Abstract
Kinetoplastid protozoa regulate their gene expression primarily through control of mRNA degradation and translation. We describe here the degradation of three reporter mRNAs in Trypanosoma brucei. One mRNA had the 3′-untranslated region (3′-UTR) from the developmentally regulated EP1 mRNA, which is abundant in the procyclic (tsetse fly) form of the parasite but is almost undetectable in the bloodstream form. This untranslated region includes a 26 nt U-rich sequence that causes extreme RNA instability in the bloodstream form. The two other RNAs, which are not developmentally regulated, had either the actin 3′-UTR, or a version of the EP1 sequence lacking the 26 nt bloodstream-form instability element. All RNAs had poly(A) tails ∼200 nt long, in both bloodstream and procyclic forms. Degradation of the two constitutively expressed mRNAs involved deadenylation and degradation by both 5′→3′ and 3′→5′ exonucleases. In contrast, in bloodstream forms, the 3′-end of the RNA bearing the bloodstream-form instability element disappeared very rapidly after transcription inhibition and partially deadenylated intermediates were not seen. The instability element may cause extremely rapid deadenylation, or it may be targeted by an endonuclease.
INTRODUCTION
African trypanosomes cause sleeping sickness in man and various diseases of domestic animals in sub-Saharan Africa. They multiply in the blood and tissue fluids of the mammal, and are transmitted from one host to the next by tsetse flies. Considerable adaptations in parasite morphology, surface composition and metabolism are required in order to survive in these disparate environments. For example, the bloodstream form of Trypanosoma brucei escapes the humoral immune response by antigenic variation, effected by frequent changes of the variant surface glycoprotein (VSG) (1). When the parasites transform to procyclic forms in the midgut of the tsetse fly, the VSG is replaced by proline-rich, acidic and repetitive EP/GPEET proteins. No VSG mRNA is detectable in procyclic forms and only minute amounts of EP mRNA have been found in bloodstream forms (2–4). Control of gene expression is crucial to parasite survival: expression of the EP/GPEET proteins in bloodstream forms would allow development of an effective immune response, and expression of developmentally regulated metabolic enzymes in the ‘wrong’ life-cycle stage can lead to cell death (5).
Nearly all trypanosomatid genes are found in very long polycistronic transcription units (6). There is no reproducible evidence for RNA polymerase II promoters or for developmental regulation of polymerase II transcription, and cotranscribed genes can have completely different expression patterns (3). After transcription, individual mRNAs are excised by a 5′ trans splicing reaction, which involves the addition of a short capped leader to the 5′-end of the RNA, and a coupled polyadenylation step (7–9). Thus capping of mRNAs is independent of the RNA polymerase used for transcription.
As a consequence of polycistronic transcription, nearly all developmental regulation of gene expression in trypanosomatids must be effected post-transcriptionally. The 5′-untranslated sequences do not play a major role in regulation, although weak 5′-splice sites result in constitutively low levels of mRNA and 5′-untranslated regions are involved in cell-cycle control of mRNA levels in the insect trypanosomatid Crithidia fasciculata (10). Most regulation is effected by sequences in the 3′-untranslated regions (3′-UTRs). This type of regulation was first shown for the VSG and EP genes (reviewed in 2,4,11,12) but in the meantime many examples of similarly regulated polymerase II transcripts have been reported (see for example 13–20). Both mRNA abundance and translation can be strongly regulated. The few relevant studies available have indicated that mRNA levels are controlled through regulated degradation (18,21–23).
Although nearly all protein-coding genes are transcribed by polymerase II, the VSG and EP/GPEET loci are transcribed by RNA polymerase I. Polymerase I transcription is about 10 times more active than transcription by RNA polymerase II (24), and initiation and/or elongation at the EP/GPEET and VSG loci is—in contrast to polymerase II—regulated by alterations in chromatin (for examples see 2,3,24–26). The EP/GPEET gene family consists of the EP1 (gene encoding the EP1 protein, previously known as PARP or procyclin), EP2, EP3 and GPEET genes (27). Transcription of the loci is only 10-fold less active in bloodstream forms than in procyclic forms (24), whereas the protein products are regulated at least 1000-fold. Post-transcriptional regulation is therefore extremely important (2,12). The 3′-UTRs of the EP and GPEET genes are quite divergent with the exception of a conserved 26mer and a 16mer stem–loop. The 16mer enhances translation in procyclic forms (28) and is not involved in developmental regulation. The sequences responsible for developmental regulation of mRNA abundance and translation are contained within the 26mer, an interrupted polyuridine tract (12,22,29), which is probably in a single-stranded conformation (30). The 26mer causes extreme instability of the EP/GPEET mRNAs and represses their translation in bloodstream forms.
In both mammalian cells and the yeast Saccharomyces cerevisiae, sequences in the 3′-UTRs of mRNAs play a decisive role in regulation of mRNA decay. One important pathway of regulated mRNA degradation in yeast involves deadenylation followed by decapping and degradation by 5′→3′ exonucleases (31,32). In mutants of the 5′→3′ degradation pathway, degradation from the 3′-end can be observed (reviewed in 32). Although the basal components of the RNA degradation machinery are conserved from yeast to mammals (for examples see 33–35), mammals have some additional mechanisms. Degradation of several mRNAs is initiated by cleavage of a specific sequence within the 3′-UTR (36,37 and references therein), and proteins that bind such sequences can inhibit the degradation. Destabilising AU-rich elements (AREs) are found in the 3′-UTRs of many mammalian mRNAs involved in cell growth and differentiation (32). The mechanism of degradation promoted by these elements is controversial (32): it could proceed either via deadenylation and decapping (38,39) or be initiated by cleavage of the ARE by an endonuclease (40–42).
Despite the vital importance of RNA stability in the survival and pathogenicity of trypanosomes and Leishmanias, virtually nothing is known about the mechanisms of mRNA degradation in these organisms. Previous studies in trypanosomes have been restricted to reports showing that various inhibitors of protein synthesis increase the abundance of EP mRNAs in bloodstream forms (43–45). These studies have generally been interpreted as implying that degradation is mediated by an unstable protein. However, because very few mRNAs have been studied in this way, it is not clear whether the postulated unstable protein is specific for particular mRNAs, or part of the general mRNA degradation machinery. The mechanisms of translation control are completely unknown. To understand regulated mRNA turnover in trypanosomes, and to define the effector molecules, it is necessary to define the pathways involved in constitutive and regulated degradation. In this paper we compare the pathway of EP1 3′-UTR-mediated mRNA degradation with that mediated by a 3′-UTR from the actin (ACT) mRNA, which is not developmentally regulated.
