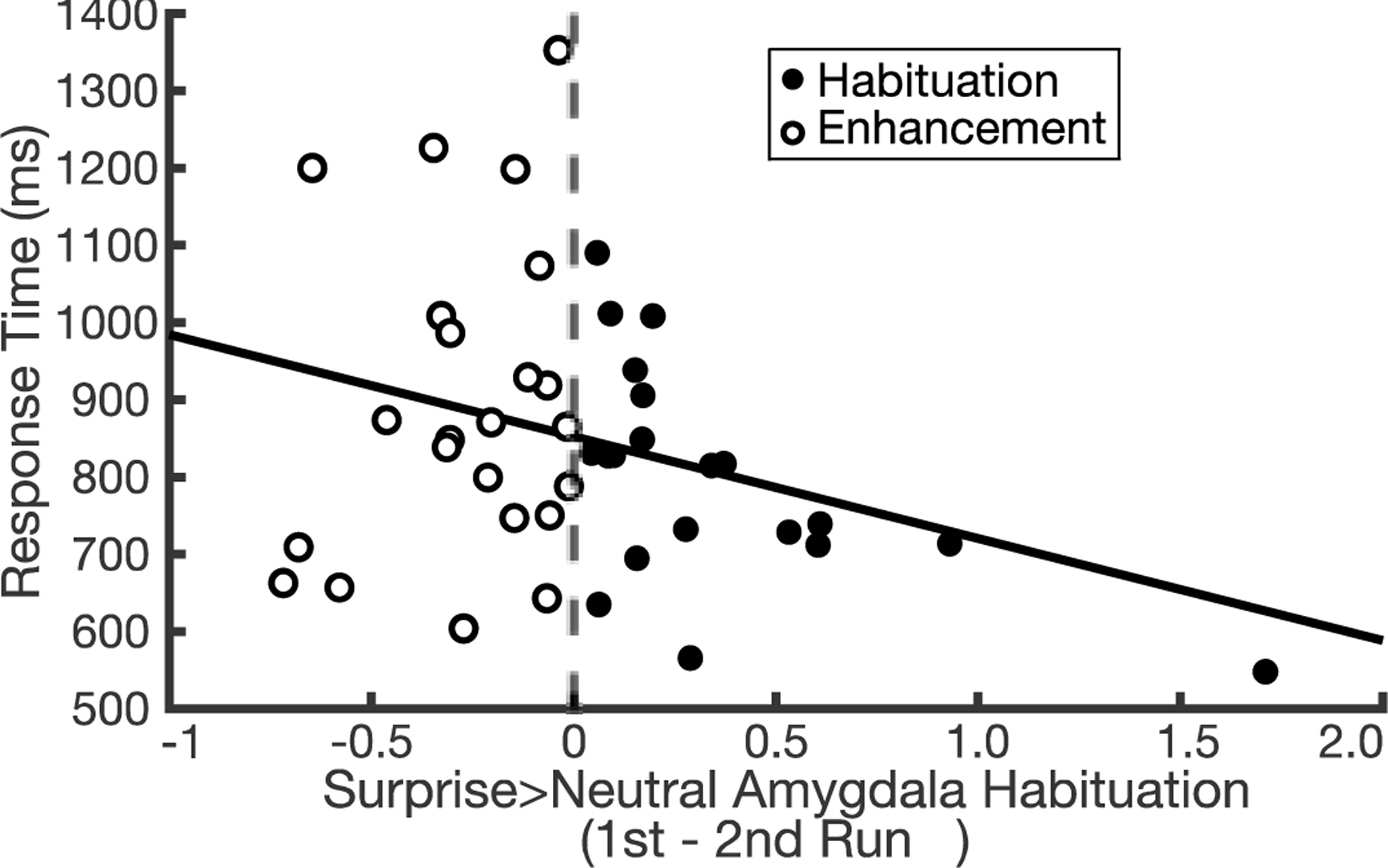
From run 1 to 2, 23 individuals showed increased amygdala activity over time (white filled dots; mean(SD) = −0.27(0.22)) and 20 showed decreased activity across runs (black filled dots; mean(SD) = 0.35(0.40)). Among this variability in amygdala change, those who showed greater habituation of amygdala activity had faster responses (B = −128.13 [95% CI, −254.80, −1.45], t41 = −2.04, p = .048, d = −0.64).
