ABSTRACT
The lack of bioactivity of conventional medical materials leads to low osseointegration ability that may result in the occurrence of aseptic loosening in the clinic. To achieve high osseointegration, surface modifications with multiple biofunctions including degradability, osteogenesis, angiogenesis and antibacterial properties are required. However, the functions of conventional bioactive coatings are limited. Thus novel biofunctional magnesium (Mg) coatings are believed to be promising candidates for surface modification of implant materials for use in bone tissue repair. By physical vapour deposition, many previous researchers have deposited Mg coatings with high purity and granular microstructure on titanium alloys, polyetheretherketone, steels, Mg alloys and silicon. It was found that the Mg coatings with high-purity could considerably control the degradation rate in the initial stage of Mg alloy implantation, which is the most important problem for the application of Mg alloy implants. In addition, Mg coating on titanium (Ti) implant materials has been extensively studied both in vitro and in vivo, and the results indicated that their corrosion behaviour and biocompatibility are promising. Mg coatings continuously release Mg ions during the degradation process, and the alkaline environment caused by Mg degradation has obvious antibacterial effects. Meanwhile, the Mg coating has beneficial effects on osteogenesis and osseointegration, and increases the new bone-regenerating ability. Mg coatings also exhibit favourable osteogenic and angiogenic properties in vitro and increased long-term bone formation and early vascularization in vivo. Inhibitory effects of Mg coatings on osteoclasts have also been proven, which play a great role in osteoporotic patients. In addition, in order to obtain more biofunctions, other alloying elements such as copper have been added to the Mg coatings. Thus, Mg-coated Ti acquired biofunctions including degradability, osteogenesis, angiogenesis and antibacterial properties. These novel multi-functional Mg coatings are expected to significantly enhance the long-term safety of bone implants for the benefit of patients. This paper gives a brief review of studies of the microstructure, degradation behaviours and biofunctions of Mg coatings, and directions for future research are also proposed.
Key Words: biofunction, coating, degradability, magnesium, osteogenesis, review
Introduction
With global ageing of the human population and economic development, the requirements are increasing for higher performance implants. Conventional medical materials, such as stainless steels, titanium (Ti) alloys and cobalt-based alloys, are still widely used for tissue repairs, especially in orthopaedics owing to their excellent, reliable mechanical performance and corrosion resistance. Over recent decades, many structural biomaterials including new types of stainless steels, Ti alloys and polymers such as polyetheretherketone (PEEK) have been developed and used in artificial joints, trauma treatment, spinal fusion and oral implantation.1-3 However, all the biomaterials mentioned above are bioinert in the human body. The lack of bioactivity leads to their low osseointegration ability that can result in the occurrence of aseptic loosening in the clinic.4, 5 Consequently, improving the bioactivity and long-term safety of implants will have clinical value.
Without reducing the performance of the matrix, surface modification technology can be used to regulate the surface performance of implants to meet clinical demands. Nowadays, almost all bone implants are modified by different surface treatments, such as sand blasting and acid etching, anodic oxidation, plasma spraying, chemical vapour deposition and physical vapour deposition.6 These treatments alter the surface state of implants to regulate their roughness, wettability and charge.7 The creation of a porous structure and porous structured surface for implants can enhance their osteoconduction.8-10 Moreover, bioactive coatings can accelerate osteogenesis, for instance, hydroxyapatite coatings prepared by plasma spraying have been extensively used on artificial joints, oral implants, etc.11, 12 However, the adhesion of ceramic coatings on metals or polymers is not perfect. Cracking and peeling off of coatings on implants occasionally occur after long-term implantation and may result in surgical failure. In fact, bone tissue repair happens during the initial stage of implantation, and temporary coatings with high bioactivity are highly desirable. However, most coatings used now are not completely absorbable. Meanwhile, between 0.5% and 15% of patients suffer from prosthetic joint infection after primary or revision joint arthroplasty, despite administration of systemic antibiotic prophylaxis as well as improvements in surgical facilities. The rates of surgical site infection can be up to 50% for open fractures.13, 14 Therefore, considering the high rate of bacterial infection after implantation, coatings with antibacterial function are also attractive.15
Magnesium (Mg) is an essential element in the human body, and half of the body’s reserve of Mg is stored in bone tissue. Due to their low corrosion potential in the physiological environment, Mg and its alloys have the feature of biodegradability. This property can avoid the need for second surgery to remove the implants. Meanwhile, the degradation of Mg increases local alkalinity on the surface of implants, and thus generates an antibacterial effect within tissue.16, 17 Besides, there is mounting evidence that Mg has excellent osteogenic inductivity,18, 19 and many cellular and molecular mechanisms have been proposed for the potential benefits of Mg ions (Figure 1).20 In contrast, deficiency of Mg leads to osteoporosis.21 With these advantages, Mg-based metals including Mg coatings are believed to be promising candidates for bone tissue repair. Recently, Mg coatings have attracted great interest from researchers. Although the control of active Mg coating fabrication is not easy, Mg coatings have been successfully deposited on Mg alloys, Ti alloys, steels, PEEK, and silicon (Si) by arc ion plating.22-26 In order to obtain better bioactivity, other elements such as copper (Cu) are added to Mg coatings.27 As summarized in Table 1, after Mg coating, implants acquire many biofunctions, including degradability,20, 22-32 osteogenesis,23, 24, 26, 27, 30-32 angiogenesis24-26, 30, 31 and antibacterial effects.24, 26, 27, 30-32 These novel coatings are expected to significantly enhance the bioactivity of implants and thus benefit patients. This paper gives a brief review of studies of the fabrication and biofunctions of Mg coatings on different substrates. The articles used in this review were identified by search terms of magnesium coating involving medical materials. This included publications prior to August, 2021, using the following conditions: magnesium (Mg) coating, biomaterials, or medical materials. An electronic search of the ScienceDirect and Web of Science databases was performed. Considering that Mg coating of medical materials is a new research area, all the search results are reviewed in this article.
