Abstract
Background
The appropriateness of using late lumen loss (LLL) as a surrogate endpoint was established in drug-eluting stent (DES) studies, but it was less supportive for drug-coated balloon (DCB) trials.
Methods
Studies published until 23 June 2021 were searched from PubMed, EMBASE, Cochrane Library, and ClinicalTrials.gov. The correlation between LLL, MLD (minimal lumen diameter), and %DS (percentage diameter stenosis) and clinical endpoints was evaluated by linear regression. Standardized effect size and its 95% CIs were used to illustrate the difference among LLL, MLD, and %DS.
Results
A total of 24 clinical trials were eligible [16 DCB vs. DES, 7 DCB vs. plain old balloon angioplasty (POBA), and 1 DCB vs. DES vs. POBA]. Thirteen (54.2%) trials used LLL as the primary endpoint. LLL, MLD, and %DS all had significant associations with clinical endpoints. For DCB vs. DES trials, the number of studies that reported inconsistent results between LLL and MLD was 12/16 (75.0%) and between LLL and %DS was 10/15 (66.7%), while in MLD and %DS, it was 1/16 (6.3%). The difference of standardized effect size between LLL and MLD was −0.47 (95% CI, −0.69 to −0.25, p < 0.001) and LLL and %DS was−0.31 (95%CI,−0.43 to−0.20, p < 0.001) while in MLD and %DS, there was no difference, 0.1 (95%CI,−0.02 to 0.22, p = 0.084).
Conclusions
For DCB trials, an appropriate surrogate is associated with the control device. The traditional LLL could be used in the DCB vs. POBA trials. However, MLD/%DS should be considered a more suitable surrogate endpoint when comparing DCB with DES.
Keywords: late lumen loss (LLL), minimal lumen diameter (MLD), percentage diameter stenosis (%DS), drug-coated balloons (DCBs), coronary artery diseases
Introduction
Drug-coated balloons (DCBs) have been extensively used for the treatment of in-stent restenosis (ISR) and de novo coronary lesions in some specific settings including small vessel disease (SVD), owing to the antiproliferative drug-eluting capability without the chronic limitations of permanent metallic implants (1). Surrogate endpoints have been widely used to demonstrate the efficacy of DCBs because of feasibility regarding the sample size. Research had proved the robustness of late lumen loss (LLL) in discriminating drug-eluting stent (DES) both in observational and randomized trials (2–4). However, the applicability of LLL to DCB is doubtful.
Late lumen loss is calculated by post-procedure minimal lumen diameter (MLD) minus follow-up MLD, reflecting the narrowing of the luminal diameter immediately after the intervention to the follow-up period. However, due to the elastic retraction, balloon angioplasty has a smaller acute gain compared with permanent scaffolds (5). A small LLL is somehow parallel with a small acute gain (6). In addition, late enlargement and vessel remodeling are achievable in a non-caged vessel in the treatment of DCB (7). Therefore, the surrogate endpoints reflecting the true status of the vessel (e.g. MLD and percentage diameter stenosis (%DS) at follow-up) might be a more ideal choice for DCB trials.
In the current study, we hypothesize that the results between LLL and MLD/%DS may not be consistent. We systematically sorted out the randomized controlled trials (RCT) focusing on DCB, assessed the relationship of these surrogate endpoints with clinical endpoints, and proved the inconsistency of LLL with MLD and %DS.
Methods
Study Subjects and Inclusion/Exclusion Criteria
A comprehensive search of studies published before 23 June 2021 was conducted on PubMed, EMBASE, Cochrane Library, and ClinicalTrials.gov, with the search terms (“drug-coated balloon” or “drug-eluting balloon”) and (“late lumen loss” or “late loss” or “late luminal loss” or “minimal lumen diameter” or “minimal luminal diameter” or “diameter stenosis”). All candidate studies were imported into Endnote (Endnote X9.3.1; Thomson Reuters, San Francisco, CA) for screening. The titles and abstracts were checked for possible relevance and duplicated studies were deleted. Further, full articles were reviewed for potential eligibility. Cross-referencing was also used to search for possibly missed articles.
The following inclusion criteria were used to assess the eligibility for each study: (1) in-stent restenosis or de novo coronary lesions; (2) RCT with DCB arm compared with DES or plain old balloon angioplasty (POBA); and (3) report at least two of LLL, MLD, or %DS. The exclusion criteria were as follows: (1) conference abstract, protocol, or review, and (2) the same clinical trial reporting long-term follow-up results.
Data Extraction
The following study level information was extracted from the recruited trials, including authors, trial name, study design, indication (ISR, de novo lesions) (8) interventions, number of patients at baseline, number of patients at follow-up, the definition of the primary endpoint. Further, the key results from each study were collected, including in-segment LLL, in-segement MLD, in-segement %DS, target lesion revascularization (TLR), target lesion failure (TLF), and major adverse cardiovascular events (MACE). For TLR, ischemia-driven TLR (ID-TLR) was preferentially selected. TLF was considered a composite of cardiac death, target vessel myocardial infarction (MI), and TLR. MACE was considered as a composite of any death, MI, and revascularization. When the study reported clinical results for more than one time-point, we chose the timepoint that was closer to the quantitative coronary angiography (QCA) results' timepoint.
Statistical Analysis
Binary variables were expressed as frequencies with percentages, and continuous variables are expressed as mean ± SD. A 2-sided p < 0.05 was considered statistically significant. All analyses were performed using R 4.0.3.
