Abstract
Obstructive sleep apnea (OSA) is the most common respiratory disease in the developed world and is commonly treated with positive airway pressure therapy (PAP). Recently, hypoglossal nerve (HNS) has been introduced as alternative treatment for OSA patients with PAP intolerance. We report the initial results with HNS treatment from Spain. Patients with OSA and PAP intolerance were screened for HNS treatment with the Inspire™ system. After implantation and activation, efficacy was evaluated with polysomnography and indication-specific questionnaires. Adherence data was recorded from the stimulation system. 18 patients (51.83 ± 11.64 years, 94% male, mean Body Mass Index 27.94 ± 3.20) received an Inspire™ UAS system and were included for analysis. Mean procedure time was 202.83 ± 64.87 min. and average hospital stay 26.67 ± 7.54 h. Mean Apnea–Hypopnea-Index was reduced by 63.44% (p ≤ 0.0001), while daytime sleepiness improved to a mean ESS score of 6.60 ± 1.25 (p ≤ 0.0001 Therapy response (AHI reduction > 50% and final AHI < 20), was reached in 64.70 and normalization of daytime sleepiness (final ESS score < 10) in 100% of patients. Therapy adherence was 6.32 ± 1.71 h per night. HNS is a safe and leads to effective OSA control and symptom normalization in selected OSA patients with PAP intolerance. Stimulation therapy is well accepted, as demonstrated by high adherence. Implementation of HNS therapy into an OSA program in Spain is feasible with acceptable learning curve and moderate resource utilization.
Keywords: Obstructive sleep apnea, Hypoglossal nerve stimulation, Upper airway stimulation, CPAP intolerance, Sleep surgery
Introduction
Obstructive sleep apnea (OSA) is the most common respiratory sleep disorder, which affects up to 34% of the adult population [1]. It is characterized by recurrent obstructions of upper airway tissue, mainly due to decrease in muscle tone during sleep. This can lead to a partial or complete cessation of air flow to the lungs, which consecutively impairs the gas exchange [2]. Patients with OSA often suffer from nightly hypoxemia as well as hypercapnia. Patients report impaired quality of life with main symptoms being excessive daytime sleepiness (EDS), due to regular arousals during sleep, followed by reduced daytime activity and depression. Left untreated, OSA can lead to development of cardiovascular and metabolic complications such as stroke, myocardial infarction, and diabetes. Patients with untreated OSA are also exposed to a higher risk of involvement in motor vehicle collisions or occupational accidents [3]. Gold-standard treatment for OSA is nocturnal application of positive airway pressure ventilation (PAP) via a mask [4]. Though highly effective, PAP treatment is associated with a significant rate of non-adherence or discontinuation, which can be up to 50% after 10 years [4].
As alternative treatments for OSA, oral appliances have shown to be non-inferior to PAP treatment in patients with mild-to-moderate OSA and absence of obesity [5]. Anatomy altering surgeries such as Uvulopalatopharyngoplasty (UPPP) are also widely available, though the longterm efficacy is often limited, due to relapse of airway tissue collapse over time [6]. Breathing synchronized electrical stimulation of the hypoglossal nerve (HNS) with the Inspire™ Upper Airway Stimulation system (Inspire™ UAS, Inspire Medical Systems, Inc. Golden Valley, MN/USA) was introduced recently as a new concept to treat upper airway obstruction and has been extensively studied over the last years [7, 8]. The treatment is based on unilateral implantation of a stimulation system, that is activating airway dilator muscles synchronized with inspiration and that is operated by the patient using an external remote control. Inclusion criteria for HNS treatment are moderate-to-severe OSA, absence of obesity with a Body Mass Index < 35 kg/m2, suitable airway anatomy and intolerance to PAP therapy [9]. After obtaining CE mark in 2010 and FDA approval in 2014, HNS was introduced in Europe and the United States and has been used in over 8,500 patients. This article reports the early results of HNS treatment using the Inspire™ UAS system in Spain, where the therapy was introduced in 2016 in a tertiary care center.
