Abstract
In March 2021, the U.S. Food and Drug Administration granted accelerated approval to axicabtagene ciloleucel, a CD19-directed chimeric antigen receptor T-cell therapy, for the treatment of adult patients with relapsed or refractory follicular lymphoma (r/r FL) after at least 2 lines of systemic therapy. Approval was based on ZUMA-5, a single-arm, open-label, multicenter trial that evaluated a single infusion of axicabtagene ciloleucel, preceded by lymphodepleting chemotherapy with cyclophosphamide and fludarabine, in this population. Efficacy was based on objective response rate (ORR) and duration of response (DOR) as determined by an independent review committee. Among 81 patients in the primary efficacy analysis, having a median of 3 (range 2-9) prior lines of systemic therapy, the ORR was 91% (95% confidence interval [CI]: 83-96) with a complete remission (CR) rate of 60% and a median time-to-response of 1 month. The median DOR was not reached, and the 1-year rate of continued remission was 76% (95% CI: 64-85). For all leukapheresed patients with FL in this trial (n = 123), the ORR was 89% (95% CI: 83-94) with a CR rate of 62%. Among 146 patients with indolent lymphoma evaluated for safety, cytokine release syndrome occurred in 84% (Grade ≥3, 8%) and neurological toxicities occurred in 77% (Grade ≥3, 21%), leading to implementation of a risk evaluation and mitigation strategy. Serious adverse reactions occurred in 48%. Post-marketing studies will further evaluate clinical benefit in patients with r/r FL and long-term safety.
Keywords: follicular lymphoma, indolent non-Hodgkin lymphoma, axicabtagene ciloleucel
In March 2021, after a priority review, the US Food and Drug Administration granted accelerated approval to axicabtagene ciloleucel for the treatment of adult patients with relapsed or refractory follicular lymphoma after at least 2 lines of systemic therapy. This article summarizes the clinical review and regulatory considerations regarding this licensing application.
Implications For Practice.
Axicabtagene ciloleucel is a new option for selected patients with relapsed or refractory follicular lymphoma in the third-line setting or beyond and is the first chimeric antigen receptor T-cell product approved for the treatment of an indolent lymphoma. The risk of serious and life-threatening adverse reactions necessitates careful patient selection, close monitoring, and a risk evaluation and mitigation strategy to help assure safe use.
Introduction
The management of relapsed or refractory follicular lymphoma (r/r FL) is highly individualized, and approaches vary widely depending upon patient fitness and comorbidities, types of prior therapies and disease responses, physician discretion, and patient preference.1 Whereas traditional cytotoxic chemotherapy and CD20-directed monoclonal antibodies were once the mainstay of FL management, the treatment landscape has expanded rapidly. Since 2017, the U.S. Food and Drug Administration (FDA) has approved 8 regimens for r/r FL over a 4 year period, with varied mechanisms of action; these regimens include rituximab with hyaluronidase, lenalidomide in combination with rituximab (R2),2-4 bendamustine alone5 or in combination with obinutuzumab,6 the phosphatidylinositol 3-kinase [PI3K] inhibitor (copanlisib), the PI3K delta and casein kinase 1 epsilon [CK1e] inhibitor umbralisib, the small-molecule enhancer of zeste homolog 2 (EZH2) inhibitor tazemetostat, and axicabtagene ciloleucel. Unmet need remains for development of improved therapies for patients with FL, particularly for patients with multiply r/r disease or poor-risk features such as disease progression within 24 months of diagnosis or initiation of first-line chemoimmunotherapy.7,8
Axicabtagene ciloleucel is an autologous, CD19-directed chimeric antigen receptor (CAR) T-cell therapy created by collecting a patient’s T cells, transducing them with a retroviral vector encoding the CAR, and infusing the modified cells back into the patient9 (Table 1). It consists of a single-chain variable fragment extracellular domain targeting CD19 proteins, with CD3ζ and CD28 intracellular domains that promote T-cell activation.9 Following anti-CD19 CAR T-cell engagement with CD19-expressing target cells, the CD28 and CD3-zeta co-stimulatory domains activate downstream signaling cascades that lead to T-cell activation, proliferation, acquisition of effector functions, and secretion of inflammatory cytokines and chemokines. This sequence of events leads to apoptosis and necrosis of CD19-expressing target cells.
Table 1.
Axicabtagene ciloleucel background information.
| Pharmacological class | CD19-directed, genetically-modified autologous T-cell immunotherapy |
| Mechanism of action | Following anti-CD19 CAR T-cell engagement with CD19-expressing target cells, the CD28 and CD3-zeta co-stimulatory domains activate downstream signaling cascades that lead to T-cell activation, proliferation, acquisition of effector functions and secretion of inflammatory cytokines and chemokines. This sequence of events leads to apoptosis and necrosis of CD19-expressing target cells. |
| Pharmacokinetics in FL | Following infusion, axicabtagene ciloleucel exhibited an initial rapid expansion phase, achieving maximal expansion 8 days following infusion and persisting in the peripheral blood for a median of 6 months. Cytokine and chemokine levels peaked within the first 14 days and generally returned to baseline within 28 days. |
| Approval in FLa | For the treatment of adult patients with relapsed or refractory FL after 2 or more lines of systemic therapy (2021) |
| Other approvals | For the treatment of adult patients with relapsed or refractory largeB-cell lymphoma after 2 or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL arising from FL (2017). Limitations of use: not indicated for the treatment of patients with primary central nervous system lymphoma. |
Under accelerated approval.
