Abstract
Although aspirin has been considered a promising agent for prevention of colorectal cancer (CRC), recent data suggest a lack of benefit among older individuals. Whether some individuals with higher risk of CRC may benefit from aspirin remains unknown. We used a 95-variant CRC polygenic risk score (PRS) to explore the association between genetic susceptibility to CRC and aspirin use in a prospective study of 12,609 individuals of European descent aged ≥70 years, enrolled in the ASPREE (ASPirin in Reducing Events in the Elderly) double-blinded, placebo-controlled randomized trial (RCT). Cox proportional hazards models were used to assess the association of aspirin use on CRC, as well as the interaction between the PRS and aspirin treatment on CRC. Over a median of 4.7 years follow-up, 143 participants were diagnosed with incident CRC. Aspirin assignment was not associated with incidence of CRC overall (hazard ratio [HR]=0.94, 95% confidence interval (CI) 0.68–1.30) or within strata of PRS (p for interaction=0.97). However, the PRS was associated with an increased risk of CRC (HR=1.28 per standard deviation [SD], 95% CI 1.09–1.51). Individuals in the top quintile of the PRS distribution had an 85% higher risk compared with individuals in the bottom quintile (HR=1.85, 95% CI 1.08–3.15). In a prospective RCT of older individuals, a PRS is associated with incident CRC risk, but aspirin use was not associated with a reduction of incident CRC, regardless of baseline genetic risk.
Introduction
Aspirin has emerged as a promising agent for CRC prevention(1–6). Although the U.S. Preventive Services Task Force (USPSTF) recommended low-dose aspirin for the primary prevention of CRC and cardiovascular disease (CVD) in adults aged 50–59 with ≥10% 10-year CVD risk in 2016, (7) they recently released draft guidelines recommending against using aspirin among adults older than age 60, largely due to concerns about increased risks of gastrointestinal bleeding (GIB) and intracranial hemorrhage (ICH) and uncertainty about aspirin’s anti-cancer benefit in older adults(7). However, one limitation of the recommendation was a lack of consideration of baseline CRC risk in weighing the risks and benefits of aspirin.
A key basis for the USPSTF’s revised guidelines were data from a recent randomized controlled trial (RCT) of daily low-dose aspirin use in generally healthy older individuals aged ≥70 - the ASPirin in Reducing Events in the Elderly (ASPREE) trial(8), which did not find a reduced risk of CRC among individuals randomized to aspirin treatment during median 4.7 years of follow-up(9). However, since ~50% of CRC occurs among adults over aged 70(10) and screening has not been universally recommended over age 75 years(11), there is an unmet need to identify potential subgroups of older populations who may benefit from aspirin prevention. Thus far, it remains unknown whether older individuals with a higher genetic susceptibility to CRC may potentially have benefits from aspirin that individuals with low CRC risk do not.
Polygenic risk scores (PRS) aggregate the effects of multiple common disease-associated genetic variants identified through genome-wide association studies into a single measure of genetic risk. PRSs have been widely used to capture genetic predispositions, including for different types of cancer (12–14), and have been evaluated as risk prediction tools for CRC. In a large study based on data from several consortia, a 95 SNP PRS was associated with increased risk of CRC(15). Thus, among participants enrolled in the ASPREE RCT, we examined whether the 95-SNP PRS for CRC(15) was associated with incident CRC risk in individuals 70 years and older, and whether individuals at higher risk of CRC, based on genetic predisposition, might have differentially benefited from aspirin use.
Materials & Methods
Study Population
ASPREE was a randomized, double-blinded placebo-controlled trial in healthy older individuals to determine whether 100mg of daily aspirin improves disability-free survival. The ASPREE trial enrolled 19,114 individuals, of which 13,349 had genotype data available. From that group, we included only individuals with non-Finnish European ancestry, and with a complete covariate dataset, resulting in 12,609 individuals (Supplementary Figure 1). The ASPREE study design(16,17), baseline characteristics(18) and trial results(8,19,20) have been published previously. The ASPREE trial is registered with Clinicaltrials.gov (NCT01038583) and approved by local ethics committees in accordance with the Belmont Report. Participants provided written informed consent for genetic research. Our report of this secondary analysis of a clinical trial follows STROBE guidelines for observational studies.
