Abstract
Introduction
Alopecia areata (AA) is an autoimmune hair loss mediated by CD8 + T cells. Treatment for moderate-to-severe AA is still challenging. Janus kinase inhibitors, such as tofacitinib, have been recently investigated as a promising treatment option for AA. Evidence on the combination use of oral tofacitinib and systemic corticosteroids (SCs) for AA is still lacking.
Objective
To compare the efficacy and safety of monotherapy of oral tofacitinib and SCs, as well as their combination in patients with moderate-to-severe AA.
Methods
Patients with moderate-to-severe AA, who have been treated with at least 3 months of monotherapy of tofacitinib or SCs, or in their combination, were included in this study. The efficacy and adverse events of these treatments were retrospectively analyzed.
Results
Sixty-one patients with moderate-to-severe AA were included in this study. There were 12 (66.7%) of 18 patients in the SCs group, 12 (60.0%) of 20 patients in the tofacitinib group, and 18 (78.3%) of 23 patients achieved SALT50, with no significant difference among the three groups. The ratio of patients who achieved SALT50 was significantly higher in patients with a short duration of current hair loss episode (≤2 years) than in those with a duration of current hair loss episode (>2 years) in all the three groups. There were 66.7% patients in the SCs group, 35.0% patients in the tofacitinib group, and 56.5% patients in the combined group that showed adverse effects.
Conclusion
Tofacitinib was an effective treatment for patients with moderate-to-severe AA, and it was more tolerated than SCs. A combination of tofacitinib and SCs may have higher efficacy than SCs alone. Efficacy significantly decreased in patients with a current episode of disease for more than 2 years.
Keywords: alopecia areata (AA), Janus kinase inhibitors, tofacitinib, corticosteroids, combination therapy
Introduction
Alopecia areata (AA) is an autoimmune non-scarring hair loss, presenting as focal hairless patches to entire scalp loss or loss involving other body hairs (1). The majority of patients with moderate-to-severe AA will experience unpredictable episodes of relapsing and remitting courses or long-term persistence. There is still no reliable therapy for severe AA. The traditional treatments, such as systemic corticosteroids (SCs) and immunosuppressants, are limited in terms of treatment efficacy and high risk of adverse effects (2, 3).
The etiology of AA is related to a complex interaction between genetic and immune abnormalities, which induced inflammation targeting the hair follicles. Breakdown of the immune privilege of hair follicles has been thought to be the prerequisite of AA (4). Interferon-γ (IFN-γ) and CD8 + NKG2D + T cells have been identified as the key contributors to the pathogenesis of AA (5). Evidence from the studies on mouse models of AA has shown that CD8 + NKG2D + T cells release IFN-γ via Janus kinase (JAK)1/2 pathways to stimulate interleukin-15 (IL-15) production in follicular epithelial cells. IL-15 then binds to the surface of CD8 + NKG2D + T cells, further promoting the production of IFN-γ via JAK1/3 pathways to amplify the inflammatory response and impair the hair growth cycle (6, 7).
Given the important role that JAK pathways play in the pathogenesis of AA, JAK inhibitors (JAKi) have been investigated as a promising therapy for AA. This therapeutic approach could block several signaling pathways and lead to a decrease of CD8 + NKG2D + T cells and a significant improvement of AA. To date, several JAKi have been reported for the treatment of AA, including tofacitinib (JAK1/3), ruxolitinib (JAK1/2), and baricitinib (JAK1/2) (8, 9). Several case reports and studies have demonstrated the impressive efficacy of monotherapy with tofacitinib (10–12), which may expand the landscape of treatment options for moderate-to-severe AA. Interestingly, the combination use of tofacitinib and SCs might induce greater hair growth in several cases resistant to tofacitinib monotherapy (13).
Up to now, evidence on the combination use of oral tofacitinib and SCs for AA is still scarce. Herein, we conducted a retrospective study to compare the efficacy and safety of monotherapy of oral tofacitinib and SCs, as well as their combination in patients with moderate-to-severe AA.
Methodology
This retrospective study was approved by the Ethics Committee of Peking University People’s Hospital (2021PHB159-001).
