Abstract
The coronavirus disease (COVID-19) pandemic caused several negative impacts on global human health and the world’s economy. Food and seafood safety and security were among the principal challenges and causes of concern for the food industry and consumers during the spread of this global pandemic. This article focused on the effects of COVID-19 pandemic on potential safety issues with seafood products and their processing methods. Moreover, the potential impacts of coronavirus transmission through seafood on human health were evaluated. The role of authenticity, traceability, and antimicrobials from natural sources to preserve seafood and the possible interaction of functional foods on the human immune system are also discussed. Although seafood is not considered a principal vector of SARS-CoV-2 transmission, the possible infections through contaminated surfaces of such food products cannot be neglected. The positive effects of seafood consumption on possible immunity built up, and COVID-19 are also summarized.
Keywords: COVID-19, SARS-CoV-2, seafood safety, natural antimicrobials, functional foods, seafood processing
Introduction
A zoonotic virus causes coronavirus disease (COVID-19) (novel coronavirus) usually originating from bats that cause severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2). It has rapidly spread worldwide from the first report of pneumonia cases in December 2019 and was declared a pandemic in March 2020 by the WHO (Tian et al., 2020). Negligible risk of contamination in foods due to contact with infected persons through hands, sneezing and coughing, or contaminated raw material and risk associated with the supply chain are considerable (Hakovirta and Hakovirta, 2020; Rizou et al., 2020). Such scenarios highlight food safety issues due to handling infected material and its unintentional person-to-person spread by food handlers in all food sectors (Shahbaz et al., 2020).
Furthermore, coronavirus 2 is proven to undergo mutation (Delta and Delta plus) on the spike protein (D614G), providing them protection against the vaccine, antibody therapeutics, and increasing pathogenicity in some cases, SARS-CoV-2 is no different (Korber et al., 2020; Kannan et al., 2021). The newest COVID-19 strain was reported to spread much rapidly, spreading across 210 countries in about a month (Harvey et al., 2021; Zeyaullah et al., 2021), enhanced replication in human lungs (Plante et al., 2021), further increasing the transmission. The mutation is reported to change amino acid positions, improving the binding affinity of the spike protein (Abdool Karim and de Oliveira, 2021). Therefore, the possibility of reduced vaccine-induced immunity, while natural immunity proved more stable to infection with replicated variants, is possible (Abdool Karim and de Oliveira, 2021; Harvey et al., 2021).
The COVID-19 pandemic has caused many countries’ total or partial closure due to the rapid spread of the virus and higher mortality rate, hampering businesses with an increase in work from home (Hamadani et al., 2020). However, many food-related activities are impossible to do from home, directly exposing the food sector to COVID-19. In addition, the pandemic has emphasized the need for optimum nutrient uptake with a role in building immunity (de Faria Coelho-Ravagnani et al., 2021). Nevertheless, severe food insecurity and lowered consumption of nutritionally rich foods were reported during the pandemic in African countries (Kansiime et al., 2021). Considering the association of COVID-19 from the seafood market, the consumption and trade related to seafood were feared in the early days of the pandemic (Eftimov et al., 2020; Meharoof et al., 2020; White E. et al., 2021; Zhang et al., 2021). However, SARS-CoV-2 was not considered to be foodborne. However, the impact of the pandemic on the food sector was dramatic and highlighted the fact that our food systems are fragile and need a transformation led by disruptive technologies and reconsideration of our consumption norms (Galanakis, 2020; Galanakis et al., 2021). Raw or undercooked foods of animal origin are not recommended as a precautionary measure. Fish and meat are rich sources of glucosaminoglycans which could anchor and transmit COVID-19 (Pressman et al., 2020). However, the high nutritional content of fish (fish oil, vitamins, mineral) are helpful as potential inhibitors and adjuvants as part of COVID-19 therapy (Ahern et al., 2021; Torrinhas et al., 2021) making the inclusion of fish potentially beneficial for COVID-19 quarantine (Abdool Karim and de Oliveira, 2021) and quality proteins from fish are known for their ability to strengthen immunity. With novel technologies being under evaluation against COVID-19 in seafood has been recently reviewed by Kulawik et al. (2022). It also covers the effects of processing and preservation methods using natural antimicrobials on COVID-19. Finally, the potential role of seafood and natural ingredients on human health, improving immunity, and reducing the risk of COVID-19 are presented.
