Abstract
Antipsychotic-induced hyperprolactinemia (AP-induced HPRL) occurs overall in up to 70% of patients with schizophrenia, which is associated with hypogonadism and sexual dysfunction. We summarized the latest evidence for the benefits of prolactin-lowering drugs. We performed network meta-analyses to summarize the evidence and applied Grading of Recommendations Assessment, Development, and Evaluation frameworks (GRADE) to rate the certainty of evidence, categorize interventions, and present the findings. The search identified 3,022 citations, 31 studies of which with 1999 participants were included in network meta-analysis. All options were not significantly better than placebo among patients with prolactin (PRL) less than 50 ng/ml. However, adjunctive aripiprazole (ARI) (5 mg: MD = −64.26, 95% CI = −87.00 to −41.37; 10 mg: MD = −59.81, 95% CI = −90.10 to −29.76; more than 10 mg: MD = −68.01, 95% CI = −97.12 to −39.72), switching to ARI in titration (MD = −74.80, 95% CI = −134.22 to −15.99) and adjunctive vitamin B6 (MD = −91.84, 95% CI = −165.31 to −17.74) were associated with significant decrease in AP-induced PRL among patients with PRL more than 50 ng/ml with moderated (adjunctive vitamin B6) to high (adjunctive ARI) certainty of evidence. Pharmacological treatment strategies for AP-induced HPRL depends on initial PRL level. No effective strategy was found for patients with AP-induced HPRL less than 50 ng/ml, while adjunctive ARI, switching to ARI in titration and adjunctive high-dose vitamin B6 showed better PRL decrease effect on AP-induced HPRL more than 50 ng/ml.
Subject terms: Schizophrenia, Clinical pharmacology
Introduction
Patients with psychosis like schizophrenia spectrum disorder and bipolar disorder could benefit from the antipsychotics (APs) [1, 2], but also suffer from side-effects of APs which prolongs the optimum treatment time and influences the prognosis.
Hyperprolactinemia (HPRL) is a common side-effect of APs, relating to the blocking of dopamine receptors in in the tuberoinfundibular dopaminergic pathway, and occurs in around 70% patients receiving APs [3].
The mostly studied consequences of HPRL are amenorrhea, galactorrhea, sexual impairment and infertility. Most clinical guidelines addressing recommended only symptomatic AP-induced HPRL need to be treated [4], but the non-symptomatic HPRL also attracts a clinical attention because of its long-term outcomes. Prolactin, a pleiotropic hormone which is secreted into circulation and acts in a wide range of tissues, involves in a wide range of physiological function such as the immune function, reproductive function and metabolic function [5]. Pathological HPRL could lead to weight gain, increased fat mass, leptin insensitivity, insulin resistance, osteoporosis and even breast cancer [6–8], which impairs the physical health of patients accepted antipsychotics treatment. Currently, clinicians often focus on the HPRL-induced short-term or directly consequences like sexual impairment amenorrhea, the long-term outcomes of HPRL are often overlooked.
Several pharmacological strategies that can improve the AP-induced HPRL, such as adding adjunctive aripiprazole (ARI) [9–11], switching to another antipsychotic (e.g., ARI, quetiapine, olanzapine, clozapine, blonanserin, and brexpiprazole) [12–20], adding dopamine agonists (DA, e.g., cabergoline, bromocriptine) [21–23], adding metformin (MET) [24], or adding the Peony-Glycyrrhiza decoction (PGD) and other traditional herb treatments [25–29]. One recent study also suggested a potential option for adjunctive high-dose vitamin B6 [30]. The most important issue associated with those strategies is the risk of worsening psychopathology of psychosis, which is particularly high in the strategies of switching to another antipsychotic or adding dopamine agonist [31]. Some previous meta-analyses have addressed the role of ARI, PGD and MET in lowering prolactin (PRL) concentration [32–38], and a recent network meta-analysis (NMA) also compared the efficacy of ARI, PGD and MET on reducing PRL which suggested that adjunctive aripiprazole (<5 mg/day) was the most effective one [39].
When multiple studies are conducted on the same research question, even if the same protocol is used, the results obtained in different medical settings are not the same. Therefore, clinical studies need to be repeated. Meta-analysis of all evidence is crucial for decision-making. Meta-analysis can ensure the reproducibility of research results by using standardized operating procedures, systematically retrieving relevant research evidence for methodological evaluation, and comprehensively analyzing the results of a certain research question. Randomized controlled trials (RCTs) and their meta-analyses provide the highest level of evidence from epidemiological studies and are ideal for testing scientific hypotheses. However, RCTs are often expensive, time-consuming, and in some cases even unethical or unfeasible. Due to various limitations of realistic conditions, RCTs are often difficult to carry out or to meet the current demand for evidence. Single-arm trials are common in medical research, and single-arm meta-analysis will conduct quantitative comprehensive analysis of single-arm trials with the same purpose. A network meta-analysis (NMA) is a method of assessing the effects of multiple interventions that leverages all direct and indirect evidence to provide a more precise estimate of the relative relationship between interventions than a single direct or indirect estimate. In addition, even if some interventions have never been compared in RCTs, NMA can use indirect evidence to estimate the relative effects of these interventions. The Bayesian hierarchical model is a statistical model with a structured hierarchy, based on interchangeability. The core idea is to add random effect parameters to the model to reflect the correlation of data within a group and the heterogeneity between data in different groups. The Bayesian Hierarchical NMA model increases the precision of parameter estimates and preserves the interpretability of the intervention.
To our knowledge, there is no systematic reviews or meta-analysis that examine all strategies for lowering prolactin levels have been conducted yet. The aim of our study is to perform NMA [40] to compare the efficacy of all the above strategies in reducing AP-induced HPRL, and to test the results according different initial PRL levels, so that to provide a reasonable treatment suggestion for AP-induced HPRL.
Methods
Search strategy and selection criteria
We did a systematic review and network meta-analysis according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol was registered in PROSPERO (CRD42022296815). Four electronic bibliographic databases were searched: PubMed, Web of Science, Embase and The Cochrane Library.
The search consisted of the following terms as Medical Subject Headings (MSH) and keywords appropriate to each database. The following search strategy was used: (schizo* OR psycho*) AND (switch* OR aripiprazole OR (bromocriptine OR cabergoline OR “dopamine agonist”) OR metformin OR (“peony-glycyrrhiza decoction” OR PGD OR herb*)) AND (prolactin OR PRL OR hyperprolactinemia).
Eligibility criteria
Language was restricted to those articles written in English or Chinese. Abstract of Chinese study must be searched in the above sources, and the abstract must be in English. Studies published since inception to November 1st, 2021 were considered for inclusion.
Two authors (ZL and YS) completed the screening and recording independently, and they will not interfere with each other’s decisions. When there are different opinions between the two authors, the third author (YZ) clarified and made final decision.
According to PICOS acronym, the selection criteria were included as follows: Participants (P): we included studies in adults with schizophrenia (as diagnosed using any recognized diagnostic criteria) who were treated with antipsychotics and experienced hyperprolactinemia induced by antipsychotics. We excluded studies in subjects with significant medical illnesses (such as liver or renal dysfunction, cardiovascular disease, organic brain disorder), pregnancy or lactation, the psychiatric diagnosis other than schizophrenia and a current history of substance use disorder; Studies in teenagers (under 18 years old) and elder people (more than 65 years) were also excluded; Interventions (I): (1) previous antipsychotics plus adjunctive medication which could reduce the prolactin level; (2) switch previous antipsychotics to another antipsychotic; Comparators (C): previous antipsychotics plus placebo or antipsychotic monotherapy (studies without controls were analyzed in single-arm meta-analysis); Outcomes (O): the mean change of PRL levels (ng/ml) after treatment (with PRL level at baseline and endpoint or the change of PRL levels); Study design (S): single-arm study and case-control studies were applied for single-arm meta-analysis. Placebo-controlled and head-to-head randomized controlled trials (RCTs) that compared different strategies were applied for network meta-analysis. The mean baseline prolactin levels must more than 25 ng/ml (HPRL was defined as prolactin levels more than 25 ng/ml). Studies with small sample size (n < 5) were excluded.
Data collection process
Two authors (ZL and YS) completed the screening and recording independently, and they could not interfere with each other’s decisions. When there are different opinions between the two authors, the third author (YZ) clarified and made final decision.
Data abstraction and synthesis
The baseline PRL level and endpoint PRL level were extracted (must include the mean and standard deviation), and the change of prolactin between baseline and endpoint was also extracted, if any.
For studies with multiple treatment arms of the same type of interventional drug, the mean/SDs were combined following methods described in the Cochrane Handbook (https://training.cochrane.org/handbook/current) and elsewhere.
Quality assessment
For single-arm and case-control studies, we applied the Methodological Index for Non-Randomized Studies (MINORS) score to assess the risk of bias [41]. For RCT studies, we applied the Cochrane Risk of Bias tool 2 (RoB 2) to assess the risk of bias [42]. For the outcomes of NMA, we applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) to evaluate the level of evidence, which provided the framework for rating the certainty of the evidence of each paired comparison as high, moderate, low, or very low [43].
Discrepancies were resolved through discussion, and if needed, a third author. The risk of bias was marked in both articles and survey results.
Data analysis
All the statistical actions were conducted on the R software, based on the meta [44] (applied for single-arm meta-analysis and RCT meta-analysis on each strategy which includes more than three studies), gemtc and rjags (applied for network meta-analysis) package [45].
We modeled the mean changes of prolactin levels with standard deviations and reported posterior mean difference (MD) with 95% confidence intervals (CIs). The concentration unit of prolactin was unified into nanogram per milliliter using a relevant conversion formula.
For single-arm meta-analysis (evaluate the effect after treatment) and RCT meta-analysis (evaluate the effect between strategy and placebo) on each strategy, use of the random or fixed effects model and the heterogeneity of meta-analysis was determined by I2 (50 was set as threshold). Meta-regression (for categorical variables, in the study, we included dosage of aripiprazole [5 mg or more than 5 mg per day] and baseline prolactin level [more than 100 ng/ml or not]; for continuous variables, we included the age, duration of trail and precent of male subjects), and sensitivity analyses (metainf code: exclude each included study individually) were preformed to examine the sources of heterogeneity. Subgroup analyses were conducted based on the result of meta-regression analysis. Funnel plot and Egger’s test were used to assess the publication bias.
