Abstract
Objectives
To estimate the marginal effectiveness of a fourth versus third dose and the vaccine effectiveness of mRNA covid-19 vaccines BNT162b2 and mRNA-1273 against any infection, symptomatic infection, and severe outcomes (hospital admission or death) related to the omicron variant.
Design
Test negative design.
Setting
Long term care facilities in Ontario, Canada, 30 December 2021 to 27 April 2022.
Participants
After exclusions, 61 344 residents aged 60 years or older across 626 long term care facilities in Ontario, Canada who were tested for SARS-CoV-2 were included.
Main outcome measures
Laboratory confirmed omicron SARS-CoV-2 infection (any and symptomatic) by reverse transcription polymerase chain reaction (RT-PCR), and hospital admission or death. Multivariable logistic regression was used to estimate marginal effectiveness (four versus three doses) and vaccine effectiveness (two, three, or four doses versus no doses) while adjusting for personal characteristics, comorbidities, week of test, and previous positive SARS-CoV-2 test result more than 90 days previously.
Results
13 654 residents who tested positive for omicron SARS-CoV-2 infection and 205 862 test negative controls were included. The marginal effectiveness of a fourth dose (95% of vaccine recipients received mRNA-1273 as the fourth dose) seven days or more after vaccination versus a third dose received 84 or more days previously was 19% (95% confidence interval 12% to 26%) against infection, 31% (20% to 41%) against symptomatic infection, and 40% (24% to 52%) against severe outcomes. Vaccine effectiveness in vaccine recipients (compared with unvaccinated) increased with each additional dose, and for a fourth dose was 49% (95% confidence interval 43% to 54%) against infection, 69% (61% to 76%) against symptomatic infection, and 86% (81% to 90%) against severe outcomes.
Conclusions
The findings suggest that compared with a third dose of mRNA covid-19 vaccine, a fourth dose improved protection against infection, symptomatic infection, and severe outcomes among long term care residents during an omicron dominant period. A fourth vaccine dose was associated with strong protection against severe outcomes in vaccinated residents compared with unvaccinated residents, although the duration of protection remains unknown.
Introduction
Residents of long term care facilities are at high risk of SARS-CoV-2 infection and severe outcomes for a range of reasons, including risk of exposure to the virus owing to reliance on care from others within a communal setting, underlying comorbidities that increase the risk of severe infection, and age related changes to the immune system (immunosenescence) that might affect the response to covid-19 vaccines.1 2 In Ontario, which comprises nearly 40% of Canada’s population, long term care facilities are publicly funded institutions that provide housing, medical support, and 24 hour access to personal and nursing care for people—mainly older adults—who are unable to live in the community because of major neurocognitive disorders or disability, or both.3 Currently, Ontario has 626 licensed long term care facilities that collectively care for about 6% of older Ontarians (≥65 years).4 5 Residents of long term care facilities in Ontario have been disproportionately affected by the covid-19 pandemic, accounting for nearly two thirds of deaths during the first two waves.2 The introduction of covid-19 vaccines noticeably improved outcomes for such residents within eight weeks, with an 89% relative reduction in infections and 96% reduction in mortality compared with unvaccinated control populations.6 However, the effectiveness of two doses declines over time, and the emergence of new variants of concern led to breakthrough infections and an increase in deaths.7 8 9 10 11 12 13 On 17 August 2021, Ontario began offering a third (first booster) vaccine dose to residents of long term care facilities.
The emergence of the omicron variant in November 2021 raised concerns for long term care populations, with early evidence suggesting increased transmissibility, greater risk of reinfection, and reduced vaccine protection against omicron compared with previous variants of concern.14 15 16 Additionally, susceptibility to infection increased owing to omicron’s partial immune evasion and to waning immunity after the third vaccine dose.15 17 To mitigate another surge in covid-19 related morbidity and mortality, Ontario began offering a fourth (second booster) vaccine dose on 30 December 2021 to long term care residents who had received their third dose at least three months previously (≥84 days).15 The preferred product was a 100 μg dose of mRNA-1273 (Spikevax; Moderna).15 The four dose schedule for long term care facilities in Ontario was a universal one, aimed at vaccinating all residents as quickly as possible, rather than a targeted or tiered programme, such as prioritising highest risk residents first. Other jurisdictions have subsequently recommended a fourth (second booster) vaccine dose for their long term care populations. Although evidence from Israel suggests that a fourth dose compared with a third dose provides additional protection against SARS-CoV-2 infection and severe covid-19 among older adults, findings have been limited to the BNT162b2 (Comirnaty; Pfizer-BioNTech) vaccine,18 19 and no studies to date have reported both marginal effectiveness and vaccine effectiveness of a fourth vaccine dose in long term care populations.
We estimated the marginal effectiveness of a fourth dose of mRNA covid-19 vaccine versus a third dose, and the vaccine effectiveness of varying numbers of doses in vaccinated residents compared with an unvaccinated group. For both objectives, we examined infection, symptomatic infection, and severe outcomes related to the omicron variant among residents of long term care facilities in Ontario.
Methods
Study design, setting, and population
We used a test negative design and linked provincial databases to estimate marginal effectiveness and vaccine effectiveness among residents aged 60 years or older as of 30 December 2021 (date eligible for fourth vaccine dose) across the 626 long term care facilities in Ontario. Residents must have had one or more reverse transcription polymerase chain reaction (RT-PCR) tests for SARS-CoV-2 between 30 December 2021 and 27 April 2022. Testing was commonplace in long term care facilities, and might have been initiated because of active screening (if a resident was experiencing covid-19 symptoms, a resident had been in contact with an individual of known positivity to SARS-CoV-2, or during an outbreak) or passive screening (among asymptomatic people without exposure to SARS-CoV-2, to create an additional layer of protection).20 We excluded residents who received a fourth vaccine dose before 30 December 2021 or had tested positive for SARS-CoV-2 within the past 90 days. Canadian and provincial guidelines recommend mRNA vaccines (mRNA-1273 or BNT162b2) versus other Health Canada approved covid-19 vaccine platforms.15 21 Few (n=165) residents received ChAdOx1-S (Vaxzevria or Covishield; Oxford-AstraZeneca) and none received Ad26.COV2.S (Janssen/Johnson & Johnson), the other vaccines available in Canada at the time. Therefore, we restricted our study population to those who received mRNA vaccines for all doses. Supplementary figure S1 is a flowchart outlining the exclusion criteria. Given B.1.1.529 (omicron) was the dominant circulating variant of concern during our study period, representing about 80.4% of samples tested on 28 December 2021 and more than 98.8% of samples tested after 30 January 2022,22 23 we estimated vaccine effectiveness against omicron only. Omicron was identified by whole genome sequencing or S gene target failure testing; the latter has 99.9% specificity, 99.5% positive predictive value, and 99.7% negative predictive value.24 If information on laboratory screening was unavailable, we assumed those who tested positive were infected with omicron unless the results confirmed B.1.617.2 (delta variant). We excluded residents infected with delta identified by whole genome sequencing or S gene target failure testing.
Data sources and outcomes
Using unique encoded identifiers, we linked provincial SARS-CoV-2 laboratory testing, covid-19 vaccination, and health administrative datasets (see supplementary table S1) and analysed the data at ICES (formerly the Institute for Clinical Evaluative Sciences).
Cohorts were created for three outcomes: any infection (SARS-CoV-2 positivity, irrespective of symptoms), symptomatic infection (one or more symptoms consistent with covid-19 that was recorded in the Ontario Laboratories Information System when tested (see supplementary table S2 for details on how symptom status was determined); many tested individuals with symptoms could have been excluded because symptom information was not recorded in the Ontario Laboratories Information System for various reasons), and severe outcomes (hospital admission or death due to, or partially due to, covid-19). Residents who tested positive at least once in a week were considered cases and those testing negative for all tests during that week were considered controls. We sampled cases and controls within each week of the study period so that time of testing was similar between groups. Among cases with multiple occurrences of the same outcome, we selected the first occurrence in the study period. Once an individual became a case, they could not re-enter the study. For controls, we randomly selected one negative test result within each week of the study period. It was possible for controls to later be considered cases if they tested negative for SARS-CoV-2 during earlier weeks of the study period and tested positive in a subsequent week. For the infection outcomes, the index date was the date of specimen collection, and for severe outcomes, the index date was the earliest of specimen collection date, hospital admission, or death.
