Abstract
Cell quotas of microcystin (QMCYST; femtomoles of MCYST per cell), protein, and chlorophyll a (Chl a), cell dry weight, and cell volume were measured over a range of growth rates in N-limited chemostat cultures of the toxic cyanobacterium Microcystis aeruginosa MASH 01-A19. There was a positive linear relationship between QMCYST and specific growth rate (μ), from which we propose a generalized model that enables QMCYST at any nutrient-limited growth rate to be predicted based on a single batch culture experiment. The model predicts QMCYST from μ, μmax (maximum specific growth rate), QMCYSTmax (maximum cell quota), and QMCYSTmin (minimum cell quota). Under the conditions examined in this study, we predict a QMCYSTmax of 0.129 fmol cell−1 at μmax and a QMCYSTmin of 0.050 fmol cell−1 at μ = 0. Net MCYST production rate (RMCYST) asymptotes to zero at μ = 0 and reaches a maximum of 0.155 fmol cell−1 day−1 at μmax. MCYST/dry weight ratio (milligrams per gram [dry weight]) increased linearly with μ, whereas the MCYST/protein ratio reached a maximum at intermediate μ. In contrast, the MCYST/Chl a ratio remained constant. Cell volume correlated negatively with μ, leading to an increase in intracellular MCYST concentration at high μ. Taken together, our results show that fast-growing cells of N-limited M. aeruginosa are smaller, are of lower mass, and have a higher intracellular MCYST quota and concentration than slow-growing cells. The data also highlight the importance of determining cell MCYST quotas, as potentially confusing interpretations can arise from determining MCYST content as a ratio to other cell components.
The microcystins (MCYSTs) are a group of cyclic heptapeptide toxins produced by several cyanobacterial species. Of the more than 60 MCYSTs characterized to date (19, 27, 29), most are potent inhibitors of protein phosphatases 1 and 2A from both plants and animals (17). One of the most common MCYST-producing cyanobacteria is the bloom-forming Microcystis aeruginosa (Kützing) Lemmermann. Due to the widespread distribution and potential toxicity of this species (toxic strains have been found worldwide), M. aeruginosa has been implicated in a number of animal-poisoning incidents (e.g., reference 7) and more recently in human fatalities (11, 23).
M. aeruginosa is a unicellular, colonial freshwater cyanobacterium which often forms blooms during warmer months in eutrophic lakes and reservoirs (37). For this reason, much research has been concerned with the environmental factors which lead to bloom formation and toxin production in this species. A wide range of batch culture studies have shown that the variables influencing MCYST content include trace metal supply (15), nitrogen (N) and phosphorus (P) (31), light and temperature (38), and pH (34). Comparative studies on MCYST production by M. aeruginosa in continuous culture, however, have been limited to examination of the effects of photon irradiance (35), N, P, and Fe3+ limitation (16, 36), and more recently P limitation (20). Despite this considerable pool of data concerning MCYST production, few studies (with the exception of the work carried out by Rapala and coworkers [25, 26]) have been able to quantitatively relate MCYST content to any growth determinant.
In a previous batch culture study, we presented data on the effect of N supply on the cellular production of MCYSTs (21). This work showed that the net specific rate of MCYST production was equal to the cell specific growth rate. The application of these findings to previously published batch culture studies suggested that the relationship held under a variety of culture conditions and that MCYST production was indirectly affected by environmental factors through their effects on cell division. A consequence of this linear relationship was that the cell quota of MCYST (QMCYST) should remain constant over a range of growth rates. However, QMCYST varied significantly, though inconsistently, throughout the growth cycle in separate batch culture experiments (21), suggesting a more complex relationship than predicted. Hence, in an attempt to specifically determine the relationships between growth rate, net rates of MCYST production, and QMCYST, we here report the results of a N-limited chemostat study using the same strain of M. aeruginosa MASH 01-A19 under growth conditions similar to those used in our previous batch culture study (21). MCYST data are expressed both as cell quotas and as a ratio to a number of biomass indicators (viz., protein, dry weight, and chlorophyll a [Chl a]) to emphasize the importance of cell quotas in determining cellular physiology of MCYST production.
MATERIALS AND METHODS
Organism and growth conditions.
M. aeruginosa MASH 01-A19 (3, 4) was provided by the CSIRO Marine Laboratories culture collection. Cells were grown in triplicate 500-ml continuous-culture vessels under constant illumination (40 ± 5 μmol of photons [PAR] m−2 s−1) using cool white fluorescent lights at 26 ± 1°C. Cultures were supplied with a continuous flow of sterile modified MLA medium (4) containing 0.2 mM NaNO3 (1/10 original concentration), 0.02 mM K2HPO4, and 3.0 mM 2-(N-cyclohexylamino)ethanesulfonic acid (pH 8.0) via a Gilson Multiplus 2 peristaltic pump. Previous batch culture studies had revealed that the concentration of NO3− used ensured N limitation (21). A single air pump provided constant airflow (through sterile, 0.45-μm-pore-size filters) to all cultures throughout the experiment. Cultures were grown at dilution rates ranging from 0.1 to 1.08 day−1 (as determined by flow rate). At steady state, dilution rate is equivalent to growth rate (24).
