Abstract
Background
Hair relaxers and skin lighteners have been commonly used by African women, with suggestions that they may have hormonal activity.
Objectives
To investigate the relationship of hair relaxer and skin lightener use to serum estrogen/estrogen metabolite levels.
Methods
We utilized the postmenopausal population-based controls of the Ghana Breast Health Study to estimate adjusted geometric means (GM) and 95% confidence intervals of individual circulating estrogen levels by hair relaxer/skin lightener exposure categories.
Results
Of the 585 postmenopausal women included in our analysis, 80.2% reported hair relaxer use and 29.4% skin lightener use. Ever hair relaxer use was positively associated with estriol (adjusted GM 95.4 pmol/L vs. never 74.5, p-value=0.02) and 16-epiestriol (20.4 vs. 16.8, p-value=0.05) particularly among users of lye-based hair relaxers. Positive associations between scalp burns and unconjugated estrogens were observed (e.g., unconjugated estrone: 5+ scalp burns 76.9 [59.6–99.2] vs. no burns 64.0 [53.7–76.3], p-trend=0.03). No association was observed between use of skin lighteners and circulating estrogens.
Significance
This study presents evidence that circulating 16-pathway estrogens (i.e., estriol and 16-epiestriol) may be increased in users of lye-based hair relaxer products. Among hair relaxer users, unconjugated estrogen levels were elevated in women with a greater number of scalp burns.
Keywords: Endogenous estrogens, hair relaxer use, skin lightener use, postmenopausal women, Ghana
INTRODUCTION
Cosmetic products, specifically hair relaxers [1–3] and skin lighteners [4–7], are commonly used by African women [8, 9]. African women have used hair straightening products since the 1940’s and home use kits became available in the mid 1960’s [2]. Hair relaxer creams came on the market in the late 1970’s and have continued to evolve as more women have sought out these products [2]. The increasing prevalence of skin lightener use among African women has been documented in recent years [5, 7, 10], particularly in younger women [10]. In the Brong Ahafo Region of Ghana skin lightener use is prevalent among female high school students (prevalence of ever use 65.6%) [9] raising concerns for possible long-term deleterious consequences of prolonged usage.
The constituents of hair relaxers and skin lighteners have long been of concern in terms of their effects on human health. Hair relaxers are classified as either lye (sodium hydroxide) or non-lye. Non-lye hair relaxers are comprised of potassium hydroxide, lithium hydroxide, guanidine carbonate or ammonium thioglycolate (also referred to as thio-relaxers) [2]. Both lye and non-lye hair relaxers can burn the scalp, which provides entrance for the chemical constituents and possible systemic exposure [8, 11]. Phthalates, suspected/known endocrine disruptors, are likely constituents of these hair relaxer formulations [12–15]; thus long-term and repeated exposure to hair relaxers may influence/disturb circulating sex steroid hormones. Skin lighteners are comprised of a mixture of substances, including topical steroids, hydroquinone, and mercury [16] and some include phthalates [17]. Thus, there are concerns that skin lightener use may have the potential to affect sex steroid hormone concentrations [17].
In the Ghana Breast Health Study, an increased breast cancer risk with hair relaxer use was previously reported, but there was no association observed with skin lightener use [8]. However, no relationship of hair relaxer use with breast cancer risk was observed among Black women in either the US Black Women’s Health Study or the Women’s Circle of Health Study [18, 19]. Recent research has identified a positive association (Hazard Ratio=1.37, 95% Confidence Interval: 1.04–1.82) between heavy-use (≥7 times/year) of lye-based hair relaxers and estrogen-receptor positive (ER+) breast cancer risk among women in the Black Women’s Health Study [20].
Prior research among predominantly White women supports the role of circulating estrogen and estrogen metabolites on breast cancer risk [21–23], with higher estrogen levels strongly associated with increased risk. Parent estrogens stimulate cell proliferation largely via estrogen receptor-mediated pathways. Hydroxylation of the parent estrogens at one of three carbon positions of the steroid pathways (i.e. 2-, 4-, and 16-hydroxylation) leads to an array of metabolites in each pathway. The ability of individual estrogen metabolites to stimulate cell proliferation via estrogen receptor-mediated mechanisms varies across these pathways of metabolism as well as by the methylation status of the metabolites. Some metabolites (i.e., catechol estrogens of 2- and 4-pathways) can stimulate cell proliferation via estrogen receptor-dependent pathways and can induce DNA damage directly by forming quinone DNA adducts or indirectly via redox cycling [24].
To date, the effect of hair relaxer or skin lightener use on circulating estrogen levels has not been explored. Taking advantage of data collected in the Ghana Breast Health Study, we investigated the role of hair relaxers and skin lighteners on estrogen metabolism among postmenopausal Ghanaian women.
MATERIALS AND METHODS
Study population
For the current analysis we utilized data from postmenopausal female controls enrolled in the Ghana Breast Health Study, a multi-disciplinary population-based case-control study conducted in two areas of Ghana, Accra and Kumasi. The methodology of the original study is described in detail elsewhere [25]. The women in the control population were frequency-matched to cases by age and district of residence in Ghana via systematic random sampling [26].
Controls were approached for in-person interviews by trained personnel who recorded information on standardized questionnaires. Interviews were generally conducted in the hospitals, although a few were administered at the subjects’ homes. Interview response rates were 91.9%. We excluded women who on their questionnaire indicated that they were still having menstrual periods (1228 reported having menstrual periods, 8 had missing information, and 15 reported they didn’t know) or were current hormone users (n=10) or who had missing information on current hormone use (n=16). Additional exclusions included women with missing age (n=3) or those who reported menstrual bleeding on the blood draw questionnaire (n=47), as well as those with insufficient sample volume (n=231), or samples damaged during laboratory preparation (n=2). The final analytic population comprised 585 postmenopausal controls.
Exposure assessment
The study questionnaire focused on established breast cancer risk factors (demographic factors, menstrual and reproductive characteristics, family history of breast cancer, medical history, occupational history, anthropometric and physical activity variables) as well as on several speculative factors, including use of hair relaxers (perms or other relaxers) and skin lighteners (any soap, cream, or other product used to lighten or brighten the skin). Women were asked detailed questions regarding their use of both practices, including ages at first use, frequency of use (<21, 21–25, 26–30, >30 years), recency of use (never, former, current), age at last usage (if a non-current user), duration of use (<10, 11–20, 21–30, >30 years for hair relaxer use; <1, 1–5, 6–10, 11–20, >20 years for skin lightener use), and types of products used (for hair relaxers only). For hair relaxers, women were asked to provide information on whether they generally used lye or non-lye products and how many times over their lives they had experienced scalp burns (i.e., not just tingling but actual skin breakage) (never had burns, 1–2 times, 3–4 times, 5+ times).
Laboratory assays
Details of the assay method have been published previously [22, 27, 28]. Briefly, stable isotope dilution liquid chromatography-tandem mass spectrometry (LC-MS/MS) was used to quantify 15 estrogens and estrogen metabolites including: estrone, estradiol, 2-pathway metabolites (2-hydroxyestrone, 2-methoxyestrone, 2-hydroxyestradiol, 2-methoxyestradiol, and 2-hydroxyestrone-3-methyl ether); 4-pathway metabolites (4-hydroxyestrone, 4-methoxyestrone, and 4-methoxyestradiol); and 16α-pathway metabolites (16α-hydroxyestrone, estriol, 16-ketoestradiol, 16-epiestriol, and 17-epiestriol). This method detects 15 estrogens and estrogen metabolites in serum which circulate, at least in part, as sulfated and/or glucuronidated conjugates to facilitate storage, transport, and excretion. Five of the estrogens (estrone, estradiol, estriol, 2-methoxyestrone and 2-methoxyestradiol) were also measured in unconjugated forms. For metabolites with both combined and unconjugated measurements, the concentration of the conjugated form was calculated as the difference between the combined estrogen measurement and the unconjugated estrogen measurement; for estradiol that calculation was (conjugated estradiol = combined estradiol – unconjugated estradiol). The limit of detection for each estrogen and estrogen metabolite measured using this LC-MS/MS assay was 10 fg on column (approximately 0.33–0.37 pmol/L) [28, 29]. There were no samples in the current study with undetectable levels for any of the hormones measured. Laboratory coefficients of variation (CV) of blinded quality control duplicates distributed within and across batches were <5% for all hormones measured. Intraclass correlation coefficients (ICCs) ranged from 0.97–0.998 with a median value of 0.99.
