Abstract
The intestinal microbiome plays an essential role in human health and disease status. So far, microbiota transplantation is considered a potential therapeutic approach for treating some chronic diseases, including inflammatory bowel disease (IBD). The diversity of gut microbiota is critical for maintaining resilience, and therefore, transplantation with numerous genetically diverse gut microbiota with metabolic flexibility and functional redundancy can effectively improve gut health than a single probiotic strain supplement. Studies have shown that natural fecal microbiota transplantation or washing microbiota transplantation can alleviate colitis and improve intestinal dysbiosis in IBD patients. However, unexpected adverse reactions caused by the complex and unclear composition of the flora limit its wider application. The evolving strain isolation technology and modifiable pre-existing strains are driving the development of microbiota transplantation. This review summarized the updating clinical and preclinical data of IBD treatments from fecal microbiota transplantation to washing microbiota transplantation, and then to artificial consortium transplantation. In addition, the factors considered for strain combination were reviewed. Furthermore, four types of artificial consortium transplant products were collected to analyze their combination and possible compatibility principles. The perspective on individualized microbiota transplantation was also discussed ultimately.
Keywords: microbiota transplantation, artificial consortium transplantation, combination principles, clinical study, inflammatory bowel disease
1 Introduction
Inflammatory bowel disease (IBD) is an inflammatory disease of the intestine, including ulcerative colitis (UC) and Crohn’s disease (CD). The disease has complex etiology, but it is generally related to genetics, immune response, environmental factors and the gut microbiome (West et al., 2017). The current IBD treatment relies on anti-inflammatory agents, immunosuppressants, and biologic agents (Van Assche et al., 2013; Gomollón et al., 2016). Even so, these agents do not achieve satisfactory outcomes in some patients, underlining the need for alternatives. Microbiome dysbiosis is an essential feature of IBD (Maloy and Powrie, 2011), which makes regulating the gut microbiome as one of the potential strategies for IBD treatment.
The human gut is home to numerous microbiota, which form a complex gut microbiome community. The gut microbiome is a highly dynamic and intricate ecosystem that differs among individuals, influenced by host genetics, age, diet, drug use, and other factors (Costello et al., 2009; Yatsunenko et al., 2012; Duffy et al., 2015; Xie et al., 2016). In a healthy state, maintaining a dynamic balance of gut microbes exists in healthy individuals. Disrupting this balance can cause several human diseases such as metabolic syndrome (Lim et al., 2017), obesity (Liu et al., 2017), infections (Petrof et al., 2013), gastrointestinal diseases [irritable bowel syndrome (IBS) (Pimentel and Lembo, 2020) and IBD (Chu et al., 2016)]. Introducing microorganisms into the intestinal tract can rapidly reverse diseases related to gut microbial diversity and abundance imbalance. Replacing the missing symbiotic microbes in the gut with corresponding strains or a mix of specific strains may prevent or treat such conditions. The gut microbiome includes bacteria, fungi, archaea and viruses. Recently, Underhill et al. reviewed the important role of fungal microbiome regulation in the development and severity of IBD in some patients (Underhill and Braun, 2022). The bacterial microbiome accounts for a large proportion of the intestinal tract which plays a major role in intestinal disturbance. Therefore, in this review, we only focused on the treatment of IBD through bacterial microbiome. Gut microbe-based therapies such as fecal microbiota transplantation (FMT) (Paramsothy et al., 2017), washed microbiota transplantation (WMT) (Zhang et al., 2020), and live biotherapeutic products (LBP) (Ye et al., 2021)have been used to treat IBD related to microbial alteration.
The essential characteristics of the gut microbiome are stability and resilience (Lozupone et al., 2012). Without interference, the gut microbiome remains stable. The gut microbiota is generally highly resilient to disturbances, and thus, the abundance of numerous key species remains stable in the host for a period of time. The stability and resilience of gut microbiota are closely related to their diversity. Higher microbial diversity increases the functional redundancy levels. It is generally thought to play a critical role in stabilizing microbial community function during disturbances (Fassarella et al., 2021). Therefore, transplantation with the combination of multiple microorganisms is more effective in modulating gut health than with a single probiotic strain supplement.
Studies have shown that fecal microbiota transplantation can alleviate IBD (Moayyedi et al., 2015; Sokol et al., 2020). However, FMT also causes adverse reactions in some patients due to the complex components in transplants (Wang et al., 2016; DeFilipp et al., 2019). A washed microbiota for transplantation that minimizes the adverse reactions caused by natural FMT has been developed (Zhang et al., 2020). Nevertheless, the precise composition of the transplantation flora is unclear, and the procedure has potential health risks. The recent technology has deepened our understanding of microbial community and its application for IBD therapy. FMT can be performed through enema, orally through freeze-dried bacterial capsules (Crothers et al., 2021), or non-freeze-dried bacterial suspension capsules (Khanna et al., 2021). Beneficial bacteria can be isolated from fermented foods or feces or engineered to obtain desirable biological characteristics (Kurtz et al., 2019; Puurunen et al., 2021).
The influence factors of microbiota transplantation outcome include recipient factors and transplant factors. The recipient parameters, such as genetics, immunity, microbiota and lifestyle, affect the efficacy of microbiota engraftment (Danne et al., 2021). Moreover, the matching between donors and recipients is essential for the long-term maintenance of disease remission (Wilson et al., 2019; Okahara et al., 2020). Given the limitation of the length of the article, we only discussed the diverse microbiota transplantation for IBD treatment.
To sum it up, numerous gut microbiota is vital to human health. Numerous studies have confirmed that intestinal flora participates in intestinal maturation and homeostasis through multiple functions, while symbiosis and interacting with human cells and organs (Backhed et al., 2005; Human Microbiome Project Consortium, 2012). Gut microbiota therapy can be performed using FMT and WMT, and this procedure effectively alleviates microbiome-related disorders, including IBD. However, those are not the best choices due to undefined composition. Microbial therapy with clear composition and standard quality monitoring may be the direction of microbiota transplantation. This review focuses on the current research on different artificial consortium transplant products and their improvement for IBD treatment. We discussed the characteristics of the strains used for combination and their possible compatibility principles, which may be necessary for the better development of individualized microbiota transplantation.
2 Undefined Consortium Transplantation
This refers to a community of microbiome with unknown composition.
2.1 Natural Microbiota Transplantation
Natural microbiota transplantation, also known as fecal microbiota transplantation (FMT), is a procedure in which stool from a healthy donor is delivered to the intestines of a recipient patient through enema or oral capsules (Gupta and Khanna, 2017). FMT is currently used primarily to treat recurrent Clostridioides difficile infections (Hvas et al., 2019). However, even though the mechanism of action of FMT is not well understood, existing findings show that it generally restores the abnormal composition and abundance of the gut microbiota. FMT is effective against microecological disorders, including IBD, hepatic encephalopathy (HE), metabolic syndrome, IBS, autism, and cancer (Bajaj et al., 2017; de Groot et al., 2017; Kang et al., 2017; Fong et al., 2020; Skrzydło-Radomańska et al., 2021).
2.1.1 Clinical Studies of FMT in IBD
Using a randomized controlled trial, Moayyedi et al. revealed that FMT induced remission of active UC. FMT is even more effective against early UC because it is easier to restore the early gut microbial imbalance (Moayyedi et al., 2015). Another study confirmed that a 2-donor fecal microbiota preparation (FMP) was also a safe and effective method for restoring the normal intestinal microbial diversity in patients with active UC (Jacob et al., 2017). In addition to direct colon FMT, oral capsule FMT (cFMT) is well tolerated in mild to moderate UC patients (Crothers et al., 2021). Also, there are currently several registered trials investigating the efficacy of cFMT in IBD, as the previous Halaweish et al. described (Halaweish et al., 2022). Oral cFMT is a more acceptable alternative for UC treatment than the direct colon FMT, and it may enhance the potential of long-term microbial-based treatment strategies. Sokol et al. conducted a pilot randomized controlled study showed that FMT significant decreased endoscopic activity and C relative protein level of CD patients (Sokol et al., 2020). A systematic review evaluated the efficacy of FMT in Crohn’s disease, involved 13 cohort studies and two RCTs between 2014 and 2020, showed that FMT may be an effective and safe therapy for CD and needed large controlled trials to confirm (Fehily et al., 2021).
The efficacy and safety of FMT have been studied in adult with IBD and children with UC and CD. Nikhil Pai et al. conducted a 6-week randomized, placebo-controlled pilot study using FMT in children with UC and CD to evaluate the safety and effectiveness of FMT supplement, providing preliminary evidence for the clinical application of FMT in children with IBD (Pai and Popov, 2017; Pai et al., 2019). Katarzyna et al. further demonstrated that FMT is also a safe and effective alternative for treating cytomegalovirus colitis in children with UC (Karolewska-Bochenek et al., 2021).
2.1.2 The Limitations of FMT
Even though FMT is effective against IBD remission, its effectiveness cannot be controlled by humans and is related to the diversity of the fecal microbiota in the donor individual, and there are no reliable and stable sources of the feces. The donor fecal microbiota mixture has many unknown ingredients, including bacteria, yeasts, parasites and viruses. It is unclear which one is responsible for beneficial effects and which may pose a risk by transferring antibiotic resistance or producing genotoxic metabolites. In addition, given the inter- and intra-individual differences in gut microbiota, the transplantation effects are not uniform even with the same donor.
Due to the complex composition of FMT, some adverse reactions often occur. Mild to moderate adverse reactions include abdominal pain, flatulence, increased stool frequency, constipation, vomiting, belching, fever, whereas serious adverse effects include viral and bacterial infections, relapse of IBD, and death (Wang et al., 2016). In 2019, the Food and Drug Administration (FDA) reported two cases of serious adverse events of Escherichia coli bacteremia that produces extended-spectrum beta-lactamase (ESBL) after FMT. Genetic sequencing revealed that both patients received FMT from the same donor. one of the patients died (DeFilipp et al., 2019). Moreover, optimized screening of fecal bacteria transplantation donors did not seem to improve the efficacy of FMT in the treatment of active UC (ECCO 2022 abstract OP03). Although studies have shown the safety and efficacy of multi-donor FMT for diseases, including IBD and obesity (Jacob et al., 2017; Wilson et al., 2021), there is no clear method for selecting multiple donors, and there is no study directly comparing the efficacy of single donor and multi-donor FMT.
2.2 Processed Microbiota Transplantation
Processed microbiota transplantation, known as washed microbiota transplantation (WMT), is the microfiltration of feces to remove fecal solids, parasites, and fungi from feces suspension. Pro-inflammatory metabolites such as leukotriene B4, corticosterone and prostaglandin G2, are also removed from the feces. Regarding safety, quality control and precise bacteria enrichment, Zhang et al. first revealed that WMT is superior to FMT through clinical results, animal experiments and in vitro trials (Zhang et al., 2020). The incidence of WMT-related adverse events in CD patients (since April 2014) was 8.7%, significantly lower than 21.7% in patients with manual FMT (from 2012 to April 2014) (Wang et al., 2018).
2.2.1 Clinical Studies of WMT in IBD
Based on a single-center, open-label prospective study, Chen et al. revealed that washed-treated FMT safely and effectively achieved a clinical response in 77.8% (7/9) of the assessed UC patients in just two weeks. At week 12, achieved clinical remission in 55.6% (5/9), whereas the endoscopic response rate was 33.3% (3/9) (Chen et al., 2020). A separate study showed that clinical remission was achieved in 53.7% of IBD patients after WMT therapy, and this therapy significantly increased the colonization rate of Akkermansia, a beneficial bacteria. Thus, the efficacy of WMT in treating IBD may be closely related to the abundance of Akkermansia bacteria (Zhang et al., 2020). Zhang and colleagues reported a case study of UC patients with recurrent fungal infections in which the antifungal therapy had failed. Interestingly, repeated WMT therapy remarkably and rapidly decreased the serum concentration of inflammatory makers and cleared the fungal during hospitalization. The fungal infection had not recurred after 6-month follow-up. However, the clinical application of WMT for recurrent fungal infections treatment needs further investigation (Wu et al., 2021). A randomized, open clinical study showed that enteral nutrition in combined with early WMT could rapidly improve the nutritional status and induce clinical remission in CD patients with malnutrition (Xiang et al., 2021).
3 Artificial Consortium Transplantation
We defined artificial consortium (AC) in a narrow sense as a combination of microbiome with clear composition in a specific manner. And the way AC are transplanted into the gut is called artificial consortium tranplantation (ACT).
