Abstract
The spread of parasites is one of the primary drivers of population decline of both managed and wild bees. Several bee parasites are transmitted by the shared use of flowers, turning floral resources into potential disease hotspots. However, we know little about how floral morphology and floral species identity affect different steps of the transmission process. Here, we used the gut parasite Crithidia bombi and its primary host, bumble bees (Bombus spp.), to examine whether floral traits or species identity better predict three basic steps of parasite transmission on flowers: feces deposition on flowers, survival of the parasite on flowers, and acquisition by a new host. We also identified which traits and/or species were most strongly associated with each step in the transmission process. We found that both trait‐ and species‐based models fit the data on deposition of feces and survival of C. bombi on flowers, but that species‐based models provided a better fit compared with trait‐based ones. However, trait‐based models were better at predicting the acquisition of C. bombi on flowers. Although different species tended to support higher fecal deposition or parasite survival, we found that floral shape provided explanatory power for each of the transmission steps. When we assessed overall transmission potential, floral shape had the largest explanatory effect, with wider, shorter flowers promoting higher transmission. Taken together, our results highlight the importance of flower species identity and floral traits in disease transmission dynamics of bee parasites, and floral shape as an important predictor of overall transmission potential. Identifying traits associated with transmission potential may help us create seed mix that presents lower parasite transmission risk for bees for use in pollinator habitat.
Keywords: bee decline, Bombus impatiens, Crithidia bombi, floral traits, transmission dynamics
INTRODUCTION
Emerging infectious diseases (EIDs) are a threat to humans as well as domestic animals and wildlife (Daszak et al., 2000). EIDs can be driven by parasites that invade a new geographic area, by spillover events or by parasites that increase in prevalence in their native range due to changes in external factors, such as alterations in environmental conditions that facilitate parasite transmission (Antonovics, 2017; Dobson & Foufopoulos, 2001). Bees (Hymenoptera: Apoidea) are hosts to a wide variety of micro‐ and macroparasites (Graystock et al., 2016; Hedtke et al., 2011; Shimanuki & Knox, 2000), and some of these parasites have been linked to the decline of both managed honey bees (Bianco et al., 2014; VanEngelsdorp & Meixner, 2010) and wild bee populations (Meeus et al., 2011; Potts et al., 2010). Given the important ecological and economic role of bees as pollinators (Losey & Vaughn, 2006), understanding the transmission dynamics of bee parasites is an important step toward developing strategies to slow parasite spread (Bonsall, 2004). Here, we investigated the role of floral morphology and floral species identity on several steps of the transmission process for the model host–parasite system of the bumble bee (Bombus spp., Apidae) and its gut parasite, Crithidia bombi (Trypanosomatida).
Parasites can exploit the use of shared resources by their hosts to infect new individuals. For example, avian mycoplasmosis spreads through bird populations via shared bird feeders (Adelman et al., 2015). In a similar vein, many bee parasites spread to new individuals via shared flowers (Graystock et al., 2015), in which 10%–30% of open flowers in a field can harbor at least one bee parasite (Figueroa et al., 2020; Graystock et al., 2020). Flower density plays an important role on bee–parasite transmission dynamics, as congregation of bees at flower‐rich spots can increase transmission (Piot et al., 2019), but an increase in flower availability can also reduce parasite transmission due to a dilution effect (Piot et al., 2021). What remains less well explored is how floral species or floral traits affect overall bee–parasite transmission potential.
The transmission process of bee parasites via flowers can be broken down into at least three basic steps: deposition of the parasite on flowers, survival of the parasite on flowers until a new individual visits the flower, and acquisition of the parasite by a new host (Figueroa et al., 2019; McArt et al., 2014). However, most studies have only focused on one of the transmission steps when comparing plant species or traits as potential transmission venues (Bodden et al., 2019; Durrer & Schmid‐Hempel, 1994; Graystock et al., 2015), even though a given floral trait could have different or even opposing effects on each step of the transmission process. For example, composite flowers with large disk flowers could collect more bee feces (Bodden et al., 2019) but, at the same time, UV radiation on these types of flowers could reduce parasite survival over time (Figueroa et al., 2019; Schmid‐Hempel et al., 1999). Additionally, studies commonly evaluate only a small number of plant species for one or more steps in the transmission process (Alger et al., 2019; Durrer & Schmid‐Hempel, 1994; Figueroa et al., 2019; Graystock et al., 2015) or evaluate a number of species but for only one transmission step (Adler et al., 2018), making it difficult to determine what species or floral traits are facilitating overall transmission and why.
The goals of this study were to quantify the deposition, survival, acquisition, and overall transmission of a bee parasite on flowers, to assess the degree to which floral traits versus species identity better explained each parasite transmission step, and to identify floral traits that were most strongly associated with parasite transmission. Identifying traits that can influence transmission would allow us to predict transmission potential of species or plant communities that have not been tested, whereas a species‐based approach would require a new study for each new species, and it would not provide insights about the mechanisms by which flowers are impacting parasite transmission (Adler et al., 2018; Han et al., 2015). We focused on the trypanosomatid gut parasite C. bombi, the host bumble bee Bombus impatiens, and 16 plant species commonly visited by bumble bees. Specifically, we asked whether species‐based or trait‐based models were better predictors of: (i) the frequency of feces deposition on flowers (Experiment 1); (ii) survival of C. bombi on flowers (Experiment 2); and (iii) acquisition of C. bombi on flowers and subsequent intensity of infection of its host (Experiment 3). Because these transmission steps are multiplicative, we also used them to assess how floral traits affected overall parasite transmission potential. We predicted that both species‐ and trait‐based models would provide a reasonable fit to the data, but that trait‐based models would be better at predicting each of the transmission steps, as trait‐based models have proven to have more predictive power in previous studies, probably because they required fewer parameters to fit the data (Adler et al., 2018; Cronin et al., 2010; Rowe et al., 2020). Identifying traits that facilitate transmission of bee parasites could allow us to select flower mixes that present a low risk of transmission to be used in pollinator habitat, which could slow down the spread of parasites in bee communities.
METHODS
Study system
Crithidia bombi (Lipa and Triggiani) (Trypanosomatida: Trypanosomatidae) reproduces in the hindgut lumen of bumble bees, and new cells are released to the environment in feces 5–10 days after parasite ingestion (Schmid‐Hempel & Schmid‐Hempel, 1993). The parasite is horizontally transmitted when individuals ingest contaminated material either on flowers or through contact with infected nest mates (Durrer & Schmid‐Hempel, 1994; Graystock et al., 2015; Imhoof & Schmid‐Hempel, 1998). When infected with C. bombi, bumble bee colonies produce fewer workers, as well as fewer new queens at the end of the colony life cycle (Brown et al., 2003). Additionally, infected overwintering queens are less likely to successfully start a nest in the spring (Brown et al., 2003; Schmid‐Hempel, 2001). We used B. impatiens as a focal bumble bee species, maintaining two or three colonies infected with C. bombi isolated from B. impatiens collected in Raleigh, NC (GPS coordinates: 35°48′26.6″ N, 78°41′58.6″ W), and used them to prepare inoculum for the survival and transmission experiments, and as a source of infected bees for the deposition experiment. We also kept two or three uninfected colonies as sources of bees for the acquisition experiments. We provide details of colony origin and maintenance in Appendix S1: Section S1.
In this study, we used 16 plant species in total across eight plant families, but we could not test every plant species in every experiment, as availability was variable (Table 1). We chose plant species that were attractive to Bombus, locally available at plant nurseries, and spanned a range of variation in floral traits. We covered flowers with mesh bags before the flowers opened to ensure that no bees had deposited any parasites on the flowers.
TABLE 1.
