Abstract
The development of in vitro toxicity assessment methods using cultured cells has gained popularity for promoting animal welfare in animal experiments. Herein, we briefly discuss the current status of hepatoxicity assessment using human- and rat-derived hepatocytes; we focus on the liver organoid method, which has been extensively studied in recent years, and discuss how toxicologic pathologists can use their knowledge and experience to contribute to the development of in vitro chemical hepatotoxicity assessment methods for drugs, pesticides, and chemicals. We also propose how toxicological pathologists should assess toxicity regarding the putative distribution of undifferentiated and differentiated cells in the organoid when liver organoids are observed in hematoxylin and eosin–stained specimens. This was done while considering the usefulness and limitations of in vitro studies for toxicologic pathology assessment.
Keywords: chemical hepatotoxicity, in vitro, organoids, rat, toxicologic pathologist
Introduction
Laboratory animals are commonly used for evaluating the safety and toxicity of pharmaceuticals, agricultural chemicals, and chemical substances. Generally, animal testing is used for drug development as well as for research on food and livelihood. However, the growing concern for animal welfare worldwide in recent years has driven countries and societies to fully commit to reduce animal testing1, 2. If the world’s major corporations made an effort to reduce the number of animal tests by a certain percentage, they could reportedly conserve 150,000 rats each year3. Based on such studies, one can expect that toxicity tests in rodents will be replaced by alternative methods in the long run; however, there are many hurdles to bridge the gap between in vitro tests that are underway and in vivo preclinical studies in rodents with complex anatomical and physiological functions.
Toxicologic pathologists can objectively and thoroughly extract pathologic findings based on basic knowledge of laboratory animal anatomy, histology, physiology, and biochemistry, as well as the applied fields of pharmacology and toxicology. Toxicologic pathologists should be able to compare the detected findings with general symptom observations, urinalysis, hematology, biochemistry, and metabolic studies. They should also be able to understand the results of various toxicologic studies from a bird’s eye view and integrate, explain, and discuss them with drug development team members with varied expertise. Thus, toxicologic pathologists play an important role in toxicity assessments and drug development. Although alternative methods continue to be developed, the question of how to utilize the knowledge and experience of toxicologic pathologists has become a very important issue.
In the case of the liver, the hepatic artery branch entering from the aorta and the portal vein entering from the gastrointestinal tract join at the portal region of the lobule4, 5. Hence, blood from both these tracts flows into sinusoids, which occur continuously, not in a single lobule but in multiple lobules, and are concentrated toward the central hepatic vein5, 6. Carbohydrates, minerals, and drugs enter hepatocytes via passive and active diffusion (including various transporters). In addition to basic metabolism, including adenosine triphosphate production by the mitochondria, phase I and II drug-metabolizing enzymes are distributed in hepatocytes, particularly in the endoplasmic reticulum, and are responsible for activating or inactivating drugs, thereby delivering active ingredients throughout the body and improving the local pathological conditions5, 7, 8. Toxicologic pathologists also understand the lobular and acinar structures containing hepatic cords5, 9 and help detect basic changes in hepatocytes, such as degeneration, necrosis, apoptosis, hypertrophy, and hyperplasia, via microscopy10. They also recognize that non-hepatocellular components, such as sinusoidal endothelial cells, Kupffer cells, and perivascular stellate cells, are distributed within the lobules. Histopathologic changes linked to various cellular reactions can occur as a secondary effect on hepatocytes or as a primary effect on these cells. An even more important part of fluid components is bile flow11. Surprisingly, bile flows in a direction opposite to that of the blood. Bile acids secreted by hepatocytes enter the capillary bile ducts located within the hepatic cord, remain concentrated in the bile ducts of the portal region, and are transported out of the liver against blood flow12. Bile stasis is an important side effect of drugs. Toxicologic pathologists understand that the distribution and coloration of bile plugs are different from those of hemosiderin and lipofuscin10.
Considering these hepatotoxicological studies, toxicologic pathologists must question whether alternative methods using hepatocytes can accurately assess toxicity based on physiologically controlled experimental conditions. Developing useful hepatocellular carcinoma cell lines, starting with short-term primary culture of hepatocytes, has enabled relatively long-term culturing and repeated replication experiments13, 14. Stem cell-derived hepatocyte-like cells were introduced, and expectations for their application in liver toxicology quickly increased. However, their limitations soon became apparent15. Spheroid and organoid research has expanded drastically and has been applied to hepatocytes, making it possible to evaluate them as a hepatocyte population rather than as a single cell population (Fig. 1). In contrast, toxicologic pathologists who focused on changes within the lobular structures of the liver also showed interest in liver slice techniques; however, they are concerned that such techniques can only be used for relatively short-term cultures13. Toxicologic pathologists would have to ask whether such an in vitro system is capable of assessing toxicity with the same quality as chemical hepatotoxicity assessment in experimental animals.
Fig. 1.

Representative image of an organoid derived from the liver of a chemical-treated rat (Uomoto et al., unpublished data).
Considering these, our review will help readers understand the current status of alternative methods for toxicological assessment using hepatocytes or hepatocyte-like cells. Our review also discusses the ways in which toxicologic pathologists should participate in various platforms for hepatotoxicity assessment.
