Abstract
Background
There is an unmet need for COVID-19 prevention in patient populations who have not mounted or are not expected to mount an adequate immune response to complete COVID-19 vaccination. We previously reported that a single subcutaneous 1200 mg dose of the monoclonal antibody combination casirivimab and imdevimab (CAS + IMD) prevented symptomatic SARS-CoV-2 infections by 81·4% in generally healthy household contacts of SARS-CoV-2-infected individuals over a 1-month efficacy assessment period. Here we present additional results, including the 7-month follow-up period (months 2–8), providing additional insights about the potential for efficacy in pre-exposure prophylaxis settings.
Methods
This was a randomised, double-blind, placebo-controlled trial done in the USA, Romania, and Moldova in 2020–2021, before the emergence of omicron (B.1.1.529) and omicron-lineage variants. Uninfected and unvaccinated household contacts of infected individuals, judged by the investigator to be in good health, were randomly assigned (1:1) to receive 1200 mg CAS + IMD or placebo by subcutaneous injection according to a central randomisation scheme provided by an interactive web response system; randomisation was stratified per site by the test results of a local diagnostic assay for SARS-CoV-2 and age group at baseline. COVID-19 vaccines were prohibited before randomisation, but participants were allowed to receive COVID-19 vaccination during the follow-up period. Participants who developed COVID-19 symptoms during the follow-up period underwent RT-PCR testing. Prespecified endpoints included the proportion of previously uninfected and baseline-seronegative participants (seronegative-modified full analysis set) who had RT-PCR-confirmed COVID-19 in the follow-up period (post-hoc for the timepoints of months 2–5 and 6–8 only) and underwent seroconversion (ie, became seropositive, considered a proxy for any SARS-CoV-2 infections [symptomatic and asymptomatic]; prespecified up to day 57, post-hoc for all timepoints thereafter). We also assessed the incidence of treatment-emergent adverse events. This study is registered with ClinicalTrials.gov, NCT04452318.
Findings
From July 13, 2020, to Oct 4, 2021, 2317 participants who were RT-PCR-negative for SARS-CoV-2 were randomly assigned, of whom 1683 (841 assigned to CAS + IMD and 842 assigned to placebo) were seronegative at baseline. During the entirety of the 8-month study, CAS + IMD reduced the risk of COVID-19 by 81·2% (nominal p<0·0001) versus placebo (prespecified analysis). During the 7-month follow-up period, protection was greatest during months 2–5, with a 100% relative risk reduction in COVID-19 (nominal p<0·0001; post-hoc analysis). Efficacy waned during months 6–8 (post-hoc analysis). Seroconversion occurred in 38 (4·5%) of 841 participants in the CAS + IMD group and in 181 (21·5%) of 842 in the placebo group during the 8-month study (79·0% relative risk reduction vs placebo; nominal p<0·0001). Six participants in the placebo group were hospitalised due to COVID-19 versus none who received CAS + IMD. Serious treatment-emergent adverse events (including COVID-19) were reported in 24 (1·7%) of 1439 participants receiving CAS + IMD and in 23 (1·6%) of 1428 receiving placebo. Five deaths were reported, none of which were due to COVID-19 or related to the study drugs.
Interpretation
CAS + IMD is not authorised in any US region as of Jan 24, 2022, because data show that CAS + IMD is not active against omicron-lineage variants. In this study, done before the emergence of omicron-lineage variants, a single subcutaneous 1200 mg dose of CAS + IMD protected against COVID-19 for up to 5 months of community exposure to susceptible strains of SARS-CoV-2 in the pre-exposure prophylaxis setting, in addition to the post-exposure prophylaxis setting that was previously shown.
Funding
Regeneron Pharmaceuticals, F Hoffmann-La Roche, US National Institute of Allergy and Infectious Diseases, US National Institutes of Health.
Research in context.
Evidence before this study
Monoclonal antibodies are being developed for the treatment and prevention of COVID-19. In patient populations who have not mounted or are not expected to mount an adequate immune response to COVID-19 vaccination, monoclonal antibodies can offer an important alternate or complementary prevention strategy. Casirivimab and imdevimab (CAS + IMD) is a combination of two neutralising monoclonal antibodies, administered together, that bind non-overlapping epitopes of the SARS-CoV-2 spike protein receptor binding domain. We searched PubMed for clinical trials of anti-SARS-CoV-2 monoclonal antibodies used for the prevention of COVID-19 published between study initiation (July 13, 2020) and study completion (Oct 4, 2021) using various combinations of the terms “COVID-19”, “SARS-CoV-2”, “monoclonal antibody”, “prophylaxis”, and “prevention.” We then filtered for the article type “clinical trial.” No language restrictions were applied. Two published trials were identified. The first trial (BLAZE-2) reported the efficacy and safety of bamlanivimab for COVID-19 prevention in residents and staff of skilled nursing and assisted living facilities over an 8-week period. The second, the report of the primary analysis of this trial, showed that a single 1200 mg dose of CAS + IMD administered subcutaneously was effective in preventing both symptomatic SARS-CoV-2 infections and any SARS-CoV-2 infections (symptomatic or asymptomatic), in household contacts of individuals with a SARS-CoV-2 infection in a high-risk transmission setting over a 1-month efficacy assessment period. After the primary analysis of the study, a question remained as to the duration of protection provided by a single 1200 mg dose of CAS + IMD.
Added value of this study
Herein, we address and further extend the findings of the previous report by assessing the rates of COVID-19 and SARS-CoV-2 infection through months 2–8 of the follow-up period, after the risks of household transmission had subsided. In post-hoc analyses, the data reveal compelling efficacy in prevention (100% relative risk reduction in COVID-19) for months 2–5, the 4-month period following the initial efficacy assessment during which study participants had ongoing community exposure to SARS-CoV-2. Efficacy waned during months 6–8. Overall, we observed an 81·2% relative risk reduction in developing COVID-19 over the entirety of the 8-month study, after receiving a single dose of CAS + IMD, compared with placebo. Additionally, because regularly scheduled surveillance RT-PCR testing was not done after week 4 of the study, we used seroconversion as a proxy for the occurrence of any SARS-CoV-2 infection. Seroconversion occurred in 38 (4·5%) of 841 participants in the CAS + IMD group and in 181 (21·5%) of 842 in the placebo group (79·0% relative risk reduction vs placebo). Furthermore, we estimated antibody concentrations and neutralising titres in serum throughout the follow-up period, providing insights about the concentrations of CAS + IMD associated with efficacy in prevention. During the entire study, none of the individuals in the CAS + IMD group were hospitalised due to COVID-19, versus six individuals in the placebo group. Of the five deaths reported during 8-month study, none were due to COVID-19 or the study drugs. These study results are applicable to SARS-CoV-2 strains that are susceptible to CAS + IMD.
Implications of all the available evidence
This 8-month study, done in 2020–21, showed the ability of a single 1200-mg subcutaneous dose of CAS + IMD to provide extended protection (approximately 5 months after a single dose) against susceptible strains of SARS-CoV-2. Estimated drug concentrations and neutralising titres in serum during the follow-up period provide initial insights about potential correlates of antibody-related immunity. This study, combined with the large database of exposure, shows that CAS + IMD is generally well tolerated. These data could be relevant to highly vulnerable populations that are immunocompromised and cannot mount an immune response against SARS-CoV-2 after natural infection or by vaccination, provided that strains of SARS-CoV-2 susceptible to the combination used are in circulation.
Introduction
COVID-19, caused by SARS-CoV-2, is now a worldwide pandemic with potential to transition to an epidemic or endemic disease.1, 2, 3, 4 Although vaccines are now available and recommended for the prevention of COVID-19,5 a substantial unmet need remains for prevention in patients who have not mounted or are not expected to mount an adequate immune response to complete COVID-19 vaccination.6 Approximately 3% of the US population is estimated to be moderately or severely immunocompromised,7 and approximately 22% of the global population is estimated to have at least one underlying condition putting them at increased risk of severe COVID-19,8 accounting for many individuals who might be unprotected by vaccination and at greatest risk of severe COVID-19 if infected. Administration of preformed neutralising antibodies to SARS-CoV-2 for pre-exposure prophylaxis has recently been authorised for emergency use,9 and additional passive immunotherapies are needed for COVID-19 prevention in vulnerable populations.
