Abstract
The right and left side of the colon derived from the midgut and hindgut, respectively. Previous studies have reported different characteristics of right-sided colon cancer (RCC) and left-sided colon cancer (LCC), but oncological outcomes remain unclear. This study compared the outcomes of RCC and LCC. This retrospective study included 1017 patients who received curative colectomy for stage I-III colon cancer at a single institute between August 2008 and December 2019. Overall survival (OS) and time to recurrence (TTR) were analyzed as outcome measurements. No significant difference in the OS or TTR of patients with RCC and LCC were observed. In subgroup analysis, RCC was associated with shorter TTR than LCC in stage II colon cancer (HR 2.36, 95% confidence interval 1.24–4.48, p < 0.01). Multivariate analysis demonstrated that right sidedness, R1 resection, low body mass index (BMI) and adjuvant chemotherapy were independent factors for poor prognosis for stage II colon cancer. Low BMI, perineural invasion, higher T stage and N2 stage were independent factors for poor prognosis for stage III colon cancer. The results were confirmed by multivariate analysis after propensity score matching. Our study revealed that RCC was an independent risk factor for recurrence in stage II colon cancer.
Subject terms: Cancer, Gastroenterology, Oncology, Risk factors
Introduction
Colorectal cancer is one of the most prevalent cancers, especially in developed countries1,2. Radical surgical resection is the standard treatment for American Joint Committee on Cancer (AJCC) stage I to III colon cancer; postoperative adjuvant chemotherapy is also administered to patients with high-risk stage II and stage III colon cancer3.
Since 1990, right-sided colon cancer (RCC) and left-sided colon cancer (LCC) have been regarded as distinct cancers based on their different embryology, epidemiology, pathology, and prognosis4,5. Patients with RCC are more likely to be older and female and present with a more advanced tumor stage, larger tumor size, and more poorly differentiated tumor cells than those with LCC6–11. The differing characteristics of RCC and LCC are thought to be caused by differences in embryologic origin, fecal exposure, and detection time6. Most previous studies have indicated that RCC was associated with a higher recurrence and lower survival rate than LCC6,12,13, although several studies have concluded that early-stage RCC had a better prognosis than LCC14,15. In light of the lack of consensus on the prognosis for RCC and LCC, this study investigated the impact of cancer sidedness and stage on outcomes.
Material and methods
Patients
The medical records of 1588 consecutive patients who received primary resection of colorectal cancer in Taipei Medical University Shuang-Ho Hospital between August 2008 and December 2019 were reviewed. The TNM staging system (AJCC Cancer Staging Manual, 8th Edition) was used for staging. Only patients with colon adenocarcinoma were included in this study. Patients who were diagnosed with rectal cancer, carcinoma in situ, synchronous colon cancer, or stage IV colon cancer were excluded, as were patients who received palliative surgery or R2 resection. The final sample comprised 1017 patients who underwent curative resection for stage I, II, or III colon cancer (Fig. 1). Metachronous cancers were defined as different individuals with different diseases instead of same patients with cancer recurrence. This study was approved by the Institutional Review Board/Ethics Committee of Taipei Medical University (approval number: N201912114). As this is a retrospective study, the informed consent is not required by TMU-JIRB.
Figure 1.
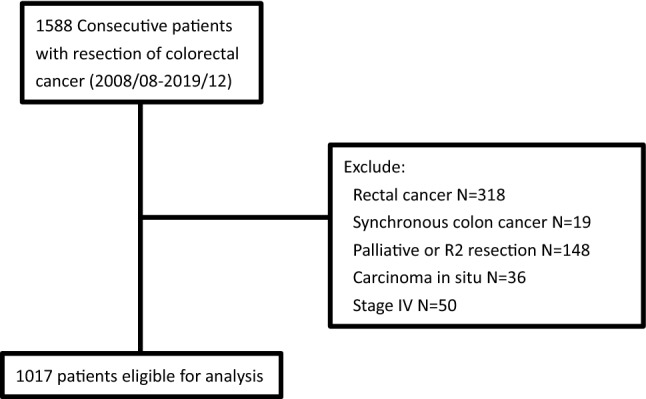
Flow diagram of colorectal cancer patients included in the study.
Outcomes measurement
Primary tumors located at the cecum, ascending colon, and transverse colon were defined as RCC, whereas primary tumors located at the splenic flexure, descending colon, and sigmoid colon were defined as LCC. Patient characteristics including age at diagnosis, gender, medical history, body mass index (BMI), carcinoembryonic antigen (CEA) value, tumor location, histological type, tumor grade, tumor stage, chemotherapy history, K-RAS status, mismatch repair (MMR) status and surgical margin status were collected from patient records. R1 resection was defined as section margin less than 2 mm. Overall survival (OS) was defined as the time from primary tumor resection to death from any cause. Time to recurrence (TTR) was defined as the time from primary tumor resection to the first recurrence confirmed by radiological or histological features.
Statistical analysis
Both K–S and S–W tests were used to confirmed the normal distribution of continuous variables. Variables normally distributed were presented as mean ± standard deviation, otherwise presented as median (Q1–Q3). Comparisons were made using independent-t test or Mann–Whitney test for analysis of continuous variables. Chi-square test was used for comparisons of categorical variables. Kaplan–Meier curves were calculated for all patients and patients with stage I, II, and III colon cancer separately to compare OS and TTR between patients with RCC and LCC. Univariate and multivariate survival analyses were performed with a Cox proportional hazards function; HRs and 95% confidence intervals (CIs) were used to estimate the impact of primary tumor location on survival outcomes. Propensity score matching (PSM) was performed with greedy nearest neighbor matching method, using 14 variables (age, sex, BMI, patient origin, operation method, CEA, LVI, PNI, tumor grade, T stage, N stage, tumor size, chemotherapy and R1 resection) that could potentially influence outcomes. The number of lymph node harvested was not included due to native difference of mesocolon taken during operation between RCC and LCC. The caliper requirement was ignored. A p value of < 0.05 was considered statistically significant.
Ethics approval
This study was approved by the Ethics Committee of Taipei Medical University (approval number: N201912114) and was performed in accordance with the Declaration of Helsinki and its subsequent amendments or comparable ethical standards.
Informed consent
Informed consent was not applicable due to the retrospective nature of this study.
Results
Clinical and pathological characteristics
We reviewed the medical records of 1588 patients, and 1017 patients were eligible for inclusion in the study. 75% of the patients were followed for more than 48 months. Ten patients were found metachronous cancers. Of the included patients, 385 (37.9%) had RCC, and 632 (62.1%) had LCC (Fig. 1). The baseline characteristics of patients with RCC and LCC are listed in Table 1. Patients with RCC were older than patients with LCC (67 (59–77) years vs. 65.3 (57–75) years, p < 0.01) and less likely to be male (47.3% vs. 56.5%, p < 0.01). A lower BMI was also noted in patients with RCC than in patients with LCC (23.7 ± 4.4 vs. 24.5 ± 4.1, p < 0.01). Minimally invasive surgeries were performed more frequently in patients with LCC than in patients with RCC (62.3% vs. 47.5%, p < 0.01). No significant differences in preoperative CEA level, the rate of emergency operations, the rate of adjuvant chemotherapy, T stage, or postoperative follow-up time were observed. More lymph nodes were harvested in patients with RCC than in patients with LCC (26 (19–35.5) vs. 21 (16–28), p < 0.01). Tumors in patients with RCC were larger (4.5 (3.15–6.4) vs. 4.0 (3.0–5.5), p < 0.01) and of a more advanced histological grade (7.8% vs. 4.4%, p = 0.03) than the tumors in patients with LCC. However, patients with LCC had a more advanced N stage (53.6% vs. 43.6% in N1 and N2 stage, p = 0.01) and AJCC cancer stage (53.6% vs. 43.6% in stage III, p < 0.01) than did patients with RCC. A higher percentage of K-RAS mutation (48.5% vs. 31.6%, p < 0.01) and deficient MMR (dMMR) (45.5% vs. 18.2%, p < 0.01) were noted in patients with RCC than in patients with LCC. No significant differences in the rate of perineural invasion or lymphovascular invasion were apparent between the two groups. R1 resection was found in 17 with RCCand 19 patients with LCC with RCC and LCC. There is no significant difference between the two groups (4.4% vs. 3.0%, p = 0.24).
Table 1.
