Abstract
Background
Some diseases have sex differences. There have been no reports on the relationship between anti‐sperm antibodies (ASA) and sex differences.
Methods
ASA are detected by sperm‐immobilization test using patients' sera in women. In men, the ASA testing is generally performed by direct‐immunobead test.
Main findings
Sperm‐immobilizing antibodies in women inhibit sperm migration in their genital tract and exert inhibitory effects on fertilization. ASA bound to sperm surface in men also show inhibitory effect on sperm passage through cervical mucus. The fertilization rate of IVF significantly decreased when sperm were coated with higher numbers of ASA. For women with the antibodies, it is important to assess individual patients' SI50 titers. In patients with continuously high SI50 titers, pregnancy can be obtained only by IVF. For men with abnormal fertilizing ability by ASA, it is necessary to select intracytoplasmic sperm injection. Production of sperm‐immobilizing antibodies is likely to occur in women with particular HLA after exposure to sperm. The risk factors for ASA production in men are still controversial.
Conclusion
Attention to sex differences in specimens, test methods and the diagnosis of ASA should be paid. For patients with ASA, treatment strategies have been established by considering sex difference for each.
Keywords: anti‐sperm antibody, autoimmune disease, sex difference, sperm‐immobilizing antibody
1. INTRODUCTION
Anti‐sperm antibodies (ASA) are one of the causes of immune infertility, and they are produced in some infertile men and women. In the infertility outpatient clinic, the ASA testing for women, such as sperm‐immobilization test (SIT). 1 , 2 is simple for the clinicians because it requires only the patients' sera. In men, however, the world health organization (WHO) recommend to perform the ASA testing for the detection of sperm‐bound antibodies simultaneously during the routine semen examination. 3 Due to the complexity, it has been suggested that the actual examinations of ASA for infertile men are rare. Therefore, the option of testing sperm‐immobilizing antibody in the infertile patients' sera is discussed for men and women. However, evaluation of sperm‐immobilizing antibody in men's sera did not reveal its clinical significance. 4
In this way, it seems to be generally hard to say that the sex difference in ASA is well recognized. For women, sperm are immunogenic and they do not exist in themselves, and ASA are strictly homologous antibodies. On the contrary, ASA are produced as autoantibodies in men. So far, there have been no reports on the relationship between ASA and sex difference. Here, the relationship between the two, which has not been discussed, will be described.
2. AUTOIMMUNE DISEASES AND SEX DIFFERENCES
In most of the diseases, standard diagnostic and treatment guidelines have been aimed at adult men. However, since epidemiological studies have shown that some diseases have sex differences, it is not always best to apply diagnostic and treatment methods based on men to women.
2.1. Sex differences in autoimmune diseases
It is reported that 8.5 million people in the United States suffer from autoimmune diseases, 80% of whom are women. In Japan as well, the proportion of women is 2 to 10 times higher than that of men in both systemic and organ‐specific autoimmune diseases. 5 , 6 The difference between sex hormones and sex chromosomes in men and women can be considered as the causes of sex differences. As for the relationship between autoimmune diseases and sex hormones, it has been shown that estrogens promote immune responses, while androgens suppress immune responses. 7 Recently, it was revealed that the X escapee Kdm6a regulates multiple immune response genes, providing a mechanism for sex differences in autoimmune disease susceptibility. 7
Systemic lupus erythematosus (SLE) and antiphospholipid antibody syndrome (APS), which are representatives of systemic autoimmune diseases, are common in the childbearing age, and there are many aggravations during pregnancy and puerperal period. Furthermore, since the risk of onset increases when hormone replacement therapy is received after menopause, the involvement of estrogen in the onset and pathogenesis of autoimmune diseases is suggested. 8 On the other hand, in rheumatoid arthritis (RA), it is difficult to believe that estrogen is involved in the onset of autoimmune diseases, rather than the peak of the onset being before and after menopause, and the symptom is improved during pregnancy, and it has been suggested that the decrease in androgen due to aging is involved in the onset. 9 , 10
2.2. Sex difference in the frequency of anti‐sperm antibody (ASA) production in patients with systemic autoimmune diseases
In patients with autoimmune diseases, autoantibodies that cause tissue damage can reduce reproductive capability, but the majority of such patients are women. In order to ascertain sex differences, the frequency of ASA production was compared between men and women with systemic autoimmune diseases.
In the initial study, our group investigated whether systemic autoimmune diseases could be one of the risk factors for developing ASA in men. 11 Serum samples obtained from 70 men with systemic autoimmune diseases and 80 healthy controls were examined, by using the indirect‐immunobead test (I‐IBT). 12 Patients in whom the effects of steroids and immunosuppressants could not be ruled out were excluded. The results of I‐IBT showed that 5 (7.1%) of 70 men with systemic autoimmune diseases had serum sperm‐binding antibodies. However, no positives existed in 80 healthy men (p < 0.05, Figure 1), indicating that systemic autoimmune diseases may be one of the risk factors for developing ASA in men. Later, our group additionally examined serum samples obtained from 78 women with systemic autoimmune diseases using the I‐IBT. 13 Among the 78 women, none had serum ASA. A significant sex difference was observed between men and women with systemic autoimmune diseases (p < 0.05; Fisher's exact test, Figure 1).
FIGURE 1.
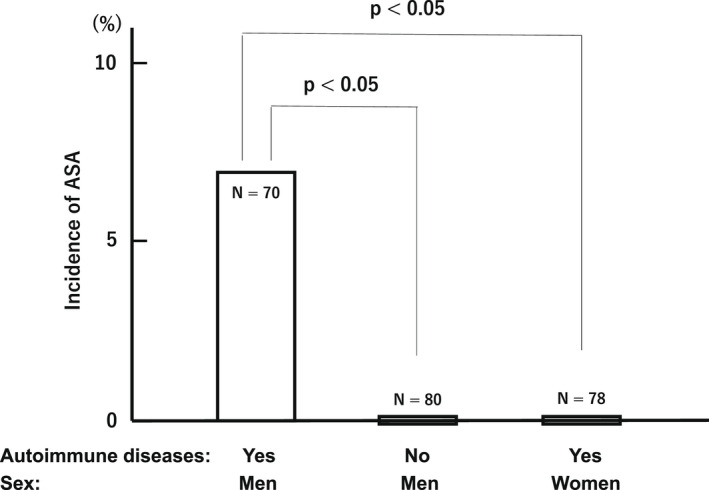
Comparison of the incidences of anti‐sperm antibodies (ASA) between men with and without systemic autoimmune diseases and those of ASA between men and women with the diseases. In order to ascertain sex differences, the frequency of ASA production was compared between men and women with systemic autoimmune diseases. Serum samples were obtained from 70 men with systemic autoimmune diseases, 80 healthy men and 78 women with systemic autoimmune diseases. These sera were examined by using the indirect‐immunobead test (I‐IBT). The results of I‐IBT showed that 5 (7.1%) of men with systemic autoimmune diseases had serum sperm‐binding antibodies. However, no positives existed in 80 healthy men (p < 0.05), indicating that systemic autoimmune diseases may be one of the risk factors for developing ASA in men. Among the 78 women, none had serum ASA. A significant sex difference was observed between men and women with systemic autoimmune diseases (p < 0.05; Fisher's exact test)
In conclusion, it was found a clear sex difference in the frequency of ASA production in patients with systemic autoimmune diseases.
3. SEX DIFFERENCES IN ANTI‐SPERM ANTIBODIES (ASA)
The frequency of ASA is about 3% in both men 14 , 15 and women. 16 ASA are generally detected by the sperm‐immobilization test (SIT) 1 , 2 using patient's serum in women. On the other hand, ASA are evaluated by the direct‐immunobead test (D‐IBT) 12 or the mixed antiglobulin reaction (MAR) test 17 using patient's sperm in men. Therefore, in the medical care of ASA, not only the detection method but also the treatment strategy is obviously different for men and women. Therefore, it is very important for the clinicians to recognize that there is sex difference in ASA when they recommend the tests for ASA to the couples and also deciding the strategy when the ASA are found to be positive in either men or women.
