Summary
Background
With the increased use of immune checkpoint inhibitors (ICIs) in advanced lung cancer, adverse events (AEs), particularly immune-related AEs (irAEs), have garnered considerable interest. We conducted a comprehensive assessment of the toxicity profile in advanced lung cancer using multi-source medical data.
Methods
First, we systematically searched the PubMed, Embase, and Cochrane Library databases (from inception to 10 August 2021) for relevant randomised controlled trials (RCTs) involving ICI-based treatments for advanced lung cancer. The primary outcomes were treatment-related AEs and irAEs, including events that were assigned grade 1–5 and 3–5. The secondary outcomes were grade 5 AEs and irAEs (grade 1–5 and grade 3–5) in specific organs. Network comparisons were conducted for 11 treatments, including chemotherapy (CT), ICI monotherapy (three regimens: programmed death-1 receptor [PD-1] inhibitors, programmed death ligand-1 [PD-L1] inhibitors, and cytotoxic T lymphocyte-associated antigen [CTLA-4] inhibitors), dual-ICI combination therapy (two regimens), and treatment using one or two ICI drugs administered in combination with CT (five regimens). We also conducted a disproportionality analysis by extracting reports of various irAEs associated with ICIs from the FDA Adverse Event Reporting System (FAERS) database. The reporting odds ratios and fatality proportions of different irAEs were calculated and compared. PROSPERO: CRD42021268650.
Findings
Overall, 41 RCTs involving 23,121 patients with advanced lung cancer were included. Treatments containing chemotherapy increased the risk of treatment-related AEs compared to ICI-based regimens without chemotherapy. Concerning irAEs, PD-L1 + CTLA-4 + CT was associated with the highest risk of grade 1–5 irAEs, followed by two regimens of dual ICI combination, three regimens of ICI monotherapy, and three regimens of one ICI combined with CT. For 3–5 irAEs, CTLA-4 accounted for most AEs. Detailed comparisons of ICI-based treatment options provided irAE profiles based on specific organs/systems and AE severity. Insights from the FAERS database revealed that signals corresponding to pneumonitis, colitis, thyroiditis, and hypophysitis were observed across all ICI regimens. Further analyses of the outcomes indicated that myocarditis (163 of 367, 44.4%), pneumonitis (1610 of 4497, 35.8%), and hepatitis (290 of 931, 31.1%) had high fatality rates.
Interpretation
Included RCTs showed heterogeneity in a few clinical factors, and reports derived from the FAERS database might have involved inaccurate data. Our results can be used as a basis for improving clinical treatment strategies and designing preventive methods for ICI treatment in advanced lung cancer.
Funding
This study was supported by the Research Project of Drug Clinical Comprehensive Evaluation and Drug Treatment Pathway (SHYXH-ZP-2021-001, SHYXH-ZP-2021-006), Clinical Research Innovation and Cultivation Fund of Ren Ji Hospital (RJPY-LX-008), Ren Ji Boost Project of National Natural Science Foundation of China (RJTJ–JX–001), and Shanghai “Rising Stars of Medical Talent” Youth Development Program – Youth Medical Talents – Clinical Pharmacist Program (SHWJRS (2019) 072).
Keywords: Immune checkpoint inhibitors, Drug adverse events, Lung cancer, Pharmacovigilance, Data mining, Real-world data
Research in context.
Evidence before this study
We searched the PubMed, Embase, and Cochrane Library databases on Aug 10, 2021 using search terms related to lung cancer and immune checkpoint inhibitor (ICI). To date, no especially ad-hoc designed randomised controlled trials (RCTs) have been conducted to assess the irAE risk in various ICI–based regimens, and several high-quality meta-analyses or disproportionality analyses were limited by number of included RCTs, ignoration of discrepancy in ICIs with different mechanisms, and inclusion of patients with unclassified cancer type, possibly introducing bias and leading to under- or over-estimation of irAE risk.
Added value of this study
We assessed toxicity profile of ten ICI–based treatments among patients with advanced lung cancer based on the network comparison of RCTs, as well as the disproportionality analysis of pharmacovigilance database. The results from pooled analysis suggested that treatments involving one ICI, especially anti–PD–1/ PD–L1 ICI, seemed to be safer options in terms of overall irAEs. Within the various organ systems and irAE severities, differences in rankings were observed among ICI regimens. Additional evidence from disproportionality analysis indicated that myocarditis, pneumonitis and hepatitis portended poorest outcomes.
Implications of all the available evidence
Comprehensive understanding of irAEs is a basis for clinical decision–making to tailor the best immunotherapy strategy for each patient with lung cancer. Furthermore, active monitoring and vigilant screening are encouraged for ICI–associated irAEs, especially those with a high incidence or poor prognosis.
Alt-text: Unlabelled box
Introduction
Immunotherapy has revolutionised the therapeutic landscape for patients with lung cancer by turning longer survival times into a reality.1 Immune checkpoint inhibitors (ICIs), including programmed death-1 receptor (PD-1) inhibitors, programmed death ligand-1 (PD-L1) inhibitors, and cytotoxic T lymphocyte-associated antigen (CTLA-4) inhibitors, are designed to block the co-inhibitory molecules of immune checkpoints to enhance immune attack on malignant cells.2 Given the impressive clinical effects of ICIs, they are administered to a substantial proportion of patients with lung cancer.
Published randomised controlled trials (RCTs) have demonstrated that the toxicity profile of ICIs appears more favorable than that of chemotherapy. However, immune-related adverse events (irAEs) are more frequently observed with ICI-based treatment regimens.3 Low incidence of irAEs may be observed in various organs or systems, including the skin, lungs, gastrointestinal tract, liver, heart, kidneys, muscles, and endocrine system.4 Without early identification and proper management, irAEs can develop into severe complications, resulting in treatment discontinuation or failure and even death, which poses a considerable challenge in daily clinical care.5,6
Isolated RCTs and their meta-analyses present the highest quality of evidence and are the basis for guidelines issued by healthcare organisations.7 However, the evaluation of entire profiles of rare irAEs derived from RCTs data is difficult owing to their stringent diagnostic standards and selection criteria, relatively small sample sizes, and limited follow-up duration.8 The Food and Drug Adverse Event Reporting System (FAERS), one of the largest pharmacovigilance databases with a large number of reported AEs and patient information, could provide data to verify and supplement the findings of RCTs.9,10 In the present study, we conducted a comprehensive assessment to examine the toxicity spectrum of ICIs in patients with advanced lung cancer by summarising evidence from RCTs and performing a disproportionality analysis with the data obtained from the FAERS database.
