Summary
Background
Cervical cancer is one of the leading causes of cancer in women of childbearing age worldwide. A substantial fraction of cervical cancer is associated with Human Papilloma Virus (HPV) infection and is preventable through vaccination and screening. The aim of the study is to describe geographic and epidemiologic trends in incidence and mortality of cervical cancer in Russia during 2007–2018.
Methods
Publicly accessible data from the P.A. Herzen Moscow Oncology Research Institute and the Ministry of Health of Russian Federation for 2007–2018 was used for this study. Cervical cancer incidence and mortality rates were analyzed using descriptive statistics and results were mapped to determine the geographic distribution. Potential contributing risk factors in the population were studied using univariate and multivariate Poisson regression analyses.
Findings
A total of 187,013 patients were diagnosed with cervical cancer in Russia between 2007 and 2018. The average age-standardized incidence (ASIR) and mortality rates (ASMR) were 15.70/100,000 and 5.76/100,000 females, respectively, with a 27% increase in the incidence observed between 2007 and 2018. The highest ASIR was observed in the Far Eastern Federal District and the lowest in the Central Federal District. Multivariate model for cervical cancer ASIR showed that daily smoking (p = 0·0003) and syphilis (p = 0.003) were significantly associated with cervical cancer incidence.
Interpretation
The incidence of cervical cancer in Russia is rising at a significant pace. This trend can in part be attributed to a lack of nationwide cervical cancer screening . The presented results are valuable for informing public health policy on HPV vaccinations, smoking prevention and cervical cancer screening as urgent interventions are needed to combat a troubling trend.
Funding
This work was supported by the Cancer Research Society (CRS)-Canadian Institutes for Health Research (CIHR) Partnership Grant #25343 to Dr. Litvinov. Canadian Dermatology Foundation research grant to Dr. Litvinov, and by the Fonds de la recherche du Québec – Santé to Dr. Sasseville (#22648) and to Dr. Litvinov (#34753 and #36769). This research was further supported by the CIHR Catalyst Grant #428712 to Dr. Litvinov.
Keywords: Squamous cell carcinoma; Cervical Cancer; Human Papilloma Virus (HPV); Vaccination; Geographic clustering; Epidemiology; Circumpolar communities; Smoking; Alcohol, Sexually Transmitted Infections (STI); Incidence; Mortality; Russian Federation
Research in context.
Evidence before this study
The Russian Federation has one of the highest cervical cancer incidence and mortality rates in Europe (8th in Europe based on the GLOBOCAN 2018 data1). Cervical cancer has the third highest age-standardized incidence rate of all cancers in the country. The etiology of cervical cancer is significantly associated with human papilloma virus (HPV) infection and, thus, many countries have introduced national HPV vaccination programs. Unfortunately, with the exception of Moscow City and other select jurisdictions, HPV vaccination rates in the Russian Federation remain low. The high incidence of cervical cancer is further exacerbated by a lack of an established, national, secondary prevention program, with only 20–25% of female population ≥18 years of age (or age of first intercourse) partaking in screening/pap test, which remains mainly opportunistic.
Added value of this study
Our study provides insight into the national burden of cervical cancer in the Russian Federation from 2007 to 2018, with a significantly increasing incidence trend observed over this time period. A non-uniform geographic distribution of incidence and mortality exists across the country. Notably, this work identifies circumpolar Inuit region as being at high-risk of cervical cancer incidence and mortality. Furthermore, regression analyses demonstrated a positive association between cervical cancer incidence among Russian females and alcohol use, cigarette smoking, tuberculosis (TB), syphilis, and gonorrhea in the general Russian population (males and females together). Particularly, smoking was significantly associated with higher cervical cancer mortality. Additionally, through demonstrating a potential association between cervical cancer and another HPV-driven cancer – oropharyngeal cancer – our study underscores the importance of expanding HPV vaccination programs. We call for organized screening and vaccination programs to reduce the burden of cervical cancer in the Russian Federation. Importantly, provided that Russia has one of the highest smoking rates globally and the trend has recently increased for women, our results serve as a call-to-action to reduce the smoking burden in addition to advocating for increased HPV vaccination and cervical cancer screening. Not only will this impact cervical cancer rates but will also lead to a reduction in other conditions such as cardiovascular disease and lung cancer, leading to an overall positive impact on health. The findings presented here reflect the need for urgent intervention in reducing cervical cancer incidence and mortality in this vast, multicultural part of the world.
Implications of all the available evidence
In August 2020, the World Health Assembly adopted a global strategy for cervical cancer elimination, which states that to globally reduce the age-standardized cervical cancer incidence to less than 4/100,000, countries should meet the ‘90-70-90’ targets by 2030 reflecting vaccination (90% of girls), screening (70% of women), and early treatment (90% of patients) of pre-cancer or invasive cancer lesions.2 These targets emphasize the importance of comprehensive HPV vaccination and secondary screening protocols for cervical cancer, which are limited across the Russian Federation. The success of global initiatives relies on the availability and access to quality data, bona fide collaboration between scientific communities of different countries and critical analysis of health resource allocation by the individual governing bodies. The Russian Federation is a vast country with diverse cultural practices/religions, different ethnicities (>190 nationalities reside in Russia), socioeconomic variability by region, which allow for the complex analysis of various risk factors that have implications on public health. Russia has the economic potential to mobilise resources required to implement the WHO objectives regarding HPV vaccination and organised screening. It is regrettable that due ongoing conflict significant resources are lost which could have been used to achieve these goals. We call on the Russian scientific community to mobilize and to focus on improving health.
Alt-text: Unlabelled box
Introduction
In 2020, the age-standardized incidence rate (ASIR) of cervical cancer worldwide was reported to be 13.3 per 100,000 and remains a significant public health burden in many countries.1,3 It is preventable through vaccination and screening, yet continues to be the fourth most common cause of cancer around the world.1,3 Human papilloma virus (HPV) infection of the genital tract is responsible for ∼99.7% of cases of cervical cancers.4 A significant number of cervical cancer cases could be prevented/managed by means of prophylactic measures through vaccination, screening, and early therapeutic interventions.
