Abstract
Cervical cancer causes many deaths in females worldwide, including in Indonesia. Several studies have reported that soursop (Annona muricata L.) leaves can be used to treat cervical cancer. This study aims to determine the use of endophytic fungi of A. muricata leaves extract as an ingredient that inhibits cervical cancer. The isolated endophytic fungi from various soursop leave accessions were grown in culture media, then extracted using ethyl acetate. The extract was then tested against anti-yeast, cervical cancer cells, and on normal cells as control using the MTT method. Five isolated fungi were selected based on the greatest inhibition in one concentration, and the inhibitory concentration 50 (IC50) value was determined. The soursop leaves endophytic fungi extracts showed cytotoxicity against cervical cancer cells by inhibiting the multiplication of HeLa cancer cells in vitro. The Sir-SM2 endophytic fungi crude ethyl acetate extract showed high cytotoxicity to cervical cancer cells (HeLa cells) but less harmful to the normal Chang cells; therefore can be a natural anticancer. Identification based on morphology shows that the isolated Sir-SM2 endophytic fungi belong to the Penicillium genus, and molecular identification based on Internal Transcribed Spacer shows high similarities with Penicillium crustosum.
Keywords: Cervical cancer, Endophytic, Cytotoxic, Ethyl acetate, Identification
1. Introduction
Cervical cancer is a type of cancer that causes the fourth highest death in women with cancer. In 2020, new cases estimated 604,000, with 342,000 deaths worldwide (WHO, 2020a). Cervical cancer can be caused by infection due to excess free radical production, apart from human papillomavirus (HPV) (WHO, 2020b).
Commonly performed treatments for patients with cancer are surgery, chemotherapy, hormone therapy, radiation therapy, targeted drug therapy, and immunotherapy (American Cancer Society, 2016). Unfortunately, some of these treatments cause side effects, such as causing hair loss, early menopause, weariness, infections, sores on the mouth and throat, and remembrance problems (Mehta and Bhargava, 2019). On the other hand, herbal medicines and phytochemical derivatives have therapeutic effects. Several studies have shown positive results from herbal medicines in improving the quality of life and increasing the immune system of cancer patients (Lee et al., 2018). Additionally, medicinal plants with secondary metabolites showing significant antioxidant activity can play a role in cancer treatment (Seca and Pinto, 2018).
Flowering plants in Indonesia consist of 30,000 species, of which 940 species are identified as having medicinal properties, one of which is soursop. The soursop plant is a type of fruit plant that contains bioactive compounds, such as tannins, phytosterols, flavonoids, saponins, and alkaloids (Agu and Okolie, 2017). Antioxidant compounds prevent the type of free radical damage that has been linked to cancer development (Meng et al., 2020). The soursop plant produces acetogenins that exhibit cytotoxic activity against cancer cells and have special affinities for some resistant cells. Acetogenins from Annonaceae are cytotoxic against several cancer and tumor cells, such as pancreatic cancer cells (PACA-2), lung cancer cells (A549), colon, lung, and pancreatic tumors (Nugraha et al., 2019). Research on Erythrina poeppigiana leaves showed an inhibitory concentration 50 (IC50) value or inhibition against cervical cancer cells (HeLa) of 7.18 μg mL−1 (Herlina et al., 2019). Bioactive compounds may be produced by endophytic microbes.
Endophytic microbes live in plant tissue by forming colonies without harming the host and producing bioactive compounds that have the potential for drug development (Gouda et al., 2016). Gouda et al. (2016) reported that endophytic microbes could synthesize bioactive compounds similar to those produced by their host due to the evolutionary exchange of genetic information. Metabolite compound production through endophytic microbes will reduce the exploitation of the soursop plant; hence, this plant can continue to produce fruit for public consumption. Minarni et al. (2017) reported that extract of endophytic fungi isolated from the A. muricata leaves could suppress the overgrowth of MCF-7 breast cancer cells with an IC50 value of 19.20 ± 7.71 g mL−1. Likewise, Arifni et al. (2017) also reported that soursop leaves endophytic fungi extract has an IC50 value for colon cancer (WiDr) of 20.80 µg mL−1. The endophytic fungi of soursop leaves show significant potential for anticancer activity. Therefore, this study aims to test the endophytic extract activity against cervical cancer cells (HeLa).
2. Material and method
This study used soursop leaves endophytic fungi isolates (from Sukabumi, Cianjur, and Garut), glucose, yeast extract, malt extract, peptone, agar, ethyl acetate, RPMI-1640 (Roswell Park Memorial Institute) cell growth medium, DMEM (Dulbecco's Modified Eagle Medium) cell growth media, doxorubicin, NaHCO3, aquabides, 96% ethanol (pro-analysis), 100% ethanol (pro-analysis), FBS, penicillin–streptomycin, trypsin, saline phosphate buffer (PBS), cervical cancer cells (HeLa, ATCC®-CCL-2™), normal cells (Chang, ATCC ®-CCL™ 13), Methyl Thyazol Tetrazolium (MTT).
2.1. Media preparation
2.1.1. Malt Glucose Yeast Peptone Agar (MGYPA) medium
Medium of MGYPA was made by dissolving in 1000 mL water, the components such as yeast extract (3 g), malt extract (3 g), glucose (10 g), peptone (5 g), and agar (20 g). The ingredients were put into an Erlenmeyer flask, covered with cotton, then heated in the oven until boiling, and all the ingredients were completely dissolved. Next, the media was autoclaved for sterilization for 15 min at a pressure of 1 atm 121 °C and poured into a Petri dish.
2.1.2. Malt Glucose Yeast Peptone Broth (MGYPB) Medium
Medium of MGYPB was made by dissolving in 1000 mL of distilled water extract of yeast (3 g), extract of malt (3 g), glucose (10 g), and peptone (5 g). All ingredients were put into the Erlenmeyer flask containing 150 mL and covered with modified cotton. The media were sterilized using an autoclave for 15 min at a pressure of 1 atm at 121 °C.
2.2. Endophytic fungi isolate cultivation (Marcellano et al., 2017)
Endophytic fungi isolates originated from the Cianjur region (with accessions codes Sir-CA1, Sir-CA2, Sir-CA3), the Garut region (accessions codes Sir-G2, Sir-G3, Sir-G4, Sir-G5), and the Sukabumi region (accessions codes Sir-SM1, Sir-SM2, Sir-SM3). Endophytic fungi isolates were inoculated aseptically in a special laminar flow. Endophytic fungi isolated from agar slant were aseptically cultured into MGYPA media and then kept for 2 days at 28 °C ± 2 °C. Secondary metabolite production was conducted by inoculating each endophytic fungi isolate using a tungsten needle into a 150 mL medium of MGYPB and incubated for 21 days at 28 °C ± 2 °C.