Casirivimab and imdevimab (CAS + IMD) is a combination of two neutralising monoclonal antibodies, administered together, that bind non-overlapping epitopes of the SARS-CoV-2 spike protein receptor binding domain. CAS + IMD was previously authorised for treatment and post-exposure prophylaxis of COVID-19 in some settings in the USA and for treatment or prevention of COVID-19 in other jurisdictions.10, 11, 12, 13, 14 Because of the high prevalence of the omicron (B.1.1.529) and omicron-lineage variants, and because data show that CAS + IMD is not active against this lineage of variants, this treatment is no longer authorised in any US region as of Jan 24, 2022.15
We previously reported that a single 1200 mg dose of CAS + IMD administered subcutaneously prevented symptomatic SARS-CoV-2 infection, with a relative risk reduction of 81·4%, in household contacts of individuals infected with SARS-CoV-2 in a high-risk transmission setting over a 1-month (28 days) efficacy assessment period (EAP).16 Because the median time from exposure to onset of symptomatic infection is approximately 4–5 days,17, 18 prevention of infections occurring in the first week (71·9% relative risk reduction with CAS + IMD vs placebo16) is considered a reasonable surrogate of post-exposure prophylaxis.19 After the incubation period of the initial close-contact SARS-CoV-2 exposure, during weeks 2–4, there was a 92·6% relative risk reduction in symptomatic SARS-CoV-2 infection with CAS + IMD versus placebo.16 Here, we present the results of the 7-month follow-up period of this trial (months 2–8).
Methods
Study design
The design of this randomised, double-blind, placebo-controlled, phase 3 trial assessing the efficacy and safety of subcutaneously administered CAS + IMD in preventing SARS-CoV-2 infections among uninfected household contacts of infected individuals (part A) was previously published.16 The trial was conducted at 112 sites in the USA, Romania, and Moldova. The study was initiated on July 13, 2020, and was completed on Oct 4, 2021 (before the emergence of omicron-lineage variants). The trial consisted of a screening–baseline period, a 28-day EAP, and a 7-month follow-up period (appendix p 21). Here, we describe the results of the entirety of the study in part A participants, including the follow-up period (months 2–8).
The trial was done in accordance with the principles of the Declaration of Helsinki, the International Council for Harmonisation Good Clinical Practice guidelines, and all applicable regulatory requirements. The central or local institutional review board or ethics committee at each study centre oversaw trial conduct and documentation.
A Data and Safety Monitoring Board convened by the US National Institutes of Health (NIH) evaluated safety data to make recommendations for trial modification or termination.
Participants
As previously described,16 asymptomatic, unvaccinated, and healthy adult (≥18 years) and adolescent (≥12 to <18 years) household contacts of the first known household member with SARS-CoV-2 infection (index case) were eligible to participate if they anticipated living with the index case for 28 or more days. Recruitment was done through several approaches, including the use of social media and websites, physician-to-physician letters, flyers, and participant-to-participant referrals. Participants were randomly assigned within 96 h of collection of the index case's positive SARS-CoV-2 diagnostic test sample. Individuals with previous SARS-CoV-2 infection were excluded from the analysis. Although COVID-19 vaccines were prohibited before randomisation, participants were allowed to receive COVID-19 vaccination during the follow-up period (after the 28-day EAP), and they were recommended to follow US Centers for Disease Control and Prevention (CDC) guidance at the time of study conduct, which was to receive the vaccine 90 or more days after receipt of monoclonal antibodies. All participants provided written informed consent before participating in the trial.
Randomisation and masking
On day 1, during the screening–baseline period, study participants were randomly assigned in a 1:1 allocation ratio to receive a single dose of CAS + IMD 1200 mg or placebo according to Parexel International's proprietary central randomisation scheme provided by an interactive web response system. On site, an unmasked pharmacist or qualified designee entered the participant's information in the interactive web response system. This system was programmed to assign treatment allocation and provide the kit numbers assigned to the unmasked team. The unmasked site team, including the pharmacist or qualified designee, had no other involvement in the trial. At the time of randomisation, participants were stratified per site according to results of the local diagnostic assay for SARS-CoV-2 (positive, negative, or undetermined), if available, and according to age group (≥12 to <18 years, ≥18 to <50 years, or ≥50 years) at baseline. The investigators, site personnel, COVID-19 Prevention Network (CoVPN) and National Institute of Allergy and Infectious Diseases (NIAID), and Regeneron Pharmaceuticals were masked to treatment-group assignments. The study drug preparation was done by the unmasked pharmacist or qualified designee. The syringes were wrapped with orange, transparent cellophane and labelled in a blinded manner before being provided to the study team for drug administration. Participants and investigators remained fully masked until the final clinical study report was published.
Procedures
At baseline (day 1), participants received 1200 mg CAS + IMD (600 mg each of casirivimab and imdevimab) or placebo through subcutaneous injection. After the 28-day EAP with weekly assessments, participants were assessed monthly during the follow-up period (days 30–225). At each scheduled visit during the follow-up period, the investigator assessed the participant's general health, adverse events, and signs and symptoms associated with SARS-CoV-2 infection since the last contact. If a participant had a positive RT-PCR SARS-CoV-2 test during the EAP, they could enter the follow-up period but continued to have weekly nasopharyngeal swabs for SARS-CoV-2 RT-PCR testing until two confirmed negative results were obtained 24 h or more apart. During the follow-up period, participants who were symptomatic were instructed to contact the investigator if symptoms occurred between visits, at which time they underwent assessment and central laboratory RT-PCR testing; if the participant was unable to have a central laboratory RT-PCR, local laboratory molecular testing was used. No scheduled surveillance SARS-CoV-2 RT-PCR testing was done during the follow-up period.
Samples for serology were collected at baseline to assess previous infection and immunity to SARS-CoV-2; serological testing included anti-spike S1 IgA (Euroimmun, Lübeck, Germany), anti-spike S1 IgG (Euroimmun), and anti-nucleocapsid IgG (Abbott, Des Plaines, IL, USA) as previously described.20 A participant was categorised as seronegative at baseline if all available serological tests were negative and was categorised as seropositive at baseline if any serological test was positive. On days 29, 57, 225 (end of study), and at the study discontinuation visit, anti-nucleocapsid IgG (Abbott) serological testing was done. Seroconversion was defined as a post-baseline positive anti-nucleocapsid IgG test one or more times during the study among participants who were initially seronegative. The presence or absence of anti-spike antibodies was not assessed post-baseline, as anti-spike serological assays would be confounded by COVID-19 vaccines and CAS + IMD administration.
Blood samples for drug concentration were collected for the first 400 participants, with more frequent (dense) sampling in the first 30 participants and less frequent (sparse) sampling in participants 31 through 400.
Outcomes
The part A primary efficacy analysis population presented in this report consisted of all enrolled participants without evidence of previous infection (RT-PCR negative and seronegative) through to the last participant visit on Oct 4, 2021, excluding participants from an initial descriptive assessment, as previously described.16 The primary and key secondary endpoints for this study have been previously described.16 The prespecified endpoints for the 7-month follow-up period included the following: the proportion of baseline-seronegative participants who had a first RT-PCR-confirmed symptomatic SARS-CoV-2 infection in the follow-up period (post-hoc for the timepoints of months 2–5 and 6–8 only); the proportion of participants with SARS-CoV-2 seroconversion from baseline-seronegative to seropositive, as assessed by an anti-nucleocapsid IgG assay (prespecified up to day 57, post-hoc for all timepoints thereafter); and the proportion of baseline-seronegative participants who had a first RT-PCR-confirmed SARS-CoV-2 infection (symptomatic or asymptomatic) in the follow-up period (post-hoc for the timepoints of months 2–5 and 6–8 only). Additional post-hoc endpoints during the follow-up period included the number of medically attended visits (MAVs) related to SARS-CoV-2—defined as hospitalisation, emergency room visits, or visits at an urgent care centre—and the number of symptomatic SARS-CoV-2 infections after COVID-19 vaccination.
