Abstract
Objective:
To test the effect of a personalized music intervention on agitated behaviors and medication use among long-stay nursing home residents with dementia.
Design:
Pragmatic, cluster-randomized controlled trial of a personalized music intervention. Staff in intervention facilities identified residents’ early music preferences and offered music at early signs of agitation or when disruptive behaviors typically occur. Usual care in control facilities may include ambient or group music.
Setting and participants:
The study was conducted between June, 2019 and February, 2020 at 54 nursing homes (27 intervention and 27 control) in 10 states owned by 4 corporations.
Methods:
Four-month outcomes were measured for each resident. The primary outcome was frequency of agitated behaviors using the Cohen-Mansfield Agitation Inventory. Secondary outcomes included frequency of agitated behaviors reported in the Minimum Data Set, and the proportion of residents using antipsychotic, antidepressant, or anti-anxiety medications.
Results:
The study included 976 residents with dementia (483 treatment and 493 control; mean age = 80.3 (12.3), 69% female, 25% African American). CMAI scores were not significantly different (treatment: 50.67; standard error, SE, 1.94 vs control: 49.34; SE 1.68) (average marginal effect, AME, 1.33; SE, 1.38; 95% CI, −1.37 to 4.03). MDS based behavior scores were also not significantly different (treatment: 0.35; SE 0.13 vs control: 0.46; SE 0.11) (AME, −0.11; SE, 0.10; 95% CI, −0.30 to 0.08). Fewer residents in intervention facilities used antipsychotics in the past week compared to controls (treatment: 26.2; SE 1.4 vs control: 29.6; SE 1.3) (AME, −3.61; SE, 1.85; 95% CI, −7.22 to 0.00) but neither this nor other measures of psychotropic drug use were statistically significant.
Conclusions and Implications:
Personalized music was not significantly effective in reducing agitated behaviors nor psychotropic drug use among long-stay residents with dementia. Barriers to full implementation included engaging frontline nursing staff and identifying resident preferred music.
Keywords: Pragmatic randomized controlled trial, Neuropsychiatric symptoms, Dementia, Nursing Homes, Agitated Behaviors, Antipsychotics
Brief Summary:
In this real-world trial conducted in 54 nursing homes with 976 residents with dementia, personalized music did not affect the frequency of agitated behaviors or receipt of psychotropic medications.
INTRODUCTION
Agitated and/or aggressive behaviors (behaviors) are one of the most common neuropsychiatric symptoms of Alzheimer’s disease and related dementias (ADRD).1 These behaviors decrease quality of life for residents living with ADRD, and are a significant source of stress for NH staff2 and other residents.3 Antipsychotic medications traditionally used to manage behaviors increase the risk of falls4 and death in people with ADRD.5 Other medication classes which are used as substitutes for antipsychotic medications, including mood stabilizers,6 also increase the risk of adverse events in this population.7 Unfortunately, there continues to be a lack of effectiveness evidence for most nonpharmaceutical alternatives for mitigating the effects of resident behaviors.8
Music & Memory® (M&M) is a potential nonpharmaceutical alternative for managing behaviors in NH residents with ADRD. In the M&M program, music a resident preferred when she or he was young is administered by NH staff at early signs of agitation or at times of day when behaviors are likely.9 It is hypothesized that musical memories are retained into later stages of ADRD,10 and, thus, early preferred music may elicit autobiographical memories11, 12 and evoke a relaxation response.13, 14 While the M&M program focuses on music residents preferred when they were young adults, songs attached to strong memories later in life may also elicit the desired reminiscence response. More research is needed to determine whether the timing of the acquisition of the musical memories affects retrieval in later stage dementia.
To date, most of the evidence for M&M is based on observational studies. A pre-post evaluation of a large M&M demonstration in California (4,107 residents from 265 NHs) found decreases in behaviors, antipsychotic use, antianxietal, and antidepressant use after residents were exposed to the M&M program.15 Decreases in behaviors, antipsychotic use, and antianxietal use were also found in a quasi-experimental study of residents with ADRD from 98 NHs which became certified in the M&M program, compared to pair-matched controls.16 However, the largest randomized trial of M&M to date (59 residents from 10 NHs) found no differences in agitation or medication use in residents with ADRD exposed to the intervention compared to usual care controls.17 Several weaknesses of this trial include small sample size and insufficient adherence to the intervention.17
In this report, we present the results from a cluster-randomized, embedded pragmatic trial (ePCT) of the M&M intervention on the frequency of agitated behaviors (primary study outcome) and medication use (pre-specified secondary study outcome) in 976 residents with ADRD from 54 NHs randomized to receive the intervention (27 NHs) or usual care (27 NHs).
METHODS
Ethics
The University’s institutional review board approved the study’s conduct with a waiver of informed consent because this was a minimal-risk trial (Clinicaltrials.gov Identifier: NCT03821844).
Facilities and Randomization
This ePCT was conducted in 54 NHs (27 treatment, 27 control) owned by four NH corporations which differed by size, ownership status, geographic location, and racial composition. Potentially eligible NHs had at least 20 residents who: had a ADRD diagnosis; had been in the NH at least 90 of the last 100 days; and, were not completely deaf. Corporate leadership also removed from consideration NHs with recent poor inspections, major leadership changes, or other significant competing demands that may negatively affect implementation. The pool of potentially eligible NHs was further restricted by geography to allow for corporate leadership and data collectors’ travel to the sites. There were 44 potentially eligible NHs contacted for corporation A, 15 for corporation B, 19 for corporation C, and 55 for corporation D. NHs were enrolled based on timing of reply until capacity was reached. NHs were allowed to opt-in and were enrolled based on when they returned their letters of commitment until capacity for each corporation was reached (corporation A - 16 NHs, corporation B – 8 NHs, corporation C – 10 NHs, corporation D - 20 NHs). Capacity was determined by corporation size.