Abbreviation: FL, follicular lymphoma.
Axicabtagene ciloleucel was originally approved in 2017 for adult patients with r/r large B-cell lymphoma after 2 or more lines of systemic therapy10 (Table 1). CD19-directed CAR T-cell therapies also have been approved for other aggressive malignancies (acute lymphoblastic leukemia and, most recently, mantle cell lymphoma), but until recently were not available for the treatment of indolent lymphoma. In March 2021, after a priority review, FDA granted accelerated approval to axicabtagene ciloleucel for the treatment of adult patients with r/r FL after 2 or more lines of systemic therapy. Herein, we summarize the FDA clinical review and regulatory considerations regarding this licensing application.
Trial Design
ZUMA-5 (NCT03105336) is a single-arm, multicenter, open-label phase II study that evaluated a single infusion of axicabtagene ciloleucel in adults with r/r indolent non-Hodgkin lymphomas (NHLs). Eligible patients had r/r FL grade 1-2 or 3a or marginal zone lymphoma (MZL) after receipt of 2 or more lines of systemic therapy, including an anti-CD20 monoclonal antibody combined with an alkylating agent. The study excluded patients with splenic MZL, transformed lymphoma, prior allogeneic hematopoietic stem cell transplantation, prior CD19-directed therapy, or any history of central nervous system (CNS) lymphoma or CNS disorders. Requirements included an Eastern Cooperative Oncology Group performance status of 0 or 1, absolute neutrophil count ≥1000/µL, absolute lymphocyte count ≥100/µL, creatinine clearance ≥60 mL/minute, hepatic transaminases ≤2.5 times the upper limit of normal, cardiac ejection fraction ≥50%, and absence of active infection.
Axicabtagene ciloleucel was administered at a one-time target dose of 2 × 106 CAR-positive viable T cells per kg body weight, with a maximum allowable dose of 2 × 108 CAR T cells. To facilitate CAR T-cell engraftment and expansion, lymphodepleting chemotherapy was administered prior to CAR T-cell infusion (cyclophosphamide 500 mg/m2 intravenously and fludarabine 30 mg/m2 intravenously, both given on the fifth, fourth, and third day before scheduled infusion). Day 0 denotes the day of CAR T-cell infusion. The trial permitted bridging therapy between leukapheresis and lymphodepleting chemotherapy at the discretion of the treating physician. All patients were hospitalized for the CAR T-cell infusion and for a minimum of 7 days afterward.
The primary endpoint was objective response rate (ORR) as determined by an independent review committee (IRC) using Lugano criteria.11 The primary efficacy analysis presented is based on the FDA’s adjudication of the IRC assessments.
Results
Study Populations
In the ZUMA-5 study, 146 patients with FL or MZL received axicabtagene ciloleucel and comprise the overall safety population. The median time from leukapheresis to site receipt of the product was 17 (range: 13-72) days. In total, 75% of products were delivered by 20 days and 92% were delivered by 30 days. There were no failures of product manufacturing.
In total, 123 patients who underwent leukapheresis informed the FL efficacy analysis, of whom 120 received axicabtagene ciloleucel; of these, 81 consecutively enrolled patients had at least 9 months of potential follow-up from the date of first response and comprised the primary efficacy population. In patients with FL, the median dose of axicabtagene ciloleucel was 2.0 × 106 CAR positive T cells/kg (range: 1.3-2.1 × 106 CAR positive T cells/kg).
Efficacy
Characteristics of the efficacy populations are described in Table 2. In the primary efficacy population (n = 81), the median age was 62 years. The median number of prior systemic therapies was 3 (range: 2-9), with 32% of patients having 2 prior lines, 22% having 3 prior lines, and 46% having ≥ 4 prior lines of systemic therapy. Thirty-one percent had received a PI3K inhibitor, 72% had progression within 6 months of their most recent regimen, and 56% had progression within 24 months of initiating their first anti-CD20 combination therapy (POD24). Between leukapheresis and administration of axicabtagene ciloleucel, one patient (1%) in the primary efficacy analysis received bridging therapy (dexamethasone).
Table 2.