Genotyping
Genotyping of DNA samples was performed using the Axiom 2.0 Precision Medicine Diversity Research Array (Thermo Fisher Scientific, CA, USA), with alignment to GRCh38. The analysis identified ASPREE participants of non-Finnish European ancestry using principal component analysis (PCA) to compare overlap with the 1000 Genomes Non-Finnish European reference population(21). The TopMED imputation server was used for imputation(22), and variants with imputation quality scores <0.3 were removed, as well as multi-allelic variants.
Endpoint
The study endpoint was invasive CRC, defined as localised (non-metastatic) or metastatic disease, confirmed by an expert panel by histopathology, imaging of metastasis or other strong clinical evidence of metastasis(9). The adjudication process used by ASPREE trial investigators to classify incident CRC diagnoses is described previously(8),(9). We excluded participants with a self-reported prior history of CRC at the time of study enrolment (23).
PRS
The PRS was calculated for genotyped participants using plink (v1.9), based on the sum of the effect sizes for each disease-associated allele found in each participant (Supplementary Figure 2). The PRS was based on 95 CRC-associated SNPs as described previously(15),(24).
Statistical Analysis
The association between the PRS and incident CRC was estimated using a multivariable Cox proportional hazards model, adjusting for age at randomisation, sex, family history of CRC from first-degree relatives, body mass index (BMI), smoking status (current or prior), alcohol consumption (current or not), diabetes (yes/no) and treatment arm (aspirin or placebo). The PRS was assessed first as a continuous variable, then divided by quintiles (q) of the distribution into low (q1), medium (q2–4), and high (q5) risk groups. The c-index was used to determine the discriminative capability of the models tested. We tested for an interaction between the PRS and aspirin treatment for incident CRC risk using the Wald test. Competing risks (death) estimates of the cumulative incidence were visualised using the survfit function from the R survival package, with competing risk of death from other causes adjusted for in plot (25). Statistical analyses were conducted using R v3.6.1(26).
Data Availability
The data underlying this article will be shared on request to the corresponding author or to ASPREE.AMS@monash.edu.
Results
Following quality control, 12,609 participants of European-descent with both genotype data and a complete phenotype data set were identified (Table 1). After excluding participants with a prior history of CRC at enrolment, 143 participants were diagnosed with invasive CRC during the median follow-up time of 4.7 years (77 cases in males, and 66 in females). For calculation of the PRS, 93 of the 95 possible common SNPs in the PRS passed imputation quality control and were available for analysis (listed in Supplementary Table 1). Individuals grouped in the high-risk PRS group (q5) score also had higher rates of family history of CRC than individuals in low (q1) and medium (q2–4) PRS groups.
Table 1.
Baseline characteristics of 12,609 genotyped participants according to PRS group.
| Characteristic | Low PRS(q1), No. (%) | Medium PRS (q2-q4), No. (%) | High PRS (q5), No. (%) |
|---|---|---|---|
| No. of participants | 2522 | 7565 | 2522 |
| PRS, mean (SD) | 7.24 (0.21) | 7.86 (0.21) | 8.49 (0.22) |
| Family history of colorectal cancer | 311 (12.3) | 1156 (15.3) | 458 (18.2) |
| Sex, female | 1395 (55.3) | 4147 (54.8) | 1391 (55.2) |
| Age at randomisation, years, mean (SD) | 74.9 (4.1) | 75.1 (4.2) | 75.0 (4.2) |
| 70–74 years | 1562 (61.9) | 4563 (60.3) | 1534 (60.8) |
| 75–79 years | 631 (25.0) | 1916 (25.3) | 633 (25.1) |
| 80–84 years | 261 (10.3) | 844 (11.2) | 268 (10.6) |
| 85+ years | 68 (2.7) | 242 (3.2) | 87 (3.4) |
| Smoker - ever | 1131 (44.8) | 3301 (43.6) | 1135 (45.0) |
| Current alcohol consumption | 2028 (80.4) | 6031 (79.7) | 1994 (79.1) |
| History of diabetes | 223 (8.8) | 696 (9.2) | 248 (9.8) |
| Mean body mass index (SD), kg/m2 | 28.1 (4.5) | 28.0 (4.6) | 28.0 (4.5) |
| Randomized to aspirin | 1276 (50.6) | 3768 (49.8) | 1244 (49.3) |
Note: Brackets are for % of the population in that PRS group unless specified.
