Abstract
Pancreatic cancer (PC) has one of the highest fatality rates and the currently available therapeutic options are not sufficient to improve its overall poor prognosis. In addition to insufficient effectiveness of anticancer treatments, the lack of clear early symptoms and early metastatic spread maintain the PC survival rates at a low level. Metabolic reprogramming is among the hallmarks of cancer and could be exploited for the diagnosis and treatment of PC. PC is characterized by its heterogeneity and, apart from molecular subtypes, the identification of metabolic subtypes in PC could aid in the development of more individualized therapeutic approaches and may lead to improved clinical outcomes. In addition to the deregulated utilization of glucose in aerobic glycolysis, PC cells can use a wide range of substrates, including branched-chain amino acids, glutamine and lipids to fulfil their energy requirements, as well as biosynthetic needs. The tumor microenvironment in PC supports tumor growth, metastatic spread, treatment resistance and the suppression of the host immune response. Moreover, reciprocal interactions between cancer and stromal cells enhance their metabolic reprogramming. PC stem cells (PCSCs) with an increased resistance and distinct metabolic properties are associated with disease relapses and cancer spread, and represent another significant candidate for therapeutic targeting. The present review discusses the metabolic signatures observed in PC, a disease with a multifaceted and often transient metabolic landscape. In addition, the metabolic pathways utilized by PC cells, as well as stromal cells are discussed, providing examples of how they could present novel targets for therapeutic interventions and elaborating on how interactions between the various cell types affect their metabolism. Furthermore, the importance of PCSCs is discussed, focusing specifically on their metabolic adaptations.
Keywords: pancreatic cancer, metabolic profiling, glycolysis, amino acid metabolism, mitochondrial oxidative metabolism, tumor microenvironment, cancer stem cells, cell metabolism, chemoresistance
1. Introduction
Pancreatic cancer (PC) is currently the seventh leading cause of cancer-related mortality, with almost as many deaths as cases; the highest incidence rates are observed in Europe and Northern America (1). With the lowest 5-year survival rate of any cancer type (10%), PC is also projected to become the second leading cause of cancer-related mortality in the United States by the year 2030 (2,3). As pancreatic ductal adenocarcinoma (PDAC) accounts for ~90% of all PC cases (4), the present review focuses primarily on this type of PC.
A genetic susceptibility for PC is related to mutations, such as BRCA2, while other risk factors for the development of this disease include smoking, obesity, diabetes and chronic pancreatitis (5,6). Due to a lack of alarming symptoms at the early stages of the disease, the majority of cases are already an advanced or metastatic stage at the time of diagnosis, and low survival rates are observed even in cases where resection is possible (7,8). Chemotherapy remains the standard treatment option, although gemcitabine monotherapy, as well as certain combination chemotherapeutic regimens, such as FOLFIRINOX and nab-paclitaxel plus gemcitabine have achieved only minimal survival benefits, while being associated with considerable toxicity issues (9,10). Moreover, advances in the field of cancer immunotherapies have not yet had an impact on PC, mainly due to the dense stromal barrier, which poses an obstacle for the infiltration of immune cells, due to the immunosuppressive environment and the shortage of effector T-cells able to eliminate tumor cells (11). Although attempts to manipulate the immune system in PC have yielded some promising results (12-14), the majority of clinical trials to date have been largely disappointing (15).
Alternative strategies to combat this lethal disease are therefore sought after and the targeting of the deregulated metabolic physiology, which is additionally connected to radio- and chemo-resistance in PDAC, is one of the promising options (16,17). The common genomic alterations, such as the activation of the KRAS oncogene, the deletion of the tumor suppressor gene, SMAD4, or the transformation of the tumor suppressor, p53, into a prooncogenic protein significantly stimulate nutrient acquisition and a variety of metabolic pathways (18). The ability to stratify PDAC tumors into subgroups with distinct metabolic requirements and vulnerabilities could offer valuable prognostic information and may aid to in the selection of specific treatments. For example, some studies have identified glycogenic and lipogenic subtypes associated with different survival outcomes and distinct sensitivity to metabolic inhibitors (19,20).
The ability of PC cells to obtain nutrients from their surroundings and through autophagic pathways, unique physiological characteristics of the tumor microenvironment, as well as the interactions of PC cells with non-cancer cells are also among the factors that markedly contribute to the extensively reprogrammed metabolism in PDAC (21). Populations of highly clonogenic, resistant and metastatic PC stem cells (PCSCs) appear to have distinct therapeutic vulnerabilities related to specific metabolic features that could be exploited. Importantly, metabolic plasticity that allows PCSCs to adapt to environmental changes appears to be connected to distinct patterns of DNA methylation and so a disruption of this metabolic-epigenetic crosstalk present within PDA could represent another therapeutic target (22).
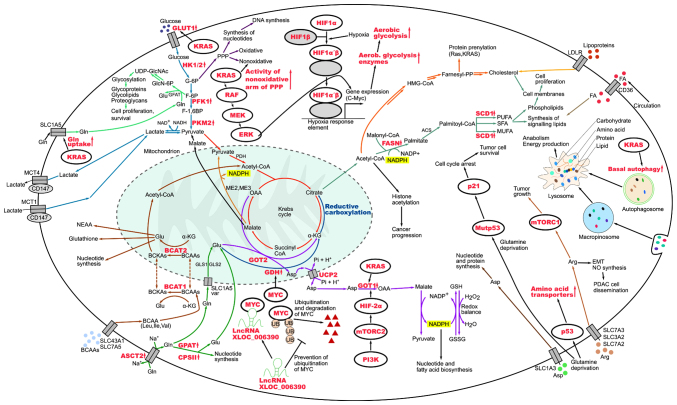
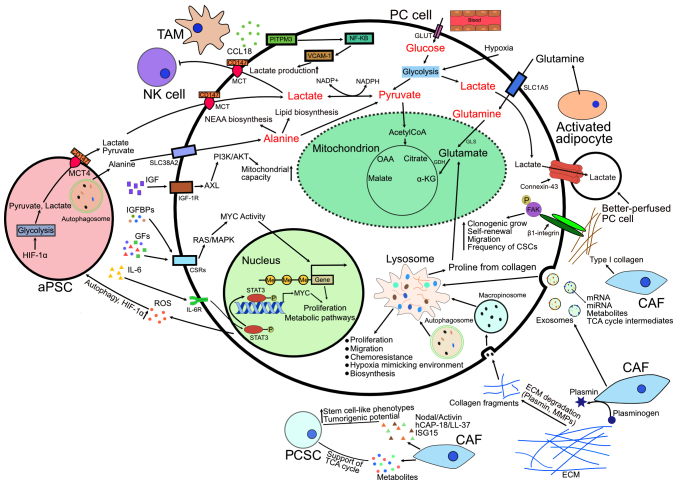
The present review discusses a range of metabolic and physiological aspects of PC cells, which play a role in the development and progression of this difficult-to-treat and aggressive malignancy. The present review also focuses on the possibility of targeting PDAC-specific metabolic pathways in order to aid in the development of more effective and personalized therapies. The metabolic pathways discussed herein as taking place in PC cells are summarized in Fig. 1 and the metabolic crosstalk within the tumor microenvironment in PC is illustrated in Fig. 2.
Figure 1.
Metabolic pathways utilized by pancreatic cancer cells. Upward arrows represent upregulation and T-bars represent inhibition. Red lettering indicates deregulated genes, proteins and processes. ACS, acetyl-CoA synthetase; Arg, arginine; ASCT2, alanine serine cysteine transporter 2; Asp, aspartate; BCAAs, branched-chain amino acids; BCAT1/2, branched-chain aminotransferase 1/2; BCKAs, branched-chain keto acids; CPSII, carbamoyl phosphate synthase II; EMT, epithelial-mesenchymal transition; F-1,6BP, fructose 1,6-bisphosphate; F-6P, fructose 6-phosphate; FA, fatty acid; FASN, fatty acid synthase; G-6P, glucose 6-phosphate; GFAT, glutamine fructose-6-phosphate amidotransferase; GlcN-6P, glucosamine-6-phosphate; Gln, glutamine; GLS1/GLS2, glutaminase 1/2; Glu, glutamate; GLUT1, glucose transporter 1; GOT1/GOT2, glutamate oxaloacetate transaminase 1/2; GPAT, glycerol-3-phosphate acyltransferase; GSH, glutathione; GSSG, glutathione disulfide; HIF1α/HIF2α, hypoxia-inducible factor 1-α/2-α; HIF1β, hypoxia-inducible factor 1-β; HK1/2, hexokinase 1/2; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; Farnesyl-PP, farnesyl pyrophosphate; Ile, isoleucine; LDLR, low-density lipoprotein receptor; Leu, leucine; lncRNA XLOC_006390, long non-coding RNA XLOC_006390; MCT1/MCT4, monocarboxylate transporter 1/4; ME2, malic enzyme 2; ME3, malic enzyme 3; mTORC1/mTORC2, mechanistic target of rapamycin complex 1/2; MUFA, monounsaturated fatty acid; Mutp53, mutant p53; NAD+, nicotinamide adenine dinucleotide; NADH, reduced form of nicotinamide adenine dinucleotide; NADP+, nicotinamide adenine dinucleotide phosphate; NADPH, reduced form of nicotinamide adenine dinucleotide phosphate; NEAA, non-essential amino acids; NO, nitric oxide; OAA, oxaloacetate; PDH, pyruvate dehydrogenase; PFK1, phosphofructokinase-1; Pi, inorganic phosphate; PI3K, phosphoinositide 3-kinase; PKM2, pyruvate kinase M2; PPP, pentose phosphate pathway; PUFA, polyunsaturated fatty acid; SCD1, stearoyl-CoA desaturase 1; SFA, saturated fatty acid; UB, ubiquitin; UCP2, uncoupling protein 2; UDP-GlcNAc, uridine diphosphate N-acetylglucosamine; Val, valine; α-KG, α-ketoglutarate.
Figure 2.
Roles of TME in PC. Upward arrows represent upregulation and T-bars represent inhibition. aPSC, activated pancreatic stellate cell; AXL, AXL receptor tyrosine kinase; CAF, cancer-associated fibroblast; CCL18, chemokine (C-C motif) ligand 18; CSCs, cancer stem cells; CSRs, cell surface receptors; ECM, extracellular matrix; FAK, focal adhesion kinase; GDH, glutamate dehydrogenase; GFs, growth factors; GLS, glutaminase; GLUT, glucose transporter; hCAP-18, human cationic antimicrobial protein 18 kDa; HIF-1α, hypoxia-inducible factor 1-alpha; IGF, insulin-like growth factor; IGF-1R, insulin-like growth factor 1 receptor; IGFBPs, insulin-like growth factor binding proteins; IL-6, Interleukin-6; IL-6R, interleukin-6 receptor; ISG15, ubiquitin-like molecule interferon-stimulated gene 15; MAPK, mitogen-activated protein kinase; MCT, monocarboxylate transporter; MMPs, matrix metalloproteinases; NEAA, non-essential amino acid; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NK cell, natural killer cell; OAA, oxaloacetate; PCSC, pancreatic cancer stem cell; PI3K/AKT, phospoinositide 3-kinase/protein kinase B; PITPNM3, membrane-associated phosphatidylinositol transfer protein 3; ROS, reactive oxygen species; STAT3, signal transducer and activator of transcription 3; TAM, tumor-associated macrophage; VCAM-1, vascular cell adhesion molecule-1; α-KG, α-ketoglutarate.
2. Search tools
The PubMed/MEDLINE, Scopus and Web of Science databases were searched with search terms including 'cell metabolism', 'metabolic phenotypes', 'metabolic profiles', 'pancreatic cancer', 'tumor microenvironment', 'stem cells', 'therapeutic/treatment resistance' and 'metabolomics' in various Boolean combinations as search strings. In order to cover the selected topic comprehensively, articles were searched without a limit on their date of publication. Only published, peer-reviewed articles in the English language were included in the search and literature analysis. The articles were then manually sorted and critically evaluated according to relevance. Based on the database searches, electronic and manual cross-referencing was used to identify additional relevant sources.
3. Distinct metabolic profiles and metabolic flexibility of pancreatic cancer cells
Pancreatic tumors are known to be heterogenic at the cellular level, with distinct metabolic traits and methods of nutrient acquisition, which can lead to issues with effective treatment (23). There is therefore a need for a better understanding of the biological characteristics of PC together with an assessment of metabolic phenotypes of individual tumors in order to lead to the development of more targeted therapeutic strategies. Patient stratification strategies are becoming increasingly useful in cancer diagnosis, prognosis assignment and in creating appropriate treatment strategies. The comparison of mutational profiles has proven problematic with questionable associations between mutational status and disease behavior (24).
Genomic and transcriptomic studies based on molecular profiling have identified subgroups of PDAC often overlapping to varying degrees (25-29). Moffitt et al (26) used PDAC gene expression microarray data to identify and validate two tumor subtypes, 'basal-like' and 'classical'. On a molecular level, the basal-like subtype was similar to basal tumors of the urinary bladder and breast cancer and had a worse outcome. Notably, using the algorithmic separation of tumor, stromal and normal gene expression, they also identified distinct stromal subtypes, which were defined as 'normal' and 'activated' and were independently prognostic. These findings demonstrate a particular significance of the stromal compartment, which has been shown to play crucial roles in PDAC biology (30-35). It is also becoming clear that further heterogeneity in expression profiles exists within cells of a single tumor (36).