Figure 1. Schematic illustrations of the biofunction mechanisms of magnesium (Mg) ions. Reprinted from Wang et al.20.
Table 1. Summary of the biofunctions of magnesium (Mg) coating on different implant materials.
| Substrate material | Fabrication method | Degradation behaviour | Osteogenic property | Angiogenic property | Antimicrobial property | References |
|---|---|---|---|---|---|---|
| AZ31 | Vapour deposition | Corrosion resistance improved | – | – | – | 28 |
| AZ31 | Vapour deposition | Comparable to the un-coated 6N-Mg | – | – | – | 20 |
| AZ31 | Vapour deposition + hot press and HIP processes | Corrosion resistance improved | – | – | – | 29 |
| Ti6Al4V | Arc ion plating | Continuous release with Mg degradation | Enhanced new bone regenerating ability in vivo | Accelerated blood vessel formation around the scaffold | Strong killing effect of pure Mg film on Staphylococcus aureus | 24, 26, 30, 31 |
| Ti6Al4V | Arc ion plating | Sustained at least for 14 days | Restrained peri-implant osteolysis | – | Cu addition enhanced the antibacterial property of Mg coatings | 27, 32 |
| Cold-rolled steel plates | Radio-frequency magnetron sputtering | Corrosion rate greatly decreased | – | – | – | 22 |
| Oxidized Si wafer | Physical vapor deposition | Grains remain intact 48 hours after implantation | Thinner fibrous capsule formed than titanium control samples | – | – | 23 |
| PEEK | Vapour deposition | Lower degradation rate without galvanic corrosion | – | The antibacterial rate reached 99% when co-cultured for 12 hours | – | 25 |
Note: Cu: copper; HIP: hot isostatic pressing; PEEK: polyetheretherketone; Si: silicon.
Fabrication and Degradation Behaviour of Magnesium Coatings
For medical Mg metals, the control of slow degradation behaviour is the main challenge, especially for the initial degradation within the first 48 hours.23 After the initial stage, a biological layer is formed on the surface of Mg metals that slows down the degradation rate. During this time, if the degradation rate is too high, but osseointegration is not enhanced, high alkaline-related cytotoxicity may develop and hydrogen gas-forming cavities may be generated.23 Therefore, for Mg coatings, researchers made efforts to fabricate the desired microstructure with a high quality and low degradation rate.
Initially, Mg coatings were fabricated to enhance the corrosion resistance of Mg alloys. As is well known, the poor corrosion resistance of Mg alloys is caused by heavy metal impurities including iron, nickel and copper. To improve corrosion resistance, high-purity Mg coatings were selected to be applied to Mg alloys.22, 28, 29, 33 In those studies, pure Mg was evaporated and deposited on the substrates by the vapour deposition technique. Then, using the retort method, the Mg coatings were further purified. There were columnar growth and planar growth types of Mg coating formation.33 By optimizing the vapour pressure and temperature, high-purity Mg coatings with high quality and high adhesion can be fabricated, which are of granular microstructure. These Mg coatings have been shown to possess superior corrosion resistance. Meanwhile, the corrosion behaviour of Mg alloys switched from filiform corrosion to general corrosion, and their corrosion rate decreased to the same level as six-nine purity bulk Mg.29, 33 Lee et al.22 also proved that Mg coatings with granular microstructure fabricated on steel by the magnetron sputtering technique exhibit good corrosion resistance. Thus, this high-purity Mg coating is really promising for the protection of biodegradable Mg alloy implants.
