Abstract
Background
Blood transfusion centres should understand the epidemiology of emerging diseases that are transmissible through the transfusion of blood components. The risk of transmission of arboviruses through this route has become apparent in recent years. The aim of our study is to summarise the reported prevalence (viraemic rate, seroprevalence and/or antigen detection) of Chikungunya (CHIKV), Dengue (DENV) and Zika (ZIKV) viruses in blood donors according to screening test used and world region.
Materials and methods
We conducted a systematic literature review and meta-analysis having searched for information in the main bibliographic databases (MEDLINE, Embase, and Scopus). The prevalence for each of the viruses was calculated according to the screening test used and geographic location.
Results
We included 18 records on CHIKV, 71 on DENV, and 27 on ZIKV. The highest prevalences of RNA for CHIKV were 1.9% in Puerto Rico (2014), 1.0% in Thailand (2009), and 1.0% in French Polynesia (2014–15). The highest prevalences of RNA for DENV were 5.5% in Saudi Arabia (2015–16), 2.3% in Madeira, Portugal (2012–13), and 0.6% in Brazil (2012). The highest prevalences of RNA for ZIKV were 2.8% in French Polynesia (2013–14), 2.7% in Brazil (2015–16), and 1.8% in Martinique (2016). Overall seroprevalence, as assessed by IgG antibodies, was 21.6% for CHIKV, 24.0% for DENV, and 5.1% for ZIKV.
Discussion
Our study shows a high proportion of donors who are viraemic and asymptomatic, especially during outbreaks, with prevalences surpassing 5% for DENV, 1% for CHIKV, and 2% for ZIKV. These data confirm a clear threat to blood transfusion safety. The elevated seroprevalence for these three arboviruses is also indicative of their wide circulation in populations, correlating with an increased risk of infected but asymptomatic donors. Health centres and institutions must address this threat, especially in tropical regions where the biggest outbreaks occur.
Keywords: Chikungunya virus, Dengue virus, Zika virus, blood transfusion, blood safety
INTRODUCTION
Emerging and re-emerging viruses have materialised as the latest challenge to blood transfusion safety. In this sense, the World Health Organisation (WHO) has called for blood transfusion centres to be informed of the epidemiology of different emerging transfusion-transmitted infections and to evaluate the possible impact on donor selection criteria and the supply of blood products1.
Among these emerging viruses, arboviruses are especially relevant because of their known or theoretical potential for transmission through blood transfusions2. Within this group, Chikungunya virus (CHIKV), Dengue virus (DENV), and Zika virus (ZIKV) stand out for their high global incidence and the wide dissemination of their vector.
CHIKV is an alphavirus in the Togaviridae family, transmitted by Aedes mosquitoes (e.g. A. albopictus, A. aegypti). Following an incubation period of 1 to 12 days, the acute phase of infection by this virus is characterised by fever; severe, incapacitating arthralgia; and other non-specific symptoms. Some patients also develop chronic illness3. Since the virus was first isolated, periodic outbreaks have been reported in Africa, Asia, and islands in the Indian Ocean, while the first cases in the Americas were reported in 2013. Since then, different outbreaks have been reported across regions of South and Central America4. In Europe, several outbreaks have occurred since 20075, including one in 2015 involving 693,489 suspected and 37,480 confirmed cases6. Although no cases of transfusion-related infections have been notified, organisations such as the American Association of Blood Banks have sounded the alarm on the theoretical potential given the high percentage of asymptomatic people infected (3% to 28%) and the high rates of viraemia that they have7. One case of iatrogenic CHIKV transmission was reported following an accidental needle puncture in France8.
For its part, DENV is a flavivirus in the Flaviviridae family. Four distinct serotypes have been documented: DEN-1, DEN-2, DEN-3, and DEN-4. Like CHIKV, DENV is transmitted by Aedes mosquitoes, usually A. aegypti. It is the main arbovirus worldwide in terms of mortality and morbidity; its incubation period is normally 4 to 7 days, although it can range from 3 to 10 days. The clinical classification of dengue divides cases into those with or without warning signs and severe dengue (including dengue shock syndrome)9. The first large epidemics date back to the 1870s10. Today, the disease is endemic in more than 100 countries from the WHO regions of Africa, the Americas, the Eastern Mediterranean, Southeast Asia, and the Western Pacific; in 2015 alone, more than 3.2 million cases were notified across the Americas, Southeast Asia, and the Western Pacific11. In Europe, 11 cases of local transmission were also reported in 201912. Since 2002, numerous cases of transfusion-transmitted infections have been described in Hong Kong, Singapore, Brazil, Pakistan, and Puerto Rico (USA)13–17.
ZIKV is another flavivirus from the Flaviridae family. Aedes spp. mosquitoes such as A. africanus, A. aegypti, and A. albopictus are the vectors of transmission, and the incubation period can be anywhere from 2 to 12 days. Although 80% of infected people remain asymptomatic, an acute presentation with non-specific symptoms, such as fever, arthralgia, and exanthema, can occur. Infection has also been related to the appearance of microcephalia in neonates (congenital Zika syndrome) and to a Guillain-Barré-type neurological presentation18. For decades, little attention was paid to this virus, as it only provoked isolated cases in Southeast Asia and Africa. However, in 2007, a large epidemic outbreak was registered on Yap Island (Micronesia), and in 2015 and 2016, another large outbreak occurred in the Americas. In 2019, the first two cases of local transmission were reported in Europe (France)19. Moreover, transmission via transfusion of platelets has been reported in Brazil20,21.
Upon performing a review of the available scientific literature on the prevalence of CHIKV, DENV, and ZIKV in blood donors, we identified only two systematic reviews: one by Liu et al., with very restrictive inclusion criteria and ten included studies on ZIKV, and one by Eick et al., with three included studies on the prevalence of ZIKV and 11 on the prevalence of DENV22,23. We did not identify any similar papers on CHIKV. There is, therefore, a lack of literature giving a broad overview of the prevalence of these three arboviruses in blood donors.
The emergence of these viruses represents a real threat to obtaining blood components and has a direct impact on donor selection criteria and the stock of components. Following WHO recommendations, the aim of this study was to summarise the reported and published prevalence of CHIKV, DENV and ZIKV in blood donors according to the screening test used (viraemic rate, seroprevalence or antigen detection) and world region (geographical region and country).
We define the research question in a PICOS (population, intervention, comparison, outcome, study) format. The population was blood donors, including conventional whole blood donors and those donating via apheresis, who were screened for the target viruses using any test and in any defined geographic region. The intervention was screening using different techniques to detect antibodies, antigens, or nucleic acids. Comparisons were not applicable to this question and the outcomes were reported and published prevalences of each virus according to the screening test used and the geographic region in which the screening was performed. Any primary studies were included.
MATERIALS AND METHODS
Design
A systematic literature review and meta-analysis were designed and conducted in accordance with the Cochrane Handbook of Systematic Reviews of Interventions and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (see Online Supplementary Content, Table SI). Although no published protocol is available, we collected and analysed data according to pre-specified outcomes and methods, performing meta-analyses, pooling the data obtained in the different studies, and establishing a single prevalence estimate for each virus. This information may be of interest from an epidemiological point of view and when considering measures with a possible impact on transfusion safety.
Sources of data
We conducted literature searches in MEDLINE, Embase, and Scopus using the University Miguel Hernández server.
Search strategy
We performed free text searches in the three bibliographic databases using the terms: “transfusion” AND “Dengue”, “transfusion” AND “Chikungunya”, “transfusion” AND “Zika”, “blood donation” AND “Dengue”, “blood donation” AND “Chikungunya”, “blood donation” AND “Zika”.
In MEDLINE, we also used Medical Subject Headings (MeSH): “blood transfusion” AND “Zika virus”, “blood donors” AND “Zika virus”, “blood transfusion” AND “Dengue”, “blood donors” AND “Dengue”, “blood transfusion” AND “Chikungunya virus”, “blood donors” AND “Chikungunya virus”.
