Abstract
Arachidonic acid metabolites epoxyeicosatrienoates (EETs) and hydroxyeicosatetraenoates (HETEs) are important regulators of myocardial blood flow and coronary vascular resistance (CVR), but their mechanisms of action are not fully understood. We applied a chemoproteomics strategy using a clickable photoaffinity probe to identify G protein-coupled receptor 39 (GPR39) as a microvascular smooth muscle cell (mVSMC) receptor selective for two endogenous eicosanoids, 15-HETE and 14,15-EET, which act on the receptor to oppose each other’s activity. The former increases mVSMC intracellular calcium via GPR39 and augments coronary microvascular resistance, and the latter inhibits these actions. Furthermore, we find that the efficacy of both ligands is potentiated by zinc acting as an allosteric modulator. Measurements of coronary perfusion pressure (CPP) in GPR39-null hearts using the Langendorff preparation indicate the receptor senses these eicosanoids to regulate microvascular tone. These results implicate GPR39 as an eicosanoid receptor and key regulator of myocardial tissue perfusion. Our findings will have a major impact on understanding the roles of eicosanoids in cardiovascular physiology and disease and provide an opportunity for the development of novel GPR39-targeting therapies for cardiovascular disease.
Keywords: EETs, eicosanoids, GPCR, GPR39, HETEs
INTRODUCTION
Myocardial oxygen demand determines coronary blood flow, which is exquisitely regulated through changing vascular resistance in coronary arterioles ranging in size from 100 to 300 µm (1). Whereas the determinants of myocardial oxygen demand are well known, the molecules responsible for the second-to-second regulation of microvascular tone and the receptors through which they act have not been identified. Two classes of signaling P450 eicosanoids, epoxyeicosatrienoates (EETs) and hydroxyeicosatetraenoates (HETEs), are potent regulators of microvascular tone and play critical roles in cardiovascular physiology and disease (2, 3). EETs are predominantly microvascular dilators and have been shown to function as endothelium-derived hyperpolarization factors (EDHFs) in the coronary circulation (4, 5), and mediating functional hyperemia in the brain (6, 7). They are anti-inflammatory, inhibit platelet aggregation, and protect against ischemia-reperfusion (I/R) injury in the heart and brain (8). HETEs, on the other hand, particularly 20-HETE, are typically vasoconstrictors that have been implicated in the generation of arteriolar myogenic tone and blood flow autoregulation in the brain and kidneys (9, 10). They promote inflammation and contribute to hypertension and I/R injury (11).
The mechanisms utilized by these eicosanoids to exert their biological actions on microvascular smooth muscle cells (mVSMCs) remain largely unknown. Ligand binding and pharmacological studies suggest that EETs signal via a G protein-coupled receptor (GPCR; 12), yet the identity of an EETs receptor has remained elusive. In the current study, we generated a clickable photo-crosslinking probe based on 14,15-EET that allowed us to isolate its binding proteins from mouse heart mVSMCs in an unbiased manner using mass spectrometry-based proteomics. This approach identified GPR39, a member of the ghrelin peptide receptor family, as a putative receptor for 14,15-EET in mVSMCs. Here, we show that GPR39 is capable of mobilizing calcium in VSMC in response to 15-HETE and serving as the site of 14,15-EET’s inhibitory action on 15-HETE-induced calcium signaling, indicating that it acts as a receptor for both eicosanoids. We also localize GPR39 immunoreactivity to mouse heart mVSMCs and demonstrate 15-HETE increases coronary perfusion pressure (CPP) using the isolated Langendorff mouse heart perfusion preparation, an effect that is inhibited by coadministration of 14,15-EET and requires GPR39 expression.
METHODS
The animal procedures described in this study were approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University and followed the NIH Guide for the Care and Use of Laboratory Animals.
Photo-Crosslinking and Modeling
To identify the molecular targets for 14,15-EET, we used a chemoproteomic approach by combining photoaffinity labeling with mass spectrometry (MS)-based proteomics. We generated a photo-crosslinking probe based on 14,15-EET, then used click chemistry to pull down its targets in mVSMCs, which were identified by MS. We confirmed binding both experimentally and by computational modeling. For computational modeling, no experimentally determined structure exists for GPR39, thus we used the closely related neurotensin receptor 1 protein (Protein Data Base ID: 4GRV) as a template. To investigate potential binding affinity, we compared predicted binding affinity of 14,15-EET to a list of long-chain fatty acids, selected based on a ligand similarity search of 14,15-EET against the human metabolome database (HMDB) long-chain fatty acid database containing 38,928 entries. Molecular dynamics (MDs) simulation was performed using the NAMD 2.10 package with CHARMM36 force-field. Detailed protocols of photo-crosslinking probe synthesis, photo-crosslinking, click chemistry and streptavidin pulldown, proteomics, and computational modeling are in Supplemental Materials (see https://doi.org/10.6084/m9.figshare.17408957.v1).
Source of the Eicosanoids
Racemic mixtures of EETs and HETEs used in the study were either purchased from Cayman Chemical (Ann Arbor, MI) or synthesized in-house, as previously described (61).
Isolated Pressurized Mesenteric Artery Preparation
Mesenteric arteries were isolated from the mesenteric vascular bed following cervical dislocation of anesthetized, 3-mo-old male C57BL6 mice and placed in ice-cold standard MOPS buffer solution containing in mM: 144 NaCl, 3.0 KCl, 2.5 CaCl2, 1.5 MgSO4, 2.0 MOPS, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, and 1.2 NaH2PO4. The mesenteric arteries (1–2 mm in length) were secured in an arteriograph perfusion chamber [Living Systems Instrumentation (LSI), Burlington, VT] between two glass micropipettes. The ends of glass micropipettes were connected to a pressure servo controller unit (LSI, PS200S) through three-way stopcocks. The cannulated vessel was pressurized to 80 mmHg and superfused with MOPS buffer solution. The vascular diameters were monitored by a video system consisting of an inverted microscope (Leica Dm IRB) with a charge-coupled device (CCD) camera (Ikegami), a television monitor, and a video dimension analyzer (LSI). The vessel diameter signal was digitized (Digidata 1440 A digitizer, Axon Instruments) and recorded (AxoScope software, Axon Instruments). The temperature of the perfusion chamber was maintained at 37°C using temperature controllers (LSI, TC-095, and Warner TC-344B). After equilibration, the arteries were precontracted with a thromboxane A2 receptor agonist, U-46619 (20–40 nM) to 26%–56% of baseline diameter, then exposed to cumulative concentrations of 14,15-epoxyeicosatrienoate probe (EET-P; 1, 3, and 10 µM). The data were expressed as % relaxation relative to contraction by U-46619.
HEK-293 Cell Transfection with GPR39
Human embryonic kidney-293 cells (HEK 293; American Type Culture Collection, Manassas, VA) were grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS). Cells were maintained in 5% CO2 at 37°C, grown for three passages after thawing, and used through passage 12. Cells grown in 12-well plates were transfected using 2.5 µg GPR39 plasmid and Lipofectamine 2000 reagent (Invitrogen) at 50%–70% confluence in media lacking antibiotics. Cells transfected with transfection solution alone without the plasmid served as controls. For subsequent experiments, untransfected and transfected HEK-293 cells were treated for 1–5 min with different concentrations of epoxyeicosatrienoates (EETs) and hydroxyeicosatetraenoates (HETEs) dissolved in dimethyl sulfoxide (DMSO) or DMSO alone.
Dot-Blot Assay
EETs and HETEs regioisomers were dissolved in DMSO, diluted in different concentrations with Tris-buffered saline (TBS) and blotted onto nitrocellulose membranes, which were allowed to dry at room temperature for 1 h. Membranes were blocked with Li-Cor Odyssey blocking buffer (Part No 927-50000) for 1 h, then incubated overnight with cell lysis proteins (in a ratio of 1 mL of cell lysis collected from a T-75 flask plus 9 mL Li-Cor blocking solution) or in the presence of EETs and HETEs regioisomers. Membranes were washed three times with Tris-buffered saline-Tween 20 (TBST), and incubated with anti-hemagglutinin (HA) epitope tag antibody (Cell Signaling Technology, Cat. No. 2367S) for 2 h at room temperature or overnight at 4°C. Membranes were then washed again three times with TBST, incubated with a secondary antibody (Li-Cor, Cat. No. 925-32210) for 1 h. After a final wash, the blot was developed and analyzed using the Li-Cor Odyssey Clx system.