Within each corporation, NHs were partitioned based on the Mahalanobis distance from the overall mean18 on the percentage of eligible residents with any agitated or aggressive behavior and the number of eligible residents within a NH. Within balanced twins, one NH was randomly chosen for each arm. Recruitment and randomization were completed in February, 2019.
Participants
Residents were enrolled in June, 2019 or October, 2019, with 4-month follow-up for each resident completed by February 29, 2020. At each enrollment period, NH staff in treatment and control NHs were asked to choose 12 residents with ADRD who were long-stay (90 of the last 100 days spent in the NH) and were not completely deaf. NHs were further given guidance to choose residents with behaviors that might be partially managed by the intervention, and to first choose residents who liked music to provide some early successes introducing the program. We did not specify which behaviors, or what intensity of behaviors, were best suited for the program, as there is no evidence to support this degree of specificity. However, several case examples were highlighted during the in-person training session including: using music to distract residents during ADL care, or using music to help manage repetitive behaviors (e.g., pacing, asking the same question over and over again) that occur at specific times of day or increase in frequency during the late afternoon or early evening hours. Residents who died or were discharged before their 4-month follow-up was complete were excluded from the analytic sample.
Intervention, Training, and Fidelity
In M&M, the music a resident preferred when s/he was a young adult is loaded onto a personal music device (e.g., a MP3 player) and used by NH staff to preempt or reduce agitation.9 Headphones or small speakers are used to deliver the music. NH staff were instructed to use the music at times of day when behaviors were likely or at early signs of agitation. Beyond this guidance, concrete examples of early signs of agitation were not provided. Instead, clinical judgement facilitated determination of appropriate timing of use. The recommended dose was 30 minutes a day. The control condition is usual care, which may include use of ambient or group music.
Each corporation provided a corporate manager to oversee the intervention and each NH provided a program champion. NH champions were typically life enrichment or social services staff. Treatment NHs received two types of training: standard M&M certification (two 1.5 hour webinars); and a half day, in-person training administered jointly by corporate leadership and study consultants. The administrator, director of nursing, activities director, nurse manager, and a certified nursing assistant were required to attend the in-person training.
To monitor fidelity, NH champions were asked to complete a form the first time the music was used with each resident. This form detailed the reason the resident was chosen for the program, how the resident’s preferred music was identified, and the resident’s initial reactions to the music. Importantly, the form also included the date the music was first used with the resident. By combining the first use date with the data downloaded from the individual music players, we were able to approximate a dose per exposed day. Additional steps taken to further enhance fidelity included: corporate managers and project consultants met monthly with NH champions to assess progress and troubleshoot; and corporate managers visited facilities to investigate reasons for nonadherence and motivate engagement.
Data Sources and Baseline Variables
Primary and secondary data sources were used to capture study baseline and outcome measures. Secondary data sources included the Minimum Data Set (MDS), derived from routine, standardized assessments of residents done by facility nurses. Primary data were obtained by trained research staff who interviewed nursing staff about enrolled residents’ behaviors using the Cohen Mansfield Agitation Inventory (CMAI).19 The CMAI has been widely used in the NH setting and has high reported interrater reliabilities (0.88 to 0.93).19, 20
Baseline characteristics of the residents were ascertained from the first MDS assessment occurring up to 90 days before or 30 days after their baseline CMAI measurement by research staff. Demographic data included age, sex, and race / ethnicity. Functional status was quantified using the MDS Activities of Daily Living scale (range, 0–28, where 28 indicates total functional dependence and 0 indicates no functional dependence).21 Cognitive status was assessed using the MDS 3.0 Cognitive Function Scale (range, 0–4, with higher scores indicating more impairment).22 Mortality risk was measured using the MDS 3.0 Changes in Health, End-Stage Disease and Symptoms and Signs Scale (CHESS) (range, 0–5, where higher scores indicate higher mortality risk).23 An ADRD diagnosis was defined as an active diagnosis of Alzheimer’s disease or non-Alzheimer’s dementia as indicated by checkbox or ICD-10 code (MDS, Section I).
Outcomes
The primary study outcome was the CMAI total agitation score at the end of 4-months of follow-up. For each eligible resident, the CMAI was administered at baseline and 4-months. The 29 items describe aggressive behaviors, physically non-aggressive behaviors, verbally agitated behaviors, and hiding and hoarding behaviors occurring in the past two weeks.24 To complete the CMAI, trained data collectors interviewed a nursing staff member who knew the resident well; all interviews were conducted in-person. Response options for each CMAI item range from never (1) to several times per hour (7). The total CMAI score ranges from 29 to 203.
Secondary study outcomes included agitated behaviors, antipsychotic, antidepressant, anxiolytic, and hypnotic medication use, as reported in the MDS. The MDS has four items describing the frequency of: physical behavioral symptoms directed toward others; verbal behavioral symptoms directed toward others; other behavioral symptoms not directed toward others; and behaviors related to resisting necessary care.25 Frequency in the past week is reported as: behavior not exhibited; behavior occurred 1–3 days; behavior occurred 4–6 days; or behavior occurred daily. These four behavioral frequency items in the MDS are summed to create the Reactive Behavior Scale (ARBS).26 The total ARBS ranges from 0 to 12. The MDS also reports the number of days out of the last seven in which a resident separately received an antipsychotic, antidepressant, anxiolytic, or hypnotic medication. We dichotomized this variable as any or no medication use in the past week.
Masking
The study principal investigator was blinded to the identity of the control and intervention NHs.