Human Hepatocyte-derived Cells for Toxicology Assessment
If pharmaceuticals, pesticides, and general chemicals are developed for human use, their toxicity can be evaluated using human-derived cells13, 14. However, ensuring a permanent supply of human cells to contribute to the development of drugs and other products worldwide is a major challenge. Cryopreserved human hepatocytes are available from several sources. However, in the future, it will be essential to increase the stock and establish a supply and sales route to obtain cells from many donors. The implication of human origin is that the cells are derived from individual patients or volunteers. Understanding and improving the fundamental aspects of drug toxicity assessment, such as gene polymorphisms, presence, and strength of drug enzymes derived from an individual, is also a major issue16. Kyffin et al.13 and Kammerer14 provided a detailed description of the current utility and shortcomings of primary cultures of human hepatocytes: HepG2 cells derived from hepatoblastomas, HepaRG cells derived from liver tumors, induced pluripotent stem cell (iPSC)-derived hepatocytes, and upcyte®-based hepatocytes. In summary, primary hepatocytes are recognized as the gold standard for assessing toxicity during the culture period, when hepatocyte characteristics can be maintained; however, they pose a major challenge in terms of cell supply. HepG2 cells express very low levels of drug-metabolizing enzymes. Given that iPSC-derived hepatocytes have many phenotypic characteristics, it may not be possible to obtain a comprehensive set of cell types that exhibit various toxic responses with the help of many collaborators. Studies on the differentiation of human skin-derived iPSCs into hepatocytes to screen for drugs that induce chemical hepatotoxicity and steatosis have been performed17, 18. In one such study, after obtaining informed consent from parents, human skin-derived iPSCs were harvested from boys aged 1–10 years. Assessment and establishment of this technique may make it relatively easier to construct large cell stocks. However, differences in hepatocyte-derived donor-dependent drug-metabolizing capacity have been investigated in primary hepatocytes from 19 donors for eight major human cytochrome P450s (CYPs). These were assessed with respect to sex- and age-related differences using acetaminophen, cyclophosphamide, ketoconazole, and tamoxifen19. Increased activity of CYP3A4, the most important CYP, is a risk factor for drug-induced liver injury. Variations in CYP expression among donors is not only caused by individual genetic polymorphisms but also by the environment where the individual has lived (possible induction of drug enzymes by various environmental factors from birth to age at donation). Second-generation upcyte® technology (https://www.upcyte.com/#technology) using lentiviral transfer of proliferation-inducing genes can greatly extend the lifespan of primary hepatocytes, enabling 21-day culture experiments and allowing drug screening, drug-to-drug interactions, and mechanism elucidation. According to the findings comparing them with HepG2 cells, upcyte®-derived hepatocytes are more comparable with primary hepatocytes20, 21, 22.
All cells need to thrive in a good microenvironment for proper functioning. Toxicologic pathologists are well aware that the hepatic cords are maintained by the extracellular matrix (ECM), which is an intrahepatic interstitial tissue5. Studies on hepatocyte differentiation based on iPSC technology have shown that it is easier to culture iPSC-derived hepatocyte-like cells using a bioplotted poly L-lactic acid (PLLA) scaffold than using other methods. The production of albumin and urea, which are indicators of hepatocyte differentiation, and the induction of various CYPs are enhanced when iPSC-derived hepatocyte-like cells are cultured using the PLLA scaffold with type I collagen infusion than with the sandwich method with type I collagen and Matrigel. This indicates that a three-dimensional (3D) environment is extremely important for hepatocytes to function well23. Similar enhancement of hepatocyte functions was observed in primary hepatocyte experiments performed using poly(L-lactide-co-glycolide) polymer scaffolds with type I collagen or fibronectin infusion24, 25.
Considering the exposure of humans to chemical substances, hepatotoxicity assessment using human-derived cells is an important research topic. Moreover, the supply route and storage of cells, stabilization and reproducibility of the culture method, and proposal of a standardized method have yet to be implemented worldwide.
Rodent Hepatocyte-derived Cells for Toxicology Assessment
Why is it necessary to conduct experiments on rodents to assess the safety and toxicity of pharmaceuticals, pesticides, and general chemical substances? The most important aspect of using laboratory animals is that there is a stable supply of rodents with decent genetic backgrounds at breeding facilities worldwide. This makes it possible to conduct experiments and confirm the reproducibility of previous studies performed by researchers in other countries or to improve test methods. However, this toxicity testing system, which uses the precious lives of laboratory animals, is undergoing a major transition. Toxicologic pathologists, whose work is based on euthanizing laboratory animals, collecting their organs, and examining those organs under a microscope, are now faced with the important challenge of contributing to the reduction of animal testing. Table 1 shows the in vitro model systems for chemical hepatotoxicity assessment that were used for histopathological examination. Toxicologic pathologists can thus enter the growing field of in vitro toxicity assessment and should collaborate with toxicologists to contribute to a more accurate detection of adverse effects and mechanisms.
Table 1. In Vitro Rat and Human Studies in Potentially Allowing for Evaluation of Hepatotoxicity with Morphological Characteristics.
Rat primary hepatocytes have long been used as an alternative for in vivo hepatotoxic assessments. The use of primary hepatocyte cultures to determine function and toxicity offers advantages over whole-animal models: 1) experimental conditions can be rigorously controlled in vitro, 2) less material is required for testing, 3) sample analysis is simplified, 4) contributions from other cell and tissue types can be avoided, and (5) a large number of samples can be obtained from a single adult animal26. Although the ability to exclude the effects of other cells has been cited as an advantage when assessing their effects on hepatocytes, it is now clear that hepatocytes alone have poor cell viability and do not fully maintain their functions, including CYP induction13, 14. Therefore, to compensate for the disadvantages of primary rat hepatocytes, improvements have been made by co-culturing them with ECM and other cells and devising new cell culture devices.
i) Modified hepatocyte culture with ECM
Two-dimensional (2D) and 3D culture systems have been widely proposed and established for rat primary hepatocytes. The 2D sandwich method is used to culture hepatocytes by sandwiching them between collagens. Rat hepatocytes were seeded in plates coated with neutralized collagen solution and overlaid with the rat tail collagen solution27. Compared with the monoculture, this method maintains CYP induction for a relatively longer period of time27 and is also effective in detecting drugs that exhibit bile stasis28, 29. Importantly, this method yielded results similar to using freshly isolated hepatocytes with cryopreserved rat hepatocytes30, thus making it an effective culture method in terms of reproducibility and versatility. Type I collagen is an essential ECM protein that is useful for cell survival, proliferation, differentiation, and adhesion. Utoh et al.31 reported that isolated hepatocytes from rat liver were mixed with fragmented collagen microfibers (average length up to 75 µm). Spheroids with collagen microfibrils for high cell-to-cell contact were obtained from non-cell-adhesion surfaces under high oxygen conditions to avoid hypoxia. From the perspective of toxicologic pathologists, hepatocytes with collagen microfibrils and bile canaliculi were closely packed in composite spheroids on hematoxylin and eosin (HE)-stained specimens. Morita et al.32 also demonstrated that fibrilized collagen microparticles, as intercellular binders, are useful for forming hepatocyte-based 3D tissues, resulting in a thick but planar morphology that is stably maintained in HE-stained sections. Future improvements in the microparticles and sizes of collagens are expected to better establish the conditions for maintaining the function and morphology of 3D hepatocytes.
