Abstract
Purpose:
Aberrant biomechanics and altered loading frequency are associated with poor knee joint health in osteoarthritis development. After ACLR, individuals demonstrate underloading (lesser vertical ground reaction force [(vGRF]) with stiffened knee gait biomechanics (lesser knee extension moment [KEM] and knee flexion angle) and take fewer daily steps as early as 6 months post-surgery. The purpose of this cross-sectional laboratory study is to compare gait biomechanics throughout stance between individuals 6-12 months post-ACLR who take the lowest, moderate, and highest daily steps.
Methods:
Individuals with primary, unilateral history of ACLR between the ages of 16-35 were included (n=36, 47% females, age=21±5 years, months since ACLR=8±2). Barefoot gait biomechanics of vGRF (body weight [BW]), KEM (BW x height), and knee flexion angle during stance were collected and time-normalized. Average daily steps were collected via a waist-mounted accelerometer in free-living settings over 7 days. Participants were separated into tertiles based on lowest daily steps (3,326-6,042 daily steps), moderate (6,043-8,198 daily steps), and highest (8,199-12,680 daily steps). Biomechanical outcomes of the ACLR limb during stance were compared between daily step groups using functional waveform gait analyses.
Results:
There were no significant differences in sex, body mass index, age, or gait speed between daily step groups. Individuals with the lowest daily steps walk with lesser vGRF and lesser KEM during weight acceptance, and lesser knee flexion angle throughout stance in the ACLR limb compared to individuals with highest and moderate daily steps.
Conclusions:
After ACLR, individuals who take the fewest daily steps also walk with lesser vGRF during weight acceptance and a stiffened knee strategy throughout stance. These results highlight complex interactions between joint loading parameters post-ACLR.
Keywords: mechanical loading, vertical ground reaction force, osteoarthritis, physical activity
INTRODUCTION
Individuals with a history of anterior cruciate ligament (ACL) injury and surgical reconstruction (ACLR) are 4-6 times more likely to develop radiographic posttraumatic osteoarthritis (PTOA) compared to uninjured individuals (1). Previous literature supports a link between aberrant mechanical loading and PTOA development (2–4). In animal studies, both excessive and insufficient mechanical loading lead to deleterious changes to joint tissue metabolism (5, 6), decreased articular cartilage proteoglycan and aggrecan content (7), and eventual articular cartilage thinning (8–10). Maintaining optimal mechanical joint loading following ACLR is likely critical for delaying or preventing PTOA onset by stimulating chondroprotective pathways that promote cartilage health (5). Multiple parameters must be addresed to optimize joint loading including lower extremity kinematics and kinetics (i.e., gait biomechanics) and the frequency with which loads are applied to an injured extremity (i.e., daily steps). Previous studies have assessed the effects of ACL injury and ACLR on changes in gait biomechanics and daily steps separately (11–13), but the interaction between these loading parameters remains understudied in individuals with ACLR.
Individuals within the first 6-12 months following ACLR demonstrate less dynamic vertical ground reaction force (vGRF) waveforms throughout the stance phase of gait characterized by a lesser peak vGRF during weight acceptance and greater vGRF during midstance compared to uninjured matched controls (11). Less dynamic vGRF waveforms are also associated with a stiffened knee strategy characterized by smaller knee flexion excursion from weight acceptance to midstance and a smaller peak internal knee extension moment (KEM) (11). These aberrant gait biomechanics of vGRF, KEM, and knee flexion excursion have been linked to deleterious joint tissue metabolism (14, 15), altered femoral cartilage composition (16, 17), and worse patient-reported outcomes (18, 19) associated with early PTOA development. Together, these studies suggest that gait strategies which incorportate a less dynamic vGRF waveform and stiffened knee strategy are associated with poor joint health.
Another parameter of loading associated with joint health is loading frequency. Loading frequency is typically measured over an extended period of time (e.g., 1 day or 1 week) via daily steps to reflect typical behavior (20). In individuals at risk for or living with idiopathic OA, those who complete fewer daily steps demonstrate worsening tibiofemoral cartilage degeneration (21) and greater risk of disability (22). Pre-operatively, patients with ACL injury who walk with a stiffened knee strategy and engage in greater daily steps demonstrate worse tibiofemoral cartilage composition (23). However, the influence of daily steps on indicators of knee joint health after ACLR surgery is unclear. Unfortunately, individuals post-ACLR demonstrate fewer daily steps as early as 6 months after surgery compared to uninjured controls (24). This behavior may persist for years post-ACLR (13) and may accelerate poor knee joint health development similar to individuals living with idiopathic OA. Furthermore, substantially different joint loading environments may develop if individuals with aberrant gait biomechanics also engage in significantly fewer daily steps, but it is unclear if individuals with ACLR who demonstrate aberrant gait biomechanics also engage in fewer daily steps.