3.3. Functional MRI – Frontal cortical activity as a function of valence bias
Older and younger adults showed no difference in surprise > neutral activation in the left middle frontal gyrus (Mdifference = 0.03 [95% CI, −0.04, 0.09], t89 = 0.81, p = .42, d = 0.17). The surprise > neutral activation in the left middle frontal gyrus was not related to valence bias in the older adults (r38 = −.18 [95% CI, −0.47, 0.14], p = .27; note that this effect was significant in younger adults in our previous report: r49 = −.28 [95% CI, −0.52, −0.01], p = .045). However, the effect in older adults was not significantly different from that in younger adults (z = 0.50, p = .31).
3.4. Age differences in amygdala functional connectivity during surprise vs neutral trials.
Amygdala activity was not inversely related to any region of the mPFC. In fact, older compared younger adults showed more positive surprise > neutral connectivity in a mPFC cluster (Mdifference = 1.42 [95% CI, 0.82, 2.02], peak-t92 = 4.67; k = 235; Talaraich (x, y, z) = −4, 69, 6), which showed peak activation in the left superior medial gyrus with coverage extending to the left and right anterior cingulate cortex (Figure S4).
4. Discussion
Older compared to younger adults categorized expressions of surprise as more positive, replicating previous work (Neta and Tong, 2016; Shuster et al., 2017) and broadly consistent with the positivity effect in aging (e.g., Mather, 2016; Mather and Knight, 2005). Importantly, older, but not younger, adults with more positive valence bias showed both 1) faster responses and 2) more amygdala habituation than those with a more negative bias, suggesting that the age-related positivity represents a shift away from the initial negativity mechanism. As predicted, valence bias was also not associated with frontal cortical activation in older adults. In other words, older adults show a less effortful or more default positivity in response to dual-valence ambiguity, replacing the more default negativity seen in younger adults.
Indeed, the notion that the positive valence bias in aging results from a default response that is more positive is supported by the age-related differences in response times. Specifically, in younger adults, positive categorizations of surprised faces were associated slower responses than negative categorizations (Neta et al., 2009), and an instruction to deliberate is sufficient for promoting greater positivity (Neta and Tong, 2016). This effect replicates extant literature and is thought to reflect additional, putatively effortful, regulatory signals (Neta and Tong, 2016; Petro et al., 2018). However, the opposite pattern was found in older adults, in which positive compared to negative categorizations were related to faster responses. This age-related difference in the relationship between valence bias and response time suggests that although younger adults appear to employ a regulatory process that overrides a default negativity in order to arrive at a more positive evaluation of valence ambiguity, a different (and less effortful) process appears to drive positive evaluations in older adults.
Moving to the neuroimaging findings, as we predicted, older (but not younger) adults showed a relationship between amygdala habituation and valence bias, such that those with a more positive bias showed greater habituation. Interestingly, patterns of persistent (as opposed to habituating) amygdala activity across stimulus presentations reflect a brain mechanism which maintains, across repeated exposures, the processing of biologically relevant information to promote continued learning (Davis et al., 2016; Herry et al., 2007; Whalen, 2007). In the context of the literature on amygdala habituation, it could be that younger adults perceive the potential (but ambiguous) negativity in response to surprised faces and thus require further learning. In contrast, there is a primacy for the potential positivity in older adults, where perhaps the potential threat is not registered, and thus no further learning is required.
To elaborate, the change in amygdala activity across runs showed wide variability in the older adults. For example, while some individuals showed habituation, others showed response enhancement. We found that the former group was more likely to have a positive valence bias, while the latter group was more likely to have a negative valence bias. Again, one possible explanation is that the amygdala increase over time is a characteristic of the latter group perceiving potential negativity or threat in response to the surprised faces. And, given there is some uncertainty about the presence of this threat, the amygdala stays active to promote further learning, consistent with the pattern previously observed in younger adults (Davis et al., 2016). Conversely, the individuals with a more positive valence bias appear to be more likely to perceive positivity in response to the surprised faces, and thus render the faces “safe” or not in need of further learning. This speculative interpretation is supported by age-related differences in the relationship between valence bias and response time. Specifically, although younger adults are faster when categorizing surprised faces as negative than positive (see also Neta et al., 2009), older adults are faster when categorizing the same faces as positive.
