ABSTRACT
Haematopoietic microenvironmental niches have been described as the ‘gatekeepers’ for the blood and immune systems. These niches change during ontogeny, with the bone marrow becoming the predominant site of haematopoiesis in post-natal life under steady state conditions. To determine the structure and function of different haematopoietic microenvironmental niches, it is essential to clearly define specific haematopoietic stem and progenitor cell subsets during ontogeny and to understand their temporal appearance and anatomical positioning. A variety of haematopoietic and non-haematopoietic cells contribute to haematopoietic stem and progenitor cell niches. The latter is reported to include endothelial cells and mesenchymal stromal cells (MSCs), skeletal stem cells and/or C-X-C motif chemokine ligand 12-abundant-reticular cell populations, which form crucial components of these microenvironments under homeostatic conditions. Dysregulation or deterioration of such cells contributes to significant clinical disorders and diseases worldwide and is associated with the ageing process. A critical appraisal of these issues and of the roles of MSC/C-X-C motif chemokine ligand 12-abundant-reticular cells and the more recently identified skeletal stem cell subsets in bone marrow haematopoietic niche function under homeostatic conditions and during ageing will form the basis of this research review. In the context of haematopoiesis, clinical translation will deal with lessons learned from the vast experience garnered from the development and use of MSC therapies to treat graft versus host disease in the context of allogeneic haematopoietic transplants, the recent application of these MSC therapies to treating emerging and severe coronavirus disease 2019 (COVID-19) infections, and, given that skeletal stem cell ageing is one proposed driver for haematopoietic ageing, the potential contributions of these stem cells to haematopoiesis in healthy bone marrow and the benefits and challenges of using this knowledge for rejuvenating the age-compromised bone marrow haematopoietic niches and restoring haematopoiesis.
Key Words: ageing, COVID-19, GvHD, haematopoietic stem cell niche, mesenchymal stromal cells, rejuvenating niche, skeletal stem cells
Introduction
The concept of specialised haematopoietic microenvironmental niches originated, and was developed, from a number of seminal studies1-10 prior to and during the 1970s. These led to or confirmed the existence of haematopoietic inductive microenvironments,11, 12 where distinct stromal microenvironments induce the differentiation of specific haematopoietic cell subsets. They led to the proposal that haematopoietic stem cells (HSCs) also reside and are maintained in specific HSC microenvironmental niches, where their fate is determined.1 HSCs, residing in adult bone marrow microenvironmental niches, were once viewed as generating all blood and immune cells required throughout mammalian adult life. Further studies have replaced this concept with one of a layered haematopoietic system, which develops in successive waves during ontogeny, with haematopoietic progenitor cells emerging first in the yolk sac before definitive HSCs emerge in the embryo proper.13, 14 Some, but not all, of the progeny of these haematopoietic precursors are now known to persist into and throughout adult life as self-renewing cells.14-16 By tracing and analysing these multiple waves of haematopoiesis during mammalian ontogeny, it is now evident that specific haematopoietic cell subsets develop over time at distinct anatomical sites in specialised microenvironments in order to meet the organism’s temporal needs. Growing evidence supports the existence of at least three sequential, but overlapping, waves of haematopoiesis during mammalian embryonic development, with haematopoietic precursors from the second and third waves of embryonic haematopoiesis colonising the foetal liver, a major haematopoietic organ in foetal life, before HSCs migrate to the foetal bone marrow where they establish their main place of residence in post-natal life.14-26
The vascular system is integral to haematopoietic stem and progenitor cell (HSPC) generation during embryonic development and plays pivotal roles in adult haematopoiesis.16-18, 24-26 In adult mammalian bone marrow, mesenchymal stromal cells (MSCs), which include C-X-C motif chemokine ligand 12 (CXCL12)-abundant reticular (CAR; LEPR+) cells and osteoblasts, are reported to constitute, though not exclusively, major components of HSPC niches.27-31 Despite this, considerable confusion and controversy has surrounded efforts to accurately define this heterogeneous group of cells and to identify the stem cells from which they originate. Here, the focus will be to first examine the evidence for the appearance of different HSPC subsets during embryonic, foetal and post-natal life. This will be followed by a review of evidence for the existence of distinct microenvironmental niches for diverse subsets of HSPCs in adult bone marrow in both mouse and man, the role played by MSCs in these niches in relation to HSPC fate decisions under homeostatic conditions and the degeneration of these niches during ageing. A vast amount of experience has been gained from the development of MSC therapies to treat a variety of clinical disorders ranging from bone and joint to immune related diseases. As an exemplar, knowledge gained and lessons learned from the therapeutic use of MSCs in treating graft versus host disease (GvHD), an immunomodulatory therapy that has benefits in the allogeneic haematopoietic transplantation arena but with its success being reliant in part on high quality, well characterised and robust MSC formulations, will be critically discussed. Given the experience gained with this and similar MSC immunosuppressive therapies, the recent adaptation of such MSC therapies to treat patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection will also be briefly reviewed. Such MSC therapies have been particularly aimed at combating, during the current coronavirus disease 2019 (COVID-19) pandemic, the SARS-CoV-2 initiated cytokine storm, a hyperinflammatory immune response resulting in significant patient morbidity and mortality and leading to significant tissue and organ damage (pneumonia, acute respiratory distress syndrome and multi-organ failure). Finally, it is estimated that, globally, one in six adults will be 60 years of age or older by 2030. This ageing process is reported to be accompanied by a decline in HSC and mesenchymal or skeletal stem cell regenerative capacities, due to intrinsic and extrinsic controls, and is associated with low grade, chronic inflammation in the bone and bone marrow ecosystems. Thus, this review concludes with a discussion of the convergence of this knowledge and the prospects to prevent or ameliorate haematological decline during ageing by rejuvenating the haematopoietic niche. For this review, electronic searches of the Medline database for literature describing haematopoietic microenvironmental niches from January 1, 1940 to December 31, 2021 were performed using the following conditions: i) haematopoietic microenvironmental niches (MeSH Terms) and/or animal models (MeSH Terms), and/or MSCs (MeSH Terms), and/or ageing (MeSH Terms); ii) HSC (MeSH Terms) and/or ontogeny (MeSH Terms); and iii) skeletal stem cells (MeSH Terms) or haematopoietic ontogeny (MeSH Terms) or haematopoietic rejuvenation (MeSH Terms) or MSC and GvHD (MeSH Terms) or MSC and COVID-19 (MeSH Terms). The results were further screened on the Medline database, and by title and abstract to include humans, mice and non-human primates only and for their particular relevance to this review.
Changing Haematopoietic Microenvironments during Development
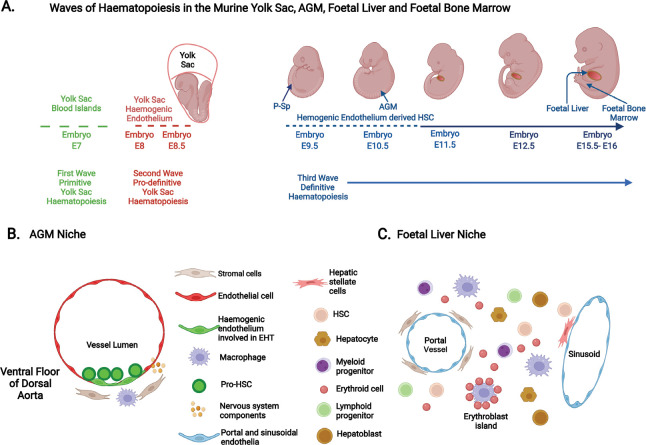
Given the limited availability of human embryonic and fetal tissues, knowledge of human haematopoiesis has relied, not exclusively but principally, on experiments using murine and in vitro pluripotent stem cell models.14, 31 Although extensive studies in mice provide invaluable insights, they do not always faithfully recapitulate human haematopoietic ontogeny.14, 31 Nevertheless, these studies will be discussed and related to the current understanding of human haematopoietic microenvironmental niches. A diagrammatic illustration of the changing locations of murine haematopoiesis is provided in Figure 1A, with examples of the cellular types that comprise the HSC niche in the AGM region and the foetal liver shown in Figure 1B and C respectively.
Figure 1. Haematopoietic ontogeny in murine models. (A) The three distinct waves of haematopoiesis that occur in the developing murine embryo. The first primitive wave sees the emergence of haematopoietic cells (nucleated erythroid cells, macrophages and megakaryocytes) at E7 (7 days post-conception) from the blood islands of the yolk sac. The second pro-definitive wave arises from haemogenic endothelium of the vascular plexus of the yolk sac by the process of endothelial-haematopoietic transition (EHT) commencing at E8-8.5 and generates erythro-myeloid progenitors and certain innate immune cells. The para-aortic splanchnopleura (P-Sp) and the aorta-gonad-mesonephros (AGM) region of the embryo proper become the first and principal site of immature hematopoietic stem cell (or pro-haematopoietic stem cell (HSC)) production between E9.5-10.5. These pro-HSCs migrate (between E10.5-11) to the foetal liver, where they mature, proliferate, self-renew and/or differentiate into lymphoid and myeloid cells. The foetal liver then becomes the major haematopoietic organ until E15.5. Foetal liver HSCs migrate to the foetal bone marrow, which becomes the main residence of HSCs in adulthood. (B) A schematic cross-section of the murine embryonic AGM region with pro-HSCs emerging from the ventral floor of the dorsal aorta from haemogenic endothelia by the process of EHT. Here components of the HSC niche include endothelia, mesenchymal stromal cells, macrophages, and sympathetic nerve components. (C) A diagrammatic representation of cells present in the murine foetal liver microenvironment at E14.5 and where HSCs expand, self-renew and differentiate. These include endothelial cells of the portal and sinusoidal vessels, perivascular stromal cells, hepatic stellate cells, hepatocytes and hepatoblasts that produce cytokines, macrophages, proliferating HSCs and various haematopoietic progenitors, both myeloid and lymphoid as well as erythroid cells. Created with BioRender.com.
In mice, it is accepted that haematopoiesis begins in the yolk sac and, in this developing environment, it is thought that this occurs in two successive waves.14, 23-26, 32-34 The first wave, termed primitive haematopoiesis, emerges at around embryonic day (E) 7, and sees the generation of nucleated erythroid cells, and, to a lesser extent megakaryocytes and macrophages (including microglia in the brain). This is closely followed by a second wave of yolk sac haematopoiesis commencing at E8–8.5 and termed pro-definitive (or transient definitive) haematopoiesis. This is characterised by the generation of erythro-myeloid progenitors (EMPs), derived from haemogenic endothelium (HE) of the early vascular plexus of the yolk sac via the process of endothelial-to-haematopoietic transition (EHT).33, 34 The yolk sac-derived EMPs seed the foetal liver between E9.5-10.5, and give rise, as a minimum, to enucleated erythroid cells, macrophages (including tissue resident macrophages in multiple organs), megakaryocytes, mast cells and granulocytes, and potentially certain innate lymphoid cells.14, 23-27, 32-41 The third wave of haematopoiesis is reported to generate multipotent progenitors (MPPs), lymphoid restricted progenitors and lympho-myeloid progenitors lacking long term in vivo repopulating ability in mice.41 These appear to originate from distinctive HE principally in the developing the ventral wall (and to a lesser extent from the dorsal endothelia) of the dorsal aorta in the aorta-gonad-mesonephros (AGM) region by the process of EHT. 14, 24-26, 33, 34, 41 Immature or pro-HSCs then emerge by a similar process (Figure 1B), but from an HE subset that is distinct from those generating MPPs, before maturing into long term repopulating HSCs at approximately E11.5.14, 41 In mice, secondary sites of de novo HSC formation from HE have been reported to follow those produced in the AGM, and these sites include the vitelline and umbilical arteries, the placenta and the embryonic head.24
The murine foetal liver then becomes the major site for differentiation of HE-derived haematopoietic progenitors and for the maturation/expansion of definitive pre-HSC and HSC, before HSCs colonise the foetal spleen and foetal bone marrow.42 Interestingly, Yvergnogeau and colleagues43 have also recently identified HE in murine foetal/young adult bone marrow as a site for de novo MPP3 and to a lesser extent HSC generation. After approximately 3-4 weeks of post-natal life, the foetal characteristics (e.g., metabolic state, cell cycle behaviour, self-renewal potential, lineage output, repopulation kinetics) of HSCs have switched to an adult bone marrow phenotype.42, 44-47 Recent single cell-RNA sequencing (sc-RNAseq), assay for transposase-accessible chromatin with high-throughput sequencing (ATACseq) and chromatin immunoprecipitation sequencing (ChIPseq) analyses support the view that this transition of HSCs from foetal-to-adult states occurs gradually, rather than abruptly.47 It is widely accepted that distinct subsets of yolk sac-derived haematopoietic cells can persist in certain adult tissues, alone or together with their HSC-derived counterparts.23, 25, 35, 36, 39, 40, 48
Based on these experimental murine models, it is presumed that human haematopoiesis in the yolk sac also occurs in two successive waves.14, 16-20, 22, 31 The first wave of primitive haematopoiesis commences in the secondary extra-embryonic yolk sac, the formation of which is unique to primates,21 at around Carnegie stage (CS) 7-8 of embryonic development (16-18.5 days post-conception (dpc)).14, 16 The haematopoietic precursors are surrounded by and closely associated with yolk sac endothelial cells, known as blood islands.19, 20 Evidence for a distinct second wave of pro-definitive haematopoiesis in the human yolk sac is limited, but is presumed to occur at CS13-15 (∼27-35 dpc)14, 16 and to be characterised by the generation of EMP and certain lymphoid progenitors derived from yolk sac HE via the process of EHT.49-51 Some of the multipotent progenitors generated demonstrate short term haematopoietic reconstituting ability following in vivo transplantation into murine models.16-18 The third wave of, or definitive, haematopoiesis sees the formation of human haematopoietic progenitor cells and immature HSCs, arising from HE in the AGM region by EHT, principally from the ventral wall of the dorsal aorta of the human embryo proper between CS13-17 (27-42 dpc).14, 16-18, 52-54 The emergence of the foetal liver rudiment at early CS10 (21 dpc) provides the next important human haematopoietic microenvironmental niche.14, 16 This is thought to be seeded first by yolk sac-derived primitive nucleated erythroid cells and CD45+ macrophages (∼late CS10, 22 dpc), then, from CS13 (27-29 dpc), by yolk sac CD34+CD45+ cells reminiscent of murine EMPs,55-57 and finally by definitive AGM-derived immature HSCs between CS13 and CS17 (27-42 dpc), thus becoming the major human foetal haematopoietic organ from 6 to 7 weeks of gestation until the mid-second trimester.58, 59 As yolk sac haematopoiesis begins to decline and shortly after bone formation commences at CS23 (56 dpc), human foetal liver HSCs seed into and colonise human foetal bone cavities, which become the dominant site of haematopoiesis after 20 post-conceptional weeks.14, 58, 59 Whether HSCs are also generated de novo by HE in human foetal/young adult bone marrow has not been reported. Recent molecular interrogation (including the use of sc-RNAseq, sc-ATACseq) of human foetal liver, and foetal, paediatric, and adult bone marrow HSPC subsets at single cell resolution, however, has extended our understanding of the developmental changes that occur in the human HSPC compartment over this time.19, 59-65 These studies demonstrate high levels of transcriptional heterogeneity in progenitor subsets, and confirm a shift from mainly erythroid-megakaryocyte lineages in the early first trimester foetal liver to lympho-myeloid lineages as haematopoiesis shifts from the foetal liver to the foetal bone marrow in the second trimester. PreProB- and ProB-progenitors are found in first trimester foetal liver, before B lymphoid cells expand in the foetal bone marrow, with PreProB-progenitors essentially being absent from adult bone marrow.61 Human HSCs, like their murine counterparts, demonstrate a progression from an actively cycling state in the foetal liver to a progressively quiescent state in foetal and then post-natal bone marrow,63, 65 a change associated with increased inflammatory signalling.63
Bone Marrow as a Specialised Niche for Adult Haematopoiesis
In adult life under normal physiological conditions, human haematopoiesis is orchestrated principally in the bone marrow, where it has become the dogma that HSCs interact with specific micro-environmental niches, which support their survival and quiescence, or promote their ability to self-renew or differentiate into multiple or lineage-biased haematopoietic lineages.66, 67 Yet, estimates for total numbers of cells in this HSC pool in normal adult human bone marrow vary widely, ranging from 384 ‘active’ HSCs to hundreds of thousands of HSCs.68-71 Despite this, it has been generally accepted that, during unperturbed haematopoiesis, the most ‘primitive’ adult bone marrow HSCs are maintained in a quiescent state, where they may remain for extended periods, while maintaining the highest regenerative potential.72, 73 More ‘mature’ HSCs are considered quiescent over shorter times before moving to an active state where they are primed to enter the cell cycle for self-renewal and/or to differentiate into multipotent or more committed lineage biased progenitors that primarily drive the production of more mature haematopoietic cells throughout adult life.71-73Although HSCs are rare, the phenotypically defined HSC pool is molecularly and functionally heterogeneous, and methodologically challenging to study. This diversity has hindered studies on the intrinsic and extrinsic regulation of both human and murine haematopoiesis.74, 75 Nevertheless, over time, increasingly sophisticated technological advances have seen a significant evolution of HSC differentiation models.74, 75
In the original classical haematopoietic lineage hierarchical model, HSCs with the highest long term haematopoietic reconstituting and self-renewal abilities are positioned at the apex of a structured hierarchy of multipotential and increasingly lineage restricted progenitors. These multi-potent and long term repopulating HSCs are upstream from HSCs or MPPs that have shorter term haematopoietic repopulating abilities, and that branch into more restricted erythro-myeloid and lymphoid progenitors, eventually giving rise to mature blood and immune cells.65 The incorporation of more sophisticated single cell selection or identification strategies, and single cell genomics and other multi-omics approaches, with or without functional analyses, barcoding, mutational analyses and mathematical/computational modelling under steady state and perturbed conditions has led to a number of iterations of the hierarchical lineage tree model for both murine and human haematopoiesis. As an example, one recent study emphasises the heterogeneity of the originally described murine ‘HSC pool’, in which at least six MPP subsets lie downstream of a CD34- multipotent HSC subset with the highest self-renewal potential.76, 77 Although differentiation trajectories from HSCs to these MPPs are still under investigation, they appear to include an HSC-like subset with more restricted in vivo haematopoietic reconstitution potential (MMP6), a metabolically active HSC-like subset (MMP1), a subset designed for both transient emergency myelopoiesis and long term stable lymphoid reconstitution potential (MPP5), myeloid biased subsets (MMP2 and MMP3), and lymphoid primed subsets capable of being reprogrammed towards the myeloid lineage (MPP4).77, 78 These and other studies suggest that the lineage tree is more fluid, more complex and more heterogeneous than predicted earlier, being more consistent with the continuum model, in which functionally mature haematopoietic cells constantly differentiate from a continuum of low-primed HSPCs with distinct and flexible transcriptional programs that can rapidly be modified to respond to haematological needs, and thus may not exist strictly as a stepwise hierarchy.60 This is reminiscent of lineage promiscuity concepts of gene expression in normal bipotent or multipotent progenitors.79 Additionally, the exact progenitor cells that generate functionally mature haematopoietic cells under homeostatic conditions in adult bone marrow are still debated, with support for the premise that mature haematopoietic cells primarily originate from MPPs, or that HSCs make consistent and significant contributions to generating mature haematopoietic cells.77, 80-83 This complexity is further enhanced by reports as defined in a recent review that cells judged to be in specific HSPC subsets may follow different pathways of differentiation before generating end cells of the same lineage.75, 84
HSCs are mobile within their specific niches, with this motility being enhanced as they proliferate or differentiate.85 With some putative HSC niches in bone marrow being unoccupied during steady state haematopoiesis, it remains possible that these become accessible for those HSCs in an altered state of activity or for their progeny. Indeed, haematopoietic niches complementary to and spatially distinct from HSC niches in adult bone marrow have been reported to regulate the proliferation, differentiation, and migration of the HSC progeny, so that haematopoietic cells can be replenished in the peripheral blood and relevant tissues throughout adult life. This extraordinary regenerative capacity is exemplified in healthy adult human bone marrow by the production of between 1 × 1011 to 1 × 1012 haematopoietic cells per day.86
Structure and Architecture of the Adult Bone Marrow and its Vasculature
Adult bone marrow
Under normal homeostatic conditions, adult murine bone marrow haematopoiesis involves all bones, whereas adult human bone marrow haematopoiesis occurs principally in the axial skeleton, which includes the vertebrae, ribs, sternum, cranium, and ilium, and to a lesser extent in the proximal areas of the long bones.67, 87 Most research on adult bone marrow haematopoietic niches, however, has been conducted in, and influenced by, studies performed on long bones (femurs, tibiae), and on flat bones (calvaria and occasionally the sternum) of mice. Given this reliance on murine studies, the architecture of the adult bone marrow haematopoietic niche in the mouse, especially in the long bones, will be briefly described and related to information available on the adult human bone marrow niche.
