Abstract
Uterine fibroids (UFs) are a common benign gynecological tumor that affect the majority of women over their lifetime. Several pharmacological agents are available to reduce the size of fibroids and ameliorate the symptoms of UF. However, these drugs are expensive and are usually associated with profound side effects. Thus, botanical drugs are gaining attention in this era due to their cost effectiveness with a comparable and more potent therapeutic efficacy while demonstrating lesser adverse effects. The objective of this review is to summarize the available information on the mechanism of various botanical drugs and polyherbal formulations with anti-uterine fibroid activity. A systematic search was performed on botanical drugs with anti-uterine fibroid activity using several search engines, which include PubMed, Google Scholar, and Science Direct. Based on the literatures identified, a total of five botanical drugs and three polyherbal formulations were included and discussed in this review, which yields useful information regarding the mechanism of different botanical drugs and polyherbal formulations in exerting anti-uterine fibroid activity for its potential use as an alternative treatment choice for uterine fibroids.
Keywords: uterine fibroids, leiomyoma, botanical drug, polyherbal, mechanism, management
Introduction
Uterine fibroids (UFs), also known as leiomyoma, are common benign gynecological tumors that proliferate from the myometrial smooth muscle cells into discrete masses (Ando et al., 2018). Generally, they affect 70–80% of women over their lifetime with the majority presenting without any symptoms (Baird et al., 2003). The clinical manifestations associated with UF include abnormal bleeding, pelvic pain, menorrhagia, infertility, recurrent miscarriages, and other obstetric-associated complications, which may lead to low quality of life. A cross-sectional study (Hervé et al., 2018) conducted among the French women reported that 64% of the participants had a moderate to significant decrease in their quality of life as a result of UF.
The incidence of UF was reported as 1.278% and 3.745% in Asia and African American women per year, respectively (Sheng et al., 2020). Moreover, studies also reported that black women have a higher lifetime prevalence of UF with more severe symptoms than white women (Stewart et al., 2013). US has reported that the cost involving uterine fibroids caused a profound impact on the nation and community, which requires a total of US$5.9 to $34.4 billion annually (Cardozo et al., 2012).
The etiology and/or the exact cause of UF are still unclear; however, genetics, cytokines, growth factors, hormones, such as estrogens and progesterone and/or their respective receptors, environmental and epigenetics, and the excessive synthesis of extracellular matrix (ECM), have been associated with the pathogenesis of UF (Flake et al., 2003; Ciavattini et al., 2013). In addition, epigenetics and microbiota were also associated with the development of UF (Li et al., 2003; Atkinson et al., 2006).
Although hysterectomy is the common and definitive treatment for UF, it is associated with several downsides, namely, the removal of the uterus and infertility. Generally, the treatment for UF depends on the size, location, symptoms, age, and fertility requirement of a patient. The available therapeutic treatment only gives temporary or partial relief from UF, whereas hormonal pills and NSAIDs produce serious side effects on the patients. Having these concerns, women are increasingly looking for an alternative option in treating fibroids.
In recent years, more research was conducted to identify natural extracts and botanical drugs for treating fibroids. With this in mind, we have conducted a detailed literature search to provide a general overview of the botanical drugs and polyherbal formulations available that may potentially treat and prevent uterine fibroids and their mechanism of action. Despite the fact that several reviews have been conducted on the use of botanical drugs on uterine fibroids, they mainly focused on the benefits and risks of using herbal or botanical drugs preparation (Liu et al., 2013; Fu et al., 2020) and on the effect of compounds on uterine fibroids (Li et al., 2020). This is the first review to our knowledge that discusses the detailed mechanisms of botanical drug extracts and polyherbal formulation in contributing to its anti-uterine fibroid activity.
Methods
Well renowned and globally accepted scientific databases, namely, PubMed, Google Scholar, and Science Direct were searched systematically to obtain relevant references up to January 2022 using the term “herb,” “plant,” “medicinal plant,” and “polyherbal formulation” alone or paired with “leiomyoma,” ‘uterine fibroids,” and “hysteromyoma.” Only research articles written in English were included.
The screening, eligibility, inclusion criteria, and exclusion criteria applied in the selection of the research articles were summarized in Figure 1. In this review, five botanical drugs and 3 polyherbal formulations have been identified based on the inclusion and exclusion criteria, with sufficient data available on their anti-uterine fibroid mechanisms.
FIGURE 1.
Schematic representation of a selection of articles.
A total of 32 articles were identified and deemed fit according to the inclusion and exclusion criteria. Among these articles, 4 studied Curcuma longa and/or its active constituents, 6 conducted on Camellia sinensis and/or its active constituents, 6 were on Scutellaria barbata, 2 were studied on Euonymus alatus, 1 study included both Scutellaria barbata and Euonymus alatus, 2 studies were conducted on the effect of Fragaria x ananassa on leiomyoma, 2 references were on Guizhi Fu Ling Wan (GZFLW), and 4 studies investigated on Lichong decoction. The rest of the studies were referenced in ‘5.3 Polyherbal Sparganii rhizoma (Sparganium stoloniferum (Buch.-Ham. Ex Graebn.) Buch.-Ham. Ex Juz [Typhaceae]) and Curcumae rhizoma (Curcuma phaeocaulis Valeton [Zingiberaceae] herb combination in Uterine fibroids.
Drawbacks of the Current Treatments for Uterine Fibroids
Apart from surgeries such as hysterectomy and myomectomy, uterine artery embolization (UAE), and focused ultrasound surgery (FUS), both ultrasound- and magnetic resonance-guided focused ultrasound surgery (USgFUS and MRgFUS) are also the main treatment strategies for UF, with hysterectomy being the only definitive solution for UF. Hysterectomy is the surgical removal of the uterus and hence is not ideal for fertile women who intend to retain their uterus. In such a condition, myomectomy, UAE, or FUS will be an alternative option for women who wish to maintain their uterus intact. However, these surgical methods are limited to their complications and cost. With the knowledge that UF is hormone-dependent, pharmacological agents that target the hormonal pathways, such as gonadotropin-releasing hormone (GnRH) agonist, selective progesterone receptor modulator (SPRM), and aromatase inhibitor, have been developed as a treatment choice for UF.
GnRH agonist acts by causing an initial increase in the secretion of gonadotropin by binding to the GnRH receptor. Following that, GnRH agonist desensitizes the receptor, causing a decrease in gonadotropin release, and subsequently reduces the estrogen level secondary to the decrease in ovary stimulation by gonadotropin. In addition, GnRH agonist was also found to have a direct antiproliferative effect on the fibroid (Khan et al., 2010). Currently, GnRH agonists are approved by FDA to be used in the preoperative treatment of UF. However, its use is generally limited to 6 months due to the high incidence of side effects reported (Matsuo, 2004; DiVasta et al., 2007; Lethaby et al., 2017).
GnRH antagonist is the newer member in the class of GnRH analog following GnRH agonist. It acts by competitively occupying the GnRH receptors, thus reducing the level of estrogen and progesterone. Unlike GnRH agonist, it does not trigger the initial surge of follicle-stimulating hormone and luteinizing hormone. In 2020, FDA have granted approval for the use of elagolix, a GnRH antagonist in combination with estradiol and norethindrone acetate for the treatment of uterine fibroid (FDA, 2020). Relugolix, the latest GnRH antagonist is currently under clinical trial for the treatment of uterine fibroid and it offers the advantage of once-a-day dosing as compared to twice-daily dosing with elagolix (Al-Hendy et al., 2021). In 2019, relugolix has received approval in Japan for being marketed as a treatment for UF symptoms (Markham, 2019). However, similar to GnRH agonist, GnRH antagonist is associated with hypoestrogenic adverse effects, which require the addition of estradiol and norethindrone acetate (add-back therapy) to attenuate the hypoestrogenic effect (Schlaff et al., 2020). Due to the risk of bone loss and fractures, FDA does not recommend the use of elagolix for more than 2 years (FDA, 2020).
Selective progesterone receptor modulator (SPRM) is another class of compounds commonly used in UF, with a mixture of agonist and antagonist activity on the progesterone receptor. The two most commonly used SPRM are mifepristone (pure antagonist) and ulipristal acetate (UPA), which have been proven to be effective against UF. Initially, UPA have been used to reduce the fibroid volume for 3 months before surgery. However, recently, it is used in patients who refuse to remove their uterus (Pazzaglia et al., 2017; Rozenberg et al., 2017). Due to the progesterone antagonist activity of UPA on the endometrium, this could cause an unopposed estrogen activity, which may increase cell proliferation. Progesterone receptor modulator-associated endometrial changes (PAEC) have been found to occur in 0.4% of patients using UPA while 41%–78.8% of patients who developed PAEC appeared to be reversible upon discontinuing UPA (De Milliano et al., 2017). Recently, the European Medicines Agency (EMA) decided to suspend the use of UPA in treating UF due to the high incidence and severity of liver toxicity, with more than 900,000 women who were given with UPA for UF requiring liver transplant. Hence, to evaluate the overall safety with regards to the use of UPA, EMA has started a new review. No patient will be prescribed UPA for the first time during this period until the review has come to a conclusion (Ekanem and Talaulikar, 2021).
Aromatase inhibitor is used to block the action of aromatase, an enzyme that is responsible for the conversion of androstenedione to estrogen, resulting in increased cell proliferation and fibrosis. The two aromatase inhibitors that have been widely studied for UF are letrozole and anastrozole. In a randomized controlled trial comparing the effect of aromatase inhibitor and GnRH agonist on UF, it was found that both interventions are able to reduce the myoma volume and symptoms; however, aromatase inhibitors possess an advantage of rapid onset of action and the absence of the initial flare associated with GnRH agonist (Parsanezhad et al., 2010). The concern regarding aromatase inhibitor is the hypoestrogenic adverse effect, which includes hot flushes and bone loss. However, it has been reported that the use of aromatase inhibitors resulted in reduced hot flushes as compared to GnRH agonist, while the adverse effect of aromatase inhibitors are usually mild and occurs more frequently with prolonged use. Nevertheless, a Cochrane Systematic Review had concluded that the evidence to support the use of aromatase inhibitors in UF was insufficient (Song et al., 2013). A summary of the benefits and drawbacks of various surgical and pharmacological interventions for uterine fibroids is shown in Table 1.
TABLE 1.
Possible benefits and drawbacks of surgical and pharmacological interventions.
| Intervention | Benefits | Drawbacks |
|---|---|---|
| Gonadotropin-releasing hormone agonist (Matsuo, 2004; DiVasta et al., 2007; Yoo et al., 2007; Donnez et al., 2012; Han et al., 2013; Lee et al., 2017) | • Greater fibroid and uterine volume reduction than SPRM | |
| • Increase preoperative hemoglobin | • Greater adverse effects, especially hot flushes and bone loss on prolonged use | |
| • Reduce blood loss during surgery | • Risk of disease recurrence | |
| • Fewer postoperative complications | ||
| • Uterus is preserved | ||
| Gonadotropin-releasing hormone antagonist (Schlaff et al., 2020; Al-Hendy et al., 2021) | • Significant reduction in menstrual blood loss | • Risk of hot flushes, metrorrhagia and bone loss on prolonged use |
| • Uterus is preserved | ||
| • Reduce uterine or fibroid volume | ||
| • Rapid symptoms improvement | ||
| • Do not trigger FSH and LH surge | ||
| Selective Progesterone Receptor Modulator (SPRM) (Donnez et al., 2012; Kulshrestha et al., 2013; De Milliano et al., 2017; Lethaby et al., 2017) | • Less hot flushes than GnRH agonist | |
| • Reduce uterine or fibroid volume | • Progesterone receptor modulator-associated endometrial changes | |
| • Increase preoperative hemoglobin | • Risk of disease recurrence | |
| • Reduce blood loss during surgery | ||
| • No hypoestrogenic side effects | ||
| Aromatase Inhibitor (Parsanezhad et al., 2010; Brito et al., 2011; Song et al., 2013) | • Reduce fibroid size | • Bone loss on prolonged use |
| • Uterus is preserved | ||
| • Fewer vasomotor adverse effect than GnRH agonist | ||
| • Rapid onset of action than GnRH agonist | ||
| Levonorgestrel-intrauterine device (Grigorieva et al., 2003; Sayed et al., 2011; Socolov et al., 2011; Jiang et al., 2014; Dhamangaonkar et al., 2015) | • Most effective for reducing blood loss | • Irregular bleeding |
| • Reduce fibroid and/or uterine volume | • Increase risk of device expulsion | |
| Hysterectomy (Pitter et al., 2014; Aarts et al., 2015) | • Definitive treatment | • Increase risk of blood loss, postoperative fever, and surgical site infection |
| • Improved quality of life and satisfaction | • Uterus is removed | |
| Myomectomy (Yoo et al., 2007; Shiota et al., 2012; Kongnyuy and Wiysonge, 2014) | • Able to preserve fertility | • Risk of myoma recurrence |
| • Lower rate of complication | • Risk of bleeding | |
| Uterine artery embolization (Moss et al., 2011; Gupta et al., 2012; Jun et al., 2012; Lethaby and Vollenhoven, 2015; Sandberg et al., 2018) | • Minimally invasive | |
| • Shorter duration of hospitalization | • Higher risk of minor complication | |
| • Shorter recovery time | • Risk of myoma recurrence and reintervention | |
| • Less major complications | ||
| Focused ultrasound surgery (USgFUS and MRgFUS) (Stewart et al., 2007; Gorny et al., 2011; Chen et al., 2018; Wong, 2020) | • Noninvasive | • Risk of skin burn, weakness, or numbness of the lower limbs |
| • Shorter duration of hospitalization | • Risk of myoma recurrence and reintervention | |
| • Less morbidity |
The table clearly summarizes that the availability of cheaper, safer, and more effective alternative treatments for uterine fibroids could greatly benefit the society and enhances their quality of life. There has been an improvement and escalated research growth that attempts to discover natural alternatives that could be used as an anti-UF agent. Botanical drug products have an increased demand in this era due to their lower cost with a comparable or more potent therapeutic efficacy while being associated with lesser adverse effects. This review will, therefore, discuss the mechanisms of action of various natural botanical drugs and herbal formulations that have been studied against UF in vitro, in vivo, and in clinical trials. A total of five botanical drugs and 3 polyherbal formulations have been included and summarized.