Materials and Methods
Patient Selection
Patients who consulted our ENT outpatient clinic with a history of OSA were evaluated for alternative treatment and, after thorough screening, offered different therapeutic modalities. Screening included in-lab polysomnography or home sleep test, clinical evaluation and drug-induced sleep endoscopy with propofol to assess airway anatomy and collapse patterns. Findings were reported using the VOTE classification [10]. The Epworth Sleepiness Scale was used to evaluate daytime symptoms of OSA. After airway evaluation, individual therapeutic options were discussed with the patient. Patients with an Apnea–Hypopnea Index (AHI) > 15 and < 65, < 25% central apnea, Body Mass Index (BMI) ≤ 35 kg/m2 and absence of soft palate concentric collapse were offered to receive HNS treatment (Table 1). All consecutive patients who received an HNS system between January 2016 and March 2020 were included in this study. The DISE revealed multi-level disease with AP soft palate obstruction in most (94%), and tongue base collapse in all cases (Table 2). Patients were excluded for severe cardiovascular or pulmonary comorbidities such as congestive heart failure NYHA class III/IV, severe COPD or recent myocardial infarction. Until August 2017, patients with a need for frequent magnetic resonance imaging (MRI) were excluded as available implantable components precluded use of MRI. Patients were thoroughly educated on the treatment with the HNS system and consent was obtained prior to treatment.
Table 1.
Baseline characteristics of patients treated with breathing-synchronized HNS therapy
| Characteristic | Value |
|---|---|
| N | 18 |
| Age (years) | 51.83 ± 11.64 |
| Male gender (%) | 94 |
| Body Mass Index (kg/m2) | 27.94 ± 3.20 |
| Apnea–Hypopnea Index (events(h) | 41.84 ± 19.43 |
| Oxygen-Desaturation Index (events(h) | 31.82 ± 47.23 |
| T90% (min.) | 10.00 ± 14.89 |
| Epworth Sleepiness Scale | 12.67 ± 3.87 |
Table 2.
Drug-induced sleep endoscopy findings according to VOTE classification
| Velum | Oropharynx | Tongue base | Epiglottis | |
|---|---|---|---|---|
| None | 11% | 89% | 0% | 72% |
| AP | 83% | 100% | 0% | |
| Lateral | 0% | 11% | 28% | |
| Concentric | 6%a |
aThis patient presented with partial concentric collapse at lower velum, which is not a contra-indication
Due to our unique position as a tertiary sleep center in Spain, referred patients have usually long histories of OSA treatment. Our cohort of HNS patients had on average 3.0 previous treatment (range 1–6, Table 3), with most common being positive airway pressure therapy (77%), tonsillectomy (55%), nasal surgery (55%) as well as soft palate surgery (44%).
Table 3.
Previous medical and surgical OSA treatment before receiving HNS treatment
| Treatment modality | % of patients receiving treatment |
|---|---|
| Positive airway pressure | 77.78 |
| Oral appliance | 22.22 |
| Sleep position trainer | 16.67 |
| Soft palate surgery | 44.44 |
| Tongue base surgery | 11.11 |
| Tonsillectomy | 55.56 |
| Genioglossus advancement | 5.56 |
| Maxillo-mandibular advancement | 5.56 |
| Nasal surgery | 55.56 |
Hypoglossal Nerve Stimulation Treatment