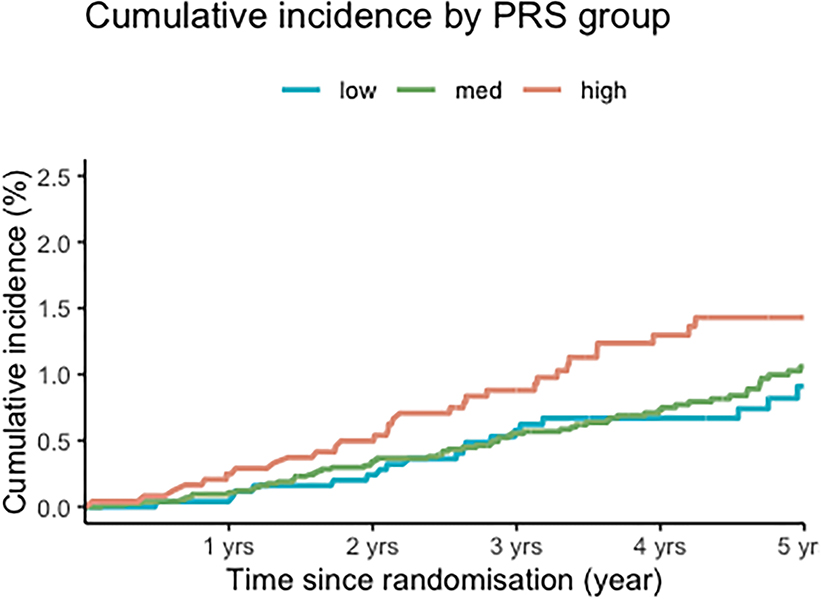
The PRS was associated with an increased risk of incident CRC (Table 2), with a HR of 1.28 per standard deviation (SD) (95% CI 1.09–1.51, p=0.003), and a c-index of 0.67 (95% CI 0.61–0.73). Compared to individuals in the low-risk PRS group (q1), individuals in the high-risk (q5) PRS group had 85% increased risk of CRC (HR: 1.85; 95% CI 1.08–3.15). After excluding participants with a family history of CRC, the PRS remained associated with incident CRC risk, with a similar HR (HR=1.31 per SD, 95% CI 1.10; 1.5, p=0.002). These findings suggest that the PRS remains associated with risk beyond age 70, even in the absence of a strong clinical risk factor like family history of CRC. The competing risks (adjusted for death) plot shows the differences in cumulative incidence between low, medium and high PRS risk groups (Figure 1).
Table 2.
Association between PRS and risk of colorectal cancer
| Low PRS | Medium PRS | High PRS | per SD | |
|---|---|---|---|---|
| Cases/Person-year | 21/11408 | 84/33598 | 38/10981 | |
| HR (unadjusted) | Ref | 1.36 (0.84–2.19) | 1.88 (1.11; 3.21) | 1.29 (1.10; 1.52) |
| HR (adjusted) | Ref | 1.32 (0.82; 2.14) | 1.85 (1.08; 3.15) | 1.28 (1.09; 1.51) |
Adjusted model includes covariates for sex, first degree family history of CRC, age at randomisation, smoker (ever), (yes/no), current alcohol intake (yes/no), history of diabetes, BMI and treatment arm. SD=Standard deviation, HR=Hazard ratio, CI=Confidence interval, BMI=Body mass index, PY=Person years.
Figure 1 – Cumulative incidence of colorectal cancer according to PRS groups.
Competing risks (death from other causes) plots showing cumulative incidence of colorectal cancer stratified by PRS group (low q1, medium q2-q4 or high q5).