Finally, in the Embase searches we used the Emtree thesaurus with the terms: “blood transfusion” AND “Zika virus”, “blood donor” AND “Zika virus”, “blood transfusion” AND “Chikungunya”, “blood donor” AND “Chikungunya”, “blood transfusion” AND “Dengue”, “blood donor” AND “Dengue”.
The records were entered into the Mendeley Desktop reference manager (Elsevier). The search was performed from the year of database inception to March 28, 2020. A weekly alert system was set up to update the search with any relevant results until August 7, 2020.
Study selection
First, we used the bibliographic reference manager to create folders containing records for each virus, eliminating duplicates. We then conducted an initial screening of titles and abstracts and retrieved the full text of all pre-selected records.
Eligible studies were publications in any language describing the prevalence of the virus in blood donor screening (both donors of conventional whole blood and those donating via different methods of apheresis). We included all studies (original articles, brief reports, letters to the editor, and conference papers) reporting the number of positive results as a proportion of total samples analysed, as long as the paper stated the type of test used for screening (serological tests, antigen tests or nucleic acid amplification tests [NAT]) and the geographic location of the study population. We excluded studies that involved people other than blood donors, such as patients, children, pregnant women, the general non-donor population, and other non-donor or unspecified populations.
A single review author selected all included articles, obviating the need for an analysis of interobserver concordance. We did perform an intraobserver concordance analysis, including in the review all records that were deemed to meet inclusion and exclusion criteria during two critical assessments of the full texts.
When a single record reported results for two different study populations, these were separated in the analysis if the participants’ characteristics differed for important variables, for example geographic region (e.g. studies evaluating one population in Africa and another in Europe), or if the prevalence was substantially different by population (i.e. we separated populations sub-nationally if the differences in prevalence were relevant). If a single population underwent screening using more than one type of test, separate analyses were performed for each. In the case of records with overlapping study populations, we selected the most relevant publication (i.e. with the largest number of screened donors).
Data extraction and analysis
A single review author extracted data on prevalence and populations from the studies included, directly entering the data into Comprehensive Meta-Analysis (CMA) software. A second review author double-checked that all data were entered correctly. We recalculated the prevalence for the three viruses, pooling all positive cases and donors screened for each to calculate an overall proportion of positive results in the blood donor screening for CHIKV, DENV, and ZIKV, according to the type of screening test used. We then stratified the results by geographic location or country as long as there were at least three included studies, the minimum number we considered capable of representing a geographic area. Moreover, when the number of publications and the nature of the screening test allowed it, we calculated the prevalence ratio according to whether or not the study had taken place in an endemic region or during an epidemic outbreak. If so, we calculated the prevalence.
Results are expressed as prevalences with 95% confidence intervals and are presented with forest plots. We evaluated the heterogeneity of the studies for each screening test using the I2 statistic. To calculate the confidence intervals, create the forest plots, and analyse the heterogeneity, we used CMA software, version 2 (Borenstein, Hedges, Higgins, & Rothstein, 2005).
Quality assessment
To evaluate the methodological quality of included records, we used the STROBE (STrengthening the Reporting of Observational studies in Epidemiology) checklists for cross-sectional studies and conference abstracts. No tools were applied to letters to the editor.
RESULTS
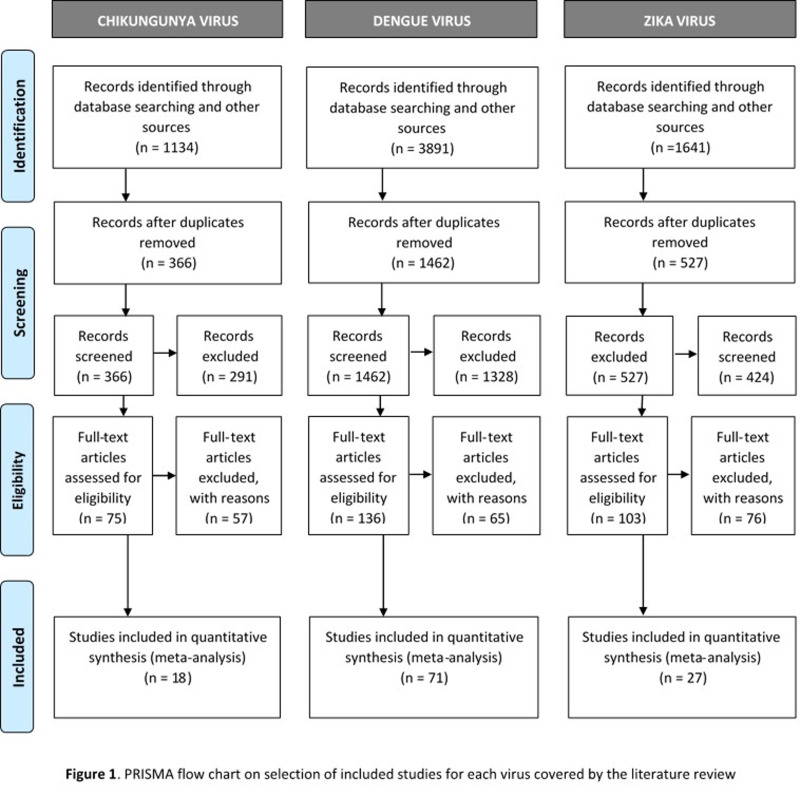
Figure 1 presents the PRISMA flow chart, describing the study selection process. Following full-text assessment, 18 studies on CHIKV24–41, 71 on DENV24,26,27,31,33,36,38,40,42–104, and 27 on ZIKV26,28,32,36,38,40,67,105–124 were included. Online Supplementary Content, Tables SII–SIV, describes the main characteristics of the studies included.
Figure 1.
PRISMA flow chart on selection of studies included for each virus covered by the literature review
Analysis of results
For each virus analysed, the prevalence varied according to the screening technique used, the geographic regions, and their characteristics.
Prevalence of Chikungunya virus
According to the assessment of IgG antibodies, the overall seroprevalence of CHIKV was 21.6% (95% CI: 20.6% to 22.5%). Several studies reported a seroprevalence of 0%, while one in Rwanda in 2015 found a seroprevalence of 63.0% (95% CI: 59.8% to 66.2%)28. By regions, the highest value was in Africa (seroprevalence 37.8%, 95% CI: 36.2% to 39.4%). The prevalence ratio between studies performed in an endemic area or during an outbreak and those in non-endemic regions was 24.4 (Table I).
Table I.
Global prevalence of Chikungunya virus in blood donors, by population
The only study we identified on the seroprevalence of IgM antibodies against CHIKV in blood donors reported a seroprevalence of 5.5% (95% CI: 3.1% to 9.7%) (Table I).
Finally, NAT showed an overall prevalence of 0.5% (95% CI: 0.4% to 0.5%). The highest rates were in the screening in Puerto Rico in 2014 (prevalence 1.9%, 95% CI: 1.4% to 2.4%)35. In populations living in endemic areas or going through epidemic outbreaks, the prevalence was 0.6% (95% CI: 0.6% to 0.7%), compared to 0% in non-endemic regions (Table I).