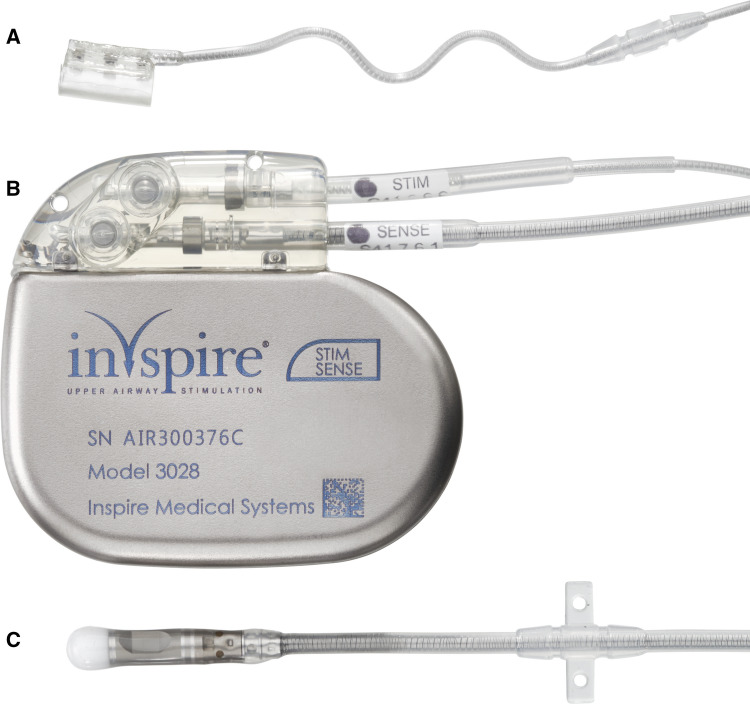
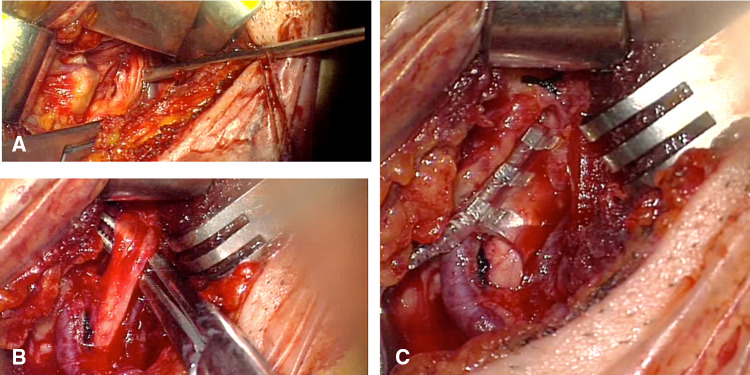
After approval by our institution to implement an HNS program, using the CE-marked Inspire™ Upper Airway Stimulation system (Inspire™ II/Inspire™ IV UAS system, Inspire Medical Systems, Inc. Golden Valley, MN USA), surgical training was conducted at a training facility from the manufacturer. The system consists of three implantable components (Fig. 1) and an external sleep remote, which is used to operate stimulation [7]. Implantation was conducted under general anesthesia in standardized manner, using previously published protocols [11]. The procedure requires three small incisions, one for each of the components. A modified submandibular incision is used to expose the distal branches of the hypoglossal nerve and those branches supplying tongue protrusor muscles, such as M. genioglossus and M. geniohyoid, are identified using two-channel nerve integrity monitoring (Natus Xltek Protektor 32 channels. Natus Excell- Tech, Oakville, Ontario, Canada) [12]. After isolating those branches, the circular cuff electrode is placed and secured on the digastric tendon to avoid dislodgement (Fig. 2). The pulse generator is placed in a small subcutaneous pocket underneath the clavicle and the breathing sensor, required to deliver stimulation synchronized with respiration, is placed between the external and internal intercostal muscles. Pulse generator and electrodes are connected using subcutaneous tunnels, which are prepared with a tunneling tool. Functioning of the system is tested prior to closure of incision to ensure good tongue protrusion with stimulation and proper detection of respiration. Patients were discharged on the second postoperative day and returned for device activation 4 weeks after implantation. At activation visit, proper device function was evaluated, and stimulation thresholds defined. Patients were educated on use of the sleep remote and stimulation parameters programmed, starting at the stimulation threshold to allow for a comfortable acclimatization. After 6–8 weeks, during which patients were asked to gradually increase stimulation intensity using the sleep remote, a fine-tune visit at our sleep lab was scheduled. Using polysomnographic monitoring according to American Academy of Sleep Medicine (AASM) recommendations, stimulation was further optimized in order to achieve highest therapeutic efficiency during all sleep phases, without creating arousals. OSA severity was evaluated after titration with AASM 2015 scoring criteria [13]. Sher criteria were further used to classify responders and non-responders [14]. Subjective response was evaluated with the Epworth Sleepiness Scale, with a reduction of at least two points considered minimally clinically relevant and a score less than 10 normalized daytime sleepiness. In addition, a structured survey was used to evaluate patient satisfaction. Procedure and device-related adverse events were recorded during follow-up, with serious adverse events being defined as events that lead to death or life-threatening injuries, permanent disabilities or sustained health impairments.
Fig. 1.