3.1 Advances in IBD
3.1.1 In Vitro Studies of ACT
Geirnaert et al. investigated the therapeutic potential of a mix of six butyric-producing bacteria against IBD, given the beneficial effects of butyric acid on epithelial barrier function and intestinal health. They found that the bacterial mix significantly enhanced the colonization of related butyric-producing bacteria and improved the integrity of the epithelial barrier in vitro ( Geirnaert et al., 2017). Pistol et al. found that a combination of grape pomace (GP) extract and a mixture of Lactobacillus bacteria modulated inflammation by regulating the expression of related genes (Pistol et al., 2019). Palócz et al. reported the effect of chlorogenic acid in combination of Lactobacillus plantarum 2142 in reducing the lipopolysaccharide (LPS)-induced intestinal inflammation in porcine IPEC-J2 cells (Palócz et al., 2016).
Cuffaro et al. developed a method of evaluating the function of 21 strains isolated from neonatal and adult gut microbiota. They found that the isolated strains regulated the immune response and enhanced the functioning of the epithelial barrier. Also, 33% of the isolates exerted various benefits (Cuffaro et al., 2021). Even so, more in vitro studies are needed to identify the specific species, strains, or metabolites important for health, which extends the selection of a limited number of bacteria considered to have clinical importance and potential health-beneficial properties.
3.1.2 Preclinical Studies of ACT
VSL#3 is the most studied probiotic combination used against IBD. Each VSL#3 dose contained 450 billion freeze-dried bacteria (Streptococcus thermophilus, Bifidobacterium longum, B. breve, B. infantis, Lactobacillus acidophilus, L. plantarum, L. casei, L. bulgaricus) and corn starch (Reiff et al., 2009). Current, VSL#3 is being used to IBD clinical treatment. Vivomixx® in EU, Visbiome® in USA, similar component to VSL#3, also alleviated canine colitis by increasing mucosal polyamine levels and TJP expression (White et al., 2017; Rossi et al., 2018). Biagioli et al. formulated a new probiotic combination by adding Bacillus subtilis to Vivomixx®. This formulation enhanced the beneficial effects of Vivomixx® on DSS and TNBS-induced colitis (Biagioli et al., 2020).
Lactobacillus and Bifidobacterium are the most common probiotics. The efficacy of Lactobacillus in combination with Bifidobacterium against IBD, such as Ultrabiotique® (Lactobacillus acidophilus, Lactobacillus Plantarum, Bifidobacterium lactis and Bifidobacterium breve), Citogenex (L. casei, Bifidobacterium lactis), PM2 (Lactobacillus acidophilus, Lactobacillus paracasei, Lactobacillus rhamnosus, and Bifidobacterium lactis) has been evaluated. Ultrabiotique® alleviated intestinal inflammation and maintained the mucosal barrier in mice with DSS-induced colitis (Toumi et al., 2013). Pre-administration of Citogenex can alleviate TNBS-induced colon injury (Traina G, 2016). PM-2 attenuated 5-FU-induced mucositis, increasing villus/crypt ratio while decreasing inflammation in the intestine (Quaresma et al., 2020). Bacterial strains from different isolated sources can be combined. Je et al. evaluated the efficacy of Lactobacillus johnsoniil DCC9203 in combination with Bifidobacterium animal subspecies lactis IDCC4301 isolated from the feces of infants with Lactobacillus plantarum IDCC3501 isolated from the pickle at a ratio of 1:1:1 to form ID-JPL934, was applied to DSS-induced colitis model. It was found that ID-JPL934 could reduce mucosal and submucosal immune cell infiltration and decrease intestinal cell loss (Je et al., 2018).
In addition to the common Lactobacillus and Bifidobacterium combinations, some specific strain combinations have also been studied in animal models of IBD. Ming Li et al. demonstrated that a combination of 10 fecal bacteria, called bacterial consortia transplantation (BCT), was comparable to FMT in reestablishing mucosal barrier function in mice with intestinal disorders and that BCT was more stable and controllable than FMT (Li et al., 2015). Further investigation revealed that BCT rapidly restored intestinal microbiome balance, renovated the interaction between symbiotic flora and intestinal γδT17 cells, and improved mucosal barrier function (Li et al., 2016; Li et al., 2017). van der Lelie et al. further reported that gut-103, a cocktail of 17 bacterial strains, rapidly colonized mice intestines and alleviated experimental colitis established in germ-free mice. They further modified unsuitable bacterial strains, including antibiotic resistance, pathogenic, and strict anaerobes, to formulate a complex cocktail of 11 bacterial strains named GUT-108. This bacterial formulation induced stable and prolonged intestinal tract colonization, providing redundancy protection (van der Lelie et al., 2021). Clinical trials of similar probiotic preparation named I3.1 and comprising Lactobacillus plantarum (CECT7484, CECT7485) and Pediococcus acidilactici (CECT7483) alleviated IBS. Lorén et al. further demonstrated that I3.1 probiotic protected against DSS-induced colitis and IL-10-deficient colitis in mice (Lorén et al., 2017). GI7, composed of four lactobacillus, two Bifidobacterium species, and Streptococcus thermophilus, significantly inhibited the production of innate pro-inflammatory cytokines and relieved DSS-induced colitis (Kim et al., 2017). Commercial products Aviguard®, which comprise ten different bacterial species, alleviated acute enterocolitis induced by campylobacter bacteria (Heimesaat et al., 2021).
3.1.3 Clinical Studies of ACT
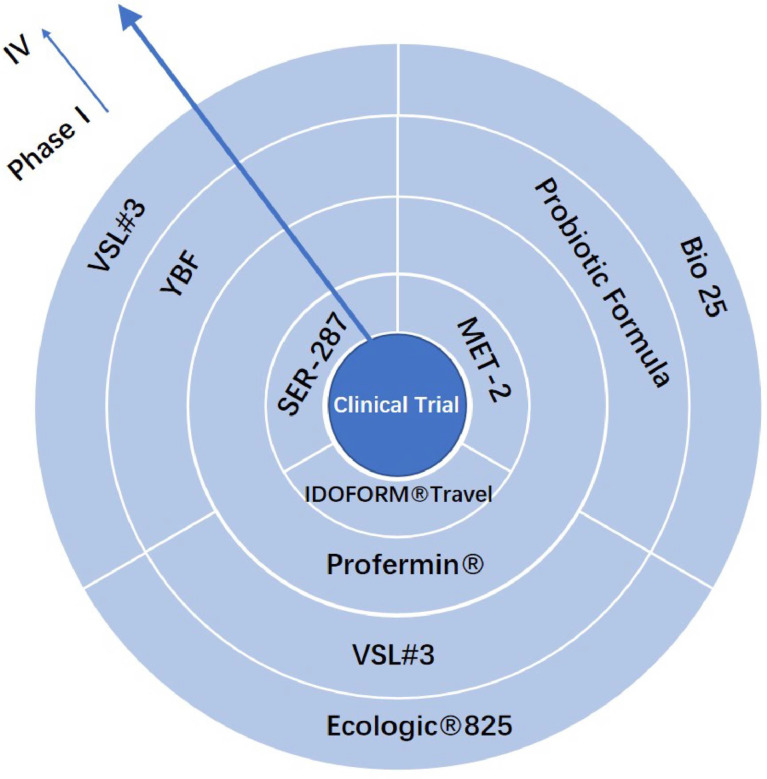
Clinical trials must be conducted to verify the safety and efficacy of treatment formulations, including bacterial combination therapy ( Figure 1 ). Several studies have demonstrated the effect of VSL#3 on IBD in mice, rats, and dogs in last decade. VSL#3 prevented the apoptosis of intestinal epithelial cells, promoted the expression of the intestinal tight junction protein (TJP), reduced the production of pro-inflammatory factors, regulated the functioning of T cells and macrophages, and changed the composition of intestinal microorganisms (Reiff et al., 2009; Mennigen et al., 2009; Hormannsperger et al., 2010; Uronis et al., 2011; Isidro et al., 2017; Liu et al., 2019). VSL#3 was effective in preventing pouch colitis and inducing remission of ulcerative colitis in clinical trials (Gionchetti et al., 2003). A double-blind, randomized, placebo-controlled study showed that VSL # 3 could be used as adjunctive therapy for IBD and can be used in combination with standard 5-ASA or immunosuppressant therapy for the remission of relapsing mild-to-moderate ulcerative colitis (Tursi et al., 2010). In a meta-analysis of 23 randomized controlled trials, Shen et al. reported that VSL#3 significantly increased the remission rates of active UC. [P¼0.01, risk ratio (RR)¼1.51], and also considerably reduced the clinical recurrence rate of pouch colitis (P, 0.00001, RR¼0.18), without additional adverse events (Shen et al., 2014). Although VSL#3 reduced the levels of mucosal inflammation in patients with CD, there was no significant difference in the rate of endoscopic recurrence between patients who received VSL#3 and placebo. Therefore, whether VSL#3 can prevent the recurrence of Crohn’s disease remains to be validated (Fedorak et al., 2015).
Figure 1.
Artificial consortium transplantation products with clinical trials.
Persborn et al. applied Ecologic®825 to UC patients with severe pouchitis founded that it normalized the permeation of E. coli K12, which was associated with active pouchitis, improved mucosal permeability, but had no effect on mucosal pouch microbiota composition (Persborn et al., 2013). Furrie et al. found that short-term synbiotic therapy (Bifidobacterium longum/Synergy 1) alleviated active UC by reducing inflammation and promoting epithelial tissue regeneration (Furrie, 2005). Steed et al. further confirmed that synbiotic therapy (Bifidobacterium longum/Synergy 1) effectively alleviated active Crohn’s disease (Steed et al., 2010). Fujimori et al. also found a combination of probiotics and prebiotics (Bifidobacterium breve, Lactobacillus casei, Bifidobacterium Longum and Psyllium) was effective against active Crohn’s disease without causing adverse events. Specifically, complete remission was observed in six patients, partial remission was observed in one patient, whereas three patients were non-responsive (Fujimori et al., 2007). In their clinical trial, Krag et al. found that Profermin® (Lactobacillus plantarum299V, fermented oats, barley malt and lecithin) was fairly tolerable and promoted remission of UC without causing serious adverse reactions. The estimated reduction mean score was 5.0 points (95% CI: 4.1-5.9, P < 0.001) (Krag, 2012). SER-287, composed of Firmicutes polyspores, is safe and well-tolerated. SER-287 induced a high remission of moderate UC after vancomycin treatment and promoted bacterial colonization of gut microbiota (Henn et al., 2021). Besides prolonged active IBD remission, microbial combination therapy can modulate inflammation and maintain remission in patients with asymptomatic or quiescent IBD. Bjarnason et al. found that a multi-strain probiotic Symprove decreased intestinal inflammation in patients with asymptomatic UC (Bjarnason et al., 2019). Yoshimatsu et al. used Bio-Three tablets containing Streptococcus faecalis, Clostridium butyricum and Bacillus mesentericus to maintain clinical remission in patients with quiescent UC (Yoshimatsu et al., 2015). Caviglia et al. found that FEEDColon® (consists of Bifidobacterium bifidum, Bifidobacterium lactis, calcium butyrate and oligosaccharides) was an effective adjunctive therapy for prolonged UC remission. Further analysis revealed that the remission rate of 5-ASA + FEEDColon® was greater than of 5-ASA alone (95% > 57%) (P = 0.009) (Caviglia et al., 2021).
There are also several bacterial combinations against IBD, such as bacterial ecosystem therapeutic-2 [MET-2] (ClinicalTrials.gov, 2019) and IDOFORM TRAVEL® (ClinicalTrials.gov, 2020a), under development ( Table 1 ).
Table 1.
Ongoing clinical trials of artificial consortium transplantation products.
| Name | Components | Indication | ClinicalTrials.gov Identifier | Ref. |
|---|---|---|---|---|
| MET-2 | comprises 40 different strains of gut bacteria from a healthy donor | Mild to moderate ulcerative colitis | NCT03832400 | (ClinicalTrials.gov, 2019) |
| IDOFORM®Travel | Lactobacillus rhamnosus (LGG), Lactobacillus acidophilus (LA-5), Bifidobacterium sp. (BB-12), Lactobacillus bulgaricus (LBY-27), and Streptococcus thermophilus (STY-31) | patients with ulcerative colitis undergoing anti-TNF treatment with insufficient clinical response | NCT04241029 | (ClinicalTrials.gov, 2020a) |
| Synbiotic | three Bifidobacterium spp.(Bifidobacterium longum spp. longum R0175, Bifidobacterium animalis spp. Lafti B94, Bifidobacterium bifidum R0071) plus three dietary fibers | Post-op Crohn’s Disease | NCT04804046 | (ClinicalTrials.gov, 2021) |
| Probiotic Mixture | contains 8 different strains of bacteria, the specific composition is unclear | Quiescent Inflammatory Bowel Disease | NCT03266484 | (ClinicalTrials.gov, 2017) |
| Probiotic Formula | Lactobacillus rhamnosus, Lactobacillus acidophilus, Lactobacillus reuteri, Lactobacillus paracasei, Lactobacillus casei, Lactobacillus gasseri, Lactobacillus plantarum, Bifidobacterium lactis, Bifidobacterium breve, Bifidobacterium bifidum, Bifidobacterium longum, Bifidobacterium infantis | Ulcerative colitis | NCT04223479 | (ClinicalTrials.gov, 2020b) |
| Peptidic+ Probiotic |
Oligomeric oral nutritional supplement (Bi1 peptidic), Bifidobacterium animalis subsp. lactis BPL1, Lactobacillus rhamnosus BPL15, Lactobacillus rhamnosus CNCM i-4036 Bifidobacterium longum ES1 |
Crohn’s Disease | NCT04305535 | (ClinicalTrials.gov, 2020c) |
4 Selection and Combination
4.1 Selection
Therapies relying on bacterial combinations usually use beneficial bacteria that have a research basis to support their benefits, most of which are recognized probiotics, such as Lactobacillus and Bifidobacterium (Araya et al., 2002). The bacteria used in combination therapy should be culturable and adaptable to the unique gastrointestinal environment. The bacteria must also stably colonize the intestinal tract.