Baseline characteristics of all patients.
| Characteristic | Right-sided cancer (N = 385) | Left-sided cancer (N = 632) | P value |
|---|---|---|---|
| Age (years) | 67(59–77) | 65.3 (57–75) | < 0.01 |
| Sex, Male, N (%) | 182 (47.3%) | 357 (56.5%) | < 0.01 |
| Body mass index | 23.7 ± 4.4 | 24.5 ± 4.1 | < 0.01 |
| Patient origin, N (%) | 0.12 | ||
| Elective operation | 301 (78.2%) | 519 (82.1%) | |
| Emergency operation | 84 (21.8%) | 113 (17.9%) | |
| Operation method, N (%) | < 0.01 | ||
| Open surgery | 202 (52.5%) | 238 (37.7%) | |
| Minimal invasive surgery | 183 (47.5%) | 394 (62.3%) | |
| CEA | 3.3 (1.7–9.4) | 3.6 (1.9–8.7) | 0.39 |
| Tumor size (cm) | 4.5 (3.2–6.4) | 4.0 (3.0–5.5) | < 0.01 |
| Lymphovascular invasion, N (%) | 185 (48.1%) | 304 (48.1%) | 0.99 |
| Perineural invasion, N (%) | 148 (38.4%) | 230 (36.4%) | 0.51 |
| Tumor grade, N (%) | 0.03 | ||
| Grade 1–2 | 355 (92.2%) | 604 (95.6%) | |
| Grade 3–4 | 30 (7.8%) | 28 (4.4%) | |
| Location | |||
| Cecum, N (%) | 70 | ||
| Ascending colon, N (%) | 166 | ||
| Hepatic flexure, N (%) | 50 | ||
| Transverse colon, N (%) | 99 | ||
| Splenic flexure, N (%) | 35 | ||
| Descending colon, N (%) | 38 | ||
| Descending-sigmoid junction, N (%) | 46 | ||
| Sigmoid colon, N (%) | 513 | ||
| T stage, N (%) | |||
| T1 | 41 (10.6%) | 86 (13.6%) | 0.05 |
| T2 | 39 (10.1%) | 83 (13.1%) | |
| T3 | 214 (55.6%) | 351 (55.5%) | |
| T4 | 91 (23.6%) | 112 (17.7%) | |
| N stage, N (%) | |||
| N0 | 217 (56.4%) | 293 (46.4%) | 0.01 |
| N1 | 106 (27.5%) | 221 (35.0%) | |
| N2 | 62 (16.1%) | 118 (18.7%) | |
| AJCC stage, N (%) | |||
| I | 66 (17.1%) | 122 (19.3%) | < 0.01 |
| II | 151 (39.2%) | 171 (27.1%) | |
| III | 168 (43.6%) | 339 (53.6%) | |
| No. of lymph node harvested | 26 (19–35.5) | 21 (16–28) | < 0.01 |
| Adjuvant chemotherapy, N (%) | 197 (51.2%) | 323 (51.1%) | 0.99 |
| K-ras mutation, No. of positive/No. of test (%) | 94/194 (48.5%) | 93/294 (31.6%) | < 0.01 |
| dMMR, No. of positive/No. of test (%) | 25/55 (45.5%) | 10/55 (18.2%) | < 0.01 |
| MSI-H, No. of positive/No. of test (%) | 5/20 (25%) | 1/13 (7.7%) | 0.21 |
| R1 resection, N (%) | 17 (4.4%) | 19 (3.0%) | 0.24 |
| Follow time (month) | 37.8 (23.5–63.5) | 43.0 (25.3–67.5) | 0.12 |
Significant values are in bold.
CEA carcinoembryonic antigen, dMMR Deficient mismatch repair, MSI-H microsatellite instability-high.
Clinical and pathological characteristics stratified by stage
The study included 188, 322, and 507 patients with stage I, II, and III colon cancer, respectively. Table 2 presents the differences in characteristics between each stage. Men made up a smaller proportion of the patients with stage I and II RCC than that of the patients with stage I and II LCC (stage I: 42.4% vs. 58.2%, p = 0.04; stage II: 45% vs. 56%, p = 0.047). Patients with stage III LCC had a higher BMI than patients with stage III RCC (23.4 ± 4.6 vs. 24.2 ± 3.8, p = 0.04). More patients with