Several HPV vaccines have been developed to date, targeting multiple strains including HPV-16 and 18 that are associated with cancer development. In fact, a Cochrane review including 73,428 participants concluded that there is high-certainty evidence that HPV vaccines protect from cervical precancer in individuals aged 15–26 years old5. A systematic review and meta-analysis found that five to eight years following vaccination in high-income countries, the prevalence of HPV-16 and 18 decreased by 83% (Relative Risk (RR) 0.17, 95% CI 0.11–0.25) in females 13–19 years of age and by 66% (RR 0.34, 95% CI 0.23–0.49) in females 20–24 years of age.6 In addition, there was a reduction in the prevalence of HPV-31, 33, and 45 with a decrease by 54% (RR 0.46, 95% CI 0.33–0.66) in females 13–19 years of age.6 A study in Sweden showed that cervical cancer incidence rate ratio of vaccinated to unvaccinated individuals was 0.12 (95% CI, 0.00 to 0.34) for women who received the HPV vaccine before 17 years of age compared to 0.47 (95% CI, 0.27 to 0.75) for those vaccinated between 17 and 30 years7. Multi-cohort vaccination programs with high population coverage have had a greater direct impact as a result of herd immunity.6 In the United States, within six years of vaccine introduction, there was a 64% decrease in 4-valent HPV prevalence among females 14–19 years of age and a 34% decrease among those 20–24 years of age. This finding extended previous observations of population impact of HPV vaccination in the United States and demonstrated the first national evidence of impact among females in their 20s.8
Recently, The Lancet Global Health highlighted that the potential health impact of HPV vaccines is higher than was previously forecasted, with health benefits such as reducing 15–19 cervical cancer cases, 12–14 deaths, and 243–306 disability-adjusted life-years for every 1000 vaccinated nine-year-old girls, with the upper and lower limits reflecting the estimates for the nonavalent vaccine and bivalent or quadrivalent vaccines, respectively.9
A report has quantified the worldwide cumulative coverage of publicly funded HPV immunization programs between 2006 and 2014 and found that over this period, 64 countries nationally, four countries sub-nationally, and 12 overseas/dependent territories had implemented HPV immunization programs.10 In more developed regions of the world, 33.6% of females 10–20 years of age received the full course of the vaccine, compared with only 2.7% in less developed regions. Interestingly, eastern Europe, which includes Russia, had by far the lowest vaccination rates compared to other developed regions of the world.10, 11, 12 As of October 2019, over 100 countries worldwide have included HPV in the national vaccination programs,13 which still does not include Russia. Additionally, one third of global HPV vaccination programs have gender-neutral approaches, however, they are almost exclusively in high-income or upper-middle-income countries.
The objective of the current populational registry-based study was to analyze the existing data to improve our knowledge on epidemiologic trends of cervical cancer in the Russian Federation.
Methods
Study design
This study was completed using an ecological study design. Study design and data reporting was performed in accordance with Strengthening the reporting of observational studies in epidemiology (STROBE) checklist.14
Setting/participants/data sources
The number of cervical cancer cases by age group and jurisdiction were only available for 2007–2018 years. Hence, this time interval was chosen for the study. The incidence and mortality data on cervical cancer (C53) was extracted from open source and publicly available, annual reports of the P.A. Herzen Moscow Oncology Research Institute, and Ministry of Health of Russian Federation for the period of the study (2007–2018), based on International Classification of Diseases (ICD).15,16 This institute is tasked with compiling this data and realizing the formal reports. C54 (malignant neoplasm of corpus uteri) and C55 (malignant neoplasm of uterus, part unspecified) were not included in the analyses due to heterogeneity of the data. The Russian Federation consists of eight Federal Districts that are conglomerates of 80 Federal Subjects (i.e., individual jurisdictions). Federal Subjects (Russian: субъекты Российской Федерации, romanized: subyekty Rossiyskoy Federatsii) include oblast, province, krai, autonomous republic, autonomous oblast and cities with a special status (e.g., Moscow and Saint Petersburg).
The P.A. Herzen Moscow Oncology Research Institute collects and analyzes the data based on ICD codes received from primary regional oncology centers/hospitals. The local organizations collect primary data (ICD codes, not registry based) and provide annual reports to higher level authorities at the national level.17 There is no information collected regarding clinical staging of the disease, clinical/pathological subtype, or treatment approaches. Additionally, there is no individual patient data with respect to demographics and other comorbidities available.
Variables (risk factors)/data sources
Risk factors including sexually transmitted infections or STIs (i.e., HIV, syphilis, gonorrhea), tuberculosis (TB), alcohol consumption, and smoking were analyzed. Data on HIV, syphilis, gonorrhea, TB, and alcohol induced psychosis and dependency by region for the years 2014–2015 (mid-point for our study period) was obtained from the Ministry of Health of the Russian Federation Monitoring and Analysis Department. Data for other years was not available. Alcohol consumption was also estimated using survey-based data reporting any alcohol or rare alcohol use. Similarly, survey-based smoking data reporting daily smoking or never smoking by region was publicly available and was obtained from the Federal State Statistics Service (Rosstat) for the year 2016 (data for other years was not available). For the above-mentioned variables, data was only available for the whole population (i.e., males and females combined). Association with other HPV-related cancers such as oropharyngeal cancer was also explored using data from The P.A. Herzen Moscow Oncology Research Institute database.
Bias
The data used in this study was retrieved from a large national database and given this approach, bias may arise if inaccuracies/errors exist in data reported by individual jurisdictions. However, as this is an official national source consisting of large patient numbers, the bias from this approach is minimized. Also, some variables are based on survey responses from individuals and hence depending on how the survey was conducted, there may be intrinsic biases associated with the reported results. For some of the variables, data assessing similar factors (e.g., alcohol intake) was derived from multiple sources (e.g., survey data, risk of psychosis and dependency due to alcohol use). Hence, when considered together we are able to optimize the accuracy and minimize reporting bias.
Statistical methods
Annual ASIRs and age-standardized mortality rates (ASMRs) were calculated based on the World Health Organization (WHO) World Population Standard 2000–2025 to allow comparison to international rates which use the same denominator.18 Age-standardization per geographic region in the Russian Federation (i.e., across jurisdictions) was performed based on the regional age population distribution from the Russian Federation Census (Rosstat) for 2008–2018 years (data for year 2007 was not available). This approach was used since the population mix within jurisdiction is more similar to the one in Russia than the WHO Standard Population.
Descriptive analyses including incidence and mortality rates by year and jurisdiction, and age at the time of diagnosis or death were conducted to report age-standardized rates. To determine trends in ASIR/ASMR over time, linear regression was performed using Prism 8.0.1 software (GraphPad Software, La Jolla, CA, USA) and SAS 9.4 software (2013, SAS Institute Inc., Cary, NC, USA). Similar to the methodology in our previous studies on melanoma in Canada19,20 and melanoma and non-melanoma skin cancers in Russia,21,22 case counts of new cases and deaths were extracted by year from the P.A. Herzen Moscow Oncology Research Institute data. Using the data on the regional populations extracted from the Russian Federation Census for 2002 and 2010 as the denominator, we re-calculated the ASIR and ASMR23 and computed the corresponding 95% Confidence Intervals (CIs) using the Poisson distribution.24 The translation of names of administrative territories of the Russian Federation was carried out according to the recommendation of the US Embassy to Russia which may differ from the Russian State Standard (GOST 7·67-2003).25
Univariate Poisson regression model analyses were performed by jurisdiction using the age-standardized incidence and mortality rates (Russian Population Standard) and the available risk factor prevalence. No offset was used since the outcome was incidence/mortality rate (not individual case counts). A two-sided dispersion test and the goodness of fit statistic was assessed for the model.