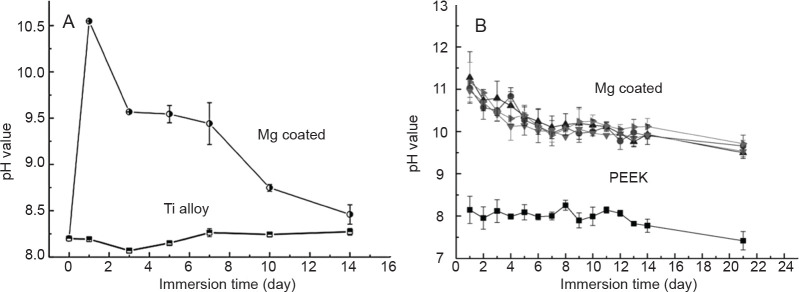
In the last few years, with the aim of enhancing the osseointegration of permanent implants, Mg coatings have started to be fabricated on Ti alloys24, 26, 30, 31 and PEEK25 by arc ion plating and vapour deposition, respectively. The Mg coatings on both substrates have a granular microstructure, with a thickness of about 5 m, as shown in Figure 2.30 To control the degradation rate, Mg coatings were of high purity. However, for Mg coatings on Ti alloys, galvanic corrosion occurred because of the large potential difference between these two metals. Thus, as shown in Figure 3, the pH value of the immersion solution increased to the maximum at the 1st day and then decreased quickly, indicating that the Mg coating degraded very fast. In contrast with PEEK, the pH value of the immersion solution remained much higher than that of the Ti alloy for a longer period.25 In addition, peeling off of coatings on Ti alloy was observed. Ultimately, the Mg coating would completely degrade in a relatively short time.
Figure 2. (A, B) Surface morphology (A) and cross section (B) of magnesium (Mg)-coated Ti6Al4V alloy. Scale bars: 50 μm.30 Copyright Wiley Periodicals, Inc. Reproduced with permission.
Figure 3. (A) Variation of the pH of immersion solutions after soaking titanium (Ti) alloy with and without magnesium (Mg) coatings. Reproduced from Du et al.30 Copyright Wiley Periodicals, Inc. (B) Variation of the pH of immersion solutions after soaking polyetheretherketone (PEEK) with and without Mg coatings. The upper four lines are all Mg coated. Reprinted from Yu et al.25 Copyright 2018, with permission from Elsevier.
As is well known, there is a large difference in the degradation rates of Mg metals between in vitro and in vivo tests. The degradation rate of Mg coatings on different substrates should be regulated according to their in vivo results. To evaluate the degradation behaviour in vitro and in vivo, Salunke et al.23 fabricated high-purity Mg coatings on oxidized Si wafers by the vapour deposition technique. After either 12-hour immersion or 48-hour implantation, Mg grains were still visible, demonstrating that the degradation rate of a Mg coating could be controlled in the initial stage of implantation. Thus, Mg coating is a promising surface modification for different implant materials.
Biofunctions of Magnesium Coating
Magnesium coatings on titanium alloys
As mentioned above, the bioinert character of Ti alloys might cause insufficient osseointegration and osteoconduction, which would increase the risks of aseptic loosening and further failure of implantation. Recent studies have extended the application of Mg alloys as biofunctional Mg coatings on Ti alloys, which makes it possible to simultaneously combine the advantages of Ti, with its better mechanical properties, with those of Mg, with its bio-functions.24, 26, 27
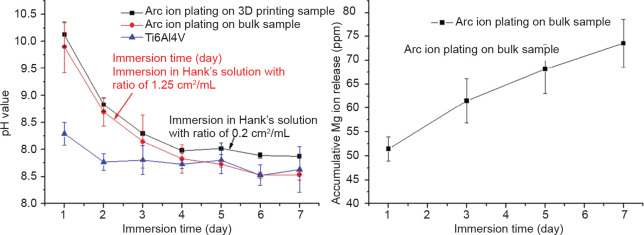
As a medical implant material, the biocompatibility of Mg coating should be the first factor to be considered. Li et al.24 reported fabrication of a biofunctional Mg coating on a Ti6Al4V substrate by arc ion plating, which resulted in uniform Mg fine grains of around 1 μm. The samples were immersed in Hank’s solution according to ISO 10993-12.34 As shown in Figure 4, there was no significant discrepancy between the pH variation of the Mg coating on the bulk Ti6Al4V substrate and the three-dimensional printed porous Ti6Al4V substrate. The accumulation of released Mg ions gradually increased to 73 ppm after 7 days of immersion, indicating a continuous release during degradation of the Mg coating. Yu et al.31 also reported the release of Mg ions from Mg coating on a Ti6Al4V substrate, and found that the dissolution of Mg ions on the 1st day was about 52 ppm, indicating good consistency.
Figure 4. (A, B) pH monitoring (A) and ion release (B) of magnesium (Mg)-coated Ti6Al4V immersed in Hank’s solution for 7 days. Reprinted from Li et al.24.
The biocompatibility of the Mg-coated Ti6Al4V scaffold was closely related to the degradation rate of the Mg coating. Li et al.24 found that Mg-coated porous Ti6Al4V might suppress MC3T3-E1 cell proliferation before day 4 compared with the bare porous Ti6Al4V. Nevertheless, the proliferation of MC3T3-E1 cells improved after day 4. The cell proliferation rate of rat bone marrow mesenchymal stem cells cultured in extracts of Mg-coated samples and Ti alloy also indicated that the cell survival rate was above 75% after the first day of culture, meeting the requirement for implant materials.31 Salunke et al.23 evaluated the degradation behaviour of Mg coating in cell culture media and in vivo, and both in vitro and in vivo results indicated that the corrosion resistance and biocompatibility of the Mg coating were promising.