3.1. Measurement and evaluation of ASA
Several assay methods have been developed to detect ASA. However, it is obvious that the most important consideration is the selection of the method. Currently available tests for detecting ASA can be classified into the three groups according to the characteristics of each test (Table 1). 16 Group 1 contains tests for the detection of bioactivity of ASA, including sperm‐agglutination test (SAT), SIT, and fertilization‐blocking test (FBT) that detects fertilization‐blocking antibodies. Group 2 contains tests for the detection of ASA bound for motile sperm, including IBT, MAR test, and Panning test. Group 3 contains tests for the detection of ASA against sperm or sperm extract, including radiolabeled antiglobulin test, indirect immunofluorescent test, fluorescent‐activated cell sorter (FACS), enzyme‐linked immunosorbent assay (ELISA), and passive hemagglutination for the evaluation of ASA.
TABLE 1.
Available tests for detecting anti‐sperm antibody (ASA) 16
| Group 1. Tests for detecting bioactivity of ASA. |
| a) SAT. |
| b) SIT. |
| c) FBT. |
| Group 2. Tests for detecting ASA bound for motile sperm. |
| a) IBT or IS. |
| b) MAR test. |
| c) Panning test. |
| Group 3. Tests for detecting ASA against sperm or sperm extract. |
| a) Radiolabeled antiglobulin test. |
| b) Indirect immunofluorescent test. |
| c) FACS. |
| d) ELISA. |
| e) Passive hemagglutination. |
Abbreviations: ELISA, enzyme‐linked immunosorbent assay; FACS, fluorescent‐activated cell sorter; FRT, fertilization‐blocking test; IBT, immunobead test; IS, ImmunoSpheres; MAR, mixed antiglobulin reaction; SAT, sperm‐agglutination test; SIT, sperm‐immobilization test.
As mentioned above, it is necessary to recognize that the selection of the ASA testing is different in men and women.
3.1.1. Measurement and evaluation of ASA in women
In those methods for the detection of ASA, it has been recommended that clinically specific test for infertile women should be chosen for detecting circulating ASA. The relationship between ASA with bioactivities, including sperm‐immobilizing antibodies, sperm‐agglutinating antibodies, and fertilization‐blocking antibodies, and those without bioactivities is drawn in a scheme of Figure 2. 16 Therefore, the tests for detecting ASA belonged to the group 1 (Table 1) are suitable for choosing as initial testing for infertile women. In them, sperm‐immobilizing antibodies are recognized as one of the causes of refractory infertility in women, and the antibodies are detected in patient's serum by the SIT, and their detection is useful for determining the strategy for infertility treatments in women.
FIGURE 2.
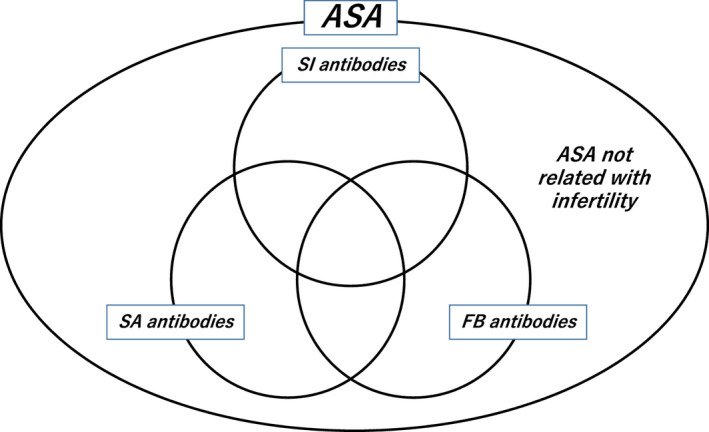
Relationship of various kinds of anti‐sperm antibodies (ASA) produced in immunologically infertile women. 16 Several assay methods have been developed to detect ASA. The relationship between ASA with bioactivities, including sperm‐immobilizing (SI) antibodies, sperm‐agglutinating (SA) antibodies, and fertilization‐blocking (FB) antibodies, and those without bioactivities is drawn in this scheme. In those methods for the detection of ASA, there are some assays for the detection of ASA that are not related with infertility. Therefore, it has been recommended that clinically specific test for infertile women should be chosen for detecting circulating ASA, that is, the tests for detecting ASA with bioactivities are suitable for choosing as initial testing for infertile women
Around the year of 1970, Isojima et al. 1 , 2 reported that positive reactions in the SIT were given by sera of 17.2% of the patients with infertility of unexplained cause and not by those of normal pregnant and unmarried women. Later, this observation was also confirmed by other studies. 18 , 19 , 20 , 21 , 22 However, it should be noted that the result obtained by the SIT, named as sperm‐immobilization value (SIV), is only to be semi‐quantitative. As the SIV cannot quantify the antibody exactly, the strategy for the treatment of infertile women with sperm‐immobilizing antibodies is not able to be decided.
For the quantitative assay for SIT, Isojima et al. 23 developed the quantitative method for this antibody. In brief, each test serum is serially diluted twofold with control serum. The sperm motility in the test serum (T %) and in control serum (C %) were calculated according to the semi‐quantitative assay method. The antibody activity for sperm immobilization is calculated by the formula (C‐T/C x 100), and the value is plotted against the dilutions of the test serum on a semi‐logarithmic chart to obtain a sigmoid dose−response curve. The dilution of the test serum at which the sigmoid curve crossed the value of 50 for the antibody activity is determined on the dose–response line and designated as 50% sperm‐immobilization unit (SI50). Thus, the amount of sperm‐immobilizing antibodies in various sera can be compared. This method is very useful for routine examination of sperm‐immobilizing antibody in the sera of infertile women.
3.1.2. Measurement and evaluation of ASA in men
On the other hand, the clinical significance of sperm‐immobilizing antibodies in serum could not be clarified in men. 4 The reason might be explained by the fact that, even if ASA are present in the sera in men, fertilization of sperm with oocyte never occur in their body.
Therefore, it is considered clinically important in men whether the ASA binds to the sperm when semen are ejaculated in the woman's vagina. It is introduced in the WHO laboratory manual that the D‐IBT or the MAR test is useful for the evaluation of ASA in sperm. 3 D‐IBT has widely been used as a screening test for ASA. 12 , 24 , 25 However, it should be noted that the positive result obtained by the D‐IBT or MAR test means that the patient has only sperm‐bound antibodies. If the detected antibodies have inhibitory bioactivities on fertility, they can be the cause of infertility in the patient.
Therefore, to establish the strategy for the treatment of infertile men with ASA, a secondary examination must be carried out. When the result of D‐IBT is positive, subsequently the inhibitory effects by ASA on sperm penetration through cervical mucus and fertilizing ability 15 should be evaluated to decide for the strategy of infertility treatments. 26
Recently, the IBT was discontinued of its production. However, the ASA test results obtained by the ImmunoSpheres (IS) assay as a substitute was shown in agreement with the results obtained with the IBT test. 27
3.2. Causes of immune infertility in patients with ASA
Some of the ASA have been shown to be highly related to reproductive failures. 28 The presence of ASA in women has been shown to inhibit sperm migration in their genital tract. Such antibodies can also exert inhibitory effects on various stages of sperm–egg interaction and subsequent embryo development in vitro.