To identify the most important risk factors from all the risk factors assessed, we performed a backwards stepwise variable selection by Akaike Information Criterion (AIC). We further checked for collinearity among predictors by assessing overall and individual variance inflation factors (VIF) with the mctest package in R version 4.0.4 for the model with the lowest AIC. Where applicable, p<0·05 was accepted as significant. Data analysis was performed using R Studio and SAS 9.4 software.
Geographic distribution of the incidence and mortality rates per jurisdiction in the Russian Federation was generated using geographic information systems software (ArcMap 10.7.1 from Environmental Systems Research Institute [ESRI], Redlands, California).
Role of Funding Sources
The funding sources played no role in the writing of the manuscript or the decision to submit it for publication. We have not been paid to write this article by a pharmaceutical company or other agency.
Results
Participants/descriptive data
The population of the Russian Federation is ∼147 million individuals (∼76.9 million females). There were 187,013 patients diagnosed with cervical cancer between 2007 and 2018 (Table 1). Mean age at the time of diagnosis was reported annually and the average of these values during 2007–2018 was 52.1 years.
Table 1.
Average number of cases and deaths from cervical cancer (C53), by age group, in the Russian Federation over the period of 2007 to 2018. Absolute number, percent per age group, and crude incidence rates per age group are presented.
| Age | Incidence Cervical Cancer |
Mortality Cervical Cancer |
||||
|---|---|---|---|---|---|---|
| # | % | CR | # | % | CR | |
| 0–19 | 83 | 0.04 | 0.1 | 8 | 0.01 | 0.0 |
| 20–29 | 8421 | 4.5 | 6.3 | 1505 | 1.97 | 1.1 |
| 30–39 | 35,370 | 18.91 | 26.6 | 9022 | 11.82 | 6.8 |
| 40–49 | 44,071 | 23.57 | 35.2 | 14,487 | 18.98 | 11.6 |
| 50–59 | 44,888 | 24 | 32.0 | 18,347 | 24.03 | 13.1 |
| 60–69 | 29,392 | 15.72 | 29.7 | 14,192 | 18.59 | 14.3 |
| 70–79 | 17,965 | 9.61 | 23.7 | 12,181 | 15.96 | 16.1 |
| ≥80 | 6823 | 3.65 | 17.5 | 6599 | 8.64 | 16.9 |
| TOTAL | 187,013 | 100 | 20.2 | 76,341 | 100 | 8.2 |
CR= Crude rate per 100,000.
Outcome data (incidence and mortality)
The average ASIR to the WHO 2000–2025 population standard of cervical cancer during the studied time period was 15.70/100,000 (95%CI 14.90–16.49), while the average crude incidence rate was 20.14/100,000 (95% CI 19.10–21.19) (Table 2). The average ASMR to the WHO 2000–2025 population standard was 5.76/100,000 (95%CI 5.68–5.84), while the average crude mortality was 8.23/100,000 (95%CI 8.13–8.33).
Table 2.
Crude and age-standardized incidence and mortality rates (ASIR and ASMR) of cervical cancer (C53) in females in the Russian Federation for the years 2007–2018. Calculations performed based on data available by age group. Age standardization performed according the World Health Organization (WHO) Standard Population 2000–2025.
| Year | C53 Crude Incidence/100,000 |
C53 ASIR/100,000 |
C53 Crude Mortality /100,000 |
C53 ASMR/100,000 |
|---|---|---|---|---|
| 2007 | 17.6 (17.3–17.9) | 13.7 (13.4–13.9) | 8.1 (7.9–8.3) | 5.7 (5.6–5.9) |
| 2008 | 18.1 (17.8–18.4) | 14.1 (13.8–14.3) | 7.9 (7.7–8.1) | 5.6 (5.4–5.7) |
| 2009 | 18.8 (18.5–19.1) | 14.7 (14.5–15.0) | 8.1 (7.9–8.3) | 5.7 (5.6–5.9) |
| 2010 | 19.3 (19.0-19.6) | 15.1 (14.8–15.3) | 8.1 (7.9–8.3) | 5.7 (5.5–5.8) |
| 2011 | 19.3 (19.0–19.6) | 15.1 (14.8–15.3) | 8.3 (8.1–8.5) | 5.8 (5.7–6.0) |
