Abstract
Autoimmune haemolytic anaemia (AIHA) is a rare autoimmune disease characterised by haemolysis associated with the presence of immunoglobulins and/or components of the complement system on red blood cells (RBCs). It is classified into warm or cold antibody-mediated AIHA according to the temperature at which autoantibodies bind optimally to RBCs. Clinicians should be familiar with the procedural tests used for a complete laboratory investigation of AIHA. Good collaboration between clinicians and laboratory specialists with correct sample handling and an exact diagnostic work-up is extremely important for the correct classification and proper therapeutic management of AIHA. Specialised serological test procedures are very complex. Problems with serological testing may be excluded with the molecular testing, which has now become a gold standard to predict a patient’s phenotype in order to secure the right antigen-matched blood for AIHA patients. More recently, genotyping has been used instead of serological typing and complex adsorption tests. This paper offers a description of various tests for differentiating between types of AIHA. A diagnostic algorithm and the problems of laboratory investigation are also presented, and an application of molecular methods for the blood group typing in AIHA is elaborated.
Keywords: autoimmune haemolytic anaemia, genotyping, molecular testing, serological testing, transfusion
INTRODUCTION
Autoimmune haemolytic anaemia (AIHA) is a rare autoimmune disease characterised by haemolysis associated with the presence of immunoglobulins (Ig)G, IgM, or IgA and/or components of the complement system on red blood cells (RBCs), usually demonstrated by a positive direct antiglobulin test (DAT). DAT alone does not define AIHA; it may be positive in up to 0.1% of healthy individuals1 as well as in many conditions without haemolysis, and may also remain positive in patients with AIHA in remission2.
The annual incidence of AIHA is estimated to be 1–3 in 100,000 of the population3. Depending on the presence of any underlying disorder, AIHA can be subdivided into two types: primary and secondary. It is classified into warm or cold antibody-mediated AIHA according to the temperature at which autoantibodies bind optimally to RBCs.
Classically, warm AIHA is characterised by the IgG class of the autoantibody optimally reactive at 37°C, while cold agglutinin disease (CAD) is caused by the IgM class of the autoantibody with optimal reactivity at 4°C. In mixed type AIHA, both IgG reactive at 37°C and IgM reactive ≥30°C are present. In paroxysmal cold haemoglobinuria (PCH), IgG biphasic haemolysin is reactive when conditions at 4°C, and then at 37°C are met. Atypically, in AIHA patients, DAT may be negative4,5. Most of the time, laboratory investigation of AIHA is very complex and time-consuming, and there are many pitfalls associated with the diagnostic work-up in AIHA patients6,7. Exact diagnostic work-up and the detection of the right type of the autoantibody involved is very important for the correct classification and the therapeutic management of AIHA.
Patients with AIHA frequently have severe anaemia that requires blood transfusion. Serum autoantibodies, that are detected by a positive indirect antiglobulin test (IAT), are often pan-reactive, making it impossible to find compatible blood. They may mask the presence of clinically significant alloantibodies, potentially resulting in a haemolytic transfusion reaction (HTR). Patients with AIHA who are chronically transfused are constantly exposed to risk of RBC alloimmunisation. The rate of alloimmunisation in this group of patients is reported to range from 12 to 40%8–10, which is comparable to other chronically transfused patients, including patients with sickle cell disease (SCD) and thalassaemia. Adsorption tests are special compatibility test procedures used to detect and identify alloantibodies in patients with autoantibodies; however, these tests are labour-intensive and not always efficient. Transfusion of the prophylactic antigen-matched blood has been suggested to avoid and replace complex adsorption testing10, particularly for patients in whom complete phenotype is determined. However, serological phenotyping may be incorrectly performed in DAT-positive patients with antiglobulin-reactive antisera, and not all RBC antigens can be determined with direct agglutinating sera (monoclonal reagents). In addition, mixed-field reaction caused by the prior transfusion may be easily interpreted as positive11. These problems may be excluded with molecular typing. In recognising its advantages over serological phenotyping, genotyping is now becoming a gold standard for testing AIHA patients12,13. Transfusion of antigen-matched blood based on genotyping is increasingly preferred to serological phenotyping14,15.
This paper offers a description of various tests for differentiating between types of AIHA. The importance of the collaboration between the laboratory and the clinic for proper diagnostics, and of timely and correct therapeutic management, is emphasised. A diagnostic algorithm and the problems of laboratory investigation are also presented, and an application of molecular methods for blood group typing in AIHA is elaborated.
TYPE OF AUTOIMMUNE HAEMOLYTIC ANAEMIA
Type of AIHA is determined by the serological characteristics of the autoantibodies detected on the patient’s RBCs and/or in the patient’s serum. Tests to be considered by clinicians for the purpose of differentiating between types of AIHA are presented in Table I.
Table I.
Tests for differentiating types of autoimmune haemolytic anaemia
| Warm IgG AIHA | Warm IgM AIHA | Warm IgA AIHA | CAD | Mixed AIHA | PCH | DAT negative AIHA | |
|---|---|---|---|---|---|---|---|
| Ig | IgG | IgM | IgA | IgM | IgG+IgM | IgG | IgG, IgA, IgM |
| DAT poly | + | − | − | + | + | + | − |
| DAT mono anti-IgG | + | − | − | − or + | + | − | − |
| DAT mono anti-C3d | + or − | + or − | + or − | + | + | + | − |
| DAT mono anti-IgM | − | + or − | − | − | + or − | − | + or − |
| DAT mono anti-IgA | + or − | − | + | − | + or − | − | + or − |
| Eluate | IgG | IgM | IgA | − | IgG (IgM) | − | IgG (IgM) or − |
| DA ggT | − | + | − | + | + | − | − |
| IAT | + | − | − | − | + | − | − |
| Thermal amplitude | Optimally at 37°C | Optimally at 37°C | Optimally at 37°C | Optimally at 4°C | Usually ≥30°C | Biphasic 4°C→ 37°C | Optimally at 37°C |
| Cold antibody titre | NA | <64 | NA | ≥64 | ≥64 (<64) | <64 | NA |
| Donath-Landsteiner test | NA | NA | NA | NA | NA | + | NA |
| Other tests * | NA | NA | NA | NA | NA | NA | + |
Enzyme-linked antiglobulin test, radiolabelled anti-IgG tests, flow-cytometry, monocyte monolayer assay, concentrated eluates, mitogen-stimulated-DAT.