Estimation of μmax.
Triplicate batch cultures were used to estimate the maximum specific growth rate (μmax) of M. aeruginosa MASH 01-A19 grown under the same medium, temperature, and light conditions as chemostats. The specific cell division rate and specific rate of dry weight accumulation were determined from a simple first-order rate law after frequent sampling of cultures postinoculation.
Sampling and analysis.
To ensure steady-state conditions at each growth rate, the stability of culture populations was determined by cell counting and dry weight analysis. Once populations had stabilized at each growth rate (not more than 3% variation in cell concentrations or dry weight between four successive samplings), cultures were allowed to grow at steady state for at least five doubling times before sampling. Approximately 75 ml of each culture was removed for analysis of cell number, cell dry weight, MCYST content, cell volume, total cellular protein, and Chl a. Cell counting was done using a hemocytometer (Neubauer) after disruption of colonies in approximately 1.0 ml of culture by heating (80°C, 20 min), using the method of Humphries and Widjaja (10). The effect of heat treatment on cell volume was found to be minimal and consistent with minor volume changes noted by Porter and Jost (22) after collapsing gas vacuoles. For the determination of cell volume, the cells were concentrated by centrifugation (13,000 × g, 5 min), and photomicrographs of the cells and a standard scale (10 μm) were taken using an Olympus compound microscope fitted with a camera. Once developed, photomicrographs were digitally scanned and cross-sectional cell area was determined using NIH Image software (National Institutes of Health, Bethesda, Md.). The cross-sectional area of between 40 and 100 cells from each culture at each growth rate was determined and used to determine mean cell volume, assuming spherical cells. Dividing and nondividing cells were treated equally.
Dry weight was determined by collecting specific volumes of culture material on preweighed 47-mm-diameter Whatman GF/C filters and allowing them to dry overnight in a vacuum desiccator before reweighing. MCYSTs were then recovered by extracting the filters four times in 2.0 ml of 80% (vol/vol) methanol. The extracts were pooled and dried in vacuo using a SpeedVac Concentrator vacuum centrifuge (Savant). The dried material was redissolved in 2.0 ml of 80% (vol/vol) methanol prior to analysis for MCYSTs by using high-pressure liquid chromatography (HPLC). MCYSTs were separated on an Alltima C18 column (250 by 4.6 mm; Alltech), using a linear gradient of 20 to 35% (vol/vol) acetonitrile in 8 mM ammonium acetate, and detected by measuring the absorbance of eluant at 238 nm according to the method of Jones and Orr (12). MCYSTs were quantified by comparison with a MCYST-LR standard (Calbiochem), and all amounts are presented as MCYST-LR molar equivalents. Cell-free culture filtrates, collected at time of sampling, were also analyzed for MCYSTs by the same method. Filtrates of the culture medium were also analyzed for dissolved NO3− by conductivity, using anion-exchange HPLC, but none was detected throughout the experiment.
For the determination of total cellular protein, cells from 5 to 10 ml of culture were collected by centrifugation (13,000 × g, 5 min) and dried in vacuo. Cells were then resuspended in 250 μl of 0.5 M NaOH, heated to 70°C for 20 min, and centrifuged (13,000 × g, 5 min) to remove cell debris. Protein in the supernatant was estimated by the method of Lowry et al. (14) as adapted by Walsh et al. (39), using bovine serum albumin as a standard. Chl a was estimated in 80% (vol/vol) acetone extracts by the method of Arnon et al. (2) in all cultures at growth rates above 0.3 day−1. At growth rates 0.3 day−1 and below, Chl a in cells was below detection limits and could not be determined by this method.
Data analysis.
Where appropriate, data were statistically analyzed by regression analysis and analysis of variance using SPSS for Windows 10.0.5 (SPSS Inc.).
RESULTS
Steady-state growth.
As determined by consistent dry weight and cell concentrations, cultures were at steady state at each growth rate prior to sampling. Steady-state cell concentration significantly increased with increasing growth rate (P < 0.001), but cell dry weight decreased from 43.4 to 17.7 pg cell−1 (Table 1). Hence, steady-state biomass concentrations increased only slightly with increasing growth rates (P < 0.03) ranging from 50.1 to 61.9 mg liter−1 between the lowest and highest growth rates (0.10 to 1.08 day−1). The reduction in cell weight with increasing growth rate was associated with a decrease in cell volume of approximately fivefold, from 111 to 19.2 μm3 (Table 1).
TABLE 1.
