Abstract
Frailty is an age-related syndrome that exposes individuals to increased vulnerability. Although it is potentially reversible, in most cases it leads to negative outcomes, including mortality. The different methods proposed identify frailty after the onset of clinical manifestations. An early diagnosis might make it possible to manage the frailty progression better. The frailty pathophysiology is still unclear although mechanisms, in particular, those linked to inflammation and immunosenescence, have been investigated. A common feature of several clinical aspects involved in senescent organisms is the increase of oxidative stress, described as one of the major causes of deoxyribonucleic acid (DNA) damage accumulation in aged cells including the adult stem cell compartment. Likely, this accumulation is implicated in frailty status. The oxidative status of our frail, pre-frail, and non-frail population was characterized. In addition, the DNA damage in hematopoietic cells was evidenced by analyzing the peripheral blood mononuclear cell and their T lymphocyte, monocyte, circulating hematopoietic progenitor stem cell (cHPSC) subpopulations. The phosphorylation of C-terminal of histone H2AX at amino acid Ser 139 (γ-H2AX), which occurs at the DNA double-strand break focus, was evaluated. In our frail population, increased oxidative stress and a high level of DNA damage in cHPSC were found. This study may have potential implications because the increment of DNA damage in cHPSC could be suggestive of an organism impairment preceding the evident frailty. In addition, it may open the possibility for attenuation of frailty progression throughout specific drugs acting on preventing DNA damage or removing damaged cells
Keywords: γ-H2AX, Biology of aging, Oxidative stress, Cellular senescence
Frailty is an age-related syndrome, which results from a decline in physiological capacities across several organ systems and exposes the individual to an increased vulnerability toward internal and external stressors (1). It is a potentially reversible condition, but in most cases it progresses toward disability and/or leads to several negative outcomes, including falls and fractures, hospitalizations, iatrogenic complications, and mortality (1–3). The risk of these adverse events can occur independently of the presence of comorbidity (4). Several methods have been proposed to assess frailty in research and in clinical practice, without clear evidence that one method is preferable to the others. Fried et al. (4) developed the phenotype model of frailty that is one of the most widely employed in clinical research. It considers frailty as a biological entity and classifies the individuals as frail when three or more of the following five criteria are present: Unintentional weight loss, exhaustion, slow walking speed, low level of physical activity, and low muscle strength. A classification of pre-frailty is also possible when only one or two of these criteria are present. The pathophysiological mechanisms underlying the frail phenotype are still unclear although mechanisms, in particular, those linked to inflammation and immunosenescence, have been investigated (5,6). However, the complex interactions between the studied molecules and these mechanisms during the aging process are yet to be defined.
A common feature of several clinical aspects involved in senescent organisms is the increase of oxidative stress that is described as one of the major causes of deoxyribonucleic acid (DNA) damage accumulation, well documented in the different tissues of older people (7). Likely, this accumulation might be implicated in frailty status (8) because DNA damage can lead to molecular and cellular alterations (genomic instability, altered gene expression, loss of cell division potential, cell death, and organ dysfunctions) (7,9) that are a good background on which frailty can arise.
The phosphorylation of the C-terminal of histone H2A, variant H2AX, at the highly conserved amino acid Ser 139 (γ-H2AX) is an early response to DNA double-strand breaks (DSBs). γ-H2AX and DSB correlate in a 1:1 manner (10), and transient and persistent γ-H2AX foci can be present in the cells. In the persistent foci, DSB are not repairable and appear to be common in senescent cells and to increase in older subjects (11–13). γ-H2AX foci detection is one of the most sensitive ways to examine DNA damage (14). In recent years, the role of DNA damage has been investigated on whole blood (15) and lymphocytes (16), evaluating the relationship between the level of persistent γ-H2AX foci and frail status of older patients.
With our study, we wanted to deepen the characterization of DNA damage in hematopoietic cells, analyzing the peripheral blood mononuclear cells (PBMCs) and the T lymphocyte, monocyte, and circulating hematopoietic progenitor stem cell (cHPSC) subpopulations present in PBMC of frail, pre-frail, and non-frail older adults.
Method
Study Population
A group of 85 subjects (40 frail, 13 pre-frail, 32 non-frail) aged 65 years and above were recruited from the Geriatric Unit, San Gerardo Hospital ASST Monza. All of them were outpatients. The exclusion criteria for the recruited subjects were: (a) illiteracy, (b) psychiatric disorders, (c) Parkinson’s disease, (d) cognitive function impairment, (e) multiple sclerosis, (f) neurodegenerative diseases, (g) hip fracture or other severe traumas affecting mobility. The identification of frail phenotype in all subjects occurred at recruitment and was based on the presence of five criteria (4): (i) shrinking, which was determined as an unintentional weight loss of 5% or more than usual, over the last year; (ii) self-reported exhaustion, which was evaluated as present or absent by asking the individual: “In the last month, do you have less energy to do the things of daily living you want?”; (iii) slow walking speed, which was defined as present when an individual took more than 7 s to cover a distance of 4 m (17); (iv) low physical activity, which was assessed using Physical Activity Scale for the Elderly (PASE) over 1 week. The PASE score combines information on leisure, household, and occupational activity (18); (v) weakness, which was measured using the dynamometer handgrip strength for three consecutive times on the dominant hand. The criterion is positive if the best value is less than the cutoff value (<27 for males and <16 for females) (19). Participants were considered frail if they fulfilled three or more criteria, pre-frail if they fulfilled one or two criteria, and robust or non-frail if they did not meet any criteria. Forty-six young subjects, aged between 25 and 35 years, without functional disability, acute pathologies in progress or abuse of drugs, alcohol, smoke, and with a healthy lifestyle, were recruited among blood donors as the control group. The study was conducted according to the principles of the Declaration of Helsinki II and approved by the Ethics Committee Brianza (Monza, Italy) code FRA-ARSC on March 22, 2018. The participation in the study was on a voluntary basis, all subjects were fully informed about the aims of the study and about the nature of their participation, and all of them signed the informed consent.