To further test the comparative effectiveness among different treatment strategies, network meta-analysis with random-effects model was conducted. We assessed the Heterogeneity by I2. Node-split method was used to calculate the inconsistency between direct and indirect evidence. We compared the efficacy of different strategies using the surface under the cumulative ranking curve (SUCRA). Subgroup analysis (we included dosage of aripiprazole (5 mg, 10 mg, more than 5 mg per day) or different switching strategies (titration of aripiprazole and tardation of previous antipsychotics reduction [switch_ari_ti_ta], fixed dosage of aripiprazole and tardation of previous antipsychotics reduction [switch_ari_fixed_ta], fixed dosage of aripiprazole and reducing previous antipsychotics immediately [switch_ari_fixed_im]), and baseline prolactin level (<50 ng/ml, 50–100 ng/ml and >100 ng/ml) were preformed to examine the sources of heterogeneity.
All statistical differences were considered significant when the P < 0.05.
Results
Study selection and characteristics
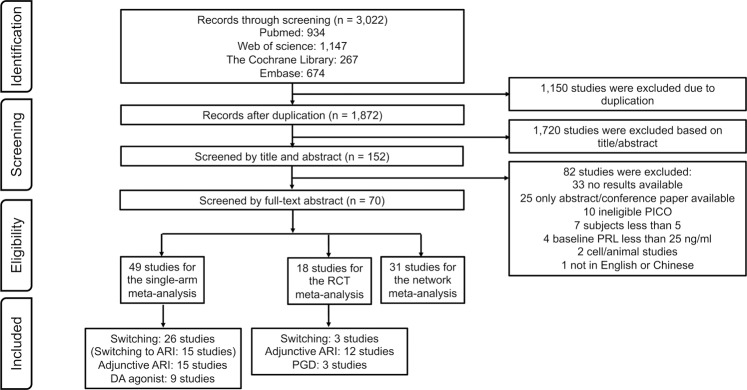
The search identified 3022 citations, including 1872 unique reports, and 152 studies were retrieved after the screening by title and abstract, finally 70 full-text articles were included in the analysis (Table 1). Then the 49 studies were included for single-arm meta-analysis (26 studies with 1273 participants for switching strategy, 15 of which with 1015 participants were for switching to ARI strategy; 15 studies with 716 participants for adjunctive ARI strategy; 9 studies with 189 participants for adjunctive dopamine agonists), 18 studies were included for RCT meta-analysis (3 studies with 63 participants for switching strategy, 12 studies with 542 participants for adjunctive ARI strategy, 3 studies with 130 participants for adjunctive PGD strategy) and 31 studies with 1999 participants were included for network meta-analysis. Figure 1 shows the flow chart of the study selection.
Table 1.
List of included studies.
| First author (Publication Year) | Blind assessment | Sample size | Age (years) Mean | Male (%) | Trial duration | Diagnostic tools | Primary APs | Treatment | References |
|---|---|---|---|---|---|---|---|---|---|
| Non-RCTs | |||||||||
| Chen CY (2011) | Open-label | 9 | 48.33 | 100 | 16 weeks | DSM-IV | RIS | Switching to ARI | [54] |
| Lu ML (2008) | Open-label | 20 | 31.70 | 0 | 8 weeks | DSM-IV | RIS/SUL | Switching to ARI | [55] |
| Lee BH (2006) | Open-label | 7 | 35 | 0 | 8 weeks | DSM-IV | RIS/AMI | Switching to ARI | [56] |
| Kinon BJ (2006) | Open-label | 54 | 39.25 | 48.14 | 16 weeks | DSM-IV | Various | Switching to OLA | [18] |
| Nakajima M (2005) | Open-label | 25 | 52.16 | 0 | 8 weeks | DSM-IV | Various | Switching to QUE | [57] |
| Takahashi H (2003) | Open-label | 16 | 25.69 | 0 | 16 weeks | DSM-IV | RIS/HAL | Switching to QUE | [19] |
| Kawabe (2013) | Open-label | 10 | 53.9 | 50 | 12 weeks | DSM-IV | Various | Switching to BLO | [58] |
| Hatzimanolis J (1998) | Open-label | 17 | 33.3 | NR | 6 weeks | DSM-III | FGAs | Switching to CLO | [59] |
| Markianos M (1999) | Open-label | 31 | 30.4 | NR | 6 weeks | DSM-III | FGAs | Switching to CLO | [20] |
| Kim KS (2002) | Open-label | 20 | 34.4 | 0 | 8 weeks | DSM-IV | RIS | Switching to OLA | [60] |
| Takeuchi H (2010) | Open-label | 32 | 54.6 | 56.3 | 56 weeks | DSM-IV | Various | Switching to ARI | [61] |
| Woo YS (2016) | Open-label | 77 | 36.2 | 37.7 | 24 weeks | DSM-IV | Various | Switching to ARI | [62] |
| Kelly DL (2021) | Open-label | 50 | 40.4 | 74 | 6 months | NR | PAL(LAI) | Switching to ARI(LAI) | [63] |
| Woo YS (2019) | Open-label | 33 | NR | NR | 12 weeks | DSM-IV | Various | Switching to BLO | [13] |
| Ichinose M (2021) | Open-label | 27 | 57.6 | 59.26 | 8 weeks | DSM-5 | Various | Switching to BRE | [12] |
| Kinon BJ (2000) | Open-label | 45 | NR | NR | 3 weeks | NR | RIS | Switching to OLA | [64] |
| Montejo AL (2009) | Open-label | 20 | 38.4 | 65 | 6 months | NR | Various | Switching to QUE | [65] |
| Jen YW (2020) | Open-label | 63 | 38.7 | 41.27 | 8 weeks | DSM-IV | Various | Switching to ARI | [66] |
| Takeuchi H (2008) | Open-label | 53 | 53.74 | 56.6 | 14 weeks | DSM-IV | Various | Switching to ARI | [16] |
| Hashimoto N (2015) | Open-label | 22 | 52.1 | 45.45 | 12 months | DSM-IV | Various | Switching to ARI | [14] |
| Kim SW (2009) | Open-label | 61 | 30.8 | 44.3 | 26 weeks | DSM-IV | Various | Switching to ARI | [67] |
| Nishimoto M (2012) | Open-label | 7 | NR | NR | NR | NR | NR | Switching to ARI | [68] |
| Fujioi J (2017) | Open-label | 21 | 41.3 | 42.86 | 24 weeks | NR | Various | Adjunctive ARI | [69] |
| Ziadi Trives M (2013) | Open-label | 13 | 41 | 12.5 | 3 months | NR | RIS(LAI) | Adjunctive ARI | [70] |
| Van Kooten M (2011) | Open-label | 12 | 47.6 | 91.7 | 16 weeks | DSM-IV | RIS(LAI) | Adjunctive ARI | [71] |
| Yasui-Furukori (2010) | Open-label | 17 | 44 | 0 | 8 weeks | DSM-IV | RIS | Adjunctive ARI | [72] |
| Chen CK (2010) | Open-label | 26 | 37.38 | 50 | 8 weeks | DSM-IV | RIS/AMI/SUL | Adjunctive ARI | [73] |
| Chen JX (2009) | Open-label | 19 | NR | NR | 8 weeks | DSM-IV | RIS | Adjunctive ARI | [74] |
| Arnaiz A (2021) | Open-label | 74 | 44.47 | 72.97 | 1 month | DSM-IV | RIS/PAL | Adjunctive ARI | [75] |
| Raveendranthan D (2018) | Open-label | 16 | 29.4 | 23.08 | 24 months | ICD-10 | RIS/AMI/OLA | Adjunctive ARI | [9] |
| Jung DU (2011) | Open-label | 24 | NR | 0 | 3 months | DSM-IV | RIS | Adjunctive ARI | [76] |
| Sajeev Kumar PB (2010) | Open-label | 10 | NR | NR | 48 weeks | NR | Various | Adjunctive ARI | [77] |
| Kalkavoura CS (2013) | Open-label | 80 | 43.6 | 56.25 | 6 months | DSM-IV | Various | Adjunctive DA | [22] |
| Coronas R (2012) | Open-label | 6 | 31.1 | 33.33 | 12 months | DSM-IV | Various | Adjunctive DA | [78] |
| Cavallaro R (2004) | Open-label | 19 | 33.7 | 31.58 | 6 months | DSM-IV | RIS | Adjunctive DA | [79] |
| Bliesener N (2004) | Open-label | 5 | NR | NR | NR | DSM-IV | AMI | Adjunctive DA | [80] |
| Hashimoto (2014) | Open-label | 20 | 42.9 | 50 | 2–4 weeks | DSM-IV | RIS/PAL | Adjunctive DA | [21] |
| Siever LJ (1981) | Open-label | 11 | NR | 12.5 | 2 weeks | NR | FGAs | Adjunctive DA | [23] |
| Cohn JB (1985) | Open-label | 11 | NR | 44.44 | 6 weeks | NR | THI | Adjunctive DA | [81] |
| RCTs | |||||||||
| Lee BJ (2013) | double | 29 | 50.78 | 72.41 | 24 weeks | DSM-IV | RIS | Switch_ARI_ti_ta/Placebo | [82] |
| Ryckmans V (2009) | Open-label | 400 | 41.10 | 56 | 12 weeks | DSM-IV | RIS | Switch_ARI_ti_ta / Switch_ARI_fixed_ta | [15] |
| Byerly MJ (2008) | double | 42 | 42.30 | 52.38 | 8 weeks | NR | RIS | Switching to QUE/Placebo | [17] |
| Byerly MJ (2009) | Open-label | 105 | 40 | 72.38 | 8 weeks | DSM-IV | RIS | Switch_ARI_fixed_im/Switch_ARI_fixed_ta/Switch_ARI_ti_ta | [11] |
| Huang P (2011) | Open-label | 67 | 23.73 | 0 | 3 months | CCMD-3 | Various | Switch_ARI_ti_ta/PGD | [83] |
| Hwang TJ (2015) | Open-label | 79 | 39.52 | 40.5 | 8 weeks | DSM-IV | Various | Switch_ARI_fixed_im/Switch_ARI_fixed_ta | [84] |
| Chen JX (2015) | double | 120 | 33.57 | 47.5 | 8 weeks | DSM-IV | RIS | ARI_5/ARI_10/ARI_more_10/Placebo | [85] |
| Shim JC (2007) | double | 54 | 39.39 | 40.74 | 8 weeks | DSM-IV | HAL | ARI_more_10/Placebo | [86] |
| Kelly DL (2018) | double | 42 | 37.03 | 0 | 16 weeks | DSM-IV | Various | ARI_10/Placebo | [87] |
| Qiao Y (2016) | single | 60 | 33.35 | 0 | 8 weeks | DSM-IV | RIS/PAL | ARI_5/Placebo | [10] |
| Zhao J (2015) | single | 107 | 29.67 | 41.12 | 8 weeks | DSM-IV | RIS | ARI_10/Placebo | [88] |
| Xu LP (2006) | single | 60 | 25 | 0 | 6 weeks | CCMD-3 | RIS/SUL | ARI_5/Placebo | [89] |
| Ji JY (2008) | single | 117 | 25 | 0 | 6 weeks | CCMD-3 | RIS | ARI_5/Placebo | [90] |
| Chen HZ (2009) | double | 65 | 30.5 | 100 | 8 weeks | CCMD-3 | RIS | ARI_5/Placebo | [91] |
| Liu ZB (2011) | Open-label | 142 | 38.75 | 61.25 | 26 weeks | CCMD-3 | Various | ARI_5/Placebo | [92] |
| Chen JX (2014) | double | 116 | 34.04 | 63.79 | 8 weeks | ICD-10 | RIS | ARI_more_10/Placebo | [93] |