The inclusion criteria were Chinese adult patients: (1) diagnosed as AA by two independent dermatologists at the dermatology clinic of the Peking University People’s Hospital between May 2019 and May 2021; (2) with AA subtypes classified as patchy AA, ophiasis, alopecia totalis (AT), and alopecia universalis (AU); (3) with the Severity of Alopecia Tool (SALT) score of 25% or over; and (4) who received at least 3 months of treatment of oral tofacitinib or SCs or combination therapy of the two. Patients with other hair loss disorders, such as androgenetic alopecia, telogen effluvium, trichotillomania, and cicatricial alopecia, were excluded.
The included patients were then divided into the three groups based on the treatment they had received: the SCs group, the tofacitinib group, and the combined therapy group. The demographic and clinical data abstracted from medical records included age, gender, duration of disease, duration of current episode of hair loss, treatment duration, subtypes, and severity of hair loss. Duration of current episode of hair loss was defined as the duration from the latest complete hair regrowth to the time the treatment of SCs and/or tofacitinib was initiated. Laboratory tests, including blood routine test, biochemical blood test, screening for infection of hepatitis B virus, and tuberculosis, were taken before treatment and every 3 months after initiating the treatments.
Alopecia severity was recorded using the SALT score (14). SALT50, SALT90, and SALT100 were defined as 50, 90, and 100% of hair regrowth. The primary endpoint was the ratio of patients who achieved SALT50. The secondary outcomes included the ratio of patients who achieved SALT90 and SALT100, as well as adverse events during the treatments.
Statistical analysis was performed using IBM SPSS software version 26 (IBM SPSS Statistics, Armonk, New York, United States). Data that were normally distributed were expressed as median (range) and non-normally distributed data expressed as median [interquartile range (IQR)]. The one-way ANOVA and the Kruskal–Wallis test were performed as appropriate to compare the continuous variables among the three groups. The chi-squared test and the Kruskal–Wallis test were used as appropriate to compare the non-continuous variables among the three groups. P-value < 0.05 was considered as statistically significant.
Results
Patient Characteristics
This study involved 61 patients (38 women and 23 men) with a median age of 29.7 years. There were 18, 20, and 23 patients in the SCs group, the tofacitinib group, and the combined group, respectively. Patients’ demographic and clinical characteristics of the three groups are given in Table 1.
TABLE 1.
Demographic data and characteristics of patients with AA in each group.
| SCs group (n = 18) | Tofacitinib group (n = 20) | Combined group (n = 23) | P-value | |
| Age, median (IQR), year | 29.4 (19.9–44.8) | 26.1 (22.8–32.6) | 35.9 (28.8–43.4) | 0.060 |
| Sex, n (%) | 0.781 | |||
| Male | 8 (44.4%) | 7 (35%) | 8 (34.8%) | |
| Female | 10 (55.6%) | 13 (65%) | 15 (65.2%) | |
| Severity, n (%) | 0.351 | |||
| Moderate AA | 8 (44.4%) | 8 (40.0%) | 7 (30.4%) | |
| Severe AA | 10 (55.6%) | 12 (60.0%) | 16 (69.6%) | |
| Duration of disease, median (IQR), year | 1.8 (0.3–14.4) | 9.7 (4.5–15.4) | 5.2 (1.0–7.8) | 0.046 |
| Duration of current disease episode, median (IQR), year | 1.1 (0.3–7.7) | 4.0 (0.8–9.6) | 2.2 (0.8–6.5) | 0.364 |
| Treatment duration, median (IQR), months | 7.1 (4.2–14.4) | 12.1 (7.0–16.5) | 9.3 (5.3–12.9) | 0.334 |
| Alopecia areata subtypes, n (%) | 0.607 | |||
| Patchy AA | 8 (44.4%) | 10 (50%) | 11 (47.8%) | |
| Ophiasis | 2 (11.1%) | 4 (20%) | 5 (21.7%) | |
| Alopecia totalis | 1 (5.6%) | 3 (15%) | 1 (4.3%) | |
| Alopecia universalis | 7 (38.9%) | 3 (15%) | 6 (26.1%) | |
| Initial SALT score, median (IQR),% | 55.0 (34.1–95.0) | 50.0 (32.5–87.5) | 60.0 (40.0–80.0) | 0.913 |
| SALT change, median (range),% | 97.5 (4.76–100.0) | 85.6 (0–100.0) | 92.0 (50.0–100.0) | 0.399 |
| Continuing treatment at study conclusion, n (%) | 1 (5.6%) | 11 (55%) | 14 (60.9%) | |
SCs, systemic corticosteroids; IQR, interquartile; AA, alopecia areata; SALT, Severity of Alopecia Tool.