Comprehensive Geriatric Assessment
After recruitment, all 85 older adults underwent a comprehensive geriatric assessment (CGA) including a survey on demographics (age and sex) and life habits (smoking and alcohol consumption), and several tools to evaluate nutritional, cognitive, functional, and somatic health status. Nutritional status was evaluated using the Mini Nutritional Assessment-short form (MNA-sf) (20) and cognitive status using the Mini-Mental State Examination (21). The functional status was assessed with the modified Barthel Index (22), the Lawton and Brody’s Instrumental Activity of Daily Living (23), and the Short Physical Performance Battery (17). Lastly, the somatic health status was assessed with the Charlson Comorbidity Index (CCI) (24). Also the number of drugs taken at home was determined.
PBMC Collection, Cell Characterization, DNA Damage Evaluation
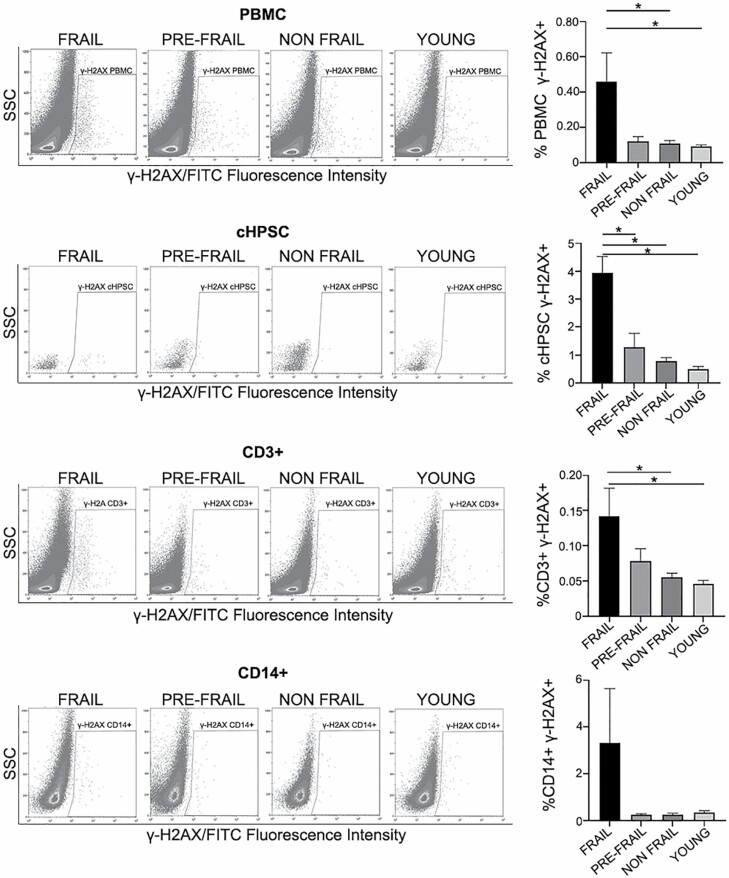
Whole blood from frail, pre-frail, non-frail, and young subjects was collected into BD Vacutainer CPT Cell Preparation Tubes with sodium heparin (Becton Dickinson, Franklin Lakes, New Jersey, USA). The isolation of PBMC by Ficoll-Paque (GE Healthcare Bio-Sciences AD, Uppsala, Sweden) density gradient separation was performed according to the manufacturer’s instructions. The isolated PBMC were suspended in blocking solution (PBS with 5% fetal bovine serum) at room temperature (RT) for 15 min then treated following a protocol modified by Beaton et al. (25). Briefly, membrane cell staining was performed for 15 min at RT with the following antibodies: APC Mouse Anti-Human CD3 (Clone UCHT1; BD Pharmingen, San Jose, CA, USA; 1:200), T lymphocyte marker; APC-H7 Mouse anti-Human CD14 (Clone MφP9; BD Pharmingen; 1:200), monocyte marker; PE Mouse Anti-Human CD34 (Clone 8G12; BD Pharmingen; 1:100), hematopoietic progenitor stem cell (HPSC) marker. The stained PBMCs were then centrifuged, suspended, fixed with 4% methanol-free formaldehyde (Thermo Fisher Scientific, Waltham, MA, USA), and permeabilized with 0.1% Triton X-100 (Sigma–Aldrich, St. Louis, MO, USA). After centrifugation, 70% cold methanol was added dropwise while vortexing the cellular pellet, then the samples were stored at −20°C for 24 h. Successively, these samples were washed and stained for 2 h at 4°C with Anti-phospho-Histone H2AX (Ser139) FITC conjugate (Clone JBW301; Millipore, Burlington, MA, USA; 2.5 mg/mL) to identify DNA DSB. The samples were then analyzed using a MoFlo Astrios cell sorter and Kaluza 2.1 software (both from Beckman Coulter, Miami, FL, USA). On the basis of the gating strategy (Supplementary Figure 1) used on the PBMC (a minimum of 105 events were acquired), the percentage of T lymphocytes CD3+, monocytes CD14+, and circulating HPSC (cHPSC) CD34+/CD3−/CD14− was evaluated as well as the respective cells positive for γ-H2AX. The mean fluorescence intensity (MFI), calculated as the median of the fluorescence intensity values of the γ-H2AX+ cells (represented on the X-axis of Figure 1 and Supplementary Figure 1), has also been evaluated. MFI provides information on the amount of DNA damaged foci in the cells (26,27).