Covid-19 vaccination
We used a centralised province-wide vaccine registry to identify receipt of covid-19 vaccines, and we classified long term care residents on the basis of number of doses received. Groups were stratified on time since the third dose (<84 days, ≥84 days) compared with the index test date to evaluate third doses over time, as well as time since the fourth dose (<7 days, ≥7 days) to account for time to expected immune response.25
Covariates
From various databases (see supplementary table S1),26 we obtained information on each resident’s age, sex, public health unit region of residence, week of test, whether they had tested positive for SARS-CoV-2 longer than 90 days ago, comorbidities, and whether there was an active SARS-CoV-2 outbreak in their long term care facility.
Statistical analysis
We calculated means (continuous variables) and frequencies (categorical variables) and compared test negative controls with test positive omicron cases using standardised differences. We also compared those vaccinated with a third dose 84 or more days before their index test with those who received no doses, one dose, two doses, or three doses less than 84 days previously, four doses less than seven days previously, or four doses seven or more days previously. We also examined descriptive facility level statistics across the 10 public health unit regions.
Multivariable logistic regression was used to estimate odds ratios, comparing the odds of vaccination among cases with the odds of vaccination among controls, while adjusting for covariates. We accounted for clustering at the facility level using a generalised estimating equations framework with an exchangeable correlation structure. The formula 1−odds ratio×100% was used to estimate marginal effectiveness and vaccine effectiveness. Geographical region was the only variable with missing data, with few observations missing (0.3%); we removed these observations from the analyses.
In the primary analysis for marginal effectiveness, we compared the effectiveness less than seven days and seven or more days after a fourth dose with a third dose received 84 or more days previously, and we included all covariates previously listed except for outbreak in the long term care facility. Age was included as a categorical variable (60-69 years, 70-79 years, ≥80 years) and the number of comorbidities as an ordinal variable. We conducted several secondary analyses: adjusted for outbreaks in long term care facilities to determine if outbreak status was a confounder (that is, a facility level outbreak might affect the vaccination and outcome status of some residents); stratified by outbreaks in long term care facilities to determine if being in an outbreak modified the effect of fourth vaccine doses on marginal effectiveness; used a third dose received less than 84 days previously as the comparator group (ie, non-exposed to the intervention of interest, a fourth dose); restricted to the peak period of omicron related infections in long term care facilities; did not adjust for residents who had a previous SARS-CoV-2 positive test result more than 90 days ago; and removed long term care facilities with ≥10% residents classified as unvaccinated to assess the impact of potential misclassification of vaccination status (eg, due to incomplete reporting to the provincial vaccine registry) in these facilities.
In the primary analysis for vaccine effectiveness, we estimated the effectiveness of two, three, or four vaccine doses compared with no vaccine doses (unvaccinated group) using the same covariates as for the marginal effectiveness analysis. We also determined the impact of potential misclassification of vaccination status on vaccine effectiveness by removing long term care facilities where ≥10% of residents were unvaccinated. Additionally, we estimated vaccine effectiveness for the most frequently occurring combinations of vaccine products among those who received a third dose (variability by product was insufficient to explore this for the fourth dose): three doses of mRNA-1273, three doses of BNT162b2, and two doses of BNT162b2 followed by one dose of mRNA-1273. Finally, we determined whether the product combination of the first three doses affected the marginal effectiveness of fourth doses of mRNA-1273.
All analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC). All tests were two sided and we used a significance level of P<0.05.
Patient and public involvement
Although study participants contributed in important ways to this research, it was not feasible to involve them effectively in the design, conduct, reporting, or dissemination plans of our research owing to time constraints. Similarly, we did not involve members of the public in this research.
Results
Between 30 December 2021 and 27 April 2022, 87.8% of residents (64 339 of 73 291) in long term care facilities in Ontario were tested for SARS-CoV-2. Across all 10 public health unit regions a high proportion of long term care residents were tested (median range 89-97%), and the median facility level SARS-CoV-2 test percentage positivity ranged from 1.8% to 5.9% by region over the study period (see supplementary table S3 and figure S2). After exclusions, 61 344 residents were included in this study (supplementary figure S1). Among those tested, we included 13 654 residents who tested positive for the omicron variant and 205 862 test negative controls. More than three quarters (80.1%) of tested residents had multiple SARS-CoV-2 tests during the study period (mean number 3.6 (standard deviation 2.4) tests per resident; see supplementary figure S3) and 9.4% of residents were immunocompromised owing to illness or treatment. At the time of testing, most of the residents who tested positive (58.1%) and the test negative controls (53.3%) had only received three covid-19 vaccine doses, and a greater proportion of controls (38.2%) than test positive residents (28.0%) had received a fourth dose (table 1). More cases lived in a facility with an active outbreak than controls (65.5% v 51.1%) and fewer had a previous positive SARS-CoV-2 test result more than 90 days ago compared with controls (7.5% v 15.6%). We observed few differences between residents who received a third dose 84 or more days previously and residents who were unvaccinated or received any other number of doses (table 2, see supplementary table S4). The mean number of comorbidities reported among vaccinated residents was similar to that reported among unvaccinated residents (see supplementary table S5).