Methods
Pooled-analysis
Search strategy and selection criteria
According to the previously established protocol (PROSPERO: CRD42021268650), as well as the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines11 and its extension statement for network meta-analyses (NMA), we systematically searched the PubMed, Embase, and Cochrane Library databases from inception to Aug 10, 2021. The detailed search strategies are listed in Table S1. Potential studies listed as references in the retrieved articles and unpublished data from ClinicalTrials.gov were also identified. Studies were included if they met the following criteria: (I) phase II and III RCTs conducted in patients with advanced lung cancer; (II) comparing two or more treatments comprising at least one ICI drug; and (III) with detailed data of AE or irAE. Studies published only in the form of posters, conference abstracts, or presentations of ongoing RCTs were excluded. If several studies were based on the same trial, the one with comprehensive safety data was included. Eligible studies were screened by two authors (YY and YZ) based on the aforementioned criteria.
Study outcomes, data extraction, and quality assessment
The primary outcomes were overall safety outcomes, viz. treatment-related AEs and irAEs, as defined in each study, including grade 1–5 and grade 3–5, respectively. The secondary outcomes were grade 5 AEs as well as irAEs (grade 1–5 and grade 3–5) in specific organ systems (pneumonitis, colitis, hepatitis, myocarditis, nephritis, and myositis) and the endocrine system (hyperthyroidism, hypothyroidism, thyroiditis, hypophysitis, and diabetes). AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE),12 as reported in each study. Two authors (YY and YZ) applied a predesigned format to extract data independently, containing study characteristics, demographics, clinical characteristics, and data of reported safety outcomes. All information was extracted from the main text and supplementary files, and only extractable data were analysed. The methodological quality of the involved trials was appraised using the Cochrane Collaboration Risk of Bias Tool.13 Disagreements during study selection, data extraction, and quality assessment processes were resolved by consultation with the corresponding investigator (ZG).
Statistical analysis
To illustrate direct and indirect comparisons among the treatments, a plot of the network geometry was generated. CT was used as the reference comparator for network comparisons among treatment regimens. Relative risks (RRs) and confidence intervals (95% CIs) were calculated using random effects and consistency models. The ranking probability was used to provide a hierarchy of treatments. For different outcomes, surface under the cumulative ranking curve (SUCRA) values were calculated to rank treatments based on the cumulative probability plots. According to the SUCRA, treatment regimens were ranked from worst (i.e., associated with the highest risk of AEs) to best (i.e., associated with the lowest risk of AEs).14 Transitivity was appraised in consistency and coherence: first, interaction analyses were performed to evaluate the comparability of results derived from consistency and inconsistency models; second, node-splitting analyses were used to assess coherence in the network.15 To further test the robustness of the findings, scenario analyses were performed based on the heterogeneity in the RCTs. When an analysis of head-to-head comparisons involved more than or equal to 10 studies, publication bias was evaluated qualitatively using funnel plots and quantitatively using Begg's test and Egger's test. The trim-and-fill method was used to assess the outcomes with potential publication bias. Data were analysed using the STATA version13.0 (StataCorp, College Station, Texas, United States).
Pharmacovigilance study
Data processing and exposure definition
A retrospective, disproportionality, pharmacovigilance study was performed from Quarter 1 (Q1) in 2004 to Q2 in 2021 using the FAERS database. MICROMEDEX (Index Nominum) was used as the dictionary for ICIs names. Both generic and brand names were used as keywords for FAERS database retrieval (Table S2). Indications and AEs in the FAERS database were coded in terms of preferred terms (PTs) from the Medical Dictionary for Regulatory Activities (MedDRA) and all AEs of interest PTs (pneumonitis, colitis, hepatitis, myocarditis, nephritis, myositis, hyperthyroidism, hypothyroidism, thyroiditis, hypophysitis, and diabetes) were substotaled in the AEs (REAC) files (Table S3). Exposure assessment was considered when ICIs were recorded as ‘primary suspect’.
Disproportionality analyses
In pharmacovigilance study, disproportionality emerges when a specific adverse event is associated with a given drug. We used the reporting odds ratio (ROR) to identify statistical associations between ICIs and AEs of interest. The ROR is the ratio of the odds of reporting one interesting event to all other events for a given drug compared to the reporting odds for other drugs present in the FAERS database.16 A statistically significant ROR was defined as a lower limit of the 95% CI (ROR025) exceeding one, with at least three cases.17 To determine the prognosis of patients with different AEs after ICI use, we assessed the outcomes in the reports. Reports of fatal events attributed to AEs were counted, and the fatality proportion for each AE was calculated by dividing the fatal outcomes by the total number of reports with outcome data. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethical statement
Ethical approval is not necessary for this study.