| Liang J (2014) | double | 40 | 30.45 | 37.5 | 4 weeks | DSM-IV | PAL | ARI_10/Placebo | [94] |
| Wang HL (2014) | double | 178 | 34.69 | 50 | 6 weeks | CCMD-3 | Various | ARI_5/ARI_10 | [95] |
| Xia SY (2014) | Open-label | 67 | 32.02 | 0 | 6 months | NR | Various | ARI_5/PGD | [27] |
| Xu CX (2015) | Open-label | 193 | 36.78 | 41.96 | 12 weeks | CCMD-3 | RIS/AMI | ARI_5/ARI_10/ARI_more_10/Placebo | [96] |
| Chen HM (2016) | double | 61 | 33.43 | 0 | 8 weeks | DSM-IV | RIS | ARI_5/ARI_10/ARI_more_10/Placebo | [97] |
| Zhang LG (2018) | double | 58 | 35.13 | 100 | 8 weeks | DSM-IV | RIS | ARI_5/ARI_10/ARI_more_10/Placebo | [98] |
| Wu RR (2012) | double | 84 | 26.4 | 0 | 6 months | DSM-IV | Various | MET/Placebo | [24] |
| Xia JX (2011) | Open-label | 143 | NR | 60.14 | 6 months | CCMD-3 | RIS | MET/Placebo | [99] |
| Yuan HN (2008) | single | 20 | 30.45 | 0 | 12 weeks | ICD-10 | RIS | DA/Placebo | [100] |
| Yu RL (2010) | Open-label | 63 | 26.15 | 0 | 12 weeks | NR | Various | DA/PGD | [28] |
| Yang P (2017) | double | 42 | 28.48 | 0 | 8 weeks | ICD-10 | AMI | PGD/Placebo | [25] |
| Man SC (2016) | double | 99 | 29.8 | 0 | 16 weeks | ICD-10 | Various | PGD/Placebo | [26] |
| Gu P (2016) | Open-label | 120 | 30.22 | 44.17 | 8 weeks | ICD-10 | OLA | PGD/Placebo | [101] |
| Zhuo C (2021) | double | 200 | 31.82 | 100 | 16 weeks | DSM-IV | Various | Vitamin B6/ARI_10 | [30] |
| Yoon HW (2016) | Open-label | 42 | 35.34 | 33.33 | 8 weeks | DSM-IV | Various | Switching to ARI /Adjunctive ARI | [102] |
ARI_5 mg adjunctive 5 mg aripiprazole, ARI_10 mg adjunctive 10 mg aripiprazole, ARI_more_10 mg adjunctive more than 10 mg aripiprazole, DA adjunctive dopamine agonist, MET adjunctive metformin, PGD adjunctive Peony-Glycyrrhiza decoction, switch_ARI_fixed_im switching to ARI with fixed dosage and reducing the previous antipsychotic immediately, switch_ARI_fixed_ta switching to ARI with fixed dosage and reducing the previous antipsychotic in tardation, switch_ARI_ti_ta switching to ARI in titration and reducing the previous antipsychotic in tardation, switch_OLA switching to olanzapine, switch_QUE switching to quetiapine, VitB6 adjunctive high-dose vitamin B6, RIS risperidone, SUL sulpiride, AIM amisulpride, HAL haloperidol, FGAs first-generation antipsychotics, PAL paliperidone, LAI long-acting injection, THI thioridazine, OLA olanzapine, DSM-IV Diagnostic and Statistical Manual of Mental Disorders, fourth version, ICD-10 International Statistical Classification of Diseases and Related Health Problems, 10th Revision, CCMD-3 Chinese Classification of Mental Disorders, 3rd version, NR not report.
Fig. 1. Flow chart of included studies.
RCT randomized controlled trials, ARI aripiprazole, DA dopamine agonist, PGD Peony-Glycyrrhiza decoction.
Single-arm meta-analysis
Switching strategy
The result of switching strategy was significant. Switching to another antipsychotic could significantly reduce the prolactin levels (Supplementary Fig. 2, MD = − 42.55 ng/ml, 95% CI = −61.47 to −37.19 ng/ml). These studies were quite heterogeneous (I2 = 98%), so a random-effect model was used to generate the pooled estimates. Egger’s test results indicate there was no statistically significant level of publication bias (Supplementary Fig. 3A, t = 0.01, df = 24, P = 0.9932). Sensitivity analyses found that the random model was stable (Supplementary Fig. 3B). Test of heterogeneity also indicated the high heterogeneity (Q = 1,119.48, df = 25, P < 0.0001), so we applied the age, sex (male participants rate), baseline PRL level, medication type and trail duration to detect the contributions of heterogeneity, the result showed that the sex and baseline PRL level was significant.
Switching to ARI
Switching to ARI could significantly reduce the prolactin levels (Supplementary Fig. 4, MD = −55.79 ng/ml, 95% CI = −72.74 to −38.85 ng/ml). These studies were quite heterogeneous (I2 = 98%), so a random-effect model was used to generate the pooled estimates. Egger’s test results indicate there was no statistically significant level of publication bias (Supplementary Fig. 5A, t = −0.03, df = 13, P = 0.9760). Sensitivity analyses found that the random model was stable (Supplementary Fig. 5B). Test of heterogeneity also indicated the high heterogeneity (Q = 773.01, df = 16, P < 0.0001), so we applied the age, sex (male participants rate), baseline PRL level, dosage of ARI and trail duration to detect the contributions of heterogeneity, the result showed that baseline PRL level was significant.
Adjunctive ARI
Adjunctive ARI could significantly reduce the prolactin levels (Supplementary Fig. 6, MD = −46.31 ng/ml, 95% CI = −57.77 to −34.84 ng/ml). These studies were quite heterogeneous (I2 = 97%), so a random-effect model was used to generate the pooled estimates. Egger’s test results indicate a statistically significant level of publication bias (Supplementary Fig. 7A, t = −2.20, df = 14, P = 0.0454). Sensitivity analyses found that the random model was stable (Supplementary Fig. 7B). Test of heterogeneity also indicated the high heterogeneity (Q = 738.30, df = 21, P < 0.0001), so we applied the age, sex (male participants rate), baseline PRL level, dosage of ARI and trail duration to detect the contributions of heterogeneity, the result showed that baseline PRL level was significant.
Adjunctive dopamine agonist
Adjunctive dopamine agonist could significantly reduce the prolactin levels (Supplementary Fig. 8, MD = −40.29 ng/ml, 95% CI = −57.19 to −23.39 ng/ml). These studies were quite heterogeneous (I2 = 85%), so a random-effect model was used to generate the pooled estimates. Egger’s test results indicate there was no statistically significant level of publication bias (Supplementary Fig. 9A, t = −0.43, df = 7, P = 0.6795). Sensitivity analyses found that the random model was stable (Supplementary Fig. 9B). Test of heterogeneity also indicated the high heterogeneity (Q = 63.95, df = 9, P < 0.0001), so we applied the age, sex (male participants rate), baseline PRL level and trail duration to detect the contributions of heterogeneity, above factors were all not significant.
RCT meta-analysis
Switching strategy
Only 3 studies were included in the analysis, and the result was not significant (P = 0.11), which might due to the high heterogeneity, their strategies were switching to ARI, OLA or QUE separately (Supplementary Fig. 10).
Adjunctive ARI
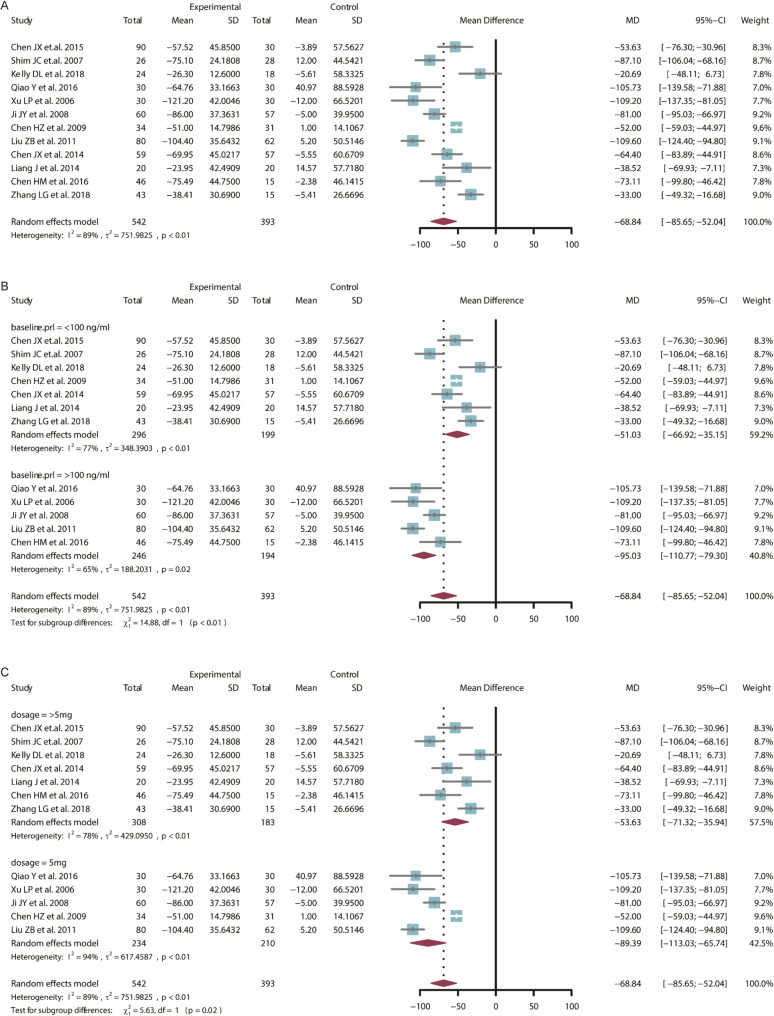
Compared to placebo, adjunctive ARI could significantly reduce the prolactin levels (Fig. 2A, MD = −68.84 ng/ml, 95% CI = −85.65 to −52.04 ng/ml). These studies were quite heterogeneous (I2 = 89%), so a random-effect model was used to generate the pooled estimates. Egger’s test results indicate there was no statistically significant level of publication bias (Supplementary Fig. 11A, t = −0.86, df = 10, P = 0.4094). Sensitivity analyses found that the random model was stable (Supplementary Fig. 11B). Test of heterogeneity also indicated the high heterogeneity (Q = 182.90, df = 14, P < 0.0001). According to the meta-regression analysis of the single-arm meta-analysis of adjunctive ARI, we conducted subgroup analysis to detect the heterogeneity. When we conducted the subgroup analysis stratified by the baseline PRL level (we set the 50 and 100 ng/ml as threshold, which were 2 times and 4 times of the normal limit; because there was no study with baseline PRL less than 50 ng/ml, we divided all the studies into two subgroup based on the 100 ng/ml), the PRL reduction after treatment in subgroup of lower baseline PRL (less than 100 ng/ml) was less than the subgroup of higher baseline PRL (more than 100 ng/ml) (Fig. 2B, C, MD: −51.03 v.s. −95.03 ng/ml). When we conducted the subgroup stratified by the dosage of ARI, the PRL reduction after treatment in subgroup of 5 mg/d was more than the subgroup of more than 5 mg/d (MD: −89.39 v.s. −53.63 ng/ml), it indicated that patients with antipsychotic-induced HPRL might be benefited from the low dosage of ARI more.