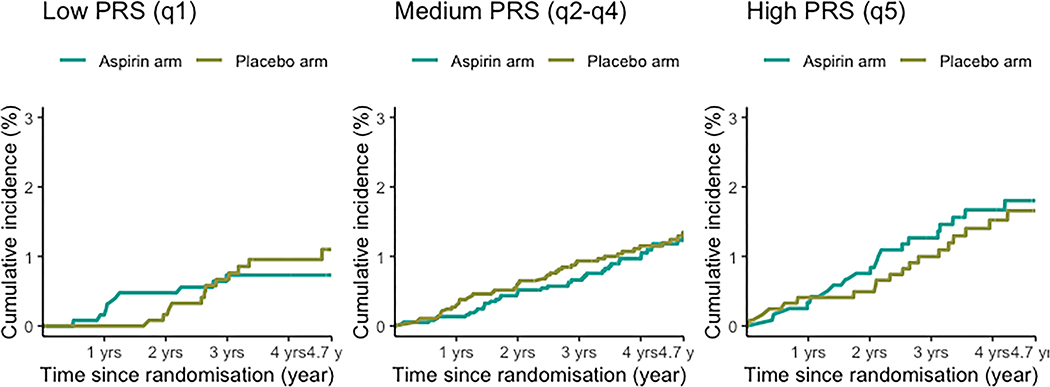
In the total study population, randomisation to aspirin treatment was not associated with a reduced incidence of CRC (HR 0.94, 95% CI 0.68–1.30, p=0.7) (Table 3). Cumulative incidence competing risk plots further show that there is no significant difference in cumulative incidence between the aspirin or placebo arms when taking into account competing risk (of death) across any of the strata (Figure 2). We did not observe any interaction of aspirin treatment and the PRS in relation to risk of CRC (p for interaction=0.97). Among individuals within strata defined by PRS, aspirin was not associated with reduced risk of CRC (Table 3) for low (HR 0.76, 95% CI 0.32; 1.81, medium (HR 0.93, 95% CI 0.60; 1.42) or high risk PRS groups (HR 1.12, 95% CI 1.12 (0.59; 2.13).
Table 3.
Interaction between aspirin treatment and PRS with risk of colorectal cancer
| Aspirin (cases/person-year) | Placebo (cases/person-year) | HR for aspirin (vs placebo) (95% CI) | |
|---|---|---|---|
| Overall | 69/27904 | 74/28083 | 0.94 (0.68; 1.30) |
| Low PRS | 9/5769 | 12/5638 | 0.76 (0.32; 1.81) |
| Medium PRS | 40/16704 | 44/16893 | 0.93 (0.60; 1.42) |
| High PRS | 20/5429 | 18/5551 | 1.12 (0.59; 2.13) |
| Interaction (PRS × Aspirin) | - | - | 0.97 (0.70; 1.34) |
Adjusted for sex, first degree family history of CRC, age at randomisation, smoking (current or former), alcohol (current/no), history of diabetes, BMI. We tested for an interaction between the PRS and aspirin treatment for incident CRC risk in the coxph model using the Wald test. SD=Standard deviation, HR=Hazard ratio, CI=Confidence interval, BMI=Body mass index.
Figure 2 -. Cumulative incidence of colorectal cancer by aspirin treatment and PRS groups.
Competing risks (death from other causes) plots showing cumulative incidence of colorectal cancer for low, medium and high PRS risk groups, with each plot stratified by treatment arm (aspirin/placebo).
Discussion
In a population of generally healthy older individuals of European descent aged >70 years, followed for a median of 4.7 years, the 95-SNP PRS continues to identify individuals at higher risk of CRC, including individuals with no family history of CRC. However, we did not observe any evidence of an association of randomized aspirin treatment with risk of incident CRC according to PRS.
Overall, the CRC PRS shows a modest association with incident colorectal cancer risk in older age, though it is not clear the extent to which this might be incorporated into current CRC risk prediction models. Current recommendations propose routine screening be offered between the ages of 45 and 75 (27),(28), with consideration of continuing screening beyond age 75 based on individual health characteristics. A PRS which can identify an increased genetic predisposition to CRC in older age may have utility in further identifying which individuals might benefit from continuing screening into older age (29).