Microvascular Smooth Muscle Cell (mVSMC) Culture
After removing the atrium, aorta, and large vessels from the heart surface of 10-wk-old male adult mice (20–25 g), the left ventricle was sliced with a mouse heart slicer matrix (Zivic Instruments, Pittsburgh, PA). To avoid epicardial coronary arteries, only four apical slices (1 mm thick) from the left ventricle were collected and placed on the surface of a collagen-coated 24-well tissue culture plate (Life Technologies, Carlsbad, CA) containing 100 µL FBS/well. The slices were incubated at 37°C with 5% CO2 for 4 h before DMEM (supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% FBS) was added to the wells. The sections were removed after mVSMCs migrated from tissue to the surface of the well. mVSMCs were trypsinized (0.05% trypsin) and replated for subsequent experiments.
Western Blot
Cells (mVSMC and HEK-293) were lysed in buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 0.25% Na-deoxycholate, 1 mM ethylenediaminetetraacetic acid, and protease inhibitor (Roche, Nutley, NJ)]. mVSMC or HEK proteins (20 µg/lane) in SDS sample buffer (2% SDS, 10% glycerol, 80 mM Tris, pH 6.8, 0.15 M β-mercaptoethanol, and 0.02% bromphenol blue) were separated on 4%–12% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat dry milk in TBST (10 mM Tris, pH 7.5, 150 mM NaCl, 0.05% Tween 20) for 30 min at room temperature and incubated overnight at 4°C with primary antibodies in dry milk as follows: 1:1,000 rabbit anti-GPR39 antibody (Cat. No. ABIN1048812, antibodies-online.com, Atlanta, GA), 1:1,000 HA-Tag mouse mAb (Cat. No. 2367, RRID:AB_10691311, Cell Signaling Technology, Beverly, MA), 1:1,000 mouse anti-extracellular signal-regulated kinase (ERK; Cat. No. 4696, RRID:AB_390780, Cell Signaling), and 1:1,000 rabbit anti-pERK (No. 4370, RRID:AB_2315112, Cell Signaling). The antigens were detected by the luminescence method (ECL-plus Western blotting detection kit) with peroxidase-linked sheep anti-mouse or donkey anti-rabbit antibodies [Cat. No. NXA931 (RRID:AB_772209) and Cat. No. NA934 (RRID:AB_772206), respectively, GE Amersham, Lafayette, CO]. To reprobe, membrane stripping was carried out by incubating membranes in stripping buffer (37.5 mM Tris, pH 6.8, 2% SDS, 1% β-mercaptoethanol at 50°C for 60 min). Stripped membranes were washed three times with TBST followed by immunoblotting with 1:2,000 mouse anti-β-actin monoclonal antibody (Cat. No. MAB 1501, RRID:AB_2223041, Millipore, Temecula, CA). In each case, the intensity of immunoblot bands was detected and quantified using the Fluor Chem FC2 Image Analysis System (Alpha Innotech).
Real-Time Quantitative Polymerase Chain Reaction (qPCR)
RNA samples from mVSMC and HEK-293 cells were treated with RNase-free DNase I (Invitrogen) for 30 min at 37°C. Reverse transcription was performed with iScriptTM reverse transcription supermix for RT-qPCR (Bio-Rad, Hercules, CA). Fluorescent signals were generated using Fast SYBR Green PCR Master Mix (Applied Biosystems). We used the Roche real-time PCR primer design software to generate the primers for GPR39 and β-actin. The specificity of these primers was confirmed by BLASTn query analysis against the GenBank database. qPCR for GPR39 and β-actin were run in triplicates on a Bio-Rad CFX96 real-time PCR system as follows: 20 s at 95°C, followed by 40 cycles of 3 s each at 95°C, then 30 s at 60°C. A melting curve analysis was performed at the end of the PCR cycles. Experimental controls included nonreverse-transcribed RNA samples. Data were analyzed by Bio-Rad CFX96 software to determine the threshold cycle (CT) for each reaction.
Immunofluorescent Staining
For immunocytochemistry (ICC), cultured cells (mVSMCs and HEK) were fixed in fresh 4% paraformaldehyde in PBS (0.1 M sodium phosphate buffer, 0.9% NaCl, pH 7.4) and subsequently blocked with 10% goat serum in PBS for 30 min, and then stained with rabbit anti-GPR39 antibody (Cat. No. ABIN1048812, antibodies-online.com, Atlanta, GA). Alexa 488-conjugated donkey anti-rabbit secondary antibody (Cat. No. A32790, RRID:AB_2762833, Invitrogen) was used to visualize primary antibodies. For immunohistochemistry (IHC), mouse hearts were perfusion-fixed through the aorta with PBS and 4% paraformaldehyde using HSE Type 801 perfusion system (Hugo Sachs Elektronik-Harvard Apparatus). Hearts were then cryoprotected in 20% sucrose in PBS overnight at 4°C. Frozen sections were cut at 10 µm on a cryostat. Sections were washed with PBS, blocked in normal goat serum (Sigma) at a concentration of 10% in PBS for 90 min, and then stained with rabbit anti-GPR39 antibody (Cat. No. ABIN1048812, antibodies-online.com, Atlanta, GA) and mouse anti-α-smooth muscle actin (α-SMA) monoclonal antibody (Cat. No. A2547, RRID:AB_476701, Sigma) for 24 h at 4°C with mild agitation. Primary antibodies were visualized using the following secondary antibodies (all from Invitrogen): Alexa 488-conjugated donkey anti-mouse secondary antibody (Cat. No. A21202, RRID:AB_141607), Alexa 568-conjugated donkey anti-rabbit antibody (Cat. No. A10042, RRID:AB_2534017), or Alexa 488-conjugated donkey anti-rabbit secondary antibody (Cat. No. A32790, RRID:AB_2762833). To reduce autofluorescence, heart sections were treated with 10 mM CuSO4 in 50 mM ammonium acetate for 30 min before they were coverslipped with ProLong Gold antifade reagent with DAPI (Life Technologies). For both ICC and IHC, confocal images were collected with a confocal microscope (Nikon Eclipse Tie-A1RSi).
Nonfluorescent Immunohistochemistry
IHC was performed on 6 μm thick paraffin sections. The sections were washed with PBS, blocked in normal horse serum (Sigma) at a concentration of 10% in PBS for 90 min, and then incubated with rabbit anti-GPR39 antibody (Cat. No. PA5-33709, RRID:AB_2551084, Thermo Fisher Scientific) for 2 h at room temperature. Sections were washed, then incubated for 30 min with Vector ImmPRESS AP anti-rabbit IgG (alkaline phosphatase) polymer detection kit (Vector No. MP-5401), then visualized with Vector Blue AP substrate (Vector SK-5300). For double labeling, the primary antibody was coincubated with mouse anti-αSMA monoclonal antibody (Cat. No. A2547, RRID:AB_476701, Sigma). The sections were incubated with Vector ImmPRESS HRP Anti-Mouse IgG (peroxidase) polymer detection kit (Vector, No. MP-7402), then visualized with Vector diaminobenzidine (DAB) substrate (Vector Cat. No. SK-4100). This was followed by the anti-rabbit IgG AP and Vector Blue as above. For GPR-39 immunohistochemistry combined with lectin staining, sections were first blocked with normal horse serum, then incubated in Carbo-Free Blocking solution (Vector Laboratories, Burlingame, CA, Cat. No. SP-5040), 50 min at room temperature. Sections were incubated with the primary anti-GPR39 antibody combined with biotinylated Griffonia (Bandeiraea) simplicifolia Lectin I (Vector Laboratories, No. B-1215) for 2 h at room temperature at a concentration of 1:500 in PBS + 0.05% Tween 20 (PBST). After washing, sections were incubated in Vectastain Elite ABC (peroxidase) reagents (Vector PK-6100) and visualized with DAB. This was followed by anti-rabbit IgG (alkaline phosphatase) and Vector Blue.
Calcium Imaging of mVSMCs
Cultured mVSMCs were incubated for 30 min at 37°C in darkness with a solution of Hank’s buffered salt solution (HBSS; Life Technologies) containing Fluo-4 AM (2 μM, Life Technologies). After washing with HBSS, cells were used for Ca2+ imaging with a confocal microscope (Nikon Eclipse Tie-A1RSi). Fluo-4 was excited by 488 nm line of an argon laser, and emission signals with 500–550 nm were collected. The brightness of the fluorescent signals represents the relative level of intracellular [Ca2+]i. For recording Ca2+ transients, a time scan mode was normally utilized. Digital image processing was performed using NIS-Elements (Nikon). The change of global Ca2+ level after stimulation was reported as % change from baseline (Fpeak/Fbase × 100).
Lentivirus Mediated RNA Interference
Eighty percent confluent mVSMCs were infected with lentivirus (10 particles of virus/cell) carrying either shRNAs targeting GPR39 (5′- ATATGTCCATCTGCACGAA-3′) or a control virus carrying the scrambled shRNA (GE Dharmacon). Knockdown of GPR39 was confirmed by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot.