2.3. Extraction metabolite compounds (Arifni et al., 2017)
An organic ethyl acetate solvent extracted bioactive compounds from endophytic fungi. Extraction was carried out from endophytic fungi cultured for 21 days at 25 °C. The fungi were cultured in 150 mL of MGYPB medium, supplemented with distillate ethyl acetate, then shaken by hand for 30 min. The fraction at the top layer was then transferred to a heating flask and put in a rotated vacuum evaporator at 36 °C. The extract was dried by simmering of nitrogen gas. The crude extract was kept at 3 °C for further analysis.
2.4. Anticancer activity against cervical cancer cells HeLa with methyl Thyazol tetrazolium (MTT) indicator (Herlina et al., 2019)
The extract was dissolved with RPMI-1640 medium and made a concentration of 100 μg mL−1. The diluted extract was then tested for its activity against HeLa cancer cells. At the first test, the cancer cells without treatment were used as the negative control. For the positive control, 6 μg mL−1 doxorubicin solution was used. The five fungi extract with the best activity according to inhibition percentage were then re-tested against HeLa cancer cells at 25, 50, 100, 200, and 400 μg mL−1 concentrations.
The cells were poured into a sterile conical tube containing RPMI 1640 medium, centrifuged, then a new RPMI 1640 medium was poured into the cell suspension and then recentrifuged until homogeneous. The cell suspension was added with 1 mL RPMI 1640 which have been supplemented with 1% penicillin–streptomycin and 5% FBS. A 100-µL medium-containing cell with a total of 5000 cells/well was added to 96 wells of tissue culture plate and kept for 24 h at 5% CO2 at 28 °C ± 2 °C to 70–80% confluent. The extract was added as much as 100 µL/well and kept for 48 h, and then washed with PBS and added with 100 µL/well MTT. Cells were kept for 4 h at 5% CO2 and 37 °C. The supernatant was separated, and 96% ethanol was added. Furthermore, an optical density was measured with an enzyme-linked immunosorbent assay (ELISA) at a wavelength of 595 nm. Data from the ELISA readings are transformed into the inhibition percentage of cells based on the following equation:
Information:
Abs. Control = Absorbance contains no extract.
Abs. Sample = Absorbance of extract solution.
The relationship between cell death and concentration was analyzed using linear regression, and then the extract concentration that could prevent 50% of the cancer cells growth was calculated (IC50). The same procedure was conducted for the cytotoxic activity assay of HeLa cells but using DMEM growth medium added to the tube and kept in the freezer for 24 h.
2.5. Morphological identification of Sir-SM2 (Minarni et al., 2017)
Identification of the Sir-SM2 isolate was conducted by observing the morphological characteristics of the colony and spore. The morphological observation was conducted macroscopically and microscopically, using the identification key (Nithiyaa et al., 2012). The purification results observed Fungi morphology using a 2-day-old endophytic fungi culture. The medium used for morphological observations was the potato dextrose agar (PDA) medium. Macroscopic observations included colony color and surface (granular, powdery, mountainous, and smooth), texture, growing area, and radial and concentric stripes (especially in Penicillium molds). Microscopic observations used a microscope with a magnification of 1000×. The observation was carried out on somatic hyphae type (septate/aseptic), hyphae pigmentation, spore stalk shape, shape, and asexual spore ornamentation (Manan et al., 2017).
2.6. Identification of Sir-SM2 based on amplification of Internal Transcribed Spacer (ITS) (Sundaresan et al., 2019)
The Sir-SM2 isolate was identified molecularly based on partial genetic analysis at the fungi’s Internal Transcribed Spacer (ITS) ribosomal deoxyribonucleic acid (DNA) locus. For DNA extraction, fungal isolates were grown in potato dextrose broth liquid medium and incubated at room temperature for 72 h. DNA was isolated from mycelia (Yang et al., 2016) with a modified solution. The hyphae grown on the cellulose membrane were taken and put into a microtube. The mycelium was mashed, and 500 µL of SDS solution was added, which had been incubated in a water bath at 65° C for 30 min and then quickly transferred to the freezer for 5 min. Chloroform: isopropanol solution of 500 µL was added to the tube, homogenized, and further centrifuged for 10 min at 4 °C 10,000 rpm.
Then the supernatant was taken and put into a new tube. Solution of 500 µL Phenol: chloroform: isopropanol (25:24:1) (v/v) was added to the tube and homogenized. The tube was centrifuged at 10,000 rpm at 4 °C for 5 min, and the supernatant was separated and put into a new tube, added with ethanol absolute, then incubated in the freezer for 24 h. The tube was centrifuged for 30 min at 4 °C 10,000 rpm, then the supernatant was removed, and only the pellet was used for the next step. Approximately 500 µL of 70% ethanol solution was added to the tube and then homogenized. The tube was centrifuged for 10 min at 4 °C 10,000 rpm. The supernatant was discarded, and only the pellet was used. Then, the tube was vacuumed at 45 °C for 30 min. The pellet containing DNA, NFW, and RNAse was added to the tube. Before the next step, the concentration and quality of the genomic DNA were determined at wavelengths of 260 and 280 nm using the Nanodrop Spectrophotometer 2000 (Thermo Scientific).
A polymerase chain reaction (PCR) was then conducted to amplify the ITS region of the extracted DNA. Two ITS primers were used, i.e., ITS Primer 4 (reverse): 5′–TCC TCC GCT TAT TGA TAT GC–3′, and ITS Primer 5 (forward): 5′–GGA AGT AAA AGT CGT AAC AAG G–3′ (Endrawati and Kusumaningtyas, 2021, Ausubel et al., 2004, McBreen et al., 2003). Amplification was conducted on 10 µL consisting of dNTP of 0.2 mM, DreamTaq 1 × buffer (containing MgCl2), ITS4 Primer of 0.5 pmol, ITS5 Primer of 0.5 pmol, Taq polymerase of 0.125 U, DNA template of 100 ng, and NFW of 5.75 µL. DNA amplification was carried with predenaturation at 95 °C for 5 min, followed by 35 cycles consisting of denaturation at 95 °C for 15 s, attachment at 57 °C for 15 s, and extension at 72 °C for 45 s. The PCR process ended with post-extension at 72 °C for 5 min and cooling at 4 °C for 5 min.
The PCR products were visualized using electrophoresis on 1% agarose gel dissolved in a TAE buffer. Electrophoresis was conducted with a TAE buffer. First, 1 µL of loading dye was mixed with 1 µL of DNA sample and then put into the agarose gel well. All samples were put in gel wells, and then a 2 µL, 1 kb ladder was inserted into one of the wells as a marker well. Samples were electrophoresed with a voltage of 100 V for 30 min. Next, the gel was soaked in EtBr solution for 30 min and then washed with distilled water for 5 min. Finally, the electrophoresis products were visualized with an ultraviolet transilluminator (Kusumaningrum et al., 2014).