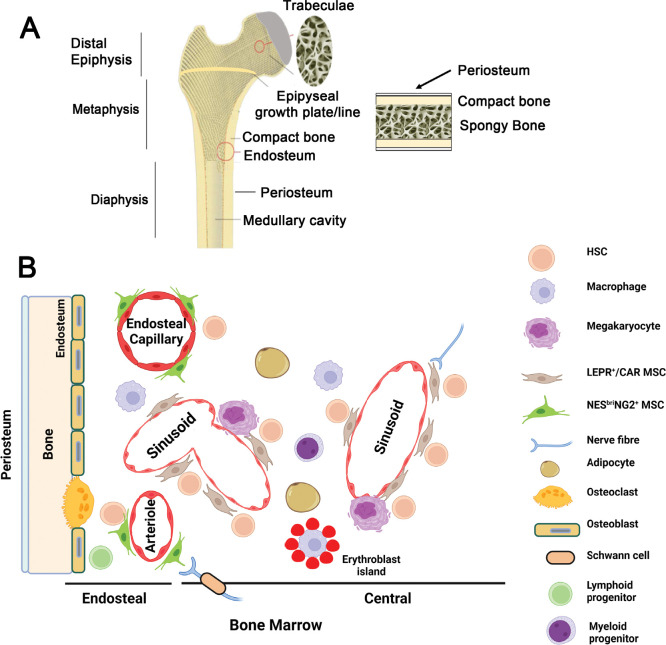
The long bones of adult mice comprise a central shaft, the diaphysis, linked to the metaphyses and epiphyses at each end of the bone (Figure 2A).88, 89 The diaphysis is primarily composed of cortical or compact bone, which separates the outer highly vascularised periosteum from the inner medullary cavity containing bone marrow. The epiphyses, metaphyses and the rim of the diaphysis in the long bones, and the flat bones (e.g., sternum and ilium) contain an anastomosing network of bony trabeculae, which extends inwards from the cortical bone (Figure 2A). Spaces between the trabeculae are filled with haematopoietic (red) bone marrow. Cortical and trabecular bone surfaces adjacent to bone marrow, the endosteum, are lined with osteoblasts and/or osteoclasts (Figure 2B).88, 89 In the adult mouse, but not the adult human, the epiphysis and metaphysis remain separated by the growth plate that forms in the foetal bone marrow.89 In human long bones from early post-natal life, there is a gradual and progressive conversion of the haematopoietic (red) bone marrow to fatty (yellow) bone marrow possessing little haematopoietic activity, a process that occurs much later in murine long bones.87
Figure 2. Architecture of long and flat bones. (A) Diagrammatic representations of an adult long bone showing the epiphyseal, metaphyseal and diaphyseal regions (left) and an adult flat bone (right). Trabeculae and the vasculature (not shown) play key roles in steady state haematopoiesis. In human adults, the epiphyseal or growth plate is replaced by an epiphyseal line. In the adult mouse, the epiphysis and metaphysis remain separated by the growth plate that forms in the foetal bone marrow. (B) A commonly accepted diagrammatic representation of cells that are proposed to have a key role in the haematopoietic stem cells (HSC) niche. Endothelia of the endosteal capillaries, arterioles and sinusoids are associated with mesenchymal stromal cells (MSCs) to varying degrees and form perivascular niches for endosteal and central bone marrow HSC subsets as described. NESbriNG2+ MSCs are associated with endosteal capillaries and arterioles. The endosteum is also lined with osteoblasts and osteoclasts which are derived from MSCs and HSCs respectively and is reported to play a role in maintaining lymphoid biased HSCs and a reserve of multi-potent long term repopulating HSCs. LEPR+/CAR MSCs are associated with sinusoids in the bone marrow, where erythropoiesis and myelopoiesis are regulated. Some recent studies suggest that the sinusoids may lie closer to the endosteum than previously indicated. MSC production of the key HSC-regulator CXCL12 is altered by the action of sympathetic nerve fibres. Adipocytes, which are also derived from MSCs, and megakaryocytes regulate HSCs in these bone marrow niches. Platelet/myeloid biased HSCs are thought to associate with the megakaryocyte-sinusoidal niche. The roles and spatial distribution of these various cells in regulating HSC fate decisions remains a matter of some debate. CXCL12: C-X-C motif chemokine ligand 12; LEPR+/CAR: C-X-C motif chemokine ligand 12-abundant reticular; NESBri: Nestinbright; NG2: neural-glial antigen 2. Created with BioRender.com.
The adult bone and bone marrow vasculature
The vascular system is pivotal to the regulation of adult haematopoiesis and integral to HSC niches. Detailed descriptions of the vasculature in murine long bones can be found in recent reviews.88-93 Although as many as 16 nutrient arteries have been reported,94 historically most studies concentrate on several arteries that enter the long bones. These include the principal nutrient artery, which was reported to supply blood to a significant portion (two-thirds) of the diaphyseal cortical bone and the medulla and to enter the murine long bones through the cortical bone via the nutrient canal or principal foramen near the mid diaphysis.88-92 This gives rise to smaller arteries and then arterioles that move from a central marrow position and radiate towards the endosteum, following the long axis of the bone. Near the endosteal and trabecular bone in the metaphyses, these arterioles together with transcortical vessels connect via interstitial (CD31hiEndomucinhi or type H) capillaries into a network of VEGFR3+CD31lo Endomucinlo sinusoidal vessels (type L), that subsequently form collecting sinuses before connecting, in the central marrow cavity, to the central venous sinus.88-93 The latter extends along the metaphyses and diaphysis and joins the nutrient vein, which exits the bone via the nutrient canal, adjacent to the incoming central artery. Two exit sites for collecting veins have been reported at the end of some murine long bones.94 Periosteal arteries have been reported to supply blood to the other one-third of the cortical bone capillaries in the metapyses.90, 91 Metaphyseal and epiphyseal arteries enter the metaphyses and epiphyses via the cortical bone towards the ends of the long bones.88-92 The blood supply to the epiphyses (important for skeletal growth) exits via the epiphysis and does not enter the diaphysis.88-92 In the metaphysis, the arteries extend longitudinally towards the diaphysis and the growth plate, transversing the trabecular surfaces, with arterial blood draining into sinusoids and capillaries near the endosteum and growth plate.88-92 Venule networks also allow the exit of blood from the metaphyses and periosteum.89 In flat bones, trabecular or cancellous bone is surrounded by cortical bone with the arterial blood supply connecting to sinusoids that lie close to the endosteum.89, 90 Recent state of the art imaging studies have emphasised the potential importance along the bone shaft of many hundreds of transcortical vessels, which are reported to provide over 80% of arterial and almost 60% of venous blood flow through the cortex of the long bones in a closed loop system and are thought to be essential for the rapid release of blood cells to the periphery.94-96 Transcortical vessels have also been detected in human long bones and cranial bones.94-96
Adult Bone Marrow Haematopoietic Stem Cell Niches
The location of microenvironmental niches for HSCs and their progeny, and the contribution of haematopoietic and non-haematopoietic cells to forming these niches, in adult bone marrow, have been the subject of intense investigations. Although the concept of the HSPC niches has evolved over time, the fine detail of these niches continues to be debated particularly in view of the evolving heterogeneity of both HSPCs and microenvironmental stromal cells, the controversy regarding the MSC terminology, and the difficulties in identifying these functional cells with specific biomarkers in situ. A commonly accepted view of the adult bone marrow niches is illustrated in Figure 2B. Knowledge of the human HSC and haematopoietic progenitor cell niches has often relied on ex vivo model systems, xenotransplantation studies in immunodeficient mice, complementary studies in non-human primates, clinical transplantation studies, and single cell molecular analyses.
Three general types of HSPC niches in adult bone marrow have been proposed historically, the endosteal ‘osteoblastic’ niche, the vascular (periarteriolar and perisinusoidal) niches and the central bone marrow niche. Attempts to decipher the importance and complexity of these haematopoietic microenvironments and niches have more recently been conducted using advanced cell and molecular technologies, and by comparing steady state conditions to those subjected to haematological stress. As well as the different endothelial cells of the vasculature, cellular components of haematopoietic niches include i) MSCs (e.g., LEPR+ cells, osteocytes, osteoblasts, adipocytes), ii) haematopoietic cells, such as macrophages, osteoclasts, megakaryocytes and regulatory T cells, and iii) nerve cells such as non-myelinating Schwann cells, sympathetic nerve fibres and nociceptive neurons.97-106 Some investigators consider that cells essential to the HSC niche include distinct endothelial cell subsets, MSCs and megakaryocytes, while other cells, such as osteoblasts, macrophages and nerve cells, act as accessory niche cells that either regulate such key cells within the HSC niche as vascular endothelial cells and their associated MSCs or regulate HSCs via long range signals.31
Here, we will concentrate on the cellular composition of HSPC niches in adult bone marrow. Notably, the heterogeneity and molecular definition of cell subsets within the adult bone marrow niches are now being explored with single cell omics, fate mapping, functional and microdissection approaches.107-110
Osteoblastic and perivascular niches in adult bone marrow
The osteoblastic endosteal niche
In 1968, Tavassoli and Crosby111 demonstrated that transplantation of autologous bone marrow fragments into extramedullary sites in rats, rabbits and dogs produced adventitial reticular cell networks that formed osteoblasts and trabecular bone, with haematopoiesis becoming established only after the formation of a sinusoidal microcirculation. Other early studies demonstrated that the so-called Dexter-type bone marrow cultures containing stromal cells could promote myelopoiesis,112 while Whitlock-Witte type bone marrow cultures using clonal MSC lines could support both myelopoiesis and B lymphopoiesis.113-115 Subsequently, osteoblasts were shown to support human haematopoietic progenitor cells and granulopoiesis in bone marrow cultures,116 with immature, rather than mature, osteo-lineage cells being more potent in promoting HSPC expansion ex vivo.28
Initial research using murine models concluded that osteo-lineage cells or osteoblasts, as descendants of MSCs, and the factors they produced were the key components of an HSC ‘osteoblastic niche’, which was located at the endosteum of the cortical and trabecular bone. This is exemplified by studies in mice which demonstrated that i) a carboxyfluorescein succinimidyl ester (CFSE)-labelled enriched ‘HSC’ subset transplanted into non-myeloablated recipients distributed preferentially to endosteal regions, while enriched lineage committed progenitors were predominantly located in the central bone marrow,117 ii) an increase in HSCs correlated with an expansion of osteo-lineage cells, in which bone morphogenetic protein receptor 1a (Bmpr1a) was conditionally inactivated, or parathyroid hormone receptor 1 (Pthr1) was activated, and iii) a decrease in HSCs correlated with conditional ablation of osteo-lineage cells via gancyclovir treatment of Col2.3ΔTK (collagen type I promoter driving thymidine kinase) tagged cells.118-120 Other research has reported that osteopontin produced by osteoblastic cells can regulate HSCs negatively, while deletion of Cxcl12 or stem cell factor (Scf) from osteo-lineage cells in Col.2.3-Cre, osteocalcin-Cre or osterix-Cre mice had no effect on HSCs.121 Limitations of these studies have varied from the use of enriched rather than highly purified HSCs and of less sophisticated imaging techniques than currently available, as well as the potentially broader cell lineage specificity of the genes selected for ablation or over-expression in osteo-lineage cells.27, 122, 123 Recent studies,124, 125 using N-cadherin-tdTomato reporter mice and conditional deletion of Scf or Cxcl12 from N-cadherin positive stromal cells, has reported, respectively, the presence of N-cadherin positive mesenchymal stem/stromal cells with osteogenic, chondrogenic and adipogenic potential in both endosteal and central bone marrow regions, as well as a reduced quantity of the HSCs in adult bone marrow after Scf, but not Cxcl12, ablation. Furthermore, quiescent CD49d- long term repopulating HSCs (considered reserve HSCs) were reported to be located at the endosteum associating with N-cadherin positive stromal cells, from whence they were able to survive chemotherapy and regenerate haematopoietic cells post-myeloablation. These studies suggested that niche cells other than osteoblasts can also supply key haematopoietic regulatory factors (e.g., SCF, CXCL12), and that osteoblasts and the endosteum may provide a niche for a subset of HSCs and protection from exhaustion of rare long lived and quiescent HSCs and may play a more significant role in stress-induced rather than steady state haematopoiesis. However, their exact mechanism of action is not well defined. Various other studies have however provided contradictory results regarding the importance of the osteoblastic niche in regulating HSC fate, with some favouring peri-sinusoidal HSC niches as discussed below, and others suggesting that, because of the proximity of sinusoids to the endosteum, a more accurate assessment would be to view the HSC niche as an osteo-vascular niche.126 Further studies support the presence in adult bone marrow of multiple spatially and functionally distinct niches for functionally heterogeneous HSC subsets, as discussed below.
Evidence for perivascular niches
Research, based on the differential expression of SLAM family cell surface receptors, CD150, CD244, and CD48, identified, in adult murine bone marrow, enriched HSPC subsets, and more specifically CD150+CD48-CD41- or CD150+CD244-CD48- HSCs, CD244+CD150-CD48- MPPs and CD48+CD244+CD150- restricted haematopoietic progenitor cells.127 Direct imaging, using these markers, demonstrated that 57% of these phenotypically defined ‘HSCs’ were in the trabecular zone and the remainder throughout the diaphysis of the long bones, with most (60%) of CD150+CD48-CD41- enriched HSCs associating with sinusoidal endothelial cells and Nestin-GFP+ perivascular cells, and fewer with the endosteum (14%). In transplantation assays, on average, 45% of the CD150+CD48-CD41- HSCs were defined as possessing long term multilineage reconstitution abilities.127 A further study by Lassailly et al.126 examined the micro-anatomical distribution of Lin-Sca1+c-Kit+(LSK)CD150+ HSPC in murine bone marrow comparing the calvaria with the epiphyses, metaphyses, and diaphysis of the long bones, and comparing steady state with transplant conditions. Under steady state conditions, similar frequencies of enriched LSKCD150+ HSCs were found to be homogeneously distributed throughout the calvaria, epiphyses, and metaphyses. However, following transplantation, these cells were initially localised in the epiphyses and calvaria with areas of high bone remodelling activity and high blood volume in preference to the diaphysis before returning to the steady state HSC distribution within approximately 3 weeks post-transplant. Notably, research126 demonstrated that sinusoidal vasculature was in proximity to the endosteum and suggested that i) the osteoblastic and vascular niches were not mutually exclusive, and ii) homeostatic HSC niches differed from reconstituting HSC niches.