The presence of ASA in men can reduce fertility as similar as that in women. However, the association between the presence of ASA in men and infertility had previously been disputed before it was clarified that there is a diversity of ASA bound to sperm. 14 , 15
3.2.1. Causes of immune infertility in women with ASA
Inhibition of sperm migration in the female genital tract by ASA possessed in women
ASA could cause infertility in female at different stages of reproduction including inhibitory effect on sperm transport in the cervical mucus (CM). There have been several reports which found the incidence of poor post‐coital test (PCT) results was significantly higher in infertile women with sperm‐immobilizing antibodies in their sera than in those without the antibodies. 29 , 30 , 31 , 32 Later, our group revealed that the SI50 titer in the serum can predict inhibitory effects on sperm migration through CM in infertile women with sperm‐immobilizing antibodies. 33 When patients with sperm‐immobilizing antibodies were divided into two groups according to the SI50 titers, the abnormal result of PCT was obtained in all 10 patients with high (≧10) SI50 titers, while that was 14 (66.7%) in 21 patients with low (<10) SI50 titers (p < 0.05). 33
To overcome the inhibitory effects of ASA on sperm transport in the CM, infertile women with ASA are generally treated by intra‐uterine insemination (IUI). However, patients with ASA, especially sperm‐immobilizing antibodies, are less likely to conceive even with IUI. 34 One of the reasons is that the sperm‐immobilizing antibodies could interfere with sperm migration at the level of the fallopian tubes. To test the possible impairment of sperm migration in the fallopian tubes, our group performed laparoscopic examinations and investigated the presence of motile sperm in the peritoneal fluid following IUI, 35 named peritoneal sperm recovery test (PSRT). 36 As a result, sperm recovery in the peritoneal fluid was not observed in 24 (88.9%) of 27 patients with sperm‐immobilizing antibodies, while it was not observed in 140 (66.0%) of 212 patients without the antibodies (p < 0.05). When the 27 patients with sperm‐immobilizing antibodies were divided into two groups according to the SI50 titers, the abnormal result of PSRT was obtained in 22 (95.7%) of 23 patients with high (>10) SI50 titers, while that was 2 (50.0%) in 4 patients with low (≦10) SI50 titers (p < 0.05). 35 These findings suggested that the SI50 titer in the serum can predict inhibitory effects on sperm migration from uterine cavity through fallopian tube in infertile women with sperm‐immobilizing antibodies.
Inhibition of various stages of fertilization by ASA possessed in women
Several diagnostic tests were developed to predict the fertilization potential of sperm, including the hemizona assay (HZA) 37 for sperm‐zona pellucida tight binding, zona pellucida penetration assay 38 for sperm penetration through zona pellucida, and zona‐free hamster egg penetration assay 39 for sperm–egg fusion. It was reported that the sperm‐immobilizing antibodies can exert inhibitory effects on sperm–egg interaction, including acrosome reaction, 40 zona pellucida recognition, and penetration. 41 , 42 , 43 , 44 , 45 , 46 , 47 Moreover, we found that the ASA block fertilization at specific stages. Some of them may inhibit sperm capacitation and thus prevent all processes of fertilization that follow. Some other antibodies may not affect capacitation and sperm binding to zona pellucida but inhibit the acrosome reaction, followed by the blocking of sperm penetration through zona pellucida and ooplasm. 48
Infertile women with sperm‐immobilizing antibodies are generally treated by in vitro fertilization–embryo transfer (IVF‐ET), 34 , 49 , 50 , 51 , 52 that is an ideal method for the treatment only if a special attention is paid to remove the antibodies adhered to cumulus cells around oocytes before insemination. The contamination of blood in the aspirated follicular fluid should be avoided as much as possible, and the antibodies in the follicular fluid are removed by transferring oocytes several times to the new culture medium. The patient's serum should never be added to the culture medium.
3.2.2. Causes of immune infertility in men with ASA
Inhibition of sperm migration in the female genital tract by ASA possessed in men
Diverse ASA are bound to sperm surfaces in men. 14 , 15 Moreover, immunologically infertile men had a relatively high incidence of asthenozoospermia. We also found that there exists significant inhibitory effect of sperm‐immobilizing antibodies in ejaculated human sperm on sperm motility in immunologically infertile men. 53 In this study, all men who were positive on the direct SIT were diagnosed as asthenozoospermia to a varying degree.
ASA bound to sperm surface in infertile men also show significant inhibitory effect on sperm passage through CM. 54 However, it was also proved by the study that the ability of sperm passage through CM is not inhibited in two‐fifths of men with ASA. It indicates there is a diversity of ASA bound to sperm surface in infertile men.
Inhibition of various stages of fertilization by ASA possessed in men
As for the blocking effects on fertilization by ASA bound to sperm surface, there have been conflicting reports. Some investigators have shown that ASA in men have an inhibitory effect on fertilization. 55 , 56 , 57 , 58 , 59 However, others have arrived at the opposite conclusion. 60 So we analyzed the diverse effects of ASA bound to the surface of ejaculated human sperm on fertilization. 15 Four (57.1%) of seven patients who had IB‐bound sperm of ≧80%, fertilizing ability was inhibited, while none of the eight patients who had <80% IB‐bound sperm had an inhibitory effect on fertilization (p = 0.01). 15
There have been some investigators who demonstrated that the fertilization rate of IVF significantly decreased when the inseminated sperm were coated with higher numbers of ASA. 56 , 58 These findings also suggest that in infertile men having positive but lower levels of ASA on the ejaculated sperm, ASA‐free sperm might contribute to higher fertilization rates in IVF. In contrast, those with higher levels of ASA have fewer ASA‐free sperm, which leads to a significant reduction in fertilization.
3.3. Treatment strategy for the infertile patients with ASA
As mentioned above, the diagnosis of ASA is different between men and women. Moreover, the causes of infertility in men and women with ASA are not completely same. Therefore, the strategy for the treatment of men and women with ASA should be different each other.
3.3.1. Strategy for the treatment of infertile women with ASA
For infertile women with ASA, treatments such as condom therapy, abstinence, and steroid suppression therapy have been applied, and they resulted a generally poor consequence. 61 IUI can overcome problems related to sperm passage through the CM, and it has been shown to be useful for the treatment of a part of infertile women with ASA. However, when the SI50 titer of sperm‐immobilizing antibodies is higher, sperm inserted in uterine cavity by IUI procedure would be impaired by the antibodies secreted from uterine cavity to fallopian tube, and even in follicular fluid. 34 , 35 Therefore, the ultrasound‐guided intra‐tubal insemination (ITI) therapy, that was developed by Jansen et al., 62 for infertile women with sperm‐immobilizing antibodies might be effective as a substitute for IUI. 63 , 64
Some success has been achieved with IUI, but not when serum ASA levels are high. To overcome the inhibitory effects of ASA on sperm migration within the CM and the fallopian tubes, IVF‐ET has been applied and satisfactory outcomes resulting from suitable culture conditions for gametes and embryo have been obtained. After removing the ASA adhered to cumulus cells around oocytes and avoiding the contamination of blood in the aspirated follicular fluid and moreover removing the antibodies in the follicular fluid by transferring oocytes several times to the new culture medium with replacement serum added to the culture medium, fertilization, and cleavage rates of mature oocytes from the patients with ASA were not different from those in patients without the antibodies. 50 It is also important that substitution of the patients' serum by replacement serum in the fertilization and embryo growth media may prove to be an effective means of improving IVF treatment for women with ASA. 51
For infertile women with sperm‐immobilizing antibodies, it is important to assess the SI50 titers by the quantitative method to select appropriate treatments. Kobayashi et al. 34 proposed a strategy for the treatment of infertile women with sperm‐immobilizing antibodies according to the undulation patterns of individual patient's SI50 titers. They divided patients with sperm‐immobilizing antibodies into three groups according to their follow‐up SI50 titers. Group A, which consisted of patients with continuously high SI50 titers (>10 units), did not conceive by ordinary or repeated IUI, however, a satisfactory pregnancy rate was obtained by IVF‐ET. Group B, in which the patients had intermediate SI50 titer patterns around 10, showed rare rates of success with IUI. In Group C, the patients with continuously low SI50 titers (<10 units), conception by repeated or ordinary IUI was achieved, although the success rates were lower than by IVF‐ET. Taken together, a strategy for the treatment of infertile women with sperm‐immobilizing antibodies is suggested in Figure 3. 65
FIGURE 3.