Table 1.
Descriptive characteristics of long term care residents tested for SARS-CoV-2 between 30 December 2021 and 27 April 2022 in Ontario, Canada, comparing omicron cases with SARS-CoV-2 negative controls. Values are numbers (percentages) unless stated otherwise
| SARS-CoV-2 negative (n=205 862) | Omicron positive (n=13 654) | Standardised difference* | |
|---|---|---|---|
| Unvaccinated | 5473 (2.7) | 572 (4.2) | 0.08 |
| Vaccination dose received: | |||
| 1 dose | 928 (0.5) | 96 (0.7) | 0.03 |
| 2 doses | 10 924 (5.3) | 1215 (8.9) | 0.14 |
| 3 doses: | |||
| ≥84 days before test | 82 567 (40.1) | 6175 (45.2) | 0.10 |
| <84 days before test | 27 137 (13.2) | 1769 (13.0) | 0.01 |
| 4 doses: | |||
| <7 days before test | 11 035 (5.4) | 646 (4.7) | 0.03 |
| ≥7 days before test | 67 798 (32.9) | 3181 (23.3) | 0.22 |
| Mean (SD) age (years) | 83.63 (9.47) | 83.95 (9.31) | 0.03 |
| Age group (years) | |||
| 60-69 | 19 996 (9.7) | 1204 (8.8) | 0.03 |
| 70-79 | 43 104 (20.9) | 2803 (20.5) | 0.01 |
| ≥80 | 142 762 (69.3) | 9647 (70.7) | 0.03 |
| Men | 65 353 (31.7) | 4749 (34.8) | 0.06 |
| Public health unit region: | |||
| Central East | 15 722 (7.6) | 1017 (7.4) | 0.01 |
| Central West | 34 407 (16.7) | 3218 (23.6) | 0.17 |
| Durham | 7670 (3.7) | 505 (3.7) | 0.00 |
| Eastern | 19 781 (9.6) | 1138 (8.3) | 0.04 |
| North | 19 647 (9.5) | 1487 (10.9) | 0.04 |
| Ottawa | 10 828 (5.3) | 839 (6.1) | 0.04 |
| Peel | 11 473 (5.6) | 494 (3.6) | 0.09 |
| South West | 28 442 (13.8) | 2109 (15.4) | 0.05 |
| Toronto | 43 581 (21.2) | 2235 (16.4) | 0.12 |
| York | 13 396 (6.5) | 578 (4.2) | 0.10 |
| Missing | 915 (0.4) | 34 (0.2) | 0.03 |
| Covid-19 outbreak in long term care facility at time of test | 105 100 (51.1) | 8940 (65.5) | 0.30 |
| Past SARS-CoV-2 positive test result (>90 days) | 32 205 (15.6) | 1021 (7.5) | 0.26 |
| Week of test†: | |||
| 30 Dec to 5 Jan | 29 986 (14.6) | 1949 (14.3) | 0.01 |
| 6 Jan to 12 Jan | 30 124 (14.6) | 2475 (18.1) | 0.09 |
| 13 Jan to 19 Jan | 23 069 (11.2) | 1938 (14.2) | 0.09 |
| 20 Jan to 26 Jan | 19 729 (9.6) | 1526 (11.2) | 0.05 |
| 27 Jan to 2 Feb | 15 607 (7.6) | 989 (7.2) | 0.01 |
| 3 Feb to 9 Feb | 10 391 (5.0) | 532 (3.9) | 0.06 |
| 10 Feb to 16 Feb | 6934 (3.4) | 269 (2.0) | 0.09 |
| 17 Feb to 23 Feb | 5808 (2.8) | 187 (1.4) | 0.10 |
| 24 Feb to 2 Mar | 6034 (2.9) | 173 (1.3) | 0.12 |
| 3 Mar to 9 Mar | 5199 (2.5) | 173 (1.3) | 0.09 |
| 10 Mar to 16 Mar | 5467 (2.7) | 193 (1.4) | 0.09 |
| 17 Mar to 23 Mar | 5595 (2.7) | 203 (1.5) | 0.09 |
| 24 Mar to 30 Mar | 6469 (3.1) | 279 (2.0) | 0.07 |
| 31 Mar to 6 Apr | 7825 (3.8) | 454 (3.3) | 0.03 |
| 7 Apr to 13 Apr | 9406 (4.6) | 584 (4.3) | 0.01 |
| 14 Apr to 20 Apr | 9005 (4.4) | 797 (5.8) | 0.07 |
| 21 Apr to 27 Apr | 9214 (4.5) | 933 (6.8) | 0.10 |
| Mean (SD) No of comorbidities | 4.09 (1.56) | 4.08 (1.55) | 0.01 |
| Type of comorbidity: | |||
| Immunocompromised | 19 226 (9.3) | 1342 (9.8) | 0.02 |
| Chronic respiratory disease | 74 633 (36.3) | 5004 (36.6) | 0.01 |
| Chronic heart disease | 77 159 (37.5) | 4998 (36.6) | 0.02 |
| Hypertension | 168 244 (81.7) | 11 144 (81.6) | 0.00 |
| Diabetes | 82 128 (39.9) | 5252 (38.5) | 0.03 |
| Autoimmune disorders | 16 811 (8.2) | 1058 (7.7) | 0.02 |
| Chronic kidney disease or dialysis‡ | 36 080 (17.5) | 2244 (16.4) | 0.03 |
| Advanced liver disease | 5351 (2.6) | 336 (2.5) | 0.01 |
| Dementia | 160 756 (78.1) | 10 825 (79.3) | 0.03 |
| History of stroke or transient ischaemic attack |
36 467 (17.7) | 2336 (17.1) | 0.02 |
| Frailty | 165 659 (80.5) | 11 157 (81.7) | 0.03 |
SD=standard deviation.
Values >0.10 are considered clinically relevant. Comparing omicron cases with SARS-CoV-2 test negative controls.
30 December, 31 December in 2021, and remaining dates in 2022.
Chronic kidney disease in past five years or dialysis for three consecutive months.
Table 2.
Descriptive characteristics of long term care residents tested for SARS-CoV-2 between 30 December 2021 and 27 April 2022 in Ontario, Canada, comparing those who received a third dose 84 or more days previously with those who received a third dose less than 84 days previously or a fourth dose. Values are numbers (percentages) unless stated otherwise
| Characteristics | Third dose ≥84 days before test (n=88 742) | Third dose <84 days before test (n=28 906) | Standardised difference* | Fourth dose <7 days before test (n=11 681) | Standardised difference* | Fourth dose ≥7 days before test (n=70 979) | Standardised difference* | ||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) age (years) | 84.06 (9.47) | 82.97 (8.93) | 0.12 | 84.29 (9.42) | 0.02 | 83.57 (9.61) | 0.05 | ||
| Age group (years): | |||||||||
| 60-69 | 8184 (9.2) | 2721 (9.4) | 0.01 | 1030 (8.8) | 0.01 | 7215 (10.2) | 0.03 | ||
| 70-79 | 17 773 (20.0) | 6460 (22.3) | 0.06 | 2237 (19.2) | 0.02 | 14 793 (20.8) | 0.02 | ||
| ≥80 | 62 785 (70.8) | 19 725 (68.2) | 0.05 | 8414 (72.0) | 0.03 | 48 971 (69.0) | 0.04 | ||
| Men | 27 500 (31.0) | 10 646 (36.8) | 0.12 | 3528 (30.2) | 0.02 | 22 004 (31.0) | 0 | ||
| Public health unit region | |||||||||
| Central East | 7336 (8.3) | 2002 (6.9) | 0.05 | 886 (7.6) | 0.03 | 5236 (7.4) | 0.03 | ||
| Central West | 16 753 (18.9) | 4666 (16.1) | 0.07 | 1794 (15.4) | 0.09 | 11 174 (15.7) | 0.08 | ||
| Durham | 3152 (3.6) | 957 (3.3) | 0.01 | 479 (4.1) | 0.03 | 2991 (4.2) | 0.03 | ||
| Eastern | 7364 (8.3) | 2249 (7.8) | 0.02 | 1232 (10.5) | 0.08 | 8772 (12.4) | 0.13 | ||
| North | 7919 (8.9) | 2885 (10.0) | 0.04 | 1090 (9.3) | 0.01 | 7866 (11.1) | 0.07 | ||
| Ottawa | 4102 (4.6) | 1513 (5.2) | 0.03 | 742 (6.4) | 0.08 | 4590 (6.5) | 0.08 | ||
| Peel | 4742 (5.3) | 1795 (6.2) | 0.04 | 615 (5.3) | 0 | 3033 (4.3) | 0.05 | ||
| South West | 12 145 (13.7) | 3560 (12.3) | 0.04 | 1553 (13.3) | 0.01 | 11 031 (15.5) | 0.05 | ||
| Toronto | 18 661 (21.0) | 6726 (23.3) | 0.05 | 2595 (22.2) | 0.03 | 12 397 (17.5) | 0.09 | ||
| York | 6222 (7.0) | 2196 (7.6) | 0.02 | 685 (5.9) | 0.05 | 3720 (5.2) | 0.07 | ||
| Missing | 346 (0.4) | 357 (1.2) | 0.09 | 10 (0.1) | 0.06 | 169 (0.2) | 0.03 | ||
| Long term care facility in outbreak at time of test | 51 090 (57.6) | 16 079 (55.6) | 0.04 | 7037 (60.2) | 0.05 | 28 600 (40.3) | 0.35 | ||