4.1.1 Environmental Adaptability
To reach therapeutic levels, the bacteria used in microbiota transplantation must be resistant to gastric acidity and bile acid toxicity (Daliri and Lee, 2015). Low pH is a primary host defense against ingested microorganisms. Compared with Bifidobacteria, Lactobacillus is more resistant to low pH (Tripathi and Giri, 2014). Acid resistance is not only genus-specific but also species-specific. For example, L.casei and L. acidophilus are better resistant to the low pH than L.delbruekiis sp.bulgaricus. Interestingly, the different Bifidobacterium strains vary in their resistance to gastrointestinal tract acidity (Daliri and Lee, 2015), with B. animalis the most acid-resistant strain (Saarela et al., 2006).
Acid tolerance is also linked to bacterial genetics. For instance, the loss of the urec gene encoding a protein associated with acid resistance reduced the ecological adaptability of L. ruteri 100-23 (Krumbeck et al., 2016). Bacterial bile salt hydrolase (BSH) protects intestinal bacteria from bile salts by breaking down conjugated bile salts into conjugated bile acids. Several bacterial genera, including Lactobacillus (Corzo and Gilliland, 1999; Wang et al., 2012), Bifidobacterium (Kim et al., 2004), Enterococcus (De Filippo et al., 2010) and Clostridium spp (Coleman and Hudson, 1995), secrete BSH. Therapeutic strains must be safe for use, even in immunocompromised individuals. And to avoid elimination by the gut immune response, probiotic strains usually have mild (not pro-inflammatory) immunomodulatory effects (Daliri and Lee, 2015). The environmental adaptability of strains also includes sensitivity to oxygen and utilization of nutrients.
4.1.2 Colonization Characteristic
Stable colonization is a vital beneficial bacterial trait. Probiotics must adhere to the human intestinal cells and intestinal mucins to colonize and proliferate in these areas and compete with potential pathogens that adhere to mucosal surfaces. Recent studies have shown that the pattern of establishment, colonization and persistence of bacteria in certain gut sites are species-and strain-specific and are related to the host factors, strains characteristics, microbial interactions, and the diet (Xiao et al., 2021).
Introducing strains early in life or after antibiotic treatment promotes colonization and persistence of the bacteria in the intestines (Denou et al., 2008; Xiao et al., 2021). From the ecological perspective and coevolution, it is possible to determine the colonization and persistence potential of a particular bacterial species in human intestines. For example, judging from the natural history, compared with Lactobacillus, Bifidobacteria can colonize the intestinal tract more easily (Xiao et al., 2020). B. longum is a perfect example of bacterial species with long-term gut colonization potential, and it is dominant in the gut throughout the human lifespan (Odamaki et al., 2018). Bacterial genes, such as luxS (Tannock et al., 2005; Christiaen et al., 2014), pili (Turroni et al., 2013), and BSH (Corzo and Gilliland, 1999), are essential in host-microbe interactions. For instance, the luxS gene in B. breve UCC2003 (Christiaen et al., 2014)and L. reuteri 100–23 (Tannock et al., 2005) participated in producing the interspecies signaling molecule autoinducer-2 (AI-2), which mediates colonization of the bacteria in the human gut. Pili is a strain-specific colonization factor in LGG (but not in LC705) support the intestinal colonization ability of bacteria was strain-specific (Kankainen et al., 2009). In addition to the bacterial characteristics that contribute to its colonization, specific strains display reciprocal colonization effect and may influence the colonization of other strains. The success of FMT in intestinal flora reconstruction after antibiotic treatment suggests that a flora with rich genetic diversity and metabolic interactions is more likely to thrive in the intestine (Suez et al., 2018). Moreover, cross-feeding of Bifidobacterium strains (B. bifidumPRL2010, B. breve12L, B. adolescentis22L, and B. InfantisATCC15697) improved the persistence of each strain in the cecum (Turroni et al., 2016). Therefore, exploring the interaction behavior of probiotic strains can enhance the development of effective co-colonization combination strains. The core effect of dietary is by adding prebiotics to provide privileged nutrition pathway for intake of strains. Prebiotics including tryptophan, GOS and polysaccharide, which enhanced the colonization of specific bacterial strains. High levels of tryptophan increased the abundance of L. reuteri in the stomach and stool (Zelante et al., 2013), whereas high levels of GOS in the human intestine significantly enrich the abundance of Bifidobacterium in the gut in a dose-dependent manner (Krumbeck et al., 2015). Despite these findings, additional synergistic dietary and specific bacterial combinations that enhance the colonization of beneficial bacteria in the gut need to be identified.
4.2 Bacterial Combination
4.2.1 Types of ACT Products
In this article, the current bacterial combinations for IBD were divided into four types ( Table 2 ). The first type is a combination of probiotics combined with prebiotics, named synbiotic. Prebiotics are dietary fiber supplementations that stimulate the growth of specific, putatively beneficial bacteria already present in the gut. Prebiotics promote the metabolism and colonization of probiotics. The most commonly used prebiotics are fermentable carbohydrates such as inulin, oligosaccharides, galactose oligosaccharides and resistant starch. In addition, polyphenols and polyunsaturated fatty acids, which act on gut microbes, are also classified as prebiotics. Example of this type of combination include Profermin® (Krag, 2012), FEEDColon® (Caviglia et al., 2021) and YBF (ClinicalTrials.gov, 2010).
Table 2.
Four categories of artificial consortium transplantation products.
| Type | Name | Components | Producer | Ref |
|---|---|---|---|---|
| Synbiotic | Lactobacillus sp. +prebiotic | grape pomace extract, L. rhamnosus (IDIBNA02), L. paracasei (ID13239), L. acidophilus (ID11692) |
Gina Cecilia Pistol et al. | (Pistol et al., 2019) |
| Bifidobacterium longum/Synergy 1 | Fructo-oligosaccharide/inulin mix, B. longum | H. Steed et al | (Steed et al., 2010) | |
| Profermin® | Fermented oats, barley malt, lecithin, L.plantarum 299v | Nordisk Rebalance | (Krag, 2012) | |
| Symprove™ | Barley extract, L.rhamnosus NCIMB 30174, L. plantarum NCIMB 30173, L. acidophilus NCIMB 30175, Enterococcus faecium NCIMB 30176 |
Symprove Ltd | (Bjarnason et al., 2019) | |
| Bio-Three tablets | Potato starch, lactose, Streptococcus faecalis T-110, Clostridium butyricum TO-A, Bacillus mesentericus TO-A |
Toa Pharmaceutical Co. | (Yoshimatsu et al., 2015) | |
| FEEDColon® | Calcium butyrate, fructo-oligosaccharides, B. bifidum, B. lactis |
Princeps | (Caviglia et al., 2021) | |
| YBF | Yogurt, soluble fiber, Bifidobacteria | Instituto Lala | (ClinicalTrials.gov, 2010) | |
| Mutualbiotic | VSL#3 |
L. plantarum, L. paracasei, L. delbrueckii subsp. bulgaricus, L. acidophilus, B. longum, B. breve, B. infantis, Streptococcus thermophilus |
VSL#3, Pharma | (Reiff et al., 2009) |
| Visbiome® |
L. plantarum DSM 24730, L. paracasei DSM 24733, L. delbrueckii subsp. bulgaricus DSM 24734, L. acidophilus DSM 24735, B. longum DSM 24736, B. breve DSM 24732, B. infantis DSM 24737, Streptococcus thermophilus DSM 24731 |
A blend produced under Prof. De Simone’s control | (Rossi et al., 2018) | |
| Five strains probiotics |
L. casei, B. breve, B. animalis subsp. Lactis, Streptococcus thermophilus, Bacillus subtilis |
Michele Biagioli et al. |
(Biagioli et al., 2020) | |
| Ultrabiotique® | L. acidophilus, L. plantarum, B. lactis, B. breve | Laboratoire Nutrisante | (Toumi et al., 2013) | |
| Citogenex | L. casei, B. animalis subspecies lactis | G Traina et al. | (Traina G, 2016) | |
| PM-2 | L. acidophilus, L. paracasei, L. rhamnosus, B. lactis | Marielle Quaresma et al. | (Quaresma et al., 2020) | |
| ID-JPL934 |
L. johnsoniiIDCC9203, L. plantarumIDCC3501, B. animalis subspecies lactisIDCC4301 |
In-Gyu Je et al. | (Je et al., 2018) | |
| I3.1 probiotic |
L. plantarum (CECT7484, CECT7485), Pediococcus acidilactici (CECT7483) |
AB-Biotics S.A | (Lorén et al., 2017) | |
| GI7 |
L. acidophilus LA1 (KCTC 11906BP), L. plantarum LP3 (KCTC 10782BP), L. rhamnosus LR5 (KCTC 12202BP), L. lactis SL6 (KCTC 11865BP), B. bifidum BF3 (KCTC 12199BP), B. breveBR3 (KCTC 12201BP), Streptococcus thermophilus ST3 (KCTC 11870BP). |
M.S. Kim et al. | (Kim et al., 2017) | |
| Ecologic®825 |
L. acidophilus, L. casei, L. paracasei, L. plantarum, L. salivarius, B. bifidum, B. lactis, Lactococcus lactis. |
Winclove Probiotics BV | (Persborn et al., 2013) | |
| IDOFORM®Travel |
L. rhamnosus (LGG), L. acidophilus (LA-5), L. bulgaricus (BY-27), Bifidobacterium sp. (BB-12), Streptococcus thermophilus (STY-31) |
Pfizer | (ClinicalTrials.gov, 2020a) | |
| SYNBIO ® | L. rhamnosus IMC 501®, L. paracasei IMC 502® | Synbiotec S.r.l. | (Coman et al., 2020) | |
| LAB mixture |
L. plantarum CRL 2130, Streptococcus thermophilus
(CRL 807, CRL 808) |
Romina Levit et al. | (Levit et al., 2019) | |
| Bifico | Bifidobacterium, Lactobacillus, Enterococcus | Shanghai Sine Pharmaceutical | (Zhang et al., 2018) | |
| Bio 25 |
L. rhamnosus LR5, L. casei LC5, L. paracasei LPC5, L. plantarum LP3, L. acidophilus LA1, L. bulgaricus LG1, B. bifidum BF3, B. longum BG7, B. breve BR3, B. infantis BT1, Streptococcus thermophilus ST3, Lactococcus lactis SL6 |
Supherb Ltd | (ClinicalTrials.gov, 2013) | |
| Diverbiotic | BCT | Lactobacillus, Eubacterium, Pediococcus, Veillonella, Streptococcus, Staphylococcus, Bifidobacterium, Bacteroide, Escherichia, Fusobacterium | Ming Li et al. | (Li et al., 2015) |
| GUT-103 |
Megamonas funiformis DSM19343, Megamonas hypermegale DSM1672, Acidaminococcus intestini DSM21505, Bacteroides massiliensis DSM17679, Bacteroides stercoris ATCC43183/DSM19555, Barnesiella intestinihominis DSM21032, Faecalibacterium prausnitzii DSM17677, Subdoligranulum variabile DSM15176, Anaerostipes caccae DSM14662, Anaerostipes hadrus DSM3319/ATCC 29173, Clostridium symbiosum ATCC14940, Akkermansia muciniphila ATCC BAA-835, Clostridium scindens ATCC35704, Clostridium bolteae ATCC BAA-613, Blautia producta DSM2950, Blautia hydrogenotrophia DSM10507, Marvinbryantia formatexigens DSM14469 |
Daniel van der Lelie et al. |
(van der Lelie et al., 2021) | |
| GUT-108 | Bacteroides xylanisolvens GGCC_0124, Clostridium butyricumGGCC_0151, Clostridium scindens GGCC_0168, Intestinimonas butyriciproducens GGCC_0179, Extibacter sp.GGCC_0201, Eubacterium callanderi GGCC_0197, Akkermansia sp. GGCC_0220, Clostridium symbiosum GGCC_0272, Bacteroides uniformisGGCC_0301, Bitterella massiliensis GGCC_0305, Barnesiella sp. GGCC_0306 | Daniel van der Lelie et al. |
(van der Lelie et al., 2021) | |
| SER-287 | Spores of Firmicutes | Matthew R. Henn et al. |
(Henn et al., 2021) | |
| MET-2 | 40 different strains of gut bacteria from a healthy donor | NuBiyota | (ClinicalTrials.gov, 2019) | |
| Ejusbiotic | Butyrate-producing bacteria | Butyricicoccus pullicaecorum 25-3T (LMG 24109 T), Butyricicoccus pullicaecorum 1.20, Faecalibacterium prausnitzii (DSM 17677), Roseburia hominis (DSM 16839), Roseburia inulinivorans (DSM 16841), Anaerostipes caccae (DSM 14662) and Eubacterium hallii (DSM 3353) | Annelies Geirnaert et al. | (Geirnaert et al., 2017) |
L., Lactobacillus; B., Bifidobacteriu.