The surprise and neutral amygdala activity evaluated separately across both runs suggests that this habituation effect in older adults with a more positive valence bias is characterized in part by an amygdala increase toward surprised faces during the first run. The finding that increased amygdala activity is related to more positivity is consistent with prior work which found that older adults show increased amygdala activity to positive relative to neutral and negative information (Leclerc and Kensinger, 2011), particularly for relatively low-arousing stimuli (Dolcos et al., 2014). In terms of the habituation effect, one straightforward explanation for this pattern of results is that the amygdala activity increases initially, and the greater increase allows for a greater change (habituation) in the second run. In contrast, the individuals with a more negative bias show no change in amygdala activity or show an increase in the second run (see more on this below). We suggest that the pattern of habituation in the subset of older adults with a positive bias is novel.
A previous literature has demonstrated that older adults are less efficient at differentiating facial features (i.e., de-differentiation) relating to identity (Goh et al., 2010). Thus, in the context of the current results, one possibility is that greater habituation of amygdala activity and more positive valence bias are associated with greater levels of de-differentiation. In other words, the amygdala may habituate more in older adults who are less able to discern the differences between face identities. However, in the current results there was a different relationship between valence bias and amygdala habituation for surprised versus neutral faces (see supplemental Figure S3), indicating that the amygdala response is indeed sensitive to the content of the expressions, rather than a dedifferentiation of face identities.
Another possibility is that the individual differences in amygdala change across runs are related to cognitive function. Indeed, cognitive function generally declines with age (Salthouse et al., 2003), and it could be that the amygdala habituation is associated with greater cognitive deficit. Although no explicit measure of cognitive function was collected in the current study, we explored the possibility that slower responses might be a useful proxy for cognitive decline. Interestingly, older adults with greater amygdala habituation showed faster (not slower) response times. While speculative, these results suggest that amygdala habituation may be putatively related to better cognitive function, consistent with work showing that those with relatively good cognitive function tend to show a stronger positivity effect (Mather and Knight, 2005). Although this prior work linking cognitive function to the positivity effect has leveraged these findings as evidence for a top-down mechanism that implies increased frontal cortical activity, we did not find any evidence for a relationship between valence bias and frontal activity in our sample of older adults. While our finding was not conclusive (i.e., it was a null effect, and also not significantly different from the pattern evident in younger adults), this potential discrepancy with prior work could be due to differences inherent to the valence bias task, given that the prior work largely focused on responses to clearly valenced stimuli (e.g., Mather and Knight, 2005; Dolcos et al., 2014). Future studies of valence bias will benefit from adding an explicit measure of cognitive ability in order to better characterize the relationships between amygdala habituation, top-down frontal signals, valence bias, and cognitive function.
The current results indicate that older adults tend to expend less effort (faster responses) in arriving at positive categorizations. Further, older adults with a more positive bias also show increased amygdala habituation, suggesting they may not perceive a potential threat that requires further learning, but rather there is a primacy for positivity in aging. It is worth noting that a primacy for positivity does not imply that this positivity effect is the result of an automatic or bottom-up process. Indeed, the responses to valence ambiguity likely involve some top-down control mechanism (at least when compared to the responses to clear valence, or well-known automatic responses in the domain of vision and attention). Instead, responses to ambiguity are relatively effortful, but within the variability of these responses, the default response in younger adults appears to be more negative, whereas the default response in older adults appears to be more positive (see also supplemental material section S6).
The interpretation of the current results, that positivity in older adults constitutes the initial, default response, is qualified by limitations of the methodology used. Foremost, the measurements of amygdala activity depend on the sluggish BOLD signal which is unable to dissociate temporally early from late processes. Future work may utilize the temporal resolution provided by EEG and eye-tracking to explore age-related differences in the response to ambiguity within precise time-windows. Second, the results should be considered within a context where there were methodological differences for collecting behavioral and BOLD data, in which surprised faces were explicitly categorized or passively viewed, respectively, and involved more phasic or tonic processes, respectively. Lastly, while a strict threshold was used to define the amygdala region across the full sample, this region showed more activation in older than younger adults, which may present a bias toward capturing older adult’s amygdala activity.