Figure 1.
DNA damage in PBMC isolated by Ficoll-Paque and in the respective cell subpopulations: cHPSC CD34+/CD3-/CD14-, T lymphocytes CD3+, monocytes CD14+. Representative FACS analysis of γ-H2AX dot plots of the different studied groups (40 frail, 13 pre-frail, 32 non-frail, 46 young samples). Histograms represent the percentage of cells positive for DNA damage (γ-H2AX) in the different groups. SSC: side scatter. Data are expressed as means ± SEM. *p < .05.
For the characterization of cHPSC on fresh whole blood, 700 µL of peripheral blood were lysed using erythrocyte lysis buffer (BioLegend, San Diego, CA, USA). Then the white blood cells underwent membrane cell staining as described above using the following antibodies: APC anti-human Lineage cocktail against CD3/CD14/CD16/CD19/CD20/CD56 (Lin1) (BioLegend; 1:5); APC/Fire 750 anti-human CD45 (Clone 2D1, BioLegend; 1:20); PE anti-human CD34 (Clone 8G12; BD Pharmingen; 1:100). Flow cytometry analysis was performed on the basis of the gating strategy described in Supplementary Figure 2. A minimum of 2 × 105 events were acquired in the PBMC region.
Evaluation of Oxidative Stress
Oxysterols as markers of oxidative stress and 8-hydroxy-2-deoxy guanosine as a marker of oxidative DNA damage were analyzed in a set of randomly chosen samples of plasma collected from frail, pre-frail, non-frail, and young individuals. 7-Keto-cholesterol (7KC), 7β-hydroxycholesterol (7βOHC), 5α6α-epoxycholesterol (5α,6αEC), 5β6β-epoxycholsterol (5β,6βEC), and 3β5α6β-3OH-cholesterol (triol) (3β,5α,6β-3OHC) were quantified using isotope dilution mass spectrometry (28) as cholesterol oxidation and cholesterol autoxidation products resulting from oxidative stress (29–31). 8-Hydroxy-2-deoxy guanosine (8-OH-dG) was analyzed in duplicate by enzyme-linked immunosorbent assay (ab201734, 8-hydroxy-2-deoxy guanosine, ELISA kit, Abcam, Cambridge, UK), according to literature (32) and the manufacturer’s instructions.
Statistical Analysis
Continuous variables were reported as mean ± standard deviation and as median with first quartile–third quartile. Categorical variables were reported as absolute and relative frequencies. The comparison between groups was performed with the one-way analysis of variance test with Tukey’s test correction and with pairwise multiple comparison procedures (Holm–Sidak method), with Wilcoxon–Mann–Whitney test in case of non-Gaussian distribution. When significant, the single pairs (frail, pre-frail, non-frail, and young) were also compared. Significance was for p < .05. Receiver operating characteristic (ROC) curve analysis was performed using one-way analysis (Wilson–Brown method) and the results were reported as a fraction. The GraphPad Prism program has been used (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Study Population
Among the 85 older adults recruited, 40 were frail (17 males, 23 females); 13 pre-frail (4 males, 9 females), 32 non-frail (21 males, 11 females), these subjects underwent the CGA (Table 1) and laboratory analyses (Supplementary Table 1). Forty-six young subjects (17 males, 29 females; mean age ± SD 29.9 ± 2.9 years; median age 29.5 years) were also recruited.
Table 1.
Comprehensive Geriatric Assessment of 85 Older Adults, According to Frail, Pre-frail, and Non-frail Status
| Characteristics | Frail, n=40 | Pre-frail, n=13 | Non-frail, n=32 | p value* | p value† | p value‡ |
|---|---|---|---|---|---|---|
| Age, years | ||||||
| Mean ± SD | 81.9 ± 6.2 | 79.5 ± 6.9 | 73.8 ± 6.7 | .197 | <.001 | .059 |
| Median (first–third quartile) | 82 (78–86) | 81 (73.5–84) | 71 (67–77) | .109 | <.001 | .004 |
| Females, n (%) | 23 (57.5) | 9 (69.2) | 11 (34.4) | .415 | .090 | .044 |
| Smoke, n (%) | ||||||
| Current smoker | 3 (7.5) | 1 (7.7) | 1 (3.1) | .872 | .397 | .337 |
| Past smoker | 12 (30) | 5 (38.5) | 7 (21.9) | |||
| Alcohol, n (%) | ||||||
| Moderate consumption | 12 (30) | 5 (38.5) | 19 (59.4) | .595 | .014 | .175 |
| Consumption of >1 unit (males) or >1/2 unit (females) | 2 (5) | |||||
| History of allergies, n (%) | 4 (10) | 2 (15.4) | 3 (9.4) | .616 | .868 | .537 |
| Mini nutritional assessment-short form | ||||||
| Median (first–third quartile) | 11 (9–12) | 13 (12–14) | 14 (13–14) | <.001 | <.001 | .800 |
| Mini-mental state examination | ||||||
| Median (first–third quartile) | 26 (23–28) | 27 (26–28.5) | 30 (28–30) | .510 | <.001 | .002 |
| Modified Barthel index | ||||||
| Median(first–third quartile) | 65 (60–90) | 98 (90–100) | 100 (100–100) | <.001 | <.001 | <.001 |
| Instrumental activity of daily living | ||||||
| Median (first–third quartile) | 4 (3–5) | 7 (5–7) | 7 (6–8) | .010 | <.001 | .083 |
| Short physical performance battery | ||||||
| Median (first–third quartile) | 5 (3–6) | 7 (6.5–8.5) | 12 (10–12) | <.001 | <.001 | .001 |
| Charlson comorbidity index | ||||||
| Median (first–third quartile) | 2 (1–4) | 1 (0–1) | 0 (0–1) | .006 | <.001 | .080 |
| Number of drugs | ||||||
| Median (first–third quartile) | 7 (5–9) | 4 (3–7) | 3 (1–3.5) | .199 | <.001 | .004 |
Note: Values are expressed as mean ± SD, unless otherwise specified.