Characteristics of the FL efficacy populations.
| Parameter | Result | |
|---|---|---|
| Primary efficacy population (N = 81) | All leukapheresed (N = 123)a,b | |
| Age, years | ||
| Median (range) | 62 (34-79) | 60 (34-79) |
| ≥ 65, n | 29 (36%) | 39 (32%) |
| Diagnosis, n | ||
| FL grade 1-2 | 59 (73%) | 93 (76%) |
| FL grade 3a | 22 (27%) | 30 (24%) |
| Stage III-IV disease, n | 71 (88%) | 105 (85%) |
| Prior lines of systemic therapy, n | ||
| Median (range) | 3 (2-9) | 3 (1-10) |
| 2 | 26 (32%) | 43 (35%) |
| 3 | 18 (22%) | 31 (25%) |
| 4 | 19 (23%) | 25 (20%) |
| 5 or more | 18 (22%) | 20 (16%) |
| Outcome of last therapy | ||
| Type of response | ||
| At least PR | 36 (44%) | 58 (47%) |
| No response | 33 (41%) | 46 (38%) |
| Not evaluable or unknown | 12 (15%) | 18 (15%) |
| Progression within 6 months | 58 (72%) | 83 (67%) |
| POD 24 | 45 (56%) | 67 (54%) |
| Prior regimens | ||
| Lenalidomide + rituximab | 19 (23%) | 26 (21%) |
| PI3K inhibitor | 25 (31%) | 33 (27%) |
Excludes 4 patients diagnosed with FL but found to have aggressive lymphoma after enrollment.
Includes 3 patients who did not receive axicabtagene ciloleucel.
Abbreviations: FL, follicular lymphoma; PI3K, phosphoinositide 3-kinase; POD24, progression of disease within 24 months of first chemoimmunotherapy.
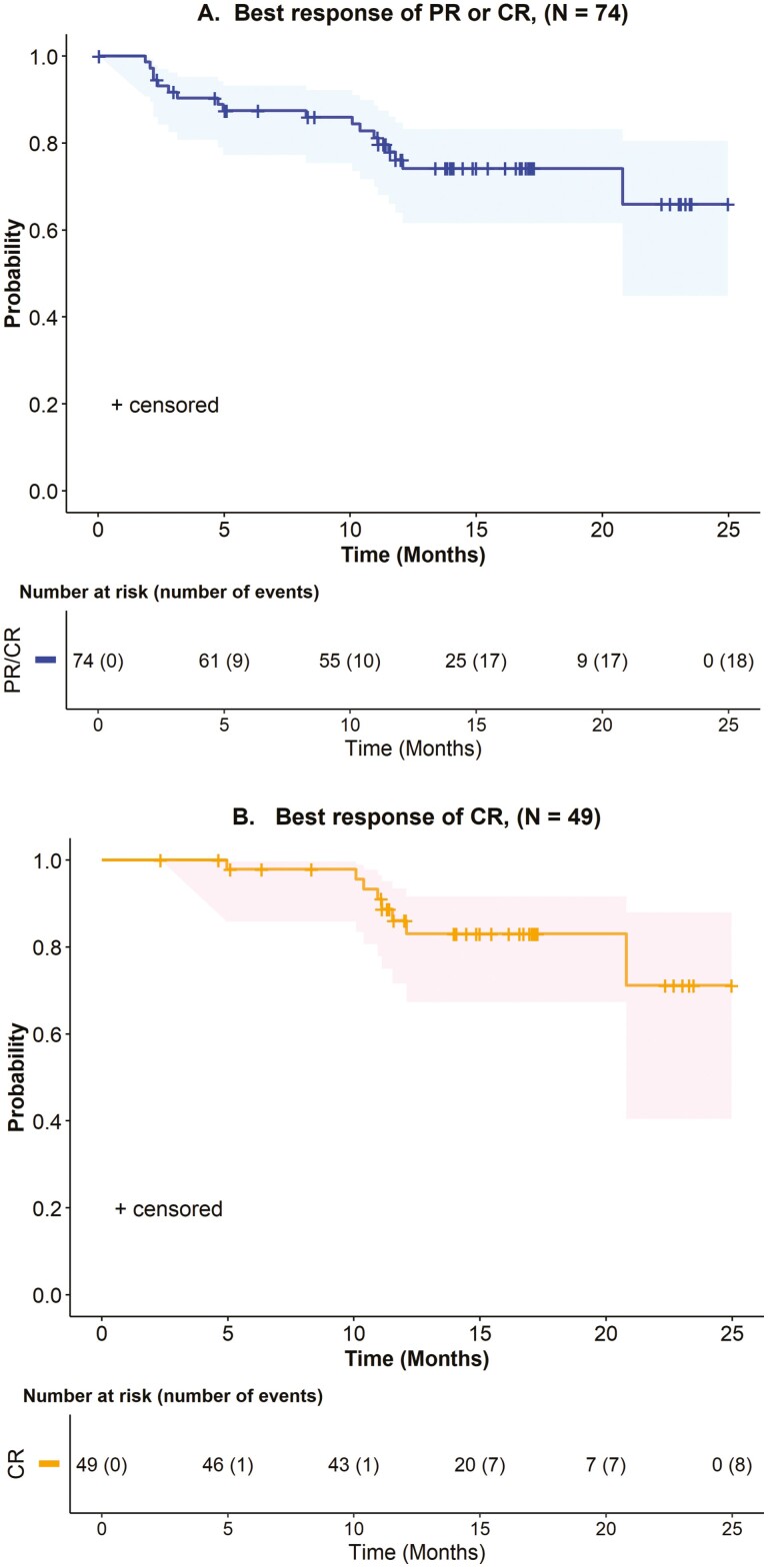
Key efficacy results are shown in Table 3. In the primary efficacy population, the ORR was 91% (95% confidence interval [CI]: 83-96), with a complete remission (CR) rate of 60%. The median time to response was 1.0 month (range: 0.8-3.1 months). After an estimated median follow-up of 14.5 months, the median duration of response (DOR) was not estimable. The estimated 1-year rate of continued remission was 76% (95% CI: 64-85) among all patients who achieved an objective response, and 83% (95% CI: 72-94) among patients who achieved CR (Table 3, Fig. 1).