Role of the funding source
Funders had no role in the study design, data collection, data analysis, data interpretation, preparation of the manuscript, or decision to publish. All authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Characteristics and quality of studies included in the pooled-analysis
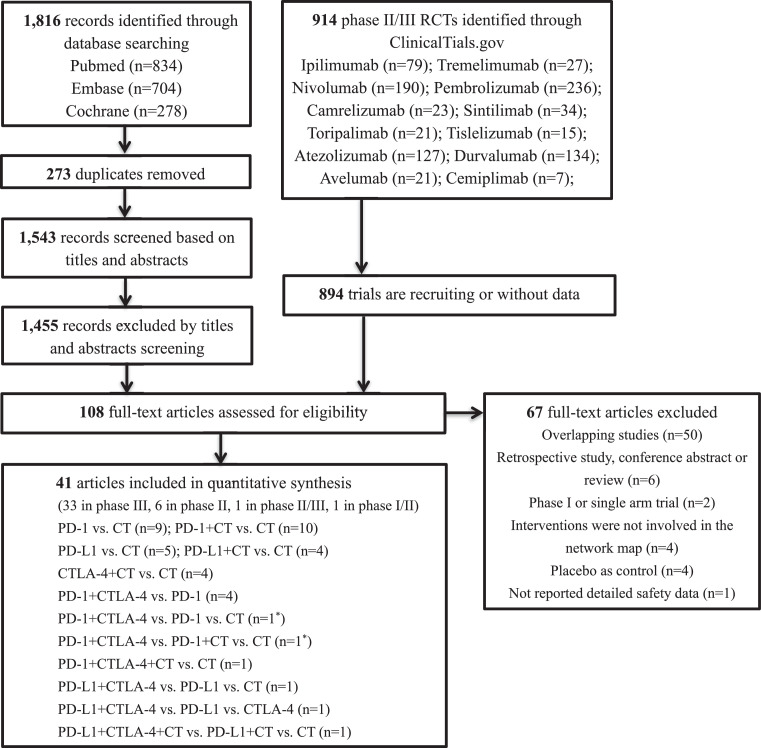
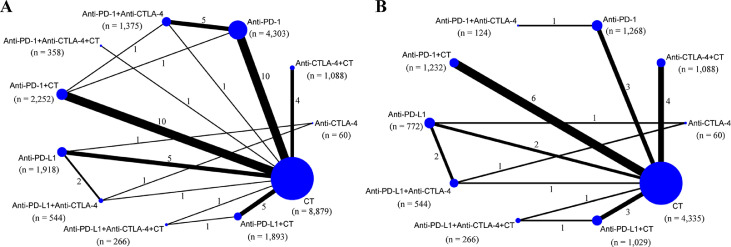
Our initial search identified 1816 articles from databases and 914 trials from the ClinicalTrials.gov platform; 1455 articles were excluded after screening titles and abstracts, and 894 trials were excluded because of unavailable data. The remaining 108 records were thoroughly reviewed, and 67 were excluded for reasons depicted in Figure 1 and Table S4. Ultimately, 41 studies fulfilled the inclusion criteria, and their characteristics are listed in Table S5. Of these 41 studies, 33 were phase III trials, six were phase II trials, one was a phase II/III trial, and one was a phase I/II trial. Regarding the indication, 32 RCTs involved patients with non-small cell lung cancer (NSCLC), and the remaining nine RCTs included patients with small cell lung cancer (SCLC). As shown in the network map (Figure 2), 11 treatment regimens were analysed in this study. A total of 23,121 patients with advanced lung cancer were included and treated with CT (9057 patients), PD-1 inhibitors (4342), PD-L1 inhibitors (1782), CTLA-4 inhibitors (60), PD-1 inhibitors + CT (2305), PD-L1 inhibitors + CT (1925), CTLA-4 inhibitors + CT (1089), PD-1 + CTLA-4 inhibitors (1386), PD-L1 + CTLA-4 inhibitors (546), PD-1 + CTLA-4 inhibitors + CT (361), and PD-L1 + CTLA-4 inhibitors + CT (268). Detailed patient demographics and clinical characteristics are summarised in Table S6. Among the included RCTs, 29 open-label trials did not meet the criteria for allocation concealment or blinding of participants and personnel, resulting in a high risk of bias in the study assessment. The remaining 12 studies were considered to have a low risk of bias. Details of the quality assessment are presented in Table S7.
Figure 1.
Flow diagram for the selection of eligible studies. RCT: randomised controlled trials; CT: chemotherapy; PD-1: programmed death–1 receptor inhibitor; PD–L1: programmed death ligand–1 inhibitor; CTLA–4: cytotoxic T lymphocyte associated antigen inhibitor; n: number; *: one study involved two groups: PD-1+CTLA–4 vs. PD–1 vs. CT (group A) and PD–1+CTLA–4 vs. PD–1+CT vs. CT (group B).
Figure 2.
Network map of comparisons based on different treatments in grade 1–5 adverse events (A) and grade 1–5 immune–related adverse events (B). Each circular node represents a type of treatment. The node size is proportional to the total number of patients administering a treatment (in parentheses). Each line represents a type of head–to–head comparison. The width of lines is proportional to the total number of studies comparing the connected treatments. CT: chemotherapy; PD–1: programmed death–1 receptor inhibitor; PD–L1: programmed death ligand–1 inhibitor; CTLA–4: cytotoxic T lymphocyte associated antigen inhibitor; n: number.
Network comparison for overall AEs
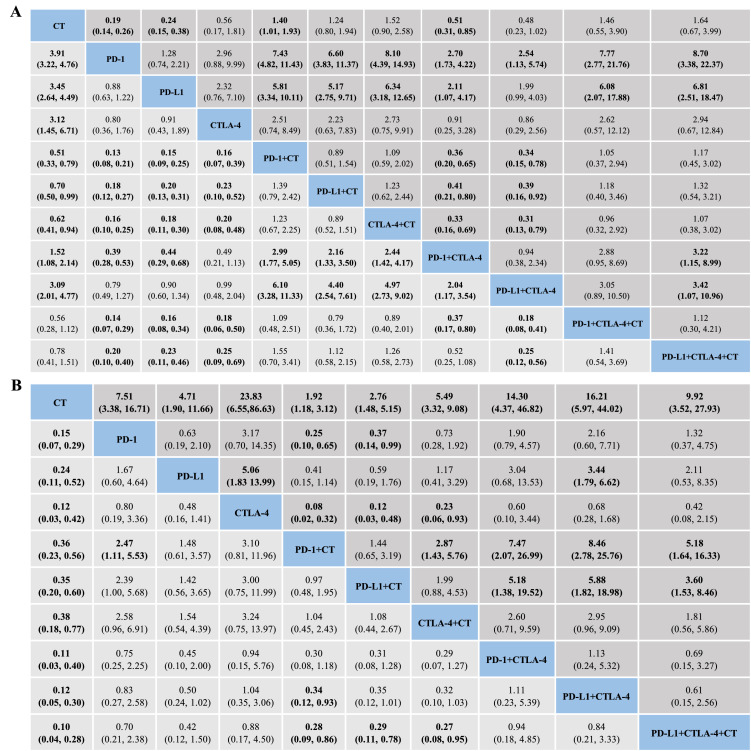
As shown in Figure 3A, the established NMA based on the consistency model indicated that CT-independent treatment or in combination with ICIs (one or two ICI agents) had a higher risk of causing grade 1–5 AEs than ICI monotherapy and dual-ICI therapy. Compared with CT, the combination of ICI monotherapy with cytotoxic drugs resulted in more AEs. Among similar treatment regimens, the PD-1 + CTLA-4 arm was associated with a higher risk of AEs than the PD-L1 + CTLA-4 arm. This significant difference was not observed in the ICI monotherapy group (PD-1, PD-L1, and CTLA-4 inhibitors), ICI monotherapy + chemotherapy group (PD-1 + CT, PD-L1 + CT, and CTLA-4 + CT), or dual-ICI therapy + chemotherapy group (PD-1 + CTLA-4 + CT and PD-L1 + CTLA-4 + CT). Regarding grade 3–5 AEs, patients using PD-1 or PD-L1 inhibitors experienced fewer AEs than those receiving CT, ICI monotherapy + CT, and dual-ICI therapy with or without CT. Compared with dual-ICI therapy, additional CT with ICI monotherapy or PD-L1 + CTLA-4 regimens resulted in more grade 3–5 AEs. The rankings of AEs ranged from least safe to safest as follows: (1), PD-1 + CT, PD-1 + CTLA-4 + CT, CTLA-4 + CT, PD-L1 + CT, PD-L1 + CTLA-4 + CT, CT, PD-1 + CTLA–4, PD-L1 + CTLA-4, CTLA-4, PD-L1, and PD-1 for grade 1–5; (2), PD-L1 + CTLA-4 + CT, CTLA-4 + CT (tied for first place), PD-1 + CT, PD-1 + CTLA-4 + CT, PD-L1 + CT, CT, CTLA-4, PD-1 + CTLA-4, PD-L1 + CTLA-4, PD-L1, and PD-1 for grade 3–5 (Figure 4, Table S8).