*Comparison between pre-frail vs. frail.
†Comparison between non-frail vs. frail.
‡Comparison between non-frail vs. pre-frail.
As expected, age was higher in frail and pre-frail adults in comparison to non-frail older adults. Furthermore, frail and pre-frail subjects had lower scores at the MNA-sf, were more impaired in cognitive and functional status, and had a higher CCI score. Lastly, non-frail subjects took a lower number of drugs when compared with both frail and pre-frail subjects. The distribution of chronic diseases (most of them used to calculate the CCI score) and the classes of drugs taken in the frail, pre-frail, and non-frail groups are described in Supplementary Table 2.
Hematological Data of Study Population
The hematological parameters were obtained on fresh blood of the 85 older and 46 younger adults (Supplementary Table 1). There were no differences in all variables considered, with two exceptions. The hemoglobin levels were lower in frail older adults (11.0 ± 1.6 g/dL) than in pre-frail (12.9 ± 1.3 g/dL), non-frail (14.1 ± 1.0 g/dL), and young (14.7 ± 1.3 g/dL) adults. The lymphocyte counts were lower in frail subjects. In a set of randomly chosen frail (5 samples), non-frail (4 samples), and young (5 samples) subjects, we analyzed the cHPSC (Lin1-/CD45+/CD34+) present in the gate of mononucleated cells (Supplementary Figure 2). The results showed a percentage of cHPSC in the range of 0.040–0.071 (Supplementary Table 1).
In all 85 older adults and 46 young adults (Table 2), we performed the separation of PBMC by Ficoll-Paque sedimentation, with a yield of around 40%. By FACS analysis, using the gating strategy shown in Supplementary Figure 1, we evaluated the PBMC, T lymphocytes as CD3+ cells, monocytes as CD14+ cells, CD3−/CD14− cells considered B-lymphocytes for the majority and cHPSC as CD34+/CD3−/CD14− cells (Table 2). In the Ficoll-Paque isolated PBMC, the ratio between the T and B lymphocytes was maintained in favor of T lymphocytes. There was a slight reduction of monocytes in the separated PBMC (Table 2) compared with monocytes of the fresh whole blood (Supplementary Table 1), probably due to their typical stickiness. In any case, there were no significant differences in the monocyte counts of the different subject groups but only a trend toward higher counts was found in frail patients. The percentage of the cHPSC CD34+/CD3−/CD14− subpopulation was around 0.068–0.099 (Table 2). This percentage is close to the one seen in the fresh whole blood, in which the more restricted Lin1-/CD45+/CD34+ phenotype has been evaluated (Supplementary Table 1).
Table 2.
Ficoll-Paque Isolated PBMC and the Respective Mononucleated Cell Subpopulations Analyzed by FACS With the Gating Strategy Shown in Supplementary Figure 1
| Frail | Pre-Frail | Non-Frail | Young | |
|---|---|---|---|---|
| N = 40 | N = 13 | n=32 | n=46 | |
| PBMC | ||||
| n* | 800 503 ± 784 393 | 942 967 ± 763 245 | 846 580 ± 696 317 | 1 068 624 ± 766 984 |
| 474 416 | 812 920 | 595 650 | 807 084 | |
| (205 072–1 168 448) | (343 009–1 315 235) | (286 251–1 321 378) | (400 997–1 856 148) | |
| T cells CD3+ | ||||
| % | 57.4 ± 14.1 | 59.2 ± 17.7 | 59.1 ± 9.5 | 62.2 ± 12.0 |
| 58.6 | 66.2 | 56.6 | 62.6 | |
| (50–69) | (49–72) | (52–67) | (56–70) | |
| Cells CD3−/CD14− | ||||
| % | 33.8 ± 12.0 | 32.2 ± 17.8 | 33.2 ± 9.7 | 31.1 ± 12.0 |
| 33.5 | 24.0 | 34.7 | 29.4 | |
| (24–40) | (17–40) | (25–39) | (23–35) | |
| Monocytes CD14+ | ||||
| % | 7.0 ± 4.6 | 5.9 ± 3.5 | 6.3 ± 4.3 | 5.5 ± 3.5 |
| 6.6 | 5.5 | 5.0 | 4.5 | |
| (4–8) | (7–3) | (3–7) | (3–7) | |
| cHPSC CD3−/CD14−/CD34+ | ||||
| % | 0.076 ± 0.070 | 0.099 ± 0.068 | 0.068 ± 0.040 | 0.081 ± 0.072 |
| 0.062 | 0.092 | 0.05 | 0.07 | |
| (0.06–0.09) | (0.03–0.14) | (0.04–0.09) | (0.03–0.10) |
Notes: PBMC = peripheral blood mononuclear cell. Samples obtained from 85 older adults divided according to the frail, pre-frail, non-frail status and from a group of 46 young subjects. Values are expressed as mean ± SD and median (first–third quartile). Statistical analysis was not significant among all groups.