Statistical analysis
Geometric means (GM) (pmol/L) of individual serum circulating estrogen levels by exposure categories were estimated using linear regression, adjusting for potential confounders: age at blood draw, blood draw year (2013, 2014, 2015), smoking status (never, former, current), diabetes (yes, no, missing), time since menopause (≤2, 3–5, 6–10, >10 years, missing), and ever use of oral contraceptives (yes, no, missing). A secondary analysis was performed in which we additionally adjusted for current measured body mass index (BMI, <18; 18.5–24.9; 25–29.9; ≥30 kg/m2). We performed a test for trend by including the exposure in the model as a continuous log-transformed hormone variable. The percent change (%Δ) in GM between the highest and the lowest categories was estimated by taking the ratio of the difference of the GM between the two categories over the reference category, multiplied by 100. We statistically tested for differences in hormone levels across exposure categories using a Wald test. We graphically plotted the most abundant estrogen from each pathway (parent, 2-, 4-, and 16-alpha-hydroxylation) across categories of exposure: never, ever, current, former, and lye-based and non-lye-based for hair relaxer use.
All statistical tests were two-sided with 5% type I error. Q-values reflecting false discovery rates (FDR) were calculated to account for multiple comparisons (25 tests per exposure) for the primary hormone-cosmetic exposures associations without adjustment for BMI or estradiol. Analyses were conducted with SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
Among 585 postmenopausal African women, the average age at blood draw was 56.8 years (±8.1 years) (Table 1). Of these women, 51.3% reported being within 10 years since the start of menopause when they completed the main questionnaire. Most women were never smokers (95.0%), did not report a history of diabetes (87.9%), and were never oral contraceptive users (84.8%). The subjects were similarly distributed across the three BMI categories, 33.0% healthy weight, 28.9% overweight, and 26.3% obese. Most women reported ever use of hair relaxers (80.2%,12.8% missing) while fewer reported ever use of skin lighteners (29.4%).
Table 1.
Descriptive characteristics of postmenopausal women in Ghana Breast Health Study (n=585 postmenopausal female controls)
| Mean (± SD) | ||
| Age at blood draw | 56.8 ± 8.1 | |
| Age at menopause | 48.3 ± 5.1 | |
| Time since menopause | 8.7 ± 7.2 | |
|
|
||
| n | Percent | |
| Year at blood draw | ||
| 2013 | 229 | 39.2 |
| 2014 | 193 | 33.0 |
| 2015 | 163 | 27.9 |
| Smoking status | ||
| Current | 0 | 0.0 |
| Former | 4 | 0.7 |
| Never | 556 | 95.0 |
| Unknown | 5 | 0.9 |
| Missing | 20 | 3.4 |
| Diabetes | ||
| Ever | 49 | 8.4 |
| Never | 514 | 87.9 |
| Unknown | 22 | 3.8 |
| Oral contraceptive use | ||
| Ever | 89 | 15.2 |
| Never | 496 | 84.8 |
| Unknown | 0 | |
| BMI (kg/m2) | ||
| Underweight (<18.5) | 20 | 3.4 |
| Healthy weight (18.5–24.9) | 193 | 33.0 |
| Overweight (25.0–29.9) | 169 | 28.9 |
| Obese (30+) | 154 | 26.3 |
| Unknown/Missing | 49 | 8.4 |
| Hair relaxer use | ||
| Never | 41 | 7.0 |
| Ever | 469 | 80.2 |
| Unknown | 75 | 12.8 |
| Recency of hair relaxer use | ||
| Never | 41 | 7.0 |
| Former | 116 | 19.8 |
| Current | 353 | 60.3 |
| Unknown | 75 | 12.8 |
| Length of hair relaxer use, years | ||
| Never | 41 | 7.0 |
| <10 | 50 | 8.6 |
| 11–20 | 68 | 11.6 |
| 21–30 | 144 | 24.6 |
| >30 | 187 | 32.0 |
| Unknown length | 20 | 3.4 |
| Missing | 75 | 12.8 |
| Age at first hair relaxer use | ||
| Never | 41 | 7.0 |
| <21 | 212 | 36.2 |
| 21–25 | 91 | 15.6 |
| 26–30 | 52 | 8.9 |
| >30 | 92 | 15.7 |
| Unknown | 22 | 3.8 |
| Missing | 75 | 12.8 |
| Type of hair relaxer most frequently used | ||
| Never | 41 | 7.0 |
| Lye | 274 | 46.8 |
| Non-Lye | 185 | 31.6 |
| Unknown | 10 | 1.7 |
| Missing | 75 | 12.8 |
| Frequency of burns from hair relaxer use (lifetime) | ||
| Never | 41 | 7.0 |
| Never had burns | 213 | 36.4 |
| 1–2 times | 111 | 19.0 |
| 3–4 times | 73 | 12.5 |
| 5+ times | 57 | 9.7 |
| Unknown | 15 | 2.6 |
| Skin lightener use | ||
| Never | 413 | 70.6 |
| Ever | 172 | 29.4 |
| Recency of skin lightener use | ||
| Never | 413 | 70.6 |
| Former | 109 | 18.6 |
| Current | 63 | 10.8 |
| Length of skin lightener use, years | ||
| Never | 413 | 70.6 |
| <1 | 27 | 4.6 |
| 1–5 | 33 | 5.6 |
| 6–10 | 12 | 2.1 |
| 11–20 | 29 | 5.0 |
| >20 | 51 | 8.7 |
| Unknown length | 20 | 3.4 |
| Age at first skin lightener use | ||
| Never | 413 | 70.6 |
| <21 | 53 | 9.1 |
| 21–25 | 34 | 5.8 |
| 26–30 | 27 | 4.6 |
| >30 | 41 | 7.0 |
| Unknown | 17 | 2.9 |
| Frequency of skin lightener use | ||
| Never | 413 | 70.6 |
| Once per day | 60 | 10.3 |
| ≥2 times per day | 104 | 17.8 |
| Unknown | 8 | 1.4 |
Hair relaxer use
In general, circulating estrogen/estrogen metabolite levels were elevated among ever hair relaxer users, compared to never users (Table 2, Figure 1); however only a few associations reached the level of statistical significance. We observed higher levels of circulating estriol (adjusted GM 95.41 pmol/L [95% CI: 79.73–114.16] users vs. 74.45 [57.78–95.93] never users, P-value=0.02), conjugated estriol (83.14 [68.02–101.62] vs. 63.02 [47.41–83.77], P-value=0.02) and 16-epiestriol (20.39 [16.11–25.81] vs.16.79 [12.69–22.21], P-value=0.05) among hair relaxer users compared with never users. In analyses with additional adjustment for BMI, the positive associations between estriol (GM 94.47 [78.76–113.32] vs. 75.12 [58.18–96.99], P-value=0.02), conjugated estriol (82.24 [67.16–100.70] vs. 63.80 [47.92–84.95], P-value=0.03), and 16-epiestriol (20.59 [16.31–26.00] vs. 16.89 [12.78–22.32], P-value=0.05) with ever (vs. never) hair relaxer use remained.
Table 2.