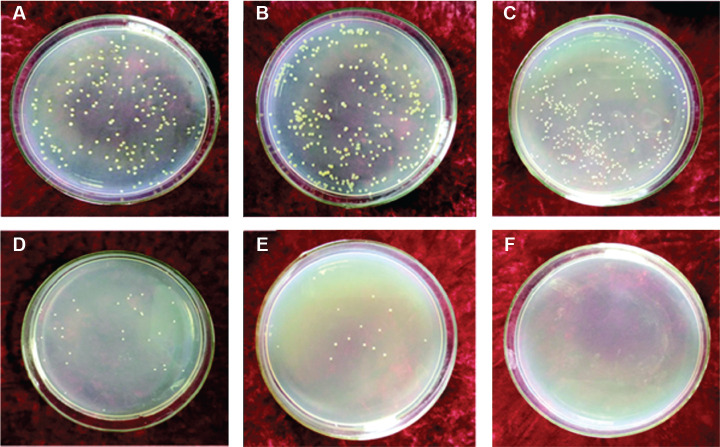
Additionally, the alkaline environment caused by degradation of the Mg coating also indicated an antibacterial effect.31 After co-cultivation of Mg-coated samples with Staphylococcus aureus for 12 hours, the sterilization rate reached 95%. When the time was extended to 24 hours, the killing effect increased to 99.99% (Figure 5). However, the number of bacteria in solid medium without Mg coating showed no decrease as the culture time was prolonged, indicating that Ti alloy itself did not have the ability to kill bacteria. Therefore, the Mg coating showed promising potential to inhibit bacterial infection in the initial stage of implantation and help to decrease the failure rate of surgery.
Figure 5. (A-F) Antibacterial effects of Ti6Al4V alloy without (A-C) and with (D-F) magnesium coating, co-cultured with Staphylococcus aureus at 37°C for 6 hours (A, D), 12 hours (B, E) and 24 hours (C, F). Reprinted with permission from Yu et al.31 Copyright © 2017 Acta Metallurgica Sinica.
Cell proliferation test results revealed that Mg coating enhanced proliferation of MC3T3-E1 cells on porous Ti6Al4V scaffolds.24 Furthermore, in vivo studies were performed based on a rabbit femoral condylar defect model. The results from fluorescent labelling, micro-computed tomography scanning and Van Gieson staining indicated higher new bone-regenerating ability of the Mg-coated porous scaffolds than the bare Ti6Al4V scaffold (Figure 6). More trabeculae of newly-regenerated bone and less connective tissue grew into the Mg-coated porous scaffolds. Gao et al.26 also found that in a bare Ti6Al4V scaffold, new bone was found mainly around the periphery of the scaffold, while the Mg-coated Ti6Al4V scaffold showed more favourable new bone ingrowth into the scaffold. All these results showed that the increased deposition of calcification and the reconstructed three-dimensional stereoscopic images of newly-formed bone growing into scaffolds indicated the promising bone-regenerating ability of the Mg-coated implants, verifying that the Mg ions released from the Mg coating could benefit the osteogenesis and osseointegration process.
Figure 6. (A, B) Micro-computed tomographic images of the porous Ti6Al4V with and without magnesium (Mg) coating at 4 and 8 weeks after implantation, where the yellow colour component was the newly-formed bone in these scaffolds. (C) Quantitative results showing the percentage of regenerated bone volume/total volume. Ti: titanium. *P < 0.01, vs. Ti Reprinted from Li et al.24.
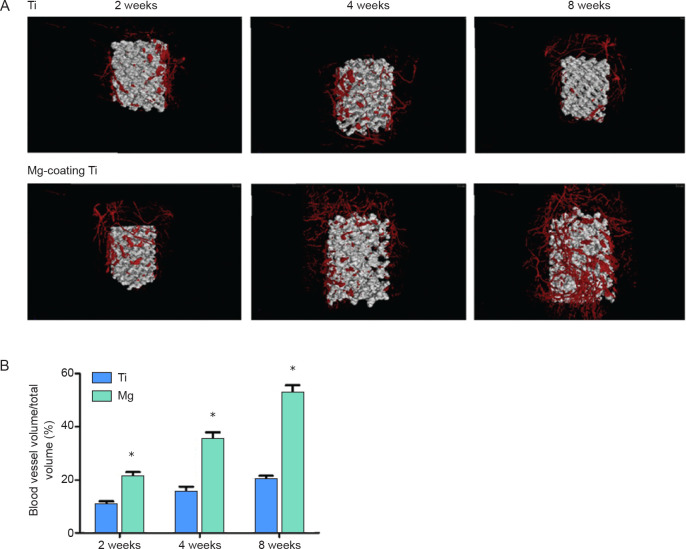
Moreover, the biofunctional Mg-coated Ti6Al4V scaffolds were also proven to enhance angiogenesis.26 Gao et al.26 emphasized the angiogenesis function of Mg-coated porous Ti scaffolds, as abundant blood vessels are indispensable to favouring osteoblastic behaviour and rapid generation of new bone. Wound healing, migration abilities, and tube formation assays, as well as angiogenesis-related gene expression (hypoxia-inducible factor-1α and vascular endothelial growth factor), showed that the Mg extract increased the angiogenesis function of human umbilical vein endothelial cells.35, 36 Microangiographic analysis revealed that the Mg-coated Ti6Al4V scaffold significantly enhanced blood vessel formation in a rabbit femoral condylar defect model (Figure 7). Their study enlarged the scope of Mg for use in weight-bearing functions in orthopaedic applications. The Mg coating on a Ti6Al4V scaffold exhibited favourable osteogenic and angiogenic properties in vitro and increased long-term bone formation and early vascularization in vivo.