*PBMC were analyzed with the non-parametric Wilcoxon–Mann–Whitney test.
In conclusion, the separation by Ficoll-Paque maintained the reciprocal ratios among the different cell populations in the different groups.
DNA Damage in PBMC and in the Respective Cell Subpopulations
We detected the percentage of cells positive for γ-H2AX foci in the frail, pre-frail, non-frail older adults and young individuals, assessing the DNA damage in the Ficoll-Paque isolated PBMC (Figure 1). We evaluated by FACS the C-terminal phosphorylation of H2AX histone at serine 139 (γ-H2AX), the early event in response of DSB, using the gating strategy shown in Supplementary Figure 1. Our data showed an evident significant increase of cells positive for γ-H2AX in the PBMC, cHPSC, and T cells of the frail patients (Figure 1). The difference between frail and pre-frail groups was significant only in cHPSC and not in PBMC and CD3+ T cells. The percentage of γ-H2AX+ cells in CD14+ monocytes increased in the frail group without a statistical significance compared with all the other groups (Figure 1). However, this finding on monocytes was also strengthened by the non-significant differences in the number of monocytes in the PBMC of all studied groups, after Ficoll-Paque sedimentation (Table 2). In monocytes, the DNA damage in pre-frail and non-frail subjects had the same level as the young individuals. The differences of age and gender, already observed among our older adult groups at recruitment, could be potential confounders for the differences observed in DNA damage. For this reason, at the initial evaluation of our data, we analyzed the frail, pre-frail, non-frail people also stratifying them according to their age class and gender. We did not have evidence that these variables had a specific influence on DNA damage, but the number of cases was low in such stratified groups. Therefore, we analyzed the groups of our older adults without additional stratification, even to achieve a more solid statistical evaluation.
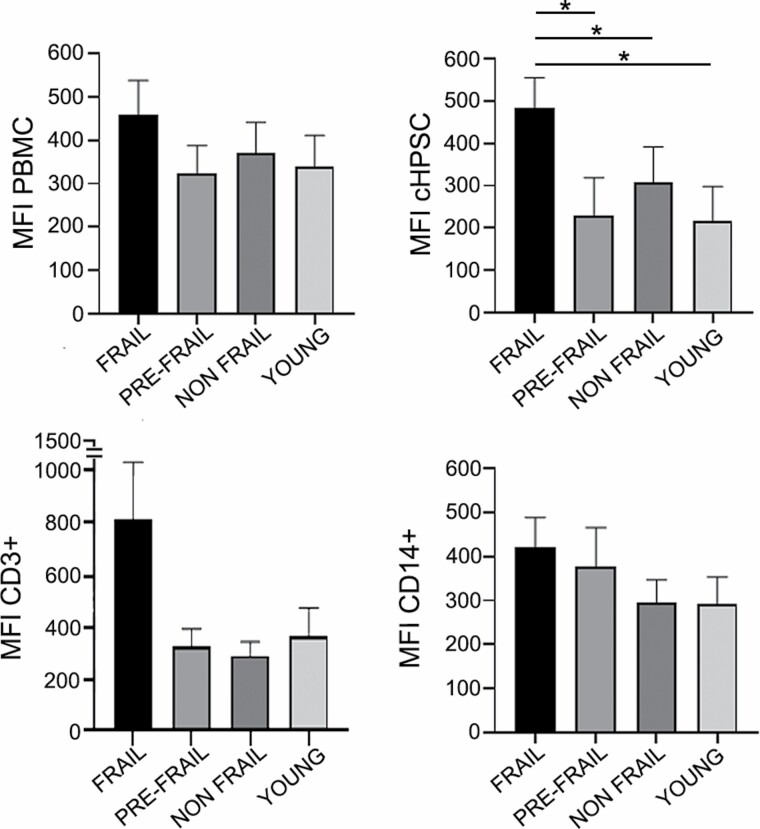
We also evaluated the mean fluorescence intensity (MFI) assessing the amount of DNA damage foci per cell in each specific cell population of the different subject groups (Figure 2). The quantification of the MFI provided a distinct and additional parameter to the percent of cells positive for DNA damage. In PBMC, CD3+, and CD14+ cells, we observed higher MFI in frail subjects compared with other groups, although nonsignificantly different (Figure 2). Instead, the MFI significantly increased in cHPSCs γ-H2AX+ of frail subjects compared with both pre-frail and non-frail groups and to young individuals (Figure 2).
Figure 2.
Mean fluorescence intensity (MFI) of DNA damage in peripheral blood mononuclear cell (PBMC) isolated by Ficoll-Paque and in the respective cell subpopulations: cHPSC CD34+/CD3-/CD14-, T lymphocytes CD3+, monocytes CD14+. Histograms represent the averaged median values of MFI in the different study groups (40 frail, 13 pre-frail, 32 non-frail, 46 young samples). Data are expressed as means ± SEM. *p < .05.
The ROC curve analysis (Supplementary Figure 3), assessing the percentage of DNA damaged cHPSC in our frail and non-frail subjects, showed that the area under the curve (AUC) was 0.835 (95% C.I. 0.740–0.931). This suggested a good discriminating power of the percentage of DNA damaged cHPSC to potentially discriminate between frail and non-frail subjects. The Youden Index that determines the optimal cutoff value was 0.611. The comparison between frail and pre-frail subjects gave an AUC of 0.780 (95% CI 0.640–0.920) with a Youden Index of 0.548.
These data showed that cHPSC of the frail group had both a higher percentage of cells with DNA damage and an increased MFI, as an indicator of the median number of γ-H2AX foci per cell, compared with the other groups.