Fig. 2. RCT meta-analysis of adjunctive aripiprazole.
A Forest plot of RCT meta-analysis; B subgroup analysis based on baseline PRL level; C subgroup analysis based on ARI dosage. RCT randomized controlled trials, PRL prolactin, ARI aripiprazole, MD mean difference, CI confidence intervals, SD standard difference.
Adjunctive PGD
The effect of PGD on reducing PRL level was not significant (Supplementary Fig. 12A, MD = −11.76, 95%CI = −31.41 to 8.07). Egger’s test results indicate there was no statistically significant level of publication bias (Supplementary Fig. 12B, t = −0.43, df = 7, P = 0.6795). Sensitivity analyses found that the random model came to stable when we omitted the study of Man SC et al. (Supplementary Fig. 12C). Test of heterogeneity also indicated the high heterogeneity (Q = 19.68, df = 3, P = 0.0002).
Network meta-analysis
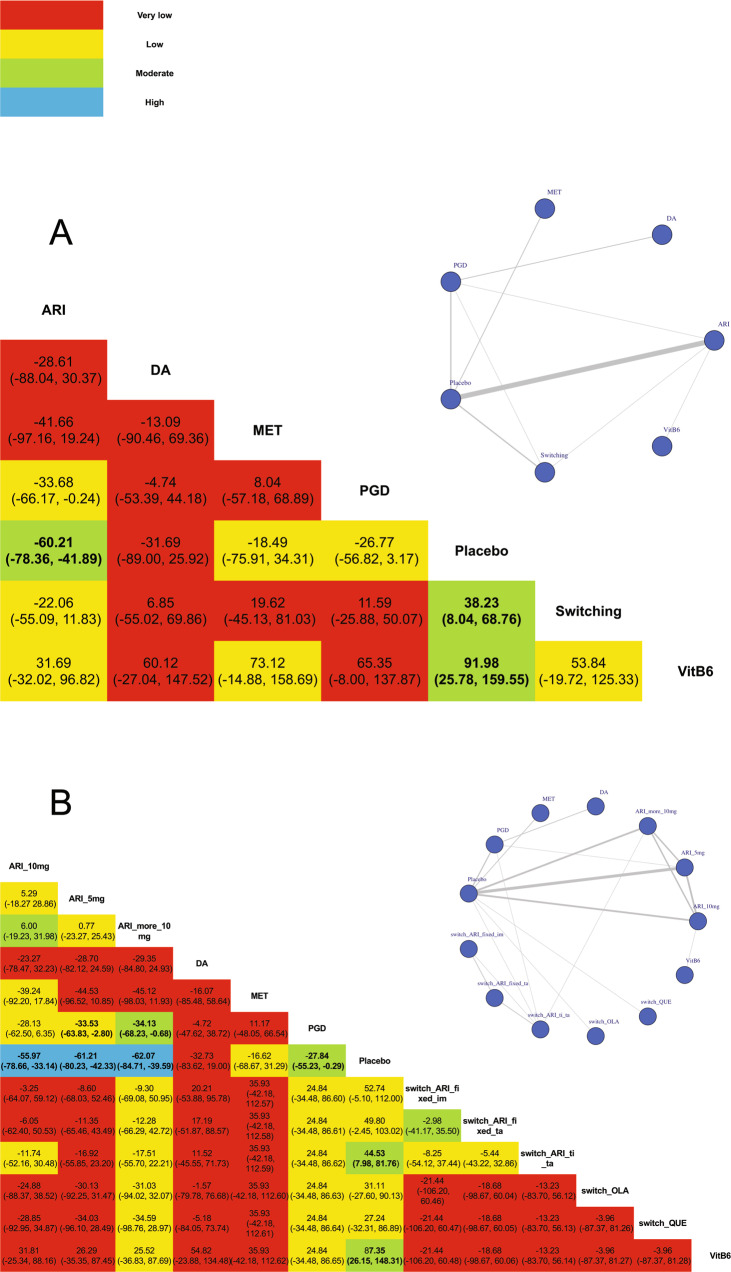
First of all, we conducted network meta-analysis on effect of different strategies (including adjunctive ARI, switching to another antipsychotic, adjunctive PGD, adjunctive MET, adjunctive DA, and adjunctive high-dose vitamin B6) on antipsychotic-induced HPRL, 26 studies with 1999 participants were included (5 studies was excluded because these head-to-head studies compared the different switching strategies or adding different dosage of ARI). When comparing to the placebo, adjunctive ARI (MD = −60.21, 95% CI = −78.36 to −41.89), switching to another antipsychotic (MD = −38.23, 95% CI = −68.76 to −8.04) and adjunction vitamin B6 (MD = −91.98, 95% CI = −159.55 to −25.78) showed the significant effect of decreasing PRL level. Furthermore, adjunctive ARI (MD = −33.68, 95% CI = −66.17 to −0.24) showed a more significant effect of decreasing PRL than adjunctive PGD. (Fig. 3A, B).
Fig. 3. Network meta-analyses of all the strategies in treatment of patients with antipsychotic-induced hyperprolactinemia.
A Network plot and league table of comparison of all the strategies in treatment of patients with antipsychotic-induced hyperprolactinemia; B network plot and league table of comparison of all re-divided strategies in treatment of patients with antipsychotic-induced hyperprolactinemia. ARI adjunctive aripiprazole, DA adjunctive dopamine agonist, MET adjunctive metformin, PGD adjunctive Peony-Glycyrrhiza decoction, Switching switch to another antipsychotic, VitB6 adjunctive high-dose vitamin B6. ARI_5 mg adjunctive 5 mg aripiprazole, ARI_10 mg adjunctive 10 mg aripiprazole, ARI_more_10 mg adjunctive more than 10 mg aripiprazole, switch_ARI_fixed_im switching to ARI with fixed dosage and reducing the previous antipsychotic immediately, switch_ARI_fixed_ta switching to ARI with fixed dosage and reducing the previous antipsychotic in tardation, switch_ARI_ti_ta switching to ARI in titration and reducing the previous antipsychotic in tardation, switch_OLA switching to olanzapine, switch_QUE switching to quetiapine. The color of each cell indicates the certainty of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation. Red color refers to very low certainty of evidence, yellow color refers to low certainty of evidence, green color refers to moderate certainty of evidence, blue color refers to high certainty of evidence. The significant outcomes were shown in bold.
Treatment strategies on adding adjunctive ARI and switching to another antipsychotic were further divided according to dosage of ARI and switching antipsychotic medications. Three ARI subgroups (5 mg ARI, adjunctive 10 mg ARI, adjunctive more than 10 mg ARI) and 5 switching subgroups (witching to ARI with fixed dosage and reducing the previous antipsychotic immediately (switch_ARI_fixed_im), switching to ARI with fixed dosage and reducing the previous antipsychotic in tardation (switch_ARI_fixed_ta), switching to ARI in titration and reducing the previous antipsychotic in tardation (switch_ARI_ti_ta), switching to OLA, switching to QUE) were performed. Finally, 31 studies with 2954 participants were included. When comparing to the placebo, adjunctive ARI (5 mg: MD = −61.21, 95% CI = −80.23 to −42.33; 10 mg: MD = −55.97, 95% CI = −78.66 to −33.14; more than 10 mg: MD = −62.21, 95% CI = −80.23 to −42.33), adjunctive PGD (MD = −27.84, 95% CI = −55.23 to −0.29), switch_ARI_ti_ta (MD = −44.53, 95% CI = −81.76 to −7.98) and adjunctive vitamin B6 (MD = −87.35, 95% CI = −148.31 to −26.15) showed the significant effect of decreasing PRL level. Furthermore, adjunctive ARI 5 mg (MD = −33.53, 95% CI = −63.83 to −2.80) and more than 10 mg (MD = −34.13, 95% CI = −68.23 to −0.68) showed more significant effect of decreasing PRL than adjunctive PGD. (Fig. 3C, D).
Subgroup analysis
When it comes to the effect of baseline PRL on the NMA model, we conducted subgroup analysis based on different baseline PRL levels (including less than 50 ng/ml and more than 50 ng/ml; furthermore, we further analyzed the subgroup of more than 100 ng/ml).