Prevalence of Dengue virus
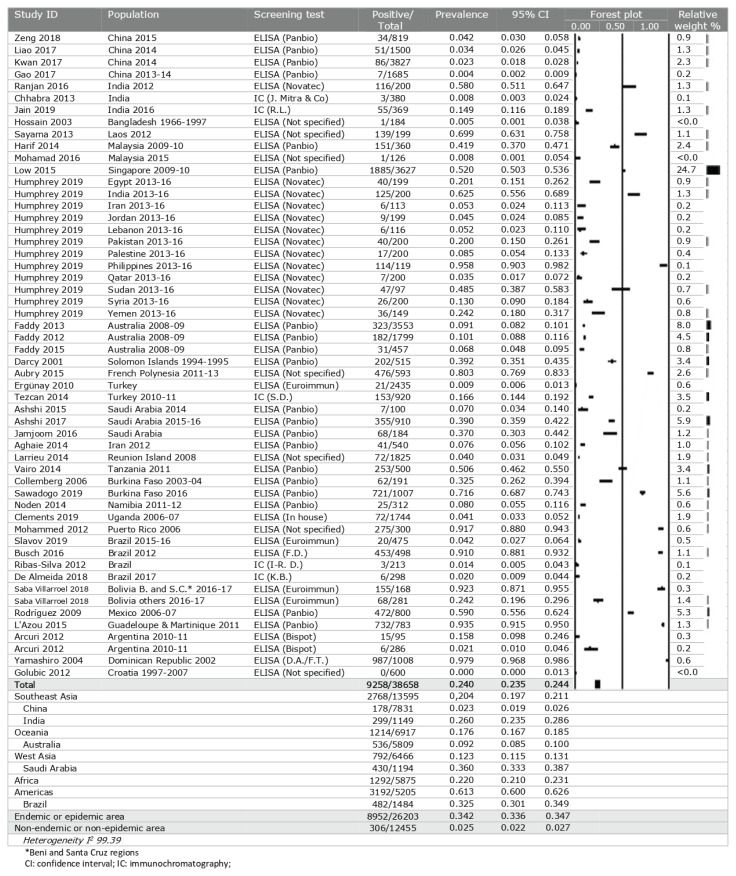
Tests for IgG antibodies showed an overall seroprevalence of DENV of 24.0% (95% CI: 23.5% to 24.4%). Several studies reported a seroprevalence of more than 90% of screened individuals, for example in the Philippines, Puerto Rico, Brazil, Guadeloupe and Martinique, and the Dominican Republic27,81,90,98,100. By geographic region, the Americas stand out for the high seroprevalence of 61.3% (95% CI: 60.0% to 62.6%), followed by Africa at 22.0% (95% CI: 21.0% to 23.1%) and Southeast Asia at 20.4% (95% CI: 19.7% to 21.1%). Saudi Arabia and Brazil were the individual countries with the highest seroprevalence (36.0% and 32.5%, respectively). The prevalence ratio between endemic and non-endemic regions was 13.9 (Table II).
Table II.
Global prevalence of IgG antibodies against Dengue virus in blood donors, by population
Tests for IgM antibodies against DENV show a seroprevalence of 1.4% (95% CI: 1.3% to 1.5%), with individual studies reporting values ranging from 0% to 13.5% (95% CI: 1.3% to 1.5%); this top value was reported in greater Delhi (India) in 201250. By geographic region, the highest percentage of positive results was in the Americas, with a seroprevalence of 3.7% (95% CI: 3.3% to 4.2%). By country, the highest seroprevalence was reported in China, at 5.5% (95% CI: 4.1% to 6.8%). Most studies took place in regions where DENV is endemic and/or had epidemic outbreaks at the time (Table III).
Table III.
Global prevalence of IgM antibodies against Dengue virus in blood donors, by population
The NAT showed an overall DENV viraemic rate of 0.2% (95% CI: 0.2% to 0.2%), with the highest results coming from Saudi Arabia in 2015 to 2016 (prevalence 5.5%, 95% CI: 4.2% to 7.2%)71. In the Americas, the prevalence was 0.2% (95% CI: 0.2% to 0.2%). The only three studies undertaken in non-endemic regions found a prevalence of 0.0% (Table IV). The highest prevalence was in Brazil, at 0.3% (95% CI: 0.3% to 0.3%).
Table IV.
Global prevalence of Dengue virus in blood donors according to nucleic acid amplification and NS1 antigen, by population
Finally, several studies tested donors for the dengue NS1 antigen, which showed an overall prevalence of 0.2% (95% CI: 0.1% to 0.2%), with results in individual studies ranging from 0% to 5.3% (95% CI: 4.0% to 6.9%). These latter results came from Saudi Arabia in 2015 to 2016, in the screening reported by Ashshi et al.72. By region, the Americas again led the ranking for the highest prevalence, with a pooled proportion of 0.1% (95% CI: 0.1% to 0.1%). All the studies took place in endemic regions or in areas with an epidemic outbreak (Table IV).
Prevalence of Zika virus
The overall seroprevalence of IgG antibodies against ZIKV was 5.1% (95% CI: 4.6% to 5.7%). The highest rate was in the donor screening programme in the Bolivian regions of Beni and Santa Cruz in 2016 to 2017, at 27.5% (95% CI: 22.8% to 32.8%)40. By region, the highest seroprevalence was again in the Americas, at 7.4% (95% CI: 6.3% to 8.7%). The prevalence ratio between endemic and non-endemic regions was 9.0 (Table V).
Table V.
Global prevalence of Zika virus in blood donors, by population
NAT showed an overall prevalence of ZIKV of 0.7×10−2% (95% CI: 0.7×10−2% to 0.8×10−2%), varying from 0% to 2.8% (95% CI: 2.1% to 3.8%). The highest viraemic rate was recorded in a study in French Polynesia in 2013 to 201426. The country with the highest prevalence estimate for ZIKV was Brazil (0.5%, 95% CI: 0.4% to 0.7%). The studies in endemic populations, with local transmission or an epidemic outbreak, documented a prevalence of 0.1% (95% CI: 0.1% to 0.1%) (Table V).
DISCUSSION
Since the advent of blood transfusions, patients’ safety has been threatened by the transmission of infectious agents125. Since the turn of the century, a high number of transfusion-transmitted arbovirus cases have been notified, in some instances ending in a fatal outcome for the patient2. The chance that an asymptomatic but viraemic person donates blood is an important concern for transfusion safety and is a possibility for all of the three arboviruses studied. To understand the magnitude of the problem, it is essential to review the published literature reporting viraemic rates in blood donors. Our study updates, collates, and summarises all the notified and published data to date.
The viraemic rates of the three arboviruses in areas experiencing outbreaks were high according to NAT screening (from 1.9% for CHIKV to 5.5% for DENV and 2.8% for ZIKV)35,71,26. Such donors are asymptomatic but infected, often with high levels of viraemia, so there is a real risk of transmission of these viruses via transfusion. NAT methods are expensive and complex, and they require a series of material and human resources that are not accessible in all settings. Health services in most countries do not make routine use of NAT assays capable of detecting these viruses during the donation process. No study in Africa used this screening technique. On the other hand, when NAT assays are used in areas with no outbreaks, the prevalence is practically zero. Consequently, it is important to select the target population appropriately for these screening tests.
Assessing seroprevalence of different arboviruses is important for understanding population exposures in the past. High rates of exposure could be correlated to a greater number of infected and asymptomatic donors capable of transmitting the infection, so this could constitute a source of information on the magnitude of the problem. Moreover, as Liu et al. pointed out in their review, quantifying the seroprevalence of these viruses is of interest from an epidemiological point of view22. In some populations, blood donor screening is the only type of seroprevalence study that has been performed.