Statistical Power and Sample Size
Assuming a two sided alpha-level of 0.05 and a conservative intraclass correlation coefficient (ICC) of 0.1,27 to achieve power of at least 90% in testing for a 6 point reduction in the total CMAI score, 24 NHs per study arm were required. To address possibly higher ICC values and non-participation, 27 NHs per study arm were recruited.
Statistical analysis
Our primary intention to treat (ITT) analysis is based on the model described by Murray & Blistein (2003)28 and Teerenstra et al (2012).29 All analyses were done at the resident level. Multilevel regression with covariates’ adjustments was used to estimate impact of the resident being in a treatment versus control NH on agitated behaviors and medication outcomes. Clustering at the facility-level was addressed using hierarchical models. Resident characteristics that differed between residents in each arm at the p≤.10 level were included in the adjusted model as covariates. To adjust for differences in baseline CMAI total agitation and ARBS scores between the two arms, we report the marginal time by intervention interaction (marginal interaction effect, MIE). This estimand is sometimes referred to as the Difference-in-Differences estimand. Formally, this interaction translates to the difference between the difference in average CMAI score at 4-months follow-up and CMAI score at baseline for the intervention arm, and the difference between 4-months follow-up and CMAI score at baseline for the control arm. Because medication use is recorded as a binary indicator, we report the average difference between the two arms after adjustment for baseline medication use (average marginal effect, AME). To compare outcomes between arms, we report the marginal averages at follow-up for each arm, and either MIE or AME with corresponding standard errors (SEs) and 95% confidence intervals (CIs).
RESULTS
A total of 2,416 long-stay residents with ADRD (1,209 treatment, 1,207 control) from the 54 enrolled NHs met basic eligibility criteria (Figure 1). From these potentially eligible residents, staff in treatment and control NHs chose 1,070 residents (532 treatment, 538 control) with behaviors to be enrolled in the intervention. In both the treatment and control conditions, 9% of the enrolled sample was excluded because they died or were discharged before their 4-month follow-up visit. The final analytic sample includes 976 residents (483 treatment, 493 control).
Figure 1.
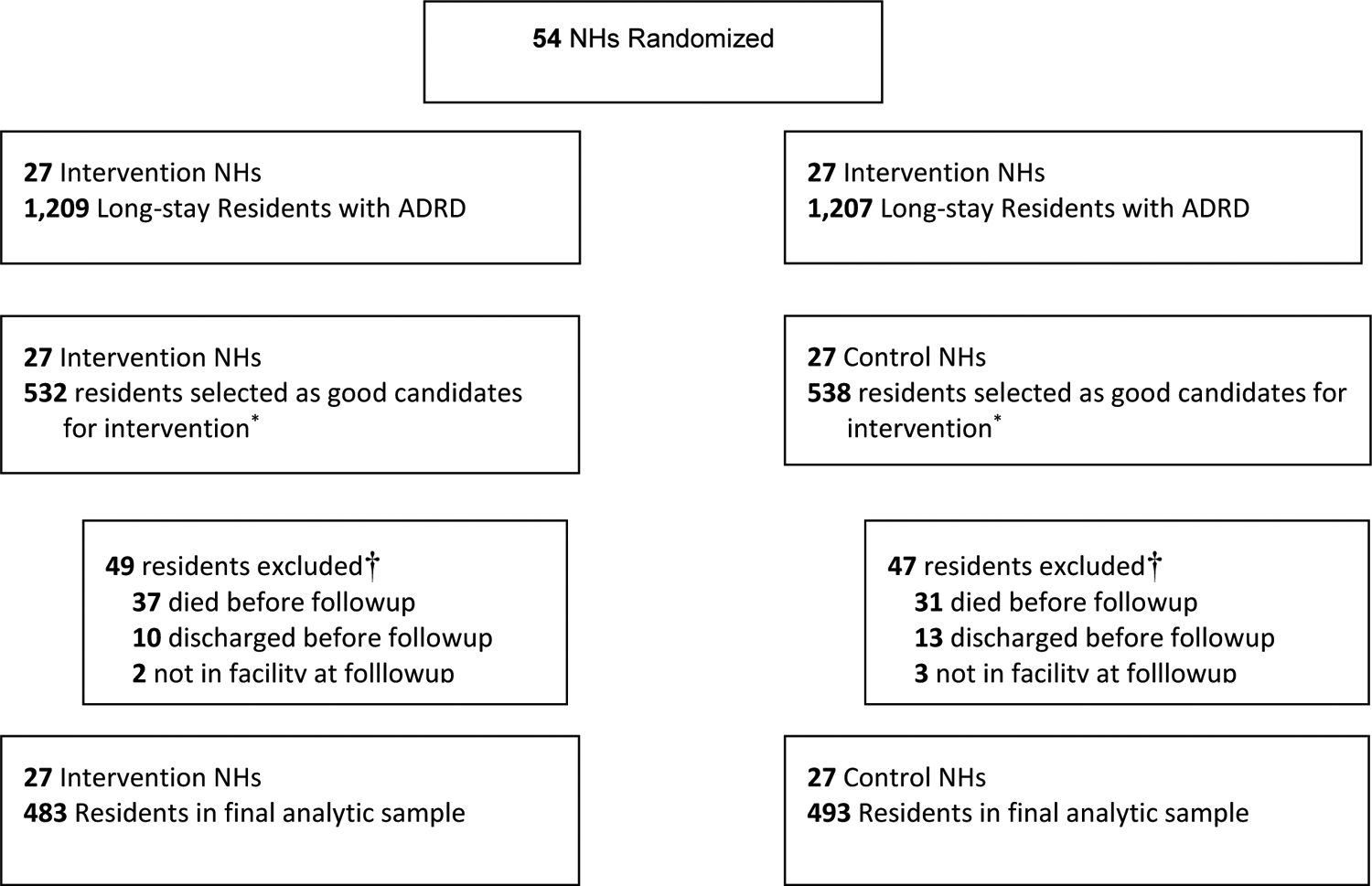
CONSORT diagram of METRIcAL nursing homes and residents
*Intervention and control sites were instructed to select residents with dementia, who were not completely deaf, who exhibited behaviors, and who enjoyed music
†Death and discharge status based on Minimum Data Set. Discharges include discharge to hospital when return not expected.
