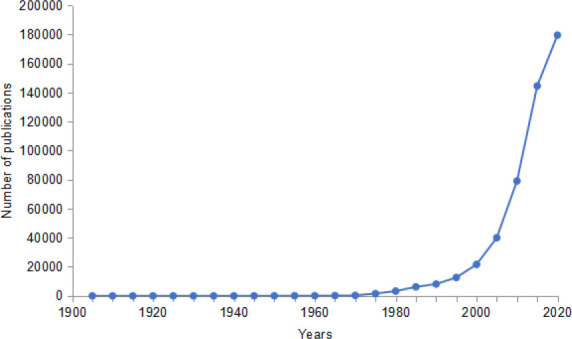
We are pleased to devote the following two issues of Biomaterials Translational to the important subject of the potential translation of stem cells for regenerative medicine and disease treatment. Stem cells with capacities to renew their own tissue are now considered present in all tissues and organs of the body. Such cells have been of great interest to biologists and clinicians over the past century, since Haeckel in his ‘Natural history of creation’ first coined the term in 1868.1 The nature of the definition of what constitutes a stem cell has changed with time, but since the 1970s there has been an explosion of research publications on stem cells ( Figure 1 ). This has involved increasing consideration of these primitive cells, that exist in the embryo and postnatally, for translation to the clinic for medical use and significant public health benefit. The trend in research output continues to increase exponentially with almost 72,000 publications on stem cells being recorded in the past year alone (searched using Web of Science databases). Hence there is great urgency and adequate justification for dedication of appreciable journal emphasis to this topic. In this issue, Stem Cells - Part I, there are a variety of topics within this theme that are covered either as viewpoint, review or research papers.
Figure 1. A topic search using Web of Science databases for publications with abstract containing the term “Stem Cells”, in five yearly intervals from 1900 - present (last checked on December 14, 2021).
In the introductory viewpoint article, a personal perspective of the historical development of our knowledge on some critical aspects of the nature of tissue-specific stem cells derived from marrow stroma and other connective tissues is given by James Triffitt.2 This is followed by a concise but embracing review by Peter Andrews3 on the nature of embryonic/induced pluripotent stem cells as related to their potential uses in regenerative medicine. This emphasizes issues of great significance regarding genetic stability and potentials for malignancy that must be considered before any potential use in humans. Jeffrey Gimble and colleagues4 continue with a review of recent development in using human adipose-derived cells as key component of novel three-dimensional microphysiological systems to replace animal models. This is an exciting research area that uses humanized micro-physiological systems and high throughput in vitro assays for rapid drug discovery. Of high relevance currently is the paper by Arnold Caplan5 on the potential use of injected mesenchymal stem cells (MSCs) for treatment of coronavirus disease 2019 (COVID-19) infections. Many clinical studies of MSCs have been initiated in the past 2 years since the global pandemic began but none with sufficient power for clear clinical benefit. This review promotes a strong proposal for the use of MSCs in combatting COVID-19, and addresses questions on how this potentially curative technology may be verified. Bo Huo and colleagues6 continue with an original research paper that considers the importance on cell morphology and differentiation of focal adhesions that connect them to the local extracellular matrices. They found that these focal adhesions may affect osteogenesis of MSCs and osteoblast-like cells. The important influences of mechanical cues derived from the extracellular matrix on the interactions of cells with biomaterials are comprehensively reviewed by Bin Li and collaborators,7 highlighting recent research progress in this extremely exciting research direction. The penultimate paper is from the laboratory of Zengwu Shao8 and emphasizes in particular the effects on tissue repair of activation of endogenous stem cells. The final contribution is by Nianguo Dong and colleagues9 and considers in critical detail the current animal, in vitro cellular and in silico models that are used to test and develop tissue engineered heart valves.
This current issue of Biomaterials Translational: Stem Cells - Part I thus embraces a wide variety of subjects relevant to the potential uses of these primitive, potentially highly proliferative cells for applications in tissue engineering and medical therapeutic procedures. The following issue Stem Cells - Part II to be published in March 2022 will continue this theme and will include papers from additional leaders in the stem cell field.
Footnotes
Editor note: James T. Triffitt and Qian Wang are Editorial Board members of Biomaterials Translational. There were blinded from reviewing or making decisions on the manuscript. The article was subject to the journal’s standard procedures, with peer review handled independently of these Editorial Board members and their research groups.
References
- 1.Haeckel E. Natürliche Schöpfungsgeschichte. Georg Reimer; Berlin: 1868. [Google Scholar]
- 2.Triffitt J. A brief history of the development of stromal stem cells (stem cells of the skeleton) Biomater Transl. 2021;2:287–293. doi: 10.12336/biomatertransl.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews P. Human pluripotent stem cells: tools for regenerative medicine. Biomater Transl. 2021;2:294–300. doi: 10.12336/biomatertransl.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frazier T., Hamel K., Wu X., Rogers E., Lassiter H., Robinson J., Mohiuddin O., Henderson M., Gimble J. Adipose-derived cells: building blocks of three-dimensional microphysiological systems. Biomater Transl. 2021;2:301–306. doi: 10.12336/biomatertransl.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caplan A. I. Mesenchymal stem cells and COVID-19: the process of discovery and of translation. Biomater Transl. 2021;2:307–311. doi: 10.12336/biomatertransl.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y., Sun Q., Huo B. Focal adhesion regulates osteogenic differentiation of mesenchymal stem cells and osteoblasts. Biomater Transl. 2021;2:312–322. doi: 10.12336/biomatertransl.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei Q., Wang S., Han F., Wang H., Zhang W., Yu Q., Liu C., Ding L., Wang J., Yu L., Zhu C., Li B. Cellular modulation by the mechanical cues from biomaterials for tissue engineering. Biomater Transl. 2021;2:323–342. doi: 10.12336/biomatertransl.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng Y., Li J., Lin H., Tian S., Liu S., Pu F., Zhao L., Ma K., Qing X., Shao Z. Endogenous repair theory enriches construction strategies for orthopaedic biomaterials: a narrative review. Biomater Transl. 2021;2:343–360. doi: 10.12336/biomatertransl.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan G., Liu Y., Xie M., Shi J., Qiao W., Dong N. Experimental and computational models for tissue-engineered heart valves: a narrative review. Biomater Transl. 2021;2:361–375. doi: 10.12336/biomatertransl.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