For the combined group, 60.9% (14/23) of patients were treated initially with tofacitinib and SCs in combination. However, 26.1% (6/23) of patients initiated with SCs alone and 13.0% (3/23) of patients initiated with tofacitinib alone did not show favorable response, and they subsequently tried combination therapy. For the dosage, 56.5% (13/23) and 43.4% (10/23) of patients in the combined group were treated initially with 5 mg tofacitinib two times daily or 5 mg one time daily. All the patients in the tofacitinib group were treated with 5 mg two times daily initially. Based on the treatment responses, the dose of tofacitinib in the two groups was then adjusted to 5 or 15 mg daily. The ways of administrating SCs included: (1) intramuscular betamethasone pulse therapy for 3–4 months, followed by tapering oral prednisone, (2) oral daily prednisone from the beginning at a dosage of 15–30 mg (tapering when observed fully hair regrowth without any dermoscopic sign of disease activity), and (3) oral prednisone daily at a dosage of 15–30 mg combined with intramuscular betamethasone at a dosage of 0.3–1 ml monthly. These ways of SC administration were done for both the SCs group and the combined group. A total of 1 ml betamethasone was estimated as equivalent to a dose of 70 mg prednisone.
Efficacy
Treatment responses in each group are shown in Figure 1. The percentage of patients who achieved SALT50, SALT90, and SALT100 was not significantly different among the three groups (P = 0.423, 0.785, and 0.245, respectively). Photos of representative responders in the three groups are shown in Figure 2.
FIGURE 1.
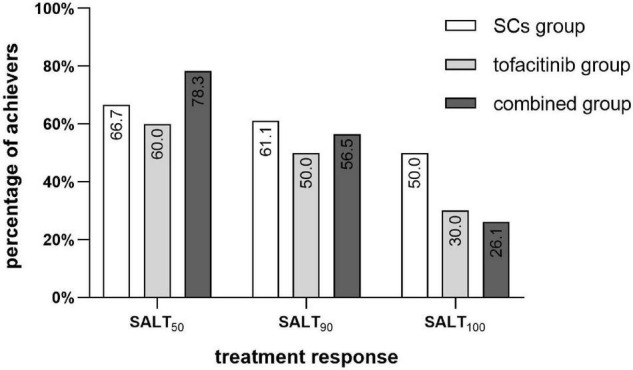
Percentage of SALT50, SALT90, and SALT100 achievers in the SCs group (n = 18), the tofacitinib group (n = 20), and the combined group (n = 23). SCs, systemic corticosteroids; SALT, Severity of Alopecia Tool.
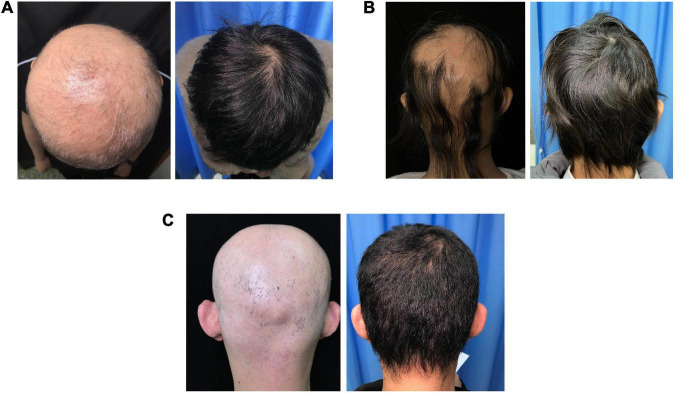
FIGURE 2.
Photographs of three representative patients in the three groups. (A) Patient in the SCs group with the SALT score of 100% prior to treatment and 0% after 18 months of treatment. (B) Patient in the tofacitinib group with the SALT score of 75% prior to treatment and 0% after 7 months of treatment. (C) Patient in the combined group with the SALT score of 100% prior to treatment and 0% after 31 months of treatment.
In the SCs group, 66.7% (12/18) of patients achieved SALT50. The median treatment duration was 6.2 months (range 3.3–15.2 months) and the median total prednisone equivalent dose was 1,105 mg (range 280–4,780 mg). 61.1% (11/18) of patients achieved SALT90 (median 5.3 months of treatment) and 50.0% of patients achieved SALT100 (median 5.1 months of treatment).