At baseline, treatment and control groups were similar with respect to age (treatment: 79.8; standard deviation, SD, 12.2 vs. control: 80.8; SD, 12.4), percent female (treatment: 67.7%; control: 70.8%) and percent Black (treatment: 25.7% vs. control: 24.3%) (Table 1). Treatment and control groups were also similar on the percent of residents with an ADRD diagnosis in the MDS 3.0 (81.6% vs. 78.3% respectively), and the percent of residents with moderate or severe ADRD (66.6% vs 65.5%). However, residents in the treatment group were more likely than residents in the control group to have severe ADL dependencies (treatment: 33.5%; control: 24.1%) and to manifest any agitated behaviors (treatment 23.4%; control 17.9%). Residents in the treatment group were less likely than controls to have any use of antipsychotics (treatment 25.3%; control 33.9%) at baseline. Our multilevel regression models adjusted for baseline differences at the resident level.
Table 1.
Characteristics of Enrolled Residents at Baseline
| Baseline characteristics | Total, n=976 | Treatment, n=483 | Control, n=493 | p-values |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD) | 80.3 (12.3) | 79.8 (12.2) | 80.8 (12.3) | 0.19 |
| Females, n (%) | 676 (69.3) | 327 (67.7) | 349 (70.8) | 0.30 |
| Race, n (%) | ||||
| White | 708 (72.8) | 345 (71.7) | 363 (73.9) | 0.54 |
| Black | 244 (25.1) | 124 (25.8) | 120 (24.4) | |
| Others | 20 (2.1) | 12 (2.5) | 8 (1.6) | |
| Missing values, n | 4 | 2 | 2 | |
| Function | ||||
| Activities of Daily Living, n (%) | ||||
| Severely impaired (≥21) | 281 (28.8) | 162 (33.5) | 119 (24.2) | <0.01 |
| Mild-moderately impaired (<21) | 694 (71.2) | 321 (66.5) | 373 (75.8) | |
| Missing values, n | 1 | 0 | 1 | |
| Cognitive function, n (%) | ||||
| Cognitively intact | 113 (11.6) | 52 (10.8) | 61 (12.4) | 0.82 |
| Mildly impaired | 206 (21.1) | 102 (21.1) | 104 (21.1) | |
| Moderately impaired | 509 (52.1) | 252 (52.2) | 257 (52.1) | |
| Severely impaired | 148 (15.2) | 77 (15.9) | 71 (14.4) | |
| CHESS Score, n (%) | ||||
| No health instability, 0 | 494 (52.6) | 240 (51.6) | 254 (53.6) | 0.26 |
| Minimal/low instability, 1–2 | 424 (45.2) | 211 (45.4) | 213 (44.9) | |
| Moderate/high instability, 3+ | 21 (2.2) | 14 (3.0) | 7 (1.5) | |
| Missing values, n | 37 | 18 | 19 | |
| Presence of comorbidities, n (%) | ||||
| Dementia | 780 (79.9) | 394 (81.6) | 386 (78.3) | 0.20 |
| Heart failure | 165 (16.9) | 74 (15.3) | 91 (18.5) | 0.19 |
| Hypertension | 781 (80.0) | 383 (79.3) | 398 (80.7) | 0.58 |
| Anxiety disorder | 394 (40.4) | 203 (42.0) | 191 (38.7) | 0.29 |
| Depression | 559 (57.3) | 264 (54.7) | 295 (59.8) | 0.10 |
| Bipolar disease | 61 (6.3) | 27 (5.6) | 34 (6.9) | 0.40 |
| Psychotic disorder | 123 (12.6) | 62 (12.8) | 61 (12.4) | 0.83 |
| Asthma / COPD | 134 (13.7) | 57 (11.8) | 77 (15.6) | 0.08 |
| Medication use, n (%) | ||||
| Any antipsychotics in past week | 289 (29.6) | 122 (25.3) | 167 (33.9) | <0.01 |
| Any antianxietals in past week | 207 (21.2) | 99 (20.5) | 108 (21.9) | 0.59 |
| Any antidepressants in past week | 573 (58.7) | 272 (56.3) | 301 (61.1) | 0.13 |
| Any hypnotics in past week | 10 (1.0) | - | - |
Abbreviations: CHESS, Changes in Health, End-Stage Disease and Symptoms and Signs Scale; COPD, chronic obstructive pulmonary disease; SD, standard deviation
Agitated Behaviors
There were no significant differences in behaviors, as measured by the CMAI total agitation score, between residents in the intervention and control arms after 4-months of follow-up (treatment: 50.67; standard error, SE, 1.94 vs control: 49.34; SE 1.68) (MIE, 1.33; SE, 1.38; 95% CI, −1.37 to 4.03) (Table 2). There were also no significant differences in behaviors, as measured by the ARBS score, between treatment and control groups (treatment: 0.35; SE 0.13 vs control: 0.46; SE 0.11) (MIE, −0.11; SE, 0.10; 95% CI, −0.30 to 0.08)
Table 2.