AIHA: autoimmune haemolytic anaemia; CAD: cold agglutinin disease; DAggT: direct agglutination test; DAT mono: direct antiglobulin test monospecific; DAT poly: direct antiglobulin test polyspecific; NA: not applicable; IAT: indirect antiglobulin test; Ig: immunoglobulin; PCH: paroxysmal cold haemoglobinuria. +positive; − negative.
Warm autoimmune haemolytic anaemia
In warm AIHA, autoantibodies show optimal reactivity at 37°C. In most cases, they are of the IgG class, and are usually directed against antigens of the Rhesus blood group system. However, other antigens may be implicated, such as: Wra, Kpb, Jka and U3. IgG is attached to RBCs usually in combination with C3d, or it is present with RBCs alone. IgG is also detected in the serum and eluate by the IAT (Table I). Warm AIHA is the most common type of AIHA (65–70%) and is usually associated with lymphoproliferative and autoimmune diseases. In the majority of warm AIHA cases, RBC destruction is extravascular. RBCs coated with IgG autoantibodies are phagocytosed primarily in the spleen because of the phagocyte’s expression of receptors for the Fc region of the IgG. Incomplete phagocytosis results in spherocytosis, which characterises the transition from a biconcave to a spherical shape of the cell, and its vulnerability to destruction during subsequent passages through the spleen. When RBCs are heavily coated with IgG autoantibodies, the classical complement pathway may be activated. Depending on the IgG subclass (where IgG3 is a more potent complement activator than IgG1), C3b-opsonised RBCs are further phagocytosed by Kupfer cells in the liver16. Cases of warm AIHA are often mild, and they are typically presented with symptoms of anaemia17. In rare atypical cases, warm autoantibodies can be of the IgM or IgA class. Rarely, only C3d (10%), and, very rarely (1%), IgM or IgA may be present on RBCs, often in association with IgG and/or complement18. Low affinity IgM autoantibodies often detach from the RBCs during washing procedures and usually only C3d is detected on RBCs. The IgM autoantibodies involved, which are often detached from the RBCs, may also explain severe cases with IgA autoantibodies19,20. Cases with warm IgM autoantibodies have been also presented as DAT-negative AIHAs21. Warm IgM autoantibodies show optimal reactivity at 37°C (and at 30°C) by a direct agglutination in the serum and eluate, and they have a low titre (<64) at 4°C (Table I). Warm IgM autoantibodies are good activators of the complement system and primarily cause intravascular RBC destruction, which is described in detail below (see CAD). There are only a few reports of warm IgM-mediated AIHA, and most of them have been associated with severe haemolysis and immune disorders22–25.
Cold agglutinin disease
Cold autoantibodies (also referred to as cold agglutinins, because of the direct agglutination they cause), that characterise CAD, react better at 4°C, but may also react in warmer conditions. Almost in all cases, they are of the IgM class, and are directed against the I/i blood group. Cases with CAD make up 20–25% of all AIHA cases and usually have an underlying lymphoproliferative disease. But recent studies have led to the conclusion that patients with CAD must have a distinct clonal B-cell lymphoproliferative disorder26,27. With the involvement of cold IgM autoantibodies, RBC destruction can be either extravascular (usually in stable cases) or intravascular (in the acute phase of the disease). RBCs coated with IgM autoantibodies are sequestered and phagocytosed in the liver. IgM is a strong complement activator which binds C1q and initiates the classical complement pathway upon C3b formation step. Complement activation may proceed beyond C3b formation and result in a membrane attack complex (MAC) formation and intravascular haemolysis. Due to regulatory proteins CD55 and CD59, complement activation is usually not sufficient to produce clinically significant activation of the terminal complement pathway16. Cold autoantibodies bind to RBCs at lower temperatures in the peripheral circulation and activate complement, but as RBCs circulate to warmer areas, IgM dissociates and C3d remains bound. Therefore, only C3d is usually attached to RBCs. The monospecific DAT can also be weakly positive for IgG in up to 20% of patients with CAD28.
The clinical significance of serum cold autoantibody is determined by the cold antibody titre and thermal amplitude of the cold autoantibody. Titration values can provide information about the relative amount of the antibody present in serum. The titre of an antibody is usually determined by testing two-fold serial dilution of the serum against selected RBCs. Results are expressed as the reciprocal of the highest serum dilution that shows macroscopic agglutination29. The thermal amplitude test is performed to determine the reactivity of cold autoantibody at varying temperatures: 4°C, 22°C, 30°C, and 37°C. The thermal amplitude of the cold autoantibody, defined as the highest temperature at which autoantibody binds to RBCs, most accurately predicts the severity of the disease. Cold autoantibodies that are reactive at temperatures >30°C have the potential to be clinically significant regardless of the cold antibody titre30. Temperature amplitude testing is quite time-consuming, and is, in most cases, not required for diagnosis because cold antibody titres are usually higher than the minimum titre required. Nevertheless, it is useful in some situations to rule out normally occurring cold autoantibodies in cases with low titres31. In addition, the pathogenicity of cold autoantibodies is more dependent on the thermal amplitude than on the antibody titer31. Clinically significant cold autoantibody is determined with a titre of ≥64 at 4°C (although it is usually >1,024) or thermal amplitude >30°C32 (Table I). Although cold autoantibodies are normally seen in healthy individuals, they are present at low titres (<64) and they have no reactivity at temperatures >30°C.