Seafood Authenticity and Traceability Concerns During COVID-19
Due to the globalization of the seafood market and modernization of the fishing industry, adulteration or substitution of cheaper and lower grade fish is a growing problem. Items may belong to entirely different species and have another place of origin, suggesting the importance of greater authenticity and traceability in the seafood industry. Post-processing, it is challenging to identify a skinless portion of fish flesh based on its morphology (Freitas et al., 2020). As seafood consumption has been associated with several food poisoning and seafood-borne illness cases in the past, authenticity and traceability are needed to manage food safety risks (Freitas et al., 2020).
Authenticity in seafood means providing detailed information on the species, origin/capture, wild or cultivated, and about any genetic modifications, if any. These must be detailed when exporting to the European Union countries, with one of the most comprehensive standards for seafood (Hofherr et al., 2016).
Methods to determine fraud include changes in fatty acids, proteomic composition, and elemental profiles (Lavilla et al., 2013). Other research studies have also provided different advanced methods. There is also a need to validate this information (Freitas et al., 2020).
Traceability can identify the exact path through the harvest area, production site, the fish’s identity and any catch certificate, the handling, food safety processing operations and standards, supply chain issues, and different regulatory protocols (Freitas et al., 2020). In addition, consumers are at risk as the substitution of fish has been related to the transmission of several zoonotic parasites, which may have several health risks (Attwood and Hajat, 2020).
Seafood Security and Safety Concerns With COVID-19
Due to lockdowns enforced by some governments, entire food supply chains were forced to shut down. Production, harvesting, processing, and marketing were severely hampered; fish/seafood was no different. As the COVID-19 originated from a seafood market, consuming all forms of meat declined as a precautionary measure, especially undercooked products (Ahmadiara, 2020; Bassett et al., 2021; White K. et al., 2021). The COVID-19 pandemic was also related to the closure of some processing plants in the United States dealing with meat and seafood (Centers for Disease Control and Prevention, 2021). Later research confirmed that infections were only found in mammals (Lam et al., 2020). However, seafood trade-related activities were reduced during the pandemic (Stoll et al., 2021). Several earlier reports on coronavirus have been reported to be stable at ambient and frozen temperature, increasing the associated risks (Tokur and Korkmaz, 2021). Also, the high stability of SARS-CoV-2 for extended periods (2 weeks) at chilled and frozen temperature conditions have been confirmed (World Health Organization, 2020).
Survival of SARS-CoV-2 at 4°C attached to salmon culture media, exploring the possibilities of transmission (end point titration assay on Vero E6 cells) in seafood markets was evaluated by Dai et al. (2020). Results proved the viability of the virus to remain active for 8 days at 4°C. The study highlights the requirement of strict regulations in the seafood trade due to the provision of lower temperatures in the seafood supply chain.
Food security and safety are always considered in any food supply channel, especially during a pandemic (Ma et al., 2021). Several modern and traditional methods for virus inactivation have been discussed by Ahmadiara (2020), such as high-temperature heating, irradiation, ultraviolet light, and high-pressure processing and using antiviral agents for disinfecting. Apart from the destruction of microorganisms, several issues focusing on food safety include the health status (e.g., sick) of those handling the product, social distance, personal hygiene (e.g., washing hands), using latex gloves, and avoiding cross-contamination from utensils and equipment using proper disinfection (Rizou et al., 2020) to prevent spreading the pandemic (Rizou et al., 2020). The intervention of many workers’ hands in fish harvesting and processing can cause a high spread of COVID-19 (Havice et al., 2020).