Examining the extent to which aberrant gait biomechanics and altered daily steps relate is a critical step towards developing comprehensive rehabilitative strategies to optimize joint loading post-ACLR. Therefore, the purpose of this study was to compare gait biomechanics (i.e. vGRF, KEM, knee flexion angle) throughout stance between those with the lowest, moderate, and highest loading frequency (i.e., daily steps) in individuals 6-12 months post-ACLR. We hypothesized that individuals with the lowest number of daily steps would demonstrate less vGRF in weight acceptance and greater vGRF through midstance (i.e. less dynamic vGRF waveform throughout stance), less knee flexion angle through midstance, and less KEM in weight acceptance in the ACLR limb during gait compared to individuals with moderate and highest daily steps.
METHODS
We conducted a cross-sectional observational study in individuals who underwent primary ACLR 6 to 12 months prior to enrollment. Daily steps were collected in free-living settings during all waking hours for 7 days using a waist-mounted triaxial Actigraph GT9X Link monitor (version 6.13.4, Actigraph LLC., Pensacola, FL). Participants also completed a gait analysis during a single laboratory session during which vGRF, KEM, and knee flexion angle were evaluated. All participants provided written informed consent or assent and permission from their parent or guardian before participating in the study as approved by the university’s Institutional Review Board.
Participants.
Participants (n=36) were recruited from a local orthopedic clinic, the university campus, and the local community through referral, flyers, and emails. Participants between the ages of 16 and 35 years who underwent primary unilateral ACLR with an autograft (bone-patellar tendon-bone: n=32, hamstring tendon: n=2, quadriceps tendon: n=2) 6 to 12 months before enrollment and had completed formalized rehabilitation were included. We included participants who had a meniscal injury or meniscal procedure at the time of ACL injury or surgery. Participants were excluded from the study if they had history of a lower extremity fracture at the time of ACL injury, multiple ligament surgery at time of ACLR, ACLR revision surgery, or clinical diagnosis of knee PTOA in either limb. Participants completed the Knee Injury and Osteoarthritis Outcome Score (KOOS) survey to determine the participant’s self-reported knee related-symptoms, pain, daily function, sport participation, and quality of life (25).
A previous study using functional waveform gait analyses reported that individuals less than 12 months post-ACLR demonstrated a moderate effect (d=0.60) for peak vGRF mean differences during the weight acceptance phase of stance between those who are symptomatic and asymptomatic (19). We determined that daily step tertile groups of 10 participants (total cohort = 30) were needed to detect a moderate effect for statistically significant differences in gait outcomes with the assumption that variability between trials is similar to the previous study (two-tailed alpha, 1-ß=0.8, α=0.05) (19). Sample size estimations were calculated using G*Power Statistics Power Analysis Software (version 3.1, Heinrich-Heine-Universität, Düsseldorf, Germany).
Daily Step Assessment.
Participants were instructed to wear a triaxial Actigraph GT9X Link monitor (version 6.13.4, Actigraph LLC., Pensacola, FL) on their right hip during all waking hours for 7 days. Data were collected at 30 Hz and processed at 60 second epochs. Wear and non-wear time were defined using the Choi et al. algorithm (26). Briefly, non-wear time was defined as any period of zero count epochs that exceeded 90 minutes. Spike tolerance of nonzero count epochs less than two consecutive minutes in duration with at least 30 minutes of zero count epochs before and after each spike were also considered non-wear time. Valid wear time over the 7-day monitoring period was defined as 4 days with at least 10 hours of wear time and at least one weekend day. Steps were calculated using Actilife’s step detection algorithm with a proprietary acceleration amplitude threshold based on uniaxial acceleration data in Actilife software (version 6.13.4, Actigraph LLC., Pensacola, FL) (27). Loading frequency was defined as the average daily steps over the monitor wear period. Participants were retrospectively assigned to groups based on tertile ranking of daily steps (i.e., lowest, moderate, and highest daily step groups).
Gait Biomechanical Assessment.
Participants were outfitted with 26 retroreflective markers and 1 sacral cluster of 3 markers. A 10-camera three-dimensional motion capture system (Vicon, Nexus, Denver, Colorado) and two embedded force plates (FP406010, Bertec Corporation, Columbus, Ohio) staggered within a 6-meter walkway were used to collect marker trajectories and kinetics at 120 and 1200 Hz, respectively. Participants were asked to walk barefoot at their habitual walking speed as if they were “comfortably walking on a sidewalk” over the 6 meter track between infrared timing gates (TF100, Trac Tronix, Lenexa, Kansas) spaced 0.97 meters a part (28). Practice trials were performed until the participant felt comfortable completing the task. Next, habitual walking speed was calculated as the average walking speed from five trials which was used to ensure that participants walked at a consistent gait speed for testing trials. Gait biomechanics were collected from five successful testing trials and used for final analysis. A trial was considered unsuccessful and repeated if participants exceeded ±5% of average habitual walking speed, demonstrated visually abnormal gait deviations (i.e., studder step), or both feet failed to make full contact with the staggered force plates during a single trial (11).