Agitated behaviors at baseline and at end of four-months follow-up among residents from intervention and control nursing homes
| Overall Baseline n=976 | Overall Follow-up n=976 | Intervention Baseline n=483 | Intervention Follow-up n=483 | Control Baseline n=493 | Control Follow-up n=493 | Marginal Interaction Effect (SE) [95% CI] | |
|---|---|---|---|---|---|---|---|
| Total CMAI score, Mean (SE) Source: Staff Interview | 50.16 (2.93) [44.14,55.91] | 49.17 (0.97) [47.27,51.06] | 50.64 (3.06) [44.65,56.63] | 50.31 (1.35) [47.66,52.97] | 49.70 (3.06) [43.71,55.69] | 48.04 (1.35) [45.40,50.68] | 1.33 (1.38) [−1.37,4.03] |
| Total ARBS score, Mean (SE) Source: Minimum Data Set | 0.45 (0.20) [0.06,0.84] | 0.41 (0.05) [0.31,0.52] | 0.52 (0.21) [0.12, 0.92] | 0.42 (0.08) [0.28,0.57] | 0.39 (0.21) [0.00,0.79] | 0.40 (0.08) [0.25,0.55] | −0.11 (0.1) [−0.30,0.08] |
Abbreviations: CMAI, Cohen-Mansfield Agitation Inventory; ARBS, Agitated and Reactive Behavior Scale; SE, standard error; MIE, marginal interaction effect – the marginal interaction of follow-up by intervention effect
Fixed effect covariates: age, sex, race, activities of daily living score, cognitive function score, psychotic disorder, bipolar disease, depression, anxiety, dementia, anti-depressant use, anti-anxietal use, antipsychotic use, nursing home corporation, time from MDS collection to CMAI score*, baseline ARBS*, baseline CMAI†, indicator of baseline/follow-up score, treatment group, interaction of baseline/follow-up measure and treatment group
Random effects: random intercept for nursing home, random intercept for the interaction of baseline/follow-up measure and nursing home, random intercept for data collector*, random intercept for individual *exclusive to CMAI model, †exclusive to ARBS and ARBS-CMAI model
Medication Use
After 4-months of follow-up, a smaller proportion of residents had any antipsychotic use in the past week in the intervention group compared to the control group, but this difference did not reach conventional levels of statistical significance (treatment: 26.2; SE 1.4 vs control: 29.6; SE 1.3) (AME, −3.61; SE, 1.85; 95% CI, −7.22 to −0.00) (Table 3). There were no significant difference between treatment and control groups in the proportion of residents with any antidepressant use in the past week (AME, −1.3; SE, 2.1; 95% CI, −5.28 to 2.76) or the proportion of resident with any anti-anxietal use in the past week (AME, −3.47; SE, 2.08; 95% CI, −7.55 to 0.06). Given the very low baseline use of hypnotics in the enrolled population (approximately 1%), we were not able to consider the effects of the intervention on this prespecified secondary trial outcome
Table 3.
Medication use at end of study follow-up among residents from intervention and control nursing homes
| Total, n=976 | Intervention, n=483 | Control, n=493 | AME (SE) [95% CI] | |
|---|---|---|---|---|
| Proportion of residents with any antipsychotic use in the past week, Mean(SE) | 28.1 (1.0) [26.2, 30.0] | 26.2 (1.4) [23.4, 29.0] | 29.6 (1.3) [27.2, 32.3] | −3.61 (1.85) [−7.22, 0.00] |
| Proportion of residents with any antidepressant use in the past week, Mean(SE) | 58.1 (1.1) [56.0, 60.3] | 57.5 (1.5) [54.6, 60.5] | 58.8 (1.5) [55.8, 61.7] | −1.26 (2.05) [−5.28, 2.76] |
| Proportion of residents with any antianxietal use in the past week, Mean(SE) | 22.6 (1.2) [20.2, 25.0] | 20.8 (1.5) [17.8, 23.8] | 24.3 (1.7) [20.9, 27.6] | −3.47 (2.08) [−7.55, 0.06] |
| Proportion of residents with any medication use in the past week, Mean(SE) | 67.1 (1.1) [65.0, 69.2] | 66.4 (1.5) [63.5, 69.3] | 67.8 (1.5) [64.9, 70.7] | −1.36 (2.02) [−5.33, 2.61] |
Abbreviations: SE, standard error; AME, average marginal effect
Variables included in the model: baseline medication use, age, sex, race, activities of daily living score, cognitive function score, psychotic diagnosis, depression, anxiety, dementia, asthma, Agitated and Reactive Behavior Scale, date of assessment, treatment group, and facility level random effect.
Intervention Fidelity
Based on the initial use forms and iPod metadata, 344 of the 483 residents (71%) with ADRD in the intervention group had their preferred music identified and the music player used at least once. Among those exposed, the median minutes of music per day exposed was 22.1 (SD: 27.6). The number of residents exposed per intervention NH ranged from 2 to 17 (mean, 12.7: SD, 3.6). Average time staff spent testing music with a resident to identify preferred songs was 1.8 hours (SD: 1.3).
DISCUSSION
In this intention-to-treat analysis of an ePCT which enrolled 976 residents from 54 NHs (27 treatment, 27 control), we find that agitated and/or aggressive behaviors were not significantly lower at 4-month follow-up among residents in NHs randomized to receive the intervention compared to residents in NHs randomized to receive usual care (primary study outcome). While residents in NHs randomized to receive the intervention had lower antipsychotic medication use compared to residents in NHs randomized to the usual care, this difference did not reach traditional levels of statistical significance. There were no differences in antidepressant or anti-anxiety medication use.
Our results contradict the findings of large observational studies,15, 16 and highlight the important contribution of large ePCTs to evaluating complex behavioral interventions implemented in NHs.30 NHs which independently adopt M&M, and other behavioral interventions, differ from NHs which do not in many ways including quality, staffing, and the capacity for organizational change.31, 32 Further, pragmatic trials enroll residents without necessarily involving proxies,33, 34, 35 further increasing generalizability.36 In this trial, we deliberately recruited racially and geographically representative NH corporations of high and low quality. This may partially explain the large variation in observed adherence. Future, as-treated analyses will attempt to elucidate for which types of residents and facilities, if any, the intervention was effective.