Safety endpoints included treatment-emergent adverse events (TEAEs) and adverse events of special interest (grade ≥3 hypersensitivity and grade ≥3 injection-site reactions) in all participants (seronegative and seropositive) during the entirety of the study.
Pharmacokinetics and neutralisation titres
To place the drug concentrations of CAS + IMD into a SARS-CoV-2 immunity context, we calculated the ability of the antibodies to neutralise SARS-CoV-2 at a given systemic concentration, expressed as a 50% neutralisation titre, using concentration versus half-maximal neutralising concentration (IC50) derived from neutralisation curves from an in-vitro assay that measured neutralisation of spike-protein-containing pseudovirus, as described in the appendix (p 19). Additional methods for pharmacokinetic analyses are described in the appendix (pp 18–19).
Statistical analysis
The sample size calculation for this study is described in the appendix (p 17). The seronegative modified full analysis set used for efficacy analyses included all randomly assigned participants aged 12 years or older who were confirmed by central laboratory testing to be negative for both SARS-CoV-2 by PCR and serology at baseline, excluding participants from the initial descriptive assessment, as previously described.16 The safety population included all enrolled part A participants, irrespective of baseline serostatus, through to the last visit on Oct 4, 2021, including individuals from the initial descriptive assessment, as previously described.16
We analysed the proportion of baseline-seronegative participants with a first RT-PCR-confirmed SARS-CoV-2 infection during each period using logistic regression. Although this endpoint was prespecified, analyses of the specific periods of months 2–5 and months 6–8 are considered post-hoc. The model included fixed category effects of treatment group (placebo vs CAS + IMD), region (USA vs not USA), and age (≥12 to <50 years vs ≥50 years of age). All p values presented are nominal. For the period with sparse RT-PCR-confirmed SARS-CoV-2 infection data, we used Fisher's exact test without stratifying by region and age group. All analyses were done with SAS, version 9.4.
This trial is registered with ClinicalTrials.gov, NCT04452318.
Role of the funding source
This study was supported by Regeneron Pharmaceuticals, and F Hoffmann-La Roche. This trial was conducted jointly with NIAID and NIH. CoVPN is supported by cooperative agreement awards from NIAID and NIH. Regeneron Pharmaceuticals designed the trial in collaboration with CoVPN and NIAID and gathered the data with the trial investigators. Regeneron Pharmaceuticals analysed the data. Manuscript authors affiliated with Regeneron Pharmaceuticals and CoVPN and NIAID also contributed to data interpretation and writing of this report.
Results
The previous primary efficacy publication included participants who were RT-PCR-negative for SARS-CoV-2 randomly assigned as of Jan 28, 2021, with a data cutoff date of March 11, 2021.16 This analysis includes all enrolled participants who were RT-PCR-negative for SARS-CoV-2, including those randomly assigned after Jan 28, 2021, with the last participant visit on Oct 4, 2021. The presented efficacy dataset includes 2317 randomly assigned participants who were RT-PCR-negative for SARS-CoV-2, of whom 1683 participants (72·6%) also showed no evidence of previous SARS-CoV-2 infection by serology testing (seronegative at baseline). These 1683 participants, without evidence of previous or ongoing infection (primary efficacy analysis population), were assigned to receive CAS + IMD (n=841) or placebo (n=842; appendix p 22).
Among the 1683 participants who were seronegative or RT-PCR-negative for SARS-CoV-2, mean age was 42·6 years (SD 15·85), mean BMI was 28·5 kg/m2 (SD 6·20), 779 (46·3%) were men and 904 (53·7%) were women, 157 (9·3%) identified as Black or African American, and 708 (42·1%) identified as Hispanic or Latino (table 1 ); 586 participants (34·8%) received at least one dose of a COVID-19 vaccine during the study (appendix p 23). Demographics and baseline characteristics for all participants who were RT-PCR-negative for SARS-CoV-2, regardless of serostatus, are presented in the appendix (p 24).
Table 1.
Demographics and baseline characteristics in patients who had a negative SARS-CoV-2 RT-PCR and were seronegative
| CAS + IMD 1200 mg (n=841) | Placebo (n=842) | Total (n=1683) | ||
|---|---|---|---|---|
| Median age, years | 43·0 (26) | 43·5 (24) | 43·0 (25) | |
| Participants aged ≥50 | 317 (37·7%) | 308 (36·6%) | 625 (37·1%) | |
| Sex | ||||
| Male | 379 (45·1%) | 400 (47·5%) | 779 (46·3%) | |
| Female | 462 (54·9%) | 442 (52·5%) | 904 (53·7%) | |
| Race | ||||
| White | 729 (86·7%) | 712 (84·6%) | 1441 (85·6%) | |
| Black or African-American | 71 (8·4%) | 86 (10·2%) | 157 (9·3%) | |
| Asian | 24 (2·9%) | 22 (2·6%) | 46 (2·7%) | |
| American Indian or Alaska Native | 3 (0·4%) | 5 (0·6%) | 8 (0·5%) | |
| Native Hawaiian or Pacific Islander | 1 (0·1%) | 2 (0·2%) | 3 (0·2%) | |
| Other | 13 (1·5%) | 15 (1·8%) | 28 (1·7%) | |
| Ethnicity | ||||
| Hispanic or Latino | 337 (40·1%) | 371 (44·1%) | 708 (42·1%) | |
| Not Hispanic or Latino | 500 (59·5%) | 465 (55·2%) | 965 (57·3%) | |
| Other | 4 (0·5%) | 6 (0·7%) | 10 (0·6%) | |
| Mean weight, kg | 81·3 (19·5) | 81·5 (19·8) | 81·4 (19·7) | |
| Mean BMI, kg/m2 | 28·5 (6·0) | 28·6 (6·4) | 28·5 (6·2) | |
| Participants with BMI ≥30 | 289 (34·4%) | 285 (33·8%) | 574 (34·1%) | |
Data are n (%), median (IQR), or mean (SD). Population includes participants who were seronegative and RT-PCR-negative for SARS-CoV-2 at baseline, excluding participants from the initial descriptive assessment. BMI=body-mass index. CAS + IMD=casirivimab and imdevimab.
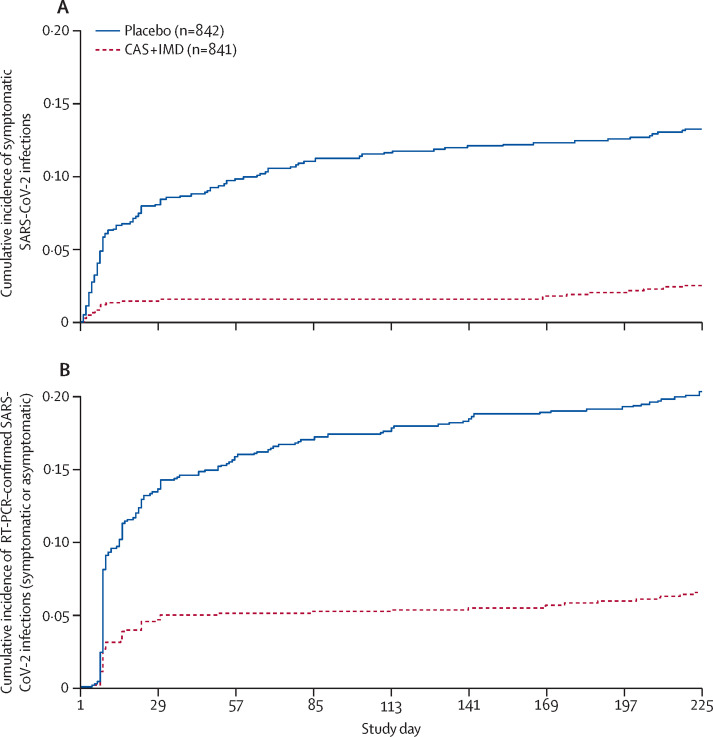
Among the 1683 participants who were seronegative and RT-PCR-negative for SARS-CoV-2, during the entirety of the 8-month study (1 month [28-day] EAP plus the 7-month follow-up period), CAS + IMD reduced the risk of symptomatic SARS-CoV-2 infections by 81·2% versus placebo (nominal p<0·0001; table 2 ). Furthermore, the overall reduction in the relative risk of symptomatic infection during the follow-up period (months 2–8) was 80·9% (nominal p<0·0001), consistent with the relative risk reduction of 81·4% during month 1 (nominal p<0·0001; table 2). As such, the cumulative incidence of symptomatic SARS-CoV-2 infections during the entire 8-month study showed a consistently slower accumulation of events for participants in the CAS + IMD group versus placebo (figure 1A ).