MATERIALS AND METHODS
Trypanosome culture and transfection
Bloodstream and procyclic trypanosomes (MiTat 1.2) were cultured and transfected, and CAT assays performed as described (22,46). Neomycin-resistant bloodstream trypanosomes expressing T7 polymerase were generated by transfecting 107 trypanosomes with 10 µg plasmid DNA linearised within β-tubulin targeting sequence, and selected with 15 µg/ml G418. These trypanosomes were transfected with plasmids that were linearised within an rRNA targeting sequence, selected with 15 µg/ml hygromycin and cloned by limiting dilution.
Plasmid constructs
The plasmid encoding T7 polymerase was kindly provided by E. Wirtz (pLew13) (47). Plasmids for stable transfection were based on pLew82 (47). These carried a T7 promoter, and chloramphenicol acetyltransferase (CAT) and hygromycin resistance (HYG) cassettes. Downstream of the CAT gene were placed various 3′-UTRs, followed by a region from 5′ of the actin (ACT) gene that ensures trans splicing of the HYG mRNA and polyadenylation of the preceding CAT mRNA (22). The initial plasmids carried the following 3′-UTRs downstream of the CAT gene: CAT-EP1 (pHD 774) CAT-ACT (pHD 789) CAT-ep1Δ26 (pHD 775). Variants of these plasmids bearing a ribosomal RNA promoter instead of a T7 promoter were also tested (in cells lacking T7 polymerase).
Secondary structures were introduced by cloning G30 oligos with restriction overhangs into available or newly created restriction sites. All constructs were sequenced in both directions (TopLab, München, Germany). In nearly every case, the sequence terminated in the middle of a G30 or C30 tract, so the size of the complete insertion was confirmed by restriction digestion. The constructs created were as follows, where G indicates that the resulting mRNA contains G30 and GC indicates that G30C30 is present at the indicated positions: CAT-ep1-G-26 (with G30 inserted at position 120 in the 3′-UTR, preceding the 26mer, pHD 879); CAT-GC-EP1 (pHD 921); 5′-G-CAT-EP1 (pHD 874); CAT-EP1-G (with G30 inserted at position 180 in the 3′-UTR, after the 26mer, pHD 891); 5′-G-CAT-GC-EP1 (pHD 922); CAT-GC-ACT (pHD 1110); 5′-G-CAT-ep1Δ26 (pHD 873); CAT-G-ep1Δ26 (pHD 1109). These constructs are illustrated in Figure 4. Additional, similar constructs bearing insertions of 18 G residues, or six BamHI linkers, were initially tested but yielded no intermediates; however, these were never analysed using RNase H.
Figure 4.
Contructs containing inserted secondary structures. The position of the inserted structures is indicated by the hairpin loop, and the nature of the insertions indicated in the captions. G indicates the presence of G30 in the mRNA, and GC indicates the presence of G30C30. A northern blot comparing steady-state CAT RNA levels from each cell line is shown below; lane numbers correspond to the clone numbers and the ribosomal RNA pattern (methylene blue stain) is again illustrated as a loading control above the CAT detection signal.
RNA preparation and manipulation
Total RNA was isolated using peqGoldTrifast (peqLab). RNA from 4 × 107 cells was separated on formaldehyde gels and blotted onto Nytran membrane (Schleicher and Schuell). In turnover experiments, 32P-labelled probes were used and bands quantitated using a PhosphorImager (Molecular Dynamics). For in vitro transcription of CAT constructs, plasmids were cut with SpeI and transcribed according to the manufacturer’s instructions, then either viewed by direct staining or blotted and detected with specific probes.
RNase H assays were carried out incubating 250 µmol oligos with 10–20 µg total RNA and RNase inhibitor (Rnasin, Stratagene) for 10 min at 45°C. RNase H (1 U; Roche, Mannheim) and RNase H buffer (50 mM Tris–HCl, pH 7.4, 10 mM MgCl2, 80 mM KCl, 1 mM DTT) were added and the mixture was incubated for 2 h at 37°C followed by a precipitation step. The RNA was run on 5% denaturing polyacrylamide gels followed by semi-dry blotting on Nytran membrane. mRNA was detected using digoxygenin-labelled RNA probes as described by the manufacturer (Roche, Mannheim).
RESULTS
Degradation of CAT mRNAs synthesised by T7 polymerase
In our previous work on post-transcriptional regulation in trypanosomes, we studied the degradation of CAT mRNAs bearing different 3′-UTRs, produced from transgenes integrated into the trypanosome genome and transcribed by RNA polymerase II. Actinomycin D was added to trypanosome cultures, to inhibit mRNA synthesis, and the levels of RNAs were measured at various times after drug addition by northern blotting. In bloodstream-form trypanosomes, CAT RNA bearing the 3′-UTR from the EP1 gene (CAT-EP1 RNA) was barely detectable and had a half-life of 5–7 min (22). An mRNA with the 3′-UTR from the ACT gene (CAT-ACT mRNA) served as an unregulated control; after actinomycin D addition, the RNA level rose for the first 10 min, then diminished with a half-life of ∼20 min. We do not know why the mRNA levels rise immediately after actinomycin D addition, but this is extremely reproducible and has been seen in similar experiments with Leishmania chagasi (see fig. 4 in ref. 48). An RNA bearing an EP1 3′-UTR with a deletion of the 26mer (CAT-ep1Δ26) behaved exactly like the CAT-ACT mRNA. Similar half-lives were observed after addition of sinefungin, which inhibits mRNA processing in trypanosomes because it inhibits cap methylation on the spliced leader RNA. In procyclic forms, the CAT-EP1 RNA, like the EP1 RNA, was much more stable than the ACT or CAT-ACT RNAs. These latter mRNAs, like other mRNAs that are expressed at a constant level throughout the life cycle, were slightly more stable in procyclic forms than in bloodstream forms. This compensates for the fact that procyclic trypanosomes have a greater volume than bloodstream forms, and a slightly longer division time.