The 16 plant species used in this study and the experiments (deposition, survival, and acquisition) that they were used in
| Family | Species | Deposition | Survival | Acquisition |
|---|---|---|---|---|
| Apocynaceae | Asclepia tuberosa | … | Anthers and petals (42) | … |
| Asteraceae | Coreopsis verticillata | 30 | Center and petal (38) | 33 |
| Solidago nemoralis | … | Center (22) | … | |
| Echinacea purpurea | … | Center and petal (40) | … | |
| Kalimeris integrifolia | … | Center and petal (44) | … | |
| Rudbeckia hirta | 32 | … | 37 | |
| Lamiaceae | Agastache foeniculum | … | Lower and upper petal (42) | … |
| Vitex agnus‐castus | 25 | Lower and upper petal (40) | 54 | |
| Plectranthus sp. a | … | Center and petal (32) | ||
| Caryopteris clandonensis | 29 | … | … | |
| Phytolaccaceae | Phytolacca americana | … | Center (20) | … |
| Plantaginaceae | Angelonia Angustifolia | … | Center and upper petal (32) | … |
| Antirrhinum majus | 16 | Center and petal (34) | … | |
| Polemoniaceae | Phlox paniculata | … | Center and petal (42) | … |
| Rubiaceae | Pentas lanceolata | 32 | … | 82 |
| Verbenaceae | Lantana camara | 29 | Center and petal (42) | 71 |
Note: Numbers indicate sample size; for the deposition and survival experiments, the replicate unit was the cage, for the acquisition experiment the replicate unit was individual bees. In the survival experiment, we also note the location where inoculum droplets were placed, and sample size indicates the number of replicates for each flower part tested. Cells with ellipses indicate species that were not tested in particular experiments.
Hybrid of P. saccatus and P. hilliardiae.
Study site
Experiments were carried out at the Honey Bee Laboratory (HBL) at the Lake Wheeler Road Field Laboratory of North Carolina State University (NCSU) (Raleigh, NC, USA; GPS coordinates: 35°43′23.5″ N, 78°40′25.2″ W). Cages for all experiments were 60 × 60 × 60 cm with white mesh (680 μm aperture; MegaView Science Co, Taiwan). When running experiments, cages with bees were kept in the shade as much as possible. When plants were not being used in trials, we kept them outdoors at the HBL with flowers and flowers buds covered by mesh bags.
Floral traits
For each plant species, we estimated floral size and shape and the number of reproductive structures (number of open flowers and flower buds) per inflorescence. We counted the number of reproductive structures on 20 inflorescences per species at peak bloom. We measured corolla length and width (Appendix S2) using digital calipers to the nearest 0.01 mm on 20 flowers per species, with not more than five flowers coming from the same individual plant. Due to collinearity among variables, we used corolla length and width in a principal component analysis using all plant species (Adler et al., 2018). The first component (PC1: 0.88 × corolla length + 0.46 × corolla width, accounting for 77% of total variance) was positively correlated with both corolla length and width, and therefore it reflected floral size. The second component (PC2: −0.46 × corolla length + 0.88 × corolla width, accounting for 23% of total variance) was positively correlated with corolla width and negatively correlated with corolla length, therefore reflecting floral shape (Appendix S3: Table S1). In this case, small values of PC2 indicate flowers that are narrow and long, whereas high values of PC2 indicate flowers that are wide and short. We present means, SDs, and sample sizes for all predictor floral traits in Appendix S3: Table S2.
Crithidia bombi inoculum preparation and estimating infection intensity
Crithidia bombi inoculum was prepared fresh every day we ran a trial. We prepared inoculum according to a standard protocol (Richardson et al., 2015). We used 5–10 workers from a source colony to prepare inoculum that had a concentration of 1200 cells/μl (details of inoculum preparation are provided in Appendix S1: Section S1). To estimate the intensity of infection of experimental bees, we dissected the guts of individual bees and prepared them using the same protocol as for the inoculum preparation (Appendix S1: Section S1), and then use a Neubauer chamber to estimate cell concentration. We also collected the right forewing from each bee we dissected and measured the length of the radial cell as an estimate of bee size (Müller et al., 1996), using ImageJ software (v.1.8).
Experiment 1: Deposition of feces on flowers
We tested how frequently the feces of bumble bees infected with C. bombi fell on the flowers of seven plant species (Table 1). This experiment was carried out between June and October 2019 at the HBL. We fed bees with sucrose that contained a non‐toxic fluorescent dye (Aurora Pink dye, product number ECO11, DayGlo Color Corp., Cleveland, OH, USA), which allowed us to find the feces droplets on the plants using a black light. The dyed sucrose was prepared by adding 0.25 g of the dye to 250 ml of 30% sucrose (as in Figueroa et al., 2019). At least 24 h before each trial, we fed workers from C. bombi infected colonies ad libitum with the dyed sucrose. On the day of the trial, in each cage we placed enough plants of a particular focal species to cover ~80% of the cage's floor (usually two to four individuals). We recorded the number of plants in the cage, number of blooming stalks, number of open flowers, and the total area that plants covered inside the cage. We placed five to seven bees from the same colony per cage, and allowed them to forage on the plants for 3 h. At 1.5 h, we checked each cage to make sure bees were foraging on the flowers and counted the number of visits to flowers in a 5‐min period. If bees were not foraging, we did not include that replicate in the data analysis. However, this only occurred in two trials. When the trial was over, we collected bees from the cages, and the following day we dissected all bees and determined whether they were infected with C. bombi and the intensity of infection (cell/μl), to calculate the average level of infection of that group of bees (Appendix S1: Section S1). We only used data from trials in which three or more bees were alive at the end of the trial and more than 50% of bees in the cage were infected, as infected bees are more likely to defecate on plants compared with uninfected bees (Figueroa et al., 2019). After excluding trials that did not meet these specifications, we had 25–32 cage replicates per plant species, except for Antirrhinum majus, for which we had 16 replicates (Table 1). After removing bees from the cages, we carefully took the cages with the plants to a dark room, and used a black light (Ustellar 100 LED, 395 nm) to find feces droplets. We counted how many flowers per cage had feces droplets on them, the number of droplets inside and outside the corolla, and on the calices, leaves and the floor of the cage. Droplets inside the corolla included droplets on the flower head of asters, inside the flower for snapdragon (A. majus), and inside the corolla tube of tubular/closed flowers. Droplets outside the corolla included droplets on the ligules of asters and outside the corolla of all other flowers. We used the number of droplets as the response variable because C. bombi has a relatively low infective dose (Davis et al., 2021), so any encounter with the fecal droplet of an infected bee is likely to cause infection. After each trial, we thoroughly cleaned the cage with 70% ethanol and removed all plant parts that had feces on them.
Statistical analyses
We performed all data analyses in R (v.4.02) (R Core Team, 2018). Our analyses assessed whether the number of fecal droplets on several flower parts was predicted by plant species identity and by floral traits, and which one was a better predictor.
Species‐based models
To test whether plant species identity predicted the number of feces droplets on flowers and on different flower parts, we constructed generalized linear models (GLM) with negative binomial distribution using the package glmmTMB (Skaug et al., 2018). We ran separate models for five biologically meaningful response variables that could affect subsequent transmission probability: the total number of droplets on flowers, total number of droplets inside the corolla, total number of droplets outside the corolla, total number droplets on the calix, and the number of flowers per cage that had droplets on them. For each response, the full model included as fixed effects plant species, initial number of bees per cage, average bee size, average intensity of infection of experimental bees, and bee activity (number of flower visits in 5 min). To account for the variable number of flowers per cage, we included it as an offset term in the models. We initially included bee colony as a random effect, but given that the random effect variance as negligibly small (<1.833e−09), we removed it from the final models. We did not include any interaction terms because they caused convergence problems in the models. We evaluated the significance of terms with a likelihood ratio χ2 test, implemented via the “drop1” function in R. We removed terms that were not significant (p > 0.05) and compared the fit of the full and the reduced models using Akaike information criterion (AIC) values. We used Tukey's honestly significant difference (HSD) tests for post hoc, pairwise comparisons using the lsmeans package (Lenth, 2018). To check model assumptions, we used QQ plots of residuals and residual versus predicted plots using the package DHARMa (Hartig & Lohse, 2020).