stage II and III LCC underwent minimally invasive surgery than did patients with stage II and III RCC (stage II: 56.1% vs. 43.0%, p = 0.02; stage III: 61.1% vs. 43.5%, p < 0.01). Lymphovascular invasion was higher in patients with stage II RCC than in patients with stage II LCC (40.4% vs. 28.7%, p = 0.03). Tumors in stage I and II RCC had more advanced histological grading than did tumors in stage I and II LCC (stage I: 6.1% vs. 0.8%, p = 0.03; stage II: 11.9% vs. 4.1%, p = 0.01). A higher rate of K-RAS mutation and dMMR was noted in stage II RCC than in stage II LCC (K-RAS: 44.2% vs. 28.9%, p = 0.04; dMMR: 46.2% vs. 15%, p = 0.03) and in stage III RCC than in stage III LCC (K-RAS: 53.2% vs. 31.2%, p < 0.01; dMMR: 47.6% vs. 10.7%, p < 0.01). More lymph nodes were harvested in RCC than LCC in all three stages (stage I: 22 (18.75–31) vs. 17 (14–24), p < 0.01; stage II: 24 (18–35) vs. 22 (17–29), p = 0.02; and stage III: 29 (21–38.75) vs. 22 (17–30), p < 0.01). More R1 resection was found in stage III RCC than in stage III LCC (7.7% vs. 3.5%, p = 0.04).
Table 2.
Comparison of clinicopathological characteristics between right-sided and left-sided in stage I, II and III colon cancer.
| Characteristic | Stage I | Stage II | Stage III | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Right-sided cancer (N = 66) |
Left-sided cancer (N = 122) |
P value | Right-sided cancer (N = 151) |
Left-sided cancer (N = 171) |
P value | Right-sided cancer (N = 168) |
Left-sided cancer (N = 339) |
P value | |
| Age (years) | 64.5 (57–74) | 62 (55.8–67.3) | 0.16 | 70 (59–79) | 66 (58–77) | 0.12 | 68 (60–77.75) | 65 (57–76) | 0.11 |
| Sex, Male, N (%) | 28 (42.4%) | 71 (58.2%) | 0.04 | 68 (45%) | 96 (56%) | 0.047 | 86 (51.1%) | 190 (56.0%) | 0.30 |
| Body mass index | 24.9 ± 3.7 | 25.9 ± 4.4 | 0.44 | 23.6 ± 4.3 | 24.2 ± 4.3 | 0.25 | 23.4 ± 4.6 | 24.2 ± 3.8 | 0.04 |
| Patient origin, N (%) | 0.74 | 0.13 | 0.65 | ||||||
| Elective operation | 62 (93.9%) | 116 (95.1%) | 111 (73.5%) | 138 (80.7%) | 128(76.1%) | 265(78.2%) | |||
| Emergency operation | 4 (6.1%) | 6 (4.9%) | 40 (26.5%) | 33 (19.3%) | 40(23.9%) | 74(21.8%) | |||
| Operation method, N (%) | 0.35 | 0.02 | < 0.01 | ||||||
| Open surgery | 21 (31.8%) | 31 (25.4%) | 86 (57.0%) | 75 (43.9%) | 95 (56.5)% | 132 (38.9%) | |||
| Minimal invasive surgery | 45 (68.2%) | 91 (74.6%) | 65 (43.0%) | 96 (56.1%) | 73 (43.5%) | 207 (61.1%) | |||
| CEA | 2.1 (1.4–3.3) | 2.0 (1.4–3.0) | 0.80 | 3.8 (1.9–10.1) | 4.4 (2.1–10.2) | 0.54 | 4.28 (2.0–13.4) | 4.3 (2.2–11.3) | 0.54 |
| Tumor size (cm) | 2.3 (1.5–3.7) | 2.4 (1.5–3.5) | 0.97 | 5.0 (3.5–7.0) | 4.5 (3.5–6.0) | 0.19 | 5.0 (4.0–6.5) | 4.2 (3.2–5.5) | < 0.01 |
| Lymphovascular invasion, N (%) | 8 (12.1%) | 23 (18.9%) | 0.24 | 61 (40.4%) | 49 (28.7%) | 0.03 | 116 (69.0%) | 232 (68.4) | 0.89 |
| Perineural invasion, N (%) | 8 (12.1%) | 6 (4.9%) | 0.07 | 48 (31.8%) | 50 (29.2%) | 0.62 | 92 (54.8%) | 174 (51.3%) | 0.47 |