Other investigations, using three-dimensional imaging, demonstrated no apparent direct association between enriched HSCs and osteoblasts.102, 128 Some studies placed cycling HSCs in the vicinity of sinusoids and their associated LEPR+ perivascular cells, while quiescent HSCs were reported to reside near small arterioles and their associated Nestinhi neural-glial antigen 2+ (NG2+) pericytes that were located near the endosteum. 102, 129 This contrasted with a report that very few HSCs were located in the peri-arteriolar region.128 In the latter study,128 90% of Lin-Sca1+c-Kit+CD48-CD41-/lo, compared to about 67% of CD150+CD48-Lin-Sca1+c-Kit+, enriched HSCs were located in peri-sinusoidal niches. These CD150+CD48-Lin-Sca1+c-Kit+ enriched HSCs were reported to contain ∼47% long term repopulating HSCs. Further evidence for multiple HSC niches was provided by Itkin and colleagues,130 who reported that different vascular niches differentially regulated the generation of reactive oxygen species (ROS) in, and the metabolism of, their adjacent HSCs, with those HSCs in the vicinity of sinusoidal niches being ROShi and those near arteriolar niches being ROSlo and hence quiescent HSC. Additionally, a subset of HSCs was found to depend on an association with megakaryocytes, which, like the arteriolar niche cells, were reported to promote HSC quiescence.101, 103, 131, 132
Another approach to a more detailed understanding of murine bone marrow HSC niches has been the use of reporter mice, in which enriched ‘HSC subsets’ are marked with fluorescent tags and imaged in vivo using state-of-the-art, Hoxb5mCherry/+technologies. Taking advantage of α-catulinGFP/+, Mds1GFP/+, Mds1GFP/+Flt3Cre, Mds1CreER Rosa26confetti/+ or Pdzk1ip1-CreER Rosa26LSC-Tom reporter mice, the fluorescently labelled enriched ‘HSC’ bone marrow subsets were found to be mostly (60-94%) located in the vicinity of sinusoidal endothelial cells and their associated LEPR+ cells, and less often with megakaryocytes.85, 100, 105, 133, 134 Using the Hoxb5mCherry reporter, Chen et al.104 found a significant association between HSCs and the sinusoidal endothelia. The use of MdsGFP/+ Flt3Cre reporter mice additionally revealed that quiescent long term repopulating HSCs, when examined in the calvaria, were essentially equidistant (< 10 μm) from the bone marrow sinusoids and the endosteum under steady state conditions but were not associated with arterioles.134 Interestingly, these researchers noted that, under steady state conditions, the long term repopulating HSCs were equally distributed over three types of endosteal bone cavities associated with bone remodelling, viz. those for bone deposition and mainly containing osteoblasts (Type D), those for bone resorption and predominantly containing osteoclasts (Type R) and those that contained mixtures of osteoblasts and osteoclasts (Type M). After cyclophosphamide/granulocyte colony stimulating factor activation, proliferating HSCs were found almost exclusively near Type M cavities, supporting the concept of different niches for quiescent and cycling HSCs. In the femurs, however, around 70% of steady state HSCs were located closer to the CD105+ sinusoidal cells and further from the endosteum, with their distribution appearing to occur at random.134 Two other studies reported that α-catulinGFP/+ tagged HSCs (where at least 50% of the α-catulinGFP/+ ‘HSC population’ examined lacked self-renewal and long term repopulating ability following transplantation) were not generally proximal to bone (< 6-10%), arteriolar cells (< 6%), adipocytes nor Schwann cells in adult murine femurs and sterna,105 and both quiescent and cycling α-catulinGFP+ HSCs appeared to have identical locations in the adult bone marrow.100, 105 These studies do not however exclude the possibilities that cell heterogeneity within the enriched ‘HSC’ pool investigated or within the niches examined obscured differential interaction of specific HSC subsets (including multipotent vs. lineage biased HSC) with specific niches.107, 108, 135-138 Indeed, Pinho et al.136 reported the differential occupation of bone marrow niches with different lineage biased HSCs. They showed that von Willebrand factor-positive platelet and myeloid-biased HSCs were located near a megakaryocytic associated sinusoidal niche, where their quiescence was maintained under steady state conditions, whereas von Willebrand factor negative lymphoid-biased HSCs resided preferentially near NG2+ peri-arteriolar niches.
These studies in general and at the current time consistently point to the importance of the peri-sinusoidal bone marrow niche, containing both sinusoidal endothelial cells and LEPR+ stromal cells, for HSC maintenance. Earlier studies had identified CAR cells as major stromal components of the HSC niche in murine bone marrow.98, 139 It has been suggested that these CAR cells are specialised MSCs, significantly overlapping with LEPR+ perivascular cells.140, 141 They also express high levels of CXCL12, SCF, interleukin (IL)-7 and early B-cell factor 3142 and are thought to play significant roles in regulating haematopoietic cell subsets including HSCs, MPPs, lymphoid progenitors, natural killer cells, B lymphoid cells, and plasmacytoid dendritic cells.143 Given the limitation that the enriched HSC pools examined are heterogeneous in their putative lineage and self-renewal potentials, a number of the studies described above have reported that sinusoidal endothelial cells and LEPR+ or CAR niche cells are spatially dominant in murine bone marrow, and that their proximity to ‘HSCs’ may not reflect active sites of HSC enrichment, but rather demonstrate the localisation of HSCs in these areas purely by chance.100, 102, 105, 134, 143
The experimental approaches described above also supported the concept that lineage biased HSPC subsets reside in distinct niches. The detailed association of at least six murine MPP subsets with specific niches has not been deciphered, although many restricted progenitors are reported to be regulated by LEPR+ or CAR cells,30, 137 while, for others, osteoblasts play an important role.144, 145 Furthermore, differential positioning of such progenitors includes peri-sinusoidal, peri-arteriolar, and endosteal niches that are spatially distinct from HSC niches. 30, 85, 137, 144-146 For example, early murine erythroid and myeloid precursors have been described as peri-sinusoidal,30 while some early murine lymphoid subpopulations are reported to be located near the osteoblastic endosteal niche and/or peri-arteriolar LEPR+ Osteolectin+ stroma.144-146
These findings have thus presented challenges for measuring the specificity of microenvironmental niches for distinct types of HSC subsets and for those of their progeny. Another challenge includes extrapolating murine studies to the human, given that adult human haematopoiesis is less prominent in the long bones, that the composition of adult human bone marrow may differ from that of the mouse (for example in adipocyte content), and that biomarkers can differ significantly between human and murine HSPCs. A further challenge is to fully understand the relative contributions of HSPC intrinsic programs and microenvironmental extrinsic controls to HSPC fate decisions, and the exact mechanisms by which these occur. For instance, it would be interesting to understand in more detail microenvironmental niche heterogeneity or the capacity of HSPCs expressing specific transcriptional modules to receive extrinsic long- or short-range signals that allow them to modify the niche in which they reside and thereby direct trajectories of lineage differentiation. Recent sc-RNAseq studies have sought to further define niche stromal cell heterogeneity (see below),106-108, 110, 147, 148 and the coupling of this to spatial, functional in vitro and in vivo studies and fate mapping will undoubtedly solve some of the unanswered questions that remain regarding microenvironmental niche specificity.
Changing Definitions for Mesenchymal Stem and Stromal Cells
MSC clonogenicity and biomarkers
Studies initiated by Friedenstein and others functionally characterised human and rodent adult bone marrow mesenchymal stem cells by their ability to form plastic-adherent clonogenic fibroblastoid colonies or colony-forming unit-fibroblasts (CFU-F), their self-renewal capacity and their multi-lineage differentiation potential in vitro and in vivo.149-159 These cells could give rise to osteogenic, adipogenic, and chondrogenic lineages and also generate MSCs that supported haematopoiesis. Given the confusion that has ensued regarding the interchangeable usage of the terms mesenchymal stem cells and stromal cells and the resulting diversity of cells defined as MSCs, four minimal criteria for defining human MSCs were introduced by the International Society for Cellular Therapy 16 years ago.160 These stromal cells were redefined as plastic adherent multipotent cells that differentiate into adipocytes, osteoblasts, and chondrocytes (AOC) in vitro, and, in humans, express CD105, CD90 (THY-1) and CD73, but not CD45, CD19 or CD79α, CD14 or CD11b, CD34 and HLA-DR surface markers. Given that stem cells are generally characterised by their ability, at the single cell level, not only to generate specialised cells but also to self-renew in vivo161 and that these criteria have not always been examined in the published literature, the MSC acronym multipotent MSCs (from Ancient Greek στρῶμα, strôma, a physical substrate to rest or lie on) was chosen in preference to mesenchymal stem cells for cells meeting the International Society for Cellular Therapy 2006 criteria and possessing haematopoietic supportive activity.162 In our own study,163 the CFU-F content of erythrocyte depleted adult bone marrow (sourced from the iliac crest of human adult donors < 40 years old) ranged from 1 in 16,000 to 1 in 33,500 bone marrow nucleated cells, with 64% possessing tri-lineage AOC potential and 95% osteogenic AOC, OC (osteogenic chondrogenic), OA (osteogenic adipocytic), O (osteogenic) potential in vitro, indicating that around 25 cells per million adult human iliac crest bone marrow nucleated cells met the International Society for Cellular Therapy 2006 criteria for MSCs. However, even these cells were heterogeneous, both in their relative ability to differentiate in AOC lineages in vitro and in their RNAseq transcriptomic profiles, but in vivo functional assays were not used to confirm their AOC potential.163 Functional in vivo analyses of these human, as well as murine, freshly isolated MSCs at limiting dilution are important for defining their self-renewal abilities, potentiality and spatial distribution.99, 164 In 2007, a subset of adult human bone marrow sinusoidal and clonogenic self-renewing CD45-CD146+ early osteoprogenitor or mesenchymal stem cells, that reportedly contained all CFU-F activity, were found by Sacchetti et al.164 to organise an haematopoietic microenvironment and generate bone in vivo, and were identified as CXCL12 and SCF-expressing adventitial reticular cells in situ. A small proportion of such perivascular CD146+ MSCs in human foetal bone marrow, like their murine counterpart, was subsequently reported to express (platelet-derived growth factor receptor (PDGFR)-α (CD140a) and CD51(ITGVA), and localise to the peri-sinusoidal area of the murine adult bone marrow HSC niche upon transplantation.165 Inherent heterogeneity of putative mesenchymal stem cell populations has been observed after adult human bone marrow CFU-F enrichment of CD45-cells using a variety of other cell surface biomarkers (e.g., CD271, mesenchymal stromal cell antigen-1, CD105, stromal antigen-1, PDGFR-β (CD140b), ErbB2 (CD340), Frizzled-9 (CD349), CD90, CD295 (LEPR), CD106, vascular cell adhesion molecule 1 (VCAM-1)CD140alow/−).163, 166-183 For example, Tormin et al.180 reported that all CFU-F activity was present in CD271 enriched adult bone marrow cells, and that the CFU-F activity of the Lineage-CD45-CD271+ subset isolated from adult human bone marrow could be segregated into CD146 positive and negative fractions, with those expressing CD146 found in perivascular locations and those lacking CD146 expression located at the endosteum.
In adult murine bone marrow, Nestin+ (Nes-GFP+) mesenchymal stem cells were initially reported to contain all the CFU-F activity, possess self-renewal capabilities, express high levels of pro-haematopoietic factors (CXCL12 and SCF) and spatially associate with HSCs and adrenergic nerve fibres in vivo.99 Difficulties in using Nestin as a marker for distinguishing perivascular stromal cell subsets in mice, where Nestinbright cells are NG2+ and Nestindim cells are LEPR+ MSC, have been described by the same laboratory,102 with current evidence indicating that LEPR+ MSCs fail to express endogenous Nestin, although they express low levels of the Nes-GFP transgene, while peri-arteriolar NG2+ stromal cells express high levels of Nes-GFP.29, 30 Murine bone marrow mesenchymal stem cells have also been enriched based on an absence of cell surface CD45, CD31 and Ter119, and positivity for CD73, CD90, CD105, CD140a, SCA-1 and CD295 (LEPR).29, 141, 184 Issues related to the lack of cell specificity of such individual cell surface makers and of genes for lineage tracing, as well as the description of certain murine bone marrow perivascular stromal cells (e.g. SCA-1+PDGFR- a(CD140a)+) as pericytes, have recently been reviewed.29, 141, 184 These will not be further discussed in detail here, except to say that Soliman et al.185 argue against the pericyte description for certain MSC subsets by virtue of their transcriptomic profiles and presence outside blood vessel basement membranes and their location near pericytes in capillaries, small blood vessels and in the adventitial layer of large blood vessels.
Molecular heterogeneity of adult bone marrowmesenchymal stem/stromal cells
Adult bone marrow MSC subsets are heterogeneous both in terms of their proliferative and differentiation potentials, and in their production of critical cytokines and chemokines which regulate the fate of distinct HSPC subpopulations that express their cognate receptors.163, 186 The latter include SCF and CXCL12, which are required for HSC maintenance and retention in adult bone marrow, and are produced at high levels by most CAR98, 139or LEPR MSCs.141, 144, 145 Another +approach to further define MSC subset heterogeneity is to examine the spatial distribution of stromal cells within adult bone marrow in the context of their single cell proteomes and transcriptomes.186 Based on negative and/or positive selection to enrich non-haematopoietic cells, multiple subsets of stromal cells have been identified in adult murine bone marrow and to a lesser extent adult human bone marrow using sc-RNAseq approaches.29, 98, 106-110, 143, 147, 186, 187 These more detailed gene signatures and transcriptional networks suggest a continuum of cell states in early mesenchymal progenitors with increasing commitment to osteogenic, chondrogenic, adipogenic and fibroblastic lineage offspring. A study from Wolock et al.109 defined the gene expression profiles of seven MSC subsets, in which MSC precursors were predicted to divide into two branches, the first being adipocyte and then pre-adipocyte offspring, while the second branched into osteoblast/ chondrocyte progenitors. The latter then differentiate into pre-osteoblasts/chondrocytes, which give rise to pro-osteoblasts and pro-chondrocytes. In some studies, Adipo-primed and Oste-primed progenitors were identified,107 while, in others, CAR cell subsets differed in their expression of osteo-lineage and adipo-lineage genes and were grouped into distinct Adipo-CAR and Osteo-CAR cells which produced the significant proportion of cytokines and chemokines for adult bone marrow haematopoiesis.110, 186 The adipocyte potential of a LEPR+ cell subset had been proposed previously,188, 189 and the study of Baccin et al.110 have shown that the Adipo-CAR cells exhibit a similar pattern of gene expression to this LEPR+ subset. Microdissection studies combined with regional cytokine/chemokine and computational analyses examining putative stromal to haematopoietic cell interactions have placed the ALPL+CXCL12+ Osteo-CAR subset closer to arterioles, and the ALPL-CXCL12+ Adipo-CAR subset near sinusoids,110 and paved the way to better define the interactions of HSPC subsets within specific bone marrow niches.190
Nevertheless, the distribution in vivo of these increasingly diverse subsets of mesenchymal stromal niche cells in relation to increasingly heterogeneous resident HSPC subsets still requires further study to support or dispute the multiple niche hypothesis, in which HSPCs occupy anatomically distinct bone marrow niches where their fate is tightly controlled.
Identifying skeletal stem cells
Over four decades on from the original CFU-F description, the use of stringent in vitro clonal assays, coupled with in vivo single cell transplantation strategies that demonstrate their multipotential and self-renewal abilities, has led to the identification, in foetal and adult bone marrow of both mice and humans, of subsets of cells defined as skeletal stem cells.191-200 A critical issue raised by these studies and which is of major importance for their clinical translation has been their relationship to mesenchymal stem cells as the mesenchymal and skeletal stem cell terminology is often used synonymously.
Murine skeletal stem cells, which are located in marrow-free regions such as near or in the avascular hypertrophic growth plates and periosteum of long bones, were identified in 2015 by Chan and colleagues.192, 196, 200 using the differential expression of multiple cell surface biomarkers to enrich CD45-, Ter119-, Tie2-, ITGAV+, Thy1-, 6C3-, CD105- and CD200+ cells, by their clonal self-renewing potential and by their ability to generate bone, cartilage and two types of haematopoietic supportive stroma, but not adipocytes, in vivo. These skeletal stem cells have been described as murine osteochondrogenic skeletal stem cells (ocSSCs) and were proposed to sit at the apex of a lineage hierarchy comprising seven downstream cell subsets, all of which contain LEPR+ cells.192 One of these seven subsets, the CD45−, Ter119−, Tie2−, ITGAV+, Thy1+, 6C3−, CD105+ subpopulation, was reported to possess characteristics of perivascular CAR, LEPR+ and Nestin+ stromal cells.192 A small subset of murine Gremlin-1+ osteochondroreticular skeletal stem cells adjacent to the growth plate in the metaphyses of long bones and with similar in vitro and in vivo characteristics was identified by Worthley et al.193 at the same time. Other researchers have attempted to identify cell subsets resembling murine skeletal stem cells using lineage tracing with such reporter genes as Nestin, Pthr1, Gli1, Ctsk (Cathepsin K), Osx (Osterix), or Lepr.99, 181, 201-204 For example, in the resting zone of the growth plate in murine post-natal long bones, such cells are reported to derive from parathyroid hormone-related protein (PTHrP)-expressing unipotent chondrocytes before differentiating in the growth plate into hypertrophic chondrocytes, that then generate such other lineages in vivo as osteoblasts and CXCL12-expressing bone marrow stromal reticular cells.201, 205 Interestingly, these appeared to overlap the ocSSC and their immediate progeny, but not the Gremlin-1 positive osteochondroreticular skeletal stem cells described above.201, 205 As another example, using lineage-tracing, Gli1+ cells expressing Pdgfra have also been located beneath the murine growth plate and to a lesser extent in the periosteum of murine long bones.203, 206, 207 They are reported to give rise to osteoblasts required for cancellous bone formation in young mice, as well as bone marrow adipocytes, osteoblasts, and stromal cells in vivo in adult mice, contributing to bone fracture repair with the generation of chondrocytes and osteoblasts.203, 206, 207 These approaches are however limited by the lack of cell specificity of these reporter genes.29
More recent research has also identified, in murine long bones, a CD45-CD31-Pdgfra+SCA-1+CD24+ perivascular skeletal stem cell (pvSSC) subset possessing the potential to form bone, cartilage, adipose tissue and stroma.200 This subset also possesses high CFU-F activity and long term self-renewal ability, distributes potentially to and shapes HSC niches throughout the bone marrow, accumulates at the ends of the long bones,200 and appears reminiscent of mesenchymal stem cells. Although both murine ocSSC and pvSSC also have a periosteal location and are both LEPR+, they are reported to be distinct skeletal stem cell types, with the former expressing higher levels of osteochondral genes (e.g., Acan, Col2a1, Pthr1, Spp1) and the latter increased Pdgfra and Cxcl12.200 Notably, sc-RNAseq studies support the view that genes used for lineage tracing (e.g., Pthrp, Ctsk, Lepr, Osx) are not restricted to either the ocSSC or pvSSC subsets, and hence would not distinguish between these subsets.200 Chan et al.195 have also purified, from hypertrophic zones of the growth plate in long bones, a subset of human PDPN+CD73+CD164+ cells, lacking CD45, CD146, CD235, Tie2 and CD31, and meeting the rigorous definition of skeletal stem cells. This includes their local restriction to the bone, their clonal self-renewal potential, and their ability to differentiate into multiple lineages (bone, cartilage, stroma) and to reconstitute a haematopoietic microenvironment in vitro, and in vivo in surrogate serial transplantation models.191, 195, 197, 208 Co-culturing these enriched human skeletal stem cells with human haematopoietic progenitors prior to transplantation into immunodeficient mice demonstrated their capability of providing haematopoietic support.195 It has been suggested, from co-transplantation (under the renal capsule of NSG mice) of murine ocSSC with pvSSC, that the former contribute to bone formation and repair, and generate reticular cells, while the latter contribute to the formation and regeneration of the haematopoietic niche potentially regulating HSPC fate decisions.200, 209 It would be pertinent to understand if these two or additional skeletal stem cell subsets exist in the human and if this affects their function, if their lineage commitment programs are flexible, if they can be found outside the bone (e.g., in adipose tissue, cord blood, umbilical cord, placenta), and if this and future knowledge can be harnessed to improve existing MSC-related therapies and to aid the development of new therapeutics and therapies.