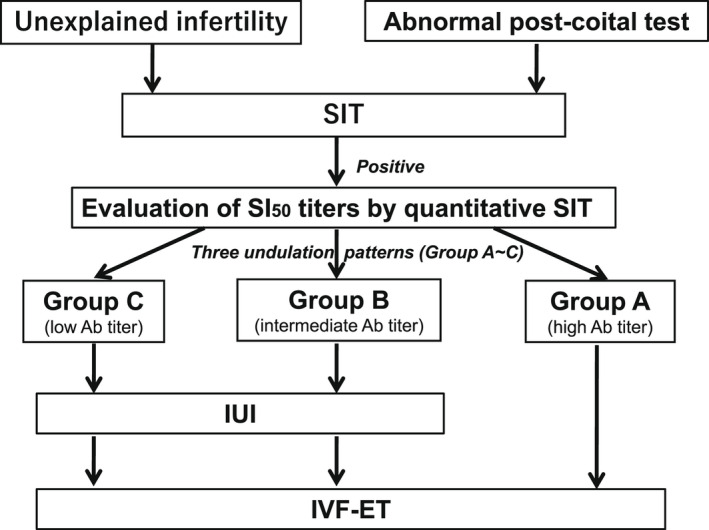
Strategy for the treatment of infertile women with sperm‐immobilizing antibodies. 65 It is important to assess the 50% sperm‐immobilization unit (SI50) titers to select treatments for infertile women with sperm‐immobilizing antibodies. A strategy for the treatments of infertile women with sperm‐immobilizing antibodies is shown according to the undulation patterns of individual patient's SI50 titers. Patients with sperm‐immobilizing antibodies are divided into three groups according to their follow‐up SI50 titers; Group A: the patients with continuously high SI50 titers (>10 units) are recommended to be treated by in vitro fertilization–embryo transfer (IVF‐ET). Group B: the patients have intermediate SI50 titer patterns around 10, and Group C; the patients with continuously low SI50 titers (<10 units) are recommended to be treated by repeated or ordinary intra‐uterine insemination (IUI). If they are not able to be conceived by several cycles of IUI, then the treatment by IVF‐ET should be considered
3.3.2. Strategy for the treatment of infertile men with ASA
Some infertile men with ASA might be treated with the classical treatments such as immunosuppressive therapies using corticosteroids or cyclosporine to reduce production of ASA. However, the randomized controlled trials (RCTs) have not found a therapeutic significance for the corticosteroid therapy for infertile men with ASA. 66 , 67 , 68 , 69 Moreover, as corticosteroids are well known to have potential side effects, important clinical considerations in the use of corticosteroids to treat infertile patients with ASA are mandatory. 70 , 71 , 72 As for the effect of cyclosporine A treatment, no definite conclusions can be drawn so far. 73
The purpose of the technique for removing ASA bound to sperm for infertile men with ASA is the effort to remove sperm‐bound antibody from sperm. However, the dissociation of tightly bound antibodies has been found to be difficult. There have been several reports, including simple sperm washing, 74 split ejaculation into medium containing serum, 75 immune absorption of ASA, 76 , 77 , 78 and enzymatic treatment. 79 , 80 , 81 , 82 The simple sperm washing did not remove ASA from the sperm surface. The addition of serum to sample collection pots may improve the oocyte fertilization rate. The latter 2 methods have not been clinically applied yet. 83
There have been reported other options for the treatments of infertile men with ASA. So far, the usefulness of IUI, 26 , 84 , 85 , 86 IVF‐ET 26 , 57 , 58 , 86 , 87 , 88 , 89 and intracytoplasmic sperm injection (ICSI) 26 , 90 , 91 for the treatments of infertile men with ASA have been reported. However, for the appropriate strategy to infertile men with ASA, it is necessary to evaluate fertilizing ability of the patient's ejaculated sperm before starting the IUI treatment. 26 If there is no inhibitory effect on fertilization by ASA, the treatment using IUI might be useful for some men with ASA who have the negative post‐coital test results. For the patient with ASA who is diagnosed to have abnormal fertilizing ability based on the HZA, it is necessary to advise the infertile couple for selecting ICSI‐ET as a primary treatment (Figure 4). 26
FIGURE 4.
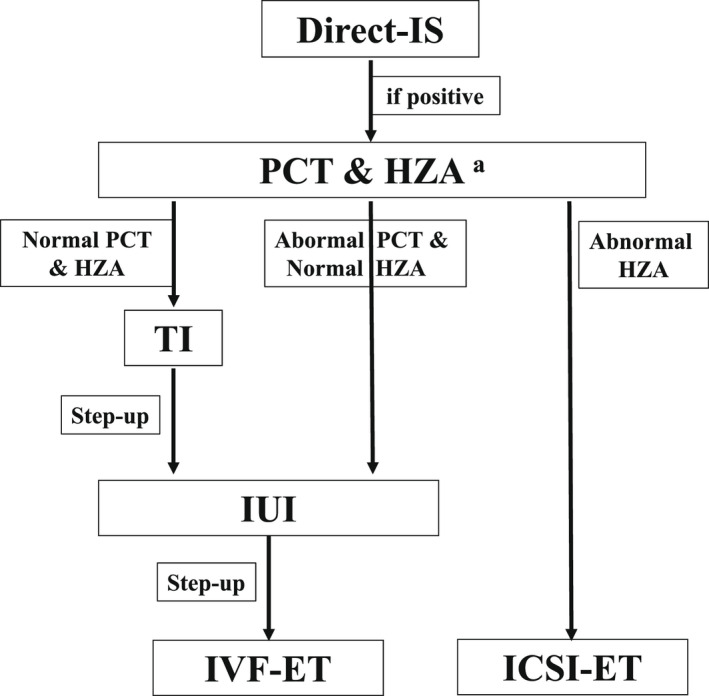
Strategy for the diagnosis and the treatment of infertile men with anti‐sperm antibodies (ASA). 26 To diagnose immunological infertility in men, the direct‐ImmunoSpheres (D‐IS) test should be carried out as a routine test in the infertility clinic. Then, the post‐coital test (PCT) and the hemizona assay (HZA) should be performed as initial screening for infertile men with ASA diagnosed by D‐IS, as a basis for decision making. If a patient with ASA has an abnormal hemizona index (HZI), it seems reasonable to advise selecting intracytoplasmic sperm injection–embryo transfer (ICSI‐ET) as the primary treatment. For the patients with ASA and normal HZI, selection of timed intercourse (TI) or intra‐uterine insemination (IUI) should be advised depending on the PCT result. a: If the HZA is not available, infertile men who have IB‐bound sperm of ≧80% had better be advised for selecting ICSI‐ET as a primary treatment
3.4. Production of ASA
Before the clinical introduction of IVF‐ET and ICSI, men and women with ASA were refractory to the classical treatment as mentioned above. If most of men and women, or male and female animals, naturally and easily produce ASA, it might be suggested that such species cease to exist. If not, there is a question what are the causes for the production of ASA. Immuno‐contraceptives could be developed in future when the reasons for the production of ASA will be clarified.
3.4.1. Production of ASA in women
Women do not generally produce antibodies against sperm, however, some infertile women have been found to possess ASA which may contribute to their infertility. 92 Moreover, the reasons why most women do not develop an immune response following exposure to sperm is not clear yet.