| 2012 | 19.6 (19.3–19.9) | 15.4 (15.1–15.6) | 8.3 (8.0–8.5) | 5.8 (5.7–6.0) |
| 2013 | 20.0 (19.7–20.4) | 15.7 (15.4–16.0) | 8.5 (8.3–8.7) | 6.0 (5.8–6.1) |
| 2014 | 20.9 (20.6–21.2) | 16.23 (16.0–16.5) | 8.3 (8.1–8.5) | 5.8 (5.6–5.9) |
| 2015 | 21.3 (21.0–21.6) | 16.6 (16.3–16.9) | 8.4 (8.2–8.7) | 6.0 (5.8–6.1) |
| 2016 | 21.9 (21.6–22.2) | 17.1 (16.8–17.3) | 8.4 (8.2–8.6) | 5.8 (5.7–6.0) |
| 2017 | 22.3 (22.0–22.7) | 17.3 (17.1–17.6) | 8.2 (8.0–8.4) | 5.7 (5.6–5.9) |
| 2018 | 22.6 (22.2–22.9) | 17.4 (17.1–17.7) | 8.1 (7.9–8.3) | 5.6 (5.4–5.7) |
| Mean | 20.1 (19.1–21.2) | 15.7 (14.9–16.5) | 8.2 (8.1–8.3) | 5.8 (5.7–5.8) |
Main results
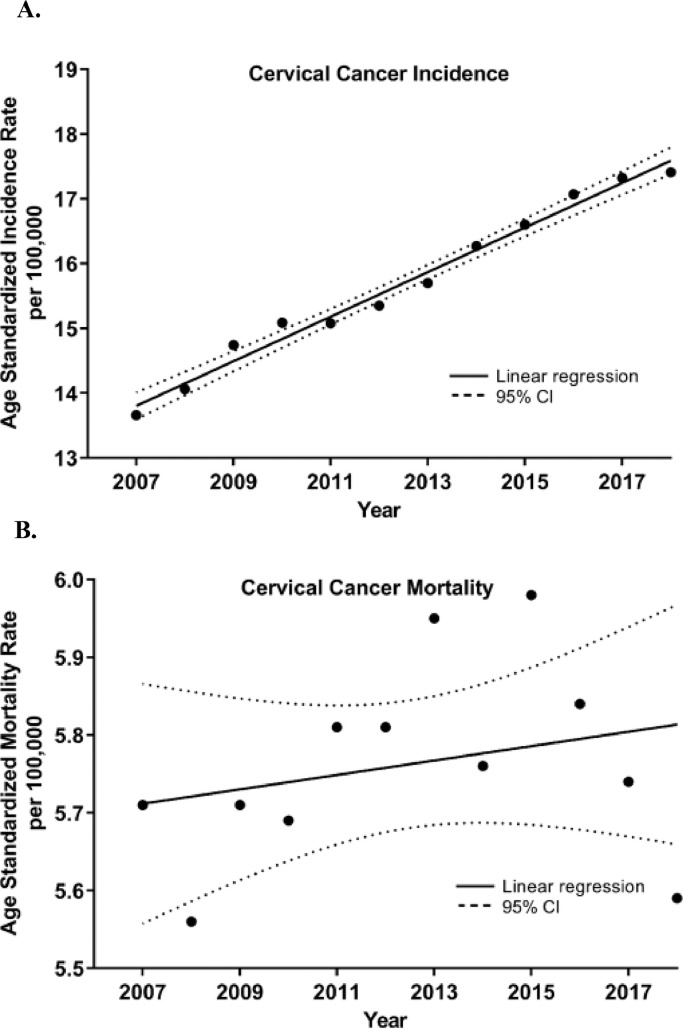
Analysis of annual ASIR rates (standardized to the WHO 2000–2025 population standard) over time revealed an increasing incidence with an annual increase of 0.34 cases/100,000 females per year (R2 = 0·98, p<0·0001, Figure 1A) where the incidence rate in 2007 was 13.66/100,000 and increased to 17.41/100,000 in 2018. Mortality trend over this time period fluctuated and no significant trends were observed (Figure 1B). The mortality rate in 2007 was 5.71/100,000 and in 2018 was found to be 5.59/100,000.
Figure 1.
Cervical cancer (a) incidence and (b) mortality trends in the Russian Federation during 2007–2018.
Of the eight Federal Districts, the highest ASIRs (Russian Population Standard) were found in the Far Eastern Federal District: 25·96 (24.22–27.70) followed by the Siberian Federal District 25.26 (24.28–26.25), and the Southern Federal District 23.18 (22.14–24.22) (Table 3). The lowest cervical cancer incidence was observed in the North Caucasian (term refers to the Caucasus Mountains, not ethnicity) Federal District 17.10 (15.87–7.30–18.4318.33).
Table 3.
Age-standardized incidence and mortality rates (ASIR and ASMR) based on the Russian Federation Standard and the WHO World Population Standard 2000–2025 of cervical cancer (C53) presented by Federal District of the Russian Federation over the period 2007–2018.
| Region | Average Female Population (2007–2018) | Total C53 | ASIR/100,000 | C53 | ASMR/100,000 | ||
|---|---|---|---|---|---|---|---|
| Cases | C53 (95%CIs) |
Deaths | C53 (95%CIs) |
||||
| Russian Federation Standard |
WHO Population Standard | Russian Federation Standard |
WHO Population Standard | ||||
| Central Federal Districta | 20,812,251 | 46,227 | 17.9 (17.3–18.4) | 12.4 (12.0– 12.8) | 18,204 | 7.5 (7.2–7.9) | 4.6 (4.4–4.8) |
| Northwestern Federal Districta | 7,407,440 | 18,941 | 20.6 (19.6–21.6) | 14.4 (13.7–15.1) | 7032 | 8.3 (7.6–8.9) | 5.1 (4.7– 5.5) |
| Southern Federal Districta | 8,240,097 | 22,819 | 23.2 (22.1–24.2) | 16.2 (15.5– 16.9) | 8881 | 9.8 (9.1–10.5) | 6.0 (5.6– 6.4) |
| North Caucasian Federal Districta | 5,041,015 | 8882 | 17.1 (15.89–18.3) | 12.7 (11.7– 13.6) | 2927 | 7.2 (6.4–8.0) | 4.7 (4.2–5.2) |
| Volga Federal Districta | 16,087,447 | 35,446 | 18.4 (17.7–19.0) | 12.8 (12.3– 13.3) | 12,473 | 7.0 (6.6–7.4) | 4.3 (4.0– 4.5) |
| Ural Federal Districta | 6,555,528 | 16,932 | 21.9 (20.7–23.0) | 15.6 (14.8–16.4) | 5692 | 8.2 (7.5–8.9) | 5.2 (4.7– 5.6) |
| Siberian Federal Districta | 10,260,075 | 30,178 | 25.3 (24.3–26.3) | 18.0 (17.3–18.7) | 11,066 | 10.3 (9.7–10.9) | 6.5 (6.1– 6.9) |
| Far Eastern Federal Districta | 3,360,632 | 10,223 | 26.0 (24.2–27.7) | 18.9 (17.6–20.1) | 3716 | 10.7 (9.6–11.9) | 7.0 (6.2–7.7) |
ASIR= age standardized incidence rate, ASMR= Age standardized mortality rate.
- Data for Federal Districts averaged over 2009–2018, as data for 2007–2008 was not available.