The seroprevalence of IgG antibodies against the three arboviruses was high, especially for CHIKV and DENV. In the case of CHIKV, we found the highest seroprevalence of IgG antibodies in sub-Saharan Africa, where periodic outbreaks have been recorded since the 1950s. Some of the most prominent occurred in the Republic of Tanzania in 1954, in the Democratic Republic of Congo in 1999 to 2000, and in Kenya in 2017. The high seroprevalence found in the regions of Beni and Santa Cruz (Bolivia)40, areas with very specific climatic, environmental, and economic conditions, was also noteworthy. We found high seroprevalence rates for IgG against DENV in hyperendemic regions or where studies took place following extensive epidemic outbreaks. Fourteen studies reported seroprevalence rates of more than 50%, with several reporting rates over 90%. DENV has been producing epidemic outbreaks for more than 200 years. This long epidemiological trajectory has translated into its wider geographic dissemination and generally higher seroprevalence rates. The seroprevalence of IgG against ZIKV in blood donors is clearly the lowest for the three arboviruses studied, reflecting the very recent appearance of this virus, which has only caused significant outbreaks since about 2007. As with CHIKV, the highest seroprevalence was found in the Beni region of Bolivia, as well as in Laos and the São Paulo region in Brazil, where outbreaks have been registered since 201640,106,114. However, the seroprevalence in African blood donors is very low, indicating the limited transmission of the virus on this continent, in contrast to DENV and CHIKV. The areas in which seroprevalence is highest have some similarities: a tropical climate with a clear, rainy season, abundant vegetation and water resources, and a low level of economic resources. All health centres and institutions should support efforts to reduce the risk of transmitting arboviruses through blood transfusions. A wide range of interventions could have an impact, from broad environmental policies directed at addressing the climate crisis or the use of water, agricultural, and forestry resources, to community-based environmental measures targeting vector control, improved conservation of wetlands and water resources, and improvements to health systems.
Blood transfusion centres also have a role to play: first, we should improve screening in potential blood donors using specific questions about the symptomology of potential infections. It is also important that donors understand the symptoms of possible infections and are encouraged to report any they experience in the days and weeks following the donation. Secondly, it may be worth establishing a quarantine period for red blood cell concentrates, postponing their release until after the incubation period for infections has passed. Implementation of these measures requires adequate training among personnel working in donor selection or haemovigilance and co-responsibility among donors in terms of monitoring their own health. However, these measures would not enable identification of asymptomatic donors126. The following measure would therefore be the suspension of blood donation collections in a region, as done during the 2007 CHIKV outbreak in Italy, although this measure is difficult to apply in low-resource areas127. Donor screening (ideally using NAT) to detect a virus or its biomarkers is another possibility. When NAT is not available, one more affordable and accessible option of interest is point-of-care testing (immunoassay, reverse transcriptase polymerase chain reaction [RT-PCR], reverse-transcription loop-mediated amplification [RT-LAMP]), which has demonstrated an acceptable sensitivity and specificity for CHIKV, DENV, and ZIKV, respectively128,129,130. Finally, where available, techniques for the deactivation of pathogens could also be applied, as these methods have proven effective against several different arboviruses 131,132,133,134.
Our study has several limitations, chief among which is the considerable heterogeneity of the diagnostic tests used by different groups on the same virus (from commercial test kits to in-house techniques). These tests have different sensitivities and specificities. Moreover, in the case of DENV, different NAT assays could fail to detect some serotypes or genotypes in naïve populations. Most commercial techniques have an acceptable sensitivity for the four serotypes: MA assay Gen-Probe (limit of detection [LoD] 95% 14.9 copies/mL; specificity 99.91%), RealStar dengue RT-PCR assay Altona Diagnostic (sensitivity 83.2%, 95% CI: 77.6% to 89.1%), Cobas CHIKV/DENV test Roche Molecular Sistems (LoD 95% 0.37 to 1.05 copies/mL, specificity 100%)104,135,33. However, in-house techniques are more variable: some are capable of detecting all four serotypes with acceptable sensitivity, while others have been designed to detect only the serotype in circulation in the specific setting in which it is being used.
Another problem is cross-reactivity between different arboviruses. Although the highest prevalence of CHIKV was in Africa, most studies did not perform neutralisation tests or only performed them on a subsample of those yielding positive results. CHIKV shows cross-reactivity with other alphaviruses such as the O’nyong-o’nyong and Mayaro viruses. Clements et al. identified 552 donors with a positive result for CHIKV, but neutralisation tests were run in just 24; of these, 23 showed higher titres for O’nyong-o’nyong virus than for Chikungunya31. Thus, the results for prevalence of IgG antibodies against CHIKV should be interpreted with caution, especially in Africa, where other alphaviruses have been shown to circulate. Although DENV also shows some cross-reactivity with other flaviviruses, authors of the studies on this virus usually did perform neutralisation tests (normally to identify the DENV serotype). In the case of ZIKV, all the studies included virus neutralisation tests. We selected studies performed in the blood donor population in order to obtain data that are representative of that population. However, the results may not be applicable to the general population. Studies are not available in all geographic areas, and a substantial proportion have been in areas known to have high prevalence, which may lead to an overestimation of results. In addition, the between-study heterogeneity was quite high (I2 >75% in all cases). As a single review author selected the studies for inclusion, we cannot rule out the risk of selection bias. Moreover, some risk of publication bias is possible, as there may have been unpublished studies finding negligent prevalence estimates. So, the external validity of the study may be limited by the real prevalence.
CONCLUSIONS
Our review has helped to elucidate the prevalence of CHIKV, DENV, and ZIKV in blood donors around the world, as determined by different screening tests. We have demonstrated that in regions where large epidemic outbreaks have occurred, the donor population has been widely exposed to the viruses, and the viraemic rate observed from donor screening may be high. This fact represents a threat to blood transfusion safety, so it is important that centres involved in these procedures understand the epidemiology driving the emergence of these transfusion-transmissible arbovirus infections. Over the next few years, it is likely that the vector will expand into new settings, increasing the risk of outbreaks worldwide. The transmission of different arboviruses through transfusion will become a global threat. Institutions, authorities, blood transfusion centres, and blood banks should make efforts to design a clear path forward.
Supplementary Information
ACKNOWLEDGEMENTS
We express our thanks to Meggan Harris for her assistance in editing.
Footnotes
AUTHORSHIP CONTRIBUTIONS
All Authors contributed to the study design and final approval of manuscript.
The Authors declare no conflicts of interest.