| Past positive SARS-CoV-2 test result (>90 days) | 14 461 (16.3) | 3051 (10.6) | 0.17 | 1889 (16.2) | 0 | 10 343 (14.6) | 0.05 | ||
| Week of test† | |||||||||
| 30 Dec to 5 Jan | 22 328 (25.2) | 5062 (17.5) | 0.19 | 224 (1.9) | 0.72 | 0 (0.0) | 0.82 | ||
| 6 Jan to 12 Jan | 22 001 (24.8) | 5232 (18.1) | 0.16 | 1300 (11.1) | 0.36 | 272 (0.4) | 0.79 | ||
| 13 Jan to 19 Jan | 14 050 (15.8) | 4222 (14.6) | 0.03 | 3044 (26.1) | 0.25 | 1100 (1.5) | 0.52 | ||
| 20 Jan to 26 Jan | 9491 (10.7) | 3495 (12.1) | 0.04 | 2735 (23.4) | 0.34 | 3655 (5.1) | 0.21 | ||
| 27 Jan to 2 Feb | 5169 (5.8) | 2764 (9.6) | 0.14 | 2101 (18.0) | 0.38 | 5282 (7.4) | 0.07 | ||
| 3 Feb to 9 Feb | 2570 (2.9) | 1903 (6.6) | 0.17 | 904 (7.7) | 0.22 | 4723 (6.7) | 0.18 | ||
| 10 Feb to 16 Feb | 1237 (1.4) | 1188 (4.1) | 0.17 | 360 (3.1) | 0.11 | 3980 (5.6) | 0.23 | ||
| 17 Feb to 23 Feb | 912 (1.0) | 962 (3.3) | 0.16 | 136 (1.2) | 0.01 | 3658 (5.2) | 0.24 | ||
| 24 Feb to 2 Mar | 981 (1.1) | 964 (3.3) | 0.15 | 110 (0.9) | 0.02 | 3801 (5.4) | 0.24 | ||
| 3 Mar to 9 Mar | 766 (0.9) | 779 (2.7) | 0.14 | 86 (0.7) | 0.01 | 3452 (4.9) | 0.24 | ||
| 10 Mar to 16 Mar | 849 (1.0) | 575 (2.0) | 0.09 | 117 (1.0) | 0 | 3791 (5.3) | 0.25 | ||
| 17 Mar to 23 Mar | 892 (1.0) | 411 (1.4) | 0.04 | 98 (0.8) | 0.02 | 4073 (5.7) | 0.26 | ||
| 24 Mar to 30 Mar | 1109 (1.2) | 369 (1.3) | 0 | 106 (0.9) | 0.03 | 4805 (6.8) | 0.28 | ||
| 31 Mar to 6 Apr | 1454 (1.6) | 336 (1.2) | 0.04 | 99 (0.8) | 0.07 | 5929 (8.4) | 0.31 | ||
| 7 Apr to 13 Apr | 1567 (1.8) | 308 (1.1) | 0.06 | 99 (0.8) | 0.08 | 7525 (10.6) | 0.37 | ||
| 14 Apr to 20 Apr | 1680 (1.9) | 179 (0.6) | 0.11 | 75 (0.6) | 0.11 | 7342 (10.3) | 0.36 | ||
| 21 Apr to 27 Apr | 1686 (1.9) | 157 (0.5) | 0.12 | 87 (0.7) | 0.1 | 7591 (10.7) | 0.37 | ||
| Mean (SD) No of comorbidities | 4.06 ±1.55 | 4.29 ±1.56 | 0.15 | 4.05 ±1.53 | 0 | 4.04 ±1.56 | 0.01 | ||
| Type of comorbidity: | |||||||||
| Immunocompromised | 7775 (8.8) | 3543 (12.3) | 0.11 | 1031 (8.8) | 0 | 6212 (8.8) | 0 | ||
| Chronic respiratory disease |
31 568 (35.6) | 10 824 (37.4) | 0.04 | 4207 (36.0) | 0.01 | 26 096 (36.8) | 0.02 | ||
| Chronic heart disease | 32 212 (36.3) | 12 253 (42.4) | 0.12 | 4147 (35.5) | 0.02 | 25 737 (36.3) | 0 | ||
| Hypertension | 72 475 (81.7) | 24 249 (83.9) | 0.06 | 9563 (81.9) | 0.01 | 57 437 (80.9) | 0.02 | ||
| Diabetes | 34 821 (39.2) | 12 202 (42.2) | 0.06 | 4533 (38.8) | 0.01 | 27 890 (39.3) | 0 | ||
| Autoimmune disorders | 7249 (8.2) | 2216 (7.7) | 0.02 | 953 (8.2) | 0 | 5939 (8.4) | 0.01 | ||
| Chronic kidney disease‡ | 14 412 (16.2) | 6687 (23.1) | 0.17 | 1794 (15.4) | 0.02 | 11 595 (16.3) | 0 | ||
| Advanced liver disease | 2129 (2.4) | 861 (3.0) | 0.04 | 264 (2.3) | 0.01 | 1868 (2.6) | 0.01 | ||
| Dementia | 71 725 (80.8) | 20 109 (69.6) | 0.26 | 9546 (81.7) | 0.02 | 56 073 (79.0) | 0.05 | ||
| History of stroke or transient ischaemic attack |
15 623 (17.6) | 4950 (17.1) | 0.01 | 2016 (17.3) | 0.01 | 12 680 (17.9) | 0.01 | ||
| Frailty | 70 002 (78.9) | 26 149 (90.5) | 0.33 | 9310 (79.7) | 0.02 | 54 938 (77.4) | 0.04 |
SD=standard deviation.
Values >0.10 are considered clinically relevant. Comparing residents who received their third dose <84 days before their index test, fourth dose <7 days previously, and fourth dose ≥7 days previously with residents who received their third dose ≥84 days before their index test.
30 December, 31 December in 2021, and remaining dates in 2022.
Chronic kidney disease in past five years or dialysis for three consecutive months.
Compared with residents who received a third vaccine dose 84 or more days before being tested, the marginal effectiveness of a fourth dose seven days or more after vaccination was 19% (95% confidence interval 12% to 26%) against infection, 31% (20% to 41%) against symptomatic infection, and 40% (24% to 52%) against severe outcomes; estimates were lower if the fourth dose was received less than seven days previously (fig 1, see supplementary table S6). Estimates remained unchanged after adjustment and stratification for covid-19 outbreaks (19-22% against infection, 26-28% against symptomatic infection, and 34-40% against severe disease; see supplementary table S7). However, the model for symptomatic infection, when a long term care facility did not have an active covid-19 outbreak, did not converge. The marginal effectiveness of a fourth vaccine dose seven days or more after vaccination versus a third dose received less than 84 days previously was 16% (95% confidence interval 9% to 23%) against infection, 20% (3% to 33%) against symptomatic infection, and 29% (8% to 46%) against severe outcomes (see supplementary figure S4 and table S6). The marginal effectiveness estimates after removing long term care facilities with ≥10% unvaccinated residents (see supplementary table S8), when restricted to the peak period of omicron infections in long term care facilities (30 December 2021 to 26 January 2022; see supplementary table S9; supplementary figure S5 shows an epidemic curve of all positive test results over the study period), and when not adjusting for residents who had a positive SARS-CoV-2 test result more than 90 days previously (see supplementary table S10) were similar to the findings of the primary analysis.
Fig 1.
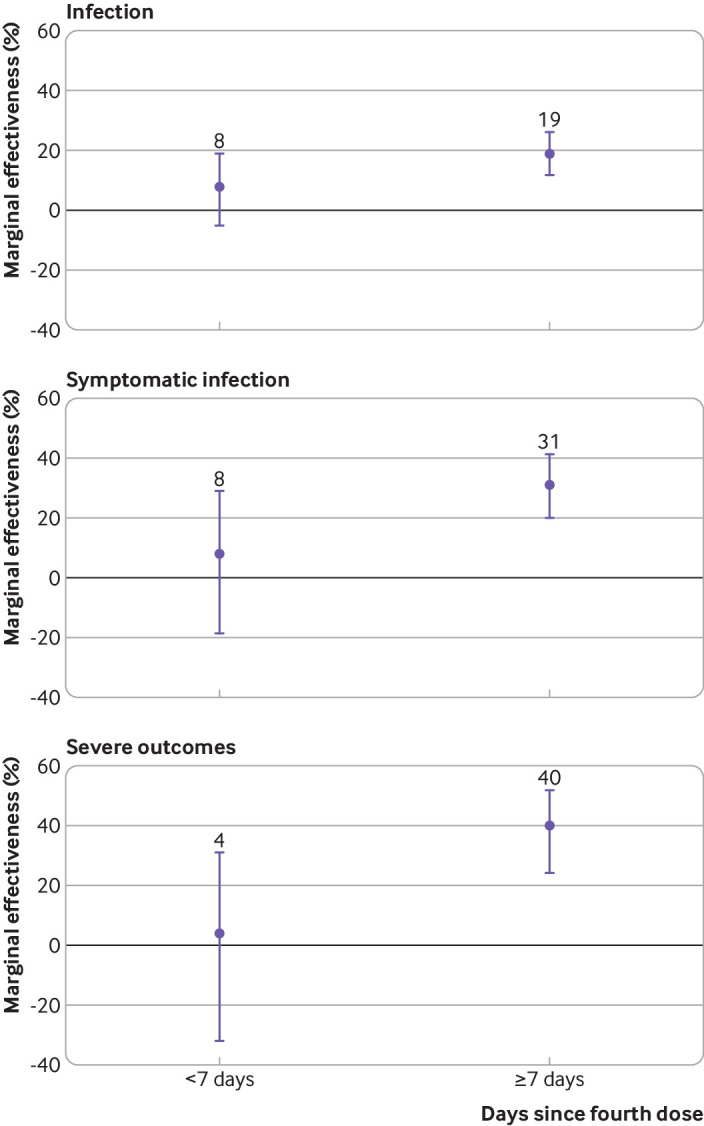
Marginal effectiveness of a fourth dose of mRNA covid-19 vaccine against omicron variant related outcomes among long term care residents in Ontario, Canada, compared with residents who received a third dose 84 or more days previously
Vaccine effectiveness in vaccinated residents compared with unvaccinated residents increased with each additional dose of vaccine but was lower for those who received a third dose 84 or more days before testing compared with those who received a third dose more recently (fig 2, see supplementary table S11). Vaccine effectiveness for a fourth dose seven days or more after vaccination was higher against infection (49%, 43% to 54%), symptomatic infection (69%, 61% to 76%), and severe outcomes (86%, 81% to 90%) than the corresponding estimates for a third dose received 84 or more days previously (37%, 31% to 43%; 55%, 45% to 64%; and 77%, 69% to 82%, respectively). Estimates for vaccine effectiveness were similar in analyses removing long term care facilities with ≥10% of unvaccinated residents (see supplementary table S12).