Visual3D software (C-Motion, Germantown, Maryland) was used to process kinematic and kinetic data using a fourth-order Butterworth filter at a cut-off of 10 Hz. The stance phase of gait was identified from heel strike (vGRF > 20 N) to toe-off (vGRF < 20 N). All biomechanical outcomes were time normalized to 101 data points (0-100%) throughout the stance phase. Gait stance phases were defined as three phases including weight acceptance (0-24%), midstance (25-62%), and late stance (63-100%) (29). Knee and ankle joint centers were estimated as half of the distance between the medial and lateral femoral epicondyles and malleoli retroreflective markers, respectively. Hip joint centers were calculated using the Bell method (30). Euler angles were used to calculate sagittal plane knee flexion based on the position of the shank relative to the thigh. An inverse dynamics approach was used to calculate internal joint moments based on anthropometrics, kinetics, and kinematics. vGRF was normalized to body weight (N) and KEM was normalized to the product of bodyweight (N) and height (m). Knee flexion angles and KEM were reported as positive values.
Statistical Analysis.
Descriptive statistics (means, standard deviations, or percentages) were calculated for participant characteristics in all study participants and for participants in each daily step group (i.e., lowest, moderate, and highest daily step groups). Age, body mass index (BMI), gait speed, daily steps, monitor wear time, and months since ACLR were compared across groups using one-way ANOVAs. If participant characteristics were significantly different, Bonferroni post-hoc tests were used to determine specific differences between groups. The percentage of women and participants with meniscal injury in each group were compared using the Fisher’s exact test. Analyses of descriptive statistics were performed using SPSS Statistics (version 26, IBM Corp, Armonk, NY).
Functional Waveform Gait Analysis.
Functional waveform analyses were conducted to allow for the evaluation of gait differences or relationships at multiple portions of stance using a single analysis (11, 31, 32). Previous studies have demonstrated that individuals post-ACLR demonstrate differences in gait biomechanics compared to uninjured controls over multiple portions of stance (11). Therefore, separate functional waveform gait analyses were conducted to compare ACLR limb vGRF, KEM, and knee flexion angle between: 1) highest and lowest daily step groups, 2) highest and moderate daily step groups, and 3) moderate and lowest daily step groups. Between group comparisons were analyzed across time-normalized percentiles of gait stance (0 to 100%). First, Bayesian functional models were fit to participants’ time-normalized gait waveforms using a P-spline model to form a representative waveform for each group (33). Next, differences between waveforms for each group and associated 95% confidence intervals were calculated. We considered between-group waveform differences to exist at proportions of stance where associated 95% confidence intervals did not include zero for greater than 3% of stance. All functional analyses were performed in R version 3.5 using the package bayesFDA which were modified from the package described in Horton et al. (32). Cohen’s d effect sizes and 95% confidence intervals were also calculated at portions of stance with the largest group differences to determine the magnitude of gait biomechanical differences between groups (see Table, Supplemental Digital Content 1, SDC 1). A post-hoc supplementary analysis using the same functional waveform analysis was completed to compare uninjured limb vGRF, KEM, and knee flexion angle between daily step groups.
RESULTS
Participant characteristics for the entire cohort and each daily step group are reported in Table 1. Daily steps were significantly different between daily step groups (all p<0.001), but there were no significant differences between groups for any other characteristic (Table 1).
Table 1.
Participant characteristics of each daily step group
| Participant Characteristics | All Participants (n=36) | Lowest Daily Step Group (n=12) | Moderate Daily Step Group (n=12) | Highest Daily Step Group (n=12) | P-value |
|---|---|---|---|---|---|
| Sex (% Females) | 47% | 50% | 50% | 42% | 0.86 |
| Age (years) | 21.4 ± 4.6 | 21.6 ± 4.8 | 23.5 ± 5.5 | 19.1 ± 2.2 | 0.06 |
| BMI (kg/m^2) | 24.3 ± 6.8 | 24.0 ± 2.7 | 24.8 ± 3.5 | 24.2 ± 2.3 | 0.78 |
| Gait Speed (m/s) | 1.23 ± 0.12 | 1.23 ± 0.14 | 1.26 ± 0.12 | 1.21 ± 0.10 | 0.56 |
| Daily Steps | 7283 ± 2294 | 4970 ± 852†Ŧ | 7030 ± 648§ | 9851 ± 1582 | <0.001* |
| Monitor Wear Time (Min.) | 4784.9 ± 804.6 | 4424.3 ± 780.4 | 5000.9 ± 906.1 | 4929.6 ± 643.8 | 0.16 |
| Months Since ACLR | 8.3 ± 1.7 | 8.6 ± 2.0 | 8.5 ± 1.7 | 7.7 ± 1.3 | 0.41 |
| Meniscal Injury (%) | 65% | 67% | 67% | 50% | 0.41 |
| Graft Type (PT/HT/QT) | 32/2/2 | 12/0/0 | 10/2/0 | 10/0/2 | 0.08 |
| KOOS Symptoms | 81.4 ± 11.4 | 84.2 ± 10.8 | 75.3 ± 9.3 | 84.8 ± 12.1 | 0.07 |
| KOOS Pain | 88.3 ± 7.3 | 87.9 ± 6.7 | 88.9 ± 5.3 | 88.0 ± 9.9 | 0.94 |
| KOOS ADL | 95.3 ± 5.8 | 95.3 ± 8.1 | 95.7 ± 2.9 | 94.7 ± 5.6 | 0.92 |
| KOOS Sport | 71.8 ± 18.5 | 71.7 ± 20.6 | 74.6 ± 18.8 | 69.2 ± 17.2 | 0.78 |
| KOOS QOL | 60.9 ± 17.2 | 63.5 ± 17.0 | 55.2 ± 18.8 | 60.9 ± 17.2 | 0.38 |
= significant difference between daily step groups using one-way ANOVA
= significant difference between lowest and moderate daily step group using Bonferroni post-hoc test
= significant difference between lowest and highest daily step group using Bonferroni post-hoc test
= significant difference between moderate and highest daily step group using Bonferroni post-hoc test
ACLR Limb Gait Biomechanics.