| Tumor grade, N (%) | 0.03 | 0.01 | 0.48 | ||||||
| Grade 1–2 | 62 (93.9%) | 121 (99.2%) | 133 (88.1%) | 164 (95.9%) | 157 (93.5%) | 322 (95.0%) | |||
| Grade 3–4 | 4 (6.1%) | 1 (0.8%) | 18 (11.9%) | 7 (4.1%) | 11 (6.5%) | 17 (5.0%) | |||
| T stage, N (%) | 0.57 | 0.13 | 0.02 | ||||||
| T1 | 35 (53.0%) | 70 (57.3%) | 6 (3.6%) | 16 (4.7%) | |||||
| T2 | 31 (47.0%) | 52 (42.7%) | 8 (4.8%) | 31 (9.1%) | |||||
| T3 | 118 (78.1%) | 145 (84.8%) | 96 (57.1%) | 206 (60.8%) | |||||
| T4 | 33 (21.9%) | 26 (15.2%) | 58 (34.5%) | 86 (25.4%) | |||||
| N stage, N (%) | 0.64 | ||||||||
| N0 | 66 | 122 | 151 | 171 | |||||
| N1 | 106 (63.1%) | 221 (65.2%) | |||||||
| N2 | 62 (36.9%) | 118 (34.8%) | |||||||
| No. of lymph node harvested | 22 (18.8–31) | 17 (14–24) | < 0.01 | 24 (18–35) | 22 (17–29) | 0.02 | 29 (21–38.8) | 22 (17–30) | < 0.01 |
| Adjuvant chemotherapy, N (%) | 1 (1.5%) | 0 (0%) | 0.18 | 72 (47.7%) | 75 (43.9%) | 0.49 | 124 (73.8%) | 248 (73.2%) | 0.88 |
| K-ras mutation, no. of positive/No. of test (%) | 10/23 (43.5%) | 13/32 (40.6) | 0.84 | 34/77 (44.2%) | 22/76 (28.9%) | 0.04 | 50/94 (53.2%) | 58/186 (31.2%) | < 0.01 |
| dMMR, no. of positive/no. of test (%) | 3/8 (37.5%) | 4/7 (57.1%) | 0.45 | 12/26 (46.2%) | 3/20 (15%) | 0.03 | 10/21 (47.6%) | 3/28 (10.7%) | < 0.01 |
| MSI-H, no. of positive/no. of test (%) | 0/1 | 0/3 | 4/10 (40%) | 1/7 (14.3) | 0.25 | 1/9 (11.1) | 0/3 | 0.55 | |
| R1 resection, N (%) | 0 | 1 (0.8%) | 0.46 | 4 (2.6%) | 6 (3.5%) | 0.66 | 13 (7.7%) | 12 (3.5%) | 0.04 |
| Follow time (month) | 43.3 (29.5–67.4) | 51.8 (30.1–78.1) | 0.34 | 42.6 (15.6–66.0) | 45.6 (28.5–73.3) | 0.43 | 35.3 (19.5–58.5) | 38.6 (22.0–61.1) | 0.12 |
Significant values are in bold.
CEA carcinoembryonic antigen, dMMR Deficient mismatch repair, MSI-H microsatellite instability-high.
Survival curves by tumor location and stage
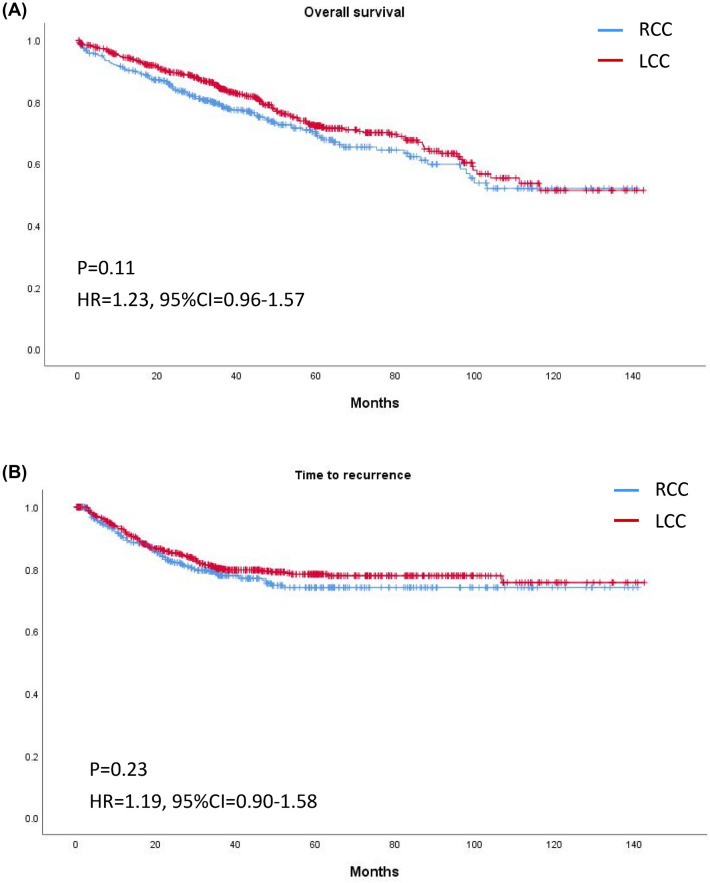
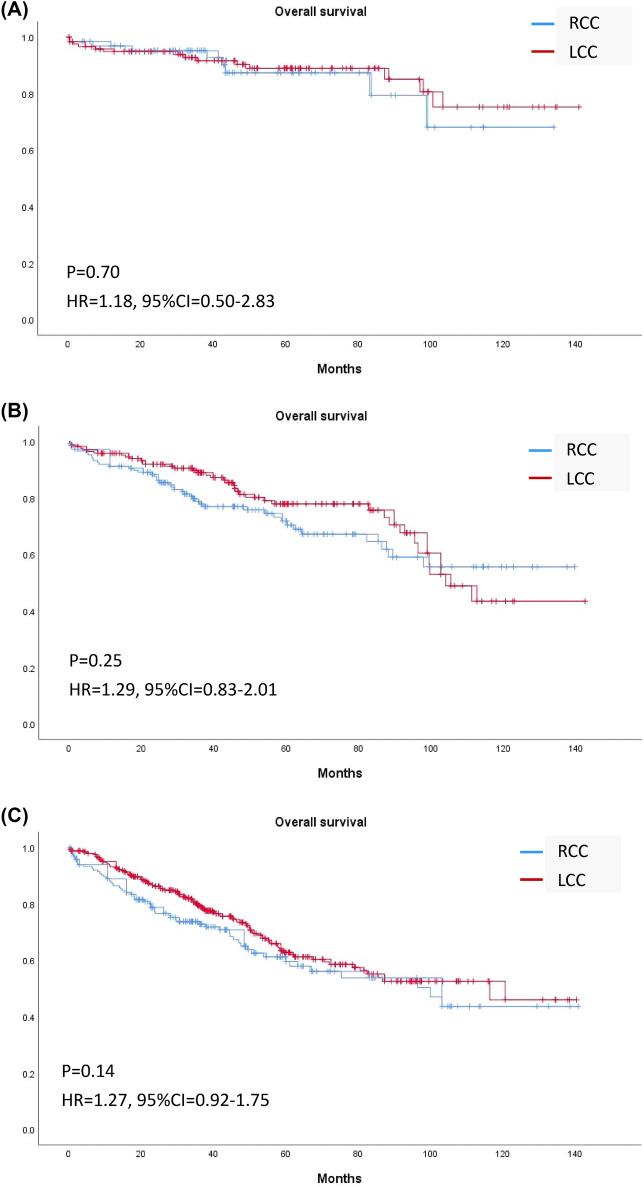
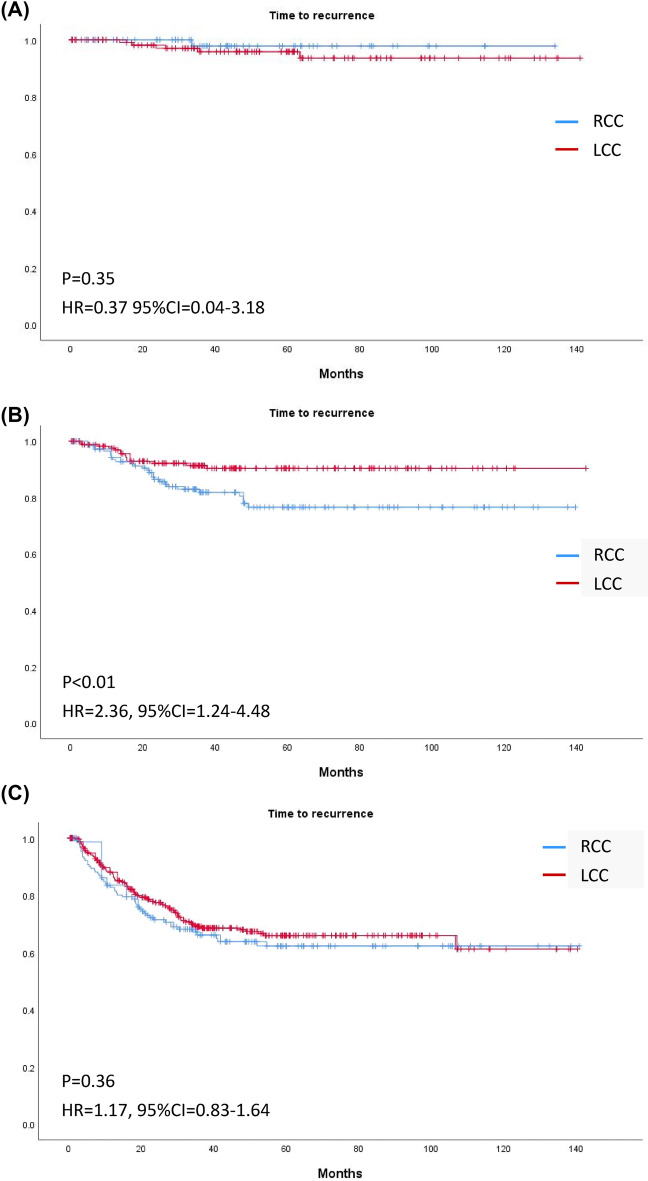
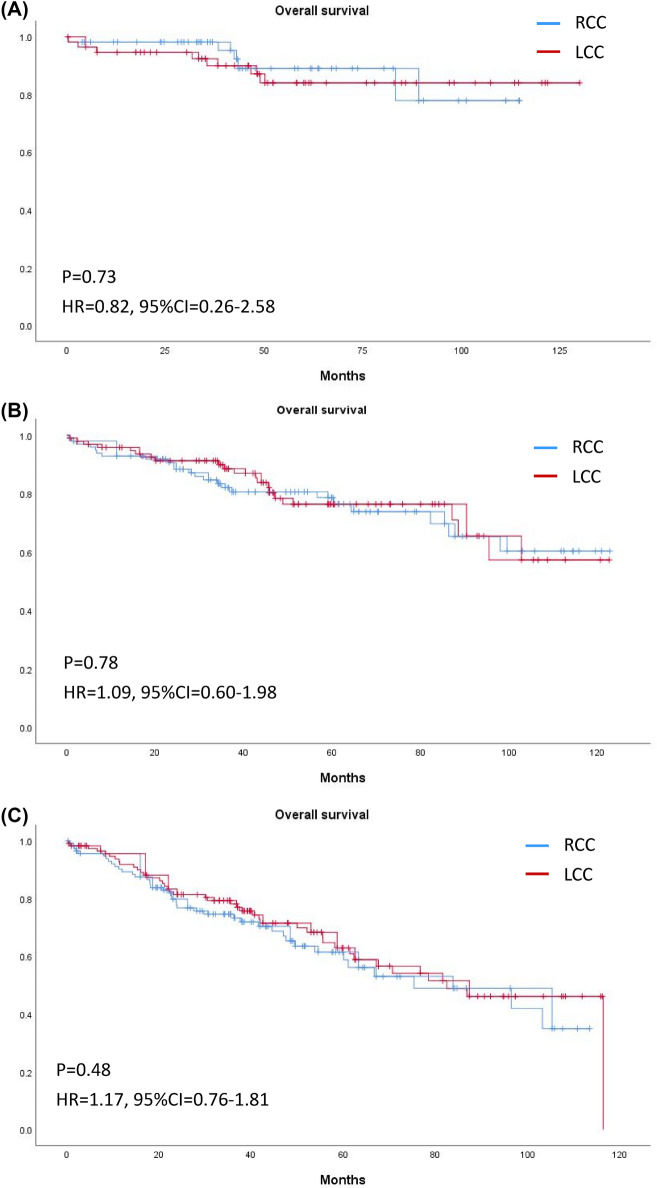
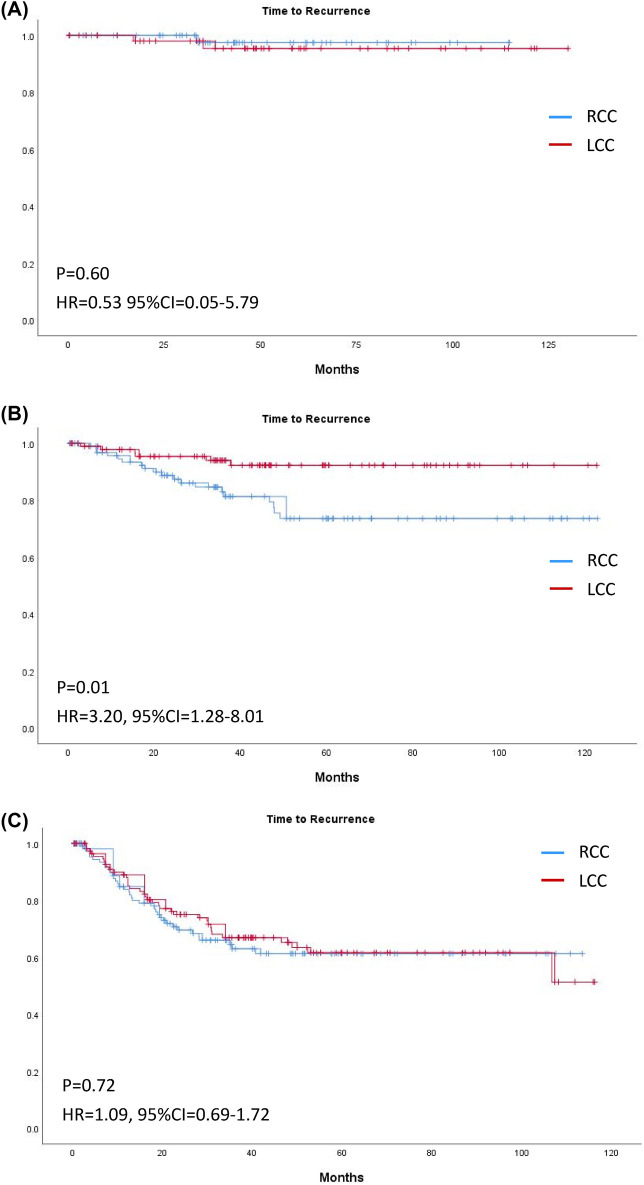
The median follow-up time was 37.8 (23.5–63.5) months in RCC and 43.0 (25.3–67.5) months in LCC (p = 0.12). Overall, Kaplan–Meier curves revealed no significant differences in OS or TTR for patients with RCC or LCC (Fig. 2). Stratification by stage revealed no significant difference in OS in RCC and LCC of any stage (Fig. 3); however, TTR in stage II RCC was shorter than that in stage II LCC (HR 2.36, 95% CI 1.24–4.48, p < 0.01; Fig. 4).
Figure 2.
Kaplan–Meier curves of (A) overall survival and (B) time to recurrence of right-sided and left-sided colon cancer of any stage.
Figure 3.
Kaplan–Meier curves of overall survival of right-sided and left-sided colon cancer in (A) Stage I, (B) Stage II, and (C) Stage III.
Figure 4.
Kaplan–Meier curves of time to recurrence of right-sided and left-sided colon cancer in (A) Stage I, (B) Stage II, and (C) Stage III.