REFERENCES
- 1.World Health Organization. Blood donor selection: guidelines on assessing donor suitability for blood donation. 2012. [accedded on 16/06/2020]. Available at: https://apps.who.int/iris/bitstream/handle/10665/76724/9789241548519_eng.pdf. [PubMed]
- 2.Petersen LR, Busch MP. Transfusion-transmitted arboviruses. Vox Sang. 2010;98:495–503. doi: 10.1111/j.1423-0410.2009.01286.x. [DOI] [PubMed] [Google Scholar]
- 3.American Association of Blood Banks. Chikungunya virus. Transfusion. 2009;49(Suppl 2):59S–61S. [Google Scholar]
- 4.Madariaga M, Ticona E, Resurrecion C. Chikungunya: bending over the Americas and the rest of the world. Braz J Infect Dis. 2016;20:91–8. doi: 10.1016/j.bjid.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autochthonous transmission of Chikungunya virus in EU/EEA, 2007–2017 [ Internet] European Centre for Disease Prevention and Control; [Accessed on 16/06/2020]. Available at: https://www.ecdc.europa.eu/en/all-topics-z/Chikungunya-virus-disease/surveillance-and-disease-data/autochthonous-transmission. [Google Scholar]
- 6.Fiebre Chikungunya - Epidemiología y situación mundial [Internet] Asociación de Médicos de Sanidad Exterior; [Accessed on 08/06/2021]. Available at: https://www.amse.es/informacion-epidemiologica/658-fiebre-chikungunya. [Google Scholar]
- 7.Chikungunya [Internet] Centers for Disease Control and Prevention CDC; [Accessed on 08/06/2021]. Yellow Book Chapter 4. Available at: https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/chikungunya. [Google Scholar]
- 8.Parola P, De Lamballerie X, Jourdan J, et al. Novel Chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis. 2006;12:1493–9. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Organización Panamericana de la Salud. Organización Mundial de la Salud. Dengue: Guías para el diagnóstico, tratamiento, prevención y control. La Paz: Bolivia. OPS/OMS; 2010. [Google Scholar]
- 10.Gubler DJ. Dengue/dengue haemorrhagic fever: history and current status. Novartis Found Symp. 2006;277:3–16. doi: 10.1002/0470058005.ch2. ; discussion 16–22, 71–3, 251–3. [DOI] [PubMed] [Google Scholar]
- 11.Dengue y Dengue grave [Internet] World Health Organization; 2020. [Accessed on 16/06/2021]. Available at: https://www.who.int/es/news-room/fact-sheets/detail/Dengue-and-severe-Dengue. [Google Scholar]
- 12.Autochthonous transmission of Dengue virus in EU/EEA, 2010/19 [Internet] European Centre for Disease Prevention and Control; [Accessed on 16/06/2021]. Available at: https://www.ecdc.europa.eu/en/all-topics-z/Dengue/surveillance-and-disease-data/autochthonous-transmission-Dengue-virus-eueea. [Google Scholar]
- 13.Tambyah PA, Koay ES, Poon ML, et al. Transfusion-Transmitted Dengue Infection Study Group. Dengue hemorrhagic fever transmitted by blood transfusion. N Engl J Med. 2008;359:1526–7. doi: 10.1056/NEJMc0708673. [DOI] [PubMed] [Google Scholar]
- 14.American Association of Blood Banks. Dengue viruses. Transfusion. 2009;49(Suppl 2):67S–9S. [Google Scholar]
- 15.Karim F, Nasir N, Moiz B. Transfusion transmitted Dengue: one donor infects two patients. Transfus Apher Sci. 2017;56:151–3. doi: 10.1016/j.transci.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Sabino EC, Loureiro P, Lopes ME, et al. Transfusion-transmitted dengue and associated clinical symptoms during the 2012 epidemic in Brazil. J Infect Dis. 2016;213:694–702. doi: 10.1093/infdis/jiv326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matos D, Tomashek KM, Perez-Padilla J, et al. Probable and possible transfusion-transmitted dengue associated with NS1 antigen-negative but RNA confirmed-positive red blood cells. Transfusion. 2016;56:215–222. doi: 10.1111/trf.13288. [DOI] [PubMed] [Google Scholar]
- 18.Slavov SN, Otaguiri KK, Kashima S, et al. Overview of Zika virus (ZIKV) infection in regards to the Brazilian epidemic. Braz J Med Biol Res [Internet] 2016;49:e5420. doi: 10.1590/1414-431X20165420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epidemiological update: second case of locally acquired Zika virus disease in Hyères, France [Internet] European Centre for Disease Prevention and Control; [Accessed on 23/06/2021]. Available at: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-second-case-locally-acquired-zika-virus-disease-hyeres-france. [Google Scholar]
- 20.Barjas-Castro ML, Angerami RN, Cunha MS, et al. Probable transfusion-transmitted Zika virus in Brazil. Transfusion. 2016;56:1684–8. doi: 10.1111/trf.13681. [DOI] [PubMed] [Google Scholar]
- 21.Motta IJ, Spencer BR, Cordeiro SG, et al. Evidence for transmission of Zika virus by platelet transfusion. N Engl J Med. 2016;375:1101–3. doi: 10.1056/NEJMc1607262. [DOI] [PubMed] [Google Scholar]
- 22.Liu R, Wang X, Ma Y, et al. Prevalence of Zika virus in blood donations: a systematic review and meta-analysis. BMC Infect Dis. 2019;19:590. doi: 10.1186/s12879-019-4226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eick SM, Dale AP, McKay B, et al. Seroprevalence of Dengue and Zika virus in blood donations: a systematic review. Transfus Med Rev. 2019;33:35–42. doi: 10.1016/j.tmrv.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Sayama Y, Suksakhone C, Keokhamphoui C, et al. Prevalence of mosquito-borne viral infection (dengue fever, Japanese encephalitis, and chikungunya fever) among blood donors in Lao PDR. Vox Sang. 2013;105:98–9. [Google Scholar]
- 25.Appassakij H, Promwong C, Rujirojindakul P, et al. The risk of blood transfusion-associated Chikungunya fever during the 2009 epidemic in Songkhla Province, Thailand. Transfusion. 2014;54:1945–52. doi: 10.1111/trf.12575. [DOI] [PubMed] [Google Scholar]
- 26.Beau F, Lastère S, Mallet HP, et al. Impact on blood safety of the last arboviruses outbreaks in French Polynesia (2012–18) Transfus Clin Biol. 2020;27:4–9. doi: 10.1016/j.tracli.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Humphrey JM, Al-Absi ES, Hamdan MM, et al. Dengue and chikungunya seroprevalence among Qatari nationals and immigrants residing in Qatar. PLoS One. 2019;14:e0211574. doi: 10.1371/journal.pone.0211574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seruyange E, Ljungberg K, Muvunyi CM, et al. Seroreactivity to Chikungunya and West Nile viruses in Rwandan blood donors. Vector Borne Zoonotic Dis. 2019;19:731–40. doi: 10.1089/vbz.2018.2393. [DOI] [PubMed] [Google Scholar]
- 29.Brouard C, Bernillon P, Quatresous I, et al. Estimated risk of Chikungunya viremic blood donation during an epidemic on Reunion Island in the Indian Ocean, 2005 to 2007. Transfusion. 2008;48:1333–41. doi: 10.1111/j.1537-2995.2008.01646.x. [DOI] [PubMed] [Google Scholar]