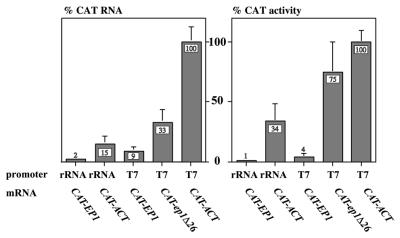
In order to study the degradation mechanism, we needed cell lines expressing more mRNA from the integrated CAT transgenes than can be obtained using RNA polymerase II. Initially, we re-engineered the plasmids to ensure transcription by trypanosome RNA polymerase I, which is approximately 10 times as active as polymerase II (24). Then we replaced the rRNA promoter with a bacteriophage T7 promoter, and transfected the plasmids into trypanosomes expressing T7 RNA polymerase (47,49). The relative mRNA levels and CAT activities obtained from the new constructs are illustrated in Figure 1. Maximum expression was obtained when the integrated transgenes were transcribed by T7 polymerase. Despite the high expression, however, the control by 3′-UTRs was retained. In our previous paper, where the CAT constructs were transcribed by RNA polymerase II, the levels of CAT-EP1 and CAT-ep1Δ26 RNAs were ∼9 and 34% relative to the CAT-ACT mRNA (22) and relative CAT activities were similar. The mRNAs expressed using T7 polymerase were present in the same ratios, but rather more translation of CAT-ep1Δ26 was seen (Fig. 1).
Figure 1.
Levels of mRNAs influenced by the RNA polymerase used for transcription, and the nature of the 3′-UTR. The plasmids used were designed for integration into the silent RRNA spacer, and had a T7 promoter (PT7) or ribosomal RNA promoter (PRRNA) preceeding a 5′-UTR (trans splicing acceptor site) and the CAT gene. The CAT gene was followed by various 3′-UTRs: actin (ACT); EP1; and a derivative of EP1 that lacked the 26mer instability element (ep1Δ26). The different 3′-UTRs were followed by a cassette allowing selection of permanent trypanosome transformants: a splice acceptor, the HYG gene (hygromycin resistance) and an actin 3′-UTR. The graphs show relative levels of CAT mRNA and CAT activity in bloodstream trypanosomes stably transformed with different constructs. The nature of the promoter, and of the 3′-UTR following the CAT gene, are shown beneath the columns. Values are expressed as a percentage, with the mean for the CAT-ACT construct with a T7 promoter set at 100%. The error bars show the standard deviation of at least three independent measurements.
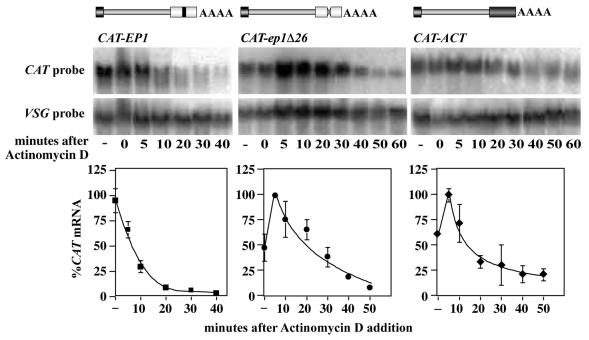
We next checked the kinetics of RNA degradation in the T7 polymerase lines. It was important to confirm that the excess production of 26mer-containing RNA had not saturated the regulation mechanism. Bloodstream trypanosomes expressing CAT-ACT, CAT-EP1 and CAT-ep1Δ26 mRNAs from the T7 promoter were treated with actinomycin D and the levels of RNA measured by northern blotting. The very stable VSG mRNA (21) was treated as a loading control. Results are shown in Figure 2. The RNAs behaved exactly as previously described. The CAT-ACT and CAT-ep1Δ26 mRNAs first accumulated then were degraded with half-lives of ∼15 min, and the CAT-EP1 mRNA disappeared with a half-life of ∼7 min. Importantly, however, the CAT-EP1 mRNA was now detectable for long enough to allow detection of degradation intermediates.
Figure 2.
Degradation kinetics of the CAT mRNAs produced by T7 polymerase. Actinomycin D was added to 10 µg/ml at time 0 and RNA prepared at the times indicated. The first time point (lane indicated –) is cells without actinomycin D and the time = 0 lane is cells placed on ice and centrifuged immediately after addition of actinomycin D. The upper panels are typical northern blots, hybridised with the probes shown. Within each panel, all lanes are from the same exposure, but some lanes were deleted from the centre of the CAT-EP1 and CAT-ep1Δ26 illustrations. The VSG mRNA is very stable in bloodstream trypanosomes so was used as a loading control. The lower panels show the relative mRNA levels, with the highest point taken as 100%. The quantitated results are expressed as the mean and standard deviation of at least three independent experiments.
Cell lines were also generated with procyclic trypanosomes using the same constructs. The stabilities of the resulting mRNAs were again similar to those previously measured for procyclic forms (22), with half-lives of 1–2 h for all 3′-UTRs.
Fate and length of the poly(A) tail during degradation
To find out which ends of the test mRNAs were attacked first by the degradation machinery, we needed to separate the 3′-end from the 5′-end. This was done by cleaving the mRNAs into three pieces: a 5′ fragment, a central piece and a 3′ fragment. To achieve this, the RNA samples were incubated with two oligonucleotides, specific for different parts of the CAT gene, together with RNase H. The RNase H cleaved each CAT RNA at the sites of oligonucleotide hybridisation, giving three fragments which are designated A, B and C in Figure 3A. The largest fragment (A in Fig. 3A) contained the 3′ portion of the CAT gene attached to the 3′-UTR and the poly(A) tail. The next largest fragment (B) was the 5′ portion of the CAT gene attached to the 5′-UTR and the spliced leader, and the smallest fragment (C) was the central portion of the CAT gene. To determine the length of the poly(A) tail, we included oligo d(T) in the RNase H incubations. This resulted in deadenylation of the 3′ fragment. The sizes and relative abundances of the fragments were analysed by denaturing polyacrylamide gel electrophoresis and blot hybridisation (Fig. 3B).
Figure 3.
Differential degradation kinetics of the 3′- and 5′-ends of the mRNAs. (A) Diagram of a generic CAT mRNA, showing the sites of RNase H cleavage after addition of targeted oligonucleotides. The fragments illustrated in (B) and (C), and also those seen in Figure 5 together with the specific probes, are indicated. (B) Northern blots displaying the mRNA fragments. RNA was isolated from the cell lines at various times after addition of actinomycin D, as in Figure 2. The RNA was subsequently digested with RNase H and specific oligonucleotides, and the resulting fragments separated on denaturing polyacrylamide gels. The identity of the bands was confirmed by hybridisation with specific probes (see Figure 5); bands marked * are partial digestion products. In (C), a methylene blue stain of the blot is shown above to indicate loading. (C) The experiment shown in (B) was repeated but the gel was run much longer, to increase resolution of the 3′-end fragment A. This better illustrates deadenylation of the CAT-ACT and CAT-ep1Δ26 mRNAs, and also shows that the CAT-EP1 mRNA is deadenylated in procyclic forms (pro), but not in bloodstream forms (bf). The panels above the blots show the stained rRNAs.