The purification of the PCR results was carried out using PEG and continued with a sequencing cycle. The results of the sequencing cycle were repurified with ethanol according to Sapula et al. (2021). Analysis of nitrogen base sequence readings using an automated DNA sequencer (ABI PRISM 3130 Genetic Analyzer) (Applied Biosystems). The raw data from the sequencing was then trimmed and assembled using the BioEdit program (https://www.mbio.ncsu.edu/BioEdit/bioedit.html). Sequence data that has been assembled is then carried out by Basic Local Alignment Search Tool (BLAST) with genomic data that has been registered at the DNA Data Bank of Japan (https://blast.ddbj.nig.ac.jp/) or NCBI/National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/BLAST/) to define which taxon/species has the greatest homology/similarity and molecularly closest. After that, several organisms were selected to be included in the phylogenetic tree. Percent of homology was calculated using Molecular Evolutionary Genetics Analysis version 5.0 software (MEGA 5) using the Maximum Likelihood parameter. The analysis was carried out with 1000 repetitions using a 2-parameter Kimura model using the Gamma distribution (+G) (Tamura et al. 2011). Monophyletic genera and species were indicated by branches having Bootstrap values (BS) > 80% (Xiao et al., 2020).
3. Results
3.1. Anticancer activity against HeLa cervical cancer cells
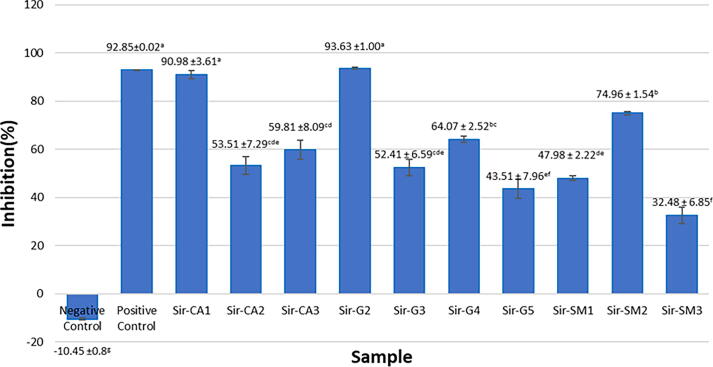
The first anticancer activity was tested using one concentration, namely 100 μg mL−1 from ten isolates (Fig. 1). The ten extracts tested at 100 µg mL−1 concentration against cervical cancer cells (HeLa cells) had a good inhibition potential based on the results shown in Fig. 1. The five best extracts were taken from the ten extracts tested based on the highest inhibition percentage (Fig. 1). The best extract was Sir-G2, with an inhibition value exceeding the positive control of 93.63% for the initial test, compared with the positive control (doxorubicin) with 92.85% inhibition.
Fig. 1.
Anticancer activity of endophytic fungi extract from soursop leaves against. HeLa cells at 100 μg mL−1 concentration.
The five best extracts (Sir-CA1, Sir-CA3, Sir-GA2, Sir-GA4, and Sir-SM2) were then tested at 25, 50, 100, 200, and 400 μg mL−1. The absorbance results of the extract cytotoxic activity inhibition test were then calculated to find the IC50 value. The IC50 value is the concentration of the extract needed to inhibit cancer cell growth by 50% (Nugraha et al., 2019). The IC50 values can be seen in Table 1. Three endophytic fungi extracts (Sir-CA1, Sir-G2, and Sir-SM2) revealed IC50 values of <30 μg mL−1 for HeLa cells. However, Sir-CA1 has the lowest IC50 value but is statistically insignificant (p < 0.05) with Sir-G2 and Sir-SM2.
Table 1.
IC50 value of ethyl acetate extract of soursop leaf endophytic fungi on HeLa cells.
| Sample | IC50 (μg mL−1) |
|---|---|
| Extract Sir-CA1 | 8.47 ± 0.25b |
| Extract Sir-CA3 | 155.34 ± 30.80a |
| Extract Sir-G2 | 11.71 ± 1.05b |
| Extract Sir-G4 | 36.48 ± 2.63b |
| Extract Sir-SM2 | 29.14 ± 5.72b |
Different letters indicate significant differences in the Tukey test (p < 0.05)
Moreover, extracts with anticancer activity are expected to have low toxic properties to normal cells (selective toxicity) (Cao, 2017). Therefore, the endophytic soursop extracts were tested against Chang’s normal cells in our research series and published elsewhere. Sir-CA1 was highly toxic to normal Chang cells, followed by Sir-G2, which produced IC50 values of 8.23 and 23.65 μg mL−1, respectively (Arifni et al., 2017). Additionally, Sir-SM2 had the lowest toxicity activity against Chang’s normal cells at 63.69 μg mL−1, compared to extracts from Cianjur (Sir-CA1) and Garut (Sir-G2). Therefore, the Sir-SM 2 was selected for further study.
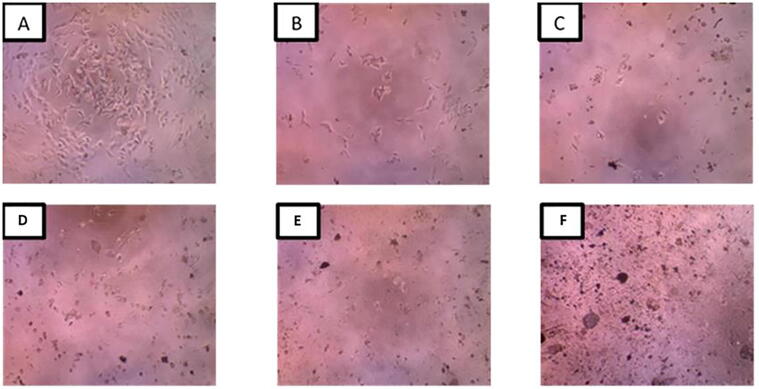
The anticancer activity of Sir-SM2 against HeLa cervical cancer cells with MTT indicator revealed that the control cells showed cell morphology in normal conditions. However, the Sir-SM2 extract can inhibit the growth of HeLa cells from extract concentrations of 25–400 μg mL−1 and successively result in an increasingly damaged cell morphology (Fig. 2). Microscopic photos with 10x magnification of cervical cancer cells treated with Sir-SM2 endophytic fungi ethyl acetate extracts from 25 to 400 μg mL−1 can be seen in Fig. 2.
Fig. 2.
Cervical cancer cells (HeLa cells) treated with soursop leaf endophytic fungi extract (Sir-SM2). Description: A = control without treatment, B = 25 μg mL−1 concentration, C = 50 μg mL−1 concentration, D = 100 μg mL−1 concentration, E = 200 μg mL−1 concentration, F = concentration 400 μg mL−1 concentration (Canon inferted microscope, Dyno Eye camera, 1280 × 1024 unit: inch) at 10× magnification).