Variation in cellular parameters, protein and Chl a in M. aeruginosa MASH 01-A19 in N-limited chemostats at different growth ratesa
| Growth rate (day−1) | Cellular changes
|
Protein content
|
Chl a content
|
||||
|---|---|---|---|---|---|---|---|
| Cells ml−1 (106) | Cell dry wt (pg cell−1) | Cell vol (μm3) | Quota (pg cell−1) | (mg g−1 [dry wt])−1 | Quota (pg cell−1) | (mg g−1 [dry wt]−1) | |
| 0.10 | 1.17 ± 0.10 | 43.4 ± 3.2 | 111 ± 49 | 6.7 ± 0.4 | 154 ± 3.0 | ND | ND |
| 0.13 | 1.36 ± 0.10 | 37.4 ± 3.5 | 99.8 ± 41 | 6.4 ± 0.3 | 173 ± 10 | ND | ND |
| 0.20 | 1.33 ± 0.02 | 36.0 ± 1.4 | 110 ± 48 | 7.6 ± 0.2 | 212 ± 6.0 | ND | ND |
| 0.23 | 1.38 ± 0.11 | 37.4 ± 1.2 | 96.4 ± 37 | 8.3 ± 0.3 | 221 ± 6.0 | ND | ND |
| 0.27 | 2.04 ± 0.10 | 33.0 ± 3.3 | 84.1 ± 32 | 8.0 ± 0.5 | 246 ± 8.0 | ND | ND |
| 0.45 | 2.70 ± 0.36 | 21.4 ± 3.3 | 61.7 ± 31 | 8.3 ± 0.6 | 397 ± 34 | 0.12 ± 0.004 | 5.64 ± 0.79 |
| 0.49 | 2.84 ± 0.34 | 19.9 ± 1.8 | 75.6 ± 29 | 6.3 ± 0.2 | 320 ± 23 | 0.15 ± 0.004 | 7.46 ± 0.61 |
| 0.59 | 4.64 ± 0.65 | 21.2 ± 0.5 | 89.4 ± 26 | 4.9 ± 0.3 | 231 ± 20 | 0.14 ± 0.010 | 6.71 ± 0.44 |
| 0.70 | 4.10 ± 0.69 | 20.2 ± 1.8 | 20.0 ± 4.9 | 5.8 ± 0.6 | 286 ± 15 | 0.18 ± 0.018 | 8.95 ± 0.64 |
| 0.96 | 3.72 ± 0.81 | 17.7 ± 0.7 | 19.2 ± 4.9 | 6.8 ± 0.5 | 387 ± 46 | 0.19 ± 0.013 | 11.0 ± 1.2 |
| 1.08 | 3.45 ± 0.14 | 18.0 ± 1.6 | 39.4 ± 14 | 8.1 ± 0.6 | 451 ± 30 | 0.22 ± 0.006 | 12.1 ± 0.83 |
Data presented are means of triplicate cultures ± SE except for cell volume; cell volumes are means from all cultures at each growth rate ± SD. All data show significant variation over the range of growth rates examined (P < 0.05). ND, not determined.
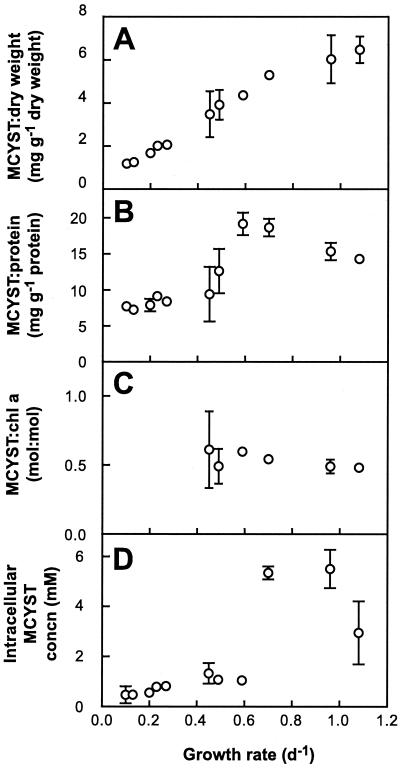
Cell protein quota showed no specific correlation with μ (Table 1). In contrast, protein expressed per unit of dry weight increased significantly from lowest to highest growth rates (P < 0.001); where Chl a was quantifiable, there was a significant increase in Chl a content with growth rate (P < 0.001) expressed both as cell quota and per unit of dry weight (Table 1). Chl a content was not determined at low growth rates (below detection limits), but the cultures were visibly more yellow, indicating low Chl a quotas in slower-growing cells.
MCYST analysis.
As described in our previous study, two MCYST peaks were determined in M. aeruginosa MASH 01-A19 by HPLC and liquid chromatography-mass spectrometry analysis (LC-MS) (21). The first of these was MCYST-LR; although it probably contains desmethyl isomers of MCYST-LR (21), it is expressed in MCYST-LR molar equivalents (assuming similar molar absorption coefficients). The second MCYST, giving the characteristic absorbance maximum at 238 nm but an uncharacteristic LC-MS spectrum with high fragmentation, has not been identified and was not included in the measurement of total MCYST. At all growth rates, this compound constituted less than 15% of total MCYST-LR equivalents.
Notably, MCYSTs were not detected in the extracellular medium of cultures at any growth rates. With a detection limit of 1.0 pmol on-column using our HPLC system, extracellular MCYST could not have exceeded a concentration of 5 nM and was therefore always less than 1% of total culture MCYST.
MCYST content and production.