Trait‐based models
For models using floral traits to predict the number of feces droplets on flowers and floral parts, we again used GLM. As with the species‐based models, we constructed separate sets of models for each response of interest. The full model had the same structure as the species‐based models, but instead of including species identity as a fixed effect, we included floral size (PC1) and shape (PC2) and number of reproductive structures per stalk (from this point forwards floral traits). We log‐transform the number of reproductive structures per stalk because one species (Caryopteris clandonensis) had values much higher than the others and therefore high leverage. We selected the best‐fit model and conducted post hoc analysis in the same way as for the species‐based models.
Comparing species versus traits models
Here and in Experiment 2 and 3, we used AIC to compare whether the species‐ or trait‐based models provided the best predictive insight into response variables. One model was considered to have substantially better empirical support than the other if its AIC value was lower by two or more units (Burnham & Anderson, 1998).
Experiment 2: Survival of C. bombi on flowers
We measured the survival of C. bombi on flowers of 14 plant species (Table 1). This experiment was carried out between June and October 2018 at the HBL. We assessed C. bombi survival after 30 min, 1 h, and 3 h of being placed on flowers, and for most species we also tested differences in survival between two locations on the flowers (Table 1; Appendix S2). Inside cages, we placed four (if testing one flower part) or eight (if testing two flower parts) flower stalks in florist water tubes to keep flowers fresh. On each flower, we placed one 10 μl drop of inoculum using a micropipette. We used 10 μl because it is within the natural range for a single B. impatiens fecal event (7 ± 5 μl, mean ± SD) (Van Wyk et al., 2021), and we did not add sucrose because B. impatiens feces typically does not contain sugar (Figueroa et al., 2019). We did not consider other compounds that may be found in feces. When testing one flower part (e.g., petal), each flower received one drop of inoculum at the location of interest. When testing two flower parts (e.g., ligules and flower head), four flowers (each on separate stalks) received the inoculum at one location, and another four flowers (again each on separate stalks) received the inoculum at the second location. At each time interval (0, 30 min, 1 h, and 3 h), we collected one drop of inoculum per location using 10‐μl microcapillary tubes, and estimated the volume of the recovered droplet. Care was taken to remove the entire liquid droplet from the specified location. If the inoculum droplet had evaporated, we placed 10 μl of distilled water on the same place the inoculum was, to try to recover any remaining C. bombi cells. We placed the recovered droplet on a Neubauer chamber and estimated the number of alive C. bombi per μl. We considered cells alive if they were “swimming” by flagellum movement (Figueroa et al., 2019), and recorded alive cells as present or absent. Each time we collected a droplet from flowers, we measured temperature and relative humidity (AcuRite Digital Humidity & Temperature Monitor). We conducted 16–22 trials per plant species (Table 1).
Statistical analyses
We conducted hazard ratio analyses using Cox proportional hazard models via the Survival package (Therneau, 2020) in R. This analysis evaluated C. bombi survival (whether there were any alive cells in the droplet) by time elapsed when the flower was inspected.
Species‐based models
These Cox models included plant species and time elapsed between inoculum preparation to the beginning of the trial as fixed effects. We did not include weather variables in the final models because they were correlated with the date of the trials, and the effect was confounded with plant species, as we tested different plant species on different days and weeks based on their natural phenology. We included Crithidia source colony as a random effect, but just as in the deposition models, the random effect variance was negligibly small, so again we left it out of the final models. To determine the significance of the terms in the models, we conducted a likelihood ratio test comparing the full model with a model that excluded plant species as an explanatory variable. Differences in survival across plant species were determined post hoc with Tukey's HSD tests using the lsmeans package.
Trait‐based models
These models had the same structure as the species‐based models, but instead of species identity, they included floral traits as fixed effects. To determine significance of each floral trait, we conducted a likelihood ratio test comparing the full model with reduced models in which we removed one of the floral traits at a time.
Effect of droplet location
As an additional analysis, we tested the effect of droplet location on the survival of C. bombi separately for each plant species. We did not include Plectranthus sp. in this analysis due to high censoring (80%) for this species. For the rest of the species for which we measured survival at two locations on the flower (Table 1), we ran a proportional hazard model testing C. bombi survival by time elapsed when the flower was inspected. For each plant species, we wrote a model that included droplet location as a fixed effect, and a second model that did not include this variable. Then, we conducted a likelihood ratio test comparing the two models.
Experiment 3: Acquisition of C. bombi from flowers to bees
We evaluated the probability of acquisition of C. bombi on flowers after a single bee visit on five plant species (Table 1), with trials carried out between June and October 2019. We prepared fresh inoculum each day to have a concentration of 1200 cells/μl. To ensure the inoculum was infective, we inoculated a control group of 10–12 bees from the same uninfected colony we used for trials (inoculations as in Richardson et al., 2015).
For control bees, we prepared the inoculum to have 25% sucrose to encourage consumption. We starved control bees for 4 h and inoculated them with 10 μl of inoculum (12,000 cells/bee). We placed them in individual containers (12 × 7 × 5 cm) with sucrose and pollen ad libitum. Seven days after inoculation, we dissected the guts of individual bees and estimated the number of C. bombi cells per μl using the same methods as for inoculum preparation (Appendix S1: Section S1). Any bees that had nonzero counts were considered infected. We only used data from days when more than 50% of the control bees were infected with C. bombi.
As we were trying to simulate transmission via a fecal–oral route, we did not add sucrose to the inoculum used in acquisition trials. For acquisition trials at the HBL, we placed four to six uninfected bees per cage and allowed them to acclimate to the cage for 5–10 min. Then, we placed one inflorescence in the cage. For Asteraceae, it was one flower head per cage. For all other plants, it was one inflorescence with multiple flowers. Then we applied 10 μl of inoculum (1200 cells/μl and 0% sucrose) to the flowers in the form of three to four small droplets. For inflorescences with multiple flowers, the inoculum droplets were applied to three to four flowers, whereas for asters the inoculum droplets were spread across the flower. We placed the droplets simulating where we most often observed feces falling in the deposition experiment, or where we considered feces would probably fall when a bee was visiting a flower of that species. We never placed the inoculum on the nectaries or inside the corolla because we typically did not find feces in these locations in the deposition trials (please refer to “Results”). If bees did not visit flowers after 2 min, we “presented” the stalks to bees by raising them in front of the bees to encourage foraging. When a bee started foraging, we allowed it to visit the flowers and captured it in a vial when the bout was over. If the bout lasted more than 10 min or if the bee stopped probing flowers but was still on the stalk, we considered the bout over and captured the bee. We then placed a new bee in the cage and repeated the procedure with a new stalk. Each bee captured was considered a replicate, and for each bee captured, we recorded the number of flowers per stalk (for asters it was considered one), length of the bout (in sec), and elapsed time between inoculum preparation and trial (in min).
We returned bees to the laboratory and placed them in individual containers with sucrose and pollen ad libitum. Seven days after each trial, we dissected out the guts of individual bees and estimated the number of C. bombi cells per μl. After removing bees from days that did not meet the selection criterion, we had between 33–82 replicate bees per plant species (Table 1).
Statistical analyses
We analyzed ‘incidence’ (presence/absence of C. bombi infection) and ‘intensity’ (count of C. bombi in infected bees) as separate components of C. bombi acquisition. We used GLMM to analyze both components and to explore the effect of plant species identity and floral traits on C. bombi acquisition.
Incidence analysis
We modeled pathogen incidence using logistic regression with the package glmmTMB using binomial error distribution. The response variable was the binary outcome of whether a bee got infected or not. For the species‐based models, we included plant species, length of the bout, time elapsed since inoculum preparation, number of flowers on the stalk used in the trial and bee size as fixed effects. We tested experimental bees' source colony and C. bombi source colony as potential random effects, but the random effect variances associated with these variables was negligibly small (<2.951e−09), so we removed them from the final models. To determine the significance of the fixed effects, we followed the same approach as with the deposition analysis. For trait‐based models, we used the same approach, but instead of using plant species as a fixed effect in the models, we included floral traits.
Intensity analysis
We modeled intensity of C. bombi infection using only data from bees that were infected, therefore we used a truncated negative binomial error distribution in the models. We used the cell count per 0.02 μl gut sample as the response variable. The full model included the same structure as the incidence model, and we tested the same potential random effects, but again we did not include them in the final models due to the small variance explained. To determine the significance of the fixed effects, we followed the same approach as with the deposition analysis. For trait‐based models, we included the same fixed effect structure, but instead of plant species, we included floral traits. We determined the significance of the fixed effects, checked for model assumptions, and compared species‐based and trait‐based models as in the incidence analyses.