Sir-SM2 has good cytotoxic activity against cervical cancer cells and the lowest cytotoxic activity against normal cells compared to other endophytic fungi ethyl acetate extracts, as reported by Arifni et al. (2017), and therefore selected as the best isolate.
3.2. Morphological identification of Sir-SM2 isolate
The selected endophytic fungi of Sir-SM2 isolate were observed on colony and spore, macroscopically and microscopically. Colonies grew well on MGYPA medium, reaching a 3.7–4.4 cm diameter within one week of incubation at 28 °C ± 2 °C. Colonies are concentric-round in shape with young hyphae white, greenish-blue, and dark green hyphae (asexual spores are green). The colony’s surface is like cotton, smooth, and does not have aerial hyphae (hyphae that rise above the colony's surface).
Microscopic observation showed that Sir-SM2 isolate had somatic hyphae with septate character. Additionally, reproductive hyphae have parts, such as leg cells, erect conidiophores, metula, bottle-shaped conidiogens (phialides), chain conidium (leading to the genus Penicillium), round conidium (globose), hyaline color (transparent), and smooth conidium surface without ornament. Furthermore, the number of conidiogenous cells in one metula is a multiple of three.
3.3. Identification of Sir-SM2 isolate based on ITS Amplification.
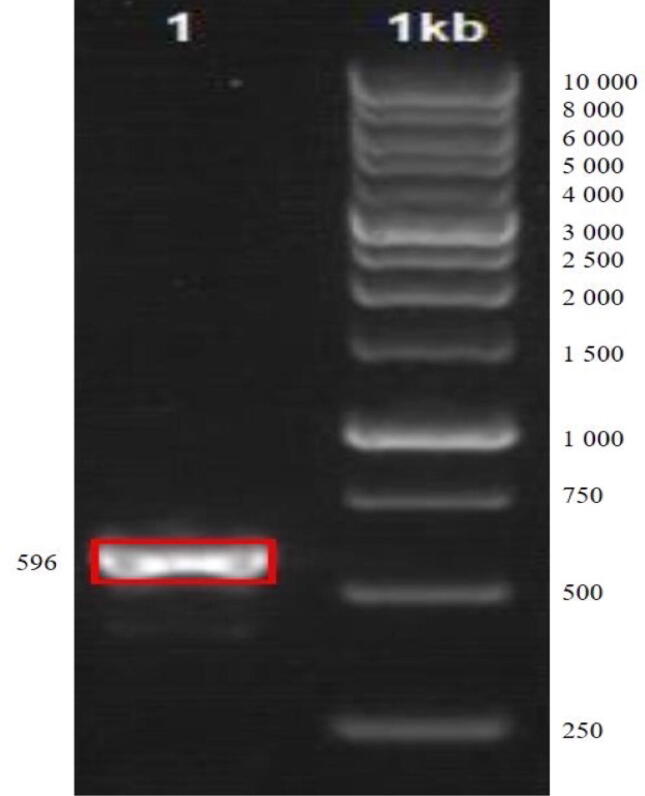
Molecular-based identification was conducted to complete the information regarding the Sir-SM2 isolate. Based on the molecular approach, it showed a clear band in the 596 base pair region of DNA isolated from the endophytic fungi Sir-SM2 (marker 1 Kb). Additionally, the band produced from the PCR resulted in 1% agarose gel electrophoresis (Fig. 3).
Fig. 3.
PCR results of endophytic fungi isolate Sir-SM2.
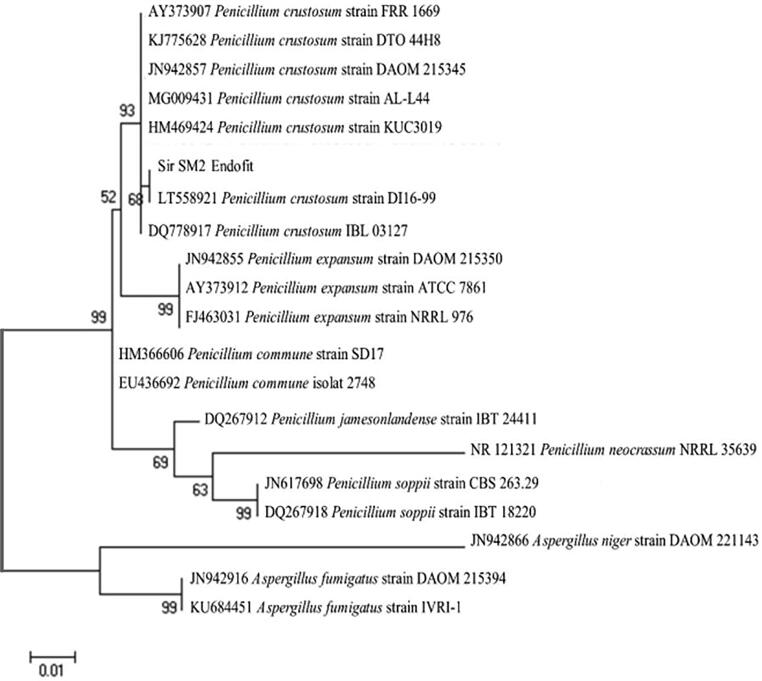
Nucleotide sequence data alignment of the Sir-SM2 isolate was used to identify isolates by looking for the homology of the closest species sequence to the sequence data in the NCBI database/Gene Bank National Center for Biotechnology Information (Benson et al., 2005). The results showed a 99% similarity rate with Penicillium crustosum (P. crustosum). The phylogenetic tree analysis results reveal that the Sir-SM2 is highly similar to P. crustosum (Fig. 4). The phylogenetic tree was designed using a single ITS sequence with Aspergillus fumigatus and Aspergillus niger species as outgroups and 15 ITS sequences from the Penicillium strains of P. crustosum, P. commune, P. expansum, P. sopii, and P. neocrassumes (Lampiron jamessum and Peniensecil). Aspergillus was chosen as the outgroup because it belongs to the group of imperfect fungi together with Penicillium. The stage of sexual reproduction in some species of Penicillium and Aspergillus has not been found (does not have a complete stage of sexual reproduction) (Nithiyaa et al., 2012).
Fig. 4.
Phylogenetic tree of endophytic fungi isolate Sir-SM2 with K2 + G model (Kimura. 2-parameter + gamma distributed).