ACLR limb gait waveforms of vGRF, KEM, and knee flexion angle during stance for each daily step group are reported in Figure 1A, 2A, and 3A.
Figure 1.
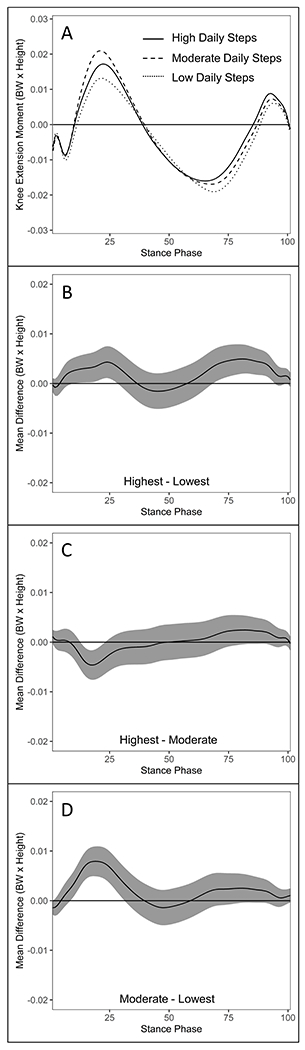
Comparisons of vertical ground reaction force ([vGRF] body weight) waveforms during stance between daily step groups in the ACLR limb of individuals 6-12 months post-ACLR (A). vGRF differences and 95% confidence intervals throughout gait stance between the highest and lowest (B), highest and moderate (C), and moderate and lowest (D) daily step groups in the ACLR limb. Positive values indicate greater vGRF in the highest (B & C) and moderate (D) daily step groups, respectively.
Figure 2.
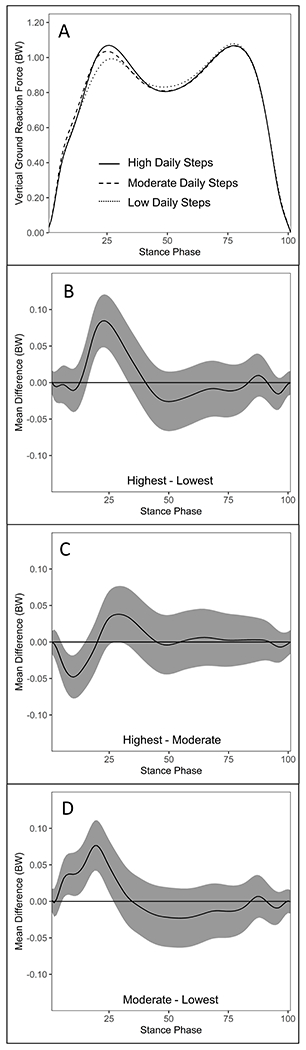
Comparisons of knee extension moment ([KEM] body weight x height) waveforms during stance between daily step groups in the ACLR limb of individuals 6-12 months post-ACLR (A). KEM differences and 95% confidence intervals throughout gait stance between the highest and lowest (B), highest and moderate (C), and moderate and lowest (D) daily step groups in the ACLR limb. Positive values indicate greater KEM in the highest (B & C) and moderate (D) daily step groups, respectively.
Figure 3.
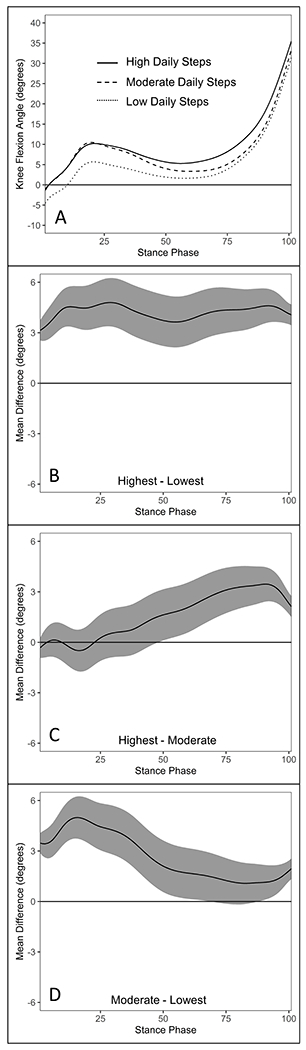
Comparisons of knee flexion angle (°) waveforms during stance between daily step groups in the ACLR limb of individuals 6-12 months post-ACLR (A). Knee flexion angle differences and 95% confidence intervals throughout gait stance between the highest and lowest (B), highest and moderate (C), and moderate and lowest (D) daily step groups in the ACLR limb. Positive values indicate greater knee flexion angle in the highest (B & C) and moderate (D) daily step groups, respectively.
vGRF.