Figure 3.
Toxicity profiles based on adverse events (A) and immune-related adverse events (B). Pooled incidences and 95% confidence intervals of grade 1–5 events for each treatment are at bottom and that of grade 3–5 events are at top of the figure. Each cell of the safety profiles shows pooled relative risks and 95% confidence intervals for grade 1–5 (light grey cell) and grade 3–5 (dark grey cell) events; significant results are in bold. For instance, as for PD-1 in Figure 3A, patients receiving PD-1 had a lower risk of grade 1-5 adverse events than those receiving PD-1 + chemotherapy (RR: 0.13, 95% CI: 0.08–0.21). CT: chemotherapy; PD–1: programmed death–1 receptor inhibitor; PD–L1: programmed death ligand–1 inhibitor; CTLA–4: cytotoxic T lymphocyte associated antigen inhibitor.
Figure 4.
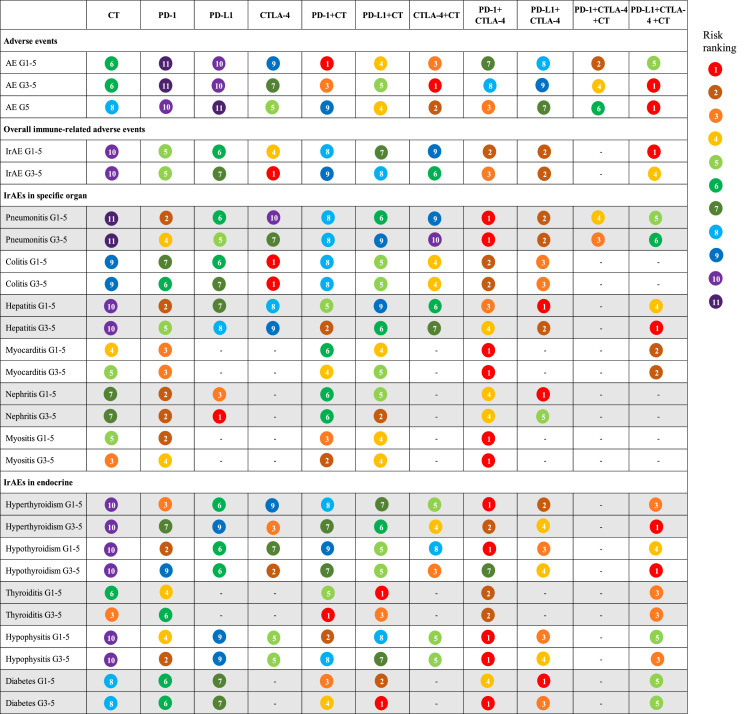
Evidence maps of adverse events based on eleven treatments. The risk ranking was generated based on the surface under the cumulative ranking curves values, with a smaller value of ranking indicating a higher risk of adverse events. For instance, as for overall adverse events, chemotherapy ranked sixth in causing grade 1-5 adverse events among the 11 treatments. AE: adverse event; irAE: immune–related adverse event; G: grade; CT: chemotherapy; PD–1: programmed death–1 receptor inhibitor; PD–L1: programmed death ligand–1 inhibitor; CTLA–4: cytotoxic T lymphocyte associated antigen inhibitor.
As for overall irAEs, the safety profiles (Figure 3B) of 10 treatment methods indicated a remarkably decreased risk favoring CT over other nine treatment strategies with respect to both grade 1–5 and grade 3–5 irAEs. Among ICI therapeutic schedules, PD-1 + CT had a lower risk of grade 1–5 irAEs than PD-1 (RR: 2.47, 95% CI: 1.11–5.53 for PD-1 vs. PD-1 + CT) and PD-L1 + CTLA-4 (RR: 0.34, 95% CI: 0.12–0.93). ICI monotherapy + CT regimens, including PD-1 + CT (RR: 0.28, 95% CI: 0.09–0.86), PD-L1 + CT (RR: 0.29, 95% CI: 0.11–0.78), and CTLA-4 + CT (RR: 0.27, 95% CI: 0.08–0.95), seemed safer than PD-L1 + CTLA-4 + CT. With respect to grade 3–5 irAEs, ICI monotherapy + CT regimens had a lower risk compared with CTLA-4 inhibitors, PD-1/ PD-L1 + CTLA-4 combination, and PD-1 + CTLA-4 + CT regimen. Similarly, CT + PD-1 (RR: 2.05, 95% CI: 0.10–0.65) or PD-L1 (RR: 0.37, 95% CI: 0.14–0.99) demonstrated less serious irAEs than anti-PD-1 agents. Compared to anti-PD-L1 agents, the addition of CTLA-4 significantly increased the risk of grade 3–5 irAEs (RR: 3.44, 95% CI: 1.79–6.62). Among similar treatments, CTLA-4 inhibitors were associated with a higher risk of irAEs than PD-L1 inhibitors (RR: 5.06, 95% CI: 1.83–13.99), and the CTLA-4 + CT arm had a higher risk of irAEs than the PD-1 + CT arm (RR: 2.87, 95% CI: 1.43–5.76). In the safety ranking, PD-L1 + CTLA-4 + CT was associated with the highest risk of grade 1–5 irAEs, followed by PD-1 + CTLA-4, PD-L1 + CTLA-4 (tied for second place), CTLA-4, PD-1, PD-L1, PD-L1 + CT, PD-1 + CT, CTLA-4 + CT, and CT. The risk of grade 3–5 irAEs was ranked from high to low as follows: CTLA-4, PD-L1 + CTLA-4, PD-1 + CTLA-4, PD-L1 + CTLA-4 + CT, PD-1, CTLA-4 + CT, PD-L1, PD-L1 + CT, PD-1 + CT, and CT (Figure 4, Table S8).