Association Between Surrogate and Clinical Endpoints
This part of the analysis was conducted at the interventional group level (the experiment and control arms from one study were used separately). Due to the systematic difference between POBA and the other two devices (DES or DCB), POBA data were excluded from the primary analysis for correlation and regression. Complete analysis (including POBA data) was conducted as sensitivity analysis. Surrogate endpoints included LLL, MLD, and %DS. Clinical endpoints focused on TLR, TLF, and MACE. The correlation coefficients were calculated for each pair of the surrogate and clinical endpoints. Linear regression analyses were performed and standardized regression coefficients (per SD increase) were used to evaluate the reflection of surrogates with the clinical endpoints. The analyses using linear regression models adjusted for the indication (ISR lesions or de novo lesions) were also performed. The adjusted β of the surrogate endpoints with clinical endpoints was considered the main indicator of the association. The higher the MLD reflected the better the vessel condition, so the opposite number of MLD was taken to maintain the same direction of benefit with LLL and %DS.
Inconsistency in LLL, MLD, and %DS
This part of the analysis was conducted at the trial level. Between-group differences were calculated for LLL, MLD, and %DS within each study. The standardized effect size (SES) was created for the comparability of effect size among the above surrogate endpoints. The SES was calculated using formula (1) (9, 10), where and were the mean of surrogate endpoints for treatment and control, respectively. n1 and n2 were the sample size, and s1 and s2 represented the standard deviation of each group.
The 95% confidence interval (CI) was calculated by SES ± 1.96 se where the se was calculated using formula (2) (11).
The SESs of three surrogate endpoints in each included trial were shown by forest plots grouped by devices: DCB vs. DES or DCB vs. POBA. Because a higher MLD indicates a better status, we took the opposite number of SES on MLD to maintain the same direction of benefit with LLL and %DS.
| (1) |
| (2) |
The inconsistency was considered if two of the surrogate endpoints gave a different result (favored DCB, favored DES, or equal), and the number of the inconsistencies was calculated and presented by n (%). Further, the difference in SES between every two surrogate endpoints was calculated. The inconsistency was recognized as the opposite direction of the observed effect size (SES) among different surrogate endpoints. A paired t-test was used to detect the potential discrepancies between LLL, MLD, or %DS. The analysis was grouped by the type of devices (DCB vs. DES/DCB vs. POBA) and compared separately. A subgroup analysis of indication (ISR/de novo lesions) was also conducted.
Results
Study Selection
The process of the literature search was illustrated in eFigure 1. A total of 1,089 articles were yielded by searching PubMed, EMBASE, Cochrane Library, and ClinicalTrials.gov. A total of 454 articles were excluded owing to duplicates. After reviewing the titles and abstracts, 138 conference abstracts, 5 reviews, 120 protocols, and 286 articles which were irrelevant to our topic were deleted. The remaining 86 studies were further evaluated for eligibility through full texts. Sixty-two articles were further deleted for the following reasons: peripheral artery diseases (n = 37), resulting from the same study (n = 24), and without a QCA result (n = 1). Finally, 24 RCTs were identified, consisting of 16 trials comparing DCB vs. DES (12–27), 7 trials comparing DCB vs. POBA (28–34), and 1 trial comparing DCB vs. DES and POBA (35). The three-arm trial was split into DCB vs. DES and DCB vs. POBA for further analysis.
Summary of the Included Trials
The 17 recruited trials comparing DCB vs. DES trials involved 3,557 patients, of which 1,822 were allocated to DCB and 1,735 were allocated to DES. The median QCA follow-up rate was 86 and 84% for DCB and DES, respectively. Of the 17 trials, 10 (58.9%) trials were indicated for ISR, and 7 (41.2%) trials were de novo lesions. Eight (47.1%) trials used LLL as the primary endpoint, 3 (17.7%) trials used MLD, and 3 (17.7%) trials used %DS (Table 1).
Table 1.
Baseline information of the included trials.
| References | Trial | Devices | Hypothesis | Indication | Na | Primary endpoint |
|---|---|---|---|---|---|---|
| Unverdorben et al. (25) | NA | DCB vs. DES | superiority | ISR | 66/65 | LLL (6 m) |
| Byrne et al. (35) | ISAR-DESIRE 3b | DCB vs. DES | noninferiority | ISR | 137/131 | %DS (6–8 m) |
| Adriaenssens et al. (13) | SEDUCE | DCB vs. DES | not specify | ISR | 25/25 | uncovered stent struts (9 m) |
| Alfonso et al. (15) | RIBS V | DCB vs. DES | superiority | ISR | 95/94 | MLD (6–9 m) |
| Xu et al. (27) | PEPCAD China ISR | DCB vs. DES | noninferiority | ISR | 109/106 | LLL (9 m) |
| Alfonso et al. (14) | RIBS IV | DCB vs. DES | superiority | ISR | 154/155 | MLD (6–9 m) |
| Pleva et al. (23) | NA | DCB vs. DES | noninferiority | ISR | 68/68 | LLL (12 m) |
| Wong et al. (26) | RESTORE | DCB vs. DES | superiority | ISR | 86/86 | LLL (9 m) |
| Baan et al. (16) | DARE | DCB vs. DES | noninferiority | ISR | 137/141 | MLD (6 m) |
| Jensen et al. (20) | BIOLUX | DCB vs. DES | noninferiority | ISR | 157/72 | LLL (6 m) |
| Cortese et al. (18) | PICCOLETO | DCB vs. DES | noninferiority | De novo | 29/31 | %DS (6 m) |
| Latib et al. (21) | BELLO | DCB vs. DES | noninferiority | De novo | 90/92 | LLL (6 m) |
| Tang et al. (24) | RESTORE SVD | DCB vs. DES | noninferiority | De novo | 116/114 | %DS (9 m) |
| Fahrni et al. (12) | BASKET-SMALL 2 | DCB vs. DES | noninferiority | De novo | 367/371 | MACE (12 m) |
| Cortese et al. (17) | PICCOLETO II | DCB vs. DES | noninferiority | De novo | 118/114 | LLL (6 m) |
| Nishiyama et al. (22) | NA | DCB vs. DES | not specify | De novo | 27/33 | 8 m |
| Gobic et al. (19) | NA | DCB vs. DES | not specify | De novo | 41/37 | LLL (6 m) |
| Habara et al. (29) | NA | DCB vs. POBA | not specify | ISR | 25/25 | LLL (6 m) |
| Scheller et al. (32) | PACCOCATH ISR I+II | DCB vs. POBA | superiority | ISR | 54/54 | LLL (6 m) |
| Rittger et al. (31) | PEPCAD-DES | DCB vs. POBA | superiority | ISR | 72/38 | LLL (6 m) |
| Byrne et al. (35) | ISAR-DESIRE 3 | DCB vs. POBA | superiority | ISR | 137/134 | %DS (6–8 m) |
| Habara et al. (34) | NA | DCB vs. POBA | superiority | ISR | 137/71 | TVF (6 m) |
| Scheller et al. (33) | PATENT-C | DCB vs. POBA | superiority | ISR | 33/28 | LLL (6 m) |
| Kleber et al. (30) | NA | DCB vs. POBA | superiority | De novo | 32/32 | LLL (9 m) |
| Funatsu et al. (28) | NA | DCB vs. POBA | superiority | De novo | 92/41 | TVF (6 m) |
aNumber of patients.