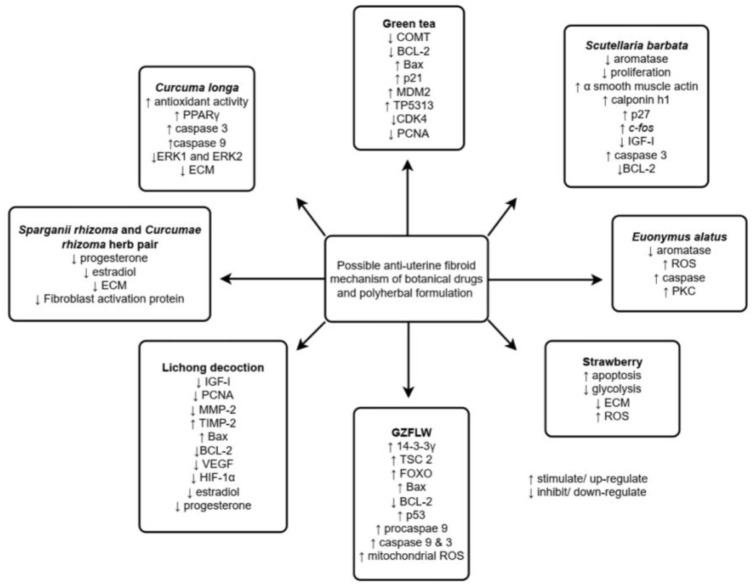
Figure 2 shows the summarized information regarding the mechanism of various botanical drugs and polyherbal formulations in exerting anti-uterine fibroid activity.
FIGURE 2.
Summarized anti-uterine fibroid mechanisms of various botanical drugs and polyherbal formulations. Adapted from (Islam et al., 2013).
Botanical Drugs With Anti-Uterine Fibroid Activity
Curcuma longa L [Zingiberaceae] as Alternative Treatment for Uterine Fibroids
Curcuma longa, commonly known as turmeric is a rhizomatous herbaceous perennial herb that belongs to the ginger family and is planted extensively throughout Asia, India, and other tropical countries. It has been used since ancient times for flavoring and coloring purposes due to its yellowish nature. In the context of Traditional Chinese Medicine (TCM), Curcuma longa was included as one of the herbs in the tumor-shrinking decoction, which is currently under phase-III clinical trials (ClinicalTrials.gov, 2014; Cheng et al., 2019). Curcuma longa was also added to tonify qi as UF is believed to be a product of vital energy deficiency and stasis of blood according to TCM theories (ClinicalTrials.gov, 2014). Likewise, Curcuma longa is also used in Ayurveda as the main ingredient for the formulation Haridra Khanda, which appeared to be effective in relieving the symptoms and size of UF in a case series (Dhiman, 2014).
The ability of turmeric extract in protecting the uterine myometrium from oxidative damage-induced UF has been reported (Eze-Steven, 2019). The reactive oxygen species (ROS) is able to induce the proliferation of leiomyoma smooth muscle cells and it is also necessary for triggering the MAPK1/MAPK3 signaling pathway, which contributes to leiomyoma smooth muscle cell proliferation (Mesquita et al., 2010). Thus, the antioxidant potential of turmeric and its protective effect on uterine myometrium shows potential to be used as a treatment or prevention for UF.
The antioxidant activity may be attributed to curcumin, the main polyphenol and active compound present in Curcuma longa which has the ability to increase the level of antioxidant enzymes, which include catalase, superoxide dismutase, and glutathione peroxidase (Panahi et al., 2012; Lin et al., 2019). Up to date, the anti-uterine fibroid activity of curcumin has been evident in many literatures. The size, volume, and proliferative activity of UF were significantly reduced when patients took 1.2 g of curcumin orally every day for 6 months, with no adverse effects observed (Sukonthanonta, 2015). Ali and Laila (Ali and Ali, 2013) reported a decrease in uterine and myoma volume followed by the significant increase in hemoglobin percentage in patients taking 1.35 g of curcumin daily for 3 months was consistent with the previous study with no side effects being reported. However, the interpretation of results from both of the studies was restricted due to a lack of information on control groups.
In vitro study with curcumin on Eker rat-derived uterine leiomyoma cell lines (ELT-3) showed the ability of curcumin to inhibit leiomyoma cell proliferation while increasing apoptosis by acting as a PPARγ ligand (Tsuiji et al., 2011). The exact signaling pathway of PPARγ in UF has not been clearly characterized despite the fact that PPARγ protein isoforms are expressed at higher levels in UF (Tsibris et al., 1999) or a relatively equal level as compared to the normal myometrium (Houston et al., 2003). Among the several mechanisms proposed in attributing the ability of PPARγ ligand in inhibiting leiomyoma cell growth, Houston suggested that the inhibition was mediated by negative cross-talk between ER and PPAR signaling pathway where the activation of PPARγ inhibits ER-mediated gene expression (Houston et al., 2003). Other possible mechanisms include the accumulation of Fas(Nam et al., 2007), downregulation of cyclooxygenase-2 (COX-2), and caspase-3 activation (Liu et al., 2007).
Apoptosis of uterine leiomyoma cells was also stimulated by the expression of caspase-3, an important mediator for apoptosis, and by caspase-9, an initiator of apoptosis (Malik et al., 2009). On top of that, ERK1 and ERK2 proteins were also decreased in a concentration-dependent manner with curcumin (Malik et al., 2009). These proteins are involved in the Ras/Raf/MEK/ERK signaling pathway which plays a role in cellular proliferation and survival and is involved in the pathophysiology of UF. Supporting this, ERK1 and ERK2 are overexpressed in leiomyoma as compared to the normal myometrium (Yu et al., 2008). Knowing the fact that UF is hormone-dependent, which is mainly associated with the estrogen hormone (Marshall et al., 1998), estradiol has been found to contribute to the growth of leiomyoma via the activation of ERK (Nierth-Simpson et al., 2009). Hence, a reduction of this protein can largely contribute to the anti-uterine fibroid activity.
Leiomyoma is a benign tumor with excessive ECM production. The ability of curcumin to down-regulate the expression of fibronectin, one of the ECM proteins, which is overexpressed in leiomyoma as compared to normal myometrium, has also been documented (Malik et al., 2009). The significant inhibitory effect of curcumin on the expression of ECM receptors in non-small-cell lung cancer (Li et al., 2017) and reducing the activity of MMP-2 and up-regulating TIMP activity in various cancer cells (Mitra et al., 2006; Hassan and Daghestani, 2012; Supriono et al., 2018) suggest the possible mechanism of curcumin in producing anti-uterine fibroid activity via reducing the ECM production, thus further study investigating the effects of curcumin on various ECM-related receptors and proteins in leiomyoma cells may be conducted.
Camellia sinensis (L.) Kuntze [Theaceae] (Green Tea) in Uterine Fibroids
A randomized controlled trial reported on the effect of green tea extract [45% epigallocatechin gallate (EGCG)] in women with symptomatic UF showed a significant reduction in the fibroid volume as compared to the placebo group after taking 800 mg of green tea extract for the duration of 4 months. The severity of symptoms associated with fibroid, average blood loss and HRQL were also significantly improved with the use of green tea extract as compared to placebo, with no adverse effects identified (Roshdy et al., 2013). The ability of green tea to exert anti-UF activity is mainly attributed by epigallocatechin gallate (EGCG), one of the main active constituents in green tea, which constitutes more than 40% of the total polyphenol of green tea catechin and is present at about 142 mg in a 200 ml of green tea (Yang and Wang, 1993). Numerous studies have identified the antiproliferative and apoptosis induction effect of EGCG on uterine fibroid cells. Although the exact mechanisms and pathways involved are unclear, a combination of mechanisms has been proposed in respective to this.
Catechol-O-methyl transferase (COMT) is an enzyme expressed at a higher level in leiomyoma as compared to the normal myometrium (Salama et al., 2006). COMT is involved in converting 2-hydroxyestradiol (an antiestrogen) to 2-methoxyestradiol (proestrogen), creating a hyper-estrogenic milieu due to the decrease in antiestrogen and an increase in proestrogen. Knowing UF is largely estrogen-dependent, the increased estrogenic environment has a great potential to increase UF proliferation and growth. In addition, those who have COMT Val/Val genotype (genotype with greater enzyme expression) were associated with a significantly increased risk of UF as compared to those with Val/Met genotype (intermediate expression) and Met/Met genotype (low expression), which could have explained the higher risk of UF in African American women (47% having Val/Val genotype) (Al-Hendy and Salama, 2006). In vitro study showed that the ability of green tea extract in inhibiting the proliferation of leiomyoma cells is in part due to the potential of EGCG in reducing the expression of the COMT enzyme (Zhang et al., 2014). Be that as it may, in vivo study showed no impairment of EGCG on COMT activity and even increase by 24% after consumption of EGCG (Lorenz et al., 2014). Hence, further study is warranted to confirm the antiproliferative effect of EGCG on leiomyoma via the COMT pathway.
Decreased BCL-2 protein and elevated Bax protein expression are other mechanisms leading to apoptosis by EGCG (Zhang et al., 2010a). BCL-2, the proto-oncogene, has the ability to block apoptosis, leading to reduced cell death and an enhanced proliferation (Omar et al., 2019). Bax protein is a proapoptotic member that acts by forming pores on the mitochondrial outer membrane, allowing cytochrome c to translocate into the cytoplasm, resulting in the loss of energy production and the activation of proteolytic cascade leading to apoptosis (Westphal et al., 2014). It was reported that EGCG significantly up-regulated BAX protein and down-regulated BCL-2 protein in a dose-dependent manner when EGCG was tested in HuLM cells. The p53 pathway genes, namely, BAX, p21, MDM2, and TP5313, which are involved in DNA repair, apoptosis, and cell cycles were also up-regulated with EGCG treatment (Zhang et al., 2010a).
The EGCG could have also exerted cell cycle arrest by causing a significant decrease in the expression of CDK4 and PCNA (Zhang et al., 2010a; 2010b). Complex formation between cyclin and CDK4 is an important driver in the cell cycle through the G1 phase to S phase transition. The reduced expression of CDK4 by EGCG, blocks the transition from G1 phase to S phase, which could cause a G0/G1 phase arrest of the cell cycle. On the other hand, PCNA is essential for cell proliferation, and it interacts with various proteins in regulating the cell cycle, DNA replication, and DNA repair process (Mansilla et al., 2020).