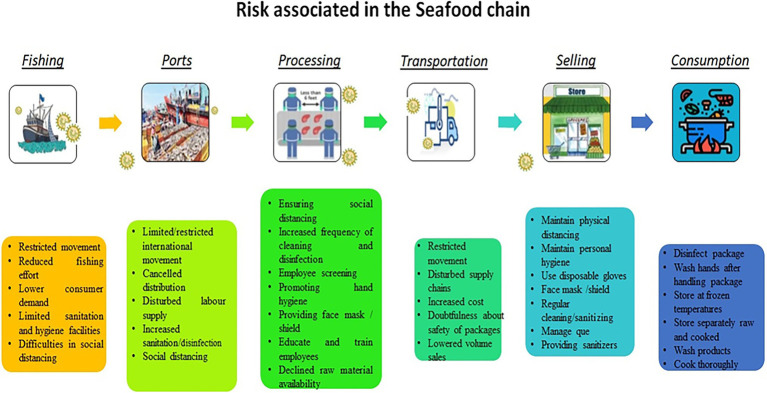
Transmission of coronavirus through fresh or processed seafood has not been reported. However, the possible transmission could be through cross-contamination from infected persons over the surfaces or packaging materials, further spreading by consuming contaminated food or by contact with the contaminated package (Rizou et al., 2020). Several risks associated with the transmission of SARS-CoV-2 through seafood chains are shown in Figure 1.
Figure 1.
Risks associated in the seafood chain.
The Effect of COVID-19 on Processing Methods for Seafood
Seafood and its products are essential because of the richness of their essential micronutrients, including several vitamins, dietary proteins as well as omega-3 fatty acids, principally long-chain ω-3 polyunsaturated fatty acids (LC n-3 PUFA), such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which are beneficial for humans (Inanli et al., 2020). In addition, antiviral compounds from marine resources have been evaluated and found helpful in COVID-19 treatment (Zahran et al., 2020; Geahchan et al., 2021). However, seafood quality can be decreased due to enzymatic, chemical, and microbiological deterioration, which can cause several safety issues (Inanli et al., 2020). Thus, these products should be preserved using different preliminary processing methods (e.g., washing and filleting) to assure their eventual quality and safety after additional processing. In addition, some recent studies have highlighted the potential of transmission of SARS-CoV-2 through the animal food product supply chain (Fu et al., 2021; Hu et al., 2021).
Many processing methods are used with seafood to inhibit or delay the growth of spoilage and pathogenic microorganisms and inactivate the alterations due to the enzymatic reactions, including freezing, chilling, packaging using a modified atmosphere (MAP), vacuum packaging, irradiation, heating, salting, smoking, and drying (Boziaris, 2014) to assure the safety and stability of seafood as well as to enhance its shelf life. Hopefully, with minimal unfavorable effects on their organoleptic attributes and the number of essential nutrients. Seafood processing methods using lower temperatures, such as freezing and chilling, are the most applied treatments to conserve seafood and its by-products. Moreover, the application of packaging methods (e.g., MAP) can extend the seafood shelf life by delaying microbial growth and decreasing oxidative reactions (Boziaris, 2014). Heating and irradiation methods are also used to inhibit the growth of spoilage and pathogenic microorganisms, reduce chemical oxidative stress, and prevent enzymatic browning in seafood (Boziaris, 2014).
However, the widespread outbreaks involving several new microorganisms, including COVID-19, impose a new challenge to global nutrition and food safety (Love et al., 2021; Nakat and Bou-Mitri, 2021). Therefore, the food and seafood sectors have required an increased focus on preventive and precautionary measures. In addition, several contradictory reports about the direct transmission of SARS-CoV-2 to humans through foods have been reported. The Centers for Disease Control and Prevention (CDC) in the United States and the WHO have rejected food transmission, while a recent study by Hu et al. (2021) suggested the possibility of increasing the infection with this virus by the consumption of contaminated food.
SARS-CoV-2 viral RNA can survive on seafood and other food products at 4°C for more extended periods than MERS-CoV RNA (Godoy et al., 2021), and SARS-CoV-2 has been found on seafood (White K. et al., 2021). As a result, SARS-CoV-2 has had huge impacts on the seafood sector, such as processor closings, shortening of fishing seasons, and disruptions in production and distribution of seafood (White E. et al., 2021) with less impact on frozen products (White K. et al., 2021).