Figure 7. (A) Microangiographic analysis of newly-formed blood vessels around porous Ti6Al4V scaffolds with and without magnesium (Mg) coating. (B) Quantitative results showing blood vessel volume/total volume. *P < 0.05, vs. bare Ti6Al4V scaffold (Ti). Ti: titanium. Reprinted from Gao et al.26.
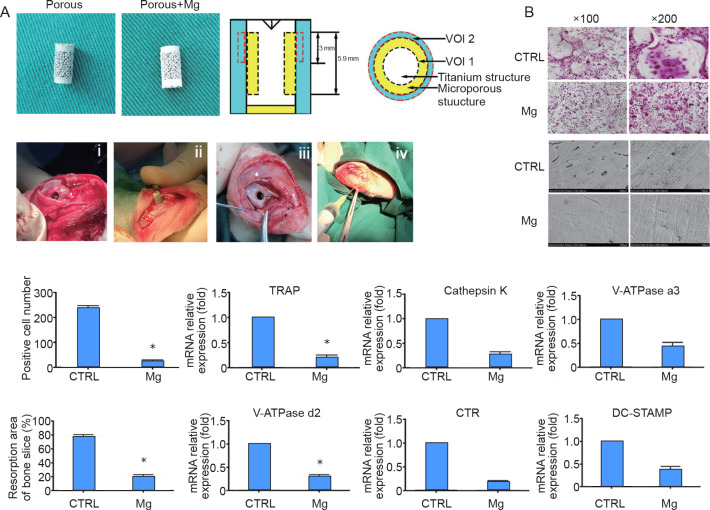
The Mg coating also showed inhibitory effects on osteoclasts. Du et al.30 found that Mg-coated porous implants might play a great role in osteoporotic patients. Both in vitro and in vivo studies supported an inhibitory effect of Mg extract on the differentiation of osteoclasts.30 For the in vivo test, wear particles of PEEK were added to generate an animal model of implant loosening.30 PEEK is a potential material for orthopaedic implants. However, the wear particles produced after implantation cause osteolysis. It was found that Mg inhibited bone resorption (Figure 8). The expression of genes related to osteoclastogenesis was also significantly decreased during osteoclast differentiation in the Mg group. When Mg was coated onto the Ti6Al4V implants with a porous structure, peri-implant osteolysis was inhibited, making it potentially favourable to treat patients with osteoporosis. However, further studies are needed to examine the precise mechanism of Mg-induced anti-osteolysis.
Figure 8. (A) General overview of the implants and implantation process. (B) Magnesium (Mg) inhibited RANKL-induced osteoclastogenesis and bone resorption. *P < 0.05, vs. control (CTRL). CTR: calcitonin receptor; DC-STAMP: dendritic cell-specific transmembrane protein; RANKL: receptor activator of nuclear factor kappa-Β ligand; TRAP: tartrate-resistant acid phosphatase; VOI: volume of interest. Du et al.30 Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.
MgCu coatings on titanium alloys
The antibacterial effect of a pure Mg-coated Ti substrate was believed to be due to the local alkaline environment caused by degradation of the Mg coating, as described above.37 Nevertheless the antibacterial effect in vivo was reduced because of the lower degradation rate compared to the in vitro environment.38 Therefore, antibacterial metal elements such as Cu39 and Ag were included to form a new Mg alloy.40, 41 The effects of Cu co-deposition in hydroxyapatite were investigated, with the aims of increasing the antibacterial properties and decreasing postoperative complications.42 In addition, it was reported that a deficiency of Cu ion could affect bone induction and osteoclast activity.32 Moreover, an in vitro study reported that a biodegradable Mg-Cu alloy could have a long-term antibacterial effect.40
To improve the bioactivity, especially the antibacterial property of the implants, Yu et al.27 deposited a MgCu coating on a Ti6Al4V substrate by arc ion plating. The in vitro degradation performance, analysed by immersion test showed that the MgCu coating degraded and gradually disappeared after up to 14 days’ immersion. Importantly, the addition of Cu enhanced the antibacterial property of the Mg coating. As analysed by inductively coupled plasma mass spectrometry, the accumulation of released Cu ions reached 20 ppb on the 1st day. With the addition of Cu to the Mg coating, the number of bacterial colonies decreased significantly. The number of Staphylococcus aureus colonies cultured with a bare Ti6Al4V substrate was 1 × 106/mL after 1 day of incubation, while with pure Mg- and MgCu-coated samples the numbers of colonies were 1 × 102/mL and 1 × 10/mL respectively. Moreover, the antibacterial efficacy of MgCu was approximately 99%, exhibiting the best antibacterial effect.27
The osteogenic effect of MgCu-coated implants was also compared with that of Mg-coated implants. Ding et al.32 fabricated Mg coating and MgCu coating on porous Ti6Al4V alloy by arc ion plating. Then, the coated implants were placed into the distal femurs of rabbits. Since Cu can accelerate the degradation of Mg coating, the release of Mg ions can be regulated by adjusting the Cu content in the coating. In their studies, Mg-0.1Cu and Mg-0.7Cu were selected to achieve different osteogenic effects and antibacterial abilities. However, the results revealed that the MgCu coating exhibited no obvious advantage with regard to bone integration compared with the porous scaffolds with or without Mg coating, which differed from a previous report that Mg-coated porous scaffolds enhanced new bone-regenerating ability more than bare Ti6Al4V.24, 26 Ding et al.32 held that the proportion of MgCu coating may not be the best, and the complicated environment in vivo might also affect the results. In the long-term the antibacterial ability of MgCu-coated porous Ti6Al4V was enhanced, demonstrating its promise for use in orthopaedic applications. However, further and deeper research involving the inclusion of functionalised alloying elements in Mg coatings as a surface treatment should be carried out.