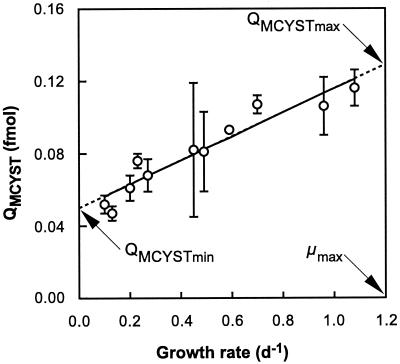
QMCYST ranged from 0.052 to 0.116 fmol cell−1 and showed a positive linear correlation with growth rate (r = 0.952) (Fig. 1). Extrapolation of the fitted regression suggests that QMCYST reaches a minimum value (QMCYSTmin) at μ = 0 and a maximum value (QMCYSTmax) at μmax (Fig. 1). The linear relationship between QMCYST and μ can be described in terms of the predicted QMCYSTmin and QMCYSTmax, and μmax as follows:
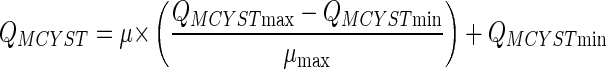 |
1 |
Since μmax cannot be achieved in chemostat cultures, this parameter was determined from analysis of separate batch culture data and estimated to be 1.2 day−1. Using this value, QMCYSTmin and QMCYSTmax were subsequently calculated from linear regression analysis to be 0.050 ± 0.004 (standard error [SE]) and 0.129 ± 0.006 (SE) fmol cell−1, respectively (Table 2).
FIG. 1.
Cellular QMCYST of M. aeruginosa grown in N-limited chemostats. Error bars represent standard errors of the means of triplicate chemostat cultures. The solid line shows cell quotas predicted from experimental growth rates (r = 0.952) using equation 1 (see text), and the dashed line represents the extrapolation of this relationship to μmax (where QMCYST = QMCYSTmax) and μ = 0 (where QMCYST = QMCYSTmin). d, day.
TABLE 2.
Derived MCYST production parameters for M. aeruginosa MASH 01-A19 grown in N-limited chemostatsa
| Measurement | QMCYSTmin | QMCYSTmax | RMCYSTmax |
|---|---|---|---|
| Cell quota basis | 0.050 fmol cell−1 | 0.129 fmol cell−1 | 0.155 fmol cell−1 day−1 |
| Dry wt basis | 0.71 mg g−1 (dry wt) | 7.6 mg g−1 (dry wt) | 9.12 mg g−1 (dry wt) day−1 |
QMCYSTmax and RMCYSTmax were calculated by using a μmax of 1.2 day−1 (estimated from batch culture experiments).
MCYST expressed per unit of dry weight increased significantly with increasing growth rate (Fig. 2A). However, because cell dry weight decreased with increasing growth rate (Table 1), the increase in MCYST/dry weight ratio was more than fivefold (1.18 to 6.47 mg g−1 [dry weight]), compared with the less than threefold increase for QMCYST. MCYST expressed per unit of protein was greater at high μ than low μ but reached a maximum at intermediate μ (Fig. 2B). MCYST normalized to Chl a was not significantly different over the growth rates examined (Fig. 2C), averaging a MCYST/Chl a ratio of 0.59 ± 0.03 (SE) on a mass (gram/gram) basis or 0.53 ± 0.02 (SE) on a molar basis.
FIG. 2.
MCYST expressed as a ratio to dry weight (A), to protein (B) to Chl a (C), and on an intracellular concentration basis (D) in M. aeruginosa grown in N-limited chemostats. Error bars represent the standard errors of the means of triplicate chemostats. d, day.
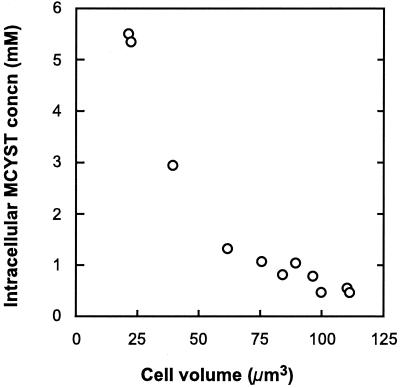
Intracellular MCYST concentration ranged from 0.47 ± 0.34 (SE) mM to 5.5 ± 0.77 (SE) mM over the growth rates examined (Fig. 2D). There was a strong negative correlation between intracellular MCYST concentration and cell volume (Fig. 3), with smaller cells containing significantly higher concentrations of MCYST (P < 0.001).
FIG. 3.
Intracellular MCYST concentration in cells of M. aeruginosa, grown in N-limited chemostats, as a function of cell volume.