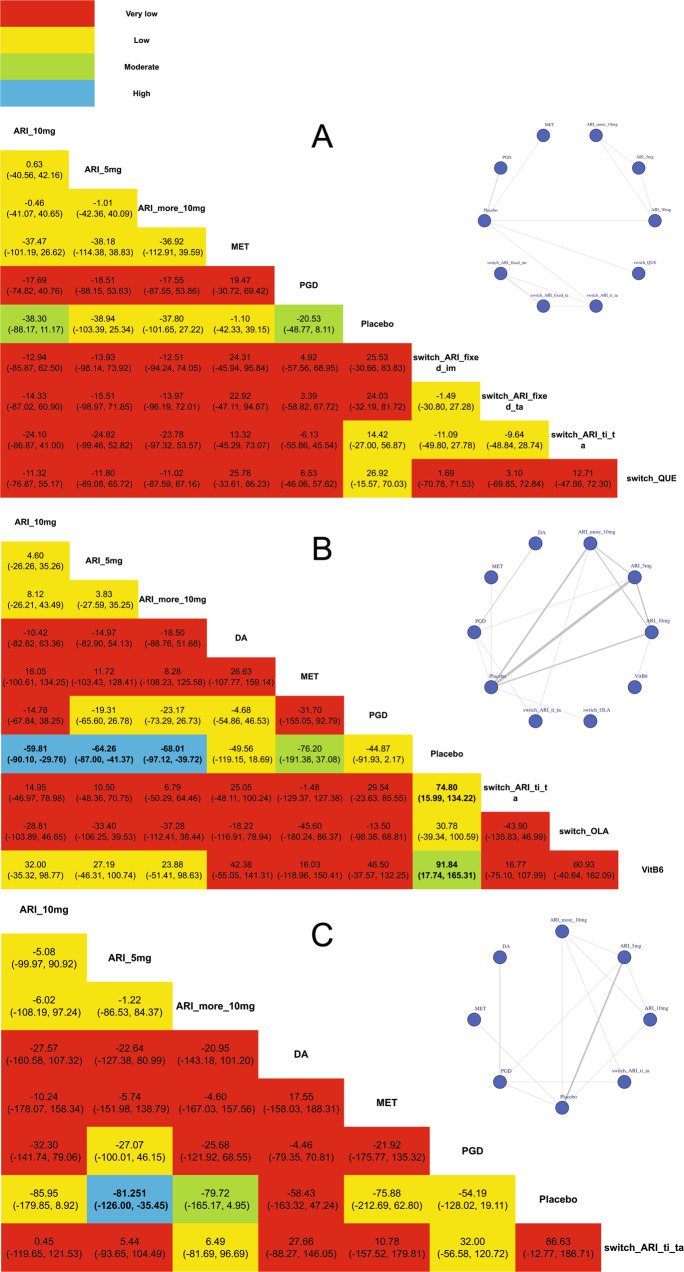
In the subgroup of less than 50 ng/ml, 9 strategies were included (9 studies with 792 participants). There was no significant difference of PRL change after treatment between therapy and placebo. (Fig. 4A, B).
Fig. 4. Subgroup analyses of network meta-analysis.
A Network plot and league table of comparison of pharmacological treatment strategies in treatment of patients with antipsychotic-induced hyperprolactinemia less than 50 ng/ml; B network plot and league table of comparison of pharmacological treatment strategies in treatment of patients with antipsychotic-induced hyperprolactinemia more than 50 ng/ml; C network plot and league table of comparison of pharmacological treatment strategies in treatment of patients with antipsychotic-induced hyperprolactinemia more than 100 ng/ml. ARI_5 mg adjunctive 5 mg aripiprazole, ARI_10 mg adjunctive 10 mg aripiprazole, ARI_more_10 mg adjunctive more than 10 mg aripiprazole, MET adjunctive metformin, PGD adjunctive Peony-Glycyrrhiza decoction, switch_ARI_fixed_im switching to ARI with fixed dosage and reducing the previous antipsychotic immediately, switch_ARI_fixed_ta switching to ARI with fixed dosage and reducing the previous antipsychotic in tardation, switch_ARI_ti_ta switching to ARI in titration and reducing the previous antipsychotic in tardation, VitB6 adjunctive high-dose vitamin B6. The color of each cell indicates the certainty of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation. Red color refers to very low certainty of evidence, yellow color refers to low certainty of evidence, green color refers to moderate certainty of evidence, blue color refers to high certainty of evidence. The significant outcomes were shown in bold.
In the subgroup of more than 50 ng/ml, 9 strategies were included (21 studies with 1762 participants). When comparing to the placebo, adjunctive ARI (5 mg: MD = −64.26, 95% CI = −87.00 to −41.37; 10 mg: MD = −59.81, 95% CI = −90.10 to −29.76; more than 10 mg: MD = −68.01, 95% CI = −97.12 to −39.72), switch_ARI_ti_ta (MD = −74.80, 95% CI = −134.22 to −15.99) and vitamin B6 (MD = −91.84, 95% CI = −165.31 to −17.74) showed the significant effect of decreasing PRL level. (Fig. 4C, D).
In the subgroup of more than 100 ng/ml, 7 strategies were included (12 studies with 881 participants). When comparing to the placebo, only adjunctive 5 mg ARI (MD = −81.25, 95% CI = −126.00 to −35.45) showed the significant effect of decreasing PRL. (Fig. 4E, F).
Safety
Finally, we evaluated the incidence of side-effects of all strategies (including ARI_5 mg, ARI_10 mg, ARI_more_10 mg, MET, PGD, switch_OLA, and VitB6), 15 studies and 844 participants were included, the result showed that only ARI_more_10 mg (OR = 2.2, 95% CI = 1.2 to 4.3) were associated with higher incidence of side-effects compared to placebo.
Discussion
To the best of our knowledge, this was the first NMA to comparing the efficacy of all strategies (6 strategies in total, including adjunctive ARI, switching to another antipsychotic, adjunctive PGD, adjunctive MET, adjunctive DA and adjunctive high-dose vitamin B6) for reducing AP-induced HPRL. Before we conducted the NMA, we firstly conducted the single-arm and RCT meta-analysis, the results showed that adjunctive ARI, switching to another antipsychotic, and adjunctive DA were associated with the significant decrease in AP-induced prolactin levels.
When we directly compared the six options, the NMA result showed that the adjunctive ARI, switching to another antipsychotic and adjunctive high-dose vitamin B6 was associated with the significant decrease in AP-induced prolactin levels compared to the placebo, and provided the moderated certainly evidence. Because there were some head-to-head RCTs which compared different dosage of ARI and switching to different antipsychotics, so we conducted the NMA included 12 options (adjunctive 5 mg ARI, adjunctive 10 mg ARI, adjunctive more than 10 mg ARI, switch_ARI_fixed_im, switch_ARI_fixed_ta, switch_ARI_ti_ta, switching to OLA, switching to QUE, adjunctive PGD, adjunctive MET, adjunctive dopamine agonist, and adjunctive vitamin B6), the result showed that the adjunctive ARI (in all subgroup), adjunctive PGD, switch_ARI_ti_ta and adjunctive vitamin B6 was associated with the significant decrease in AP-induced PRL compared to the placebo, and provided the high (adjunctive ARI) to moderated (adjuntive vitamin B6) certainly evidence; furthermore, the paired comparation indicated that the adjunctive ARI 5 mg was more efficacious than adjunctive PGD, while the certainly evidence was low. Baseline PRL was also a key-factor which influenced the change of PRL after treatment. We divided those studies into 2 subgroups based on the baseline PRL level (less than 50 ng/ml and more than 50 ng/ml), then we want to explore the best strategy for high PRL level (more than 100 ng/ml) and we set the studies whose baseline PRL more than 100 ng/ml as a subgroup. The result showed that all the options were not significant compared placebo for the patients with PRL level less than 50 ng/ml, which indicated that the intervention for patient with AP-induced HPRL less than 50 ng/ml might be not necessary; adjunctive ARI (all the subgroups: 5 mg, 10 mg and more than 10 mg), switch_ARI_ti_ta (low certainly evidence) and adjunctive high-dose vitamin B6 were associated with the significant decrease in AP-induced PRL compared to the placebo for the patients with PRL more than 50 ng/ml, and provided the high (adjunctive ARI) to moderated (adjunctive vitamin B6) certainly evidence; only the adjunctive 5 mg ARI was associated with the significant decrease in AP-induced PRL compared to the placebo for the patients with PRL more than 100 ng/ml.
The advantage of ARI, and PGD in reducing HPRL is consistent with previous researches [33, 35, 38]. The NMA of Zhang L et al. compared the efficacy among ARI, PGD and MET, it indicated that adjunctive ARI (<5 mg) was associated with the most significant reduction in prolactin levels compared to placebo; they also found that adjunctive PGD had the most significant effect in reducing risperidone-induced HPRL; furthermore, adjunctive aripiprazole (<5 mg) had the most significant effect in reducing amisulpride-induced HPRL, while the result was imprecise [39]. In our study, adjunctive ARI was also significantly associated with decrease in AP-induced HPRL, especially adjunctive ARI 5 mg in subgroup of baseline PRL more than 100 ng/ml, while this option was not significant in the subgroup of baseline PRL less than 50 ng/ml; adjunctive PGD was associated with AP-induced HPRL when we subdivided all the strategies. PGD is prepared from peony and glycyrrhiza in a certain proportion, which has been applied to improve HPRL in China and Japan. Its effect is associated with the modulation of dopamine D2 receptor. However, the preparation of PGD is various, and this strategy should be replicated in other countries. Adjunctive MET had no significant effect on reducing PRL compared to placebo, which was inconsistent with previous reviews [36, 37].
As for the switching strategy, there was no previous study, we did the single-arm meta-analysis firstly in this study, the result showed that the option was significant, but the result of RCT meta-analysis was not significant. The main reason might be switching to different antipsychotics. In the NMA, switching strategy was significant compared to placebo, especially for the switch_ARI_ti_ta option in the subgroup of PRL more than 50 ng/ml, while this option was not effective in the subgroup of PRL more than 100 ng/ml.
When it comes to the strategy of adjunctive DA, because the RCT number was too small, we just conducted the single-arm meta-analysis and it indicated this option was associated with decrease of AP-induced HPRL. However, the NMA result showed this strategy was not significantly associated with decrease of AP-induced HPRL.
Adjunctive high-dose vitamin B6, a recent novel attempt of old drug, was applied to treat the AP-induced HPRL, which showed a significant benefit for the participants. From 1970s, several case reports showed that high-dose vitamin B6 (from 200 to 1200 mg/day) could improve the galactorrhea-amenorrhea syndrome with or without hyperprolactinemia (including the drug-induced hyperprolactinemia) [46, 47], while there were some opposite results [48–50]. Due to the inconsistent curative effect and considering the adverse effect of high-dose vitamin B6, few studies were conducted to evaluate the potential efficacy. However, vitamin B6 is supposed that it works by promoting dopamine production and then activates dopamine receptors to reduce the secretion of pituitary prolactin [51], and the side-effects of high-dose vitamin B6 are fewer than expected [52], re-attempts of it are conducted recently and the results indicated that it could improve the AP-induced HPRL with few side-effects [30, 53]. In our study, the results of NMA showed that this option might be a very effective method for the patients with AP-induced HPRL and provide a moderate certainly evidence. However, there was only one study about it, this moderate evidence needed to be further researched in the future [30].
Because the strategies act in different mechanisms, the side effects of different strategies are various and affect a wide range of system including nervous system (insomnia, somnolence, agitation, anxiety/depression, psychosis, sedation, weakness, akathisia, tremor), metabolic system (liver dysfunction, elevated blood sugar), autonomic nervous system (nausea, salivate, constipation, dry mouth, rhinitis, diarrhea, stomachache) and cardiovascular system (tachycardia, electrocardiogram ST segment elevation). In order to evaluate the safety of each strategy, we extracted the total side-effect incidence rate and conducted the NMA. The result showed that ARI_more_10 mg were associated with higher incidence of side-effects compared to placebo. Combined the result of efficacy, low-dose ARI might be a better choice.