Components that are implanted in the body. a Stimulation lead with electrode cuff. b IPG. c Sensing lead
Fig. 2.
a Dissection of the hypoglossal nerve fibers. b Selection of protrude fibers of the hypoglossal nerve. c Stimulation cuff placed around protrude fibers
Statistical Analysis
Demographic and anthropometric variables were analyzed using descriptive statistics. A paired t-test was used to calculate the difference between baseline values and post-titration outcomes. Results were reported as mean ± standard deviation. P-values ≤ 0.05 were considered to be statistically significant. Patient satisfaction scores were calculated using the means of five-level Likert scales.
Results
Between January 2016 and March 2020, 18 patients received HNS treatment with the Inspire™ UAS device at our institution. The cohort consisted mainly of middle-aged (51.83 ± 11.64 years), primarily male patients (94%) with a history of severe OSA (AHI 41.84 ± 19.43) and pathologic daytime sleepiness (ESS 12.67 ± 3.87).
Surgical Results and Safety Outcomes
The average procedure time was 202 ± 64 min., and the average total hospital stay was 26.6 ± 7.5 h. With improvements in surgical processes, the procedure time was reduced meaningfully by 40 min, comparing the first nine patients (223.1 ± 76.0 min) to the latter nine (182.5 ± 42.5 min). Surgical success, defined as sufficient tongue motion (right- or bi-lateral protrusion) and adequate detection of respiratory signal was achieved in 100% of patients. Adverse events during the implantation procedure were reported in 5.5% of patients (n = 1, placement on wrong nerve with intraoperative replacement). Hypoglossal nerve injuries or palsy was not reported in any patient. Over the course of follow-up, one patient required replacement of a breathing sensor.
Reduction of OSA Severity
At the follow-up visits, which occurred 86.0 ± 29.6 days after implantation, the mean AHI decreased 64% compared to baseline (41.84 ± 19.43 vs. 13.12 ± 9.61, p < 0.0001, Figs. 3, 4). According to Sher criteria (AHI reduction > 50% from baseline to a value < 20), 65% of patients were classified responders (Table 4). At least 50% reduction in AHI was reported in 67% of treated patients. Nocturnal oxygen saturation improved compared to baseline. A 55% reduction in the Oxygen desaturation index (ODI, 31.82 ± 47.23 vs. 14.17 ± 23.31, p = n.s.) and a 69% reduction in Sleep time with < 90% O2 saturation (T90%, 10.00 ± 14.89 vs. 3.18 ± 5.12, p < 0.03) were observed, leading to clinically relevant reductions of hypoxic load in the majority of patients (78%).
Fig. 3.
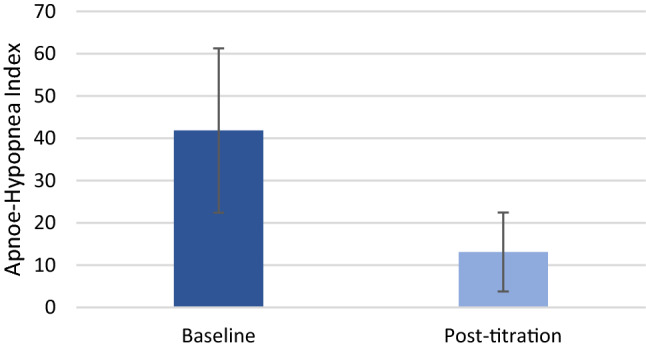
Baseline and post-titration Apnea–Hypopnea Index (41.84 ± 19.43 vs. 13.12 ± 9.61, p < 0.0001)
Fig. 4.
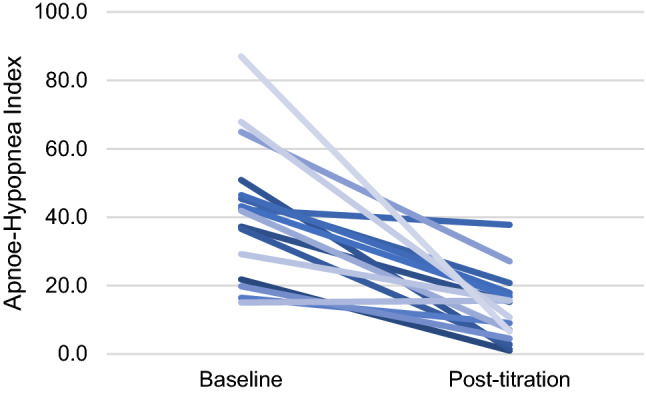
Decrease of OSA severity (AHI) on individual level
Table 4.