The highest daily step group demonstrated greater vGRF compared to the lowest daily step group (15-32%) (Figure 1B), but lesser vGRF compared to the moderate daily step group during weight acceptance (4-13%) (Figure 1C). The moderate daily step group demonstrated greater vGRF during weight acceptance compared to the lowest daily step group (5-25%) (Figure 1D).
Knee Extension Moment.
The highest daily step group demonstrated greater KEM during weight acceptance (9-27%) and late stance (68-93%) compared to the lowest daily step group (Figure 2B), but lesser KEM during weight acceptance compared to the moderate daily step group (12-22%) (Figure 2C). The moderate daily step group demonstrated greater KEM during weight acceptance compared to the lowest daily step group (8-30%) (Figure 2D).
Knee Flexion Angle.
The highest daily step group demonstrated greater knee flexion angle throughout stance compared to the lowest daily step group (0-100%) (Figure 3B) as well as during mid- and late stance (48-100%) compared to the moderate daily step group (Figure 3C). The moderate daily step group demonstrated greater knee flexion angle throughout most of stance compared to the lowest daily step group (0-67% and 87-100%) (Figure 3D).
Uninjured Limb Gait Biomechanics.
While gait biomechanics of the uninjured limb were not part of the primary analysis, a post hoc supplementary analyses found that between-group differences for vGRF, KEM, and knee flexion angle were similar to those reported in the ACLR limb (see Table, Figures, Supplemental Data Files 1-4, SDC 1, SDC 2, SDC 3, SDC 4).
DISCUSSION
In support of our hypothesis, individuals engaging in the fewest daily steps demonstrated lesser vGRF during the weight acceptance phase of stance, lesser KEM during weight acceptance and late stance, and lesser knee flexion angle throughout all of stance during walking compared to individuals taking the highest daily steps. Our novel findings suggest that individuals who load the ACLR limb less frequently during the day (i.e. fewer daily steps) also exert lesser magnitudes of force (i.e. vGRF normalized to body weight) through the ACLR extremity with stiffer knee kinematics during each step. Contrary to our hypothesis, individuals engaging in the fewest daily steps did not demonstrate greater vGRF during midstance. The first vGRF impact peak during the weight acceptance phase of stance is typically characterized as a portion of stance when individuals must attenuate higher magnitudes of force (34). Adequate lower extremity muscle strength and neuromuscular control is needed to properly attenuate loads exerted on the lower extremity during gait (35). ACLR patients often demonstrate decreased lower extremity muscle strength (36) and aberrant neuromuscular control (37), which may limit the ability to control force across the joint. It is possible that with the inability to properly attenuate loading that patients attempt to minimize peak vGRF early after ACLR and thus develop aberrant gait biomechanics (38). If not addressed with rehabilitation, gait compensations used to initially attenuate load may persist as profiles characterized by underloaded, less dynamic vGRF waveforms and stiffened knee flexion angles (11). Similar between group differences were reported in the uninjured limb. These findings are not surprising because previous work has demonstrated that the uninjured limb may develop altered gait patterns that are similar to the ACLR limb (11). These data suggest that multiple loading parameters (daily steps and biomechanics) are simultaneously affected in some individuals following ACL injury and ACLR (2, 15, 16). Our study indicates that future efforts should be made to measure both gait biomechanics and loading frequency following ACLR in order to best understand the cumulative loading changes in specific patients.
While we did not directly compare gait biomechanics in our ACLR cohort to uninjured individuals in the current study, all group averages demonstrate lesser peak vGRF (uninjured controls=1.12±0.09 x BW), peak KEM (uninjured controls=0.03±0.01 BW x height), and peak knee flexion angle (uninjured controls= 11.6±5.2°) during the loading phase of gait compared to previously reported uninjured sex, age, and BMI matched control participants (see Table, Supplemental Data File 5, SDC 5) (11). Likewise, individuals in the lowest (4970±852 daily steps) and moderate daily step groups (7030±648 daily steps) took fewer daily steps than previously reported uninjured individuals (uninjured controls=9769±2785 daily steps) (12). Daily step groupings reported in this study should be interpretated with caution as thresholds of high moderate and low daily steps are specific to our particular cohort. Our results indicate that, in general, individuals who engage in low daily steps also demonstrate gait underloading 6-12 months post-ACLR. Future work is needed to determine if a specific range of daily steps would also predict specific changes in gait biomechanics. Underloading of the ACLR limb in the first months to years following ACL injury is hypothesized to result in insufficient loading of some regions of knee joint articular cartilage which may contribute to poor long-term knee health outcomes (2, 14, 16). Both underloading combined with a stiffened knee strategy during gait (i.e. lesser vGRF, KEM, and knee flexion angle) and lesser daily steps have been associated with deleterious aspects of knee joint health including poor joint metabolism, poor cartilage composition, and cartilage degeneration in individuals at risk for PTOA or idiopathic OA (2, 14, 16, 21). The aforementioned gait pattern may result in underloading in some cartilage regions, while reduced knee flexion range of motion may facilitate sustained contact force patterned across other cartilage regions. Future longitudinal research studies should evaluate the relative contributions of persistent aberrant loading parameters to poor knee joint health development and potential PTOA risk after ACLR.