Fig 2.
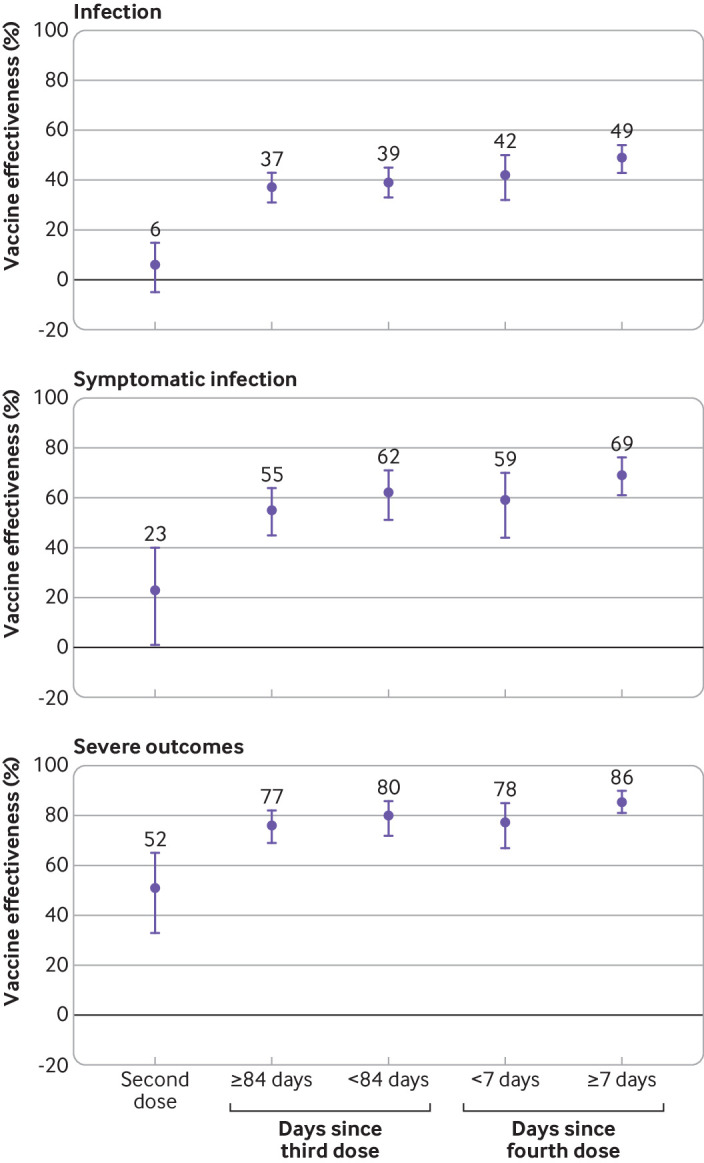
Vaccine effectiveness of two, three, and four doses of mRNA covid-19 vaccine against omicron variant related outcomes among long term care residents in Ontario, Canada, compared with unvaccinated residents
Vaccine effectiveness against infection was similar among residents who received three doses of mRNA-1273 (infection: 44%, 95% confidence interval 38% to 49%; symptomatic infection: 61%, 50% to 69%; severe outcomes: 81%, 74% to 86%) and those who received two doses of BNT162b2 with a third dose of mRNA-1273 (infection: 36%, 28% to 44%; symptomatic infection: 57%, 40% to 69%; severe outcomes: 81%, 67% to 89%), although time from vaccination to testing was shorter for the latter schedule (table 3). Vaccine effectiveness was lower among residents who received three doses of BNT162b2 (infection: 32%, 24% to 38%; symptomatic infection: 53%, 39% to 63%; severe outcomes: 77%, 67 to 83%). Almost all long term care residents (95%) who received a fourth dose received mRNA-1273, and vaccine effectiveness against infection and severe outcomes with a fourth dose of mRNA-1273 was similar across all combinations of vaccine products (see supplementary table S13). However, vaccine effectiveness against symptomatic infection was higher among those who received either four doses of mRNA-1273 or three doses of BNT162b2 followed by one dose of mRNA-1273 compared with those who received two doses of BNT162b2 followed by two doses of mRNA-1273. Few residents received the last of these vaccination schedules, and confidence intervals were wide and overlapped with other schedules, making it difficult for conclusions to be drawn.
Table 3.
Vaccine effectiveness of three doses of mRNA covid-19 vaccines against omicron variant related outcomes by vaccine product among long term care residents in Ontario, Canada, compared with unvaccinated residents
| Outcome by product used for first three doses | Mean (SD) time from third dose to SARS-CoV-2 test* (days) | No of SARS-CoV-2 negative controls | No of residents positive for omicron | Vaccine effectiveness, % (95% CI) |
|---|---|---|---|---|
| Infection | ||||
| 3 doses of mRNA-1273 | 107 (37.3) | 54 515 | 3089 | 44 (38 to 49) |
| 3 doses of BNT162b2 | 104 (40.5) | 44 647 | 4059 | 32 (24 to 38) |
| 2 doses of BNT162b2, mRNA-1273 booster | 57 (41.6) | 6,102 | 442 | 36 (28 to 44) |
| Symptomatic infection | ||||
| 3 doses of mRNA-1273 | 112 (39.7) | 1,357 | 474 | 61 (50 to 69) |
| 3 doses of BNT162b2 | 109 (38.1) | 1,420 | 719 | 53 (39 to 63) |
| 2 doses of BNT162b2, mRNA-1273 booster | 65 (45.8) | 208 | 78 | 57 (40 to 69) |
| Severe outcomes | ||||
| 3 doses of mRNA-1273 | 111 (39.2) | 1357 | 161 | 81 (74 to 86) |
| 3 doses of BNT162b2 | 108 (39.0) | 1420 | 218 | 77 (67 to 83) |
| 2 doses of BNT162b2, mRNA-1273 booster | 68 (44.3) | 208 | 21 | 81 (67 to 89) |
SD=standard deviation; CI=confidence interval.
Time period from vaccination to testing was considerably shorter for two doses of BNT162b2 with an mRNA-1273 booster compared with the other two schedules for all outcomes. It is unknown how much of the vaccine effectiveness is attributed to the booster product versus shorter time period.
Discussion
In this study of older residents of long term care facilities in Ontario, Canada, we found that compared with a third dose of mRNA vaccine received 84 or more days previously, a fourth dose offered increased effectiveness against any SARS-CoV-2 infection (19%), symptomatic infection (31%), and severe outcomes (40%) related to the omicron variant. Marginal effectiveness against all outcomes was lower when comparing fourth doses with third doses received less than 84 days previously, which broadly supports a three month minimum interval between third and fourth doses, although the optimal dosing interval remains unknown. Whether the long term care facility experienced an outbreak of covid-19 at the time of testing neither confounded nor modified marginal effectiveness estimates. Vaccine effectiveness estimates (compared with unvaccinated residents) against infection (49%), symptomatic infection (69%), and severe outcomes (86%) related to the omicron variant were consistently higher for a fourth dose than for a third dose received 84 or more days previously.