Clinical Translation
It is approximately 26 years since Lazarus and colleagues210 first infused cultured human bone marrow MSCs into patients to assess their safety. Since then, MSC clinical trials have grown exponentially as exemplified in a recent review by Kouchakian et al.211 who identified 1240 MSC clinical trials by searching the US Library of Medicine (https://clinicaltrials.gov) between 2016-2020. A further search showed that 290 trials were listed as completed, active, or recruiting. Interventions using MSCs have ranged from treating haematological diseases/ disorders to treatments for GvHD, Crohn’s disease, diabetes, ischaemic or congenital heart disease, stroke, cystic fibrosis, bone and joint disorders, gingival disorders, autoimmune disorders, certain malignancies, skin ulcers and chronic wounds, multiple sclerosis, other neurological disorders, acute respiratory distress syndrome, and, more recently, COVID-19 related acute respiratory distress syndrome, and to enhance solid organ transplants. Depending on the medical condition, these therapies use autologous or allogeneic MSCs sourced principally from adipose tissue, the umbilical cord or bone marrow. Despite this, few MSC products (10 by 2020 globally) have received regulatory and marketing approvals as reviewed recently.212-215 The major challenges to the therapeutic use of MSCs in the human setting have been described in detail recently.216
With clearer descriptions of human bone marrow skeletal stem cell subsets becoming available, it might be expected that there will be a particular benefit in using this knowledge in the first instance to improve treatments for chronic or life-threatening disorders that include acute, congenital and degenerative bone and joint disorders, and those which affect the haematopoietic microenvironmental niche, haematopoiesis and the immune system, and that represent substantial burdens on global healthcare provision and patient quality of life. Here, the advantages, and disadvantages of MSCs in treating certain haematological and immune related disorders will be discussed in the light that recent advances described above will further improve their usage.
Treating graft versus host disease and COVID-19
Haematopoietic cell transplants have been the most successful regenerative therapy to date with over 1.5 million being performed worldwide.214 Such transplants, which can be autologous or allogeneic, are potentially curative therapies for a wide variety of diseases including severe anaemias (thalassaemia, sickle cell disease), bone marrow failures, immunodeficiencies and certain haematological malignancies.214 Serious complications of allogeneic transplants, in terms of morbidity and mortality, are post-transplant relapse, GvHD, and conditioning regimen-related toxicities.214 A major objective for treating haematopoietic malignancies with allogeneic haematopoietic cell transplants is to prevent GvHD without enhancing the risk of relapse by compromising graft versus leukaemia effects.214
GvHD can be acute or chronic and occurs in an estimated 30-70% of recipients receiving allogeneic transplants.217, 218 Acute GvHD results from an inflammatory response, is initiated by alloreactive donor T cell subsets (with the early phase being predominantly Th1/Tc1 cell mediated), and can damage the skin, gut, liver, lung, and central nervous system. Both T and B cell subsets are implicated in chronic GvHD, which may affect most organs, and result in autoimmune-like syndromes characteristically resembling scleroderma and bronchiolitis obliterans, and/or fibrosis.218 The complex immune mechanisms and pathophysiology of acute and chronic GvHD have been elegantly and extensively reviewed recently by Hill et al.218 A first-line therapy for moderate to severe chronic GvHD is the use of systemic high dose corticosteroids, with significant adverse effects when used for extended periods and with response rates in 50-60% of patients often lacking durability, and leading to 80% requiring second-line therapy.218, 219 Corticosteroids may also constitute a first-line treatment for acute GvHD,213 with various other approaches being reviewed by Hill et al.218
Administration of MSCs has been one approach in the treatment of GvHD, especially to steroid refractory GvHD. The first report of this approach in 2004 used a third party haploidentical bone marrow MSCs (two doses) to successfully treat a pediatric patient with treatment-resistant grade IV acute GvHD of the liver and gut following allogeneic haematopoietic cell transplantation for acute lymphoblastic leukemia.220 Kelly and Rasko219 have recently summarised 27 published studies using MSCs (mostly allogeneic and sourced from bone marrow) for the treatment of acute or chronic GvHD, and which were registered on clinicaltrials.com or published outside the USA where this registration is not required. Approximately two-thirds of these published studies included recipients with steroid refractory acute GvHD. While the outcomes were encouraging, the day 28 overall and complete response rates using bone marrow MSCs for steroid refractory acute GvHD (n = 797 recipients) varied from 50% to 93% and 8 to 75% respectively, while, for those with steroid refractory chronic GvHD (n = 43 recipients), two studies (n = 5 recipients) recorded no response, and, for the remainder, day 28 overall and complete responses varied from 50 to 80% and 13% to 80% respectively.219 These outcomes appeared to compare well to the clinical trials which used the Janus kinases 1 and 2 inhibitor ruxolitinib, etanercept or extracorporeal photopheresis (which has been proposed to promote regulatory T cell activity) as second-line therapies for steroid refractory acute GvHD, where day 28 complete responses were reported as < 35%.219-221 In the phase III multi-centre randomised clinical trial with ruxolitinib (n = 310 patients recruited),221 the day 28 overall and complete responses were 62% and 34% compared to 34% and 19% for the control group who were mostly treated with extracorporeal photopheresis. However, there was a reported increased incidence of adverse events, including thrombocytopenia, anemias and infections in ruxolitinib-treated compared to control recipients.221 A number of systematic reviews or meta-analyses for the prevention and/ or treatment of GvHD with MSCs have been conducted in the past decade. These confirmed the safety of MSCs for clinical use in haematopoietic cell transplants and gave some indication of efficacy.222-227 Of these, only two were restricted to randomised clinical trials, one for chronic GvHD225 and one for both acute and chronic GvHD.226 These provided support for prophylactic MSC use to treat chronic GvHD, although evidence was considered low and additional well-powered randomised controlled trials were recommended, particularly concerning use of MSCs in preventing or treating acute GvHD. Given the substantial number of clinical trials in this area, the number of MSC treatments provided for compassionate use, and an exceptional safety profile for MSC therapies, regulatory agencies in Japan, Canada, New Zealand and Europe (orphan designation EU3/18/2044) have approved the use of MSCs for treating pediatric GvHD, with approval being considered by the US FDA,213, 215-217 while ruxolitinib has received FDA approval for steroid refractory acute GvHD treatment of recipients 12 years of age or older.218
It is important to understand the reasons that some MSC studies described above show poor outcomes and poor complete responses, and why variability exists amongst different clinical centres. The clinical studies presented are difficult to compare, given that they are not all randomised and may often be for compassionate use, that MSCs, protocols and recipients differ significantly between clinical centres, and that individual or pooled MSC donors of varying ages may be used to treat many GvHD recipients or an individual donor derived MSC preparation may be limited to the treatment of one or a few recipients. It is well documented that MSCs lose their potency by extensive ex vivo culture and with ageing, vary when sourced from different individual donors and when derived from different tissues, and when protocols for different studies are not standardised amongst the clinical transplant centres.65, 198, 199, 212-220, 228-231 Thus, poor outcomes may reflect the poor or variable quality of some batches of MSCs, the clinical strategy used therapeutically, and/or the condition or immune characteristics or responses of the recipient.213 Therefore, it is important to standardise MSC sourcing, characterisation and subset analyses, manufacturing including the use of freshly isolated or off-the-shelf cryopreserved products, release criteria, route of injection, dosage, the therapeutic approach, predictors of outcome, potency assays related to the therapeutic endpoints and efficacy, and informed by the intended therapeutic mechanism of action,198, 199, 212, 213, 216, 228-231 but, although a consensus for this is generally still lacking, these issues are being actively promoted.212, 213
It is also particularly important, in respect to GvHD therapies, to understand the mechanisms of action of MSCs in modulating GvHD, their biodistribution in vivo following therapeutic administration and whether MSCs from tissues other than bone marrow, that may be easier to source, possess comparable efficacy for their intended therapeutic use. Krampera and Le Blanc213 have recently reviewed the experimental evidence related to mechanisms of action. To summarise their conclusions in the context of GvHD therapy, it has been proposed that, once activated by an inflammatory environment, MSCs exert their immunoregulatory therapeutic effects on GvHD by paracrine mechanisms which may be mediated more efficiently or partly via extracellular vesicle (exosomes, microvesicles) delivery in vivo.213, 216, 229, 231 However, it is currently thought that over 95% of MSCs, whether allogeneic or autologous, administered intravenously are trapped intravascularly in the lungs transiently before undergoing apoptosis or alternatively efferocytosis whereby apoptotic MSCs are phagocytosed by tissue resident macrophages, leading to systemic immunomodulatory effects. 213, 231, 232 In contrast if administered into the extravascular space, autologous MSCs can persist for longer periods of time.228 These immunoregulatory effects include suppressing effector T and B lymphocytes, augmenting the proliferation of regulatory T cells, secreting anti-inflammatory cytokines, and enhancing macrophage polarisation towards an M2-phenotype and immature dendritic cells.213, 218, 228, 231 It has also been reported that the response to MSC therapies in acute GvHD patients correlates with robust CD8+ T and CD56+ natural killer cell cytotoxicity responses against MSCs in vitro.232 Whether MSC therapies can also ameliorate damage to tolerogenic fibroblastic reticular cells in lymph nodes caused by GvHD233 has not to my knowledge been examined. It is obvious that understanding the mechanism of action of MSCs in treating GvHD is key to the development of functional or relevant potency assays, and guide MSC therapeutic design.
The experience garnered from understanding the anti-inflammatory and immunomodulatory properties of and in the manufacturing of MSCs as advanced therapy medicinal products described here and elsewhere has led to an assessment of MSCs and their products in treating patients with severe COVID-19. The aim has been to reduce, for example, tissue and organ damage that results from the cytokine storm induced in such patients by SARS-CoV-2 infection, which can lead to pneumonia, acute respiratory distress syndrome and multi-organ failure.234 Since the World Health Organization declared COVID-19 a Public Health Emergency of International Concern on January 30, 2020, and a pandemic on March 11, 2020, over 257 million people worldwide have acquired this infection, with mortality exceeding 5.14 million.235 As of November 21, 2021, there are currently 68 studies using MSC therapies for COVID-19 patients listed as active, recruiting, or completed on clinicaltrials.gov. Recent reviews have summarised such studies which use MSCs sourced from umbilical cord, bone marrow or adipose tissue.234, 236-238 While some initial reports and a systematic review are encouraging in terms of safety and efficacy,234, 236-238 concerns regarding the safety profile particularly of poorly defined MSC products that are administered intravenously to seriously ill COVID-19 patients and that potentially express high procoagulant tissue factor levels have been expressed.239, 240 To mitigate such risks in this respect, Moll et al.240 have proposed the following (to quote these authors): ‘(1) updated clinical guidelines for cell and tissue transplantation, (2) updated minimal criteria for characterisation of cellular therapeutics, and (3) updated cell therapy routines reflecting specific patient needs.’ Outcomes of existing and potential additional well powered and high quality double-blind multi-centre clinical trials are awaited as are assessments to compare the results of these MSC clinical studies with other new therapeutics for treating or preventing these highly infectious viral diseases.
Mesenchymal stromal cells and skeletal stem cells in the ageing bone marrow niche and opportunities for rejuvenation
The World Health Organisation predicts that by 2030 an estimated 17% of the world’s population will be aged 60 years of age or older, the equivalent of around 1.4 billion people.241 In terms of haematopoiesis, ageing in man, as well as mouse, although temporally different, is characterised by increased HSC numbers and increased adipogenesis in the bone marrow, and is associated with a shift in haematopoietic output from lymphopoiesis to myelopoiesis, with some functional losses.188, 189, 209, 242-247 The causes involve both HSPC intrinsic and extrinsic regulatory mechanisms. MSCs and skeletal stem cells in the bone and bone marrow work in concert with haematopoietic cells and, in ageing, this may have immune and autoimmune consequences.247 Thus, MSC and skeletal stem cell ageing in the bone and bone marrow impacts on haematopoiesis, as well as on bone fragility, and haematopoietic ageing impacts on MSC and skeletal stem cell driven osteogenesis, as well as on haematopoiesis, potentially contributing to the increased incidence of haematopoietic and immune disorders, including clonal haematopoiesis of indeterminate potential, acute and chronic leukaemias, multiple myeloma, non-Hodgkin lymphomas, myeloproliferative neoplasms, myelodysplastic syndrome.247-251 Although of great importance, the changes to or remodelling of the haematopoietic niche or development of a pre-metastatic niche in such haematological disorders has been well reviewed recently247-251 and will not be reiterated here. Instead, this section will concentrate on recent, and briefly summarise, studies on the influence of MSC or skeletal stem cell ageing on haematopoiesis.
With ageing, senescent MSCs have been reported to accumulate in the bone marrow.247 The inhibition of proliferation with the onset of senescence has been attributed, at least in part, to dysregulation of epigenetic control mechanisms (DNA methylation, histone modifications, chromatin reorganisation), metabolic changes (e.g., in autophagosome formation) and alterations in the secretome (with the development of a senescent-associated secretory phenotype) in resident bone marrow MSCs.247, 252, 253 Epigenetic regulators contributing to MSC ageing have been reported to include such histone deacetylases as the Sirtuins, SIRT1 and SIRT6, both of which promote MSC senescence by modulating cell proliferation254 and differentiation255 or altering redox metabolism and oxidative stress sensitivity256 respectively, while SIRT3 over-expression in cultured MSCs reduced their senescence and oxidative stress, and enhanced their ability to differentiate.257 Epigenetic regulators of MSCs are also reported to include ten-eleven translocation 2 (TET2), additional sex combs-like 1 (ASXL1) and DNA methyltransferase 3.247, 252 The DNA dioxygenase TET2 catalyses 5-methylcytosine conversion to 5-hydroxymethylcytosine eventually leading to DNA demethylation and promoting proliferation and osteogenic and adipogenic differentiation of bone marrow MSCs and their ability to support haematopoiesis.258, 259 The role of ASXL1 in epigenetic regulation is to activate or repress transcription of genes involved in differentiation and proliferation via its histone methylation effects. ASXL1 deletion in bone marrow MSCs favours a myeloid bias in haematopoiesis, with loss of ASXL1 causing RNA polymerase II transcriptional deregulation and altering HSPC maintenance gene expression (e.g., VCAM1) in these bone marrow MSCs.260 Ageing or senescent MSCs adopt a senescent-associated secretory phenotype which importantly incorporates into its secretome such proinflammatory cytokines as IL-1β, IL-6 and IL-8.253 The resultant low grade but chronic inflammation, termed inflamm-ageing, can bias haematopoietic output towards myelopoiesis.248, 253, 261-264 Ageing and senescence are also associated with modulation of MSC extracellular vesicle contents (such as miRNAs, proteins, lipids) and an increase in their transport via tunnelling nanotubes to adjacent cells (e.g., HSPCs and MSCs) thus also modulating the niche and haematopoiesis.247, 251, 259 Shen and colleagues146 have demonstrated that a reduction in murine Osteolectin-positive periarteriolar cells during ageing contributes to a decrease in lymphoid progenitor cells but does not affect the maintenance of HSCs. The latter observation most likely reflects the unique preservation of bone marrow sinusoids and peri-sinusoidal niches during ageing, although sinusoidal function is disrupted long term by myeloablation with resultant haematopoietic failure.146
Recently, by comparing young versus old mice, Ambrosi et al.265 have demonstrated that in aged mice, the ocSSC and their offspring, the bone-cartilage-stromal progenitors, are intrinsically compromised in their ability to form robust bone grafts with an ectopic bone marrow cavity in vivo, generating instead stromal cells producing high amounts of pro-resorptive and pro-inflammatory cytokines. Transplantation of these enriched aged stem and progenitor cell subsets into young mice, and exposure to a juvenile circulation and environment, for example in parabiosis experiments, did not alter this outcome. Notably, however, aged murine ocSSC enhanced osteoclast activity in part by the increased secretion of colony stimulating factor-1 and skewed haematopoiesis towards myelopoiesis. This led to the suggestion that aged ocSSC might drive haematopoietic ageing and that it might be possible to delay or reverse haematopoietic decline. Of note was the observation that reactivating aged ocSSC in mice by addition of bone morphogenetic factor 2 and blocking the pro-inflammatory effects of colony stimulating factor-1 reversed defective fracture healing, although it was unclear if this was from direct effects on the skeletal stem cell proliferative and differentiation capacities and/or on the function of their mature osteoblastic offspring, and if similar strategies modulate haematopoiesis.265 This provides a stem cell basis for degenerative skeletal diseases266, 267 that may impact haematopoiesis.
In conclusion, a huge amount of research has been conducted on MSCs for many decades, both in terms of basic science and their clinical translation. Major technological advances have, during this time, provided the basis for significant advances in defining the heterogeneity of both haematopoietic lineages and the cells that form the bone marrow niches while identifying both haematopoietic and skeletal stem cells, and deciphering their response to inflammatory conditions and the crosstalk that exists between the different cell types. The results of further research and the definition of safe and effective strategies for translating this more recent research to the clinic in terms of healthy ageing while combating immune and degenerative disorders are keenly awaited.