The results obtained via the inconsistency model showed a generally satisfactory fit compared to those obtained using the consistency model, except for minor comparisons based on CT (Table S9). Following the node-splitting analysis, no significant inconsistencies were observed (Table S10). Given the heterogeneity in terms of the trial phase, subtypes of cancer, and treatment line, scenario analyses were conducted with respect to phase III RCTs, patients with NSCLC, and first-line treatment. The ranking orders were consistent with those of the original NMA after removing phase I/II RCTs, studies involving patients with SCLC, or studies with previously treated patients, irrespective of overall AEs and irAEs (Table S11). Visual inspection of the funnel plots and the results of Egger's and Begg's tests showed relative asymmetry for AE when comparing PD-1 + CT vs. CT, suggesting that publication bias existed (Figure S1, Table S12). The trim-and-fill method was adopted to mitigate publication bias, and the outcomes were consistent with the primary results (P for interaction > 0.05).
Network comparison for grade 5 AEs and specific irAEs
NMAs and rankings for different treatment strategies in grade 5 AEs and subgroups of irAEs are presented in Figures S2–5, Figure 4 and Table S8. The top three regimens that were most likely to cause grade 5 AEs were PD-L1 + CTLA-4 + CT, CTLA-4 + CT, and PD-1 + CTLA-4. Among ICI monotherapy, anti-CTLA-4 agents had the highest risk of fatal AEs.
Among individual irAEs, the ranking results varied according to organ system and severity, whereas traditional CT consistently presented the lowest risk for the majority of irAEs. Among ICI-based treatments for irAEs in specific organs, the PD-1 + CTLA-4 regimen ranked first with respect to the risk of pneumonitis, myocarditis, and myositis, and the PD-L1 + CTLA-4 regimen ranked first in the risk of grade 1–5 hepatitis and nephritis. Notably, patients treated with CTLA-4 inhibitors monotherapy had the highest risk of colitis, even higher than those treated with dual-ICI therapy. For endocrine irAEs, a dual-ICI regimen with or without CT was associated with a high risk of hyperthyroidism, hypothyroidism, hypophysitis, and diabetes. However, a different trend was detected for irAEs associated with thyroiditis. PD-L1 + CT ranked first in grade 1–5 thyroiditis, and PD-1 + CT ranked first in grade 3–5 thyroiditis.
Toxicity profiling of immunotherapy in disproportionality analysis
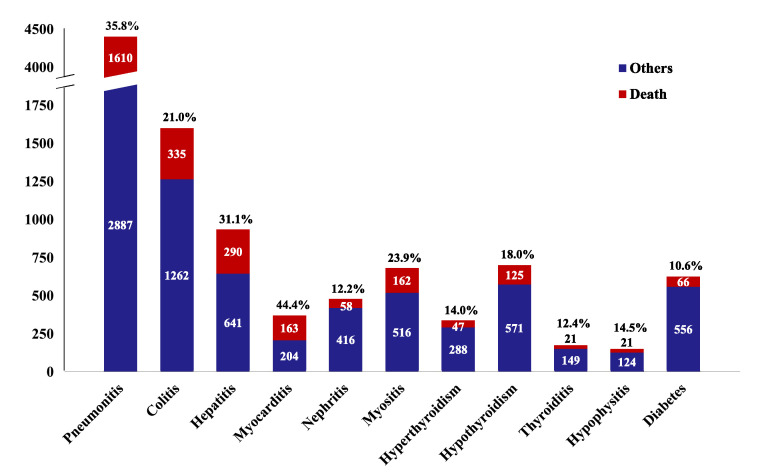
A total of 37,915 reports related to ICI immunotherapy were documented in the FAERS database. Of these reports, 4685 were pneumonitis, 1638 were colitis, 1160 were hepatitis, 407 were myocarditis, 486 were nephritis, 868 were myositis, 409 were hyperthyroidism, 849 were hypothyroidism, 236 were thyroiditis, 186 were hypophysitis, and 998 were diabetes. As shown in Table 1, the majority of the selected complications were associated with the use of PD-1 inhibitors (n = 8519, 71.5%), and strong signals were observed for these irAEs. As for anti-PD-L1 agents (n = 2388, 20.0%), disproportionality analysis also detected strong signals in different irAEs, except for myositis and hypophysitis. In contrast, anti-CTLA-4 drugs contributed a small proportion (n = 183, 1.5%), and only held strong signals in pneumonitis, colitis, thyroiditis, and hypophysitis. For combination therapy with PD-1 and CTLA-4 inhibitors (n = 832, 7.0%), disproportionality analysis failed to detect signals of nephritis, myositis, or hypothyroidism. With respect to the outcome of ICI-associated AEs (Figure 5), myocarditis accounted for the highest fatality rate (44.4%, 163 deaths in 367 cases), followed by pneumonitis (35.8%, 1610 deaths in 4497 cases), hepatitis (31.1%, 290 deaths in 931 cases), and the lowest one was diabetes (10.6%, 66 deaths in 622 cases). The detailed fatality rates of irAEs associated with various ICIs are summarised in Table S13.
Table 1.