Post-titration objective and subjective results
| Parameter | Value |
|---|---|
| Apnea–Hypopnea Index (events(h) | 13.12 ± 9.61 |
| AHI reduction (%) | −63.44 |
| Sher response (%) | 64.70 |
| AHI < 15 (%) | 47.05 |
| Oxygen-Desaturation Index (events(h) | 14.17 ± 23.31 |
| ODI reduction (%) | −55.46 |
| T90% (min.) | 3.18 ± 5.13 |
| T90% reduction (%) | −68.20 |
| Epworth Sleepiness Scale | 6.60 ± 1.25 |
| ESS improvement (%) | 42% |
| ESS normalization (%) | 100% |
| Therapy adherence (h/night) | 6.32 ± 1.71 |
Subjective Response and Therapy Adherence
Daytime sleepiness measured with the ESS questionnaire significantly improved from 12.67 ± 3.87 at baseline to 6.60 ± 1.25 (p < 0.0001, Figs. 5, 6). The minimal clinically important difference for the ESS of 2 points was reached in 89% of patients. Normalization of daytime sleepiness, defined as ESS < 10, occurred in all patients under treatment. Objective adherence to UAS treatment was interrogated from the device and showed 6.32 ± 1.71 h per night. All patients, except one, were using stimulation for more than four hours per night. For patient satisfaction, 91% reported superior experience compared to PAP treatment, 89% would choose HNS again, 89% would recommend HNS to family and friends, and 86% reported to be generally satisfied with HNS therapy (Fig. 7).
Fig. 5.
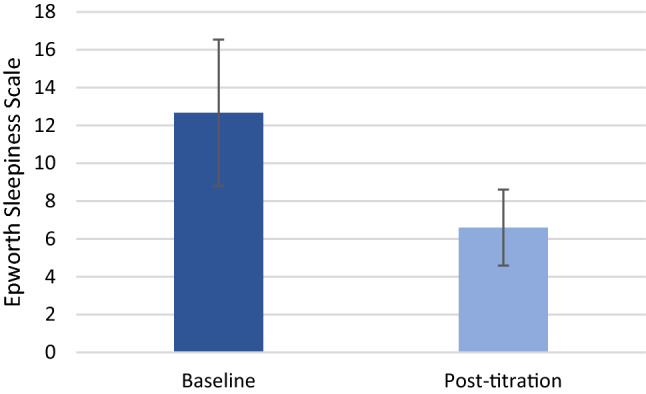
Baseline and post-titration Epworth Sleepiness Scale (12.67 ± 3.87 at baseline to 6.60 ± 1.25, p < 0.0001)
Fig. 6.
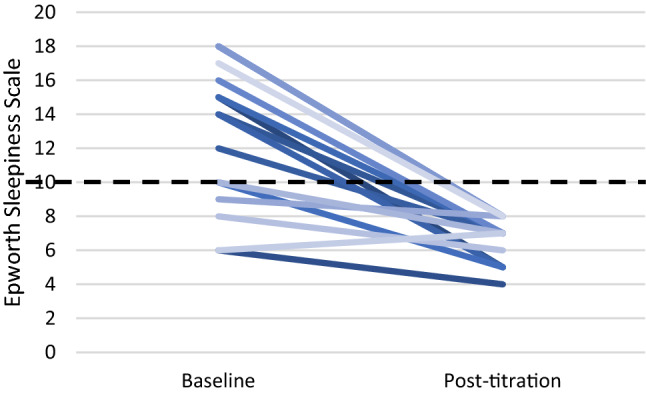
Decrease of daytime sleepiness (ESS) on individual level, values < 10 are considered normal (dashed line)
Fig. 7.