Generation of GPR39 Knockout Mice
The Gpr39 mutant mice were generated by Cyagen Biosciences (Santa Clara, CA) using a CRISPR/Cas9 deletion strategy and zygotic injections (Supplemental Fig. S7A). Briefly, two guide RNAs ( GTGGGCAACAGCGTCACCATC and GCATGGGTATCGAGTACCCTC) were designed to delete a portion of exon 1 in the mouse Gpr39 gene that includes multiple transmembrane domains, rendering the gene nonfunctional. Genotyping of founders and progeny was conducted using a primer pair (P1- CCGAAAAACTAAGTCACTCCTGGC and P2- CCCTGCTTGCTCTTCATTAGCACTT; Supplemental Fig. S7B) producing a 911 bp band from the wild-type (WT) allele and a 524 bp band from the null allele (Supplemental Fig. S7C). Heterozygous breeder pairs were mated to generate the GPR39 knockout, heterozygous and wild-type control animals used for this study. Mice lacking both copies of the wild-type GPR39 allele did not exhibit any overt phenotypes including altered viability.
Langendorff Mouse Heart Coronary Perfusion
GPR39 knockout mice and their WT littermates were maintained on the C57Black6/N strain. Both male and female mice were used between 3 and 6 mo of age. GPR39 knockout (KO) and WT mice were injected with ketamine (100 mg/kg ip), and hearts were immediately excised and perfused retrogradely at a constant flow with Hank’s buffered salt solution (HBSS) of the following composition (mM): 137 NaCl, 5.3 KCl, 1.26 CaCl2, 0.4 MgSO4, 0.49 MgCl2, 4.1 NaHCO3, 0.44 KH2PO4, 0.33 Na2HPO4, 5.5 d-glucose. The perfusion solution was oxygenated by bubbling carbogen (95% O2, 5% CO2) through the solution. The pH was set to 7.4, and the temperature was kept at 37°C using Hugo Sachs Elektronik Langendorff Perfusion System (Harvard Apparatus). The hearts were equilibrated for at least 1 h before drug treatments (200 nM of 14,15-EET or 15-HETE in the presence of 10 μM zinc, which had no effect when applied alone; 200 nM prostaglandin I2; 100 nM angiotensin II; 1:10,000 ethanol as a vehicle). The coronary perfusion pressure (CPP) was recorded with Pressure Monitor PM-4 (Living System Instrumentation). The signals were digitized with AxonCNS Digidata 440 A and analyzed with the AxoScope 10.7 (Molecular Devices).
Data Analysis
Data analysis was performed using the Prism statistical software package (version 6.0 GraphPad, San Diego, CA). Sample size (n) represents independent measurements taken from distinct samples. For normally distributed samples, group differences were assessed by a two-tailed t test or ANOVA with a Student–Newman–Keuls post hoc test. Statistical significance was assumed for P values < 0.05.
Data availability statement.
Complete MS data have been deposited to the ProteomeXchange Consortium via the PRIDE database (data set identifier PXD013952). The data that support other findings of this study are available from the corresponding author upon reasonable request.
RESULTS
Identification of GPR39 as a Putative Receptor for 14,15-EET
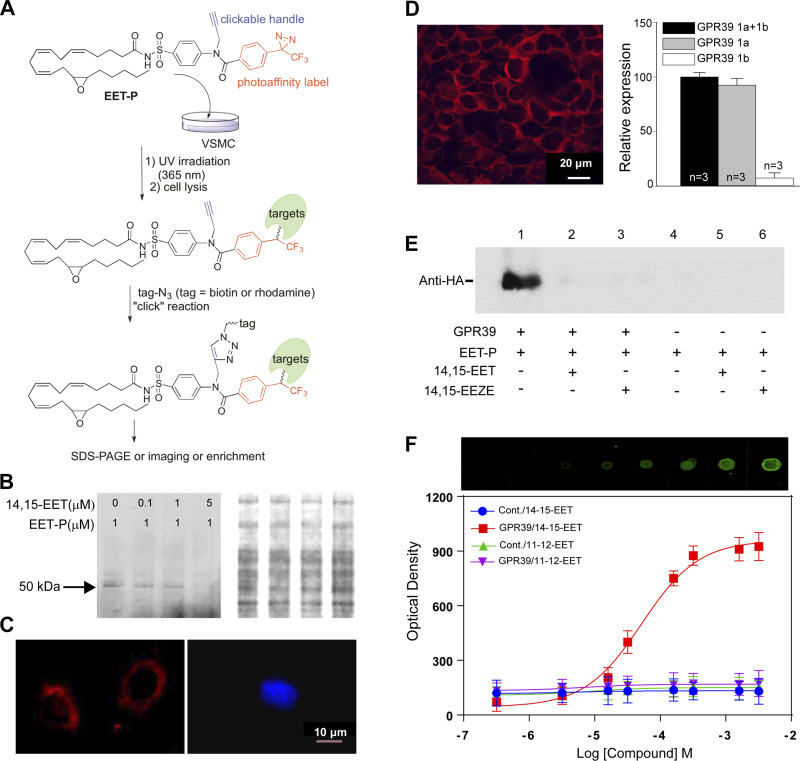
We implemented a chemical biology approach to purify 14,15-EET-binding proteins by producing a modified form of 14,15-EET (EET-P, Fig. 1A and Supplementary Material; see https://doi.org/10.6084/m9.figshare.17408957) that covalently cross links to target proteins following exposure to ultraviolet (UV) light. The probe also incorporates a click chemistry moiety that allows subsequent fluorophore labeling or biotin-streptavidin for affinity purification and identification of linked proteins by mass spectrometry (Fig. 1A). Despite these additional functional groups, we were able to demonstrate that EET-P mimics 14,15-EET’s previously reported ability to dilate mouse mesenteric arteries preconstricted with the thromboxane A2 agonist U46619 (13; Supplemental Fig. S1). We next used this probe to identify putative mVSMC 14,15-EET receptor(s) by treating cultured mouse heart mVSMCs with EET-P (1 µM) in the presence or absence of 14,15-EET followed by a 5 min UV (365 nm) exposure. Cell lysates were reacted with biotin-azide for affinity purification and subsequent mass spectrometry analysis that yielded a number of intracellular and membrane-associated proteins that could be competitively displaced by 14,15-EET, including a known 14,15-EET metabolizing enzyme, epoxide hydrolase, an indication of probe specificity (Supplemental Table S1; complete MS data has been deposited to the ProteomeXchange Consortium via the PRIDE database; data set identifier PXD013952). A single GPCR was detected in the screen, GPR39, a 50 kDa orphan member of the ghrelin receptor family previously reported to be activated by zinc ions (Zn2+; 14). We confirmed membrane-bound targets of EET-P by using rhodamine-azide labeling followed by membrane purification and SDS-PAGE analysis to detect EET-P-labeled proteins and observed a single band that was dose-dependently displaced by 14,15-EET and exhibited approximately the same molecular weight as GPR39 (Fig. 1B). We also found that cultured VSMCs could be labeled with EET-P using a rhodamine azide in-cell click reaction. Importantly, this membrane labeling can be displaced by pretreatment with 14,15-EET (Fig. 1C). The Gpr39 genomic locus encodes two isoforms, GPR39 1a [a full-length 7-transmembrane (7TM) isoform] and 1b (a truncated 5TM 1b isoform that lacks TM6 and 7; 15). Using immunocytochemistry and real-time quantitative PCR, we confirmed the expression of GPR39 1a, but not 1b in cultured mouse heart mVSMCs (Fig. 1D). We also determined that human embryonic kidney (HEK)-293 cells express only the 1b isoform (Supplemental Fig. S2B). Probe binding specificity was confirmed by cross-linking EET-P to HEK-293 cells transiently transfected with HA-tagged human GPR39 1a (Supplemental Fig. S2, C–E). Western blots probed with HA-antisera (Fig. 1E) indicate that HA-tagged GRP39 1a copurifies with EET-P (lane 1), and this band is absent in lysates of untransfected cells (lanes 4–6). We further confirmed probe specificity by eliminating probe binding to HA-tagged GPR39 by pretreating GPR39-transfected HEK-293 cells with either 14,15-EET (5 µM, lane 2) or EETs antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE, 5 µM, lane 3; 16) before EET-P exposure. Finally, dot-blot assay demonstrated dose-dependent saturable binding of 14,15-EET, but not 11,12-EET, to protein extracts from HEK-293 cells expressing GPR39 1a but not control cells (Fig. 1F).
Figure 1.