bISAR-DESIRE 3 trial compared DCB with DES and POBA.
BMS, bare-mental stents; DCB, drug-coated balloons; DES, drug-eluting stents; %DS, percentage diameter stenosis; HS, healing score; ISR, in-stent restenosis; LLL, late lumen loss; MLD, minimal lumen diameter; MACE, major adverse cardiovascular events; NA, not available; POBA, plain old balloon angioplasty; QCA, quantitative coronary angiography; SV, small vessels; TVF, target vessel failure.
The 8 recruited studies comparing DCB vs. POBA trials involved 1,005 patients, of which 582 were allocated to DCB, and 423 were allocated to POBA. The median QCA follow-up rate was 88 and 89% among the DCB and POBA groups, respectively. Of the 8 trials, 6 (75.0%) trials were ISR, and 2 (25.0%) trials were de novo lesions. Five (62.5%) trials used LLL as the primary endpoint, no trial used MLD as the primary endpoint, and 1 (12.5%) trial used %DS as the primary endpoint (Table 1).
Association Between LLL, MLD, and %DS vs. Clinical Endpoints
Among 23 two-arm trials, 20 trials reported three QCA results, 18 trials reported TLR results, 11 trials reported TLF results, and 17 trials reported MACE results and the three-arm trial gave 3 QCA, TLR, and MACE results (eTable 1; eTable 2). All LLL, MLD, and %DS were associated with the incidence of TLR, TLF, and MACE for the included devices and for the subgroup of DCB devices (eTable 3; Figure 1). For DCB devices, after adjusting the indication (ISR or de novo lesions), MLD and %DS attributed more clinical endpoints compared with LLL. There were a 6.1% increase in TLR per SD decrease in in-segment MLD but a 4.7% increase in TLR per SD increase in in-segment %DS and a 3.4% increase in TLR per SD increase in in-segment LLL. Similar results were observed on TLF and MACE. For ISR, the LLL, MLD, and %DS showed significant association with TLR and MACE. However, for de novo lesions, LLL did not show significant association with TLR, TLF, and MACE, while MLD and %DS showed significant association with TLR and MACE (eFigure 2). Sensitivity analysis (including POBA data) results were shown in the eTable 4, eFigure 3 in (Supplementary Appendix).
Figure 1.
Regression of LLL, MLD, or %DS vs. clinical endpoints (TLR, TLF, and MACE) (Excluding POBA devices). (A) For 30 devices reporting the LLL and TLR values, there was a significant relationship between LLL and TLR (R-squared = 0.388 y = 22.891x+2.169, p < 0.001). (B) For 32 devices reporting the MLD and TLR values, there was a significant relationship between MLD and TLR (R-squared = 0.195, y = 10.854x+27.688, p = 0.011). (C) For 31 devices reporting the %DS and TLR values, there was a significant relationship between %DS and TLR (R-squared = 0.304, y = 0.546x-8.438, p = 0.001). (D) For 17 devices reporting the LLL and TLF values, there was a significant relationship between LLL and TLF (R-squared = 0.348, y = 18.973x+5.288, p = 0.013). (E) For 32 devices reporting the MLD and TLF values, there was a significant relationship between MLD and TLF (R-squared = 0.253, y = 16.665x+40.904, p = 0.028). (F) For 31 devices reporting the %DS and TLF values, there was a significant relationship between %DS and TLF (R-squared = 0.258, y = 0.799x-13.904, p = 0.026). (G) For 30 devices reporting the LLL and MACE values, there was a significant relationship between LLL and MACE (R-squared.352, y = 26.093x+6.686, p = 0.001). (H) For 32 devices reporting the MLD and MACE values, there was a significant relationship between MLD and MACE (R-squared = 0.235, y = 11.185x+33.359, p = 0.005). (I) For 31 devices reporting the %DS and MACE values, there was a significant relationship between %DS and MACE (R-squared = 0.211, y = 0.542x-3.7, p = 0.012). In order to maintain the same direction of benefit, the standardized effect size of MLD here took the opposite number. DCB, drug-coated balloons; DES, drug-eluting stents; %DS, percentage diameter stenosis; ISR, in-stent restenosis; LLL, late lumen loss; MLD, minimal lumen diameter; MACE, major adverse cardiovascular events; POBA, plain old balloon angioplasty; QCA, quantitative coronary angiography; TLF, target lesion failure; TLR, target lesion revascularization.