In the tofacitinib group, 60.0% (12/20) of patients achieved SALT50. The median treatment duration was 12.5 months (range 3.3–20.7 months) and the median total prednisone equivalent dose was 3,150 mg (range 900–7,800 mg). 50.0% (10/20) of patients achieved SALT90 (median 13.2 months of treatment) and 30.0% of patients achieved SALT100 (median 14.4 months of treatment).
In the combined group, 78.3% (18/23) of patients achieved SALT50. The median treatment duration was 10.4 months (range 4.1–39.0 months) with a median total prednisone equivalent dose of 880 mg (range 154–3,300 mg) and median total tofacitinib dose of 2,100 mg (range 600–11,400 mg). 56.5% of patients achieved SALT90 (median 8.0 months of treatment) and 26.1% of patients achieved SALT100 (median 6.4 months of treatment).
In the SCs group, the ratio of SALT50 achievers was significantly higher in patients with a short duration of current hair loss episode (≤2 years) than in those with a duration of current hair loss episode (>2 years) (90.9 vs. 28.6%, P = 0.006). Similarly, the ratio of SALT50 achievers was significantly higher in patients with a shorter duration of current hair loss episode in the tofacitinib group (100.0 in ≤ 2 years subgroup vs. 33.3% in > 2 years subgroup, P = 0.003) and in the combined group (100.0 vs. 58.3%, P = 0.016).
In addition, for patients who had more than 10 years of current episode of scalp hair loss, there were 0 (0/4), 75.0 (3/4), and 66.7% (2/3) of patients in the SCs group, the tofacitinib group, and the combined group that achieved SALT50, respectively.
For patients with AT and AU, there were 62.5 (5/8), 60.0 (3/5), and 100% (7/7) of patients in the SCs group, the tofacitinib group, and the combined group that achieved SALT50, respectively (Figure 3). For patients with ophiasis, there were 50.0 (1/2), 0 (0/4), and 40.0% (2/5) of patients in the SCs group, the tofacitinib group, and the combined group that achieved SALT50, respectively.
FIGURE 3.
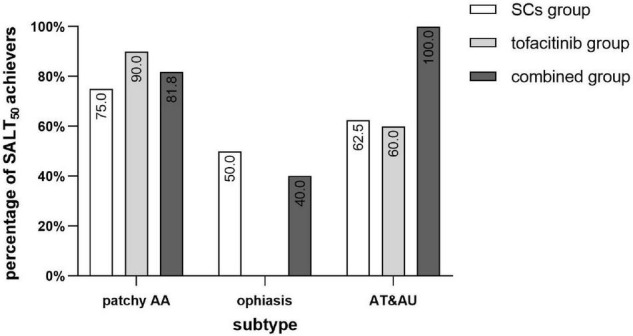
Percentage of SALT50 achievers of different subtypes of AA in the three groups. SCs, systemic corticosteroids; SALT, Severity of Alopecia Tool; AA, alopecia areata; AT, alopecia totalis; AU, alopecia universalis.
In the combined group, 80.0% (4/5) of patients who achieved SALT50 and discontinued the treatment experienced recurrence after a mean duration of 3.3 months. In the tofacitinib group, 66.7% (2/3) of patients who achieved SALT50 had completely stopped the treatment and recurred after a mean duration of 2 months. In the SCs group, 60.0% (6/10) of patients who achieved SALT50 showed recurrence after stopping the treatment in a mean duration of 2 months.
Safety
The adverse effects (AEs) are given in Table 2. All of the AEs were mild in the three groups and none of the patients discontinued the treatments because of the AEs. The proportions of patients who experienced AEs were not significantly different between the three groups (P = 0.132).
TABLE 2.