The lack of association between randomization to aspirin and the PRS is consistent with other CRC studies, which have also not observed a significant interaction between aspirin use and PRS(30)(31). Although the USPSTF does not currently recommend prophylactic use of aspirin for the primary prevention of CRC for individuals aged ≥60 years(32), other expert panels have continued to support consideration of its use for those aged <70 (33)(34). Multiple randomized clinical trials (RCT)s (35) have shown aspirin use resulting in a reduction in risk of colorectal adenomas among individuals with history of adenoma or CRC. For example, the CAPP2 RCT showed a reduction in risk of incident CRC among individuals with Lynch syndrome on higher doses of aspirin. However, the effect observed in CAPP2 was not evident with short-term follow-up (2.5 years of intervention) but only emerged after longer-term follow-up (35). The intervention phase of ASPREE was ceased after a median of 4.7 years of follow up, during which aspirin therapy was found to increase risk of late-stage cancer incidence and cancer mortality. However, longer term follow up, in line with previously reported studies, may reveal a protective effect in older adults. There are numerous other studies also reporting evidence of aspirin reducing incident CRC diagnosis (36) and mortality(37)(38) in younger populations, particularly with longer duration of aspirin use(39,40). In the Nurses’ Health Study and Health Professionals Follow-up Study, we recently found that aspirin use was associated with a lower risk of CRC among individuals aged over 70, but only if aspirin use was initiated at a younger age (41).
Indeed, the overall lack of benefit of aspirin observed in the ASPREE trial regardless of baseline genetic risk may also be explained by a differential effect of aspirin when initiated at an older age (the vast majority of ASPREE participants commenced aspirin after age 70) (42,43) or differential metabolism of aspirin at older ages (44) (45). Finally, the ASPREE trial also utilized a relatively low dose of aspirin (100mg) daily, which could account for some variability in effects compared with other RCTs. However, it is noteworthy that other RCTs, including the Women’s Health Study and meta-analyses of RCTs led by Rothwell et. al., observed benefits of aspirin use after long-term follow-up at low doses (46,47).
The strengths of our study are the well-characterised, older population followed prospectively, and the ability to examine aspirin effects alongside PRS in a randomized trial population. All in-trial CRC diagnoses were adjudicated by an expert panel utilising evidentiary documentation. The median age at the end of follow-up in ASPREE was 78 years, making the study well-suited to assessing CRC risk in an older age group where a large proportion of CRC diagnoses occur(10).
This analysis also has several limitations. First, the study had relatively short duration of treatment and follow-up and the vast majority of participants did not initiate aspirin until after age 70. It remains possible that a benefit for aspirin use overall, and potentially by PRS, may emerge with longer treatment duration, longer follow-up, or earlier age of initiation. Second, although the trial cohort was reasonably large, we had a limited number of incident CRC cases, limiting our statistical power, including to examine the effect of aspirin. Third, because this study only examines individuals of non-Finnish European descent, using a PRS derived from a similar ancestral population, it is unclear whether the results would be consistent in populations of more diverse genetic ancestry. Fourth, we did not examine specific genetic variants not captured by the 95 SNP PRS, including rare monogenic variants associated with Lynch syndrome and other CRC-associated syndromes. The literature suggests the possibility that individual genetic variants (distinct from the aggregated effect of many common variants in a PRS) may interact with aspirin treatment for modifying the risk of CRC(48)(49). The majority of these studies have found associations in genes or pathways associated with the development of CRC, such as the WNT pathway(50)(51) the prostaglandin synthesis pathways(52), and the ornithine decarboxylase gene(53). Thus, individual variability in response to aspirin for the primary prevention of CRC according to genetic variation may exist, but independently of the current PRS.
In conclusion, we present an assessment of an established CRC PRS in a group of healthy older individuals participating in an aspirin RCT. Although the PRS was associated with CRC risk among those aged over 70, we found no evidence that aspirin use benefited those at higher genetic risk of CRC according to their PRS. Further studies are needed to assess a potential biological basis for the difference in the association of aspirin with risk of CRC among older adults observed in this trial.
Supplementary Material
Prevention Relevance Statement.
There is strong evidence to support prophylactic aspirin use for the prevention of colorectal cancer. However recent recommendations suggest the risk of bleeding in older individuals outweighs the benefit. We sought to determine whether some older individuals might still benefit from aspirin based on their genetic susceptibility.