Mixed-type autoimmune haemolytic anaemia
Mixed-type AIHA is characterised by the presence of both warm IgG and cold IgM autoantibodies. Mixed-type AIHA is rare (8–10%) and is usually associated with systemic lupus erythematosus or lymphoma. Patients with mixed AIHA often present with severe haemolysis33–38.
Both C3d and IgG are usually attached to RBCs; however, C3d, IgG or IgA alone may be detected on the RBCs18. Warm IgG is also detected in the serum and eluate by the IAT. Cold IgM autoantibody has a thermal range of at least up to 30°C; it usually has a cold antibody titre of >1,024, but may have a low antibody titre (<64) (Table I).
Paroxysmal cold haemoglobinuria
Paroxysmal cold haemoglobinuria (PCH), also known as Donath-Landsteiner syndrome is activated by IgG biphasic autoantibodies or Donath-Landsteiner haemolysins. IgG biphasic autoantibodies are usually directed against the P antigen. PCH is very rare (1%), and is often detected in paediatric cases characterised with frequently severe attacks of haemolysis following a viral illness39,40. Biphasic IgG autoantibodies bind to RBCs and fix complement in colder areas of the body, but they activate complement and dissociate from RBCs when blood circulates to warmer areas. This is also the principle of a diagnostic test used for the diagnosis of biphasic haemolysins, termed the Donath-Landsteiner test. Only C3d is usually attached to RBCs, or a DAT is negative. IgG biphasic autoantibodies are not reactive above 4°C, so antibody screening test by IAT is usually negative29. Cold antibody titre is low and is rarely >16 (Table I).
Direct antiglobulin test-negative autoimmune haemolytic anaemia
On rare occasions (5%), DAT may be negative in patients with haemolysis, which is explained with the very low quantity of RBC-bound IgG, or an immunoglobulin not tested in IgA- or IgM-only AIHA6,41,42. Another possible reason for the absence of a positive DAT might be the low IgG affinity. The majority of DAT-negative cases are severe and refractory, and may be fatal5,43. The threshold for the IgG detection by DAT varies with the commercial antiglobulin reagent used, ranging from 150 to 500 IgG molecules/RBC. However, as shown from the earlier studies of Gilliland using the complement-fixing, antibody consumption test for quantification of IgG molecules bound to RBCs, fewer than 150 molecules/RBC may induce haemolysis44,45. Kamesaki et al. analysed the quantity of RBC-bound IgG molecules in DAT-negative AIHA cases by using an immunoradiometric assay. They concluded that IgG levels on RBCs should be measured for the diagnosis of DAT-negative AIHA, and a cut-off of 78.5 IgG molecules/RBC should be used to distinguish AIHA from non-specific DAT-positive cases46.
Monospecific antisera (IgG, IgA, IgM) or more sensitive tests (e.g., enzyme-linked antiglobulin test, radiolabelled anti-IgG tests, flow-cytometry, monocyte monolayer assay, concentrated eluates, mitogen-stimulated-DAT) than traditional DAT tube, microcolumn and solid-phase, may detect autoantibodies in these cases7,47,48 (Table I). In most cases with warm IgM where only C3d bound to RBCs is detected, IgM may be detected with the Dual Direct Antiglobulin Test, which is based on the agglutination of the non-agglutinable RBCs coated with low IgM concentration using a second antiglobulin reagent49. Most laboratories do not have these tests or reagents available; they are only available at referral laboratories.
LABORATORY INVESTIGATION
Clinicians need to be familiar with the procedural tests used for a complete laboratory investigation of AIHA and understand the principles of the tests, the complexities of specialised serological procedures to remove interferences caused by autoantibodies, and the required duration of the tests. Serological findings need to be clearly communicated to clinicians so they can decide on the therapeutic management.
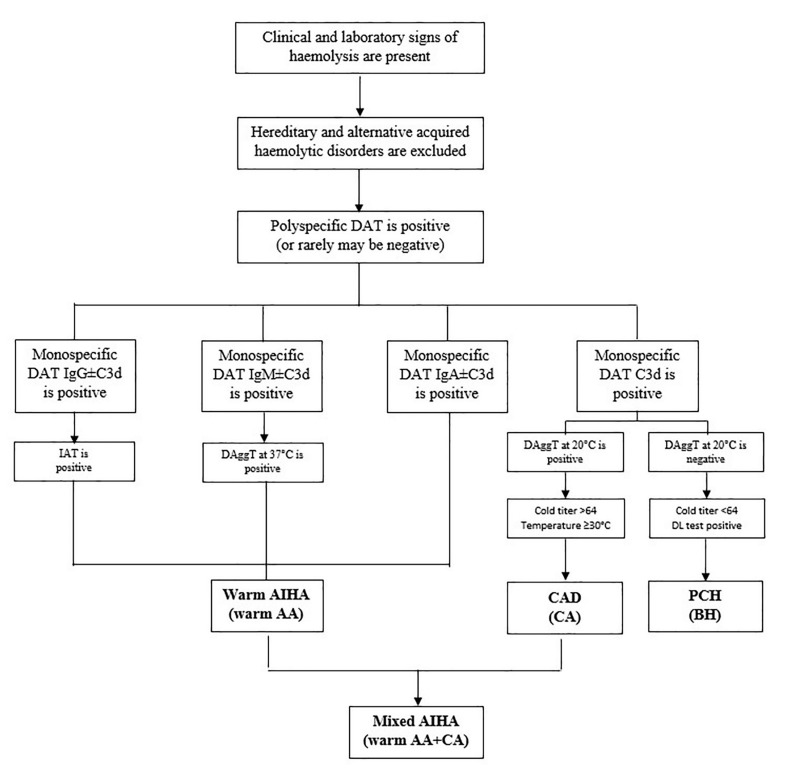
For the patients with a suspected AIHA, routine tests requested by clinicians are DAT and antibody screening test by IAT. Should clinical and laboratory signs of haemolysis be confirmed, patients should undergo further investigation to exclude hereditary or other acquired haemolytic disorders. Firstly, a positive polyspecific DAT indicates that IgG and/or C3d are present on RBCs. Monospecific DAT further indicates which immunoglobulin class (IgG, IgM, IgA) of the autoantibody and/or the component of the complement system (C3c, C3d) is present on the RBCs. At least a monospecific DAT for anti-IgG and C3d is suggested. A positive IAT detects IgG auto- and/or alloantibodies present in serum and/or eluate. According to the preliminary results, additional complex testing can be performed to characterise the type of autoantibody involved. An algorithm for laboratory investigation of AIHA is proposed in Figure 1. The type of AIHA determined according to the results of serological investigation is shown in Table II.