Purification and validation of GPR39 as a putative receptor for 14,15-EET. A: chemoproteomics strategy to identify 14,15-EET receptor in mVSMCs. B: photo-crosslinking GPR39 with EET-P in mVSMCs. Left: representative SDS-PAGE gel revealing the presence of a ∼50 kDa band. 14,15-EET pretreatment reduced protein binding to EET-P in a dose-dependent manner. Right: total protein determined by Coomassie Blue staining of the same gel. C: representative confocal images demonstrating binding of EET-P to outer surface of mVSMCs (left, red). Pretreatment with 1 μM 14,15-EET for 10 min prevented EET-P surface binding (right; blue color is nuclear stain DAPI). D: a representative immunofluorescent confocal image illustrating GPR39 expression (left, red) in primary cultured mouse heart mVSMCs (scale bar = 20 μm), and expression of GPR39 1a, but not 1b, confirmed by qPCR (right), n = 3. E: a representative Western blot of EET-P cross linking to epitope-tagged GPR39 1a in transfected HEK cells. HEK cells were treated with 5 μM 14,15-EET or 14,15-EEZE for 10 min, 1 μM EET-P for 15 min, and then irradiated with UV for 5 min at 4oC. After clicking with biotin and purification with streptavidin Dynabeads, protein extracts were probed by anti-HA antibody. F: a representative dot-blot assay and quantification of dose-dependent binding of 14,15-EET, but not 11,12-EET, to membrane protein extracts from HEK-293 cells stably expressing GPR39 1a, but not untransfected control cells (top, raw image, and optical density quantification, bottom; n = 3). EET, epoxyeicosatrienoate; EET-P, epoxyeicosatrienoate probe; GPR39, G protein-coupled receptor 39; HEK, human embryonic kidney; mVSMCs, microvascular smooth muscle cells; 14,15-EEZE, 14,15-epoxyeicosa-5(Z)-enoic acid.
Isoform-Specific Activation of GPR39 Signaling by 14,15-EET and 15-HETE
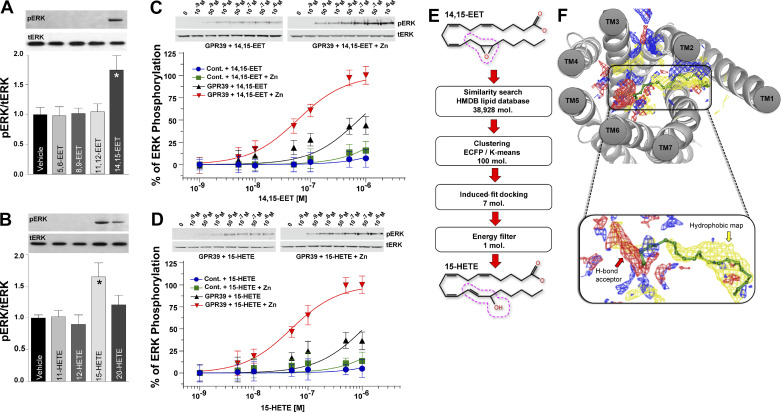
We transfected HEK-293 cells with GPR39 1a to confirm that 14,15-EET binding activates GPR39 signaling and that 14,15-EET activation is specific with the receptor not being activated by related eicosanoids. Cells were subsequently stimulated with one of four regioisomers of EETs (5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET), HETEs (11-HETE, 12-HETE, 15-HETE, and 20-HETE), or vehicle. 14,15-EET and 15-HETE are the only regioisomers that significantly increase GPCR activation as monitored by extracellular signal-regulated kinase (ERK) phosphorylation in cells transfected with GPR39 1a (Fig. 2, A and B) but not in untransfected cells (Fig. 2, C–D), with both eicosanoids displaying a concentration-dependent activation of GPR39 (Fig. 2, C and D). Dot-blot assays using lysates from GPR39 1a stably expressing HEK-293 cells also demonstrate dose-dependent, saturable binding of 15-HETE, similar to 14,15-EET, that is not observed for 12-HETE (Supplemental Fig. S3A). Importantly, binding competition experiments indicate that 15-HETE and 14,15-EET can displace each other, whereas 12-HETE (Supplemental Fig. S3B) and 11,12-EET (Supplemental Fig. S3C), respectively, fail to do so. Zinc has been reported to be either a GPR39 agonist (14) or an allosteric modulator for synthetic ligands of the receptor (17). Therefore, we repeated the dose-response curves for 14,15-EET and 15-HETE in the presence of 1 µM zinc, which has no effect on ERK phosphorylation by itself. Zinc significantly potentiates the effects of both eicosanoids, consistent with its role as an allosteric modulator of GPR39 (Fig. 2, C and D).
Figure 2.
Functional activation and modeling of GPR39 ligand binding. ERK phosphorylation induced by 1 min treatment with 1 μM of each one of four regioisomers of EETs (A) or HETEs (B) , n = 3 for each regioisomer (A, *P < 0.05; B, *P < 0.05). Total was used as a protein loading control. Dose-dependent ERK phosphorylation induced by 1 min treatment with either 14,15-EET (C; n = 3) or 15-HETE (D; n = 3) in HEK cells expressing GPR39 1a, but not untransfected cells, in the presence and absence of 1 μM zinc. E: virtual screening of 14,15-EET-like compounds from HMDB database revealed comparable energies for 15-HETE and 14,15-EET. The 14,15-EET and 15-HETE structures exhibit a high degree of structural similarity. The dotted red line around carbons 13–15 highlights the structural difference between the two eicosanoids. F: GPR39 7TM core binding pocket and an enlarged view of the predicted binding pose of 14,15-EET. Blue, red, and yellow maps correspond to hydrogen bond donor, hydrogen bond acceptor, and hydrophobic areas within the binding pocket, respectively. N-terminal residues contributing to minor pocket are not shown for the sake of clarity. The carboxylate group of 14,15-EET interacts with polar residues in TM6, and the lipid portion interacts with the hydrophobic site formed at the minor-binding pocket, as shown in site mapping. EET, epoxyeicosatrienoate; ERK, extracellular signal-regulated kinase; GPR39, G protein-coupled receptor 39; HETE, hydroxyeicosatetraenoate; HMDB, human metabolome database; TM6, transmembrane domain 6.
GPR39-Ligand Interaction Modeling Predicts Selectivity for 14,15-EET and 15-HETE
We next used a homology model to investigate GPR39 1a-ligand specificity in silico (Supplemental Fig. S4, A and B) that predicted structural complementarity between 14,15-EET and GPR39, as indicated by a low docking energy (−8.7 kcal/mol). A fingerprint similarity search for other GPR39 ligands using 14,15-EET, as a reference structure resulted in a list of long-chain fatty acids (Table S2), including 15-HETE, which had a predicted docking energy of −8.6 kcal/mol, similar to 14,15-EET. This likely reflects the fact that both compounds have a polar group at the 15th carbon position and maintain double bonds in the cis configuration at the 5th, 8th, and 11th positions (Fig. 2E) unlike the other EETs and HETEs regioisomers. A potential orthostatic pocket was identified using SiteMap version 3.2, which revealed a hydrophilic major pocket and a hydrophobic minor pocket (Supplemental Fig. S4C) in GPR39 1a with the tail portion of these lipids extending into the minor binding pocket formed by transmembrane domains 1 (TM1), TM2, TM7, and the N-terminal loop (Fig. 2F and Supplemental Fig. S4, D–E). The major pocket accommodates the carboxylate moiety of 14,15-EET and 15-HETE by forming ionic interactions with positively charged residues from TM6 (Fig. 2F).
GPR39 Mediates Effects of 14,15-EET and 15-HETE on mVSMC Calcium Transients
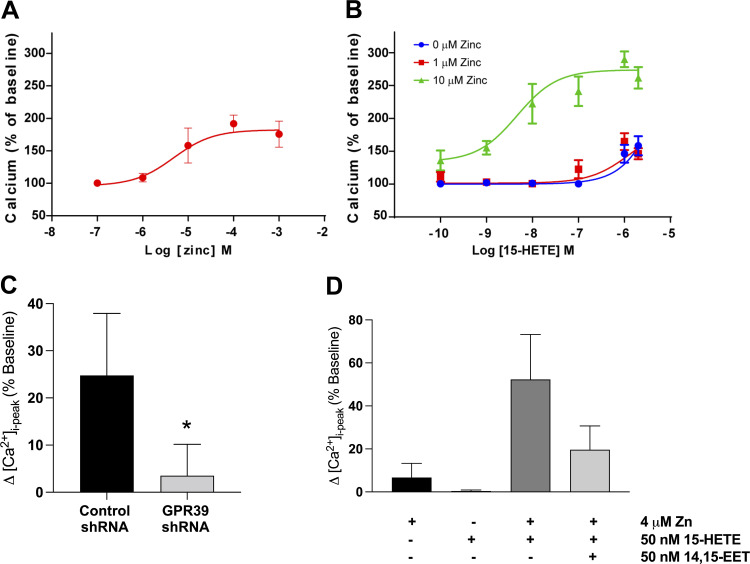
We next inquired whether 14,15-EET and 15-HETE regulate the signaling of mVSMC via GPR39 and if their actions are modulated by zinc. Using live-cell fluorescent imaging to monitor calcium transients, we first evaluated the effect of Zn2+ alone in mVSMCs and found that it increases intracellular calcium with an EC50 of 7.8 µM (Fig. 3A and Supplemental Fig. S5A). We then established the dose-response relationship for 15-HETE in the presence and absence of 1, 4, and 10 µM zinc. We observed that zinc, at 4 or 10 µM, significantly potentiates the response of GPR39 to 15-HETE stimulation (Fig. 3B; only 1 and 10 µM are shown). Application of 15-HETE alone elicits a calcium response in mVSMCs only at concentrations above 100 nM (Fig. 3B). However, in the presence of 10 µM (Fig. 3B) or 4 µM (Fig. 3C) zinc, 15-HETE elicits a significant calcium response in mVSMCs at low nanomolar concentrations (Fig. 3B), suggesting that zinc serves as a positive allosteric modulator for 15-HETE in mVSMCs. Importantly, GPR39 RNAi knockdown in mVSMCs attenuated the increase in mVSMC intracellular calcium produced by 15-HETE (Fig. 3C and Supplemental Fig. S5B), confirming a significant role for GPR39 in mediating the effect of 15-HETE on mVSMC calcium transients. 14,15-EET had no effect on intracellular calcium in mVSMCs at concentrations between 1 pM–10 µM (data not shown), but it was observed to inhibit 15-HETE-dependent increases in intracellular calcium (Fig. 3D), consistent with GPR39 acting as a dual sensor for both 14,15-EET and 15-HETE. Taken together, our modeling data along with pERK, calcium imaging, and binding assays indicate a competitive structure-activity relationship between 14,15-EET and 15-HETE.