BMP2 gene appeared to have a 14-fold greater expression upon EGCG treatment on HuLM cells (Zhang et al., 2010a). BMP2 is a member of the TGF-β superfamily, which plays a vital role in the regulation of cell proliferation, differentiation, and apoptosis. A recent study on colorectal cancer demonstrated the role of BMP2 in reducing growth, enhancing apoptosis, and decreasing the development of tumors in vivo (Vishnubalaji et al., 2016), supporting that the decreased expression of BMP2 also leads to the progression of prostate cancer (Horvath et al., 2004). These data, therefore, propose the possibility of BMP2 as a potential target choice for UF treatments.
EGCG was also identified to differentially inhibit TNF-α and LPS-mediated activation of NF-κB (Ahmad et al., 2000), and also reduce the expression of bcl2A1 (Zhang et al., 2010a), the key factor of NF-κB pathway. NF-κB is a transcription factor involved in promoting angiogenesis, cell proliferation, and inhibiting apoptosis and is often activated in cancer cells (Taniguchi and Karin, 2018). Noteworthy, EGCG only reduces cell proliferation and induces apoptosis in cancer cells but not in normal cells, demonstrating its invulnerability/safeness to be used as a potential alternative treatment (Ahmad et al., 2000).
Apart from producing promising effects, EGCG has several drawbacks, such as low stability, poor bioavailability, and highly metabolized under physiological conditions. Taking these drawbacks into consideration, the prodrugs of EGCG analogs were synthesized with the compounds identified as 2a and 4a having lower susceptibility to be metabolized or inhibited by COMT and having an amplified antiproliferative, antiangiogenic, and antifibrotic effect in HuLM (Ahmed et al., 2016).
Scutellaria barbata D. Don [Lamiaceae] for Uterine Fibroids
Scutellaria barbata (SB) is a perennial herb, known in TCM, and traditional Korean Medicine as “Ban-Zhi-Lian” and “Ban-Ji-Ryun,” respectively. This botanical drug exhibits anti-inflammatory, antitumor activity, and antimutagenic effects. Its effects on UF were established by several studies with multiple mechanisms of action being reported.
In 2004, aromatase inhibitory activity of SB in leiomyoma cells was reported and the enzyme was inhibited in a time- and dose-dependent manner (Lee et al., 2004b). Aromatase is an enzyme responsible for converting androstenedione to estrogen. With the ability of SB to inhibit intracellular aromatase activity in leiomyoma cells, it potentially reduces the production of estrogen in the fibroids and thus reduces the stimulation of estrogen in causing cell proliferation and subsequently fibrosis or increased size of the fibroid.
SB also exhibited a dose-dependent inhibition on the proliferation of leiomyoma and normal myometrial cells. SB increases the fraction of cells in the G1 phase of the cell cycle and was suggested to exhibit its antiproliferative action via blocking the transition of the cell cycle from the G1 phase to the S phase or by arresting the cell cycle at the G1 phase (Lee et al., 2004d). In addition, the expression of smooth muscle cell differentiation markers, which include α-smooth muscle actin, calponin h1, and cell cycle inhibitor p27, in both leiomyoma and normal myometrial cells was increased after treatment with SB. On the contrary, SB has no effect on cyclin E and cdk2, which are gene products associated with G1 phase of the cell cycle (Lee et al., 2004d). P27 protein is considered a potent tumor suppressor, which is able to induce apoptosis and reduce the viability and proliferation of UF cells (Ramachandran et al., 2008). With this, it is possible that SB induces its antiproliferative effect via an induced differentiation of smooth muscle cells and an up-regulation of p27.
When human uterine leiomyoma cells were being treated with SB, it was observed that c-fos mRNA expression was being induced by SB via the cAMP/PKA pathway which was activated by the increased in cAMP level (Lee et al., 2004a). This was evident by the fact that the PKA inhibitor inhibited the SB-induced c-fos gene expression (Lee et al., 2004a). c-fos is a proto-oncogene that is closely involved in cell growth and differentiation and was also suggested to be involved in smooth muscle cell differentiation (Raimundo et al., 2009). c-fos gene expression has been identified to be significantly lower in leiomyoma cells as compared to normal myometrium which is consistent with the previous study that has suggested that a decrease in the smooth muscle cells progenitors could have contributed to the formation of UF (Arslan et al., 2005). Hence, further studies are needed to determine whether the increased c-fos expression in leiomyoma cells induced by SB is associated with its antiproliferative actions (Lee et al., 2004a).
The higher incidence of UF and the greater fibroid size during the first trimester of pregnancy have suggested the role of hCG in the pathogenesis of UF (Benaglia et al., 2014; Ciavattini et al., 2016). Supporting this, hCG significantly increases the proliferation in both leiomyoma and normal myometrial cells. In this context, SB demonstrated an ability to reduce the proliferating effect of hCG in leiomyoma and myometrial cells. On top of that, treatment with SB also reduces the expression of PCNA, cyclin E, and cdc2 in hCG-treated leiomyoma cells (Lee et al., 2004c).
SB has also been observed to down-regulate the expression of IGF-I in leiomyoma cells which were overexpressed in UF as compared to normal myometrium (Párrizas et al., 1997). IGF-I functions as a survival factor that is able to block apoptosis in a number of cell types; therefore, the over-expression of IGF-I has been associated with its tumorigenic activity, protecting the cancer cells from apoptosis (Párrizas et al., 1997). Studies have also demonstrated the role of IGF-I in promoting mitosis of cells, and its ability to particularly promote the uterine smooth muscle cells proliferation, therefore making it a potential contributor to the formation of UF (Maruo et al., 2007; Baird et al., 2009). Besides the aforementioned mechanisms, SB was also reported to exhibit its antiproliferative effect and apoptosis inductive effect by causing the release of cytochrome c from mitochondrial into the cytosol, with a subsequent increase in caspase-3-like activity and causing a decrease in BCL-2 protein in leiomyoma cells, all of this contributing to its anti-uterine fibroid activity (Lee et al., 2006; Kim et al., 2008).
Euonymus alatus (Thunb.) Siebold [Celastraceae] for Uterine Fibroids
Euonymus alatus (EA) is a medicinal plant used for the treatment of tumors in TCM and traditional Korean medicine. Similar to Scutellaria barbata, Euonymus alatus is also able to inhibit the aromatase enzyme activity and was found to be 10 to 30 times more potent as compared to SB. EA exhibited a time- and dose-dependent inhibition of intracellular aromatase activity in leiomyoma cells, which potently inhibit the capability of the tumor cell in self-supplying estrogen, the hormone that results in the proliferation of leiomyoma cells (Lee et al., 2004b). EA was observed to induce apoptosis by acting as a prooxidant and induced the release of cytochrome c from mitochondria, followed by the activation of caspase in leiomyoma cells. Interestingly, the apoptotic effect of EA was observed only in leiomyoma cells without any effect on the peripheral blood mononuclear cells, which greatly reduces the possibility of causing an adverse effect (Kim et al., 2006). EA also demonstrated the ability to increase PKC activity in uterine leiomyoma cells and a slight increase in the normal myometrial cell. The activity of PKC is lesser in leiomyoma cell as compared to normal myometrium; however, the exact role of PKC in the pathogenesis of UF is not well established as of now (Lee et al., 2005). Even so, it was proposed that PKC could be involved in the downstream pathway of TGF-beta2, which is known to contribute to fibroid development (Laping et al., 2007).
Fragaria x Ananassa (Duchesne Ex Weston) Duchesne Ex Rozier [Rosaceae] (Strawberry) as Alternative Treatment for Uterine Fibroids
In recent years, strawberry extract has also exhibited potential as a therapeutic and/or preventive agent against UF. Islam et al.(Islam et al., 2017) discovered that anthocyanin-rich strawberry extract is able to induce apoptosis, inhibit glycolysis, and significantly reduce ECM components, namely, collagen 1A1, fibronectin, and versican in leiomyoma cells. The author has suggested that the possible mechanism at which strawberry extract induces apoptosis is due to the increased ROS production. It is worth noting that the ROS production and the percentage of apoptotic and dead cells are significantly higher in leiomyoma cells; however, the ROS production was decreased instead while there was no significant difference in the percentage of apoptotic and dead cells in the normal myometrial cell, which could have indicated the possibility of strawberry extract to only target the UF cells while maintaining a homeostatic condition in the normal myometrial cells, hence lesser side effect could be achieved with the use of the strawberry extract.
Activin A, a member of the TGF- β superfamily was thought to be involved in the pathogenesis of UF by increasing the ECM components, such as versican, fibronectin, and collagen 1A1, at least in part through activating the Smad-2/3 and p38-MAPK signaling pathway (Islam et al., 2014; Bao et al., 2018). The treatment of the Alba cultivar of the strawberry extract was found to inhibit the induction of collagen 1A1, fibronectin, and versican mRNA expression by activin A in leiomyoma cells (Islam et al., 2017).
Following that, the same group of researchers investigated the anti-UF activity of five different cultivars of strawberry. It was identified that Romina followed by the Alba cultivar produced the most promising anti-UF activity, which suggests its potential in using strawberry extract as an alternative treatment for UF (Giampieri et al., 2019).
Polyherbal Formulation for Uterine Fibroids
It has been observed that the etiology and pathophysiology of uterine fibroid are multifactorial and the apoptosis and reduction in fibroid volume and size can be achieved via several pathways. Hence, to obtain a multimodal activity in the management of uterine fibroid, certain purposeful mixtures of herbs have been evaluated on their effect on the uterine fibroid. Such a polyherbal is able to target different pathways and pathological events from several approaches and potentially provide a more effective anti-uterine fibroid activity while improving the quality of life of the patients. In this context, we have reviewed several polyherbal formulations that have been studied on leiomyoma cells, with their anti-uterine fibroid mechanism being described.
Polyherbal GZFLW in Uterine Fibroids
Guizhi Fu Ling Wan (GZFLW) is a famous traditional Chinese herbal formula and is the most frequently prescribed traditional Chinese formula in Taiwan (Yen et al., 2015). GZLFW is a formula consisting of five herbs in the ratio of 1:1:1:1:1 (g/g), namely Cinnamomi Ramulus (the dried twig of Cinnamomum cassia (L.) J. Presl), Poria (the dried sclerotia of Poria cocos (Schw.) Wolf), Moutan Cortex (the dried root bark of Paeonia x suffruticosa Andrews [Paeoniaceae]), Persicae Semen (the dried mature seed of Prunus persica (L.) Batsch [Rosaceae]) and Paeoniae Radix Alba (the dried root of Paeonia lactiflora Pall [Paeoniaceae]) (Xiao et al., 2012). Several studies that have investigated the effect of GZLFW on leiomyoma cells found that GZLFW is able to reduce cell proliferation and viability in a dose-dependent manner (Shen et al., 2016; Lee et al., 2019). In addition, a meta-analysis revealed that the combination of GZLFW with mifepristone was more effective in reducing the fibroid volume as compared to mifepristone alone. GZFLW is also able to improve the symptoms of dysmenorrhea in UF patients with no serious adverse effects being found (Chen et al., 2014).
The mechanism of GZFLW in inhibiting the proliferation and the induction of apoptosis has not been clearly defined; however, several possible mechanisms have been suggested. 14-3-3γ protein is a phosphoserine or phosphothreonine binding protein that is involved in various cellular processes, such as cell proliferation, apoptosis, and cell cycle (Morrison, 2009). 14-3-3γ is able to associate with FOXO and TSC2, preventing them from being dephosphorylated which controls the transcription of cytoplasmic and nuclear proteins subsequently affecting cell proliferation and apoptosis (Morrison, 2009; Khorrami et al., 2017). GZFLW is able to significantly increase the expression of 14-3-3γ, TSC2, and FOXO in human primary uterine leiomyoma cells as compared to the nontreatment group, which suggests the possible pathway of GZFLW in inhibiting proliferation and inducing apoptosis (Shen et al., 2016). Although 14-3-3γ signal transduction pathway has been associated with the formation of several cancer, its role in uterine fibroid formation is not well understood. However, the significant downregulation of 14-3-3γ identified in UF as compared to the normal myometrium suggests its role in the origin or growth of UF (Lv et al., 2008; Wang et al., 2012).