Increment of Oxidative Stress in Frail Population
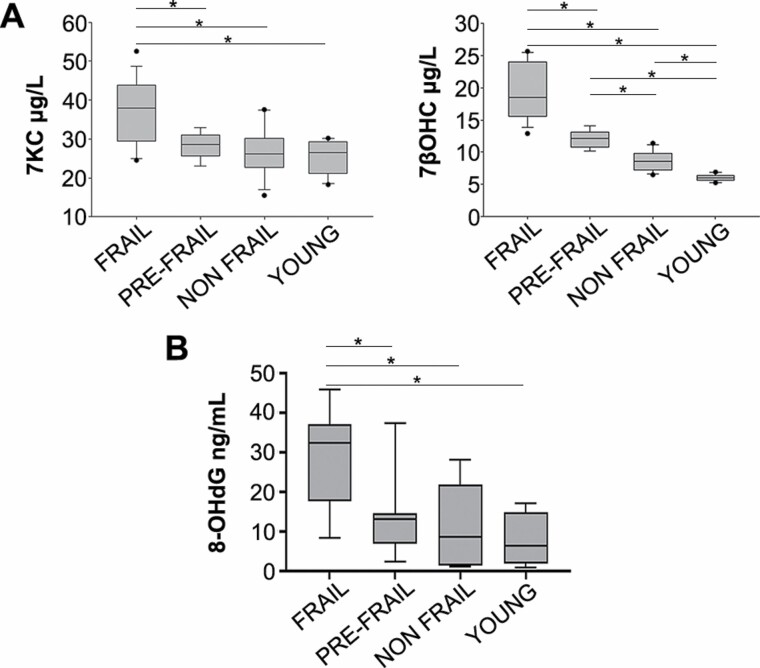
We evaluated the oxidative status in a set of plasma of our subjects to verify whether in the frail subjects an increase of oxidative stress was detectable. We observed significant increments of auto-oxidation of oxysterols 7KC and 7βOHC, markers of oxidative stress (29–31) in the frail plasma with respect to pre-frail and non-frail plasma (Figure 3A). Even the oxysterols 5α6αEC, 3β5α6β-3OHC, and 5β6βEC significantly increased in plasma of frail subjects (Supplementary Figure 4). These findings were suggestive for increased oxidative stress in frail compared with pre-frail, non-frail, and young individuals.
Figure 3.
Representation of oxidative stress evaluated in plasma. (A) Level of 7-keto-cholesterol (7KC, left) and 7β-hydroxycholesterol (7βOHC, right) in 17 frail, 9 pre-frail, 18 non-frail, 10 young samples. (B) Level of 8-hydroxy-2-deoxy Guanosine (8-OH-dG) in 10 frail, 9 pre-frail, 10 non-frail, 10 young samples. *p < .01. Data are expressed as means ± SD.
To reinforce the hypothesis that the DNA damage found in the hematopoietic cells had an oxidative origin, we also evaluated in a different set of plasma the 8-OH-dG, which is produced by the oxidative damage of DNA (32). We observed a significant increase of 8-OH-dG in frail compared with pre-frail, non-frail, and young subjects (Figure 3B) as with 7KC and 7βOHC.
Discussion
The daily intake of drugs, malnutrition, together with comorbidity, are significantly represented in our frail patients and these may contribute to the shift to a higher level of their oxidative metabolism. In fact, the oxysterols analysis evidenced increased oxidative stress in frail individuals. We found that the increased oxidative status and the increased 8-OH-dG matched with DNA damage in the hematopoietic cells of frail patients. We observed a trend of an increased percentage of PBMC, CD34+ cHPSC, and CD3+ T lymphocytes with DNA damage in non-frail older adults compared with young controls. This finding was in accordance with the literature (33,34) and can be considered as an event associated with aging. However, our work also showed a significant percentage increment of PBMC, CD34+ cHPSC, and CD3+ T cells with DNA damage in frail in comparison to non-frail individuals. In case of cHPSC, this significant increment was evident even in comparison to the pre-frail group. Instead, the CD14+ monocytes in the frail group showed only a trend to the increment of DNA damage compared with the other older adult groups. It was similarly very interesting that the number of DNA damage foci per cell, evidenced by the MFI, was significantly higher in the CD34+ cHPSC of frail patients compared with pre-frail, non-frail, and young subjects. In PBMC, CD3+ T lymphocytes, and CD14+ monocytes, the MFI value was higher in the frail group but not significantly. It has to be noted that T lymphocytes are considered long-lived and quiescent cells (35) and their high DNA damage level may represent the accumulation of DNA damage foci that occurred over the lifespan. Instead, the monocytes are typically proliferating short-lived cells (36,37) and DNA damage foci occur in these cells that die precociously. Likely, monocytes do not have time to accumulate foci during lifespan, except in the frail period in which the risk for DNA damage increases.
In aging, genomic instability has raised a great and persistent interest and it has also been recognized as a determinant of age-related stem cell decline that accelerates age-related pathologies in several tissues and organs (38,39). It is known that genomic alterations accumulate at a linear rate in human HPSC during life, similarly to adult stem cells of other tissues (33). It is also known that the long-lived CD34+ hematopoietic stem cells accumulate DNA damages, particularly during aging, when they come out of their dormant state by cell-intrinsic mechanisms (40) and by the shift of the basal metabolism to a higher level of oxidative metabolism (41,42). Both, the loss of dormant status and the increment of ROS, may have a direct consequence in the progressive increase of γ-H2AX leading toward frailty status. It has to be mentioned that, in mice, part of the γ-H2AX foci found in aged hematopoietic stem cells can be due to ineffective dephosphorylation rather than persistent DNA damage. In any case, this situation pushes the cells to transcriptional silencing and functional decline (43).