Assessing overall transmission
We combined the results of the deposition, survival, and acquisition experiments multiplicatively to provide a relative comparison of how much different traits might contribute to overall transmission potential. We used the relative number of droplets per flower per infected bee from the deposition experiment, the relative droplet lifetime from the survival experiment, and the relative infection probability per contaminated inflorescence visited from the acquisition experiment. Because the same plant species were not used across all three experiments, we compared overall transmission by floral traits only. First, to avoid overextrapolation, we restricted each trait to the intersection between the ranges of values used in the three experiments. Next, for every set of trait values, we generated predictions of the relevant responses from the best trait‐based models of the three experiments, and then multiplied the results together (please refer to Appendix S1: Section S1 for details on how the Cox proportional hazards were used to assess relative droplet infectious lifetimes). Finally, we rescaled the overall transmission values so that the maximum value within the trait space was 1. We did not utilize information about the intensity of infection from the acquisition experiments. This is because we did not know exactly how level of infection and droplet infectivity were related.
RESULTS
Here we focus on the effect of the main factors of interest (species identity and floral traits). A detailed description of the effect of other covariates is found in Table 2 and Appendix S1: Section S2.
TABLE 2.
Experiment 1, deposition: Summary of the species‐based and trait‐based models for the deposition of bumble bee feces on flowers
| Response variable | Fixed effects terms left in the model | Effect | χ2 | df | p | AIC |
|---|---|---|---|---|---|---|
| Species models | ||||||
| No. droplets on flowers/cage | Species ID | 286.45 | 6 | <0.0001 | 1208 | |
| No. bees in the cage | + | 6.407 | 1 | 0.0114 | ||
| No. droplets inside the corolla/cage | Species ID | 71.72 | 6 | <0.0001 | 610 | |
| No. droplets outside the corolla/cage | Species ID | 125.95 | 6 | <0.0001 | 830 | |
| No. bees in the cage | + | 8.320 | 1 | 0.0039 | ||
| No. droplets on the calix/cage | Species ID | 81.76 | 6 | <0.0001 | 306 | |
| No. bees in the cage | + | 4.20 | 2 | <0.0001 | ||
| No. flowers with droplets in the cage | Species ID | 359.30 | 6 | <0.0001 | 963 | |
| No. bees in the cage | + | 9.99 | 2 | 0.0016 | ||
| Trait‐based models | ||||||
| No. droplets on flowers | Floral shape | + | 86.70 | 1 | <0.0001 | 1278 |
| Length of trial | + | 16.38 | 1 | <0.0001 | ||
| Bee size | − | 3.88 | 1 | 0.0488 | ||
| Level of infection | − | 15.66 | 1 | <0.0001 | ||
| No. droplets inside the corolla/cage | Floral shape | + | 68.16 | 1 | <0.0001 | 666 |
| Bee size | + | 4.57 | 1 | 0.0330 | ||
| Start time | − | 5.63 | 1 | 0.0180 | ||
| No. droplets outside the corolla/cage | Corolla size | + | 4.57 | 1 | 0.0324 | 993 |
| Flowers/inflorescence | − | 10.86 | 1 | 0.0009 | ||
| Length of trial | + | 18.46 | 1 | <0.0001 | ||
| No. bees in the cage | − | 2.23 | 1 | 0.1351 | ||
| Level of infection | − | 16.63 | 1 | <0.0001 | ||
| Plant area | − | 4.74 | 1 | 0.029 | ||
| No. droplets on the calix/cage | Floral shape | + | 105.00 | 1 | <0.0001 | 327 |
| Bee size | + | 4.01 | 1 | 0.029 | ||
| No. flowers with droplets in the cage | Floral shape | + | 3.94 | 1 | 0.0470 | 1046 |
| Flowers/inflorescence | − | 15.46 | 1 | <0.0001 | ||
| Length of trial | + | 15.55 | 1 | <0.0001 | ||
| Length of trial | − | 6.87 | 1 | 0.0088 | ||
| Level of infection | − | 19.60 | 1 | <0.0001 | ||
Note: Effect refers to factors that have a positive (+) or negative (−) effect on response variables. AIC, Akaike information criterion.
Experiment 1: Deposition of feces on flowers
Across all plant species, most of the fecal droplets we observed were on the floor of the cage (mean percentage range across all species and trials: 63%–90% of droplets on the cage floor), followed by plant leaves (mean range: 1.4%–19.9% on leaves), and droplets on flowers (mean range: 1.9%–16.6% on flowers) (Appendix S3: Figure S1).
Species‐based models
For all variables (number of droplets on flowers, number of droplets inside and outside the corolla and on the calix, and the number of flowers with droplets), species identity was a significant predictor (p < 0.0001 in all cases; Table 2). We observed differences between species (Appendix S3: Tables S3–S7), with Rudbeckia hirta having the most droplets on flowers, the most flowers with droplets per cage (Figure 1a), and the most droplets inside the corolla (Appendix S3: Figure S2a) relative to all other plant species. R. hirta was also the species with the most droplets outside the corolla (Appendix S3: Figure S2b) and on the calix (Appendix S3: Figure S2c) in most comparisons with other species.
FIGURE 1.
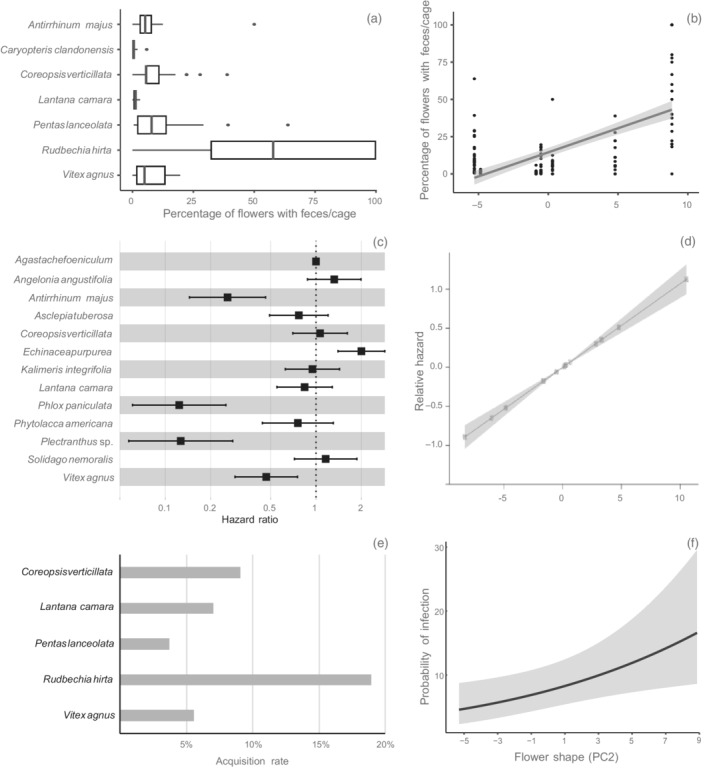
Effect of species and floral shape on the three steps of Crithidia bombi transmission on flowers. (a) Boxplot of the percentage of flowers per cage that had bee feces on them. Rudbeckia hirta had significantly more flowers with feces than other species. (b) Effect of floral shape on the percentage of flowers per cage that had bee feces on them. As flowers get wider and shorter they collect more feces. (c) Hazard ratio of each plant species to C. bombi. Plant species that present high hazard reduce C. bombi survival, compared with the reference species Agastache foeniculum. (d) Effect of floral shape on the relative hazard. Wider and shorter flowers increase the hazard for C. bombi. (e) Probability of a bee getting infected with C. bombi in a single visit to a contaminated flower. (f) Effect of floral shape on the estimated probability of infection in a single visit to a contaminated flower. As flowers get wider and shorter, the probability of infection increases. For figures (b), (d), and (f), the shaded area represents the 95% CI.