In yeast and mammals, mRNA degradation is frequently initiated by degradation of the poly(A) tail. To see if this was true in trypanosomes, we inhibited transcription using actinomycin D as before, and isolated RNA at different time points. The mRNAs were then cleaved into three pieces as described. Experiments were performed at least four times on each RNA; representative results are shown in Figure 3B. Often, additional fragments were present in amounts that varied considerably between experiments; these are marked with asterisks in the figures. After blot hybridisation with specific fragments of the CAT gene, and testing a variety of incubation conditions (data not shown), we concluded that these were mainly products of incomplete RNase H cleavage.
The steady-state lengths of the poly(A) tails on all three RNAs were 150–200 nt, as judged by comparing the size of fragment A in the zero time-point lane without oligo d(T) with the size of fragment A incubated with oligo d(T). (These lanes are on the extreme right of the panels in Fig. 3B.) The degradation pattern of the CAT-ACT and CAT-ep1Δ26 mRNAs (centre and right-hand panels in Fig. 3B) followed a pattern indicating that degradation is preceded by deadenylation. After actinomycin D addition, the sizes of the 5′ (B) and central (C) fragments remained constant for 40 min, although their abundance decreased. In contrast, the 3′ fragment (A) gradually became shorter. The reduction in size can be seen in Figure 3B by comparing the migration of the 3′ fragment A at the 40 min time point with the migration of deadenylated fragment A and fragment A at time = 0. Deadenylation was more clearly seen when the gel electrophoresis was prolonged to improve resolution of the longer fragments (Fig. 3C, see the panels for CAT-ep1Δ26 and CAT-ACT).
The CAT-EP1 mRNA degradation pattern in bloodstream forms was clearly different from that of the other two transcripts (Fig. 3B and C). The full-length 3′ fragment A disappeared rapidly. Its disappearance preceded the disappearance of the other two fragments and there was no sign of deadenylation intermediates. This was also true 5 min after actinomycin D addition (not shown). This implied that degradation was initiated by extremely rapid destruction of the 3′-UTR, possibly through an endonuclease cleavage followed by 3′→5′ digestion. Throughout the incubation, however, there was a faint band that corresponded in length to the fully deadenylated species (seen clearest in Fig. 3C, CAT-EP1 bf). This result suggested to us that a very minor portion of the CAT-EP1 mRNA was subject to rapid deadenylation.
When we repeated these experiments in procyclic forms, we saw that the degradation of the CAT-EP1 mRNA now followed the same pathway as that for the CAT-ACT and CAT-ep1Δ26 mRNAs (Fig. 3C, CAT-EP1 pro, and data not shown). As previously shown, the CAT-EP1 mRNA was much more stable in procyclic than in bloodstream forms. The length of the poly(A) tail on CAT-EP1 mRNA was not developmentally regulated (compare the bf and pro lanes at zero time), but deadenylation was clearly visible after transcription inhibition. Thus the rapid destruction of the EP1 3′-UTR correlated with mRNA instability, while more stable mRNAs were degraded by a more classical pathway involving deadenylation.
The direction of regulated degradation
As already noted, a possible mechanism for EP1 mRNA degradation in bloodstream trypanosomes would be an endonuclease cleavage at or near the 26mer. We therefore tried to find specific cleavage products by RNase protection assays. Despite considerable efforts, using a variety of probes and including clear positive controls, the results were consistently negative (not shown) (50). This result has two possible explanations. First, there may indeed be no cleavage in or near the 26mer, but it is also possible that cleavage occurs and the products are very rapidly degraded.
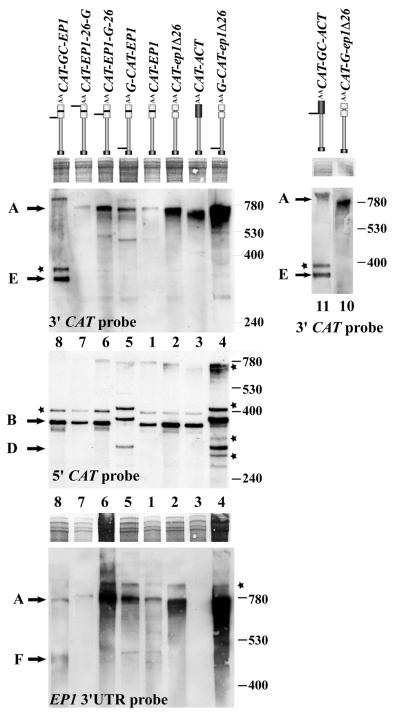
As an alternative we adopted an approach that had already successfully been used in yeast (31,51) and in Chlamydomonas reinhardtii (52). Here, the direction of degradation was determined by examining the fate of modified transcripts containing poly(G) tracts, which form stable secondary structures that impede the progress of exonucleases and lead to the accumulation of stable intermediates. We inserted secondary structures into the CAT constructs, and made transgenic bloodstream trypanosomes. The relevant constructs are illustrated in Figure 4. The minimal sequence inserted was G30 (indicated by G in the plasmid names), but some plasmids contained G30C30 (indicated by GC in the plasmid names). To detect 5′→3′ exonuclease activity, G30 was inserted immediately preceding the CAT gene (5′-G-CAT-EP1, construct 5; 5′-G-CAT-ep1Δ26, construct 4). Similarly, to detect 3′→5′ exonuclease action, secondary structure was inserted at the junction between the CAT gene and the 3′-UTR (CAT-GC-EP1, construct 8; CAT-GC-ACT, construct 11; CAT-G-ep1Δ26, construct #10). In order to look for endonuclease cleavage in the EP1 3′-UTR, structures were also inserted either immediately preceding the 26mer (CAT-EP1-G-26, construct 6) or within the 16mer stem–loop, downstream of the 26mer (CAT-EP1-26-G, construct 7). Selected constructs were also transfected into procyclic forms.
We intially looked for intermediates by northern blotting. The blot at the foot of Figure 4 displays RNA from each line, with each lane number corresponding to the number of the transfected construct. The relative abundances of the starting RNAs are clearly seen (lanes 1–3). With one exception, the structures did not reproducibly influence the mRNA abundance. (Compare for example lanes 11 and 3, and lanes 1, 5, 7, 8 and 9.) The exception was the mRNA in which a poly(G) had been inserted immediately upstream of the 26mer (CAT-EP1-G-26, compare lanes 6 and 7). This showed increased mRNA levels, half-life and CAT activity (data not shown), generally behaving as if the 26mer had been deleted. The insertion could have disrupted the secondary structure, diminishing the accessibility of the 26mer to the degradation machinery. G30 structures preceding the CAT coding region dimished CAT activity, presumably by inhibiting translation (not shown).