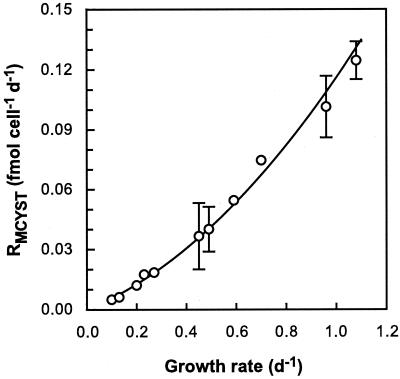
The net MCYST production rate (RMCYST) was determined from the product of μ and QMCYST. Minimum net rates of MCYST production were 0.005 ± 0.0005 fmol of MCYST cell−1 day−1 and 0.11 ± 0.002 mg g−1 (dry weight) day−1 at 0.1 day−1, and maximum net rates were 0.13 ± 0.01 fmol cell−1 day−1 and 6.9 ± 0.07 mg g−1 (dry weight) day−1 at 1.08 day−1. The net rates of MCYST production reported in previous studies for M. viridis TAC44 (0.175 mg g−1 [dry weight] day−1) and M. aeruginosa M228-12 (1.13 mg g−1 [dry weight] day−1) (40) and for M. aeruginosa UTEX 2388 (0.11 to 0.44 mg g−1 [dry weight] day−1) (20) fall within the range reported here. RMCYST shows a positive correlation with growth rate (Fig. 4) and can also be described in terms of QMCYSTmin, QMCYSTmax, μ, and μmax by the following equation:
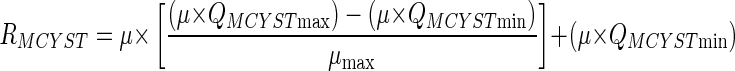 |
2 |
This relationship predicts that RMCYST is 0 in cells at stationary phase and reaches a maximum (RMCYSTmax) of 0.155 fmol cell−1 day−1 (9.1 mg g−1 [dry weight] day−1) at μmax (Table 2). The hyperbolic shape of the relationship (Fig. 4) results from QMCYSTmax/QMCYSTmin being greater than 1—the higher this ratio, the greater will be the curvature in the RMCYST-versus-μ plot.
FIG. 4.
RMCYST as a function of growth rate in M. aeruginosa grown in N-limited chemostats. Error bars represent standard errors of the means of triplicate cultures. The solid line shows net rates of MCYST production calculated using equation 2 (see the text) and the values in Table 2.
DISCUSSION
This study shows, for the first time, that the cellular MCYST content of N-limited M. aeruginosa can be predicted from growth rate, with faster-growing cells containing higher intracellular concentrations of MCYST. We believe that these results were achievable by ensuring that cultures were maintained in steady-state, nitrogen-limited growth conditions at all times. Our data also highlight the importance of determining cellular MCYST quotas in experiments examining MCYST content and production. Clearly there are difficulties in interpretation that may arise when MCYST content is measured as a ratio to another cell component that itself may be varying independently in response to a change in growth rate or experimental treatment. This is evidenced by our observations that with increasing growth rate, MCYST increased linearly as a ratio to cell dry weight, generally increased but reached a maximum at intermediate growth rate as a ratio to protein content, and yet was invariant as a ratio to Chl a.
The model put forward in equation 1 proposes that QMCYST at any growth rate is dependent on the constants QMCYSTmax, QMCYSTmin, and μmax in N-limited cultures (Table 2; Fig. 1). The parameters QMCYSTmax and QMCYSTmin determine a fixed range of cellular MCYST quotas. This implies that toxigenic strains will always contain, at least, a minimum QMCYST and that they will not exceed a maximum QMCYST determined by the nutrient saturated μmax (for the given temperature and light growth conditions). Although a growth rate of zero cannot be achieved in a chemostat, our predicted QMCYSTmin is very similar to that observed for batch cultures of this strain at stationary phase, where QMCYST remained stable for at least 2 weeks (21). In the same study, QMCYSTmax ranged from 0.13 to 0.16 fmol cell−1, again similar to the value predicted from this chemostat study (Table 2). Collectively, the data are consistent with the generalization that MCYST production is constitutive and that toxigenic strains remain so under a variety of growth conditions (33). In support of this conclusion, data from other researchers suggest that minimum and maximum cell quotas of MCYSTs exist in other strains, and even in different Microcystis spp. (34, 40). We also note that the maximum MCYST/dry weight ratio reported in this study (7.6 mg g−1 [dry weight]) is very similar to that found in late log-phase of the original strain MASH 01 (the parent culture) by Bolch et al. (5) (i.e., 7.24 mg g−1 [dry weight]), indicating a conserved process of toxin production in this strain for several years.
Böttcher et al. (6) recently found QMCYST to remain constant while μ increased with increasing irradiance in turbidostat experiments. These results may at first appear to contradict ours. However, light-limited turbidostats differ from chemostats in that μ at any irradiance is always nutrient saturated μmax, and therefore QMCYST always equals nutrient saturated QMCYSTmax. Thus, their findings suggest a constant QMCYSTmax while nutrient saturated μmax increases with increasing irradiance. In contrast, recent batch culture studies under nutrient-replete conditions over a range of temperatures revealed that QMCYSTmin decreased in response to increasing temperature (B. M. Long, unpublished data). Further work is needed to confirm the exact details of how QMCYSTmin or QMCYSTmax may vary in response to physical conditions limiting growth (temperature, irradiance, etc.).