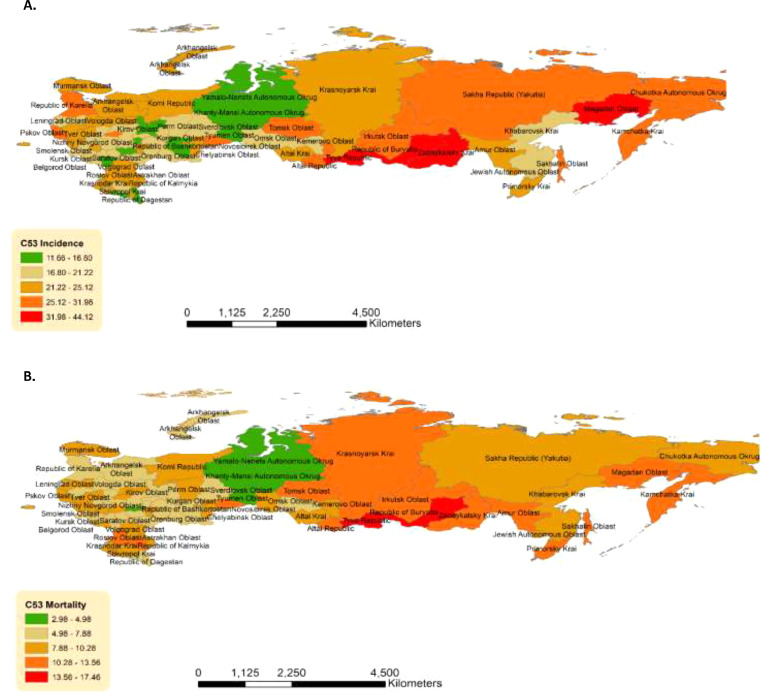
Of the individual jurisdictions, the highest ASIR (Russian Population Standard) rates were noted in the Tyva Republic (Siberian Federal District) 44.12 (32.67–55.57), Zabaykalsky Krai (Siberian Federal District) 43.72 (38.04–49·39), Magadan Oblast (Far Eastern Federal District) 42·54 (28·24–56·84). This was followed by Republic of Buryatia (Siberian Federal District) 36·92 (31.40–42.44), and Kamchatka Krai (Far Eastern Federal District) 31.98 (23.27–40.68). These jurisdictions have ASIRs of 1.5–2 times the national average. The corresponding mortality findings are presented in Supplementary Table 1. Non-uniform geographic distribution of cervical cancer incidence and mortality across the Russian Federation was documented (Figure 2A-B).
Figure 2.
Geographic distribution, by jurisdiction, of cervical cancer (a) incidence and (b) mortality over the time period of the study (2007–2018).
Univariate Poisson regression analysis revealed that any alcohol use (p = 0.021), alcohol induced psychosis and dependency (p = 0.0012), and alcohol induced psychosis alone (p = 0.0017) in any given jurisdiction were associated with higher cervical cancer incidence. In terms of smoking, high daily smoking (p<0.0001) in a given jurisdiction was associated with increased cervical cancer incidence. Never smoking rates for a jurisdiction were negatively associated (p<0.0001) with the incidence of cervical cancer. For infectious causes, prevalence of TB (p<0.0001), syphilis (p<0.0001), and gonorrhea (p<0.0001) in a given jurisdiction were also associated with higher incidence of cervical cancer. There was also a significant correlation by jurisdiction of oropharyngeal cancer incidence (p<0.0001) and mortality (p<0.0001) with cervical cancer incidence. In the multivariate analyses, stepwise regression revealed that daily smoking (p = 0.0003) and syphilis (p = 0.003) remained significantly associated with cervical cancer incidence (Supplementary Table 2).
For mortality, univariate analysis revealed no association with alcohol use. A significant positive association was observed for daily smoking (p<0.0001) and a negative one for never smoking rates (p = 0.0024). For infectious diseases, TB (p<0.0001), syphilis (p<0.0001), and gonorrhea (p = 0.0005) were also significantly associated with cervical cancer mortality rates. Strong association with oropharyngeal cancer mortality was similarly observed (p<0.0001). In a multivariate analysis, stepwise regression revealed significant association between cervical cancer mortality and daily smoking (p = 0.026) as well as TB (p = 0.0027) (Supplementary Table 3).
In the present model (Supplementary Tables 2–3), observations were individual jurisdictions, and the outcomes were ASIR and ASMR for cervical cancer over the 12-year period. Covariates were expressed as percent of people in the jurisdiction that belong to the risk factor category. Model performance was assessed using a two-sided dispersion test with the AER package in R 4.0.4. Incidence and mortality models demonstrated dispersion statistics of 0.89 and 0.37, respectively. The goodness of fit statistic was assessed and the likelihood-ratio-based R-squared calculated for the incidence and mortality rate models were 0.55 and 0.31, respectively.
Discussion
The Russian Federation is a vast country with a diverse culture/religion, different ethnicities (>190 nationalities reside in Russia), socioeconomic variability by region, which allow for the complex analysis of various risk factors. Our study demonstrated a 27% increase in the incidence rate of cervical cancer between 2007 and 2018 and non-uniform geographic distribution of cases/deaths. No significant increase in mortality was observed over the study period despite increase in incidence. This could be attributable to improvement in screening or treatment of the disease. As the incidence of this potentially preventable cancer has been steadily increasing over the years, the concerns surrounding lack of national vaccination or screening programs have become more prominent. National HPV immunization programs currently exist in >100 countries as part of the national vaccination schedule, however the Russian Federation is, regrettably, not one of them.13 This work identified several regions in the country with higher rates of cervical cancer (i.e., Tyva Republic, Zabaykalsky Krai, Magadan oblast, and Buryatia Republic) where HPV vaccination and screening programs need to be urgently introduced/strengthened to help reduce the risk of this common and potentially preventable malignancy. Currently, in Russia, only a limited number of vaccination programs were initiated for females 12–13 years old. Such programs are in place in the Moscow Region and Moscow City since 2009 and were recently expanded to include the following cities: Ekaterinburg, Perm, Smolensk, Tyumen, Novosibirsk, Tomsk, and Kemerovo.26 Additionally, out of 80, the following Federal Subject regions have also been added: Khanty-Mansiysk Okrug, Primorski Krai, Sakha Republic, and Sakhalin.26
Several factors which could account for the current observed regional rates include smoking, alcohol consumption, and STI rates. As data on HPV infection rates are not available, the rate of STIs such as syphilis and gonorrhea, which could serve as surrogate markers for HPV transmission, correlated with cervical cancer incidence and mortality, as expected27, 28, 29, 30 Given there may be inaccuracies in data reporting and patients could be affected by both syphilis and gonorrhea as well as either infection alone, we considered both infections in the analyses. The study by Menezes et al. in 2019 found a coinfection of up to 47% of HPV and other STIs, such as herpes simplex virus and/or chlamydia in young women from South Africa.31 The presence of STIs increases the risk of acquiring HPV which could be attributed to increased risk of sexual practices with multiple partners.32,33 Co-occurring STIs also increase the persistence of high-risk HPV subtypes as well as cervical cancer development.34,35,27, 28, 29 Thus, as part of cervical cancer prevention, it is important to screen for and treat other STIs early. High-incidence cervical cancer regions in the western part of Russia included Republic of Karelia (30.82 cases/100,000), Kaliningrad Oblast (23.51/100,000), Pskov Oblast (28.17/100,000), Novgorod Oblast (23.69/100,000), Smolensk Oblast (22.98/100,000), Tver Oblast (23.63/100,000), and Vologda Oblast (24.57/100,000 females). These are the regions that mostly follow a western lifestyle, where people have several sexual partners, facilitating HPV transmission.36 Some of the lowest incidence rates of cervical cancer in the country were seen in the Republic of Dagestan, Tyumen Oblast, Chuvash Republic, and the Republic of Ingushetia, which with exception of Tyumen, correspond to Muslim/Chuvash regions where there are, perhaps, more conservative sexual practices, and therefore, lower risk for HPV acquisition.23
Objective data for TB prevalence was available and was used as a possible surrogate marker for poor general health status. In our study TB rates correlated with cervical cancer incidence in the univariate analyses and mortality in both univariate and multivariate analyses. However, this has not been well described in the literature. One study in Botswana found that prior TB disease was prevalent in patients with cervical cancer and those infected with HIV.37 The increased rate of TB may reflect the general poor health status of individuals in higher risk communities (in our case jurisdictions) and correspond with increasing cervical cancer rates, although it is possible that both TB and cervical cancer pathogenesis is impacted by the HIV due to immunosuppression. Notably, a recent case-control study showed that patients with TB have an elevated cancer risk of cervical cancer, possibly due to chronic inflammation, but the etiopathogenesis has not been fully elucidated.38 Hence, the association between TB and cervical cancer requires further investigation.