As mentioned earlier, Bax and BCL-2 play an opposite role in the regulation of apoptosis and thus the ratio of Bax to BCL-2 is often used as an indicator for apoptosis. GZFLW was observed to increase the ratio of Bax to BCL-2 in a dose-dependent manner and at the same time up-regulating the tumor suppressor gene p53 (Lee et al., 2019). The p53 mediates apoptosis by inducing the release of mitochondria cytochrome c with subsequent caspase activation along with the cleavage of caspase substrates. Following the release of cytochrome c into the cytosol, with the presence of ATP, it facilitates the activation of caspase-9 which then activates caspase-3 and the other effector caspases (Schuler et al., 2000). Besides affecting Bax, BCL-2, and p53, GZFLW exhibits a dose-dependent increase in the expression of procaspase-9, cleaved-caspase-9, and cleaved-caspase-3 (Lee et al., 2019).
GZFLW is also able to induce mitochondrial ROS production in human uterine leiomyoma cells (Lee et al., 2019). Mitochondrial ROS plays a critical role in apoptosis. A high level of mitochondrial ROS can lead to the release of mitochondrial apoptogenic factors, such as cytochrome c, which initiates intrinsic apoptosis (El-Osta and Circu, 2016). All these mechanisms, therefore, show that GZFLW induces apoptosis via the mitochondria-intrinsic mechanism.
Polyherbal Lichong Decoction in Uterine Fibroids
Lichong decoction (LD) is another Chinese herbal formulation that has been studied for its effect on UF. The herbs included in this decoction includes 9 g of the root of Astragalus mongholicus Bunge [Fabaceae], 6 g of root of Codonopsis pilosula (Franch.) Nannf [Campanulaceae], 6 g of rhizome of Atractylodes macrocephala Koidz [Asteraceae], 15 g of rhizome of Dioscorea opposita Thunb [Dioscoreaceae], 12 g of root of Trichosanthes kirilowii Maxim [Cucurbitaceae], 12 g of rhizome of Anemarrhena asphodeloides Bunge [Asparagaceae], 9 g of rhizome of Sparganium stoloniferum (Buch.-Ham. Ex Graebn.) Buch.-Ham. Ex Juz [Typhaceae] , 9 g of rhizome of Curcuma phaeocaulis Valeton [Zingiberaceae] and 9 g of Endothelium Coreneum Gigeriae (chicken gizzard). This decoction was traditionally formulated for the treatment of UF by strengthening the healthy Qi, enhancing blood flow, and eliminating disease-causing pathogens (Wang et al., 2016).
In recent studies, several mechanisms have been proposed for the ability of LD to be used as a treatment of UF. According to Li et al., it was found that when LD is used on UF-induced rats, the expression of IGF-I and PCNA mRNA were significantly reduced as compared to the model group, suggesting its ability to reduce leiomyoma cell proliferation via this mechanism. The cells appeared ordered with the organelles appearing almost normal under a transmission electron microscope with the collagen fibers arranged relatively regularly as compared to the model control group where the collagen fibers were irregular and disordered with the organelles being malformed (Li et al., 2012). In line with the effect of LD on the collagen fibers, this group, later on, discovered that the MMP-2 protein expression was significantly reduced in rats treated with LD with an increased tissue inhibitor of metalloproteinase-2 (TIMP-2) expression, suggesting the influence of LD on the ECM (Wang et al., 2016). When there is a change in the ECM component, it will affect the stiffness and thus affect the development of fibroid. In the normal myometrium, homeostasis is achieved with the assistance of matrix metalloproteinases (MMP), which are involved in remodeling and degrading certain constituents of the extracellular matrices, such as collagen, which contributes to the stiffness of ECM (Curry and Osteen, 2003). Studies throughout the years have proved that the activity of MMP-2 is significantly higher in UF as compared to control (Bodner-Adler et al., 2004; Bogusiewicz et al., 2007a; Korompelis et al., 2015). MMP-2 mediates the degradation of collagen type IV and other components of ECM consequently, interfering with differentiation and proliferation (Moiseeva, 2001; Bogusiewicz et al., 2007b). On the other hand, TIMP-2 plays an opposite role to MMP-2 by inhibiting the protease activity in tissues undergoing remodeling of the ECM (Edelstein, 2017), thus a balance between MMP-2 and TIMP-2 plays a key role in maintaining the proper development and metabolism of the ECM. The ability of LD in reducing MMP-2 and increasing TIMP-2 expression, therefore, suggests that it may play a role in the protection of the uterus, and may also play a preventive and treatment role in UF.
In addition, when the UF animal model was being treated with LD the BCL-2 expression in the uterine tissue was significantly decreased with an increase in Bax mRNA expression, thus showing the apoptotic potential of LD in the treatment of UF (Li et al., 2013). LD was also proposed to reduce the size of the uterine in UF by suppressing angiogenesis via the reduction of VEGF expression and the downregulation of HIF-1α. The level of estradiol and progesterone, which are associated with the pathogenesis of UF, was also lowered with high-dose LD (Wang et al., 2020). Regardless of the promising effect of Lizhong decoction as proposed by the literatures, it is worth noting that the literatures do not provide the full botanical taxonomic names for the individual herb present in the formulation, while the common names given can be misidentified with the presence of various species and family available for the same herb as with the name provided.
Polyherbal Sparganii rhizoma (Sparganium stoloniferum (Buch.-Ham. Ex Graebn.) Buch.-Ham. Ex Juz [Typhaceae]) and Curcumae rhizoma (Curcuma phaeocaulis Valeton [Zingiberaceae] Herb Combination in Uterine Fibroids
Another well-studied traditional Chinese herb is Sparganii rhizoma, which is one of the most frequently prescribed herbs and Curcumae rhizoma. There were studies that have been conducted on the effect of this herb pair on UF rats. When Sparganii rhizoma was studied in mice, it was observed that there was a significant decrease in the fibroblast growth factor-1 (FGF-1) and VEGF level, suggesting that Sparganii rhizoma may have an effect on angiogenesis (Sun et al., 2011). On the other hand, studies have shown and proven the antiangiogenic activity of Curcumae rhizoma essential oil and also its ability in inhibiting cell proliferation. In addition, as mentioned previously that MMP-2 was significantly increased in leiomyoma cells, Curcumae rhizoma was also reported to inhibit the expression of MMP-2 which could also contribute to its use in the treatment of UF (Chen et al., 2011). The essential oil from Curcumae rhizoma was also able to induce apoptosis by decreasing the level of BCL-2 and at the same time inhibit the phosphorylation of the AKT/NF-κB pathway (Chen et al., 2013).
When both of these herbs are used together (known as CRSR, CR:SR = 1:1) in the treatment of UF, the uterine mass was found to be decreased significantly with a significantly lowered progesterone and estradiol level. Moreover, CRSR was found to affect various ECM-associated genes, which may be responsible for its ability to decrease uterine mass (Yu et al., 2019). In line with the hypothesis that CRSR produces an effect on the ECM, another independent study reported a reduced expression of fibroblast activation protein, which is a collagen component of the ECM and TGF-b (Feng et al., 2021).
Future Prospect
At present, although conventional pharmacological agents used for uterine fibroid continue to exhibit numerous drawbacks, it is still regarded as the ultimate choice of treatment for patients who prefer a noninvasive approach. Natural herbs and botanical drug products which have been widely reported in vitro showed favorable outcomes in the treatment of uterine fibroid. However, there are certain limitations that hindered its application in clinical practice. It is important to take into consideration that many phytoconstituents have a low bioavailability when they are consumed orally as the body considered them xenobiotics (Karaś et al., 2017) and, thus, may produce a significantly different result as that demonstrated from in vitro studies. In addressing the bioavailability issue, several novel delivery systems incorporating natural herbs or compounds have been developed (Zheng et al., 2019; Wang et al., 2021). Accordingly, more clinical studies are needed to determine their safety and efficacy in a well-designed clinical trial before they can be introduced and incorporated into clinical guidelines for the treatment or prevention of uterine fibroids.
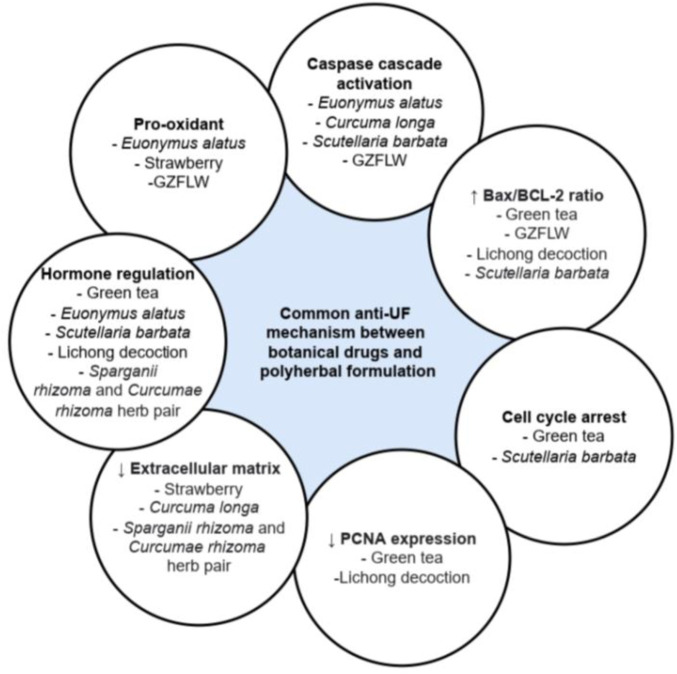
Among the botanical drugs and polyherbal formulations reviewed, only Curcuma longa (Ali and Ali, 2013; Sukonthanonta, 2015), Camellia sinensis (Roshdy et al., 2013), and GZFLW (Chen et al., 2014) have been investigated in clinical trials on their effect against UF. Among these, the clinical studies against Curcuma longa were limited without the presence of a negative control group, while a systematic review of randomized clinical trials conducted on GZFLW revealed that the trials have a high risk of bias, with no description of the allocation concealment of the participants. Hence, a well-designed clinical trial should be conducted in the future before these botanical drugs can be recommended for the treatment of UF. In addition, different botanical drugs may exert anti-UF activity via the same mechanism, as shown in Figure 3. Thus, combining the different botanical drugs to promote synergistic effect via the same or different pathway can be considered and investigated. It is also possible for future studies to investigate the efficacy and safety of using botanical drugs and conventional pharmacological agents in combination, which may allow the dose of the pharmacological agents to be reduced, subsequently reducing the adverse effects experienced by the patients. The inclusion of only articles acquired from online platform and in English only presents limitation in this review. However, most of the recently published articles that fit the inclusion and exclusion criteria were all reviewed comprehensively and included in the present review.
FIGURE 3.
Common anti-uterine fibroid mechanism between botanical drugs and polyherbal formulation.
Conclusion
The use of botanical drugs as a part of treatment has been a practice since ancient times, and it is being imposed in this era with the urge of evaluating their mechanism of action and their associated safety profile. Many drugs available in the market are derived from plants and play an important role in today’s modern medicine. The ideal pharmacological agent for UF should be effective and affordable, with a short treatment duration and minimal adverse effects. Although pharmacological treatment and intervention are already present in the market, they are far behind from achieving a balanced state and have brought the treatment of UF to a rough path. Botanical drugs are always preferrable by many patients due to their affordability and they are associated with lesser side effects. Therefore, researchers have been investigating various botanical drugs that could be used as an alternative for UF. As UF remains a significant health condition for many women all over the world, the discovery of an effective, safe, and less costly treatment could greatly benefit the society. However, a validated testing protocol is needed to standardize the active constituents in the botanical drug products for use as an anti-UF treatment in ensuring a reproducible therapeutic effect.
Acknowledgments
We would like to thank the Director General of Health Malaysia for his permission to publish this article.
Author Contributions
MS involved in original draft preparation, review, and editing. VY prepared the original draft. MR conceptualized, reviewed, and edited the manuscript. MA, SC, and DK were involved in reviewing.
Funding
This work was supported by the UCSI University Research Excellence and Innovation Grant (REIG) with project code REIG-FPS-2022/005.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMP, bone morphogenetic proteins; ECM, extracellular matrix; ER, estrogen receptor; FOXO, forkhead box O; HuLM, human leiomyoma; IGF-I, insulin-like growth factor-I; MMP, metalloproteinase; NF-κB, nuclear factor kappa B; PKC, protein kinase C; PPARγ, peroxisome proliferator-activated receptor-gamma; TGF, transforming growth factor; TIMP, tissue inhibitor of metalloproteinase; TSC2, tuberous sclerosis complex 2; VEGF, vascular endothelial growth factor.