Table 3.
IRC-assessed efficacy results in relapsed or refractory FL.
| Outcome | Result | |
|---|---|---|
| Primary efficacy population (N = 81) | All leukapheresed (N = 123)d | |
| Best overall response, n (%) | ||
| Objective response | 74 (91%) | 110 (89%) |
| (95% CI for ORR) | (83-96) | (83-94) |
| CRa | 49 (60%) | 76 (62%) |
| (95% CI for CR rate) | (49-71) | (53-70) |
| PR | 25 (31%) | 34 (28%) |
| (95% CI for PR rate) | (21-42) | (20-36) |
| Stable or progressive disease | 4 (5%) | 7 (6%) |
| Not evaluableb | 3 (4%) | 6 (5%) |
| DOR c | ||
| Estimated median DOR (95% CI), months | NE (20.8-NE) | — |
| Rate of DOR [95% CI], % | ||
| At 6 months | 88 (77-93) | — |
| At 12 months | 76 (64-85) | — |
| At 18 months | 74 (62-83) | — |
| Rate of DOR if CR achieved (95% CI), % | ||
| At 6 months | 98 (94-100) | — |
| At 12 months | 86 (76-97) | — |
| At 18 months | 83 (72-96) | — |
CR required documentation of a negative bone marrow biopsy after treatment, in patients who did not have a negative bone marrow biopsy between their most recent disease progression prior to ZUMA-5 and initiation of lymphodepleting chemotherapy.
Includes indeterminate cases.
Kaplan-Meier estimates, after 14.5 month median follow-up (by reverse Kaplan-Meier method) for all responders in the primary efficacy population. DOR is measured from the date of first objective response to the date of progression or death from any cause.
Includes 3 patients who did not receive axicabtagene ciloleucel.
Abbreviations: CR, complete remission; DOR, duration of response; FL, follicular lymphoma; IRC, independent review committee; NE, not estimable; ORR, objective response rate; PR, partial remission.
Figure 1.
Kaplan-Meier estimates (95% CI) of duration of response in the primary efficacy population. (A) Patients with FL who achieved an objective response. (B) Patients with FL who achieved a best overall response of CR. CI, confidence interval; CR, complete remission; FL, follicular lymphoma.
In the overall FL efficacy population (n = 123), the ORR was 89%, with a CR rate of 62% (Table 3). On exploratory subgroup analysis, the ORR was consistent across multiple disease-related subgroups, including presence of POD24 (ORR 87%; 95% CI: 76-94), receipt of 3 or more prior lines of systemic therapy (ORR 89%; 95% CI: 80-95), and receipt of prior R2 (ORR 88%; 95% CI: 70-98). These assessments were limited by sample size (Table 2).
Of 20 patients with MZL who informed efficacy, the IRC-assessed ORR was 85% (95% CI: 62-97).
Safety
An overview of safety is next provided, with a focus on adverse reactions (ARs) of special interest.
Safety Overview
Table 4 summarizes the most common ARs in the ZUMA-5 safety population (n = 146). ARs affecting ≥ 30% of patients, excluding laboratory terms, included fever, cytokine release syndrome (CRS), hypotension, encephalopathy, fatigue, headache, tachycardia, infections with pathogen unspecified, febrile neutropenia, musculoskeletal pain, nausea, and tremor (Table 4).
Table 4.
Selected adverse reactions in patients with indolent NHL.