Importantly, one of the main factors identified in this study was smoking. We have observed strong positive association of daily smoking with both incidence and mortality of cervical cancer in univariate and the multivariate analyses. Similarly, negative association with no smoking was observed, confirming the protective effect of not smoking. A meta-analysis evaluating impact of smoking on cervical cancer risk established that the relative risk for individuals, who had ever smoked compared to never smokers was 2.03, and some studies showed a dose-dependent relationship.39 In fact, smoking is designated by the International Agency for Research on Cancer (IARC) as having adequate evidence for a relationship with cervical SCC.40,41 In the Russian Federation a prominent increase in smoking rates in females during 1992–2003 was observed, and age‐adjusted prevalence of smoking more than doubled from 6.9% to 14.8% (p<0.001) during that time.41 This increase was significant for all age groups except those ≥65 years.42 Thus, smoking cessation should serve as a key component of public health efforts to help decrease rates for cervical and other cancers.
The role of alcohol consumption in cervical cancer pathogenesis remains largely unknown, with conflicting results reported in the literature,43,44 hence we included this variable for further assessment. Alcohol use, alcohol induced psychosis and dependency in the affected jurisdictions were generally associated with increased cervical cancer incidence in our study. Notably, alcohol consumption has previously been associated with Cervical Intraepithelial Neoplasia (CIN)1 amongst HPV-positive Korean women, though the relationship was not reproduced for CIN2/3 disease or cervical cancer.45 The patients who consume alcohol may have increased HPV infection rates given lifestyle related reasons such as having higher number of sexual partners.44 Despite select registry-based studies44 showing an increased risk of cervical cancer pathogenesis in women who consume alcohol, there is paucity of population-based data confirming such findings.43 Provided that Russia has significant alcohol consumption rates per capita, it is particularly interesting to observe the statistically significant association of alcohol consumption and cervical cancer incidence.
We observed a correlation for jurisdictions between incidence of oropharyngeal cancer in females and cervical cancer. This could perhaps be attributed to the spread of HPV between sexual partners. A prior systematic review and meta-analysis found an increased risk of a second HPV-associated malignancy following identification and treatment of the first.46 In fact, recent evidence suggests that the global burden of HPV-related oropharyngeal cancers is surpassing that of cervical cancer in countries where cervical cancer has been largely controlled.47 Our findings suggest that after identification of the initial malignancy, regular screening should be considered to ensure detection of any additional possible HPV-related cancers.
Analysis of geographic trends showed that mortality in the southern and eastern areas of the country maintained high rates, whereas the western part of Russia had significantly lower mortality rates (Figure 2B, Supplementary Table 1). This could be explained by increased knowledge about HPV and its consequences, more people taking advantage of the opportunistic screening and due to better access to care and management of early detected cervical lesions.
A review paper found that despite over half of the world's countries having HPV vaccination programs in 2019, the global full-dose coverage for eligible girls was only 15%.48 This observation was hypothesized to be due to the fact that 70% of girls globally live in some of the world's most populous countries without national HPV programs, including Russia.
In terms of secondary prevention, cervical cancer/CIN screening in Russia occurs on an opportunistic basis. Only 20–25% of women undergo regular screening with a pap smear.49 This indicates the absence of preventive examinations for the population and the inadequacy of screening measures despite our modern medical capabilities. Low vaccination rates together with opportunistic screening programs can account for the high burden of cervical cancer in Russia.
Notably, Magadan Oblast, displayed the third highest average ASIR of 42.5 cases/100,000 females (2.71-fold greater than national ASIR rate) along with Karelia Republic ranked 7th and Chukotka ranked with the 9th highest incidence rates of >30.4/100,000. These regions belong to the circumpolar Inuit group.50 While the study period of interest was earlier (2000–2009) and a mild decreasing trend in cervical cancer was observed, it is important to note that the general trend towards increasing cancer risk among circumpolar populations calls for effective preventative strategies to be implemented to reduce the public health impact of cancer in these regions.50
Similar to the results of our study, another report observed a high incidence rate of all cancers in the Siberian and Far Eastern Federal districts between 2005 and 2018.51 It also found that the age of diagnosis decreased for both sexes and percent of increase in the ASIR rate was higher in females than in males.
In conclusion, identified regions in Russia with high cervical cancer rates defined in this study should be prioritized for HPV vaccination and screening programs. Additional populations to consider include circumpolar groups as they have been shown to have higher rates of cervical cancer while at times having limited access to healthcare resources. Moreover, screening should include close follow up for possible occurrence of another HPV-related malignancy. Importantly, smoking cessation remains a key risk factor for this disease and requires concerted effort to address. Russia has the economic potential to mobilise resources required to implement the WHO objectives regarding HPV vaccination and organised screening. It is regrettable that due ongoing conflict significant resources are lost which could have been used to achieve these goals. We call on the Russian scientific community to mobilize and to focus on improving health.
This was a retrospective study based on open-source government reports for the Russian Federation population during 2007–2018. The limitations of this approach include missed cases, not reported in the databases, or incorrectly classified.52 However, the use of large populational databases allows analysis of large sets of data, assessment of geographic patterns across the country and evaluation multiple risk factors across jurisdictions. Data quality control procedures and verification of completeness are in place at every stage of information compilation. The automated data verification procedure prevents the erroneous re-entry of patient information. The method for recording new cases in the cancer registry system meets the requirements of the IARC for collecting and analyzing data on the population and hence, allows the Russian Federation to obtain reliable information. Information about the location of the cancer (e.g., exo vs. endocervix), subtype, and treatment approaches were not available for the study. This is a population-based study and hence, the detailed above risk factors do not belong to each individual patient but rather to the population residing in each jurisdiction. Given this, only association can be interpreted, and causation cannot be established. Another limitation is that the available risk factors pertained to the population as a whole (males and females combined), while cervical cancer can only affect the female population, hence this could explain the weak associations observed between risk factors and cervical cancer incidence.