PC cells need to be able to proliferate rapidly, which is complicated by the dense stroma environment, which lacks adequate vasculature and therefore presents a nutrient- and oxygen-poor environment. Cells in such conditions undergo extensive oncogene-directed metabolic reprogramming, which includes a higher nutrient acquisition, increased glucose utilization through aerobic glycolysis, an upregulated biosynthesis of lipids and amino acids, alterations in the redox balance, as well as an activation of recycling and scavenging pathways (37-41).
In addition to the molecular signatures of PDAC afore-mentioned, metabolic subtypes with specific metabolic requirements have also been identified that are of prognostic value and could aid in the selection of treatments and may thus lead to more favorable therapeutic outcomes. Based on broad metabolic profiling, Daemen et al (19) described 'slow proliferating', 'glycolytic' and 'lipogenic' PDAC subtypes, which exhibited different metabolite levels associated with glycolysis, lipogenesis and redox pathways. Studying the differences between glycolytic and lipogenic lines, theys found particularly significant high levels of phosphoenolpyruvate (PEP) and of the ENO2 mRNA coding for the glycolytic enzyme neuron-specific enolase (ENO2), which converts 2-phosphoglycerate to PEP. The two metabolic subtypes also responded differently to inhibitors of glycolysis and lipid metabolism. Based on these findings, those authors suggested ENO2 inhibitors as a possible therapeutic option for aggressive, fast growing glycolytic tumors where ENO2 was found to be strongly expressed. They also observed a correlation between these subtypes and those identified by Collisson et al (25), indicating that links indeed exist between molecular and metabolic subtypes. They used their findings to create a model, in which epithelial tumors use glucose for the tricarboxylic acid (TCA) cycle and de novo lipogenesis, whereas mesenchymal tumors preferentially fuel the TCA cycle by glutamine with a potentially enhanced vulnerability to ROS-inducing agents (19).
Yu et al (42) performed an analysis of the expression of metabolism-related proteins and classified pancreatic tumors derived from patients into categories based on the utilization of glucose and glutamine: Warburg type, reverse Warburg type, mixed type, and null type in glucose-dependent metabolism and canonical type, and non-canonical type, mixed type and null type in glutamine-dependent metabolism. The Warburg type, non-canonical type and mixed types of these two metabolic branches were represented by metabolically active, biologically aggressive and tumors with a poor prognosis. Those authors came to the conclusion that a higher number of metabolic subtypes and categories leads to major differences in survival outcome: The more subtypes and categories PCs employ, the worse the outcome (42). There are other studies focusing on the molecular and metabolic stratification of PDAC tumors (20,43); however, the two important phenotype categories which can be taken out of those are more aggressive, often metastatic glycolytic subtypes commonly featuring the amplified oncogenes, KRAS and Myc, with less favorable clinical outcomes, and lipogenic subtypes with the more prominent utilization of lipid metabolism pathways and a better prognosis. In addition to those studies, an association of particular lipid metabolites with a worse prognosis in PDAC has recently been highlighted. Performing metabolomic profiling of patient-derived tumor xenografts (PDTX) Kaoutari et al (44) linked increased levels of triacylglycerols with a poor prognosis. They also managed to improve the sensitivity of PDTX-derived primary cells to the cytotoxic drugs gemcitabine, oxaliplatin and 5-fluorouracil by blocking the synthesis of glycerophospholipids (44).
It should be kept in mind that interactions involving distinct cell populations within the tumor microenvironment may also contribute to the complex nature of PDAC metabolic phenotypes. Single-cell metabolomics can be used to distinguish different cell types in a heterogeneous cell mixture and to provide information about metabolic specificities of tumor cells from clinical cancer tissues (45). Specifically, a high-throughput, label-free and sensitive dielectric barrier discharge ionization-mass spectrometry (DBDI-MS) platform has been developed for the analysis of single-cell metabolites, which identified deregulated lipid metabolism in PDAC cells and detected a reduction in lipid content after the inhibition of ATP citrate lyase, a rate-limiting enzyme for lipid synthesis, which has high mRNA levels in both patients with PDAC from The Cancer Genome Atlas dataset, as well as PC cell lines (45). Distinguishing metabolic variations among the subsets of cell populations on a single-cell level is also possible via a complex metabolic profiling method single cell energetic metabolism by profiling translation inhibition (SCENITH) or by measuring multiple enzymatic activities reflecting different metabolic pathways at saturating substrate conditions at single cell resolution (46,47). SCENITH is based around puromycin labeling and flow cytometry measurements of protein synthesis, reflective of global cellular metabolic activity. It can be used directly in heterogeneous human tumor samples to study metabolic profiles of multiple cell types. The analysis of single cell enzymatic activities within a native tissue microenvironment relies on enzyme histochemistry and automated histocytometry to identify particular cell types and simultaneously characterize intra- and intercellular metabolic configurations (46,47).
Metabolic phenotypes of cancer cells can be transient. For example, progressively de-differentiated PC cells have been shown to undergo a shift from glycolytic to oxidative metabolism, resulting in a quiescent state. Following the re-differentiation, these cells regained their proliferative capacity and glycolytic metabolism, commonly associated with a greater aggressiveness (48). Metabolic switches in PDAC cells can occur also as a response to disruptions caused by treatments (39,49). It is thus of utmost significance to understand the underlying mechanisms, as it could help either to prevent, overcome or even exploit such metabolic plasticity.
4. Glucose, lactate and the metabolic collaboration in the tumor microenvironment
The enhanced utilization of glucose by tumor cells even under normal oxygen levels was observed by Otto Warburg as early as the 1920s and was later defined as the Warburg effect (50). Since then, the importance of the Warburg phenotype in cancer cells has been well-documented. Its advantages lie in the quick supply of ATP, the support of increased biosynthetic needs, the modification of chromatin structure and redox regulation (51,52). Moreover, the produced and exported lactate contributes to immunosuppression within the tumor microenvironment (53). To compensate for the relatively low ATP output compared to mitochondrial oxidative phosphorylation (OXPHOS), the uptake of glucose can be increased by an overexpression of the glucose transporter 1 (GLUT1) (54). Additionally, phosphorylation on Ser226 in GLUT1 was identified as a key event for the enhanced cell surface localization of GLUT1 and for the regulation of glucose transport (55). A sustained elevated glycolytic flux in PDAC is markedly connected with an increased expression of rate-limiting glycolytic enzymes like phosphofructokinase 1 and lactate dehydrogenase A (LDHA) (56-58), and there is also evidence of glycolytic enzymes regulating the epithelial-mesenchymal transition (EMT) in PC cells, inducing PC cell invasion and metastasis as well as promoting tumor angiogenesis (59-63). The Raf/MEK/extracellular signal-regulated kinase (ERK) signaling pathway activated by oncogenic KRAS together with hypoxic conditions known to be present in PDAC increase glucose uptake and the expression of glycolytic enzymes, which then leads to the establishment of an acidic microenvironment and the promotion of tumorigenesis (57,64).
The transport of lactate in and out of cells is a key factor in metabolic adaptations to deal with the unequal access to vasculature and differing oxygen levels within a tumor. Out of the group of monocarboxylate transporters (MCTs) belonging to the SLC16A gene family, which are crucial for controlling acidity levels within the tumor and the microenvironment, MCT-1 and MCT-4 were previously shown to be upregulated in PC (65). To correctly function, MCT1 and MCT4 need to be properly inserted into the plasma membrane, which is facilitated by CD147 glycoprotein (66). MCT-4 is upregulated by hypoxia, promotes glucose uptake and the production of lactate via glycolysis. MCT-1 expression on the other hand is dependent on oxygen and is repressed by hypoxia (67). It facilitates lactate uptake by cells, which is then used as a substrate in the TCA cycle for ATP production in OXPHOS (68). Cells expressing MCT-1 have a well-developed mitochondrial network and produce large amounts of TCA intermediates and ATP (41). This led to the development of a model of PDAC where aerobic cells in normoxic regions utilize OXPHOS fed by lactate produced by hypoxic cells and imported through MCT-1, leaving glucose to be preferentially used in aerobic glycolysis by cells growing at low oxygen pressure (65).
A similar collaboration appears to exist between cancer cells and stromal resident cells. Cancer cells induce oxidative stress and aerobic glycolysis in cancer-associated fibroblasts (CAFs) and subsequently use energy-rich metabolites lactate and pyruvate secreted by CAFs to sustain their own increased proliferation (69,70). The Gi-coupled receptor GPR81 was confirmed to be expressed in several cancer cell lines, including those of PDAC and to play a critical role in sensing extracellular lactate (71). Its activation leads to an increased expression of MCTs, CD147 and peroxisome proliferator-activated receptor-γ co-activator PGC-1α which, apart from its ability to increase MCT1 expression, engages in a spectrum of biological processes (72). PGC-1α is strongly linked to the regulation of glucose and fatty acid metabolism, fiber type switching in skeletal muscle, adaptive thermogenesis and heart development as well as the stimulation of mitochondrial biogenesis (73). The silencing of GPR81 has been found to negatively affect the mitochondrial activity of cancer cells grown in cell culture conditions, with only lactate as an available energy source. Roland et al (71) also observed that the loss of GPR81 was associated with a reduced PC tumor growth and metastasis in vivo. Following these findings regarding the key role of lactate metabolism in cancer progression, researchers have begun to explore potential benefits of the inhibition of MCTs and CD147 in PDAC and beyond (74-78).
Finally, Lau et al (79) demonstrated the importance of studying tumor cellular metabolism within heterogeneous systems, which contain different cell types to identify dependencies that may not be evident from studying isolated cells in culture. They used isotope-labeled nutrient tracing in macromolecules to detect increased pyruvate carboxylation of PC cells relative to fibroblasts in murine PC organoid-fibroblast co-cultures and tumors and then demonstrated that a loss of enzymes with pyruvate carboxylation activity (malic enzyme 1 and pyruvate carboxylase) had a minimal effect on PC cell proliferation in monoculture, whereas it reduced the growth of organoid co-cultures compared to the controls (79).
5. Lipids as essential metabolites and possible clinical markers of PDAC
Lipids support cancer progression via multiple mechanisms, including the formation of biomembranes, participation in cellular signaling and as an energy source (80). Depending on the microenvironmental conditions, as well as currently active metabolic pathways, PC cells can direct glucose or glutamine-derived carbons to increase the synthesis of fatty acids (19). Notably, increased levels of fatty acid synthase (FASN) involved in de novo lipid synthesis have been shown to be associated with a worse prognosis of patients with PC (81). As for the possible underlying reasons behind this association, FASN was previously associated with the HER2-PI3K/AKT signaling axis and shown to exert a prominent effect on the proliferation and migration of cancer cells (82). Additionally, acidic extracellular conditions could lie behind the transcriptional upregulation of FASN by means of epigenetic modifications (83). It is not unreasonable to suggest that similar processes could be taking place in PC, where the increased expression of other lipogenic genes has been found (19). Furthermore, FASN knockdown has been found to lead to an increased responsiveness of PC cells to gemcitabine, as well as radiation treatments, thus creating a link between the overexpression of FASN and treatment-resistant phenotypes (16). In a more recent study, a paclitaxel-poly(lactic-co-glycolic acid) nanoparticle (PPNPs) formulation was able to inhibit de novo lipid synthesis, alter membrane stability and improve anticancer efficacy of gemcitabine in PC cells, further emphasizing the importance of lipid metabolic signaling in PC chemoresistance (84). Aside from FASN, stearoyl-CoA desaturase1 (SCD1) is another central lipogenic enzyme that contributes to the progression of cancer. SCD1 is important for keeping the correct composition of cancer cell membranes, where monounsaturated phospholipids provide protection from oxidative stress (85,86). Taken together with the fact that the inhibition of SCD1 appears to cause obstructions in aberrant RAS and AKT signaling often involved in the development and progression of PDAC, it could represent a potential therapeutic target (87,88).
An increased amount of free, newly synthesized fatty acids, such as palmitate could be toxic for cancer cells with apoptosis as the likely outcome. Therefore, a controlled release of fatty acids from intracellular lipid stores, where they are stored in the form of triglycerides, by intracellular lipolysis is crucial for preventing that scenario (89). Apart from de novo synthesis, PC cells can obtain fatty acids also from the circulation from food digestion or from fatty acid release from the adipose tissue. This may present an obstacle, as it could render the aforementioned inhibition of the endogenous fatty acid synthesis in PDAC insufficient with regard to antitumor effects, making it hard to justify as an isolated therapeutic approach. The ability of cancer cells to use fatty acid uptake in addition to de novo fatty acid synthesis could help to explain why obesity with characteristically elevated fatty acid plasma levels and a high-fat diet are among the potential risk factors for cancer development and worse clinical outcomes of malignancies (38,90-94).
Cholesterol and its synthetic pathway have numerous functions in cancer cells, supporting their growth, proliferation and migration (95). Both the targeting of exogenous cholesterol uptake in PC cells through the shRNA silencing of low-density lipoprotein (LDL) receptor (96) and the inhibition of 3-hydroxy-methylglutaryl-CoA reductase (HMG-CoA reductase), a key enzyme in cholesterol synthesis, by statins has exhibited promising anticancer effects (97). An inhibition of HMG-CoA reductase can also disrupt the process of protein prenylation, an essential process for the activation of signaling proteins including KRAS, which could be exploited to further target cancer cells (98). It is, however, uncertain whether statins may be used as a monotherapy for cancer, as their therapeutic efficacy in clinical trials is controversial (99). For example, pravastatin, a potent HMG-CoA reductase inhibitor, has been found to significantly prolong the survival of patients with advanced hepatocellular carcinoma (HCC) in one study (100), while in another clinical study, the same statin did not improve the survival of patients with advanced HCC, despite the high doses (40-80 mg/day) administered (101). Nevertheless, their use, specifically in combination with other agents, such as FASN inhibitors, remains of pharmacological interest.