Inconsistency in LLL, MLD, and %DS
The standardized effect size of LLL, MLD, and %DS between the treatment group and control in each recruited study was displayed through forest plots. For trials comparing DCB vs. DES, the number of studies that reported inconsistent results between LLL and MLD was 12/16 (75.0%), between LLL and %DS was 10/15 (66.7%), and between MLD and %DS was 1/16 (6.3%). The subgroup analysis of ISR and de novo lesions gave a similar result (Figure 2A; Table 2). The difference of the standardized effect size in LLL and MLD, LLL and %DS were significant, with the means of −0.47 (95%CI, −0.69 to −0.25, p < 0.001) and −0.31 (95%CI, −0.43 to −0.20, p < 0.001), respectively (Table 2). The subgroup analysis of ISR and de novo lesions gave a similar result, except that the standardized effect size of MLD and %DS had a slight difference in de novo lesions, 0.28 (95%CI, 0.01 to 0.55, p = 0.044).
Figure 2.
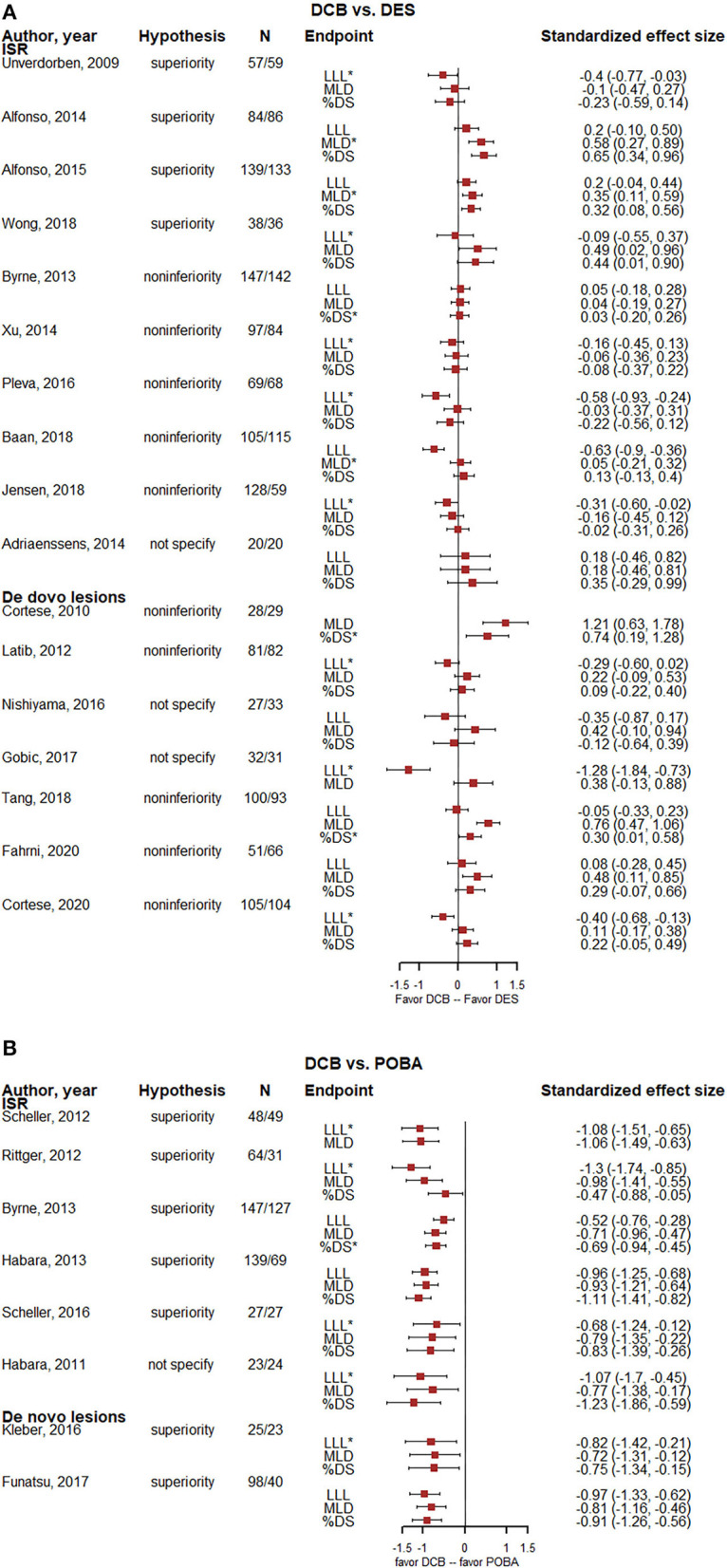
Forest plots of the standardized effect size of LLL, MLD, and %DS. (A) Forest plots for 17 DCB vs. DES trials. (B) Forest plots for 8 DCB vs. POBA trials. In order to maintain the same direction of benefit, the MLD here took the opposite number. N means the number of lesions. *Means the primary endpoint of the trial. DCB, drug-coated balloons; DES, drug-eluting stents; %DS, percentage diameter stenosis; ISR, in-stent restenosis; LLL, late lumen loss; MLD, minimal lumen diameter; POBA, plain old balloon angioplasty.
Table 2.
Inconsistencies among the surrogate endpoints.