Trait‐based models
Floral shape was a significant predictor for all response variables (Figure 1b) evaluated except the number of droplets outside the corolla, with all variables increasing as flowers became shorter and wider. Floral size was only a significant predictor of the number of droplets outside the corolla, with more droplets deposited as the flowers got longer and wider. The number of reproductive structures per inflorescence was a significant predictor for the number of droplets outside the corolla and number of flowers with droplets per cage (Table 2), with both variables decreasing as the number of flowers per inflorescence increased.
Comparing species‐based versus trait‐based models
Species‐based models were better predictors than trait‐based models for the total number of droplets on flowers, on all flower parts (inside corolla, outside corolla, calix) and the number of flowers with droplets per cage (ΔAIC > 21; Table 2).
Experiment 2: Survival of C. bombi on flowers
In 69% of trials across all plant species, all C. bombi cells became immobile after 3 h. However, for three plant species (A. majus, Phlox paniculate, and Plectranthus sp.), C. bombi survived longer than 3 h in more than 85% of trials (Appendix S3: Table S8). The estimated restricted mean survival time across all species ranged from 117 to 180 min.
Species‐based models
Species identity was a significant predictor of C. bombi survival on flowers (χ2 12 = 171.08, p < 0.0001). A. majus, P. paniculate, and Plectranthus sp. represented lower hazard ratios, and therefore higher survival of C. bombi (p < 0.001 in all cases from pairwise comparisons) whereas Echinacea purpurea represented a higher hazard ratio, and therefore lowest survival of C. bombi (Appendix S3: Figure S3). All other species showed intermediate levels of C. bombi survival that did not differ significantly from one another (Figure 1c; Appendix S3: Table S9).
Trait‐based models
Floral size and shape were significant predictors of the survival of C. bombi on flowers (χ2 1 = 11.34, p < 0.0001; χ2 1 = 90.30, p < 0.0001, respectively). Larger flowers supported higher parasite survival (or a lower hazard rate; 0.98), whereas an increase in flower shape (wider and shorter flowers) was associated with a reduction in parasite survival (higher hazard rate: 1.16) (Figure 1d). The number of flowers per inflorescence was a marginally significant predictor (χ2 1 = 3.57, p = 0.058) of C. bombi survival, with survival decreasing (higher hazard rate: 1.12) as the number of flowers per inflorescence increased.
Comparing species‐based versus trait‐based models
The species‐based model was a better predictor of the survival of C. bombi on flowers than the trait‐based model (ΔAIC = 41.5).
Location of the droplets on flowers
Location of the inoculum droplet on flowers only had a significant effect for E. purpurea (Appendix S3: Table S10, Figure S4). The mean survival time was 1.5‐times higher on the flower head than on the ligules (mean survival time ± SE: 146 ± 8.3 min (flower head) and 93 ± 8.8 min (ligules); χ2 1 = 22.1, p < 0.0001).
Experiment 3: Acquisition of C. bombi on flowers
Across all plant species and trials, C. bombi acquisition rates ranged between 4%–19% (Figure 2). Given this low acquisition rate, there were few infected bees that we could use in our analyses of infection intensity (range: 3–7 infected bees per plant species tested).
FIGURE 2.
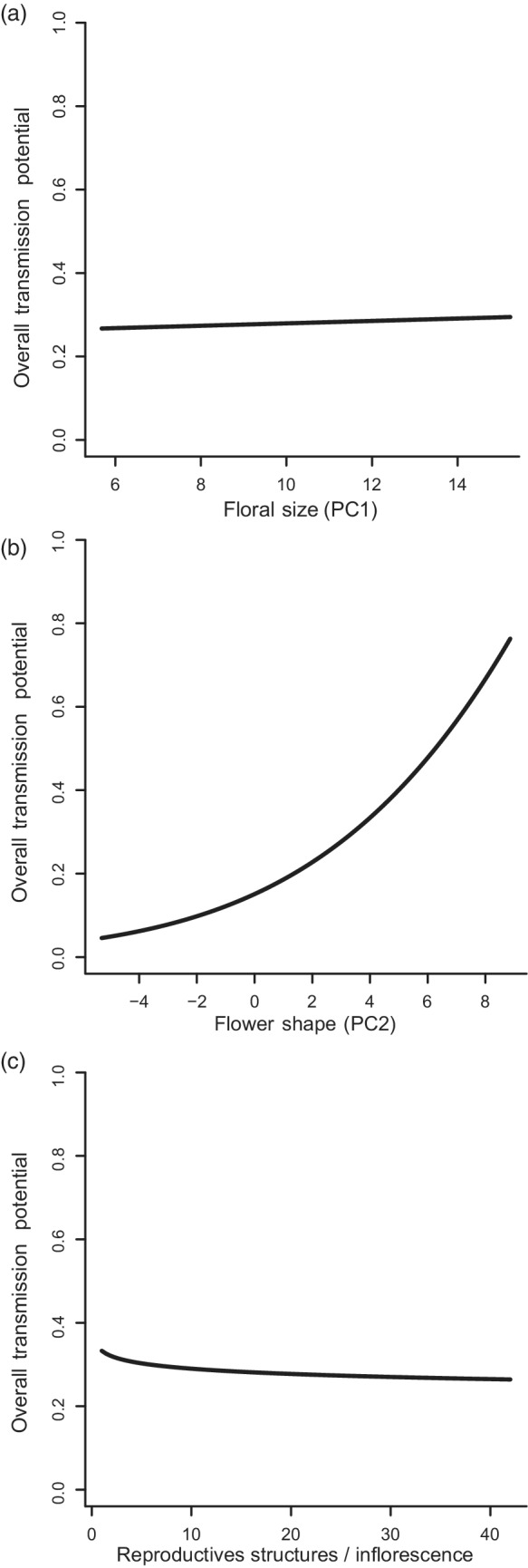
Overall transmission potential varies with floral traits. Transmission potential was assessed by combining the best‐fit trait‐based models from the three experiments multiplicatively, and then rescaled to a maximum of 1 for the range of trait values being considered. (a–c) Dependence on flower size, shape, and number of reproductive structures per inflorescence respectively, each marginalized across the other two trait values.
Species‐based models
Species identity was the only factor retained in the final model for incidence, but it was not a significant predictor (χ2 4 = 7.83, p = 0.098; Figure 1e). For the intensity of infection, species identity, length of bout and time elapsed since inoculum preparation were retained in the final model, but again, none of these factors were significant predictors (χ2 4 = 8.48, p = 0.075; χ2 1 = 2.22, p = 0.13; χ2 1 = 3.10, p = 0.078, respectively; Appendix S3: Figure S5).
Trait‐based models
Floral shape was a significant predictor for incidence (χ2 1 = 6.27, p = 0.012); the probability of acquisition increased as flowers got wider and shorter (Figure 1f). Floral shape was not a significant predictor for the intensity of infection (χ2 1 = 1.29, p = 0.105).
Comparing species‐based versus trait‐based models
For incidence of infection, the trait‐based model was a better fit (ΔAIC = 8.5), whereas for the intensity of infection, both species‐based and trait‐based models produced similar fits (ΔAIC = 0.6). However, the best trait‐based model required fewer parameters than the best species‐based model.
Overall effect of floral traits on transmission
Despite having found that all three traits were statistically significant in at least one of the three models, the marginal effect sizes of floral size and number of reproductive structures per inflorescence (Figure 2a,c) were small. In contrast, floral shape had a large marginal effect on transmission potential, with large values (wider and shorter flowers) leading to higher transmission (Figure 2b). Looking at the individual models, a large value for floral shape reduced the droplet infectious lifetime, but this was more than compensated for by the higher deposition and acquisition rates. Plotting the conditional dependence on the three traits revealed the same patterns (Appendix S3: Figure S8; with only floral shape having a large effect on transmission potential).
DISCUSSION
Here, we present the first combined analysis of the effects of floral traits on three main steps of bee–parasite transmission on flowers. In general, species‐based models provided better fits than trait‐based models for parasite deposition and survival, whereas trait‐based models performed better for parasite acquisition. Floral shape had a strong overall effect on transmission potential, with wide and short flowers having higher transmission potential.