Recurrent risks analysis in stage II and III colon cancer
In Table 3, we demonstrated recurrent risks (shorter TTR) by univariate and multivariate analysis. In univariate analysis, stage II RCC was associated with shorter TTR than was stage II LCC (HR 2.36, CI 1.24–4.48, p < 0.01). The risk factors for shorter TTR in stage II colon cancer were a higher BMI (HR: 0.88 for every increment in BMI, CI 0.81–0.97, p = 0.01), lymphovascular invasion (HR 2.19, CI 1.20–4.01, p = 0.01), perineurial invasion (HR 1.99, CI 1.08–3.66, p = 0.03), advanced T stage (HR 2.85, CI 1.52–5.36, p < 0.01), adjuvant chemotherapy (HR 2.28, CI 1.20–4.33, p = 0.01), and R1 resection (HR 6.35, CI 2.49–16.21, p < 0.01). The risk factors for shorter TTR in stage III colon cancer were higher BMI (HR 0.92 for every increment in BMI, CI 0.88–0.96, p < 0.01), older age (HR 1.02, CI 1.01–1.03, p = 0.01), emergency surgery (HR 2.36, CI 1.67–3.33, p < 0.01), open surgery (HR 1.39, CI 1.01–1.92, p = 0.046), perineural invasion (HR 2.15, CI 1.53–3.02, p < 0.01), advanced T stage (T2 vs T1 HR: 1.12, CI 0.1–12.33; T3 vs T1 HR 6.50 CI 0.9–46.96; T4 vs T1 HR 15.56 CI 2.16–112.2, p < 0.01), and N stage (HR 2.18, CI 1.59–3.02, p < 0.01; Table 3).
Table 3.
Univariate and multivariate analysisa for TTR by Cox Proportional Hazards Regression in stage II and stage III colon cancer.
| Characteristic | Unadjusted analysis | Adjusted analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Stage II | Stage III | Stage II | Stage III | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Location | ||||||||
| Right | 2.36 (1.24–4.48) | < 0.01 a | 1.17 (0.83–1.64) | 0.36 | 2.35 (1.14–4.85) | 0.02 | ||
| Left | 1 | 1 | 1 | 1 | ||||
| Age (for every 1 additional year in age) | 0.99 (0.97–1.01) | 0.45 | 1.02 (1.01–1.03) | 0.01a | 1.02 (0.99–1.03) | 0.07 | ||
| Gender | ||||||||
| Male | 0.82 (0.45–1.51) | 0.52 | 0.999 (0.72–1.38) | 0.99 | ||||
| Female | 1 | 1 | 1 | |||||
| BMI (for every 1 additional in BMI) | 0.88 (0.81–0.97) | 0.01a | 0.92 (0.88–0.96) | < 0.01 a | 0.88 (0.80–0.97) | 0.01 | 0.94 (0.89–0.99) | 0.03 |
| Patient origin | ||||||||
| Elective operation | 1 | 1 | 1 | 1 | ||||
| Emergency operation | 1.73 (0.90–3.32) | 0.10a | 2.36 (1.67–3.33) | < 0.01 a | 0.99 (0.42–2.35) | 0.99 | 1.65 (1.04–2.60) | 0.03 |
| Operation method | ||||||||
| Open surgery | 1.65 (0.59–3.05) | 0.11a | 1.39 (1.01–1.92) | 0.046a | 0.79 (0.35–1.79) | 0.57 | 1.14 (0.75–1.74) | 0.53 |
| Minimal invasive surgery | 1 | 1 | 1 | 1 | ||||
| CEA (for every 1 additional in CEA) | 0.999 (0.99–1.01) | 0.74 | 1.01 (1.00–1.01) | < 0.01 a | 1.002 (0.99–1.01) | 0.23 | ||
| Lymphovascular invasion (LVI) | ||||||||
| LVI (−) | 1 | 1 | 1 | |||||
| LVI (+) | 2.19 (1.20–4.01) | 0.01a | 1.20 (0.84–1.70) | 0.32a | 1.70 (0.83–3.48) | 0.15 | ||
| Perineural invasion (PNI) | ||||||||
| PNI (−) | 1 | 1 | 1 | 1 | ||||
| PNI (+) | 1.99 (1.08–3.66) | 0.03a | 2.15 (1.53–3.02) | < 0.01 a | 1.11 (0.54–2.27) | 0.78 | 1.75 (1.14–2.67) | 0.01 |
| Surgical margin | ||||||||
| R0 resection | 1 | 1 | 1 | |||||
| R1 resection | 6.35 (2.49–16.21) | < 0.01 a | 1.16 (0.57–2.37) | 0.68 | 20.36 (5.96–69.61) | < 0.01 | ||
| Tumor grade | ||||||||
| Grade 1–2 | 1 | 1 | 1 | |||||
| Grade 3–4 | 0.79 (0.25–2.57) | 0.70 | 1.67 (0.88–3.18) | 0.12a | 0.73 (0.28–1.88) | 0.51 | ||
| T stage | ||||||||
| T1 | 1 | < 0.01 a | 1 | < 0.01 | ||||
| T2 | 1.12 (0.10–12.33) | 1.18 (0.11–13.03) | ||||||
| T3 | 1 | 6.50 (0.90–46.69) | 1 | 3.53 (0.48–26.03) | ||||
| T4 | 2.85 (1.52–5.36) | < 0.01 a | 15.56 (2.16–112.2) | 1.68 (0.76–3.73) | 0.20 | 6.59 (0.87–49.72) | ||
| N stage | ||||||||
| N0 | ||||||||
| N1 | 1 | 1 | ||||||
| N2 | 2.18 (1.59–3.02) | < 0.01 a | 2.00 (1.36–2.94) | < 0.01 | ||||
| Tumor size (for every additional 1 cm) | 0.97 (0.85–1.10) | 0.61 | 1.08 (1.00–1.16) | 0.06a | 1.02 (0.92–1.13) | 0.75 | ||
| Lymph node harvested (for every additional 1 lymph node harvested) | 0.98 (0.95–1.01) | 0.20 | 0.996 (0.98–1.01) | 0.55 | ||||
| Adjuvant chemotherapy | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 2.28 (1.20–4.33) | 0.01 a | 0.74 (0.51–1.08) | 0.12 a | 3.38 (1.49–7.66) | < 0.01 | 0.98 (0.58–1.68) | 0.95 |
Significant values are in bold.
BMI Body mass index, CEA carcinoembryonic antigen.
aOnly variables with p < 0.2 in univariate analysis were included to multivariate analysis.
In multivariate analysis, RCC (HR 2.35, CI 1.14–4.85, p = 0.02), R1 resection (HR 20.36, CI 5.96–69.61, p < 0.01), and adjuvant chemotherapy (HR 3.38, CI 1.49–7.66, p < 0.01) were risk factors for shorter TTR in stage II colon cancer. On the other hand, emergent operation (HR 1.65, CI 1.04–2.60, p = 0.03), perineurial invasion (HR 1.75, CI 1.14–2.67, p = 0.01), advanced T stage (T2 vs T1 HR 1.18, CI 0.11–13.03; T3 vs T1 HR 3.53 CI 0.48–26.03; T4 vs T1 HR 6.59 CI 0.87–49.72, p < 0.01) and N2 stage (HR 2.00, CI 1.36–2.94, p < 0.01) were risk factors for shorter TTR in stage III colon cancer. BMI was associated with lower risk of recurrence in both stage II (HR 0.88, CI 0.80–0.97, p = 0.02) and stage III (HR 0.94, CI 0.89–0.99, p = 0.03) colon cancer.
Propensity score matching
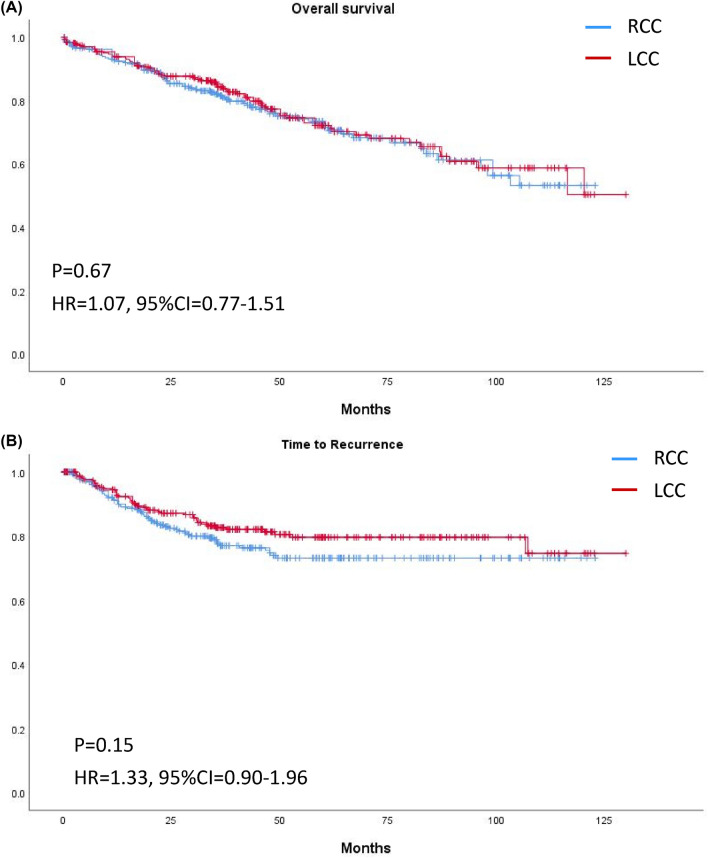
After PSM, 271 pairs of patients were successfully matched and were listed in Table 4. All variables showed no significance except of number of lymph node harvested (26 (20–36) vs. 22 (17–29), p < 0.01). Figure 5, 6, 7 demonstrated survival curves of OS and TTR between RCC and LCC in all stage and stage stratified. Significant difference was found in TTR of stage II colon cancer patients between RCC and LCC (Fig. 7B, HR 3.20, CI 1.28–8.01, p = 0.01). Table 5 demonstrated recurrent risks by univariate and multivariate analysis after PSM. The results of multivariate analysis were mostly compatible with results before PSM, except emergent operation did not show increased risk for recurrence in stage III colon cancer patients (HR 1.37, CI 0.72–2.61, p = 0.34).