References
- Aarts J. W., Nieboer T. E., Johnson N., Tavender E., Garry R., Mol B. W. J., et al. (2015). Surgical Approach to Hysterectomy for Benign Gynaecological Disease. Cochrane Database Syst. Rev. 8(3) CD003677. 10.1002/14651858.CD003677.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N., Gupta S., Mukhtar H. (2000). Green Tea Polyphenol Epigallocatechin-3-Gallate Differentially Modulates Nuclear Factor kappaB in Cancer Cells versus Normal Cells. Arch. Biochem. Biophys. 376, 338–346. 10.1006/ABBI.2000.1742 [DOI] [PubMed] [Google Scholar]
- Ahmed R. S., Liu G., Renzetti A., Farshi P., Yang H., Soave C., et al. (2016). Biological and Mechanistic Characterization of Novel Prodrugs of Green Tea Polyphenol Epigallocatechin Gallate Analogs in Human Leiomyoma Cell Lines. J. Cell. Biochem. 117, 2357–2369. 10.1002/jcb.25533 [DOI] [PubMed] [Google Scholar]
- Al-Hendy A., Salama S. A. (2006). Catechol-O-methyltransferase Polymorphism Is Associated with Increased Uterine Leiomyoma Risk in Different Ethnic Groups. J. Soc. Gynecol. Investig. 13, 136–144. 10.1016/J.JSGI.2005.10.007 [DOI] [PubMed] [Google Scholar]
- Al-Hendy A., Venturella R., Arjona Ferreira J. C., Li Y., Wagman R. B., Lukes A. S. (2021). Liberty Randomized Withdrawal Study: 2-year Efficacy and Safety of Relugolix Combination Therapy in Women with Heavy Menstrual Bleeding Associated with Uterine Fibroids. Fertil. Steril. 116, e2. 10.1016/j.fertnstert.2021.07.014 [DOI] [PubMed] [Google Scholar]
- Ali A. F. M., Ali L. (2013). Curcumin a new modality for treatment of uterine myoma. J. Am. Sci. 1545–1003. Available at: http://www.jofamericanscience.orghttp//www.americanscience.org.33 (Accessed October 8, 2021). [Google Scholar]
- Ando T., Kato H., Furui T., Morishige K. I., Goshima S., Matsuo M. (2018). Uterine Smooth Muscle Tumours with Hyperintense Area on T1 Weighted Images: Differentiation between Leiomyosarcomas and Leiomyomas. Br. J. Radiol. 91, 20170767. 10.1259/bjr.20170767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan A. A., Gold L. I., Mittal K., Suen T. C., Belitskaya-Levy I., Tang M. S., et al. (2005). Gene Expression Studies Provide Clues to the Pathogenesis of Uterine Leiomyoma: New Evidence and a Systematic Review. Hum. Reprod. 20, 852–863. 10.1093/HUMREP/DEH698 [DOI] [PubMed] [Google Scholar]
- Atkinson C., Lampe J. W., Scholes D., Chen C., Wähälä K., Schwartz S. M. (2006). Lignan and Isoflavone Excretion in Relation to Uterine Fibroids: A Case-Control Study of Young to Middle-Aged Women in the United States. Am. J. Clin. Nutr. 84, 587–593. 10.1093/ajcn/84.3.587 [DOI] [PubMed] [Google Scholar]
- Baird D. D., Dunson D. B., Hill M. C., Cousins D., Schectman J. M. (2003). High Cumulative Incidence of Uterine Leiomyoma in Black and White Women: Ultrasound Evidence. Am. J. Obstet. Gynecol. 188, 100–107. 10.1067/mob.2003.99 [DOI] [PubMed] [Google Scholar]
- Baird D. D., Travlos G., Wilson R., Dunson D. B., Hill M. C., D'Aloisio A. A., et al. (2009). Uterine Leiomyomata in Relation to Insulin-like Growth Factor-I, Insulin, and Diabetes. Epidemiology 20, 604–610. 10.1097/EDE.0b013e31819d8d3f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H., Sin T. K., Zhang G. (2018). Activin A Induces Leiomyoma Cell Proliferation, Extracellular Matrix (ECM) Accumulation and Myofibroblastic Transformation of Myometrial Cells via P38 MAPK. Biochem. Biophys. Res. Commun. 504, 447–453. 10.1016/J.BBRC.2018.08.171 [DOI] [PubMed] [Google Scholar]
- Benaglia L., Cardellicchio L., Filippi F., Paffoni A., Vercellini P., Somigliana E., et al. (2014). The Rapid Growth of Fibroids during Early Pregnancy. PLoS One 9, e85933. 10.1371/journal.pone.0085933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodner-Adler B., Bodner K., Kimberger O., Czerwenka K., Leodolter S., Mayerhofer K. (2004). Expression of Matrix Metalloproteinases in Patients with Uterine Smooth Muscle Tumors: An Immunohistochemical Analysis of MMP-1 and MMP-2 Protein Expression in Leiomyoma, Uterine Smooth Muscle Tumor of Uncertain Malignant Potential, and Leiomyosarcoma. J. Soc. Gynecol. Investig. 11, 182–186. 10.1016/j.jsgi.2003.09.004 [DOI] [PubMed] [Google Scholar]
- Bogusiewicz M., Stryjecka-Zimmer M., Postawski K., Jakimiuk A. J., Rechberger T. (2007a). Activity of Matrix Metalloproteinase-2 and -9 and Contents of Their Tissue Inhibitors in Uterine Leiomyoma and Corresponding Myometrium. Gynecol. Endocrinol. 23, 541–546. 10.1080/09513590701557416 [DOI] [PubMed] [Google Scholar]
- Bogusiewicz M., Stryjecka-Zimmer M., Rechberger T. (2007b). Activity of Matrix Metalloproteinases -2 and -9 (MMP-2 and MMP-9) and Content of Their Tissue Inhibitors in Endometrial Cancer-Aa Preliminary Study. Ginekol. Pol. 78, 366–372. [PubMed] [Google Scholar]
- Brito L. G., Candido-Dos-Reis F. J., Magario F. A., Sabino-De-Freitas M. M. (2011). Effect of the Aromatase Inhibitor Anastrozole on Uterine and Leiomyoma Doppler Blood Flow in Patients Scheduled for Hysterectomy: A Pilot Study. Ultrasound Obstet. Gynecol. 40, 119–120. 10.1002/uog.10145 [DOI] [PubMed] [Google Scholar]
- Cardozo E. R., Clark A. D., Banks N. K., Henne M. B., Stegmann B. J., Segars J. H. (2012). The Estimated Annual Cost of Uterine Leiomyomata in the United States. Am. J. Obstet. Gynecol. 206, 211–9. 10.1016/j.ajog.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Lu Y., Gao M., Wu J., Wang A., Shi R. (2011). Anti-angiogenesis Effect of Essential Oil from Curcuma Zedoaria In Vitro and In Vivo . J. Ethnopharmacol. 133, 220–226. 10.1016/j.jep.2010.09.031 [DOI] [PubMed] [Google Scholar]
- Chen C. C., Chen Y., Hsi Y. T., Chang C. S., Huang L. F., Ho C. T., et al. (2013). Chemical Constituents and Anticancer Activity of Curcuma Zedoaria Roscoe Essential Oil against Non-small Cell Lung Carcinoma Cells In Vitro and In Vivo . J. Agric. Food Chem. 61, 11418–11427. 10.1021/jf4026184 [DOI] [PubMed] [Google Scholar]
- Chen N. N., Han M., Yang H., Yang G. Y., Wang Y. Y., Wu X. K., et al. (2014). Chinese Herbal Medicine Guizhi Fuling Formula for Treatment of Uterine Fibroids: A Systematic Review of Randomised Clinical Trials. BMC Complement. Altern. Med. 14, 2. 10.1186/1472-6882-14-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Li Y., Wang Z., McCulloch P., Hu L., Chen W., et al. (2018). Evaluation of High-Intensity Focused Ultrasound Ablation for Uterine Fibroids: an IDEAL Prospective Exploration Study. BJOG 125, 354–364. 10.1111/1471-0528.14689 [DOI] [PubMed] [Google Scholar]
- Cheng I. C., Li R. K., Leung G. P., Li S. L., Kong M., Lao L. X., et al. (2019). Application of UPLC-MS/MS to Simultaneously Detect Four Bioactive Compounds in the Tumour-Shrinking Decoction (FM1523) for Uterine Fibroids Treatment. Phytochem. Anal. 30, 447–455. 10.1002/PCA.2827 [DOI] [PubMed] [Google Scholar]
- Ciavattini A., Di Giuseppe J., Stortoni P., Montik N., Giannubilo S. R., Litta P., et al. (2013). Uterine Fibroids: Pathogenesis and Interactions with Endometrium and Endomyometrial Junction. Obstetrics Gynecol. Int. 2013, 1–11. 10.1155/2013/173184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciavattini A., Delli Carpini G., Clemente N., Moriconi L., Gentili C., Di Giuseppe J. (2016). Growth Trend of Small Uterine Fibroids and Human Chorionic Gonadotropin Serum Levels in Early Pregnancy: an Observational Study. Fertil. Steril. 105, 1255–1260. 10.1016/J.FERTNSTERT.2016.01.032 [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov. (2014). Study of Tumor-Shrinking Decoction (TSD) to Treat Symptomatic Uterine Fibroids. U.S. Natl. Libr. Med. Available at: https://clinicaltrials.gov/ct2/show/NCT02189083 (Accessed October 8, 2021). [Google Scholar]
- Curry T. E., Osteen K. G. (2003). The Matrix Metalloproteinase System: Changes, Regulation, and Impact throughout the Ovarian and Uterine Reproductive Cycle. Endocr. Rev. 24, 428–465. 10.1210/er.2002-0005 [DOI] [PubMed] [Google Scholar]
- De Milliano I., Van Hattum D., Ket J. C. F., Huirne J. A. F., Hehenkamp W. J. K. (2017). Endometrial Changes during Ulipristal Acetate Use: A Systematic Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 214, 56–64. 10.1016/j.ejogrb.2017.04.042 [DOI] [PubMed] [Google Scholar]
- Dhamangaonkar P. C., Anuradha K., Saxena A. (2015). Levonorgestrel Intrauterine System (Mirena): An Emerging Tool for Conservative Treatment of Abnormal Uterine Bleeding. J. Midlife. Health 6, 26–30. 10.4103/0976-7800.153615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman K. (2014). Ayurvedic Intervention in the Management of Uterine Fibroids: A Case Series. Ayu 35, 303–308. 10.4103/0974-8520.153750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiVasta A. D., Laufer M. R., Gordon C. M. (2007). Bone Density in Adolescents Treated with a GnRH Agonist and Add-Back Therapy for Endometriosis. J. Pediatr. Adolesc. Gynecol. 20, 293–297. 10.1016/j.jpag.2007.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnez J., Tomaszewski J., Vázquez F., Bouchard P., Lemieszczuk B., Baró F., et al. (2012). Ulipristal Acetate versus Leuprolide Acetate for Uterine Fibroids. N. Engl. J. Med. 366, 421–432. 10.1056/nejmoa1103180 [DOI] [PubMed] [Google Scholar]
- Edelstein C. L. (2017). “Chapter Six - Biomarkers in Acute Kidney Injury,” in. Biomarkers of Kidney Disease 1. 241–315. 10.1016/B978-0-12-803014-1.00006-6 [DOI] [Google Scholar]
- Ekanem E., Talaulikar V. (2021). Medical Therapy for Fibroids: What Next for Ulipristal Acetate? Adv. Ther. 38, 137–148. 10.1007/S12325-020-01555-Z/TABLES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Osta H., Circu M. L. (2016). “Mitochondrial ROS and Apoptosis,” in Mitochondrial Mechanisms of Degeneration and Repair in Parkinson’s Disease. Editor Buhlman L. (Cham: Springer; ), 1–23. 10.1007/978-3-319-42139-1_1 [DOI] [Google Scholar]