Few studies to date have explored the effect of the fourth dose of covid-19 vaccines. In Israel, among adults aged 60 years or older, the marginal effectiveness 7-30 days after a fourth dose versus a third dose of BNT162b2 received four or more months earlier was 45% against any infection, 55% against symptomatic infection, 68% against hospital admission, and 74% against death.27 In our study we also found that a fourth dose was associated with additional protection compared with a third dose; however, our marginal effectiveness estimates were lower than those observed in Israel. Nevertheless, findings cannot be directly compared owing to differences in study design, outcome definitions, population characteristics, settings, vaccine products, time since vaccination, and dosing intervals. Notably, the study from Israel excluded residents of long term care facilities.
We observed higher vaccine effectiveness with each dose for all outcomes. When interpreting marginal effectiveness estimates, differences in vaccine effectiveness between doses should be taken into consideration.28 Although the marginal effectiveness estimate against infection might seem low at 19%, vaccine effectiveness was 12 percentage points higher (49% v 37%) seven or more days after a fourth dose compared with a third dose received 84 or more days previously. Against symptomatic infection, a marginal effectiveness of 31% corresponded to a 14 percentage point difference in vaccine effectiveness (69% v 55%). A boost in vaccine effectiveness against infection among long term care residents is still important because the consequences of infection, including extended social isolation, disruptions to care, risk of severe disease, and mortality, are higher than among the general population.1 2 29 The difference in vaccine effectiveness against severe outcomes was lower, at 9 percentage points (86% v 77%), but nonetheless translated to a 40% marginal effectiveness.28 Given the high baseline incidence of severe outcomes in this population,25 if SARS-CoV-2 transmission is high, a 9 percentage point increase in vaccine effectiveness might still reduce covid-19 related morbidity and mortality substantially. For example, if the incidence of severe outcomes among unvaccinated long term care residents is 10 per 1000 resident weeks, vaccine effectiveness from a fourth dose is 86%, and vaccine effectiveness from a third dose is 77%, then vaccinating all residents who had received a third dose 84 or more days previously with a fourth dose would avert 0.9 severe outcomes per 1000 resident weeks (ie, 2.3 per 1000 resident weeks minus 1.4 per 1000 resident weeks). If the baseline incidence is 100 per 1000 resident weeks, fourth doses administered to all residents would avert nine severe outcomes per 1000 resident weeks.
Past studies of vaccine effectiveness of two doses of mRNA vaccines in long term care populations conducted earlier in the pandemic have reported higher vaccine effectiveness estimates (71-82%) against infection compared with the estimates we observed for fourth doses.30 31 However, vaccine effectiveness studies conducted later against predominating variants of concern have reported similar estimates to our fourth dose estimates against the omicron variant; two dose vaccine effectiveness against beta infection in long term care facilities in France was 49% and against delta infection in US facilities was 53%.12 32 Vaccine effectiveness against omicron, particularly against infection, has also been found to be lower than for any previous variant of concern.16 33 34 Our vaccine effectiveness estimate against hospital admission or death was similar to two dose vaccine effectiveness against similar outcomes due to the beta variant in France (86%).12 Vaccine effectiveness estimates might also be slightly lower in our study because we reported estimates for a longer time after vaccination (ie, up to three months), and protection may have already started waning among residents who received a vaccine dose shortly after implementation of the programme. Nonetheless, our observed increases in vaccine effectiveness with a fourth dose were still considerable for a vulnerable population at high risk of severe outcomes and living in a setting with increased risks of transmission.
Similar to recent studies outside Ontario among adult populations,33 35 36 we also observed waning of a third dose based on lower vaccine effectiveness estimates for residents who received a third dose 84 or more days previously versus less than 84 days previously, but not enough time has elapsed to explore waning or duration of protection of fourth doses among residents of long term care facilities in Ontario. Recent studies in Israel among adults aged 60 years or older suggest that effectiveness of the fourth dose of BNT162b2 against infection may wane faster than the third dose, but similar to third doses, the degree of waning against severe disease is lower.18 19 Canadian studies have found that immune protection among long term care residents wanes much faster than among younger, healthier adults after two doses; similar patterns might be expected for booster doses.37 38
Studies from the United Kingdom among adults suggest similar levels of protection from a third dose of either mRNA vaccine against symptomatic omicron infection irrespective of the mRNA product used for the primary series.16 36 Among adults aged 65 years or older in the UK, vaccine effectiveness against hospital admission was also similar for a third dose of either mRNA vaccine after two doses of BNT162b2.39 We found that among Ontario long term care residents, a third dose of mRNA-1273 after a homologous two dose primary series of either mRNA vaccine was more effective against all outcomes than three doses of BNT162b2. For those receiving a primary course of BNT162b2 with an mRNA-1273 booster, the time between vaccination and testing was shorter compared with the other schedules, making it difficult to determine the relative impact of the booster product versus the shorter period. Additionally, a 100 μg dose of mRNA-1273 is now recommended as the booster for long term care residents in Ontario,15 40 whereas other jurisdictions (eg, UK41 and US42) use a 50 μg dose for boosters, which could have influenced our findings.
Strengths and limitations of this study
This study has some limitations. Firstly, our cohort assessing symptomatic infection was limited to residents with symptoms recorded in the Ontario Laboratories Information System and therefore might be incomplete. Secondly, Ontario laboratories discontinued routine screening using S gene target failure testing of all positive samples in late December 2021, therefore some cases with the delta variant might be misclassified as having the omicron variant, potentially biasing estimates away from the null. It is, however, unlikely that this would affect our estimates considerably because the prevalence of the delta variant was low in Ontario during our study period. Thirdly, we classified outbreaks at the facility level because we did not have data on whether the outbreak was on a resident’s floor or if it was more contained; therefore, we could have overestimated the impact of outbreaks at the individual level. Fourthly, residual confounding is possible because we were limited to the covariates available in the study databases. Fifthly, information was not available on why residents might have delayed or refused vaccination, which could have introduced some bias. Finally, we did not have access to staff vaccination records in the long term care facilities. Staff vaccination strongly influences SARS-CoV-2 transmission in long term care facilities.43 At the time of this study, all staff working in long term care facilities in Ontario were required to be vaccinated with two doses of a covid-19 vaccine,44 but two dose vaccine effectiveness against omicron infection is low.16 33 34 Although a mandate for required third doses was also implemented, staff had until 14 March 2022 (well into our study period) to comply (although this might not have been enforced because the province shifted from a provincial long term care vaccination mandate to supporting employer led policies on the same day).44 This study also has many strengths, such as its test negative design, which helps mitigate selection bias from differences in healthcare seeking behaviours between vaccinated and unvaccinated individuals, and our large sample size. Our study included more than 60 000 residents across all 626 long term care facilities in Ontario, increasing the generalisability of these findings.
Conclusions
Our findings indicate that a fourth dose of a covid-19 mRNA vaccine (95% of our study participants received mRNA-1273) successfully increased protection against any SARS-CoV-2 infection, symptomatic infection, and severe outcomes among long term care residents in an omicron dominant period. Nevertheless, many unknowns about fourth doses still remain in this population, including the duration of protection, particularly for the mRNA-1273 vaccine. Layering other public health measures with vaccination in long term care facilities, including mask wearing, increased ventilation, and physical distancing might help optimise protection against SARS-CoV-2 for this highly vulnerable population.
What is already known on this topic
Real world effectiveness studies among adults aged ≥60 years in Israel suggest that a fourth dose of BNT162b2 (Pfizer-BioNTech) provides considerable additional protection against infection with the omicron variant and severe covid-19 compared with a third dose received four or more months previously
What this study adds?