The second type of ACT products comprises beneficial bacteria of different phyla or genera paired based on their social interactions, is a mutual effect combination (mutualbiotic). The most common match includes a combination of Bifidobacteria or Lactobacillus and other strains. The strains establish a symbiotic interaction that promotes reciprocal colonization in the harsh and dynamic human gut environment, including ID-JPL934 (Je et al., 2018), GI7 (Kim et al., 2017), VSL#3 (Tursi et al., 2010) and IDOFORM TRAVEL® (ClinicalTrials.gov, 2020a).
The third type of diverse bacterial combination consists of strains from multiple phyla, named diversified combination(diverbiotic). These strains are isolated from feces then combined in an optimum manner to form a diverse community. This type of consortia includes BCT (Li et al., 2016), GUT-103, GUT-108 (van der Lelie et al., 2021) and MET-2 (ClinicalTrials.gov, 2019).
The fourth type of bacterial consortia includes a combination of bacteria grouped together according to metabolic characteristics, named ejusdem combination (ejusbiotic), comprises bacteria that produce the same metabolic products beneficial for IBD patients. An example is the butyrate-producing bacteria (Geirnaert et al., 2017).
4.2.2 The Principles of the ACT Products
ACT shall be developed in accordance with the principles of complementarity, reciprocity, specificity and stability (CRSS).
The function of each strain is limited, and the strains complement the effects of each strain. For example, the butyrate-producing bacteria consortium competed with resident microbiota for substrates (e.g., acetate) and, thus, promoted the production of butyrate (Geirnaert et al., 2017).
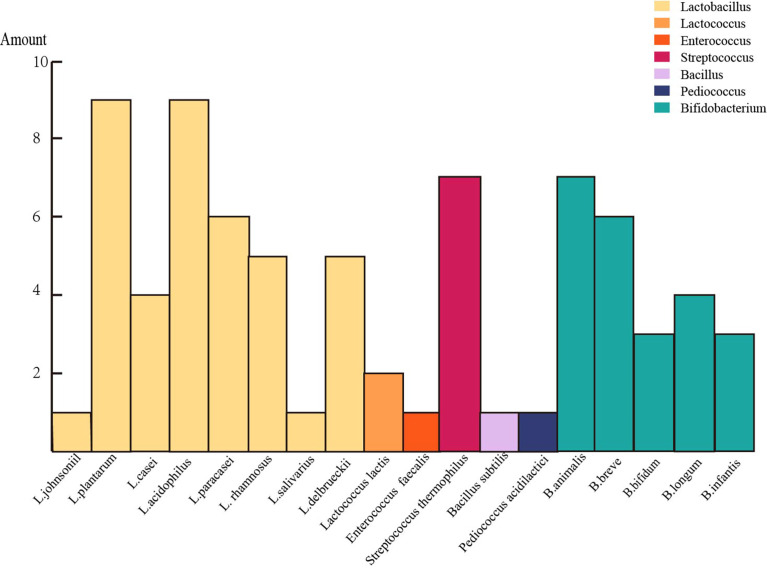
The gut microbiome must establish a reciprocal interaction to remain stable and functional in the dynamic gut environment. Given that exogenous acetate is critical in maintaining butyrate production, the cross-feed between the acetic acid-producing bacteria and butyrate-producing bacteria enhances the continued functioning of butyrate-producing bacteria (Duncan et al., 2004). The cross-feeding observed among the intestinal microbiota suggests the inter-dependence of the strains. Lactobacillus and Bifidobacterium, the most commonly used probiotics, are the most common artificial consortium bacteria ( Table 3 ; Figure 2 . each corresponding). Most Bifidobacteria display reciprocal carbohydrate metabolism capability in vitro ( Riviere et al., 2018) and persistence in the gut in vivo ( Turroni et al., 2016). In addition to their symbiosis association within the genus, Bifidobacterium promotes the activities of other gut bacteria (e.g., Bacteroidetes), including carbohydrate metabolism, thus, enhancing the environmental adaptability of these bacteria (Sonnenburg et al., 2006; Turroni et al., 2016).
Table 3.
A list of each product components of mutulbiotics applied in IBD.
| Phylum | Firmicutes | Actinobacteria | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genus | Lactobacillus | Lactococcus | Entero-coccus | Streptococcus | Bacillus | Pediococcus | Bifidobacterium | |||||||||||
| Species/Name | johnsonii | plantarum | casei | acidophilus | paracasei | rhamnosus | salivarius | delbrueckii | lactis | faecalis | thermophilus | subtilis | acidilactici | animalis | breve | bifidum | longum | infantis |
| VSL#3 | O | O | O | O | O | O | O | O | ||||||||||
| Visbiome® | DSM 24730 | DSM 24735 | DSM 24733 | Bulgaricus DSM 24734 | DSM 24731 | DSM 24732 | DSM 24736 | DSM 24737 | ||||||||||
| Five strains probiotics | O(30%) | O(30%) | O(10%) | lactis15% | O(15%) | |||||||||||||
| Ultrabiotique® | O | O | lactis | O | ||||||||||||||
| Citogenex | O | lactis | ||||||||||||||||
| PM-2 | O | O | O | lactis | ||||||||||||||
| ID-JPL934 (1:1:1) | DCC9203 | DCC3501 | lactisDCC4301 | |||||||||||||||
| I3.1probiotic formula | CECT7484+ CECT7485 | CECT7483 | ||||||||||||||||
| GI7 | LP3(KCTC 10782BP) | LA1(KCTC 11906BP) | LR5 (KCTC 12202BP) | LactisSL6 (KCTC 11865BP) | ST3(KCTC 11870BP) | BR3(KCTC12201BP) | BF3 (KCTC12199BP) | |||||||||||
| Ecologic®825 | W62 | W56 | W22 | W20 | W24 | W19 | lactisW51+W52 | W23 | ||||||||||
| IDOFORM® Travel | LA-5 | O | bulgaricusLBY-27 | STY-31 | BB-12 | |||||||||||||
| SYNBIO® | IMC50® | IMC501® | ||||||||||||||||
| LAB mixture | CRL 2130 | CRL808+ CRL 807 | ||||||||||||||||
| Bifico | O | O | O | |||||||||||||||
| Bio 25 | LP3 | LC5 | LA1 | LPC5 | LR5 | LG1 | SL6 | ST3 | BR3 | BF3 | BG7 | BT1 | ||||||
“O” indicates the existence of the strain in this product, but the specific strain is not clear.
Figure 2.
A chart listed the number of occurrences of each strain in these mutualbiotics (one color represents a genus).
The strain-specific and disease-specific effects of bacteria on disease have been extensively reported. In addition to the inter-species differences, the diversity within species should be considered. For example, in describing two different F. prausnitzii phylogroups (Lopez-Siles et al., 2012), Lopez-Siles et al. found that whereas the abundance of phylogroup I was significantly low in the gut of CD, UC, and colorectal cancer patients, depletion of phylogroup II was explicitly related to CD (Lopez-Siles et al., 2016). A decrease in Lactobacillus is predominant in active ulcerative colitis, whereas a similar phenomenon is observed for Bifidobacteria in Crohn’s disease.
Conditions may also influence the effect of the final ACT products, such as production method or strain ratio. Even if the composition of strains in the artificial consortium products is similar, their effect may differ. For example, VSL#3 and Visbiome® are two products composed of the same bacteria species. However, Visbiome® activated Treg cells (CD4+FoxP3+) and T lymphocytes to produce anti-inflammatory cytokines IL-10 and short-chain fatty acids more effectively than VSL#3, which may be due to different production methods or composition ratios of individual bacterial species (Biagioli et al., 2019). Therefore, more stable production standards should be maintained.
5 Perspectives of Engineered Consortium Transplantation
Naturally isolated strains aside, genetically modified bacteria can be used for microbiota transplantation (Gao et al., 2022). The engineered strains perform different functions from the original strain or possess modified metabolic characteristics. For example, Puurunen et al. inserted genes encoding phenylalanine ammonia-lyase and L-amino acid deaminase into the E. coli Nissle 1917 genome, generating the modified SYNB1618 strain. The engineered strain could degrade phenylalanine in the gastrointestinal tract (Puurunen et al., 2021). A butyrate-producing bacteria, recombinant B. subtilis BsS-RS06550 with high butyric acid production was constructed using synthetic biological strategies could effectively regulate body metabolism and intestinal flora disruption (Bai et al., 2020; Wang et al., 2022).The modified strains can increase the variety and number of available strains to the bacterial combination. So far, the engineered strain alone against certain diseases, such as phenylketonuria (Puurunen et al., 2021), liver cirrhosis (https://clinicaltrials.gov/ct2/show/NCT03447730?term=NCT03447730&draw=2&rank=1), has been assessed.
Lactococcus lactis (LL-Thy12), in which the thymidylate synthase gene was replaced with a synthetic sequence encoding mature human interleukin-10 (Braat et al., 2006), was found to alleviate Crohn’s disease. Yeast expressing human P2Y2 purinergic receptor and ATP-degrading enzyme, creating self-regulating yeast probiotics system capable of sensing pro-inflammatory molecules inhibits intestinal inflammation in IBD mice (Scott et al., 2021). However, clinical trials on the efficacy of engineered strains against IBD are limited, and even few engineered strains have been transplanted together.
Bacterial combinations promote metabolic characteristics and colonization and increase species diversity. Therefore, the efficacy of the combination of engineered bacteria is a promising research direction for microbiota transplantation.
6 Discussion
Autochthonous strains are more likely to exert beneficial effects, while allochthonous strains may stimulate the immune system to some extent (Tannock et al., 2000; Yamashita et al., 2020). The sources of the strains are various, such as fermented food and feces. While from the co-evolution perspective, it is better to select strains isolated from human feces for transplantation into the human intestine. Different species display distinctly varied gut fitness. For instance, autochthonous lactobacilli showed better gut colonization ability than the allochthonous (Duar et al., 2017). Microbiota transplantation research is gravitating toward using specific FMT or engineered fecal microbiota, which generates a superior effect to the natural fecal microbiota.
The efficacy of strain combinations can be assessed based on the degree and duration of in vivo colonization. Most of the data on intestinal colonization of bacteria have been derived from animal models. Given the differences between animal and human systems, more clinical trials are needed to validate the effectiveness of combined microbiota transplantation. Moreover, the functional characterization of most symbiotic strains in the gut is still in infancy, and more research is needed to identify new strains with high potential for health benefits. Identification of novel health-associated gut bacteria allows better insight into the functionality of the different species and strains (Cuffaro et al., 2021). The findings extend the number of potential candidates for personalized probiotics, taking individual host variations and specific responses into account.
The diversity of gut microbiota is critical for maintaining resilience, and therefore, the transplantation of microbiota combinations is a potentially effective alternative for IBD treatment. Previous articles on microbiota transplantation were mainly limited to FMT, and most of them focused on the application of FMT in IBD, or emphasized the importance of the gut microbes in the pathogenesis and treatment of IBD (Zuo and Ng, 2018; Ooijevaar et al., 2019; Tan et al., 2020; Lee and Chang, 2021; Underhill and Braun, 2022; Halaweish et al., 2022). We mainly analyzed different microbiome-based interventions currently applied in IBD clinical trials, including FMT, WMT (a method that removes adverse factors in natural FMT by special washing manner), as well as ACT, which combines different and limited microorganisms, and analyzed the possible combination principles of ACT. In particular, engineered single bacteria have been used against IBD in recent years (Braat et al., 2006; Scott et al., 2021), we imaged that the artificial consortium combined with engineered bacteria is expected to bring revolutionary mutations to microbiota transplantation.