In addition to describing the cell quota of MCYST, the constants QMCYSTmin, QMCYSTmax, and μmax also determine the net rate of MCYST production (equation 2). RMCYST is the product of QMCYST and μ, and as a consequence, equation 2 predicts no net MCYST production at μ = 0 (or stationary phase in batch culture). Also, RMCYST is constrained by the maximum cell division rate, as QMCYST will not exceed that which is achieved at μmax (i.e., RMCYSTmax = μmax × QMCYSTmax). This is inconsistent with the regression model advanced by Oh et al. (20), however, which predicted a net production of MCYST at μ = 0 (0.082 mg g−1 [dry weight] day−1). This implies that when cell division stops, MCYST production continues, resulting in increasingly toxic nondividing cells. We can find no other published data to support this proposition. In addition, Oh et al. (20) found that the MCYST/dry weight ratio correlated negatively with μ in P-limited chemostats. When MCYST data are expressed as a ratio to another cell constituent (e.g., protein) or group of constituents (e.g., dry weight) which may be under independent and varying cellular regulation in response to the limiting nutrient, it is very difficult to understand the cellular regulation of MCYST content and production. Cyanobacterial dry weight is affected differentially by N and P limitation (1), demonstrating that the physiological regulation of dry weight production is quite different under different nutrient limitations. We suggest that the observed differences between our findings and those of Oh et al. (20) may result from differential dry weight changes under N and P limitation. Hence, simple comparisons of MCYST/dry weight ratio data cannot be made between cultures grown under different nutrient limitations. The near absence of MCYST cell quotas from the existing literature makes comparison of our data with other studies almost impossible.
Shi et al. (30) found that MCYST was associated with the thylakoid membranes of M. aeruginosa PCC 7820, suggesting a close physical association between MCYSTs and the photosynthetic machinery of the cell. The constant ratio of MCYST to Chl a (1:2 [mol:mol] [Fig. 2C]) found in this study supports this contention and suggests that MCYST synthesis and or function could be linked to photosynthetic processes. The absence of reports of major perturbations in the photosynthetic activity of M. aeruginosa PCC 7806 after knocking out MCYST production (8), however, would suggest that MCYSTs are not essential in photosynthesis. Nevertheless, the MCYST synthetase knockout mutant of strain PCC 7806 was found to have slightly altered thylakoid structure and also to exhibit irregularities in the structure of gas vesicles (E. Dittmann and T. Börner, personal communication).
The decrease in cell size with increasing growth rate is consistent with cell volume variations reported previously for M. aeruginosa by Krüger and Eloff (13). The same authors suggest that cell size is a likely indicator of the physiological state of a cell, with stressed cells being larger. This supports the generally held view that MCYST production is greatest when conditions are favorable for growth (32, 33), as larger cells occur at lowest growth rates (Table 1).
Our finding that smaller cells contain more MCYST than larger ones (Fig. 3) may have implications for toxicity toward grazing zooplankton. Since some daphnids, though notably not all (18), are sensitive to toxic strains of M. aeruginosa (28), it is conceivable that zooplankton could ingest a greater number of smaller Microcystis spp. cells, thus receiving a considerably larger dose of toxin. This is speculation, however, and further work is required to determine the importance of cell size and toxin ingestion rates.
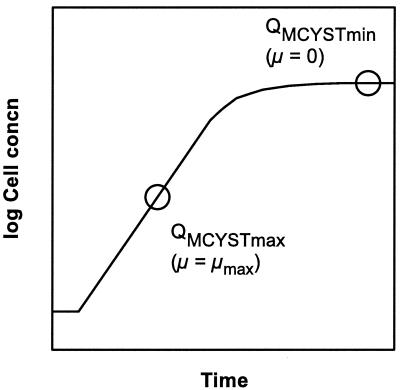
Given the observed relationship between μ and QMCYST, our model predicts that QMCYST can be determined for any value of μ if μmax, QMCYSTmax, and QMCYSTmin are known. Since obtaining these constants in a chemostat study is time-consuming, we suggest that a more practical approach can be made with less complicated apparatus. As QMCYSTmin represents QMCYST at nitrogen-limited stationary phase in a batch culture, and QMCYSTmax represents QMCYST during nitrogen-saturated logarithmic growth, these parameters can be determined from a single batch culture experiment (Fig. 5). The only caveat is that the initial nitrogen concentration in the batch culture medium must be sufficient to ensure nitrogen-saturated growth during logarithmic phase but not so high as to allow high biomass development to the point of self-shading (light limitation) or CO2 limitation; i.e., stationary phase must arise only due to nitrogen depletion.
FIG. 5.
Theory for the determination of the constants QMCYSTmax and QMCYSTmin from batch cultures. QMCYSTmax occurs shortly after inoculation when cells are in log phase (μ = μmax). QMCYSTmin will occur in batch cultures at stationary phase (μ = 0). The determination of cell quotas of MCYST at these times should permit the relationship between μ and QMCYST to be calculated according to equation 1 (see text).
Our findings quantitatively demonstrate that under N-limited growth, QMCYST in M. aeruginosa is a function of μ. As μ is controlled by cellular N quota (QN) under N-limited growth (9), this is consistent with QMCYST also being regulated by QN. Whether this is the case, or whether there is a more general relationship between QMCYST and growth limitation by any environmental factor, remain to be elucidated.