Geometric means (pmol/L) and 95% CIs of serum estrogens/estrogen metabolites by hair relaxer use (ever vs never) in postmenopausal control women not using menopausal hormone therapy in the Ghana Breast Health Study
| Geometric Means (95% CI) Model 1 | Model 1 + adjusted for categorical current BMI | |||||||
|---|---|---|---|---|---|---|---|---|
| Never | Ever | p-value* | %Δ¥ | Never | Ever | p-value* | %Δ¥ | |
| Estrone | 308.60 (197.92–481.16) | 393.63 (278.47–556.42) | 0.11 | 27.6 | 306.37 (203.93–460.26) | 366.01 (262.89–509.59) | 0.21 | 19.5 |
| Unconjugated | 57.24 (45.43–72.12) | 68.39 (57.67–81.10) | 0.05 | 19.5 | 57.27 (46.67–70.26) | 66.07 (56.63–77.07) | 0.09 | 15.4 |
| Conjugated | 233.42 (136.48–399.21) | 310.57 (208.76–462.03) | 0.14 | 33.1 | 230.62 (140.54–378.45) | 283.08 (192.88–415.47) | 0.25 | 22.7 |
| Estradiol | 32.28 (21.88–47.62) | 36.86 (27.40–49.59) | 0.36 | 14.2 | 32.12 (23.09–44.67) | 36.78 (29.21–46.33) | 0.34 | 14.5 |
| Unconjugated | 20.96 (14.81–29.67) | 24.24 (18.65–31.50) | 0.29 | 15.6 | 20.87 (15.91–27.38) | 24.34 (20.21–29.32) | 0.21 | 16.6 |
| Conjugated | 5.78 (2.65–12.62) | 6.85 (3.64–12.88) | 0.53 | 18.4 | 5.68 (2.68–12.06) | 6.64 (3.69–11.97) | 0.59 | 17 |
| 2-Hydroxyestrone | 56.76 (48.74–66.10) | 58.13 (52.05–64.93) | 0.70 | 2.4 | 56.50 (48.85–65.35) | 57.29 (51.59–63.61) | 0.82 | 1.4 |
| 2-Hydroxyestradiol | 8.78 (6.28–12.28) | 9.28 (7.11–12.11) | 0.63 | 5.7 | 8.93 (6.43–12.39) | 9.62 (7.42–12.48) | 0.52 | 7.8 |
| 2-Methoxyestrone | 21.10 (16.86–26.40) | 22.95 (19.37–27.19) | 0.33 | 8.8 | 21.02 (16.77–26.34) | 22.97 (19.42–27.17) | 0.30 | 9.3 |
| Unconjugated | 8.05 (6.14–10.54) | 9.11 (7.54–11.00) | 0.27 | 13.2 | 8.01 (6.11–10.50) | 9.17 (7.55–11.14) | 0.23 | 14.5 |
| Conjugated | 11.75 (8.29–16.67) | 11.28 (8.75–14.53) | 0.77 | −4.1 | 11.79 (8.31–16.74) | 11.13 (8.68–14.27) | 0.69 | −5.6 |
| 2-Methoxyestradiol | 11.45 (8.69–15.09) | 13.41 (10.86–16.56) | 0.12 | 17.1 | 11.29 (8.56–14.89) | 13.19 (10.68–16.29) | 0.12 | 16.9 |
| Unconjugated | 2.97 (2.33–3.79) | 3.15 (2.59–3.83) | 0.44 | 6.1 | 2.95 (2.30–3.78) | 3.22 (2.64–3.94) | 0.26 | 9.4 |
| Conjugated | 7.19 (4.69–11.04) | 8.82 (6.53–11.92) | 0.24 | 22.6 | 7.06 (4.59–10.86) | 8.44 (6.23–11.44) | 0.29 | 19.6 |
| 2-Hydroxyestrone-3-methyl ether | 3.33 (2.59–4.29) | 3.85 (3.13–4.73) | 0.12 | 15.4 | 3.32 (2.59–4.27) | 3.86 (3.15–4.74) | 0.11 | 16.2 |
| 4-Hydroxyestrone | 7.47 (5.20–10.73) | 8.21 (6.12–11.00) | 0.37 | 9.9 | 7.50 (5.24–10.75) | 8.15 (6.10–10.88) | 0.43 | 8.6 |
| 4-Methoxyestrone | 3.29 (2.59–4.19) | 3.68 (3.04–4.46) | 0.18 | 11.8 | 3.31 (2.60–4.22) | 3.76 (3.09–4.56) | 0.13 | 13.4 |
| 4-Methoxyestradiol | 1.52 (1.17–1.99) | 1.54 (1.26–1.87) | 0.92 | 1 | 1.52 (1.16–1.99) | 1.55 (1.27–1.89) | 0.83 | 1.9 |
| 16α-Hydroxyestrone | 32.08 (22.60–45.53) | 38.75 (29.18–51.46) | 0.14 | 20.8 | 32.17 (22.99–45.01) | 36.95 (28.29–48.26) | 0.26 | 14.8 |
| Estriol | 74.45 (57.78–95.93) | 95.41 (79.73–114.16) | 0.02 | 28.2 | 75.12 (58.18–96.99) | 94.47 (78.76–113.32) | 0.02 | 25.8 |
| Unconjugated | 9.68 (8.25–11.36) | 9.81 (8.77–10.98) | 0.81 | 1.4 | 9.65 (8.19–11.37) | 9.78 (8.70–10.99) | 0.81 | 1.4 |
| Conjugated | 63.02 (47.41–83.77) | 83.14 (68.02–101.62) | 0.02 | 31.9 | 63.80 (47.92–84.95) | 82.24 (67.16–100.70) | 0.03 | 28.9 |
| 16-Ketoestradiol | 16.44 (12.42–21.75) | 19.62 (15.95–24.14) | 0.11 | 19.4 | 16.35 (12.53–21.35) | 18.54 (15.37–22.37) | 0.24 | 13.4 |
| 16-Epiestriol | 16.79 (12.69–22.21) | 20.39 (16.11–25.81) | 0.049 | 21.5 | 16.89 (12.78–22.32) | 20.59 (16.31–26.00) | 0.05 | 21.9 |
| 17-Epiestriol | 23.50 (17.29–31.96) | 27.73 (21.57–35.66) | 0.10 | 18 | 23.78 (17.53–32.26) | 28.24 (21.97–36.29) | 0.09 | 18.7 |
Model 1: adjusted for age at blood draw (continuous), blood draw year (2013, 2014, 2015), smoking status (never, former, current, missing), diabetes (yes, no, missing), time since menopause (≤2, 3–5, 6–10, >10, missing), ever used oral contraceptives (yes, no, missing).
p-value was estimated using the Wald test
%Δ indicates the percentage change in estrogen/estrogen metabolite levels, comparing women reporting ever hair relaxer use vs never use and was estimated by taking the ratio of the geometric mean difference in estrogen/estrogen metabolite levels between women ever and never hair relaxer users to the geometric mean of women reporting never use, multiplied by 100
indicates FDR q-value <0.1
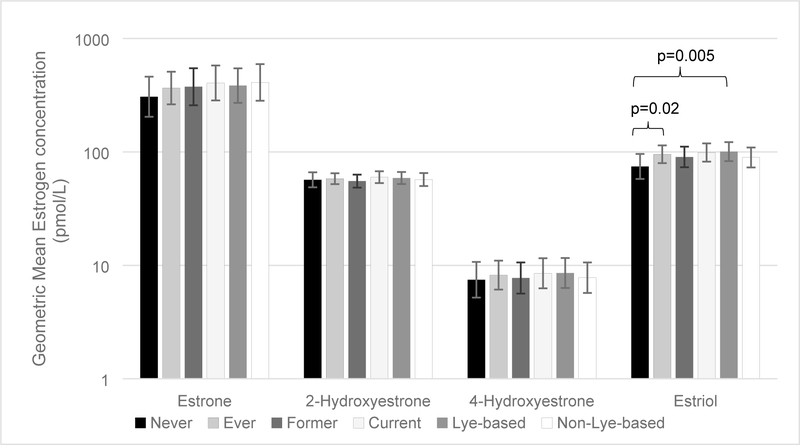
Figure 1.