| N | LLL-MLDa | P-value | N | LLL-%DS | P-value | N | MLD-%DS | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| The number of inconsistencies, n (%) | |||||||||
| DCB vs. DES | |||||||||
| Total | 16 | 12/16 (75.0) | NA | 15 | 10/15 (66.7) | NA | 16 | 1/16 (6.3) | NA |
| ISR | 10 | 7/10 (70.0) | NA | 10 | 7/10 (70.0) | NA | 10 | 0/10 (0.0) | NA |
| De novo | 6 | 5/6 (83.3) | NA | 5 | 3/5 (60.0) | NA | 6 | 1/6 (16.7) | NA |
| DCB vs. POBA | |||||||||
| Total | 8 | 0/8 (0.0) | NA | 7 | 0/7 (0.0) | NA | 7 | 0/7 (0.0) | NA |
| ISR | 6 | 0/6 (0.0) | NA | 5 | 0/5 (0.0) | NA | 5 | 0/5 (0.0) | NA |
| De novo | 2 | 0/2 (0.0) | NA | 2 | 0/2 (0.0) | NA | 2 | 0/2 (0.0) | NA |
| Difference of SES, mean (95%CI) | |||||||||
| DCB vs. DES | |||||||||
| Total | 16 | −0.47 (−0.69, −0.25) | <0.001 | 15 | −0.31 (−0.43, −0.2) | <0.001 | 16 | 0.1 (−0.02, 0.22) | 0.084 |
| ISR | 10 | −0.29 (−0.47, −0.11) | 0.006 | 10 | −0.29 (−0.46, −0.12) | 0.004 | 10 | 0 (−0.08, 0.08) | 0.941 |
| De novo | 6 | −0.78 (−1.26, −0.29) | 0.009 | 5 | −0.36 (−0.56, −0.15) | 0.008 | 6 | 0.28 (0.01, 0.55) | 0.044 |
| DCB vs. POBA | |||||||||
| Total | 8 | −0.08 (−0.23, 0.07) | 0.259 | 7 | −0.05 (−0.38, 0.29) | 0.741 | 7 | 0.04 (−0.23, 0.31) | 0.726 |
| ISR | 6 | −0.06 (−0.28, 0.16) | 0.504 | 5 | −0.04 (−0.59, 0.51) | 0.852 | 5 | 0.03 (−0.41, 0.47) | 0.854 |
| De novo | 2 | −0.13 (−0.52, 0.26) | 0.147 | 2 | −0.07 (−0.12, −0.02) | 0.038 | 2 | 0.06 (−0.37, 0.5) | 0.319 |
aIn order to maintain the same direction of benefit, the standardized effect size of MLD here took the opposite number.
CI, confidence interval; DCB, drug-coated balloons; DES, drug-eluting stents; %DS, percentage diameter stenosis; ISR, in-stent restenosis; LLL, late lumen loss; MLD, minimal lumen diameter; NA, not applicable; POBA, plain old balloon angioplasty.
For trials comparing DCB vs. POBA, no evidence showed discrepancy among the observed effect sizes between LLL, MLD, and %DS (Figure 2B; Table 2). The subgroup analysis of ISR and de novo lesions gave a similar result.
Discussion
Late lumen loss was still the most commonly used surrogate endpoint for DCB in coronary heart disease trials. Our study showed that MLD and %DS, together with LLL, were all correlated with TLR, TLF, and MACE. After adjusting the indication (ISR lesions or de novo lesions), MLD and %DS gave more attribution to clinical endpoints compared with LLL. In addition, among studies that compared DCB with POBA, the three QCA surrogate endpoints showed similar observed results. However, for DCB vs. DES trials, the inconsistency of LLL with MLD or %DS was obvious, and the standardized effect sizes in LLL with %DS or MLD were significantly different. However, MLD and %DS gave consistent results and did not have significantly different standardized effect sizes.
The inconsistent observed effect size between LLL and MLD/%DS should be noticed. It indicated that an inappropriate surrogate endpoint may lead to a wrong estimation of the efficacy of DCB. LLL was commonly used as the primary endpoint for DES devices, because the failure of DES was mostly caused by neointimal hyperplasia (36). The validity of LLL had been demonstrated in the era of DES vs. BMS trials (2–4). The acute gain was comparable between experimental and control devices. Therefore, LLL, a direct angiographic measure of neointimal hyperplasia, became an appropriate surrogate endpoint. However, the vessel condition after DCB treatment was different, and the appropriateness of LLL should be re-evaluated accordingly (37–47).
For DCB devices, polymer materials failed to provide effective support equivalent to that of metal stents, which led to acute recoil of blood vessels, and this was the main reason for the restenosis instead of neointimal hyperplasia (7). More importantly, for native vessels, especially in the case of small vessels and distal lesions, the late lumen of some vessels enlarged without reduction (48, 49). The vessels were free from the continuous stimulus caused by the stent, which led to intimal hyperplasia and negative vascular remodeling. Taking the above considerations, the mechanism of changes in vessel diameter was more complex after DCB treatment. The joint effect of acute recoiling, intimal hyperplasia, and vascular remodeling would be attributed to the differences among QCA measurements.
In our study, for DCB vs. DES trials, 12/16 (75%) studies reported inconsistent results between LLL and MLD, and 10/15 (66.7%) studies reported inconsistent results between LLL and %DS, while only one study reported inconsistent results between MLD and %DS. In addition, among the studies which gave an inconsistent QCA result, all LLL indicated better performance of DCB than DES, while MLD and %DS, which directly reflected the state of vessels, did not. Kang et al. (49) conducted a multivariate analysis and demonstrated that post-MLD and %DS were helpful to get optimal results in de novo lesions after DCB. It could be partly explained that, after the adjustment of lesion type (ISR or de novo lesion), the association between LLL and clinical endpoints were diluted, due to the decision on repeated revascularization which may have systematic difference for patients with or without the stent in their vessel. In general, the relationship with clinical endpoint (TLR, TLF, and MACE) also supported the usage of MLD and %DS as appropriate surrogate endpoints. Thus, we considered that MLD and %DS might be more reliable discriminators of DCB performance.
The strength of our study is that we systematically summarized the relationship between QCA indexes and clinical endpoints in DCB trials and assessed the consistency of LLL, MLD, and %DS using standardized effect sizes.