ACKNOWLEDGMENTS
We extend thanks to John Beardall for advice with chemostat cultures and constructive comments and to John Anderson, Nicole Morcom, Ingrid Chorus, and Peter Crouch for reading drafts of the manuscript. We also thank Seamus Ward for aid in statistical analysis of the data and Trevor Phillips for help with photography.
REFERENCES
- 1.Ahlgren G. Growth of Oscillatoria agardhii in chemostat culture 3. Simultaneous limitation of nitrogen and phosphorus. Br Phycol J. 1985;20:249–261. [Google Scholar]
- 2.Arnon D I, McSwain B D, Tsujimoto H Y, Wada K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974;357:231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- 3.Bolch C, Blackburn S, Neilan B, Grewe P. Genetic characterization of strains of cyanobacteria using PCR-RFLP of the cpcBA intergenic spacer and flanking regions. J Phycol. 1996;32:445–451. [Google Scholar]
- 4.Bolch C J S, Blackburn S I. Isolation and purification of Australian isolates of the toxic cyanobacterium Microcystis aeruginosa Kütz. J Appl Phycol. 1996;8:5–13. [Google Scholar]
- 5.Bolch C J S, Blackburn S I, Jones G J, Orr P T, Grewe P M. Plasmid content and distribution in the toxic cyanobacterial genus Microcystis Kützing ex Lemmermann (Cyanobacteria: Chroococcales) Phycologia. 1997;36:6–11. [Google Scholar]
- 6.Böttcher, G., I. Chorus, S. Ewald, T. Hinze, and N. Walz. Light-limited growth and microcystin content of Planktothrix agardhii and Microcystis aeruginosa in turbidostats. In I. Chorus (ed.), Cyanotoxins: occurrences, causes, consequences, in press. Springer Press, Berlin, Germany.
- 7.Carbis C R, Simons J A, Mitchell G F, Anderson J W, McCauley I. A biochemical profile for predicting the chronic exposure of sheep to Microcystis aeruginosa, an hepatotoxic species of blue-green alga. Res Vet Sci. 1994;57:310–316. doi: 10.1016/0034-5288(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 8.Dittmann E, Neilan B A, Erhard M, von Döhren H, Börner T. Insertional mutagenesis of a peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Mol Microbiol. 1997;26:779–787. doi: 10.1046/j.1365-2958.1997.6131982.x. [DOI] [PubMed] [Google Scholar]
- 9.Droop M R. Some thoughts on nutrient limitation in algae. J Phycol. 1973;9:264–272. [Google Scholar]
- 10.Humphries S E, Widjaja F. A simple method for separating cells of Microcystis aeruginosa for counting. Br Phycol J. 1979;14:313–316. [Google Scholar]
- 11.Jochimsen E M, Carmichael W W, An J S, Cardo D M, Cookson S T, Holmes C E, Antunes M B, de Melo Filho D A, Lyra T M, Barreto V S, Azevedo S M, Jarvis W R. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. N Engl J Med. 1998;338:873–878. doi: 10.1056/NEJM199803263381304. [DOI] [PubMed] [Google Scholar]
- 12.Jones G J, Orr P T. Release and degradation of microcystin following algicide treatment of a Microcystis aeruginosa bloom in a recreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Res. 1994;28:871–876. [Google Scholar]
- 13.Krüger G H J, Eloff J N. The effect of physico-chemical factors on growth relevant to the mass culture of axenic Microcystis. In: Carmichael W W, editor. The water environment—algal toxins and health. New York, N.Y: Plenum Press; 1981. pp. 193–222. [Google Scholar]
- 14.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Lukač M, Aegerter R. Influence of trace metals on growth and toxin production of Microcystis aeruginosa. Toxicon. 1993;31:293–305. doi: 10.1016/0041-0101(93)90147-b. [DOI] [PubMed] [Google Scholar]
- 16.Lyck S, Gjølme N, Utkilen H. Iron starvation increases toxicity of Microcystis aeruginosa CYA 228/1 (Chroococcales, Cyanophyceae) Phycologia. 1996;35:120–124. [Google Scholar]
- 17.MacKintosh C, Beattie K A, Klumpp S, Cohen P, Codd G A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990;264:187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- 18.Matveev V, Matveeva L, Jones G J. Study of the ability of Daphnia carinata King to control phytoplankton and resist cyanobacterial toxicity—implications for biomanipulation in Australia. Aust J Mar Freshw Res. 1994;45:889–904. [Google Scholar]
- 19.Namikoshi M, Yuan M, Sivonen K, Carmichael W W, Rinehart K L, Rouhiainen L, Sun F, Brittain S, Otsuki A. Seven new microcystins possessing two l-glutamic acid units, isolated from Anabaena sp. strain 186. Chem Res Toxicol. 1998;11:143–149. doi: 10.1021/tx970120t. [DOI] [PubMed] [Google Scholar]