Species‐based models
Although other studies have not found differences in feces deposition between plant species (Figueroa et al., 2019), we found that R. hirta, an aster with platform‐like flowers, was the species most likely to collect feces droplets. It has been observed that flowers with large area are more likely to collect feces than tubular flowers (Bodden et al., 2019). Although there were no significant differences between species, R. hirta was also the species with the highest acquisition rate. Unfortunately, we did not test R. hirta for C. bombi survival on flowers, but other asters of similar size, such as E. purpurea, reduced the survival of the parasite, so the overall effect of platform‐like flowers and R. hirta still needs to be tested.
For C. bombi survival on flowers, the maximum survival time we observed for most species was 3 h, which is similar to previous reports (Figueroa et al., 2019). However, for three plant species (A. majus, P. paniculate and Plectranthus sp.), C. bombi lived longer than 3 h in 85% of trials. This indicates that the maximum time a contaminated flower can remain infective could depend on the plant species and potentially environmental variables. Earlier studies have speculated on the role of desiccation and exposure to UV light in causing C. bombi mortality (Figueroa et al., 2019; Schmid‐Hempel et al., 1999). In an additional analysis based on our data of cell counts in the droplets (Appendix S1: Section S1; Appendix S3: Figures S6, S7), we found that droplets with 100% cell loss had almost always completely evaporated, whereas the losses before evaporation were typically much more gradual. This suggests that although UV exposure or other processes behind the gradual losses can somewhat reduce the infectivity of a droplet, rapid desiccation after evaporation is nonetheless required for a complete loss of infectivity.
Parasite acquisition by hosts can vary widely. For acquisition of the dengue virus by Aedes aegypti mosquitos, it can range from 5% to 20% depending on the mosquito and virus genotype (Gloria‐Soria et al., 2017). Reports of acquisition of C. bombi on flowers range from 20% to 80% depending on the flower species (Adler et al., 2018; Durrer & Schmid‐Hempel, 1994), whereas our acquisition rate ranged from 4% to 19%. This lower rate is probably due to the difference that previous studies used a sugar solution as a medium for the inoculum placed on flowers, which could inflate acquisition rates as the presence of sugar can encourage consumption by bees. This is unrealistic, as C. bombi is rarely found in the nectar of wild plants (Cisarovsky & Schmid‐Hempel, 2014), and bumble bee feces contains little to no sugar (Figueroa et al., 2019). As we did not add sugar to the inoculum, we suspect that our acquisition rate is closer to natural acquisition levels. We note, however, that although prior studies are likely to report unnaturally high parasite acquisition rates, they may still provide comparative insight into which species result in higher transmission risk.
Trait‐based models
Floral shape was a predictor of almost all measures of feces deposition on flowers, survival of the parasite, and acquisition by new bees. Wider and shorter flowers had a higher percentage of flowers in the cage with droplets, more droplets per flower, and increased the probability of a bee of getting infected when visiting a flower contaminated with C. bombi. Wider and shorter flowers (platform‐like) could collect more feces given the larger surface area. Floral morphology can also shape the behavior of floral visitors (Laverty, 1994), so it is possible that wide and short flowers could encourage a behavior or body positioning on the flower that would increase the chances of coming into contact with the parasite. In platform‐like flowers, such as R. hirta and E. purpurea, bees usually walk on the flower head probing multiple florets, which could increase the chances of encountering a feces droplet. Conversely, opposite to the effect on deposition and acquisition, wide and short flowers reduced the survival of C. bombi on flowers. This is consistent with the idea that platform‐like flowers can increase exposure to UV light and provide less protection against environmental factors that reduce parasite survival or increase the evaporation of the fecal droplet (Figueroa et al., 2019, Schmid‐Hempel et al., 1999).
Understanding each step of parasite transmission is crucial to understand parasite dynamics. For example, for malaria, in order to transmit it to humans, a mosquito first needs to feed on an infected host, then survive long enough for the parasite to develop, before feeding on a susceptible host (Killeen, 2014). Additionally, decomposing these steps can also allow us to identify nonlinearities in the transmission process and to achieve a more realistic estimate of β (transmission coefficient) for transmission models (McCallum et al., 2017). For C. bombi, we found that wide and short flowers decreased parasite survival, but at the same time increased deposition of feces and acquisition rate. This shows that to be able to determine whether a particular trait is going to have an overall positive effect on parasite transmission, we need to assess its effect not only on the acquisition of parasites on flowers. Although our analysis indicates that wide and short flowers are more likely to contribute to parasite transmission, our conclusions are limited to the trait range of the species we tested. Before making general recommendations about what type of flowers could be used to slow down parasite spread in managed landscapes, we need to assess a wider span of floral traits, to confirm that the pattern we observed is consistent as we increase trait variation.
For floral size and the number of flowers per inflorescence, they were significant predictors of feces deposition on some flower parts and survival of the parasite on flowers, but they were not predictors of acquisition on flowers, and their overall effect on parasite transmission potential was small compared with floral shape. Other studies have found that traits such as the arrangement of the flowers on the inflorescence and the number of reproductive structures can also influence parasite acquisition rates (Adler et al., 2018; Durrer & Schmid‐Hempel, 1994). This suggests that we need to test a wider range of trait variation on each step of transmission and that the likelihood of detecting patterns may be a function of where in trait space the species reside, interactions among traits, and experimental conditions.
Comparing species‐based versus trait‐based models
We found that species‐based models provided a better fit for the deposition of feces on flowers and survival of C. bombi on flowers, whereas trait‐based models provided a better fit for the acquisition of the parasite. Trait‐based models could be preferred for predicting parasite transmission because within‐species variation can be included, results can be generalized across communities due to taxonomic independence, and we could predict transmission potential of flower species that have not been tested (Dobson, 2004; Truitt et al., 2019). That species‐based models were a better fit for two out of three transmission steps in our study could be due to limited trait variation in the plants we tested. Moreover, it is also possible that the traits that we measured were not the most relevant traits for each of the transmission steps, or that there are additional traits that should be included to improve the fit of the trait‐based models. For example, adding six additional traits to a model that predicted rodent species as zoonotic reservoirs increased accuracy from 75% to >90% (Han et al., 2015).
It is important to note that floral traits or species identity can also influence other factors that affect parasite transmission on flowers, including bee visitation rates to flowers and bee behavior. Floral traits such as floral area, flower height, color and scent can influence visitation rates (Gumbert, 2000; Rowe et al., 2020) and bee behavior could interact with floral traits to determine the probability of parasite deposition and acquisition. For example, flowers that receive longer visits by honey bees are more likely to become contaminated with viruses (Alger et al., 2019). Additionally, bumble bees can recognize and avoid flowers that are contaminated with C. bombi (Fouks & Lattorff, 2011), which would ultimately reduce the risk of transmission on flowers. Another area that should be considered in future studies is the interaction of floral traits with environmental factors, as some transmission steps, such as parasite survival on flowers, are likely to be affected by atmospheric conditions such as relative humidity, possibly leading to changes in the relative effect of a particular trait over seasons. Models that include traits that influence both transmission as well as visitation, behavior, and environmental factors will be important extensions of this research.
Future directions
One approach commonly used to improve habitat for bee pollinators is wildflower strips on private and public lands or near agricultural settings (Goulson, 2009; Hatfield et al., 2012), but in most cases, this approach primarily focuses on maximizing forage for pollinators (Blaauw & Isaacs, 2014; Landis, 2017). However, flower plantings could turn into transmission hotspots for bee parasites, as increased density of bees foraging in those areas can lead to higher transmission rates (Bailes et al., 2020; Theodorou et al., 2016). Identifying floral traits or species that discourage parasite transmission could help us select flower mixes that reduce the risk of parasite transmission in flower plantings while providing floral resources for bees. Although our results suggest that wide and short flowers are more likely to transmit C. bombi, we need to increase the trait variation and the number of traits tested to provide clear seed mix guidelines. Large‐scale experiments in managed pollinator habitat would be an important next step.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1
Appendix S2
Appendix S3
ACKNOWLEDGMENTS
We thank the HBL at Lake Wheeler Laboratories for access to field and laboratory space. We also thank Bridger Johnston and Melanie Handley for help with the field experiments and sample processing. This research was improved by the advice of Seema Sheth, Quinn McFrederick, Charles Mitchell, and David Tarpy. Funding for the undergraduate students came from the BeeMORE program by the USDA–NIFA Research and Extension Experiential Learning for Undergraduates (REEU) Fellowships Program. The lead author received support from the Southeast Climate Adaptation Science Center. This project was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award no. R01GM122062.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the funding agencies.