- Eze-Steven P. (2019). Histopathological Investigations of Curcuma Longa (Turmeric) and Zingiber Officinale (Ginger) on Rats with Monosodium Glutamate-Induced Leiomyoma. J. Exp. Res. 7, 9–15. [Google Scholar]
- FDA. FDA Approves New Option to Treat Heavy Menstrual Bleeding Associated with Fibroids in Women | FDA (2020). U.S. Food Drug Adm. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-new-option-treat-heavy-menstrual-bleeding-associated-fibroids-women (Accessed May 1, 2022). [Google Scholar]
- Feng Y., Zhao Y., Li Y., Peng T., Kuang Y., Shi X., et al. (2021). Inhibition of Fibroblast Activation in Uterine Leiomyoma by Components of Rhizoma Curcumae and Rhizoma Sparganii. Front. Public Health 9. 650022. 10.3389/fpubh.2021.650022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flake G. P., Andersen J., Dixon D. (2003). Etiology and Pathogenesis of Uterine Leiomyomas: A Review. Environ. Health Perspect. 111, 1037–1054. 10.1289/ehp.5787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Fan Y., Fan W., Lv Y., Ai S., Yu C. (2020). Efficacy and Safety of Traditional Chinese Herbal Formula Combined with Western Medicine for Uterine Fibroid: A Protocol for Systematic Review and Meta-Analysis. Med. Baltim. 99, e22039. 10.1097/MD.0000000000022039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampieri F., Islam M. S., Greco S., Gasparrini M., Forbes Hernandez T. Y., Delli Carpini G., et al. (2019). Romina: A Powerful Strawberry with In Vitro Efficacy against Uterine Leiomyoma Cells. J. Cell. Physiol. 234, 7622–7633. 10.1002/jcp.27524 [DOI] [PubMed] [Google Scholar]
- Gorny K. R., Woodrum D. A., Brown D. L., Henrichsen T. L., Weaver A. L., Amrami K. K., et al. (2011). Magnetic Resonance-Guided Focused Ultrasound of Uterine Leiomyomas: Review of a 12-month Outcome of 130 Clinical Patients. J. Vasc. Interv. Radiol. 22, 857–864. 10.1016/J.JVIR.2011.01.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorieva V., Chen-Mok M., Tarasova M., Mikhailov A. (2003). Use of a Levonorgestrel-Releasing Intrauterine System to Treat Bleeding Related to Uterine Leiomyomas. Fertil. Steril. 79, 1194–1198. 10.1016/S0015-0282(03)00175-4 [DOI] [PubMed] [Google Scholar]
- Gupta J. K., Sinha A., Lumsden M. A., Hickey M. (2012). Uterine Artery Embolization for Symptomatic Uterine Fibroids. Cochrane Database Syst. Rev. 16, CD005073. 10.1002/14651858.CD005073.pub3 [DOI] [PubMed] [Google Scholar]
- Han Y., Zou S. E., Long Q. Q., Zhang S. F. (2013). The Incidence and Characteristics of Uterine Bleeding during Postoperative GnRH Agonist Treatment Combined with Estrogen-Progestogen Add-Back Therapy in Endometriosis Patients of Reproductive Age. Int. J. Clin. Exp. Med. 6, 583–588. [PMC free article] [PubMed] [Google Scholar]
- Hassan Z. K., Daghestani M. H. (2012). Curcumin Effect on MMPs and TIMPs Genes in a Breast Cancer Cell Line. Asian pac. J. Cancer Prev. 13, 3259–3264. 10.7314/APJCP.2012.13.7.3259 [DOI] [PubMed] [Google Scholar]
- Hervé F., Katty A., Isabelle Q., Céline S. (2018). Impact of Uterine Fibroids on Quality of Life: a National Cross-Sectional Survey. Eur. J. Obstetrics Gynecol. Reproductive Biol. 229, 32–37. 10.1016/j.ejogrb.2018.07.032 [DOI] [PubMed] [Google Scholar]
- Horvath L. G., Henshall S. M., Kench J. G., Turner J. J., Golovsky D., Brenner P. C., et al. (2004). Loss of BMP2, Smad8, and Smad4 Expression in Prostate Cancer Progression. Prostate 59, 234–242. 10.1002/PROS.10361 [DOI] [PubMed] [Google Scholar]
- Houston K. D., Copland J. A., Broaddus R. R., Gottardis M. M., Fischer S. M., Walker C. L. (2003). Inhibition of Proliferation and Estrogen Receptor Signaling by Peroxisome Proliferator-Activated Receptor Gamma Ligands in Uterine Leiomyoma. Cancer Res. 63, 1221–1227. [PubMed] [Google Scholar]
- Islam M. S., Protic O., Giannubilo S. R., Toti P., Tranquilli A. L., Petraglia F., et al. (2013). Uterine Leiomyoma: Available Medical Treatments and New Possible Therapeutic Options. J. Clin. Endocrinol. Metab. 98, 921–934. 10.1210/jc.2012-3237 [DOI] [PubMed] [Google Scholar]
- Islam M. S., Catherino W. H., Protic O., Janjusevic M., Gray P. C., Giannubilo S. R., et al. (2014). Role of Activin-A and Myostatin and Their Signaling Pathway in Human Myometrial and Leiomyoma Cell Function. J. Clin. Endocrinol. Metab. 99, E775–E785. 10.1210/JC.2013-2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. S., Giampieri F., Janjusevic M., Gasparrini M., Forbes-Hernandez T. Y., Mazzoni L., et al. (2017). An Anthocyanin Rich Strawberry Extract Induces Apoptosis and ROS while Decreases Glycolysis and Fibrosis in Human Uterine Leiomyoma Cells. Oncotarget 8, 23575–23587. 10.18632/oncotarget.15333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Shen Q., Chen M., Wang Y., Zhou Q., Zhu X., et al. (2014). Levonorgestrel-releasing Intrauterine System Use in Premenopausal Women with Symptomatic Uterine Leiomyoma: A Systematic Review. Steroids 86, 69–78. 10.1016/j.steroids.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Jun F., Yamin L., Xinli X., Zhe L., Min Z., Bo Z., et al. (2012). Uterine Artery Embolization versus Surgery for Symptomatic Uterine Fibroids: A Randomized Controlled Trial and a Meta-Analysis of the Literature. Arch. Gynecol. Obstet. 285, 1407–1413. 10.1007/s00404-011-2065-9 [DOI] [PubMed] [Google Scholar]
- Karaś M., Jakubczyk A., Szymanowska U., Złotek U., Zielińska E. (2017). Digestion and Bioavailability of Bioactive Phytochemicals. Int. J. Food Sci. Technol. 52, 291–305. 10.1111/IJFS.13323 [DOI] [Google Scholar]
- Khan K. N., Kitajima M., Hiraki K., Fujishita A., Nakashima M., Ishimaru T., et al. (2010). Cell Proliferation Effect of GnRH Agonist on Pathological Lesions of Women with Endometriosis, Adenomyosis and Uterine Myoma. Hum. Reprod. 25, 2878–2890. 10.1093/humrep/deq240 [DOI] [PubMed] [Google Scholar]
- Khorrami A., Sharif Bagheri M., Tavallaei M., Gharechahi J. (2017). The Functional Significance of 14-3-3 Proteins in Cancer: Focus on Lung Cancer. Horm. Mol. Biol. Clin. Investig. 32. 10.1515/hmbci-2017-0032 [DOI] [PubMed] [Google Scholar]
- Kim C. H., Kim D. I., Kwon C. N., Kang S. K., Jin U. H., Suh S. J., et al. (2006). Euonymus Alatus (Thunb.) Sieb Induces Apoptosis via Mitochondrial Pathway as Prooxidant in Human Uterine Leiomyomal Smooth Muscle Cells. Int. J. Gynecol. Cancer 16, 843–848. 10.1111/J.1525-1438.2006.00524.X [DOI] [PubMed] [Google Scholar]
- Kim K. W., Jin U. H., Kim D. I., Lee T. K., Kim M. S., Oh M. J., et al. (2008). Antiproliferative Effect of Scutellaria Barbata D. Don. On Cultured Human Uterine Leiomyoma Cells by Down-Regulation of the Expression of Bcl-2 Protein. Phytother. Res. 22, 583–590. 10.1002/PTR.1996 [DOI] [PubMed] [Google Scholar]
- Kongnyuy E. J., Wiysonge C. S. (2014). Interventions to Reduce Haemorrhage during Myomectomy for Fibroids. Cochrane Database Syst. Rev. 15, CD005355. 10.1002/14651858.CD005355.pub5 [DOI] [PubMed] [Google Scholar]
- Korompelis P., Piperi C., Adamopoulos C., Dalagiorgou G., Korkolopoulou P., Sepsa A., et al. (2015). Expression of Vascular Endothelial Factor-A, Gelatinases (MMP-2, MMP-9) and TIMP-1 in Uterine Leiomyomas. Clin. Chem. Lab. Med. 53, 1415–1424. 10.1515/cclm-2014-0798 [DOI] [PubMed] [Google Scholar]
- Kulshrestha V., Kriplani A., Agarwal N., Sareen N., Garg P., Hari S., et al. (2013). Low Dose Mifepristone in Medical Management of Uterine Leiomyoma - an Experience from a Tertiary Care Hospital from North India. Indian J. Med. Res. 137, 1154–1162. [PMC free article] [PubMed] [Google Scholar]
- Laping N. J., Everitt J. I., Frazier K. S., Burgert M., Portis M. J., Cadacio C., et al. (2007). Tumor-specific Efficacy of Transforming Growth Factor-Beta RI Inhibition in Eker Rats. Clin. Cancer Res. 13, 3087–3099. 10.1158/1078-0432.CCR-06-1811 [DOI] [PubMed] [Google Scholar]
- Lee T. K., Cho H. L., Kim D. I., Lee Y. C., Kim C. H. (2004a). Scutellaria Barbata D. Don Induces C-Fos Gene Expression in Human Uterine Leiomyomal Cells by Activating Beta2-Adrenergic Receptors. Int. J. Gynecol. Cancer 14, 526–531. 10.1111/J.1048-891X.2004.014315.X [DOI] [PubMed] [Google Scholar]
- Lee T. K., Kim D. I., Han J. Y., Kim C. H. (2004b). Inhibitory Effects of Scutellaria Barbata D. Don. And Euonymus Alatus Sieb. on Aromatase Activity of Human Leiomyomal Cells. Immunopharmacol. Immunotoxicol. 26, 315–327. 10.1081/IPH-200026840 [DOI] [PubMed] [Google Scholar]
- Lee T. K., Kim D. I., Song Y. L., Lee Y. C., Kim H. M., Kim C. H. (2004c). Differential Inhibition of Scutellaria Barbata D. Don (Lamiaceae) on HCG-Promoted Proliferation of Cultured Uterine Leiomyomal and Myometrial Smooth Muscle Cells. Immunopharmacol. Immunotoxicol. 26, 329–342. 10.1081/IPH-200026841 [DOI] [PubMed] [Google Scholar]
- Lee T. K., Lee D. K., Kim D. I., Lee Y. C., Chang Y. C., Kim C. H. (2004d). Inhibitory Effects of Scutellaria Barbata D. Don on Human Uterine Leiomyomal Smooth Muscle Cell Proliferation through Cell Cycle Analysis. Int. Immunopharmacol. 4, 447–454. 10.1016/J.INTIMP.2003.12.012 [DOI] [PubMed] [Google Scholar]
- Lee T. K., Lee J. Y., Kim D. I., Lee Y. C., Kim C. H. (2005). Differential Regulation of Protein Kinase C Activity by Modulating Factors and Euonymus Alatus (Thunb.) Sieb in Human Myometrial and Uterine Leiomyomal Smooth Muscle Cells. Int. J. Gynecol. Cancer 15, 349–358. 10.1111/J.1525-1438.2005.15228.X [DOI] [PubMed] [Google Scholar]
- Lee T. K., Lee Y. J., Kim D. I., Kim H. M., Chang Y. C., Kim C. H. (2006). Pharmacological Activity in Growth Inhibition and Apoptosis of Cultured Human Leiomyomal Cells of Tropical Plant Scutellaria Barbata D. Don (Lamiaceae). Environ. Toxicol. Pharmacol. 21, 70–79. 10.1016/J.ETAP.2005.07.015 [DOI] [PubMed] [Google Scholar]