It has been demonstrated that susceptibility to various immune disorders including autoimmune diseases has a genetic background. Therefore, our group examined the frequencies of human leukocyte antigen (HLA)‐DQA1, HLA‐DQB1, and HLA‐DRB1 genes and found that the high frequency of HLA‐DRB1*0901 and DQB1*0303 genes in the Japanese population may account for higher frequency of sperm‐immobilizing antibody. 92 Another immune disease such as juvenile‐onset myasthenia gravis 93 and systemic lupus erythematosus with antiphospholipid syndrome 94 are reported to be linked to these genotypes. These immune diseases also occur at a higher frequency in the Japanese population.
Then, the following studies were conducted to clarify the source of immunity for the production of ASA by using severe combined immunodeficient (SCID) mice. 95 Successful heterotransplantation with human peripheral blood lymphocytes from infertile women with sperm‐immobilizing antibodies into SCID mice indicate that the immune response resulting in the production of the antibodies in SCID mice might require the ongoing presence of the eliciting human sperm antigens. 96
In clinical practice, there was a significant difference in the incidence in sperm‐immobilizing antibodies between the women treated by ICSI because of a severe male factor and those whose husbands had a normal sperm count. 97 Moreover, it was also found that infertile women with past Chlamydia trachomatis infection has significantly higher incidence of sperm‐immobilizing antibodies. 98 Chlamydia trachomatis infection could play a role in the production of sperm‐immobilizing antibodies in infertile women.
Therefore, the production of sperm‐immobilizing antibodies is likely to occur in women with particular HLA haplotypes after repeated exposure to a large enough amount of sperm. Inflammatory disease of the genital tract, such as Chlamydia trachomatis infection, might be a trigger at least for women to produce sperm‐immobilizing antibodies.
3.4.2. Production of ASA in men
In men, the blood–testis barrier (BTB) and immune privilege are critical for fertility, as breakdown can result in the generation of autoimmune T and B lymphocytes, and production of ASA. 99 This leads to autoimmune‐mediated testicular damage, germ cell loss, and impaired sperm function. 99 , 100 ASA can affect male fertility by various mechanisms. 101 Some of them relate to sperm agglutination and/or complement‐dependent sperm immobilization. 53 ASA also impair sperm penetration through cervical mucus and interfere with sperm–egg interaction. 15 , 54
The possible risk factors of ASA production in men have been shown such as congenital or acquired chronic obstruction of the male reproductive tract (MRT), infection in the MRT, varicocele, cryptorchidism, testicular injuries, testicular tumors, homosexual men, spinal cord injury, and autoimmune diseases. 102 However, the relationship between these risk factors and the production of ASA is still controversial. Recently, it was suggested that vasectomy followed by vasovasostomy is the only clinical condition that shows almost permanently high titers of ASA in numerous clinical series. 103
4. CONCLUSIONS AND FUTURE DIRECTIONS
A summary of the previously unreported sex differences in ASA is shown in Table 2. Since ASA are allogeneic antibodies in women and autoantibodies in men, it is characterized by having a background different from that of other general diseases. At the time of diagnosis, it is necessary to pay attention to the difference in specimens, the difference in test methods, the mechanism of infertility onset and to treat immune infertility by ASA. Treatment strategies are established for each.
TABLE 2.
Sex difference of anti‐sperm antibodies (ASA) in infertile couples
| Women | Men | |
|---|---|---|
| Classification | Homologous antibody | Autoantibody |
| Samples | Serum/CM/FF | Motile sperm |
| Assay | Sperm‐immobilization test (Indirect method) | ImmunoSpheres (direct method) |
| Frequency | Approximately 3% | Approximately 3% |
| Causes of infertility |
|
|
| Treatment strategy | IUI or IVF is selected according to the SI50 titers |
|
| Production of ASA |
|
Breakdown of the BTB such as vasectomy followed by vasovasostomy |
Abbreviations: CM, cervical mucus; Ct, Chlamydia trachomatis; FF, follicular fluid; ICSI, intracytoplasmic sperm injection; IUI, intra‐uterine insemination; IVF, in vitro fertilization; SI50, 50% sperm‐immobilization unit; TI, timed intercourse.
It is strongly expected that the development of immunological contraceptive vaccines using sperm antigens will be materialized in the future as the elucidation of the production mechanism of ASA progresses.
CONFLICT OF INTEREST
Nothing to declare.
Shibahara H, Chen Y, Honda H, Wakimoto Y, Fukui A, Hasegawa A. Sex difference in anti‐sperm antibodies. Reprod Med Biol. 2022;21:e12477. doi: 10.1002/rmb2.12477
REFERENCES
- 1. Isojima S, Li T, Ashitaka Y. Immunologic analysis of sperm‐immobilizing factor found in sera of women with unexplained sterility. Am J Obstet Gynecol. 1968;101:677–83. [Google Scholar]
- 2. Isojima S, Tsuchiya K, Koyama K, Tanaka C, Naka O, Adachi H. Further studies on sperm‐ immobilizing antibody found in sera of unexplained cases of sterility in women. Am J Obstet Gynecol. 1972;112:199–207. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization (ed) . WHO laboratory manual for the examination and processing of human semen. 6th ed. Switzerland: WHO; 2021. [Google Scholar]
- 4. Henmi T, Shibahara H, Kobayashi S, Shigeta M, Koyama K, Isojima S. Efficacy of corticosteroid therapy for infertile men with sperm‐immobilizing antibodies in their sera. Adv Obstet Gynecol. 1990;42:461 (In Japanese). [Google Scholar]
- 5. Oertelt‐Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev. 2012;11:A479–85. [DOI] [PubMed] [Google Scholar]
- 6. McCarthy M. The “gender gap” in autoimmune disease. Lancet. 2000;356:1088. [DOI] [PubMed] [Google Scholar]
- 7. Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35:347–69. [DOI] [PubMed] [Google Scholar]
- 8. Itoh Y, Golden LC, Itoh N, Matsusaka MA, Ren E, Tse V, et al. The X‐linked histone demethylase Kdm6a in CD4+ T lymphocytes modulates autoimmunity. J Clin Invest. 2019;129:3852–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cutolo M, Castagnetta L. Immunomodulatory mechanisms mediated by sex hormones in rheumatoid arthritis. Ann N Y Acad Sci. 1996;784:237–51. [DOI] [PubMed] [Google Scholar]
- 10. Verthelyi D. Sex hormones as immunomodulators in health and disease. Int Immunopharmacol. 2001;1:983–93. [DOI] [PubMed] [Google Scholar]
- 11. Shiraishi Y, Shibahara H, Koriyama J, Hirano Y, Okazaki H, Minota S, et al. Incidence of antisperm antibodies in males with systemic autoimmune diseases. Am J Reprod Immunol. 2009;61:183–9. [DOI] [PubMed] [Google Scholar]