In contrast with the results obtained with both yeast and Chlamydomonas, no stable degradation intermediates were detected if single G30 structures were placed in any position. The only clear effect seen was with construct 9, which has G30 preceding the CAT gene and G30C30 between the CAT gene and the EP1 3′-UTR (G-CAT-GC-EP1) (Fig. 4). The most abundant mRNA was full-length, but a weaker band of ∼700 nt, the size of the CAT gene without UTRs, was clearly present (Fig. 4, lane 9). This suggested that the RNA was subject to degradation from both ends simultaneously. If secondary structures inhibit exonucleases, but degradation is bidirectional, the insertion of a single secondary structure will give no stable intermediates. In fact, for clones 9 and 11, which have G30C30 between the CAT gene and either the EP1 3′-UTR (clone 9) or the ACT 3′-UTR (clone 11), a shorter band was also just perceptible on the blot shown, but the abundance was extremely low and the band was not reproducibly detected. Construct 10, which has G30 between the CAT gene and the ep1Δ26 3′-UTR, never showed intermediates in any experiment. In experiments with plant and animal cells, poly(G) tracts failed to stabilise mRNA degradation intermediates, even if two tracts were included to stabilise a central portion (summarised in 53); the evidence so far suggests that plant cells must contain additional proteins (such as helicases) that can unwind secondary structures and allow digestion (53).
The result obtained with the construct containing two secondary structures indicated that in vivo, G30 could inhibit 5′→3′ exonucleases, and G30C30 could inhibit 3′→5′ exonucleases. This suggested that it might be possible to see degradation intermediates from constructs with an appropriate single secondary structure if we cut the mRNAs within the CAT gene. To characterise the products of 5′→3′ or 3′→5′ degradation in more detail we therefore once again subjected the mRNAs to RNase H digestion, as in Figure 3. All constructs were analysed in at least three independent experiments. The most important results are shown in Figure 5.
Figure 5.
Identification of the degradation intermediates stabilised by secondary structures. RNA was prepared from cell lines expressing the different CAT mRNAs. The RNA map is above the lanes, and the clone number beneath or between the blots. RNA was digested with RNase H and separated as in Figure 3. A methylene blue stain of a portion of the gel is included to indicate the loading, and the nature of the probes used indicated on each panel. Fragments detected correspond to those illustrated in Figure 3A and asterisks again indicate partial digestion products.
A diagram showing the CAT mRNA and the position of the different hybridisation probes used is presented in Figure 3A. Again, partial digestion products that were present in very variable amounts are marked with asterisks (above bands B and A). First, as already shown, cleavage of the CAT-EP1 mRNA yielded three fragments. Band A represents the 3′ portion of the CAT gene joined to the 3′-UTR. It is shown here hybridising with the EP1 3′-UTR probe and the 3′ CAT probe. Band B contains the 5′-UTR and the 5′-portion of the CAT gene and is shown here hybridising with the 5′ CAT probe. The fragment C (centre CAT fragment) is not seen in Figure 5 because it did not hybridise to any of the probes used. Similar patterns were seen for CAT-ep1Δ26 and CAT-ACT, except that the CAT-ACT of course did not hybridise with the EP1 3′-UTR probe. The pattern for the RNA containing G30 just preceding the 26mer (CAT-ep1-G-26) was just like that for CAT-ep1Δ26 except that the 3′ fragment was slightly longer, as expected. The RNA with an insertion of G30 downstream of the 26mer (CAT-EP1-26-G) gave a pattern similar to CAT-EP1; no intermediates were seen. This could be because there is endonuclease cleavage in the 26mer, but it could also reflect an inability of the G30 structure to halt 3′→5′ exonucleases.
Two constructs had G30 inserted between the 5′-UTR and the CAT coding region, either with an EP1 3′-UTR (G-CAT-EP1, construct 5 in Fig. 4) or with ep1Δ26mer (mutant version of EP1 3′-UTR with deletion of regulatory 26mer sequence) 3′-UTR (5′-G-CAT-ep1Δ26, construct 4). These were designed to detect 5′→3′ exonuclease activity. The results are best seen on the blot hybridised with the 5′ CAT probe in Figure 5. In both cases, the intact 5′ portion (fragment B) was longer than for the control, as expected due to the insertion. But in addition, a fragment (D) corresponding in length to the 5′-end of the CAT gene without the 5′-UTR was observed. This additional fragment, which hybridised only to the CAT 5′ probe, could be the product of 5′→3′ exonuclease action, digesting the 5′-UTR but halting at the inserted G30 structure.
As could be predicted from the northern blot in Figure 4, the pattern with CAT-GC-EP1 (construct 8), which has G30C30 between the CAT gene and the EP1 3′-UTR, also had an additional band, which is labelled E in Figures 4 and 5. The fragment was ∼300 bp long and hybridised with the CAT 3′ probe but not with the 5′ probe or the EP1 3′-UTR probe (Fig. 5), indicating that it corresponded to the 3′-end of the CAT gene, terminating at G30C30. This could be created by 3′→5′ digestion terminating at the secondary structure. This fragment was also seen in procyclic forms, indicating that the 3′→5′ activities were active throughout the life cycle (data not shown). Similar blots of bloodstream-form mRNA hybridised with a probe specific for the EP1 3′-UTR showed a faint smear running at approximately the size of a (partially) deadenylated EP1 3′-UTR, lacking the 5′-UTR and CAT gene portion (fragment F in the figures). This smear was reproducible and clone-specific, so could be a product of 5′→3′ exonuclease action. A similar construct for the ACT 3′-UTR containing G30C30 (construct 11) gave analogous results with the CAT probe, but construct 10 in which G30 had been inserted between the CAT gene and the ep1Δ26mer, yielded no additional bands (Fig. 5), suggesting that G30 was insufficent to inhibit 3′→5′ exonuclease activity in trypanosomes.