4. Discussion
The statistical test results from the first screening showed that almost all samples had anticancer activity (p < 0.05) (Fig. 1). Five extracts, i.e., Sir-CA1, Sir-CA3, Sir-G2, Sir-G4, and Sir-SM2, were taken among the ten tested extracts based on the highest inhibition percentage. However, the five best extracts showed large variation in IC50 values following the five concentration variation testing, namely 25, 50, 100, 200, and 400 g mL−1. Therefore, extracts from Cianjur, Garut, and Sukabumi with isolates names Sir-CA1, Sir-G2, and Sir-SM2, among the five best extracts, produced the highest IC50 values and were chosen for further study. The selection was based on the criteria that a crude extract should be able to inhibit 50% of the cancer cells at a concentration below 30 μg mL−1 (IC50 < 30 g mL−1) (Itharat et al., 2007).
The extracts of Sir-CA1, Sir-G2, and Sir-SM2 showed the highest anticancer activity with different values (p < 0.05) of 8.47, 11.71, and 29.14 μg mL−1, respectively. Differences in values may be due to content differences of secondary metabolites in plants. The difference in metabolite compound contents from different areas may be attributed to differences in geological or environmental conditions. Temperature, water availability, solar energy, atmospheric quality, structure, air and soil composition, and reactions of soil and organisms will affect the content compounds in plants as hosts of these microbes form colonies (Nugraha et al., 2019).
Elisya and Murtini (2015) produced an IC50 of 35.98 μg mL−1 following testing of the soursop leaves ethyl acetate extract tablets on HeLa cells. At least 10 kg of soursop leaves is required to make tablet formulations with the best activity. Dewangga (2015) showed an IC50 value of 77.096 μg ml−1 against HeLa cells, using the chloroform–ethyl acetate fraction of soursop leaves. Chloroform extract from soursop leaves by Artanti et al. (2016) produced an IC50 value of 127.3 μg mL−1. Mardiyaningsih and Ismiyati (2014) showed that the IC50 value or inhibition of cervical cancer cells (HeLa) was 10.016 ± 3.770 μg mL−1. Suyatmi et al. (2012) found that soursop leaf extract inhibited the growth of 50% of HeLa cervical cancer cells at 97 μg mL−1 concentration. All these researchers used soursop leave extracts to test against HeLa cells with chloroform or ethyl acetate solvents, except Dewangga (2015), who used the fraction of soursop leave extracts. Our study, which used endophytic fungi ethyl acetate extract, is different from these studies. Different materials and solvents may result in different extracted chemical compounds, affecting cytotoxicity. However, the cytotoxicity against HeLa cancer cells obtained in our study was better than Dewangga, 2015, Zuraida and Mariya, 2019. Furthermore, the research from Herlina et al., 2019, Mardiyaningsih and Ismiyati, 2014 produced a better IC50 value compared to our study.
Soursop leaves have been traditionally used to treat cancer. The types and contents of biochemical compounds from soursop leaves grown in the natural condition are influenced by the environmental and plant growth conditions. Therefore, endophytic fungi isolated from soursop leaves and cultured on bioreactors under controlled conditions should maintain the continuity of chemical compound production with relative stability. Sir-SM2 fermentation using a bioreactor with controlled conditions showed that similar secondary metabolites were produced at both the laboratory and the factory scales and contained chemical compounds that act as anticancer (Hasan et al., 2020).
The extracts of Sir-SM2 was further tested using the MTT method for cytotoxic activity. The reduction by tetrazolium salts can reliably check proliferation. The basic of the MTT method is that the yellow tetrazolium salt is metabolically reduced by the active cells. This is partly caused by the dehydrogenase enzyme’s action which reduces the equivalent amount of nicotinamide adenine dinucleotide (NAD) hydride and nicotinamide adenine dinucleotide phosphate (NADP). The resulting intracellular purple formazan can be dissolved and measured by spectrophotometer (American Type Culture Collection, 2017). The MTT can measure the rate of cell proliferation, and conversely, cell survival is reduced when a metabolic event causes apoptosis or necrosis. Furthermore, the MTT reagent produces a low absorbance value in the absence of living cells. Therefore, a linear relationship is found between the number of living cells and the signal generated, allowing accurate quantification of changes in cell proliferation rates (American Type Culture Collection, 2017).
The MTT test result supported the IC50 data that the Sir-SM2 endophytic fungi extract can inhibit cancer cells. The HeLa cell inhibition mechanism can occur in three ways: the cell cycle arrest mechanism, cell cycle delay, and apoptosis. Apoptosis is the process of programmed cell death, a body regulation to get rid of unneeded or abnormal cells (Meng et al., 2020). Sir-SM2 endophytic fungi extract can induce cancer cell apoptosis, which may be caused by the metabolite compounds contained in the Sir-SM2 extract.
The Sir-SM2 isolate extract contained metabolite compounds that belong to the group of alkaloid i.e., (hexahydro-pyrrolo[1,2-a]pyrazine-1,4-dione, and hexahydro-3-[2-methyl propyl]-pyrrolo[1,2-a]pyrazine-1,4-dione) (Arifni et al., 2017). These compounds have alkylation ability, which can break DNA strands so that cells are damaged and cause cell death (Ser et al., 2015). The hexahydro-3-(2-methyl propyl)-Pyrrolo[1,2-a]pyrazine-1,4-dione compound can induce changes in cancer cell morphology due to DNA fragmentation, which indicates the ability of these alkaloid compounds to induce apoptosis in A549, and HeLa cancer cells (Lalitha et al., 2016). Hexahydro-pyrrolo[1,2-a]pyrazine-1,4-dione also has excellent antioxidant activity (Balakrishnan et al., 2015). Antioxidants have been studied to trigger damage to the DNA double-strand of cancer cells, causing apoptosis (Meng et al., 2020).
The extracts of Sir-CA1, Sir-G2, and Sir-SM2 showed inhibition against HeLa cancer cells with IC50 values of <30 μg mL−1. The IC50 data is quite interesting because endophytic fungi extract is more toxic to cancer cells than normal cells. Therefore, the endophytic fungi extract is supposedly safer with an IC50 of more than the limit set by Bray et al. (2018) (IC50 of <30 μg mL−1).
These three extracts were tested against normal Chang cells to select the best candidate for their toxicity. An extract with the greatest IC50 value against Chang’s normal cells will be chosen as the best extract. Sir-CA1 and Sir-G2 showed the best cancer inhibition values, but they were also toxic to Chang’s normal cells (Arifni et al., 2017). The Sir-SM2 showed the lowest toxicity against Chang’s normal cells at 63.69 μg mL−1, and its inhibition against HeLa cells was 29.14 μg mL−1. Therefore, Sir-SM2 is selected as the best isolate.
This study used doxorubicin as a positive control because of its wide use as chemotherapy in cancer treatment (Meredith and Dass, 2016). Doxorubicin at a lower concentration (3 μg/mL) in the study resulted in a large inhibition percentage against Chang’s normal cells of 72.52%. Sir-SM2 extract can inhibit HeLa cell proliferation for >50% but damage normal cells at <50% at 25 μg mL−1 concentration. Therefore, the use of Sir-SM2 extract as an anticancer drug must be designed to specifically directly act on cancer cells for them to be less toxic to normal cells. Traditionally, patients with cancer are given a dose of cancer cytotoxic drugs in the trial phase to the maximum tolerated dose because it is assumed as the most effective dose (Thaker et al., 2017).