Table 2.
SARS-CoV-2 infection by study period
| CAS + IMD 1200 mg (n=841) | Placebo (n=842) | ||
|---|---|---|---|
| Symptomatic SARS-CoV-2 infections (COVID-19) | |||
| Month 1 (28-day EAP) | .. | .. | |
| Participants | 13 (1·5%) | 70 (8·3%) | |
| Relative risk reduction | 81·4% | .. | |
| Odds ratio (95% CI)* | 0·17 (0·09–0·31) | .. | |
| Nominal p value* | <0·0001 | .. | |
| Follow-up (months 2 to 5)† | .. | .. | |
| Participants | 0 | 32 (3·8%) | |
| Relative risk reduction | 100% | .. | |
| Odds ratio (95% CI) | 0·00 (0·00–0·09)‡ | .. | |
| Nominal p value | <0·0001‡ | .. | |
| Follow-up (months 6 to 8)† | .. | .. | |
| Participants | 8 (1·0%) | 10 (1·2%) | |
| Relative risk reduction | 19·9% | .. | |
| Odds ratio (95% CI)* | 0·80 (0·31–2·04) | .. | |
| Nominal p value* | 0·64 | .. | |
| Follow-up (months 2 to 8) | .. | .. | |
| Participants | 8 (1·0%) | 42 (5·0%) | |
| Relative risk reduction | 80·9% | .. | |
| Odds ratio (95% CI)* | 0·18 (0·09–0·39) | .. | |
| Nominal p value* | <0·0001 | .. | |
| Entire study (months 1 to 8) | .. | .. | |
| Participants | 21 (2·5%) | 112 (13·3%) | |
| Relative risk reduction | 81·2% | .. | |
| Odds ratio (95% CI)* | 0·17 (0·10–0·27) | .. | |
| Nominal p value* | <0·0001 | .. | |
| RT-PCR-confirmed SARS-CoV-2 infections (symptomatic or asymptomatic) | |||
| Month 1 (28-day EAP) | .. | .. | |
| Participants | 42 (5·0%) | 122 (14·5%) | |
| Relative risk reduction | 65·5% | .. | |
| Odds ratio (95% CI)* | 0·31 (0·22–0·45) | .. | |
| Nominal p value* | <0·0001 | .. | |
| Follow-up (months 2 to 5)† | .. | .. | |
| Participants | 4 (0·5%) | 38 (4·5%) | |
| Relative risk reduction | 89·5% | .. | |
| Odds ratio (95% CI)* | 0·10 (0·04–0·29) | .. | |
| Nominal p value* | <0·0001 | .. | |
| Follow-up (months 6 to 8)† | .. | .. | |
| Participants | 9 (1·1%) | 13 (1·5%) | |
| Relative risk reduction | 30·7% | .. | |
| Odds ratio (95% CI)* | 0·69 (0·29–1·63) | .. | |
| Nominal p value* | 0·40 | .. | |
| Follow-up (months 2 to 8) | .. | .. | |
| Participants | 13 (1·5%) | 51 (6·1%) | |
| Relative risk reduction | 74·5% | .. | |
| Odds ratio (95% CI)* | 0·24 (0·13–0·45) | .. | |
| Nominal p value* | <0·0001 | .. | |
| Entire study (months 1 to 8) | .. | .. | |
| Participants | 55 (6·5%) | 173 (20·5%) | |
| Relative risk reduction | 68·2% | .. | |
| Odds ratio (95% CI)* | 0·27 (0·20–0·37) | .. | |
| Nominal p value* | <0·0001 | .. | |
Data are n (%) unless otherwise specified. Population includes participants who were seronegative and RT-PCR-negative for SARS-CoV-2 at baseline, excluding participants from the initial descriptive assessment. SARS-CoV-2 infection was confirmed by central or local SARS-CoV-2 RT-PCR. CAS + IMD=casirivimab and imdevimab. EAP=efficacy assessment period.
Based on a logistic regression model adjusted by region (USA vs not USA) and age group (12 to <50 years vs ≥50 years).
Post-hoc analysis.
Unadjusted odds ratio and the exact 95% CI are reported due to sparse events; p value is based on the Fisher's exact test.
Figure 1.
Cumulative incidence of symptomatic (A) or RT-PCR-confirmed (symptomatic or asymptomatic; B) SARS-CoV-2 infections by study day
Population includes participants who were RT-PCR-negative for SARS-CoV-2 and seronegative at baseline. Infection was confirmed by central or local laboratory. After the 28-day efficacy assessment period, no scheduled SARS-CoV-2 RT-PCR testing was done (no surveillance testing during the follow-up period). Central laboratory RT-PCR (or local laboratory molecular testing) was done and reported if symptoms occurred or when participants had a local positive test for any reason. CAS + IMD=casirivimab and imdevimab.
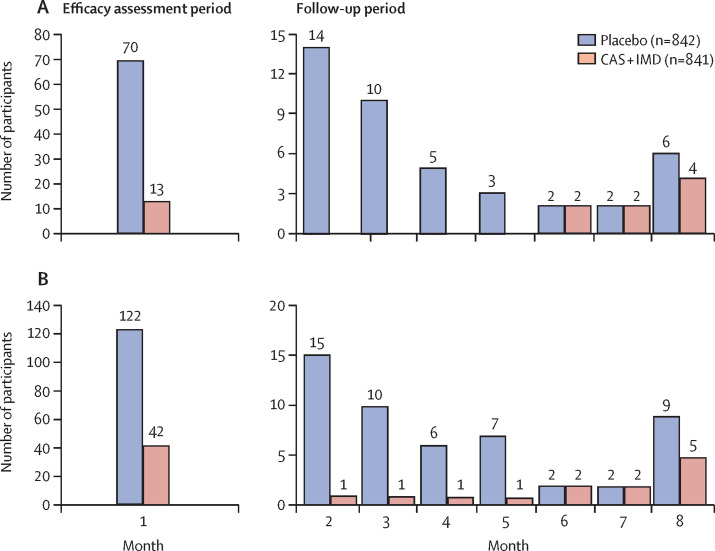
We also assessed efficacy in the months after the initial 1-month EAP separately to provide an additional estimate of efficacy as pre-exposure prophylaxis when the initial acute high risk of exposure in the household had subsided. When the data were assessed by monthly intervals, a reduction in the risk of symptomatic SARS-CoV-2 infections was maintained in a post-hoc analysis combining data for the subsequent 4 months, during follow-up months 2–5 (100% relative risk reduction; nominal p<0·0001; table 2). After 5 months, efficacy appeared to wane, with a resumption of symptomatic SARS-CoV-2 infections in the CAS + IMD group from months 6–8 (19·9% relative risk reduction; nominal p=0·64; table 2, figure 2A ). An additional analysis of symptomatic SARS-CoV-2 infections in all baseline participants who were SARS-CoV-2 RT-PCR negative regardless of serostatus is presented in the appendix (p 20).
Figure 2.
Participants with symptomatic (A) or RT-PCR-confirmed (symptomatic or asymptomatic; B) SARS-CoV-2 infection over time
Figures depict the number of participants with a first positive SARS-COV-2 RT-PCR test result by central or local laboratory over time during the efficacy assessment period (the first month) or the follow-up period (the subsequent 7 months). The population includes participants who were RT-PCR-negative for SARS-CoV-2 and seronegative at baseline. Symptomatic SARS-CoV-2 infection was defined as a positive RT-PCR result with symptoms reported within 14 days of the positive test result. Cases were assigned to study week on the basis of the nominal week of the positive RT-PCR test. Each study month consists of a 4-week period. Infection was confirmed by central or local laboratory. CAS + IMD=casirivimab and imdevimab.