One way to confirm that these fragments represent degradation intermediates would be to perform a transcriptional pulse-chase experiment. As transcription of an mRNA was induced, the full-length RNA would increase in abundance; after transcriptional repression, the amount of full-length RNA would diminish and the abundance of intermediates would transiently increase before they too were degraded. Unfortunately this is currently not technically possible in trypanosomes. Nevertheless, we could exclude some potential artifacts, as follows. (i) The extra bands did not result from anomalous migration in the polyacrylamide gel due to strong secondary structure. This can be completely excluded for the fragment E, because it hybridised to the CAT probe but not the 3′-UTR probe. (ii) Fragment E (but not fragment D) could have been the product of transcriptional termination by T7 RNA polymerase at or just after G30C30. This was unlikely, as termination by T7 polymerase is usually very sequence specific. Nevertheless, to check, three plasmids, CAT-GC-EP1, CAT-EP1 and CAT-G-ep1Δ26, were cleaved at a restriction site downstream of the normal polyadenylation site, and transcribed in vitro by T7 polymerase. Each reaction yielded a full-length band of ∼1.3 kb and a smear of shorter products (not shown), but there was no evidence for specific termination at either G30 or G30C30. (iii) The G30 or G30C30 might have been targeted by RNase H or have acted as a self-cleaving ribozyme. This is again unprecedented, and can be ruled out for G30 as no additional band was seen with the CAT-G-ep1Δ26 construct. To control for in vitro cleavage at G30C30, the in vitro transcripts described above were incubated under standard RNase H digestion conditions, with or without RNase H and without oligonucleotides. All products were analysed by polyacrylamide gel electrophoresis and results were again negative (not shown).
We still cannot rule out the possibility that fragments D, E and F are generated by in vivo T7 polymerase termination or by trypanosome endonuclease activity at the secondary structures. It is however more likely that they are products of trypanosome exonuclease action on full-length RNAs.
DISCUSSION
The results described in this manuscript suggest that constitutive mRNA degradation in trypanosomes involves gradual deadenylation followed by rapid degradation by both 5′→3′ exonucleases, as seen in yeast. The very rapid degradation induced by the U-rich 26mer instability element exhibits features consistent with either very rapid deadenylation or endonuclease cleavage.
The predominant mechanism of both regulated and constitutive mRNA degradation in yeast is deadenylation followed by decapping and 5′→3′ degradation, and a similar pathway has been described in Chlamydomonas (52). The principal enzyme involved in cytoplasmic 5′→3′ degradation in yeast is Xrn1p; the dependence on deadenylation is at least partially explained by the formation of a degradation complex which also includes components that can interact with the poly(A) tail (54). The trypanosome genome database contains at least four potential homologues of Xrn1p or the similar nuclear 5′→3′ exonuclease Rat1p (H. Irmer, S. Freese and A. Estévez, unpublished results).
Yeast strains that are mutant in the deadenylation-dependent decapping pathway are viable and degrade mRNA, but at lower rates (55). Degradation now occurs in the 3′→5′ direction (56) and is effected by a complex called the exosome (57). In yeast, this degradation seems to be considerably less active than that in the 5′→3′ direction. Our results suggested that in trypanosomes 3′→5′ degradation and 5′→3′ degradation are equally active. For example, RNAs that were degraded from the 5′-end could only be detected if they were internally cleaved to create uniform 3′-termini. Failure to detect stabilised degradation intermediates by insertion of single secondary structures in mRNAs in mammalian cells has also been reported (38); this could perhaps also be due to simultaneous degradation from 3′- and 5′-ends. Trypanosomes contain several 3′→5′ exonucleases that are similar to the components of the yeast exosome, but only a subset of these are incorporated into the trypanosome exosome complex (58). It may be that the differences between the trypanosome and yeast 3′→5′ exonuclease complements, and their regulation, results in higher activity in trypanosomes. The accelerated degradation of the EP mRNA in trypanosomes appears to be initiated by rapid destruction of the 3′-UTR. Although a minor portion may be subject to deadenylation, the results would be more consistent with an endonuclease cleavage within the U-rich instability element. After attack on the 3′-UTR, the mRNA is digested from both ends. In order to detect any products of endonuclease cleavage, we will have to create conditional mutants in the exonuclease activities. These experiments are in progress (58).
Overall, the pattern of EP1-mediated mRNA decay is rather reminiscent of the regulated decay of a number of mammalian mRNAs. Degradation of some mammalian mRNAs is clearly initiated by endonuclease cleavage (32). For early response mRNAs containing AREs, there is conflicting information supporting roles for both deadenylation and endonuclease cleavage (see for example 40–42,59,60). Interestingly, AU-element-mediated decay has recently been reported in Trypanosoma cruzi (23), and a protein mediating the decay shows homology to mammalian proteins that regulated AU-mediated decay (61). Since many of the genes encoding the basal degradation machinery are found in trypanosomes, it is possible that the regulatory mechanisms also have ancient evolutionary origins.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Elisabetta Ullu (Yale University) and Antonio Estévez for useful discussions, Claudia Hartmann for technical assistance, and George Cross and Elizabeth Wirtz (Rockefeller University) for plasmids and cell lines. This work was supported by the Deutsche Foschungsgemeinschaft.