Conclusion and Future Research Directions
By physical vapour deposition, Mg coatings with high purity and granular microstructure have been deposited on Ti alloys, PEEK, steels, Mg alloys and Si. Besides, their degradation rates can be controlled in the initial stage after implantation. in vitro and in vivo investigations demonstrated that Mg-coated implant materials acquired biofunctions including degradability, osteogenesis, angiogenesis and antibacterial properties. Thus, novel biofunctional Mg coatings are promising candidates for surface modification of implant materials to be used for bone tissue repair. In addition, these novel multi-functional Mg coatings are expected to significantly enhance the long-term safety of bone implants and thus benefit the patients.
However, studies on biofunctional Mg coatings are still in the early stage. There are many scientific and technological issues that need to be further studied. For instance, the degradation rate and thickness of Mg coatings on different implant materials need to be accurately controlled. The degradation time should be a good match for the bone tissue repair period, especially when there is galvanic corrosion between Mg coatings and implant substrate materials. Meanwhile, degradation behaviour in vivo also needs to be further clarified. Additionally, to maintain the integrity of a coating during implantation, the wear resistance and adhesion ability should be properly regulated. To obtain more effective biofunctions, some other bioactive elements can be considered for addition to Mg coatings. As for the mechanisms responsible for these biofunctions, further studies are needed to examine the precise mechanism of Mg-induced anti-osteolysis. Further, in vivo study of the possible long-term antibacterial effect of biodegradable Mg or MgCu coatings should be further investigated.
These novel Mg or Mg alloy coatings are metallic materials, which differ from conventional coatings including ceramic and polymer materials. As with other new materials, the combined properties of Mg coatings still need to be further regulated and systematically studied. The multi-functions of Mg coatings are unique in surface modification technology, which is attractive to researchers and implant manufacturers. All in all, after further optimisation, these novel Mg coatings are expected to significantly enhance the bioactivity of implants and bring added benefits for patients.
Funding Statement
This work was supported by the National Key Research and Development Program of China (Nos. 2016YFC1101804, 2016YFC1100604), National Natural Science Foundation of China (Nos. 51971222, 51631009), Natural Science Foundation of Liaoning Province of China (No. 2019-MS-326), Dongguan Innovative Research Team Program of China (No. 2020607234007) and China Postdoctoral Science Foundation (No. 2021M690494).
Footnotes
Acknowledgement: None.
Conflicts of interest statement: We confirm that all the authors have checked the manuscript and have agreed to the submission and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication. If this manuscript can be accepted, it will not be published elsewhere in the same form, in English or in any other language, without the written consent of the copyright-holder.