Plot of Geometric Mean and 95% Confidence Intervals (error bars) of the most abundant estrogen from each pathway parent (estrone), 2-hydroxylation (2-hydroxyestrone), 4-hydroxylation (4-hydroxyestrone), and 16-alpha-hydroxylation (estriol) pathways and hair relaxer use exposure.
We did not observe differences in circulating estrogen levels when comparing former versus never or current versus never users of hair relaxers (Table 3). However, we observed higher levels of estriol, conjugated estriol, and 16-epiestriol when comparing lye hair relaxer users with never users (lye use vs. never user: estriol GM 100.74 [83.08–122.17] vs. 74.45 [57.78–95.93], P-value=0.005; conjugated estriol 88.47 [71.29–109.79] vs. 63.02 [47.41–83.77], P-value=0.006; 16-episteriol 21.35 [16.69–27.32] vs. 16.79 [12.69–22.21], P-value=0.02). Lye users had higher conjugated estradiol levels than non-lye hair relaxer users (GM 8.07 [4.16–15.65] vs. 5.56 [2.80–11.02], P-value=0.04).
Table 3.
Geometric means (pmol/L) and 95% CIs of serum estrogens/estrogen metabolites by hair relaxer use (recency: current, former, never, and formulation: lye, non-lye) in postmenopausal control women not using menopausal hormone therapy in the Ghana Breast Health Study
| Geometric Means (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Never | Former | Current | pvalue* former vs never | p-value* current vs former | Lye | Non-lye | pvalue* lye vs never | pvalue* lye vs nonlye | |
| Estrone | 308.60 (197.92–481.16) | 375.54 (257.75–547.17) | 405.56 (284.75–577.63) | 0.28 | 0.47 | 384.59 (270.78–546.21) | 410.25 (282.94–594.82) | 0.16 | 0.55 |
| Unconjugated | 57.24 (45.43–72.12) | 64.87 (53.15–79.18) | 70.75 (58.92–84.97) | 0.29 | 0.24 | 67.05 (56.05–80.20) | 70.54 (58.24–85.44) | 0.10 | 0.51 |
| Conjugated | 233.42 (136.48–399.21) | 295.44 (191.58–455.61) | 320.57 (213.73–480.81) | 0.29 | 0.52 | 302.13 (202.00–451.90) | 325.73 (212.88–498.40) | 0.19 | 0.54 |
| Estradiol | 32.28 (21.88–47.62) | 34.70 (24.80–48.56) | 38.30 (28.02–52.37) | 0.73 | 0.41 | 40.81 (29.71–56.07) | 32.49 (23.05–45.78) | 0.14 | 0.06 |
| Unconjugated | 20.96 (14.81–29.67) | 21.97 (16.20–29.79) | 25.83 (19.55–34.13) | 0.87 | 0.16 | 25.89 (19.54–34.31) | 22.32 (16.39–30.40) | 0.15 | 0.23 |
| Conjugated | 5.78 (2.65–12.62) | 7.32 (3.76–14.25) | 6.56 (3.45–12.48) | 0.41 | 0.56 | 8.07 (4.16–15.65) | 5.56 (2.80–11.02) | 0.26 | 0.04 |
| 2-Hydroxyestrone | 56.76 (48.74–66.10) | 55.34 (48.52–63.13) | 59.98 (53.18–67.63) | 0.64 | 0.14 | 58.92 (52.18–66.53) | 57.11 (50.04–65.18) | 0.59 | 0.60 |
| 2-Hydroxyestradiol | 8.78 (6.28–12.28) | 8.52 (6.45–11.24) | 9.80 (7.49–12.80) | 0.72 | 0.09 | 9.80 (7.48–12.83) | 8.65 (6.51–11.48) | 0.37 | 0.16 |
| 2-Methoxyestrone | 21.10 (16.86–26.40) | 21.35 (17.56–25.95) | 24.03 (19.92–29.00) | 0.98 | 0.08 | 22.14 (18.51–26.49) | 24.36 (20.19–29.39) | 0.54 | 0.19 |
| Unconjugated | 8.05 (6.14–10.54) | 8.50 (6.87–10.51) | 9.52 (7.73–11.73) | 0.75 | 0.14 | 8.81 (7.18–10.81) | 9.58 (7.78–11.79) | 0.41 | 0.32 |
| Conjugated | 11.75 (8.29–16.67) | 10.34 (7.67–13.95) | 11.91 (9.08–15.63) | 0.38 | 0.23 | 11.20 (8.53–14.70) | 11.49 (8.64–15.28) | 0.76 | 0.83 |
| 2-Methoxyestradiol | 11.45 (8.69–15.09) | 13.13 (10.45–16.49) | 13.60 (10.91–16.95) | 0.24 | 0.61 | 13.50 (10.85–16.79) | 13.19 (10.51–16.54) | 0.12 | 0.75 |
| Unconjugated | 2.97 (2.33–3.79) | 2.96 (2.39–3.68) | 3.28 (2.66–4.04) | 0.87 | 0.13 | 3.25 (2.65–4.00) | 3.05 (2.46–3.77) | 0.25 | 0.33 |
| Conjugated | 7.19 (4.69–11.04) | 8.88 (6.41–12.31) | 8.78 (6.45–11.96) | 0.26 | 0.91 | 8.89 (6.49–12.18) | 8.58 (6.19–11.89) | 0.24 | 0.75 |
| 2-Hydroxyestrone-3-methyl ether | 3.33 (2.59–4.29) | 3.58 (2.86–4.49) | 4.02 (3.23–5.01) | 0.57 | 0.10 | 3.71 (2.99–4.61) | 4.06 (3.25–5.08) | 0.24 | 0.21 |
| 4-Hydroxyestrone | 7.47 (5.20–10.73) | 7.74 (5.64–10.61) | 8.52 (6.28–11.56) | 0.85 | 0.26 | 8.57 (6.32–11.62) | 7.79 (5.71–10.62) | 0.23 | 0.27 |
| 4-Methoxyestrone | 3.29 (2.59–4.19) | 3.56 (2.89–4.37) | 3.77 (3.08–4.60) | 0.47 | 0.35 | 3.68 (3.02–4.49) | 3.76 (3.06–4.63) | 0.18 | 0.74 |
| 4-Methoxyestradiol | 1.52 (1.17–1.99) | 1.59 (1.28–1.97) | 1.51 (1.23–1.84) | 0.65 | 0.47 | 1.52 (1.24–1.86) | 1.56 (1.26–1.93) | 0.97 | 0.67 |
| 16α-Hydroxyestrone | 32.08 (22.60–45.53) | 37.05 (27.04–50.77) | 39.87 (29.49–53.90) | 0.36 | 0.49 | 39.06 (29.01–52.59) | 38.68 (28.36–52.76) | 0.14 | 0.93 |
| Estriol | 74.45 (57.78–95.93) | 90.28 (73.23–111.30) | 98.81 (82.04–119.00) | 0.13 | 0.28 | 100.74 (83.08–122.17) | 89.40 (73.00–109.49) | 0.0051 ¶ | 0.18 |
| Unconjugated | 9.68 (8.25–11.36) | 10.13 (8.94–11.48) | 9.61 (8.55–10.81) | 0.45 | 0.26 | 10.02 (8.93–11.24) | 9.60 (8.46–10.90) | 0.57 | 0.32 |
| Conjugated | 63.02 (47.41–83.77) | 77.47 (61.25–97.99) | 86.95 (70.62–107.05) | 0.16 | 0.23 | 88.47 (71.29–109.79) | 76.98 (61.21–96.83) | 0.006 ¶ | 0.17 |
| 16-Ketoestradiol | 16.44 (12.42–21.75) | 19.19 (15.14–24.32) | 19.91 (16.02–24.73) | 0.24 | 0.68 | 19.56 (15.68–24.39) | 19.77 (15.73–24.85) | 0.13 | 0.90 |
| 16-Epiestriol | 16.79 (12.69–22.21) | 19.65 (15.22–25.36) | 20.88 (16.36–26.64) | 0.20 | 0.46 | 21.35 (16.69–27.32) | 19.26 (15.04–24.66) | 0.02 | 0.18 |
| 17-Epiestriol | 23.50 (17.29–31.96) | 26.42 (20.16–34.64) | 28.59 (21.90–37.34) | 0.36 | 0.31 | 27.72 (21.33–36.02) | 28.05 (21.61–36.41) | 0.11 | 0.87 |
Geometric means adjusted for age at blood draw (continuous), blood draw year (2013, 2014, 2015), smoking status (never, former, current, missing), diabetes (yes, no, missing), time since menopause (≤2, 3–5, 6–10, >10, missing), ever used oral contraceptives (yes, no, missing).