Given the enormous prospective of microbiota transplantation, the review of different combinations and principles will help to provide a theoretical basis for the generation of more artificial consortium transplantation in the future. The application of microbiota combination in different disease states is differ, even distinct in different individuals. The artificial consortium should not just be a simple combination of strains. However, it should be oriented by engineering ideas and form a systematic whole with the combined characteristics of strains, classification of recipient microbes, disease stages and other factors. Such a consortium could be the next generation of microbiota transplants. Finding rules or principles on the basis of existing research is helpful in exploring the optimal solution of the microbiota approach applied to IBD patients, that is, fewer adverse effects and better clinical outcomes. Recently, Gianluca Ianiro published a comment on the treatment of recurrent Clostridioides difficile Infection by SER-109, an artificial microbiome consortium Product, which was similar to our idea in this review, that ACT, to a certain extent, overcome issues related to donor safety and maintenance associated with classical FMT. However, still needed more studies to compare synthetic microbial complexes with standard FMT. If these are better or equivalent to classic FMT, then this will herald the era of FMT2.0 (Feuerstadt et al., 2022; Ianiro, 2022).
The limitations of this review are that neither the fungal microbiome is taken into account nor receptor factors are combined. In most clinical trials, only limited information about the receptors has been mentioned. Generally, there is only a classification of IBD severity. However, the classification of the intestinal microbiota of the recipients is always absent and the age and gender information is also ominous, which is not conducive to our more comprehensive analysis. Therefore, we appeal to record more complete and comprehensive information in clinical trials which could provide a foundation for more comprehensive analysis of microbiota transplantation in the future.
7 Conclusion
In this review, we summarized clinical studies on various microbiota combinations applied to IBD ( Figure 3 ) and emphasized the application of artificial microbiota combination transplantation against IBD. The advantages of the bacterial combination were discussed, the types and the possible principles of ACT products were summarized, while the prospect of microbiota transplantation was discussed. The combination of microbiota needs to take the complementary relevance between strains, individual strains’ specificity and stable conditions into account. Future research should identify combinations of strains that display metabolic interactions based on ecological knowledge, bacterial genomic data and in vitro experimental results, and then validate them in vivo, ultimately contributing to mutually beneficial human implantation. The transplantation of microbiota combinations is a potentially safe and effective alternative for IBD treatment. Compared with classical FMT, ACT reduces safety concerns and diversified the options available for different disease states to some extent. Furthermore, the artificial consortium combined with engineered bacteria is expected to bring revolutionary mutations to the microbiota transplantation, which has a bright foreground against IBD. These will probably herald the arrival of the new era of microbial therapy!
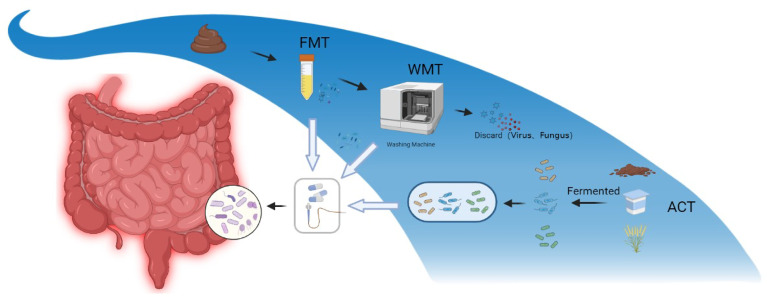
Figure 3.
Diagram of three different modes of microbiota transplantation. FMT, fecal microbiota transplantation; WMT, washed microbiota transplantation; ACT, artificial consortium transplantation.
Author Contributions
XC, GK, XW, and JZ contributed to the conception of this review. XW and JZ wrote the first draft of the manuscript. YF, ZF, YY, and LL is responsible for literature retrieval. XC, GK, XW, JZ, YF, ZF, YY, and LL wrote sections of the manuscript. XC and GK supervise the project administration. All authors contributed to the article and approved the submitted version.
Funding
The present study was supported by grants from the National Key Research and Development Project (GrantNo.2019YFA0905600); the Tianjin Health Science and Technology Research Project (GrantNo.TJWJ2021MS005); the Major State Basic Research Development Program of the Natural Science Foundation of Shandong Province in China (GrantNo.ZR2020ZD11), We thank Shaoxing “Ming Shi Zhi Xiang” Meritocrat Project and Program of Introducing Talents of Discipline to University Ministry of Education, China-111 Project (GrantNo.BP0618007) for its support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to Professor Faming Zhang (The Second Affiliated Hospital of Nanjing Medical University) for advise and critical reading the manuscript.
References
- Araya M., Morelli L., Reid G., Sanders M. E., Stanton C., Pineiro M., et al. (2002). Guidelines for the Evaluation of Probiotics in Food (pp. 1–11). Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food London, Ontario, Canada, April 30 and May 1, 2002. [Google Scholar]
- Backhed F., Ley R. E., Sonnenburg J. L., Peterson D. A., Gordon J. I. (2005). Host-Bacterial Mutualism in the Human Intestine. Science 307, 1915–1920. doi: 10.1126/science.1104816 [DOI] [PubMed] [Google Scholar]
- Bai L., Gao M., Cheng X., Kang G., Cao X., Huang H. (2020). Engineered Butyrate-Producing Bacteria Prevents High Fat Diet-Induced Obesity in Mice. Microb. Cell Fact. 19, 94. doi: 10.1186/s12934-020-01350-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj J. S., Kassam Z., Fagan A., Gavis E. A., Liu E., Cox I. J., et al. (2017). Fecal Microbiota Transplant From a Rational Stool Donor Improves Hepatic Encephalopathy: A Randomized Clinical Trial. Hepatology 66, 1727–1738. doi: 10.1002/hep.29306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagioli M., Capobianco D., Carino A., Marchiano S., Fiorucci C., Ricci P., et al. (2019). Divergent Effectiveness of Multispecies Probiotic Preparations on Intestinal Microbiota Structure Depends on Metabolic Properties. Nutrients 11, 325. doi: 10.3390/nu11020325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagioli M., Carino A., Di Giorgio C., Marchianò S., Bordoni M., Roselli R., et al. (2020). Discovery of a Novel Multi-Strains Probiotic Formulation With Improved Efficacy Toward Intestinal Inflammation. Nutrients 12, 1945. doi: 10.3390/nu12071945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., Sission G., Hayee B. H. (2019). A Randomised, Double-Blind, Placebo-Controlled Trial of a Multi-Strain Probiotic in Patients With Asymptomatic Ulcerative Colitis and Crohn’s Disease. Inflammo.Pharmacol 27, 465–473. doi: 10.1007/s10787-019-00595-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braat H., Rottiers P., Hommes D. W., Huyghebaert N., Remaut E., Remon J. P., et al. (2006). A Phase I Trial With Transgenic Bacteria Expressing Interleukin-10 in Crohn’s Disease. Clin. Gastroenterol. Hepatol. 4, 754–759. doi: 10.1016/j.cgh.2006.03.028 [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov (2018). Safety, Tolerability and Pharmacodynamics of SYNB1020. NCT03447730. U.S. National Library of Medicine, Bethesda, MD, UDA. Available at: https://clinicaltrials.gov/ct2/show/NCT03447730?term=NCT03447730&draw=2&rank=1 [Google Scholar]
- ClinicalTrials.gov (2019). Safety and Efficacy of Microbial Ecosystem Therapeutic-2 (MET-2) in Patients With Ulcerative Colitis (UC). NCT03832400. U.S. National Library of Medicine, Bethesda, MD, UDA. Available at https://clinicaltrials.gov/ct2/show/NCT03832400?term=NCT03832400&draw=2&rank=1. [Google Scholar]
- ClinicalTrials.gov (2020. a). Boosting Biologics in UC. NCT04241029. U.S. National Library of Medicine, Bethesda, MD, UDA. Available at: https://clinicaltrials.gov/ct2/show/NCT04241029?term=IDOFORM%C2%AETravel&draw=2&rank=1. [Google Scholar]
- ClinicalTrials.gov (2021). Synbiotics and Post-op Crohn's Disease. NCT04804046. U.S. National Library of Medicine, Bethesda, MD, UDA. Available at: https://clinicaltrials.gov/ct2/show/NCT04804046?term=strains&cond=inflammatory+bowel+disease&draw=2. [Google Scholar]
- ClinicalTrials.gov (2017). Effect of a Probiotic Mixture on the Gut Microbiome and Fatigue in Patients With Quiescent Inflammatory Bowel Disease. NCT03266484. U.S. National Library of Medicine, Bethesda, MD, UDA. Available at: https://clinicaltrials.gov/ct2/show/NCT03266484?term=strains&cond=IBD&draw=2. [Google Scholar]
- ClinicalTrials.gov (2020. b). Effect of Probiotic Supplementation on the Immune System in Patients With Ulcerative Colitis in Amman, Jordan. NCT04223479. U.S. National Library of Medicine, Bethesda, MD, UDA. Available at: https://clinicaltrials.gov/ct2/show/NCT04223479?term=Probiotic&cond=Inflammatory+Bowel+Diseases&draw=2&rank=22. [Google Scholar]
- ClinicalTrials.gov (2020. c). Impact of an Oligomeric Diet in Intestinal Absorption and Inflammatory Markers in Patients With Crohn Disease. NCT04305535. U.S. National Library of Medicine, Bethesda, MD, UDA. Available at: https://clinicaltrials.gov/ct2/show/NCT04305535?term=Probiotic&cond=Crohn+Disease&draw=2. [Google Scholar]
- ClinicalTrials.gov (2010). Effect of Yogurt Added With Bifidobacteria and Soluble Fiber on Bowel Function. NCT01173588. U.S. National Library of Medicine, Bethesda, MD, UDA. Available at: https://clinicaltrials.gov/ct2/show/NCT01173588?term=Probiotic&cond=Inflammatory+Bowel+Diseases&draw=2&rank=11. [Google Scholar]
- ClinicalTrials.gov (2013). The Effect of Probiotics on Exacerbation of Inflammatory Bowel Disease Exacerbation (Crohn's Disease). NCT01765998. U.S. National Library of Medicine, Bethesda, MD, UDA. Available at: https://clinicaltrials.gov/ct2/show/NCT01765998?term=NCT01765998&draw=2&rank=1. [Google Scholar]
- Caviglia G. P., De Blasio F., Vernero M., Armandi A., Rosso C., Saracco G. M., et al. (2021). Efficacy of a Preparation Based on Calcium Butyrate, Bifidobacterium Bifidum, Bifidobacterium Lactis, and Fructooligosaccharides in the Prevention of Relapse in Ulcerative Colitis: A Prospective Observational Study. J. Clin. Med. 10, 4961. doi: 10.3390/jcm10214961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Liu X. L., Zhang Y. J., Nie Y. Z., Wu K. C., Shi Y. Q. (2020). Efficacy and Safety of Fecal Microbiota Transplantation by Washed Preparation in Patients With Moderate to Severely Active Ulcerative Colitis. J. Digest. Dis. 21, 621–628. doi: 10.1111/1751-2980.12938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaen S. E., O’Connell M. M., Bottacini F., Lanigan N., Casey P. G., Huys G., et al. (2014). Autoinducer-2 Plays a Crucial Role in Gut Colonization and Probiotic Functionality of Bifidobacterium Breve UCC2003. PloS One 9, e98111. doi: 10.1371/journal.pone.0098111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H., Khosravi A., Kusumawardhani I. P., Kwon A. H. K., Vasconcelos A. C., Cunha L. D., et al. (2016). Gene-Microbiota Interactions Contribute to the Pathogenesis of Inflammatory Bowel Disease. Science 352, 1116–1120. doi: 10.1126/science.aad9948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. P., Hudson L. L. (1995). Cloning and Characterization of a Conjugated Bile Acid Hydrolase Gene From Clostridium Perfringens. Appl. Environ. Microbiol. 61, 2514–2520. doi: 10.1128/aem.61.7.2514-2520.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman M. M., Mazzotti L., Silvi S., Scalise A., Orpianesi C., Cresci A., et al. (2020). Antimicrobial Activity of SYNBIO((R)) Probiotic Formulation in Pathogens Isolated From Chronic Ulcerative Lesions: In Vitro Studies. J. Appl. Microbiol. 128, 584–597. doi: 10.1111/jam.14482 [DOI] [PubMed] [Google Scholar]