Limitations of Review
This review has principally been limited to the contribution of MSCs and skeletal stem cells to adult bone marrow HSC niches under homeostatic conditions, while briefly describing those in the AGM region of the developing embryo and in the foetal liver. MSCs described in other tissues or in circulating umbilical cord blood have not been discussed. There have also been various challenges in reviewing data from experimental studies conducted over many decades, particularly in the light of the continued development of advanced technologies. Although the limitations of various studies have been described throughout this review, they will be summarised briefly here. First, there has been considerable debate regarding the location, spatial distribution and specificity of niches for HSCs and their progeny in adult bone marrow, and regarding the identity of cells that contribute to these niches. Many of the cell populations studied, including HSCs and MSCs, are heterogeneous, and not easily nor specifically identified in situ with the limited set of the biomarkers or gene-markers often used or available, while in vitro studies generally fail to accurately reflect the cellular and molecular complexity of the in vivo niches and hematopoietic tissues. A second challenge has involved extrapolating murine studies to the human, as the latter is much more limited and the control of murine haematopoiesis within specific niches may not recapitulate that in the human. For example, adult human haematopoiesis is less prominent in the long bones, the composition of adult human bone marrow can differ from that of the mouse (for example in adipocyte content), and cellular biomarkers differ significantly between human and murine systems. A further challenge has been in fully deciphering the relative contributions of HSC intrinsic programs and microenvironmental extrinsic controls to HSC fate decisions. The lack of a standardised terminology to describe mesenchymal stem cells, skeletal stem cells and their progeny has added a further layer of complexity to these analyses, although recent single cell transcriptomic studies, coupled to spatial, functional in vitro and in vivo studies and fate mapping, are seeking to better define niche cell and HSC heterogeneity. These and further technological advances will undoubtedly resolve some of the unanswered questions that remain, including microenvironmental niche specificity for HSPC subsets and the validity of the multiple HSPC niche hypothesis. Finally, the translation of MSC research into the clinic has also been challenging and difficult to compare because of the lack of standardised GMP-manufacturing and clinical protocols, the variability in sources and potency of MSCs used and the variety of disorders treated across different clinical centres. Additionally, some MSC-based clinical studies are not randomised, and MSC therapies may have been designed for compassionate use. Despite these restrictions from limitations, enormous efforts have been and are still being made to clearly identify the structure and function of human haematopoietic niches in health and disease, and to improve the relevant clinical strategies by actively promoting the availability of standardised clinical guidelines.
Footnotes
Author contributions: SMW has drafted manuscript, critically reviewed published studies, revised the manuscript and approved the final manuscript.
Financial support: None.
Acknowledgement: I wish to thank the Nuffield Department of Clinical Laboratory Sciences, Radcliffe Department of Medicine, University of Oxford, Oxford, UK, and the Faculty of Health and Medical Sciences, University of Adelaide, and South Australian Health and Medical Research Institute, Adelaide, Australia, for their support.
Conflicts of interest statement: The author declares no competing conflicts of interest.
References
- 1.Guest I., Ilic Z., Sell S. Origin of the stem cell niche concept. Exp Hematol. 2016;44:809–810. doi: 10.1016/j.exphem.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Röhlich K. Über die Beziehungen zwischen der Knochensubstanz und der Blut-bildung im Knochenmark. Z Mikr Anat Forsch. 1941;49:425–464. [Google Scholar]
- 3.Curry J., Trentin J., Wolf N. Control of spleen colony histology by erythropoietin, cobalt and hypertransfusion. Exp Hematol. 1964;7:80. [Google Scholar]
- 4.Curry J. L., Trentin J. J. Hemopoietic spleen colony studies. I. Growth and differentiation. Dev Biol. 1967;15:395–413. doi: 10.1016/0012-1606(67)90034-6. [DOI] [PubMed] [Google Scholar]
- 5.Wolf N. S., Trentin J. J. Effect of hemopoietic organ matrix on stem cell differentiation. Fed Proc. 1966;26:746. [Google Scholar]
- 6.Curry J. L., Trentin J. J., Wolf N. Hemopoietic spleen colony studies. II. Erythropoiesis. J Exp Med. 1967;125:703–720. doi: 10.1084/jem.125.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crosby W. H. Experience with injured and implanted bone marrow: relation of function to structure. In: Stohlman F. Jr, editor. Hemopoietic cellular proliferation. Grune & Stratton; New York: 1970. pp. 87–96. [Google Scholar]
- 8.Maloney M. A., Patt H. M. Bone marrow restoration after localized depletion. Cell Prolif. 1969;2:29–38. [PubMed] [Google Scholar]
- 9.Wolf N. S. Dissecting the hematopoietic microenvironment. I. Stem cell lodgment and commitment, and the proliferation and differentiation of erythropoietic descendants in the S1-S1d mouse. Cell Tissue Kinet. 1974;7:89–98. doi: 10.1111/j.1365-2184.1974.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 10.Fliedner T. M., Calvo W., Haas R., Forteza J., Bohne F. Morphologic and cytokinetic aspects of bone marrow stroma. In: Stohlman F., editor. Haemopoietic cellular proliferation. Grune & Stratton; New York: 1970. pp. 67–86. [Google Scholar]
- 11.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 12.Trentin J. J. Determination of bone marrow stem cell differentiation by stromal hemopoietic inductive microenvironments (HIM) Am J Pathol. 1971;65:621–628. [PMC free article] [PubMed] [Google Scholar]
- 13.Herzenberg L. A., Herzenberg L. A. Toward a layered immune system. Cell. 1989;59:953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- 14.Elsaid R., Soares-da-Silva F., Peixoto M., Amiri D., Mackowski N., Pereira P., Bandeira A., Cumano A. Hematopoiesis: a layered organization across chordate species. Front Cell Dev Biol. 2020;8:606642. doi: 10.3389/fcell.2020.606642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin S. B., McNagny K. M. ILC-you in the thymus: a fresh look at innate lymphoid cell development. Front Immunol. 2021;12:681110. doi: 10.3389/fimmu.2021.681110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Easterbrook J., Rybtsov S., Gordon-Keylock S., Ivanovs A., Taoudi S., Anderson R. A., Medvinsky A. Analysis of the spatiotemporal development of hematopoietic stem and progenitor cells in the early human embryo. Stem Cell Reports. 2019;12:1056–1068. doi: 10.1016/j.stemcr.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanovs A., Rybtsov S., Welch L., Anderson R. A., Turner M. L., Medvinsky A. Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J Exp Med. 2011;208:2417–2427. doi: 10.1084/jem.20111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivanovs A., Rybtsov S., Ng E. S., Stanley E. G., Elefanty A. G., Medvinsky A. Human haematopoietic stem cell development: from the embryo to the dish. Development. 2017;144:2323–2337. doi: 10.1242/dev.134866. [DOI] [PubMed] [Google Scholar]
- 19.Crosse E. I., Gordon-Keylock S., Rybtsov S., Binagui-Casas A., Felchle H., Nnadi N. C., Kirschner K., Chandra T., Tamagno S., Webb D. J., Rossi F., Anderson R. A., Medvinsky A. Multi-layered spatial transcriptomics identify secretory factors promoting human hematopoietic stem cell development. Cell Stem Cell. 2020;27:822–839. doi: 10.1016/j.stem.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park J. E., Jardine L., Gottgens B., Teichmann S. A., Haniffa M. Prenatal development of human immunity. Science. 2020;368:600–603. doi: 10.1126/science.aaz9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross C., Boroviak T. E. Origin and function of the yolk sac in primate embryogenesis. Nat Commun. 2020;11:3760. doi: 10.1038/s41467-020-17575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack R., Zhang L., Breslin Sj P., Zhang J. The fetal-to-adult hematopoietic stem cell transition and its role in childhood hematopoietic malignancies. Stem Cell Rev Rep. 2021;17:2059–2080. doi: 10.1007/s12015-021-10230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wittamer V., Bertrand J. Y. Yolk sac hematopoiesis: does it contribute to the adult hematopoietic system? Cell Mol Life Sci. 2020;77:4081–4091. doi: 10.1007/s00018-020-03527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heck A. M., Ishida T., Hadland B. Location, location, location: how vascular specialization influences hematopoietic fates during development. Front Cell Dev Biol. 2020;8:602617. doi: 10.3389/fcell.2020.602617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neo W. H., Lie A. L. M., Fadlullah M. Z. H., Lacaud G. Contributions of embryonic HSC-independent hematopoiesis to organogenesis and the adult hematopoietic system. Front Cell Dev Biol. 2021;9:631699. doi: 10.3389/fcell.2021.631699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canu G., Ruhrberg C. First blood: the endothelial origins of hematopoietic progenitors. Angiogenesis. 2021;24:199–211. doi: 10.1007/s10456-021-09783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szade K., Gulati G. S., Chan C. K. F., Kao K. S., Miyanishi M., Marjon K. D., Sinha R., George B. M., Chen J. Y., Weissman I. L. Where hematopoietic stem cells live: the bone marrow niche. Antioxid Redox Signal. 2018;29:191–204. doi: 10.1089/ars.2017.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh J., Koussa R. E., Mohamad S. F., Liu J., Kacena M. A., Srour E. F. Cellular components of the hematopoietic niche and their regulation of hematopoietic stem cell function. Curr Opin Hematol. 2021;28:243–250. doi: 10.1097/MOH.0000000000000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mabuchi Y., Okawara C., Méndez-Ferrer S., Akazawa C. Cellular Heterogeneity of mesenchymal stem/stromal cells in the bone marrow. Front Cell Dev Biol. 2021;9:689366. doi: 10.3389/fcell.2021.689366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comazzetto S., Shen B., Morrison S. J. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev Cell. 2021;56:1848–1860. doi: 10.1016/j.devcel.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Pel M., Fibbe W. E., Schepers K. The human and murine hematopoietic stem cell niches: are they comparable? Ann N Y Acad Sci. 2016;1370:55–64. doi: 10.1111/nyas.12994. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson G., Sommarin M. N. E., Böiers C. Defining the emerging blood system during development at single-cell resolution. Front Cell Dev Biol. 2021;9:660350. doi: 10.3389/fcell.2021.660350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen M. J., Li Y., De Obaldia M. E., Yang Q., Yzaguirre A. D., Yamada-Inagawa T., Vink C. S., Bhandoola A., Dzierzak E., Speck N. A. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell. 2011;9:541–552. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palis J., Yoder M. C. Endothelial cells transition to blood cells but probably not back again. Circ Res. 2020;127:1233–1235. doi: 10.1161/CIRCRESAHA.120.318113. [DOI] [PubMed] [Google Scholar]
- 35.Ghosn E., Yoshimoto M., Nakauchi H., Weissman I. L., Herzenberg L. A. Hematopoietic stem cell-independent hematopoiesis and the origins of innate-like B lymphocytes. Development. 2019;146:dev170571. doi: 10.1242/dev.170571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soares-da-Silva F., Freyer L., Elsaid R., Burlen-Defranoux O., Iturri L., Sismeiro O., Pinto-do-= P., Gomez-Perdiguero E., Cumano A. Yolk sac, but not hematopoietic stem cell-derived progenitors, sustain erythropoiesis throughout murine embryonic life. J Exp Med. 2021;218:e20201729. doi: 10.1084/jem.20201729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshimoto M. The ontogeny of murine B-1a cells. Int J Hematol. 2020;111:622–627. doi: 10.1007/s12185-019-02787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azevedo Portilho N., Scarfò R., Bertesago E., Ismailoglu I., Kyba M., Kobayashi M., Ditadi A., Yoshimoto M. B1 lymphocytes develop independently of Notch signaling during mouse embryonic development. Development. 2021;148:dev199373. doi: 10.1242/dev.199373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGrath K. E., Frame J. M., Palis J. Early hematopoiesis and macrophage development. Semin Immunol. 2015;27:379–387. doi: 10.1016/j.smim.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dege C., Fegan K. H., Creamer J. P., Berrien-Elliott M. M., Luff S. A., Kim D., Wagner J. A., Kingsley P. D., McGrath K. E., Fehniger T. A., Palis J., Sturgeon C. M. Potently cytotoxic natural killer cells initially emerge from erythro-myeloid progenitors during mammalian development. Dev Cell. 2020;53:229–239.e7. doi: 10.1016/j.devcel.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vo L. T., Kinney M. A., Liu X., Zhang Y., Barragan J., Sousa P. M., Jha D. K., Han A., Cesana M., Shao Z., North T. E., Orkin S. H., Doulatov S., Xu J., Daley G. Q. Regulation of embryonic haematopoietic multipotency by EZH1. Nature. 2018;553:506–510. doi: 10.1038/nature25435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C., Yu W., Tober J., Gao P., He B., Lee K., Trieu T., Blobel G. A., Speck N. A., Tan K. Spatial genome re-organization between fetal and adult hematopoietic stem cells. Cell Rep. 2019;29:4200–4211.e7. doi: 10.1016/j.celrep.2019.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yvernogeau L., Gautier R., Petit L., Khoury H., Relaix F., Ribes V., Sang H., Charbord P., Souyri M., Robin C., Jaffredo T. In vivo generation of haematopoietic stem/progenitor cells from bone marrow-derived haemogenic endothelium. Nat Cell Biol. 2019;21:1334–1345. doi: 10.1038/s41556-019-0410-6. [DOI] [PubMed] [Google Scholar]
- 44.Bowie M. B., Kent D. G., Dykstra B., McKnight K. D., McCaffrey L., Hoodless P. A., Eaves C. J. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci U S A. 2007;104:5878–5882. doi: 10.1073/pnas.0700460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Copley M. R., Eaves C. J. Developmental changes in hematopoietic stem cell properties. Exp Mol Med. 2013;45:e55. doi: 10.1038/emm.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benz C., Copley M. R., Kent D. G., Wohrer S., Cortes A., Aghaeepour N., Ma E., Mader H., Rowe K., Day C., Treloar D., Brinkman R. R., Eaves C. J. Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell. 2012;10:273–283. doi: 10.1016/j.stem.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Li Y., Magee J. A. Transcriptional reprogramming in neonatal hematopoietic stem and progenitor cells. Exp Hematol. 2021;101-102:25–33. doi: 10.1016/j.exphem.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Y., Hirschi K. K. Tissue-resident macrophage development and function. Front Cell Dev Biol. 2020;8:617879. doi: 10.3389/fcell.2020.617879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slukvin II. Deciphering the hierarchy of angiohematopoietic progenitors from human pluripotent stem cells. Cell Cycle. 2013;12:720–727. doi: 10.4161/cc.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ayllón V., Bueno C., Ramos-Mejía V., Navarro-Montero O., Prieto C., Real P. J., Romero T., García-León M. J., Toribio M. L., Bigas A., Menendez P. The Notch ligand DLL4 specifically marks human hematoendothelial progenitors and regulates their hematopoietic fate. Leukemia. 2015;29:1741–1753. doi: 10.1038/leu.2015.74. [DOI] [PubMed] [Google Scholar]
- 51.Zeng Y., He J., Bai Z., Li Z., Gong Y., Liu C., Ni Y., Du J., Ma C., Bian L., Lan Y., Liu B. Tracing the first hematopoietic stem cell generation in human embryo by single-cell RNA sequencing. Cell Res. 2019;29:881–894. doi: 10.1038/s41422-019-0228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tavian M., Coulombel L., Luton D., Clemente H. S., Dieterlen-Liàvre F., Péault B. Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood. 1996;87:67–72. [PubMed] [Google Scholar]
- 53.Tavian M., Biasch K., Sinka L., Vallet J., Péault B. Embryonic origin of human hematopoiesis. Int J Dev Biol. 2010;54:1061–1065. doi: 10.1387/ijdb.103097mt. [DOI] [PubMed] [Google Scholar]
- 54.Tavian M., Cortés F., Charbord P., Labastie M. C., Péault B. Emergence of the haematopoietic system in the human embryo and foetus. Haematologica. 1999;84(Suppl EHA-4):1–3. [PubMed] [Google Scholar]
- 55.Migliaccio G., Migliaccio A. R., Petti S., Mavilio F., Russo G., Lazzaro D., Testa U., Marinucci M., Peschle C. Human embryonic hemopoiesis. Kinetics of progenitors and precursors underlying the yolk sac----liver transition. J Clin Invest. 1986;78:51–60. doi: 10.1172/JCI112572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H., He J., Xu C., Chen X., Yang H., Shi S., Liu C., Zeng Y., Wu D., Bai Z., Wang M., Wen Y., Su P., Xia M., Huang B., Ma C., Bian L., Lan Y., Cheng T., Shi L., Liu B., Zhou J. Decoding human megakaryocyte development. Cell Stem Cell. 2021;28:535–549.e8. doi: 10.1016/j.stem.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Migliaccio A. R., Hoffman R. An outline of the outset of thrombopoiesis in human embryos at last. Cell Stem Cell. 2021;28:363–365. doi: 10.1016/j.stem.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holt P. G., Jones C. A. The development of the immune system during pregnancy and early life. Allergy. 2000;55:688–697. doi: 10.1034/j.1398-9995.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 59.Popescu D. M., Botting R. A., Stephenson E., Green K., Webb S., Jardine L., Calderbank E. F., Polanski K., Goh I., Efremova M., Acres M., Maunder D., Vegh P., Gitton Y., Park J. E., Vento-Tormo R., Miao Z., Dixon D., Rowell R., McDonald D., Fletcher J., Poyner E., Reynolds G., Mather M., Moldovan C., Mamanova L., Greig F., Young M. D., Meyer K. B., Lisgo S., Bacardit J., Fuller A., Millar B., Innes B., Lindsay S., Stubbington M. J. T., Kowalczyk M. S., Li B., Ashenberg O., Tabaka M., Dionne D., Tickle T. L., Slyper M., Rozenblatt-Rosen O., Filby A., Carey P., Villani A. C., Roy A., Regev A., Chédotal A., Roberts I., Göttgens B., Behjati S., Laurenti E., Teichmann S. A., Haniffa M. Decoding human fetal liver haematopoiesis. Nature. 2019;574:365–371. doi: 10.1038/s41586-019-1652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Velten L., Haas S. F., Raffel S., Blaszkiewicz S., Islam S., Hennig B. P., Hirche C., Lutz C., Buss E. C., Nowak D., Boch T., Hofmann W. K., Ho A. D., Huber W., Trumpp A., Essers M. A., Steinmetz L. M. Human haematopoietic stem cell lineage commitment is a continuous process. Nat Cell Biol. 2017;19:271–281. doi: 10.1038/ncb3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Byrne S., Elliott N., Rice S., Buck G., Fordham N., Garnett C., Godfrey L., Crump N. T., Wright G., Inglott S., Hua P., Psaila B., Povinelli B., Knapp D., Agraz-Doblas A., Bueno C., Varela I., Bennett P., Koohy H., Watt S. M., Karadimitris A., Mead A. J., Ancliff P., Vyas P., Menendez P., Milne T. A., Roberts I., Roy A. Discovery of a CD10-negative B-progenitor in human fetal life identifies unique ontogeny-related developmental programs. Blood. 2019;134:1059–1071. doi: 10.1182/blood.2019001289. [DOI] [PubMed] [Google Scholar]