Acknowledgements
We thank the trial staff in Australia and the United States, the participants who volunteered for this trial, and the general practitioners and staff of the medical clinics who cared for the participants.
Funding
This work was supported by an ASPREE Flagship cluster grant (including the Commonwealth Scientific and Industrial Research Organisation, Monash University, Menzies Research Institute, Australian National University, University of Melbourne); and grants U01AG029824 (J. McNeil) and U19AG062682 (A.T. Chan, J. McNeil) from the National Institute on Aging and the National Cancer Institute at the National Institutes of Health, by grants 334047 and 1127060 (J. McNeil) from the National Health and Medical Research Council of Australia, and by Monash University and the Victorian Cancer Agency. A.T. Chan is supported by an NCI Outstanding Investigator Award (R35 CA253185) and is the Stuart and Suzanne Steele MGH Research Scholar. J. McNeil is supported by a NHMRC Leadership Fellowship Investigator Grant (ID 1173690). P. Lacaze is supported by a National Heart Foundation Future Leader Fellowship (ID 102604). Y. Cao is supported by NCI project grant K07CA218377.
Footnotes
Notes
Role of the funder
No sponsor had any role in the study design, data collection, analysis, interpretation, the writing, and decision to submit the manuscript
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Chang DT, Pai RK, Rybicki LA, Dimaio MA, Limaye M, Jayachandran P, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol 2012;25:1128–39. [DOI] [PubMed] [Google Scholar]
- 2.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–7. [DOI] [PubMed] [Google Scholar]
- 3.Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. Eur J Epidemiol. 2015;30:5–18. [DOI] [PubMed] [Google Scholar]
- 4.Flossmann E British Doctors Aspirin Trial and the UK-TIA Aspirin Trial: Effect of aspirin on long-term risk of colorectal cancer: Consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–13. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Price JF, Fowkes FGR, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–12. [DOI] [PubMed] [Google Scholar]
- 6.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9:259–67. [DOI] [PubMed] [Google Scholar]
- 7.McNeil JJ. Aspirin and Primary Prevention of Colorectal Cancer: A Still-Evolving Story. J Natl Cancer Inst. 2021;113:801–2. [DOI] [PubMed] [Google Scholar]
- 8.McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N Engl J Med. 2018;379:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNeil JJ, Gibbs P, Orchard SG, Lockery JE, Bernstein WB, Cao Y, et al. Effect of Aspirin on Cancer Incidence and Mortality in Older Adults. J Natl Cancer Inst. 2021;113:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colorectal and other digestive-tract cancers [Internet]. [cited 2021 Sep 30]. Available from: https://www.aihw.gov.au/reports/cancer/colorectal-other-digestive-tract-cancers/contents/table-of-contents
- 11.Dehmer SP, Maciosek MV, Flottemesch TJ, LaFrance AB, Whitlock EP. Aspirin for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: A Decision Analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:777–86. [DOI] [PubMed] [Google Scholar]
- 12.Bakshi A, Riaz M, Orchard SG, Carr PR, Joshi AD, Cao Y, et al. A Polygenic Risk Score Predicts Incident Prostate Cancer Risk in Older Men but Does Not Select for Clinically Significant Disease. Cancers [Internet]. 2021;13. Available from: 10.3390/cancers13225815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacaze P, Bakshi A, Riaz M, Orchard SG, Tiller J, Neumann JT, et al. Genomic Risk Prediction for Breast Cancer in Older Women. Cancers [Internet]. 2021;13. Available from: 10.3390/cancers13143533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakshi A, Yan M, Riaz M, Polekhina G, Orchard SG, Tiller J, et al. Genomic Risk Score for Melanoma in a Prospective Study of Older Individuals. J Natl Cancer Inst [Internet]. 2021; Available from: 10.1093/jnci/djab076,1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Archambault AN, Su Y-R, Jeon J, Thomas M, Lin Y, Conti DV, et al. Cumulative Burden of Colorectal Cancer-Associated Genetic Variants Is More Strongly Associated With Early-Onset vs Late-Onset Cancer. Gastroenterology. 