Figure 1.
Diagnostic algorithm for the investigation of autoimmune haemolytic anaemia
AA: autoantibodies; AIHA: autoimmune haemolitic anaemia; BH: biphasic haemolysins; CA: cold autoantibodies; CAD: cold agglutinin disease; DAggT: direct agglutinin test; DAT: direct antiglobulin test; DL: Donath-Landsteimer test; IAT: indirect antiglobulin test; PCH: paroxysmal cold haemoglobinuria.
Table II.
Types of autoimmune haemolytic anaemia according to laboratory investigation
| Type of AIHA | Laboratory investigation |
|---|---|
| Warm AIHA | DAT IgG±C3d (rarely IgA, IgM) positive, and IgG reactive by IAT in serum and eluate, or rarely DAT C3d±IgM positive (or negative), IgM optimally reactive at 37°C in serum and eluate, and a cold antibody titre <64 at 4°C |
| CAD | DAT C3d positive (IgG negative or weakly positive), DAggT positive, and a cold antibody titre ≥64 at 4°C |
| Mixed AIHA | DAT IgG±C3d (rarely IgA, IgM) positive, IgG reactive by IAT in serum and eluate by warm technique using anti-IgG, and IgM with a thermal amplitude ≥30°C, and a cold antibody titre ≥64 at 4°C (but may be <64) |
| PCH | DAT C3d positive, DAggT negative, a cold antibody titer <64 at 4°C, and a Donath-Landsteiner test positive |
| DAT negative AIHA | DAT IgG or C3d negative, DAT IgA, IgM positive or negative, IgG reactive by IAT in eluate, or IgG, IgA, IgM detected by more sensitive tests |
AIHA: autoimmune haemolytic anaemia; CAD: cold agglutinin disease; DAggT: direct agglutination test; DAT: direct antiglobulin test; IAT: indirect antiglobulin test; PCH: paroxysmal cold haemoglobinuria
Should DAT be IgG±C3d (rarely IgA) positive, the IAT will usually detect and identify IgG autoantibodies in serum and eluate at 37°C. When DAT is C3d positive, a DAggT performed at room temperature is suggested for the detection of serum IgM autoantibodies. If positive, the clinical significance of autoantibodies is then determined by a cold antibody titre performed at 4°C, and a temperature amplitude reading at 4°C, 20°C, 30°C, and 37°C. DAT is seldom C3d±IgM positive, or DAT may be even negative, and warm IgM autoantibodies, optimally reactive at 37°C, are detected by a direct agglutination in serum and eluate, with a cold agglutinin titre <64. Warm IgG, IgA, or IgM autoantibodies characterise warm AIHA. In addition to warm IgG, cold IgM autoantibodies (reactive at 20°C) may also be present, but they are not clinically significant (Figure 1).
In cases in which DAT is only C3d positive and DAggT at room temperature is positive, CAD is confirmed when the titre of the cold antibody is ≥64 and/or thermal amplitude of autoantibodies is ≥30°C. However, when DAT is C3d positive and haemoglobinuria is also present, but a DAggT at room temperature is negative or the cold antibody titre is <64, a positive Donath Landsteiner test will confirm PCH. When the criteria for the presence of both, warm AIHA and CAD are met, mixed-type AIHA is confirmed. (Figure 1).
PROBLEMS WITH SEROLOGICAL TESTING
Autoantibodies may interfere with standard pre-transfusion testing primarily related to blood group typing and detection of clinically significant alloantibodies. Therefore, in order to avoid any interference with warm or cold autoantibodies, specialised sample handling and serological testing procedures are required.
A phenomenon called “spontaneous agglutination” is usually observed from the sample in which patient’s RBCs are heavily coated with IgM or IgG autoantibodies. If cold autoantibodies are involved, it may be sufficient to collect, maintain, and separate the sample at 37°C so that IgM is detached from the RBCs. The problem may be further managed by washing the patient’s RBCs several times with 37ºC saline, or by treating RBCs with 0.01 M dithiothreitol (DTT), or 2-mercaptoethanol (ME) reagent, which denature bound IgM. More complex problems with warm autoantibodies may be resolved in a variety of ways: (i) by washing RBCs several times; (ii) by using low-protein antisera (monoclonal reagents that do not require an IAT) for the phenotyping; or (iii) by adopting methods to dissociate IgG from RBCs, such as treatment with ZZAP (a combination of DTT and papain), chloroquine diphosphate (CPD) and EDTA glycine acid.
Patients with warm serum autoantibodies present a challenge in standard pre-transfusion testing. Warm autoantibodies will most likely react with all RBCs tested thus masking the presence of clinically significant alloantibodies. Alloantibodies, if undetected, may cause increased HTR, which may then be falsely attributed to autoantibodies. Specialised serological testing used for the detection of clinically significant alloantibodies when autoantibodies are also present includes adsorption studies and phenotyping. Adsorption tests remove the autoantibody from the patient’s serum leaving alloantibodies in the adsorbed serum where they can be detected and identified. Phenotyping is useful to predict which clinically significant alloantibodies could potentially be present in the patient’s serum50.