Finally, we did not replicate previous work in younger adults showing that a more negative valence bias is associated with greater surprise-related amygdala activity than those with a positive bias (Kim, Somerville, Johnstone, et al., 2003). Methodological differences may account for this inconsistency. For instance, Kim, Somerville, McLean et al. (2003) used a whole-brain correlation conducted on the surprise > baseline beta values to identify an amygdala region of interest, whereas we defined the region as voxels showing activation to all faces. Further, Kim, Somerville, McLean et al. (2003) measured valence bias using only a single trial immediately following the MRI session, whereas we relied on an entire behavioral task (consisting of approximately 24 trials) administered approximately a week before the MRI session. This discrepancy in findings may need to be explored in future work.
4.1. Conclusions
To summarize, in older, but not younger, adults, valence bias was associated with amygdala habituation to surprised faces. Specifically, the magnitude of habituation in older adults was associated with a more positive valence bias and faster responses when categorizing the valence of surprised faces. These results suggest that, whereas a positive valence bias in younger adults putatively relies on an additional regulatory mechanism, older adults show evidence for a primacy of positivity in response to dual-valence ambiguity.
Supplementary Material
Highlights.
Older adults show a more positive bias in response to ambiguity than younger adults
Faster positive ratings in older than younger adults, suggesting default positivity
Amygdala habituates to ambiguity more so in older adults with a positive bias
Greater positivity in aging does not appear to be related to cognitive decline
Potential shift in mechanism from default negativity to default positivity with age
Acknowledgements
This work was supported by the National Institutes of Health (NIMH111640; PI: Neta), the National Science Foundation (CAREER Award; PI: Neta), and by Nebraska Tobacco Settlement Biomedical Research Enhancement Funds. We thank Elizabeth Kensinger for helpful comments on the manuscript. We also thank Kayla Clark, Daniel J. Henley, and Tien T. Tong for assistance in data collection and management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare that they have no competing interests.
References
- Allard ES, Wadlinger HA, Isaacowitz DM, 2010. Positive gaze preferences in older adults: assessing the role of cognitive effort with pupil dilation. Aging Neuropsychol. Cogn, 17(3), 296–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F, LeDoux J, Clugnet MC, Pavlides C, 1993. Single-unit activity in the lateral nucleus of the amygdala and overlying areas of the striatum in freely behaving rats: Rates, discharge patterns, and responses to acoustic stimuli. Behav. Neurosci 107, 757–769. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR, 1996. Response and habituation of the human amygdala during visual processing of facial expression. Neuron 17, 875–887. [DOI] [PubMed] [Google Scholar]
- Bucks RS, Garner M, Tarrant L, Bradley BP, Mogg K, 2008. Interpretation of emotionally ambiguous faces in older adults. J. Gerontol. B. Psychol. Sci. Soc. Sci 63, P337–P343. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, 2006. The Influence of a Sense of Time on Human Development. Science 312, 1913–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, 2010. Strength and vulnerability integration: A model of emotional well-being across adulthood. Psychol. Bull 136, 1068–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL, 2003. Aging and emotional memory: The forgettable nature of negative images for older adults. J. Exp. Psychol. Gen 132, 310–324. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CHY, 2008. Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Res. Rev 58, 57–70. [DOI] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Davis FC, Neta M, Kim MJ, Moran JM, Whalen PJ, 2016. Interpreting ambiguous social cues in unpredictable contexts. Soc. Cogn. Affect. Neurosci 11, 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos S, Katsumi Y, Dixon RA, 2014. The role of arousal in the spontaneous regulation of emotions in healthy aging: a fMRI investigation. Front. Psychol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RS, Mazziotta JC, Evans AC, 1994. Assessing the significance of focal activations using their spatial extent. Hum. Brain Mapp 1, 210–220. [DOI] [PubMed] [Google Scholar]