- Lee M. J., Yun B. S., Seong S. J., Kim M. L., Jung Y. W., Kim M. K., et al. (2017). Uterine Fibroid Shrinkage after Short-Term Use of Selective Progesterone Receptor Modulator or Gonadotropin-Releasing Hormone Agonist. Obstet. Gynecol. Sci. 60, 69–73. 10.5468/ogs.2017.60.1.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. M., Choi E. S., Ha E., Ji K. Y., Shin S. J., Jung J. (2019). Gyejibongnyeong-hwan (Gui Zhi Fu Ling Wan) Ameliorates Human Uterine Myomas via Apoptosis. Front. Pharmacol. 10, 1105. 10.3389/fphar.2019.01105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lethaby A., Vollenhoven B. (2015). Fibroids (Uterine Myomatosis, Leiomyomas). BMJ Clin. Evid. 2015. 0814. [PMC free article] [PubMed] [Google Scholar]
- Lethaby A., Puscasiu L., Vollenhoven B. (2017). Preoperative Medical Therapy before Surgery for Uterine Fibroids. Cochrane Database Syst. Rev. 11. CD000547. 10.1002/14651858.CD000547.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Chiang T. C., Richard-Davis G., Barrett J. C., Mclachlan J. A. (2003). DNA Hypomethylation and Imbalanced Expression of DNA Methyltransferases (DNMT1, 3A, and 3B) in Human Uterine Leiomyoma. Gynecol. Oncol. 90, 123–130. 10.1016/S0090-8258(03)00194-X [DOI] [PubMed] [Google Scholar]
- Li D., Zhang Y., Han H., Geng J., Xie X., Zheng J., et al. (2012). Effect of Lichong Decoction on Expression of IGF-I and Proliferating Cell Nuclear Antigen mRNA in Rat Model of Uterine Leiomyoma. J. Tradit. Chin. Med. 32, 636–640. 10.1016/s0254-6272(13)60084-9 [DOI] [PubMed] [Google Scholar]
- Li D., Xu X., Qian R., Geng J., Zhang Y., Xie X., et al. (2013). Effect of Lichong Decoction on Expression of Bcl-2 and Bcl-2-Associated X Protein mRNAs in Hysteromyoma Model Rat. J. Tradit. Chin. Med. 33, 238–242. 10.1016/s0254-6272(13)60132-6 [DOI] [PubMed] [Google Scholar]
- Li H., Wu H., Zhang H., Li Y., Li S., Hou Q., et al. (2017). Identification of Curcumin-Inhibited Extracellular Matrix Receptors in Non-small Cell Lung Cancer A549 Cells by RNA Sequencing. Tumour Biol. 39, 1010428317705334. 10.1177/1010428317705334 [DOI] [PubMed] [Google Scholar]
- Li Z.-L., Huang T.-Y., Ho Y., Shih Y.-J., Chen Y.-R., Tang H.-Y., et al. (2020). “Herbal Medicine in Uterine Fibroid,” in Fibroids. Editor Abduljabbar H. (London: IntechOpen; ). 10.5772/INTECHOPEN.94101 [DOI] [Google Scholar]
- Lin X., Bai D., Wei Z., Zhang Y., Huang Y., Deng H., et al. (2019). Curcumin Attenuates Oxidative Stress in RAW264.7 Cells by Increasing the Activity of Antioxidant Enzymes and Activating the Nrf2-Keap1 Pathway. PLoS One 14, e0216711. 10.1371/journal.pone.0216711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. J., Liu P. Q., Lin D. J., Xiao R. Z., Huang M., Li X. D., et al. (2007). Downregulation of Cyclooxygenase-2 Expression and Activation of Caspase-3 Are Involved in Peroxisome Proliferator-Activated Receptor-Gamma Agonists Induced Apoptosis in Human Monocyte Leukemia Cells In Vitro . Ann. Hematol. 86, 173–183. 10.1007/S00277-006-0205-2 [DOI] [PubMed] [Google Scholar]
- Liu J. P., Yang H., Xia Y., Cardini F. (2013). Herbal Preparations for Uterine Fibroids. Cochrane Database Syst. Rev. 2013. CD005292. 10.1002/14651858.CD005292.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz M., Paul F., Moobed M., Baumann G., Zimmermann B. F., Stangl K., et al. (2014). The Activity of Catechol-O-Methyltransferase (COMT) Is Not Impaired by High Doses of Epigallocatechin-3-Gallate (EGCG) In Vivo . Eur. J. Pharmacol. 740, 645–651. 10.1016/J.EJPHAR.2014.06.014 [DOI] [PubMed] [Google Scholar]
- Lv J., Zhu X., Dong K., Lin Y., Hu Y., Zhu C. (2008). Reduced Expression of 14-3-3 Gamma in Uterine Leiomyoma as Identified by Proteomics. Fertil. Steril. 90, 1892–1898. 10.1016/j.fertnstert.2007.08.039 [DOI] [PubMed] [Google Scholar]
- Malik M., Mendoza M., Payson M., Catherino W. H. (2009). Curcumin, a Nutritional Supplement with Antineoplastic Activity, Enhances Leiomyoma Cell Apoptosis and Decreases Fibronectin Expression. Fertil. Steril. 91, 2177–2184. 10.1016/j.fertnstert.2008.03.045 [DOI] [PubMed] [Google Scholar]
- Mansilla S. F., de la Vega M. B., Calzetta N. L., Siri S. O., Gottifredi V. (2020). Cdk-independent and Pcna-dependent Functions of P21 in Dna Replication. Genes (Basel) 11. 593. 10.3390/genes11060593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham A., Keam S. J. (2019). Camrelizumab: First Global Approval. Drugs 79, 1355–1361. 10.1007/S40265-019-01105-010.1007/s40265-019-01167-0 [DOI] [PubMed] [Google Scholar]
- Marshall L. M., Spiegelman D., Goldman M. B., Manson J. E., Colditz G. A., Barbieri R. L., et al. (1998). A Prospective Study of Reproductive Factors and Oral Contraceptive Use in Relation to the Risk of Uterine Leiomyomata. Fertil. Steril. 70, 432–439. 10.1016/S0015-0282(98)00208-8 [DOI] [PubMed] [Google Scholar]
- Maruo T., Ohara N., Matsuo H., Xu Q., Chen W., Sitruk-Ware R., et al. (2007). Effects of Levonorgestrel-Releasing IUS and Progesterone Receptor Modulator PRM CDB-2914 on Uterine Leiomyomas. Contraception 75, S99–S103. 10.1016/j.contraception.2007.01.025 [DOI] [PubMed] [Google Scholar]
- Matsuo H. (2004). Prediction of the Change in Bone Mineral Density Induced by Gonadotropin-Releasing Hormone Agonist Treatment for Endometriosis. Fertil. Steril. 81, 149–153. 10.1016/j.fertnstert.2003.05.022 [DOI] [PubMed] [Google Scholar]
- Mesquita F. S., Dyer S. N., Heinrich D. A., Bulun S. E., Marsh E. E., Nowak R. A. (2010). Reactive Oxygen Species Mediate Mitogenic Growth Factor Signaling Pathways in Human Leiomyoma Smooth Muscle Cells. Biol. Reprod. 82, 341–351. 10.1095/biolreprod.108.075887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A., Chakrabarti J., Banerji A., Chatterjee A., Das B. R. (2006). Curcumin, a Potential Inhibitor of MMP-2 in Human Laryngeal Squamous Carcinoma Cells HEp2. J. Environ. Pathol. Toxicol. Oncol. 25, 679–690. 10.1615/jenvironpatholtoxicoloncol.v25.i4.70 [DOI] [PubMed] [Google Scholar]
- Moiseeva E. P. (2001). Adhesion Receptors of Vascular Smooth Muscle Cells and Their Functions. Cardiovasc. Res. 52, 372–386. 10.1016/S0008-6363(01)00399-6 [DOI] [PubMed] [Google Scholar]
- Morrison D. K. (2009). The 14-3-3 Proteins: Integrators of Diverse Signaling Cues that Impact Cell Fate and Cancer Development. Trends Cell Biol. 19, 16–23. 10.1016/J.TCB.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J. G., Cooper K. G., Khaund A., Murray L. S., Murray G. D., Wu O., et al. (2011). Randomised Comparison of Uterine Artery Embolisation (UAE) with Surgical Treatment in Patients with Symptomatic Uterine Fibroids (REST Trial): 5-Year Results. BJOG 118, 936–944. 10.1111/j.1471-0528.2011.02952.x [DOI] [PubMed] [Google Scholar]
- Nam D. H., Ramachandran S., Song D. K., Kwon K. Y., Jeon D. S., Shin S. J., et al. (2007). Growth Inhibition and Apoptosis Induced in Human Leiomyoma Cells by Treatment with the PPAR Gamma Ligand Ciglitizone. Mol. Hum. Reprod. 13, 829–836. 10.1093/MOLEHR/GAM071 [DOI] [PubMed] [Google Scholar]
- Nierth-Simpson E. N., Martin M. M., Chiang T. C., Melnik L. I., Rhodes L. V., Muir S. E., et al. (2009). Human Uterine Smooth Muscle and Leiomyoma Cells Differ in Their Rapid 17beta-Estradiol Signaling: Implications for Proliferation. Endocrinology 150, 2436–2445. 10.1210/EN.2008-0224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar M., Laknaur A., Al-Hendy A., Yang Q. (2019). Myometrial Progesterone Hyper-Responsiveness Associated with Increased Risk of Human Uterine Fibroids. BMC Womens. Health 19, 92. 10.1186/s12905-019-0795-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Párrizas M., Saltiel A. R., LeRoith D. (1997). Insulin-like Growth Factor 1 Inhibits Apoptosis Using the Phosphatidylinositol 3′-Kinase and Mitogen-Activated Protein Kinase Pathways. J. Biol. Chem. 272, 154–161. 10.1074/JBC.272.1.154 [DOI] [PubMed] [Google Scholar]
- Panahi Y., Sahebkar A., Amiri M., Davoudi S. M., Beiraghdar F., Hoseininejad S. L., et al. (2012). Improvement of Sulphur Mustard-Induced Chronic Pruritus, Quality of Life and Antioxidant Status by Curcumin: Results of a Randomised, Double-Blind, Placebo-Controlled Trial. Br. J. Nutr. 108, 1272–1279. 10.1017/S0007114511006544 [DOI] [PubMed] [Google Scholar]
- Parsanezhad M. E., Azmoon M., Alborzi S., Rajaeefard A., Zarei A., Kazerooni T., et al. (2010). A Randomized, Controlled Clinical Trial Comparing the Effects of Aromatase Inhibitor (Letrozole) and Gonadotropin-Releasing Hormone Agonist (Triptorelin) on Uterine Leiomyoma Volume and Hormonal Status. Fertil. Steril. 93, 192–198. 10.1016/j.fertnstert.2008.09.064 [DOI] [PubMed] [Google Scholar]
- Pazzaglia E., Praet J., Vandromme J., Rozenberg S. (2017). Medical or Surgical Management of Fibroids? an Internet Survey of Gynecologists' Views. Maturitas 95, 6–10. 10.1016/j.maturitas.2016.10.012 [DOI] [PubMed] [Google Scholar]
- Pitter M. C., Simmonds C., Seshadri-Kreaden U., Hubert H. B. (2014). The Impact of Different Surgical Modalities for Hysterectomy on Satisfaction and Patient Reported Outcomes. Interact. J. Med. Res. 3, e11. 10.2196/ijmr.3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimundo N., Vanharanta S., Aaltonen L. A., Hovatta I., Suomalainen A. (2009). Downregulation of SRF-FOS-JUNB Pathway in Fumarate Hydratase Deficiency and in Uterine Leiomyomas. Oncogene 28, 1261–1273. 10.1038/onc.2008.472 [DOI] [PubMed] [Google Scholar]
- Ramachandran S., Kwon K. Y., Shin S. J., Kwon S. H., Cha S. D., Bae I., et al. (2008). Cyclin-dependent Kinase Inhibitor p27Kip1 Controls Growth and Cell Cycle Progression in Human Uterine Leiomyoma. J. Korean Med. Sci. 23, 667–673. 10.3346/JKMS.2008.23.4.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshdy E., Rajaratnam V., Maitra S., Sabry M., Allah A. S., Al-Hendy A. (2013). Treatment of Symptomatic Uterine Fibroids with Green Tea Extract: A Pilot Randomized Controlled Clinical Study. Int. J. Womens. Health 5, 477–486. 10.2147/IJWH.S41021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenberg S., Praet J., Pazzaglia E., Gilles C., Manigart Y., Vandromme J. (2017). The Use of Selective Progestin Receptor Modulators (SPRMs) and More Specifically Ulipristal Acetate in the Practice of Gynaecology. Aust. N. Z. J. Obstet. Gynaecol. 57, 393–399. 10.1111/ajo.12641 [DOI] [PubMed] [Google Scholar]