In conclusion, in our frail population, the clinical characteristics (age, malnutrition, and drug intake) and comorbidity (diabetes mellitus, cardiovascular diseases, kidney diseases, and obstructive pulmonary diseases) causing oxidative stress can justify the high level of DNA damage in cHPSC. In addition, the oxidative DNA damage probably affects several other adult stem cells and the DNA damage, as a process interconnected with other biological processes, may induce an amplification of dangerous effects. Therefore, our findings suggest that, in the tissues of older adults, the occurrence of DNA damage accumulation, the DNA damage repair deficiency, as well as the cellular functional decline can limit the adult stem cell compartment functions (44). All these factors may be implicated in the decline of tissue renewal capacity, in the appearance of aging-related pathology (45) and in a more severe and progressive frailty.
Our study has potential implications: (i) the increment of DNA damage in cHPSC might be a suggestive signal of organism impairment that precedes the evident frailty in older people. The evaluation of cHPSC CD34+/γ-H2AX+ can be improved for its eventual clinical use. However, it is promising that the ROC curve analysis showed the possibility to distinguish the frail from non-frail and pre-frail subjects by the percentage of DNA-damaged cells in the hematopoietic adult stem cell compartment. (ii) Future studies could replicate or corroborate our findings, also recruiting individuals of similar age and gender and stratifying them according to the presence of frailty. In this case, it would be possible to hypothesize that progression of frailty may be attenuated through specific drugs that act on preventing DNA damage or on removing damaged cells. Indeed, drug therapies (eg, geroprotectors, senolytic drugs, and repurposed drugs) have been shown to attenuate frailty in preclinical and/or clinical studies (46).
Supplementary Material
Acknowledgments
We thank Prof. Cattoretti G. from the University of Milano-Bicocca for the helpful discussion.
Contributor Information
Chiara Grasselli, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Silvia Bombelli, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Stefano Eriani, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Giulia Domenici, Acute Geriatric Unit, San Gerardo Hospital, ASST-Monza, Monza, Italy.
Riccardo Galluccio, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Acute Geriatric Unit, San Gerardo Hospital, ASST-Monza, Monza, Italy.
Chiara Tropeano, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Laboratory of Clinical Chemistry, Hospital of Desio , ASST-Brianza, Desio, Italy.
Sofia De Marco, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Maddalena M Bolognesi, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Barbara Torsello, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Cristina Bianchi, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Laura Antolini, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Fabio Rossi, Immunotransfusional Unit, San Gerardo Hospital, ASST-Monza, Monza, Italy.
Paolo Mazzola, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Acute Geriatric Unit, San Gerardo Hospital, ASST-Monza, Monza, Italy.
Valerio Leoni, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Laboratory of Clinical Chemistry, Hospital of Desio , ASST-Brianza, Desio, Italy.
Giuseppe Bellelli, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy; Acute Geriatric Unit, San Gerardo Hospital, ASST-Monza, Monza, Italy.
Roberto A Perego, School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
Funding
This work was supported by Fondazione Cariplo, Milano (Italy) (grant 2017-0577 to R.A.P.).
Conflict of Interest
None declared.
References
- 1. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394:1376–1386. doi: 10.1016/S0140-6736(19)31785-4 [DOI] [PubMed] [Google Scholar]
- 2. Vermeiren S, Vella-Azzopardi R, Beckwee D, et al. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17:1163.e1–1163.e17. doi: 10.1016/j.jamda.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 3. Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology. 2009;55(5):539–549. doi: 10.1159/000211949 [DOI] [PubMed] [Google Scholar]
- 4. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 5. Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133(6):456–466. doi: 10.1016/j.mad.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 6. Mitnitski A, Collerton J, Martin-Ruiz C, et al. Age-related frailty and its association with biological markers of ageing. BMC Med. 2015;13:161. doi: 10.1186/s12916-015-0400-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sánchez-Flores M, Marcos-Pérez D, Costa S, et al. Oxidative stress, genomic features and DNA repair in frail elderly: a systematic review. Ageing Res Rev. 2017;37:1–15. doi: 10.1016/j.arr.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 8. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. doi: 10.1016/j.ejim.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 9. Rattan SIS. Increased molecular damage and heterogeneity as the basis of aging. Biol Chem. 2008;389(3):267–272. doi: 10.1515/BC.2008.030 [DOI] [PubMed] [Google Scholar]
- 10. Siddiqui MS, Francois M, Fenech MF, Leifert WR. Persistent gammaH2AX: a promising molecular marker of DNA damage and aging. Mutat Res Rev Mutat Res. 2015;766:1–19. doi: 10.1016/j.mrrev.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 11. Bouquet F, Muller C, Salles B. The loss of gammaH2AX signal is a marker of DNA double strand breaks repair only at low levels of DNA damage. Cell Cycle. 2006;5(10):1116–1122. doi: 10.4161/cc.5.10.2799 [DOI] [PubMed] [Google Scholar]
- 12. Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol. 2004;6(2):168–170. doi: 10.1038/ncb1095 [DOI] [PubMed] [Google Scholar]
- 13. Sedelnikova OA, Horikawa I, Redon C, et al. Delayed kinetics of DNA double-strand break processing in normal and pathological aging. Aging Cell. 2008;7:89–100. doi: 10.1111/j.1474-9726.2007.00354.x [DOI] [PubMed] [Google Scholar]