- Geissberger N, Tik M, Sladky R, Woletz M, Schule A-L, Willinger D, Windischberger C, 2020. Reproducibility of amygdala activation in facial emotion processing at 7T. NeuroImage 116585. [DOI] [PubMed]
- Goeleven E, De Raedt R, Leyman L, Verschuere B, 2008. The Karolinska directed emotional faces: a validation study. Cogn. Emot 22, 1094–1118. [Google Scholar]
- Goh JO, Suzuki A, Park DC, 2010. Reduced neural selectivity increases fMRI adaptation with age during face discrimination. NeuroImage 51, 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronchi G, Righi S, Pierguidi L, Giovannelli F, Murasecco I, Viggiano MP, 2018. Automatic and controlled attentional orienting in the elderly: A dual-process view of the positivity effect. Acta Psychol. (Amst.) 185, 229–234. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D, 2011. ERPs and the Study of Emotion Oxford University Press. [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR, 2003. Neocortical modulation of the amygdala response to fearful stimuli. Biol. Psychiatry 53, 494–501. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Luthi A, Seifritz E, 2007. Processing of temporal unpredictability in human and animal amygdala. J. Neurosci 27, 5958–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilimire MR, Mienaltowski A, Blanchard-Fields F, Corballis PM, 2014. Age-related differences in event-related potentials for early visual processing of emotional faces. Soc. Cogn. Affect. Neurosci 9, 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ, 1998. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philos. Trans. R. Soc. Londz. B. Biol. Sci 353, 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston JR, Pollock JW, Lien M-C, Allen PA, 2018. Emotional arousal deficit or emotional regulation bias? An electrophysiological study of age-related differences in emotion perception. Exp. Aging Res 44, 187–205. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, Kalin NH, Whalen PJ, 2005. Stability of amygdala BOLD response to fearful faces over multiple scan sessions. NeuroImage 25, 1112–1123. [DOI] [PubMed] [Google Scholar]
- Kennedy BL, Huang R, Mather M, 2019. Age differences in emotion-induced blindness: Positivity effects in early attention. Emotion 20, 1266–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ, 2003. Inverse amygdala and medial prefrontal cortex responses to surprised faces. NeuroReport 14, 2317. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, McLean AA, Johnstone T, Shin LM, Whalen PJ, 2003. Functional MRI responses of the human dorsal amygdala/substantia innominata region to facial expressions of emotion. Annals of the New York Academy of Sciences 985, 533–535. [Google Scholar]
- LaBar KS, LeDoux JE, 1996. Partial disruption of fear conditioning in rats with unilateral amygdala damage: Correspondence with unilateral temporal lobectomy in humans. Behav. Neurosci 110, 991–997. [DOI] [PubMed] [Google Scholar]
- Langeslag SJE, van Strien JW, 2009. Aging and emotional memory: The co-occurrence of neurophysiological and behavioral positivity effects. Emotion 9, 369–377. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA, 2011. Neural processing of emotional pictures and words: a comparison of young and older adults. Dev. Neuropsychol 36, 519–538. [DOI] [PubMed] [Google Scholar]
- Mather M, 2016. The affective neuroscience of aging. Annu. Rev. Psychol 67, 213–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, John DEG, Carstensen LL, 2004. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychol. Sci 15, 259–263. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL, 2003. Aging and attentional biases for emotional faces. Psychol. Sci 14, 409–415. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M, 2005. Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychol. Aging 20, 554–570. [DOI] [PubMed] [Google Scholar]
- Maus B, van Breukelen GJP, Goebel R, Berger MPF, 2012. Optimal design for nonlinear estimation of the hemodynamic response function. Hum. Brain Mapp 33, 1253–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Yang J, Cai Ay., Ding X, Liu W, Li H, Yuan J, 2015. The neural mechanisms underlying the aging-related enhancement of positive affects: electrophysiological evidences. Front. Aging Neurosci 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Negreira A, Weierich M, Dautoff R, Dickerson BC, Wright CI, Barrett LF, 2011. Differential Hemodynamic Response in Affective Circuitry with Aging: An fMRI Study of Novelty, Valence, and Arousal. J. Cogn. Neurosci 23, 1027–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MM, Andersen SK, Keil A, 2008. Time course of competition for visual processing resources between emotional pictures and foreground task. Cereb. Cortex 18, 1892–1899. [DOI] [PubMed] [Google Scholar]