REFERENCES
- 1.Cross G.A.M. (1997) Antigenic variation in trypanosomes: secrets surface slowly. Bioessays, 18, 283–291. [DOI] [PubMed] [Google Scholar]
- 2.Roditi I., Furger,A., Ruepp,S., Schurch,N. and Butikofer,P. (1998) Unravelling the procyclin coat of Trypanosoma brucei. Mol. Biochem. Parasitol., 91, 117–130. [DOI] [PubMed] [Google Scholar]
- 3.Vanhamme L. and Pays,E. (1995) Control of gene expression in trypanosomes. Microbiol. Rev., 59, 223–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanhamme L. and Pays,E. (1998) Controls of the expression of the vsg in Trypanosoma brucei. Mol. Biochem. Parasitol., 91, 107–116. [DOI] [PubMed] [Google Scholar]
- 5.Blattner J., Helfert,S., Michels,P. and Clayton,C.E. (1998) Compartmentation of phosphoglycerate kinase in Trypanosoma brucei plays a critical role in parasite energy metabolism. Proc. Natl Acad. Sci. USA, 95, 11596–11600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myler P.J., Audleman,L., deVos,T., Hixson,G., Kiser,P., Lemley,C., Magness,C., Rickel,E., Sisk,E., Sunkin,S., Swartzell,S., Westlake,T., Bastien,P., Fu,G., Ivens,A. and Stuart,K. (1999) Leishmania major Friedlin chromosome 1 has an unusual distribution of protein-coding genes. Proc. Natl Acad. Sci. USA, 96, 2902–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews K.R., Tschudi,C. and Ullu,E. (1994) A common pyrimidine-rich motif governs trans-splicing and polyadenylation of tubulin polycistronic pre-mRNA in trypanosomes. Genes Dev., 8, 491–501. [DOI] [PubMed] [Google Scholar]
- 8.Schürch N., Hehl,A., Vassella,E., Braun,R. and Roditi,I. (1994) Accurate polyadenylation of procyclin mRNAs in Trypanosoma brucei is determined by pyrimidine-rich elements in the intergenic regions. Mol. Cell. Biol., 14, 3668–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hug M., Hotz,H.R., Hartmann,C. and Clayton,C.E. (1994) Hierarchies of RNA processing signals in a trypanosome surface antigen mRNA precursor. Mol. Cell. Biol., 14, 7428–7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasion S.G., Hines,J.C., Ou,X., Mahmood,R. and Ray,D.S. (1996) Sequences within the 5′ untranslated region regulate the levels of a kinetoplast DNA topoisomerase mRNA during the cell cycle. Mol. Cell. Biol., 16, 6724–6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clayton C. and Hotz,H.-R. (1996) Post-transcriptional control of PARP gene expression. Mol. Biochem. Parasitol., 77, 1–6. [DOI] [PubMed] [Google Scholar]
- 12.Hotz H.-R., Biebinger,S., Flaspohler,J. and Clayton,C.E. (1998) PARP gene expression: regulation at many levels. Mol. Biochem. Parasitol., 91, 131–143. [DOI] [PubMed] [Google Scholar]
- 13.Aly R., Argaman,M., Halman,S. and Shapira,M. (1994) A regulatory role for the 5′ and 3′ untranslated regions in differential expression of hsp83 in Leishmania. Nucleic Acids Res., 22, 2922–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argaman M., Aly,R. and Shapira,M. (1994) Expression of the heat shock protein 83 in Leishmania is regulated post-transcriptionally. Mol. Biochem. Parasitol., 64, 95–110. [DOI] [PubMed] [Google Scholar]
- 15.Blattner J. and Clayton,C.E. (1995) The 3′-untranslated regions from the Trypanosoma brucei phosphoglycerate kinase genes mediate developmental regulation. Gene, 162, 153–156. [DOI] [PubMed] [Google Scholar]
- 16.Häusler T. and Clayton,C.E. (1996) Post-transcriptional control of hsp 70 mRNA in Trypanosoma brucei. Mol. Biochem. Parasitol., 76, 57–72. [DOI] [PubMed] [Google Scholar]
- 17.Hotz H.-R., Lorenz,P., Fischer,R., Krieger,S. and Clayton,C.E. (1995) Developmental regulation of hexose transporter mRNAs in Trypanosoma brucei. Mol. Biochem. Parasitol., 75, 1–14. [DOI] [PubMed] [Google Scholar]
- 18.Charest H., Zhang,W.-W. and Matlashewski,G. (1996) The developmental expression of Leishmania donovani A2 amastigote-specific genes is post-transcriptionally mediated and involves elements in the 3′-untranslated region. J. Biol. Chem., 271, 17081–17090. [DOI] [PubMed] [Google Scholar]
- 19.Teixeira S.M.R., Kirchhoff,L.V. and Donelson,J.E. (1995) Post-transcriptional elements regulating expression of mRNAs from the amastin/tuzin gene cluster of Trypanosoma cruzi. J. Biol. Chem., 270, 22586–22594. [DOI] [PubMed] [Google Scholar]
- 20.Nozaki T. and Cross,G.A.M. (1995) Effects of 3′-untranslated and intergenic regions on gene expression in Trypanosoma cruzi. Mol. Biochem. Parasitol., 75, 55–68. [DOI] [PubMed] [Google Scholar]
- 21.Berberof M., Vanhamme,L., Tebabi,P., Pays,A., Jefferies,D., Welburn,S. and Pays,E. (1995) The 3′-terminal region of the mRNAs for VSG and procyclin can confer stage specificity to gene expression in T. brucei. EMBO J., 14, 2925–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotz H.-R., Hartmann,C., Huober,K., Hug,M. and Clayton,C.E. (1997) Mechanisms of developmental regulation in Trypanosoma brucei: A polypyrimidine tract in the 3′-untranslated region of a trypanosome surface protein mRNA affects RNA abundance and translation. Nucleic Acids Res., 25, 3017–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Noia J.M., D’Orso,I., Sánchez,D.O. and Frasch,A.C.C. (2000) AU-rich elements in the 3′-untranslated region of a new mucin-type gene family of Trypanosoma cruzi confers mRNA instability and modulates translation efficiency. J. Biol. Chem., 275, 10218–10227. [DOI] [PubMed] [Google Scholar]
- 24.Biebinger S., Rettenmaier,S., Flaspohler,J., Hartmann,C., Peña-Diaz,J., Wirtz,L.E., Hotz,H.R., Barry,J.D. and Clayton,C.E. (1996) The PARP promoter of Trypanosoma brucei is developmentally regulated in a chromosomal context. Nucleic Acids Res., 24, 1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro M., Cross,G.A.M. and Wirtz,E. (1999) Trypanosoma brucei variant surface glycoprotein regulation involves coupled activation/inactivation and chromatin remodeling of expression sites. EMBO J., 18, 2265–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horn D. and Cross,G.A.M. (1997) Position-dependent and promoter-specific regulation of gene expression in Trypanosoma brucei.EMBO J., 16, 7422–7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roditi I. and Clayton,C.E. (1999) An unambiguous nomenclature for the major surface glycoprotein genes of the procyclic form of Trypanosoma brucei. Mol. Biochem. Parasitol., 103, 99–100. [DOI] [PubMed] [Google Scholar]
- 28.Hehl A., Vassella,E., Braun,R. and Roditi,I. (1994) A conserved stem-loop structure in the 3′ untranslated region of procyclin mRNAs regulates expression in Trypanosoma brucei. Proc. Natl Acad. Sci. USA, 91, 370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furger A., Schürch,N., Kurath,U. and Roditi,I. (1997) Elements in the 3′ untranslated region of procyclin mRNA regulate expression in insect forms of Trypanosoma brucei by modulating RNA stability and translation. Mol. Cell. Biol., 17, 4372–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drozdz M. and Clayton,C.E. (1999) Structure of a regulatory 3′-untranslated region from Trypanosoma brucei. RNA, 5, 1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Decker C.J. and Parker,R. (1993) A turnover pathway for both stable and unstable mRNAs in yeast: evidence for a requirement for deadenylation. Genes Dev., 7, 1632–1643. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell P. and Tollervey,D. (2000) mRNA stability in eucaryotes. Curr. Opin. Genet. Dev., 10, 193–198. [DOI] [PubMed] [Google Scholar]