Detecting alloantibodies in patients with warm autoantibodies includes autologous or allogeneic warm adsorptions. Autologous adsorption is the best procedure for detecting alloantibodies. Prior to the procedure, patient’s RBCs are first treated with ZZAP, chloroquine diphosphate or EDTA glycine acid, in order to free bound IgG, but these procedures are demanding and not always successful51. The main limitation of testing is that autologous adsorption test may only be performed if the patient has not recently been transfused and when there are enough of the patient’s RBCs available. For allogeneic warm adsorption, phenotype-matched RBCs should be used if the patient’s profile is known; otherwise, if this is not the case, antigen combinations (CcEe, K, Jka, Jkb) must be chosen. There are certain limitations connected with an allogeneic adsorption test because alloantibodies to high-prevalence antigens will be adsorbed and not detected50. The main problem with adsorption tests is that they take many hours to complete, causing significant delays in the patient being transfused, and they may also be comprimised by incomplete adsorption, autoantibodies mimicking alloantibodies, or weak alloantibodies.
Strong cold autoantibodies may also interfere with standard pre-transfusion testing and mask the presence of underlying clinically significant alloantibodies. Interferences with cold antibodies may be avoided by using a pre-warming method. Here, RBCs and patient’s plasma are warmed to 37°C separately before testing so that cold antibodies are prevented from binding once the test RBCs and plasma are combined; this allows clinically significant antibodies that bind at 37°C and/or at antihuman-globulin (AHG) phase to be identified. Anti-IgG is preferred instead of AHG serum to avoid reactions with RBCs-bound complement52. However, caution is needed because weak alloantibodies and alloantibodies that activate the complement system might not be detected29. On rare occasions, a cold autologous or allogeneic adsorption test must be performed in order to remove cold autoantibodies, while leaving clinically significant alloantibodies in the plasma for their detection and identification at 37°C by the IAT53.
Warm sample collection, transport and handling until serum and RBCs are separated is required in a diagnostic workup of cold AIHA to investigate the clinical significance of cold autoantibodies. In this way, IgM autoantibodies are detached from the RBCs so true titre or thermal amplitude of IgM can be determined. It is also important to handle the sample in conditions as close as possible to the physiological temperature of 37°C to avoid haemolysis. This is not common practice in the clinic, but the importance of providing a warm sample is worth remembering and should be recognised by clinicians in order to obtain precise test results. Alternatively, if the sample is warmed at 37°C for 10–15 minutes, adsorbed antibody to RBCs may be released back into plasma29. This may be hard to achieve in cases of strongly reacting cold autoantibodies, even with longer incubation times. Such cases of cold autoantibodies may affect the thermal amplitude by carrying over autoantibody agglutination. This interference can be avoided by carrying out titrations with separate sets of tubes at different temperatures, instead of moving tubes from one temperature to another (37°C, 30°C, 20°C, and 4°C)29.
In mixed-typed AIHA, cases of strongly reacting cold autoantibodies, or those with a broader range of reactivity, may interfere with the reactivity of warm autoantibodies. Reactivity at all phases is tested, so serological testing for warm autoantibodies must be carried out by a pre-warming method using anti-IgG, or, more rarely, a cold adsorption test must be performed to detect both the warm and the cold autoantibodies separately.
Rarely, in cases with a negative DAT or in a case of PCH, autoantibodies cannot be detected by standard testing. In DAT-negative AIHA cases, washing RBCs at 4°C or with low ionic strength solution allows low affinity IgG to be detected49. In PCH, antibody screening by IAT is usually negative and only components of complement are bound to RBCs. Biphasic IgG may only be detected if RBCs are washed with cold saline and tested with a cold reagent18. A Donath-Landsteiner test is suggested to detect the biphasic IgG autoantibody, but false negative results may be caused by low levels of complement present, so the addition of the complement is crucial for testing54. Since there are great variabilities in test results, testing is best performed in reference laboratories.
APPLICATION OF MOLECULAR METHODS IN BLOOD GROUP TYPING
Determination of the patient’s complete or extensive phenotype (CcEe, K, Jka, Jkb, Fya, Fyb and Ss) or a blood group genotype is recommended prior to transfusion in highly alloimmunised chronically transfused patients with SCD and thalassaemia. In order to prevent alloimmunisation, prophylactic antigen-matched blood is suggested for their transfusion55,56. Shirley et al. suggested a similar transfusion management in AIHA patients to prevent alloimmunisation, but also to avoid complex and expensive adsorption studies10. In addition, patients with warm autoantibodies are transfused with incompatible blood and therefore they have a greater risk of HTR. Although extensive antigen-matched blood is preferred for their transfusion, if unavailable, or if only a patient’s partial phenotype (CcEe, K) is known, partial antigen-matched blood units must be selected, and adsorption studies must be performed prior to transfusion.
Precise serological phenotyping is not always possible in AIHA patients since AHG-reactive antisera are not suitable for DAT-positive patients. In addition, methods applied to free bound IgG from the patient’s RBCs are not always successful, and direct agglutination typing sera (monoclonal reagents) are not available for all RBC antigens (e.g., Fya, Fyb, s and k). Often, these patients are also frequently transfused, so mixed-field reaction caused by the donor RBCs may easily be interpreted as positive11. Likewise, the capillary tube method which may be used to distinguish between the patient’s and donor’s RBCs depends on the percentage of the patient’s reticulocytes, and can also give false results57. Furthermore, if depressed, some antigens may be serologically phenotyped as negative in AIHA patients: AnWj, Co3, Ena, Ge3, JMH, Jka, Jkb, Kpb, LW, Rh, Sc1, Sc3, U and Vel58.
By understanding the molecular mechanism behind the blood group antigens, most of the blood group systems have been sequenced and assays for blood group genotyping to predict the phenotype have been developed. The molecular methods currently in use predict the blood group antigen phenotype by detecting blood group polymorphisms and rare alleles, which determine the expression of blood group antigens. However, caution is needed in interpretating these results since the presence of a particular genotype does not guarantee expression of this antigen on RBCs (e.g., in the case of a null allele or a hybrid gene).
Today, molecular testing has become the gold standard for complex problems at reference laboratories, including suspected variants of blood group antigens, and phenotypes obscured by recent transfusions, or a positive DAT12. With the application of mass-scale genotyping, molecular testing has become routine for identifying donors with rare phenotypes or rare combinations of a large number of antigen-negative types to secure the availability of rare blood units in the inventory59,60.