Figure 3.
A: concentration-dependent increase in [Ca2+]i in mVSMCs in response to extracellular zinc (n = 5 for each concentration). B: dose-dependent increase in mVSMC [Ca2+]i in response to 15-HETE with and without zinc (n = 5–7 for each concentration). C: summary of [Ca2+]i response to 1 μM 15-HETE in mVSMCs treated with a lentivirus containing either a scrambled or GPR39-targeting shRNA for 72 h (n = 5, *P = 0.0122). D: summary of changes in mVSMCs [Ca2+]i in response to 50 nM of 14,15-EET and 15-HETE, separately and in combination, and with and without zinc (n = 4 for zinc alone, and n = 6). When applied alone, neither 4 μM zinc nor 50 nM 15-HETE had an effect on [Ca2+]i. Zinc (4 μM) potentiates the effect of 15-HETE (50 nM) on [Ca2+]i in mVSMCs, and the increase in mVSMCs [Ca2+]i by 15-HETE is abolished by pretreatment with 14,15-EET (*P < 0.00011). EET, epoxyeicosatrienoate; GPR39, G protein-coupled receptor 39; HETE, hydroxyeicosatetraenoate; mVSMCs, microvascular smooth muscle cells.
GPR39 Localization and Function in Myocardial Microvessels
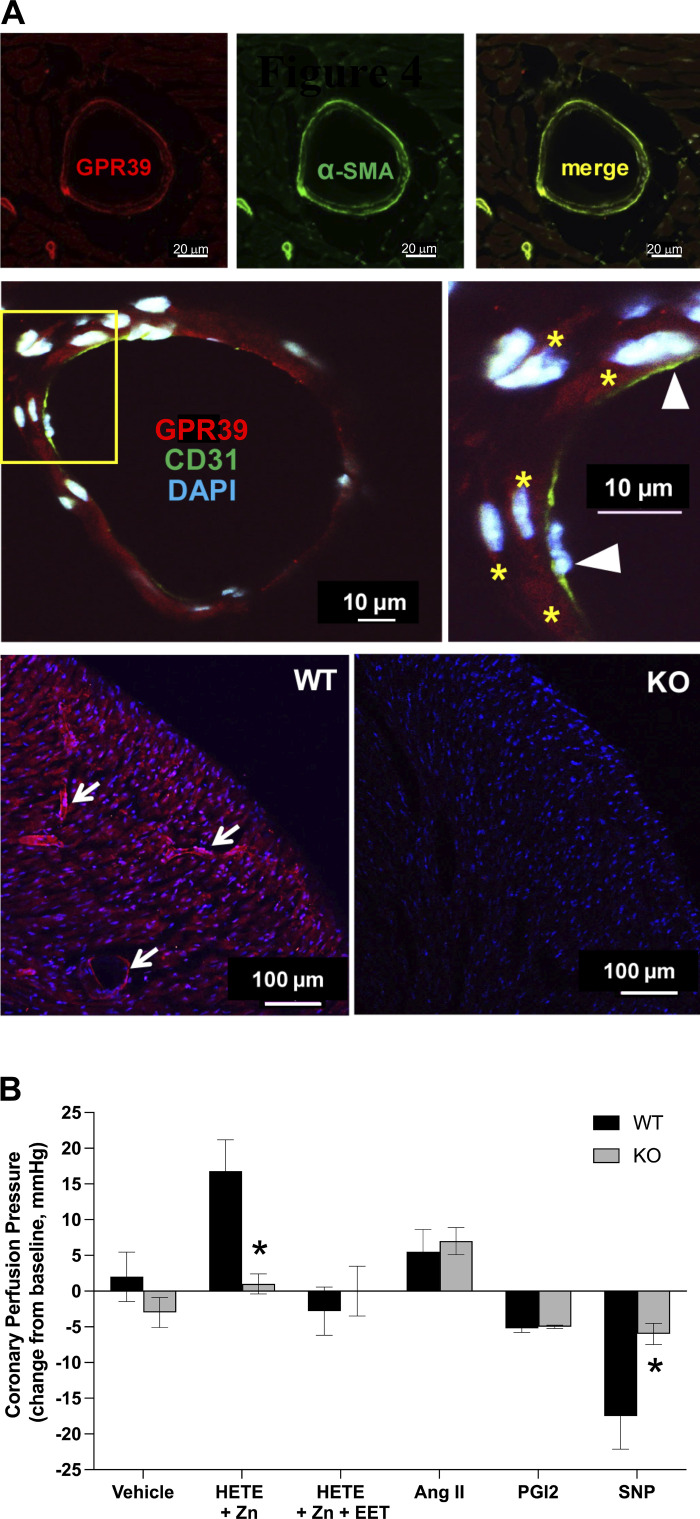
Consistent with a role in regulating coronary vascular resistance, immunofluorescence indicates that GPR39 expression is predominantly restricted to microvessels in the mouse heart (Fig. 4A). Costaining with α-smooth muscle actin (α-SMA, top) and CD31 (middle) confirms expression in arteriolar smooth muscle cells, but not endothelial cells (Fig. 4A). Antibody specificity was confirmed using heart tissue from GPR39 knockout (KO) mice, which lacked microvascular immunoreactivity observed in hearts from wild-type littermates (Fig. 4A, bottom). This microvascular pattern of expression was further confirmed using nonfluorescent immunostaining where we also observed GPR39 expression in both mouse and human hearts within perivascular cells that were consistently adjacent to microvessels including capillaries (Supplemental Fig. S6), suggestive of GPR39 expression in pericytes as well.
Figure 4.
Microvascular localization and function of GPR39 in mouse heart. A: immunofluorescent imaging of GPR39 shows microvascular pattern of expression in mouse heart (top left, red; scale bar = 20 μm), which colocalizes with VSMC markers α-smooth muscle actin (α-SMA, green, top middle and right; scale bar = 20 μm), but not endothelial marker CD31 (middle; green and arrow heads in magnified view; yellow stars point to GPR39-positive mVSMCs within vascular wall in red; blue is DAPI nuclear staining; scale bar = 10 μm). The bottom panel shows GPR39 immunoreactivity in wild-type (WT, left; arrows point to immuno-reactive microvessels in red) and GPR39 knockout (KO; blue is nuclear stain DAPI) mouse heart tissue sections (scale bar = 100 μm). B: changes in coronary perfusion pressure, at a constant flow rate, in response to infusion of 15-HETE (1 μM; in presence of 10 µM zinc), 15-HETE plus 14,15-EET (1 μM; in presence of 10 µM zinc), prostaglandin I2 (PGI2, 200 nM), angiotensin II (AngII, 100 nM), sodium nitroprusside (SNP; 10 µM), or vehicle in isolated mouse heart preparation from WT and GPR39 KO mice (n = 5, *P < 0.05). EET, epoxyeicosatrienoate; GPR39, G protein-coupled receptor 39; HETE, hydroxyeicosatetraenoate; mVSMCs, microvascular smooth muscle cells.