2020;158:1274–86.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ASPREE Investigator Group. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials. 2013;36:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson MR, Reid CM, Ames D, Beilin LJ, Donnan GA, Gibbs P, et al. Feasibility of conducting a primary prevention trial of low-dose aspirin for major adverse cardiovascular events in older people in Australia: results from the ASPirin in Reducing Events in the Elderly (ASPREE) pilot study [Internet]. Medical Journal of Australia. 2008. page 105–9. Available from: 10.5694/j.1326-5377.2008.tb01932.x [DOI] [PubMed] [Google Scholar]
- 18.McNeil JJ, Woods RL, Nelson MR, Murray AM, Reid CM, Kirpach B, et al. Baseline Characteristics of Participants in the ASPREE (ASPirin in Reducing Events in the Elderly) Study [Internet]. The Journals of Gerontology: Series A. 2017. page 1586–93. Available from: 10.1093/gerona/glw342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N Engl J Med. 2018;379:1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, et al. Effect of Aspirin on Disability-free Survival in the Healthy Elderly. N Engl J Med. 2018;379:1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Consortium T 1000 GP, The 1000 Genomes Project Consortium. A global reference for human genetic variation [Internet]. Nature. 2015. page 68–74. Available from: 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taliun D, Harris DN, Kessler MD, Carlson J, Szpiech ZA, Torres R, et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature. 2021;590:290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orchard SG, Lockery JE, Gibbs P, Polekhina G, Wolfe R, Zalcberg J, et al. Cancer history and risk factors in healthy older people enrolling in the ASPREE clinical trial. Contemp Clin Trials. 2020;96:106095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert SA, Gil L, Jupp S, Ritchie SC, Xu Y, Buniello A, et al. The Polygenic Score Catalog as an open database for reproducibility and systematic evaluation. Nat Genet. 2021;53:420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Therneau T (2022). A Package for Survival Analysis in R. R package version 3.3–1, Cited Mar 24 2022. Available from: https://CRAN.R-project.org/package=survival. [Google Scholar]
- 26.R Core Team (2021). Version 4.4 R. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Cited Mar 24 2022. Available from: https://www.R-project.org/. [Google Scholar]
- 27.US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:1965–77. [DOI] [PubMed] [Google Scholar]
- 28.Ma W, Wang K, Nguyen LH, Joshi A, Cao Y, Nishihara R, et al. Association of Screening Lower Endoscopy With Colorectal Cancer Incidence and Mortality in Adults Older Than 75 Years. JAMA Oncol. 2021;7(7), 985–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeon J, Du M, Schoen RE, Hoffmeister M, Newcomb PA, Berndt SI, et al. Determining Risk of Colorectal Cancer and Starting Age of Screening Based on Lifestyle, Environmental, and Genetic Factors. Gastroenterology. 2018;154:2152–64.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim AE, Drew DA, Lin Y, Qu C, Morrison JP, Bien S, et al. Abstract 820: NSAIDs and colorectal cancer risk: Results from genome-wide interaction scans. Cancer Res. American Association for Cancer Research; 2021;81:820–820. [Google Scholar]
- 31.Chen X, Guo F, Hoffmeister M, Chang-Claude J, Brenner H. Non-steroidal anti-inflammatory drugs, polygenic risk score and colorectal cancer risk. Aliment Pharmacol Ther. 2021;54:167–75. [DOI] [PubMed] [Google Scholar]
- 32.Chan AT, Ladabaum U. Where Do We Stand With Aspirin for the Prevention of Colorectal Cancer? The USPSTF Recommendations. Gastroenterology. 2016;150:14–8. [DOI] [PubMed] [Google Scholar]
- 33.Macrae F, Chetcuti A, Clarke Julie, Emery J, Jenkins Mark, Lockett Trevor, et al. , Cancer Council Australia Colorectal Cancer Guidelines Working Party. Clinical question:Aspirin for prevention of colorectal cancer. In: Clinical practice guidelines for the prevention, early detection and management of colorectal cancer. Sydney: Cancer Council Australia; [Internet]. [cited 2021 Nov 11]. Available from: https://wiki.cancer.org.au/australia/Clinical_question:Aspirin_for_prevention_of_colorectal_cancer [Google Scholar]