As soon as molecular methods were applied into blood transfusion, differences between the molecular and serological typing were detected in chronically transfused patients with SCD and thalassaemia, and molecular blood group phenotype prediction has been proven to be superior to serological phenotyping for the prevention of alloantibody formation in these groups of patients61–64. Patients who were switched to the correct antigen-matched RBCs had better RBC survival after transfusion65,66. Except for multiply-transfused patients, its application is preferable in DAT-positive patients with AIHA, whose RBCs are coated with IgG and who, therefore, cannot be typed for antigens if direct agglutinating sera are not available. An additional advantage of molecular typing in patients with autoantibodies is that it can show the patient’s status for some high-prevalence antigens, as these may be missed with allogeneic adsorption. Genotyping is also preferable when antigen expression on RBCs is depressed, such as may be the case in AIHA patients. Results of genotyping may not be immediately available, but they are useful for any future antigen-matched transfusions. In addition, in urgent cases, genotyping facilitates the selection of compatible blood for transfusion of AIHA patients13.
Although blood group genotyping is recognised to be beneficial and cost efficient for chronically transfused patients with SCD, thalassaemia and AIHA, its more widespread use will only be achieved with higher and faster high throughput at a lower cost60. Next generation sequencing will allow even more accurate blood group predictions in both donors and patients to be obtained, while it may detect variants that are not predefined by the assays in the molecular methods currently in use15,67, 68.
CONCLUSIONS
Good collaboration between clinicians and laboratory specialists with correct sample handling and an exact diagnostic work-up is extremely important for the correct classification and proper therapeutic management of AIHA. There are certain limitations in the routine pre-transfusion testing. Specialised serological test procedures are very complex. Molecular blood group typing has now become a gold standard to predict a patient’s phenotype in order to secure the right antigen-matched blood for AIHA patients. More recently, genotyping has been used instead of serological typing and complex adsorption tests.
Footnotes
The Authors declare no conflicts of interest.
REFERENCES
- 1.Hannon JL. Management of blood donors and blood donations from individuals found to have a positive direct antiglobulin test. Transfus Med Rev. 2012;26:142–52. doi: 10.1016/j.tmrv.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Parker V, Tormey CA. The direct antiglobulin test. Indication, Interpretation, and Pitfalls. Arch Pathol Lab Med. 2017;141:305–10. doi: 10.5858/arpa.2015-0444-RS. [DOI] [PubMed] [Google Scholar]
- 3.Petz LD, Garratty C. Immune hemolytic anemia. 2nd ed. Philadelphia: Churchill Livingstone; 2004. [Google Scholar]
- 4.Jäger U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the First International Consensus Meeting. Blood Rev. 2020;41:100648. doi: 10.1016/j.blre.2019.100648. [DOI] [PubMed] [Google Scholar]
- 5.Barcelini W, Fattizzo B, Zaninoni A, et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood. 2014;124:2930–6. doi: 10.1182/blood-2014-06-583021. [DOI] [PubMed] [Google Scholar]
- 6.Barcellini W. The clinical dilemma and management of red cell autoantibodies. Expert Rev Hematol. 2016;9:325–7. doi: 10.1586/17474086.2016.1152885. [DOI] [PubMed] [Google Scholar]
- 7.Barcellini W. Pitfalls in the diagnosis of autoimmune haemolytic anaemia. Blood Transfus. 2015;13:3–5. doi: 10.2450/2014.0252-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branch DR, Petz LD. Detecting alloantibodies in patients with autoantibodies. Transfusion. 1997;39:6–10. doi: 10.1046/j.1537-2995.1999.39199116888.x. [DOI] [PubMed] [Google Scholar]
- 9.Leger RM, Garraty G. Evaluation of methods for detecting alloantibodies underlying warm autoantibodies. Transfusion. 1999;39:11–6. doi: 10.1046/j.1537-2995.1999.39199116889.x. [DOI] [PubMed] [Google Scholar]
- 10.Shirey RS, Boyd JS, Parwani AV, et al. Prophylactic antigen-matched donor blood for patients with warm autoantibodies: an algorithm for transfusion management. Transfusion. 2002;42:1435–41. doi: 10.1046/j.1537-2995.2002.00234.x. [DOI] [PubMed] [Google Scholar]
- 11.Wagner FF. Why do we use serological blood group phenotype determination in chronically transfused patients? Blood Transfus. 2014;12:1–2. doi: 10.2450/2013.0186-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flegel WA, Castilho L, Heaton WAL, et al. Molecular immunohematology round table discussion at the AABB Anual Meeting, Anaheim 2015. Blood Transfus. 2016;14:557–63. doi: 10.2450/2016.0063-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sapatnekar S, Figueroa PJ. How to use molecular red blood cell antigen typing to supplement pretransfusion testing? Transfusion. 2014;54:1452–8. doi: 10.1111/trf.12623. [DOI] [PubMed] [Google Scholar]
- 14.Cobianchi da Costa D, Pellegrino J, Guwlsin GAS, et al. Molecular matching of red blood cells is superior to serological matching in sickle cell disease patients. Rev bras Hematol Hemoter. 2013;25:35–8. doi: 10.5581/1516-8484.20130012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutner JM, Mota M, Conti F, et al. Blood genotyping for improved outcomes in chronic transfusion patients: current and future perspectives. International Journal of Clinical Transfusion Medicine. 2014;2:65–72. [Google Scholar]