Looking at the metabolic rewiring in PDAC and specifically, at the alterations of lipid metabolism in the context of genomic alterations, inactivating mutations of the TP53 gene that often precede the emergence of metastatic tumors stand out in particular. Recently, mass-spectrometry-based lipidome profiling in combination with the transcriptomics of in vitro models and patients with PDAC revealed that the loss of p53 caused a deregulation of intracellular and secreted lysophospholipids with the suggestion that this class of lipids may play a role in p53-mediated non-cell-autonomous molecular signaling, which causes the remodeling of the cancer microenvironment and contributes to immune evasion during PDAC pathogenesis (102).
In addition to rendering the treatment of PDAC more effective, it is crucial to spot its onset early. Biomarkers with optimal specificity and sensitivity for an early diagnosis of PC are therefore much sought after. Within that context, altered serum levels of several lipid metabolites, such as very-low-density lipoprotein, LDL, high-density lipoprotein, 3-hydroxybyturate, lysophosphatidylcholine, phosphatidylethanolamine and bile acids, such as taurocholic acid were identified (103,104). Recently, Wang et al (105) combined machine learning and metabolomics to select lipids, such as diacylglycerol and lysophosphatidylethanolamine for their detection in PDAC patients' serum and proposed the potential clinical application of their liquid chromatography-mass spectrometry-based targeted lipidomics assay for the effective and accurate auxiliary diagnosis of PDAC. Analyzing the lipid content in pancreatic tissue could also be used to distinguish chronic pancreatitis from PC, e.g., through the employment of NMR spectroscopy (106).
6. Amino acids are involved in the regulation of pancreatic cancer cell proliferation and dissemination
As a consequence of the use of aerobic glycolysis for ATP production and the conversion of pyruvate to lactate, cancer cells use other mechanisms to secure the fueling of the citric acid cycle than the conversion of pyruvate of glycolytic origin to acetyl-CoA and then to citrate. An increased dependence on glutaminolysis, which is accompanied by nicotinamide-adenine dinucleotide phosphate (NADPH) production and changes in the activity of corresponding enzymes is one particular mechanism through which cancer cells maintain the proper function of the Krebs cycle (107). This allows such cells to reserve glucose-derived carbon for the use in biosynthetic pathways, such as the pentose phosphate pathway, which ultimately yields NADPH and substrates for the synthesis of ribonucleotides (108,109). NADPH, as a glutathione reductase (GR) co-substrate, plays a role in maintaining the reduced pool of glutathione important for reactive oxygen species (ROS) scavenging (110). Stepping aside from the important role of glutamine metabolism in maintaining the appropriate levels of ROS in cancer cells, the metabolism of other amino acids has also been identified as a possible target for the disruption of the redox homeostasis in PC cells. The inhibition of the import of oxidized cysteine has been shown to cause a redox imbalance and subsequent ferroptosis in targeted cells (111). This points at a critical dependency of PDAC cells on cysteine contribution to the synthesis of glutathione and coenzyme A to endure elevated lipid ROS production, typically elicited by oncogenic signaling to stimulate tumor growth (111).
The mitochondrial production of citrate from glutamine in a multistep process and its subsequent conversion to cytosolic acetyl-CoA links glutaminolysis with fatty acid synthesis to support the growth of cancer cells in vivo (112). Glutamine also serves as a substrate in the hexosamine phosphate synthesis and is critical for the replenishment of nucleotides, as well as amino acids pools (113-115). Bott et al (116) found that in glutamine-deprived PDAC cells, the glutamate ammonia ligase-mediated de novo synthesis of glutamine was critical for the transfer of the terminal amide nitrogen to nucleotides and hexosamines and subsequently, for their growth. Among the enzymes present in cancer cells that are responsible for the production of glutamine-derived α-ketoglutarate are glutamate-dehydrogenase (GDH), alanine aminotransferase (ALT or GTP), phosphoserine aminotransferase 1 and aspartate transaminase (AST or GOT) (117). In PC, a so-called non-canonical glutamine metabolism pathway is prominently used, where glutamine is first converted to aspartate, which is then converted to oxaloacetate by GOT1 in the cytoplasm. This NADPH-producing pathway concludes with the formation of malate and later pyruvate. An oncogenic form of the KRAS protein has been shown to be associated with the establishment of this non-canonical pathway of glutamine metabolism in a study on PDAC (118). This growth advantage-conferring pathway can also be stimulated by hypoxia, often observed in PDAC cells: The PI3K/mammalian target of rapamycin complex (mTORC) 2 pathway targets hypoxia-inducible factor 2-α (HIF2-α) with a consequent transcriptional activation of GOT1 (119). Recently, it has also been confirmed in PDAC cells that the uncoupling protein 2 (UCP2)-mediated transport of glutamine-derived aspartate from mitochondria to cytosol is critical for the generation of NADPH in this pathway. Notably, UCP2 silencing has been shown to markedly suppress the growth of KRASmut PDAC cells (120).
Multiple research teams have tried to confirm the critical importance of this non-canonical pathway of glutamine metabolism in PC. In a previous study, a pan-inhibitor of transaminases, aminooxyacetate (AOA) negatively affected the growth of PDAC cell lines; however, non-transformed human pancreatic ductal cells and human diploid fibroblasts remained largely unaffected (118). The authors of that study suggested, however, that the sensitivity of cells to AOA was dependent on whether or not oncogenic KRAS was present to support the anabolic metabolism of glutamine. Encouragingly, the decreased ability of PDAC cells to combat oxidative stress after an impairment of Gln metabolism could significantly increase the success rate of the whole approach (118). In another study, the inhibition of mitochondrial GOT2 led to a marked induction of cyclin-dependent kinase inhibitor p27-mediated cellular senescence and the growth suppression of PDAC cells. This effect was not observed in non-transformed cells (121).
Apart from oncogenic KRAS signaling, other regulatory mechanisms exist in PDAC that modulate the metabolism of amino acids and provide for the metabolic needs of cancer cells. The MYC oncogene positively regulates glutamine uptake and causes a suppression of microRNAs (miRNAs/miRs) with an inhibitory effect towards glutaminase (GLS) (122,123). Son et al (118) demonstrated that a chemical inhibition of GLS had a growth-suppressive effect on both human and mouse PDAC cells. However, Roux et al (124) observed a decreased activity and mRNA levels of GLS in the majority of the analyzed PDAC cells. Those cells had an increased activity, mRNA and protein expression of glycerol-3-phosphate acyltransferase and carbamoyl phosphate synthetase II, which allowed them to produce nucleotides and glutamate, as well as upregulated levels of alanine serine cysteine transporter 2 responsible for glutamine import. GDH and AST/GOT were other enzymes with an elevated activity. Another study demonstrated that the activity of Myc itself could be regulated by RNA and that expression levels of long non-coding RNA (lncRNA) XLOC_006390 were specifically associated with changes in glutamate metabolism, proliferation and migration of PC cells (125). It was found that this specific lncRNA functioned by binding to c-Myc and preventing ubiquitination with the final result being the increased stability of the transcription factor that binds to the promoter of GDH1 and acts as its transcriptional activator. Of note, He et al (125) suggested that it was the XLOC_006390/c-Myc signaling axis resulting in the upregulation of GDH1 expression that was associated with worse survival times of patients with PC rather than the expression of other enzymes involved in the α-ketoglutarate supply.
In conditions with a low glutamine availability, the transcription factor p53 is a chief participant in pro-survival signaling and can help cancer cells to overcome such a nutritional challenge by stimulating the expression of transporters, facilitating the import of other amino acids. Aspartate and arginine can be taken up by cells more robustly due to the involvement of p53, with the latter amino acid capable of activating the mTORC1, and in turn promoting tumor growth (126,127). In addition to wild-type p53, mutated p53 can also play a role upon a glutamine withdrawal to promote tumor cell survival via an induction of p21 resulting in cell cycle arrest (128). Taken together, p53 can act in conjunction with mTORC1 either towards stopping or promoting cell proliferation in a context-dependent manner, with factors such as the type of stress stimulus and the mutational status of p53 likely to be involved.
Other tumor suppressors frequently mutated in cancer cells have been linked to the altered cellular metabolism of various types of cancer, including PDAC (18). A homozygous deletion of the tumor suppressor gene locus SMAD4, which occurs in PDAC and can cause the loss of the malic enzyme 2 (ME2), was previously exploited to disrupt NADPH production, mitochondrial redox homeostasis and amino acid metabolism. The simultaneous deletion of both mitochondrial malic acid enzymes ME2 and ME3 led to a suppressed transcription of branched-chain aminotransferase ½ and thus impeded the transfer of amino groups from branched chain amino acids to α-ketoglutarate. As a consequence, glutamate could not be resynthesized efficiently and the de novo nucleotide synthesis was also impaired (129). This approach could be potentially therapeutically beneficial in patients with PDAC with a homozygous deletion of the SMAD4 locus, who constitute approximately one-third of PDAC cases and who, at the same time, have ME2 deletions (129).
Aberrant metabolism can lead to epigenetic alterations associated with an uncontrolled proliferation, as the activity of chromatin-modulating enzymes involved in the regulation of DNA transcription is dependent on the presence of cellular metabolites, such as acetyl-coenzyme A and S-adenosylmethionine (130-132). Kottakis et al (133) established how a concrete metabolic state interconnected with epigenetic processes contributes to the oncogenic transformation in pancreatic cells with concurrent LKB1 and KRAS mutations. An interrelation was found between the generation of excess S-adenosylmethionine induced by the mTOR-dependent channeling of glucose and glutamine-derived intermediates into the serine-glycine-one carbon pathway, and transcriptional silencing through DNA methylation (133).
The importance of amino acids is also apparent when it comes to the transcriptional regulation of the Snail family of EMT transcription factors (EMT-TFs). These EMT-TFs suppress E-cadherin, and other epithelial markers and adhesion molecules, and increase the expression of matrix metalloproteinase (MMPs), thus stimulating EMT, which often precedes the metastatic spread of cancer cells (134). Arginine additionally supports PDAC cell dissemination by serving as the precursor for the synthesis of nitric oxide (NO), while glutamate has been shown to stimulate the metastatic spread in PC, functioning through its neurotransmitter receptors (135,136). Specifically, the depletion of arginine has been found to impede the migration, adhesion and invasion of PC cells with a lower expression of EMT-TFs, extracellular matrix-rebuilding MMPs and an increased expression of the epithelial marker, E-cadherin (134). The use of arginine deprivation in the treatment of PC could also achieve more prominent antitumor effects. It may thus combat the development of therapeutic resistance with a simultaneous use of cytotoxic agents, such as gemcitabine or when combined with the inhibition of other metabolic pathways in PDAC cells, including those belonging to glutamine metabolism, serine biosynthesis or polyamine biosynthesis (137,138).
Finally, to be able to cope with a situation when nutritional deficiencies arise, PDAC cells are well-adapted to take advantage of extracellular nutrient sources available in the microenvironment. When needed, they can utilize protein-macropinocytosis to obtain important amino acids by lysosomal degradation, as well as autophagy to recycle proteins, macromolecules and whole organelles and to refill essential nucleotide pools (139-143). Additionally, autophagy in PC supports immune evasion and is required for proper cystine transport and cysteine homeostasis by promoting the localization of the cystine transporter SLC7A11 at the plasma membrane (144,145). Notably, Maertin et al (140) found that while the basal autophagic activity of PC cells was relatively high, amino acid depletion and hypoxia activated autophagy only weakly, if at all. As regards the precise regulation of the autophagic process, they confirmed the stimulation of basal autophagy by the oncogenic activation mutation in KRAS GTPase and by the consequently stimulated OXPHOS. A limitation of amino acid supply to suppress OXPHOS was also mentioned as a potential option for the treatment of PDAC (140).
7. Mitochondrial oxidative metabolism as a therapeutic target
It is no longer controversial that numerous cancer cells have fully functional mitochondria performing OXPHOS, depending on the oxygen availability in the particular tumor site, the amount of available metabolites capable of fueling the TCA cycle and the required regulation state. This is contrary to the previous hypothesis of universally diminished OXPHOS activity with defective mitochondria whenever glycolysis was found to be upregulated in tumors (146).
Substrates produced by fatty acid oxidation, glycolysis and glutaminolysis enter the TCA cycle, which extracts electrons passed on to NADH and flavoproteins. A constant supply of electrons is necessary for the creation of a transmembrane proton gradient and the efficient production of ATP by the electron transport chain complexes and ATP synthase in the inner mitochondrial membrane (147). The transport of these electrons can lead to the generation of ROS in mitochondrial complexes of the electron transport chain (148). Oncogene-inducible ROS can support anchorage-independent cancer cell growth by decreasing the phosphorylation of ERK1/2 in the ERK MAPK signaling pathway to levels that are compatible with cellular proliferation and as such, were described as a factor supporting KRAS-driven tumorigenicity (149). Attempts to target the TCA cycle in PDAC to suppress mitochondrial metabolism are currently ongoing, with a protocol that includes the lipoic acid analogue, devimistat, which selectively inhibits the TCA cycle by impairing the activity of pyruvate dehydrogenase and α-ketoglutarate dehydrogenase, combined with the modified FOLFIRINOX regimen (150).
