Abstract
Vestibular organs consist of the maculae staticae, which are located in both the utricle and saccule, as well as the semicircular ducts and their ampullas. There have been no reports on specimen preparation methods for vestibular organs, including maculae staticae or semicircular ducts. In this study, we investigated highly reproducible methods of preparing vestibular organ specimens for histopathological examinations. We established a method that allows researchers to observe the utricle and saccule, including otoliths, the ampulla of a semicircular duct, and parts of semicircular ducts. This highly reproducible method is useful for histopathological analysis of mice with symptoms of abnormal equilibrium caused by medical toxicity and genetic modification.
Keywords: specimen preparation, maculae staticae, semicircular ducts, vestibular organs, mice
The inner ear consists of auditory and vestibular organs. The vestibular organs include maculae staticae, which are located in both the utricle and saccule, as well as semicircular ducts and their ampullas1. Although there are many reports of drug-induced hearing loss, there are few reports on toxicity affecting the sense of equilibrium2, 3. Among these, streptomycin is reportedly toxic to the auditory and equilibrium sensory organs, resulting in the degeneration of the otolithic membrane and loss of otoliths in experimental animals4, 5, 6. The clinical failure of the sense of equilibrium and the loss of otoliths, the giant otolith in maculae staticae, and the ectopic otolith in semicircular ducts, have been reported in ATP2b2KO and Pendred’s syndrome model mice7, 8. However, specimen preparation methods for vestibular organs, including maculae staticae or semicircular ducts, have not been reported. In this study, we investigated highly reproducible methods to prepare vestibular organ specimens for histopathological examinations.
Five 10-week-old female ICR mice (Charles River Laboratories Japan, Inc., Kanagawa, Japan) were used for the preliminary examination. The head was separated from the body for each mouse, and the brain, mandible, eyes, skin, and muscles were removed from the head. The heads were fixed in 10% neutral-buffered formalin (NBF) and decalcified with K-CX solution. The following five excision methods (Fig. 1) were compared:
Fig. 1.
Schema of dissecting direction (red line) of methods A–E (left figure). Structure of labyrinth (right). Labels: Pituitary gland (p), Tympanic cavity (t), Trigeminal nerve (tn). Maculae staticae (blue dots).
Method A: The head was dissected from the tympanic cavity to the contralateral anterior edge of the orbit and sectioned inwardly (Fig. 2A).
Fig. 2.
(A–E) Schema of methods A–E, respectively. (A–D) Dissecting (dotted line) and sectioning directions (arrow) (left). Dissected surface (right). (E) Dissecting (dotted line) and sectioning directions (arrow) (left). Dissected surface (right). Labels: Pituitary gland (p), Tympanic cavity (t), Trigeminal nerve (tn), Orbit (o). Bar=5 mm.
Method B: The head was dissected along the trigeminal nerve and sectioned inwardly (Fig. 2B).
Method C: The head was dissected from the tympanic cavity to the ipsilateral anterior edge of the orbit and sectioned inwardly (Fig. 2C).
Method D: The head was dissected transversely 2 mm from the posterior edge of the pituitary gland and sectioned rostrally (Fig. 2D).
Method E: The head was dissected transversely from the posterior edge of the pituitary gland and sectioned caudally (Fig. 2E).
With methods A, B, and C, the utricle, saccule, and parts of semicircular ducts were observable; however, the ampulla of a semicircular duct was not (Fig. 3A–C). With method D, parts of semicircular ducts were observable at the dissected surface (data not shown). The utricle, saccule, ampulla of a semicircular duct, and parts of semicircular ducts were all simultaneously observable at 150 μm sectioned from the dissected surface (Fig. 3D). However, the maculae staticae disappeared after further 300 μm sectioning (Fig. 3D-300 μm). With method E, the maculae staticae were not observable from the dissected surface (data not shown).
Fig. 3.
Specimens in low magnification (left) and the areas highlighted by squares in high magnification (right). (A) Saccule and parts of semicircular ducts stained with hematoxylin and eosin (HE). (B) Saccule, parts of semicircular ducts, and cochlea stained with HE. (C) Utricle, saccule, and parts of semicircular ducts stained with HE. (D) Utricle, saccule, the ampulla of a semicircular duct (arrowhead), and parts of semicircular ducts stained with HE. (D-300 μm) All compartments of vestibular organs disappeared after further 300 μm sectioning; stained with HE. Labels: Tympanic cavity (t), Utricle (u), Saccule (s), Semicircular ducts (d), Cochlea (c).
Dissecting the heads transversely along the posterior edge of the pituitary gland as in methods D and E was technically easier than dissecting them longitudinally or diagonally as in methods A, B, and C. The results from methods D and E demonstrated that the maculae staticae were located between the respective dissection sites. With method D, parts of semicircular ducts were observable, and the maculae staticae disappeared immediately after further sectioning. Therefore, sectioning from the posterior edge of the pituitary gland to the caudal side, as in method E, was better for observing the maculae staticae with high reproducibility. Using methods D and E, the morphologies of the left and right sides were different due to the slope of the dissection. Therefore, we decided to use method Eʹ for the main study, which involves dissecting the heads 1 mm from the posterior edge of the pituitary gland, dividing them into right and left parts, and sectioning them towards the caudal side (Fig. 4).
Fig. 4.