- 16.Berentsen S. Role of Complement in Autoimmune Hemolytic Anemia. Transf Med Hemother. 2015;42:303–10. doi: 10.1159/000438964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packman CH. The Clinical Pictures of Autoimmune Hemolytic Anemia. Transf Med Hemother. 2015;42:317–24. doi: 10.1159/000440656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein HG, Anstee DJ. Mollison’s Blood Transfusion in Clinical Medicine. 12th ed. Chichester: John Wiley & Sons, Ltd; 2014. [Google Scholar]
- 19.Bardill B, Mengis C, Tschopp M, Wuillemin WA. Severe IgA-mediated autoimmune haemolytic anemia in a 48-yr-old woman. Eur J Haematol. 2003;70:60–3. doi: 10.1034/j.1600-0609.2003.02846.x. [DOI] [PubMed] [Google Scholar]
- 20.Wagner-Ballon O, Michel M. A rare case of autoimmune hemolytic anemia. Blood. 2017;130:559. doi: 10.1182/blood-2017-05-783514. [DOI] [PubMed] [Google Scholar]
- 21.Arndt PA, Leger RM, Garratty G. Serologic findings in autoimmune hemolytic anemia associated with immunoglobulin M warm autoantibodies. Transfusion. 2009;49:235–42. doi: 10.1111/j.1537-2995.2008.01957.x. [DOI] [PubMed] [Google Scholar]
- 22.Shinoda K, Taki H, Hounoki H, et al. Severe autoimmune hemolytic anemia associated with IgM warm auto-antibodies in primary Sjögren’s syndrome. Int J Rheum Dis. 2010;13:94–6. doi: 10.1111/j.1756-185X.2009.01450.x. [DOI] [PubMed] [Google Scholar]
- 23.Chao MP, Hong J, Kunder C, et al. Refractory warm IgM-mediated autoimmune hemolytic anemia associated with Churg-Strauss syndrome responsive to eculitzumab and rituximab. Am J Hematol. 2015;90:78–81. doi: 10.1002/ajh.23791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tajima K, Yamamoto H, Suzuki I, et al. Autoimmune hemolytic anemia with warm-reactive immunoglobulin M antibody in multicentric Castleman disease. Ann Hematol. 2013;92:849–51. doi: 10.1007/s00277-012-1626-8. [DOI] [PubMed] [Google Scholar]
- 25.Ito T, Tojo N, Takashiro K, et al. IgM-mediated warm autoimmune hemolytic anemia: an autopsy report. Intern Med. 2019;58:999–1002. doi: 10.2169/internalmedicine.1291-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Randen U, Trøen G, Tierens A, et al. Primary cold agglutinin-associated lymphoproliferative disease: A B-cell lymphoma of the bone marrow distinct from lymphoplasmacytic lymphoma. Hematologica. 2014;99:497–504. doi: 10.3324/haematol.2013.091702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berentsen S, Randen U, Tjønnfjord GE. Cold agglutinin-mediated autoimmune hemolytic anemia. Hematol Oncol Clin N Am. 2015;29:455–71. doi: 10.1016/j.hoc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Chandesris MO, Schleinitz N, Ferrrera V, et al. Cold agglutinins, clinical presentation and significance: retrospective analysis of 58 patients. Rev Med Interne. 2004;25:856–65. doi: 10.1016/j.revmed.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Fung MK, Grossman BJ, Hillyer CD, Westhoff CM. AABB Technical Manual. 18th ed. Bethesda, Maryland: AABB; 2014. [Google Scholar]
- 30.Hopkins C, Walters TK. Thermal amplitude test. Immunohematology. 2013;29:49–50. [PubMed] [Google Scholar]
- 31.Berentsen S. Cold agglutinin disease. Hematology Am Soc Hematol Educ Program. 2016;1:226–31. doi: 10.1182/asheducation-2016.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berentsen S, Tjønnfjord GE. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rew. 2012;26:107–15. doi: 10.1016/j.blre.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Sokol RJ, Hewitt S, Stamps BK. Autoimmune hemolysis: mixed warm and cold antibody type. Acta Haematol. 1983;69:266–74. doi: 10.1159/000206903. [DOI] [PubMed] [Google Scholar]
- 34.Shulman IA, Branch DR, Nelson JM, et al. Autoimmune hemolytic anemia with both cold and warm autoantibodies. JAMA. 1985;253:1746–8. [PubMed] [Google Scholar]
- 35.Das SS, Chakrabarty R, Zaman RU. Immunohematological and clinical characterization of mixed autoimmune hemolytic anemia. Asian J Transfus Sci. 2018;12:99–104. doi: 10.4103/ajts.AJTS_105_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rai P, Sharma G, Singh D, Garg J. Rare presentation of mixed autoimmune hemolytic anemia in children: Report of 2 cases. J Lab Physicians. 2017;9:332–6. doi: 10.4103/JLP.JLP_95_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Win N, Tiwari D, Keevil VL, et al. Mixed-type autoimmune haemolytic anaemia: Unusual cases and a case associated with splenic T cell angioimmunoblastic non-Hodgkins lymphoma. Hematology. 2007;12:159–62. doi: 10.1080/110245330601111466. [DOI] [PubMed] [Google Scholar]
- 38.Xu Q, Luo H, Zuo X, Liu S. Case report of mixed-type autoimmune hemolytic anemia in a patient with relapsing polychondritis. Medicine. 2018;94:1–3. doi: 10.1097/MD.0000000000012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petz LD. Cold antibody autoimmune hemolytic anemias. Blood Rev. 2008;22:1–15. doi: 10.1016/j.blre.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Prince SD, Winestone LE, Nance SJ, Friedman DF. Recurrent Donath-Landsteiner hemolytic anemia. A pediatric case report. Transfusion. 2017;57:1401–6. doi: 10.1111/trf.14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garratty G. Immune hemolytic anemia associated with negative routine serology. Semin Hematol. 2005;42:156–64. doi: 10.1053/j.seminhematol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Karafin MS, Denomme GA, Schanen M, Gottschall JL. Clinical and reference lab characteristics of patients with suspected direct antiglobulin test (DAT)-negative immune hemolytic anemia. Immunohematology. 2015;31:108–15. [PubMed] [Google Scholar]