Scheduled surveillance RT-PCR testing was not done after week 4 of the study. Therefore, SARS-CoV-2 infections during the follow-up period could have been missed in the case of asymptomatic infection or if participants did not seek medical attention for symptomatic infections. We thus used seroconversion as a proxy for the occurrence of any SARS-CoV-2 infections. The rates of seroconversion (the detection of SARS-CoV-2 anti-nucleocapsid IgG) were 4·5% (38 of 841) for participants in the CAS + IMD group and 21·5% (181 of 842) for the participants in the placebo group (79·0% relative risk reduction versus placebo; nominal p<0·0001; table 3 ). We observed higher rates of seroconversion in the placebo group at each period evaluated, including after day 60 and overall, providing evidence of sustained benefit with use of CAS + IMD (table 3).
Table 3.
Proportion of baseline-seronegative participants who seroconverted during the study
| CAS + IMD 1200 mg (n=841) | Placebo (n=842) | Relative risk reduction | Odds ratio (95% CI)* | Nominal p value* | ||
|---|---|---|---|---|---|---|
| Participants with ≥1 post-baseline seropositivity† | 38 (4·5%) | 181 (21·5%) | 79·0% | 0·17 (0·12–0·25) | <0·0001 | |
| Interval of first seropositivity | ||||||
| ≤day 32 | 10 (1·2%) | 90 (10·7%) | 88·9% | 0·10 (0·05–0·19) | <0·0001 | |
| >day 32 to ≤day 60‡ | 7 (0·8%) | 32 (3·8%) | 78·1% | 0·21 (0·09–0·48) | 0·0002 | |
| >day 60§ | 21 (2·5%) | 59 (7·0%) | 64·4% | 0·34 (0·20–0·56) | <0·0001 | |
Data are n (%) unless otherwise specified. Population includes participants who were seronegative and RT-PCR-negative for SARS-CoV-2 at baseline, excluding participants from the initial descriptive assessment. Seroconversion was based on positive anti-SARS-CoV-2 nucleocapsid IgG testing post-baseline. CAS + IMD=casirivimab and imdevimab.
Based on a logistic regression model adjusted by region (USA vs not USA) and age group (12 to <50 years vs ≥50 years).
Includes both prespecified and post-hoc timepoints.
≤day 60 is a prespecified timepoint of day 57 ± 3.
Post-hoc analysis.
Some individuals in the study had unscheduled SARS-CoV-2 RT-PCR testing even though they did not have symptoms consistent with COVID-19. To complement the data provided by the seroconversion testing, we used the proportion of SARS-CoV-2 infections, as detected by any RT-PCR-positive test, as an additional sensitivity analysis for any SARS-CoV-2 infection (symptomatic or asymptomatic). CAS + IMD reduced the risk of any RT-PCR-confirmed SARS-CoV-2 infections by 68·2% versus placebo during the 8-month study (nominal p<0·0001; table 2). Reduction in the risk of RT-PCR-confirmed SARS-COV-2 infections with CAS + IMD administration was also similar during the initial 1-month high-risk period of household exposure (28-day EAP; 65·5% relative risk reduction) and the follow-up period of months 2–8 (74·5% relative risk reduction; table 2). Therefore, these RT-PCR data are consistent with the observed reduction in any SARS-CoV-2 infection as measured by seroconversion.
A marked reduction in the risk of RT-PCR-confirmed SARS-CoV-2 infections (symptomatic and asymptomatic) was also maintained in a post-hoc analysis of months 2–5 (89·5% relative risk reduction; nominal p<0·0001), with waning efficacy after 5 months and resumption of RT-PCR-confirmed SARS-CoV-2 infections in the CAS + IMD group from months 6 to 8 (30·7% relative risk reduction; nominal p=0·40; table 2, figure 2B). An additional analysis of any SARS-CoV-2 infection (symptomatic or asymptomatic) in all baseline participants who were SARS-CoV-2 RT-PCR negative regardless of serostatus is presented in the appendix (p 20).
Fewer participants reported a MAV related to SARS-CoV-2—hospitalisation, emergency room visit, or visit at an urgent care centre—in the CAS + IMD group (one [0·1%] of 841) versus placebo (16 [1·9%] of 842) during the 8-month study (appendix p 25). One participant in the CAS + IMD group reported a visit to an urgent care centre due to a symptomatic SARS-CoV-2 infection during the initial 28-day EAP; no participants in the CAS + IMD group were hospitalised due to COVID-19. Ten participants in the placebo group visited an emergency room or an urgent care centre, and six participants in the placebo group were hospitalised due to COVID-19. Of the six hospitalised participants in the placebo group, events occurred throughout the study on days 16, 63, 87, 99, 135, and 197. Compared with placebo, in the follow-up period, study participants in the CAS + IMD group had no MAVs, including hospitalisation, through month 8.
Participants were allowed to be vaccinated against COVID-19 during the follow-up period, with a 90-day waiting period after study drug administration, as recommended by the US CDC at the time the study was done. The number of participants vaccinated was balanced between the study groups, with 290 (34·5%) of 841 participants in the CAS + IMD group and 296 (35·2%) of 842 participants in the placebo group receiving at least one dose of a COVID-19 vaccine. Additionally, the median time to first COVID-19 vaccination was balanced between the study groups: 108·5 days (IQR 62) in the CAS + IMD group and 109·0 days (60) in the placebo group (appendix p 23). The majority of participants in the CAS + IMD (201 [69·3%] of 290) and placebo (212 [71·6%] of 296) groups waited the recommended 90 days before vaccination. Only three participants developed symptomatic infection after vaccination (all others occurred before vaccination): one participant in the CAS + IMD group received vaccine doses on days 121 and 145 and reported a symptomatic infection on day 176, one participant in the placebo group received a vaccine on day 189 and reported a symptomatic infection on day 206, and one participant in the placebo group received a vaccine on day 95 and reported a symptomatic infection on day 219.
During the entirety of the 8-month study, in all participants irrespective of baseline serostatus (n=2867), TEAEs were reported in 405 (28·1%) of 1439 participants in the CAS + IMD group and in 512 (35·9%) of 1428 in the placebo group (table 4 ). The higher frequency of TEAEs in the placebo group was primarily driven by a higher proportion of participants with COVID-19 than that in the CAS + IMD group. Non-COVID-19 TEAEs were similar across the CAS + IMD and placebo groups (table 4). The most common TEAEs reported were COVID-19 (29 [2·0%] of 1439 in CAS + IMD and 149 [10·4%] of 1428 in placebo), asymptomatic SARS-CoV-2 infection (71 [4·9%] in CAS + IMD and 119 [8·3%] in placebo), and injection site reactions (60 [4·2%] in CAS + IMD and 26 [1·8%] in placebo; appendix p 26). All injection site reactions reported were mild-to-moderate in intensity and resolved either spontaneously without treatment or with over-the-counter medications. No adverse events of special interest (grade ≥3 hypersensitivity and grade ≥3 injection-site reactions) were reported during the entirety of the study, including the follow-up period.
Table 4.