One of the major barriers to implementing M&M, and other non-pharmacological alternatives, is obtaining nursing and frontline staff “buy-in.”37 In general, nursing staff do not receive training on how to identify ADRD-related behaviors or to consider alternatives to medication management.38 While ADRD-specific training programs exist,39 most nursing staff are not certified in these techniques and understaffing and turnover limit the ability of NHs to provide time for training.40
Another major challenge to implementation is the amount of time spent identifying residents preferred music.37 To implement the M&M program using the standardized protocol, NH staff identify the music a resident preferred when s/he was a young adult.9 This is particularly difficult to accomplish for residents who are unable to communicate verbally and do not have a living relative to communicate preferences. Music identification becomes a trial-and-error process performed by activities staff or volunteers,41 who are perceived to “own” the intervention. Activities staff are not likely to use the intervention in response to behaviors, but, rather, during prescribed 1:1 activity therapy sessions or when staff happen to be on shift.
Our pragmatic trial also had limitations. First, although NHs were balanced on the percent of residents with agitated behaviors and antipsychotic use, and instructions for choosing eligible residents were standardized across treatment and control sites, intervention sites chose residents with more frequent behaviors. We attribute this selection bias to learning in the treatment arm, as NHs nominated residents at baseline and mid-intervention. To address this limitation, all analyses controlled for baseline differences in resident populations. Second, we faced challenges scaling our measurement strategy between the pilot26 and the full pragmatic trial. Using routinely-collected administrative data to assess outcomes for participants is one way to increase pragmatism in study eligibility and contain study costs.42, 43 However, NH staff tend to normalize behaviors and only the most severe behaviors are recorded in the EMR, resulting in underdetection.44 For this reason, we decided to collect CMAI as the study primary outcome. The CMAI takes 15–20 minutes to administer for each resident. Many interviews were hurried and completed by non-nursing staff, potentially affecting the quality and reliability of these data. A shorter, more focused CMAI might be helpful45 or it may be possible to create a model to equate CMAI data to available ARBS data thus reducing the need for primary data collection in future pragmatic trials. Finding a suitable behavioral outcome for trials of nonpharmacological interventions in NHs is a challenge facing many ADRD care researchers conducting pragmatic research.46 Finally, one hallmark of pragmatic trials is that they should enroll participants who are similar to those who would receive the intervention as part of usual care.47 In this trial, 20% of participants identified by NH staff as having dementia did not have an active dementia diagnosis in the available administrative data (Minimum Data Set). Understanding this gap between potentially eligible residents, as identified in the available administrative data, and potentially eligible residents identified by practitioners, is part of a larger research agenda for pragmatic trials.46, 48
CONCLUSION AND IMPLICATIONS
Based on our findings, we are planning a second ePCT of the M&M intervention with two major modifications to the implementation strategy. First, we will initiate the intervention with a nursing champion, who will identify residents with behaviors that might be addressed by the music. Second, using music play data from the first trial, we will preload players with the music that residents are likely to enjoy. This preloading relies upon a predictive algorithm to identify popular songs based on a resident’s age, race / ethnicity, gender, and geographic location. By delivering preloaded music players directly to the nursing champion, who has already identified the clinical need and timing of the intervention, we anticipate greater music use. This approach decreases personalization of the intervention (intervention fidelity) but may increase the use of music to target agitated behaviors (intervention adherence and clinical targeting). This adaptive pragmatic design49, 50 studies the comparative effectiveness of alternate strategies for implementing a promising intervention under real-world conditions in fully functioning health care systems.31
Acknowledgements:
We would like to acknowledge our nursing home partners whose commitment to providing innovative care to their residents living with dementia made this work possible. We would also like to thank the data collectors whose professionalism and passion for their work was unmatched. The sponsor did not have a role in the design, methods, subject recruitment, data collection, analysis or preparation of paper.
Funding:
This work is supported by the National Institute on Aging (R33AG057451).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: There are no conflicts of interest
ClinicalTrials.gov identifier: NCT03821844
REFERENCES
- 1.Zuidema S, Koopmans R and Verhey F. Prevalence and predictors of neuropsychiatric symptoms in cognitively impaired nursing home patients. J Geriatr Psychiatry Neurol 2007; 20: 41–49. 2007/03/08. DOI: 10.1177/0891988706292762. [DOI] [PubMed] [Google Scholar]
- 2.Kandelman N, Mazars T and Levy A. Risk factors for burnout among caregivers working in nursing homes. J Clin Nurs 2018; 27: E147–E153. DOI: 10.1111/jocn.13891. [DOI] [PubMed] [Google Scholar]
- 3.DeBois KA, Evans SD and Chatfield SL. Resident-to-Resident Aggression in Long-Term Care: Analysis of Structured and Unstructured Data From the National Violent Death Reporting System, 2003–2016. J Appl Gerontol 2019: 733464819863926. 2019/07/20. DOI: 10.1177/0733464819863926. [DOI] [PubMed] [Google Scholar]
- 4.Fraser L-A, Liu K, Naylor KL, et al. Falls and Fractures With Atypical Antipsychotic Medication Use: A Population-Based Cohort Study. JAMA internal medicine 2015; 175: 450–452. DOI: 10.1001/jamainternmed.2014.6930. [DOI] [PubMed] [Google Scholar]
- 5.Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry 2015; 72: 438–445. 2015/03/19. DOI: 10.1001/jamapsychiatry.2014.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlach LB, Kales HC, Kim HM, et al. Trends in Antipsychotic and Mood Stabilizer Prescribing in Long-Term Care in the U.S.: 2011–2014. J Am Med Dir Assoc 2020; 21: 1629–1635.e1628. 2020/07/23. DOI: 10.1016/j.jamda.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh Y-C and Ouyang W-C. Mood stabilizers for the treatment of behavioral and psychological symptoms of dementia: An update review. The Kaohsiung Journal of Medical Sciences 2012; 28: 185–193. DOI: 10.1016/j.kjms.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Brasure M, Jutkowitz E, Fuchs E, et al. AHRQ Comparative Effectiveness Reviews. Nonpharmacologic Interventions for Agitation and Aggression in Dementia. Rockville (MD): Agency for Healthcare Research and Quality (US), 2016. [PubMed] [Google Scholar]
- 9.Why Get Certified. Music & Memory., https://musicandmemory.org (accessed December 1 2019).