- 62.Ranzoni A. M., Tangherloni A., Berest I., Riva S. G., Myers B., Strzelecka P. M., Xu J., Panada E., Mohorianu I., Zaugg J. B., Cvejic A. Integrative single-cell RNA-seq and ATAC-seq analysis of human developmental hematopoiesis. Cell Stem Cell. 2021;28:472–487.e7. doi: 10.1016/j.stem.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roy A., Wang G., Iskander D., O’Byrne S., Elliott N., O’Sullivan J., Buck G., Heuston E. F., Wen W. X., Meira A. R., Hua P., Karadimitris A., Mead A. J., Bodine D. M., Roberts I., Psaila B., Thongjuea S. Transitions in lineage specification and gene regulatory networks in hematopoietic stem/progenitor cells over human development. Cell Rep. 2021;36:109698. doi: 10.1016/j.celrep.2021.109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hua P., Roy N., de la Fuente J., Wang G., Thongjuea S., Clark K., Roy A., Psaila B., Ashley N., Harrington Y., Nerlov C., Watt S. M., Roberts I., Davies J. O. J. Single-cell analysis of bone marrow-derived CD34+ cells from children with sickle cell disease and thalassemia. Blood. 2019;134:2111–2115. doi: 10.1182/blood.2019002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Belyavsky A., Petinati N., Drize N. Hematopoiesis during ontogenesis, adult life, and aging. Int J Mol Sci. 2021;22:9231. doi: 10.3390/ijms22179231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao X., Xu C., Asada N., Frenette P. S. The hematopoietic stem cell niche: from embryo to adult. Development. 2018;145:dev139691. doi: 10.1242/dev.139691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinho S., Frenette P. S. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20:303–320. doi: 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Busch K., Klapproth K., Barile M., Flossdorf M., Holland-Letz T., Schlenner S. M., Reth M., Höfer T., Rodewald H. R. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015;518:542–546. doi: 10.1038/nature14242. [DOI] [PubMed] [Google Scholar]
- 69.Catlin S. N., Busque L., Gale R. E., Guttorp P., Abkowitz J. L. The replication rate of human hematopoietic stem cells in vivo. Blood. 2011;117:4460–4466. doi: 10.1182/blood-2010-08-303537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abkowitz J. L., Catlin S. N., McCallie M. T., Guttorp P. Evidence that the number of hematopoietic stem cells per animal is conserved in mammals. Blood. 2002;100:2665–2667. doi: 10.1182/blood-2002-03-0822. [DOI] [PubMed] [Google Scholar]
- 71.Lee-Six H., Øbro N. F., Shepherd M. S., Grossmann S., Dawson K., Belmonte M., Osborne R. J., Huntly B. J. P., Martincorena I., Anderson E., O’Neill L., Stratton M. R., Laurenti E., Green A. R., Kent D. G., Campbell P. J. Population dynamics of normal human blood inferred from somatic mutations. Nature. 2018;561:473–478. doi: 10.1038/s41586-018-0497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson A., Laurenti E., Oser G., van der Wath R. C., Blanco-Bose W., Jaworski M., Offner S., Dunant C. F., Eshkind L., Bockamp E., Lió P., Macdonald H. R., Trumpp A. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 73.Wilson A., Laurenti E., Trumpp A. Balancing dormant and self-renewing hematopoietic stem cells. Curr Opin Genet Dev. 2009;19:461–468. doi: 10.1016/j.gde.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 74.Lee-Six H., Kent D. G. Tracking hematopoietic stem cells and their progeny using whole-genome sequencing. Exp Hematol. 2020;83:12–24. doi: 10.1016/j.exphem.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liggett L. A., Sankaran V. G. Unraveling Hematopoiesis through the Lens of Genomics. Cell. 2020;182:1384–1400. doi: 10.1016/j.cell.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pietras E. M., Reynaud D., Kang Y. A., Carlin D., Calero-Nieto F. J., Leavitt A. D., Stuart J. M., Göttgens B., Passegué E. Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell. 2015;17:35–46. doi: 10.1016/j.stem.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sommerkamp P., Romero-Mulero M. C., Narr A., Ladel L., Hustin L., Schönberger K., Renders S., Altamura S., Zeisberger P., Jäcklein K., Klimmeck D., Rodriguez-Fraticelli A., Camargo F. D., Perié L., Trumpp A., Cabezas-Wallscheid N. Mouse multipotent progenitor 5 cells are located at the interphase between hematopoietic stem and progenitor cells. Blood. 2021;137:3218–3224. doi: 10.1182/blood.2020007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cabezas-Wallscheid N., Klimmeck D., Hansson J., Lipka D. B., Reyes A., Wang Q., Weichenhan D., Lier A., von Paleske L., Renders S., Wünsche P., Zeisberger P., Brocks D., Gu L., Herrmann C., Haas S., Essers M. A. G., Brors B., Eils R., Huber W., Milsom M. D., Plass C., Krijgsveld J., Trumpp A. Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell. 2014;15:507–522. doi: 10.1016/j.stem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Greaves M. F., Chan L. C., Furley A. J., Watt S. M., Molgaard H. V. Lineage promiscuity in hemopoietic differentiation and leukemia. Blood. 1986;67:1–11. [PubMed] [Google Scholar]
- 80.Sawai C. M., Babovic S., Upadhaya S., Knapp D., Lavin Y., Lau C. M., Goloborodko A., Feng J., Fujisaki J., Ding L., Mirny L. A., Merad M., Eaves C. J., Reizis B. Hematopoietic stem cells are the major source of multilineage hematopoiesis in adult animals. Immunity. 2016;45:597–609. doi: 10.1016/j.immuni.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Säwen P., Eldeeb M., Erlandsson E., Kristiansen T. A., Laterza C., Kokaia Z., Karlsson G., Yuan J., Soneji S., Mandal P. K., Rossi D. J., Bryder D. Murine HSCs contribute actively to native hematopoiesis but with reduced differentiation capacity upon aging. eLife. 2018;7:e41258. doi: 10.7554/eLife.41258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chapple R. H., Tseng Y. J., Hu T., Kitano A., Takeichi M., Hoegenauer K. A., Nakada D. Lineage tracing of murine adult hematopoietic stem cells reveals active contribution to steady-state hematopoiesis. Blood Adv. 2018;2:1220–1228. doi: 10.1182/bloodadvances.2018016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pucella J. N., Upadhaya S., Reizis B. The source and dynamics of adult hematopoiesis: insights from lineage tracing. Annu Rev Cell Dev Biol. 2020;36:529–550. doi: 10.1146/annurev-cellbio-020520-114601. [DOI] [PubMed] [Google Scholar]
- 84.Weinreb C., Rodriguez-Fraticelli A., Camargo F. D., Klein A. M. Lineage tracing on transcriptional landscapes links state to fate during differentiation. Science. 2020;367:eaaw3381. doi: 10.1126/science.aaw3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Upadhaya S., Krichevsky O., Akhmetzyanova I., Sawai C. M., Fooksman D. R., Reizis B. Intravital imaging reveals motility of adult hematopoietic stem cells in the bone marrow niche. Cell Stem Cell. 2020;27:336–345.e4. doi: 10.1016/j.stem.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fliedner T. M., Friesecke I., Graessle D., Paulsen C., Weiss M. Hematopoietic cell renewal as the limiting factor in low-level radiation exposure: diagnostic implications and therapeutic options. Mil Med. 2002;167:46–48. [PubMed] [Google Scholar]
- 87.Kricun M. E. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14:10–19. doi: 10.1007/BF00361188. [DOI] [PubMed] [Google Scholar]
- 88.Tuckermann J., Adams R. H. The endothelium-bone axis in development, homeostasis and bone and joint disease. Nat Rev Rheumatol. 2021;17:608–620. doi: 10.1038/s41584-021-00682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prisby R. D. Bone Marrow Microvasculature. Compr Physiol. 2020;10:1009–1046. doi: 10.1002/cphy.c190009. [DOI] [PubMed] [Google Scholar]
- 90.Chen J., Hendriks M., Chatzis A., Ramasamy S. K., Kusumbe A. P. Bone vasculature and bone marrow vascular niches in health and disease. J Bone Miner Res. 2020;35:2103–2120. doi: 10.1002/jbmr.4171. [DOI] [PubMed] [Google Scholar]
- 91.Ramasamy S. K. Structure and functions of blood vessels and vascular niches in bone. Stem Cells Int. 2017;2017:5046953. doi: 10.1155/2017/5046953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tortora G. J., Derrickson B. H. Principles of anatomy and physiology. John Wiley & Sons Press; New York: 2021. [Google Scholar]
- 93.Kopp H. G., Hooper A. T., Avecilla S. T., Rafii S. Functional heterogeneity of the bone marrow vascular niche. Ann N Y Acad Sci. 2009;1176:47–54. doi: 10.1111/j.1749-6632.2009.04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grüneboom A., Hawwari I., Weidner D., Culemann S., Müller S., Henneberg S., Brenzel A., Merz S., Bornemann L., Zec K., Wuelling M., Kling L., Hasenberg M., Voortmann S., Lang S., Baum W., Ohs A., Kraff O., Quick H. H., Jäger M., Landgraeber S., Dudda M., Danuser R., Stein J. V., Rohde M., Gelse K., Garbe A. I., Adamczyk A., Westendorf A. M., Hoffmann D., Christiansen S., Engel D. R., Vortkamp A., Krönke G., Herrmann M., Kamradt T., Schett G., Hasenberg A., Gunzer M. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat Metab. 2019;1:236–250. doi: 10.1038/s42255-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chanavaz M. Anatomy and histophysiology of the periosteum: quantification of the periosteal blood supply to the adjacent bone with 85Sr and gamma spectrometry. J Oral Implantol. 1995;21:214–219. [PubMed] [Google Scholar]
- 96.Herisson F., Frodermann V., Courties G., Rohde D., Sun Y., Vandoorne K., Wojtkiewicz G. R., Masson G. S., Vinegoni C., Kim J., Kim D. E., Weissleder R., Swirski F. K., Moskowitz M. A., Nahrendorf M. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat Neurosci. 2018;21:1209–1217. doi: 10.1038/s41593-018-0213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gao X., Zhang D., Xu C., Li H., Caron K. M., Frenette P. S. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature. 2021;589:591–596. doi: 10.1038/s41586-020-03057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sugiyama T., Kohara H., Noda M., Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 99.Méndez-Ferrer S., Michurina T. V., Ferraro F., Mazloom A. R., Macarthur B. D., Lira S. A., Scadden D. T., Ma’ayan A., Enikolopov G. N., Frenette P. S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Acar M., Kocherlakota K. S., Murphy M. M., Peyer J. G., Oguro H., Inra C. N., Jaiyeola C., Zhao Z., Luby-Phelps K., Morrison S. J. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526:126–130. doi: 10.1038/nature15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakamura-Ishizu A., Takubo K., Kobayashi H., Suzuki-Inoue K., Suda T. CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. J Exp Med. 2015;212:2133–2146. doi: 10.1084/jem.20150057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kunisaki Y., Bruns I., Scheiermann C., Ahmed J., Pinho S., Zhang D., Mizoguchi T., Wei Q., Lucas D., Ito K., Mar J. C., Bergman A., Frenette P. S. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bruns I., Lucas D., Pinho S., Ahmed J., Lambert M. P., Kunisaki Y., Scheiermann C., Schiff L., Poncz M., Bergman A., Frenette P. S. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20:1315–1320. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen J. Y., Miyanishi M., Wang S. K., Yamazaki S., Sinha R., Kao K. S., Seita J., Sahoo D., Nakauchi H., Weissman I. L. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature. 2016;530:223–227. doi: 10.1038/nature16943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kokkaliaris K. D., Kunz L., Cabezas-Wallscheid N., Christodoulou C., Renders S., Camargo F., Trumpp A., Scadden D. T., Schroeder T. Adult blood stem cell localization reflects the abundance of reported bone marrow niche cell types and their combinations. Blood. 2020;136:2296–2307. doi: 10.1182/blood.2020006574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Desterke C., Petit L., Sella N., Chevallier N., Cabeli V., Coquelin L., Durand C., Oostendorp R. A. J., Isambert H., Jaffredo T., Charbord P. Inferring gene networks in bone marrow hematopoietic stem cell-supporting stromal niche populations. iScience. 2020;23:101222. doi: 10.1016/j.isci.2020.101222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tikhonova A. N., Dolgalev I., Hu H., Sivaraj K. K., Hoxha E., Cuesta-Domínguez Á., Pinho S., Akhmetzyanova I., Gao J., Witkowski M., Guillamot M., Gutkin M. C., Zhang Y., Marier C., Diefenbach C., Kousteni S., Heguy A., Zhong H., Fooksman D. R., Butler J. M., Economides A., Frenette P. S., Adams R. H., Satija R., Tsirigos A., Aifantis I. The bone marrow microenvironment at single-cell resolution. Nature. 2019;569:222–228. doi: 10.1038/s41586-019-1104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baryawno N., Przybylski D., Kowalczyk M. S., Kfoury Y., Severe N., Gustafsson K., Kokkaliaris K. D., Mercier F., Tabaka M., Hofree M., Dionne D., Papazian A., Lee D., Ashenberg O., Subramanian A., Vaishnav E. D., Rozenblatt-Rosen O., Regev A., Scadden D. T. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell. 2019;177:1915–1932.e16. doi: 10.1016/j.cell.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wolock S. L., Krishnan I., Tenen D. E., Matkins V., Camacho V., Patel S., Agarwal P., Bhatia R., Tenen D. G., Klein A. M., Welner R. S. Mapping distinct bone marrow niche populations and their differentiation paths. Cell Rep. 2019;28:302–311.e5. doi: 10.1016/j.celrep.2019.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baccin C., Al-Sabah J., Velten L., Helbling P. M., Grünschläger F., Hernández-Malmierca P., Nombela-Arrieta C., Steinmetz L. M., Trumpp A., Haas S. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat Cell Biol. 2020;22:38–48. doi: 10.1038/s41556-019-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tavassoli M., Crosby W. H. Transplantation of marrow to extramedullary sites. Science. 1968;161:54–56. doi: 10.1126/science.161.3836.54. [DOI] [PubMed] [Google Scholar]
- 112.Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 113.Whitlock C. A., Witte O. N. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci U S A. 1982;79:3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Collins L. S., Dorshkind K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol. 1987;138:1082–1087. [PubMed] [Google Scholar]
- 115.Deryugina E. I., Müller-Sieburg C. E. Stromal cells in long-term cultures: keys to the elucidation of hematopoietic development? Crit Rev Immunol. 1993;13:115–150. [PubMed] [Google Scholar]
- 116.Zhu J., Garrett R., Jung Y., Zhang Y., Kim N., Wang J., Joe G. J., Hexner E., Choi Y., Taichman R. S., Emerson S. G. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 117.Nilsson S. K., Johnston H. M., Coverdale J. A. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 118.Calvi L. M., Adams G. B., Weibrecht K. W., Weber J. M., Olson D. P., Knight M. C., Martin R. P., Schipani E., Divieti P., Bringhurst F. R., Milner L. A., Kronenberg H. M., Scadden D. T. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 119.Zhang J., Niu C., Ye L., Huang H., He X., Tong W. G., Ross J., Haug J., Johnson T., Feng J. Q., Harris S., Wiedemann L. M., Mishina Y., Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 120.Visnjic D., Kalajzic Z., Rowe D. W., Katavic V., Lorenzo J., Aguila H. L. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 121.Stier S., Ko Y., Forkert R., Lutz C., Neuhaus T., Grünewald E., Cheng T., Dombkowski D., Calvi L. M., Rittling S. R., Scadden D. T. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201:1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Joseph C., Quach J. M., Walkley C. R., Lane S. W., Lo Celso C., Purton L. E. Deciphering hematopoietic stem cells in their niches: a critical appraisal of genetic models, lineage tracing, and imaging strategies. Cell Stem Cell. 2013;13:520–533. doi: 10.1016/j.stem.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 123.Haltalli M. L. R., Lo Celso C. Intravital imaging of bone marrow niches. Methods Mol Biol. 2021;2308:203–222. doi: 10.1007/978-1-0716-1425-9_16. [DOI] [PubMed] [Google Scholar]
- 124.Zhao M., Tao F., Venkatraman A., Li Z., Smith S. E., Unruh J., Chen S., Ward C., Qian P., Perry J. M., Marshall H., Wang J., He X. C., Li L. N-cadherin-expressing bone and marrow stromal progenitor cells maintain reserve hematopoietic stem cells. Cell Rep. 2019;26:652–669.e6. doi: 10.1016/j.celrep.2018.12.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakamura-Ishizu A., Suda T. Dynamic changes in the niche with N-cadherin revisited: the HSC “niche herein”. Cell Stem Cell. 2019;24:355–356. doi: 10.1016/j.stem.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 126.Lassailly F., Foster K., Lopez-Onieva L., Currie E., Bonnet D. Multimodal imaging reveals structural and functional heterogeneity in different bone marrow compartments: functional implications on hematopoietic stem cells. Blood. 2013;122:1730–1740. doi: 10.1182/blood-2012-11-467498. [DOI] [PubMed] [Google Scholar]