References
- 1.Wang Q. C., Zhang B. C., Ren Y. B., Yang K. Research and application of biomedical nickel-free stainless steels. Jinshu Xuebao. 2017;53:1311–1316. [Google Scholar]
- 2.Chen Q., Thouas G. A. Metallic implant biomaterials. Mater Sci Eng R Rep. 2015;87:1–57. [Google Scholar]
- 3.Najeeb S., Zafar M. S., Khurshid Z., Siddiqui F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J Prosthodont Res. 2016;60:12–19. doi: 10.1016/j.jpor.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Drago C., Howell K. Concepts for designing and fabricating metal implant frameworks for hybrid implant prostheses. J Prosthodont. 2012;21:413–424. doi: 10.1111/j.1532-849X.2012.00835.x. [DOI] [PubMed] [Google Scholar]
- 5.Xue W., Krishna B. V., Bandyopadhyay A., Bose S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 2007;3:1007–1018. doi: 10.1016/j.actbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Ratner B., Hoffman A., Schoen F., Lemons J. Biomaterials science: an introduction to materials in medicine. 2nd ed. Academic Press; 2004. [Google Scholar]
- 7.Asri R. I. M., Harun W. S. W., Samykano M., Lah N. A. C., Ghani S. A. C., Tarlochan F., Raza M. R. Corrosion and surface modification on biocompatible metals: a review. Mater Sci Eng C Mater Biol Appl. 2017;77:1261–1274. doi: 10.1016/j.msec.2017.04.102. [DOI] [PubMed] [Google Scholar]
- 8.Li Y., Yang W., Li X., Zhang X., Wang C., Meng X., Pei Y., Fan X., Lan P., Wang C., Li X., Guo Z. Improving osteointegration and osteogenesis of three-dimensional porous Ti6Al4V scaffolds by polydopamine-assisted biomimetic hydroxyapatite coating. ACS Appl Mater Interfaces. 2015;7:5715–5724. doi: 10.1021/acsami.5b00331. [DOI] [PubMed] [Google Scholar]
- 9.Li Y., Jahr H., Zhou J., Zadpoor A. A. Additively manufactured biodegradable porous metals. Acta Biomater. 2020;115:29–50. doi: 10.1016/j.actbio.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Geetha M., Singh A. K., Asokamani R., Gogia A. K. Ti based biomaterials, the ultimate choice for orthopaedic implants - A review. Prog Mater Sci. 2009;54:397–425. [Google Scholar]
- 11.Rafieerad A. R., Ashra M. R., Mahmoodian R., Bushroa A. R. Surface characterization and corrosion behavior of calcium phosphate-base composite layer on titanium and its alloys via plasma electrolytic oxidation: A review paper. Mater Sci Eng C Mater Biol Appl. 2015;57:397–413. doi: 10.1016/j.msec.2015.07.058. [DOI] [PubMed] [Google Scholar]
- 12.Le V. Q., Pourroy G., Cochis A., Rimondini L., Abdel-Fattah W. I., Mohammed H. I., Carradò A. Alternative technique for calcium phosphate coating on titanium alloy implants. Biomatter. 2014;4:e28534. doi: 10.4161/biom.28534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romanò C. L., Tsuchiya H., Morelli I., Battaglia A. G., Drago L. Antibacterial coating of implants: are we missing something? Bone Joint Res. 2019;8:199–206. doi: 10.1302/2046-3758.85.BJR-2018-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J., Qin H., Chai Y., Zhang P., Chen Y., Yang K., Qin M., Zhang Y., Xia H., Ren L., Yu B. Molecular mechanisms of osteogenesis and antibacterial activity of Cu-bearing Ti alloy in a bone defect model with infection in vivo. J Orthop Translat. 2021;27:77–89. doi: 10.1016/j.jot.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang K., Zhou C., Fan H., Fan Y., Jiang Q., Song P., Fan H., Chen Y., Zhang X. Bio-Functional design, application and trends in metallic biomaterials. Int J Mol Sci. 2017;19:24. doi: 10.3390/ijms19010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson D. A., Griffith R. W., Shechtman D., Evans R. B., Conzemius M. G. In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Acta Biomater. 2010;6:1869–1877. doi: 10.1016/j.actbio.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Ren L., Lin X., Tan L., Yang K. Effect of surface coating on antibacterial behavior of magnesium based metals. Mater Lett. 2011;65:3509–3511. [Google Scholar]
- 18.Zhai Z., Qu X., Li H., Yang K., Wan P., Tan L., Ouyang Z., Liu X., Tian B., Xiao F., Wang W., Jiang C., Tang T., Fan Q., Qin A., Dai K. The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-κB and NFATc1 signaling. Biomaterials. 2014;35:6299–6310. doi: 10.1016/j.biomaterials.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z., Mao X., Tan L., Friis T., Wu C., Crawford R., Xiao Y. Osteoimmunomodulatory properties of magnesium scaffolds coated with β-tricalcium phosphate. Biomaterials. 2014;35:8553–8565. doi: 10.1016/j.biomaterials.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 20.Wang J. L., Xu J. K., Hopkins C., Chow D. H., Qin L. Biodegradable Magnesium-Based Implants in Orthopedics-A General Review and Perspectives. Adv Sci (Weinh) 2020;7:1902443. doi: 10.1002/advs.201902443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belluci M. M., Giro G., Del Barrio R. A. L., Pereira R. M. R., Marcantonio E., Jr, Orrico S. R. P. Effects of magnesium intake deficiency on bone metabolism and bone tissue around osseointegrated implants. Clin Oral Implants Res. 2011;22:716–721. doi: 10.1111/j.1600-0501.2010.02046.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee M. H., Bae I. Y., Kim K. J., Moon K. M., Oki T. Formation mechanism of new corrosion resistance magnesium thin films by PVD method. Surf Coat Technol. 2003;169-170:670–674. [Google Scholar]