We believe that the results presented for cervical cancer in the Russian Federation would be valuable for policy advocacy in that country, in Eastern Europe and beyond. This work provides further evidence for the urgent need for inclusion of the HPV vaccination in national immunization programs, as well as the need for patient education in high incidence areas.
Contributors
Anastasiya Muntyanu, Vladimir Nechaev, Andrei Zubarev, Elena Netchiporouk, and Ivan V. Litvinov designed the study. Anastasiya Muntyanu, Elena Pastukhova, James Logan, Andrei Zubarev, Elham Rahme and Vladimir Nechaev obtained and analyzed the data. Elham Rahme provided guidance about statistical analyses. Anastasiya Muntyanu, Andrei Zubarev, Vladimir Nechaev and Elham Rahme have verified the underlying data; Anastasiya Muntyanu prepared the figures and wrote the manuscript. All the authors reviewed and edited the final manuscript. Ivan V. Litvinov supervised the study. Ivan V. Litvinov obtained funding for the study.
Data sharing statement
Data for analysis was derived from public domain resources which can be accessible online.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
No conflicts of interest to declare.
Acknowledgments
Not applicable.
Funding
None.
Footnotes
Key message: This work identifies regions in the Russian Federation disproportionately affected by the cervical cancer incidence and mortality including circumpolar communities, where HPV vaccination and cervical cancer screening as well as assessment for other HPV-driven cancers and smoking cessation programs should be prioritised.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2022.100454.
Appendix. Supplementary materials
References
- 1.Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Cervical cancer elimination initiative. 2020. https://www.who.int/initiatives/cervical-cancer-elimination-initiative.
- 3.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochr Database Syst Rev. 2018;5 doi: 10.1002/14651858.CD009069.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drolet M, Benard E, Perez N, Brisson M, Group HPVVIS. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394(10197):497–509. doi: 10.1016/S0140-6736(19)30298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383(14):1340–1348. doi: 10.1056/NEJMoa1917338. [DOI] [PubMed] [Google Scholar]
- 8.Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics. 2016;137(3) doi: 10.1542/peds.2015-1968. [DOI] [PubMed] [Google Scholar]
- 9.Abbas KM, van Zandvoort K, Brisson M, Jit M. Effects of updated demography, disability weights, and cervical cancer burden on estimates of human papillomavirus vaccination impact at the global, regional, and national levels: a PRIME modelling study. Lancet Glob Health. 2020;8(4):e536–e544. doi: 10.1016/S2214-109X(20)30022-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruni L, Diaz M, Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453–e463. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 11.Arbyn M, Antoine J, Magi M, et al. Trends in cervical cancer incidence and mortality in the Baltic countries, Bulgaria and Romania. Int J Cancer. 2011;128(8):1899–1907. doi: 10.1002/ijc.25525. [DOI] [PubMed] [Google Scholar]
- 12.Arbyn M, Primic-Zakelj M, Raifu AO, et al. The burden of cervical cancer in south-east Europe at the beginning of the 21st century. Coll Antropol. 2007;31(suppl 2):7–10. [PubMed] [Google Scholar]
- 13.Major milestone reached as 100 countries have introduced HPV vaccine into national schedule. 2019.https://www.who.int/news/item/31-10-2019-major-milestone-reached-as-100-countries-have-introduced-hpv-vaccine-into-national-schedule.
- 14.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Kaprina AD, Starinsky VV, Petrova GV. Malignant neoplasma in Russia in 2017 (morbidity and mortality). Russia, Moscow: P.A. Herzen branch of the FGBU “NMITS of radiology” of the Ministry of Health; 2018.
- 16.Petrova GV, Kaprina AD, Gretsova OP, Starinsky VV. P.A. Herzen branch of the FSBI “NMIRTS” of the Ministry of Health Russia; Moscow: 2015. Malignant Neoplasms in Russia Review of Statistical Information for 1993–2013. [Google Scholar]
- 17.VS AK, Gretsova O, Petrova G, et al. Population-based registry of cancer patients in the Russian Federation. Public Health Panorama. 2019;5(1):95–98. [Google Scholar]
- 18.Spiegelman M. Introduction to demography, Revised Edition - Formula 4.29; 1969.
- 19.Ghazawi FM, Cyr J, Darwich R, et al. Cutaneous malignant melanoma incidence and mortality trends in Canada: a comprehensive population-based study. J Am Acad Dermatol. 2019;80(2):448–459. doi: 10.1016/j.jaad.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 20.Conte S, Ghazawi FM, Le M, et al. Population-based study detailing cutaneous melanoma incidence and mortality trends in Canada. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.830254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muntyanu A, Savin E, Ghazawi FM, Alakel A, Zubarev A, Litvinov IV. Geographic variations in cutaneous melanoma distribution in the Russian Federation. Dermatology. 2020;236(6):500–507. doi: 10.1159/000507617. [DOI] [PubMed] [Google Scholar]
- 22.Muntyanu A, Ghazawi FM, Nedjar H, et al. Non-melanoma skin cancer distribution in the Russian Federation. Dermatology. 2021;237(6):1007–1015. doi: 10.1159/000512454. [DOI] [PubMed] [Google Scholar]
- 23.Extension of the All-Russian Population Census (Всероссийской переписи населения 2010) and in collaboration with the Russian Ministry of Justice (Минюста РФ). 2010.
- 24.Petrova GV, Gretsova OP, Kaprina AD, Starinsky VV. Characteristics and methods of calculating medical and statistical indicators used in oncology. Moscow: FGBU MNII P.A. Herzen of the Ministry of Health of the Russian Federation; 2014.
- 25.GOST 7.67-2003. System of standards on information LAPCNCFT.http://protect.gost.ru/v.aspx?control=8&baseC=-1&page=0&month=-1&year=-1&search=&RegNum=1&DocOnPageCount=15&id=121716&pageK=E38E821F-AB20-4B3B-9445-4E14301C2C07. Accessed 21 August 2019.
- 26.Bruni L AG, Serrano B, Mena M, et al. Human papillomavirus and related diseases in Russian Federation. Summary Report 17 June 2019. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). 2019.