- 12. Bronson RA, Cooper GW, Rosenfeld D. Detection of sperm specific antibodies on the spermatozoa surface by immunobead binding. Arch Androl. 1982;9:61. [Google Scholar]
- 13. Koriyama J, Shibahara H, Shiraishi Y, Hirano Y, Suzuki M. Gender difference in the frequency of anti‐sperm antibody production in patients with autoimmune diseases. Jpn J Reprod Med. 2008;53:338 (in Japanese). [Google Scholar]
- 14. Shibahara H, Tsunoda T, Taneichi A, Hirano Y, Ohno A, Takamizawa S, et al. Diversity of antisperm antibodies bound to sperm surface in male immunological infertility. Am J Reprod Immunol. 2002;47:146–50. [DOI] [PubMed] [Google Scholar]
- 15. Shibahara H, Shiraishi Y, Hirano Y, Suzuki T, Takamizawa S, Suzuki M. Diversity of the inhibitory effects on fertilization by anti‐sperm antibodies bound to the surface of ejaculated human sperm. Hum Reprod. 2003;18:1469–73. [DOI] [PubMed] [Google Scholar]
- 16. Shibahara H. Detection of anti‐sperm antibody (ASA) in women. In: Shibahara H, Hasegawa A, editors. Gamete immunology. Singapore: Springer; 2022. [Google Scholar]
- 17. Jager S, Kremer J, van Slochteren‐Draaisma T. A simple method of screening for antisperm antibodies in the human male. Detection of spermatozoal surface IgG with the direct mixed antiglobulin reaction carried out on untreated fresh human semen. Int J Fertil. 1978;23:12–21. [PubMed] [Google Scholar]
- 18. Ansbacher R, Keung‐Yeung K, Behrman SJ. Clinical significance of sperm antibodies in infertile couples. Fertil Steril. 1973;24:305–8. [DOI] [PubMed] [Google Scholar]
- 19. Jones WR, Ing RMY, Kaye MD. A comparison of screening tests for anti‐sperm activity in the serum of infertile women. J Reprod Fertil. 1973;32:357–64. [DOI] [PubMed] [Google Scholar]
- 20. Petrunia DM, Taylor PJ, Watson JI. A comparison of methods of screening for sperm antibodies in the serum of women with otherwise unexplained infertility. Fertil Steril. 1976;27:655–61. [DOI] [PubMed] [Google Scholar]
- 21. Cantuiria AA. Sperm immobilizing antibodies in the serum and cervicovaginal secretions in infertile and normal women. Br J Obstet Gynecol. 1977;84:865–8. [PubMed] [Google Scholar]
- 22. Blumenfeld Z, Gershon H, Makler A, Stoler J, Brandes JM. Detection of antisperm antibodies: a cytotoxicity immobilization test. Int J Fertil. 1986;31:207–12. [PubMed] [Google Scholar]
- 23. Isojima S, Koyama K. Quantitative estimation of sperm immobilizing antibody in the sera of women with sterility of unknown etiology; the 50% sperm immobilization unit (SI50). Excerpta Medica Intl Cong Series. 1976;370:10–5. [Google Scholar]
- 24. Clarke GN, Baker HWG. Treatment of sperm antibodies in infertile men: sperm antibody coating and mucus penetration. In: Bratanov K, editor. Proc Int Symp Immunology of Reproduction. Bulgaria: Bulgarian Academy of Sciences Press; 1982. p. 337. [Google Scholar]
- 25. Clarke GN, Stojanoff A, Cauchi MN. Immunoglobulin class of sperm‐bound antibodies in semen. In: Bratanov K, editor. Proc Int Symp Immunology of Reproduction. Bulgaria: Bulgarian Academy of Sciences Press; 1982. p. 482. [Google Scholar]
- 26. Shibahara H, Shiraishi Y, Suzuki M. Diagnosis and treatment of immunologically infertile males with antisperm antibodies. Reprod Med Biol. 2005;4:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Centola GM, Andolina E, Deutsch A. Comparison of the immunobead binding test (IBT) and immunospheres (IS) assay for detecting serum antisperm antibodies. Am J Reprod Immunol. 1997;37:300–3. [DOI] [PubMed] [Google Scholar]
- 28. Shibahara H, Wakimoto Y, Fukui A, Hasegawa A. Anti‐sperm antibodies and reproductive failures. Am J Reprod Immunol. 2021;85:e13337. [DOI] [PubMed] [Google Scholar]
- 29. Cantuaria AA. Sperm immobilizing antibodies in the serum and cervicovaginal secretions of infertile and normal women. Br J Obstet Gynaecol. 1977;84:865–8. [PubMed] [Google Scholar]
- 30. Koyama K, Ikuma K, Kubota K, Isojima S. Effects of antisperm antibodies on sperm migration through cervical mucus. Excerpta Med Int Congr Ser. 1980;512:705–8. [Google Scholar]
- 31. Chen C, Jones WR. Application of a sperm micro‐immobilization test to cervical mucus in the investigation of immunologic infertility. Fertil Steril. 1981;35:542–5. [DOI] [PubMed] [Google Scholar]
- 32. Jager S, Kremer J, De Wilde‐Janssen IW. Are sperm immobilizing antibodies in cervical mucus an explanation for a poor post‐coital test? Am J Reprod Immunol. 1984;5:56–60. [DOI] [PubMed] [Google Scholar]
- 33. Shibahara H, Shiraishi Y, Hirano Y, Kasumi H, Koyama K, Suzuki M. Relationship between level of serum sperm immobilizing antibody and its inhibitory effect on sperm migration through cervical mucus in immunologically infertile women. Am J Reprod Immunol. 2007;57:142–6. [DOI] [PubMed] [Google Scholar]
- 34. Kobayashi S, Bessho T, Shigeta M, Koyama K, Isojima S. Correlation between quantitative antibody titers of sperm immobilizing antibodies and pregnancy rates by treatments. Fertil Steril. 1990;54:1107–13. [DOI] [PubMed] [Google Scholar]
- 35. Shibahara H, Shigeta M, Toji H, Koyama K. Sperm immobilizing antibodies interfere with sperm migration from the uterine cavity through the Fallopian tubes. Am J Reprod Immunol. 1995;34:120–4. [DOI] [PubMed] [Google Scholar]
- 36. Templeton AA, Mortimer D. Laparoscopic sperm recovery in infertile women. Br J Obstet Gynecol. 1980;87:1128–31. [DOI] [PubMed] [Google Scholar]
- 37. Burkman LJ, Coddington CC, Franken DR, Kruger TF, Rosenwaks Z, Hodgen GD. The hemizona assay (HZA)‐ development of a diagnostic test for the binding of human spermatozoa to the human hemizona pellucida to predict fertilization potential. Fertil Steril. 1988;49:688–97. [PubMed] [Google Scholar]
- 38. Yanagimachi R, Lopata A, Odom CB, Bronson RA, Mahi CA. Penetration of biologic characteristics of zona pellucida in highly concentrated salt solution the use of salt stored eggs for assessing the fertilizing capacity of spermatozoa. Fertil Steril. 1979;35:562–74. [DOI] [PubMed] [Google Scholar]
- 39. Yanagimachi R, Yanagimachi H, Rogers BJ. The use of zona‐free animal ova as a test‐system for the assessment of the fertilizing capacity of human spermatozoa. Biol Reprod. 1976;15:471–6. [DOI] [PubMed] [Google Scholar]
- 40. Bandoh R, Yamano S, Kamada M, Daitoh T, Aono T. Effect of sperm‐immobilizing antibodies on the acrosome reaction of human spermatozoa. Fertil Steril. 1992;57:387–92. [PubMed] [Google Scholar]
- 41. Bronson RA, Cooper GW, Rosenfeld DL. Sperm‐specific isoantibodies and autoantibodies inhibit the binding of human sperm to the human zona pellucida. Fertil Steril. 1982;38:724–9. [DOI] [PubMed] [Google Scholar]