- 23.Salunke P., Shanov V., Witte F. High purity biodegradable magnesium coating for implant application. Mater Sci Eng B. 2011;176:1711–1717. [Google Scholar]
- 24.Li X., Gao P., Wan P., Pei Y., Shi L., Fan B., Shen C., Xiao X., Yang K., Guo Z. Novel bio-functional magnesium coating on porous Ti6Al4V orthopaedic implants: in vitro and in vivo study. Sci Rep. 2017;7:40755. doi: 10.1038/srep40755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X., Ibrahim M., Liu Z., Yang H., Tan L., Yang K. Biofunctional Mg coating on PEEK for improving bioactivity. Bioact Mater. 2018;3:139–143. doi: 10.1016/j.bioactmat.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao P., Fan B., Yu X., Liu W., Wu J., Shi L., Yang D., Tan L., Wan P., Hao Y., Li S., Hou W., Yang K., Li X., Guo Z. Biofunctional magnesium coated Ti6Al4V scaffold enhances osteogenesis and angiogenesis in vitro and in vivo for orthopedic application. Bioact Mater. 2020;5:680–693. doi: 10.1016/j.bioactmat.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu X., Ibrahim M., Lu S., Yang H., Tan L., Yang K. MgCu coating on Ti6Al4V alloy for orthopedic application. Mater Lett. 2018;233:35–38. [Google Scholar]
- 28.Yamamoto A., Watanabe A., Sugahara K., Fukumoto S., Tsubakino H. Deposition coating of magnesium alloys with pure magnesium. Mater Trans. 2001;42:1237–1242. [Google Scholar]
- 29.Fukumoto S., Sugahara K., Yamamoto A., Tsubakino H. Improvement of corrosion resistance and adhesion of coating layer for magnesium alloy coated with high purity magnesium. Mater Trans. 2003;44:518–523. [Google Scholar]
- 30.Du Z., Yu X., Nie B., Zhu Z., Ibrahim M., Yang K., Tan L., Wang Y. Effects of magnesium coating on bone-implant interfaces with and without polyether-ether-ketone particle interference: A rabbit model based on porous Ti6Al4V implants. J Biomed Mater Res B Appl Biomater. 2019;107:2388–2396. doi: 10.1002/jbm.b.34332. [DOI] [PubMed] [Google Scholar]
- 31.Yu X. M., Tan L. L., Liu Z. Y., Yang K., Zhu Z. L., Li Y. D. Preparation and properties of biological functional magnesium coating on Ti6Al4V substrate. Jinshu Xuebao. 2018;54:943–949. [Google Scholar]
- 32.Ding Y., Du Z., Zhu Z., Yu X., Wang Y. Effect of biodegradable magnesium-copper coatings on bone integration based on the porous structures in a rabbit model. RSC Adv. 2018;8:25127–25132. doi: 10.1039/c8ra03157f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsubakino H., Yamamoto A., Fukumoto S., Watanabe A., Sugahara K., Inoue H. High-purity magnesium coating on magnesium alloys by vapor deposition technique for improving corrosion resistance. Mater Trans. 2003;44:504–510. [Google Scholar]
- 34.International Organization for Standardization. ISO 10993-12:2021. Biological evaluation of medical devices — Part 12: Sample preparation and reference materials. 2021 [Google Scholar]
- 35.Wang C., Lin K., Chang J., Sun J. Osteogenesis and angiogenesis induced by porous β-CaSiO(3)/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials. 2013;34:64–77. doi: 10.1016/j.biomaterials.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y., Jin G., Xue Y., Wang D., Liu X., Sun J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017;49:590–603. doi: 10.1016/j.actbio.2016.11.067. [DOI] [PubMed] [Google Scholar]
- 37.Tan L., Yu X., Wan P., Yang K. Biodegradable materials for bone repairs: a review. J Mater Sci Technol. 2013;29:503–513. [Google Scholar]
- 38.Hou P., Zhao C., Cheng P., Wu H., Ni J., Zhang S., Lou T., Wang C., Han P., Zhang X., Chai Y. Reduced antibacterial property of metallic magnesium in vivo. Biomed Mater. 2016;12:015010. doi: 10.1088/1748-605X/12/1/015010. [DOI] [PubMed] [Google Scholar]
- 39.Jin X., Gao L., Liu E., Yu F., Shu X., Wang H. Microstructure, corrosion and tribological and antibacterial properties of Ti-Cu coated stainless steel. J Mech Behav Biomed Mater. 2015;50:23–32. doi: 10.1016/j.jmbbm.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Liu C., Fu X., Pan H., Wan P., Wang L., Tan L., Wang K., Zhao Y., Yang K., Chu P. K. Biodegradable Mg-Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Sci Rep. 2016;6:27374. doi: 10.1038/srep27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y., Liu L., Wan P., Zhai Z., Mao Z., Ouyang Z., Yu D., Sun Q., Tan L., Ren L., Zhu Z., Hao Y., Qu X., Yang K., Dai K. Biodegradable Mg-Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: in vitro and in vivo evaluations. Biomaterials. 2016;106:250–263. doi: 10.1016/j.biomaterials.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 42.He Y., Zhang Y., Zhang J., Jiang Y., Zhou R. Fabrication and characterization of Ti-13Nb-13Zr alloy with radial porous Ti-HA coatings for bone implants. Mater Lett. 2017;209:543–546. [Google Scholar]