Primary disproportionality analysis of treatments.
| Outcomes | Anti-PD-1 |
Anti-PD-L1 |
Anti-CTLA-4 |
Anti-PD-1+Anti-CTLA-4 |
||||
|---|---|---|---|---|---|---|---|---|
| N | ROR (95% CI) | N | ROR (95% CI) | N | ROR (95% CI) | N | ROR (95% CI) | |
| Pneumonitis | 2994 | 1.60 (1.53, 1.68) | 1347 | 3.7 (3.47, 3.95) | 68 | 1.69 (1.31, 2.18) | 276 | 1.65 (1.45, 1.88) |
| Colitis | 1202 | 2.81 (2.58, 3.06) | 216 | 1.51 (1.31, 1.74) | 63 | 6.22 (4.77, 8.12) | 157 | 3.30 (2.79, 3.90) |
| Hepatitis | 795 | 1.45 (1.32, 1.58) | 248 | 1.94 (1.70, 2.23) | 12 | 1.08 (0.61, 1.91) | 105 | 2.24 (1.83, 2.74) |
| Myocarditis | 315 | 7.44 (5.95, 9.30) | 64 | 2.35 (1.80, 3.08) | 0 | - | 28 | 3.04 (2.06, 4.46) |
| Nephritis | 373 | 2.48 (2.15, 2.87) | 91 | 2.05 (1.64, 2.56) | 1 | 0.27 (0.04, 1.93) | 21 | 1.23 (0.80, 1.91) |
| Myositis | 728 | 2.33 (2.10, 2.58) | 110 | 1.18 (0.97, 1.44) | 5 | 0.66 (0.27, 1.59) | 25 | 0.71 (0.48, 1.06) |
| Hyperthyroidism | 325 | 8.80 (6.96, 11.13) | 50 | 1.84 (1.37, 2.48) | 2 | 1.01 (0.25, 4.08) | 32 | 3.54 (2.46, 5.09) |
| Hypothyroidism | 722 | 10.56 (8.92, 12.50) | 95 | 1.58 (1.27, 1.96) | 3 | 0.70 (0.22, 2.17) | 29 | 1.42 (0.98, 2.05) |
| Thyroiditis | 177 | 6.53 (4.91, 8.68) | 29 | 1.78 (1.21, 2.63) | 5 | 4.47 (1.84, 10.88) | 25 | 4.86 (3.21, 7.37) |
| Hypophysitis | 114 | 4.26 (3.14, 5.79) | 21 | 1.79 (1.13, 2.83) | 12 | 15.28 (8.46, 27.62) | 39 | 11.92 (8.34, 17.05) |
| Diabetes | 774 | 2.57 (2.32, 2.85) | 117 | 1.20 (1.00, 1.46) | 12 | 1.49 (0.84, 2.65) | 95 | 2.90 (2.35, 3.59) |
PD-1: programmed death–1 receptor inhibitor; PD-L1: programmed death ligand–1 inhibitor; CTLA-4: cytotoxic T lymphocyte associated antigen inhibitor; N: number; ROR: reporting odds ratio; CI: confidence interval.
Figure 5.
Fatality proportion for ICI-associated adverse events from FAERS database. Each irAE contain cases resulted in death (red bar) and others (blue bar), and the fatality proportions are at top of the bars.
Discussion
The current study highlights the toxicity profile of ICI-based treatments in patients with advanced lung cancer based on the pooled analysis of 41 RCTs involving 23,121 patients, as well as the disproportionality analysis of the pharmacovigilance database containing 37,915 reports. The results of the pooled analysis suggested that ICI monotherapy (anti-PD-1, anti-PD-L1, and anti-CTLA-4 agents) was associated with relatively fewer AEs among ICI-based treatments, whereas the combination of ICI monotherapy with CT appeared to be a safer option in terms of irAEs. Differences in rankings were observed among ten ICI regimens in various organ systems and the severity of irAEs. Additional evidence from the FAERS database indicated that signals corresponding to pneumonitis, colitis, thyroiditis, and hypophysitis were generated across all ICI regimens. Of the irAEs, myocarditis and pneumonitis portended poor outcomes, with deaths reported in more than one third of the cases.
Extensive clinical application of ICIs in advanced lung cancer results in a noticeable increase in irAEs that can affect any tissue or organ. Several meta-analyses have evaluated profiles of ICI-associated irAEs. Berti et al. compared irAEs between immunotherapy and immunochemotherapy in lung cancer based on 16 phase III RCTs and demonstrated that ICI alone showed a significantly lower risk of irAEs.18 However, the investigators excluded phase II RCTs and ignored discrepancies between individual ICIs with different immune mechanisms. In 2021, an NMA of 12 RCTs was conducted for five ICI agents (nivolumab, pembrolizumab durvalumab, atezolizumab, and ipilimumab) in advanced NSCLC, and compared their irAEs, including colitis, endocrine irAEs, pneumonitis and hepatitis.19 Another updated NMA involving 14 RCTs further assessed severe irAEs across these ICI agents.20 These two studies was considered flawed because of the limited number of included trials and omission of ICI agents and specific irAEs, which inevitably led to instability in network construction as well as insufficient evidence for a conclusion. Given these limitations, we previously performed an NMA involving 38 RCTs to compare the risk of irAEs across different ICI-based regimens and found that ICI monotherapy + CT might be a better choice in advanced lung cancer.21 Nevertheless, the characteristics of irAEs in various ICIs with different immunological mechanisms are yet to be elucidated.
As some delayed or rare irAEs cannot be observed within the clinical timeframe, evidence from large-sample real-world data could fill this gap. To date, several disproportionality analyses have investigated irAEs associated with ICIs with respect to pulmonary,22 endocrine,23 renal,24 neurological,25 cardiovascular,26 and musculoskeletal27 toxicity. However, these studies focused on one specific irAE, rendering the assessment of toxicity associated with ICIs difficult. Recently, Chen et al. characterised overall irAEs related to each ICI regimen and found that pembrolizumab had the highest fatality proportion.28 Notably, the aforementioned disproportionality analyses involved all patients with ICI treatment, whose risk of irAEs may vary according to cancer type. Thus, the current study comprehensively estimated the overall and organ-specific toxicity spectrum of 10 ICI-based regimens by pooling available phase II/III clinical trials and performing a disproportionality analysis from the FAERS database in patients with advanced lung cancer.