The best results from endophytic extract as an anticancer agent without damaging normal cells may be obtained through dosage, administration route, and treatment procedure modifications, which are important in research and further utilization. Chemical structure transformation and drug administration system application can reduce the toxicity of compounds from this extract, especially in normal cells. Additionally, isolation and use of selective compounds against cancer cells can be pharmacologically useful for further research.
Sir-SM2 was identified by observing several morphological characters, both macroscopically and microscopically. Macroscopic identification showed that the endophytic colony of Sir-SM2 was concentric spherical with young hyphae greenish-blue to white on the outside and dark green hyphae (asexual spores in green). Indrawati et al. (2021) reported that endophytes with dark green colonies with white surroundings on the PDA medium could be classified as Penicillium sp.
Microscopic identification showed that the endophytic fungi Sir-SM2 had rough cylindrical conidia, a characteristic specific to the genus Penicillium (Indrawati et al., 2021). Conidiophores singly arise from the mycelium or appear in synnemata, branching at the ends, penicillate, three-branched metula on one conidiophore, beam-shaped, and supporting three conidiogens on one metula. Conidiogen shaped like a bottle without any special ornaments (called phialid): conidia (phialospores) hyaline (transparent), conidia mostly globose to ovoid, in basipetal chains, and single-celled (no bulkhead in conidium). These microscopic characteristics point to the genus Penicillium sp. following the identification key of the genus (Nithiyaa et al., 2012).
Penicillium is one genus that can adapt to the most diverse environmental conditions (Nicoletti et al., 2014). Penicillium is commonly found in soil or as a contaminant in food, fruit, fiber, and other starchy materials. However, several studies have shown that Penicillium has evolved into endophytic fungi and has the potential as a new bioactive source and inhibit plants' stress tolerance (Yeshi et al., 2022). Penicillium is also known capable of synthesizing a large number of biochemical components and therefore has spurred numerous studies aimed at investigating its relationship with plant protection and opportunities for medicinal applications in humans, given the relevance of natural products for the development of new antibiotics, immunosuppressants, and medicines for anticancer (Nicoletti et al., 2014).
Fungal species identification at the DNA level uses Ribosomal DNA (rDNA) sequences in the ITS region. It has been used to establish phylogenetic relationships in many fungal groups (Nithiyaa et al., 2012). rDNA is the genomic coding region for the components of ribosomal RNA. The rDNA strands in eukaryotic organisms are located in the nucleus and mitochondria. Fungi are eukaryotic organisms. Conservative regions are found in fungal rDNA, namely the 18S, 5.8S, and 28S rRNA encoding genes, including the ITS region. The ITS area displays sufficient sequence variability to identify many fungal species down to the species level (Nilsson et al., 2008). DNA barcodes use standard sequences of 500–800 base pairs to identify species of the eukaryotic Kingdom using primers suitable for that taxonomic group (Yang et al., 2018).
The endophytic fungi isolate Sir-SM2 has a close relationship with Penicillium crustosum (P. crustosum) based on the phylogenetic tree (Fig. 4). The phylogenetic tree shows a strong clade formation in several species of P. crustosum, and Sir-SM2 belongs to that clade. The confidence level is 93%; thus, Sir-SM2 can be classified as a species of P. crustosum. In the clade P. crustosum, the Sir-SM2 showed a 68% similarity with P. crustosum strain DI16-99. However, Sir-SM2 is possibly a new strain different from P. crustosum strain DI16-9 because this value is still below the standard bootstrap value (<80%). Therefore, further testing is needed by sequencing the gene regions other than ITS4 and ITS5, namely ITS1, ITS2, ITS3, tubulin, actin, small subunit, and large subunit, and other gene regions distinguish P. crustosum and Sir-SM2 in strain levels.
The score value, the sequence alignment value accuracy is an unknown nucleotide with the nucleotide sequence found in the GeneBank, was 1094. The higher the score obtained, the higher the level of homology of the two sequences. Query coverages, the percentage of nucleotide lengths consistent with the BLAST database, was 100%. Max identity, the highest value of percentage match between sequence query with an aligned database sequence, was 99%, and the E-value of 0 (zero) implies that the two sequences are indistinguishable (Pearson, 2013). The morphological identification showed that the soursop leaves endophytic fungi Sir-SM2 had similarities with the morphology of P. crustosum (Visagie et al., 2014).
P. crustosum is a cosmopolitan fungal species that can be isolated from meat, cheese, feed, vegetables, pomegranates, nuts, and seeds and is commonly found in rhizosphere soils from vegetable crops (Overy and Frisvad, 2003). P. crustosum is also found in the medicinal plant Teucrium polium L. (Hassan, 2017). Penicillium species are a widespread presence and are important due to the ability to synthesize mycotoxins and other active ingredients (Sonjak et al., 2005). In addition, P. crustosum synthesizes many volatile and non-volatile compounds (Overy and Frisvad, 2003).
5. Conclusion
Soursop leaves endophytic fungi ethyl acetate extracts are toxic to cervical cancer cells as indicated by inhibiting the proliferation of HeLa cancer cells in vitro. Crude ethyl acetate extract of isolated endophytic fungi Sir-SM2 has the potential as an anticancer. The Sir-SM2 shows high cytotoxicity to cervical cancer cells (HeLa cells) and produces the lowest toxicity on Chang’s normal cells. Morphological identification suggested that the endophytic fungi Sir-SM2 belongs to the Penicillium genus. Furthermore, molecular identification using ITS shows that Sir-SM2 is similar to P. crustosum. The endophytic fungi Sir-SM2 is the potential for developing anticancer drugs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was funded by the Agricultural Research and Development Agency, Ministry of Agriculture of the Republic of Indonesia, which the author would like to thank.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agu K.C., Okolie P.N. Proximate composition, phytochemical analysis, and in vitro antioxidant potentials of extracts of Annona muricata (Soursop) Food Sci. Nutr. 2017;5(5):1029–1036. doi: 10.1002/fsn3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuraida, Mariya S. In vitro cytotoxicity of Alstonia scholaris (R.Br) bark on Vero and HeLa cell lines. IOP Conf. Ser. Earth Environ. Sci. 2019;374:12065. doi: 10.1088/1755-1315/374/1/012065. [DOI] [Google Scholar]
- American Cancer Society, 2016. Chemotherapy for breast cancer [WWW Document]. URL http://www.cancer.org/cancer/breast-cancer-treating-chemotherapy/.
- American Type Culture Collection, 2017. MTT cell proliferation assay [WWW Document]. URL https://www.atcc.org/~/media/DA5285A1F52C414E864C966FD78C9A79.ashx/.