- Corzo G., Gilliland S. E. (1999). Bile Salt Hydrolase Activity of Three Strains of Lactobacillus Acidophilus1. J. Dairy Sci. 82, 472–480. doi: 10.3168/jds.S0022-0302(99)75256-2 [DOI] [PubMed] [Google Scholar]
- Costello E. K., Lauber C. L., Hamady M., Fierer N., Gordon J. I., Knight R. (2009). Bacterial Community Variation in Human Body Habitats Across Space and Time. Science 326, 1694–1697. doi: 10.1126/science.1177486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers J. W., Chu N. D., Nguyen L. T. T., Phillips M., Collins C., Fortner K., et al. (2021). Daily, Oral FMT for Long-Term Maintenance Therapy in Ulcerative Colitis: Results of a Single-Center, Prospective, Randomized Pilot Study. BMC Gastroenterol. 21, 281. doi: 10.1186/s12876-021-01856-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuffaro B., Assohoun A. L. W., Boutillier D., Peucelle V., Desramaut J., Boudebbouze S., et al. (2021). Identification of New Potential Biotherapeutics From Human Gut Microbiota-Derived Bacteria. Microorganisms 9, 565. doi: 10.3390/microorganisms9030565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daliri E. B., Lee B. H. (2015). New Perspectives on Probiotics in Health and Disease. Food Sci. Hum. Wellness 4, 56–65. doi: 10.1016/j.fshw.2015.06.002 [DOI] [Google Scholar]
- Danne C., Rolhion N., Sokol H. (2021). Recipient Factors in Faecal Microbiota Transplantation: One Stool Does Not Fit All. Nat. Rev. Gastroenterol. Hepatol. 18, 503–513. doi: 10.1038/s41575-021-00441-5 [DOI] [PubMed] [Google Scholar]
- DeFilipp Z., Bloom P. P., Torres S. M., Mansour M. K., Sater M., Huntley M. H., et al. (2019). Drug-Resistant E. Coli Bacteremia Transmitted by Fecal Microbiota Transplant. N Engl. J. Med. 381, 2043–2050. doi: 10.1056/NEJMoa1910437 [DOI] [PubMed] [Google Scholar]
- De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J. B., Massart S., et al. (2010). Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children From Europe and Rural Africa. Proc. Natl. Acad. Sci. 107, 14691–14696. doi: 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot P. F., Frissen M. N., de Clercq N. C., Nieuwdorp M. (2017). Fecal Microbiota Transplantation in Metabolic Syndrome: History, Present and Future. Gut Microbes 8, 253–267. doi: 10.1080/19490976.2017.1293224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denou E., Pridmore R. D., Berger B., Panoff J., Arigoni F., Br̈ssow H. (2008). Identification of Genes Associated With the Long-Gut-Persistence Phenotype of the Probiotic Lactobacillus Johnsonii Strain NCC533 Using a Combination of Genomics and Transcriptome Analysis. J. Bacteriol. 190, 3161–3168. doi: 10.1128/JB.01637-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duar R. M., Lin X. B., Zheng J., Martino M. E., Grenier T., Perez-Munoz M. E., et al. (2017). Lifestyles in Transition: Evolution and Natural History of the Genus Lactobacillus. FEMS Microbiol. Rev. 41, S27–S48. doi: 10.1093/femsre/fux030 [DOI] [PubMed] [Google Scholar]
- Duffy L. C., Raiten D. J., Hubbard V. S., Starke-Reed P. (2015). Progress and Challenges in Developing Metabolic Footprints From Diet in Human Gut Microbial Cometabolism. J. Nutr. 145, 1123S–1130S. doi: 10.3945/jn.114.194936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S. H., Holtrop G., Lobley G. E., Calder A. G., Stewart C. S., Flint H. J. (2004). Contribution of Acetate to Butyrate Formation by Human Faecal Bacteria. Br. J. Nutr. 91, 915–923. doi: 10.1079/BJN20041150 [DOI] [PubMed] [Google Scholar]
- Fassarella M., Blaak E. E., Penders J., Nauta A., Smidt H., Zoetendal E. G. (2021). Gut Microbiome Stability and Resilience: Elucidating the Response to Perturbations in Order to Modulate Gut Health. Gut 70, 595–605. doi: 10.1136/gutjnl-2020-321747 [DOI] [PubMed] [Google Scholar]
- Fedorak R. N., Feagan B. G., Hotte N., Leddin D., Dieleman L. A., Petrunia D. M., et al. (2015). The Probiotic VSL3 Has Anti-Inflammatory Effects and Could Reduce Endoscopic Recurrence After Surgery for Crohn’s Disease. Clin. Gastroenterol. H. 13, 928–935. doi: 10.1016/j.cgh.2014.10.031 [DOI] [PubMed] [Google Scholar]
- Fehily S. R., Basnayake C., Wright E. K., Kamm M. A. (2021). Fecal Microbiota Transplantation Therapy in Crohn’s Disease: Systematic Review. J. Gastroen. Hepatol. 36, 2672–2686. doi: 10.1111/jgh.15598 [DOI] [PubMed] [Google Scholar]
- Feuerstadt P., Louie T. J., Lashner B., Wang E., Diao L., Bryant J. A., et al. (2022). SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides Difficile Infection. N Engl. J. Med. 386, 220–229. doi: 10.1056/NEJMoa2106516 [DOI] [PubMed] [Google Scholar]
- Fong W., Li Q., Yu J. (2020). Gut Microbiota Modulation: A Novel Strategy for Prevention and Treatment of Colorectal Cancer. Oncogene 39, 4925–4943. doi: 10.1038/s41388-020-1341-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori S., Tatsuguchi A., Gudis K., Kishida T., Mitsui K., Ehara A., et al. (2007). High Dose Probiotic and Prebiotic Cotherapy for Remission Induction of Active Crohn’s Disease. J. Gastroen. Hepatol. 22, 1199–1204. doi: 10.1111/j.1440-1746.2006.04535.x [DOI] [PubMed] [Google Scholar]
- Furrie E. (2005). Synbiotic Therapy (Bifidobacterium Longum/Synergy 1) Initiates Resolution of Inflammation in Patients With Active Ulcerative Colitis: A Randomised Controlled Pilot Trial. Gut 54, 242–249. doi: 10.1136/gut.2004.044834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Wang L., Huang H. (2022). Advances in Synthetic Biology Assisted Intestinal Microecological Therapy. Synthetic Biol. J. 3, 35–52. doi: 10.12211/2096-8280.2021-097 [DOI] [Google Scholar]
- Geirnaert A., Calatayud M., Grootaert C., Laukens D., Devriese S., Smagghe G., et al. (2017). Butyrate-Producing Bacteria Supplemented In Vitro to Crohn’s Disease Patient Microbiota Increased Butyrate Production and Enhanced Intestinal Epithelial Barrier Integrity. Sci. Rep. UK 7, 11450. doi: 10.1038/s41598-017-11734-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gionchetti P., Rizzello F., Helwig U., Venturi A., Lammers K. M., Brigidi P., et al. (2003). Prophylaxis of Pouchitis Onset With Probiotic Therapy: A Double-Blind, Placebo-Controlled Trial. Gastroenterology 124, 1202–1209. doi: 10.1016/S0016-5085(03)00171-9 [DOI] [PubMed] [Google Scholar]
- Gomollón F., Dignass A., Annese V., Tilg H., Van Assche G., Lindsay J. O., et al. (2016). 3rd European Evidence-Based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohn’s Colitis 11, 3–25. doi: 10.1093/ecco-jcc/jjw168 [DOI] [PubMed] [Google Scholar]
- Gupta A., Khanna S. (2017). Fecal Microbiota Transplantation. JAMA 318, 102. doi: 10.1001/jama.2017.6466 [DOI] [PubMed] [Google Scholar]
- Halaweish H. F., Boatman S., Staley C. (2022). Encapsulated Fecal Microbiota Transplantation: Development, Efficacy, and Clinical Application. Front. Cell. Infect. Mi 12. doi: 10.3389/fcimb.2022.826114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimesaat M. M., Weschka D., Mousavi S., Bereswill S. (2021). Treatment With the Probiotic Product Aviguard® Alleviates Inflammatory Responses During Campylobacter Jejuni-Induced Acute Enterocolitis in Mice. Int. J. Mol. Sci. 22, 6683. doi: 10.3390/ijms22136683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn M. R., O’Brien E. J., Diao L., Feagan B. G., Sandborn W. J., Huttenhower C., et al. (2021). A Phase 1b Safety Study of SER-287, a Spore-Based Microbiome Therapeutic, for Active Mild to Moderate Ulcerative Colitis. Gastroenterology 160, 115–127. doi: 10.1053/j.gastro.2020.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormannsperger G., Clavel T., Hoffmann M., Reiff C., Kelly D., Loh G., et al. (2010). Posttranslational Inhibition of Proinflammatory Chemokine Secretion in Intestinal Epithelial Cells: Implications for Specific IBD Indications. J. Clin. Gastroenterol. 44 Suppl 1, S10–S15. doi: 10.1097/MCG.0b013e3181e102c1 [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium (2012). Structure, Function and Diversity of the Healthy Human Microbiome. Nature 486, 207–214. doi: 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvas C. L., Dahl Jørgensen S. M., Jørgensen S. P., Storgaard M., Lemming L., Hansen M. M., et al. (2019). Fecal Microbiota Transplantation Is Superior to Fidaxomicin for Treatment of Recurrent Clostridium Difficile Infection. Gastroenterology 156, 1324–1332. doi: 10.1053/j.gastro.2018.12.019 [DOI] [PubMed] [Google Scholar]
- Ianiro G. (2022). An Artificial Microbiome Consortium Prevents Recurrence of Clostridioides Difficile Infection: Paving the Way for Fecal Microbiota Transplantation 2.0. Gastroenterology S16–S5085. doi: 10.1053/j.gastro.2022.05.002 [DOI] [PubMed] [Google Scholar]
- Isidro R. A., Lopez A., Cruz M. L., Gonzalez Torres M. I., Chompre G., Isidro A. A., et al. (2017). The Probiotic VSL3 Modulates Colonic Macrophages, Inflammation, and Microflora in Acute Trinitrobenzene Sulfonic Acid Colitis. J. Histochem. Cytochem. 65, 445–461. doi: 10.1369/0022155417718542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob V., Crawford C., Cohen-Mekelburg S., Viladomiu M., Putzel G. G., Schneider Y., et al. (2017). Single Delivery of High-Diversity Fecal Microbiota Preparation by Colonoscopy Is Safe and Effective in Increasing Microbial Diversity in Active Ulcerative Colitis. Inflamm. Bowel Dis. 23, 903–911. doi: 10.1097/MIB.0000000000001132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Je I., Lee D., Jeong D., Hong D., Yoon J., Moon J. S., et al. (2018). ID-JPL934, Attenuates Dextran Sulfate Sodium-Induced Colitis in Mice Through Inhibition of Proinflammatory Cytokines Expression. J. Med. Food 21, 858–865. doi: 10.1089/jmf.2017.4152 [DOI] [PubMed] [Google Scholar]
- Kang D., Adams J. B., Gregory A. C., Borody T., Chittick L., Fasano A., et al. (2017). Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study. Microbiome 5, 10. doi: 10.1186/s40168-016-0225-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankainen M., Paulin L., Tynkkynen S., von Ossowski I., Reunanen J., Partanen P., et al. (2009). Comparative Genomic Analysis of Lactobacillus Rhamnosus GG Reveals Pili Containing a Human-Mucus Binding Protein. Proc. Natl. Acad. Sci. U.S.A. 106, 17193–17198. doi: 10.1073/pnas.0908876106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolewska-Bochenek K., Lazowska-Przeorek I., Grzesiowski P., Dziekiewicz M., Dembinski L., Albrecht P., et al. (2021). Faecal Microbiota Transfer - a New Concept for Treating Cytomegalovirus Colitis in Children With Ulcerative Colitis. Ann. Agric. Environ. Med. 28, 56–60. doi: 10.26444/aaem/118189 [DOI] [PubMed] [Google Scholar]
- Khanna S., Pardi D. S., Jones C., Shannon W. D., Gonzalez C., Blount K. (2021). RBX7455, a Non-Frozen, Orally Administered Investigational Live Biotherapeutic, Is Safe, Effective, and Shifts Patients’ Microbiomes in a Phase 1 Study for Recurrent Clostridioides Difficile Infections. Clin. Infect. Dis. 73, e1613–e1620. doi: 10.1093/cid/ciaa1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. S., Byun J. S., Yoon Y. S., Yum D. Y., Chung M. J., Lee J. C. (2017). A Probiotic Combination Attenuates Experimental Colitis Through Inhibition of Innate Cytokine Production. Benef. Microbes 8, 231–241. doi: 10.3920/BM2016.0031 [DOI] [PubMed] [Google Scholar]