Pinilla‐Gallego, Mario S. , Ng Wee Hao, Amaral Victoria E., and Irwin Rebecca E.. 2022. “Floral Shape Predicts Bee–Parasite Transmission Potential.” Ecology 103(7): e3730. 10.1002/ecy.3730
Handling Editor: Nicholas J. Gotelli
Funding information National Institute of Food and Agriculture; National Institute of General Medical Sciences of the National Institutes of Health, Grant/Award Number: R01GM122062; Southeast Climate Adaptation Science Center
DATA AVAILABILITY STATEMENT
Data (Irwin et al., 2022a) are available in Dryad at https://doi.org/10.5061/dryad.kkwh70s6k. Code (Irwin et al., 2022b) is available in Zenodo at https://doi.org/10.5281/zenodo.6298912.
REFERENCES
- Adelman, J. S. , Moyers S. C., Farine D. R., and Hawley D. M.. 2015. “Feeder Use Predicts both Acquisition and Transmission of a Contagious Pathogen in a North American Songbird.” Proceedings of the Royal Society B: Biological Sciences 282: 20151429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler, L. S. , Michaud K. M., Ellner S. P., McArt S. H., Stevenson P. C., and Irwin R. E.. 2018. “Disease where you Dine: Plant Species and Floral Traits Associated with Pathogen Transmission in Bumble Bees.” Ecology 99: 2535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger, S. A. , Burnham P. A., and Brody A. K.. 2019. “Flowers as Viral Hot Spots: Honey Bees (Apis mellifera) Unevenly Deposit Viruses across Plant Species.” PLoS One 14: e0221800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonovics, J. 2017. “Transmission Dynamics: Critical Questions and Challenges.” Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 372: 20160087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailes, E. J. , Bagi J., Coltman J., Fountain M. T., Wilfert L., and Brown M. J. F.. 2020. “Host Density Drives Viral, but Not Trypanosome, Transmission in a Key Pollinator.” Proceedings of the Royal Society B: Biological Sciences 287: 20191969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco, M. , Cooper J., and Fournier M.. 2014. “Honey Bee Population Decline in Michigan: Causes, Consequences, and Responses to Protect the state's Agriculture and Food System.” Michigan Journal of Public Affairs 11: 4–26. [Google Scholar]
- Blaauw, B. R. , and Isaacs R.. 2014. “Larger Patches of Diverse Floral Resources Increase Insect Pollinator Density, Diversity, and their Pollination of Native Wildflower.” Basic and Applied Ecology 15: 701–11. [Google Scholar]
- Bodden, J. M. , Hazlehurst J. A., and Wilson Rankin E. E.. 2019. “Floral Traits Predict Frequency of Defecation on Flowers by Foraging Bumble Bees.” Journal of Insect Science 19: 4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsall, M. B. 2004. “The Impact of Diseases and Pathogens on Insect Population Dynamics.” Physiological Entomology 29: 223–36. [Google Scholar]
- Brown, M. J. F. , Schmid‐Hempel R., and Schmid‐Hempel P.. 2003. “Strong Context‐Dependent Virulence in a Host–Parasite System: Reconciling Genetic Evidence with Theory.” Journal of Animal Ecology 72: 994–1002. [Google Scholar]
- Burnham, K. , and Anderson D.. 1998. Model Selection and Multimodel Inference. New York: Springer. [Google Scholar]
- Cisarovsky, G. , and Schmid‐Hempel P.. 2014. “Combining Laboratory and Field Approaches to Investigate the Importance of Flower Nectar in the Horizontal Transmission of a Bumblebee Parasite.” Entomologia Experimentalis et Applicata 152: 209–15. [Google Scholar]
- Cronin, J. P. , Welsh M. E., Dekkers M. G., Abercrombie S. T., and Mitchell C. E.. 2010. “Host Physiological Phenotype Explains Pathogen Reservoir Potential.” Ecology Letters 13: 1221–32. [DOI] [PubMed] [Google Scholar]
- Daszak, P. , Cunningham A. A., and Hyatt A. D.. 2000. “Emerging Infectious Diseases of Wildlife‐ Threats to Biodiversity and Human Health.” Science 287: 443–9. [DOI] [PubMed] [Google Scholar]
- Davis, A. E. , Deutsch K. R., Torres A. M., Mata Loya M. J., Cody L. V., Harte E., Sossa D., Muñiz P. A., Ng W. H., and McArt S. H.. 2021. “ Eristalis Flower Flies Can Be Mechanical Vectors of the Common Trypanosome Bee Parasite, Crithidia bombi .” Scientific Reports 11: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, A. 2004. “Population Dynamics of Pathogens with Multiple Host Species.” The American Naturalist 164: S64–78. [DOI] [PubMed] [Google Scholar]
- Dobson, A. , and Foufopoulos J.. 2001. “Emerging Infectious Pathogens of Wildlife.” Philosophical Transactions of the Royal of London, Society B: Biological Sciences 356: 1001–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrer, S. , and Schmid‐Hempel P.. 1994. “Shared Use of Flowers Leads to Horizontal Pathogen Transmission.” Proceedings of the Royal Society B: Biological Sciences 258: 299–302. [Google Scholar]
- Figueroa, L. L. , Blinder M., Grincavitch C., Jelinek A., Mann E. K., Merva L. A., Metz L. E., et al. 2019. “Bee Pathogen Transmission Dynamics: Deposition, Persistence and Acquisition on Flowers.” Proceedings of the Royal Society B: Biological Sciences 286: 20190603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa, L. L. , Grab H., Ng W. H., Myers C. R., Graystock P., McFrederick Q. S., and McArt S. H.. 2020. “Landscape Simplification Shapes Pathogen Prevalence in Plant‐Pollinator Networks.” Ecology Letters 23: 1212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouks, B. , and Lattorff H. M. G.. 2011. “Recognition and Avoidance of Contaminated Flowers by Foraging Bumblebees (Bombus terrestris).” PLoS One 6: e26328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloria‐Soria, A. , Armstrong P. M., Powell J. R., and Turner P. E.. 2017. “Infection Rate of Aedes aegypti Mosquitoes with Dengue Virus Depends on the Interaction between Temperature and Mosquito Genotype.” Proceedings of the Royal Society B: Biological Sciences 284: 20171506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson, D. 2009. “Conservation of Bumblebees.” In Species Management: Challenges and Solutions for the 21st Century, edited by Baxter J. and Galbraith C.. Edinburgh: Scottish Natural Heritage. [Google Scholar]
- Graystock, P. , Blane E. J., McFrederick Q. S., Goulson D., and Hughes W. O. H.. 2016. “Do Managed Bees Drive Parasite Spread and Emergence in Wild Bees?” International Journal for Parasitology: Parasites and Wildlife 5: 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graystock, P. , Goulson D., and Hughes W. O. H.. 2015. “Parasites in Bloom: Flowers Aid Dispersal and Transmission of Pollinator Parasites within and between Bee Species.” Proceedings of the Royal Society B: Biological Sciences 282: 20151371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graystock, P. , Ng W. H., Parks K., Tripodi A. D., Muñiz P. A., Fersch A. A., Myers C. R., McFrederick Q. S., and McArt S. H.. 2020. “Dominant Bee Species and Floral Abundance Drive Parasite Temporal Dynamics in Plant‐Pollinator Communities.” Nature Ecology & Evolution 4: 1358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbert, A. 2000. “Color Choices by Bumble Bees (Bombus terrestris): Innate Preferences and Generalization after Learning.” Behavioral Ecology and Sociobiology 48: 36–43. [Google Scholar]
- Han, B. A. , Schmidt J. P., Bowden S. E., and Drake J. M.. 2015. “Rodent Reservoirs of Future Zoonotic Diseases.” Proceedings of the National Academy of Sciences of the United States of America 112: 7039–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig, F. , and Lohse L.. 2020. “Residual Diagnostics for Hierarchical (Multi‐Level / Mixed) Regression Models.” https://cran.r-project.org/web/packages/DHARMa/vignettes/DHARMa.html.