Role of GPR39 in Microvascular Regulation
We next determined the contribution of GPR39 activation by eicosanoids to microvascular tone regulation. This was accomplished using a mouse heart Langendorff preparation where changes in coronary perfusion pressure (CPP) are determined by microvascular resistance at a constant flow rate. Accordingly, 15-HETE (1 µM) increases CPP (Fig. 4B), and this effect is inhibited by coadministration of 14,15-EET (1 µM) or abolished in GPR39-null hearts (Fig. 4B, and Supplemental Fig. S7). 14,15-EET alone had no effect on CPP (not shown). Importantly, the effects of two other vasoactive agents on CPP, angiotensin II (Ang II) and prostaglandin I2 (PGI2), were unaffected by GPR39 deletion. Interestingly, CPP response to sodium nitroprusside (SNP) was significantly attenuated in GPR39 KO.
DISCUSSION
We developed a chemical cross-linking mimetic to identify 14,15-EET as an endogenous ligand of GPR39, canonically considered to be a zinc receptor. We further show that 14,15-EET and 15-HETE can regulate mVSMC calcium dynamics and coronary vascular resistance via their opposing actions on GPR39.
Models of 14,15-EET signaling mediated by a GPCR have been proposed by several groups (13, 18, 19), yet the receptor has remained unidentified. Photoaffinity labeling was previously used to demonstrate a ∼47 kDa high-affinity binding protein of unknown identity for 14,15-EET in membrane fractions from U937 and vascular cells (13). Unlike previous approaches, our clickable photo-crosslinking probe allowed enrichment of cross-linked proteins from mVSMCs, permitting target identification by mass spectrometry. GPR39 represents the first high-affinity receptor for any EET regioisomer, although several membrane proteins have been shown to respond to high micromolar concentrations of 14,15-EET including large-conductance calcium-activated (BKCa) and ATP-sensitive potassium channels, the transient receptor potential cation channel subfamily V member 4 (TRPV4), and some GPCRs including GPR40 (20) and the prostaglandin E (EP2; 19) and thromboxane (TP; 18) receptors as well as our own recent work screening a variety of GPCRs for 14,15-EET responses (21). Our modeling predictions also led us to test and confirm that 15-HETE is an additional endogenous ligand of GPR39. No high-affinity receptor has been identified for 15-HETE, although receptors have been identified for other HETE regioisomers, including 12-HETE (22) and 20-HETE (23).
Endogenous ligands for GPR39 have remained elusive as early reports suggested obestatin, a ghrelin-derived peptide, but this has since been refuted (14, 24). Multiple studies have linked GPR39 with metabolism, including two studies of GPR39 KO mice, one where body weight and food intake were reported to be normal (25), whereas the other noted higher body weight and fat composition with no change in food intake (26). A report has also linked GPR39 loss with impaired insulin secretion (27), yet synthetic agonists of GPR39 fail to drive insulin secretion (28). GPR39 has also been implicated a variety of neurovascular, neurological, and neuropsychiatric disorders (29), including seizures (30) and depression (31). GPR39 has also been proposed to function as a Zn2+ receptor (14). Zinc also modulates receptor activity by synthetic GPR39 agonists (17). Furthermore, Zn2+ is known to be an allosteric modulator for multiple receptors and ion channels (32). Here, we confirm GPR39 sensitivity to high concentrations of Zn2+, and critically provide a novel function for physiologic levels of Zn2+ as an allosteric modulator enhancing the efficacy of the natural GPR39 ligands 14,15-EET and 15-HETE. Thus, Zn2+ may also play a role in the regulation and disorders of the microcirculation through GPR39 modulation. Recent reports suggested that zinc regulates endothelial function (33) and inhibits phosphate-induced vascular calcification (34), presumably through its action on GPR39.
The concentrations of 14,15-EET, 15-HETE, and zinc used in our study are similar to levels measured in human blood under physiological and pathophysiological conditions. The concentration of 15-HETE in normal plasma ranges from 0.8 ± 0.023 nM (35) to 0.1 µM (36). Higher levels have been measured in serum (37–39). Importantly, 15-HETE levels are increased in patients with chronic inflammatory conditions (40) and vascular diseases, including coronary artery disease (41–43) and pulmonary arterial hypertension (44). Similarly, the concentration of EETs in plasma from healthy volunteers was reported to be in the low nanomolar range (median of 0.6 nM), with patients with coronary artery disease (CAD) having higher EETs (median 1 nM; 45). Among patients with CAD, plasma EETs were inversely related to disease severity, with lower EETs found in patients with obstructive compared with nonobstructive CAD or in patients with no apparent CAD (46). Finally, levels of eicosanoids are even higher in heart tissues compared with plasma (41), likely reaching effective concentrations at the receptor level. Mean serum zinc concentration is around 10 µM, with lower levels in young children, peak concentrations in young adults, and a slow decline with age (47). Zinc is present in all body tissues and fluids. Plasma zinc is only ∼0.1% of total body zinc content, with ∼30% of total body zinc content in skeletal muscle (48).
The high regioselectivity of GPR39 for 14,15-EET and 15-HETE indicates that this receptor is probably not involved in the vasodilator or anti-inflammatory actions of other EET regioisomers, such as 11,12-EET, or in the vasoconstrictor actions of other HETEs, such as 20-HETE. Moreover, the present study identified GPR39 in mVSMCs and pericytes, and it cannot be excluded that other EETs receptors with other binding and signaling characteristics exist in endothelial cells and other vascular beds. Expression in pericytes has recently been confirmed in postmortem human brain tissue (49). It is also not clear if 14,15-EET vasoactivity is solely due to its antagonism of 15-HETE binding to GPR39, if GPR39 is also necessary for the ability of 14,15-EET to relax thromboxane receptor agonist (U46619)-preconstricted arteries, and whether there are GPR39-independent vasodilator actions of EETs in other vascular beds. Similarly, it is known that nitric oxide (NO) inhibits 20-HETE synthesis, which augments its vasodilator effect (50, 51). Our observation that dilation to NO is attenuated in GPR39 KO mice suggests that NO-mediated dilation in the coronary microcirculation is in part mediated via GPR39, likely linked to NO-mediated inhibition of 20-HETE or 15-HETE. In other words, GPR39 deletion eliminates the HETE-mediated component of NO vasodilation.
In conclusion, our study is the first to propose a role for GPR39 in microvascular regulation, in part by sensing the relative concentrations of 14,15-EET and 15-HETE. It is possible that the imbalance between these two eicosanoids could alter GPR39 activity, contributing to myocardial ischemia, and predisposing individuals to microvascular complications of systemic diseases such as diabetes, hypertension, and septic shock. In support of this idea, two recent studies demonstrated a role for GPR39 in cerebral (52) and myocardial (53) ischemia. Work by numerous investigators over the past three decades has established a critical role for P450 eicosanoids in cardiovascular physiology and disease (54–60). Further progress in this field has been hampered by the lack of understanding of the molecular mechanisms of actions of these important lipid mediators. Our identification of the receptor for two important endogenous vasoactive eicosanoids and their unique mode of dual and opposing regulation of receptor function is a major breakthrough in the field. These findings will have a significant impact on our understanding of how P450 eicosanoids control microcirculation, their roles in microvascular disease, and the development of novel therapeutic agents for vascular disease.
SUPPLEMENTAL DATA
Supplemental Methods, Supplemental Tables S1 and S2, and Supplemental Figs. S1-S7: https://doi.org/10.6084/m9.figshare.17408957.
GRANTS
This work was supported by the Knight Cardiovascular Institute. Mass spectrometric analysis was performed by the OHSU Proteomics Shared Resource with partial support from National Institutes of Health (NIH) core Grants P30EY010572, P30CA069533, and shared instrumentation Grant S10OD012246.
DISCLOSURES
This research involves technology of which N. J. Alkayed, S. Kaul, and S. Nagarajan are co-inventors and which has been licensed, in part by OHSU, to Vasocardea. OHSU and S. Kaul have a financial interest in Vasocardea, a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by Oregon Health and Science University. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
This article is part of the special collection "Advances in GPCRs: Structure, Mechanisms, Disease, and Pharmacology." Wei Kong, MD, PhD, and Jinpeng Sun, PhD, served as Guest Editors of this collection.
AUTHOR CONTRIBUTIONS
N.J.A., Z.C., Z.Y.Q., S.N., X.L., L.L., M.R.G., X.X., A.P.B., and S.K. conceived and designed research; Z.C., Z.Y.Q., S.N., X.L., J.W.N., F.X., B.L., W.F., L.L., C.M.D., and A.P.B. performed experiments; Z.C., Z.Y.Q., S.N., X.L., F.X., B.L., W.F., L.L., M.R.G., X.X., and A.P.B. analyzed data; N.J.A., Z.C., Z.Y.Q., S.N., F.X., B.L., W.F., M.R.G., X.X., A.P.B., and S.K. interpreted results of experiments; Z.C., Z.Y.Q., S.N., X.L., W.F., L.L., M.R.G., X.X., and A.P.B. prepared figures; N.J.A., S.N., X.L., X.X., A.P.B., and S.K. drafted manuscript; N.J.A., M.R.G., C.M.D., X.X., A.P.B., and S.K. edited and revised manuscript; N.J.A., Z.C., Z.Y.Q., S.N., X.L., J.W.N., F.X., B.L. W.F., L.L., M.R.G., C.M.D., X.X., A.P.B., and S.K. approved final version of manuscript.