- 30.Moyen N, Thiberville SD, Pastorino B, et al. First reported chikungunya fever outbreak in the Republic of Congo, 2011. PLoS One. 2014;9:e115938. doi: 10.1371/journal.pone.0115938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clements TL, Rossi CA, Irish AK, et al. Chikungunya and O’nyong-nyong viruses in Uganda: implications for diagnostics. Open Forum Infect Dis. 2019;6:ofz001. doi: 10.1093/ofid/ofz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benites BD, Rocha D, Andrade E, et al. Zika virus and the safety of blood supply in Brazil: a retrospective epidemiological evaluation. Am J Trop Med Hyg. 2019;100:174–7. doi: 10.4269/ajtmh.17-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stramer SL, Stanley J, Nguyen ML, et al. Duplex nucleic acid test for the detection of chikungunya and dengue RNA viruses in blood donations. Transfusion. 2019;59:1283–90. doi: 10.1111/trf.15128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu CY, Bres V, Yu G, et al. Genomic assays for identification of Chikungunya virus in blood donors, Puerto Rico, 2014. Emerg Infect Dis. 2015;21:1409–13. doi: 10.3201/eid2108.150458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simmons G, Brès V, Lu K, et al. High incidence of Chikungunya virus and frequency of viremic blood donations during epidemic, Puerto Rico, USA, 2014. Emerg Infect Dis. 2016;22:1221–8. doi: 10.3201/eid2207.160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saa P, Chiu C, Grimm K, et al. Acute Zika virus infection in an asymptomatic blood donor at the onset of the Puerto Rico epidemic. Transfusion. 2019;59:3164–70. doi: 10.1111/trf.15484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slavov SN, Otaguiri KK, Bianquini ML, et al. Seroprevalence of Chikungunya virus in blood donors from Northern and Southeastern Brazil. Hematol Transfus Cell Ther. 2018;40:358–62. doi: 10.1016/j.htct.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma R, Costa Santos L, da Silva RA, et al. Surveillance of donated blood during the 2016 arbovirus outbreak in Brazil. J Med Virol. 2018;90:1406–10. doi: 10.1002/jmv.25193. [DOI] [PubMed] [Google Scholar]
- 39.Gallian P, Leparc-Goffart I, Richard P, et al. Epidemiology of Chikungunya virus outbreaks in Guadeloupe and Martinique, 2014: an observational study in volunteer blood donors. PLoS Negl Trop Dis. 2017;11:e0005254. doi: 10.1371/journal.pntd.0005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saba Villarroel PM, Nurtop E, Pastorino B, et al. Zika virus epidemiology in Bolivia: a seroprevalence study in volunteer blood donors. PLoS Negl Trop Dis. 2018;12:e0006239. doi: 10.1371/journal.pntd.0006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sargento C, Silva E, Ferreira C, et al. Is chikungunya virus a blood transfusion problem in north Portugal? Vox Sang. 2017;112:168. [Google Scholar]
- 42.Zeng P, Liao Q, Gao Z, et al. Sero-prevalence and viremia status of dengue virus among asymptomatic blood donors post epidemic outbreak in Chinese Guangzhou in 2015. Transfus Med. 2018;28:468–9. doi: 10.1111/tme.12551. [DOI] [PubMed] [Google Scholar]
- 43.Liao Q, Shan Z, Wang M, et al. An evaluation of asymptomatic Dengue infections among blood donors during the 2014 Dengue outbreak in Guangzhou, China. J Med Virol. 2017;89:2037–40. doi: 10.1002/jmv.24883. [DOI] [PubMed] [Google Scholar]
- 44.Chen JY, Liao Q, You RR, et al. Serum epidemiological investigation of dengue virus infection in blood donors from Guangzhou. J Trop Med. 2015;8:1014–6. [Google Scholar]
- 45.Kwan TH, Lee SS, Chan DPC, et al. Assessing the risk of dengue virus transmission in a non-endemic city surrounded by endemic and hyperendemic areas. Int J Infect Dis. 2017;55:99–101. doi: 10.1016/j.ijid.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Gao Z, Zhang Y, Yang Y, et al. Dengue virus infections among blood donors in Guangxi of China, 2013–14. Transfus Med. 2018;28:236–42. doi: 10.1111/tme.12448. [DOI] [PubMed] [Google Scholar]
- 47.Tsai J-J, Lin P-C, Tsai C-Y, et al. Low frequency of asymptomatic dengue virus-infected donors in blood donor centers during the largest dengue outbreak in Taiwan. PLoS One. 2018;13:e0205248. doi: 10.1371/journal.pone.0205248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin MH, Chen JW, Yeh CC. Lesson learned from the 2015 dengue outbreak in Taiwan. Vox Sang. 2016;111:74. [Google Scholar]
- 49.Lu C, Chen Y, Tsai M, et al. Contribution of alanine aminotransferase to prevent DENV transfusion infection among asymptomatic blood donors in Taiwan during 2015 dengue epidemic. Vox Sang. 2018;113:217. [Google Scholar]
- 50.Ranjan P, Natarajan V, Bajpai M, et al. High seroprevalence of dengue virus infection in blood donors from Delhi: a single centre study. J Clin Diagn Res. 2016;10:DC08–DC10. doi: 10.7860/JCDR/2016/21262.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chhabra C, David R, Singh Swaroop N, et al. Additional dengue testing helps in prevention transfusion transmited infections-experience from a tertiary care hospital in North India. Vox Sang. 2013;105:165. [Google Scholar]
- 52.Mangwana S. Dengue viremia in blood donors in Northern India: challenges of emerging dengue outbreaks to blood transfusion safety. Asian J Transfus Sci. 2015;9:177–80. doi: 10.4103/0973-6247.154253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain A, Jain S, Chowdhury N. Seroprevalence of dengue in blood donors in an outbreak: experience of a blood bank in north India. Trop Doct. 2019;49:212–5. doi: 10.1177/0049475519848947. [DOI] [PubMed] [Google Scholar]
- 54.Kulkarni R, Tiraki D, Wani D, et al. Risk of transfusion-associated dengue: screening of blood donors from Pune, western India. Transfusion. 2019;59:458–62. doi: 10.1111/trf.15007. [DOI] [PubMed] [Google Scholar]
- 55.Hossain MA, Khatun M, Arjumand F, et al. Serologic evidence of dengue infection before onset of epidemic, Bangladesh. Emerg Infect Dis. 2003;9:1411–4. doi: 10.3201/eid0911.030117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harif NF, Kader ZS, Joshi SR, et al. Seropositive status of dengue virus infection among blood donors in North Malaysia. Asian J Transfus Sci. 2014;8:64. doi: 10.4103/0973-6247.126702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohamad NN. Prevalence of positive Dengue serology among the blood donors in Hospital University Sains Malaysia (HUSM): a preliminary study. Malays J Pathol. 2016;38:208. [Google Scholar]
- 58.Yusuf MFM, Fauzi HM, Noordin SS, et al. NS1 Dengue antigen among blood donors in two blood collection centers in North Malaysia. Mal J Med Health Sci. 2018;14:17–22. [Google Scholar]
- 59.Low SL, Lam S, Wong WY, et al. Dengue seroprevalence of healthy adults in Singapore: serosurvey among blood donors, 2009. Am J Trop Med Hyg. 2015;93:40–5. doi: 10.4269/ajtmh.14-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fellizar A. Dengue NS1 antigen detection among selected blood donors in a dengue-endemic province in Northern Philippines. Vox Sang. 2012;103:190–1. [Google Scholar]
- 61.Rooks K, Seed CR, Fryk JJ, et al. Mitigating the risk of transfusion-transmitted dengue in Australia. J Blood Transfus. 2016;2016:3059848. doi: 10.1155/2016/3059848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flower RLP, Fryk J, Hyland C, et al. Dengue fever viral exposure rates among Australian blood donors during local outbreaks. Vox Sang. 2011;101:96. [Google Scholar]
- 63.Faddy HM, Seed CR, Fryk JJ, et al. Implications of dengue outbreaks for blood supply, Australia. Emerg Infect Dis. 2013;19:787–9. doi: 10.3201/eid1905.121664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faddy H, Fryk J, Hyland C, et al. Are Australian blood donors exposed to dengue virus during local outbreaks? Vox Sang. 2012;103:185. [Google Scholar]
- 65.Faddy HM, Fryk JJ, Seed CR, et al. The search for dengue virus in Australian blood donations during local outbreaks. Vox Sang. 2015;109:67. [Google Scholar]
- 66.Darcy A, Clothier H, Phillips D, et al. Solomon Islands dengue seroprevalence study--previous circulation of dengue confirmed. P N G Med J. 2001;44:43–7. [PubMed] [Google Scholar]