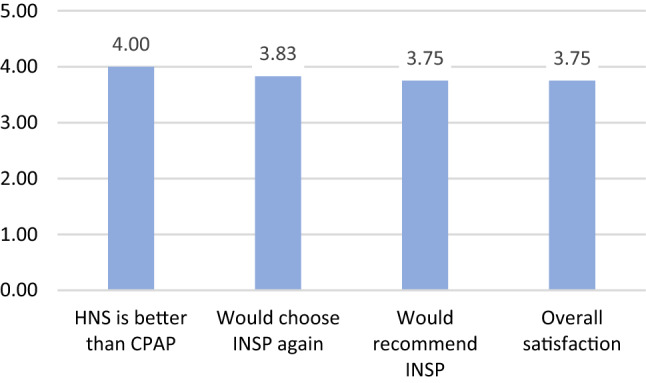
Patient satisfaction after HNS treatment, average agreement with each statement on a five-level Likert scale (1: strongly disagree—5: strongly agree)
Discussion
Our results show that HNS therapy using the breathing synchronized Inspire™ UAS system can be implemented in a multidisciplinary OSA program without major learning curve and it is well adopted by patients in search for alternative treatments after PAP intolerance. Safety and perioperative morbidity is favorable compared to other surgical interventions for OSA, as confirmed by other groups already [15, 16]. Treatment with HNS resulted in significant reduction of OSA severity and clinically meaningful improvements in quality of life. Improvement in daytime sleepiness, the most common symptom in OSA which often leads to further impairments in general quality of life, were more pronounced than commonly observed with nightly ventilation therapy [17]. Adverse events and side-effects were uncommon and did not impact therapy utilization among our patients, as reported by objective interrogation of the stimulation system. Our results reported demonstrate the need to broaden the portfolio of OSA interventions, as most of our patients had three or more previous treatments which did not relieved symptoms sufficiently or that could be adhered to. In a chronic disease such as OSA, physicians and providers will have to accept, that there will not be one treatment for the complete disease journey of a patient. PAP remains the gold-standard for treating OSA due to its relatively low costs and ubiquitous availability. Innovative treatments like HNS though will become more important, as many patients on PAP will require alternative treatments over the course of time [4]. HNS with the Inspire UAS system requires thorough screening and patient education in order to achieve good outcomes. Previously published studies evaluated predictors of success, which have been widely integrated into routine practice [18]. Our results confirm, that the results from larger multicentric studies can be repeated in a newly established program. DISE is a critical part of the screening and thorough airway assessment is crucial to rule out patients with soft palate concentric collapse.
One limitation of our study is that we could enroll only patients who were able to cover most of the costs for HNS treatment themselves. A significant financial contribution by the patient itself could lead to higher motivation to adhere to therapy. Evidence from markets where HNS treatment is fully covered by public payers as in Germany show comparable results though with regards to adherence and patient satisfaction [19, 20]. Another limitation of our study is the short follow-up of only three months after implantation. For data completeness we decided to focus on the short-term post-titration results which were available for all treated patients. Additional research is though required to provide information on chronic utilization in our cohort. Published results from other groups show though, that patient maintain high adherence once they acclimatized well to HNS and effective long-term OSA control is provided with stimulation treatment [21, 22].
Conclusions
Breathing-synchronized hypoglossal nerve stimulation leads to significant reductions in OSA severity and is associated with a normalization of daytime sleepiness in a cohort of Spanish patients with PAP intolerance. Our results show the need for alternative treatments for effective long-term OSA control and demonstrate the potential of HNS as such an alternative for selected patients with favorable airway anatomy. Implementation of a HNS program into routine practice is feasible and outcomes, comparable to those obtained in controlled clinical trials, can be achieved.
Funding
None.
Compliance with Ethical Standards
Conflict of interest
Dr Peter Baptista has received honoraria for giving presentation on Hypoglossal nerve stimulation by INSPIRE. The rest of authors have no disclosures.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Peter Michael Baptista, Email: peterbaptista@gmail.com.
Carlos Prieto-Matos, Email: cprietom@unav.es.