- Neta M, Berkebile MM, Freeman JB, 2020. The dynamic process of ambiguous emotion perception. Cognition and Emotion 1–8. [DOI] [PMC free article] [PubMed]
- Neta M, Kelley WM, Whalen PJ, 2013. Neural responses to ambiguity involve domain-general and domain-specific emotion processing systems. J. Cogn. Neurosci 25, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Norris CJ, Whalen PJ, 2009. Corrugator muscle responses are associated with individual differences in positivity-negativity bias. Emotion 9, 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Tong TT, 2016. Don’t like what you see? Give it time: Longer reaction times associated with increased positive affect. Emotion 16, 730–739. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F, 2001. Emotion drives attention: Detecting the snake in the grass. J. Exp. Psychol. Gen 130, 466–478. [DOI] [PubMed] [Google Scholar]
- Pehlivanoglu D, Verhaeghen P, 2019. Now you feel it, now you don’t: Motivated attention to emotional content is modulated by age and task demands. Cogn Affect Behav Neurosci 19, 1299–1316. [DOI] [PubMed] [Google Scholar]
- Petro NM, Tong TT, Henley DJ, Neta M, 2018. Individual differences in valence bias: fMRI evidence of the initial negativity hypothesis. Soc. Cogn. Affect. Neurosci 13, 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petro NM, Tottenham N, Neta M, 2021. Exploring valence bias as a metric for frontoamygdalar connectivity and depressive symptoms in childhood. Dev Psychobiol, 1–16. [DOI] [PMC free article] [PubMed]
- Plichta MM, Grimm O, Morgen K, Mier D, Sauer C, Haddad L, Tost H, Esslinger C, Kirsch P, Schwarz AJ, Meyer-Lindenberg A, 2014. Amygdala habituation: A reliable fMRI phenotype. NeuroImage 103, 383–390. [DOI] [PubMed] [Google Scholar]
- Proctor RW, Vu K-PL, Pick DF, 2005. Aging and response selection in spatial choice tasks. Hum. Factors J. Hum. Factors Ergon. Soc 47, 250–270. [DOI] [PubMed] [Google Scholar]
- Reed AE, Carstensen LL, 2012. The theory behind the age-related positivity effect. Front. Psychol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfroe JB, Bradley MM, Sege CT, Bowers D, 2016. Emotional Modulation of the Late Positive Potential during Picture Free Viewing in Older and Young Adults. PLoS ONE 11, e0162323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Nga L, Mather M, 2013. Amygdala Functional Connectivity with Medial Prefrontal Cortex at Rest Predicts the Positivity Effect in Older Adults’ Memory. Journal of Cognitive Neuroscience 25, 1206–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE, 2003. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. J. Exp. Psychol. Gen 132, 566–594. [DOI] [PubMed] [Google Scholar]
- Samuelsson H, Jarnvik K, Henningsson H, Andersson J, Carlbring P, 2012. The Umeå university database of facial expressions: A validation study. J Med Internet Res 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster MM, Mikels JA, Camras LA, 2017. Adult age differences in the interpretation of surprised facial expressions. Emotion 17, 191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach P, Tournoux J, 1988. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging Thieme Medical, New York. [Google Scholar]
- Tanovic E, Gee DG, Joormann J, 2018. Intolerance of uncertainty: Neural and psychophysiological correlates of the perception of uncertainty as threatening. Clin. Psychol. Rev 60, 87–99. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, et al. , 2009. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res 168, 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh KA, Breitner JCS, Magruder-Habib KM, 1993. Detection of dementia in the elderly using telephone screening of cognitive status. Cogn. Behav. Neurol 6, 103–110. [Google Scholar]
- Whalen PJ, 2007. The uncertainty of it all. Trends Cogn. Sci 11, 499–500. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Davis FC, Oler JA, Kim H, Kim MJ, Neta M, 2009. Human amygdala responses to facial expressions of emotion., in: The Human Amygdala Guilford Press, New York, NY, US, pp. 265–288. [Google Scholar]
- Wilcox RR, 2016. Introduction to robust estimation and hypothesis testing, 4th edition. ed. Elsevier, Waltham, MA. [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, Peduto A, Gordon E, 2006. The mellow years?: neural basis of improving emotional stability over age. J. Neurosci 26, 6422–6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