Within a cohort of patients with PDAC, tumors with heterogeneous OXPHOS rates have been discovered and the high OXPHOS activity with high amounts of complex I mRNAs and proteins could be used as a helpful biomarker to identify tumors vulnerable to substances, such as phenformin in combination with traditional chemotherapy (151). The cause for optimism with this type of treatments is the fact that they could be applied independently of the main genetic alterations forming the causal background of the majority of PDAC cases, such as KRAS mutations, and that the antitumor effects of phenformin with gemcitabine were successfully replicated in vivo (151). It was hypothesized that after inhibiting OXPHOS at complex I with phenformin, the affected cells would lose the protection from the stress caused by the inhibition of DNA synthesis by gemcitabine (151). The clinical confirmation of those results remains to be performed, at least to the best of our knowledge. To improve the results of OXPHOS inhibition, combined treatments using several compounds will likely be required. For example, Candido et al (152) based their experiments on the findings of metformin-mediated autophagy induction through the suppression of mTORC1 activity. Using metformin, they increased the anti-proliferative effects of the mTOR inhibitor, rapamycin, in PDAC cell lines (152).
Attention should be paid to the possibility of potential disease relapses when cancer treatments targeting mitochondrial metabolism are used. An adaptation to OXPHOS inhibition by a fraction of tumor cells that can switch to glycolysis and vice versa was previously described in PCSCs, where their metabolic phenotype observed at a particular time depended on MYC/PGC-1α balance (39). These highly metastatic and treatment-resistant cells capable of tumor repopulation were previously described as more reliant on the OXPHOS pathway and thus could represent an interesting cell subpopulation in PC to be targeted by OXPHOS inhibiting drugs (17). The inhibition of OXPHOS could further benefit cancer patients by lowering tumor hypoxia, which can interfere with the efficacy of treatments. An oxygen consumption decrease in better accessible normoxic regions could lead to a higher oxygen tension across the microenvironment of the whole tumor, leading to an improvement of the anticancer effects in the initially hypoxic regions (146). Finally, carefully selected medications aimed at increasing tumor oxygenation could help in the quest to identify strategies with which to suppress treatment resistance in a number of cancer cases, as exemplified by an improved response to radiotherapy following the administration of metformin in a prostate cancer in a study using xenografts (153).
8. Interactions of pancreatic cancer cells with the stromal environment
The extracellular environment in PC consists of a desmoplastic tissue composed of collagen fibers, dissolved growth factors, metabolites and diverse stromal cells, among which CAFs, stellate cells and activated macrophages are strongly represented (154). Drug penetration through this dense, fibrotic tissue barrier can be problematic, which together with frequently occurring hypovascularity, adds to the intrinsic chemoresistance of PDAC (154). This desmoplastic environment can be advantageous for cancer cells, while at the same time forming an obstacle to their access to growth factors and metabolites and for the metastatic spread. A proteolytic breakdown of stromal tissue facilitated by plasminogen activation system, as well as MMPs expressed by CAFs and tumor cells can help tumor growth and invasion (155). SerpinB2 was previously identified as a regulator of stromal remodeling of collagen in PDAC, with its enzymatic target urokinase plasminogen activator (uPA) emerging as a novel PDAC prognostic marker (156). The non-universally beneficial implications of the fibrotic stroma on PDAC cells have been reflected in reported findings regarding the role of tumor microenvironment in PDAC (157-163). Olive et al (160) discovered that the depletion of tumor-associated stromal tissue by the inhibition of the Hedgehog cellular signaling pathway enhanced the delivery of chemotherapy in a mouse model of PC, which led to a transient stabilization of the disease. By contrast, Croucher et al (155) pointed out the association between reduced stromal integrity and tumor growth, as well as local invasion in PC.
Pancreatic stellate cells (PSCs) are primarily responsible for the maintenance of normal stromal tissue architecture; however, in the case of a repeated or sustained pancreatic injury, as well as during PC, they become perpetually activated. Apart from causing pathological fibrosis, important supporting roles of activated PSCs towards adjacent cancer cells have been uncovered (164). Signals derived from stromal cells in the form of secreted factors regulate the expression of various genes in PC cells, including those connected with the cell cycle, DNA replication and metabolic pathways. Those signals can increase glycolytic metabolites, the pentose phosphate pathway, nucleic acid synthesis and the TCA cycle, which translates into the increased viability of PDAC cells under nutrient-deprived conditions (33). Of note, genes activated by stromal cues have been found to overlap with genes activated by the KrasG12D allele, suggesting a cooperation of stromal components and oncogenes in transcriptionally driven PC progression (33). Furthermore, oncogenic KRAS switches on Sonic hedgehog secretion by PDAC cells to activate PSCs and triggers heterocellular crosstalk, which leads, together with multiple phosphorylation events via an IGFR1/AXL-AKT axis, to an increased mitochondrial capacity in tumor cells (35).
In an effort to elucidate the mechanisms through which the stromal secretome can alter gene expression in PDAC cells and to identify potential novel therapeutic targets, Sherman et al (33) uncovered the histone acetylation at promoter and enhancer regions of functionally relevant genes as a key mediating event. In their concluding remarks, they also proposed the hepatocyte growth factor, insulin-like growth factor binding proteins 2, 3, 7 and MYC-activating, PSCs-produced Il-6 as possible microenvironment-derived factors inducing transcriptional and metabolic changes in the epithelial compartment.
The cooperation between stromal and cancer cells can take on a different form as PDAC cells can manipulate PSCs to actively use autophagy and secrete non-essential amino acids (NEAAs). Those alternative carbon sources are then captured by PDAC cells and serve to support TCA anaplerosis, lipid and NEAA biosynthesis when other drivers of these pathways such as glucose or glutamine become scarce (34). It has been determined that oxidative stress induced by cancer cells in the adjacent stromal cells may be behind the autophagy activation in PSCs with the resultant stromal overproduction of recycled nutrients benefiting cancer cells by driving mitochondrial biogenesis and anabolic growth (165). Additionally, the excess stromal ROS production enhances the antioxidant defense in nearby cancer cells and contributes to their genomic instability (165). It has also been suggested that autophagy in CAFs could be induced by ammonia produced by cancer cells during glutaminolysis and diffused into the microenvironment, which could lead to a positive feedback, with subsequent high levels of glutamine secreted into the TME by CAFs increasing the conversion of glutamine to glutamate and ammonia along with mitochondrial activity in cancer cells (166,167). A supportive role of the metabolic scavenging in PDAC is further evidenced by the confirmed existence of CAF-derived exosomes containing mRNA, miRNA, intact metabolites and TCA cycle intermediates, which promote PC cell proliferation, migration and chemoresistance in a mechanism similar to macropinocytosis under nutrient-depleted conditions (31,168-170). Aside from acting as a direct source of metabolites, such exosomes could create a hypoxia-mimicking microenvironment stimulating the reductive carboxylation of glutamine in cancer cells, the pathway previously observed in rapidly growing malignant cells and identified to be an alternative to oxidative metabolism as the major source of citrate and precursors for macromolecular synthesis (168,171).
Under conditions of nutrient stress, PDAC cells can also take up collagen fragments from the surrounding stroma either through macropinocytosis-dependent or independent mechanisms. Following the breakdown of extracellular matrix proteins, collagen-derived proline is further metabolized to support the TCA cycle and to promote PDAC cell survival and proliferation (172). Moreover, following their activation by PDAC cells, fibroblasts start to produce higher amount of type I collagen, which enhances integrin-FAK signaling in PDAC cells, resulting in an increased PDAC clonogenic growth, self-renewal and the frequency of cancer stem cells (CSCs) (173).
In addition to stromal fibroblasts, PC cells can interact with different cell types within the tumor microenvironment. Adipocytes can acquire a mesenchymal phenotype characterized by decreased lipid content and energy utilization after a de-differentiation caused by a co-culture with PC cells. Adipocytes with this phenotype can increase PC cell aggressiveness in vitro and can also contribute to matrix remodeling (174). Meyer et al (175) established a model of adipocyte-induced proliferation of PC cells enhanced by nutrient-poor conditions, demonstrating that adipocytes secreted glutamine and initiated its transfer to cancer cells after their own catabolism of the same metabolite was downregulated by PC cells (175).
Other intercellular interplay found to contribute to the malignant progression of PDAC involves tumor-associated macrophages (TAMs). By secreting the cytokine, CCL18, M2 TAMs can activate the NF-κB signal transduction pathway in PDAC cells and thus induce their expression of the adhesion molecule, vascular cell adhesion molecule-1, which subsequently enhances aerobic glycolysis in PDAC cells. Lactate produced by PC cells reciprocally facilitates the M2-like polarization of macrophages, thus creating a positive feedback loop (176). Due to the ability of lactate to modify the host antitumor immune response, its increased production contributes to the formation of an immunosuppressive tumor niche and the improved survival of metastatic cells, as shown by the improvement of the natural killer cell cytolytic function towards LDHA-deficient PanO2 cells in mice (53).
Intercellular interactions in the pancreatic tumor stroma may also include short-range interactions through gap junctions or the transfer of mitochondria between PC cells and other stromal cells, including mesenchymal stem cells (MSCs), as has been shown for other types of cancer (177). CAFs have been shown to influence the metabolism and support the malignant progression of non-small cell lung cancer (NSCLC) cells through connexin-43 gap junctions (178). Of note, the outward transfer of mitochondria mediated by cell adhesion and tunneling nanotubes from Jurkat cells, which had an increased level of ROS after a cytotoxic treatment, to MSCs was shown to cause chemoresistance in Jurkat cells (179). Multiple myeloma cells can also receive mitochondria from their microenvironment, which can lead to changes in their metabolism, resulting in a more prominent use of OXPHOS (180). It is possible that similar intercellular interactions may be present in pancreatic tumors. In fact, the role of connexin-43 channels in exporting lactate from glycolytic PDAC cells to sustain their metabolism and growth when an outward MCT transport is inhibited by extracellular acidity, as well as to provide substrate for OXPHOS of cells in better-perfused areas, where there is also a more favorable transmembrane gradient for MCT-facilitated lactate off-loading, has already been described (181). It would thus be of interest to examine whether other such mechanisms play a role in PC tumors.
9. Pancreatic cancer stem cells
CSCs are a highly-chemoresistant subpopulation of cells within tumors capable to self-renew and replenish the cancer cell population following chemotherapy, which leads to the recurrence of the disease. The importance of eradicating these cells to successfully treat cancer has become clear (182-184). A tremendous effort has been place in identifying potential biomarkers for PCSCs over the years, so they could be isolated and targeted. For this purpose, multiple surface markers expressed by these undifferentiated cells were identified, such as CD44, CD24, EpCAM, CD133, CXCR4, ALDH1 and hepatocyte growth factor receptor c-MET (185-189). To further expand the options of the detection and isolation of CSCs in different types of cancer, Miranda-Lorenzo et al (190) used autofluorescence as an exclusive marker of CSCs from solid human tumors, including that of PDAC. Such cells expressed pluripotency-associated genes and displayed chemoresistance, long-term tumorigenicity and invasiveness in vivo, traits suggestive of their stem-like identity. As to what potential advantage could these cells gain from having this autofluorescent phenotype, e.g., during hostile conditions after chemotherapy, it was hypothesized that riboflavin stored in the CSC autofluorescent vesicles could be used for the synthesis of flavin-dependent coenzymes and flavin nucleotides, factors important for the setting up of an antioxidant defense system (191,192). Additionally, certain genes that are differentially expressed and distinguish stem cells in the normal pancreas could be potentially used to identify stem-like cells in various tumors (193).
Advances in the identification and isolation of PCSCs uncovered their association with PSCs and CAFs. These stromal cells create a paracrine niche for PCSCs by secreting factors, including Nodal/Activin, hCAP-18/LL-37 and ISG15, and enhance their stem cell-like phenotypes along with their tumorigenic potential (30,194,195).
In keeping with the exceptional ability of PCSCs to survive stressful conditions, one would predict the occurrence of specific metabolic adaptations in these cells. Indeed, an increased utilization of OXPHOS by PCSCs could confer to them some resistance in situations when glucose or glutamine is limited, as well as during other situations affecting their specifically adjusted metabolism, such as the ablation of K-Ras oncogene (17,39). Importantly, oxygen deprivation typical for the tumor microenvironment of PDAC does not prevent the ability of PCSCs to use OXPHOS; instead, hypoxia supports autophagy and favors their survival and migration (196,197). A higher reliance on OXPHOS could, however, be an issue for PCSCs as the ROS produced by this pathway need to be detoxified to maintain their stemness. Jagust et al (198) demonstrated their dependency on glutathione metabolism, which was confirmed by the significantly diminished CSC self-renewal and chemoresistance after a pharmacological targeting of this antioxidant pathway. Similar to their non-CSCs counterparts, PCSCs can utilize multiple seemingly crucial metabolic pathways. The targeting of the non-canonical glutamine metabolism pathway in PCSCs was shown to negatively affect their self-renewal, elevate their intracellular levels of ROS and subsequently increase their radiosensitivity (199). Apart from glutamine, the inhibition of fatty acid synthesis and mevalonate pathways caused an anti-proliferative effect in PCSCs that was greater than that in parental PC cells, suggesting that their utilization of metabolic substrates could be heterogeneous, often dependent on the microenvironmental context (200).