Due to the cross-sectional nature of the study, we cannot determine cause and effect relationships between mechanical loading parameters. However, it is possible that changes in gait biomechanics and daily steps may influence each other. For example, individuals who take fewer daily steps between 6 to 12 months post-ACLR may inadvertently limit the amount of time they practice normalizing walking gait biomechanics preventing the re-adoption of optimal gait biomechanics following ACL injury and ACLR. Alternatively, the adoption of aberrant gait biomechanics following ACL injury and ACLR may lead to less engagement in physical activity resulting in fewer daily steps. Previous work has linked aberrant gait biomechanics to cartilage compositional changes (2, 28) and symptomatic knee function after ACLR (19), thus individuals with worse underlying joint tissue changes or increased symptoms may simultaneously adopt compensatory gait biomechanics and choose not to engage in activities that require greater daily steps. Ultimately, the mechanistic link between gait biomechanical movement patterns and loading frequency are undefined and should be explored in future research to devise best treatment approaches to aberrant joint loading.
Gait retraining interventions are effective for improving aberrant gait biomechanics (39, 40). Encouraging research highlights the beneficial effects of a single session of real-time gait biofeedback to improve biomechanical underloading and deleterious joint tissue metabolism in patients after ACLR (15, 41). Additionally, physical activity promotion is effective in increasing daily steps in individuals with low daily step counts (42). It remains unknown whether increasing steps in individuals with low daily steps who also demonstrate gait biomechanical underloading is beneficial or detrimental to knee joint health in individuals post-ACLR. Increasing steps without first addressing compensatory gait behavior may subject the knee to greater loading frequency that is similar to uninjured individuals, but also result in more steps completed with aberrant biomechanical loading. The order in which these loading parameters are addressed during a rehabilitation protocol to maximize joint health requires further investigation. Given the association between gait biomechanics and daily steps reported in the current study, it would be important to determine if addressing one parameter of loading results in improvements in the other. For example, patients with idiopathic knee OA who participated in an exercise intervention over 18 months demonstrated improvements in knee compressive forces during gait (43). Alternatively, individuals participating in gait retraining interventions multiple times per week over 2-6 weeks may accumulate up to 3,000 steps each session (39) potentially resulting in short-term increases in daily steps. However, long-term improvements in daily steps often require interventions that target behavior change and maintenance (i.e. goal setting or social support) (44) which is not a typical component of gait retraining interventions. Both aberrant gait biomechanics and insufficient daily step counts may need to be addressed separately or at different time points in rehabilitation. Trials investigating interventions targeting either aberrant gait biomechanics or daily step promotion should include assessments of both aspects of joint loading to determine transfer between these two parameters of overall joint loading in individuals with an ACLR.
Limitations.
The results of the current study provide unique insights into the relationships between different aspects of joint loading after ACLR by combining laboratory and free-living assessments. However, limitations of the current study should be considered to inform future research. The current study design is cross-sectional with a conservative sample size and cannot determine mechanistic links between aberrant gait biomechanics and daily step frequencies. Understanding the causal relationship between gait biomechanical underloading and low daily step accumulation in a larger cohort may help develop comprehensive assessments and interventions targeting aberrant mechanical loading and should be an area of focus for future research. Additionally, participants in the current study were recruited after their ACLR surgery. We were unable collect pre-injury biomechanical or daily step data and cannot determine whether the gait biomechanics and/or daily steps measured post ACLR existed prior to ACL injury. We currently lack the ability to control for covariates related to joint loading in functional waveform analyses (i.e. age (45), gait speed (46), and BMI (47)), but there were no differences in participants characteristics between groups (Table 1). Participants with concomitant meniscal pathology and subsequent surgical procedures at time of ACL injury were included in study and concomitant meniscal injury may impact gait biomechanics after ACLR (48). The percentage of participants with meniscal pathology were not statistically significantly different between step groups, suggesting that that the potential for meniscal injury may have been distributed similar across the three groups in our study. Future studies should specifically determine the role that meniscal pathology has on altered gait biomechanics and daily steps following ACLR.
CONCLUSIONS
These data suggest a link between aberrant gait biomechanics and fewer daily steps following ACLR. Specifically, individuals 6 to 12 months post-ACLR who take fewer daily steps demonstrate biomechanical gait strategies characterized by underloading during weight-acceptance (i.e. lesser peak vGRF) and a stiffened knee strategy (i.e. lesser KEM and knee flexion angle) during weight acceptance and late stance in the ACLR extremity.
Supplementary Material
Conflict of Interest and Funding Statement:
This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (1R21AR074094-01). The authors have no conflicts of interest to declare. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by ACSM.