- 127.Kiel M. J., Yilmaz O. H., Iwashita T., Yilmaz O. H., Terhorst C., Morrison S. J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 128.Nombela-Arrieta C., Pivarnik G., Winkel B., Canty K. J., Harley B., Mahoney J. E., Park S. Y., Lu J., Protopopov A., Silberstein L. E. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Asada N., Kunisaki Y., Pierce H., Wang Z., Fernandez N. F., Birbrair A., Ma’ayan A., Frenette P. S. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol. 2017;19:214–223. doi: 10.1038/ncb3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Itkin T., Gur-Cohen S., Spencer J. A., Schajnovitz A., Ramasamy S. K., Kusumbe A. P., Ledergor G., Jung Y., Milo I., Poulos M. G., Kalinkovich A., Ludin A., Kollet O., Shakhar G., Butler J. M., Rafii S., Adams R. H., Scadden D. T., Lin C. P., Lapidot T. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;532:323–328. doi: 10.1038/nature17624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nakamura-Ishizu A., Takubo K., Fujioka M., Suda T. Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochem Biophys Res Commun. 2014;454:353–357. doi: 10.1016/j.bbrc.2014.10.095. [DOI] [PubMed] [Google Scholar]
- 132.Zhao M., Perry J. M., Marshall H., Venkatraman A., Qian P., He X. C., Ahamed J., Li L. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. 2014;20:1321–1326. doi: 10.1038/nm.3706. [DOI] [PubMed] [Google Scholar]
- 133.Zhang Y., McGrath K. E., Ayoub E., Kingsley P. D., Yu H., Fegan K., McGlynn K. A., Rudzinskas S., Palis J., Perkins A. S. Mds1(CreERT2), an inducible Cre allele specific to adult-repopulating hematopoietic stem cells. Cell Rep. 2021;36:109562. doi: 10.1016/j.celrep.2021.109562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Christodoulou C., Spencer J. A., Yeh S. A., Turcotte R., Kokkaliaris K. D., Panero R., Ramos A., Guo G., Seyedhassantehrani N., Esipova T. V., Vinogradov S. A., Rudzinskas S., Zhang Y., Perkins A. S., Orkin S. H., Calogero R. A., Schroeder T., Lin C. P., Camargo F. D. Live-animal imaging of native haematopoietic stem and progenitor cells. Nature. 2020;578:278–283. doi: 10.1038/s41586-020-1971-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Silberstein L., Goncalves K. A., Kharchenko P. V., Turcotte R., Kfoury Y., Mercier F., Baryawno N., Severe N., Bachand J., Spencer J. A., Papazian A., Lee D., Chitteti B. R., Srour E. F., Hoggatt J., Tate T., Lo Celso C., Ono N., Nutt S., Heino J., Sipilä K., Shioda T., Osawa M., Lin C. P., Hu G. F., Scadden D. T. Proximity-based differential single-cell analysis of the niche to identify stem/progenitor cell regulators. Cell Stem Cell. 2016;19:530–543. doi: 10.1016/j.stem.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pinho S., Marchand T., Yang E., Wei Q., Nerlov C., Frenette P. S. Lineage-biased hematopoietic stem cells are regulated by distinct niches. Dev Cell. 2018;44:634–641.e4. doi: 10.1016/j.devcel.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cordeiro Gomes A., Hara T., Lim V. Y., Herndler-Brandstetter D., Nevius E., Sugiyama T., Tani-Ichi S., Schlenner S., Richie E., Rodewald H. R., Flavell R. A., Nagasawa T., Ikuta K., Pereira J. P. Hematopoietic stem cell niches produce lineage-instructive signals to control multipotent progenitor differentiation. Immunity. 2016;45:1219–1231. doi: 10.1016/j.immuni.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bourgine P. E., Klein T., Paczulla A. M., Shimizu T., Kunz L., Kokkaliaris K. D., Coutu D. L., Lengerke C., Skoda R., Schroeder T., Martin I. In vitro biomimetic engineering of a human hematopoietic niche with functional properties. Proc Natl Acad Sci U S A. 2018;115:E5688–e5695. doi: 10.1073/pnas.1805440115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Omatsu Y., Sugiyama T., Kohara H., Kondoh G., Fujii N., Kohno K., Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 140.Coutu D. L., Kokkaliaris K. D., Kunz L., Schroeder T. Three-dimensional map of nonhematopoietic bone and bone-marrow cells and molecules. Nat Biotechnol. 2017;35:1202–1210. doi: 10.1038/nbt.4006. [DOI] [PubMed] [Google Scholar]
- 141.Ding L., Saunders T. L., Enikolopov G., Morrison S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Seike M., Omatsu Y., Watanabe H., Kondoh G., Nagasawa T. Stem cell niche-specific Ebf3 maintains the bone marrow cavity. Genes Dev. 2018;32:359–372. doi: 10.1101/gad.311068.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gomariz A., Helbling P. M., Isringhausen S., Suessbier U., Becker A., Boss A., Nagasawa T., Paul G., Goksel O., Székely G., Stoma S., Nørrelykke S. F., Manz M. G., Nombela-Arrieta C. Quantitative spatial analysis of haematopoiesis-regulating stromal cells in the bone marrow microenvironment by 3D microscopy. Nat Commun. 2018;9:2532. doi: 10.1038/s41467-018-04770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ding L., Morrison S. J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Greenbaum A., Hsu Y. M., Day R. B., Schuettpelz L. G., Christopher M. J., Borgerding J. N., Nagasawa T., Link D. C. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shen B., Tasdogan A., Ubellacker J. M., Zhang J., Nosyreva E. D., Du L., Murphy M. M., Hu S., Yi Y., Kara N., Liu X., Guela S., Jia Y., Ramesh V., Embree C., Mitchell E. C., Zhao Y. C., Ju L. A., Hu Z., Crane G. M., Zhao Z., Syeda R., Morrison S. J. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature. 2021;591:438–444. doi: 10.1038/s41586-021-03298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhong L., Yao L., Tower R. J., Wei Y., Miao Z., Park J., Shrestha R., Wang L., Yu W., Holdreith N., Huang X., Zhang Y., Tong W., Gong Y., Ahn J., Susztak K., Dyment N., Li M., Long F., Chen C., Seale P., Qin L. Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. eLife. 2020;9:e54695. doi: 10.7554/eLife.54695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Matsushita Y., Nagata M., Kozloff K. M., Welch J. D., Mizuhashi K., Tokavanich N., Hallett S. A., Link D. C., Nagasawa T., Ono W., Ono N. A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat Commun. 2020;11:332. doi: 10.1038/s41467-019-14029-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Friedenstein A. J., Petrakova K. V., Kurolesova A. I., Frolova G. P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 150.Friedenstein A. J., Chailakhyan R. K., Latsinik N. V., Panasyuk A. F., Keiliss-Borok I. V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 151.Friedenstein A. J., Chailakhyan R. K., Gerasimov U. V. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 152.Friedenstein A. J., Chailakhjan R. K., Lalykina K. S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 153.Castro-Malaspina H., Gay R. E., Resnick G., Kapoor N., Meyers P., Chiarieri D., McKenzie S., Broxmeyer H. E., Moore M. A. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- 154.Kuznetsov S. A., Friedenstein A. J., Robey P. G. Factors required for bone marrow stromal fibroblast colony formation in vitro. Br J Haematol. 1997;97:561–570. doi: 10.1046/j.1365-2141.1997.902904.x. [DOI] [PubMed] [Google Scholar]
- 155.Owen M., Friedenstein A. J. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 156.Kuznetsov S. A., Krebsbach P. H., Satomura K., Kerr J., Riminucci M., Benayahu D., Robey P. G. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 157.Wakitani S., Imoto K., Yamamoto T., Saito M., Murata N., Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 158.Caplan A. I. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 159.Pittenger M. F., Discher D. E., Péault B. M., Phinney D. G., Hare J. M., Caplan A. I. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 161.Dos Santos F., Andrade P. Z., Boura J. S., Abecasis M. M., da Silva C. L., Cabral J. M. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol. 2010;223:27–35. doi: 10.1002/jcp.21987. [DOI] [PubMed] [Google Scholar]
- 162.Krebsbach P. H., Kuznetsov S. A., Bianco P., Robey P. G. Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med. 1999;10:165–181. doi: 10.1177/10454411990100020401. [DOI] [PubMed] [Google Scholar]
- 163.Merryweather-Clarke A. T., Cook D., Lara B. J., Hua P., Repapi E., Ashley N., Lim S. Y., Watt S. M. Does osteogenic potential of clonal human bone marrow mesenchymal stem/stromal cells correlate with their vascular supportive ability? Stem Cell Res Ther. 2018;9:351. doi: 10.1186/s13287-018-1095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., Tagliafico E., Ferrari S., Robey P. G., Riminucci M., Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 165.Pinho S., Lacombe J., Hanoun M., Mizoguchi T., Bruns I., Kunisaki Y., Frenette P. S. PDGFRα and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210:1351–1367. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aslan H., Zilberman Y., Kandel L., Liebergall M., Oskouian R. J., Gazit D., Gazit Z. Osteogenic differentiation of noncultured immunoisolated bone marrow-derived CD105+ cells. Stem Cells. 2006;24:1728–1737. doi: 10.1634/stemcells.2005-0546. [DOI] [PubMed] [Google Scholar]
- 167.Battula V. L., Treml S., Bareiss P. M., Gieseke F., Roelofs H., de Zwart P., Müller I., Schewe B., Skutella T., Fibbe W. E., Kanz L., Bühring H. J. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica. 2009;94:173–184. doi: 10.3324/haematol.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Boiret N., Rapatel C., Veyrat-Masson R., Guillouard L., Guérin J. J., Pigeon P., Descamps S., Boisgard S., Berger M. G. Characterization of nonexpanded mesenchymal progenitor cells from normal adult human bone marrow. Exp Hematol. 2005;33:219–225. doi: 10.1016/j.exphem.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 169.Bühring H. J., Battula V. L., Treml S., Schewe B., Kanz L., Vogel W. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 2007;1106:262–271. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- 170.Churchman S. M., Ponchel F., Boxall S. A., Cuthbert R., Kouroupis D., Roshdy T., Giannoudis P. V., Emery P., McGonagle D., Jones E. A. Transcriptional profile of native CD271+ multipotential stromal cells: evidence for multiple fates, with prominent osteogenic and Wnt pathway signaling activity. Arthritis Rheum. 2012;64:2632–2643. doi: 10.1002/art.34434. [DOI] [PubMed] [Google Scholar]
- 171.Churchman S. M., Boxall S. A., McGonagle D., Jones E. A. Predicting the remaining lifespan and cultivation-related loss of osteogenic capacity of bone marrow multipotential stromal cells applicable across a broad donor age range. Stem Cells Int. 2017;2017:6129596. doi: 10.1155/2017/6129596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Crisan M., Yap S., Casteilla L., Chen C. W., Corselli M., Park T. S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P. N., Traas J., Schugar R., Deasy B. M., Badylak S., Buhring H. J., Giacobino J. P., Lazzari L., Huard J., Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 173.Gronthos S., Zannettino A. C., Hay S. J., Shi S., Graves S. E., Kortesidis A., Simmons P. J. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 174.Harkness L., Zaher W., Ditzel N., Isa A., Kassem M. CD146/ MCAM defines functionality of human bone marrow stromal stem cell populations. Stem Cell Res Ther. 2016;7:4. doi: 10.1186/s13287-015-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jones E., English A., Churchman S. M., Kouroupis D., Boxall S. A., Kinsey S., Giannoudis P. G., Emery P., McGonagle D. Large-scale extraction and characterization of CD271+ multipotential stromal cells from trabecular bone in health and osteoarthritis: implications for bone regeneration strategies based on uncultured or minimally cultured multipotential stromal cells. Arthritis Rheum. 2010;62:1944–1954. doi: 10.1002/art.27451. [DOI] [PubMed] [Google Scholar]
- 176.Li H., Ghazanfari R., Zacharaki D., Ditzel N., Isern J., Ekblom M., Méndez-Ferrer S., Kassem M., Scheding S. Low/negative expression of PDGFR-α identifies the candidate primary mesenchymal stromal cells in adult human bone marrow. Stem Cell Reports. 2014;3:965–974. doi: 10.1016/j.stemcr.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mabuchi Y., Morikawa S., Harada S., Niibe K., Suzuki S., Renault-Mihara F., Houlihan D. D., Akazawa C., Okano H., Matsuzaki Y. LNGFR(+)THY-1(+)VCAM-1(hi+) cells reveal functionally distinct subpopulations in mesenchymal stem cells. Stem Cell Reports. 2013;1:152–165. doi: 10.1016/j.stemcr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Quirici N., Soligo D., Bossolasco P., Servida F., Lumini C., Deliliers G. L. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 179.Simmons P. J., Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 180.Tormin A., Li O., Brune J. C., Walsh S., Schütz B., Ehinger M., Ditzel N., Kassem M., Scheding S. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117:5067–5077. doi: 10.1182/blood-2010-08-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhou B. O., Yue R., Murphy M. M., Peyer J. G., Morrison S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang Y., Sivakumaran P., Newcomb A. E., Hernandez D., Harris N., Khanabdali R., Liu G. S., Kelly D. J., Pébay A., Hewitt A. W., Boyle A., Harvey R., Morrison W. A., Elliott D. A., Dusting G. J., Lim S. Y. Cardiac repair with a novel population of mesenchymal stem cells resident in the human heart. Stem Cells. 2015;33:3100–3113. doi: 10.1002/stem.2101. [DOI] [PubMed] [Google Scholar]
- 183.Ghazanfari R., Li H., Zacharaki D., Lim H. C., Scheding S. Human non-hematopoietic CD271(pos)/CD140a(low/neg) bone marrow stroma cells fulfill stringent stem cell criteria in serial transplantations. Stem Cells Dev. 2016;25:1652–1658. doi: 10.1089/scd.2016.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ambrosi T. H., Longaker M. T., Chan C. K. F. A revised perspective of skeletal stem cell biology. Front Cell Dev Biol. 2019;7:189. doi: 10.3389/fcell.2019.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Soliman H., Theret M., Scott W., Hill L., Underhill T. M., Hinz B., Rossi F. M. V. Multipotent stromal cells: One name, multiple identities. Cell Stem Cell. 2021;28:1690–1707. doi: 10.1016/j.stem.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 186.Dolgalev I., Tikhonova A. N. Connecting the dots: resolving the bone marrow niche heterogeneity. Front Cell Dev Biol. 2021;9:622519. doi: 10.3389/fcell.2021.622519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Balzano M., De Grandis M., Vu Manh T. P., Chasson L., Bardin F., Farina A., Sergé A., Bidaut G., Charbord P., Hérault L., Bailly A. L., Cartier-Michaud A., Boned A., Dalod M., Duprez E., Genever P., Coles M., Bajenoff M., Xerri L., Aurrand-Lions M., Schiff C., Mancini S. J. C. Nidogen-1 contributes to the interaction network involved in pro-B cell retention in the peri-sinusoidal hematopoietic stem cell niche. Cell Rep. 2019;26:3257–3271.e8. doi: 10.1016/j.celrep.2019.02.065. [DOI] [PubMed] [Google Scholar]
- 188.Yue R., Zhou B. O., Shimada I. S., Zhao Z., Morrison S. J. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell. 2016;18:782–796. doi: 10.1016/j.stem.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 189.Zhou B. O., Yu H., Yue R., Zhao Z., Rios J. J., Naveiras O., Morrison S. J. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19:891–903. doi: 10.1038/ncb3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fröbel J., Landspersky T., Percin G., Schreck C., Rahmig S., Ori A., Nowak D., Essers M., Waskow C., Oostendorp R. A. J. The hematopoietic bone marrow niche ecosystem. Front Cell Dev Biol. 2021;9:705410. doi: 10.3389/fcell.2021.705410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bianco P., Robey P. G. Skeletal stem cells. Development. 2015;142:1023–1027. doi: 10.1242/dev.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chan C. K., Seo E. Y., Chen J. Y., Lo D., McArdle A., Sinha R., Tevlin R., Seita J., Vincent-Tompkins J., Wearda T., Lu W. J., Senarath-Yapa K., Chung M. T., Marecic O., Tran M., Yan K. S., Upton R., Walmsley G. G., Lee A. S., Sahoo D., Kuo C. J., Weissman I. L., Longaker M. T. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160:285–298. doi: 10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Worthley D. L., Churchill M., Compton J. T., Tailor Y., Rao M., Si Y., Levin D., Schwartz M. G., Uygur A., Hayakawa Y., Gross S., Renz B. W., Setlik W., Martinez A. N., Chen X., Nizami S., Lee H. G., Kang H. P., Caldwell J. M., Asfaha S., Westphalen C. B., Graham T., Jin G., Nagar K., Wang H., Kheirbek M. A., Kolhe A., Carpenter J., Glaire M., Nair A., Renders S., Manieri N., Muthupalani S., Fox J. G., Reichert M., Giraud A. S., Schwabe R. F., Pradere J. P., Walton K., Prakash A., Gumucio D., Rustgi A. K., Stappenbeck T. S., Friedman R. A., Gershon M. D., Sims P., Grikscheit T., Lee F. Y., Karsenty G., Mukherjee S., Wang T. C. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kassem M., Bianco P. Skeletal stem cells in space and time. Cell. 2015;160:17–19. doi: 10.1016/j.cell.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 195.Chan C. K. F., Gulati G. S., Sinha R., Tompkins J. V., Lopez M., Carter A. C., Ransom R. C., Reinisch A., Wearda T., Murphy M., Brewer R. E., Koepke L. S., Marecic O., Manjunath A., Seo E. Y., Leavitt T., Lu W. J., Nguyen A., Conley S. D., Salhotra A., Ambrosi T. H., Borrelli M. R., Siebel T., Chan K., Schallmoser K., Seita J., Sahoo D., Goodnough H., Bishop J., Gardner M., Majeti R., Wan D. C., Goodman S., Weissman I. L., Chang H. Y., Longaker M. T. Identification of the human skeletal stem cell. Cell. 2018;175:43–56.e21. doi: 10.1016/j.cell.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gulati G. S., Murphy M. P., Marecic O., Lopez M., Brewer R. E., Koepke L. S., Manjunath A., Ransom R. C., Salhotra A., Weissman I. L., Longaker M. T., Chan C. K. F. Isolation and functional assessment of mouse skeletal stem cell lineage. Nat Protoc. 2018;13:1294–1309. doi: 10.1038/nprot.2018.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tichy E. D., Mourkioti F. Human skeletal stem cells: the markers provide some clues in the hunt for hidden treasure. Cell Stem Cell. 2018;23:462–463. doi: 10.1016/j.stem.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 198.Viswanathan S., Shi Y., Galipeau J., Krampera M., Leblanc K., Martin I., Nolta J., Phinney D. G., Sensebe L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019–1024. doi: 10.1016/j.jcyt.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 199.Bianco P., Cao X., Frenette P. S., Mao J. J., Robey P. G., Simmons P. J., Wang C. Y. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ambrosi T. H., Sinha R., Steininger H. M., Hoover M. Y., Murphy M. P., Koepke L. S., Wang Y., Lu W. J., Morri M., Neff N. F., Weissman I. L., Longaker M. T., Chan C. K. Distinct skeletal stem cell types orchestrate long bone skeletogenesis. eLife. 