The present study confirmed the findings of our prior NMA that the extra addition of CT to ICI monotherapy decreased the risk of irAEs compared to ICI monotherapy and dual ICIs therapy, even if ICIs were sorted by different mechanisms. This finding might be attributed to the immunosuppression mediated by CT, as discussed in the previous study.21 Focusing specifically on ICIs with different mechanisms, we revealed that anti-CTLA-4 agent monotherapy was associated with the highest risk of irAEs, followed by anti-PD-1 and anti-PD-L1 agents. Following analyses based on specific irAEs, we recognised that the toxicity of the anti-CTLA-4 agents was primarily attributed to colitis. The mechanisms of anti-CTLA-4 and anti-PD-1/PD-L1 differ in terms of the stage of T-cell activation, impact on downstream pathways and localisation of action. Recently, anti-CTLA-4 and anti-PD-1/PD-L1 antibodies have been termed ‘immune enhancers’ and ‘immune normalisers’, respectively.29 The latter terminology is in line with the hypothesis that anti-PD-1/PD-L1 agents ‘normalise’ T-cell immunity in the tumour microenvironment.30 Consistent with the differences in their mechanisms, anti-PD-1/PD-L1 ICIs showed more potent effects and less toxicity than anti-CTLA-4 ICIs in clinical trials of melanoma.31,32
The disproportionality analysis provided unique insights. In the present study, pneumonitis was the most frequently reported ICI-associated irAE, and generated signals in all ICI regimens. However, Chen et al. indicated that colitis was the most commonly reported AE in patients with unclassified cancers. This finding may be related to patients with lung cancer, with attributes such as a high percentage of patients with smoking habits, underlying pulmonary conditions such as chronic obstructive pulmonary disease, and prior anti-cancer treatments, such as thoracic radiation.33,34 With respect to outcomes in the spectrum of irAEs, pneumonitis with a 35.8% (1610 of 4497) fatality rate ranked next to myocarditis. Although myocarditis is a relatively rare irAE, it is a serious outcome with death reported in approximately half of the cases (163 of 367, 44.4%), which is similar to the conclusions of previous studies (43–51%).26,35
As the application of immune therapies continues to rise in oncology, our results provide insights into the safety of therapies, thereby aiding in clinical decision-making to develop tailored immunotherapy strategies for each patient with lung cancer. For instance, administration of anti-PD-1/PD-L1 ICI plus anti-CTLA-4 ICI appeared to significantly enhance the risk of pneumonitis (grade 1–5 and grade 3–5) compared to ICI monotherapy with or without CT and could be avoided in selected cases of lung fibrosis or severe chronic obstructive pulmonary disease. In addition, treatments without anti-CTLA-4 ICI were associated with the lowest risk for colitis and may be preferred in selected patients for whom gastrointestinal irAEs could be a concern. These results should be confirmed in prospective registries or cohorts to better understand the safety of novel ICI–based options in this subset of patients. After deciding to administer immunotherapy, active monitoring and vigilant screening for ICI-associated irAEs are encouraged, particularly for those with a high incidence or poor prognosis. The present findings should be considered in clinical decisions regarding ICI treatment and in further clinical trials.
The main strength of this study was the scientific and systematic quantification of the potential risks associated with ICIs, with the steady support of big data in clinical trials and real-world databases. Second, we provided a full view of the toxicity profile of ICIs in different dimensions, including the type, severity, location, and prognosis of AEs. Third, given the differences in toxicity spectra based on cancer type, we focused on a specific population of patients (i.e., those with lung cancer). Fourth, except for the combination of ICIs and targeted agents, we comprehensively evaluated and characterised AEs in all available ICI-based regimens, serving as a reference for the further prevention and management of irAEs in patients with lung cancer.
However, our study had several limitations. In the pooled analysis, descriptions of irAEs differed among the included RCTs. In clinical settings, AEs or irAEs are typically recognised based on the evaluation of a physician and diagnosed based on their experience. Therefore, the identification of irAEs might not be completely accurate and may lead to bias in the assessment. Second, the included studies showed heterogeneity in cancer subtypes, pharmacological strategies, treatment line, and other factors. Thus, scenario analyses were performed to control the effect of possible confounders. Third, we could not access comorbidity data, which may be a high-risk factor for certain irAEs. In addition, several inherent limitations in pharmacovigilance studies were also noted. Primarily, data mining from the FAERS database cannot avoid the shortcomings of under- or over-reporting, false reporting, missing data, inaccuracy, and arbitrariness. Meanwhile, disproportionality analysis can neither quantify the risk nor prove causality. The ROR is an indicator of an increased risk of AE reporting. Given these limitations, further studies are needed to confirm our findings.
This study contributes to the clarification of the characteristics of AEs associated with ICI-based regimens in patients with advanced lung cancer. In general, the combination of two ICIs was associated with a higher risk of irAEs than the use of a single ICI. In addition, treatments involving anti-CTLA-4 ICI contributed to more irAEs, especially colitis. After the initiation of immunotherapy, irAEs that are frequently reported (pneumonitis and colitis) or those associated with high mortality (myocarditis, pneumonitis, and hepatitis) should be closely monitored by clinicians.
Contributors
Zhi-Chun Gu and Hou-Wen Lin are the guarantors of the manuscript. Zhi-Chun Gu, Yi–Dan Yan, and Xiang-Li Cui contributed to the study conception and design and critical revision of the manuscript for important intellectual content. Yi–Dan Yan, Ying Zhao, Chi Zhang and Jie Fu contributed to data collection, analysis, and interpretation. Zhi-Chun Gu, Hou-Wen Lin, Ying-Jie Su and Xiang-Li Cui verified the underlying data. Er-Li Ma and Bing-Long Liu provided important guidance for this study. All authors had full access to the data and had the final responsibility for the decision to submit for publication.
Data sharing statement
The data that support the findings of this study are available on reasonable request from the corresponding authors.
Declaration of interests
The authors have no conflicts of interest to declare.
Acknowledgements
This study was supported by the Research Project of Drug Clinical Comprehensive Evaluation and Drug Treatment Pathway (SHYXH-ZP-2021-001, SHYXH-ZP-2021-006), Clinical Research Innovation and Cultivation Fund of Ren Ji Hospital (RJPY-LX-008), Ren Ji Boost Project of National Natural Science Foundation of China (RJTJ–JX–001), and Shanghai “Rising Stars of Medical Talent” Youth Development Program – Youth Medical Talents – Clinical Pharmacist Program (SHWJRS (2019) 072).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101535.
Contributor Information
Zhi-Chun Gu, Email: guzhichun213@163.com.
Hou-Wen Lin, Email: linhouwen@renji.com.