REFERENCES
- 1.Poulsen E, Goncalves GH, Bricca A, Roos EM, Thorlund JB, Juhl CB. Knee osteoarthritis risk is increased 4-6 fold after knee injury-a systematic review and meta-analysis. Br J Sports Med. 2019;53(23):1454–63. [DOI] [PubMed] [Google Scholar]
- 2.Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS, Snyder-Mackler L. Decreased knee joint loading associated with early knee osteoarthritis after anterior cruciate ligament injury. Am J Sports Med. 2016;44(1):143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu CR, Andriacchi TP. Dance between biology, mechanics, and structure: A systems-based approach to developing osteoarthritis prevention strategies. J Orthop Res. 2015;33(7):939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart HF, Collins NJ, Ackland DC, Cowan SM, Crossley KM. Gait characteristics of people with lateral knee osteoarthritis after ACL reconstruction. Med Sci Sports Exerc. 2015;47(11):2406–15. [DOI] [PubMed] [Google Scholar]
- 5.Sun HB. Mechanical loading, cartilage degradation, and arthritis. Ann N Y Acad Sci. 2010;1211:37–50. [DOI] [PubMed] [Google Scholar]
- 6.Castrogiovanni P, Di Rosa M, Ravalli S et al. Moderate physical activity as a prevention method for knee osteoarthritis and the role of synoviocytes as biological key. Int J Mol Sci. 2019;20(3):511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace IJ, Bendele AM, Riew G et al. Physical inactivity and knee osteoarthritis in guinea pigs. Osteoarthritis Cartilage. 2019;27(11):1721–8. [DOI] [PubMed] [Google Scholar]
- 8.Nomura M, Sakitani N, Iwasawa H et al. Thinning of articular cartilage after joint unloading or immobilization. An experimental investigation of the pathogenesis in mice. Osteoarthritis Cartilage. 2017;25(5):727–36. [DOI] [PubMed] [Google Scholar]
- 9.Hagiwara Y, Ando A, Chimoto E, Saijo Y, Ohmori-Matsuda K, Itoi E. Changes of articular cartilage after immobilization in a rat knee contracture model. J Orthop Res 2009;27(2):236–42. [DOI] [PubMed] [Google Scholar]
- 10.Maldonado DC, Silva MCPd, Neto SE-R, de Souza MR, de Souza RR. The effects of joint immobilization on articular cartilage of the knee in previously exercised rats. J Anat. 2013;222(5):518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis-Wilson HC, Pfeiffer SJ, Johnston CD et al. Bilateral gait 6 and 12 months post-anterior cruciate ligament reconstruction compared with controls. Med Sci Sports Exerc. 2020;52(4):785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell DR, Pfeiffer KA, Cadmus-Bertram LA et al. Objectively measured physical activity in patients after anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(8):1893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisee C, Montoye AH, Lewallen NF, Hernandez M, Bell DR, Kuenze CM. Assessment of free-living cadence using actigraph accelerometers among individuals with and without ACL reconstruction. J Athl Train. 2020;55(9):994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietrosimone B, Loeser RF, Blackburn JT et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(10):2288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luc-Harkey BA, Franz J, Hackney AC et al. Immediate biochemical changes after gait biofeedback in individuals with anterior cruciate ligament reconstruction. J Athl Train. 2020;55(10):1106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeiffer SJ, Spang J, Nissman D et al. Gait mechanics and T1rho MRI of tibiofemoral cartilage 6 months post ACL reconstruction. Med Sci Sports Exerc. 2018;51(4):630–639. [DOI] [PubMed] [Google Scholar]
- 17.Teng HL, Wu D, Su F et al. Gait characteristics associated with a greater increase in medial knee cartilage T1rho and T2 relaxation times in patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(14):3262–71. [DOI] [PubMed] [Google Scholar]
- 18.Pietrosimone B, Blackburn JT, Padua DA et al. Walking gait asymmetries 6 months following anterior cruciate ligament reconstruction predict 12-month patient-reported outcomes.J Orthop Res. 2018;36(11):2932–40. [DOI] [PubMed] [Google Scholar]
- 19.Pietrosimone B, Seeley MK, Johnston C, Pfeiffer SJ, Spang JT, Blackburn JT. Walking ground reaction force post-ACL reconstruction: Analysis of time and symptoms. Med Sci Sports Exerc. 2019;51(2):246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. [DOI] [PubMed] [Google Scholar]
- 21.Voinier D, Neogi T, Stefanik JJ et al. Using cumulative load to explain how body mass index and daily walking relate to worsening knee cartilage damage over two years: The MOST Study. Arthritis Rheumatol. 2020;72(6):957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White DK, Tudor-Locke C, Zhang Y et al. Daily walking and the risk of incident functional limitation in knee osteoarthritis: an observational study. Arthritis Care Res (Hoboken). 2014;66(9):1328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wellsandt E, Kallman T, Golightly Y et al. Knee joint unloading and daily physical activity associate with cartilage T2 relaxation times 1 month after ACL injury. J Orthop Res. 2021;29: 10.1002/jor.25034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuenze C, Collins K, Triplett A et al. Comparison of free living step accumulation among adolescent patients six months after ACL reconstruction and healthy controls. J Athl Train. 2020;55(6s):S-1-S-378. [Google Scholar]