- 34.Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021;116:458–79. [DOI] [PubMed] [Google Scholar]
- 35.Burn J, Sheth H, Elliott F, Reed L, Macrae F, Mecklin J-P, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. 2020;395:1855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hull MA, Rees CJ, Sharp L, Koo S. A risk-stratified approach to colorectal cancer prevention and diagnosis. Nat Rev Gastroenterol Hepatol. 2020;17:773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Figueiredo JC, Jacobs EJ, Newton CC, Guinter MA, Cance WG, Campbell PT. Associations of Aspirin and Non-Aspirin Non-Steroidal Anti-Inflammatory Drugs With Colorectal Cancer Mortality After Diagnosis. J Natl Cancer Inst. 2021;113:833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao S, Xie W, Fan Y, Zhou L. Timing of Aspirin Use Among Patients with Colorectal Cancer in Relation to Mortality: A Systematic Review and Meta-Analysis. JNCI Cancer Spectr. 2021. Jul 14;5(5):pkab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Chan AT, Meyerhardt JA, Giovannucci EL. Timing of Aspirin Use in Colorectal Cancer Chemoprevention: A Prospective Cohort Study. J Natl Cancer Inst. 2021;113:841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drew DA, Chan AT. Aspirin in the Prevention of Colorectal Neoplasia. Annu Rev Med. 2021;72:415–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo C-G, Ma W, Drew DA, Cao Y, Nguyen LH, Joshi AD, et al. Aspirin Use and Risk of Colorectal Cancer Among Older Adults. JAMA Oncol. 2021;7:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang PS, Shaukat A, Crockett SD. AGA Clinical Practice Update on Chemoprevention for Colorectal Neoplasia: Expert Review. Clin Gastroenterol Hepatol. 2021;19:1327–36. [DOI] [PubMed] [Google Scholar]
- 43.Chan AT, McNeil J. Aspirin and cancer prevention in the elderly: Where do we go from here? Gastroenterology. Elsevier BV; 2019. page 534–8. [DOI] [PubMed] [Google Scholar]
- 44.Bigler J, Whitton J, Lampe JW, Fosdick L, Bostick RM, Potter JD. CYP2C9 and UGT1A6 genotypes modulate the protective effect of aspirin on colon adenoma risk. Cancer Res. 2001;61:3566–9. [PubMed] [Google Scholar]
- 45.Chan AT, Tranah GJ, Giovannucci EL, Hunter DJ, Fuchs CS. Genetic variants in the UGT1A6 enzyme, aspirin use, and the risk of colorectal adenoma. J Natl Cancer Inst. 2005;97:457–60. [DOI] [PubMed] [Google Scholar]
- 46.Cook NR, Lee I-M, Zhang SM, Moorthy MV, Buring JE. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;159:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothwell PM, Fowkes FGR, Belch JFF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. [DOI] [PubMed] [Google Scholar]
- 48.Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16:173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song N, Lee J, Cho S, Kim J, Oh JH, Shin A. Evaluation of gene-environment interactions for colorectal cancer susceptibility loci using case-only and case-control designs. BMC Cancer. 2019;19:1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nan H, Hutter CM, Lin Y, Jacobs EJ, Ulrich CM, White E, et al. Association of aspirin and NSAID use with risk of colorectal cancer according to genetic variants. JAMA. 2015;313:1133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nan H, Morikawa T, Suuriniemi M, Imamura Y, Werner L, Kuchiba A, et al. Aspirin use, 8q24 single nucleotide polymorphism rs6983267, and colorectal cancer according to CTNNB1 alterations. J Natl Cancer Inst. 2013;105:1852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Resler AJ, Makar KW, Heath L, Whitton J, Potter JD, Poole EM, et al. Genetic variation in prostaglandin synthesis and related pathways, NSAID use and colorectal cancer risk in the Colon Cancer Family Registry. Carcinogenesis. 2014;35:2121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barry EL, Mott LA, Sandler RS, Ahnen DJ, Baron JA. Variants downstream of the ornithine decarboxylase gene influence risk of colorectal adenoma and aspirin chemoprevention. Cancer Prev Res. 2011;4:2072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on request to the corresponding author or to ASPREE.AMS@monash.edu.