Table 4.
Baseline characteristics after propensity score matching.
| Characteristic | Right-sided cancer (N = 271) |
Left-sided cancer (N = 271) |
P value |
|---|---|---|---|
| Age (years) | 66(58–76) | 65 (59–76) | 0.67 |
| Sex, Male, N (%) | 129 (47.6%) | 129 (47.6%) | 1 |
| Body mass index | 24.1 ± 4.1 | 24.1 ± 4.0 | 0.96 |
| Patient origin, N (%) | 0.66 | ||
| Elective operation | 246 (90.8%) | 243 (89.7%) | |
| Emergency operation | 25 (9.2%) | 28 (10.3%) | |
| Operation method, N (%) | 0.48 | ||
| Open surgery | 160 (59.0%) | 168 (62.0%) | |
| Minimal invasive surgery | 111 (41.0%) | 103 (38.0%) | |
| CEA | 3.5 (1.9–9.6) | 3.8 (1.9–8.4) | 0.68 |
| Tumor size (cm) | 4.5 (3.0–6.1) | 4.3 (3.0–6.0) | 0.41 |
| Lymphovascular invasion, N (%) | 128 (47.2%) | 132 (48.7%) | 0.73 |
| Perineural invasion, N (%) | 106 (39.1%) | 107 (39.5%) | 0.93 |
| Tumor grade, N (%) | 0.21 | ||
| Grade 1–2 | 251 (92.6%) | 258 (95.2%) | |
| Grade 3–4 | 20 (7.4%) | 13 (4.8%) | |
| Location | |||
| Cecum, N (%) | 42 | ||
| Ascending colon, N (%) | 129 | ||
| Hepatic flexure, N (%) | 33 | ||
| Transverse colon, N (%) | 67 | ||
| Splenic flexure, N (%) | 17 | ||
| Descending colon, N (%) | 14 | ||
| Descending-sigmoid junction, N (%) | 16 | ||
| Sigmoid colon, N (%) | 224 | ||
| T stage, N (%) | |||
| T1 | 32 (11.8%) | 31 (11.4%) | 0.84 |
| T2 | 36 (13.3%) | 39 (14.4%) | |
| T3 | 143 (52.8%) | 149 (55.0%) | |
| T4 | 60 (22.1%) | 52 (19.2%) | |
| N stage, N (%) | |||
| N0 | 154 (56.8%) | 153 (56.5%) | 0.99 |
| N1 | 72 (26.6%) | 72 (26.6%) | |
| N2 | 45 (16.6%) | 46 (17.0%) | |
| AJCC stage, N (%) | |||
| I | 55 (20.3%) | 57 (21.0%) | 0.96 |
| II | 99 (36.5%) | 96 (35.4%) | |
| III | 117 (43.2%) | 118 (43.5%) | |
| No. of lymph node harvested | 26 (20–36) | 22 (17–29) | < 0.01 |
| Adjuvant chemotherapy, N (%) | 136 (50.2%) | 133 (49.1%) | 0.80 |
| K-ras mutation, no. of positive/no. of test (%) | 68/149 (45.6%) | 37/116 (31.9%) | 0.02 |
| dMMR, no. of positive/no. of test (%) | 17/42 (40.5%) | 5/27 (18.5%) | 0.06 |
| MSI-H, no. of positive/no. of test (%) | 5/16 (31.3%) | 1/11 (9.1%) | 0.17 |
| R1 resection, N (%) | 13 (4.8%) | 10 (3.7%) | 0.52 |
| Follow time (month) | 38.5 (23.7–62.6) | 43.1 (24.0–65.7) | 0.32 |
Significant values are in bold.
CEA carcinoembryonic antigen, dMMR Deficient mismatch repair, MSI-H microsatellite instability-high.
Figure 5.
Kaplan–Meier curves of (A) overall survival and (B) time to recurrence of right-sided and left-sided colon cancer of any stage after PSM.
Figure 6.
Kaplan–Meier curves of overall survival of right-sided and left-sided colon cancer in (A) Stage I, (B) Stage II, and (C) Stage III after PSM.
Figure 7.
Kaplan–Meier curves of time to recurrence of right-sided and left-sided colon cancer in (A) Stage I, (B) Stage II, and (C) Stage III after PSM.
Table 5.
Univariate and multivariate analysisa for TTR by Cox Proportional Hazards Regression in stage II and stage III colon cancer after PSM.
| Characteristic | Unadjusted analysis | Adjusted analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Stage II | Stage III | Stage II | Stage III | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Location | ||||||||
| Right | 3.20 (1.28–4.48) | < 0.01a | 1.09 (0.69–1.72) | 0.72 | 4.26 (1.61–11.28) | < 0.01 | ||
| Left | 1 | 1 | 1 | |||||
| Age (for every 1 additional year in age) | 0.99 (0.96–1.02) | 0.42 | 1.01 (0.99–1.02) | 0.55 | ||||
| Gender | ||||||||
| Male | 0.74 (0.33–1.64) | 0.45 | 0.997 (0.63–1.57) | 0.99 | ||||
| Female | 1 | 1 | 1 | |||||
| BMI (for every 1 additional in BMI) | 0.90 (0.81–1.00) | 0.049a | 0.93 (0.87–0.99) | 0.02a | 0.87 (0.77–0.98) | 0.02 | 0.91 (0.85–0.97) | <0.01 |
| Patient origin | ||||||||
| Elective operation | 1 | 1 | 1 | 1 | ||||
| Emergency operation | 2.57 (0.88–7.50) | 0.08a | 1.84 (1.01–3.35) | 0.04a | 1.12 (0.34–3.70) | 0.85 | 1.37 (0.72–2.61) | 0.34 |
| Operation method | ||||||||
| Open surgery | 1.75 (0.80–3.83) | 0.16a | 1.45 (0.92–2.29) | 0.11a | 0.95 (0.38–2.38) | 0.91 | 1.21 (0.73–20.1) | 0.47 |
| Minimal invasive surgery | 1 | 1 | 1 | |||||
| CEA (for every 1 additional in CEA) | 0.997 (0.97–1.03) | 0.78 | 1.00 (0.997–1.01) | 0.37 | ||||
| Lymphovascular invasion (LVI) | ||||||||
| LVI (−) | 1 | 1 | 1 | |||||
| LVI(+) | 1.91 (0.88–4.19) | 0.11a | 1.21 (0.72–2.03) | 0.48 | 1.83 (0.78–4.31) | 0.17 | ||
| Perineural invasion (PNI) | ||||||||
| PNI (−) | 1 | 1 | 1 | 1 | ||||
| PNI (+) | 1.71 (0.78–3.78) | 0.18a | 2.01 (1.22–3.31) | < 0.01a | 1.60 (0.70–3.65) | 0.27 | 1.79 (1.04–3.06) | 0.04 |
| Surgical margin | ||||||||
| R0 resection | 1 | 1 | 1 | |||||
| R1 resection | 14.80 (4.87–45.01) | < 0.01a | 1.01 (0.41–2.49) | 0.99 | 25.06 (5.59–112.46) | < 0.01 | ||
| Tumor grade | ||||||||
| Grade 1–2 | 1 | 1 | ||||||
| Grade 3–4 | 0.44 (0.06–3.24) | 0.42 | 0.99 (0.36–2.71) | 0.98 | ||||
| T stage | ||||||||
| T1 | 1 | < 0.01a | 1 | <0.01 | ||||
| T2 | 0.64 (0.04–10.30) | 0.84 (0.05–13.52) | ||||||
| T3 | 1 | 3.43 (0.47–24.99) | 1 | 2.85 (0.38–21.332) | ||||
| T4 | 3.79 (1.70–8.44) | < 0.01a | 7.42 (1.01–54.32) | 2.04 (0.81–5.14) | 0.13 | 5.82 (0.77–44.28) | ||
| N stage | ||||||||
| N0 | ||||||||
| N1 | 1 | 1 | ||||||
| N2 | 2.01 (1.27–3.17) | < 0.01a | 1.74 (1.09–2.77) | 0.02 | ||||
| Tumor size (for every additional 1 cm) | 0.94 (0.79–1.12) | 0.49 | 1.07 (0.97–1.18) | 0.195a | 1.01 (0.90–1.13) | 0.83 | ||
| Lymph node harvested (for every additional 1 lymph node harvested) | 0.99 (0.96–1.03) | 0.74 | 0.997 (0.98–1.02) | 0.72 | ||||
| Adjuvant chemotherapy | ||||||||
| No | 1 | 1 | 1 | |||||
| Yes | 2.37 (0.99–5.68) | 0.053a | 1.05 (0.59–1.84) | 0.88 | 2.69 (1.06–6.83) | 0.04 | ||
Significant values are in bold.