REFERENCES
- 1.Muller JM, Davis MJ, Chilian WM. Integrated regulation of pressure and flow in the coronary microcirculation. Cardiovasc Res 32: 668–678, 1996. [PubMed] [Google Scholar]
- 2.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res 41: 163–181, 2000. [PubMed] [Google Scholar]
- 3.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 4.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 5.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 6.Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke 29: 229–234, 1998. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- 7.Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, Traystman RJ, Koehler RC. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol 283: H2029–H2037, 2002. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- 8.Davis CM, Liu X, Alkayed NJ. Cytochrome P450 eicosanoids in cerebrovascular function and disease. Pharmacol Ther 179: 31–46, 2017. doi: 10.1016/j.pharmthera.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gebremedhin D, Gopalakrishnan S, Harder DR. Endogenous events modulating myogenic regulation of cerebrovascular function. Curr Vasc Pharmacol 12: 810–817, 2014. doi: 10.2174/15701611113116660153. [DOI] [PubMed] [Google Scholar]
- 10.Zou AP, Imig JD, Kaldunski M, Ortiz de Montellano PR, Sui Z, Roman RJ. Inhibition of renal vascular 20-HETE production impairs autoregulation of renal blood flow. Am J Physiol Renal Physiol 266: F275–F282, 1994. doi: 10.1152/ajprenal.1994.266.2.F275. [DOI] [PubMed] [Google Scholar]
- 11.Hoopes SL, Garcia V, Edin ML, Schwartzman ML, Zeldin DC. Vascular actions of 20-HETE. Prostaglandins Other Lipid Mediat 120: 9–16, 2015. doi: 10.1016/j.prostaglandins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li PL, Campbell WB. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ Res 80: 877–884, 1997. doi: 10.1161/01.res.80.6.877. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Falck JR, Manthati VL, Jat JL, Campbell WB. 20-Iodo-14,15-epoxyeicosa-8(Z)-enoyl-3-azidophenylsulfonamide: photoaffinity labeling of a 14,15-epoxyeicosatrienoic acid receptor. Biochemistry 50: 3840–3848, 2011. doi: 10.1021/bi102070w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach LO, Storjohann L, Stidsen CE, Jones R, Beck-Sickinger AG, Schwartz TW. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology 148: 13–20, 2007. doi: 10.1210/en.2006-0933. [DOI] [PubMed] [Google Scholar]
- 15.Egerod KL, Holst B, Petersen PS, Hansen JB, Mulder J, Hökfelt T, Schwartz TW. GPR39 splice variants versus antisense gene LYPD1: expression and regulation in gastrointestinal tract, endocrine pancreas, liver, and white adipose tissue. Mol Endocrinol 21: 1685–1698, 2007. doi: 10.1210/me.2007-0055. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res 90: 1028–1036, 2002. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- 17.Sato S, Huang XP, Kroeze WK, Roth BL. Discovery and characterization of novel GPR39 agonists allosterically modulated by zinc. Mol Pharmacol 90: 726–737, 2016. doi: 10.1124/mol.116.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behm DJ, Ogbonna A, Wu C, Burns-Kurtis CL, Douglas SA. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: identification of a novel mechanism of vasodilation. J Pharmacol Exp Ther 328: 231–239, 2009. doi: 10.1124/jpet.108.145102. [DOI] [PubMed] [Google Scholar]
- 19.Yang C, Kwan YW, Au AL, Poon CC, Zhang Q, Chan SW, Lee SM, Leung GP. 14,15-Epoxyeicosatrienoic acid induces vasorelaxation through the prostaglandin EP(2) receptors in rat mesenteric artery. Prostaglandins Other Lipid Mediat 93: 44–51, 2010. doi: 10.1016/j.prostaglandins.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Ma SK, Wang Y, Chen J, Zhang MZ, Harris RC, Chen JK. Overexpression of G-protein-coupled receptor 40 enhances the mitogenic response to epoxyeicosatrienoic acids. PLoS One 10: e0113130, 2015. doi: 10.1371/journal.pone.0113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Qian ZY, Xie F, Fan W, Nelson JW, Xiao X, Kaul S, Barnes AP, Alkayed NJ. Functional screening for G protein-coupled receptor targets of 14,15-epoxyeicosatrienoic acid. Prostaglandins Other Lipid Mediat 132: 31–40, 2017. doi: 10.1016/j.prostaglandins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Y, Zhang W, Giroux C, Cai Y, Ekambaram P, Dilly AK, Hsu A, Zhou S, Maddipati KR, Liu J, Joshi S, Tucker SC, Lee MJ, Honn KV. Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. J Biol Chem 286: 33832–33840, 2011. doi: 10.1074/jbc.M110.216564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia V, Gilani A, Shkolnik B, Pandey V, Zhang FF, Dakarapu R, Gandham SK, Reddy NR, Graves JP, Gruzdev A, Zeldin DC, Capdevila JH, Falck JR, Schwartzman ML. 20-HETE signals through G-protein-coupled receptor GPR75 (Gq) to affect vascular function and trigger hypertension. Circ Res 120: 1776–1788, 2017. doi: 10.1161/CIRCRESAHA.116.310525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauwers E, Landuyt B, Arckens L, Schoofs L, Luyten W. Obestatin does not activate orphan G protein-coupled receptor GPR39. Biochem Biophys Res Commun 351: 21–25, 2006. doi: 10.1016/j.bbrc.2006.09.141. [DOI] [PubMed] [Google Scholar]
- 25.Tremblay F, Perreault M, Klaman LD, Tobin JF, Smith E, Gimeno RE. Normal food intake and body weight in mice lacking the G protein-coupled receptor GPR39. Endocrinology 148: 501–506, 2007. doi: 10.1210/en.2006-1275. [DOI] [PubMed] [Google Scholar]
- 26.Moechars D, Depoortere I, Moreaux B, de Smet B, Goris I, Hoskens L, Daneels G, Kass S, Ver Donck L, Peeters T, Coulie B. Altered gastrointestinal and metabolic function in the GPR39-obestatin receptor-knockout mouse. Gastroenterology 131: 1131–1141, 2006. doi: 10.1053/j.gastro.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Holst B, Egerod KL, Jin C, Petersen PS, Østergaard MV, Hald J, Sprinkel AM, Størling J, Mandrup-Poulsen T, Holst JJ, Thams P, Orskov C, Wierup N, Sundler F, Madsen OD, Schwartz TW. G protein-coupled receptor 39 deficiency is associated with pancreatic islet dysfunction. Endocrinology 150: 2577–2585, 2009. doi: 10.1210/en.2008-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fjellström O, Larsson N, Yasuda S, Tsuchida T, Oguma T, Marley A, Wennberg-Huldt C, Hovdal D, Fukuda H, Yoneyama Y, Sasaki K, Johansson A, Lundqvist S, Brengdahl J, Isaacs RJ, Brown D, Geschwindner S, Benthem L, Priest C, Turnbull A. Novel Zn2+ modulated GPR39 receptor agonists do not drive acute insulin secretion in rodents. PLoS One 10: e0145849, 2015. doi: 10.1371/journal.pone.0145849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, Barnes AP, Alkayed NJ. Role of GPR39 in neurovascular homeostasis and disease. Int J Mol Sci 22: 8200, 2021. doi: 10.3390/ijms22158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilad D, Shorer S, Ketzef M, Friedman A, Sekler I, Aizenman E, Hershfinkel M. Homeostatic regulation of KCC2 activity by the zinc receptor mZnR/GPR39 during seizures. Neurobiol Dis 81: 4–13, 2015. doi: 10.1016/j.nbd.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mlyniec K, Budziszewska B, Holst B, Ostachowicz B, Nowak G. GPR39 (zinc receptor) knockout mice exhibit depression-like behavior and CREB/BDNF down-regulation in the hippocampus. Int J Neuropsychopharmacol 18: pyu002, 2014. doi: 10.1093/ijnp/pyu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peralta FA, Huidobro-Toro JP. Zinc as allosteric ion channel modulator: ionotropic receptors as metalloproteins. Int J Mol Sci 17: 1059, 2016. doi: 10.3390/ijms17071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu D, Su Y, Zheng Y, Fu B, Tang L, Qin YX. Zinc regulates vascular endothelial cell activity through zinc-sensing receptor ZnR/GPR39. Am J Physiol Cell Physiol 314: C404–C414, 2018. doi: 10.1152/ajpcell.00279.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voelkl J, Tuffaha R, Luong TTD, Zickler D, Masyout J, Feger M, Verheyen N, Blaschke F, Kuro OM, Tomaschitz A, Pilz S, Pasch A, Eckardt KU, Scherberich JE, Lang F, Pieske B, Alesutan I. Zinc inhibits phosphate-induced vascular calcification through TNFAIP3-mediated suppression of NF-κB. J Am Soc Nephrol 29: 1636–1648, 2018. doi: 10.1681/ASN.2017050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, Sullards CM, Wang E, Murphy RC, Barkley RM, Leiker TJ, Raetz CR, Guan Z, Laird GM, Six DA, Russell DW, McDonald JG, Subramaniam S, Fahy E, Dennis EA. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res 51: 3299–3305, 2010. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walenga RW, Boone S, Stuart MJ. Analysis of blood HETE levels by selected ion monitoring with ricinoleic acid as the internal standard. Prostaglandins 34: 733–748, 1987. doi: 10.1016/0090-6980(87)90296-6. [DOI] [PubMed] [Google Scholar]