- 14. Sharma A, Singh K, Almasan A. Histone H2AX phosphorylation: a marker for DNA damage. Methods Mol Biol. 2012;920:613–626. doi: 10.1007/978-1-61779-998-3_40 [DOI] [PubMed] [Google Scholar]
- 15. Teixeira-Gomes A, Lage B, Esteves F, et al. Frailty syndrome, biomarkers and environmental factors – a pilot study. Toxicol Lett. 2020;330:14–22. doi: 10.1016/j.toxlet.2020.04.023 [DOI] [PubMed] [Google Scholar]
- 16. Valdiglesias V, Sánchez-Flores M, Marcos-Pérez D, et al. Exploring genetic outcomes as frailty biomarkers. J Gerontol A Biol Sci Med Sci. 2019;74(2):168–175. doi: 10.1093/gerona/gly085 [DOI] [PubMed] [Google Scholar]
- 17. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 18. Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The Physical Activity Scale for the Elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52(7):643–651. doi: 10.1016/s0895-4356(99)00049-9 [DOI] [PubMed] [Google Scholar]
- 19. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaiser MJ, Bauer JM, Ramsch C, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. Nov 2009;13(9):782–788. doi: 10.1007/s12603-009-0214-7 [DOI] [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 22. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 23. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. doi: 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 24. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 25. Beaton LA, Marro L, Malone S, et al. Investigating γ H2AX as a biomarker of radiosensitivity using flow cytometry methods. ISRN Radiol. 2013;2013:704659. doi: 10.5402/2013/704659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johansson P, Fasth A, Ek T, Hammarsten O. Validation of a flow cytometry-based detection of g-H2AX, to measure DNA damage for clinical applications. Cytometry B. 2017;92B:534–540. doi: 10.1002/cyto.b.21374 [DOI] [PubMed] [Google Scholar]
- 27. Porcedda P, Turinetto V, Brusco A, et al. A rapid flow cytometry test based on histone H2AX phosphorylation for the sensitive and specific diagnosis of ataxia telangiectasia. Cytometry A. 2008;73A:508–516. doi: 10.1002/cyto.a.20566 [DOI] [PubMed] [Google Scholar]
- 28. Marcello A, Civra A, Bonotto R, et al. The cholesterol metabolite 27-hydroxycholesterol inhibits SARS-CoV-2 and is markedly decreased in COVID-19 patients. Redox Biol. 2020;36:101682. doi: 10.1016/j.redox.2020.101682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown AJ, Jessup W. Oxysterols: sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol Aspects Med. 2009;30(3):111–122. doi: 10.1016/j.mam.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 30. Iuliano L. Pathways of cholesterol oxidation via non-enzymatic mechanisms. Chem Phys Lipids. 2011;164(6):457–468. doi: 10.1016/j.chemphyslip.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 31. Kulig W, Cwiklik L, Jurkiewicz P, Rog T, Vattulainen I. Cholesterol oxidation products and their biological importance. Chem Phys Lipids. 2016;199:144–160. doi: 10.1016/j.chemphyslip.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 32. Diaz-De la Cruz EN, Cerillos-Gutiérrez JI, Garcìa-Sànchez A, et al. The alteration of pro-inflammatory cytokines and oxidative stress markers at six-month post-living kidney donation. Front Med. 2020;7:382. doi: 10.3389/fmed.2020.00382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Osorio FG, Rosendahl Huber A, Oka R, et al. Somatic mutations reveal lineage relationships and age-related mutagenesis in human hematopoiesis. Cell Rep. 2018;25(9):2308.e4–2316.e4. doi: 10.1016/j.celrep.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moehrle BM, Geiger H. Aging of hematopoietic stem cells: DNA damage and mutations? Exp Hematol. 2016;44(10):895–901. doi: 10.1016/j.exphem.2016.06.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Di Rosa F. Two niches in the bone marrow: a hypothesis on life-long T cell memory. Trends Immunol. 2016;37:503–512. doi: 10.1016/j.it.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 36. Patel AA, Zhang Y, Fullerton JN, et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med. 2017;214(7):1913–1923. doi: 10.1084/jem.20170355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yona S, Kim KW, Wolf Y, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park Y, Gerson ST. DNA repair defects in stem cell function and aging. Annu Rev Med. 2005;56:495–508. doi: 10.1146/annurev.med.56.082103.104546 [DOI] [PubMed] [Google Scholar]
- 40. Pang WW, Price EA, Sahoo D, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci USA. 2011;108(50):20012–20017. doi: 10.1073/pnas.1116110108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Verovskaya EV, Dellorusso PV, Passegué E. Losing sense of self and surroundings: hematopoietic stem cell aging and leukemic transformation. Trends Mol Med. 2019;25(6):494–515. doi: 10.1016/j.molmed.2019.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bigarella CL, Liang R, Ghaffari S. Stem cells and the impact of ROS signaling. Development. 2014;141(22):4206–4218. doi: 10.1242/dev.107086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Flach J, Bakker ST, Mohrin M, et al. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature. 2014;512:198–202. doi: 10.1038/nature13619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mani C, Reddy PH, Palle K. DNA repair fidelity in stem cell maintenance, health, and disease. Biochim Biophys Acta Mol Basis Dis. 2020;1866(4):165444. doi: 10.1016/j.bbadis.2019.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rossi JD, Bryder D, Nussenzweig A, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of hematopoietic stem cells with age. Nature. 2007;447(7145):725–729. doi: 10.1038/nature05862 [DOI] [PubMed] [Google Scholar]
- 46. Rockwood K, Howlett SE. Age-related deficit accumulation and the diseases of ageing. Mech Ageing Dev. 2019;180:107–116. doi: 10.1016/j.mad.2019.04.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.