Cell-to-cell interactions between hepatocytes are essential for maintaining hepatocyte function26. Ye et al.33 reported that hepatocytes with heparin-immobilized gelatin gel particle-embedded supportive polyurethane foam showed high local density and strong cell–cell contacts, as evident from HE stains. Heparin-modified thermoresponsive surfaces bound to heparin-binding epidermal growth factor-like growth factor were also designed to create hepatocyte sheets and maintain cell functions34. Cell-to-cell interactions were enhanced using fibrous scaffolds of polystyrene and poly(styrene-co-maleic acid) to obtain 3D hepatocytes. These were confirmed to be useful for evaluating the toxicity of acetaminophen (APAP)35.
ii) Co-culture of hepatocytes and non-hepatocytes
The production of ECM and cultures with non-hepatocytes is also a useful method for maintaining hepatocyte function. Yamada et al.12 reported that a microfluidic system for fabricating sandwich-type alginate hydrogel microfibers can incorporate rat hepatocytes and feeder cells (Swiss 3T3 cells). This results in hepatic cord-like hepatocytes surrounded by feeder cells and maintenance of albumin secretion and urea synthesis for up to 50 days. Lu et al.36 also demonstrated that the co-cultures of rat primary hepatocyte spheroids with NIH/3T3 mouse fibroblast cells on a galactosylated poly(vinylidene difluoride) surface self-assembled into multicellular spheroids. These that the hepatocyte spheroids were surrounded by fibroblasts, enhanced and prolonged albumin synthesis and CYP1 activity. Although the positional relationship between hepatocytes and non-hepatocytes is important for constructing hepatic cords from the perspective of toxicologic pathologists, co-culture systems of primary rat hepatocytes and rat stellate cells using silk porous scaffolds with ECM incorporation have stellate cells located in the central part and primary hepatocytes located at the periphery of the organoid tissues37. According to known concepts in toxicologic pathology, it is important to always observe the hepatic lobules or lobules with a focus on the hepatic cords, which are composed of hepatocytes. Moreover, it is not strange to consider the histological structure with a focus on the constituent cells of the sinusoids.
Thus, several methods have been devised to control the distribution of cells in co-cultures using unique cell culture devices. A unique method for creating micropatterned surfaces on dishes was proposed by Kang et al.38, who demonstrated that rat hepatocytes in hepatic cord-like zonal structures grew on regions with poly(allylamine) containing azidophenyl and β-galactose moieties in the side chains. Furthermore, human fibroblasts grew on regions with poly(methyl methacrylate) between hepatic cord-like structures. Fibroblasts produce ECM, including fibronectin, to maintain hepatocyte function. Micropatterned co-culture cell sheets were also used to prepare endothelial cell sheets, which adhered to the intervals of hepatocyte zonal structures39. Kim et al.40 reported that two-layered rat hepatocytes and bovine endothelial cell sheets are useful for maintaining hepatocyte function. By applying the sandwich method to endothelial cells instead of collagen, the same research group constructed functional triple-layered hepatic tissues comprising a rat hepatocyte sheet sandwiched between two bovine aortic endothelial cell sheets. This could maintain albumin secretion for up to 30 days9. Importantly, the cultured hepatocytes were repolarized with apical–basolateral poles and structurally resembled the liver microstructure. As failure to re-establish normal cell polarity and architecture is highly disadvantageous in initial studies involving rat primary hepatocyte culture26, co-culture with hepatocytes and endothelial cells should establish the surface of endothelial cells and canalicular networks. The micropatterning technique relying on a polydimethylsiloxane membrane demonstrated that hepatocytes were arranged on 2-mm-diameter circular islands, distanced 0.5 mm apart. Kupffer cells were seeded within the cap, resulting in Kupffer cell–hepatocyte interactions, which might have allowed the assessment of drug-induced inflammatory reactions41. Such unique ideas that are not limited to lobular or acinar structures may originate from novel ideas that toxicologists and cell biologists other than toxicologic pathologists can provide.
iii) Decellularized liver tissues
Toxicologic pathologists would be intrigued by the idea that using the liver as a scaffold for hepatocyte culture has been proposed. This method involves liver decellularization by refluxing hepatocytes and non-hepatocytes with Triton-X and/or sodium dodecyl sulfate and using the remaining stromal tissue as a template to redistribute the cells to be cultured42. In this method, the remaining hepatocytes and DNA interfere with the analysis after redistribution; therefore, reflux and validation methods were modified43, 44. Using this method, several researchers have investigated the differentiation of bone marrow-derived mesenchymal cells into hepatocytes45. Differentiated rat hepatocytes in decellularized miniature pig liver samples were confirmed in sections with HE stain and Periodic acid–Schiff (PAS) reaction (glycogen production)46. This has the potential to create a convenient microenvironment for the proliferation and differentiation of primary cultured hepatocytes. Monolayers of rat primary hepatocytes were cultured on films containing hepatocyte growth factor-immobilizable, soluble ECM derived from decellularized rat liver47. As HepG2 cells and human aortic endothelial cells can be maintained in decellularized liver tissue scaffolds derived from rats for up to five weeks48, they can be applied as a method for evaluating chemical hepatotoxicity. Toxicologic pathologists have always considered animal species differences in toxicity assessments and may suspect mixed-species microenvironments; however, pioneering cell biologists have made no such barriers and have contributed to the construction of “new livers”.
iv) Transplanted hepatocytes
To observe the morphology of hepatocytes, there is a limit to the culture dishes, and it is possible to observe the morphology more appropriately by transplanting them into mice. Ohashi et al.49 cultured 2D murine hepatocytes with cell–cell contacts and ECM deposition using a temperature-responsive polymer, poly(N-isopropylacrylamide), resulting in a real hepatic cord-like 3D morphology on HE staining and PAS reaction after transplantation in the subcutaneous tissues of mice. Surprisingly, they confirmed that the transplanted tissue persisted for at least 235 days in vivo. Although transplantation of cultured hepatocytes into animals may provide an optimal microenvironment for histopathological observation, it may be preferable to focus on establishing the liver microenvironment in a cultured environment as much as possible given the reduced number of donor animals.