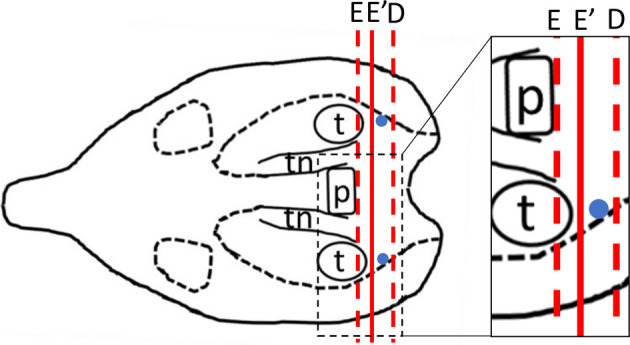
Schema of dissecting direction of methods E and E′. The dissection site for method E′ was 1 mm posterior to that of method E. Dissection direction (red line). Maculae staticae (blue dots).
Two 28-week-old female FVB/N mice (CLEA Japan, Inc., Tokyo, Japan) were used in the main study. The mice were perfusion-fixed with 4% paraformaldehyde systemically after systemic perfusion with heparinized saline under isoflurane anesthesia. For each mouse, the head was separated from the body, and the brain, mandible, eyes, skin, and muscles were removed from the head. The heads were fixed with 10% NBF and decalcified using a K-CX solution. The heads were dissected transversely 1 mm from the posterior edge of the pituitary gland (Fig. 5A) and sectioned caudally. All specimens were processed routinely, and intermittent, thin sections every 20 μm from the dissected surface were stained with hematoxylin and eosin (HE). All animal procedures were conducted in accordance with the Chugai Pharmaceutical Guide for the Care and Use of Laboratory Animals, and all experimental protocols were approved by the Institutional Animal Care and Use Committee.
Fig. 5.
Histology at 160 μm sectioned from the dissected surface. (A) Dissecting direction (dotted line) and sectioning direction (arrow) of method Eʹ. (B) The specimen in loupe view stained with HE. (C) Overview of the vestibular organ; stained with HE. (D) The saccule, including sensory epithelium, otolithic membrane, and otoliths stained with HE. The utricle, including sensory epithelium, otolithic membrane, and otoliths stained with HE. (F) The ampulla of a semicircular duct, including sensory epithelium stained with HE. (G) Parts of semicircular ducts stained with HE. Labels: Utricle (u), Saccule (s), Ampulla of semicircular duct (a), Semicircular ducts (d), Sensory epithelium (se), Otoliths (o), Otolithic membrane (om).
In both animals, the utricle and saccule, including otoliths, the ampulla of a semicircular duct, and parts of semicircular ducts, were observed on the dissected surface or at 260 μm sectioned from the dissected surface, as in method D (Fig. 5B–G).
By sectioning from the posterior edge of the pituitary gland to the caudal side, vestibular organs, including the utricle, saccule, ampulla of a semicircular duct, and parts of semicircular ducts were observed. When the head was dissected 1 mm from the posterior edge of the pituitary gland, the maculae staticae were observed without deep sectioning. The sensory epithelium at the maculae staticae and ampulla was evaluated in the preliminary and main study. Since the maculae staticae are extremely small organs, intermittent serial sections from the posterior edge of the pituitary gland should be made to avoid any loss of the maculae staticae. This method is helpful for histopathological analysis of mice with symptoms of abnormal equilibrium caused by medical toxicity and genetic modification.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Acknowledgments
We thank Jacob Davis at Chugai Pharmaceutical Co., Ltd. for proofreading the manuscript.
References
- 1.Ekdale EG. Form and function of the mammalian inner ear. J Anat. 228: 324–337. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paken J, Govender CD, Pillay M, and Sewram V. Cisplatin-associated ototoxicity: a review for the health professional. J Toxicol. 2016: 1809394. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding D, Liu H, Qi W, Jiang H, Li Y, Wu X, Sun H, Gross K, and Salvi R. Ototoxic effects and mechanisms of loop diuretics. J Otol. 11: 145–156. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schirmer M, Kaiser A, Lessenich A, Lindemann S, Fedrowitz M, Gernert M, and Löscher W. Auditory and vestibular defects and behavioral alterations after neonatal administration of streptomycin to Lewis rats: Similarities and differences to the circling (ci2/ci2) Lewis rat mutant. Brain Res. 1155: 179–195. 2007. [DOI] [PubMed] [Google Scholar]
- 5.Harada Y, and Sugimoto Y. Metabolic disorder of otoconia after streptomycin intoxication. Acta Otolaryngol. 84: 65–71. 1977. [DOI] [PubMed] [Google Scholar]
- 6.Johnsson LG, Wright CG, Preston RE, and Henry PJ. Streptomycin-induced defects of the otoconial membrane. Acta Otolaryngol. 89: 401–406. 1980. [DOI] [PubMed] [Google Scholar]
- 7.Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, and Shull GE. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem. 273: 18693–18696. 1998. [DOI] [PubMed] [Google Scholar]
- 8.Dror AA, Politi Y, Shahin H, Lenz DR, Dossena S, Nofziger C, Fuchs H, Hrabé de Angelis M, Paulmichl M, Weiner S, and Avraham KB. Calcium oxalate stone formation in the inner ear as a result of an Slc26a4 mutation. J Biol Chem. 285: 21724–21735. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]