- 20.Oh H-M, Lee S J, Jang M-H, Yoon B-D. Microcystin production by Microcystis aeruginosa in a phosphorus-limited chemostat. Appl Environ Microbiol. 2000;66:176–179. doi: 10.1128/aem.66.1.176-179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orr P T, Jones G J. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol Oceanogr. 1998;43:1604–1614. [Google Scholar]
- 22.Porter J, Jost M. Physiological effects of the presence and absence of gas vacuoles in the blue-green alga, Microcystis aeruginosa Kuetz. emend. Elenkin Arch Microbiol. 1976;110:225–231. doi: 10.1007/BF00690231. [DOI] [PubMed] [Google Scholar]
- 23.Pouria S, de Andrade A, Barbosa J, Cavalcanti R L, Barreto V T, Ward C J, Preiser W, Poon G K, Neild G H, Codd G A. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet. 1998;352:21–26. doi: 10.1016/s0140-6736(97)12285-1. [DOI] [PubMed] [Google Scholar]
- 24.Pritchard R H, Tempest D W. Growth: cells and populations. In: Mandelstam J, McQuillen K, Dawes I, editors. Biochemistry of bacterial growth. 3rd ed. Oxford, England: Blackwell Scientific Publications; 1982. pp. 99–123. [Google Scholar]
- 25.Rapala J, Sivonen K, Lyra C, Niemelä S I. Variation of microcystins, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimuli. Appl Environ Microbiol. 1997;63:2206–2212. doi: 10.1128/aem.63.6.2206-2212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rapala J, Sivonen K. Assessment of environmental conditions that favor hepatotoxic and neurotoxic Anabaena spp. strains cultured under light limitation at different temperatures. Microb Ecol. 1998;36:181–192. doi: 10.1007/s002489900105. [DOI] [PubMed] [Google Scholar]
- 27.Rinehart K L, Namikoshi M, Choi B W. Structure and biosynthesis of toxins from blue-green algae (cyanobacteria) J Appl Phycol. 1994;6:159–176. [Google Scholar]
- 28.Rohrlack T, Dittmann E, Henning M, Börner T, Kohl J G. Role of microcystins in poisoning and food ingestion inhibition of Daphnia galeata caused by the cyanobacterium Microcystis aeruginosa. Appl Environ Microbiol. 1999;65:737–739. doi: 10.1128/aem.65.2.737-739.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sano T, Kaya K. Two new (E)-2-amino-2-butenoic acid (Dhb)-containing microcystins isolated from Oscillatoria agardhii. Tetrahedron. 1998;54:463–470. [Google Scholar]
- 30.Shi L, Carmichael W W, Miller I. Immuno-gold localization of hepatotoxins in cyanobacterial cells. Arch Microbiol. 1995;163:7–15. doi: 10.1007/BF00262197. [DOI] [PubMed] [Google Scholar]
- 31.Sivonen K. Effects of light, temperature, nitrate, orthophosphate, and bacteria on growth of and hepatotoxin production by Oscillatoria agardhii strains. Appl Environ Microbiol. 1990;56:2658–2666. doi: 10.1128/aem.56.9.2658-2666.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sivonen K. Cyanobacterial toxins and toxin production. Phycologia. 1996;35:12–24. [Google Scholar]
- 33.Sivonen K, Jones G J. Cyanobacterial toxins. In: Chorus I, Bartram J, editors. Toxic cyanobacteria in water—a guide to their public health consequences, monitoring and management. E & FN Spon. 1999. pp. 41–111. London, England. [Google Scholar]
- 34.Song L, Sano T, Li R, Watanabe M M, Liu Y, Kaya K. Microcystin production of Microcystis viridis (cyanobacteria) under different culture conditions. Phycol Res. 1998;46:19–23. [Google Scholar]
- 35.Utkilen H, Gjølme N. Toxin production by Microcystis aeruginosa as a function of light in continuous cultures and its ecological significance. Appl Environ Microbiol. 1992;58:1321–1325. doi: 10.1128/aem.58.4.1321-1325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Utkilen H, Gjølme N. Iron-stimulated toxin production in Microcystis aeruginosa. Appl Environ Microbiol. 1995;61:797–800. doi: 10.1128/aem.61.2.797-800.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Utkilen H, Skulberg O M, Underdal B, Gjølme N, Skulberg R, Kotai J. The rise and fall of a toxigenic population of Microcystis aeruginosa (cyanophyceae/cyanobacteria): a decade of observations in Lake Akersvatnet, Norway. Phycologia. 1996;35:189–197. [Google Scholar]
- 38.van der Westhuizen A J, Eloff J N. Effect of temperature and light on the toxicity and growth of the blue-green alga Microcystis aeruginosa (UV-006) Planta. 1985;163:55–59. doi: 10.1007/BF00395897. [DOI] [PubMed] [Google Scholar]
- 39.Walsh K, Jones G J, Dunstan R H. Effect of irradiance on fatty acid, carotenoid, total protein composition and growth of Microcystis aeruginosa. Phytochemistry. 1997;44:817–824. [Google Scholar]
- 40.Watanabe M F, Harada K, Matsuura K, Watanabe M, Suzuki M. Heptapeptide toxin production during the batch culture of two Microcystis species (Cyanobacteria) J Appl Phycol. 1989;1:161–165. [Google Scholar]