- 25.Collins NJ, Prinsen CAC, Christensen R, Bartels EM, Terwee CB, Roos EM. Knee Injury and Osteoarthritis Outcome Score (KOOS): systematic review and meta-analysis of measurement properties. Osteoarthritis Cartilage. 2016;24(8):1317–29. [DOI] [PubMed] [Google Scholar]
- 26.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.John D, Morton A, Arguello D, Lyden K, Bassett D. “What is a step?” Differences in how a step is detected among three popular activity monitors that have impacted physical activity research. Sensors (Basel). 2018;18(4):1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeiffer S, Harkey MS, Stanley LE et al. Associations between slower walking speed and T1rho magnetic resonance imaging of femoral cartilage following anterior cruciate ligament reconstruction. Arthritis Care Res (Hoboken). 2018;70(8):1132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry J, Burnfield JM. Gait analysis. Normal and pathological function 2nd ed. California: Slack. 2010. [Google Scholar]
- 30.Bell AL, Brand RA, Pedersen DR. Prediction of hip joint centre location from external landmarks. Hum Mov Sci. 1989;8(1):3–16. [Google Scholar]
- 31.Evans-Pickett A, Longobardi L, Spang JT et al. Synovial fluid concentrations of matrix Metalloproteinase-3 and Interluekin-6 following anterior cruciate ligament injury associate with gait biomechanics 6 months following reconstruction. Osteoarthritis Cartilage. 2021;29(7):1006–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horton WZ, Page GL, Reese CS, Lepley LK, White M. Template Priors in Bayesian Curve Registration. Technometrics. 2021;63(4):487–499. [Google Scholar]
- 33.Lang S, Brezger A. Bayesian P-Splines. J Comput Graph Stat. 2004;13(1):183–212. [Google Scholar]
- 34.Müller B, Wolf S, Brueggemann GP, Deng Z, Miller F, Selbie WS. Handbook of Human Motion. Springer International Publishing; 2018. [Google Scholar]
- 35.Jefferson RJ, Collins JJ, Whittle MW, Radin EL, O’Connor JJ. The role of the quadriceps in controlling impulsive forces around heel strike. Proc Inst Mech Eng H. 1990;204(1):21–8. [DOI] [PubMed] [Google Scholar]
- 36.Blackburn JT, Pietrosimone B, Harkey MS, Luc BA, Pamukoff DN. Quadriceps function and gait kinetics after anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 2016;48(9):1664–70. [DOI] [PubMed] [Google Scholar]
- 37.Di Stasi SL, Snyder-Mackler L. The effects of neuromuscular training on the gait patterns of ACL-deficient men and women. Clin Biomech (Bristol, Avon). 2012;27(4):360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knoll Z, Kiss RM, Kocsis L. Gait adaptation in ACL deficient patients before and after anterior cruciate ligament reconstruction surgery. J Electromyogr Kinesiol. 2004;14(3):287–94. [DOI] [PubMed] [Google Scholar]
- 39.Davis IS, Tenforde AS, Neal BS, Roper JL, Willy RW. Gait retraining as an intervention for patellofemoral pain. Curr Rev Musculoskelet Med. 2020;13(1):103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung RTH, Ho KKW, Au IPH et al. Immediate and short-term effects of gait retraining on the knee joint moments and symptoms in patients with early tibiofemoral joint osteoarthritis: a randomized controlled trial. Osteoarthritis Cartilage. 2018;26(11):1479–86. [DOI] [PubMed] [Google Scholar]
- 41.Evans-Pickett A, Davis-Wilson HC, Luc-Harkey BA et al. Biomechanical effects of manipulating peak vertical ground reaction force throughout gait in individuals 6-12 months after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon). 2020;76:105014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams MA, Sallis JF, Norman GJ, Hovell MF, Hekler EB, Perata E. An adaptive physical activity intervention for overweight adults: a randomized controlled trial. PLoS One. 2013;8(12):e82901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Messier SP, Mihalko SL, Legault C et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howlett N, Trivedi D, Troop NA, Chater AM. Are physical activity interventions for healthy inactive adults effective in promoting behavior change and maintenance, and which behavior change techniques are effective? A systematic review and meta-analysis. Transl Behav Med. 2019;9(1):147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tudor-Locke C, Craig CL, Brown WJ et al. How many steps/day are enough? For adults. Int J Behav Nutr Phys Act. 2011;8:79.21798015 [Google Scholar]
- 46.Fukuchi CA, Fukuchi RK, Duarte M. Effects of walking speed on gait biomechanics in healthy participants: a systematic review and meta-analysis. Syst Rev. 2019;8(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pamukoff DN, Lewek MD, Blackburn JT. Greater vertical loading rate in obese compared to normal weight young adults. Clin Biomech (Bristol, Avon). 2016;33:61–5. [DOI] [PubMed] [Google Scholar]
- 48.Capin JJ, Khandha A, Zarzycki R, Manal K, Buchanan TS, Snyder-Mackler L. Gait mechanics after ACL reconstruction differ according to medial meniscal treatment. J Bone Joint Surg Am. 2018;100(14):1209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