- 33.Körner C.G., Wormington,M., Muckenthaler,M., Schneider,S., Dehlin,E. and Wahle,E. (1998) The deadenylating nuclease (DAN) is involved in poly(A) tail removal during the meiotic maturation of Xenopus oocytes. EMBO J., 17, 5427–5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bashkirov V.I., Scherthan,H., Solinger,J.A., Buerstedde,J.M. and Heyer,W.D. (1997) A mouse cytoplasmic exoribonuclease (mXRN1p) with preference for G4 tetraplex substrates. J. Cell Biol., 136, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiomi T., Fukushima,K., Suzuki,N., Nakashima,N., Noguchi,E. and Nishimoto,T. (1998) Human dis3p, which binds to either GTP- or GDP-Ran, complements Saccharomyces cerevisiae dis3.J. Biochem., 123, 883–890. [DOI] [PubMed] [Google Scholar]
- 36.Binder R., Horowitz,J.A., Basilion,J.P., Koeller,D.M., Klausner,R.D. and Harford,J.B. (1994) Evidence that the pathway of transferrin receptor mRNA degradation involves an endonucleolytic cleavage within the 3′-UTR and does not involve poly(A) tail shortening. EMBO J., 13, 1969–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z. and Kiledjian,M. (2000) Identification of an erythroid-enriched endoribonuclease activity involved in specific mRNA cleavage. EMBO J., 19, 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curatola A.M., Nadal,M.S. and Schneider,R.J. (1995) Rapid degradation of AU-rich element (ARE) mRNAs is activated by ribosome transit and blocked by secondary structure at any position 5′ to the ARE. Mol. Cell Biol., 15, 6331–6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng S.S., Chen,C.Y. and Shyu,A.B. (1996) Functional characterization of a non-AUUUA AU-rich element from the c-jun proto-oncogene mRNA: evidence for a novel class of AU-rich elements. Mol. Cell Biol., 16, 1490–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng S.S., Chen,C.Y., Xu,N. and Shyu,A.B. (1998) RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J., 17, 3461–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan X.C. and Steitz,J.A. (1998) Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J., 17, 3448–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Z., Chang,F.-C. and Furneaux,H.M. (2000) The identification of an endonuclease that cleaves within an HuR binding site in mRNA. Nucleic Acids Res., 28, 2695–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorn P.L., Aman,R.A. and Boothroyd,J.C. (1991) Inhibition of protein synthesis results in super-induction of procyclin (PARP) RNA levels. Mol. Biochem. Parasitol., 44, 133–140. [DOI] [PubMed] [Google Scholar]
- 44.Graham S.V. and Barry,J.D. (1996) Polysomal, procyclin mRNAs accumulate in bloodstream forms of monomorphic and pleomorphic trypanosomes treated with protein synthesis inhibitors. Mol. Biochem. Parasitol., 80, 179–192. [DOI] [PubMed] [Google Scholar]
- 45.Schürch N., Furger,A., Kurath,U. and Roditi,I. (1997) Contribution of the procyclin 3′ untranslated region and coding region to the regulation of expression in bloodstream forms of Trypanosoma brucei. Mol. Biochem. Parasitol., 89, 109–121. [DOI] [PubMed] [Google Scholar]
- 46.Wirtz L.E., Hartmann,C. and Clayton,C.E. (1994) Gene expression mediated by bacteriophage T3 and T7 RNA polymerases in transgenic trypanosomes. Nucleic Acids Res., 22, 3887–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wirtz E., Hoek,M. and Cross,G.A.M. (1998) Regulated processive transcription of chromatin by T7 RNA polymerase in Trypanosoma brucei. Nucleic Acids Res., 26, 4626–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brittingham A., Miller,M.A., Donelson,J.E. and Wilson,M.E. (2001) Regulation of GP63 mRNA stability in promastigotes of virulent and attenuated Leishmania chagasi. Mol. Biochem. Parasitol., 112, 51–59. [DOI] [PubMed] [Google Scholar]
- 49.Wirtz L.E. and Clayton,C.E. (1995) Inducible gene expression in trypanosomes mediated by a procaryotic repressor. Science, 268, 1179–1183. [DOI] [PubMed] [Google Scholar]
- 50.Irmer H. (2001) mRNA-degradation in Trypanosoma brucei. PhD thesis, University of Heidelberg, Germany.
- 51.Muhlrad D., Decker,C.J. and Parker,R. (1994) Deadenylation of the unstable mRNA encoded by the yeast MFA2 gene leads to decapping followed by 5′-3′ digestion of the transcript. Genes Dev., 8, 855–866. [DOI] [PubMed] [Google Scholar]
- 52.Gera J.F. and Baker,E.J. (1998) Deadenylation-dependent and -independent decay pathways for alpha1-tubulin mRNA in Chlamydomonas reinhardtii.Mol. Cell. Biol., 18, 1498–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kastenmeier J.P. and Green,P.J. (2000) Novel features of the XRN-family in Arabidopsis: evidence that ATXRN4, one of several orthologs of nuclear Xrn2p/rat1p, functions in the cytoplasm. Proc. Natl Acad. Sci. USA, 97, 13985–13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouveret E., Rigaut,G., Shevchenko,A., Wilm,M. and Séraphin,B. (2000) A Sm-like protein complex that participates in mRNA degradation. EMBO J., 19, 1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beelman C.A. and Parker,R. (1995) Degradation of mRNA in eukaryotes. Cell, 81, 179–184. [DOI] [PubMed] [Google Scholar]
- 56.Muhlrad D., Decker,C.J. and Parker,R. (1995) Turnover mechanisms of the stable yeast PGK1 mRNA. Mol. Cell. Biol., 15, 2145–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobs-Anderson J.S. and Parker,R.P. (1998) The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J., 17, 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Estévez A., Kempf,T. and Clayton,C.E. (2001) The exosome of Trypanosoma brucei. EMBO J., 20, 3831–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen C.Y., Xu,N. and Shyu,A.B. (1995) mRNA decay mediated by two distinct AU-rich elements from c-fos and granulocyte-macrophage colony-stimulating factor transcripts: different deadenylation kinetics and uncoupling from translation. Mol. Cell Biol., 15, 5777–5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voeltz G.K. and Steitz,J.A. (1998) AUUUA sequences direct mRNA deadenylation uncoupled from decay during Xenopus early development. Mol. Cell Biol., 18, 7537–7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.D’Orso I. and Frasch,A.C.C. (2001) TcUBP-1, a developmentally regulated U-rich RNA-binding protein involved in selective mRNA destabilization in trypanosomes. J. Biol. Chem., 276, 34801–34809. [DOI] [PubMed] [Google Scholar]