- 42. Alexander NJ. Antibodies to human spermatozoa impede sperm penetration of cervical mucus or hamster eggs. Fertil Steril. 1984;41:433–9. [PubMed] [Google Scholar]
- 43. Kamada M, Daitoh T, Hasebe H, Irahara M, Yamano S, Mori T. Blocking of human fertilization in vitro by sera with sperm‐immobilizing antibodies. Am J Obstet Gynecol. 1985;153:328–31. [DOI] [PubMed] [Google Scholar]
- 44. Mahony MC, Blackmore PF, Bronson RA, Alexander NJ. Inhibition of human sperm‐zona pellucida tight binding in the presence of antisperm antibody positive polyclonal patient sera. J Reprod Immunol. 1991;19:287–301. [DOI] [PubMed] [Google Scholar]
- 45. Tsukui S, Noda Y, Fukuda A, Matsumoto H, Tatsumi K, Mori T. Blocking effect of sperm immobilizing antibodies on sperm penetration of human zonae pellucidae. In Vitro Fert Embryo Transf. 1988;5:123–8. [DOI] [PubMed] [Google Scholar]
- 46. Shibahara H, Shigeta M, Koyama K, Burkman LJ, Alexander NJ, Isojima S. Inhibition by the hemizona assay (HZA). Acta Obstet Gynaecol Jpn. 1991;43:237–8. [PubMed] [Google Scholar]
- 47. Shibahara H, Burkman LJ, Isojima S, Alexander NJ. Effects of sperm‐immobilizing antibodies on sperm‐zona pellucida tight binding. Fertil Steril. 1993;60:533–9. [PubMed] [Google Scholar]
- 48. Shibahara H, Shigeta M, Inoue M, Hasegawa A, Koyama K, Alexander NJ, et al. Diversity of the blocking effects of antisperm antibodies on fertilization in human and mouse. Hum Reprod. 1996;11:2595–9. [DOI] [PubMed] [Google Scholar]
- 49. Sugimoto Y, Hasegawa A, Yokoyama K, Ikeda Y, Bessho T, Shigeta M, et al. Successful application of in vitro fertilization and embryo replacement in the treatment of infertile women with sperm immobilizing antibody. Acta Obstet Gynaecol Jpn. 1987;38:1135–6. [PubMed] [Google Scholar]
- 50. Shibahara H, Mitsuo M, Ikeda Y, Shigeta M, Koyama K. Effects of sperm immobilizing antibodies on pregnancy outcome in infertile women treated with IVF‐ET. Am J Reprod Immunol. 1996;36:96–100. [DOI] [PubMed] [Google Scholar]
- 51. Taneichi A, Shibahara H, Hirano Y, Suzuki T, Obara H, Fujiwara H, et al. Sperm immobilizing antibodies in the sera of infertile women cause low fertilization rates and poor embryo quality in vitro. Am J Reprod Immunol. 2002;47:46–51. [DOI] [PubMed] [Google Scholar]
- 52. Shibahara H, Hirano Y, Shiraishi Y, Shimada K, Kikuchi K, Suzuki T, et al. Effects of in vivo exposure to eggs with sperm‐immobilizing antibodies in follicular fluid on subsequent fertilization and embryo development in vitro. Reprod Med Biol. 2006;5:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shibahara H, Hirano Y, Takamizawa S, Sato I. Effect of sperm‐immobilizing antibodies bound to the surface of ejaculated human spermatozoa on sperm motility in immunologically infertile men. Fertil Steril. 2003;79:641–2. [DOI] [PubMed] [Google Scholar]
- 54. Shibahara H. Causes of immune infertility in men with anti‐sperm antibody (ASA). In: Shibahara H, Hasegawa A, editors. Gamete immunology. Singapore: Springer; 2022. [Google Scholar]
- 55. Mandelbaum SL, Diamond SP, DeCherney AH. Relationship of antisperm antibodies to oocyte fertilization in in‐vitro fertilization‐embryo transfer. Fertil Steril. 1987;644–651:644–51. [PubMed] [Google Scholar]
- 56. de Almeida M, Gazagne I, Jeulin C, Herry M, Belaisch‐Allart J, Frydman R, et al. In‐vitro processing of sperm with autoantibodies and in‐vitro fertilization results. Hum Reprod. 1989;4:49–53. [DOI] [PubMed] [Google Scholar]
- 57. Rajah SV, Parslow JM, Howell RJ, Hendry WF. The effects on in‐vitro fertilization of autoantibodies to spermatozoa in subfertile men. Hum Reprod. 1993;8:1079–82. [DOI] [PubMed] [Google Scholar]
- 58. Lahteenmaki A. In‐vitro fertilization in the presence of antisperm antibodies detected by the mixed antiglobulin reaction (MAR) and the tray agglutination test (TAT). Hum Reprod. 1993;8:84–8. [DOI] [PubMed] [Google Scholar]
- 59. Yeh WR, Acosta AA, Seltman HJ, Doncel G. Impact of immunoglobulin isotype and sperm surface location of antisperm antibodies. Fertil Steril. 1995;63:1287–92. [DOI] [PubMed] [Google Scholar]
- 60. Sukcharoen N, Keith J. The effect of the antisperm auto‐antibody bound sperm on in vitro fertilization outcome. Andrologia. 1995;27:281–9. [DOI] [PubMed] [Google Scholar]
- 61. Shibahara H. Non‐ART Treatments for infertile women with anti‐sperm antibody. In: Shibahara H, Hasegawa A, editors. Gamete immunology. Singapore: Springer; 2022. [Google Scholar]
- 62. Jansen RP, Anderson JC. Catheterisation of the fallopian tubes from the vagina. Lancet. 1987;2:309–10. [DOI] [PubMed] [Google Scholar]
- 63. Shibahara H, Hayashi T, Yamada Y, Shiotani T, Ikuma K. Ultrasound‐guided intratubal insemination as a treatment for the infertile patients who were refractory to intrauterine insemination. Jpn J Fertil Steril. 1994;39:198–203. [in Japanese]. [Google Scholar]
- 64. Asada K, Shibahara H, Shiraishi Y, Shimada K, Kikuchi K, Hirano Y, et al. Effectiveness of the intra‐tubal insemination for infertile women with sperm‐immobilizing antibodies. Acta Obstet Gynaecol Jpn. 2008;60(Suppl):510 (S‐230). [Google Scholar]
- 65. Shibahara H, Koriyama J, Shiraishi Y, Hirano Y, Suzuki M, Koyama K. Diagnosis and treatment of immunologically infertile women with sperm‐immobilizing antibodies in their sera. J Reprod Immunol. 2009;83:139–44. [DOI] [PubMed] [Google Scholar]
- 66. De Almeida M, Feneux D, Rigaud C, Jouannet P. Steroid therapy for male infertility associated with antisperm antibodies. Results of a small randomized clinical trial. Int J Androl. 1985;8:111–7. [DOI] [PubMed] [Google Scholar]
- 67. Haas GG Jr, Manganiello P. A double‐blind, placebo‐controlled study of the use of methylprednisolone in infertile men with sperm‐associated immunoglobulins. Fertil Steril. 1987;47:295–301. [PubMed] [Google Scholar]
- 68. Bals‐Pratsch M, Doren M, Karbowski B, Schneider HP, Nieschlag E. Cyclic corticosteroid immunosuppression is unsuccessful in the treatment of sperm antibody‐related male infertility: a controlled study. Hum Reprod. 1992;7:99–104. [DOI] [PubMed] [Google Scholar]
- 69. Rasanen M, Lahteenmaki A, Agrawal YP, Saarikoski S, Hovatta O. A placebo‐controlled flow cytometric study of the effect of low‐dose prednisolone treatment on sperm‐bound antibody levels. Int J Androl. 1996;19:150–4. [DOI] [PubMed] [Google Scholar]
- 70. Hendry WF, Hughes L, Scammell G, Pryor JP, Hargreave TB. Comparison of prednisolone and placebo in subfertile men with antibodies to spermatozoa. Lancet. 1990;335:85–8. [DOI] [PubMed] [Google Scholar]
- 71. Hendry WF. Bilateral aseptic necrosis of femoral heads following intermittent high‐dose steroid therapy. Fertil Steril. 1982;38:120. [DOI] [PubMed] [Google Scholar]