References
- 1.Gottlieb DJ, Punjabi NM. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA. 2020;323(14):1389–1400. doi: 10.1001/jama.2020.3514. [DOI] [PubMed] [Google Scholar]
- 2.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knauert M, Naik S, Gillespie MB, Kryger M. Clinical consequences and economic costs of untreated obstructive sleep apnea syndrome. W J Otorhinolaryngol Head Neck Surg. 2015;1(1):17–27. doi: 10.1016/j.wjorl.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoch OD, Baty F, Niedermann J, Rüdiger JJ, Brutsche MH. Baseline predictors of adherence to positive airway pressure therapy for sleep apnea: a 10-year single-center observational cohort study. Respiration. 2014;87(2):121–128. doi: 10.1159/000354186. [DOI] [PubMed] [Google Scholar]
- 5.Ramar K, et al. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J Clin Sleep Med. 2015;11(07):773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caples SM, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33(10):1396–1407. doi: 10.1093/sleep/33.10.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strollo PJJ, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 8.A. Costantino et al. (2019) Hypoglossal nerve stimulation long-term clinical outcomes: a systematic review and meta-analysis. Sleep Breath Schlaf Atm. doi: 10.1007/s11325-019-01923-2. [DOI] [PubMed]
- 9.Vanderveken OM, et al. Development of a Clinical Pathway and Technical Aspects of Upper Airway Stimulation Therapy for Obstructive Sleep Apnea. Front. Neurosci. 2017;11:523. doi: 10.3389/fnins.2017.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vito A, Carrasco Llatas M, Ravesloot MJ, Kotecha B, De Vries N, Hamans E, Maurer J, Bosi M, Blumen M, Heiser C, Herzog M, Montevecchi F, Corso RM, Braghiroli A, Gobbi R, Vroegop A, Vonk PE, Hohenhorst W, Piccin O, Sorrenti G, Vanderveken OM, Vicini C. European position paper on drug-induced sleep endoscopy: 2017 Update. Clin Otolaryngol. 2018;43(6):1541–1552. doi: 10.1111/coa.13213. [DOI] [PubMed] [Google Scholar]
- 11.Heiser C, Hofauer B, Lozier L, Woodson BT, Stark T. Nerve monitoring-guided selective hypoglossal nerve stimulation in obstructive sleep apnea patients. Laryngoscope. 2016;126(12):2852–2858. doi: 10.1002/lary.26026. [DOI] [PubMed] [Google Scholar]
- 12.Maurer JT, et al. Operative technique of upper airway stimulation: an implantable treatment of obstructive sleep apnea. Oper Tech Otolaryngol Head Neck Surg. 2012;2012(23):227–233. doi: 10.1016/j.otot.2012.07.002. [DOI] [Google Scholar]
- 13.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV (2015) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.2. Am Acad Sleep Med. www.aasmnet.org (online)
- 14.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19(2):156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 15.Huntley C, Chou DW, Doghramji K, Boon M. Comparing upper airway stimulation to expansion sphincter pharyngoplasty: a single university experience. Ann Otol Rhinol Laryngol. 2018;127(6):379–383. doi: 10.1177/0003489418771395. [DOI] [PubMed] [Google Scholar]
- 16.Shah J, Russell JO, Waters T, Kominsky AH, Trask D. Uvulopalatopharyngoplasty vs CN XII stimulation for treatment of obstructive sleep apnea: a single institution experience. Am J Otolaryngol. 2018;39(3):266–270. doi: 10.1016/j.amjoto.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163(5):565–571. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 18.Van de Heyning PH, et al. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope. 2012;122(7):1626–1633. doi: 10.1002/lary.23301. [DOI] [PubMed] [Google Scholar]
- 19.Steffen A, Sommer JU, Hofauer B, Maurer JT, Hasselbacher K, Heiser C (2017) Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. Laryngoscope 2017. doi: 10.1002/lary.26688. [DOI] [PubMed]
- 20.Hasselbacher K, Hofauer B, Maurer JT, Heiser C, Steffen A, Sommer JU. Patient-reported outcome: results of the multicenterGerman post-market study. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol Head Neck Surg. 2018;275(7):1913–1919. doi: 10.1007/s00405-018-5017-1. [DOI] [PubMed] [Google Scholar]
- 21.Woodson BT, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Neck Surg. 2018;159(1):194–202. doi: 10.1177/0194599818762383. [DOI] [PubMed] [Google Scholar]
- 22.Steffen A, Sommer UJ, Maurer JT, Abrams N, Hofauer B, Heiser C (2019) Long-term follow-up of the German post-market study for upper airway stimulation for obstructive sleep apnea. Sleep Breath Schlaf Atm. doi: 10.1007/s11325-019-01933-0. [DOI] [PubMed]