However, our study has several limitations. First, our study was based on published literature, there were no individual-level patient data. Although we included the RCT trials to partly ensure the quality of recruited studies, besides the LLL, MLD, and %DS, potential other surrogate endpoints such as acute gain were not considered. Second, the period of the eligible studies is > 10 years, and the interventional technology of DES, DCB, and POBA may have undergone major changes. However, according to our inconsistency test of the three QCA indexes, the result did not differ across publication years. Third, the number of eligible studies is not enough for a subgroup analysis (ISR and de novo lesions, paclitaxel, and sirolimus-coated balloon). We suggested that the heterogeneity in the mechanism of restenosis after DCB treatment was low. Fourth, the methodology of LLL calculation in DCB trials was inconsistent, which could cause systematic bias. The definition of clinical outcomes was not the same in the original studies. TLR is influenced by both clinical symptoms and QCA results, and using ID-TLR could get a more accurate result (50, 51). Lastly, eligible research lacked intraluminal imaging data, and it is limited to inferring the mechanism of difference between LLL and MLD/%DS based on QCA measurement alone. Therefore, further studies based on patient-level data and with identical definitions of clinical endpoints are still needed to determine the robustness of MLD and %DS for DCB trials.
Conclusions
For DCB trials, the appropriate surrogate endpoint selection depends on the type of control. The traditional LLL could be used in the DCB vs. POBA trials. However, MLD or %DS should be considered as a more suitable surrogate endpoint instead of LLL when comparing DCB with DES devices.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
XLa analyzed data and wrote the manuscript. YW came up with the conception and design of the paper. CW, XL, YZha, ML, XLi, and YZhu contributed to reviewing, discussing, and amending the manuscript. LS and BX reviewed the manuscript and gave clinical advice. YW and WL did the final review and provided approval of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the members of the Medical Research and Biometrics Center for their help.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.897365/full#supplementary-material
References
- 1.Liu W, Zhang M, Chen G, Li Z, Wei F. Drug-coated balloon for de novo coronary artery lesions: a systematic review and trial sequential meta-analysis of randomized controlled trials. Cardiovasc Ther. (2020) 2020:4158363. 10.1155/2020/4158363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mauri L, Orav EJ, Candia SC, Cutlip DE, Kuntz RE. Robustness of late lumen loss in discriminating drug-eluting stents across variable observational and randomized trials. Circulation. (2005) 112:2833–9. 10.1161/CIRCULATIONAHA105.570093 [DOI] [PubMed] [Google Scholar]
- 3.Mauri L, Orav EJ, Kuntz RE. Late loss in lumen diameter and binary restenosis for drug-eluting stent comparison. Circulation. (2005) 111:3435–42. 10.1161/CIRCULATIONAHA.104.513952 [DOI] [PubMed] [Google Scholar]
- 4.Mauri L, Orav EJ, O'Malley AJ, Moses JW, Leon MB, Holmes DR Jr, et al. Relationship of late loss in lumen diameter to coronary restenosis in sirolimus-eluting stents. Circulation. (2005) 111:321–7. 10.1161/01.CIR.0000153356.72810.97 [DOI] [PubMed] [Google Scholar]
- 5.Bangalore S, Mauri L. Late loss in a disappearing frame of reference: is it still applicable to fully absorbable scaffolds? EuroIntervention. (2009) 5:F43–8. 10.4244/EIJV5IFA7 [DOI] [PubMed] [Google Scholar]
- 6.Byrne RA, Kastrati A. Lesions in small coronary vessels disease: should drug-coated balloons replace drug-eluting stents as the treatment of choice? EuroIntervention. (2011) 7:K47–52. 10.4244/EIJV7SKA8 [DOI] [PubMed] [Google Scholar]
- 7.Cortese B, Silva Orrego P, Agostoni P, Buccheri D, Piraino D, Andolina G, et al. Effect of drug-coated balloons in native coronary artery disease left with a dissection. JACC Cardiovasc Interv. (2015) 8:2003–9. 10.1016/j.jcin.2015.08.029 [DOI] [PubMed] [Google Scholar]
- 8.Jeger RV, Eccleshall S, Wan Ahmad WA, Ge J, Poerner TC, Shin ES, et al. Drug-coated balloons for coronary artery disease: third report of the international dcb consensus Group. JACC Cardiovasc Interv. (2020) 13:1391–402. 10.1016/j.jcin.2020.02.043 [DOI] [PubMed] [Google Scholar]
- 9.Flury BK, Riedwyl H. Standard distance in univariate and multivariate analysis. Am Stat. (1986) 40:249–51. 10.1080/00031305.1986.10475403 [DOI] [Google Scholar]
- 10.Killeen PR. An alternative to null-hypothesis significance tests. Psychol Sci. (2005) 16:345–53. 10.1111/j.0956-7976.2005.01538.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. (2007) 82:591–605. 10.1111/j.1469-185X.2007.00027.x [DOI] [PubMed] [Google Scholar]
- 12.Fahrni G, Scheller B, Coslovsky M, Gilgen N, Farah A, Ohlow MA, et al. Drug-Coated Balloon Versus Drug-Eluting Stent in Small Coronary Artery Lesions: Angiographic Analysis from the Basket-Small 2 Trial. Clin res cardiol. (2020) 109:1114–24. 10.1007/s00392-020-01603-2 [DOI] [PubMed] [Google Scholar]
- 13.Adriaenssens T, Dens J, Ughi G, Bennett J, Dubois C, Sinnaeve P, et al. Optical coherence tomography study of healing characteristics of paclitaxel-eluting balloons vs. everolimus-eluting stents for in-stent restenosis: the seduce (safety and efficacy of a drug eluting balloon in coronary artery restenosis) randomised clinical trial. EuroIntervention. (2014) 10:439–48. 10.4244/EIJV10I4A77 [DOI] [PubMed] [Google Scholar]