- 10.Jacobsen JH, Stelzer J, Fritz TH, et al. Why musical memory can be preserved in advanced Alzheimer’s disease. Brain 2015; 138: 2438–2450. DOI: 10.1093/brain/awv135. [DOI] [PubMed] [Google Scholar]
- 11.Irish M, Cunningham CJ, Walsh JB, et al. Investigating the enhancing effect of music on autobiographical memory in mild Alzheimer’s disease. Dementia and geriatric cognitive disorders 2006; 22: 108–120. 2006/05/24. DOI: 10.1159/000093487. [DOI] [PubMed] [Google Scholar]
- 12.El Haj M, Fasotti L and Allain P. The involuntary nature of music-evoked autobiographical memories in Alzheimer’s disease. Consciousness and cognition 2012; 21: 238–246. 2012/01/24. DOI: 10.1016/j.concog.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Peck KJ, Girard TA, Russo FA, et al. Music and Memory in Alzheimer’s Disease and The Potential Underlying Mechanisms. Journal of Alzheimer’s disease : JAD 2016; 51: 949–959. 2016/03/12. DOI: 10.3233/jad-150998. [DOI] [PubMed] [Google Scholar]
- 14.de la Rubia Ortí JE, García-Pardo MP, Iranzo CC, et al. Does Music Therapy Improve Anxiety and Depression in Alzheimer’s Patients? Journal of alternative and complementary medicine (New York, NY) 2018; 24: 33–36. 2017/07/18. DOI: 10.1089/acm.2016.0346. [DOI] [PubMed] [Google Scholar]
- 15.Bakerjian D, Bettega K, Cachu AM, et al. The Impact of Music and Memory on Resident Level Outcomes in California Nursing Homes. J Am Med Dir Assoc 2020; 21: 1045–1050.e1042. 2020/03/09. DOI: 10.1016/j.jamda.2020.01.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas KS, Baier R, Kosar C, et al. Individualized Music Program is Associated with Improved Outcomes for U.S. Nursing Home Residents with Dementia. Am J Geriatr Psychiatry 2017; 25: 931–938. 2017/05/10. DOI: 10.1016/j.jagp.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwak J, Anderson K and O’Connell Valuch K. Findings From a Prospective Randomized Controlled Trial of an Individualized Music Listening Program for Persons With Dementia. J Appl Gerontol 2018: 733464818778991. 2018/06/07. DOI: 10.1177/0733464818778991. [DOI] [PubMed] [Google Scholar]
- 18.Rencher AC and Schimek M. Methods of multivariate analysis. Computational Statistics 1997; 12: 422–422. [Google Scholar]
- 19.Cohen-Mansfield J, Marx MS and Rosenthal AS. A description of agitation in a nursing home. J Gerontol 1989; 44: M77–84. 1989/05/01. DOI: 10.1093/geronj/44.3.m77. [DOI] [PubMed] [Google Scholar]
- 20.Cohen-Mansfield J, Werner P and Marx MS. An observational study of agitation in agitated nursing home residents. Int Psychogeriatr 1989; 1: 153–165. 1989/01/01. DOI: 10.1017/s1041610289000165. [DOI] [PubMed] [Google Scholar]
- 21.Morris JN, Fries BE and Morris SA. Scaling ADLs within the MDS. The journals of gerontology Series A, Biological sciences and medical sciences 1999; 54: M546–553. 2000/01/05. DOI: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 22.Thomas KS, Dosa D, Wysocki A, et al. The Minimum Data Set 3.0 Cognitive Function Scale. Medical care 2017; 55: e68–e72. 2015/03/13. DOI: 10.1097/mlr.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogarek JA, McCreedy EM, Thomas KS, et al. Minimum Data Set Changes in Health, End-Stage Disease and Symptoms and Signs Scale: A Revised Measure to Predict Mortality in Nursing Home Residents. J Am Geriatr Soc 2018; 66: 976–981. 2018/03/04. DOI: 10.1111/jgs.15305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saliba DBJ. Development and validation of a revised nursing home assessment tool: MDS 3.0. 2008.
- 25.Medicare Cf and Services M. MDS 3.0 RAI Manual. 2017.