Strengths of our review included the most comprehensive synthesis of evidence to date on benefits of pharmacological therapies for adults with AP-induced HPRL, capturing all recent publications. And we conducted subgroup analysis based on the baseline PRL, which was more reasonable for clinical practice. We used state-of-the-art approaches to categorize and present the findings using GRADE frameworks. Limitations of our review included the absence of individual patient data pooling, which particularly reduced the precision of synthesis for subgroup effects. Studies varied in population characteristics and duration of follow-up. However, our meta-regression analyses showed no important differences in results across age and follow-up durations. In addition, some options like adjunctive vitamin B6 only included one publication, it might lack the representative.
In conclusion, patients with AP-induced HPRL could benefit from the strategies of adjunctive ARI, adjunctive Vitamin B6, adjunctive PGD and switching to ARI in titration. Patients with initial PRL less than 50 ng/ml might not need special intervention; adjunctive ARI, switch_ARI_ti_ta and adjunctive high-dose vitamin B6 proved to be the better PRL decrease effect for AP-induced HPRL more than 50 ng/ml; only adjunctive ARI 5 mg showed the significant effect of reduction PRL when the patients with AP-induced HPRL more than 100 ng/ml. Most comparative medium or high certainty evidence requires the confident application of these findings as clinical practice guidelines.
Supplementary information
Acknowledgements
We would like to thank colleagues in our lab for their feedbacks and technical assistances.
Author contributions
WY and ZL conceived and designed the study. ZL, YS and YZ screened and selected the articles. ZL, YS and YZ extracted the data. ZL, YS and YZ assessed the risk of bias. ZL analyzed the data. WY supervised the data analyses. ZL, YS and YZ rated the certainty of evidence. ZL, YS, YZ, YC, LG, YL, ZK and XF interpreted the data. ZL drafted the manuscript. ZL, YS and WY contributed to revising the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
This work was supported by the National Key R&D Program of China (2021YFF1201103; 2016YFC1307000), National Natural Science Foundation of China (81825009), Collaborative Research Fund of Chinese Institute for Brain Research Beijing (2020-NKX-XM-12), CAMS Innovation Fund for Medical Sciences (2021-I2M-C&T-B-099; 2019-I2M-5-006), PKUHSC-KCL Joint Medical Research (BMU2020KCL001) (WY). This study was also supported by Innovation Fund for Outstanding Doctoral Students of Peking University Health Science Center (ZL).
Data availability
The data and codes in this study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-022-02027-4.
References
- 1.McIntyre RS, Berk M, Brietzke E, Goldstein BI, López-Jaramillo C, Kessing LV, et al. Bipolar disorders. Lancet. 2020;396:1841–56. doi: 10.1016/S0140-6736(20)31544-0. [DOI] [PubMed] [Google Scholar]
- 2.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97. doi: 10.1016/S0140-6736(15)01121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery J, Winterbottom E, Jessani M, Kohegyi E, Fulmer J, Seamonds B, et al. Prevalence of hyperprolactinemia in schizophrenia: association with typical and atypical antipsychotic treatment. J Clin Psychiatry. 2004;65:1491–8. doi: 10.4088/JCP.v65n1108. [DOI] [PubMed] [Google Scholar]
- 4.Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:273–88. doi: 10.1210/jc.2010-1692. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Vicchi F, De Winne C, Brie B, Sorianello E, Ladyman SR, Becu-Villalobos D. Metabolic functions of prolactin: Physiological and pathological aspects. J Neuroendocrinol. 2020;32:e12888. doi: 10.1111/jne.12888. [DOI] [PubMed] [Google Scholar]
- 6.Inder WJ, Castle D. Antipsychotic-induced hyperprolactinaemia. Aust N. Z J Psychiatry. 2011;45:830–7. doi: 10.3109/00048674.2011.589044. [DOI] [PubMed] [Google Scholar]
- 7.Bernichtein S, Touraine P, Goffin V. New concepts in prolactin biology. J Endocrinol. 2010;206:1–11. doi: 10.1677/JOE-10-0069. [DOI] [PubMed] [Google Scholar]
- 8.Taipale H, Solmi M, Lähteenvuo M, Tanskanen A, Correll CU, Tiihonen J. Antipsychotic use and risk of breast cancer in women with schizophrenia: a nationwide nested case-control study in Finland. Lancet Psychiatry. 2021;8:883–91. doi: 10.1016/S2215-0366(21)00241-8. [DOI] [PubMed] [Google Scholar]
- 9.Raveendranthan D, Rao NP, Rao MG, Mangot AG, Varambally S, Kesavan M, et al. Add-on Aripiprazole for Atypical Antipsychotic-induced, Clinically Significant Hyperprolactinemia. Indian J Psychol Med. 2018;40:38–40. doi: 10.4103/IJPSYM.IJPSYM_147_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiao Y, Yang F, Li C, Guo Q, Wen H, Zhu S, et al. Add-on effects of a low-dose aripiprazole in resolving hyperprolactinemia induced by risperidone or paliperidone. Psychiatry Res. 2016;237:83–9. doi: 10.1016/j.psychres.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 11.Byerly MJ, Marcus RN, Tran QV, Eudicone JM, Whitehead R, Baker RA. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. 2009;107:218–22. doi: 10.1016/j.schres.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Ichinose M, Miura I, Horikoshi S, Yamamoto S, Kanno-Nozaki K, Watanabe K, et al. Effect of Switching to Brexpiprazole on Plasma Homovanillic Acid Levels and Antipsychotic-Related Side Effects in Patients with Schizophrenia or Schizoaffective Disorder. Neuropsychiatr Dis Treat. 2021;17:1047–53.. doi: 10.2147/NDT.S306573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo YS, Yoon BH, Jeon BH, Seo JS, Nam B, Lee SY, et al. Switching Antipsychotics to Blonanserin in Patients with Schizophrenia: An Open-label, Prospective, Multicenter Study. Clin Psychopharmacol Neurosci. 2019;17:423–31. doi: 10.9758/cpn.2019.17.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto N, Toyomaki A, Honda M, Miyano S, Nitta N, Sawayama H, et al. Long-term efficacy and tolerability of quetiapine in patients with schizophrenia who switched from other antipsychotics because of inadequate therapeutic response-a prospective open-label study. Ann Gen Psychiatry. 2015;14:1.. doi: 10.1186/s12991-014-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryckmans V, Kahn JP, Modell S, Werner C, McQuade RD, Kerselaers W, et al. Switching to aripiprazole in outpatients with schizophrenia experiencing insufficient efficacy and/or safety/tolerability issues with risperidone: a randomized, multicentre, open-label study. Pharmacopsychiatry. 2009;42:114–21. doi: 10.1055/s-0028-1112134. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi H, Suzuki T, Uchida H, Nakajima S, Nomura K, Kikuchi T, et al. A randomized, open-label comparison of 2 switching strategies to aripiprazole treatment in patients with schizophrenia: add-on, wait, and tapering of previous antipsychotics versus add-on and simultaneous tapering. J Clin Psychopharmacol. 2008;28:540–3. doi: 10.1097/JCP.0b013e3181842586. [DOI] [PubMed] [Google Scholar]
- 17.Byerly MJ, Nakonezny PA, Rush AJ. Sexual functioning associated with quetiapine switch vs. risperidone continuation in outpatients with schizophrenia or schizoaffective disorder: A randomized double-blind pilot trial. Psychiatry Res. 2008;159:115–20. doi: 10.1016/j.psychres.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Kinon BJ, Ahl J, Liu-Seifert H, Maguire GA. Improvement in hyperprolactinemia and reproductive comorbidities in patients with schizophrenia switched from conventional antipsychotics or risperidone to olanzapine. Psychoneuroendocrinology. 2006;31:577–88. doi: 10.1016/j.psyneuen.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi H, Higuchi H, Kamata M, Naitoh S, Yoshida K, Shimizu T, et al. Effectiveness of switching to quetiapine for neuroleptic-induced amenorrhea. J Neuropsychiatry Clin Neurosci. 2003;15:375–7. doi: 10.1176/jnp.15.3.375. [DOI] [PubMed] [Google Scholar]
- 20.Markianos M, Hatzimanolis J, Lykouras L. Switch from neuroleptics to clozapine does not influence pituitary-gonadal axis hormone levels in male schizophrenic patients. Eur Neuropsychopharmacol. 1999;9:533–6. doi: 10.1016/S0924-977X(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto K, Sugawara N, Ishioka M, Nakamura K, Yasui-Furukori N. The effects of additional treatment with terguride, a partial dopamine agonist, on hyperprolactinemia induced by antipsychotics in schizophrenia patients: a preliminary study. Neuropsychiatr Dis Treat. 2014;10:1571–6. doi: 10.2147/NDT.S68298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalkavoura CS, Michopoulos I, Arvanitakis P, Theodoropoulou P, Dimopoulou K, Tzebelikos E, et al. Effects of cabergoline on hyperprolactinemia, psychopathology, and sexual functioning in schizophrenic patients. Exp Clin Psychopharmacol. 2013;21:332–41. doi: 10.1037/a0033448. [DOI] [PubMed] [Google Scholar]
- 23.Siever LJ. The effect of amantadine on prolactin levels and galactorrhea on neuroleptic-treated patients. J Clin Psychopharmacol. 1981;1:2–7. doi: 10.1097/00004714-198101000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Wu RR, Jin H, Gao K, Twamley EW, Ou JJ, Shao P, et al. Metformin for treatment of antipsychotic-induced amenorrhea and weight gain in women with first-episode schizophrenia: a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2012;169:813–21. doi: 10.1176/appi.ajp.2012.11091432. [DOI] [PubMed] [Google Scholar]