- Salama S. A., Ho S. L., Wang H. Q., Tenhunen J., Tilgmann C., Al-Hendy A. (2006). Hormonal Regulation of Catechol-O-Methyl Transferase Activity in Women with Uterine Leiomyomas. Fertil. Steril. 86, 259–262. 10.1016/J.FERTNSTERT.2005.12.049 [DOI] [PubMed] [Google Scholar]
- Sandberg E. M., Tummers F. H. M. P., Cohen S. L., van den Haak L., Dekkers O. M., Jansen F. W. (2018). Reintervention Risk and Quality of Life Outcomes after Uterine-Sparing Interventions for Fibroids: a Systematic Review and Meta-Analysis. Fertil. Steril. 109, 698–e1. 10.1016/j.fertnstert.2017.11.033 [DOI] [PubMed] [Google Scholar]
- Sayed G. H., Zakherah M. S., El-Nashar S. A., Shaaban M. M. (2011). A Randomized Clinical Trial of a Levonorgestrel-Releasing Intrauterine System and a Low-Dose Combined Oral Contraceptive for Fibroid-Related Menorrhagia. Int. J. Gynaecol. Obstet. 112, 126–130. 10.1016/j.ijgo.2010.08.009 [DOI] [PubMed] [Google Scholar]
- Schlaff W. D., Ackerman R. T., Al-Hendy A., Archer D. F., Barnhart K. T., Bradley L. D., et al. (2020). Elagolix for Heavy Menstrual Bleeding in Women with Uterine Fibroids. N. Engl. J. Med. 382, 328–340. 10.1056/NEJMOA1904351 [DOI] [PubMed] [Google Scholar]
- Schuler M., Bossy-Wetzel E., Goldstein J. C., Fitzgerald P., Green D. R. (2000). p53 Induces Apoptosis by Caspase Activation through Mitochondrial Cytochrome C Release. J. Biol. Chem. 275, 7337–7342. 10.1074/JBC.275.10.7337 [DOI] [PubMed] [Google Scholar]
- Shen Q., Ye W., Hu X., Zhao C., Zhou L., Zhu X. (2016). The Effects of Guizhi Fuling Capsule Drug Serum on Uterine Leiomyoma Cells and its Mechanism. Evidence-Based Complementary Altern. Med. 2016, 1–9. 10.1155/2016/2393640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng B., Song Y., Liu Y., Jiang C., Zhu X. (2020). Association between Vitamin D and Uterine Fibroids: A Study Protocol of an Open-Label, Randomised Controlled Trial. BMJ Open 10, e038709. 10.1136/bmjopen-2020-038709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota M., Kotani Y., Umemoto M., Tobiume T., Hoshiai H. (2012). Recurrence of Uterine Myoma after Laparoscopic Myomectomy: What Are the Risk Factors? Gynecol. Minim. Invasive Ther. 1, 34–36. 10.1016/j.gmit.2012.08.003 [DOI] [Google Scholar]
- Socolov D., Blidaru I., Tamba B., Miron N., Boiculese L., Socolov R. (2011). Levonorgestrel Releasing-Intrauterine System for the Treatment of Menorrhagia And/or Frequent Irregular Uterine Bleeding Associated with Uterine Leiomyoma. Eur. J. Contracept. Reprod. Health Care 16, 480–487. 10.3109/13625187.2011.614028 [DOI] [PubMed] [Google Scholar]
- Song H., Lu D., Navaratnam K., Shi G. (2013). Aromatase Inhibitors for Uterine Fibroids. Cochrane Database Syst. Rev. 2013. CD009505. 10.1002/14651858.CD009505.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart E. A., Gostout B., Rabinovici J., Kim H. S., Regan L., Tempany C. M. (2007). Sustained Relief of Leiomyoma Symptoms by Using Focused Ultrasound Surgery. Obstet. Gynecol. 110, 279–287. 10.1097/01.AOG.0000275283.39475.F6 [DOI] [PubMed] [Google Scholar]
- Stewart E. A., Nicholson W. K., Bradley L., Borah B. J. (2013). The Burden of Uterine Fibroids for African-American Women: Results of a National Survey. J. Womens Health (Larchmt) 22, 807–816. 10.1089/jwh.2013.4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukonthanonta A. (2015). Curcumin in Reduction Size of Myoma Uteri. Thai J. Obstet. Gynaecol. 23, 165–171. 10.14456/tjog.2015.14 [DOI] [Google Scholar]
- Sun J., Wang S., Wei Y. H. (2011). Reproductive Toxicity of Rhizoma Sparganii (Sparganium Stoloniferum Buch.-Ham.) in Mice: Mechanisms of Anti-angiogenesis and Anti-estrogen Pharmacologic Activities. J. Ethnopharmacol. 137, 1498–1503. 10.1016/j.jep.2011.08.026 [DOI] [PubMed] [Google Scholar]
- Supriono S., Pratomo B., Kriestian M. (2018). Effects of Curcumin against Matrix Metalloproteinase-2 (MMP-2) and Tissue Inhibitor Metalloproteinase-2 (TIMP-2) Serum Level on Rat Model of Liver Fibrosis Resolution Process. Indones. J. Gastroenterol. Hepatol. Dig. Endosc. 19, 10–15. 10.24871/191201810-15 [DOI] [Google Scholar]
- Taniguchi K., Karin M. (2018). NF-κB, Inflammation, Immunity and Cancer: Coming of Age. Nat. Rev. Immunol. 18, 309–324. 10.1038/NRI.2017.142 [DOI] [PubMed] [Google Scholar]
- Tsibris J. C., Porter K. B., Jazayeri A., Tzimas G., Nau H., Huang H., et al. (1999). Human Uterine Leiomyomata Express Higher Levels of Peroxisome Proliferator-Activated Receptor Gamma, Retinoid X Receptor Alpha, and All-Trans Retinoic Acid Than Myometrium. Cancer Res. 59, 5737–5744. [PubMed] [Google Scholar]
- Tsuiji K., Takeda T., Li B., Wakabayashi A., Kondo A., Kimura T., et al. (2011). Inhibitory Effect of Curcumin on Uterine Leiomyoma Cell Proliferation. Gynecol. Endocrinol. 27, 512–517. 10.3109/09513590.2010.507287 [DOI] [PubMed] [Google Scholar]
- Vishnubalaji R., Yue S., Alfayez M., Kassem M., Liu F. F., Aldahmash A., et al. (2016). Bone Morphogenetic Protein 2 (BMP2) Induces Growth Suppression and Enhances Chemosensitivity of Human Colon Cancer Cells. Cancer Cell Int. 16, 77–12. 10.1186/S12935-016-0355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Huang H., Liu D., Fang S., Xian Y., Zhou J., et al. (2012). Evaluation of 14-3-3 Protein Family Levels and Associated Receptor Expression of Estrogen and Progesterone in Human Uterine Leiomyomas. Gynecol. Endocrinol. 28, 665–668. 10.3109/09513590.2012.650768 [DOI] [PubMed] [Google Scholar]
- Wang Y., Li D., Xu X., Qian R., Zhang Y., Huang Y., et al. (2016). Lichong Decoction Reduces Matrix Metalloproteinases-2 Expression but Increases Tissue Inhibitors of Matrix Metalloproteinases-2 Expression in a Rat Model of Uterine Leiomyoma. J. Tradit. Chin. Med. 36, 479–485. 10.1016/s0254-6272(16)30065-6 [DOI] [PubMed] [Google Scholar]
- Wang W., Zhang W., Li D., Qian R., Zhu L., Liu Y., et al. (2020). Lichong Decoction Inhibits Micro-angiogenesis by Reducing the Expressions of Hypoxia Inducible Factor-1α and Vascular Endothelial Growth Factor in Hysteromyoma Mouse Model. J. Tradit. Chin. Med. 40, 928–937. 10.19852/j.cnki.jtcm.2020.06.005 [DOI] [PubMed] [Google Scholar]
- Wang L., Huang X., Jing H., Ma C., Wang H. (2021). Bilosomes as Effective Delivery Systems to Improve the Gastrointestinal Stability and Bioavailability of Epigallocatechin Gallate (EGCG). Food Res. Int. 149, 110631. 10.1016/j.foodres.2021.110631 [DOI] [PubMed] [Google Scholar]
- Westphal D., Kluck R. M., Dewson G. (2014). Building Blocks of the Apoptotic Pore: How Bax and Bak Are Activated and Oligomerize during Apoptosis. Cell Death Differ. 21, 196–205. 10.1038/cdd.2013.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W. S. F. (2020). Focused Ultrasound Surgery for Uterine Fibroids: Potential Impact on Fertility and Pregnancy Outcome-A Review. J. Gynecol. 5, 1–6. 10.23880/oajg-16000203 [DOI] [Google Scholar]
- Xiao F., Li Q., Liang K., Zhao L., He B., Ji W., et al. (2012). Comparative Pharmacokinetics of Three Triterpene Acids in Rat Plasma after Oral Administration of Poria Extract and its Formulated Herbal Preparation: GuiZhi-FuLing Capsule. Fitoterapia 83, 117–124. 10.1016/J.FITOTE.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Yang C. S., Wang Z. Y. (1993). Tea and Cancer. J. Natl. Cancer Inst. 85, 1038–1049. 10.1093/JNCI/85.13.1038 [DOI] [PubMed] [Google Scholar]
- Yen H. R., Chen Y. Y., Huang T. P., Chang T. T., Tsao J. Y., Chen B. C., et al. (2015). Prescription Patterns of Chinese Herbal Products for Patients with Uterine Fibroid in Taiwan: A Nationwide Population-Based Study. J. Ethnopharmacol. 171, 223–230. 10.1016/j.jep.2015.05.038 [DOI] [PubMed] [Google Scholar]
- Yoo E. H., Lee P. I., Huh C. Y., Kim D. H., Lee B. S., Lee J. K., et al. (2007). Predictors of Leiomyoma Recurrence after Laparoscopic Myomectomy. J. Minim. Invasive Gynecol. 14, 690–697. 10.1016/j.jmig.2007.06.003 [DOI] [PubMed] [Google Scholar]
- Yu L., Saile K., Swartz C. D., He H., Zheng X., Kissling G. E., et al. (2008)., 14. Yu, 264–275. 10.2119/2007-0010110.2119/2007-00101.Yu Differential Expression of Receptor Tyrosine Kinases (RTKs) and IGF-I Pathway Activation in Human Uterine Leiomyomas Mol. Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. H., Zhao J. S., Zhao H., Peng T., Shen D. C., Xu Q. X., et al. (2019). Transcriptional Profiling of Uterine Leiomyoma Rats Treated by a Traditional Herb Pair, Curcumae Rhizoma and Sparganii Rhizoma . Braz J. Med. Biol. Res. 52, e8132. 10.1590/1414-431x20198132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Al-Hendy M., Richard-Davis G., Montgomery-Rice V., Rajaratnam V., Al-Hendy A. (2010a). Antiproliferative and Proapoptotic Effects of Epigallocatechin Gallate on Human Leiomyoma Cells. Fertil. Steril. 94, 1887–1893. 10.1016/j.fertnstert.2009.08.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Al-Hendy M., Richard-Davis G., Montgomery-Rice V., Sharan C., Rajaratnam V., et al. (2010b). Green Tea Extract Inhibits Proliferation of Uterine Leiomyoma Cells In Vitro and in Nude Mice. Am. J. Obstet. Gynecol. 202, 289.e1–9. 10.1016/J.AJOG.2009.10.885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Rajaratnam V., Al-Hendy O., Halder S., Al-Hendy A. (2014). Green Tea Extract Inhibition of Human Leiomyoma Cell Proliferation Is Mediated via Catechol-O-Methyltransferase. Gynecol. Obstet. Invest. 78, 109–118. 10.1159/000363410 [DOI] [PubMed] [Google Scholar]
- Zheng B., Zhang X., Peng S., Julian McClements D. (2019). Impact of Curcumin Delivery System Format on Bioaccessibility: Nanocrystals, Nanoemulsion Droplets, and Natural Oil Bodies. Food Funct. 10, 4339–4349. 10.1039/C8FO02510J [DOI] [PubMed] [Google Scholar]