- 72. Shulman JF, Shulman S. Methylprednisolone treatment of immunologic infertility. Fertil Steril. 1982;38:591–9. [DOI] [PubMed] [Google Scholar]
- 73. Bouloux PM, Wass JA, Parslow JM, Hendry WF, Besser GM. Effect of cyclosporine A in male autoimmune infertility. Fertil Steril. 1986;46:81–5. [DOI] [PubMed] [Google Scholar]
- 74. Haas GG Jr, D'Cruz OJ. Effect of repeated washing on sperm‐bound immunoglobulin G. J Androl. 1988;9:190–6. [DOI] [PubMed] [Google Scholar]
- 75. Elder KT, Wick KL, Edwards RG. Seminal plasma anti‐sperm antibodies and IVF; the effect of semen sample collection into 50% serum. Hum Reprod. 1990;5:179–84. [DOI] [PubMed] [Google Scholar]
- 76. Kiser GC, Alexander NJ, Fuchs EF, Fulgham DL. In vitro immune absorption of antisperm antibodies with immunobead‐rise, immunomagnetic, and immunocolumn separation techniques. Fertil Steril. 1987;47:466–74. [DOI] [PubMed] [Google Scholar]
- 77. Foresta C, Varotto A, Caretto A. Immunomagnetic method to select human sperm without sperm surface‐bound autoantibodies in male autoimmune infertility. Arch Androl. 1990;24:221–5. [DOI] [PubMed] [Google Scholar]
- 78. Viganò P, Fusi FM, Brigante C, Busacca M, Vignali M. Immunomagnetic separation of antibody‐labelled from antibody‐free human spermatozoa as a treatment for immunologic infertility. A preliminary report. Andrologia. 1991;23:367–71. [DOI] [PubMed] [Google Scholar]
- 79. Pattinson HA, Mortimer D, Curtis EF, Leader A, Taylor PJ. Treatment of spermagglutination with proteolytic enzymes. I. Sperm motility, vitality, longevity and successful disagglutination. Hum Reprod. 1990;5:167–73. [DOI] [PubMed] [Google Scholar]
- 80. Pattinson HA, Mortimer D, Taylor PJ. Treatment of spermagglutination with proteolytic enzymes, II. Sperm function after enzymatic disagglutination. Hum Reprod. 1990;5:174–8. [DOI] [PubMed] [Google Scholar]
- 81. Bronson RA, Cooper GW. Documentation of IgA1 and IgA2 antisperm antibodies within seminal fluid. Am J Reprod Immunol Microbiol. 1988;18:7–10. [DOI] [PubMed] [Google Scholar]
- 82. Kutteh WH, Kilian M, Ermel LD, Byrd EW, Mestecky J. Antisperm antibodies (ASAs) in infertile males: subclass distribution of lgA antibodies and the effect of an lgA1 protease on sperm‐bound antibodies. Am J Reprod Immunol. 1994;31:77–83. [DOI] [PubMed] [Google Scholar]
- 83. Shibahara H. Non‐ART Treatments for infertile men with anti‐sperm antibody. In: Shibahara H, Hasegawa A, editors. Gamete immunology. Singapore: Springer; 2022. [Google Scholar]
- 84. Haas GG Jr. Male infertility and immunity. In: Lipshutz LI, Howards SS Jr, editors. Infertility in the Male, 2. St. Louis: Mosby Yearbook; 1991. p. 277–96. [Google Scholar]
- 85. Francavilla F, Romano R, Santucci R, Marrone V, Corrao G. Failure of intrauterine insemination in male immunological infertility in cases in which all spermatozoa are antibody‐coated. Fertil Steril. 1992;58:587–92. [PubMed] [Google Scholar]
- 86. Ombelet W, Vandeput H, Janssen M, Cox A, Vossen C, Pollet H, et al. Treatment of male infertility due to sperm surface antibodies: IUI or IVF? Hum Reprod. 1997;12:1165–70. [DOI] [PubMed] [Google Scholar]
- 87. Sukcharoen N, Keith J. The effect of the antisperm autoantibody bound sperm on in vitro fertilization outcome. Andrologia. 1995;27:281–9. [DOI] [PubMed] [Google Scholar]
- 88. Pagidas K, Hemmings R, Falcone T, Miron P. The effect of antisperm autoantibodies in male or female partners undergoing in vitro fertilization‐embryo transfer. Fertil Steril. 1994;62:363–9. [DOI] [PubMed] [Google Scholar]
- 89. Zouari R, DeAlmeida M, Rodrigues D, Jouannet P. Localization of antibodies on spermatozoa and sperm movement characteristics are good predictors of in vitro fertilization success in cases of male autoimmune infertility. Fertil Steril. 1993;59:606–12. [DOI] [PubMed] [Google Scholar]
- 90. Lahteenmaki A, Reima I, Hovatta O. Treatment of severe male immunological infertility by intracytoplasmic sperm injection. Hum Reprod. 1995;10:2824–8. [DOI] [PubMed] [Google Scholar]
- 91. Nagy ZP, Verheyen G, Liu J, Joris H, Janssenswillen C, Wisanto A, et al. Results of 55 intracytoplasmic sperm injection cycles in the treatment of male immunological infertility. Hum Reprod. 1995;10:1775–80. [DOI] [PubMed] [Google Scholar]
- 92. Tsuji Y, Mitsuo M, Yasunami R, Sakata K, Shibahara H, Koyama K. HLA‐DR and HLA‐DQ gene typing of infertile women possessing sperm‐immobilizing antibody. J Reprod Immunol. 2000;46:31–8. [DOI] [PubMed] [Google Scholar]
- 93. Matsuki K, Juji T, Tokunaga K, Takamizawa M, Maeda H, Soda M, et al. HLA antigens in Japanese patients with myasthenia gravis. J Clin Invest. 1990;86:392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Matsushita S, Fujisao S, Nishimura Y. Molecular mechanisms underlying HLA‐DR‐associated susceptibility to autoimmunity. Int J Cardiol. 1996;54:S81–90. [DOI] [PubMed] [Google Scholar]
- 95. Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335(6187):256–9. [DOI] [PubMed] [Google Scholar]
- 96. Shibahara H, Tsuji Y, Koyama K. Production of human sperm immobilizing antibodies in severe combined immunodeficient (SCID) mice reconstituted with human peripheral blood lymphocytes from infertile women. Am J Reprod Immunol. 1996;35:57–60. [PubMed] [Google Scholar]
- 97. Shibahara H, Kikuchi K, Shiraishi Y, Suzuki M, Shigeta M, Koyama K. Infertile women without sensitization to an appropriate amount of sperm do not produce sperm‐immobilizing antibodies in their sera. Reprod Med Biol. 2003;2:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hirano Y, Shibahara H, Koriyama J, Tokunaga M, Shimada K, Suzuki M. Incidence of sperm‐immobilizing antibodies in women with past Chlamydia trachomatis infection. Am J Reprod Immunol. 2010;65:127–32. [DOI] [PubMed] [Google Scholar]
- 99. Fijak M, Iosub R, Schneider E, Linder M, Respondek K, Klug J, et al. Identification of immunodominant autoantigens in rat autoimmune orchitis. J Pathol. 2005;207:127–38. [DOI] [PubMed] [Google Scholar]
- 100. Redgrove KA, McLaughlin EA. The role of the immune response in chlamydia trachomatis infection of the male genital tract: a double edged sword. Front Immunol. 2014;5:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. WHO . World Health Organization manual for the standardized investigation and diagnosis of the infertile couple. 2nd ed. Cambridge: Cambridge University Press; 2021. [Google Scholar]
- 102. Shibahara H. Production of ASA in men. In: Shibahara H, Hasegawa A, editors. Gamete immunology. Singapore: Springer; 2022. [Google Scholar]
- 103. Marconi M, Weidner W. Site and risk factors of antisperm antibodies production in the male population. In: WKH Krause, Naz RK, ediotrs. Immune infertility. Switzerland: Springer; 2017; p. 133–147. [Google Scholar]