- 25.Yang P, Li L, Yang D, Wang C, Peng H, Huang H, et al. Effect of Peony-Glycyrrhiza Decoction on Amisulpride-Induced Hyperprolactinemia in Women with Schizophrenia: A Preliminary Study. Evid Based Complement Altern Med. 2017;2017:7901670. doi: 10.1155/2017/7901670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Man SC, Li XB, Wang HH, Yuan HN, Wang HN, Zhang RG, et al. Peony-Glycyrrhiza Decoction for Antipsychotic-Related Hyperprolactinemia in Women With Schizophrenia: A Randomized Controlled Trial. J Clin Psychopharmacol. 2016;36:572–9. doi: 10.1097/JCP.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 27.Xia S-Y, Zhang Y-R, Yu H, Meng X, Zhang P, Liu J. Treatment of antipsychotic drug-induced phlegm dampness type amenorrhea by Wuji Powder and a small dose aripiprazole: a clinical study. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi = Chin J Integr traditional West Med / Zhongguo Zhong xi yi jie he xue hui, Zhongguo Zhong yi yan jiu yuan zhu ban. 2014;34:1440–3. [PubMed] [Google Scholar]
- 28.Yu R, Qi Y, Hou C, Jia F. Efficacy of Bupleurum Reconcile Soup in treatment of antipsychotics-induced hyperprolactinemia. Guangdong Med J. 2010;31:2587–9. [Google Scholar]
- 29.Yamada K, Kanba S, Yagi G, Asai M. Herbal medicine (Shakuyaku-kanzo-to) in the treatment of risperidone-induced amenorrhea. J Clin Psychopharmacol. 1999;19:380–1. doi: 10.1097/00004714-199908000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Zhuo C, Xu Y, Wang H, Fang T, Chen J, Zhou C, et al. Safety and Efficacy of High-Dose Vitamin B6 as an Adjunctive Treatment for Antipsychotic-Induced Hyperprolactinemia in Male Patients With Treatment-Resistant Schizophrenia. Front Psychiatry. 2021;12:681418. doi: 10.3389/fpsyt.2021.681418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snellen M, Power J, Blankley G, Galbally M. Pharmacological lactation suppression with D2 receptor agonists and risk of postpartum psychosis: A systematic review. Aust N. Z J Obstet Gynaecol. 2016;56:336–40. doi: 10.1111/ajo.12479. [DOI] [PubMed] [Google Scholar]
- 32.Labad J, Montalvo I, González-Rodríguez A, García-Rizo C, Crespo-Facorro B, Monreal JA, et al. Pharmacological treatment strategies for lowering prolactin in people with a psychotic disorder and hyperprolactinaemia: A systematic review and meta-analysis. Schizophr Res. 2020;222:88–96. doi: 10.1016/j.schres.2020.04.031. [DOI] [PubMed] [Google Scholar]
- 33.Meng M, Li W, Zhang S, Wang H, Sheng J, Wang J, et al. Using aripiprazole to reduce antipsychotic-induced hyperprolactinemia: meta-analysis of currently available randomized controlled trials. Shanghai Arch Psychiatry. 2015;27:4–17. doi: 10.11919/j.issn.1002-0829.215014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labad J, Montalvo I, González-Rodríguez A, García-Rizo C, Crespo-Facorro B, Monreal JA, et al. Data of a meta-analysis on pharmacological treatment strategies for lowering prolactin in people with a psychotic disorder and hyperprolactinaemia. Data Brief. 2020;31:105904. doi: 10.1016/j.dib.2020.105904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng W, Cai DB, Li HY, Wu YJ, Ng CH, Ungvari GS, et al. Adjunctive Peony-Glycyrrhiza decoction for antipsychotic-induced hyperprolactinaemia: a meta-analysis of randomised controlled trials. Gen Psychiatr. 2018;31:e100003. doi: 10.1136/gpsych-2018-100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng W, Yang XH, Cai DB, Ungvari GS, Ng CH, Wang N, et al. Adjunctive metformin for antipsychotic-related hyperprolactinemia: A meta-analysis of randomized controlled trials. J Psychopharmacol. 2017;31:625–31. doi: 10.1177/0269881117699630. [DOI] [PubMed] [Google Scholar]
- 37.Bo QJ, Wang ZM, Li XB, Ma X, Wang CY, de Leon J. Adjunctive metformin for antipsychotic-induced hyperprolactinemia: A systematic review. Psychiatry Res. 2016;237:257–63. doi: 10.1016/j.psychres.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PloS one. 2013;8:e70179. doi: 10.1371/journal.pone.0070179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, Qi H, Xie YY, Zheng W, Liu XH, Cai DB, et al. Efficacy and Safety of Adjunctive Aripiprazole, Metformin, and Paeoniae-Glycyrrhiza Decoction for Antipsychotic-Induced Hyperprolactinemia: A Network Meta-Analysis of Randomized Controlled Trials. Front Psychiatry. 2021;12:728204. doi: 10.3389/fpsyt.2021.728204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. Bmj. 2005;331:897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 42.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 43.Brignardello-Petersen R, Florez ID, Izcovich A, Santesso N, Hazlewood G, Alhazanni W, et al. GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. Bmj. 2020;371:m3900. doi: 10.1136/bmj.m3900. [DOI] [PubMed] [Google Scholar]
- 44.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–60. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012;3:285–99. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 46.McIntosh EN. Treatment of women with the galactorrhea-amenorrhea syndrome with pyridoxine (vitamin B6) J Clin Endocrinol Metab. 1976;42:1192–5. doi: 10.1210/jcem-42-6-1192. [DOI] [PubMed] [Google Scholar]
- 47.Vescovi PP, Gerra G, Rastelli G, Ceresini G, Moccia G. Pyridoxine (Vit. B6) decreases opioids-induced hyperprolactinemia. Horm Metab Res. 1985;17:46–7. doi: 10.1055/s-2007-1013447. [DOI] [PubMed] [Google Scholar]
- 48.Spiegel AM, Rosen SW, Weintraub BD, Marynick SP. Effect of intravenous pyridoxine on plasma prolactin in hyperprolactinemic subjects. J Clin Endocrinol Metab. 1978;46:686–8. doi: 10.1210/jcem-46-4-686. [DOI] [PubMed] [Google Scholar]
- 49.de Waal JM, Steyn AF, Harms JH, Slabber CF, Pannall PR. Failure of pyridoxine to suppress raised serum prolactin levels. S Afr Med J. 1978;53:293–4. [PubMed] [Google Scholar]
- 50.Brambilla F, Penati G, Freni S, Bottinelli S, Maffei C, Maiella V. Failure of pyridoxine to effect neuroleptic-induced hyperprolactinemia in psychotic patients. J Endocrinol Investig. 1979;2:299–302. doi: 10.1007/BF03350421. [DOI] [PubMed] [Google Scholar]
- 51.Mooney S, Leuendorf JE, Hendrickson C, Hellmann H. Vitamin B6: a long known compound of surprising complexity. Molecules. 2009;14:329–51. doi: 10.3390/molecules14010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calderon-Ospina CA, Nava-Mesa MO, Paez-Hurtado AM. Update on Safety Profiles of Vitamins B1, B6, and B12: A Narrative Review. Ther Clin Risk Manag. 2020;16:1275–88. doi: 10.2147/TCRM.S274122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao Y, Li M, Luo J, Yang Y, Li Z, Li Y, et al. Pyridoxine for the treatment of quetiapine-induced hyperprolactinemia and amenorrhea: A case report. Schizophr Res. 2019;206:448–9. doi: 10.1016/j.schres.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Chen CY, Lin TY, Wang CC, Shuai HA. Improvement of serum prolactin and sexual function after switching to aripiprazole from risperidone in schizophrenia: a case series. Psychiatry Clin Neurosci. 2011;65:95–7. doi: 10.1111/j.1440-1819.2010.02156.x. [DOI] [PubMed] [Google Scholar]
- 55.Lu ML, Shen WW, Chen CH. Time course of the changes in antipsychotic-induced hyperprolactinemia following the switch to aripiprazole. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1978–81. doi: 10.1016/j.pnpbp.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 56.Lee BH, Kim YK, Park SH. Using aripiprazole to resolve antipsychotic-induced symptomatic hyperprolactinemia: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:714–7. doi: 10.1016/j.pnpbp.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Nakajima M, Terao T, Iwata N, Nakamura J. Switching female schizophrenic patients to quetiapine from conventional antipsychotic drugs: effects on hyperprolactinemia. Pharmacopsychiatry. 2005;38:17–9. doi: 10.1055/s-2005-837766. [DOI] [PubMed] [Google Scholar]
- 58.Kawabe K, Horiuchi F, Ueno SI. Blonanserin, a novel antipsychotic, is suitable for treating schizophrenia associated with hyperprolactinemia: A case series. Clin Neuropharmacol. 2013;36:239–41. doi: 10.1097/WNF.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 59.Hatzimanolis J, Lykouras L, Markianos M, Oulis P. Neurochemical variables in schizophrenic patients during switching from neuroleptics to clozapine. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:1077–85. doi: 10.1016/S0278-5846(98)00064-5. [DOI] [PubMed] [Google Scholar]
- 60.Kim KS, Pae CU, Chae JH, Bahk WM, Jun TY, Kim DJ, et al. Effects of olanzapine on prolactin levels of female patients with schizophrenia treated with risperidone. J Clin Psychiatry. 2002;63:408–13. doi: 10.4088/JCP.v63n0506. [DOI] [PubMed] [Google Scholar]
- 61.Takeuchi H, Uchida H, Suzuki T, Watanabe K, Kashima H. Changes in metabolic parameters following a switch to aripiprazole in Japanese patients with schizophrenia: One-year follow-up study. Psychiatry Clin Neurosci. 2010;64:104–6. doi: 10.1111/j.1440-1819.2009.02036.x. [DOI] [PubMed] [Google Scholar]
- 62.Woo YS, Bahk WM, Park YM, Chung S, Yoon BH, Won S, et al. Effects of switching to aripiprazole from current atypical antipsychotics on subsyndromal symptoms and tolerability in patients with bipolar disorder. Int Clin Psychopharmacol. 2016;31:275–86. doi: 10.1097/YIC.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 63.Kelly DL, Claxton A, Bidollari I, Du Y. Analysis of prolactin and sexual side effects in patients with schizophrenia who switched from paliperidone palmitate to aripiprazole lauroxil. Psychiatry Res. 2021;302:114030. doi: 10.1016/j.psychres.2021.114030. [DOI] [PubMed] [Google Scholar]
- 64.Kinon BJ, Basson B, Wang J, Malcolm SK, Stauffer VL. Rapid reduction in hyperprolactinemia upon switching treatment to olanzapine from conventional antipsychotic drugs or risperidone. Eur Neuropsychopharmacol. 2000;10:S306–S. doi: 10.1016/S0924-977X(00)80349-1. [DOI] [Google Scholar]