v) Precision-cut liver tissue slice (PCLS)
Ex vivo PCLSs are beneficial for observing the pathology of hepatic cords and lobular structures in the liver (Fig. 2). As PCLSs contain all major cell types of the liver parenchyma and preserve the original cell–cell and cell–matrix contacts, toxicologic pathologists would observe PCLSs in HE stains, as seen in in vivo toxicity studies (Fig. 3). Moronvalle-Halley et al.50 demonstrated that thioacetamide induced apoptosis in HE-stained sections derived from PCLSs; the effects were correlated with activated caspase-3, as determined by immunohistochemistry and western blotting. They reported that apoptotic hepatocytes were either scattered or clustered around the central veins and were accompanied by mild inflammatory cell infiltration in in vivo toxicity studies; however, no inflammatory reaction was detected in the PCLSs. In contrast, morphological changes in hepatocytes, which are specific to PCLS, have been reported in detail by Granitzny et al51. After treatment with a low dose of APAP as a model compound, a large number of hepatocytes with red-colored areas within the cytoplasm were observed in the slices. This was associated with increased adenosine triphosphate content and higher synthesis rates of urea and albumin. Middle doses of APAP induced extensive necrosis, with small areas containing viable cells of a darker color and preserved polygonal shape. Viable but mostly rounded or disintegrated cells that were light red and homogenous with dark nuclei were detected. At high doses of APAP, hepatocytes showed reduced connections with other cells, and larger free spaces were visible between the cells. Although the pathological significance of these cellular changes remains uncertain, further analysis may be performed by toxicologic pathologists to define these findings and clarify their significance. Because the incubation time was limited to approximately 24 h, it was necessary to observe the changes that were unique to this method, along with the cell degeneration and necrosis in the control slices. By improving the culture conditions, PCLSs may be observed for longer periods of time52. In line with this, we observed histopathological changes in control PCLSs after 72 h. Eosinophilic hepatocytes were observed in the centrilobular and periportal regions, which might have been caused by the decreased deposition of glycogen (Katoh et al., unpublished data) (Fig. 3). Toxicologic pathologists know that fasting prior to autopsy in in vivo toxicity studies can cause differences in the accumulation of glycogen in hepatocytes. This may affect the assessment of drug-induced hepatocellular changes. Glycogen content in the PCLSs varied depending on the culture conditions of each experiment. Therefore, toxicologic pathologists always compared the findings with those of the control group, and there is a need to take these background changes into account for toxicity assessment in PCLSs.
Fig. 2.

Representative image of precision-cut liver tissue slices obtained from the liver of a control rat (Katoh et al., unpublished data)
Fig. 3.

Representative histopathological image of a precision-cut liver tissue slice obtained from rat livers (Katoh et al., unpublished data). Eosinophilic hepatocytes are evident in the centrilobular (C) and periportal regions (P), whereas clear hepatocytes possibly rich in glycogen deposition are noted in other regions. Hematoxylin and eosin staining. Magnification 200×.
Organoids for Hepatotoxicity Assessment
Here, we reiterate the advantages of 3D cell culture over other cell culture methods. Research using cultured cells has undergone a dramatic shift from conventional monolayer and 2D cell culture methods to 2.5D cell culture and 3D-embedded culture systems53. When comparing 2D cell culture with 3D cell culture, 2D culture has been found to demonstrate sparse cell-to-cell contact, with limited cell-to-matrix contact and no diffusion gradient of nutrients, oxygen, or drugs54. The 2.5D method is a relatively simple method in which cells are seeded on Matrigel and cultured in a Matrigel-enriched culture medium. However, the cell-to-cell paracrine system does not work effectively due to the large surface area of cells in contact with the culture medium53. The 3D cell culture method overcomes the disadvantages of the 2D and 2.5D methods because the cells are in contact with the Matrigel uniformly, and cell differentiation, which is inherently a complex process, can be achieved naturally in a 3D microenvironment55. Thus, human stem cell research can now be performed in a 3D microenvironment by observing stem cells as highly differentiated, functional cells. This is a very useful experimental technique for basic and applied research, drug discovery and screening, and reduction of animal studies53, 55. In addition, 3D cell culture using scaffolds made from natural or synthetic materials allows the construction of more sophisticated tissue-like structures13, 55.
Several reviews have shown the advantages and limitations of 3D culture in assessing hepatotoxicity of human hepatic cancer-derived cell lines and iPSC-derived hepatocyte-like cells. However, these studies have the disadvantage of having immaturity cells and necrotic regions due to oxygen supply discrepancies when evaluating drug sensitivity13, 56. The review by Godoy et al.5 is very helpful for more details regarding concepts and techniques of isolation and 3D culture from human and rat liver. To the best of our knowledge, no high-throughput experiments in 3D rat hepatocyte culture have been reported, and critical findings have been obtained from studies using a small number of drugs only. Richert et al.27 compared 2D cell culture on the collagen-collagen sandwich method with 3D cell culture on Matrigel in primary hepatocytes of rats and showed a similar favorable response in terms of CYP2B induction by phenobarbital exposure; however, 2D cell culture has better results than 3D cell culture in glutathione production, where matrix-cell interactions are required. Therefore, we recommend that drug screening studies be conducted using both 2D and 3D cultures to avoid conflicting results. Importantly, well-differentiated hepatocytes might reduce drug sensitivity in 3D cell culture, where the expression of multidrug resistance-associated protein 2 (Mrp2) is upregulated and the excretion of methotrexate from cells is enhanced. This results in reduced hepatotoxicity compared to that seen with 2D cell culture57. Mrp2 is a transporter for biliary excretion of drugs, and its expression in hepatocyte organoids suggests its differentiation into capillary bile ducts5, 31. Alternatively, in anticancer drug sensitivity testing of cancer cells, 3D cell culture can be a useful experimental tool for assessing drug resistance54. Thus, cell differentiation in 3D culture can be advantageous or disadvantageous, depending on the research objective.
Proposed Histopathological Organoid Observation
Histopathological analysis of 3D cell cultures help visualize the expression of local cell adhesion molecules and cell differentiation markers, as well as the detection of apoptosis due to drug exposure31, 58. As toxicologic pathologists enter the field of in vitro hepatotoxicity assessment, histopathological analysis of organoids will provide them with a new venue for their work.