Epigenetic alterations connected with distinct metabolic pathways in PCSCs probably predetermine their metabolic plasticity, which allows them to respond promptly to different environmental challenges and enhances their tumorigenicity. Notably, chromatin modifications linked to distant metastatic subclones was reversed by targeting a specific metabolic enzyme in a PDAC study, with those results further supporting metabolism-epigenome links in cancer (201). Currently, knowledge is expanding regarding the factors behind the metabolic plasticity of PCSCs, which needs to be overcome if this highly resistant cellular subpopulation is to be eliminated in PDAC tumors. The impairment of mitochondrial ISGylation critical for the recycling of dysfunctional mitochondria disrupts PaCSC mitochondrial metabolism, consequently affecting their metabolic plasticity and rendering them susceptible to a prolonged inhibition with metformin in vivo (202). Recently, it was also demonstrated that the unique metabolic signatures of PCSCs mediate organ-specific metastasis, which further points to the importance of metabolic programming of this cellular entity (203).
10. Conclusions and future perspectives
Therapeutic options for PDAC are still limited mainly to surgery and cytotoxic chemotherapy. The targeting of the reprogrammed cancer metabolism provides an innovative therapeutic strategy to be used also in combination with cytotoxic agents to achieve greater effects. Such applications already exist. Devimistat, a drug that inhibits key enzymes for the functioning of the TCA cycle in tumor cells in combination with the modified FOLFIRINOX, protocol led to a response rate of 61% in patients with metastatic PC in an open-label, phase I trial (NCT01835041) (204) and a phase-III clinical trial to evaluate efficacy and safety of the same combination of drugs in patients with metastatic adenocarcinoma of the pancreas is ongoing (205). The cell autophagy inhibitor, hydroxychloroquine, in combination with cytotoxic agents or ERK/MAPK pathway inhibitors is currently being tested for effectiveness in PC clinical trials (NCT01506973; NCT04145297; NCT03825289 and NCT04132505).
It is likely that further such therapeutic strategies can be developed based on a detailed understanding of the metabolic flexibility and needs of cancer cells together with the metabolic cooperation and signaling within the tumor microenvironment.
Acknowledgments
Not applicable.
Funding Statement
No external funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
Both authors (MZ and JT) conceived the present review article. MZ collected the literature and drafted the manuscript. JT made critical revisions to the manuscript. Both authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Both authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Sarantis P, Koustas E, Papadimitropoulou A, Papavassiliou AG, Karamouzis MV. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J Gastrointest Oncol. 2020;12:173–181. doi: 10.4251/wjgo.v12.i2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu JX, Zhao CF, Chen WB, Liu QC, Li QW, Lin YY, Gao F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J Gastroenterol. 2021;27:4298–4321. doi: 10.3748/wjg.v27.i27.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846–4861. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picozzi VJ, Oh SY, Edwards A, Mandelson MT, Dorer R, Rocha FG, Alseidi A, Biehl T, Traverso LW, Helton WS, Kozarek RA. Five-year actual overall survival in resected pancreatic cancer: A contemporary single-institution experience from a multidisciplinary perspective. Ann Surg Oncol. 2017;24:1722–1730. doi: 10.1245/s10434-016-5716-z. [DOI] [PubMed] [Google Scholar]
- 9.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 11.Upadhrasta S, Zheng L. Strategies in developing immunotherapy for pancreatic cancer: Recognizing and correcting multiple immune 'defects' in the tumor microenvironment. J Clin Med. 2019;8:1472. doi: 10.3390/jcm8091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beatty GL, O'Hara MH, Lacey SF, Torigian DA, Nazimuddin F, Chen F, Kulikovskaya IM, Soulen MC, McGarvey M, Nelson AM, et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology. 2018;155:29–32. doi: 10.1053/j.gastro.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Hara M, O'Reilly E, Rosemarie M, Varadhachary G, Wainberg ZA, Ko A, Fisher GA, Rahma O, Lyman JP, Cabanski CR, et al. Abstract CT004: A phase Ib study of CD40 agonistic monoclonal antibody APX005M together with gemcitabine (Gem) and nab-paclitaxel (NP) with or without nivolumab (Nivo) in untreated metastatic ductal pancreatic adenocarcinoma (PDAC) patients. Cancer Res. 2019;79(Suppl 13):CT004. doi: 10.1158/1538-7445.AM2019-CT004. [DOI] [Google Scholar]
- 14.Bahary N, Garrido-Laguna I, Cinar P, O'Rourke MA, Somer BG, Nyak-Kapoor A, Lee JS, Munn D, Paul Kennedy E, Vahanian NN, et al. Phase 2 trial of the indoleamine 2,3-dioxygenase pathway (IDO) inhibitor indoximod plus gemcitabine/nab-paclitaxel for the treatment of metastatic pancreas cancer: Interim analysis. J Clin Oncol. 2016;34(Suppl 15):S3020. doi: 10.1200/JCO.2016.34.15_suppl.3020. [DOI] [Google Scholar]
- 15.Di Federico A, Tateo V, Parisi C, Formica F, Carloni R, Frega G, Rizzo A, Ricci D, Di Marco M, Palloni A, Brandi G. Hacking pancreatic cancer: Present and future of personalized medicine. Pharmaceuticals (Basel) 2021;14:677. doi: 10.3390/ph14070677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Liu H, Li Z, Zhao Z, Yip-Schneider M, Fan Q, Schmidt CM, Chiorean EG, Xie J, Cheng L, et al. Role of fatty acid synthase in gemcitabine and radiation resistance of pancreatic cancers. Int J Biochem Mol Biol. 2011;2:89–98. [PMC free article] [PubMed] [Google Scholar]
- 17.Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sánchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M, Liu W, Qin Y, Xu X, Yu X, Zhuo Q, Ji S. Regulation of metabolic reprogramming by tumor suppressor genes in pancreatic cancer. Exp Hematol Oncol. 2020;9:23. doi: 10.1186/s40164-020-00179-x. [DOI] [Google Scholar]
- 19.Daemen A, Peterson D, Sahu N, McCord R, Du X, Liu B, Kowanetz K, Hong R, Moffat J, Gao M, et al. Metabolite profiling stratifies pancreatic ductal adenocarcinomas into subtypes with distinct sensitivities to metabolic inhibitors. Proc Natl Acad Sci USA. 2015;112:E4410–E4417. doi: 10.1073/pnas.1501605112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karasinska JM, Topham JT, Kalloger SE, Jang GH, Denroche RE, Culibrk L, Williamson LM, Wong HL, Lee MK, O'Kane GM, et al. Altered gene expression along the glycolysis-cholesterol synthesis axis is associated with outcome in pancreatic cancer. Clin Cancer Res. 2020;26:135–146. doi: 10.1158/1078-0432.CCR-19-1543. [DOI] [PubMed] [Google Scholar]
- 21.Halbrook CJ, Lyssiotis CA. Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell. 2017;31:5–19. doi: 10.1016/j.ccell.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Perusina Lanfranca M, Thompson JK, Bednar F, Halbrook C, Lyssiotis C, Levi B, Frankel TL. Metabolism and epigenetics of pancreatic cancer stem cells. Semin Cancer Biol. 2019;57:19–26. doi: 10.1016/j.semcancer.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perera RM, Bardeesy N. Pancreatic cancer metabolism: Breaking it down to build it back up. Cancer Discov. 2015;5:1247–1261. doi: 10.1158/2159-8290.CD-15-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dal Molin M, Zhang M, De Wilde RF, Ottenhof NA, Rezaee N, Wolfgang CL, Blackford A, Vogelstein B, Kinzler KW, Papadopoulos N, et al. Very long-term survival following resection for pancreatic cancer is not explained by commonly mutated genes: Results of whole-exome sequencing analysis. Clin Cancer Res. 2015;21:1944–1950. doi: 10.1158/1078-0432.CCR-14-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung AH, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47:1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao L, Zhao H, Yan H. Gene expression profiling of 1200 pancreatic ductal adenocarcinoma reveals novel subtypes. BMC Cancer. 2018;18:603. doi: 10.1186/s12885-018-4546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurer C, Holmstrom SR, He J, Laise P, Su T, Ahmed A, Hibshoosh H, Chabot JA, Oberstein PE, Sepulveda AR, et al. Experimental microdissection enables functional harmonisation of pancreatic cancer subtypes. Gut. 2019;68:1034–1043. doi: 10.1136/gutjnl-2018-317706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan-Seng-Yue M, Kim JC, Wilson GW, Ng K, Figueroa EF, O'Kane GM, Connor AA, Denroche RE, Grant RC, McLeod J, et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat Genet. 2020;52:231–240. doi: 10.1038/s41588-019-0566-9. [DOI] [PubMed] [Google Scholar]
- 30.Lonardo E, Frias-Aldeguer J, Hermann PC, Heeschen C. Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle. 2012;11:1282–1290. doi: 10.4161/cc.19679. [DOI] [PubMed] [Google Scholar]
- 31.Richards KE, Zeleniak AE, Fishel ML, Wu J, Littlepage LE, Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770–1778. doi: 10.1038/onc.2016.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mejia I, Bodapati S, Chen KT, Díaz B. Pancreatic adenocarcinoma invasiveness and the tumor microenvironment: From biology to clinical trials. Biomedicines. 2020;8:401. doi: 10.3390/biomedicines8100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherman MH, Yu RT, Tseng TW, Sousa CM, Liu S, Truitt ML, He N, Ding N, Liddle C, Atkins AR, et al. Stromal cues regulate the pancreatic cancer epigenome and metabolome. Proc Natl Acad Sci USA. 2017;114:1129–1134. doi: 10.1073/pnas.1620164114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;536:479–483. doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tape CJ, Ling S, Dimitriadi M, McMahon KM, Worboys JD, Leong HS, Norrie IC, Miller CJ, Poulogiannis G, Lauffenburger DA, Jørgensen C. Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell. 2016;165:910–920. doi: 10.1016/j.cell.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juiz N, Elkaoutari A, Bigonnet M, Gayet O, Roques J, Nicolle R, Iovanna J, Dusetti N. Basal-like and classical cells coexistence in pancreatic cancer revealed by single cell analysis. bioRxiv. 2020 doi: 10.1096/fj.202000363RR. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki T, Otsuka M, Seimiya T, Iwata T, Kishikawa T, Koike K. The biological role of metabolic reprogramming in pancreatic cancer. MedComm (2020) 2020;1:302–310. doi: 10.1002/mco2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaidi N, Lupien L, Kuemmerle NB, Kinlaw WB, Swinnen JV, Smans K. Lipogenesis and lipolysis: The pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res. 2013;52:585–589. doi: 10.1016/j.plipres.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sancho P, Burgos-Ramos E, Tavera A, Bou Kheir T, Jagust P, Schoenhals M, Barneda D, Sellers K, Campos-Olivas R, Graña O, et al. MYC/PGC-1α balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metab. 2015;22:590–605. doi: 10.1016/j.cmet.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA, Bar-Sagi D, et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75:544–553. doi: 10.1158/0008-5472.CAN-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshida GJ. Metabolic reprogramming: The emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res. 2015;34:111. doi: 10.1186/s13046-015-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu M, Zhou Q, Zhou Y, Fu Z, Tan L, Ye X, Zeng B, Gao W, Zhou J, Liu Y, et al. Metabolic phenotypes in pancreatic cancer. PLoS One. 2015;10:e0115153. doi: 10.1371/journal.pone.0115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lomberk G, Blum Y, Nicolle R, Nair A, Gaonkar KS, Marisa L, Mathison A, Sun Z, Yan H, Elarouci N, et al. Distinct epigenetic landscapes underlie the pathobiology of pancreatic cancer subtypes. Nat Commun. 2018;9:1978. doi: 10.1038/s41467-018-04383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaoutari AE, Fraunhoffer NA, Hoare O, Teyssedou C, Soubeyran P, Gayet O, Roques J, Lomberk G, Urrutia R, Dusetti N, Iovanna J. Metabolomic profiling of pancreatic adenocarcinoma reveals key features driving clinical outcome and drug resistance. EBioMedicine. 2021;66:103332. doi: 10.1016/j.ebiom.2021.103332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Q, Ge W, Wang T, Lan J, Martínez-Jarquín S, Wolfrum C, Stoffel M, Zenobi R. High-throughput single-cell mass spectrometry reveals abnormal lipid metabolism in pancreatic ductal adenocarcinoma. Angew Chem Int Ed Engl. 2021;60:24534–24542. doi: 10.1002/anie.202107223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Argüello RJ, Combes AJ, Char R, Gigan JP, Baaziz AI, Bousiquot E, Camosseto V, Samad B, Tsui J, Yan P, et al. SCENITH: A flow cytometry-based method to functionally profile energy metabolism with single-cell resolution. Cell Metab. 2020;32:1063–1075.e7. doi: 10.1016/j.cmet.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller A, Nagy C, Knapp B, Laengle J, Ponweiser E, Groeger M, Starkl P, Bergmann M, Wagner O, Haschemi A. Exploring metabolic configurations of single cells within complex tissue microenvironments. Cell Metab. 2017;26:788–800.e6. doi: 10.1016/j.cmet.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 48.Ambrosini G, Dalla Pozza E, Fanelli G, Di Carlo C, Vettori A, Cannino G, Cavallini C, Carmona-Carmona CA, Brandi J, Rinalducci S, et al. Progressively de-differentiated pancreatic cancer cells shift from glycolysis to oxidative metabolism and gain a quiescent stem state. Cells. 2020;9:1572. doi: 10.3390/cells9071572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biancur DE, Paulo JA, Małachowska B, Quiles Del Rey M, Sousa CM, Wang X, Sohn ASW, Chu GC, Gygi SP, Harper JW, et al. Compensatory metabolic networks in pancreatic cancers upon perturbation of glutamine metabolism. Nat Commun. 2017;8:15965. doi: 10.1038/ncomms15965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 51.Liu XS, Little JB, Yuan ZM. Glycolytic metabolism influences global chromatin structure. Oncotarget. 2015;6:4214–4225. doi: 10.18632/oncotarget.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liberti MV, Locasale JW. The Warburg effect: How does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: Effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191:1486–1495. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- 54.Du J, Gu J, Deng J, Kong L, Guo Y, Jin C, Bao Y, Fu D, Li J. The expression and survival significance of glucose transporter-1 in pancreatic cancer: Meta-analysis, bioinformatics analysis and retrospective study. Cancer Invest. 2021;39:741–755. doi: 10.1080/07357907.2021.1950755. [DOI] [PubMed] [Google Scholar]