- 14.Alfonso F, Perez-Vizcayno MJ, Cardenas A, Garcia del Blanco B, Garcia-Touchard A, Lopez-Minguez JR, et al. A prospective randomized trial of drug-eluting balloons versus everolimus-eluting stents in patients with in-stent restenosis of drug-eluting stents: the Ribs IV randomized clinical trial. J Am Coll Cardiol. (2015) 66:23–33. 10.1016/j.jacc.2015.04.063 [DOI] [PubMed] [Google Scholar]
- 15.Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, García del Blanco B, Seidelberger B, Iñiguez A, et al. A randomized comparison of drug-eluting balloon versus everolimus-eluting stent in patients with bare-metal stent–in-stent restenosis: the RIBS V clinical trial (restenosis intra-stent of bare metal stents: paclitaxel-eluting balloon vs everolimus-eluting stent). J Am Coll Cardiol. (2014) 63:1378–86. 10.1016/j.jacc.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 16.Baan J, Claessen BE, Dijk K-V, Vendrik J, van der Schaaf RJ, Meuwissen M, et al. A randomized comparison of paclitaxel-eluting balloon versus everolimus-eluting stent for the treatment of any in-stent restenosis: the dare trial. JACC. (2018) 11:287–97. 10.1016/j.jcin.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 17.Cortese B, Di Palma G, Guimaraes MG, Piraino D, Orrego PS, Buccheri D, et al. Drug-coated balloon versus drug-eluting stent for small coronary vessel disease: piccoleto Ii randomized clinical trial. JACC. (2020) 13:2840–9. 10.1016/j.jcin.2020.08.035 [DOI] [PubMed] [Google Scholar]
- 18.Cortese B, Micheli A, Picchi A, Coppolaro A, Bandinelli L, Severi S, et al. Paclitaxel-coated balloon versus drug-eluting stent during pci of small coronary vessels, a prospective randomised clinical trial. the piccoleto study. Heart. (2010) 96:1291–6. 10.1136/hrt.2010.195057 [DOI] [PubMed] [Google Scholar]
- 19.Gobić D, Tomulić V, Lulić D, Židan D, Brusich S, Jakljević T, et al. Drug-coated balloon versus drug-eluting stent in primary percutaneous coronary intervention: a feasibility study. Am J Med Sci. (2017) 354:553–60. 10.1016/j.amjms.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 20.Jensen CJ, Richardt G, Tölg R, Erglis A, Skurk C, Jung W, et al. Angiographic and clinical performance of a paclitaxel-coated balloon compared to a second-generation sirolimus-eluting stent in patients with in-stent restenosis: the biolux randomised controlled trial. EuroIntervention. (2018) 14:1096–103. 10.4244/EIJ-D-17-01079 [DOI] [PubMed] [Google Scholar]
- 21.Latib A, Colombo A, Castriota F, Micari A, Cremonesi A, De Felice F, et al. A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: the bello (balloon elution and late loss optimization) study. J Am Coll Cardiol. (2012) 60:2473–80. 10.1016/j.jacc.2012.09.020 [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama N, Komatsu T, Kuroyanagi T, Fujikake A, Komatsu S, Nakamura H, et al. Clinical value of drug-coated balloon angioplasty for de novo lesions in patients with coronary artery disease. Int J Cardiol. (2016) 222:113–8. 10.1016/j.ijcard.2016.07.156 [DOI] [PubMed] [Google Scholar]
- 23.Pleva L, Kukla P, Kusnierova P, Zapletalova J, Hlinomaz O. Comparison of the efficacy of paclitaxel-eluting balloon catheters and everolimus-eluting stents in the treatment of coronary in-stent restenosis: the treatment of in-stent restenosis study. Circ Cardiovasc Interv. (2016) 9:e003316. 10.1161/CIRCINTERVENTIONS.115.003316 [DOI] [PubMed] [Google Scholar]
- 24.Tang Y, Qiao S, Su X, Chen Y, Jin Z, Chen H, et al. Drug-coated balloon versus drug-eluting stent for small-vessel disease: the restore SVD China randomized trial. JACC Cardiovasc Interv. (2018) 11:2381–92. 10.1016/j.jcin.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 25.Unverdorben M, Vallbracht C, Cremers B, Heuer H, Hengstenberg C, Maikowski C, et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation. (2009) 119:2986–94. 10.1161/CIRCULATIONAHA.108.839282 [DOI] [PubMed] [Google Scholar]
- 26.Wong YTA, Kang DY, Lee JB, Rha SW, Hong YJ, Shin ES, et al. Comparison of drug-eluting stents and drug-coated balloon for the treatment of drug-eluting coronary stent restenosis: a randomized restore trial. Am Heart J. (2018) 197:35–42. 10.1016/j.ahj.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 27.Xu B, Gao R, Wang J, Yang Y, Chen S, Liu B, et al. A prospective, multicenter, randomized trial of paclitaxel-coated balloon versus paclitaxel-eluting stent for the treatment of drug-eluting stent in-stent restenosis: results from the pepcad China Isr trial. JACC Cardiovasc Interv. (2014) 7:204–11. 10.1016/j.jcin.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 28.Funatsu A, Nakamura S, Inoue N, Nanto S, Nakamura M, Iwabuchi M, et al. A multicenter randomized comparison of paclitaxel-coated balloon with plain balloon angioplasty in patients with small vessel disease. Clin Res Cardiol. (2017) 106:824–32. 10.1007/s00392-017-1126-x [DOI] [PubMed] [Google Scholar]
- 29.Habara S, Mitsudo K, Kadota K, Goto T, Fujii S, Yamamoto H, et al. Effectiveness of paclitaxel-eluting balloon catheter in patients with sirolimus-eluting stent restenosis. JACC Cardiovasc Interv. (2011) 4:149–54. 10.1016/j.jcin.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 30.Kleber FX, Rittger H, Ludwig J, Schulz A, Mathey DG, Boxberger M, et al. Drug eluting balloons as stand alone procedure for coronary bifurcational lesions: results of the randomized multicenter pepcad-bif trial. Clin Res Cardiol. (2016) 105:613–21. 10.1007/s00392-015-0957-6 [DOI] [PubMed] [Google Scholar]