AEs observed in each group*.
| SCs group (n = 18) | Tofacitinib group (n = 20)a, c | Combined group (n = 23)c | |
| Patients who experienced AEs, n(%) | 12 (66.7%) | 7 (35.0%) | 13 (56.5%) |
| Infections (n) | Acne (3) | Acneiform eruption (4)b | Folliculitis (4) |
| Zoster (1) | Acneiform eruption (6)b | ||
| Wart (1) | |||
| Upper respiratory infection (2) | |||
| Other AEs (n) | Weight gain (8) | Fatigue (1) | Liver enzyme abnormalities (2) |
| Moon facies (2) | Hyperlipidemia (2) | Hyperlipidemia (2) | |
| Arthralgia (1) | Arthralgia (1) | Hirsutism (3) | |
| Hirsutism (1) | Weight gain (5) | ||
| Headache (1) | Proteinuria (1) | ||
| Menstrual irregularity (1) | Fatigue (1) | ||
| Hypertension (1) | Moon facies (1) | ||
| Sleep disturbance (1) | Menstrual irregularity (1) | ||
| Frequent urination (1) |
*The proportions of patients who experienced AEs were not significantly different between the three groups (P = 0.132).
aOne patient was diagnosed with asymptomatic liver enzyme elevation before treatment, which didn’t increase during the therapy.
bPatients who experienced acneiform eruption mostly performed as whitehead.
cTwo patients in the combined group and the tofacitinib group were diagnosed with hepatitis before the treatment. Both of them experienced elevated HBV DNA during the treatment without any liver enzyme abnormalities and liver symptoms, one of whom is taking anti-virus medication for now.
SCs, systemic corticosteroids; AEs, adverse effects.
In the SCs group, 66.7% (12/18) of patients experienced AEs, including weight gain (8), acne (3), moon facies (2), hirsutism (1), arthralgia (1), headache (1), menstrual irregularity (1), hypertension (1), sleep disturbance (1), and frequent urination (1).
In the tofacitinib group, 35.0% (7/20) of patients showed AEs, including acneiform eruption (4), recurrent herpes zoster (1), fatigue (1), hyperlipidemia (2), and arthralgia (1). One patient in the tofacitinib group was diagnosed with asymptomatic liver enzyme elevation before treatment, which did not increase during the therapy with tofacitinib.
In the combined group, 56.5% (13/23) of patients showed AEs. Folliculitis (4) and acneiform eruption (6) were the most common infectious AEs, followed by upper respiratory infection (URI) (2) and wart (1). Weight gain (5), hirsutism (3), hyperlipidemia (2), liver enzyme abnormalities (2), proteinuria (1), fatigue (1), and moon facies (1) were also reported in the combined group of patients. It is worth mentioning that two patients in the combined group and the tofacitinib group had been diagnosed with hepatitis B before treatment, and after being refused tofacitinib by our dermatologists, they started the treatment by themselves with illegally imported tofacitinib for 39 and 16.6 months. One of the two patients had been taking antiviral medication and both of them experienced elevated hepatitis B virus (HBV) DNA levels during the treatment, without liver enzyme abnormalities and liver symptoms.
Discussion
To date, despite the numerous options that have been proposed for systemic treatment of AA, the majority of them are of limited efficacy with a high risk of side effects and high recurrence rates, especially for patients with severe AA. Previous studies have shown favorable results of SCs in patients with AA, but a lower response rate in ophiasis and AU (15, 16). Additionally, the response may not be maintained and the majority of patients will relapse within 4–9 weeks after discontinuing SCs treatment (2), which is consistent with our findings.
Some studies and case series have demonstrated promising efficacy of tofacitinib as a monotherapy of 10 mg/day or higher doses in adult patients with severe AA (11, 17, 18). In a clinical trial of 66 adults with severe AA treated with 10 mg of tofacitinib daily, it was shown that SALT50 was achieved in 32.0% of patients after 3 months (11). In a retrospective study of 18 patients with refractory AT or AU, 44.4% of patients achieved SALT50 with a 10–15 mg daily dose of tofacitinib after 6 months (17). In this study, patients in the tofacitinib group presented slightly higher efficacy (60.0% of patients achieved SALT50). It was probably due to that, moderate AA was included and treatment duration was longer in this study.
Combined treatment showed probably a higher response rate in SALT50 than monotherapy of tofacitinib or SCs; however, they did not achieve any statistical significance. Studies with a larger sample size are needed to clarify this. Interestingly, our limited data did not show higher SALT90 and SALT100 in combined treatment than in monotherapy.
Some previous reports indicated that patients resistant to SCs may respond to tofacitinib (17, 19, 20). Meanwhile, it has been reported that some patients that resistant to tofacitinib monotherapy could respond to combination therapy of tofacitinib and prednisone (13). Notably, in the combined group of this study, 26.1% (6/23) of patients and 13.0% (3/23) of patients who did not show significant hair regrowth when receiving monotherapy of SCs or tofacitinib, respectively, achieved SALT50 after receiving combined therapy. This implicated that the immunological pathogenesis of AA is still far from clear.