2021;10:e66063. doi: 10.7554/eLife.66063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mizuhashi K., Ono W., Matsushita Y., Sakagami N., Takahashi A., Saunders T. L., Nagasawa T., Kronenberg H. M., Ono N. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature. 2018;563:254–258. doi: 10.1038/s41586-018-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Debnath S., Yallowitz A. R., McCormick J., Lalani S., Zhang T., Xu R., Li N., Liu Y., Yang Y. S., Eiseman M., Shim J. H., Hameed M., Healey J. H., Bostrom M. P., Landau D. A., Greenblatt M. B. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562:133–139. doi: 10.1038/s41586-018-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shi Y., He G., Lee W. C., McKenzie J. A., Silva M. J., Long F. Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat Commun. 2017;8:2043. doi: 10.1038/s41467-017-02171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mizoguchi T., Pinho S., Ahmed J., Kunisaki Y., Hanoun M., Mendelson A., Ono N., Kronenberg H. M., Frenette P. S. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29:340–349. doi: 10.1016/j.devcel.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hallett S. A., Matsushita Y., Ono W., Sakagami N., Mizuhashi K., Tokavanich N., Nagata M., Zhou A., Hirai T., Kronenberg H. M., Ono N. Chondrocytes in the resting zone of the growth plate are maintained in a Wnt-inhibitory environment. eLife. 2021;10:e64513. doi: 10.7554/eLife.64513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hojo H., Ohba S., Yano F., Saito T., Ikeda T., Nakajima K., Komiyama Y., Nakagata N., Suzuki K., Takato T., Kawaguchi H., Chung U. I. Gli1 protein participates in Hedgehog-mediated specification of osteoblast lineage during endochondral ossification. J Biol Chem. 2012;287:17860–17869. doi: 10.1074/jbc.M112.347716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhao H., Feng J., Ho T. V., Grimes W., Urata M., Chai Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat Cell Biol. 2015;17:386–396. doi: 10.1038/ncb3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Watt S. M., Bühring H. J., Simmons P. J., Zannettino A. W. C. The stem cell revolution: on the role of CD164 as a human stem cell marker. NPJ Regen Med. 2021;6:33. doi: 10.1038/s41536-021-00143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ambrosi T. H., Scialdone A., Graja A., Gohlke S., Jank A. M., Bocian C., Woelk L., Fan H., Logan D. W., Schürmann A., Saraiva L. R., Schulz T. J. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20:771–784.e6. doi: 10.1016/j.stem.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lazarus H. M., Haynesworth S. E., Gerson S. L., Rosenthal N. S., Caplan A. I. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. [PubMed] [Google Scholar]
- 211.Kouchakian M. R., Baghban N., Moniri S. F., Baghban M., Bakhshalizadeh S., Najafzadeh V., Safaei Z., Izanlou S., Khoradmehr A., Nabipour I., Shirazi R., Tamadon A. The clinical trials of mesenchymal stromal cells therapy. Stem Cells Int. 2021;2021:1634782. doi: 10.1155/2021/1634782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Galipeau J., Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Krampera M., Le Blanc K. Mesenchymal stromal cells: putative microenvironmental modulators become cell therapy. Cell Stem Cell. 2021;28:1708–1725. doi: 10.1016/j.stem.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 214.Granot N., Storb R. History of hematopoietic cell transplantation: challenges and progress. Haematologica. 2020;105:2716–2729. doi: 10.3324/haematol.2019.245688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang L. L., Janes M. E., Kumbhojkar N., Kapate N., Clegg J. R., Prakash S., Heavey M. K., Zhao Z., Anselmo A. C., Mitragotri S. Cell therapies in the clinic. Bioeng Transl Med. 2021;6:e10214. doi: 10.1002/btm2.10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Levy O., Kuai R., Siren E. M. J., Bhere D., Milton Y., Nissar N., De Biasio M., Heinelt M., Reeve B., Abdi R., Alturki M., Fallatah M., Almalik A., Alhasan A. H., Shah K., Karp J. M. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6:eaba6884. doi: 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Galipeau J. Mesenchymal stromal cells for graft-versus-host disease: a trilogy. Biol Blood Marrow Transplant. 2020;26:e89–e91. doi: 10.1016/j.bbmt.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 218.Hill G. R., Betts B. C., Tkachev V., Kean L. S., Blazar B. R. Current concepts and advances in graft-versus-host disease immunology. Annu Rev Immunol. 2021;39:19–49. doi: 10.1146/annurev-immunol-102119-073227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kelly K., Rasko J. E. J. Mesenchymal stromal cells for the treatment of graft versus host disease. Front Immunol. 2021;12:761616. doi: 10.3389/fimmu.2021.761616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Le Blanc K., Rasmusson I., Sundberg B., Götherström C., Hassan M., Uzunel M., Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 221.Zeiser R., von Bubnoff N., Butler J., Mohty M., Niederwieser D., Or R., Szer J., Wagner E. M., Zuckerman T., Mahuzier B., Xu J., Wilke C., Gandhi K. K., Socié G. REACH2 Trial Group. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382:1800–1810. doi: 10.1056/NEJMoa1917635. [DOI] [PubMed] [Google Scholar]
- 222.Chen X., Wang C., Yin J., Xu J., Wei J., Zhang Y. Efficacy of mesenchymal stem cell therapy for steroid-refractory acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. PLoS One. 2015;10:e0136991. doi: 10.1371/journal.pone.0136991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hashmi S., Ahmed M., Murad M. H., Litzow M. R., Adams R. H., Ball L. M., Prasad V. K., Kebriaei P., Ringden O. Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: systematic review and meta-analysis. Lancet Haematol. 2016;3:e45–52. doi: 10.1016/S2352-3026(15)00224-0. [DOI] [PubMed] [Google Scholar]
- 224.Kallekleiv M., Larun L., Bruserud Ø., Hatfield K. J. Co-transplantation of multipotent mesenchymal stromal cells in allogeneic hematopoietic stem cell transplantation: A systematic review and meta-analysis. Cytotherapy. 2016;18:172–185. doi: 10.1016/j.jcyt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 225.Wang L., Zhu C. Y., Ma D. X., Gu Z. Y., Xu C. C., Wang F. Y., Chen J. G., Liu C. J., Guan L. X., Gao R., Gao Z., Fang S., Zhuo D. J., Liu S. F., Gao C. J. Efficacy and safety of mesenchymal stromal cells for the prophylaxis of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: a meta-analysis of randomized controlled trials. Ann Hematol. 2018;97:1941–1950. doi: 10.1007/s00277-018-3384-8. [DOI] [PubMed] [Google Scholar]
- 226.Fisher S. A., Cutler A., Doree C., Brunskill S. J., Stanworth S. J., Navarrete C., Girdlestone J. Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition. Cochrane Database Syst Rev. 2019;1:Cd009768. doi: 10.1002/14651858.CD009768.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Li T., Luo C., Zhang J., Wei L., Sun W., Xie Q., Liu Y., Zhao Y., Xu S., Wang L. Efficacy and safety of mesenchymal stem cells co-infusion in allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. Stem Cell Res Ther. 2021;12:246. doi: 10.1186/s13287-021-02304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Galipeau J., Krampera M., Leblanc K., Nolta J. A., Phinney D. G., Shi Y., Tarte K., Viswanathan S., Martin I. Mesenchymal stromal cell variables influencing clinical potency: the impact of viability, fitness, route of administration and host predisposition. Cytotherapy. 2021;23:368–372. doi: 10.1016/j.jcyt.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 229.Nolta J. A., Galipeau J., Phinney D. G. Improving mesenchymal stem/ stromal cell potency and survival: Proceedings from the International Society of Cell Therapy (ISCT) MSC preconference held in May 2018, Palais des Congràs de Montréal, Organized by the ISCT MSC Scientific Committee. Cytotherapy. 2020;22:123–126. doi: 10.1016/j.jcyt.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 230.Ménard C., Dulong J., Roulois D., Hébraud B., Verdiàre L., Pangault C., Sibut V., Bezier I., Bescher N., Monvoisin C., Gadelorge M., Bertheuil N., Flécher E., Casteilla L., Collas P., Sensebé L., Bourin P., Espagnolle N., Tarte K. Integrated transcriptomic, phenotypic, and functional study reveals tissue-specific immune properties of mesenchymal stromal cells. Stem Cells. 2020;38:146–159. doi: 10.1002/stem.3077. [DOI] [PubMed] [Google Scholar]
- 231.Forsberg M. H., Kink J. A., Hematti P., Capitini C. M. Mesenchymal stromal cells and exosomes: progress and challenges. Front Cell Dev Biol. 2020;8:665. doi: 10.3389/fcell.2020.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Galleu A., Riffo-Vasquez Y., Trento C., Lomas C., Dolcetti L., Cheung T. S., von Bonin M., Barbieri L., Halai K., Ward S., Weng L., Chakraverty R., Lombardi G., Watt F. M., Orchard K., Marks D. I., Apperley J., Bornhauser M., Walczak H., Bennett C., Dazzi F. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9:eaam7828. doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- 233.Carrington L. J., Maillard I. One-two punch injury to tolerance mechanisms in graft-versus-host disease. J Clin Invest. 2020;130:1625–1628. doi: 10.1172/JCI136139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cheng X., Jiang M., Long L., Meng J. Potential roles of mesenchymal stem cells and their exosomes in the treatment of COVID-19. Front Biosci (Landmark Ed) 2021;26:948–961. doi: 10.52586/4999. [DOI] [PubMed] [Google Scholar]
- 235.World Health Organization. WHO coronavirus (COVID-19) dashboard. [Accessed November 21, 2021]. https://covid19.who.int
- 236.Abdelgawad M., Bakry N. S., Farghali A. A., Abdel-Latif A., Lotfy A. Mesenchymal stem cell-based therapy and exosomes in COVID-19: current trends and prospects. Stem Cell Res Ther. 2021;12:469. doi: 10.1186/s13287-021-02542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zanirati G., Provenzi L., Libermann L. L., Bizotto S. C., Ghilardi I. M., Marinowic D. R., Shetty A. K., Da Costa J. C. Stem cell-based therapy for COVID-19 and ARDS: a systematic review. NPJ Regen Med. 2021;6:73. doi: 10.1038/s41536-021-00181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shetty A. K., Shetty P. A., Zanirati G., Jin K. Further validation of the efficacy of mesenchymal stem cell infusions for reducing mortality in COVID-19 patients with ARDS. NPJ Regen Med. 2021;6:53. doi: 10.1038/s41536-021-00161-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Moll G., Hoogduijn M. J., Ankrum J. A. Editorial: safety, efficacy and mechanisms of action of mesenchymal stem cell therapies. Front Immunol. 2020;11:243. doi: 10.3389/fimmu.2020.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Moll G., Drzeniek N., Kamhieh-Milz J., Geissler S., Volk H. D., Reinke P. MSC therapies for COVID-19: importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efficacy. Front Immunol. 2020;11:1091. doi: 10.3389/fimmu.2020.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.World Health Organization. Ageing and health. [Accessed November 21, 2021]. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health
- 242.Rossi D. J., Bryder D., Zahn J. M., Ahlenius H., Sonu R., Wagers A. J., Weissman I. L. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Pang W. W., Price E. A., Sahoo D., Beerman I., Maloney W. J., Rossi D. J., Schrier S. L., Weissman I. L. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Beerman I., Bhattacharya D., Zandi S., Sigvardsson M., Weissman I. L., Bryder D., Rossi D. J. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wahlestedt M., Pronk C. J., Bryder D. Concise review: hematopoietic stem cell aging and the prospects for rejuvenation. Stem Cells Transl Med. 2015;4:186–194. doi: 10.5966/sctm.2014-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Young K., Borikar S., Bell R., Kuffler L., Philip V., Trowbridge J. J. Progressive alterations in multipotent hematopoietic progenitors underlie lymphoid cell loss in aging. J Exp Med. 2016;213:2259–2267. doi: 10.1084/jem.20160168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Massaro F., Corrillon F., Stamatopoulos B., Meuleman N., Lagneaux L., Bron D. Aging of bone marrow mesenchymal stromal cells: hematopoiesis disturbances and potential role in the development of hematologic cancers. Cancers (Basel) 2020;13:68. doi: 10.3390/cancers13010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Batsivari A., Haltalli M. L. R., Passaro D., Pospori C., Lo Celso C., Bonnet D. Dynamic responses of the haematopoietic stem cell niche to diverse stresses. Nat Cell Biol. 2020;22:7–17. doi: 10.1038/s41556-019-0444-9. [DOI] [PubMed] [Google Scholar]
- 249.Mian S. A., Bonnet D. Nature or nurture? Role of the bone marrow microenvironment in the genesis and maintenance of myelodysplastic syndromes. Cancers (Basel) 2021;13:4116. doi: 10.3390/cancers13164116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Vandyke K. Seed and soil revisited in multiple myeloma. Blood. 2021;137:2282–2283. doi: 10.1182/blood.2020009555. [DOI] [PubMed] [Google Scholar]
- 251.Khoo W. H., Ledergor G., Weiner A., Roden D. L., Terry R. L., McDonald M. M., Chai R. C., De Veirman K., Owen K. L., Opperman K. S., Vandyke K., Clark J. R., Seckinger A., Kovacic N., Nguyen A., Mohanty S. T., Pettitt J. A., Xiao Y., Corr A. P., Seeliger C., Novotny M., Lasken R. S., Nguyen T. V., Oyajobi B. O., Aftab D., Swarbrick A., Parker B., Hewett D. R., Hose D., Vanderkerken K., Zannettino A. C. W., Amit I., Phan T. G., Croucher P. I. A niche-dependent myeloid transcriptome signature defines dormant myeloma cells. Blood. 2019;134:30–43. doi: 10.1182/blood.2018880930. [DOI] [PubMed] [Google Scholar]
- 252.Cakouros D., Gronthos S. Epigenetic regulation of bone marrow stem cell aging: revealing epigenetic signatures associated with hematopoietic and mesenchymal stem cell aging. Aging Dis. 2019;10:174–189. doi: 10.14336/AD.2017.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Neri S., Borzì R. M. Molecular mechanisms contributing to mesenchymal stromal cell aging. Biomolecules. 2020;10:340. doi: 10.3390/biom10020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yuan H. F., Zhai C., Yan X. L., Zhao D. D., Wang J. X., Zeng Q., Chen L., Nan X., He L. J., Li S. T., Yue W., Pei X. T. SIRT1 is required for long-term growth of human mesenchymal stem cells. J Mol Med (Berl) 2012;90:389–400. doi: 10.1007/s00109-011-0825-4. [DOI] [PubMed] [Google Scholar]
- 255.Simic P., Zainabadi K., Bell E., Sykes D. B., Saez B., Lotinun S., Baron R., Scadden D., Schipani E., Guarente L. SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating β-catenin. EMBO Mol Med. 2013;5:430–440. doi: 10.1002/emmm.201201606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pan H., Guan D., Liu X., Li J., Wang L., Wu J., Zhou J., Zhang W., Ren R., Zhang W., Li Y., Yang J., Hao Y., Yuan T., Yuan G., Wang H., Ju Z., Mao Z., Li J., Qu J., Tang F., Liu G. H. SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res. 2016;26:190–205. doi: 10.1038/cr.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Denu R. A. SIRT3 enhances mesenchymal stem cell longevity and differentiation. Oxid Med Cell Longev. 2017;2017:5841716. doi: 10.1155/2017/5841716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yang R., Yu T., Kou X., Gao X., Chen C., Liu D., Zhou Y., Shi S. Tet1 and Tet2 maintain mesenchymal stem cell homeostasis via demethylation of the P2rX7 promoter. Nat Commun. 2018;9:2143. doi: 10.1038/s41467-018-04464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Cakouros D., Hemming S., Gronthos K., Liu R., Zannettino A., Shi S., Gronthos S. Specific functions of TET1 and TET2 in regulating mesenchymal cell lineage determination. Epigenetics Chromatin. 2019;12:3. doi: 10.1186/s13072-018-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhang P., Chen Z., Li R., Guo Y., Shi H., Bai J., Yang H., Sheng M., Li Z., Li Z., Li J., Chen S., Yuan W., Cheng T., Xu M., Zhou Y., Yang F. C. Loss of ASXL1 in the bone marrow niche dysregulates hematopoietic stem and progenitor cell fates. Cell Discov. 2018;4:4. doi: 10.1038/s41421-017-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fulop T., Witkowski J. M., Olivieri F., Larbi A. The integration of inflammaging in age-related diseases. Semin Immunol. 2018;40:17–35. doi: 10.1016/j.smim.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 262.Lee B. C., Yu K. R. Impact of mesenchymal stem cell senescence on inflammaging. BMB Rep. 2020;53:65–73. doi: 10.5483/BMBRep.2020.53.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gnani D., Crippa S., Della Volpe L., Rossella V., Conti A., Lettera E., Rivis S., Ometti M., Fraschini G., Bernardo M. E., Di Micco R. An early-senescence state in aged mesenchymal stromal cells contributes to hematopoietic stem and progenitor cell clonogenic impairment through the activation of a pro-inflammatory program. Aging Cell. 2019;18:e12933. doi: 10.1111/acel.12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Whitehead J., Zhang J., Harvestine J. N., Kothambawala A., Liu G. Y., Leach J. K. Tunneling nanotubes mediate the expression of senescence markers in mesenchymal stem/stromal cell spheroids. Stem Cells. 2020;38:80–89. doi: 10.1002/stem.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ambrosi T. H., Marecic O., McArdle A., Sinha R., Gulati G. S., Tong X., Wang Y., Steininger H. M., Hoover M. Y., Koepke L. S., Murphy M. P., Sokol J., Seo E. Y., Tevlin R., Lopez M., Brewer R. E., Mascharak S., Lu L., Ajanaku O., Conley S. D., Seita J., Morri M., Neff N. F., Sahoo D., Yang F., Weissman I. L., Longaker M. T., Chan C. K. F. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature. 2021;597:256–262. doi: 10.1038/s41586-021-03795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Saçma M., Pospiech J., Bogeska R., de Back W., Mallm J. P., Sakk V., Soller K., Marka G., Vollmer A., Karns R., Cabezas-Wallscheid N., Trumpp A., Méndez-Ferrer S., Milsom M. D., Mulaw M. A., Geiger H., Florian M. C. Haematopoietic stem cells in perisinusoidal niches are protected from ageing. Nat Cell Biol. 2019;21:1309–1320. doi: 10.1038/s41556-019-0418-y. [DOI] [PubMed] [Google Scholar]
- 267.Greenblatt M. B., Debnath S. A stem-cell basis for skeletal ageing. Nature. 2021;597:182–183. doi: 10.1038/d41586-021-02118-0. [DOI] [PubMed] [Google Scholar]