| Adverse reactions by categorya | Result (N = 146)b | ||
|---|---|---|---|
| Any grade, % | Grade 3 or higher, % | ||
| Adverse reactions (≥20% any grade or ≥5% Grade ≥3), excluding laboratory terms c | |||
| General | Fever | 85 | 8 |
| Fatigue | 49 | 0.7 | |
| Chills | 29 | 0 | |
| Immune system | CRS | 84 | 8 |
| Cardiac or vascular | Hypotension | 51 | 4.1 |
| Tachycardia | 44 | 1.4 | |
| Arrhythmia | 21 | 2.1 | |
| Hypertension | 13 | 6 | |
| Nervous system or psychiatric | Encephalopathy | 50 | 16 |
| Headache | 45 | 1.4 | |
| Tremor | 31 | 0.7 | |
| Dizziness | 20 | 0 | |
| Delirium | 16 | 5 | |
| Infections | Infections, pathogen unspecified | 42 | 14 |
| Pneumonia | 13 | 8 | |
| Blood and lymphatic system | Febrile neutropenia | 41 | 19 |
| Gastrointestinal | Nausea | 40 | 0 |
| Diarrhea | 29 | 0.7 | |
| Constipation | 28 | 0 | |
| Vomiting | 24 | 0.7 | |
| Musculoskeletal | Musculoskeletal pain | 40 | 1.4 |
| Metabolism and nutrition | Decreased appetite | 26 | 1.4 |
| Respiratory | Cough | 25 | 0 |
| Hypoxia | 23 | 8 | |
| New or worsening laboratory abnormalities (≥20% Grade 3 or 4) c | |||
| Hematologic | Neutrophil count decrease | 98 | 92 |
| Platelet count decrease | 79 | 35 | |
| Hemoglobin decrease | 81 | 30 | |
| Lymphocyte count decrease | 23 | 23 | |
| Chemistry | Phosphate decrease | 79 | 25 |
Includes grouped preferred terms. Refer to prescribing information for definitions. Toxicities were graded using NCI CTCAE version 4.03.
Includes 4 patients with FL diagnosis at enrollment, who were identified as having transformed lymphoma or DLBCL on central pathology review. These patients were excluded from the efficacy analysis.
Baseline for adverse reactions and laboratory values was assessed immediately prior to axicabtagene ciloleucel infusion.
Abbreviations: DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; NCI, National Cancer Institute Common Terminology Criteria for Adverse Events; NHL, non-Hodgkin lymphoma.
Grade ≥ 3 ARs occurred in 86% of patients, with Grade ≥ 3 ARs of special interest including febrile neutropenia (41%), prolonged cytopenias (32%), neurological toxicities (21%), infections (16%), and CRS (8%). Serious ARs occurred in 48% of patients; serious ARs in ≥10% of patients were encephalopathy (17%), fever (14%), CRS (14%), and pneumonia (7%). Two (1.4%) patients had fatal ARs (CRS with multiorgan failure; coccidiomycosis). Blindness, likely attributable to fludarabine, occurred in one patient. Grade 3 and 4 laboratory abnormalities in ≥10% of patients included neutropenia, leukopenia, thrombocytopenia, and anemia (Table 4).
ARs of Special Interest
CRS and neurological toxicity are leading safety concerns of axicabtagene ciloleucel. In the ZUMA-5 study, CRS of any grade, based on modified Lee criteria,12 occurred in 123 (84%) patients, including an 8% incidence of Grade ≥ 3 CRS. Manifestations of CRS in ≥ 20% of patients included fever, hypotension, tachycardia, chills, and hypoxia. The median time to CRS onset was 4 days (range: 1-20), with a median time to onset of maximum grade CRS of 5 days (range: 1-20). The median duration of CRS was 6 days (range: 1-27).
Neurological toxicity, as defined by FDA, was reported in 77% of patients, with a 21% incidence of Grade ≥ 3 neurological toxicity. In total, 9% of the safety population developed neurological toxicity without developing CRS. The most common neurological toxicities included encephalopathy, headache, tremor, dizziness, delirium, insomnia, affective disorder, and ataxia. The median time to onset of any neurological toxicity was 6 days (range: 1-79), with 74% of cases identified within the first 7 days of product infusion and virtually all (99%) occurring within the first 8 weeks of infusion. Neurological toxicities resolved in 96% of affected patients, with a median time to resolution of 16 days (Q1, Q3: 7, 50). Of the 124 patients with FL, neurological toxicities developed in 76% (Grade ≥ 3, 18%), and CRS developed in 81% (Grade ≥ 3, 6%).
Among all 146 patients in the ZUMA-5 safety population, 75 (51%) received tocilizumab, 23 of whom received more than one dose (range: 2-4 doses). Systemic steroids were used to manage neurological toxicities in 54 (37%) patients and to manage CRS in 29 (20%) patients.
In registrational studies of axicabtagene ciloleucel, among all patients with aggressive or indolent NHL (n = 254), CRS occurred in 88% (Grade ≥ 3, 10%) and neurological toxicities occurred in 81% (Grade ≥ 3, 26%).13 The most common non-laboratory ARs (incidence ≥20%) in patients with NHL included CRS, fever, hypotension, encephalopathy, tachycardia, fatigue, headache, febrile neutropenia, nausea, infections with pathogen unspecified, decreased appetite, chills, diarrhea, tremor, musculoskeletal pain, cough, hypoxia, constipation, vomiting, arrhythmias, and dizziness.13
The U.S. prescribing information (USPI) for axicabtagene ciloleucel contains boxed warnings for CRS and neurological toxicities, which in both cases have included fatal and life-threatening reactions. Other Warnings and Precautions in the prescribing information include hypersensitivity reactions, serious infections, prolonged cytopenias, hypogammaglobulinemia, secondary malignancies, and, due to the potential for neurological events, restrictions on driving and other activities.