- 67.Aubry M, Finke J, Teissier A, et al. Seroprevalence of arboviruses among blood donors in French Polynesia, 2011/13. Int J Infect Dis. 2015;41:11–2. doi: 10.1016/j.ijid.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 68.Ergünay K, Saygan MB, Aydoğan S, et al. Investigation of dengue virus and yellow fever virus seropositivities in blood donors from Central/ Northern Anatolia, Turkey. Mikrobiyol Bul. 2010;44:415–24. [PubMed] [Google Scholar]
- 69.Tezcan S, Kizildamar S, Ülger M, et al. Mersin İli Kan Donörlerinde Flavivirus Seroepidemiyolojisi. Mikrobiyol Bul. 2014;48:606–17. doi: 10.5578/mb.8301. [DOI] [PubMed] [Google Scholar]
- 70.Ashshi AM. Serodetection of Dengue virus and its antibodies among blood donors in the western region of Saudi Arabia: a preliminary study. Blood Transfus. 2015;13:135–8. doi: 10.2450/2014.0134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ashshi AM. The prevalence of dengue virus serotypes in asymptomatic blood donors reveals the emergence of serotype 4 in Saudi Arabia. Virol J. 2017;14:107. doi: 10.1186/s12985-017-0768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ashshi AM, Alghamdi S, El-Shemi AG, et al. Seroprevalence of asymptomatic dengue virus infection and its antibodies among healthy/ eligible Saudi blood donors: findings from Holy Makkah City. Virology (Auckl) 2017;8:1–5. doi: 10.1177/1178122X17691261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jamjoom GA, Azhar EI, Kao MA, et al. Seroepidemiology of asymptomatic dengue virus infection in Jeddah, Saudi Arabia. Virology (Auckl) 2016;7:1–7. doi: 10.4137/VRT.S34187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aghaie A, Aaskov J, Chinikar S, et al. Frequency of dengue virus infection in blood donors in Sistan and Baluchestan province in Iran. Transfus Apher Sci. 2014;50:59–62. doi: 10.1016/j.transci.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 75.Larrieu S, Alain Michault A, Polycarpe D, et al. Dengue outbreaks: a constant risk for Reunion Island. Results from a seroprevalence study among blood donors. Trans R Soc Trop Med Hyg. 2014;108:57–9. doi: 10.1093/trstmh/trt110. [DOI] [PubMed] [Google Scholar]
- 76.Vairo F, Nicastri E, Yussuf SM, et al. IgG against dengue virus in healthy blood donors, Zanzibar, Tanzania. Emerg Infect Dis. 2014;20:465–8. doi: 10.3201/eid2003.130150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collenberg E, Ouedraogo T, Ganamé J, et al. Seroprevalence of six different viruses among pregnant women and blood donors in rural and urban Burkina Faso: a comparative analysis. J Med Virol. 2006;78:683–92. doi: 10.1002/jmv.20593. [DOI] [PubMed] [Google Scholar]
- 78.Sawadogo S, Baguiya A, Yougbare F, et al. Seroprevalence and factors associated with IgG anti-DENV positivity in blood donors in Burkina Faso during the 2016 dengue outbreak and implications for blood supply. Transfus Med. 2020;30:37–45. doi: 10.1111/tme.12646. [DOI] [PubMed] [Google Scholar]
- 79.Noden BH, Musuuo M, Aku-Akai L, et al. Risk assessment of flavivirus transmission in Namibia. Acta Trop. 2014;137:123–9. doi: 10.1016/j.actatropica.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 80.Stramer SL, Foster GA, Boucher CB, et al. Comparing the yield of dengue viremic blood donations using NS1 antigen (AG) and NAT. Transfusion. 2011;51:4A. [Google Scholar]
- 81.Mohammed H, Tomashek KM, Stramer SL, et al. Prevalence of anti-dengue immunoglobulin Gantibodies among American Red Cross blood donors in Puerto Rico, 2006. Transfusion. 2012;52:1652–6. doi: 10.1111/j.1537-2995.2011.03492.x. [DOI] [PubMed] [Google Scholar]
- 82.Mohammed H, Linnen JM, Muñoz-Jordán JL, et al. Dengue virus in blood donations, Puerto Rico, 2005. Transfusion. 2008;48:1348–54. doi: 10.1111/j.1537-2995.2008.01771.x. [DOI] [PubMed] [Google Scholar]
- 83.Stramer SL, Foster GA, Brodsky J, et al. Investigational dengue testing yields high rates of ribonucleic acid (RNA)-positive donors in Puerto Rico. Transfusion. 2013;53:215A. [Google Scholar]
- 84.Stramer SL, Linnen M, Carrick M, et al. Dengue donor viremia determined by RNA and NS1 antigen, and detection of dengue transfusion transmission during the 2007 dengue outbreak in Puerto Rico. Vox Sang. 2010;99:32. [Google Scholar]
- 85.Slavov SN, Cilião-Alves DC, Gonzaga FAC, et al. Dengue seroprevalence among asymptomatic blood donors during an epidemic outbreak in Central-West Brazil. PLoS One. 2019;14:e0213793. doi: 10.1371/journal.pone.0213793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Slavov SN, Hespanhol MR, Ferreira AR, et al. Silent dengue virus circulation among asymptomatic blood donors from a hyperendemic Brazilian region. Transfus Med. 2018;28:465–7. doi: 10.1111/tme.12521. [DOI] [PubMed] [Google Scholar]
- 87.Sabino E, Loureiro P, Lopes M, et al. Dengue RNA among blood donors and recipients during large epidemics of DENV-4 in Rio de Janeiro and Recife, Brazil. Vox Sang. 2013;105:39. [Google Scholar]
- 88.Lavezzo LC, Dos Santos Santana V, Terzian AC, et al. Arboviruses in blood donors: a study in the Amazon region and in a small city with a dengue outbreak. Transfus Med. 2010;20:278–9. doi: 10.1111/j.1365-3148.2010.00996.x. [DOI] [PubMed] [Google Scholar]
- 89.Dias LL, Amarilla AA, Poloni TR, et al. Detection of dengue virus in sera of Brazilian blood donors. Transfusion. 2012;52:1667–71. doi: 10.1111/j.1537-2995.2012.03729.x. [DOI] [PubMed] [Google Scholar]
- 90.Busch MP, Sabino EC, Brambilla D, et al. Duration of dengue viremia in blood donors and relationships between donor viremia, infection incidence and clinical case reports during a large epidemic. J Infect Dis. 2016;214:49–54. doi: 10.1093/infdis/jiw122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ribas-Silva RC, Eid AA. Dengue antibodies in blood donors. Rev Bras Hematol Hemoter. 2012;34:193–5. doi: 10.5581/1516-8484.20120048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Levi J, Wendel SW, Rodrigues CL. Absence of dengue viremia in blood donors from São Paulo - Brazil. Vox Sang. 2009;97:124. [Google Scholar]
- 93.Ribas-Silva RC, Eid AA. Dengue antibodies in blood donors. Rev Bras Hematol Hemoter. 2012;34:193–5. doi: 10.5581/1516-8484.20120048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patavino GM, Salles NA, Barreto AE, et al. Use of EIA antigen assay to screen blood donors for dengue virus. Transfusion. 2009;49:208A. [Google Scholar]
- 95.De Carvalho SC, Schatzmayr HS, Dos Santos F, et al. Dengue virus in blood donors from an endemic area in Brazil. Vox Sang. 2010;99:319. [Google Scholar]
- 96.Arellanos-Soto D, Cruz V, Mendoza-Tavera N, et al. Constant risk of dengue virus infection by blood transfusion in an endemic area in Mexico. Transfus Med. 2015;25:122–4. doi: 10.1111/tme.12198. [DOI] [PubMed] [Google Scholar]
- 97.Rodríguez D, Garza M, Chavarria AM, et al. Dengue virus antibodies in blood donors from an endemic area. Transfus Med. 2009;19:125–31. doi: 10.1111/j.1365-3148.2009.00922.x. [DOI] [PubMed] [Google Scholar]
- 98.L’Azou M, Jean-Marie J, Bessaud M, et al. Dengue seroprevalence in the French West Indies: a prospective study in adult blood donors. Am J Trop Med Hyg. 2015;92:1137–40. doi: 10.4269/ajtmh.14-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arcuri A, Salim J, Krakowiesky C, et al. Determination of IgG/IgM anti dengue virus antibodies in blood blanks of two different geographic areas of Argentina, the city of Buenos Aires vs the city of Santo Tome, Corrientes. Vox Sang. 2012;103:189. [Google Scholar]
- 100.Yamashiro T, Disla M, Petit A, et al. Seroprevalence of IgG specific for dengue virus among adults and children in Santo Domingo, Dominican Republic. Am J Trop Med Hyg. 2004;71:138–43. [PubMed] [Google Scholar]