- 31.Rittger H, Brachmann J, Sinha AM, Waliszewski M, Ohlow M, Brugger A, et al. A randomized, multicenter, single-blinded trial comparing paclitaxel-coated balloon angioplasty with plain balloon angioplasty in drug-eluting stent restenosis: the pepcad-des study. J Am Coll Cardiol. (2012) 59:1377–82. 10.1016/j.jacc.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 32.Scheller B, Clever YP, Kelsch B, Hehrlein C, Bocksch W, Rutsch W, et al. Long-Term Follow-up after Treatment of Coronary in-Stent Restenosis with a Paclitaxel-Coated Balloon Catheter. JACC Cardiovasc Interv. (2012) 5:323–30. 10.1016/j.jcin.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 33.Scheller B, Fontaine T, Mangner N, Hoffmann S, Bonaventura K, Clever YP, et al. A novel drug-coated scoring balloon for the treatment of coronary in-stent restenosis: results from the multi-center randomized controlled patent-C first in human trial. Catheter Cardiovasc Interv. (2016) 88:51–9. 10.1002/ccd.26216 [DOI] [PubMed] [Google Scholar]
- 34.Habara S, Iwabuchi M, Inoue N, Nakamura S, Asano R, Nanto S, et al. A multicenter randomized comparison of paclitaxel-coated balloon catheter with conventional balloon angioplasty in patients with bare-metal stent restenosis and drug-eluting stent restenosis. Am Heart J. (2013) 166:527–33. 10.1016/j.ahj.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 35.Byrne RA, Neumann FJ, Mehilli J, Pinieck S, Wolff B, Tiroch K, et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (isar-desire 3): a randomised, open-label trial. Lancet. (2013) 381:461–7. 10.1016/S0140-6736(12)61964-3 [DOI] [PubMed] [Google Scholar]
- 36.Nestelberger T, Kaiser C, Jeger R. Drug-coated balloons in cardiovascular disease: benefits, challenges, and clinical applications. Expert Opin Drug Deliv. (2020) 17:201–11. 10.1080/17425247.2020.1714590 [DOI] [PubMed] [Google Scholar]
- 37.Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, et al. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. (2014) 100:153–9. 10.1136/heartjnl-2013-304933 [DOI] [PubMed] [Google Scholar]
- 38.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics−2014 update: a report from the american heart association. Circulation. (2014) 129:e28–e292. 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kastrati A, Mehilli J, Pache J, Kaiser C, Valgimigli M, Kelbaek H, et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. (2007) 356:1030–9. 10.1056/NEJMoa067484 [DOI] [PubMed] [Google Scholar]
- 40.Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D'Ascenzo F, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. (2012) 379:1393–402. 10.1016/S0140-6736(12)60324-9 [DOI] [PubMed] [Google Scholar]
- 41.Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent study group. N Engl J Med. (1994) 331:489–95. 10.1056/NEJM199408253310801 [DOI] [PubMed] [Google Scholar]
- 42.Silber S. Which parameter should be chosen as primary endpoint for randomized drug-eluting stent studies? J Interv Cardiol. (2004) 17:375–85. 10.1111/j.1540-8183.2004.04079.x [DOI] [PubMed] [Google Scholar]
- 43.Siontis GC, Stefanini GG, Mavridis D, Siontis KC, Alfonso F, Perez-Vizcayno MJ, et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet. (2015) 386:655–64. 10.1016/S0140-6736(15)60657-2 [DOI] [PubMed] [Google Scholar]
- 44.Stefanini GG, Baber U, Windecker S, Morice MC, Sartori S, Leon MB, et al. Safety and efficacy of drug-eluting stents in women: a patient-level pooled analysis of randomised trials. Lancet. (2013) 382:1879–88. 10.1016/S0140-6736(13)61782-1 [DOI] [PubMed] [Google Scholar]
- 45.Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schomig A, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. (2007) 370:937–48. 10.1016/S0140-6736(07)61444-5 [DOI] [PubMed] [Google Scholar]
- 46.Taniwaki M, Stefanini GG, Silber S, Richardt G, Vranckx P, Serruys PW, et al. 4-Year clinical outcomes and predictors of repeat revascularization in patients treated with new-generation drug-eluting stents: a report from the resolute all-comers trial (a randomized comparison of a zotarolimus-eluting stent with an everolimus-eluting stent for percutaneous coronary intervention). J Am Coll Cardiol. (2014) 63:1617–25. 10.1016/j.jacc.2013.12.036 [DOI] [PubMed] [Google Scholar]
- 47.Waliszewski M, Rittger H. Surrogate and clinical endpoints in interventional cardiology: are statistics the brakes? Ther Adv Cardiovasc Dis. (2016) 10:314–26. 10.1177/1753944716656150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleber FX, Schulz A, Waliszewski M, Hauschild T, Böhm M, Dietz U, et al. Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin Res Cardiol. (2015) 104:217–25. 10.1007/s00392-014-0775-2 [DOI] [PubMed] [Google Scholar]
- 49.Kang WC, Park SM, Jang AY, Oh PC, Shin ES, Yu CW, et al. Predictors of favorable angiographic outcomes after drug-coated balloon use for de novo small vessel coronary disease (DCB-Only). Angiology. (2021):33197211015534. 10.1177/00033197211015534 [DOI] [PubMed] [Google Scholar]
- 50.Kurihara K, Ashikaga T, Sasaoka T, Yoshikawa S, Isobe M. Incidence and predictors of early and late target lesion revascularization after everolimus-eluting stent implantation in unselected patients in Japan. Catheter Cardiovasc Interv. (2017) 90:78–86. 10.1002/ccd.26964 [DOI] [PubMed] [Google Scholar]
- 51.Gao RL, Xu B, Lansky AJ, Yang YJ, Ma CS, Han YL, et al. a randomised comparison of a novel abluminal groove-filled biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: clinical and angiographic follow-up of the target i trial. EuroIntervention. (2013) 9:75–83. 10.4244/EIJV9I1A12 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.