p-value was estimated using the Wald test
%Δ indicates the percentage change in estrogen/estrogen metabolite levels, comparing women reporting ever hair relaxer use vs never use and was estimated by taking the ratio of the geometric mean difference in estrogen/estrogen metabolite levels between women ever and never hair relaxer users to the geometric mean of women reporting never use, multiplied by 100
Indicates FDR q-value <0.1
History of scalp burns from hair relaxer use was positively associated with circulating unconjugated estrogens (Table 4). We observed higher levels of circulating unconjugated estrone (5+ burns vs. never burned GM 76.88 [59.59–99.21] vs. 64.01 [53.67–76.34], P-value=0.03), unconjugated estradiol (30.44 [20.36–45.52] vs. 22.59 [17.03–29.97], P-value=0.04), unconjugated 2-methoxyestrone (11.00 [8.22–14.73] vs. 8.69 [7.10–10.63], P-value=0.04), and unconjugated 2-methoxyestradiol (4.01 [3.08–5.22] vs. 3.05 [2.47–3.77], P-value=0.008). The positive relationship between scalp burns and circulating unconjugated 2-methoxyestrone (5+ burns vs. never GM 11.05 [8.23–14.85] vs. 8.78 [7.15–10.79], P-value=0.04) and 2-methoxyestradiol (5+ burns vs. never burned GM 4.04 [3.12–5.22] vs. 3.15 [2.55–3.90], P-value=0.01) remained in analyses adjusted for BMI (Supplemental Table 1). In contrast, with additional adjustment for unconjugated estradiol, the association was only present for unconjugated 2-methoxyestradiol (5+ burns vs. never burned GM 3.91 [3.03–4.04] vs. 3.17 [2.56–3.94], P-value=0.04) (results not tabled). We did not observe any association between circulating estrogens and either age at first hair relaxer use or length of use.
Table 4.
Geometric means (pmol/L) and 95% CIs of serum estrogens/estrogen metabolites by burns associated with hair relaxer frequency of burns in postmenopausal control women not using menopausal hormone therapy in the Ghana Breast Health Study
| Geometric Means (95% CI) | |||||
|---|---|---|---|---|---|
| Never had burns | 1–2 times burned | 3–4 times burned | 5+ times burned | ptrend | |
| Estrone | 372.31 (259.95–533.23) | 454.26 (310.96–663.59) | 364.34 (242.89–546.52) | 442.46 (284.79–687.43) | 0.16 |
| Unconjugated | 64.01 (53.67–76.34) | 78.37 (64.17–95.71) | 65.79 (53.90–80.30) | 76.88 (59.59–99.21) | 0.03 |
| Conjugated | 292.08 (193.19–441.59) | 363.74 (234.55–564.07) | 287.27 (179.96–458.56) | 346.91 (209.23–575.21) | 0.19 |
| Estradiol | 34.74 (25.21–47.88) | 40.43 (28.45–57.44) | 35.85 (24.85–51.71) | 42.75 (27.99–65.29) | 0.11 |
| Unconjugated | 22.59 (17.03–29.97) | 26.56 (19.29–36.58) | 23.30 (16.74–32.43) | 30.44 (20.36–45.52) | 0.04 |
| Conjugated | 6.05 (3.13–11.69) | 7.81 (3.93–15.53) | 7.54 (3.69–15.41) | 7.61 (3.61–16.02) | 0.14 |
| 2-Hydroxyestrone | 57.89 (51.24–65.41) | 58.21 (50.39–67.24) | 56.67 (48.78–65.84) | 62.61 (51.12–76.70) | 0.48 |
| 2-Hydroxyestradiol | 9.59 (7.31–12.58) | 8.67 (6.49–11.58) | 9.11 (6.70–12.40) | 9.61 (6.98–13.23) | 0.91 |
| 2-Methoxyestrone | 22.91 (19.05–27.55) | 22.96 (18.57–28.40) | 21.58 (17.65–26.39) | 26.50 (20.53–34.21) | 0.23 |
| Unconjugated | 8.69 (7.10–10.63) | 9.15 (7.25–11.56) | 9.21 (7.38–11.51) | 11.00 (8.22–14.73) | 0.04 |
| Conjugated | 11.77 (8.87–15.63) | 10.76 (7.80–14.85) | 10.91 (7.96–14.96) | 10.96 (7.29–16.47) | 0.55 |
| 2-Methoxyestradiol | 13.32 (10.70–16.59) | 13.22 (10.50–16.64) | 13.93 (10.89–17.82) | 13.08 (10.06–17.01) | 0.34 |
| Unconjugated | 3.05 (2.47–3.77) | 2.99 (2.39–3.75) | 3.17 (2.53–3.96) | 4.01 (3.08–5.22) | 0.01 |
| Conjugated | 9.00 (6.56–12.36) | 9.33 (6.71–12.97) | 8.67 (5.92–12.70) | 7.40 (5.01–10.92) | 0.83 |
| 2-Hydroxyestrone-3-methyl ether | 3.72 (3.01–4.60) | 4.14 (3.24–5.30) | 3.80 (2.99–4.83) | 4.01 (3.09–5.20) | 0.17 |
| 4-Hydroxyestrone | 8.43 (6.17–11.53) | 8.28 (6.00–11.44) | 7.63 (5.45–10.67) | 8.22 (5.68–11.89) | 0.94 |
| 4-Methoxyestrone | 3.69 (3.03–4.50) | 3.50 (2.81–4.36) | 3.67 (2.93–4.59) | 4.06 (3.18–5.19) | 0.17 |
| 4-Methoxyestradiol | 1.51 (1.23–1.85) | 1.55 (1.24–1.94) | 1.59 (1.25–2.02) | 1.52 (1.18–1.96) | 0.64 |
| 16α-Hydroxyestrone | 38.54 (28.35–52.39) | 38.33 (27.56–53.31) | 36.67 (26.25–51.22) | 44.83 (30.41–66.10) | 0.26 |
| Estriol | 94.62 (76.84–116.52) | 106.57 (84.34–134.66) | 84.69 (67.28–106.60) | 103.27 (78.47–135.92) | 0.25 |
| Unconjugated | 9.54 (8.44–10.79) | 10.04 (8.77–11.49) | 9.63 (8.42–11.03) | 11.10 (9.29–13.27) | 0.07 |
| Conjugated | 82.87 (65.70–104.52) | 93.89 (72.22–122.08) | 72.75 (56.05–94.43) | 88.87 (65.44–120.69) | 0.34 |
| 16-Ketoestradiol | 19.83 (15.87–24.78) | 20.69 (16.24–26.36) | 17.84 (13.96–22.78) | 20.59 (15.20–27.89) | 0.62 |
| 16-Epiestriol | 21.21 (16.31–27.57) | 20.46 (15.63–26.79) | 17.34 (13.04–23.07) | 24.10 (17.65–32.90) | 0.42 |
| 17-Epiestriol | 27.26 (20.87–35.59) | 30.41 (23.04–40.13) | 27.41 (20.54–36.56) | 25.80 (18.93–35.17) | 0.73 |
Geometric means adjusted for age at blood draw (continuous), blood draw year (2013, 2014, 2015), smoking status (never, former, current, missing), diabetes (yes, no, missing), time since menopause (≤2, 3–5, 6–10, >10, missing), ever used oral contraceptives (yes, no, missing).
p-value for trend was estimated using the Wald test, all FDR q-values ≥ 0.20
Skin lightener use
Circulating estrogen levels were not largely different comparing women who reported ever skin lightener use versus never use (Table 5). When comparing current users versus former users of skin lighteners we observed a modest association for 4-methoxyestrone, with levels being approximately 10% lower in current skin lightener users compared with former users (GM current 3.29 [2.73–3.96] vs. former 3.67 [3.09–4.37], P value=0.006).