- 101.Golubic D, Dobler G. Flaviviruses in the north-west Croatia. Infektoloski Glasnik. 2012;32:153–7. [Google Scholar]
- 102.Escoval MA, Sousa G, Freitas B, et al. Dengue outbreak in Madeira Island (Portugal) blood safety measures. Vox Sang. 2013;105:192–3. [Google Scholar]
- 103.Sargento C, Ferreira C, Achando P, et al. Choosing a molecular test to screen dengue infection. Vox Sang. 2017;112:168–9. [Google Scholar]
- 104.Linnen JM, Vinelli E, Sabino EC, et al. Dengue viremia in blood donors from Honduras, Brazil, and Australia. Transfusion. 2008;48:1355–62. doi: 10.1111/j.1537-2995.2008.01772.x. [DOI] [PubMed] [Google Scholar]
- 105.Zheng X, Zeng J, Xu X, et al. A preliminary survey of Zika virus infection by nucleic acid test in the volunteer blood donor samples in Shenzhen China. J Med Virol. 2020;92:1326–9. doi: 10.1002/jmv.25654. [DOI] [PubMed] [Google Scholar]
- 106.Pastorino B, Sengvilaipaseuth O, Chanthongthip A, et al. Low Zika virus seroprevalence in Vientiane, Laos, 2003–2015. Am J Trop Med Hyg. 2019;100:639–42. doi: 10.4269/ajtmh.18-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lam S, Chua SS, Stone M, et al. Universal Zika screening for blood donors in Singapore. Transfusion. 2017;57:27A. [Google Scholar]
- 108.Gake B, Vernet MA, Leparc-Goffart I, et al. Low seroprevalence of Zika virus in Cameroonian blood donors. Braz J Infect Dis. 2017;21:481–3. doi: 10.1016/j.bjid.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nurtop E, Moyen N, Dzia-Lepfoundzou A, et al. A report of Zika virus seroprevalence in Republic of the Congo. Vector Borne Zoonotic Dis. 2020;20:40–2. doi: 10.1089/vbz.2019.2466. [DOI] [PubMed] [Google Scholar]
- 110.Diarra I, Nurtop E, Sangaré A, et al. Zika virus circulation in Mali. Emerg Infect Dis. 2020;26:945–52. doi: 10.3201/eid2605.191383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Borena W, Hofer T, Stiasny K, et al. No molecular or serological evidence of Zikavirus infection among healthy blood donors living in or travelling to regions where Aedes albopictus circulates. PLoS One. 2017;12:e0178175. doi: 10.1371/journal.pone.0178175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Slavov SN, Gonzaga FAC, Pimentel BMS, et al. Zika virus RNA surveillance in blood donors in the Federal District of Brazil during the 2016 outbreak. Hematol Transfus Cell Ther. 2020;42:394–6. doi: 10.1016/j.htct.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Magnus MM, Espósito DLA, da Costa VA, et al. Risk of Zika virus transmission by blood donations in Brazil. Hematol Transfus Cell Ther. 2018;40:250–254. doi: 10.1016/j.htct.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Slavov SN, Guaragna, Machado R, et al. Zika virus seroprevalence in blood donors from the Northeastern region of São Paulo State, Brazil, between 2015 and 2017. J Infect. 2020;80:111–115. doi: 10.1016/j.jinf.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 115.Slavov SN, Hespanhol MR, Rodrigues ES, et al. Zika virus RNA detection in asymptomatic blood donors during an outbreak in the northeast region of São Paulo State, Brazil, 2016. Transfusion. 2017;57:2897–901. doi: 10.1111/trf.14322. [DOI] [PubMed] [Google Scholar]
- 116.Gallian P, Cabié A, Richard P, et al. Zika virus in asymptomatic blood donors in Martinique. Blood. 2017;129:263–6. doi: 10.1182/blood-2016-09-737981. [DOI] [PubMed] [Google Scholar]
- 117.Williamson P, Simmons G, Biggerstaff B, et al. Characterization of evolving viral and serological stages of ZIKV RNA positive blood donors and estimation of population incidence of infections during the Puerto Rico Zika epidemic, 2016. Vox Sang. 2018;113:81. [Google Scholar]
- 118.Pate LL, Williamson PC, Busch MP, et al. Detection of Zika virus RNA in United States blood donations using Cobas® Zika on the Cobas® 6800/8800 systems. Transfusion. 2017;57:26A. [Google Scholar]
- 119.Fedyk CG, Shahin G, Cap AP. Screening of donated blood products for Zika virus in US armed services blood program donors. Transfusion. 2019;59:54A–55A. doi: 10.1111/trf.17375. [DOI] [PubMed] [Google Scholar]
- 120.Galel SA, Williamson PC, Busch MP, et al. First Zika-positive donations in the continental United States. Transfusion. 2017;57:762–9. doi: 10.1111/trf.14029. [DOI] [PubMed] [Google Scholar]
- 121.Williamson P, Linnen J, Kessler D, et al. Us blood donors with evidence of Zika virus infection outside areas of active transmission. Vox Sang. 2017;112:45. [Google Scholar]
- 122.Saá P, Proctor M, Foster G, et al. Investigational testing for Zika Virus among U.S. blood donors. N Engl J Med. 2018;378:1778–88. doi: 10.1056/NEJMoa1714977. [DOI] [PubMed] [Google Scholar]
- 123.Covin R, Nguyen K-AT. Zika virus donor screening-first year experience of a community blood center. Transfusion. 2017;57:205A–206A. [Google Scholar]
- 124.Diefenbach CF, Slavov SN, Kashima S, et al. Prevalence of Zika virus (ZIKV) in blood donors from a hemotherapy service of the southern region of Brazil. Vox Sang. 2019;14:157–62. [Google Scholar]
- 125.Perkins HA, Busch MP. Transfusion-associated infections: 50 years of relentless challenges and remarkable progress. Transfusion. 2010;50:2080–99. doi: 10.1111/j.1537-2995.2010.02851.x. [DOI] [PubMed] [Google Scholar]
- 126.Petersen LR, Epstein JS. Chikungunya virus: new risk to transfusion safety in the Americas. Transfusion. 2014;54:1911–5. doi: 10.1111/trf.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liumbruno GM, Calteri D, Petropulacos K, et al. The Chikungunya epidemic in Italy and its repercussion on the blood system. Blood Transfus. 2008;6:199–210. doi: 10.2450/2008.0016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arya SC, Agarwal N. Rapid point–of–care diagnosis of chikungunya virus infection. Asian Pac J Trop Dis. 2011;1:230–1. [Google Scholar]
- 129.Tsai JJ, Liu LT, Lin PC, et al. Validation of the Pockit dengue virus reagent set for rapid detection of dengue virus in human serum on a field-deployable PCR system. J Clin Microbiol. 2018;56:e01865–17. doi: 10.1128/JCM.01865-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mauk MG, Song J, Bau HH, et al. Point-of-care molecular test for Zika infection. Clin Lab Int. 2017;41:25–7. [PMC free article] [PubMed] [Google Scholar]
- 131.Fryk JJ, Marks DC, Hobson-Peters J, et al. Dengue and chikungunya viruses in plasma are effectively inactivated after treatment with methylene blue and visible light. Transfusion. 2016;56:2278–85. doi: 10.1111/trf.13729. [DOI] [PubMed] [Google Scholar]
- 132.Wang Y, Ren K, Liao X, et al. Inactivation of Zika virus in plasma and derivatives by four different methods. J Med Virol. 2019;91:2059–65. doi: 10.1002/jmv.25538. [DOI] [PubMed] [Google Scholar]
- 133.Faddy HM, Fryk JJ, Hall RA, et al. Inactivation of yellow fever virus in plasma after treatment with methylene blue and visible light and in platelet concentrates following treatment with ultraviolet C light. Transfusion. 2019;59:2223–7. doi: 10.1111/trf.15332. [DOI] [PubMed] [Google Scholar]
- 134.Papin JF, Floyd RA, Dittmer DP. Methylene blue photoinactivation abolishes West Nile virus infectivity in vivo. Antiviral Res. 2005;68:84–7. doi: 10.1016/j.antiviral.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 135.Najioullah F, Viron F, Césaire R. Evaluation of four commercial real-time RT-PCR kits for the detection of dengue viruses in clinical samples. Virol J. 2014;11:164. doi: 10.1186/1743-422X-11-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.