- 65.Montejo AL, Majadas S, Franco M, Prieto N, Alvarez P, Gordo R, et al. Changes in hyperprolactinaemia and related adverse events after switching antipsychotic treatment to quetiapine. Eur Neuropsychopharmacol. 2009;19:S544–S5. doi: 10.1016/S0924-977X(09)70867-3. [DOI] [Google Scholar]
- 66.Jen YW, Hwang TJ, Chan HY, Hsieh MH, Liu CC, Liu CM, et al. Abnormally low prolactin levels in schizophrenia patients after switching to aripiprazole in a randomized trial: a biomarker for rebound in psychotic symptoms? BMC Psychiatry. 2020;20:552. doi: 10.1186/s12888-020-02957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim SW, Lee JH, Lee YH, Chung KH, Ryu JH, Yoon JS, et al. Switching to aripiprazole from atypical antipsychotics in patients with schizophrenia. Eur Neuropsychopharmacol. 2009;19:S488–S9. doi: 10.1016/S0924-977X(09)70770-9. [DOI] [PubMed] [Google Scholar]
- 68.Nishimoto M, Ishigaki T, Koyama Y, Nishimoto K, Funaki K, Matsunaga Y, et al. Sexual dysfunction and changes in satisfaction with switch to aripiprazole. Int J Neuropsychopharmacol. 2012;15:59. [Google Scholar]
- 69.Fujioi J, Iwamoto K, Banno M, Kikuchi T, Aleksic B, Ozaki N. Effect of Adjunctive Aripiprazole on Sexual Dysfunction in Schizophrenia: A Preliminary Open-Label Study. Pharmacopsychiatry. 2017;50:74–8. doi: 10.1055/s-0042-116323. [DOI] [PubMed] [Google Scholar]
- 70.Ziadi Trives M, Bonete Llácer JM, García Escudero MA, Martínez, Pastor CJ. Effect of the addition of aripiprazole on hyperprolactinemia associated with risperidone long-acting injection. J Clin Psychopharmacol. 2013;33:538–41. doi: 10.1097/JCP.0b013e3182970431. [DOI] [PubMed] [Google Scholar]
- 71.van Kooten M, Arends J, Cohen D. Preliminary report: a naturalistic study of the effect of aripiprazole addition on risperidone-related hyperprolactinemia in patients treated with risperidone long-acting injection. J Clin Psychopharmacol. 2011;31:126–8. doi: 10.1097/JCP.0b013e318205e1aa. [DOI] [PubMed] [Google Scholar]
- 72.Yasui-Furukori N, Furukori H, Sugawara N, Fujii A, Kaneko S. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol. 2010;30:596–9. doi: 10.1097/JCP.0b013e3181ee832d. [DOI] [PubMed] [Google Scholar]
- 73.Chen CK, Huang YS, Ree SC, Hsiao CC. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1495–9. doi: 10.1016/j.pnpbp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 74.Chen JX, Su YA, Wang SL, Bian QT, Liu YH, Wang N, et al. Aripiprazole Treatment of Risperidone-Induced Hyperprolactinemia. J Clin Psychiatry. 2009;70:1058–9. doi: 10.4088/JCP.08l04671. [DOI] [PubMed] [Google Scholar]
- 75.Arnaiz A, Zumárraga M, Erkoreka L, Olivas O, Arrue A, Zamalloa MI, et al. Factors influencing the effect of aripiprazole on prolactin levels in patients treated with risperidone or paliperidone. Sex seems to matter. Schizophr Res. 2021;228:382–4. doi: 10.1016/j.schres.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 76.Jung DU, Kelly DL, Kong BG, Kang JW, Oh MK, Seo BJ, et al. Adjunctive treatment with aripiprazole for risperidone-induced amenrrhea. Schizophrenia Bull. 2011;37:307. doi: 10.1093/schbul/sbp151. [DOI] [Google Scholar]
- 77.Sajeev Kumar PB, Rao NP, Venkatasubramanian G, Arasappa R, Behere RV, Vishwanath BV, et al. Aripiprazole in treatment of antipsychotic-induced hyperprolactinemia. Indian J Psychiatry. 2010;52:S53. [Google Scholar]
- 78.Coronas R, Cobo J, Giménez-Palop O, Ortega E, Márquez M. Safety of cabergoline in the management of pituitary prolactin-induced symptoms with patients treated with atypical neuroleptics. Curr Drug Saf. 2012;7:92–8. doi: 10.2174/157488612802715753. [DOI] [PubMed] [Google Scholar]
- 79.Cavallaro R, Cocchi F, Angelone SM, Lattuada E, Smeraldi E. Cabergoline treatment of risperidone-induced hyperprolactinemia: a pilot study. J Clin Psychiatry. 2004;65:187–90. doi: 10.4088/JCP.v65n0207. [DOI] [PubMed] [Google Scholar]
- 80.Bliesener N, Yokusoglu H, Quednow BB, Klingmüller D, Kühn KU. Usefulness of bromocriptine in the treatment of amisulpride-induced hyperprolactinemia: A case report. Pharmacopsychiatry. 2004;37:189–91. doi: 10.1055/s-2004-827176. [DOI] [PubMed] [Google Scholar]
- 81.Cohn JB, Brust J, DiSerio F, Singer J. Effect of bromocriptine mesylate on induced hyperprolactinemia in stabilized psychiatric outpatients undergoing neuroleptic treatment. Neuropsychobiology. 1985;13:173–9. doi: 10.1159/000118184. [DOI] [PubMed] [Google Scholar]
- 82.Lee BJ, Lee SJ, Kim MK, Lee JG, Park SW, Kim GM, et al. Effect of aripiprazole on cognitive function and hyperprolactinemia in patients with schizophrenia treated with risperidone. Clin Psychopharmacol Neurosci. 2013;11:60–6. doi: 10.9758/cpn.2013.11.2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang P, Yang Z. Controlled observation of traditional Chinese medicine Xuefuzhuyu decoction combined with aripiprazole in treatment of schizophrenic patients with antipsychotic-induced amenorrhea. Acta Academiae Medicinae Militaris Tertiae. 2011;33:625–7. [Google Scholar]
- 84.Hwang TJ, Lo WM, Chan HY, Lin CF, Hsieh MH, Liu CC, et al. Fast versus slow strategy of switching patients with schizophrenia to aripiprazole from other antipsychotics. J Clin Psychopharmacol. 2015;35:635–44. doi: 10.1097/JCP.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 85.Chen JX, Su YA, Bian QT, Wei LH, Zhang RZ, Liu YH, et al. Adjunctive aripiprazole in the treatment of risperidone-induced hyperprolactinemia: A randomized, double-blind, placebo-controlled, dose-response study. Psychoneuroendocrinology. 2015;58:130–40. doi: 10.1016/j.psyneuen.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 86.Shim JC, Shin JG, Kelly DL, Jung DU, Seo YS, Liu KH, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am J Psychiatry. 2007;164:1404–10. doi: 10.1176/appi.ajp.2007.06071075. [DOI] [PubMed] [Google Scholar]
- 87.Kelly DL, Powell MM, Wehring HJ, Sayer MA, Kearns AM, Hackman AL, et al. Adjunct Aripiprazole Reduces Prolactin and Prolactin-Related Adverse Effects in Premenopausal Women With Psychosis: results From the DAAMSEL Clinical Trial. J Clin Psychopharmacol. 2018;38:317–26. doi: 10.1097/JCP.0000000000000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao J, Song X, Ai X, Gu X, Huang G, Li X, et al. Adjunctive Aripiprazole Treatment for Risperidone-Induced Hyperprolactinemia: An 8-Week Randomized, Open-Label, Comparative Clinical Trial. PloS One. 2015;10:e0139717. doi: 10.1371/journal.pone.0139717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu LPJJ, Shi H. A control study of aripiprazole in the treatment of hyperprolactinemia by antipsychotics origin. Chin J Behav Med Sci. 2006;15:718–20. [Google Scholar]
- 90.Ji JY, Song ZX, Lp X. Aripiprazole in treatment of female schizophrenics with risperidone induced hyperprolactinemia. Chin J Psychiatry. 2008;41:169–71. [Google Scholar]
- 91.Chen HZ, Niu FRQM. Effect of aripiprazole on the hyperprolactinmia induced by risperidone in male schizophrenia patients. Chin J Psychiatry. 2009;42:224–7. [Google Scholar]
- 92.Liu Z, Cao B, Jiao F, Li D, Chen H, Liu W. A control study of aripiprazole in the treatment for antipsychotics-induced hyperprolactinemia. Chin J Hospital Pharm. 2011;31:843–6. [Google Scholar]
- 93.Chen J, Zhang R, Li W, Liu Y, Jiang L, Bian Q, et al. Adjunctive treatment of risperidone-induced hyperprolactinemia with aripiprazole: a randomized, double-blind, placebo-controlled study. Chin J N. Drugs. 2014;23:811–4. [Google Scholar]
- 94.Liang J, Yan J, Zhang X. Aripirazole reduces paliperidone-induced increase of prolactin in patients with schizophrenia: a randomized, double blind and placebo-controlled study. Chin J N. Drugs. 2014;23:1300–3,10. [Google Scholar]
- 95.Wang H, Zhang J, Wen S, Zhao J. Effects of various doses of aripiprazole on antipsychotics-induced hyperprolactinemia: an add-on therapy study. Chin J Neuromed. 2014;13:1035–8. [Google Scholar]
- 96.Xu C, Huang W, Zhao X, Liang Z, Deng W. Effect of various doses aripiprazole on hyperprolactinemia induced by amisulpride and risperidone. Chin J Psychiatry. 2015;48:297–302. [Google Scholar]
- 97.Chen H, Zhang R, Chen J, Liu Y, Li W, Tan Y, et al. Dose-effect relationship of aripiprazole on hyperprolactinemia induced by risperidone in female patients. Chin J N. Drugs. 2016;25:569–73. [Google Scholar]
- 98.Zhang L, Li Y, Liu Y, Chen J, Liu Y, Zhang R, et al. Dose-effect relationship of aripiprazole on hyperprolactinemia induced by risperidone in male patients. Chin J N. Drugs. 2018;27:334–8. [Google Scholar]
- 99.Xia J, Wang Y, Gan J, Cao S, Duan D, Qian P, et al. Efficacy of metformin combined behavior intervention in the treatment of metabolic disorders caused by risperidone. Chin J Clin Pharmacol. 2011;27:417–9. [Google Scholar]
- 100.Yuan HN, Wang CY, Sze CW, Tong Y, Tan QR, Feng XJ, et al. A randomized, crossover comparison of herbal medicine and bromocriptine against risperidone-induced hyperprolactinemia in patients with schizophrenia. J Clin Psychopharmacol. 2008;28:264–370. doi: 10.1097/JCP.0b013e318172473c. [DOI] [PubMed] [Google Scholar]
- 101.Gu P, Jin X, Li X, Wu YQ, Mao FQ. [Treatment of Olanzapine-induced Hyperprolactinemia by Shaoyao Gancao Decoction] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2016;36:1456–9. [PubMed] [Google Scholar]
- 102.Yoon HW, Lee JS, Park SJ, Lee SK, Choi WJ, Kim TY, et al. Comparing the Effectiveness and Safety of the Addition of and Switching to Aripiprazole for Resolving Antipsychotic-Induced Hyperprolactinemia: A Multicenter, Open-Label, Prospective Study. Clin Neuropharmacol. 2016;39:288–94. doi: 10.1097/WNF.0000000000000175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and codes in this study are available from the corresponding author on reasonable request.