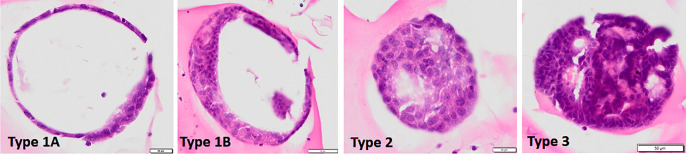
Here, we demonstrated liver organoids derived from rat hepatocytes using HE staining (Uomoto et al., unpublished data) (Fig. 4). If you are a toxicologic pathologist, you will notice several types of HE organoid images. We proposed dividing rat hepatocyte organoids into three types. Type 1A cells have an almost circular shape with a hollow interior lined with one or more layers of cells. Type 1B is similar to type 1A in that it is nearly round and has a hollow interior. However, it is characterized by a multilayered inner lining of cells. Cells of this type are flat or oval, with large or small nuclei, and the cytoplasm is acidophilic. Type 2 cells are nearly round and almost completely filled with cells. Cells of this type have an oval nucleus, acidophilic cytoplasm, and relatively clear cell boundaries. Type 3 is nearly round, with cells filling the interior of a filled or glandular tubular structure. The constituent cells had a slightly smaller nucleus, round shape, and immature morphology. How appropriate is histopathological examination for these types? Whether to consider all types or focus on one type needs to be discussed. We believe that most toxicologic pathologists would agree that they should target type 2 cells, which are relatively large and differentiated according to the HE images. However, immunostaining or fluorescence staining may be needed to detect immature and mature hepatocyte markers (hepatocyte nuclear factor 4, alpha-fetoprotein, and albumin) and drug-metabolizing enzyme expression (CYPs) with glycogen accumulation by PAS reaction59, 60, 61. Type 3 cells might appear as adenomas or adenocarcinomas62, right?
Fig. 4.
Several types of organoids derived from hepatocytes in a chemical-treated rat (Uomoto et al., unpublished data). Type 1 has a hollow interior and is subdivided into type 1A and 1B: Type 1A is lined with a single cell layer or a few cell layers, and type 1B is lined with a multicellular layer. Type 2 has an interior almost completely filled with cells. Type 3 has a full or glandular interior. Hematoxylin and eosin staining. Bar=20 (Type 1A and 2) or 50 µm (Type 1B and 3).
Thus, several morphological variations exist in liver organoids, probably depending on the degree of cell differentiation; however, branching morphogenesis seems obscure53. When evaluating hepatocytotoxicity in vitro, the most common method is to collect whole treated cells and detect CYP induction, cell death, and cell proliferation while checking the degree of hepatocyte differentiation (albumin and urea production)31, 32, 61, 63. However, in the case of organoids, it may be possible to detect more detailed toxic reactions by histopathologically confirming the type of organoid (Fig. 4) with toxic or reactive changes. During the development of organoids, progenitor/daughter cells are generated from a single stem cell and various differentiated cells are generated (Fig. 5). In such cases, how would each cell with varying degrees of differentiation be distributed within the organoid? If stem cells are on the margins of the organoid, progenitor/daughter cells may also be distributed on the margins, and differentiated cells that respond to chemicals may be distributed near the center. Not all differentiated cells respond to chemicals; depending on the degree of expression of drug transporters and CYPs, some cells may respond, whereas others may not. If stem cells are located in the center of the organoid, progenitor/daughter cells may be randomly distributed near the stem cell. When examining a liver organoid specimen, the degree of cell differentiation in the organoid, in addition to the differences in drug concentrations must be considered to determine whether the type 2 organoid is equally affected or only some cells are affected. Moreover, unlike spheroids, the size of organoids is difficult to control, and larger organoids, which are probably easier for toxicologic pathologists to observe, tend to be hypoxic in the center15, 64. Toxicologic pathologists are well aware that the lobular center of the liver is physiologically prone to hypoxia and malnutrition10; however, we must not forget that the same phenomenon occurs in organoids.
Fig. 5.
Hypothetical distribution of several types of cells in an organoid. An organoid might be derived from one stem cell, which can divide into a stem cell and progenitor cell. This will further differentiate into various cell distribution patterns. After exposure to a chemical, some mature cells with expression of transporters and drug- metabolizing enzymes are expected to exhibit a toxic response, depending on their respective cellular distribution patterns. Progenitor cell counts might increase in response to chemical toxicity, when reduced cell number. The organoid is assumed to be type 2, as shown in Fig. 4.
Conclusion
In summary, we reviewed in vitro chemical hepatotoxicity assessment methods using human and rat hepatocytes. It would be difficult to assess hepatitis and fibrosis in a system constructed using only hepatocytes because the involvement of non-hepatocytes, including Kupffer cells and hepatic satellite cells, cannot be assessed. Even when co-cultured with non-hepatocytes, ECM, and engineered scaffolds, it takes a lot of time, effort, wisdom, and verification to reconstruct the lobule structure of the organism accurately. When observing HE-stained specimens of liver organoids under the microscope (Fig. 4), toxicologic pathologists may notice that their structure is far removed from the lobular structure of the liver of a living organism (Fig. 3). Li et al.65 discussed several limitations in constructing human organoids, including the diversity in the nature and morphology of the organoids produced. Toxicologic pathologists are expected to actively participate in finding solutions to address the diversity of ex vivo-constructed organoids. They are also expected to use their experience with morphological changes in a large number of normal tissues and tumors to extract organoids with morphologies appropriate for toxicity assessment and to exchange opinions with cell culture experts on how to produce organoids with specific properties more efficiently and reproducibly. Li et al.65 also suggested establishing guidelines for evaluating the quality and effectiveness of organoids to minimize the differences in organoids among laboratories. In humans, genetic and environmental diversity cannot be eliminated from donor-derived organoids. However, in the case of organoids derived from experimental animals, it is possible to construct a useful drug screening tool by minimizing genetic and environmental diversity and considering extrapolation to humans. Therefore, it is necessary to examine the toxicity response of each drug, including the type of CYPs induced in both human and experimental animal-derived cells66. Reducing toxicity testing in animals is a proposition that must be promoted by toxicologic pathologists as well. They need to be extensively involved in developing in vitro chemical hepatotoxicity assessment systems by utilizing their knowledge and experience.
Disclosure of Potential Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We would like to thank Editage (www.editage.com) for the English language editing. This work was supported by a Grant-in-Aid for Scientific Research (Grant No. 20H03146) from the Ministry of Education, Culture, Sports, Science.