Among long term care residents in Ontario, Canada, a fourth dose of an mRNA (BNT162b2 or mRNA-1273 (Moderna) vaccine was associated with increased protection against omicron infection, symptomatic infection, and severe outcomes compared with a third dose, particularly if the third dose was received three or more months previously; 95% of residents received mRNA-1273 as their fourth dose
Compared with unvaccinated residents, vaccine effectiveness increased with each additional dose of an mRNA vaccine, and a fourth dose provided strong protection against severe outcomes due to the omicron variant
Acknowledgments
We thank Public Health Ontario for access to vaccination data from COVaxON, case level data from CCM (Public Health Case and Contact Management Solution), and covid-19 laboratory data, as well as assistance with data interpretation; the staff of Ontario’s public health units who are responsible for covid-19 case and contact management and data collection within CCM; IQVIA Solutions Canada for use of its Drug Information File; the Ontario residents without whom this research would be impossible; and Sharifa Nasreen for producing the original figures for this manuscript.
Web extra.
Extra material supplied by authors
Supplementary information: additional figures S1-S5 and tables S1-S13
Supplementary information: Excel file showing model coefficients
Contributors: RG and SAK contributed equally to the work and are presented in alphabetical order. SAK and LN obtained the data and conducted the analyses (dataset and variable creation and statistical modelling). RG, SAK, and JCK drafted the manuscript. RG, SAK, LN, SAB, SEW, APC, and JCK contributed to the analysis plan, interpreted the results, critically reviewed and edited the manuscript, approved the final version, and agreed to be accountable for all aspects of the work. JCK is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by the Applied Health Research Questions (AHRQ) Portfolio at ICES, which is funded by the Ontario Ministry of Health (MOH). For more information on AHRQ and how to submit a request, please visit https://www.ices.on.ca/DAS/AHRQ. This work was also supported by the Ontario Health Data Platform (OHDP), a Province of Ontario initiative to support Ontario’s ongoing response to covid-19 and its related impacts. This work was supported by Public Health Ontario. This study was also supported by ICES, which is funded by an annual grant from the Ontario MOH and the Ministry of Long term Care (MLTC). The study sponsors did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI), and by Ontario Health (OH). However, the analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement by ICES, MOH, MLTC, OHDP, its partners, the Province of Ontario, CIHI, or OH is intended or should be inferred.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from the Applied Health Research Questions Portfolio at ICES, which is funded by the Ontario Ministry of Health, for the submitted work, and no financial relationships with any organisations that might have an interest in the submitted work in the previous three years. SEW is a member of Canada’s National Advisory Committee on Immunization (NACI) and APC is a member of the Congregate Care Setting Working Group of the Ontario Science Table.
The corresponding author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: At the time of writing, results from this study have been shared with provincial public health officials overseeing Ontario’s covid-19 vaccination programme, expert immunisation advisory committees for Ontario and Canada, national long term care based vaccine surveillance programmes, and with the global community by posting on a preprint server. Findings have helped inform Ontario’s Spring covid-19 second booster programme45 and are also informing Autumn booster planning. Additionally, these findings were used to inform the World Health Organization interim statement on additional booster doses.46 Our findings will also be shared through peer reviewed publication and Twitter. Finally, we will work with national long term care based vaccine surveillance programmes to disseminate findings to long term care operators, resident and family councils, and international long term care policy networks.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
ICES is a prescribed entity under Ontario’s Personal Health Information Protection Act (PHIPA). Section 45 of PHIPA authorises ICES to collect personal health information, without consent, for the purpose of analysis or compiling statistical information with respect to the management of, evaluation or monitoring of, the allocation of resources to or planning for, all or part of the health system. Projects that use data collected by ICES under section 45 of PHIPA, and use no other data, are exempt from research ethics board review. The use of the data in this project is authorised under section 45 and approved by ICES’ Privacy and Legal Office.
Data availability statement
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (eg, healthcare organisations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at https://www.ices.on.ca/DAS/AHRQ (email: das@ices.on.ca). The full dataset creation plan and underlying analytical code are available from the authors on request, understanding that the computer programs might rely on coding templates or macros that are unique to ICES and are therefore either inaccessible or require modification.
References
- 1. Wingert A, Pillay J, Gates M, et al. Risk factors for severity of COVID-19: a rapid review to inform vaccine prioritisation in Canada. BMJ Open 2021;11:e044684. 10.1136/bmjopen-2020-044684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canadian Institute for Health Information. The Impact of COVID-19 on Long-Term Care in Canada: Focus on the First 6 Months. Ottawa, ON: CIHI; 2021 [cited 2022 Mar 10]. https://www.cihi.ca/sites/default/files/document/impact-covid-19-long-term-care-canada-first-6-months-report-en.pdf
- 3. Tanuseputro P, Chalifoux M, Bennett C, et al. Hospitalization and mortality rates in long-term care facilities: Does for-profit status matter? J Am Med Dir Assoc 2015;16:874-83. 10.1016/j.jamda.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 4. Wilkinson A, Haroun V, Wong T, Cooper N, Chignell M. Overall quality performance of long-Term care homes in Ontario. Healthc Q 2019;22:55-62. 10.12927/hcq.2019.25903 [DOI] [PubMed] [Google Scholar]
- 5.Canadian Institute for Health Information. Long-term care and COVID-19: International comparisons. 2020 [cited 2022 May 19]. https://www.cihi.ca/en/long-term-care-and-covid-19-international-comparisons
- 6. Brown KA, Stall NM, Vanniyasingam T, et al. Early impact of Ontario’s COVID-19 vaccine rollout on long-term care home residents and health care workers. Science Briefs of the Ontario COVID-19 Science Advisory Table. 2021;2. https://covid19-sciencetable.ca/sciencebrief/early-impact-of-ontarios-covid-19-vaccine-rollout-on-long-term-care-home-residents-and-health-care-workers/ [Google Scholar]
- 7. Abe KT, Hu Q, Mozafarihashjin M, et al. Neutralizing antibody responses to SARS-CoV-2 variants in vaccinated Ontario long-term care home residents and workers. medRxiv. 2021. 10.1101/2021.08.06.21261721. [DOI]
- 8. Canaday DH, Carias L, Oyebanji OA, et al. Reduced BNT162b2 mRNA vaccine response in SARS-CoV-2-naive nursing home residents. medRxiv 2021. 10.1101/2021.03.19.21253920. [DOI] [PMC free article] [PubMed]
- 9. Vanker A, McGeer AJ, O’Byrne G, et al. Adverse outcomes associated with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) variant B.1.351 infection in vaccinated residents of a long-term care home, Ontario, Canada. Clin Infect Dis 2022;74:751-2. 10.1093/cid/ciab523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williams C, Al-Bargash D, Macalintal C, et al. Coronavirus Disease 2019 (COVID-19) outbreak associated with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) P.1 lineage in a long-term care home after implementation of a vaccination program - Ontario, April-May 2021. Clin Infect Dis 2022;74:1085-8. 10.1093/cid/ciab617 [DOI] [PubMed] [Google Scholar]
- 11. Kertes J, Gez SB, Saciuk Y, et al. Effectiveness of mRNA BNT162b2 vaccine 6 months after vaccination among patients in large health maintenance organization, Israel. Emerg Infect Dis 2022;28:338-46. 10.3201/eid2802.211834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant — National Healthcare Safety Network, March 1–August 1, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1163-6. 10.15585/mmwr.mm7034e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Breznik JA, Zhang A, Huynh A, et al. Antibody responses 3-5 months post-vaccination with mRNA-1273 or BNT163b2 in nursing home residents. J Am Med Dir Assoc 2021;22:2512-4. 10.1016/j.jamda.2021.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ito K, Piantham C, Nishiura H. Relative instantaneous reproduction number of Omicron SARS-CoV-2 variant with respect to the Delta variant in Denmark. J Med Virol 2022;94:2265-8. 10.1002/jmv.27560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ontario Agency for Health Protection and Promotion (Public Health Ontario). Recommendations: Fourth COVID-19 Vaccine Dose for Long-Term Care Home Residents and Older Adults in Congregate Settings. Toronto, ON: Queen’s Printer for Ontario; 2021 Dec [cited 2022 Mar 10]. https://www.publichealthontario.ca/-/media/documents/ncov/vaccines/2022/01/covid-19-oiac-4th-dose-recommendations-older-adults-ltc.pdf?sc_lang=en