Table 5.
Geometric means (pmol/L) and 95% CIs of serum estrogens/estrogen metabolites by skin lightener use (ever, former, current, vs. never) characteristics in postmenopausal control women not using menopausal hormone therapy in the Ghana Breast Health Study
| Geometric Means (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Never | Ever | p-value* ever vs never | %Δ¥ | Former | Current | p-value* former vs never | p-value* current vs former | |
| Estrone | 360.42 (251.55–516.41) | 360.56 (255.06–509.69) | 0.99 | 0 | 360.33 (251.89–515.46) | 399.76 (276.54–577.86) | 0.37 | 0.08 |
| Unconjugated | 64.59 (54.24–76.93) | 66.36 (55.95–78.70) | 0.68 | 2.7 | 64.58 (54.06–77.17) | 72.13 (59.27–87.79) | 0.14 | 0.05 |
| Conjugated | 285.37 (188.23–432.63) | 279.15 (187.20–416.25) | 0.84 | −2.2 | 285.30 (188.63–431.53) | 306.05 (200.48–467.23) | 0.61 | 0.18 |
| Estradiol | 36.26 (26.90–48.87) | 34.21 (25.60–45.71) | 0.57 | −5.7 | 36.25 (27.02–48.65) | 36.38 (26.60–49.75) | 0.98 | 0.34 |
| Unconjugated | 23.80 (18.16–31.17) | 22.70 (17.50–29.43) | 0.65 | −4.6 | 23.79 (18.25–31.01) | 24.06 (18.05–32.08) | 0.93 | 0.38 |
| Conjugated | 6.63 (3.51–12.54) | 6.74 (3.61–12.58) | 0.92 | 1.6 | 6.63 (3.51–12.53) | 7.17 (3.75–13.70) | 0.66 | 0.50 |
| 2-Hydroxyestrone | 57.63 (51.28–64.76) | 55.98 (49.95–62.73) | 0.55 | −2.9 | 57.62 (51.27–64.77) | 57.65 (50.57–65.72) | 0.99 | 0.34 |
| 2-Hydroxyestradiol | 9.24 (7.05–12.12) | 8.58 (6.54–11.26) | 0.26 | −7.2 | 9.24 (7.03–12.15) | 8.38 (6.29–11.16) | 0.17 | 0.58 |
| 2-Methoxyestrone | 22.05 (18.60–26.13) | 22.70 (19.06–27.03) | 0.64 | 2.9 | 22.05 (18.63–26.10) | 22.20 (18.23–27.04) | 0.93 | 0.59 |
| Unconjugated | 8.69 (7.13–10.58) | 8.93 (7.33–10.89) | 0.69 | 2.8 | 8.69 (7.13–10.58) | 8.72 (7.01–10.83) | 0.97 | 0.61 |
| Conjugated | 11.22 (8.65–14.54) | 10.50 (8.23–13.40) | 0.54 | −6.4 | 11.22 (8.52–14.77) | 11.59 (8.65–15.52) | 0.78 | 0.20 |
| 2-Methoxyestradiol | 12.87 (10.40–15.94) | 13.41 (10.91–16.50) | 0.48 | 4.2 | 12.88 (10.40–15.93) | 13.21 (10.63–16.42) | 0.69 | 0.69 |
| Unconjugated | 3.02 (2.48–3.67) | 3.23 (2.66–3.94) | 0.18 | 7.2 | 3.02 (2.51–3.62) | 3.06 (2.53–3.70) | 0.80 | 0.13 |
| Conjugated | 8.80 (6.45–12.00) | 8.68 (6.46–11.67) | 0.89 | −1.3 | 8.80 (6.45–11.99) | 8.99 (6.61–12.22) | 0.84 | 0.59 |
| 2-Hydroxyestrone-3-methyl ether | 3.69 (3.02–4.52) | 3.84 (3.12–4.71) | 0.55 | 3.8 | 3.69 (3.02–4.52) | 3.70 (2.95–4.63) | 1.00 | 0.36 |
| 4-Hydroxyestrone | 7.37 (5.49–9.90) | 8.41 (6.28–11.27) | 0.07 | 14.1 | 7.37 (5.48–9.91) | 8.47 (6.26–11.47) | 0.12 | 0.88 |
| 4-Methoxyestrone | 3.67 (3.04–4.44) | 3.60 (2.99–4.35) | 0.72 | −1.9 | 3.67 (3.09–4.37) | 3.29 (2.73–3.96) | 0.07 | 0.006 |
| 4-Methoxyestradiol | 1.47 (1.20–1.81) | 1.57 (1.28–1.92) | 0.30 | 6.4 | 1.47 (1.20–1.82) | 1.59 (1.29–1.96) | 0.30 | 0.74 |
| 16α-Hydroxyestrone | 35.26 (26.58–46.77) | 35.64 (26.81–47.39) | 0.90 | 1.1 | 35.26 (26.57–46.80) | 34.22 (25.21–46.45) | 0.78 | 0.50 |
| Estriol | 85.85 (71.24–103.47) | 95.51 (79.89–114.18) | 0.14 | 11.2 | 85.86 (71.10–103.68) | 94.29 (77.52–114.68) | 0.24 | 0.79 |
| Unconjugated | 9.67 (8.60–10.87) | 10.05 (8.98–11.24) | 0.35 | 3.9 | 9.67 (8.60–10.87) | 10.05 (8.90–11.34) | 0.44 | 0.99 |
| Conjugated | 73.25 (59.48–90.22) | 83.14 (68.17–101.40) | 0.11 | 13.5 | 73.25 (59.34–90.43) | 81.99 (65.99–101.88) | 0.21 | 0.79 |
| 16-Ketoestradiol | 17.89 (14.36–22.30) | 19.17 (15.54–23.65) | 0.35 | 7.1 | 17.89 (14.35–22.32) | 18.98 (15.16–23.77) | 0.50 | 0.84 |
| 16-Epiestriol | 19.14 (15.17–24.16) | 19.65 (15.64–24.69) | 0.69 | 2.6 | 19.14 (15.16–24.17) | 19.49 (15.25–24.92) | 0.82 | 0.85 |
| 17-Epiestriol | 28.42 (22.25–36.29) | 27.53 (21.51–35.22) | 0.61 | −3.1 | 28.42 (22.27–36.28) | 25.53 (19.74–33.01) | 0.15 | 0.05 |
Geometric means adjusted for age at blood draw (continuous), blood draw year (2013, 2014, 2015), smoking status (never, former, current, missing), diabetes (yes, no, missing), time since menopause (≤2, 3–5, 6–10, >10, missing), ever used oral contraceptives (yes, no, missing).
p-value for trend was estimated using the Wald test, all FDR q-values ≥ 0.2
%Δ indicates the percentage change in estrogen/estrogen metabolite levels, comparing women reporting ever skin lightener use vs never use and was estimated by taking the ratio of the geometric mean difference in estrogen/estrogen metabolite levels between women ever and never skin lightener users to the geometric mean of women reporting never use, multiplied by 100
Circulating estrogen levels related to skin lightener use were mostly similar across the categories of years of use (<1, 1–5, 6–10, 11–20, >20 years) (Supplemental Table 2). Estriol was 2.7% higher for women who used skin lighteners for >20 years (GM 84.27 [58.21–121.98]) than women that used skin lighteners for <1 year (GM 82.02 [67.56–99.58]). Unconjugated estriol was 3.2% higher for women who used skin lighteners for >20 years (GM 72.10 [48.21–107.84]) than women that used skin lighteners for <1 year (GM 69.89 [56.25–86.85]). We did not observe any associations between circulating estrogens and frequency of skin lightener use or age at first use.