References
- 1.Bayne K, Ramachandra GS, Rivera EA, and Wang J. The evolution of animal welfare and the 3Rs in Brazil, China, and India. J Am Assoc Lab Anim Sci. 54: 181–191. 2015. [PMC free article] [PubMed] [Google Scholar]
- 2.Lewejohann L, Schwabe K, Häger C, and Jirkof P. Impulse for animal welfare outside the experiment. Lab Anim. 54: 150–158. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Törnqvist E, Annas A, Granath B, Jalkesten E, Cotgreave I, and Öberg M. Strategic focus on 3R principles reveals major reductions in the use of animals in pharmaceutical toxicity testing. PLoS One. 9: e101638. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majno P, Mentha G, Toso C, Morel P, Peitgen HO, and Fasel JHD. Anatomy of the liver: an outline with three levels of complexity--a further step towards tailored territorial liver resections. J Hepatol. 60: 654–662. 2014. [DOI] [PubMed] [Google Scholar]
- 5.Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Böttger J, Braeuning A, Budinsky RA, Burkhardt B, Cameron NR, Camussi G, Cho CS, Choi YJ, Craig Rowlands J, Dahmen U, Damm G, Dirsch O, Donato MT, Dong J, Dooley S, Drasdo D, Eakins R, Ferreira KS, Fonsato V, Fraczek J, Gebhardt R, Gibson A, Glanemann M, Goldring CE, Gómez-Lechón MJ, Groothuis GM, Gustavsson L, Guyot C, Hallifax D, Hammad S, Hayward A, Häussinger D, Hellerbrand C, Hewitt P, Hoehme S, Holzhütter HG, Houston JB, Hrach J, Ito K, Jaeschke H, Keitel V, Kelm JM, Kevin Park B, Kordes C, Kullak-Ublick GA, LeCluyse EL, Lu P, Luebke-Wheeler J, Lutz A, Maltman DJ, Matz-Soja M, McMullen P, Merfort I, Messner S, Meyer C, Mwinyi J, Naisbitt DJ, Nussler AK, Olinga P, Pampaloni F, Pi J, Pluta L, Przyborski SA, Ramachandran A, Rogiers V, Rowe C, Schelcher C, Schmich K, Schwarz M, Singh B, Stelzer EH, Stieger B, Stöber R, Sugiyama Y, Tetta C, Thasler WE, Vanhaecke T, Vinken M, Weiss TS, Widera A, Woods CG, Xu JJ, Yarborough KM, and Hengstler JG. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 87: 1315–1530. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman SL. Liver fibrosis—from bench to bedside. J Hepatol. 38(Suppl 1): S38–S53. 2003. [DOI] [PubMed] [Google Scholar]
- 7.Zanger UM, and Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 138: 103–141. 2013. [DOI] [PubMed] [Google Scholar]
- 8.Singh D, Cho WC, and Upadhyay G. Drug-induced liver toxicity and prevention by herbal antioxidants: an overview. Front Physiol. 6: 363. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim K, Utoh R, Ohashi K, Kikuchi T, and Okano T. Fabrication of functional 3D hepatic tissues with polarized hepatocytes by stacking endothelial cell sheets in vitro. J Tissue Eng Regen Med. 11: 2071–2080. 2017. [DOI] [PubMed] [Google Scholar]
- 10.Thoolen B, Maronpot RR, Harada T, Nyska A, Rousseaux C, Nolte T, Malarkey DE, Kaufmann W, Küttler K, Deschl U, Nakae D, Gregson R, Vinlove MP, Brix AE, Singh B, Belpoggi F, and Ward JM. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 38(Suppl): 5S–81S. 2010. [DOI] [PubMed] [Google Scholar]
- 11.Nathanson MH, and Boyer JL. Mechanisms and regulation of bile secretion. Hepatology. 14: 551–566. 1991. [PubMed] [Google Scholar]
- 12.Yamada M, Utoh R, Ohashi K, Tatsumi K, Yamato M, Okano T, and Seki M. Controlled formation of heterotypic hepatic micro-organoids in anisotropic hydrogel microfibers for long-term preservation of liver-specific functions. Biomaterials. 33: 8304–8315. 2012. [DOI] [PubMed] [Google Scholar]
- 13.Kyffin JA, Sharma P, Leedale J, Colley HE, Murdoch C, Mistry P, and Webb SD. Impact of cell types and culture methods on the functionality of in vitro liver systems—a review of cell systems for hepatotoxicity assessment. Toxicol In Vitro. 48: 262–275. 2018. [DOI] [PubMed] [Google Scholar]
- 14.Kammerer S. Three-dimensional liver culture systems to maintain primary hepatic properties for toxicological analysis in vitro. Int J Mol Sci. 22: 10214. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asthana A, and Kisaalita WS. Microtissue size and hypoxia in HTS with 3D cultures. Drug Discov Today. 17: 810–817. 2012. [DOI] [PubMed] [Google Scholar]
- 16.Jennings P. The future of in vitro toxicology. 29: 1217–1221. 2015. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues RM, De Kock J, Branson S, Vinken M, Meganathan K, Chaudhari U, Sachinidis A, Govaere O, Roskams T, De Boe V, Vanhaecke T, and Rogiers V. Human skin-derived stem cells as a novel cell source for in vitro hepatotoxicity screening of pharmaceuticals. Stem Cells Dev. 23: 44–55. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodrigues RM, Branson S, De Boe V, Sachinidis A, Rogiers V, De Kock J, and Vanhaecke T. In vitro assessment of drug-induced liver steatosis based on human dermal stem cell-derived hepatic cells. Arch Toxicol. 90: 677–689. 2016. [DOI] [PubMed] [Google Scholar]
- 19.Utkarsh D, Loretz C, and Li AP. In vitro evaluation of hepatotoxic drugs in human hepatocytes from multiple donors: Identification of P450 activity as a potential risk factor for drug-induced liver injuries. Chem Biol Interact. 255: 12–22. 2016. [DOI] [PubMed] [Google Scholar]
- 20.Ramachandran SD, Vivarès A, Klieber S, Hewitt NJ, Muenst B, Heinz S, Walles H, and Braspenning J. Applicability of second-generation upcyte® human hepatocytes for use in CYP inhibition and induction studies. Pharmacol Res Perspect. 3: e00161. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tolosa L, Gómez-Lechón MJ, López S, Guzmán C, Castell JV, Donato MT, and Jover R. Human Upcyte hepatocytes: Characterization of the hepatic phenotype and evaluation for acute and long-term chemical hepatotoxicity routine testing. Toxicol Sci. 152: 214–229. 2016. [DOI] [PubMed] [Google Scholar]