Overview of TEAEs during the 8-month study by baseline serology status
|
Combined serostatus |
Seronegative |
Seropositive |
Other |
|||||
|---|---|---|---|---|---|---|---|---|
| CAS + IMD 1200 mg (n=1439) | Placebo (n=1428) | CAS + IMD 1200 mg (n=1028) | Placebo (n=1067) | CAS + IMD 1200 mg (n=337) | Placebo (n=296) | CAS + IMD 1200 mg (n=74) | Placebo (n=65) | |
| Participants with ≥1 TEAE | 405 (28·1%) | 512 (35·9%) | 298 (29·0%) | 411 (38·5%) | 83 (24·6%) | 79 (26·7%) | 24 (32·4%) | 22 (33·8%) |
| Participants with ≥1 non-COVID-19 TEAE | 342 (23·8%) | 326 (22·8%) | 254 (24·7%) | 249 (23·3%) | 66 (19·6%) | 57 (19·3%) | 22 (29·7%) | 20 (30·8%) |
| Participants with ≥1 serious TEAE | 24 (1·7%) | 23 (1·6%) | 15 (1·5%) | 17 (1·6%) | 6 (1·8%) | 5 (1·7%) | 3 (4·1%) | 1 (1·5%) |
| Participants with ≥1 serious non-COVID-19 TEAE | 24 (1·7%) | 16 (1·1%) | 15 (1·5%) | 10 (0·9%) | 6 (1·8%) | 5 (1·7%) | 3 (4·1%) | 1 (1·5%) |
| Participants with any TEAE resulting in death* | 3 (0·2%) | 2 (0·1%) | 1 (<0·1%) | 0 | 2 (0·6%) | 2 (0·7%) | 0 | 0 |
Data are n (%). Population includes participants who were RT-PCR-negative for SARS-CoV-2 at baseline, including participants from the initial descriptive assessment. Serological testing included anti-spike S1 IgA, anti-spike S1 IgG, and anti-nucleocapsid IgG. A participant was categorised at baseline as seronegative if all available serological tests were negative and as seropositive if any serological test was positive. Participants who had only borderline serology test results or no data were categorised as “other”. CAS + IMD=casirivimab and imdevimab. TEAE=treatment-emergent adverse event.
None due to COVID-19 or study drug; the five deaths occurred outside of the 28-day efficacy assessment period; four of the deaths were previously described;16 the fifth death occurred in a participant in the CAS + IMD group who died in a road traffic accident.
Occurrence of serious TEAEs (including COVID-19) was similar between groups (table 4; appendix pp 27–28). Except for COVID-19 and COVID-19 pneumonia, no other serious TEAE was reported in more than two participants in either group. No participant in the CAS + IMD group had a COVID-19-related serious TEAE, and no serious TEAEs were considered related to study drug by the investigator. Five deaths were reported during the study: three (0·2%) of 1439 participants in the CAS + IMD group and two (0·1%) of 1428 in the placebo group. The five deaths occurred outside of the 28-day EAP. Four of the deaths were previously described.16 The fifth death occurred in a participant in the CAS + IMD group who died in a road traffic accident. None of the deaths were related to study drug or due to COVID-19 in either group.
We also evaluated safety by serostatus. Safety findings in participants who were seronegative were consistent with the overall population, with 298 (29·0%) of 1028 in the CAS + IMD group and 411 (38·5%) of 1067 in the placebo group reporting TEAEs, with a higher frequency of COVID-19 TEAEs in the placebo group than in the CAS + IMD group (table 4). In participants who were seropositive, 83 (24·6%) of 337 in the CAS + IMD group and 79 (26·7%) of 296 in the placebo group reported TEAEs. The most common TEAE in participants who were seropositive was asymptomatic COVID-19 (appendix p 26). Serious TEAEs and non-COVID-19 TEAEs in participants who were seropositive were similar across the study groups (table 4).
Protection from symptomatic SARS-CoV-2 infection with CAS + IMD was maintained for approximately 5 months (table 2). For the timepoint with available data in the closest proximity to month 5 (day 140), the observed mean concentration of CAS + IMD combined in serum was 3·60 (SD 2·19) mg/L (9 participants); there was good agreement between the observed concentration and the population-pharmacokinetic predicted median (90% prediction interval) concentration of 3·50 (0·47–12·9) mg/L of CAS + IMD in serum. On the basis of the population-pharmacokinetic model, the predicted concentration of CAS + IMD combined in serum at the end of the 5-month period (day 150) was 2·73 mg/L, which corresponded to an estimated 50% neutralising titre against the reference SARS-CoV-2 variant (Asp614Gly) of 1:963 using a pseudovirus neutralisation assay (appendix p 29).
Discussion
Despite the changing landscape of the COVID-19 pandemic, with emerging SARS-CoV-2 variants showing differing characteristics with regards to transmissibility and severity of illness, widespread prevention is crucial to control the pandemic and to allow for a return to relative normalcy. Although vaccination has resulted in a decreased incidence of severe COVID-19, many individuals,21 especially those who are immunocompromised without appropriate B-cell function, remain unprotected despite vaccination. Findings from our study show that CAS + IMD might be useful as pre-exposure prophylaxis against susceptible variants, with a single subcutaneous administration of 1200 mg providing protection against COVID-19, in a variant-dependent manner, for approximately 5 months.
We previously reported primary analysis data over a 1-month period showing that a single subcutaneous 1200 mg dose of CAS + IMD reduced the relative risk of symptomatic SARS-CoV-2 infections (COVID-19) in a high-risk household contact setting.16 Data were consistent with efficacy both in a post-exposure prophylaxis setting (acutely during the first week after exposure to an infected household member) and during weeks 2–4 when community exposure was also the source of infection. We now support these findings of pre-exposure prophylaxis by assessing prevention of COVID-19 in the subsequent 7 months of follow-up when the risk of SARS-CoV-2 infection was presumably primarily through community exposure.
In the prespecified analysis looking at the entire 8-month study, efficacy in prevention was observed, with an 81·2% relative risk reduction of developing COVID-19 after receipt of a single dose of CAS + IMD. However, when evaluated month by month, efficacy waned after month 5. Protection against COVID-19 was most compelling during months 2–5 of the follow-up, with a 100% relative risk reduction of developing COVID-19, suggesting that this was driving the observed efficacy during the follow-up period. The 5-month duration of protection observed in this study was an unexpected finding and suggests that only a very low serum concentration of CAS + IMD was required to effectively neutralise SARS-CoV-2 and protect against symptomatic infection. The neutralisation titre is a consequence of the potency of the antibodies against the spike protein and absolute drug concentrations over time, which is informed by both the half-life of the antibodies and the dose administered. These data suggest that despite half-lives of only approximately 30 days,16 a single 1200 mg dose provided sufficient drug exposure concentrations, above those required to effectively neutralise the virus in target tissues for 5 months. This observation is consistent with a 2022 meta-analysis,22 which also suggests that many SARS-CoV-2 antibodies have been administered at doses much higher than needed to achieve sufficient neutralisation for efficacy in prevention or treatment settings.
Additionally, although scheduled surveillance RT-PCR testing was not done after the first 4 weeks of the study, seroconversion used as a proxy to identify any SARS-CoV-2 infection (including asymptomatic infections) supported the sustained benefit of CAS + IMD. During the entirety of the 8-month study, none of the individuals in the CAS + IMD group were hospitalised due to COVID-19, compared with six individuals in the placebo group. Although five deaths occurred during the study, none were attributed to the study drugs, and none were due to COVID-19. Taken together with the primary analysis that was previously reported,16 these data provide additional support for the efficacy of CAS + IMD in pre-exposure and post-exposure prophylaxis settings.
This study was done before vaccines were widely available. 35% of participants were vaccinated during the follow-up period, allowing us to explore extended protection with a single dose of CAS + IMD in this population where a minority had vaccine-related immunity to SARS-CoV-2. However, only three participants developed symptomatic infection after vaccination. Therefore, we had insufficient data to quantify risk reduction in those who were vaccinated.
No new or serious safety signals were observed in the follow-up period of the study. Overall, CAS + IMD was associated with a lower incidence of TEAEs versus placebo, driven by fewer COVID-19-associated TEAEs with CAS + IMD than with placebo. Consistent with previous findings,16 injection site reactions were reported more frequently in the CAS + IMD group, but these reactions were mild-to-moderate in severity.
These data provide some insights about correlates of antibody-mediated immunity when the concentrations of CAS + IMD are expressed as SARS-CoV-2 viral neutralisation capability, the 50% neutralising titre. Although, at the time of the study, the viral landscape was dominated by the pre-variant of concern lineages (collectively referred to as Asp614Gly) and not the delta (B.1.617.2) variant,23 the in-vitro neutralisation IC50s for CAS + IMD against delta and Asp614Gly are nearly identical,24 suggesting similar estimated 50% neutralising titres over time against these two SARS-CoV-2 variants.