- 43.Fattizo B, Zaninoni A, Nesa E, et al. Lessons from very severe refractory and fatal primary autoimmune hemolytic anemias. Am J Hematol. 2015;90:E149–150. doi: 10.1002/ajh.24047. [DOI] [PubMed] [Google Scholar]
- 44.Gilliland BC, Baxter E, Evans RS. Red-cell antibodies in acquired hemolytic anemia with negative antiglobulin serum tests. N Engl J Med. 1971;285:252–6. doi: 10.1056/NEJM197107292850503. [DOI] [PubMed] [Google Scholar]
- 45.Gilliland RC. Coombs-negative immune hemolytic anemia. Semin Hematol. 1976;13:267–75. [PubMed] [Google Scholar]
- 46.Kamesaki T, Oyamada T, Omine M, et al. Cut-off value of red-blood-cell-bound IgG for the diagnosis of Coombs-negative autoimmune hemolytic anemia. Am J Hematol. 2009;84:98–101. doi: 10.1002/ajh.21336. [DOI] [PubMed] [Google Scholar]
- 47.Fayek MH, Saad AA, Eissa DG, et al. Role of gel test and flow cytometry in diagnosis of Coombs’ negative autoimmune haemolytic anaemia. Int Jnl Lab Hem. 2012;34:311–9. doi: 10.1111/j.1751-553X.2011.01397.x. [DOI] [PubMed] [Google Scholar]
- 48.Barcellini W, Revelli N, Imperial FG, et al. Comparison of traditional methods and mitogen-stimulated direct antiglobulin test for detection of anti-red blood cell autoimmunity. Int J Hematol. 2010;91:762–9. doi: 10.1007/s12185-010-0578-9. [DOI] [PubMed] [Google Scholar]
- 49.Bartolomäs T, Salama A. A dual antiglobulin test for the detection of weak or nonagglutinating immunoglobulin M warm autoantibodies. Transfusion. 2010;50:1131–4. doi: 10.1111/j.1537-2995.2009.02533.x. [DOI] [PubMed] [Google Scholar]
- 50.Nobles JR, Wong C. Warm autoantibodies: time for a change. Immunohematology. 2013;29:5–9. [PubMed] [Google Scholar]
- 51.Aye T, Arndt PA. Utility of chloroquine diphosphate in the blood bank laboratory. Immunohematology. 2018;34:98–102. [PubMed] [Google Scholar]
- 52.Dupuis S. Use of the prewarm method for detecting clinically significant alloantibodies in the presence of cold autoantibodies. Immunohematology. 2018;34:148–50. [PubMed] [Google Scholar]
- 53.Ekema EM. Cold autoadsorption. Immunohematology. 2018;34:158–60. [PubMed] [Google Scholar]
- 54.Judd WJ, Susan TJ, Storry JR. Judd’s Methods in Immunohematology. 3rd ed. Bethesda: AABB Press; 2008. [Google Scholar]
- 55.Compernolle V, Chou ST, Tanael S, et al. Red blood cell specifications for patients with hemoglobinopathies: a systematic review and guideline. Transfusion. 2018;58:1555–66. doi: 10.1111/trf.14611. [DOI] [PubMed] [Google Scholar]
- 56.Milkins C, Berryman J, Cantwell C, et al. Guidelines for pre-transfusion compatibility procedures in blood transfusion laboratories. British Committee for Standards in Haematology. Transfus Med. 2013;23:3–35. doi: 10.1111/j.1365-3148.2012.01199.x. [DOI] [PubMed] [Google Scholar]
- 57.Ye Z, Zhang D, Boral L, et al. Comparison of blood group molecular genotyping to traditional serological phenotyping in patients with chronic or recent blood transfusion. JBM. 2016;4:1–8. [Google Scholar]
- 58.Issit D Applied Blood Group. Serology. 3rd ed. Miami: Montgomery Scientific Publications; 1985. [Google Scholar]
- 59.Denomme GA. Prospects for the provision of genotyped blood for transfusion. Br J Haematol. 2013;163:3–9. doi: 10.1111/bjh.12476. [DOI] [PubMed] [Google Scholar]
- 60.Flegel WA, Jerome L, Gottschall JL, Denomme GA. Implementing mass-scale red cell genotyping at a blood center. Transfusion. 2015;55:2610–15. doi: 10.1111/trf.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Legler TJ, Eber SW, Lakomek M, et al. Application of RHD and RHCE genotyping for correct blood group determination in chronically transfused patients. Transfusion. 1999;39:852–5. doi: 10.1046/j.1537-2995.1999.39080852.x. [DOI] [PubMed] [Google Scholar]
- 62.Bakanay SM, Ozturk A, Ileri T, et al. Blood group genotyping in multitransfused patients. Transfus Apher Sci. 2013;48:257–61. doi: 10.1016/j.transci.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 63.Chou ST, Evans P, Vege S, et al. RH genotype matching for transfusion support in sickle cell disease. Blood. 2018;132:1198–207. doi: 10.1182/blood-2018-05-851360. [DOI] [PubMed] [Google Scholar]
- 64.Menegati SFP, Santos TD, Marcedo MD, et al. Discrepancies between red cell phenotyping and genotyping in daily immunohematology laboratory practice. Transfus Apher Sci. 2020;59:102585. doi: 10.1016/j.transci.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 65.Belsitto A, Costa D, Napoli C. Blood group genotyping for patients with autoimmune hemolytic anemia. Transl Res. 2014;164:177–8. doi: 10.1016/j.trsl.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Kenz El, Efira A, Le PQ, et al. Transfusion support of autoimmune hemolytic anemia: how could the blood group genotyping help? Transl Res. 2014;163:36–42. doi: 10.1016/j.trsl.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Liu Z, LM, Mercado T, et al. Extended blood group molecular typing and next-generation sequencing. Transfus Med Rev. 2014;28:177–86. doi: 10.1016/j.tmrv.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 68.Tilley L, Grimsey S. Is Next Generation Sequencing the future of blood group testing? Transfus Apher Sci. 2014;50:183–8. doi: 10.1016/j.transci.2014.02.013. [DOI] [PubMed] [Google Scholar]