- 22.Tolosa L, Jiménez N, Pelechá M, Castell JV, Gómez-Lechón MJ, and Donato MT. Long-term and mechanistic evaluation of drug-induced liver injury in Upcyte human hepatocytes. Arch Toxicol. 93: 519–532. 2019. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Jakus AE, Baptista PM, Soker S, Soto-Gutierrez A, Abecassis MM, Shah RN, and Wertheim JA. Functional maturation of induced pluripotent stem cell hepatocytes in extracellular matrix—a comparative analysis of bioartificial liver microenvironments. Stem Cells Transl Med. 5: 1257–1267. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JH, Das P, DiVito MD, Ivancic D, Tan LP, and Wertheim JA. Nanofibrous PLGA electrospun scaffolds modified with type I collagen influence hepatocyte function and support viability in vitro. Acta Biomater. 73: 217–227. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das P, DiVito MD, Wertheim JA, and Tan LP. Collagen-I and fibronectin modified three-dimensional electrospun PLGA scaffolds for long-term in vitro maintenance of functional hepatocytes. Mater Sci Eng C. 111: 110723. 2020. [DOI] [PubMed] [Google Scholar]
- 26.LeCluyse EL, Bullock PL, and Parkinson A. Strategies for restoration and maintenance of normal hepatic structure and function in long-term cultures of rat hepatocytes. Adv Drug Deliv Rev. 22: 133–186. 1996. [Google Scholar]
- 27.Richert L, Binda D, Hamilton G, Viollon-Abadie C, Alexandre E, Bigot-Lasserre D, Bars R, Coassolo P, and LeCluyse E. Evaluation of the effect of culture configuration on morphology, survival time, antioxidant status and metabolic capacities of cultured rat hepatocytes. Toxicol In Vitro. 16: 89–99. 2002. [DOI] [PubMed] [Google Scholar]
- 28.Chatterjee S, Richert L, Augustijns P, and Annaert P. Hepatocyte-based in vitro model for assessment of drug-induced cholestasis. Toxicol Appl Pharmacol. 274: 124–136. 2014. [DOI] [PubMed] [Google Scholar]
- 29.Oorts M, Baze A, Bachellier P, Heyd B, Zacharias T, Annaert P, and Richert L. Drug-induced cholestasis risk assessment in sandwich-cultured human hepatocytes. Toxicol In Vitro. 34: 179–186. 2016. [DOI] [PubMed] [Google Scholar]
- 30.Oorts M, Richert L, and Annaert P. Drug-induced cholestasis detection in cryopreserved rat hepatocytes in sandwich culture. J Pharmacol Toxicol Methods. 73: 63–71. 2015. [DOI] [PubMed] [Google Scholar]
- 31.Utoh R, Enomoto S, Yamada M, Yamanaka K, Yajima Y, Furusawa K, and Seki M. Polyanion-induced, microfluidic engineering of fragmented collagen microfibers for reconstituting extracellular environments of 3D hepatocyte culture. Mater Sci Eng C. 129: 112417. 2021. [DOI] [PubMed] [Google Scholar]
- 32.Morita A, Yamada M, Utoh R, Momiyama K, Iwadate H, and Seki M. Formation of 3D tissues of primary hepatocytes using fibrillized collagen microparticles as intercellular binders. J Biosci Bioeng. 133: 265–272. 2022. [DOI] [PubMed] [Google Scholar]
- 33.Ye J, Shirakigawa N, and Ijima H. Hybrid organoids consisting of extracellular matrix gel particles and hepatocytes for transplantation. J Biosci Bioeng. 120: 231–237. 2015. [DOI] [PubMed] [Google Scholar]
- 34.Arisaka Y, Kobayashi J, Ohashi K, Tatsumi K, Kim K, Akiyama Y, Yamato M, and Okano T. A heparin-modified thermoresponsive surface with heparin-binding epidermal growth factor-like growth factor for maintaining hepatic functions in vitro and harvesting hepatocyte sheets. Regen Ther. 3: 97–106. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan S, Wei J, Liu Y, Zhang H, Chen J, and Li X. Hepatocyte spheroid culture on fibrous scaffolds with grafted functional ligands as an in vitro model for predicting drug metabolism and hepatotoxicity. Acta Biomater. 28: 138–148. 2015. [DOI] [PubMed] [Google Scholar]
- 36.Lu H-F, Chua K-N, Zhang P-C, Lim W-S, Ramakrishna S, Leong KW, and Mao H-Q. Three-dimensional co-culture of rat hepatocyte spheroids and NIH/3T3 fibroblasts enhances hepatocyte functional maintenance. Acta Biomater. 1: 399–410. 2005. [DOI] [PubMed] [Google Scholar]
- 37.Wei G, Wang J, Lv Q, Liu M, Xu H, Zhang H, Jin L, Yu J, and Wang X. Three-dimensional coculture of primary hepatocytes and stellate cells in silk scaffold improves hepatic morphology and functionality in vitro. J Biomed Mater Res A. 106: 2171–2180. 2018. [DOI] [PubMed] [Google Scholar]
- 38.Kang I-K, Kim GJ, Kwon OH, and Ito Y. Co-culture of hepatocytes and fibroblasts by micropatterned immobilization of beta-galactose derivatives. Biomaterials. 25: 4225–4232. 2004. [DOI] [PubMed] [Google Scholar]
- 39.Elloumi Hannachi I, Itoga K, Kumashiro Y, Kobayashi J, Yamato M, and Okano T. Fabrication of transferable micropatterned-co-cultured cell sheets with microcontact printing. Biomaterials. 30: 5427–5432. 2009. [DOI] [PubMed] [Google Scholar]
- 40.Kim K, Ohashi K, Utoh R, Kano K, and Okano T. Preserved liver-specific functions of hepatocytes in 3D co-culture with endothelial cell sheets. Biomaterials. 33: 1406–1413. 2012. [DOI] [PubMed] [Google Scholar]
- 41.Zinchenko YS, and Coger RN. Engineering micropatterned surfaces for the coculture of hepatocytes and Kupffer cells. J Biomed Mater Res A. 75: 242–248. 2005. [DOI] [PubMed] [Google Scholar]
- 42.Hillebrandt K, Polenz D, Butter A, Tang P, Reutzel-Selke A, Andreou A, Napierala H, Raschzok N, Pratschke J, Sauer IM, and Struecker B. Procedure for decellularization of rat livers in an oscillating-pressure perfusion device. J Vis Exp. 102: e53029. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Struecker B, Butter A, Hillebrandt K, Polenz D, Reutzel-Selke A, Tang P, Lippert S, Leder A, Rohn S, Geisel D, Denecke T, Aliyev K, Jöhrens K, Raschzok N, Neuhaus P, Pratschke J, and Sauer IM. Improved rat liver decellularization by arterial perfusion under oscillating pressure conditions. J Tissue Eng Regen Med. 11: 531–541. 2017. [DOI] [PubMed] [Google Scholar]