Clinical Pharmacology
In patients with r/r FL, axicabtagene ciloleucel exhibited an initial rapid expansion phase achieving maximal expansion (Cmax) around 8 days post-infusion, followed by a bi-phasic decline. CAR T cells were detected in peripheral blood for a median of 26 weeks (range: 1 week to 2.2 years), demonstrating the potential for long-term persistence. There was no apparent association identified between axicabtagene ciloleucel expansion and age or sex. Higher percentages of both total CCR7+ T cells and CCR7+ CD45RA+ naïve T cells in the infused product were associated with greater expansion of the CAR T cells.
Higher axicabtagene ciloleucel exposures were observed in patients with FL who developed severe CRS (Grade ≥ 3) or severe neurological toxicity. Patients with Grade ≥ 3 CRS (n = 8) had a 7.7 fold higher median Cmax and median AUC0-28d of CAR T cells compared to patients with Grade 2, Grade 1, or no CRS (n = 126). Patients with Grade ≥ 3 neurological toxicity (n = 22) had a 2.4 fold higher median Cmax and 1.7 fold higher median AUC0-28d of CAR T cells compared to patients with Grade 2, Grade 1, or no neurological toxicity (n = 102). The CAR T cells continued to expand following administration of tocilizumab, corticosteroids, or both after infusion. Data on the rate of expansion in relation to receipt of tocilizumab were not available.
Due to the on-target effect of axicabtagene ciloleucel, a period of B-cell aplasia is expected. At 12 months post axicabtagene ciloleucel infusion, the B-cell levels recovered in 53% of evaluable patients (26 of 49), as assessed by peripheral blood flow cytometry.
Regulatory Considerations
Based on the ORR and DOR observed in ZUMA-5’s primary efficacy analysis population, in conjunction with an acceptable safety profile, axicabtagene ciloleucel was granted accelerated approval for the treatment of adult patients with r/r FL after 2 or more lines of systemic therapy. FDA’s benefit-risk analysis is summarized in Table 5. This application was granted priority review, breakthrough therapy designation, and orphan drug designation. There were multiple regulatory considerations throughout the review process, including those summarized below.
Table 5.
FDA benefit-risk analysis axicabtagene ciloleucel for r/r FL.
| Parameter | Summary |
|---|---|
| Unmet medical need | Patients with relapsed or refractory FL after 2 or more regimens have unmet medical needs. |
| Clinical Benefit | Of 81 patients in the primary efficacy population, ORR was 91% (95% CI: 83-96) and CR rate 60%, with a 1-year rate of continued remission of 76% (95% CI: 64-85). |
| Risks | • The USPI for axicabtagene ciloleucel has Warnings and Precautions for CRS, neurological toxicity, serious infections, prolonged cytopenias, hypogammaglobulinemia, and secondary malignancies. • Of 146 patients with iNHL treated with axicabtagene ciloleucel: - ARs in ≥ 30%, excluding laboratory terms, included fever, CRS, hypotension, encephalopathy, fatigue, headache, tachycardia, infections with pathogen unspecified, febrile neutropenia, musculoskeletal pain, nausea, and tremor. - Grade ≥ 3 ARs occurred in 86%; the most common Grade ≥ 3 ARs of interest included febrile neutropenia(41%), prolonged cytopenias (32%), neurological toxicities (21%), infections (16%), and CRS (8%). - Any grade of CRS occurred in 84% patients, neurological toxicity in 77%, serious ARs in 48%, and fatal ARs in 1.4%. |
| Uncertainties | • Verification of clinical benefit in a confirmatory trial(s) in FL is pending. • Long-term safety after treatment with axicabtagene ciloleucel, particularly for secondary malignancies, is undefined. |
| Conclusions | • The ZUMA-5 study provides substantial evidence of effectiveness in patients with r/r FL after 2 or more regimens, with an acceptable safety profile. • CRS and neurological toxicity can be life-threatening or fatal, supporting boxed warnings in labeling and a REMS. |
Abbreviations: CI, confidence interval; FL, follicular lymphoma; REMS, risk evaluation and mitigation strategy.
Adjudication of Efficacy
During review of the application, FDA reclassified 16% (13/81) patients in the primary efficacy analysis as having a best overall response (BOR) of partial remission (PR), rather than complete remission (CR), due to missing bone marrow assessments. These patients achieved radiographic CR, but did not have a documented negative bone marrow biopsy within 4 weeks of screening or the confirmatory negative bone marrow biopsy required per study protocol to meet CR criteria. Moreover, protocol requirements for the timing of baseline bone marrow examination changed during the course of the study. However, to meaningfully analyze study data, the same analysis rules should be consistently applied. According to the Lugano criteria11 upon which the study’s primary endpoint was based, the data in lymphoma histologies other than Hodgkin lymphoma or diffuse large B-cell lymphoma are insufficient to change the standard practice of evaluating bone marrow involvement through bone marrow biopsy. Accordingly, FDA’s approach for the primary efficacy analysis was to require a bone marrow biopsy/aspirate negative for lymphoma to confirm CR, except for patients having a negative bone marrow examination performed between diagnosis of their most recent pre-trial disease progression and initiation of ZUMA-5 study treatment.