This study has several limitations. It was done from July, 2020, to October, 2021, with the delta variant becoming dominant in the summer of 2021 and before the emergence of omicron-lineage variants. Although CAS + IMD is not expected to be effective against omicron-lineage variants,25 it retains viral neutralisation activity against all other historical variants of concern before omicron. Furthermore, the study enrolled a generally healthy population before the widespread availability of vaccines; therefore, most participants did not have SARS-CoV-2 antibody response (ie, were seronegative). Future use of SARS-CoV-2 monoclonal antibodies for prevention is likely to be focused on the immunocompromised, particularly individuals without B-cell function who cannot mount an appropriate antibody response. Because the efficacy analysis was done in the seronegative population, results might be applicable to immunocompromised populations. Supportive evidence of CAS + IMD efficacy in patients with B-cell deficiency and dysfunction was previously reported.26 Additionally, regarding the seroconversion data presented in this analysis, we recognise that anti-nucleocapsid IgG response wanes, and that some participants who were infected might not have developed an antibody response. To compensate, we collected serology samples at multiple timepoints during the study to capture even a transient anti-nucleocapsid IgG response. Nevertheless, it is important to interpret the serology results as a supportive analysis to the RT-PCR results, each of which capture different aspects of infection, with a consistent trend of prevention regardless of the assay used.
The evolution of SARS-CoV-2 is challenging to predict. Proactive epidemiological surveillance to assess the predominant circulating strains in different regions combined with assessment of neutralisation activity against emerging variants—and with safe and active SARS-CoV-2 antibodies at our disposal, in addition to CAS + IMD—will probably guide decisions on which monoclonal antibodies should be chosen for prophylaxis and treatment in the future.
As a whole, this 8-month prevention study showed the benefit of CAS + IMD as post-exposure prophylaxis in a high-risk household transmission setting and as pre-exposure prophylaxis in a broader community setting during the COVID-19 pandemic, at a time when susceptible variants were circulating. These data are important for the many individuals who might not mount an effective immune response against SARS-CoV-2, whether by active vaccination or natural infection. Providing protective levels of SARS-CoV-2 antibodies passively through administration of CAS + IMD might be an effective option for the prevention of COVID-19 in these vulnerable populations when circulating variants are susceptible to CAS + IMD.
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on July 15, 2022
Data sharing
Qualified researchers can request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, and statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymised participant data will be considered for sharing once the product and indication has been approved by major health authorities (eg, US Food and Drug Administration, European Medicines Agency, Pharmaceuticals and Medical Devices Agency, and so on), if there is legal authority to share the data and there is not a reasonable likelihood of participant re-identification. Requests should be submitted to https://vivli.org/.
Declaration of interests
GAH, MPO, FI, KCT, and JDH are Regeneron Pharmaceuticals employees and stockholders who report having a patent pending (International Patent application number PCT/US2021/035556), which has been licensed and provides royalties, with Regeneron Pharmaceuticals. EF-N, NSa, PH, K-CC, BJM, JDD, AM, JP, DS, ATD, NB, and DMW are Regeneron Pharmaceuticals employees and stockholders. MSC reports a relationship with National Institute for Health for study funding and Regeneron Pharmaceuticals for drug studies and manuscript writing support, and has a leadership or fiduciary role for HIV Prevention Trials Network, COVID-29 Prevention Network, Fogart, and McGill. CBH reports support for manuscript writing from Regeneron Pharmaceuticals, salary support from University of North Carolina at Chapel Hill, activities sponsored by Gilead Sciences, and receiving honorarium for developing content for a continuing education programme with PRIME Education. MAM reports being a federal employee who, as part of the US Government through Operation Warp Speed (later transferred into the responsibilities of the White House COVID-19 Response Team), supported the clinical trial network involved in implementation of this effort. ATH is a Regeneron Pharmaceuticals employee and stockholder, a former Pfizer employee and current stockholder, and reports having a patent pending (International Patent application number PCT/US2021/035556), which has been licensed and provides royalties, with Regeneron Pharmaceuticals. AB, CAK, NSt, and GDY have issued patents (US patent number 10 787 501, 10 954 289, and 10 975 139) and pending patents (International Patent application number PCT/US2020/039707), which have been licensed and provide royalties, with Regeneron Pharmaceuticals. KJB and DRB declare no competing interests.
Acknowledgments
Acknowledgments
We thank the study participants, their families, the investigational site members involved in this trial (listed in appendix pp 4–16), the COVID-19 Phase 3 Prevention Trial Team (listed in appendix pp 4–13), the members of the Data and Safety Monitoring Board, Caryn Trbovic from Regeneron Pharmaceuticals for assistance with development of the manuscript, and Prime (Knutsford, UK), for formatting and copy editing suggestions. This study was supported by Regeneron Pharmaceuticals and F Hoffmann-La Roche. This trial was done jointly with NIAID and NIH. CoVPN is supported by cooperative agreement awards from NIAID and NIH. The work on this study was supported by awards UM1AI068619 and UM1AI148684. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Acknowledgments
Contributors
GAH, MPO, EF-N, NSa, FI, PH, K-CC, KJB, RVB, DHB, MSC, CBH, DRB, MAM, BJM, JDD, KCT, AM, ATH, JDH, JP, DS, AB, CAK, ATD, NSt, NB, GDY, and DMW contributed to study concept and design. KJB, RVB, DHB, MSC, CBH, ATH, and JDH were involved in data collection. DRB, MAM, JDD, KCT, DS, AB, and CAK provided administrative, technical, or material support. NSa, FI, PH, and K-CC provided statistical analysis. GAH, MPO, EF-N, NSa, FI, PH, K-CC, BJM, DRB, MAM, JDD, KCT, AM, ATH, JDH, JP, DS, AB, CAK, ATD, NSt, NB, GDY, and DMW provided analysis and interpretation of the data. K-CC and RVB accessed and verified all the data. All authors provided critical revision of the manuscript for intellectual content and provided approval to submit.