- 44.Felgendreff P, Schindler C, Mussbach F, Xie C, Gremse F, Settmacher U, and Dahmen U. Identification of tissue sections from decellularized liver scaffolds for repopulation experiments. Heliyon. 7: e06129. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji R, Zhang N, You N, Li Q, Liu W, Jiang N, Liu J, Zhang H, Wang D, Tao K, and Dou K. The differentiation of MSCs into functional hepatocyte-like cells in a liver biomatrix scaffold and their transplantation into liver-fibrotic mice. Biomaterials. 33: 8995–9008. 2012. [DOI] [PubMed] [Google Scholar]
- 46.Bao J, Wu Q, Wang Y, Li Y, Li L, Chen F, Wu X, Xie M, and Bu H. Enhanced hepatic differentiation of rat bone marrow-derived mesenchymal stem cells in spheroidal aggregate culture on a decellularized liver scaffold. Int J Mol Med. 38: 457–465. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura S, and Ijima H. Solubilized matrix derived from decellularized liver as a growth factor-immobilizable scaffold for hepatocyte culture. J Biosci Bioeng. 116: 746–753. 2013. [DOI] [PubMed] [Google Scholar]
- 48.Caires-Júnior LC, Goulart E, Telles-Silva KA, Araujo BHS, Musso CM, Kobayashi G, Oliveira D, Assoni A, Carvalho VM, Ribeiro-Jr AF, Ishiba R, Braga KAO, Nepomuceno N, Caldini E, Rangel T, Raia S, Lelkes PI, and Zatz M. Pre-coating decellularized liver with HepG2-conditioned medium improves hepatic recellularization. Mater Sci Eng C. 121: 111862. 2021. [DOI] [PubMed] [Google Scholar]
- 49.Ohashi K, Yokoyama T, Yamato M, Kuge H, Kanehiro H, Tsutsumi M, Amanuma T, Iwata H, Yang J, Okano T, and Nakajima Y. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 13: 880–885. 2007. [DOI] [PubMed] [Google Scholar]
- 50.Moronvalle-Halley V, Sacré-Salem B, Sallez V, Labbe G, and Gautier J-C. Evaluation of cultured, precision-cut rat liver slices as a model to study drug-induced liver apoptosis. Toxicology. 207: 203–214. 2005. [DOI] [PubMed] [Google Scholar]
- 51.Granitzny A, Knebel J, Schaudien D, Braun A, Steinberg P, Dasenbrock C, and Hansen T. Maintenance of high quality rat precision cut liver slices during culture to study hepatotoxic responses: acetaminophen as a model compound. Toxicol In Vitro. 42: 200–213. 2017. [DOI] [PubMed] [Google Scholar]
- 52.Behrsing HP, Vickers AEM, and Tyson CA. Extended rat liver slice survival and stability monitored using clinical biomarkers. Biochem Biophys Res Commun. 312: 209–213. 2003. [DOI] [PubMed] [Google Scholar]
- 53.Shamir ER, and Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol. 15: 647–664. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Białkowska K, Komorowski P, Bryszewska M, and Miłowska K. Spheroids as a type of three-dimensional cell cultures-examples of methods of preparation and the most important application. Int J Mol Sci. 21: 6225. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knight E, and Przyborski S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J Anat. 227: 746–756. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsui T, and Shinozawa T. Human organoids for predictive toxicology research and drug development. Front Genet. 12: 767621. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin J, Meng Q, Zhang G, and Sun Y. Differential methotrexate hepatotoxicity on rat hepatocytes in 2-D monolayer culture and 3-D gel entrapment culture. Chem Biol Interact. 180: 368–375. 2009. [DOI] [PubMed] [Google Scholar]
- 58.Li R, Liu J, Ma J, Sun X, Wang Y, Yan J, Yu Q, Diao J, Yang C, Reid LM, and Wang Y. Fibrinogen improves liver function via promoting cell aggregation and fibronectin assembly in hepatic spheroids. Biomaterials. 280: 121266. 2022. [DOI] [PubMed] [Google Scholar]
- 59.Piryaei A, Valojerdi MR, Shahsavani M, and Baharvand H. Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis model. Stem Cell Rev Rep. 7: 103–118. 2011. [DOI] [PubMed] [Google Scholar]
- 60.Tsuchida T, Murata S, Matsuki K, Mori A, Matsuo M, Mikami S, Okamoto S, Ueno Y, Tadokoro T, Zheng Y-W, and Taniguchi H. The regenerative effect of portal vein injection of liver organoids by retrorsine/partial hepatectomy in rats. Int J Mol Sci. 21: 178. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He Y-T, Zhu X-L, Li S-F, Zhang B-Q, Li Y, Wu Q, Zhang Y-L, Zhou Y-Y, Li L, Qi Y-N, Bao J, and Bu H. Creating rat hepatocyte organoid as an in vitro model for drug testing. World J Stem Cells. 12: 1184–1195. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ganesh K, Wu C, O’Rourke KP, Szeglin BC, Zheng Y, Sauvé CG, Adileh M, Wasserman I, Marco MR, Kim AS, Shady M, Sanchez-Vega F, Karthaus WR, Won HH, Choi S-H, Pelossof R, Barlas A, Ntiamoah P, Pappou E, Elghouayel A, Strong JS, Chen C-T, Harris JW, Weiser MR, Nash GM, Guillem JG, Wei IH, Kolesnick RN, Veeraraghavan H, Ortiz EJ, Petkovska I, Cercek A, Manova-Todorova KO, Saltz LB, Lavery JA, DeMatteo RP, Massagué J, Paty PB, Yaeger R, Chen X, Patil S, Clevers H, Berger MF, Lowe SW, Shia J, Romesser PB, Dow LE, Garcia-Aguilar J, Sawyers CL, and Smith JJ. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med. 25: 1607–1614. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hou Y-T, and Hsu C-C. Development of a 3D porous chitosan/gelatin liver scaffold for a bioartificial liver device. J Biosci Bioeng. 129: 741–748. 2020. [DOI] [PubMed] [Google Scholar]
- 64.Anada T, Fukuda J, Sai Y, and Suzuki O. An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials. 33: 8430–8441. 2012. [DOI] [PubMed] [Google Scholar]
- 65.Li H, Gao L, Du J, Ma T, Ye Z, and Li Z. To better generate organoids, what can we learn from teratomas? Front Cell Dev Biol. 9: 700482. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mueller SO, Guillouzo A, Hewitt PG, and Richert L. Drug biokinetic and toxicity assessments in rat and human primary hepatocytes and HepaRG cells within the EU-funded Predict-IV project. Toxicol In Vitro. 30(1 Pt A): 19–26. 2015. [DOI] [PubMed] [Google Scholar]