- Arifni F., Hasan A., Hasim, Julistiono H., Husnawaty, Bermawie N., Riyanti E. Anticancer Activities of Endophytic Fungi Isolated from Soursop Leaves (Annona muricata L.) against WiDr Cancer Cells. Annu. Res. Rev. Biol. 2017;18(5):1–11. doi: 10.9734/ARRB/2017/34657. [DOI] [Google Scholar]
- Artanti A.N., Astirin O.P., Prayitno A. Cytotoxic Activity Of Non Polar Fraction From Annona Muricata L. Leaves On Hela And Raji Cell Line. J. Pharm. Sci. Clin1. Cytotoxic Activity Of Non-Polar Fraction From Annona Muricata L. Leaves On Hela And Raji Cell Line. JPSCR J. Pharm. Sci. Clin. Res. 2016;1:112. doi: 10.20961/jpscr.v1i2.1944. [DOI] [Google Scholar]
- Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K. Current protocols in molecular biology: Preface. Curr. Protoc. Mol. Biol. 2010 doi: 10.1002/0471142727.mbprefs66. [DOI] [Google Scholar]
- Balakrishnan D., Bibiana A.S., Vijayakumar A., Santhosh R.S., Dhevendaran K., Nithyanand P. Antioxidant Activity of Bacteria Associated with the Marine Sponge Tedania anhelans. Indian J. Microbiol. 2015;55(1):13–18. doi: 10.1007/s12088-014-0490-8. [DOI] [Google Scholar]
- Benson D.A., Karsch-Mizrachi I., Lipman D.J., Ostell J., Wheeler D.L. GenBank. Nucleic Acids Res. 2005;33:34–38. doi: 10.1093/nar/gki063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Cao Y. Tumorigenesis as a process of gradual loss of original cell identity and gain of properties of neural precursor/progenitor cells. Cell Biosci. 2017;7:61. doi: 10.1186/s13578-017-0188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewangga V.S. Sebelas Maret University; 2015. Karakterisasi isolat Aktif Daun Sirsak (Annona muricata L.) dan Uji Sitotoksisitas isolat. [Google Scholar]
- Elisya Y., Murtini G. Effect of soursop leaf extract tablets (Annona muricata L.) against cancer cells. Asian J. Appl. Sci. 2015;03:244–248. [Google Scholar]
- Endrawati D., Kusumaningtyas E. Molecular profile of Trichophyton mentagrophytes and Microsporum canis based on PCR-RFLP of internal transcribed spacer. J. Ilmu Ternak Vet. 2021;26:10. doi: 10.14334/jitv.v26i1.2546. [DOI] [Google Scholar]
- Gouda S., Das G., Sen S.K., Shin H.S., Patra J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016;7:1538. doi: 10.3389/fmicb.2016.01538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A.E.Z., -, Husnawati H, -, Andrianto D, -, Julistiono H, -, Mahsunnah Anis H., - Stabilitas Metabolit Sekunder Kapang Endofit Daun Sirsak (Annona Muricata L.) pada Skala Pabrik. Prosiding Seminar Nasional Himpunan Mahasiswa KimiaFMIPA UNMUL 2020Jurusan Kimia FMIPA Universitas Mulawarman. 2020;1(1):1–6. 14. In this issue. [Google Scholar]
- Hassan S.-D. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J. Adv. Res. 2017;8(6):687–695. doi: 10.1016/j.jare.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlina T., Haraswati N., Apriani R., Nishinarizki V., Gaffar S., Supratman U. Cytotoxic activity of alpinum isoflavone from Erythrina poeppigiana (Leguminosae) against colon cancer (WiDr), cervical cancer (Hela), and hepatoma cancer (Hep G2) cells. HAYATI J. Biosci. 2019;26:96–100. doi: 10.4308/hjb.26.2.96. [DOI] [Google Scholar]
- Indrawati I., Rossiana N., Fathurrohim M.F. Diversity of endophytic bacteria and microfungi in Syzygium cumini fruit from west java, Indonesia. Biodiversitas. 2021;22:3943–3948. doi: 10.13057/biodiv/d220941. [DOI] [Google Scholar]
- Itharat A., Plubrukan A., Kaewpradub N., Chuchom T., Ratanasuwan P., Houghton P.J. Selective cytotoxicity and antioxidant effects of compounds from Dioscorea membranacea rhizomes. Nat. Prod. Commun. 2007;2:643–648. doi: 10.1177/1934578X0700200605. [DOI] [Google Scholar]
- Karma Yeshi D.C., Ritmejeryte E., Wangchuk P. Plant secondary metabolites produced in response to abiotic product development. Molecules. 2022;27:1–31. doi: 10.3390/molecules27010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumaningrum H.P., Budi W.S., Azam M., Bawono A. Design of electrophoresis device for Optimation of DNA visualization and dna concentration using software. J. Pendidik Fis. Indones. 2014;10:194–202. doi: 10.15294/jpfi.v10i2.3357. [DOI] [Google Scholar]
- Lalitha P., Veena V., Vidhyapriya P., Lakshmi P., Krishna R., Sakthivel N. Anticancer potential of pyrrole (1, 2, a) pyrazine 1, 4, dione, hexahydro 3-(2-methyl propyl) (PPDHMP) extracted from a new marine bacterium, Staphylococcus sp. strain MB30. Apoptosis. 2016;21(5):566–577. doi: 10.1007/s10495-016-1221-x. [DOI] [PubMed] [Google Scholar]
- Lee Y.K., Bae K., Yoo H.-S., Cho S.-H. Benefit of adjuvant traditional herbal medicine with chemotherapy for resectable gastric cancer. Integr. Cancer Ther. 2018;17(3):619–627. doi: 10.1177/1534735417753542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manan M.A., Rosfarizan M., Ariff A. The morphology and structure of red pigment producing fungus: Monascus Purpureus. Microbiol. Exp. 2017;5:1–5. doi: 10.15406/jmen.2017.05.00138. [DOI] [Google Scholar]
- Marcellano J.P., Collanto A.S., Fuentes R.G. Antibacterial activity of endophytic fungi isolated from the bark of Cinnamomum mercadoi. Pharmacogn. J. 2017;9(3):405–409. doi: 10.5530/pj.2017.3.69. [DOI] [Google Scholar]
- Mardiyaningsih A., Ismiyati N. Cytotoxic activity of ethanolic extract of Persea Americana mill.eaves on Hela cervical cancer cell. Trad. Med. J. 2014;19:24–28. [Google Scholar]
- McBreen K., Lockhart P.J., McLenachan P.A., Scheele S., Robertson A.W. The use of molecular techniques to resolve relationships among traditional weaving cultivars of Phormium. J. Bot. 2003;41(2):301–310. doi: 10.1080/0028825X.2003.9512849. [DOI] [Google Scholar]
- Mehta P., Bhargava R. Personalized medicine in diffuse large B-cell lymphoma. Indian J. Med. Paediatr. Oncol. 2019;40(04):463–464. doi: 10.4103/ijmpo.ijmpo_211_19. [DOI] [Google Scholar]