Appendix. Supplementary materials
References
- 1.Mielgo-Rubio X, Uribelarrea EA, Cortés LQ, Moyano MS. Immunotherapy in non-small cell lung cancer: update and new insights. J Clin Transl Res. 2021;7(1):1–21. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang H, Dai Z, Wu W, et al. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J Exp Clin Cancer Res: CR. 2021;40(1):184. doi: 10.1186/s13046-021-01987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee DJ, Lee HJ, Jr., Farmer JR, Reynolds KL. Mechanisms driving immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Curr Cardiol Rep. 2021;23(8):98. doi: 10.1007/s11886-021-01530-2. [DOI] [PubMed] [Google Scholar]
- 4.Neelapu SS, Adkins S, Ansell SM, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of lymphoma. J Immunother Cancer. 2020;8(2):e001235. doi: 10.1136/jitc-2020-001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durrechou Q, Domblides C, Sionneau B, et al. Management of immune checkpoint inhibitor toxicities. Cancer Manage Res. 2020;12:9139–9158. doi: 10.2147/CMAR.S218756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence - what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 8.Maughan BL, Bailey E, Gill DM, Agarwal N. Incidence of immune-related adverse events with program death receptor-1- and program death receptor-1 ligand-directed therapies in genitourinary cancers. Front Oncol. 2017;7:56. doi: 10.3389/fonc.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Z, Sun X, Zhao Z, et al. Risk of pneumonitis in cancer patients treated with PARP inhibitors: a meta-analysis of randomized controlled trials and a pharmacovigilance study of the FAERS database. Gynecol Oncol. 2021;162(2):496–505. doi: 10.1016/j.ygyno.2021.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Jing Y, Zhang Y, Wang J, et al. Association between sex and immune-related adverse events during immune checkpoint inhibitor therapy. J Natl Cancer Inst. 2021;113(10):1396–1404. doi: 10.1093/jnci/djab035. [DOI] [PubMed] [Google Scholar]
- 11.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (Clinical research ed) 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr. 2021;112(1):90–92. doi: 10.1016/j.ad.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang P, Wei S, Zhai K, et al. Efficacy of left ventricular unloading strategies during venoarterial extracorporeal membrane oxygenation in patients with cardiogenic shock: a protocol for a systematic review and Bayesian network meta-analysis. BMJ Open. 2021;11(10) doi: 10.1136/bmjopen-2020-047046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu C, Chen YP, Du XJ, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ (Clinical research ed) 2018;363:k4226. doi: 10.1136/bmj.k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13(8):519–523. doi: 10.1002/pds.1001. [DOI] [PubMed] [Google Scholar]
- 17.Zhai Y, Ye X, Hu F, et al. Endocrine toxicity of immune checkpoint inhibitors: a real-world study leveraging US Food and Drug Administration adverse events reporting system. J Immunother Cancer. 2019;7(1):286. doi: 10.1186/s40425-019-0754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berti A, Bortolotti R, Dipasquale M, et al. Meta-analysis of immune-related adverse events in phase 3 clinical trials assessing immune checkpoint inhibitors for lung cancer. Crit Rev Oncol Hematol. 2021;162 doi: 10.1016/j.critrevonc.2021.103351. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Gu J, Bian C, Huang G. Immune-related adverse events associated with immune checkpoint inhibitors for advanced non-small cell lung cancer: a network meta-analysis of randomized clinical trials. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.686876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu J, Shi L, Jiang X, et al. Severe immune-related adverse events of immune checkpoint inhibitors for advanced non-small cell lung cancer: a network meta-analysis of randomized clinical trials. Cancer Immunol Immunother. 2022 doi: 10.1007/s00262-022-03140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan YD, Cui JJ, Fu J, et al. A network comparison on safety profiling of immune checkpoint inhibitors in advanced lung cancer. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.760737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reese SW, Cone E, Marchese M, et al. Lessons from pharmacovigilance: pulmonary immune-related adverse events after immune checkpoint inhibitor therapy. Lung. 2021;199(2):199–211. doi: 10.1007/s00408-021-00425-x. [DOI] [PubMed] [Google Scholar]
- 23.Bai X, Lin X, Zheng K, et al. Mapping endocrine toxicity spectrum of immune checkpoint inhibitors: a disproportionality analysis using the WHO adverse drug reaction database, VigiBase. Endocrine. 2020;69(3):670–681. doi: 10.1007/s12020-020-02355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, Qin Y, Fan QQ, Zhao B, Mei D, Li XM. Renal adverse effects following the use of different immune checkpoint inhibitor regimens: a real-world pharmacoepidemiology study of post-marketing surveillance data. Cancer Med. 2020;9(18):6576–6585. doi: 10.1002/cam4.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato K, Mano T, Iwata A, Toda T. Neurological and related adverse events in immune checkpoint inhibitors: a pharmacovigilance study from the Japanese Adverse Drug Event Report database. J Neurooncol. 2019;145(1):1–9. doi: 10.1007/s11060-019-03273-1. [DOI] [PubMed] [Google Scholar]
- 26.Fan Q, Hu Y, Yang C, Zhao B. Myocarditis following the use of different immune checkpoint inhibitor regimens: a real-world analysis of post-marketing surveillance data. Int Immunopharmacol. 2019;76 doi: 10.1016/j.intimp.2019.105866. [DOI] [PubMed] [Google Scholar]
- 27.Allenbach Y, Anquetil C, Manouchehri A, et al. Immune checkpoint inhibitor-induced myositis, the earliest and most lethal complication among rheumatic and musculoskeletal toxicities. Autoimmun Rev. 2020;19(8) doi: 10.1016/j.autrev.2020.102586. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Wu B, Zhang C, Xu T. Immune-related adverse events associated with immune checkpoint inhibitors: an updated comprehensive disproportionality analysis of the FDA adverse event reporting system. Int Immunopharmacol. 2021;95 doi: 10.1016/j.intimp.2021.107498. [DOI] [PubMed] [Google Scholar]
- 29.Weinmann SC, Pisetsky DS. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology (Oxford) 2019;58(suppl 7):vii59–vii67. doi: 10.1093/rheumatology/kez308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175(2):313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ascierto PA, Del Vecchio M, Mandalá M, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(11):1465–1477. doi: 10.1016/S1470-2045(20)30494-0. [DOI] [PubMed] [Google Scholar]
- 32.Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239–1251. doi: 10.1016/S1470-2045(19)30388-2. [DOI] [PubMed] [Google Scholar]
- 33.Bouros D, Hatzakis K, Labrakis H, Zeibecoglou K. Association of malignancy with diseases causing interstitial pulmonary changes. Chest. 2002;121(4):1278–1289. doi: 10.1378/chest.121.4.1278. [DOI] [PubMed] [Google Scholar]
- 34.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen C, Chen T, Liang J, et al. Cardiotoxicity induced by immune checkpoint inhibitors: a pharmacovigilance study from 2014 to 2019 based on FAERS. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.616505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.