- Kim G. B., Miyamoto C. M., Meighen E. A., Lee B. H. (2004). Cloning and Characterization of the Bile Salt Hydrolase Genes (Bsh) From Bifidobacterium Bifidum Strains. Appl. Environ. Microbiol. 70, 5603–5612. doi: 10.1128/AEM.70.9.5603-5612.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krag A. (2012). Safety and Efficacy of Profermin® to Induce Remission in Ulcerative Colitis. World J. Gastroentero. 18, 1773. doi: 10.3748/wjg.v18.i15.1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbeck J. A., Maldonado-Gomez M. X., Martínez I., Frese S. A., Burkey T. E., Rasineni K., et al. (2015). In Vivo Selection To Identify Bacterial Strains With Enhanced Ecological Performance in Synbiotic Applications. Appl. Environ. Microb. 81, 2455–2465. doi: 10.1128/AEM.03903-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbeck J. A., Marsteller N. L., Frese S. A., Peterson D. A., Ramer-Tait A. E., Hutkins R. W., et al. (2016). Characterization of the Ecological Role of Genes Mediating Acid Resistance in Lactobacillus Reuteri During Colonization of the Gastrointestinal Tract. Environ. Microbiol. 18, 2172–2184. doi: 10.1111/1462-2920.13108 [DOI] [PubMed] [Google Scholar]
- Kurtz C. B., Millet Y. A., Puurunen M. K., Perreault M., Charbonneau M. R., Isabella V. M., et al. (2019). Coli Nissle Improves Hyperammonemia and Survival in Mice and Shows Dose-Dependent Exposure in Healthy Humans. Sci. Transl. Med. 11, u7975. doi: 10.1126/scitranslmed.aau7975 [DOI] [PubMed] [Google Scholar]
- Lee M., Chang E. B. (2021). Inflammatory Bowel Diseases (IBD) and the Microbiome—Searching the Crime Scene for Clues. Gastroenterology 160, 524–537. doi: 10.1053/j.gastro.2020.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levit R., Savoy De Giori G., de Moreno De LeBlanc A., LeBlanc J. G. (2019). Beneficial Effect of a Mixture of Vitamin-Producing and Immune-Modulating Lactic Acid Bacteria as Adjuvant for Therapy in a Recurrent Mouse Colitis Model. Appl. Microbiol. Biot 103, 8937–8945. doi: 10.1007/s00253-019-10133-5 [DOI] [PubMed] [Google Scholar]
- Li M., Liang P., Li Z., Wang Y., Zhang G., Gao H., et al. (2015). Fecal Microbiota Transplantation and Bacterial Consortium Transplantation Have Comparable Effects on the Re-Establishment of Mucosal Barrier Function in Mice With Intestinal Dysbiosis. Front. Microbiol. 6. doi: 10.3389/fmicb.2015.00692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Li Z., Wen S., Liu Y., Wang Y., Tang L. (2016). Transplantation of a Bacterial Consortium Ameliorates Trinitrobenzenesulfonic Acid-Induced Colitis and Intestinal Dysbiosis in Rats. Future Microbiol. 11, 887–902. doi: 10.2217/fmb-2015-0002 [DOI] [PubMed] [Google Scholar]
- Lim M. Y., You H. J., Yoon H. S., Kwon B., Lee J. Y., Lee S., et al. (2017). The Effect of Heritability and Host Genetics on the Gut Microbiota and Metabolic Syndrome. Gut 66, 1031–1038. doi: 10.1136/gutjnl-2015-311326 [DOI] [PubMed] [Google Scholar]
- Liu R., Hong J., Xu X., Feng Q., Zhang D., Gu Y., et al. (2017). Gut Microbiome and Serum Metabolome Alterations in Obesity and After Weight-Loss Intervention. Nat. Med. 23, 859–868. doi: 10.1038/nm.4358 [DOI] [PubMed] [Google Scholar]
- Liu X., Yu R., Zou K. (2019). Probiotic Mixture VSL3 Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice by Downregulating T Follicular Helper Cells. Curr. Med. Sci. 39, 371–378. doi: 10.1007/s11596-019-2045-z [DOI] [PubMed] [Google Scholar]
- Li M., Wang B., Sun X., Tang Y., Wei X., Ge B., et al. (2017). Upregulation of Intestinal Barrier Function in Mice With DSS-Induced Colitis by a Defined Bacterial Consortium Is Associated With Expansion of IL-17a Producing Gamma Delta T Cells. Front. Immunol. 8. doi: 10.3389/fimmu.2017.00824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Siles M., Khan T. M., Duncan S. H., Harmsen H. J. M., Garcia-Gil L. J., Flint H. J. (2012). Cultured Representatives of Two Major Phylogroups of Human Colonic Faecalibacterium Prausnitzii Can Utilize Pectin, Uronic Acids, and Host-Derived Substrates for Growth. Appl. Environ. Microb. 78, 420–428. doi: 10.1128/AEM.06858-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Siles M., Martinez-Medina M., Surís-Valls R., Aldeguer X., Sabat-Mir M., Duncan S. H., et al. (2016). Changes in the Abundance of Faecalibacterium Prausnitzii Phylogroups I and II in the Intestinal Mucosa of Inflammatory Bowel Disease and Patients With Colorectal Cancer. Inflamm. Bowel Dis. 22, 28–41. doi: 10.1097/MIB.0000000000000590 [DOI] [PubMed] [Google Scholar]
- Lorén V., Manyé J., Fuentes M. C., Cabré E., Ojanguren I., Espadaler J. (2017). Comparative Effect of the I3.1 Probiotic Formula in Two Animal Models of Colitis. Probiotics Antimicrob. Proteins 9, 71–80. doi: 10.1007/s12602-016-9239-5 [DOI] [PubMed] [Google Scholar]
- Lozupone C. A., Stombaugh J. I., Gordon J. I., Jansson J. K., Knight R. (2012). Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 489, 220–230. doi: 10.1038/nature11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy K. J., Powrie F. (2011). Intestinal Homeostasis and its Breakdown in Inflammatory Bowel Disease. Nature 474, 298–306. doi: 10.1038/nature10208 [DOI] [PubMed] [Google Scholar]
- Mennigen R., Nolte K., Rijcken E., Utech M., Loeffler B., Senninger N., et al. (2009). Probiotic Mixture VSL3 Protects the Epithelial Barrier by Maintaining Tight Junction Protein Expression and Preventing Apoptosis in a Murine Model of Colitis. Am. J. Physiol. Gastr. L. 296, G1140–G1149. doi: 10.1152/ajpgi.90534.2008 [DOI] [PubMed] [Google Scholar]
- Moayyedi P., Surette M. G., Kim P. T., Libertucci J., Wolfe M., Onischi C., et al. (2015). Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 149, 102–109. doi: 10.1053/j.gastro.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Odamaki T., Bottacini F., Kato K., Mitsuyama E., Yoshida K., Horigome A., et al. (2018). Genomic Diversity and Distribution of Bifidobacterium Longum Subsp. Longum Across Hum. Lifespan. Sci. Rep. 8, 85. doi: 10.1038/s41598-017-18391-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahara K., Ishikawa D., Nomura K., Ito S., Haga K., Takahashi M., et al. (2020). Matching Between Donors and Ulcerative Colitis Patients Is Important for Long-Term Maintenance After Fecal Microbiota Transplantation. J. Clin. Med. 9, 1650. doi: 10.3390/jcm9061650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooijevaar R. E., Terveer E. M., Verspaget H. W., Kuijper E. J., Keller J. J. (2019). Clinical Application and Potential of Fecal Microbiota Transplantation. Annu. Rev. Med. 70, 335–351. doi: 10.1146/annurev-med-111717-122956 [DOI] [PubMed] [Google Scholar]
- Pai N., Popov J. (2017). Protocol for a Randomised, Placebo-Controlled Pilot Study for Assessing Feasibility and Efficacy of Faecal Microbiota Transplantation in a Paediatric Ulcerative Colitis Population: PediFETCh Trial. BMJ Open 7, e16698. doi: 10.1136/bmjopen-2017-016698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai N., Popov J., Hill L., Hartung E. (2019). Protocol for a Double-Blind, Randomised, Placebo-Controlled Pilot Study for Assessing the Feasibility and Efficacy of Faecal Microbiota Transplant in a Paediatric Crohn’s Disease Population: PediCRaFT Trial. BMJ Open 9, e30120. doi: 10.1136/bmjopen-2019-030120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palócz O., Pászti-Gere E., Gálfi P., Farkas O. (2016). Chlorogenic Acid Combined With Lactobacillus Plantarum 2142 Reduced LPS-Induced Intestinal Inflammation and Oxidative Stress in IPEC-J2 Cells. PloS One 11, e166642. doi: 10.1371/journal.pone.0166642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramsothy S., Paramsothy R., Rubin D. T., Kamm M. A., Kaakoush N. O., Mitchell H. M., et al. (2017). Faecal Microbiota Transplantation for Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Crohn’s Colitis 11, 1180–1199. doi: 10.1093/ecco-jcc/jjx063 [DOI] [PubMed] [Google Scholar]
- Persborn M., Gerritsen J., Wallon C., Carlsson A., Akkermans L. M. A., Söderholm J. D. (2013). The Effects of Probiotics on Barrier Function and Mucosal Pouch Microbiota During Maintenance Treatment for Severe Pouchitis in Patients With Ulcerative Colitis. Aliment. Pharm. Ther. 38, 772–783. doi: 10.1111/apt.12451 [DOI] [PubMed] [Google Scholar]
- Petrof E. O., Gloor G. B., Vanner S. J., Weese S. J., Carter D., Daigneault M. C., et al. (2013). Stool Substitute Transplant Therapy for the Eradication of Clostridium Difficile Infection: ‘Repoopulating’ the Gut. Microbiome 1, 3. doi: 10.1186/2049-2618-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel M., Lembo A. (2020). Microbiome and Its Role in Irritable Bowel Syndrome. Digest. Dis. Sci. 65, 829–839. doi: 10.1007/s10620-020-06109-5 [DOI] [PubMed] [Google Scholar]
- Pistol G. C., Marin D. E., Dragomir C., Taranu I. (2019). Synbiotic Combination of Prebiotic Grape Pomace Extract and Probiotic Lactobacillus Sp. Reduced Important Intestinal Inflammatory Markers and in-Depth Signalling Mediators in Lipopolysaccharide-Treated Caco-2 Cells. Brit. J. Nutr. 121, 291–305. doi: 10.1017/S0007114518003410 [DOI] [PubMed] [Google Scholar]
- Puurunen M. K., Vockley J., Searle S. L., Sacharow S. J., Phillips J. A., Denney W. S., et al. (2021). Safety and Pharmacodynamics of an Engineered E. Coli Nissle for the Treatment of Phenylketonuria: A First-in-Human Phase 1/2a Study. Nat. Metab. 3, 1125–1132. doi: 10.1038/s42255-021-00430-7 [DOI] [PubMed] [Google Scholar]
- Quaresma M., Damasceno S., Monteiro C., Lima F., Mendes T., Lima M., et al. (2020). Probiotic Mixture Containing Lactobacillus Spp. And Bifidobacterium Spp. Attenuates 5-Fluorouracil-Induced Intestinal Mucositis in Mice. Nutr. Cancer 72, 1355–1365. doi: 10.1080/01635581.2019.1675719 [DOI] [PubMed] [Google Scholar]
- Reiff C., Delday M., Rucklidge G., Reid M., Duncan G., Wohlgemuth S., et al. (2009). Balancing Inflammatory, Lipid, and Xenobiotic Signaling Pathways by VSL#3, a Biotherapeutic Agent, in the Treatment of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 15, 1721–1736. doi: 10.1002/ibd.20999 [DOI] [PubMed] [Google Scholar]
- Riviere A., Selak M., Geirnaert A., Van den Abbeele P., De Vuyst L. (2018). Complementary Mechanisms for Degradation of Inulin-Type Fructans and Arabinoxylan Oligosaccharides Among Bifidobacterial Strains Suggest Bacterial Cooperation. Appl. Environ. Microbiol. 84, e2817–e2893. doi: 10.1128/AEM.02893-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G., Cerquetella M., Scarpona S., Pengo G., Fettucciari K., Bassotti G., et al. (2018). Effects of Probiotic Bacteria on Mucosal Polyamines Levels in Dogs With IBD and Colonic Polyps: A Preliminary Study. Benef. Microbes 9, 247–255. doi: 10.3920/BM2017.0024 [DOI] [PubMed] [Google Scholar]
- Saarela M., Virkajärvi I., Alakomi H., Sigvart-Mattila P., Mättö J. (2006). Stability and Functionality of Freeze-Dried Probiotic Bifidobacterium Cells During Storage in Juice and Milk. Int. Dairy J. 16, 1477–1482. doi: 10.1016/j.idairyj.2005.12.007 [DOI] [Google Scholar]
- Scott B. M., Gutierrez-Vazquez C., Sanmarco L. M., Da S. P. J., Li Z., Plasencia A., et al. (2021). Self-Tunable Engineered Yeast Probiotics for the Treatment of Inflammatory Bowel Disease. Nat. Med. 27, 1212–1222. doi: 10.1038/s41591-021-01390-x [DOI] [PubMed] [Google Scholar]
- Shen J., Zuo Z., Mao A. (2014). Effect of Probiotics on Inducing Remission and Maintaining Therapy in Ulcerative Colitis, Crohn's Disease, and Pouchitis. Inflamm. Bowel Dis. 20, 21–35. doi: 10.1097/01.MIB.0000437495.30052.be [DOI] [PubMed] [Google Scholar]