- 55.Lee EE, Ma J, Sacharidou A, Mi W, Salato VK, Nguyen N, Jiang Y, Pascual JM, North PE, Shaul PW, et al. A protein kinase C phosphorylation motif in GLUT1 affects glucose transport and is mutated in GLUT1 deficiency syndrome. Mol Cell. 2015;58:845–853. doi: 10.1016/j.molcel.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng CS, Tan HY, Wang N, Chen L, Meng Z, Chen Z, Feng Y. Functional inhibition of lactate dehydrogenase suppresses pancreatic adenocarcinoma progression. Clin Transl Med. 2021;11:e467. doi: 10.1002/ctm2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang W, Qiao L, Zuo D, Qin D, Xiao J, An H, Wang Y, Zhang X, Jin Y, Ren L. Aberrant lactate dehydrogenase A signaling contributes metabolic signatures in pancreatic cancer. Ann Transl Med. 2021;9:358. doi: 10.21037/atm-21-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yalcin A, Solakoglu TH, Ozcan SC, Guzel S, Peker S, Celikler S, Balaban BD, Sevinc E, Gurpinar Y, Chesney JA. 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase-3 is required for transforming growth factor β1-enhanced invasion of Panc1 cells in vitro. Biochem Biophys Res Commun. 2017;484:687–693. doi: 10.1016/j.bbrc.2017.01.178. [DOI] [PubMed] [Google Scholar]
- 60.Ji S, Zhang B, Liu J, Qin Y, Liang C, Shi S, Jin K, Liang D, Xu W, Xu H, et al. ALDOA functions as an oncogene in the highly metastatic pancreatic cancer. Cancer Lett. 2016;374:127–135. doi: 10.1016/j.canlet.2016.01.054. [DOI] [PubMed] [Google Scholar]
- 61.Principe M, Borgoni S, Cascione M, Chattaragada MS, Ferri-Borgogno S, Capello M, Bulfamante S, Chapelle J, Di Modugno F, Defilippi P, et al. Alpha-enolase (ENO1) controls alpha v/beta 3 integrin expression and regulates pancreatic cancer adhesion, invasion, and metastasis. J Hematol Oncol. 2017;10:16. doi: 10.1186/s13045-016-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Principe M, Ceruti P, Shih NY, Chattaragada MS, Rolla S, Conti L, Bestagno M, Zentilin L, Yang SH, Migliorini P, et al. Targeting of surface alpha-enolase inhibits the invasiveness of pancreatic cancer cells. Oncotarget. 2015;6:11098–11113. doi: 10.18632/oncotarget.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azoitei N, Becher A, Steinestel K, Rouhi A, Diepold K, Genze F, Simmet T, Seufferlein T. PKM2 promotes tumor angiogenesis by regulating HIF-1α through NF-κB activation. Mol Cancer. 2016;15:3. doi: 10.1186/s12943-015-0490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thews O, Riemann A. Tumor pH and metastasis: A malignant process beyond hypoxia. Cancer Metastasis Rev. 2019;38:113–129. doi: 10.1007/s10555-018-09777-y. [DOI] [PubMed] [Google Scholar]
- 65.Guillaumond F, Leca J, Olivares O, Lavaut MN, Vidal N, Berthezène P, Dusetti NJ, Loncle C, Calvo E, Turrini O, et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc Natl Acad Sci USA. 2013;110:3919–3924. doi: 10.1073/pnas.1219555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19:3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Semenza GL. Tumor metabolism: Cancer cells give and take lactate. J Clin Invest. 2008;118:3835–3837. doi: 10.1172/JCI37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun X, Wang M, Wang M, Yao L, Li X, Dong H, Li M, Sun T, Liu X, Liu Y, Xu Y. Role of proton-coupled monocarboxylate transporters in cancer: From metabolic crosstalk to therapeutic potential. Front cell Dev Biol. 2020;8:651. doi: 10.3389/fcell.2020.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 70.Benny S, Mishra R, Manojkumar MK, Aneesh TP. From Warburg effect to reverse Warburg effect; the new horizons of anti-cancer therapy. Med Hypotheses. 2020;144:110216. doi: 10.1016/j.mehy.2020.110216. [DOI] [PubMed] [Google Scholar]
- 71.Roland CL, Arumugam T, Deng D, Liu SH, Philip B, Gomez S, Burns WR, Ramachandran V, Wang H, Cruz-Monserrate Z, Logsdon CD. Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res. 2014;74:5301–5310. doi: 10.1158/0008-5472.CAN-14-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benton CR, Yoshida Y, Lally J, Han X-X, Hatta H, Bonen A. PGC-1alpha increases skeletal muscle lactate uptake by increasing the expression of MCT1 but not MCT2 or MCT4. Physiol Genomics. 2008;35:45–54. doi: 10.1152/physiolgenomics.90217.2008. [DOI] [PubMed] [Google Scholar]
- 73.Cheng CF, Ku HC, Lin H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int J Mol Sci. 2018;19:3447. doi: 10.3390/ijms19113447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schneiderhan W, Scheler M, Holzmann KH, Marx M, Gschwend JE, Bucholz M, Gress TM, Seufferlein T, Adler G, Oswald F. CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models. Gut. 2009;58:1391–1398. doi: 10.1136/gut.2009.181412. [DOI] [PubMed] [Google Scholar]
- 75.Lee SH, Hwang HK, Lee WJ, Kang CM. MCT4 as a potential therapeutic target to augment gemcitabine chemosensitivity in resected pancreatic cancer. Cell Oncol (Dordr) 2021;44:1363–1371. doi: 10.1007/s13402-021-00643-8. [DOI] [PubMed] [Google Scholar]
- 76.Benjamin D, Robay D, Hindupur SK, Pohlmann J, Colombi M, El-Shemerly MY, Maira SM, Moroni C, Lane HA, Hall MN. Dual inhibition of the lactate transporters MCT1 and MCT4 Is synthetic lethal with metformin due to NAD+ depletion in cancer cells. Cell Rep. 2018;25:3047–3058.e4. doi: 10.1016/j.celrep.2018.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu DH, Liang H, Lu SN, Wang H, Su ZL, Zhang L, Ma JQ, Guo M, Tai S, Yu S. miR-124 suppresses pancreatic ductal adenocarcinoma growth by regulating monocarboxylate transporter 1-mediated cancer lactate metabolism. Cell Physiol Biochem. 2018;50:924–935. doi: 10.1159/000494477. [DOI] [PubMed] [Google Scholar]
- 79.Lau AN, Li Z, Danai LV, Westermark AM, Darnell AM, Ferreira R, Gocheva V, Sivanand S, Lien EC, Sapp KM, et al. Dissecting cell-type-specific metabolism in pancreatic ductal adenocarcinoma. Elife. 2020;9:e56782. doi: 10.7554/eLife.56782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koundouros N, Poulogiannis G. Reprogramming of fatty acid metabolism in cancer. Br J Cancer. 2020;122:4–22. doi: 10.1038/s41416-019-0650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alo PL, Amini M, Piro F, Pizzuti L, Sebastiani V, Botti C, Murari R, Zotti G, Di Tondo U. Immunohistochemical expression and prognostic significance of fatty acid synthase in pancreatic carcinoma. Anticancer Res. 2007;27:2523–2527. [PubMed] [Google Scholar]
- 82.Li N, Lu H, Chen C, Bu X, Huang P. Loss of fatty acid synthase inhibits the 'hER2-PI3K/Akt axis' activity and malignant phenotype of Caco-2 cells. Lipids Health Dis. 2013;12:83. doi: 10.1186/1476-511X-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Menendez JA, Decker JP, Lupu R. In support of fatty acid synthase (FAS) as a metabolic oncogene: Extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J Cell Biochem. 2005;94:1–4. doi: 10.1002/jcb.20310. [DOI] [PubMed] [Google Scholar]
- 84.Shetty A, Nagesh PKB, Setua S, Hafeez BB, Jaggi M, Yallapu MM, Chauhan SC. Novel paclitaxel nanoformulation impairs de novo lipid synthesis in pancreatic cancer cells and enhances gemcitabine efficacy. ACS Omega. 2020;5:8982–8991. doi: 10.1021/acsomega.0c00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tracz-Gaszewska Z, Dobrzyn P. Stearoyl-CoA desaturase 1 as a therapeutic target for the treatment of cancer. Cancers (Basel) 2019;11:948. doi: 10.3390/cancers11070948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniëls VW, Machiels J, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70:8117–8126. doi: 10.1158/0008-5472.CAN-09-3871. [DOI] [PubMed] [Google Scholar]
- 87.Fritz V, Benfodda Z, Rodier G, Henriquet C, Iborra F, Avancès C, Allory Y, de la Taille A, Culine S, Blancou H, et al. Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol Cancer Ther. 2010;9:1740–1754. doi: 10.1158/1535-7163.MCT-09-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eser S, Schnieke A, Schneider G, Saur D. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer. 2014;111:817–822. doi: 10.1038/bjc.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Menendez JA. Fine-tuning the lipogenic/lipolytic balance to optimize the metabolic requirements of cancer cell growth: Molecular mechanisms and therapeutic perspectives. Biochim Biophys Acta. 2010;1801:381–391. doi: 10.1016/j.bbalip.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 90.Xu M, Jung X, Hines OJ, Eibl G, Chen Y. Obesity and pancreatic cancer: Overview of epidemiology and potential prevention by weight loss. Pancreas. 2018;47:158–162. doi: 10.1097/MPA.0000000000000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, Abbruzzese JL. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kasenda B, Bass A, Koeberle D, Pestalozzi B, Borner M, Herrmann R, Jost L, Lohri A, Hess V. Survival in overweight patients with advanced pancreatic carcinoma: A multicentre cohort study. BMC Cancer. 2014;14:728. doi: 10.1186/1471-2407-14-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anderson AS, Renehan AG, Saxton JM, Bell J, Cade J, Cross AJ, King A, Riboli E, Sniehotta F, Treweek S, et al. Cancer prevention through weight control-where are we in 2020? Br J Cancer. 2021;124:1049–1056. doi: 10.1038/s41416-020-01154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jacobs EJ, Newton CC, Patel AV, Stevens VL, Islami F, Flanders WD, Gapstur SM. The association between body mass index and pancreatic cancer: Variation by age at body mass index assessment. Am J Epidemiol. 2020;189:108–115. doi: 10.1093/aje/kwz230. [DOI] [PubMed] [Google Scholar]
- 95.Chimento A, Casaburi I, Avena P, Trotta F, De Luca A, Rago V, Pezzi V, Sirianni R. Cholesterol and its metabolites in tumor growth: Therapeutic potential of statins in cancer treatment. Front Endocrinol (Lausanne) 2019;9:807. doi: 10.3389/fendo.2018.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guillaumond F, Bidaut G, Ouaissi M, Servais S, Gouirand V, Olivares O, Lac S, Borge L, Roques J, Gayet O, et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc Natl Acad Sci USA. 2015;112:2473–2478. doi: 10.1073/pnas.1421601112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nam GH, Kwon M, Jung H, Ko E, Kim SA, Choi Y, Song SJ, Kim S, Lee Y, Kim GB, et al. Statin-mediated inhibition of RAS prenylation activates ER stress to enhance the immunogenicity of KRAS mutant cancer. J Immunother cancer. 2021;9:e002474. doi: 10.1136/jitc-2021-002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang W, Hu JW, He XR, Jin WL, He XY. Statins: A repurposed drug to fight cancer. J Exp Clin Cancer Res. 2021;40:241. doi: 10.1186/s13046-021-02041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Osmak M. Statins and cancer: Current and future prospects. Cancer Lett. 2012;324:1–12. doi: 10.1016/j.canlet.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 100.Kawata S, Yamasaki E, Nagase T, Inui Y, Ito N, Matsuda Y, Inada M, Tamura S, Noda S, Imai Y, Matsuzawa Y. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br J Cancer. 2001;84:886–891. doi: 10.1054/bjoc.2000.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lersch C, Schmelz R, Erdmann J, Hollweck R, Schulte-Frohlinde E, Eckel F, Nader M, Schusdziarra V. Treatment of HCC with pravastatin, octreotide, or gemcitabine-a critical evaluation. Hepatogastroenterology. 2004;51:1099–1103. [PubMed] [Google Scholar]