It is worth noting that 100% of patients with AT and AU achieved SALT50 in the combined group, while only about 60% of patients with AT and AU achieved SALT50 in the SCs or tofacitinib monotherapy group. It was considered that the larger the area of hair loss, the poorer the treatment response, especially in AT and AU (21). In patients with any monotherapy, patchy AA responded better than AT and AU; however, in the combined group, patients with AT and AU seemed to show a better treatment response than patchy AA. Ophiasis is a special subtype of AA associated with poor prognosis and reduced efficacy to traditional systemic treatment (2). In a previous clinical trial, three patients with ophiasis who were treated with tofacitinib for 3 months showed a median SALT improvement of 68% and were more responsive than patients with AT and AU (11). For ophiasis in this study, though 50% in the SCs group and 40% in the combined group achieved SALT50, no patients in the tofacitinib group showed favorable efficacy. Interestingly, patients with AT and AU showed more favorable responses than patients with ophiasis in all the three different treatment groups.
Duration of current episode of hair loss has been recognized as a probable clinical indicator to predict treatment response in some previous reports (12). In this study, we found that the efficacy of patients with AA who had more than 2 years of current episode of disease in all the three groups was significantly poorer compared with patients who had less than 2 years of current episode of disease. Interestingly, for patients with more than 10 years of duration of current episode of disease, there was no patient in the SCs group who achieved SALT50, while there were 75.0 and 66.7% of patients in the tofacitinib group and the combined group who achieved SALT50, respectively. It seemed that tofacitinib with or without SCs might be a better choice than SCs alone for moderate or severe patients with AA with long disease duration.
Relapse occurred after cessation of therapy in monotherapy of tofacitinib or SCs or their combination, which indicated that maintenance therapy might be necessary for obtaining continued remission.
Though different application strategies of SCs have shown fair efficacy in severe AA, high rates of AEs have restricted the long-term application of SCs (2, 3). In a placebo-controlled trial, 23 patients with severe AA were treated with weekly oral prednisolone pulse therapy (200 mg). AEs were seen in 55.0% of patients after 3 months of treatment and 3 months of follow-up, including general weakness, acneiform eruption, weight gain, facial mooning, and gastrointestinal upset (22). In this study, though no serious AEs were recorded, patients in the SCs monotherapy group also showed the highest rate of AEs (66.7%) among the three groups.
The AEs occurred in patients with tofacitinib monotherapy seemed to be mild, self-limited, and more acceptable for patients than SCs. Severe AEs reported in tofacitinib treatment, including severe infections, venous thromboembolic events, and malignancy, were not reported in our study. The most frequently reported AE of tofacitinib is the increased risk of infection (23), such as nasopharyngitis and URI (24). Interestingly, in this study, only two patient in the combined group experienced URI, while none in the monotherapy group with SCs or tofacitinib experienced URI. One possible reason for the low incidence of URI might be the increasing use of facial masks for preventing COVID-19 in China since January 2020. In our experience, patients who are undergoing treatments of immunosuppressant and/or systemic corticosteroids should be encouraged to wear a face mask in assembly occupancies for preventing URI. The incidence of acneiform eruption in the tofacitinib group and the combined group was remarkably higher than it was reported in previous studies. In a retrospective study, no HBV reactivation was observed in four patients with rheumatoid arthritis with resolved HBV infection treated with tofacitinib within 3 years of follow-up (25). It is worth noting that two patients with hepatitis B infection were included in the combined group and the tofacitinib group, one of whom accepted antiviral medication. Both of them experienced elevated HBV DNA levels during the treatment, without liver enzyme abnormalities and liver symptoms. Tofacitinib appears safe in patients with resolved HBV infection (26) and reactivation of HBV infection could be prevented by antiviral prophylaxis. However, it is important to beware of the high incidence rate of HBV reactivation in patients with HBsAg + receiving tofacitinib and close monitoring of HBV DNA and alanine aminotransferase should be suggested (27). In clinical trials of tofacitinib for rheumatoid arthritis, no new AEs were observed in 10 years (28). However, concerns still exist regarding its long-term safety profile, and longer observational periods are still needed.