- Skrzydło-Radomańska B., Prozorow-Król B., Cichoż-Lach H., Majsiak E., Bierła J. B., Kanarek E., et al. (2021). The Effectiveness and Safety of Multi-Strain Probiotic Preparation in Patients With Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized Controlled Study. Nutrients 13, 756. doi: 10.3390/nu13030756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H., Landman C., Seksik P., Berard L., Montil M., Nion-Larmurier I., et al. (2020). Fecal Microbiota Transplantation to Maintain Remission in Crohn’s Disease: A Pilot Randomized Controlled Study. Microbiome 8, 12. doi: 10.1186/s40168-020-0792-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg J. L., Chen C. T., Gordon J. I. (2006). Genomic and Metabolic Studies of the Impact of Probiotics on a Model Gut Symbiont and Host. PloS Biol. 4, e413. doi: 10.1371/journal.pbio.0040413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed H., Macfarlane G. T., Blackett K. L., Bahrami B., Reynolds N., Walsh S. V., et al. (2010). Clinical Trial: The Microbiological and Immunological Effects of Synbiotic Consumption - a Randomized Double-Blind Placebo-Controlled Study in Active Crohn’s Disease. Aliment. Pharm. Ther. 32, 872–883. doi: 10.1111/j.1365-2036.2010.04417.x [DOI] [PubMed] [Google Scholar]
- Suez J., Zmora N., Zilberman-Schapira G., Mor U., Dori-Bachash M., Bashiardes S., et al. (2018). Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 174, 1406–1423. doi: 10.1016/j.cell.2018.08.047 [DOI] [PubMed] [Google Scholar]
- Tan P., Li X., Shen J., Feng Q. (2020). Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease: An Update. Front. Pharmacol. 11. doi: 10.3389/fphar.2020.574533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Ghazally S., Walter J., Loach D., Brooks H., Cook G., et al. (2005). Ecological Behavior of Lactobacillus Reuteri 100-23 Is Affected by Mutation of the luxS Gene. Appl. Environ. Microbiol. 71, 8419–8425. doi: 10.1128/AEM.71.12.8419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Munro K., Harmsen H. J., Welling G. W., Smart J., Gopal P. K. (2000). Analysis of the Fecal Microflora of Human Subjects Consuming a Probiotic Product Containing Lactobacillus Rhamnosus DR20. Appl. Environ. Microbiol. 66, 2578–2588. doi: 10.1128/AEM.66.6.2578-2588.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toumi R., Abdelouhab K., Rafa H., Soufli I., Raissi-Kerboua D., Djeraba Z., et al. (2013). Beneficial Role of the Probiotic Mixture Ultrabiotique on Maintaining the Integrity of Intestinal Mucosal Barrier in DSS-Induced Experimental Colitis. Immunopharm. Immunot. 35, 403–409. doi: 10.3109/08923973.2013.790413 [DOI] [PubMed] [Google Scholar]
- Traina G M. L. R. F. (2016). Probiotic Mixture Supplementation in the Preventive Management of Trinitrobenzenesulfonic Acid-Induced Inflammation in a Murine Model. J. Biol. Regul. Homeost. Agents 30, 895–901. [PubMed] [Google Scholar]
- Tripathi M. K., Giri S. K. (2014). Probiotic Functional Foods: Survival of Probiotics During Processing and Storage. J. Funct. Foods 9, 225–241. doi: 10.1016/j.jff.2014.04.030 [DOI] [Google Scholar]
- Turroni F., Milani C., Duranti S., Mancabelli L., Mangifesta M., Viappiani A., et al. (2016). Deciphering Bifidobacterial-Mediated Metabolic Interactions and Their Impact on Gut Microbiota by a Multi-Omics Approach. ISME J. 10, 1656–1668. doi: 10.1038/ismej.2015.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turroni F., Serafini F., Foroni E., Duranti S., Connell Motherway M. O., Taverniti V., et al. (2013). Role of Sortase-Dependent Pili of Bifidobacterium Bifidum PRL2010 in Modulating Bacterium–Host Interactions. Proc. Natl. Acad. Sci. 110, 11151–11156. doi: 10.1073/pnas.1303897110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursi A., Brandimarte G., Papa A., Giglio A., Elisei W., Giorgetti G. M., et al. (2010). Treatment of Relapsing Mild-to-Moderate Ulcerative Colitis With the Probiotic VSL3 as Adjunctive to a Standard Pharmaceutical Treatment: A Double-Blind, Randomized, Placebo-Controlled Study. Am. J. Gastroenterol. 105, 2218–2227. doi: 10.1038/ajg.2010.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill D. M., Braun J. (2022). Fungal Microbiome in Inflammatory Bowel Disease: A Critical Assessment. J. Clin. Invest. 132, e155786. doi: 10.1172/JCI155786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uronis J. M., Arthur J. C., Keku T., Fodor A., Carroll I. M., Cruz M. L., et al. (2011). Gut Microbial Diversity Is Reduced by the Probiotic VSL3 and Correlates With Decreased TNBS-Induced Colitis. Inflamm. Bowel Dis. 17, 289–297. doi: 10.1002/ibd.21366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Assche G., Dignass A., Bokemeyer B., Danese S., Gionchetti P., Moser G., et al. (2013). Second European Evidence-Based Consensus on the Diagnosis and Management of Ulcerative Colitis Part 3: Special Situations. J. Crohn’s Colitis 7, 1–33. doi: 10.1016/j.crohns.2012.09.005 [DOI] [PubMed] [Google Scholar]
- van der Lelie D., Oka A., Taghavi S., Umeno J., Fan T., Merrell K. E., et al. (2021). Rationally Designed Bacterial Consortia to Treat Chronic Immune-Mediated Colitis and Restore Intestinal Homeostasis. Nat. Commun. 12, 3105. doi: 10.1038/s41467-021-23460-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Cheng X., Bai L., Gao M., Kang G., Cao X., et al. (2022). Positive Interventional Effect of Engineered Butyrate-Producing Bacteria on Metabolic Disorders and Intestinal Flora Disruption in Obese Mice. Microbiol. Spectr. 10, e114721. doi: 10.1128/spectrum.01147-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Cui B., Li Q., Ding X., Li P., Zhang T., et al. (2018). The Safety of Fecal Microbiota Transplantation for Crohn’s Disease: Findings From A Long-Term Study. Adv. Ther. 35, 1935–1944. doi: 10.1007/s12325-018-0800-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Xu M., Wang W., Cao X., Piao M., Khan S., et al. (2016). Systematic Review: Adverse Events of Fecal Microbiota Transplantation. PloS One 11, e161174. doi: 10.1371/journal.pone.0161174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zeng X., Mo Y., Smith K., Guo Y., Lin J. (2012). Identification and Characterization of a Bile Salt Hydrolase From Lactobacillus Salivarius for Development of Novel Alternatives to Antibiotic Growth Promoters. Appl. Environ. Microb. 78, 8795–8802. doi: 10.1128/AEM.02519-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West N. R., Hegazy A. N., Owens B. M. J., Bullers S. J., Linggi B., Buonocore S., et al. (2017). Oncostatin M Drives Intestinal Inflammation and Predicts Response to Tumor Necrosis Factor–Neutralizing Therapy in Patients With Inflammatory Bowel Disease. Nat. Med. 23, 579–589. doi: 10.1038/nm.4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R., Atherly T., Guard B., Rossi G., Wang C., Mosher C., et al. (2017). Randomized, Controlled Trial Evaluating the Effect of Multi-Strain Probiotic on the Mucosal Microbiota in Canine Idiopathic Inflammatory Bowel Disease. Gut Microbes 8, 451–466. doi: 10.1080/19490976.2017.1334754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. C., Vatanen T., Cutfield W. S., O’Sullivan J. M. (2019). The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front. Cell. Infect. Mi. 9. doi: 10.3389/fcimb.2019.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. C., Vatanen T., Jayasinghe T. N., Leong K. S. W., Derraik J. G. B., Albert B. B., et al. (2021). Strain Engraftment Competition and Functional Augmentation in a Multi-Donor Fecal Microbiota Transplantation Trial for Obesity. Microbiome 9, 107. doi: 10.1186/s40168-021-01060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Cui B., Zhang F. (2021). Washed Microbiota Transplantation for the Treatment of Recurrent Fungal Infection in a Patient With Ulcerative Colitis. Chin. Med. J. (Engl) 134, 741–742. doi: 10.1097/CM9.0000000000001212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L., Yu Y., Ding X., Zhang H., Wen Q., Cui B., et al. (2021). Exclusive Enteral Nutrition Plus Immediate vs. Delayed Washed Microbiota Transplantation in Crohn’s Disease With Malnutrition: A Randomized Pilot Study. Front. Med. 8. doi: 10.3389/fmed.2021.666062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Zhai Q., Zhang H., Chen W., Hill C. (2021). Gut Colonization Mechanisms of Lactobacillus and Bifidobacterium: An Argument for Personalized Designs. Annu. Rev. Food Sci. Technol. 12, 213–233. doi: 10.1146/annurev-food-061120-014739 [DOI] [PubMed] [Google Scholar]
- Xiao Y., Zhao J., Zhang H., Zhai Q., Chen W. (2020). Mining Lactobacillus and Bifidobacterium for Organisms With Long-Term Gut Colonization Potential. Clin. Nutr. 39, 1315–1323. doi: 10.1016/j.clnu.2019.05.014 [DOI] [PubMed] [Google Scholar]
- Xie H., Guo R., Zhong H., Feng Q., Lan Z., Qin B., et al. (2016). Shotgun Metagenomics of 250 Adult Twins Reveals Genetic and Environmental Impacts on the Gut Microbiome. Cell Syst. 3, 572–584. doi: 10.1016/j.cels.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M. M., Ferrarezi J. V., Pereira G. D. V., Bandeira G., Côrrea Da Silva B., Pereira S. A., et al. (2020). Autochthonous vs Allochthonous Probiotic Strains to Rhamdia Quelen. Microb. Pathogen. 139, 103897. doi: 10.1016/j.micpath.2019.103897 [DOI] [PubMed] [Google Scholar]
- Yatsunenko T., Rey F. E., Manary M. J., Trehan I., Dominguez-Bello M. G., Contreras M., et al. (2012). Human Gut Microbiome Viewed Across Age and Geography. Nature 486, 222–227. doi: 10.1038/nature11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Erland L. A. E., Gill S. K., Bishop S. L., Verdugo-Meza A., Murch S. J., et al. (2021). Metabolomics-Guided Hypothesis Generation for Mechanisms of Intestinal Protection by Live Biotherapeutic Products. Biomolecules 11, 738. doi: 10.3390/biom11050738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimatsu Y., Yamada A., Furukawa R., Sono K., Osamura A., Nakamura K., et al. (2015). Effectiveness of Probiotic Therapy for the Prevention of Relapse in Patients With Inactive Ulcerative Colitis. World J. Gastroentero. 21, 5985–5994. doi: 10.3748/wjg.v21.i19.5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T., Iannitti R. G., Cunha C., De Luca A., Giovannini G., Pieraccini G., et al. (2013). Tryptophan Catabolites From Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity 39, 372–385. doi: 10.1016/j.immuni.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Zhang T., Li P., Wu X., Lu G., Marcella C., Ji X., et al. (2020). Alterations of Akkermansia Muciniphila in the Inflammatory Bowel Disease Patients With Washed Microbiota Transplantation. Appl. Microbiol. Biot 104, 10203–10215. doi: 10.1007/s00253-020-10948-7 [DOI] [PubMed] [Google Scholar]
- Zhang T., Lu G., Zhao Z., Liu Y., Shen Q., Li P., et al. (2020). Washed Microbiota Transplantation vs. Manual Fecal Microbiota Transplantation: Clinical Findings, Animal Studies and In Vitro Screening. Protein Cell 11, 251–266. doi: 10.1007/s13238-019-00684-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhao X., Zhu Y., Ma J., Ma H., Zhang H. (2018). Probiotic Mixture Protects Dextran Sulfate Sodium-Induced Colitis by Altering Tight Junction Protein Expressions and Increasing Tregs. Mediat. Inflamm. 2018, 1–11. doi: 10.1155/2018/9416391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T., Ng S. C. (2018). The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.02247 [DOI] [PMC free article] [PubMed] [Google Scholar]