- 102.Butera A, Roy M, Zampieri C, Mammarella E, Panatta E, Melino G, D'Alessandro A, Amelio I. p53-driven lipidome influences non-cell-autonomous lysophospholipids in pancreatic cancer. Biol Direct. 2022;17:6. doi: 10.1186/s13062-022-00319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang L, Jin H, Guo X, Yang Z, Zhao L, Tang S, Mo P, Wu K, Nie Y, Pan Y, Fan D. Distinguishing pancreatic cancer from chronic pancreatitis and healthy individuals by (1)H nuclear magnetic resonance-based metabonomic profiles. Clin Biochem. 2012;45:1064–1069. doi: 10.1016/j.clinbiochem.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 104.Urayama S, Zou W, Brooks K, Tolstikov V. Comprehensive mass spectrometry based metabolic profiling of blood plasma reveals potent discriminatory classifiers of pancreatic cancer. Rapid Commun Mass Spectrom. 2010;24:613–620. doi: 10.1002/rcm.4420. [DOI] [PubMed] [Google Scholar]
- 105.Wang G, Yao H, Gong Y, Lu Z, Pang R, Li Y, Yung Y, Song H, Liu J, Jin Y, et al. Metabolic detection and systems analyses of pancreatic ductal adenocarcinoma through machine learning, lipidomics, and multi-omics. Sci Adv. 2021;7:eabh2724. doi: 10.1126/sciadv.abh2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fang F, He X, Deng H, Chen Q, Lu J, Spraul M, Yu Y. Discrimination of metabolic profiles of pancreatic cancer from chronic pancreatitis by high-resolution magic angle spinning 1H nuclear magnetic resonance and principal components analysis. Cancer Sci. 2007;98:1678–1682. doi: 10.1111/j.1349-7006.2007.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Z, Liu F, Fan N, Zhou C, Li D, Macvicar T, Dong Q, Bruns CJ, Zhao Y. Targeting glutaminolysis: New perspectives to understand cancer development and novel strategies for potential target therapies. Front Oncol. 2020;10:589508. doi: 10.3389/fonc.2020.589508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghanem N, El-Baba C, Araji K, El-Khoury R, Usta J, Darwiche N. The pentose phosphate pathway in cancer: Regulation and therapeutic opportunities. Chemotherapy. 2021;66:179–191. doi: 10.1159/000519784. [DOI] [PubMed] [Google Scholar]
- 110.Ju HQ, Lin JF, Tian T, Xie D, Xu RH. NADPH homeostasis in cancer: Functions, mechanisms and therapeutic implications. Signal Transduct Target Ther. 2020;5:231. doi: 10.1038/s41392-020-00326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Badgley MA, Kremer DM, Maurer HC, DelGiorno KE, Lee HJ, Purohit V, Sagalovskiy IR, Ma A, Kapilian J, Firl CEM, et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85–89. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 113.Akella NM, Ciraku L, Reginato MJ. Fueling the fire: Emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 2019;17:52. doi: 10.1186/s12915-019-0671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tong X, Zhao F, Thompson CB. The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr Opin Genet Dev. 2009;19:32–37. doi: 10.1016/j.gde.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y, Bai C, Ruan Y, Liu M, Chu Q, Qiu L, Yang C, Li B. Coordinative metabolism of glutamine carbon and nitrogen in proliferating cancer cells under hypoxia. Nat Commun. 2019;10:201. doi: 10.1038/s41467-018-08033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bott AJ, Shen J, Tonelli C, Zhan L, Sivaram N, Jiang YP, Yu X, Bhatt V, Chiles E, Zhong H, et al. Glutamine anabolism plays a critical role in pancreatic cancer by coupling carbon and nitrogen metabolism. Cell Rep. 2019;29:1287–1298.e6. doi: 10.1016/j.celrep.2019.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li T, Le A. Glutamine metabolism in cancer. In: Le A, editor. The Heterogeneity of Cancer Metabolism. Springer International Publishing; Cham: 2018. pp. 13–32. [DOI] [Google Scholar]
- 118.Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li W, Chen C, Zhao X, Ye H, Zhao Y, Fu Z, Pan W, Zheng S, Wei L, Nong T, et al. HIF-2α regulates non-canonical glutamine metabolism via activation of PI3K/mTORC2 pathway in human pancreatic ductal adenocarcinoma. J Cell Mol Med. 2017;21:2896–2908. doi: 10.1111/jcmm.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Raho S, Capobianco L, Malivindi R, Vozza A, Piazzolla C, De Leonardis F, Gorgoglione R, Scarcia P, Pezzuto F, Agrimi G, et al. KRAS-regulated glutamine metabolism requires UCP2-mediated aspartate transport to support pancreatic cancer growth. Nat Metab. 2020;2:1373–1381. doi: 10.1038/s42255-020-00315-1. [DOI] [PubMed] [Google Scholar]
- 121.Yang S, Hwang S, Kim M, Seo SB, Lee JH, Jeong SM. Mitochondrial glutamine metabolism via GOT2 supports pancreatic cancer growth through senescence inhibition. Cell Death Dis. 2018;9:55. doi: 10.1038/s41419-017-0089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wise DR, Deberardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roux C, Riganti C, Borgogno SF, Curto R, Curcio C, Catanzaro V, Digilio G, Padovan S, Puccinelli MP, Isabello M, et al. Endogenous glutamine decrease is associated with pancreatic cancer progression. Oncotarget. 2017;8:95361–95376. doi: 10.18632/oncotarget.20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.He J, Li F, Zhou Y, Hou X, Liu S, Li X, Zhang Y, Jing X, Yang L. LncRNA XLOC_006390 promotes pancreatic carcinogenesis and glutamate metabolism by stabilizing c-Myc. Cancer Lett. 2020;469:419–428. doi: 10.1016/j.canlet.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 126.Lowman XH, Hanse EA, Yang Y, Ishak Gabra MB, Tran TQ, Li H, Kong M. p53 Promotes cancer cell adaptation to glutamine deprivation by upregulating Slc7a3 to increase arginine uptake. Cell Rep. 2019;26:3051–3060.e4. doi: 10.1016/j.celrep.2019.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tajan M, Hock AK, Blagih J, Robertson NA, Labuschagne CF, Kruiswijk F, Humpton TJ, Adams PD, Vousden KH. A role for p53 in the adaptation to glutamine starvation through the expression of SLC1A3. Cell Metab. 2018;28:721–736.e6. doi: 10.1016/j.cmet.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tran TQ, Lowman XH, Reid MA, Mendez-Dorantes C, Pan M, Yang Y, Kong M. Tumor-associated mutant p53 promotes cancer cell survival upon glutamine deprivation through p21 induction. Oncogene. 2017;36:1991–2001. doi: 10.1038/onc.2016.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dey P, Baddour J, Muller F, Wu CC, Wang H, Liao WT, Lan Z, Chen A, Gutschner T, Kang Y, et al. Genomic deletion of malic enzyme 2 confers collateral lethality in pancreatic cancer. Nature. 2017;542:119–123. doi: 10.1038/nature21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dai Z, Ramesh V, Locasale JW. The evolving metabolic landscape of chromatin biology and epigenetics. Nat Rev Genet. 2020;21:737–753. doi: 10.1038/s41576-020-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang G, Han JJ. Connections between metabolism and epigenetic modifications in cancer. Med Rev. 2021;1:199–221. doi: 10.1515/mr-2021-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Etchegaray JP, Mostoslavsky R. Interplay between metabolism and epigenetics: A nuclear adaptation to environmental changes. Mol Cell. 2016;62:695–711. doi: 10.1016/j.molcel.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kottakis F, Nicolay BN, Roumane A, Karnik R, Gu H, Nagle JM, Boukhali M, Hayward MC, Li YY, Chen T, et al. LKB1 loss links serine metabolism to DNA methylation and tumorigenesis. Nature. 2016;539:390–395. doi: 10.1038/nature20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang H, Li QF, Chow H, Choi S, Leung YC. Arginine deprivation inhibits pancreatic cancer cell migration, invasion and EMT via the down regulation of snail, slug, twist, and MMP1/9. J Physiol Biochem. 2020;76:73–83. doi: 10.1007/s13105-019-00716-1. [DOI] [PubMed] [Google Scholar]
- 135.Wang J, Yang S, He P, Schetter AJ, Gaedcke J, Ghadimi BM, Ried T, Yfantis HG, Lee DH, Gaida MM, et al. Endothelial nitric oxide synthase traffic inducer (NOSTRIN) is a negative regulator of disease aggressiveness in pancreatic cancer. Clin Cancer Res. 2016;22:5992–6001. doi: 10.1158/1078-0432.CCR-16-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Herner A, Sauliunaite D, Michalski CW, Erkan M, De Oliveira T, Abiatari I, Kong B, Esposito I, Friess H, Kleeff J. Glutamate increases pancreatic cancer cell invasion and migration via AMPA receptor activation and Kras-MAPK signaling. Int J Cancer. 2011;129:2349–2359. doi: 10.1002/ijc.25898. [DOI] [PubMed] [Google Scholar]
- 137.Gitto SB, Pandey V, Oyer JL, Copik AJ, Hogan FC, Phanstiel O, IV, Altomare DA. Difluoromethylornithine combined with a polyamine transport inhibitor is effective against gemcitabine resistant pancreatic cancer. Mol Pharm. 2018;15:369–376. doi: 10.1021/acs.molpharmaceut.7b00718. [DOI] [PubMed] [Google Scholar]
- 138.Kremer JC, Prudner BC, Lange SES, Bean GR, Schultze MB, Brashears CB, Radyk MD, Redlich N, Tzeng SC, Kami K, et al. Arginine deprivation inhibits the Warburg effect and upregulates glutamine anaplerosis and serine biosynthesis in ASS1-deficient cancers. Cell Rep. 2017;18:991–1004. doi: 10.1016/j.celrep.2016.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Davidson SM, Jonas O, Keibler MA, Hou HW, Luengo A, Mayers JR, Wyckoff J, Del Rosario AM, Whitman M, Chin CR, et al. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat Med. 2017;23:235–241. doi: 10.1038/nm.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maertin S, Elperin JM, Lotshaw E, Sendler M, Speakman SD, Takakura K, Reicher BM, Mareninova OA, Grippo PJ, Mayerle J, et al. Roles of autophagy and metabolism in pancreatic cancer cell adaptation to environmental challenges. Am J Physiol Gastrointest Liver Physiol. 2017;313:G524–G536. doi: 10.1152/ajpgi.00138.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saliakoura M, Sebastiano MR, Nikdima I, Pozzato C, Konstantinidou G. Restriction of extracellular lipids renders pancreatic cancer dependent on autophagy. J Exp Clin Cancer Res. 2022;41:16. doi: 10.1186/s13046-021-02231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Piffoux M, Eriau E, Cassier PA. Autophagy as a therapeutic target in pancreatic cancer. Br J Cancer. 2021;124:333–344. doi: 10.1038/s41416-020-01039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li J, Chen X, Kang R, Zeh H, Klionsky DJ, Tang D. Regulation and function of autophagy in pancreatic cancer. Autophagy. 2021;17:3275–3296. doi: 10.1080/15548627.2020.1847462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, Sohn ASW, Mukhopadhyay S, Lin EY, Parker SJ, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581:100–105. doi: 10.1038/s41586-020-2229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mukhopadhyay S, Biancur DE, Parker SJ, Yamamoto K, Banh RS, Paulo JA, Mancias JD, Kimmelman AC. Autophagy is required for proper cysteine homeostasis in pancreatic cancer through regulation of SLC7A11. Proc Natl Acad Sci USA. 2021;118:e2021475118. doi: 10.1073/pnas.2021475118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ashton TM, Gillies McKenna W, Kunz-Schughart LA, Higgins GS. Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res. 2018;24:2482–2490. doi: 10.1158/1078-0432.CCR-17-3070. [DOI] [PubMed] [Google Scholar]
- 147.Zong WX, Rabinowitz JD, White E. Mitochondria and cancer. Mol Cell. 2016;61:667–676. doi: 10.1016/j.molcel.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lambert A, Brand M. Reactive oxygen species production by mitochondria. Methods Mol Biol. 2009;554:165–181. doi: 10.1007/978-1-59745-521-3_11. [DOI] [PubMed] [Google Scholar]
- 149.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol. 2020;17:108–123. doi: 10.1038/s41571-019-0281-6. [DOI] [PubMed] [Google Scholar]
- 151.Masoud R, Reyes-Castellanos G, Lac S, Garcia J, Dou S, Shintu L, Abdel Hadi N, Gicquel T, El Kaoutari A, Diémé B, et al. Targeting mitochondrial complex I overcomes chemoresistance in high OXPHOS pancreatic cancer. Cell Rep Med. 2020;1:100143. doi: 10.1016/j.xcrm.2020.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Candido S, Abrams SL, Steelman L, Lertpiriyapong K, Martelli AM, Cocco L, Ratti S, Follo MY, Murata RM, Rosalen PL, et al. Metformin influences drug sensitivity in pancreatic cancer cells. Adv Biol Regul. 2018;68:13–30. doi: 10.1016/j.jbior.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 153.Zannella VE, Dal Pra A, Muaddi H, McKee TD, Stapleton S, Sykes J, Glicksman R, Chaib S, Zamiara P, Milosevic M, et al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin Cancer Res. 2013;19:6741–6750. doi: 10.1158/1078-0432.CCR-13-1787. [DOI] [PubMed] [Google Scholar]
- 154.Neesse A, Bauer CA, Öhlund D, Lauth M, Buchholz M, Michl P, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: Ready for clinical translation? Gut. 2019;68:159–171. doi: 10.1136/gutjnl-2018-316451. [DOI] [PubMed] [Google Scholar]