- Hatfield, R. , Jepsen S., Mader E., Hoffman S., and Shepherd M.. 2012. Conserving Bumble Bees. Guidelines for creating and managing habitat for America's declining pollinators. Portland: The Xerces Society for Invertebrate Conservation. [Google Scholar]
- Hedtke, K. , Jensen P. M., Jensen A. B., and Genersch E.. 2011. “Evidence for Emerging Parasites and Pathogens Influencing Outbreaks of Stress‐Related Diseases like Chalkbrood.” Journal of Invertebrate Pathology 108: 167–73. [DOI] [PubMed] [Google Scholar]
- Imhoof, B. , and Schmid‐Hempel P.. 1998. “Patterns of Local Adaptation of a Protozoan Parasite to its Bumblebee Host.” Oikos 82: 59–65. [Google Scholar]
- Irwin, R. , Pinilla‐Gallego M. S., Ng W., and Amaral V.. 2022a. “Floral Shape Predicts Bee‐Parasite Transmission Potential.” Dryad, dataset. 10.5061/dryad.kkwh70s6k. [DOI] [PMC free article] [PubMed]
- Irwin, R. , Pinilla‐Gallego M. S., Ng W., and Amaral V.. 2022b. “Floral Shape Predicts Bee‐Parasite Transmission Potential.” Zenodo, software. 10.5281/zenodo.6298912. [DOI] [PMC free article] [PubMed]
- Killeen, G. F. 2014. “Characterizing, Controlling and Eliminating Residual Malaria Transmission.” Malaria Journal 13: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis, D. A. 2017. “Designing Agricultural Landscapes for Biodiversity‐Based Ecosystem Services.” Basic and Applied Ecology 18: 1–12. [Google Scholar]
- Laverty, T. M. 1994. “Bumble Bee Learning and Flower Morphology.” Animal Behaviour 47: 531–45. [Google Scholar]
- Lenth, R. 2018. “lsmeans: Least‐Squares Means. R Package Version 2.3.” https://cran.r-project.org/web/packages/lsmeans/lsmeans.pdf.
- Losey, J. , and Vaughn M.. 2006. “The Economic Value of Ecological Services Provided by Insects.” Bioscience 56: 311–23. [Google Scholar]
- McArt, S. H. , Koch H., Irwin R. E., and Adler L. S.. 2014. “Arranging the Bouquet of Disease: Floral Traits and the Transmission of Plant and Animal Pathogens.” Ecology Letters 17: 624–36. [DOI] [PubMed] [Google Scholar]
- McCallum, H. , Fenton A., Hudson P. J., Lee B., Levick B., Norman R., Perkins S. E., Viney M., Wilson A. J., and Lello J.. 2017. “Breaking Beta: Deconstructing the Parasite Transmission Function.” Philosophical Transactions of the Royal Society B: Biological Sciences 372: 20160084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeus, I. , Brown M. J. F., De Graaf D. C., and Smagghe G.. 2011. “Effects of Invasive Parasites on Bumble Bee Declines.” Conservation Biology 25: 662–71. [DOI] [PubMed] [Google Scholar]
- Müller, C. B. , Blackburn T. M., and Schmid‐Hempel P.. 1996. “Field Evidence that Host Selection by Conopid Parasitoids Is Related to Host Body Size.” Insectes Sociaux 43: 227–33. [Google Scholar]
- Piot, N. , Meeus I., Kleijn D., Scheper J., Linders T., and Smagghe G.. 2019. “Establishment of Wildflower Fields in Poor Quality Landscapes Enhances Micro‐Parasite Prevalence in Wild Bumble Bees.” Oecologia 189: 149–58. [DOI] [PubMed] [Google Scholar]
- Piot, N. , Eeraerts M., Pisman M., Claus G., Meeus I., and Smagghe G.. 2021. “More Is Less: Mass‐Flowering Fruit Tree Crops Dilute Parasite Transmission between Bees.” International Journal for Parasitology 51(9): 777–85. [DOI] [PubMed] [Google Scholar]
- Potts, S. G. , Biesmeijer J. C., Kremen C., Neumann P., Schweiger O., and Kunin W. E.. 2010. “Global Pollinator Declines: Trends, Impacts and Drivers.” Trends in Ecology & Evolution 25: 345–53. [DOI] [PubMed] [Google Scholar]
- Richardson, L. L. , Adler L. S., Leonard A. S., Andicoechea J., Regan K. H., Anthony W. E., Manson J. S., and Irwin R. E.. 2015. “Secondary Metabolites in Floral Nectar Reduce Parasite Infections in Bumblebees.” Proceedings of the Royal Society B: Biological Sciences 282: 20142471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe, L. , Gibson D., Bahlai C. A., Gibbs J., Landis D. A., and Isaacs R.. 2020. “Flower Traits Associated with the Visitation Patterns of Bees.” Oecologia 193: 511–22. [DOI] [PubMed] [Google Scholar]
- Schmid‐Hempel, P. 2001. “On the Evolutionary Ecology of Host–Parasite Interactions: Addressing the Question with Regard to Bumblebees and their Parasites.” Naturwissenschaften 88: 147–58. [DOI] [PubMed] [Google Scholar]
- Schmid‐Hempel, P. , Puhr K., Krüger N., Reber C., and Schmid‐Hempel R.. 1999. “Dynamic and Genetic Consequences of Variation in Horizontal Transmission for a Microparasitic Infection.” Evolution 53: 426–34. [DOI] [PubMed] [Google Scholar]
- Schmid‐Hempel, P. , and Schmid‐Hempel R.. 1993. “Transmission of a Pathogen in Bombus terrestris, with a Note on Division of Labour in Social Insects.” Behavioral Ecology and Sociobiology 33: 319–27. [Google Scholar]
- Shimanuki, H. , and Knox D. A.. 2000. Diagnosis of Honey Bee Diseases. Springfield, VA: USDA. [Google Scholar]
- Skaug, H. , Nielsen A., Berg C., Kristensen K., Maechler M., van Bentham K., Bolker B., and Brooks M.. 2018. “Generalized Linear Mixed Models Using Template Model Builder.” R package version 1.0.2.
- R Core Team . 2018. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Therneau, T. 2020. “Mixed Effects Cox Models.” R package version 2.2.0. https://cran.r-project.org/web/packages/survival/survival.pdf.
- Theodorou, P. , Radzevičiūtė R., Settele J., Schweiger O., Murray T. E., and Paxton R. J.. 2016. “Pollination Services Enhanced with Urbanization despite Increasing Pollinator Parasitism.” Proceedings of the Royal Society B: Biological Sciences 283: 20160561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt, L. L. , McArt S. H., Vaughn A. H., and Ellner S. P.. 2019. “Trait‐Based Modeling of Multihost Pathogen Transmission: Plant‐Pollinator Networks.” The American Naturalist 193: E149–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanEngelsdorp, D. , and Meixner M. D.. 2010. “A Historical Review of Managed Honey Bee Populations in Europe and the United States and the Factors that May Affect them.” Journal of Invertebrate Pathology 103: S80–95. [DOI] [PubMed] [Google Scholar]
- Van Wyk, J. , Amponsah E., Ng W. H., and Adler L. S.. 2021. “Big Bees Spread Disease: Body Size Mediates Transmission of a Bumble Bee Pathogen.” Ecology 102: e03429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Data Availability Statement
Data (Irwin et al., 2022a) are available in Dryad at https://doi.org/10.5061/dryad.kkwh70s6k. Code (Irwin et al., 2022b) is available in Zenodo at https://doi.org/10.5281/zenodo.6298912.