Discussion
Our analysis of cosmetic product use in postmenopausal women in Ghana suggests that hair relaxers, specifically lye-based products, are associated with increased levels of circulating 16-pathway estrogens estriol and 16-epiestriol when compared to never users. We also observed a higher level of circulating conjugated estradiol for users of lye-based products compared with non-lye-based products. Additionally, we noted increasing levels of many unconjugated estrogens with increasing frequency of scalp burns among women using hair relaxers. In contrast, we generally did not see a difference in hormone levels with ever versus never skin lightener use or with patterns of frequency or age at first use. We did note some patterns among skin lightener users, specifically higher levels of 4-methoxyestrone in current users versus former users and higher estriol levels for women who reported longer lengths of skin lightener use (20+ years) compared to very short durations of use (<1 year).
Our results are relevant in possibly shedding light on prior studies that have examined the role of these cosmetics products in relation to breast cancer risk [8]. Specifically, in the Ghana Breast Health Study there was an increased risk of breast cancer with hair relaxer use, that did not differ by menopausal status at diagnosis [8]. Given that this analytic sample was approximately 50% postmenopausal, our results are likely not generalizable to these previous findings. However, this prior study did not find any associations of overall breast cancer risk with lye-based products [8]. Skin lightener use was not associated with overall breast cancer risk in the Ghana Breast Health Study [8]. Our results suggest that select 16-hydroxylation pathway estrogen metabolites are higher in hair relaxer users compared with non-users and may help to explain part of the association between hair relaxer use and increased breast cancer risk in postmenopausal women given that these estrogens and metabolites have been associated with increased postmenopausal breast cancer risk in the US [21–23]. However, the current study cannot directly evaluate the potential mediation of the hair relaxer-breast cancer association. It is possible that hair relaxers may have other effects on endocrine function that were not measured in the current study. Additional investigation among Black women of both the estrogen metabolite-breast cancer risk association and the hair relaxer use-estrogen metabolite associations are necessary to better understand how the observed differences in estrogen metabolism with hair relaxer use may affect breast cancer risk.
The observed associations with hair relaxer use in the current study further support that lye-based products may be involved with increased concentrations of select 16-hydroxylation pathway estrogen metabolites. However, no distinctive differences in breast cancer risk between lye-and non-lye users were previously noted in the Ghana Breast Health Study, among an analytic sample that was comprised of approximately 50% postmenopausal women [8]. The increased levels of circulating 16-pathway estrogens with lye-based products in our study could reflect possible effects on estrogen metabolism from the constituents of these products, including phthalates [30], or additional contaminants ranging from formaldehyde [31] to metals [32, 33].
We acknowledge several limitations of this study. We used a single measurement of circulating estrogens among postmenopausal women; however, moderate to high 1-year ICCs reported in a previous study for most estrogens/estrogen metabolites (range 0.35–0.72) [34] suggest that our data may adequately represent levels over at least 1 year. It is also important to note that product use was based entirely on self-report which may result in misclassification; and it is unclear how well women can recall the composition of the products they use (e.g., lye vs. non-lye based hair relaxers). However, measurement error in this context is unlikely to be related to serum hormone levels.
Despite these limitations, this study has important strengths. Use of a high-performance LC-MS/MS assay allowed comprehensive evaluations of individual circulating estrogen metabolites with high reliability, sensitivity, and specificity. Use of data from a population-based study with careful adjustment for potential confounders assessed at blood collection increases the validity of the results. Another strength was our ability to examine various characteristics of hair relaxer and skin lightener use due to the available responses on history of use, recency of use, length of use, age at first use, type of hair relaxer used, and location of skin lightener use.
Our results support a possible association between circulating estrogen metabolites in postmenopausal African women that used lye-based hair relaxer products. Additionally, our findings indicate a possible association between circulating unconjugated estrogens and number of scalp burns among hair relaxer users. In contrast, skin lightener use was not strongly associated with differences in estrogen levels. Further examination into the effects of hair relaxer use on circulating hormone levels in Black women is merited to better understand possible biological mechanisms underlying breast cancer risk.
Supplementary Material
Impact Statement.
In this population-based study of hair relaxer and skin lightener use among postmenopausal women in Ghana, altered estrogen metabolism was observed with hair relaxer use, particularly among women using lye-based products or with a greater number of scalp burns. In contrast, skin lightener use was not associated with differences in estrogen metabolism in this population. Continued investigation of the potential biological impact on breast cancer risk of hair relaxer use is warranted.
Acknowledgments:
The success of this investigation would not have been possible without exceptional teamwork and the diligence of the field staff who oversaw the recruitment, interviews and collection of data from study subjects. Special thanks are due to the following individuals: Korle Bu Teaching Hospital,Accra—Dr. Adu-Aryee, Obed Ekpedzor, Angela Kenu, Victoria Okyne, Naomi Oyoe Ohene Oti, Evelyn Tay; Komfo Anoyke Teaching Hospital, Kumasi— Marion Alcpaloo, Bernard Arhin, Emmanuel Asiamah, Isaac Boakye, Samuel Ka-chungu and; Peace and Love Hospital, Kumasi—Samuel Amanama, Emma Abaidoo, Prince Agyapong, Thomas Agyei-Ansong, Debora Boateng, Margaret Frempong, Bridget Nortey Mensah, Richard Opoku, and Kofi Owusu Gyimah. The study was further enhanced by surgical expertise provided by Dr. Lisa Newman of the University of Michigan and by pathological expertise provided by Drs. Stephen Hewitt and Petra Lenz of the National Cancer Institute, and Dr. Maire A. Duggan from the Cumming School of Medicine, University of Calgary, Canada. Study management assistance was received from Ricardo Diaz, Shelley Niwa, Usha Singh, Ann Truelove and Michelle Brotzman at Westat, Inc. Appreciation is also expressed to the many women who agreed to participate in the study and to provide information and biospecimens in hopes of preventing and improving outcomes of breast cancer in Ghana.
Funding:
This research was supported in part by funds from the intramural research program of the National Cancer Institute, National Institutes of Health.
Footnotes
Competing interests: All authors declare no conflicts of interest
Ethics approval and consent to participate: All questionnaires were administered after obtaining written informed consent on forms approved by institutional review boards in the U.S. and Ghana.
Data Availability:
The datasets generated or analyzed for the current study are not publicly available due to data privacy of patients but are available from the corresponding author upon reasonable request.