- 26.McCreedy E, Ogarek JA, Thomas KS, et al. The Minimum Data Set Agitated and Reactive Behavior Scale: Measuring Behaviors in Nursing Home Residents With Dementia. J Am Med Dir Assoc 2019; 20: 1548–1552. 2019/11/05. DOI: 10.1016/j.jamda.2019.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fossey J, Ballard C, Juszczak E, et al. Effect of enhanced psychosocial care on antipsychotic use in nursing home residents with severe dementia: cluster randomised trial. BMJ 2006; 332: 756–761. 2006/03/18. DOI: 10.1136/bmj.38782.575868.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray DM and Blistein JL. Methods to reduce the impact of intraclass correlation in group-randomized trials. Evaluation review 2003; 27: 79–103. 2003/02/06. DOI: . [DOI] [PubMed] [Google Scholar]
- 29.Teerenstra S, Eldridge S, Graff M, et al. A simple sample size formula for analysis of covariance in cluster randomized trials. Stat Med 2012; 31: 2169–2178. 2012/04/13. DOI: 10.1002/sim.5352. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell SL, Mor V, Harrison J, et al. Embedded Pragmatic Trials in Dementia Care: Realizing the Vision of the NIA IMPACT Collaboratory. J Am Geriatr Soc 2020; 68 Suppl 2: S1–s7. 2020/06/27. DOI: 10.1111/jgs.16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuzzio L, Hanson LR, Reuben DB, et al. Transforming Dementia Care Through Pragmatic Clinical Trials Embedded in Learning Healthcare Systems. J Am Geriatr Soc 2020; 68 Suppl 2: S43–s48. 2020/06/27. DOI: 10.1111/jgs.16629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glasgow RE, Lichtenstein E and Marcus AC. Why don’t we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition. Am J Public Health 2003; 93: 1261–1267. DOI: 10.2105/ajph.93.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shepherd V, Wood F, Griffith R, et al. Protection by exclusion? The (lack of) inclusion of adults who lack capacity to consent to research in clinical trials in the UK. Trials 2019; 20: 474. 2019/08/07. DOI: 10.1186/s13063-019-3603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baskin SA, Morris J, Ahronheim JC, et al. Barriers to obtaining consent in dementia research: implications for surrogate decision-making. J Am Geriatr Soc 1998; 46: 287–290. 1998/03/26. DOI: 10.1111/j.1532-5415.1998.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 35.McCreedy E, Loomer L, Palmer JA, et al. Representation in the Care Planning Process for Nursing Home Residents With Dementia. J Am Med Dir Assoc 2018; 19: 415–421. 2018/03/15. DOI: 10.1016/j.jamda.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Largent EA, Hey SP, Harkins K, et al. Ethical and Regulatory Issues for Embedded Pragmatic Trials Involving People Living with Dementia. J Am Geriatr Soc 2020; 68 Suppl 2: S37–s42. 2020/06/27. DOI: 10.1111/jgs.16620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwak J, Ha JH and O’Connell Valuch K. Lessons learned from the statewide implementation of the Music & Memory program in nursing homes in Wisconsin in the USA. Dementia (London, England) 2020: 1471301220962234. 2020/09/25. DOI: 10.1177/1471301220962234. [DOI] [PubMed] [Google Scholar]
- 38.Sengupta M, Harris-Kojetin LD and Ejaz FK. A national overview of the training received by certified nursing assistants working in U.S. nursing homes. Gerontology & geriatrics education 2010; 31: 201–219. 2010/08/24. DOI: 10.1080/02701960.2010.503122. [DOI] [PubMed] [Google Scholar]
- 39.Peterson D, Berg-Weger M, McGillick J, et al. Basic Care I: The effect of dementia-specific training on certified nursing assistants and other sraff. American Journal of Alzheimer’s Disease & Other Dementiasr 2002; 17: 154–164. DOI: 10.1177/153331750201700309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck C, Ortigara A, Mercer S, et al. Enabling and empowering certified nursing assistants for quality dementia care. International journal of geriatric psychiatry 1999; 14: 197–211. [DOI] [PubMed] [Google Scholar]
- 41.Gerdner LA and Schoenfelder DP. Evidence-based guideline. Individualized music for elders with dementia. Journal of gerontological nursing 2010; 36: 7–15. 2010/06/12. DOI: 10.3928/00989134-20100504-01. [DOI] [PubMed] [Google Scholar]
- 42.Zimmerman S and Sloane PD. Making Pragmatic Trials Pragmatic in Post-acute and Long-term Care Settings. J Am Med Dir Assoc 2019; 20: 107–109. 2019/01/30. DOI: 10.1016/j.jamda.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Lipman PD, Loudon K, Dluzak L, et al. Framing the conversation: use of PRECIS-2 ratings to advance understanding of pragmatic trial design domains. Trials 2017; 18: 532. 2017/11/12. DOI: 10.1186/s13063-017-2267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bharucha AJ, Vasilescu M, Dew MA, et al. Prevalence of behavioral symptoms: comparison of the minimum data set assessments with research instruments. J Am Med Dir Assoc 2008; 9: 244–250. 2008/05/07. DOI: 10.1016/j.jamda.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paudel A, Resnick B and Galik E. Factor Analysis of the Short Form Cohen Mansfield Agitation Inventory and Measurement Invariance by Gender. Innov Aging 2020; 4: 377–377. DOI: 10.1093/geroni/igaa057.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bynum JPW, Dorr DA, Lima J, et al. Using Healthcare Data in Embedded Pragmatic Clinical Trials among People Living with Dementia and Their Caregivers: State of the Art. J Am Geriatr Soc 2020; 68 Suppl 2: S49–s54. 2020/06/27. DOI: 10.1111/jgs.16617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. Bmj-Brit Med J 2015; 350. DOI: ARTN h2147 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 48.McCreedy E, Gilmore-Bykovskyi A, Dorr DA, et al. Barriers to identifying residents with dementia for embedded pragmatic trials: A call to action. J Am Geriatr Soc 2021. 2021/11/03. DOI: 10.1111/jgs.17539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhatt DL and Mehta C. Adaptive Designs for Clinical Trials. The New England journal of medicine 2016; 375: 65–74. 2016/07/15. DOI: 10.1056/NEJMra1510061. [DOI] [PubMed] [Google Scholar]
- 50.Chow SC and Chang M. Adaptive design methods in clinical trials - a review. Orphanet journal of rare diseases 2008; 3: 11. 2008/05/06. DOI: 10.1186/1750-1172-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