BMI Body mass index, CEA carcinoembryonic antigen.
aOnly variables with p<0.2 in univariate analysis were included to multivariate analysis.
Discussion
This study was a large-scale retrospective study. We followed more than 1000 patients who underwent curative resection for stage I, II, and III colon cancer, with the longest follow-up time more than 10 years. This study demonstrated that the sidedness of colon cancer did not influence OS but influenced TTR in stage II colon cancer. We also observed that RCC, lower BMI, R1 resection and chemotherapy were risk factors for shorter TTR in stage II colon cancer, whereas lower BMI, perineurial invasion, advanced T and N stage were risk factors for shorter TTR in stage III colon cancer. To compensate for the possible bias in this retrospective study, we performed propensity score matching. The results of multivariate analysis were mostly compatible with the results before PSM, which makes this study more reliable.
In previous population-based studies, the OS rate was highly different between stage I, II, and III RCC and LCC. A study of 91,416 patients demonstrated that OS in stage I and II RCC was longer than in stage I and II LCC14. In a study of 77,978 patients, Meguid et al. reported that OS in stage II and III RCC was worse than in stage II and III LCC6. However, in a study of 53,801 patients over 65 years of age, Weiss et al. reported that RCC had longer OS than LCC in stage II but worse OS in stage III16. The age at diagnosis of RCC is approximately 3.0 to 4.6 years older than that for LCC6,17,18, and only one-third of patients with stage I–III colon cancer die from the cancer19. Therefore, OS does not truly reflect the difference in prognosis between RCC and LCC20; hence, we used TTR to compare the risk factors of RCC and LCC.
This study also noted differences between RCC and LCC and reinforced the consensus that RCC and LCC can be regarded as distinct cancers. However, our results contradicted previous studies regarding their specific differences. First, many previous population-based studies have reported a higher proportion of women with RCC than LCC6,14,16; this was also true in our study. However, in our results, women only composed a higher proportion of patients with stage I and II RCC, not stage III. Second, several studies have noted that RCC has a larger tumor size and presents as a later stage than LCC6,21. In our study, tumor size was significantly larger in stage III RCC than in stage III LCC but not in stage II RCC. It is generally believed that LCC tends to manifest in symptoms such as changes in bowel habits and bleeding earlier than RCC, and consequently, LCC is diagnosed earlier than RCC22. However, the results of this study indicate that the consensus that LCC is often detected at an earlier stage than RCC may need to be reevaluated. A relatively small tumor size with lymph node metastasis may be the typical representation of LCC at the time of diagnosis. Third, lymphovascular invasion and tumor grade are powerful prognostic factors in colon cancer23, and many studies have reported that RCC has a higher rate of lymphovascular invasion and a higher tumor grade than LCC12,20,21,24. However, we observed that tumor grade and the incidence of lymphovascular invasion are significantly higher in stage II RCC than in stage II LCC but not in stage III RCC. The high rate of lymphovascular invasion and the presence of grade 3–4 tumor cells shortened TTR in stage II RCC compared with that for stage II LCC. In our study, although stage III RCC had a larger tumor size and more advanced T stage than stage III LCC, cancer recurrence did not differ if patients underwent appropriate radical resection and adjuvant chemotherapy; moreover, we observed no difference in tumor size between stage II RCC and LCC. However, RCC had a shorter TTR because of a higher proportion of lymphovascular invasion and tumor grade. Therefore, we believe that stage II RCC can be regarded as a unique category of colorectal cancer, and postoperative treatment and follow-up strategies need to be accordingly tailored.
Several studies have also suggested that colon cancer heterogeneity is different between RCC and LCC. Microsatellite instability (MSI) and dMMR are more common in RCC. Patients with stage II colon cancer and MSI or dMMR tumors had a better prognosis in RCC than LCC, but in patients with stage III colon cancer, MSI or dMMR provided no benefit to prognosis12,25,26. A recent study reported that in patients with dMMR stage II colon cancer, 5-FU based chemotherapy did not improve survival and could even worsen prognosis27. The National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (2017) recommended that MMR status be checked in stage II colon cancer3. Our institute follows this treatment guideline and has tested MMR status for colon cancer since 2017. According to the limited MMR test data, nearly half of patients with RCC had dMMR (45.5%), which is 10–20% higher than in previous studies12,28. This also explains why chemotherapy was a risk factor for stage II RCC because many patients underwent adjuvant chemotherapy without an MMR status test before 2017.
In previous studies, no differences in BMI were reported between patients with RCC and LCC23,24,29. In this study, however, patients with RCC and stage III RCC had significantly lower BMI than did patients with LCC and stage III LCC, respectively. This may be because patients with RCC are predominantly female and older, which are two groups that generally have a lower BMI. We observed increased BMI to be a positive prognostic factor for patients with both stage II and stage III cancer. By contrast, previous studies have reported obesity or a BMI of > 30 kg/m2 to be risk factors for cancer recurrence and cancer-related mortality in colorectal cancer30–33. In agreement with our study, Sinicrope et al. reported that overweight patients (BMI 25–29.9 kg/m2) had better OS in stage II and III colon cancer34; the authors speculated that relatively healthy patients in this BMI range can tolerate operations and adjuvant chemotherapy34. We speculate that patients with a low BMI have too little visceral fat to cover the tumor, and thus, the tumor can easily invade the surrounding organs or spread. Patients with obesity usually have comorbid diseases, and complications often occur during surgery or chemotherapy. Most patients (91%) in this study had a BMI below 30, and we observed that increased BMI was associated with longer TTR.
This study had several limitations. First, this was a retrospective study at a single institution, which makes our study susceptible to bias and confounding factors despite the large study population. Second, only 33 patients (0.3%), 110 patients (11%) and 488 patients (48%) had an MSI status, MMR status or K-RAS mutation test, respectively. Because of the low number of patients with an MSI status, MMR status or K-RAS mutation test, we did not analyze the effect of these variables on prognosis in this study. Third, detailed chemotherapy data were not available in this study. Patients who received oral chemotherapy, intravenous chemotherapy, and incomplete chemotherapy courses were all classified as having received adjuvant chemotherapy. This may underestimate the effects of chemotherapy. Finally, complete mesocolic excision (CME) in RCC increases OS and DFS, and cancer stage may be underdiagnosed in patients without CME35–37. CME is not routinely performed for RCC at our institute, and CME status is not always recorded in medical records, thus affecting the analysis of prognosis.
In conclusion, sidedness is an independent risk factor for cancer recurrence in stage II colon cancer; patients with stage II RCC had shorter TTR than did those with stage II LCC. Furthermore, stage III RCC had a more advanced T stage, but this did not influence TTR in stage III colon cancer. Further research is needed to evaluate the differences of sidedness by using clinicopathological and genetic factors.
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Author contributions
Conception and design: C.-Y.Y., M.-H.Y., and T.-C.C. Data acquisition: C.-Y.Y., M.-H.Y., K.-T.K., Y.-T.C., and T.-C.C. Analysis and interpretation data: C.-Y.Y. and T.-C.C. Writing, review, and revision of the manuscript: C.-Y.Y. and T.-C.C.