- 27.Paba P, Bonifacio D, Di Bonito L, et al. Co-expression of HSV2 and Chlamydia trachomatis in HPV-positive cervical cancer and cervical intraepithelial neoplasia lesions is associated with aberrations in key intracellular pathways. Intervirology. 2008;51(4):230–234. doi: 10.1159/000156481. [DOI] [PubMed] [Google Scholar]
- 28.Smith JS, Herrero R, Bosetti C, et al. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J Natl Cancer Inst. 2002;94(21):1604–1613. doi: 10.1093/jnci/94.21.1604. [DOI] [PubMed] [Google Scholar]
- 29.Silva J, Cerqueira F, Medeiros R. Chlamydia trachomatis infection: implications for HPV status and cervical cancer. Arch Gynecol Obstet. 2014;289(4):715–723. doi: 10.1007/s00404-013-3122-3. [DOI] [PubMed] [Google Scholar]
- 30.Jha PK, Beral V, Peto J, et al. Antibodies to human papillomavirus and to other genital infectious agents and invasive cervical cancer risk. Lancet (London, England) 1993;341(8853):1116–1118. doi: 10.1016/0140-6736(93)93128-n. [DOI] [PubMed] [Google Scholar]
- 31.Menezes LJ, Pokharel U, Sudenga SL, et al. Patterns of prevalent HPV and STI co-infections and associated factors among HIV-negative young Western Cape, South African women: the EVRI trial. Sex Transm Infect. 2018;94(1):55–61. doi: 10.1136/sextrans-2016-053046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bray F, Loos AH, McCarron P, et al. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev. 2005;14(3):677–686. doi: 10.1158/1055-9965.EPI-04-0569. [DOI] [PubMed] [Google Scholar]
- 33.Vaccarella S, Franceschi S, Herrero R, et al. Sexual behavior, condom use, and human papillomavirus: pooled analysis of the IARC human papillomavirus prevalence surveys. Cancer Epidemiol Biomarkers Prev. 2006;15(2):326–333. doi: 10.1158/1055-9965.EPI-05-0577. [DOI] [PubMed] [Google Scholar]
- 34.Xie L, Li Q, Dong X, et al. Investigation of the association between ten pathogens causing sexually transmitted diseases and high-risk human papilloma virus infection in Shanghai. Mol Clin Oncol. 2021;15(1):132. doi: 10.3892/mco.2021.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson AM, Mercer CH, Beddows S, et al. Epidemiology of, and behavioural risk factors for, sexually transmitted human papillomavirus infection in men and women in Britain. Sex Transm Infect. 2012;88(3):212. doi: 10.1136/sextrans-2011-050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wellings K, Collumbien M, Slaymaker E, et al. Sexual behaviour in context: a global perspective. Lancet. 2006;368(9548):1706–1728. doi: 10.1016/S0140-6736(06)69479-8. [DOI] [PubMed] [Google Scholar]
- 37.Zetola NM, Grover S, Modongo C, et al. Collision of three pandemics: the coexistence of cervical cancer, HIV infection, and prior tuberculosis in the Sub-Saharan Country of Botswana. J Glob Oncol. 2016;2(1):47–50. doi: 10.1200/JGO.2015.001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen GL, Guo L, Yang S, Ji DM. Cancer risk in tuberculosis patients in a high endemic area. BMC Cancer. 2021;21(1):679. doi: 10.1186/s12885-021-08391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugawara Y, Tsuji I, Mizoue T, et al. Cigarette smoking and cervical cancer risk: an evaluation based on a systematic review and meta-analysis among Japanese women. Jpn J Clin Oncol. 2018;49(1):77–86. doi: 10.1093/jjco/hyy158. [DOI] [PubMed] [Google Scholar]
- 40.Secretan B, Straif K, Baan R, et al. A review of human carcinogens—Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10(11):1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 41.Cogliano VJ, Baan R, Straif K, et al. Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;103(24):1827–1839. doi: 10.1093/jnci/djr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perlman F, Bobak M, Gilmore A, McKee M. Trends in the prevalence of smoking in Russia during the transition to a market economy. Tob Control. 2007;16(5):299–305. doi: 10.1136/tc.2006.019455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hjartaker A, Meo MS, Weiderpass E. Alcohol and gynecological cancers: an overview. Eur J Cancer Prev. 2010;19(1):1–10. doi: 10.1097/CEJ.0b013e328333fb3a. [DOI] [PubMed] [Google Scholar]
- 44.Weiderpass E, Ye W, Tamimi R, et al. Alcoholism and risk for cancer of the cervix uteri, vagina, and vulva. Cancer Epidemiol Biomarkers Prev. 2001;10(8):899–901. [PubMed] [Google Scholar]
- 45.Min KJ, Lee JK, Lee S, Kim MK. Alcohol consumption and viral load are synergistically associated with CIN1. PLoS One. 2013;8(8):e72142. doi: 10.1371/journal.pone.0072142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilbert DC, Wakeham K, Langley RE, Vale CL. Increased risk of second cancers at sites associated with HPV after a prior HPV-associated malignancy, a systematic review and meta-analysis. Br J Cancer. 2019;120(2):256–268. doi: 10.1038/s41416-018-0273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gougousis S, Mouchtaropoulou E, Besli I, Vrochidis P, Skoumpas I, Constantinidis I. HPV-related oropharyngeal cancer and biomarkers based on epigenetics and microbiome profile. Front Cell Dev Biol. 2021;8 doi: 10.3389/fcell.2020.625330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruni L, Saura-Lazaro A, Montoliu A, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. 2021;144 doi: 10.1016/j.ypmed.2020.106399. [DOI] [PubMed] [Google Scholar]
- 49.Rogovskaya SI, Shabalova IP, Mikheeva IV, et al. Human papillomavirus prevalence and type-distribution, cervical cancer screening practices and current status of vaccination implementation in Russian Federation, the Western countries of the former Soviet Union, Caucasus region and Central Asia. Vaccine. 2013;31(suppl 7):H46–H58. doi: 10.1016/j.vaccine.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 50.Young TK, Kelly JJ, Friborg J, Soininen L, Wong KO. Cancer among circumpolar populations: an emerging public health concern. Int J Circumpolar Health. 2016;75 doi: 10.3402/ijch.v75.29787. 29787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cancer incidence in Siberia and Russian Far East. SIBIRSKIJ ONKOLOGIČESKIJ ŽURNAL. 2020;18(6):5–11. [Google Scholar]
- 52.Sanders CM, Saltzstein SL, Schultzel MM, Nguyen DH, Stafford HS, Sadler GR. Understanding the limits of large datasets. J Cancer Educ. 2012;27(4):664–669. doi: 10.1007/s13187-012-0383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.