- 16. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med 2022;386:1532-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022;602:676-81. 10.1038/s41586-021-04388-0 [DOI] [PubMed] [Google Scholar]
- 18. Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Amir O, Freedman L, et al. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med 2022;386:1712-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gazit S, Saciuk Y, Perez G, Peretz A, Pitzer VE, Patalon T. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. BMJ 2022;377:e071113. 10.1136/bmj-2022-071113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ontario Ministry of Health. COVID-19 Guidance: Long-Term Care Homes and Retirement Homes for Public Health Units. Ontario, Canada: Government of Ontario; 2022 Apr [cited 2022 May 19]. https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/2019_LTC_homes_retirement_homes_for_PHUs_guidance.pdf
- 21.National Advisory Committee on Immunization. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI): Recommendations on the use of COVID-19 vaccines. Ottawa, ON: National Advisory Committee on Immunization; 2021 Oct [cited 2022 Mar 23]. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html
- 22.Ontario Agency for Health Protection and Promotion (Public Health Ontario). SARS-CoV-2 Whole Genome Sequencing in Ontario, March 15, 2022. Toronto, ON: Queen’s Printer for Ontario; 2022 Mar [cited 2022 Mar 23]. https://www.publichealthontario.ca/-/media/documents/ncov/epi/covid-19-sars-cov2-whole-genome-sequencing-epi-summary.pdf?sc_lang=en
- 23.Ontario Agency for Health Protection and Promotion (Public Health Ontario). COVID-19 Variant of Concern Omicron (B.1.1.529): Risk Assessment, January 12, 2022. Toronto, ON: Queen’s Printer for Ontario; 2022 Jan [cited 2022 Mar 23]. https://www.publichealthontario.ca/-/media/documents/ncov/voc/2022/01/covid-19-omicron-b11529-risk-assessment-jan-12
- 24.Public Health Ontario. SARS-CoV-2 (COVID-19 Virus) Variant of Concern (VoC) Screening and Genomic Sequencing for Surveillance. Public Health Ontario. 2022 [cited 2022 Apr 11]. https://www.publichealthontario.ca/en/Laboratory-Services/Test-Information-Index/COVID-19-VoC
- 25. Falsey AR, Frenck RW, Jr, Walsh EE, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med 2021;385:1627-9. 10.1056/NEJMc2113468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chung H, He S, Nasreen S, et al. Canadian Immunization Research Network (CIRN) Provincial Collaborative Network (PCN) Investigators . Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ 2021;374:n1943. 10.1136/bmj.n1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magen O, Waxman JG, Makov-Assif M, et al. Fourth dose of BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl J Med 2022;386:1603-14. 10.1056/NEJMoa2201688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewis NM, Chung JR, Uyeki TM, Grohskopf L, Ferdinands JM, Patel MM. Interpretation of relative efficacy and effectiveness for influenza vaccines. Clin Infect Dis 2021;ciab1016. Published online 7 Dec. 10.1093/cid/ciab1016 [DOI] [PubMed] [Google Scholar]
- 29. Savage RD, Rochon PA, Na Y, et al. Excess mortality in long-term care residents with and without personal contact with family or friends during the COVID-19 pandemic. J Am Med Dir Assoc 2022;23:441-443.e1. 10.1016/j.jamda.2021.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Starrfelt J, Danielsen AS, Kacelnik O, Børseth AW, Seppälä E, Meijerink H. High vaccine effectiveness against coronavirus disease 2019 (COVID-19) and severe disease among residents and staff of long-term care facilities in Norway, November 2020–June 2021. Antimicrob Steward Healthc Epidemiol. 2022;2:e10 10.1017/ash.2021.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mazagatos C, Monge S, Olmedo C, et al. Working Group for the surveillance and control of COVID-19 in Spain. Working group for the surveillance and control of COVID-19 in Spain . Effectiveness of mRNA COVID-19 vaccines in preventing SARS-CoV-2 infections and COVID-19 hospitalisations and deaths in elderly long-term care facility residents, Spain, weeks 53 2020 to 13 2021. Euro Surveill 2021;26:2100452. 10.2807/1560-7917.ES.2021.26.24.2100452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lefèvre B, Tondeur L, Madec Y, et al. Beta SARS-CoV-2 variant and BNT162b2 vaccine effectiveness in long-term care facilities in France. Lancet Healthy Longev 2021;2:e685-7. 10.1016/S2666-7568(21)00230-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med 2022;28:1063-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buchan SA, Chung H, Brown KA, et al. Effectiveness of COVID-19 vaccines against Omicron or Delta symptomatic infection and severe outcomes. medRxiv. 2022. 10.1101/2021.12.30.21268565. [DOI] [PMC free article] [PubMed]
- 35. Regev-Yochay G, Gonen T, Gilboa M, et al. Efficacy of a fourth dose of Covid-19 mRNA vaccine against Omicron. N Engl J Med 2022;386:1377-80. 10.1056/NEJMc2202542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.UK Health and Security Agency. COVID-19 vaccine surveillance report: Week 12. United Kingdom: UK Health and Security Agency; 2022 Mar [cited 2022 Mar 25]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1063023/Vaccine-surveillance-report-week-12.pdf
- 37.COVID-19 Immunity Task Force. Protecting Canada’s Long-Term Care Residents from COVID-19: The Evidence Behind the Policies. Canada: COVID-19 Immunity Task Force; 2021 Oct [cited 2022 Mar 15]. https://www.covid19immunitytaskforce.ca/wp-content/uploads/2021/10/CITF_LTC-summary_2021-EN.pdf
- 38. Walmsley S, Szadkowski L, Wouters B, et al. Safety and efficacy of preventative COVID vaccines: The StopCoV Study. medRxiv 2022. [DOI] [PMC free article] [PubMed]
- 39. Stowe J, Andrews N, Kirsebom F, Ramsay M, Bernal JL. Effectiveness of COVID-19 vaccines against Omicron and Delta hospitalisation: test negative case-control study. medRxiv. 2022. 10.1101/2022.04.01.22273281. [DOI] [PMC free article] [PubMed]
- 40.Ontario Ministry of Health. COVID-19 Vaccine Third Dose and Booster Recommendations. Ontario, Canada: Government of Ontario; 2022 Apr [cited 2022 Apr 7]. https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/vaccine/COVID-19_vaccine_third_dose_recommendations.pdf
- 41.Joint Committee on Vaccination and Immunisation (JCVI). JCVI statement regarding a COVID-19 booster vaccine programme for winter 2021 to 2022. 2021 [cited 2022 Apr 13]. https://www.gov.uk/government/publications/jcvi-statement-september-2021-covid-19-booster-vaccine-programme-for-winter-2021-to-2022/jcvi-statement-regarding-a-covid-19-booster-vaccine-programme-for-winter-2021-to-2022
- 42.Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. Clinical Guidance for COVID-19 Vaccination. 2022 [cited 2022 May 19]. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
- 43. McGarry BE, Barnett ML, Grabowski DC, Gandhi AD. Nursing home staff vaccination and COVID-19 outcomes. N Engl J Med 2022;386:397-8. 10.1056/NEJMc2115674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Government of Ontario. Minister’s Directive: Long-term care home COVID-19 immunization policy. [cited 2022 Apr 7]. https://www.ontario.ca/page/ministers-directive-long-term-care-home-covid-19-immunization-policy
- 45.National Advisory Committee on Immunization. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI): Initial guidance on a second booster dose of COVID-19 vaccines in Canada. Ottawa, ON: National Advisory Committee on Immunization; 2022 Apr [cited 2022 May 19]. https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/naci-guidance-second-booster-dose-covid-19-vaccines.pdf
- 46.Interim statement on the use of additional booster doses of Emergency Use Listed mRNA vaccines against COVID-19. 2022 [cited 2022 May 20]. https://www.who.int/news/item/17-05-2022-interim-statement-on-the-use-of-additional-booster-doses-of-emergency-use-listed-mrna-vaccines-against-covid-19
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional figures S1-S5 and tables S1-S13
Supplementary information: Excel file showing model coefficients
Data Availability Statement
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (eg, healthcare organisations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at https://www.ices.on.ca/DAS/AHRQ (email: das@ices.on.ca). The full dataset creation plan and underlying analytical code are available from the authors on request, understanding that the computer programs might rely on coding templates or macros that are unique to ICES and are therefore either inaccessible or require modification.