Contributor Information
COVID-19 Phase 3 Prevention Trial Team:
Achint Chani, Adebiyi Adepoju, Adnan Mahmood, Aisha Mortagy, Ajla Dupljak, Alina Baum, Alison Brown, Amy Froment, Andrea Hooper, Andrea Margiotta, Andrew Bombardier, Anita Islam, Anne Smith, Arvinder Dhillon, Audra McMillian, Aurora Breazna, Ayesha Aslam, Barabara Carpentino, Bari Kowal, Barry Siliverstein, Benjamin Horel, Bo Zhu, Bret Musser, Brian Bush, Brian Head, Brian Snow, Bryan Zhu, Camille Debray, Careta Phillips, Carmella Simiele, Carol Lee, Carolyn Nienstedt, Caryn Trbovic, Casey (Kuo-Chen) Chan, Catherine Elliott, Chad Fish, Charlie Ni, Christa Polidori, Christine Enciso, Christopher Caira, Christopher Powell, Christos A. Kyratsous, Cliff Baum, Colin McDonald, Cynthia Leigh, Cynthia Pan, Dana Wolken, Danielle Manganello, David Liu, David Stein, David M. Weinreich, Dawlat Hassan, Daya Gulabani, Deborah Fix, Deborah Leonard, Deepshree Sarda, Denise Bonhomme, Denise Kennedy, Devin Darcy, Dhanalakshmi Barron, Diana Hughes, Diana Rofail, Dipinder Kaur, Divya Ramesh, Dona Bianco, Donna Cohen, Eduardo Forleo-Neto, Edward Jean-Baptiste, Ehsan Bukhari, Eileen Doyle, Elizabeth Bucknam, Emily Labriola-Tomkins, Emily Nanna, Esther Huffman O'Keefe, Evelyn Gasparino, Evonne Fung, Flonza Isa, Fung-Yee To, Gary Herman, George D. Yancopoulos, Georgia Bellingham, Giane Sumner, Grainne Moggan, Grainne Power, Haixia Zeng, Hazel Mariveles, Heath Gonzalez, Helen Kang, Hibo Noor, Ian Minns, Ingeborg Heirman, Izabella Peszek, James Donohue, Jamie Rusconi, Janice Austin, Janie Parrino, Jeannie Yo, Jenna McDonnell, Jennifer D. Hamilton, Jessica Boarder, Jianguo Wei, Jingchun Yu, Joanne Malia, Joanne Tucciarone, Jodie Tyler-Gale, John D. Davis, John Strein, Jonathan Cohen, Jonathan Meyer, Jordan Ursino, Joseph Im, Joseph Tramaglini, Joseph Wolken, Kaitlyn Potter, Kaitlyn Scacalossi, Kamala Naidu, Karen Browning, Karen Rutkowski, Karen Yau, Katherine Woloshin, Kelly Lewis-Amezcua, Kenneth Turner, Kimberly Dornheim, Kit Chiu, Kosalai Mohan, Kristina McGuire, Kristy Macci, Kurt Ringleben, Kusha Mohammadi, Kyle Foster, Latora Knighton, Leah Lipsich, Lindsay Darling, Lisa Boersma, Lisa Cowen, Lisa Hersh, Lisa Jackson, Lisa Purcell, Lisa Sherpinsky, Livia Lai, Lori Faria, Lori Geissler, Louise Boppert, Lyra Fiske, Marc Dickens, Marco Mancini, Maria C. Leigh, Meagan P. O'Brien, Michael Batchelder, Michael Klinger, Michael Partridge, Michel Tarabocchia, Michelle Wong, Mivianisse Rodriguez, Moetaz Albizem, Muriel O'Byrne, Ned Braunstein, Neena Sarkar, Neil Stahl, Nicole Deitz, Nicole Memblatt, Nirav Shah, Nitin Kumar, Olga Herrera, Oluchi Adedoyin, Ori Yellin, Pamela Snodgrass, Patrick Floody, Paul D'Ambrosio, Paul (Xiaobang) Gao, Peijie Hou, Philippa Hearld, Qin Li, Rachel Kitchenoff, Rakiyya Ali, Ramya Iyer, Ravikanth Chava, Rinol Alaj, Rita Pedraza, Robert Hamlin, Romana Hosain, Ruchin Gorawala, Ryan White, Ryan Yu, Rylee Fogarty, S. Balachandra Dass, Sagarika Bollini, Samit Ganguly, Sandra DeCicco, Sanket Patel, Sarah Cassimaty, Selin Somersan-Karakaya, Shane McCarthy, Sharon Henkel, Shazia Ali, Shelley Geila Shapiro, Somang Kim, Soraya Nossoughi, Stephanie Bisulco, Steven Elkin, Steven Long, Sumathi Sivapalasingam, Susan Irvin, Susan Wilt, Tami Min, Tatiana Constant, Theresa Devins, Thomas DiCioccio, Thomas Norton, Travis Bernardo, Tzu-Chien Chuang, Victor (Jianguo) Wei, Vinh Nuce, Vishnu Battini, Wilson Caldwell, Xiaobang Gao, Xin Chen, Yanmei Tian, Yasmin Khan, Yuming Zhao, Yunji Kim, Bonnie Dye, Christopher B. Hurt, Dale R. Burwen, Dan H. Barouch, David Burns, Elizabeth Brown, Katharine J. Bar, Mary Marovich, Meredith Clement, Myron S. Cohen, Nirupama Sista, Ruanne V. Barnabas, and Sheryl Zwerski
Supplementary Material
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO WHO Director-General's opening remarks at the media briefing on COVID-19—11 March 2020. 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020
- 3.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Telenti A, Arvin A, Corey L, et al. After the pandemic: perspectives on the future trajectory of COVID-19. Nature. 2021;596:495–504. doi: 10.1038/s41586-021-03792-w. [DOI] [PubMed] [Google Scholar]
- 5.COVID-19 Treatment Guidelines Panel . National Institutes of Health; 2021. Coronavirus disease 2019 (COVID-19) treatment guidelines.https://www.covid19treatmentguidelines.nih.gov/ [PubMed] [Google Scholar]
- 6.Yan Z, Yang M, Lai C-L. COVID-19 vaccinations: a comprehensive review of their safety and efficacy in special populations. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Centers for Disease Control and Prevention COVID-19 vaccines for people who are moderately or severely immunocompromised. 2020. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html
- 8.Clark A, Jit M, Warren-Gash C, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8:e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration Fact sheet for healthcare providers: emergency use authorization for EVUSHELD™ (tixagevimab co-packaged with cilgavimab) 2021. https://www.fda.gov/media/154701/download
- 10.US Food and Drug Administration Fact sheet for health care providers: emergency use authorization (EUA) of REGEN-COV® (casirivimab with imdevimab) 2021. https://www.fda.gov/media/145611/download
- 11.European Medicines Agency Ronapreve: annex I summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/ronapreve-epar-product-information_en.pdf
- 12.Roche Products Ronapreve® (casirivimab and imdevimab) Australian product information. 2021. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2021-PI-02271-1&d=20220107172310101
- 13.Pharmaceuticals and Medical Devices Agency Report on the deliberation results for ronapreve for intravenous infusion. 2021. https://www.pmda.go.jp/files/000244786.pdf
- 14.Medicines & Healthcare Products Regulatory Agency Summary of product characteristics for Ronapreve. 2021. https://www.gov.uk/government/publications/regulatory-approval-of-ronapreve/summary-of-product-characteristics-for-ronapreve
- 15.US Food and Drug Administration Emergency use authorization 091. 2022. https://www.fda.gov/media/145610/download
- 16.O'Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV antibody combination to prevent COVID-19. N Engl J Med. 2021;385:1184–1195. doi: 10.1056/NEJMoa2109682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alene M, Yismaw L, Assemie MA, Ketema DB, Gietaneh W, Birhan TY. Serial interval and incubation period of COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:257. doi: 10.1186/s12879-021-05950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAloon C, Collins Á, Hunt K, et al. Incubation period of COVID-19: a rapid systematic review and meta-analysis of observational research. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones A, Fialkowski V, Prinzing L, Trites J, Kelso P, Levine M. Assessment of day-7 postexposure testing of asymptomatic contacts of COVID-19 patients to evaluate early release from quarantine—Vermont, May–November 2020. MMWR Morb Mortal Wkly Rep. 2021;70:12–13. doi: 10.15585/mmwr.mm7001a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao SY, Gerber AN, Zelarney P, Make B, Wechsler ME. Impaired SARS-CoV-2 mRNA vaccine antibody response in chronic medical conditions: a real-world analysis. Chest. 2022;161:1490–1493. doi: 10.1016/j.chest.2021.12.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stadler E, Chai KL, Schlub TE, et al. Determinants of passive antibody effectiveness in SARS-CoV-2 infection. medRxiv. 2022 doi: 10.1101/2022.03.21.22272672. published online March 22. (preprint). [DOI] [Google Scholar]
- 23.Nextstrain team Genomic epidemiology of SARS-CoV-2 with global subsampling. 2022. https://nextstrain.org/ncov/gisaid/global
- 24.Copin R, Baum A, Wloga E, et al. The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies. Cell. 2021;184:3949. doi: 10.1016/j.cell.2021.06.002. 61.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regeneron Pharmaceuticals Regeneron's next generation monoclonal antibodies are active against all known variants of concern, including both Omicron and Delta. 2021. https://investor.regeneron.com/static-files/4aed42a1-3d26-48af-bd01-3f0c92938c11
- 26.Stein D, Oviedo-Orta E, Kampman WA, et al. Compassionate use of REGEN-COV® in patients with COVID-19 and immunodeficiency-associated antibody disorders. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab1059. published online Dec 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers can request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, and statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymised participant data will be considered for sharing once the product and indication has been approved by major health authorities (eg, US Food and Drug Administration, European Medicines Agency, Pharmaceuticals and Medical Devices Agency, and so on), if there is legal authority to share the data and there is not a reasonable likelihood of participant re-identification. Requests should be submitted to https://vivli.org/.