References
- 1.Aryiku SA, Salam A, Dadzie OE & Jablonski NG, Clinical and anthropological perspectives on chemical relaxing of afro-textured hair. J Eur Acad Dermatol Venereol, 2015. 29(9): p. 1689–95. [DOI] [PubMed] [Google Scholar]
- 2.de Sá Dias TC, Baby AR, Kaneko TM & Robles Velasco MV, Relaxing/straightening of Afro-ethnic hair: historical overview. J Cosmet Dermatol, 2007. 6(1): p. 2–5. [DOI] [PubMed] [Google Scholar]
- 3.Sishi VNB, Van Wyk JC & Khumalo NP, The pH of lye and no-lye hair relaxers, including those advertised for children, is at levels that are corrosive to the skin. S Afr Med J, 2019. 109(12): p. 941–946. [DOI] [PubMed] [Google Scholar]
- 4.Benn EK, Alexis A, Mohamed N, Wang YH, Khan IA & Liu B, Skin Bleaching and Dermatologic Health of African and Afro-Caribbean Populations in the US: New Directions for Methodologically Rigorous, Multidisciplinary, and Culturally Sensitive Research. Dermatol Ther (Heidelb), 2016. 6(4): p. 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dlova NC, Hamed SH, Tsoka-Gwegweni J & Grobler A, Skin lightening practices: an epidemiological study of South African women of African and Indian ancestries. Br J Dermatol, 2015. 173 Suppl 2: p. 2–9. [DOI] [PubMed] [Google Scholar]
- 6.Ladizinski B, Mistry N & Kundu RV, Widespread use of toxic skin lightening compounds: medical and psychosocial aspects. Dermatol Clin, 2011. 29(1): p. 111–23. [DOI] [PubMed] [Google Scholar]
- 7.Lartey M, Krampa FD, Abdul-Rahman M, Quarcoo NL, Yamson P, Hagan PG, et al. , Use of skin-lightening products among selected urban communities in Accra, Ghana. Int J Dermatol, 2017. 56(1): p. 32–39. [DOI] [PubMed] [Google Scholar]
- 8.Brinton LA, Figueroa JD, Ansong D, Nyarko KM, Wiafe S, Yarney J, et al. , Skin lighteners and hair relaxers as risk factors for breast cancer: results from the Ghana breast health study. Carcinogenesis, 2018. 39(4): p. 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osei M, Ali M, Owusu A & Baiden F, Skin-lightening practices among female high school students in Ghana. Public Health, 2018. 155: p. 81–87. [DOI] [PubMed] [Google Scholar]
- 10.Peltzer K, Pengpid S & James C, The globalization of whitening: prevalence of skin lighteners (or bleachers) use and its social correlates among university students in 26 countries. Int J Dermatol, 2016. 55(2): p. 165–72. [DOI] [PubMed] [Google Scholar]
- 11.Crawford K & Hernandez C, A review of hair care products for black individuals. Cutis, 2014. 93(6): p. 289–93. [PubMed] [Google Scholar]
- 12.Hauser R & Calafat AM, Phthalates and human health. Occup Environ Med, 2005. 62(11): p. 806–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darbre PD, Environmental oestrogens, cosmetics and breast cancer. Best Pract Res Clin Endocrinol Metab, 2006. 20(1): p. 121–43. [DOI] [PubMed] [Google Scholar]
- 14.Giulivo M, Lopez de Alda M, Capri E & Barceló D, Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ Res, 2016. 151: p. 251–264. [DOI] [PubMed] [Google Scholar]
- 15.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. , Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev, 2009. 30(4): p. 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maneli MH, Wiesner L, Tinguely C, Davids LM, Spengane Z, Smith P, et al. , Combinations of potent topical steroids, mercury and hydroquinone are common in internationally manufactured skin-lightening products: a spectroscopic study. Clin Exp Dermatol, 2016. 41(2): p. 196–201. [DOI] [PubMed] [Google Scholar]
- 17.Romero-Franco M, Hernández-Ramírez RU, Calafat AM, Cebrián ME, Needham LL, Teitelbaum S, et al. , Personal care product use and urinary levels of phthalate metabolites in Mexican women. Environ Int, 2011. 37(5): p. 867–71. [DOI] [PubMed] [Google Scholar]
- 18.Llanos AAM, Rabkin A, Bandera EV, Zirpoli G, Gonzalez BD, Xing CY, et al. , Hair product use and breast cancer risk among African American and White women. Carcinogenesis, 2017. 38(9): p. 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg L, Boggs DA, Adams-Campbell LL & Palmer JR, Hair relaxers not associated with breast cancer risk: evidence from the black women’s health study. Cancer Epidemiol Biomarkers Prev, 2007. 16(5): p. 1035–7. [DOI] [PubMed] [Google Scholar]
- 20.Coogan PF, Rosenberg L, Palmer JR, Cozier YC, Lenzy YM & Bertrand KA, Hair product use and breast cancer incidence in the Black Women’s Health Study. Carcinogenesis, 2021. 42(7): p. 924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinton LA, Trabert B, Anderson GL, Falk RT, Felix AS, Fuhrman BJ, et al. , Serum Estrogens and Estrogen Metabolites and Endometrial Cancer Risk among Postmenopausal Women. Cancer Epidemiol Biomarkers Prev, 2016. 25(7): p. 1081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trabert B, Brinton LA, Anderson GL, Pfeiffer RM, Falk RT, Strickler HD, et al. , Circulating Estrogens and Postmenopausal Ovarian Cancer Risk in the Women’s Health Initiative Observational Study. Cancer Epidemiol Biomarkers Prev, 2016. 25(4): p. 648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampson JN, Falk RT, Schairer C, Moore SC, Fuhrman BJ, Dallal CM, et al. , Association of Estrogen Metabolism with Breast Cancer Risk in Different Cohorts of Postmenopausal Women. Cancer Res, 2017. 77(4): p. 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yager JD & Davidson NE, Estrogen carcinogenesis in breast cancer. N Engl J Med, 2006. 354(3): p. 270–82. [DOI] [PubMed] [Google Scholar]
- 25.Brinton LA, Awuah B, Nat Clegg-Lamptey J, Wiafe-Addai B, Ansong D, Nyarko KM, et al. , Design considerations for identifying breast cancer risk factors in a population-based study in Africa. Int J Cancer, 2017. 140(12): p. 2667–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyante SJ, Biritwum R, Figueroa J, Graubard B, Awuah B, Addai BW, et al. , Recruiting population controls for case-control studies in sub-Saharan Africa: The Ghana Breast Health Study. PLoS One, 2019. 14(4): p. e0215347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD & Ziegler RG, Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem, 2007. 79(20): p. 7813–21. [DOI] [PubMed] [Google Scholar]
- 28.Loud JT, Gierach GL, Veenstra TD, Falk RT, Nichols K, Guttmann A, et al. , Circulating estrogens and estrogens within the breast among postmenopausal BRCA1/2 mutation carriers. Breast Cancer Res Treat, 2014. 143(3): p. 517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD & Ziegler RG, Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal.Chem, 2007. 79(20): p. 7813–7821. [DOI] [PubMed] [Google Scholar]
- 30.James-Todd T, Senie R & Terry MB, Racial/ethnic differences in hormonally-active hair product use: a plausible risk factor for health disparities. J Immigr Minor Health, 2012. 14(3): p. 506–11. [DOI] [PubMed] [Google Scholar]
- 31.Galli CL, Bettin F, Metra P, Fidente P, De Dominicis E & Marinovich M, Novel analytical method to measure formaldehyde release from heated hair straightening cosmetic products: Impact on risk assessment. Regul Toxicol Pharmacol, 2015. 72(3): p. 562–8. [DOI] [PubMed] [Google Scholar]
- 32.Bocca B, Pino A, Alimonti A & Forte G, Toxic metals contained in cosmetics: a status report. Regul Toxicol Pharmacol, 2014. 68(3): p. 447–67. [DOI] [PubMed] [Google Scholar]
- 33.Kaličanin B & Velimirović D, A Study of the Possible Harmful Effects of Cosmetic Beauty Products on Human Health. Biol Trace Elem Res, 2016. 170(2): p. 476–84. [DOI] [PubMed] [Google Scholar]
- 34.Falk RT, Brinton LA, Dorgan JF, Fuhrman BJ, Veenstra TD, Xu X, et al. , Relationship of serum estrogens and estrogen metabolites to postmenopausal breast cancer risk: a nested case-control study. Breast Cancer Res, 2013. 15(2): p. R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated or analyzed for the current study are not publicly available due to data privacy of patients but are available from the corresponding author upon reasonable request.