Data availability
The data generated in this study are available on request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global Burden of Disease Cancer Collaboration et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA. Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, et al. Colorectal cancer statistics, 2017. CA. Cancer. J. Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 3.Benson AB, 3rd, et al. Colon cancer, version 12017, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer. Netw. 2017;15:370–398. doi: 10.6004/jnccn.2017.0036. [DOI] [PubMed] [Google Scholar]
- 4.Bufill JA. Colorectal cancer: Evidence for distinct genetic categories based on proximal or distal tumor location. Ann. Intern. Med. 1990;113:779–788. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 5.Iacopetta B. Are there two sides to colorectal cancer? Int. J. Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 6.Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right-versus left-sided colon cancers? Ann. Surg. Oncol. 2008;15:2388–2394. doi: 10.1245/s10434-008-0015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moritani K, Hasegawa H, Okabayashi K, Ishii Y, Endo T, Kitagawa Y. Difference in the recurrence rate between right- and left-sided colon cancer: A 17-year experience at a single institution. Surg. Today. 2014;44:1685–1691. doi: 10.1007/s00595-013-0748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JM, et al. Impact of tumor sidedness on survival and recurrence patterns in colon cancer patients. Ann. Surg. Treat. Res. 2019;96:296–304. doi: 10.4174/astr.2019.96.6.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saltzstein SL, Behling CA. Age and time as factors in the left-to-right shift of the subsite of colorectal adenocarcinoma: A study of 213,383 cases from the California Cancer Registry. J. Clin. Gastroenterol. 2007;41:173–177. doi: 10.1097/01.mcg.0000225550.26751.6a. [DOI] [PubMed] [Google Scholar]
- 10.Powell AG, et al. The relationship between tumour site, clinicopathological characteristics and cancer-specific survival in patients undergoing surgery for colorectal cancer. Colorectal. Dis. 2012;14:1493–1499. doi: 10.1111/j.1463-1318.2012.03048.x. [DOI] [PubMed] [Google Scholar]
- 11.Benedix F, et al. Comparison of 17,641 patients with right- and left-sided colon cancer: Differences in epidemiology, perioperative course, histology, and survival. Dis. Colon. Rectum. 2010;53:57–64. doi: 10.1007/DCR.0b013e3181c703a4. [DOI] [PubMed] [Google Scholar]
- 12.Qin Q, et al. Comparison of 627 patients with right- and left-sided colon cancer in China: Differences in clinicopathology, recurrence, and survival. Chronic. Dis. Transl. Med. 2017;3:51–59. doi: 10.1016/j.cdtm.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bustamante-Lopez LA, Nahas SC, Nahas C, Pinto RA, Marques C, Cecconello I. Is there a difference between right-versus left-sided colon cancers? Does side make any difference in long term follow-up? Arq. Bras. Cir. Dig. 2019;32:e1479. doi: 10.1590/0102-672020190001e1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warschkow R, et al. Better survival in right-sided versus left-sided stage I–III colon cancer patients. BMC Cancer. 2016;16:554. doi: 10.1186/s12885-016-2412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, et al. Characteristics of differently located colorectal cancers support proximal and distal classification: A population-based study of 57,847 patients. PLoS ONE. 2016;11:e0167540. doi: 10.1371/journal.pone.0167540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss JM, et al. Mortality by stage for right-versus left-sided colon cancer: Analysis of surveillance, epidemiology, and end results–medicare data. J. Clin. Oncol. 2011;29:4401–4409. doi: 10.1200/JCO.2011.36.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suttie SA, Shaikh I, Mullen R, Amin AI, Daniel T, Yalamarthi S. Outcome of right- and left-sided colonic and rectal cancer following surgical resection. Colorectal. Dis. 2011;13:884–889. doi: 10.1111/j.1463-1318.2010.02356.x. [DOI] [PubMed] [Google Scholar]
- 18.Mik M, Berut M, Dziki L, Trzcinski R, Dziki A. Right- and left-sided colon cancer: Clinical and pathological differences of the disease entity in one organ. Arch. Med. Sci. 2017;13:157–162. doi: 10.5114/aoms.2016.58596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarantino I, et al. Relative survival is an adequate estimate of cancer-specific survival: Baseline mortality-adjusted 10-year survival of 771 rectal cancer patients. Ann. Surg. Oncol. 2013;20:3877–3884. doi: 10.1245/s10434-013-3173-5. [DOI] [PubMed] [Google Scholar]
- 20.Klose J, et al. Does side really matter? Survival analysis among patients with right- versus left-sided colon cancer: A propensity score-adjusted analysis. Ann. Surg. Oncol. 2021;28:2768–2778. doi: 10.1245/s10434-020-09116-y. [DOI] [PubMed] [Google Scholar]
- 21.Turner MC, et al. The side of the primary tumor affects overall survival in colon adenocarcinoma: An analysis of the national cancer database. Tech. Coloproctol. 2019;23:537–544. doi: 10.1007/s10151-019-01997-w. [DOI] [PubMed] [Google Scholar]
- 22.Yahagi M, Okabayashi K, Hasegawa H, Tsuruta M, Kitagawa Y. The worse prognosis of right-sided compared with left-sided colon cancers: A systematic review and meta-analysis. J. Gastrointest. Surg. 2016;20:648–655. doi: 10.1007/s11605-015-3026-6. [DOI] [PubMed] [Google Scholar]
- 23.Kwak HD, Ju JK, Lee SY, Kim CH, Kim YJ, Kim HR. Comparison of right-side and left-side colon cancers following laparoscopic radical lymphadenectomy. J. Invest. Surg. 2021;34:142–147. doi: 10.1080/08941939.2019.1608334. [DOI] [PubMed] [Google Scholar]
- 24.Lim DR, Kuk JK, Kim T, Shin EJ. Comparison of oncological outcomes of right-sided colon cancer versus left-sided colon cancer after curative resection: Which side is better outcome? Medicine. 2017;96:e8241. doi: 10.1097/MD.0000000000008241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinicrope FA, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J. Clin. Oncol. 2013;31:3664–3672. doi: 10.1200/JCO.2013.48.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natsume S, et al. Clinicopathological and molecular differences between right-sided and left-sided colorectal cancer in Japanese patients. Jpn. J. Clin. Oncol. 2018;48:609–618. doi: 10.1093/jjco/hyy069. [DOI] [PubMed] [Google Scholar]
- 27.Sargent DJ, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J. Clin. Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchins G, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J. Clin. Oncol. 2011;29:1261–1270. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
- 29.Campana JP, Pellegrini PA, Rossi GL, Ojea Quintana G, Mentz RE, Vaccaro CA. Right versus left laparoscopic colectomy for colon cancer: Does side make any difference? Int. J. Colorectal. Dis. 2017;32:907–912. doi: 10.1007/s00384-017-2776-x. [DOI] [PubMed] [Google Scholar]
- 30.Dignam JJ, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J. Natl. Cancer. Inst. 2006;98:1647–1654. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 31.Scarpa M, et al. Obesity is a risk factor for multifocal disease and recurrence after colorectal cancer surgery: A case-control study. Anticancer. Res. 2014;34:5735–5741. [PubMed] [Google Scholar]
- 32.Harriss DJ, et al. Lifestyle factors and colorectal cancer risk (1): Systematic review and meta-analysis of associations with body mass index. Colorectal. Dis. 2009;11:547–563. doi: 10.1111/j.1463-1318.2009.01766.x. [DOI] [PubMed] [Google Scholar]
- 33.Gribovskaja-Rupp I, Kosinski L, Ludwig KA. Obesity and colorectal cancer. Clin. Colon. Rectal. Surg. 2011;24:229–243. doi: 10.1055/s-0031-1295686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinicrope FA, Foster NR, Sargent DJ, O'Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin. Cancer. Res. 2010;16:1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotake K, et al. Impact of D3 lymph node dissection on survival for patients with T3 and T4 colon cancer. Int. J. Colorectal. Dis. 2014;29:847–852. doi: 10.1007/s00384-014-1885-z. [DOI] [PubMed] [Google Scholar]
- 36.Storli KE, et al. Short term results of complete (D3) vs standard (D2) mesenteric excision in colon cancer shows improved outcome of complete mesenteric excision in patients with TNM stages I–II. Tech. Coloproctol. 2014;18:557–564. doi: 10.1007/s10151-013-1100-1. [DOI] [PubMed] [Google Scholar]
- 37.Hashiguchi Y, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020;25:1–42. doi: 10.1007/s10147-019-01485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in this study are available on request from the corresponding author.