- Meng D.-F., Guo L.-L., Peng L.-X., Zheng L.-S., Xie P., Mei Y., Li C.-Z., Peng X.-S., Lang Y.-H., Liu Z.-J., Wang M.-D., Xie D.-H., Shu D.-T., Hu H., Lin S.-T., Li H.-F., Luo F.-F., Sun R., Huang B.-J., Qian C.-N. Antioxidants suppress radiation-induced apoptosis via inhibiting MAPK pathway in nasopharyngeal carcinoma cells. Biochem. Biophys. Res. Commun. 2020;527(3):770–777. doi: 10.1016/j.bbrc.2020.04.093. [DOI] [PubMed] [Google Scholar]
- Meredith A.M., Dass C.R. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J. Pharm. Pharmacol. 2016;68:729–741. doi: 10.1111/jphp.12539. [DOI] [PubMed] [Google Scholar]
- Minarni, Artika I.M., Julistiono H., Bermawie N., Riyanti E.I., Hasim, Hasan A.E.Z. Anticancer activity test of ethyl acetate extract of endophytic fungi isolated from soursop leaf (Annona muricata L.) Asian Pac. J. Tropical Med. 2017;10(6):566–571. doi: 10.1016/j.apjtm.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Nicoletti D., Casandruc E., Laplace Y., Khanna V., Hunt C.R., Kaiser S., Dhesi S.S., Gu G.D., Hill J.P., Cavalleri A. Optically induced superconductivity in striped La 2–x Ba x CuO 4 by polarization-selective excitation in the near infrared. Phys. Rev. B. 2014;90 [Google Scholar]
- Nilsson R.H., Kristiansson E., Ryberg M., Hallenberg N., Larsson K.H. Intraspecific ITS variability in the Kingdom Fungi as expressed in the international sequence databases and its implications for molecular species identification. Evol. Bioinform. Online. 2008;4:193–201. doi: 10.4137/EBO.S653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithiyaa P., Izzati N.A., Umi Kalsom Y., Salleh B. Diversity and morphological characteristics of Aspergillus species and Fusarium species isolated from cornmeal in Malaysia. Pertanika J. Trop. Agric. Sci. 2012;35:103–116. [Google Scholar]
- Nugraha A.S., Damayanti Y.D., Wangchuk P., Keller P.A. Anti-infective and anticancer properties of the Annona species: Their ethnomedicinal uses, alkaloid diversity, and pharmacological activities. Molecules. 2019;24(23):4419. doi: 10.3390/molecules24234419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overy D.P., Frisvad J.C. New Penicillium species associated with bulbs and root vegetables. Syst. Appl. Microbiol. 2003;26:631–639. doi: 10.1078/072320203770865945. [DOI] [PubMed] [Google Scholar]
- Pearson W.R. An introduction to sequence similarity (‘homology’) searching. Curr. Protoc. Bioinform. 2013;1:1–9. doi: 10.1002/0471250953.bi0301s42.An. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapula S.A., Whittall J.J., Pandopulos A.J., Gerber C., Venter H. An optimized and robust PEG precipitation method for detection of SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;785:147270. doi: 10.1016/j.scitotenv.2021.147270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seca A., Pinto D. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018;19(1):263. doi: 10.3390/ijms19010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ser H.L., Palanisamy U.D., Yin W.F., Abd Malek S.N., Chan K.G., Goh B.H., Lee L.H. Presence of antioxidative agent, pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- in newly isolated Streptomyces mangrovisoli sp. nov. Front. Microbiol. 2015;6:854. doi: 10.3389/fmicb.2015.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonjak S., Frisvad J.C., Gunde-Cimerman N. Comparison of secondary metabolite production by Penicillium crustosum strains, isolated from the Arctic and other various ecological niches. FEMS Microbiol. Ecol. 2005;53:51–60. doi: 10.1016/j.femsec.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Sundaresan N., Jagan E.G., Kathamuthu GokulRaj, Pandi M., Papp T. Internal transcribed spacer 2 (ITS2) molecular, morphometric analysis based species delimitation of foliar endophytic fungi from Aglaia elaeagnoidea, Flacourtia inermis, and Premna serratifolia. PLOS ONE. 2019;14(4):e0215024. doi: 10.1371/journal.pone.0215024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyatmi Y.H., Jusuf S.A. The selective cytotoxicity of ethanolic extract of Annona muricata leaf on HeLa cervical cancer cells. Res. Appl. Tradit. Complement. Altern. Med. Heal. Care. 2012:24–27. [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker P.H., Salani R., Brady W.E., Lankes H.A., Cohn D.E., Mutch D.G., Mannel R.S., Bell-McGuinn K.M., Di Silvestro P.A., Jelovac D., Carter J.S., Duan W., Resnick K.E., Dizon D.S., Aghajanian C., Fracasso P.M. A phase I trial of paclitaxel, cisplatin, and veliparib in the treatment of persistent or recurrent carcinoma of the cervix: An NRG Oncology Study (NCT#01281852). Ann. Oncol. 28, 505–511. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017;28(3):505–511. doi: 10.1093/annonc/mdw635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie C.M., Houbraken J., Frisvad J.C., Hong S.B., Klaassen C.H.W., Perrone G., Seifert K.A., Varga J., Yaguchi T., Samson R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014;78:343–371. doi: 10.1016/j.simyco.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2020a. Cancer [WWW Document]. URL http://www.who.int/mediacentre/factsheets/fs297/en.
- WHO, 2020b. Human papilloma virus (HPV) and cervical cancer [WWW Document]. URL https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer.
- Xiao T.-W., Xu Y., Jin L.u., Liu T.-J., Yan H.-F., Ge X.-J. Conflicting phylogenetic signals in plastomes of the tribe Laureae (Lauraceae) PeerJ. 2020;8:e10155. doi: 10.7717/peerj.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R.-H., Su J.-H., Shang J.-J., Wu Y.-Y., Li Y., Bao D.-P., Yao Y.-J., Cullen D. Evaluation of the ribosomal DNA internal transcribed spacer (ITS), specifically ITS1 and ITS2, for the analysis of fungal diversity by deep sequencing. PLOS ONE. 2018;13(10):e0206428. doi: 10.1371/journal.pone.0206428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zuzak K., Feng J. An improved simple method for DNA extraction from fungal mycelia. Can. J. Plant Pathol. 2016;38(4):476–482. doi: 10.1080/07060661.2016.1243585. [DOI] [Google Scholar]