- 37.Mazaleuskaya LL, Salamatipour A, Sarantopoulou D, Weng L, FitzGerald GA, Blair IA, Mesaros C. Analysis of HETEs in human whole blood by chiral UHPLC-ECAPCI/HRMS. J Lipid Res 59: 564–575, 2018. doi: 10.1194/jlr.D081414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The human serum metabolome. PLoS One 6: e16957, 2011. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walenga RW, Sunderji S, Stuart MJ. Formation of hydroxyeicosatetraenoic acids (HETE) in blood from adults versus neonates: reduced production of 12-HETE in cord blood. Pediatr Res 24: 563–567, 1988. doi: 10.1203/00006450-198811000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Lu S, Herzlinger M, Cao W, Noble L, Yang D, Shapiro J, Kurtis J, LeLeiko N, Resnick M. Utility of 15(S)-HETE as a serological marker for eosinophilic esophagitis. Sci Rep 8: 14498, 2018. doi: 10.1038/s41598-018-32944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lundqvist A, Sandstedt M, Sandstedt J, Wickelgren R, Hansson GI, Jeppsson A, Hultén LM. The arachidonate 15-lipoxygenase enzyme product 15-HETE is present in heart tissue from patients with ischemic heart disease and enhances clot formation. PLoS One 11: e0161629, 2016. doi: 10.1371/journal.pone.0161629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shishehbor MH, Zhang R, Medina H, Brennan ML, Brennan DM, Ellis SG, Topol EJ, Hazen SL. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Radic Biol Med 41: 1678–1683, 2006. doi: 10.1016/j.freeradbiomed.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le DE, García-Jaramillo M, Bobe G, Alcazar Magana A, Vaswani A, Minnier J, Jump DB, Rinkevich D, Alkayed NJ, Maier CS, Kaul S. Plasma oxylipins: a potential risk assessment tool in atherosclerotic coronary artery disease. Front Cardiovasc Med 8: 645786, 2021. doi: 10.3389/fcvm.2021.645786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Naamani N, Sagliani KD, Dolnikowski GG, Warburton RR, Toksoz D, Kayyali U, Hill NS, Fanburg BL, Roberts KE, Preston IR. Plasma 12- and 15-hydroxyeicosanoids are predictors of survival in pulmonary arterial hypertension. Pulm Circ 6: 224–233, 2016. doi: 10.1086/686311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theken KN, Schuck RN, Edin ML, Tran B, Ellis K, Bass A, Lih FB, Tomer KB, Poloyac SM, Wu MC, Hinderliter AL, Zeldin DC, Stouffer GA, Lee CR. Evaluation of cytochrome P450-derived eicosanoids in humans with stable atherosclerotic cardiovascular disease. Atherosclerosis 222: 530–536, 2012. doi: 10.1016/j.atherosclerosis.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oni-Orisan A, Edin ML, Lee JA, Wells MA, Christensen ES, Vendrov KC, Lih FB, Tomer KB, Bai X, Taylor JM, Stouffer GA, Zeldin DC, Lee CR. Cytochrome P450-derived epoxyeicosatrienoic acids and coronary artery disease in humans: a targeted metabolomics study. J Lipid Res 57: 109–119, 2016. doi: 10.1194/jlr.M061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976-1980). Am J Clin Nutr 78: 756–764, 2003. doi: 10.1093/ajcn/78.4.756. [DOI] [PubMed] [Google Scholar]
- 48.Food and Agricultural Organization (FAO) and World Health Organization (WHO). Human Vitamin and Mineral Requirements: Report of a Joint FAO/WHO Expert Consultation. Rome: Food and Nutrition Division, 2001. [Google Scholar]
- 49.Davis CM, Bah TM, Zhang WH, Nelson JW, Golgotiu K, Nie X, Alkayed FN, Young JM, Woltjer RL, Silbert LC, Grafe MR, Alkayed NJ. GPR39 localization in the aging human brain and correlation of expression and polymorphism with vascular cognitive impairment. Alzheimers Dement (NY) 7: e12214, 2021. doi: 10.1002/trc2.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alonso-Galicia M, Hudetz AG, Shen H, Harder DR, Roman RJ. Contribution of 20-HETE to vasodilator actions of nitric oxide in the cerebral microcirculation. Stroke 30: 2727–2734, 1999. doi: 10.1161/01.str.30.12.2727. [DOI] [PubMed] [Google Scholar]
- 51.Sun CW, Falck JR, Okamoto H, Harder DR, Roman RJ. Role of cGMP versus 20-HETE in the vasodilator response to nitric oxide in rat cerebral arteries. Am J Physiol Heart Circ Physiol 279: H339–H350, 2000. doi: 10.1152/ajpheart.2000.279.1.H339. [DOI] [PubMed] [Google Scholar]
- 52.Xie S, Jiang X, Doycheva DM, Shi H, Jin P, Gao L, Liu R, Xiao J, Hu X, Tang J, Zhang L, Zhang JH. Activation of GPR39 with TC-G 1008 attenuates neuroinflammation via SIRT1/PGC-1α/Nrf2 pathway post-neonatal hypoxic-ischemic injury in rats. J Neuroinflammation 18: 226, 2021. doi: 10.1186/s12974-021-02289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Methner C, Cao Z, Mishra A, Kaul S. Mechanism and potential treatment of the “no reflow” phenomenon after acute myocardial infarction: role of pericytes and GPR39. Am J Physiol Heart Circ Physiol 321: H1030–H1041, 2021. doi: 10.1152/ajpheart.00312.2021. [DOI] [PubMed] [Google Scholar]
- 54.Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, Alkayed NJ. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab 27: 1931–1940, 2007. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siler DA, Martini RP, Ward JP, Nelson JW, Borkar RN, Zuloaga KL, Liu JJ, Fairbanks SL, Raskin JS, Anderson VC, Dogan A, Wang RK, Alkayed NJ, Cetas JS. Protective role of p450 epoxyeicosanoids in subarachnoid hemorrhage. Neurocrit Care 22: 306–319, 2015. doi: 10.1007/s12028-014-0011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, Gooch R, Foley J, Newman J, Mao L, Rockman HA, Hammock BD, Murphy E, Zeldin DC. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res 95: 506–514, 2004. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- 57.Renic M, Klaus JA, Omura T, Kawashima N, Onishi M, Miyata N, Koehler RC, Harder DR, Roman RJ. Effect of 20-HETE inhibition on infarct volume and cerebral blood flow after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab 29: 629–639, 2009. doi: 10.1038/jcbfm.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285: 1276–1279, 1999. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crago EA, Thampatty BP, Sherwood PR, Kuo CW, Bender C, Balzer J, Horowitz M, Poloyac SM. Cerebrospinal fluid 20-HETE is associated with delayed cerebral ischemia and poor outcomes after aneurysmal subarachnoid hemorrhage. Stroke 42: 1872–1877, 2011. doi: 10.1161/STROKEAHA.110.605816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W, Davis CM, Zeppenfeld DM, Golgotiu K, Wang MX, Haveliwala M, Hong D, Li Y, Wang RK, Iliff JJ, Alkayed NJ. Role of endothelium-pericyte signaling in capillary blood flow response to neuronal activity. J Cereb Blood Flow Metab 41: 1873–1885, 2021. doi: 10.1177/0271678X211007957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie F, Li BX, Alkayed NJ, Xiao X. Synthesis of 14,15-EET from arachidonic acid using urea–hydrogen peroxide as the oxidant. Synthetic Comm 45: 105–110, 2015. doi: 10.1080/00397911.2014.956369. 34623177 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Methods, Supplemental Tables S1 and S2, and Supplemental Figs. S1-S7: https://doi.org/10.6084/m9.figshare.17408957.
Data Availability Statement
Complete MS data have been deposited to the ProteomeXchange Consortium via the PRIDE database (data set identifier PXD013952). The data that support other findings of this study are available from the corresponding author upon reasonable request.