- 155.Croucher DR, Saunders DN, Lobov S, Ranson M. Revisiting the biological roles of PAI2 (SERPINB2) in cancer. Nat Rev Cancer. 2008;8:535–545. doi: 10.1038/nrc2400. [DOI] [PubMed] [Google Scholar]
- 156.Harris NLE, Vennin C, Conway JRW, Vine KL, Pinese M, Cowley MJ, Shearer RF, Lucas MC, Herrmann D, Allam AH, et al. SerpinB2 regulates stromal remodelling and local invasion in pancreatic cancer. Oncogene. 2017;36:4288–4298. doi: 10.1038/onc.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jiang B, Zhou L, Lu J, Wang Y, Liu C, You L, Guo J. Stroma-targeting therapy in pancreatic cancer: One coin with two sides? Front Oncol. 2020;10:576399. doi: 10.3389/fonc.2020.576399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.de la Fouchardière C, Gamradt P, Chabaud S, Raddaz M, Blanc E, Msika O, Treilleux I, Bachy S, Cattey-Javouhey A, Guibert P, et al. A promising biomarker and therapeutic target in patients with advanced PDAC: The stromal protein βig-h3. J Pers Med. 2022;12:623. doi: 10.3390/jpm12040623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bulle A, Lim KH. Beyond just a tight fortress: Contribution of stroma to epithelial-mesenchymal transition in pancreatic cancer. Signal Transduct Target Ther. 2020;5:249. doi: 10.1038/s41392-020-00341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Malchiodi ZX, Cao H, Gay MD, Safronenka A, Bansal S, Tucker RD, Weinberg BA, Cheema A, Shivapurkar N, Smith JP. Cholecystokinin receptor antagonist improves efficacy of chemotherapy in murine models of pancreatic cancer by altering the tumor microenvironment. Cancers (Basel) 2021;13:4949. doi: 10.3390/cancers13194949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee JJ, Perera RM, Wang H, Wu DC, Liu XS, Han S, Fitamant J, Jones PD, Ghanta KS, Kawano S, et al. Stromal response to hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci USA. 2014;111:E3091–E3100. doi: 10.1073/pnas.1411679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu Y, Zhang C, Jiang K, Werner J, Bazhin AV, D'Haese JG. The role of stellate cells in pancreatic ductal adenocarcinoma: Targeting perspectives. Front Oncol. 2021;10:621937. doi: 10.3389/fonc.2020.621937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lisanti MP, Martinez-Outschoorn UE, Chiavarina B, Pavlides S, Whitaker-Menezes D, Tsirigos A, Witkiewicz A, Lin Z, Balliet R, Howell A, Sotgia F. Understanding the 'lethal' drivers of tumor-stroma co-evolution: Emerging role(s) for hypoxia, oxidative stress and autophagy/mitophagy in the tumor micro-environment. Cancer Biol Ther. 2010;10:537–542. doi: 10.4161/cbt.10.6.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ko YH, Lin Z, Flomenberg N, Pestell RG, Howell A, Sotgia F, Lisanti MP, Martinez-Outschoorn UE. Glutamine fuels a vicious cycle of autophagy in the tumor stroma and oxidative mitochondrial metabolism in epithelial cancer cells: Implications for preventing chemotherapy resistance. Cancer Biol Ther. 2011;12:1085–1097. doi: 10.4161/cbt.12.12.18671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mariño G, Kroemer G. Ammonia: A diffusible factor released by proliferating cells that induces autophagy. Sci Signal. 2010;3:pe19. doi: 10.1126/scisignal.3124pe19. [DOI] [PubMed] [Google Scholar]
- 168.Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5:e10250. doi: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Waldenmaier M, Seibold T, Seufferlein T, Eiseler T. Pancreatic cancer small extracellular vesicles (exosomes): A tale of short- and long-distance communication. Cancers (Basel) 2021;13:4844. doi: 10.3390/cancers13194844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Takikawa T, Masamune A, Yoshida N, Hamada S, Kogure T, Shimosegawa T. Exosomes derived from pancreatic stellate cells: MicroRNA signature and effects on pancreatic cancer cells. Pancreas. 2017;46:19–27. doi: 10.1097/MPA.0000000000000722. [DOI] [PubMed] [Google Scholar]
- 171.Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2011;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Olivares O, Mayers JR, Gouirand V, Torrence ME, Gicquel T, Borge L, Lac S, Roques J, Lavaut MN, Berthezène P, et al. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat Commun. 2017;8:16031. doi: 10.1038/ncomms16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Begum A, McMillan RH, Chang YT, Penchev VR, Rajeshkumar NV, Maitra A, Goggins MG, Eshelman JR, Wolfgang CL, Rasheed ZA, Matsui W. Direct interactions with cancer-associated fibroblasts lead to enhanced pancreatic cancer stem cell function. Pancreas. 2019;48:329–334. doi: 10.1097/MPA.0000000000001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cai Z, Liang Y, Xing C, Wang H, Hu P, Li J, Huang H, Wang W, Jiang C. Cancer-associated adipocytes exhibit distinct phenotypes and facilitate tumor progression in pancreatic cancer. Oncol Rep. 2019;42:2537–2549. doi: 10.3892/or.2019.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Meyer KA, Neeley CK, Baker NA, Washabaugh AR, Flesher CG, Nelson BS, Frankel TL, Lumeng CN, Lyssiotis CA, Wynn ML, et al. Adipocytes promote pancreatic cancer cell proliferation via glutamine transfer. Biochem Biophys Rep. 2016;7:144–149. doi: 10.1016/j.bbrep.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ye H, Zhou Q, Zheng S, Li G, Lin Q, Wei L, Fu Z, Zhang B, Liu Y, Li Z, Chen R. Tumor-associated macrophages promote progression and the Warburg effect via CCL18/NF-kB/VCAM-1 pathway in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018;9:453. doi: 10.1038/s41419-018-0486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Asencio-Barría C, Defamie N, Sáez JC, Mesnil M, Godoy AS. Direct intercellular communications and cancer: A snapshot of the biological roles of connexins in prostate cancer. Cancers (Basel) 2019;11:1370. doi: 10.3390/cancers11091370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Luo M, Luo Y, Mao N, Huang G, Teng C, Wang H, Wu J, Liao X, Yang J. Cancer-associated fibroblasts accelerate malignant progression of non-small cell lung cancer via connexin 43-formed unidirectional gap junctional intercellular communication. Cell Physiol Biochem. 2018;51:315–336. doi: 10.1159/000495232. [DOI] [PubMed] [Google Scholar]
- 179.Wang J, Liu X, Qiu Y, Shi Y, Cai J, Wang B, Wei X, Ke Q, Sui X, Wang Y, et al. Cell adhesion-mediated mitochondria transfer contributes to mesenchymal stem cell-induced chemoresistance on T cell acute lymphoblastic leukemia cells. J Hematol Oncol. 2018;11:11. doi: 10.1186/s13045-018-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Marlein CR, Piddock RE, Mistry JJ, Zaitseva L, Hellmich C, Horton RH, Zhou Z, Auger MJ, Bowles KM, Rushworth SA. CD38-driven mitochondrial trafficking promotes bioenergetic plasticity in multiple myeloma. Cancer Res. 2019;79:2285–2297. doi: 10.1158/0008-5472.CAN-18-0773. [DOI] [PubMed] [Google Scholar]
- 181.Dovmark TH, Saccomano M, Hulikova A, Alves F, Swietach P. Connexin-43 channels are a pathway for discharging lactate from glycolytic pancreatic ductal adenocarcinoma cells. Oncogene. 2017;36:4538–4550. doi: 10.1038/onc.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44:2144–2151. doi: 10.1016/j.biocel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mummery CL, van de Stolpe A, Roelen B, Clevers H. Chapter 12-Cancer stem cells: Where do they come from and where are they going? In: Mummery CL, van de Stolpe A, Roelen B, Clevers HB, editors. Stem Cells. 3rd. Academic Press; Boston: 2021. pp. 299–328. [DOI] [Google Scholar]
- 184.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: An evolving concept. Nat Rev Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 185.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1038. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 186.Gzil A, Zarębska I, Bursiewicz W, Antosik P, Grzanka D, Szylberg Ł. Markers of pancreatic cancer stem cells and their clinical and therapeutic implications. Mol Biol Rep. 2019;46:6629–6645. doi: 10.1007/s11033-019-05058-1. [DOI] [PubMed] [Google Scholar]
- 187.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 188.Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, Murter C, Hong SM, Koorstra JB, Rajeshkumar NV, He X, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141:2218–2227.e5. doi: 10.1053/j.gastro.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 190.Miranda-Lorenzo I, Dorado J, Lonardo E, Alcala S, Serrano AG, Clausell-Tormos J, Cioffi M, Megias D, Zagorac S, Balic A, et al. Intracellular autofluorescence: A biomarker for epithelial cancer stem cells. Nat Methods. 2014;11:1161–1169. doi: 10.1038/nmeth.3112. [DOI] [PubMed] [Google Scholar]
- 191.Sancho P, Alcala S, Usachov V, Hermann PC, Sainz B., Jr The ever-changing landscape of pancreatic cancer stem cells. Pancreatology. 2016;16:489–496. doi: 10.1016/j.pan.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 192.Depeint F, Bruce WR, Shangari N, Mehta R, O'Brien PJ. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact. 2006;163:94–112. doi: 10.1016/j.cbi.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 193.Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci USA. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sainz BJ, Jr, Alcala S, Garcia E, Sanchez-Ripoll Y, Azevedo MM, Cioffi M, Tatari M, Miranda-Lorenzo I, Hidalgo M, Gomez-Lopez G, et al. Microenvironmental hCAP-18/LL-37 promotes pancreatic ductal adenocarcinoma by activating its cancer stem cell compartment. Gut. 2015;64:1921–1935. doi: 10.1136/gutjnl-2014-308935. [DOI] [PubMed] [Google Scholar]
- 195.Sainz B, Jr, Martín B, Tatari M, Heeschen C, Guerra S. ISG15 is a critical microenvironmental factor for pancreatic cancer stem cells. Cancer Res. 2014;74:7309–7320. doi: 10.1158/0008-5472.CAN-14-1354. [DOI] [PubMed] [Google Scholar]
- 196.Rausch V, Liu L, Apel A, Rettig T, Gladkich J, Labsch S, Kallifatidis G, Kaczorowski A, Groth A, Gross W, et al. Autophagy mediates survival of pancreatic tumour-initiating cells in a hypoxic microenvironment. J Pathol. 2012;227:325–335. doi: 10.1002/path.3994. [DOI] [PubMed] [Google Scholar]
- 197.Zhu H, Wang D, Zhang L, Xie X, Wu Y, Liu Y, Shao G, Su Z. Upregulation of autophagy by hypoxia-inducible factor-1α promotes EMT and metastatic ability of CD133+ pancreatic cancer stem-like cells during intermittent hypoxia. Oncol Rep. 2014;32:935–942. doi: 10.3892/or.2014.3298. [DOI] [PubMed] [Google Scholar]
- 198.Jagust P, Alcalá S, Sainz B, Jr, Heeschen C, Sancho P. Glutathione metabolism is essential for self-renewal and chemoresistance of pancreatic cancer stem cells. World J Stem Cells. 2020;12:1410–1428. doi: 10.4252/wjsc.v12.i11.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li D, Fu Z, Chen R, Zhao X, Zhou Y, Zeng B, Yu M, Zhou Q, Lin Q, Gao W, et al. Inhibition of glutamine metabolism counteracts pancreatic cancer stem cell features and sensitizes cells to radiotherapy. Oncotarget. 2015;6:31151–31163. doi: 10.18632/oncotarget.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Brandi J, Dando I, Pozza ED, Biondani G, Jenkins R, Elliott V, Park K, Fanelli G, Zolla L, Costello E, et al. Proteomic analysis of pancreatic cancer stem cells: Functional role of fatty acid synthesis and mevalonate pathways. J Proteomics. 2017;150:310–322. doi: 10.1016/j.jprot.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 201.McDonald OG, Li X, Saunders T, Tryggvadottir R, Mentch SJ, Warmoes MO, Word AE, Carrer A, Salz TH, Natsume S, et al. Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat Genet. 2017;49:367–376. doi: 10.1038/ng.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Alcalá S, Sancho P, Martinelli P, Navarro D, Pedrero C, Martín-Hijano L, Valle S, Earl J, Rodríguez-Serrano M, Ruiz-Cañas L, et al. ISG15 and ISGylation is required for pancreatic cancer stem cell mitophagy and metabolic plasticity. Nat Commun. 2020;11:2682. doi: 10.1038/s41467-020-16395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nimmakayala RK, Leon F, Rachagani S, Rauth S, Nallasamy P, Marimuthu S, Shailendra GK, Chhonker YS, Chugh S, Chirravuri R, et al. Metabolic programming of distinct cancer stem cells promotes metastasis of pancreatic ductal adenocarcinoma. Oncogene. 2021;40:215–231. doi: 10.1038/s41388-020-01518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Alistar A, Morris BB, Desnoyer R, Klepin HD, Hosseinzadeh K, Clark C, Cameron A, Leyendecker J, D'Agostino R, Jr, Topaloglu U, et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: A single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017;18:770–778. doi: 10.1016/S1470-2045(17)30314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Philip PA, Buyse ME, Alistar AT, Rocha Lima CM, Luther S, Pardee TS, Van Cutsem E. A phase III open-label trial to evaluate efficacy and safety of CPI-613 plus modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) in patients with metastatic adenocarcinoma of the pancreas. Future Oncol. 2019;15:3189–3196. doi: 10.2217/fon-2019-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.