Although ORR, and thus the study’s primary endpoint, was not impacted by these missing data, the extent of missing bone marrow exams reduced the CR rate and limited estimation of the magnitude of efficacy in patients with r/r FL. Given the uncertainty surrounding the depth of response, the USPI describes DOR for all responding patients in the FL primary efficacy population, without sub-analyses of DOR according to BOR. This contrasts with the labeling for axicabtagene ciloleucel for patients with r/r large B-cell lymphoma.13 Importantly, in patients with r/r large B-cell lymphoma, durable remissions to axicabtagene ciloleucel are largely restricted to patients who achieve CR.10,13 Because of the extent of incomplete response data in ZUMA-5, assessment of the durability of response is limited in patients with FL who achieve PR only, as some such patients may have had CR.
Considerations for Accelerated Approval
Criteria for accelerated approval stipulate that the data must demonstrate a meaningful advantage in the context of available therapies.14 Because available therapy (defined as therapy with regular approval for the proposed indication) is determined at the time of regulatory action on the licensing or marketing application, seeking accelerated approval can be especially challenging in a disease area such as indolent NHL with multiple ongoing development programs. In contrast to the FL data, there were insufficient data to support an indication for axicabtagene ciloleucel in patients with r/r MZL, in part due to the small sample size and immature follow-up.
For the FL indication, the basis of the efficacy determination was ORR supported by durability of response. There is not enough information to assess the longer-term durability of the treatment effect with axicabtagene ciloleucel in patients with r/r FL, in comparison to that observed with currently available therapies. Because the ZUMA-5 eligibility criteria require prior treatment with an alkylating agent, the most relevant available therapy for consideration was R2, which has regular approval for the treatment of FL and MZL in the second-line setting and beyond. In the primary efficacy population, of the 19 (23%) patients with FL treated previously with R2, the ORR with axicabtagene ciloleucel was 89% (95% CI: 67-99), with 10/17 responses lasting ≥ 12 months. Although more data would be helpful, the ZUMA-5 study provided evidence that axicabtagene ciloleucel can have meaningful clinical activity in patients with FL that have relapsed after or is refractory to R2.
ORR and DOR are intermediate endpoints that are reasonably likely to predict clinical benefit.14 Continued approval of the FL indication may be contingent upon verification of clinical benefit in a confirmatory trial(s), which is being pursued as a post-marketing requirement (PMR).
Adjudication of ARs of Special Interest
FDA identified higher rates and severity of CRS and neurological toxicity than identified by the applicant. CRS was reported and graded as a syndrome using a modification of the grading system proposed by Lee and colleagues.12 It should be noted that in the original Lee grading scale, neurological ARs were included as symptoms of CRS. In the applicant’s modification of the Lee grading scale, neurological ARs were reported separately using the National Cancer Institute Common Terminology Criteria for Adverse Events criteria v4.03. Although the applicant’s CRS grading was largely concordant with the FDA’s findings, FDA adjudicated 4 (2.7%) other patients as having CRS and 2 (1.4%) other patients as having Grade ≥ 3 CRS. FDA’s review strategy included identifying fever, hypotension, or hypoxia between Day 0 and Day 30 in the patients who were not recognized by the applicant as having CRS. FDA also identified patients who received tocilizumab, vasopressors, intravenous fluids, or oxygen and who were not in the applicant’s CRS database. Cytokine and laboratory data (ferritin, C-reactive protein, IL-6 levels) were used for supportive evidence. Patients identified as having isolated symptoms such as hypotension without other symptoms suggestive of CRS were not included.
Based on FDA’s review, any grade neurological toxicity increased from 60%, as reported by the applicant, to 77%, and Grade ≥ 3 cases increased from 19% to 21%. The Applicant defined neurological toxicities as per Topp et al.15 In contrast, neurological toxicities were more broadly defined by FDA as all events classified as nervous system disorders or psychiatric disorders, excluding sleep disorders and disturbances and peripheral neuropathies. However, ARs such as insomnia when occurring in setting of other neurological events were included in FDA’s definition. Furthermore, other ARs under other body systems (system organ classes) that were not classified as neurological toxicity, but that overlapped with other neurological events, were also included in the FDA’s definition of neurological toxicity. Isolated events (not overlapping with other neurological or psychiatric symptoms) or ARs that started relatively late post axicabtagene ciloleucel infusion and were unlikely to represent neurological toxicity were not included in this definition.
Risk Mitigation and Post-marketing Studies
During the ZUMA-5 study, life-threatening and fatal ARs caused by axicabtagene ciloleucel were mitigated by mandated site and investigator training, careful site selection and monitoring, instructions for early detection and management of the most serious complications, and a requirement for in-patient administration and in-patient monitoring for 7 days following the infusion. The life-threatening and fatal ARs warranted warnings in the labeling, including a boxed warning for CRS and neurological toxicity, and a risk evaluation and mitigation strategy with elements to assure safe use.