Tofacitinib is a non-specific JAK inhibitor, and it can target more than one single JAK molecule and may lead to a relatively broad immune suppression (29). More selective JAKi are expected to increase efficacy and improve safety in the future. Topical JAKi are currently under investigation in patients with AA for the sake of minimizing the risk of systemic side effects, especially for the long-term treatment (30). However, the efficacy of topical JAKi has not yet been shown as encouraging as oral tofacitinib for severe AA (24).
In summary, tofacitinib was an effective treatment for patients with moderate-to-severe AA and it was more tolerated than corticosteroids. Oral tofacitinib combined with SCs seems to have higher efficacy with lower dosage and less AEs than SCs alone. Efficacy decreased in patients with current episode of disease for more than 2 years, regardless of treatments with oral tofacitinib, SCs, or their combination.
Limitation
An intrinsic limitation of this study is that it is a retrospective study. Though we had tried to minimize the differences in baseline characteristics of the groups, there was still difference in duration of disease among the three groups, which had the potential to impact the results biaswise. The SALT scores in some follow-ups during the treatment were missing; therefore, the association of the SALT score changes and treatment duration were not evaluated in this study. The sample size of this study is relatively small, and significant difference in the efficacy was not identified among the three treatment groups. Studies with larger sample sizes will still be needed in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
CZ and WZ contributed to the conception and design of this study, collected the data, and wrote and edited the manuscript. WZ and XL performed the statistical analysis. CZ reviewed the literature. KT-C edited the manuscript. All authors have contributed to the article and approved the submitted version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82073459).
References
- 1.Pratt CH, King LE, Jr, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. (2017) 3:17011. 10.1038/nrdp.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. (2018) 78:15–24. 10.1016/j.jaad.2017.04.1142 [DOI] [PubMed] [Google Scholar]
- 3.Zhou C, Li X, Wang C, Zhang J. Alopecia areata: an update on etiopathogenesis, diagnosis, and management. Clin Rev Allergy Immunol. (2021) 61:403–23. 10.1007/s12016-021-08883-0 [DOI] [PubMed] [Google Scholar]
- 4.Bertolini M, McElwee K, Gilhar A, Bulfone-Paus S, Paus R. Hair follicle immune privilege and its collapse in alopecia areata. Exp Dermatol. (2020) 29:703–25. 10.1111/exd.14155 [DOI] [PubMed] [Google Scholar]
- 5.Gilhar A, Laufer-Britva R, Keren A, Paus R. Frontiers in alopecia areata pathobiology research. J Allergy Clin Immunol. (2019) 144:1478–89. 10.1016/j.jaci.2019.08.035 [DOI] [PubMed] [Google Scholar]
- 6.Simakou T, Butcher JP, Reid S, Henriquez FL. Alopecia areata: a multifactorial autoimmune condition. J Autoimmun. (2019) 98:74–85. 10.1016/j.jaut.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. (2018) 78:1–12. 10.1016/j.jaad.2017.04.1141 [DOI] [PubMed] [Google Scholar]
- 8.Pourang A, Mesinkovska NA. New and emerging therapies for alopecia areata. Drugs. (2020) 80:635–46. 10.1007/s40265-020-01293-0 [DOI] [PubMed] [Google Scholar]
- 9.Ramot Y, Zlotogorski A. [Jak inhibitors for the treatment of alopecia areata]. Harefuah. (2020) 159:38–42. [PubMed] [Google Scholar]
- 10.Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. (2014) 134:2988–90. 10.1038/jid.2014.260 [DOI] [PubMed] [Google Scholar]
- 11.Kennedy Crispin M, Ko JM, Craiglow BG, Li S, Shankar G, Urban JR, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. (2016) 1:e89776. 10.1172/jci.insight.89776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park HS, Kim MW, Lee JS, Yoon HS, Huh CH, Kwon O, et al. Oral tofacitinib monotherapy in Korean patients with refractory moderate-to-severe alopecia areata: a case series. J Am Acad Dermatol. (2017) 77:978–80. 10.1016/j.jaad.2017.06.027 [DOI] [PubMed] [Google Scholar]