The genetic modification of axicabtagene ciloleucel triggers an additional safety concern. Generation of replication-competent retrovirus during the manufacturing process for axicabtagene ciloleucel is a theoretical safety concern. Additionally, insertional mutagenesis due to vector integration is a potential risk for inducing secondary malignancies. Integration of the vector into the patient’s cells might inadvertently activate a cellular protooncogene or disrupt a tumor suppressor gene, leading to malignant transformation events.
Long-term safety following axicabtagene ciloleucel treatment remains important due to the limited duration of follow-up duration, and the potential risk of insertional mutagenesis from the retrovirus. Therefore, the PMR study that was issued with the original approval was amended to include a separate protocol for patients with FL. This PMR requires 15 years of follow-up for all patients treated with axicabtagene ciloleucel to assess its long-term toxicities.
Conclusion
The results of the ZUMA-5 study support the determination that a single dose of axicabtagene ciloleucel has clinically meaningful efficacy and is reasonably likely to confer clinical benefit in patients with r/r FL after 2 or more lines of systemic therapy. Careful patient selection and close monitoring for toxicities are, however, of utmost importance. Post-marketing study data are needed to confirm the clinical benefit of axicabtagene ciloleucel in patients with r/r FL. As a PMR, a randomized phase III trial will evaluate axicabtagene ciloleucel as compared to standard of care therapy in patients with r/r FL, with a primary endpoint of progression-free survival.
Contributor Information
Najat Bouchkouj, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD, USA.
Megan Zimmerman, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD, USA.
Yvette L Kasamon, Center for Drug Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD, USA.
Cong Wang, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD, USA.
Tianjiao Dai, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD, USA.
Zhenzhen Xu, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD, USA.
Xiaofei Wang, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD, USA.
Marc Theoret, Center for Drug Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD, USA; Oncology Center of Excellence, U.S. Food and Drug Administration, Silver Spring, MD, USA.
Tejashri Purohit-Sheth, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD, USA.
Bindu George, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Silver Spring, MD, USA.
Conflict of Interest
The authors indicated no financial relationships.
Author Contributions
Conception/design: N.B., M.Z., Y.L.K. Collection and/or assembly of data: N.B., M.Z., Y.L.K., C.W., T.D., and X.W. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors.
References
- 1. Zelenetz AD, Gordon LI, Abramson JS, et al. NCCN Guidelines: B-Cell Lymphomas, Version 1.2022; 2021. Accessed May 13, 2021. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf.
- 2. Leonard JP, Trneny M, Izutsu K, et al. AUGMENT: a phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol. 2019;37:1188-1199. 10.1200/JCO.19.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108:2366-2372. 10.1182/blood-2006-04-015545 [DOI] [PubMed] [Google Scholar]
- 4. Andorsky DJ, Coleman M, Yacoub A, et al. MAGNIFY: Phase IIIb interim analysis of induction R2 followed by maintenance in relapsed/refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37:abstract 7513. [Google Scholar]
- 5. Kahl BS, Bartlett NL, Leonard JP, et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study. Cancer. 2010;116:106-114. 10.1002/cncr.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gibiansky E, Gibiansky L, Buchheit V, et al. Pharmacokinetics, exposure, efficacy and safety of obinutuzumab in rituximab-refractory follicular lymphoma patients in the GADOLIN phase III study. Br J Clin Pharmacol. 2019;85:1935-1945. 10.1111/bcp.13974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol. 2015;33:2516-2522. 10.1200/JCO.2014.59.7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sortais C, Lok A, Tessoulin B, et al. Progression of disease within 2 years (POD24) is a clinically relevant endpoint to identify high-risk follicular lymphoma patients in real life. Ann Hematol. 2020;99:1595-1604. 10.1007/s00277-020-04025-2 [DOI] [PubMed] [Google Scholar]
- 9. Locke FL, Go WY, Neelapu SS.. Development and use of the anti-CD19 chimeric antigen receptor T-cell therapy axicabtagene ciloleucel in large B-cell lymphoma: a review. JAMA Oncol. 2020;6:281-290. 10.1001/jamaoncol.2019.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bouchkouj N, Kasamon YL, de Claro RA, et al. FDA approval summary: axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma. Clin Cancer Res. 2019;25:1702-1708. 10.1158/1078-0432.CCR-18-2743 [DOI] [PubMed] [Google Scholar]
- 11. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-3068. 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188-195. 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kite Pharma, Inc. YESCARTA (Axicabtagene Ciloleucel) Suspension for Intravenous Infusion: Prescribing Information. Accessed October 27, 2021. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/yescarta-axicabtagene-ciloleucel.
- 14. U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for Industry: Expedited Programs for Serious Conditions—Drugs And Biologics; 2014. Accessed October 20, 2021. https://www.fda.gov/media/86377/download.
- 15. Topp MS, Gokbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57-66. 10.1016/S1470-2045(14)71170-2 [DOI] [PubMed] [Google Scholar]