- 13.Liu LY, Craiglow BG, Dai F, King BA. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. (2017) 76:22–8. 10.1016/j.jaad.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 14.Olsen E, Hordinsky M, McDonald-Hull S, Price V, Roberts J, Shapiro J, et al. Alopecia areata investigational assessment guidelines. National alopecia areata foundation. J Am Acad Dermatol. (1999) 40(2 Pt 1):242–6. 10.1016/s0190-9622(99)70195-7 [DOI] [PubMed] [Google Scholar]
- 15.Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part II. Treatment. J Am Acad Dermatol. (2010) 62:191–202; quiz 3–4. 10.1016/j.jaad.2009.10.031 [DOI] [PubMed] [Google Scholar]
- 16.Friedli A, Labarthe MP, Engelhardt E, Feldmann R, Salomon D, Saurat JH. Pulse methylprednisolone therapy for severe alopecia areata: an open prospective study of 45 patients. J Am Acad Dermatol. (1998) 39(4 Pt 1):597–602. 10.1016/s0190-9622(98)70009-x [DOI] [PubMed] [Google Scholar]
- 17.Shin JW, Huh CH, Kim MW, Lee JS, Kwon O, Cho S, et al. Comparison of the treatment outcome of oral tofacitinib with other conventional therapies in refractory alopecia totalis and universalis: a retrospective study. Acta Derm Venereol. (2019) 99:41–6. 10.2340/00015555-3057 [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim O, Bayart CB, Hogan S, Piliang M, Bergfeld WF. Treatment of alopecia areata with tofacitinib. JAMA Dermatol. (2017) 153:600–2. 10.1001/jamadermatol.2017.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta AK, Carviel JL, Abramovits W. Efficacy of tofacitinib in treatment of alopecia universalis in two patients. J Eur Acad Dermatol Venereol. (2016) 30:1373–8. 10.1111/jdv.13598 [DOI] [PubMed] [Google Scholar]
- 20.Scheinberg M, de Lucena Couto Ocea RA, Cruz BA, Ferreira SB. Brazilian experience of the treatment of alopecia universalis with the novel antirheumatic therapy tofacitinib: a case series. Rheumatol Ther. (2017) 4:503–8. 10.1007/s40744-017-0069-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. (2015) 61:751–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Kar BR, Handa S, Dogra S, Kumar B. Placebo-controlled oral pulse prednisolone therapy in alopecia areata. J Am Acad Dermatol. (2005) 52:287–90. 10.1016/j.jaad.2004.10.873 [DOI] [PubMed] [Google Scholar]
- 23.Phan K, Sebaratnam DF. JAK inhibitors for alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. (2019) 33:850–6. 10.1111/jdv.15489 [DOI] [PubMed] [Google Scholar]
- 24.Ismail FF, Sinclair R. JAK inhibition in the treatment of alopecia areata – a promising new dawn? Expert Rev Clin Pharmacol. (2020) 13:43–51. 10.1080/17512433.2020.1702878 [DOI] [PubMed] [Google Scholar]
- 25.Serling–Boyd N, Mohareb AM, Kim AY, Hyle EP, Wallace ZS. The use of tocilizumab and tofacitinib in patients with resolved hepatitis b infection: a case series. Ann Rheum Dis. (2021) 80:274–6. 10.1136/annrheumdis-2020-218289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YM, Huang WN, Wu YD, Lin CT, Chen YH, Chen DY, et al. Reactivation of hepatitis B virus infection in patients with rheumatoid arthritis receiving tofacitinib: a real-world study. Ann Rheum Dis. (2018) 77:780–2. 10.1136/annrheumdis-2017-211322 [DOI] [PubMed] [Google Scholar]
- 27.Wang ST, Tseng CW, Hsu CW, Tung CH, Huang KY, Lu MC, et al. Reactivation of hepatitis B virus infection in patients with rheumatoid arthritis receiving tofacitinib. Int J Rheum Dis. (2021) 24:1362–9. 10.1111/1756-185X.14217 [DOI] [PubMed] [Google Scholar]
- 28.Alvaro-Gracia JM, Garcia-Llorente JF, Valderrama M, Gomez S, Montoro M. Update on the safety profile of tofacitinib in rheumatoid arthritis from clinical trials to real-world studies: a narrative review. Rheumatol Ther. (2021) 8:17–40. 10.1007/s40744-020-00258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. (2019) 10:2847. 10.3389/fimmu.2019.02847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. (2017) 76:736–44. 10.1016/j.jaad.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.