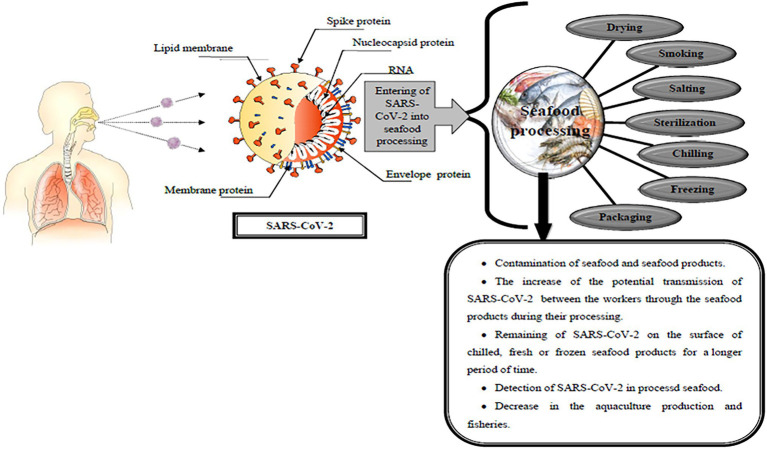
During the COVID-19 pandemic, food products were contaminated with SARS-CoV-2, particularly meat and seafood products, which can transmit this virus during seafood processing (Chitrakar et al., 2021). In addition, seafood workers can be contaminated with COVID-19 due to the virus spreading during extended periods of confined work (White E. et al., 2021). Fresh seafood can also be contaminated by infected persons who prepare or serve seafood at home or in restaurants (Duda-Chodak et al., 2020). On the other hand, SARS-CoV-2 can be transmitted through contact with fish products contaminated and stored at different temperatures: 4°C, −20°C, and −80°C due to the ability of the virus to survive on the surface for 3 weeks in all of these samples (Ceniti et al., 2021). The impact of SARS-CoV2 on seafood processing methods is shown in Figure 2.
Figure 2.
The impacts of SARS-CoV-2 on seafood processing methods.
Fresh Seafood
Fresh seafood is the most preferred form (Nguyen et al., 2015). Fresh seafood can be sold in different forms depending on the species (e.g., whole, gutted, headed and gutted, steak and fillet with skin on or off; Nguyen et al., 2015). Unfortunately, the pandemic has led to a decrease in fresh sales in many countries and a 30%–40% decrease in exports (White E. et al., 2021). Thus, the pandemic has strongly negatively impacted communities dependent on seafood products.
The virus can remain on the surface of chilled fresh seafood, such as salmon, for more than a week, increasing the international transmission of this virus. Thus, the experimental studies have confirmed that the SARS-CoV-2 can survive on seafood products for 8 days at 4°C (Ceniti et al., 2021). Another study showed that SARS-CoV-2 could survive on fish for more than a week in refrigerated rooms at 4°C (Godoy et al., 2021). The SAS-CoV-2 can also remain viable on salmon for 8 days at 4°C and 2 days at 25°C (Han S. et al., 2021). Thus, contaminated seafood products in the cold chain with coronavirus can be considered the amplifying source of the virus through transport over long distances (Han J. et al., 2021), although most public health agencies have confirmed that food is not a vector for the transmission of the virus (World Health Organization, 2019).
Frozen Seafood
Freezing has a vital role in inhibiting the growth of diverse microbial species and generally reduces the chemical reactions in the food matrix. Hence, freezing can extend the shelf life of food products.
However, SARS-CoV-2 has been found in frozen seafood during processing. For example, this virus has been detected on the packaging materials of frozen shrimp (Chitrakar et al., 2021) and survives for a long time when frozen, as described above. Another study showed that both frozen (−20°C) and refrigerated (4°C) salmon samples after 3 weeks retained the virus. In Beijing’s Xinfadi market, SARS-CoV-2 was found on salmon surfaces (Liu et al., 2020). This led to China examining all imported seafood for SARS-CoV-2 RNA (Godoy et al., 2021).
Other Processing Methods
Although both thermal processing (such as pasteurization, sterilization, and high-temperature drying) or non-thermal processing methods (such as ionizing radiation, ultraviolet light, high-intensity ultrasound, and high hydrostatic pressure) are used to extend shelf life and preserve seafood, the quality and safety of these products concerning COVID-19 needs to be considered. The transmission of COVID-19 between seafood workers raised concerns about the safety of aquatic food products. Thus, the seafood sector has suffered recently from a systemic problem due to the COVID-19 pandemic. Besides finding the virus on the product, at one seafood market, the virus was found on the cutting boards used to process the imported seafood, e.g., salmon (Olatunde et al., 2021). Contamination can be through respiratory droplets from infected humans or the contact between their hands and foods, leading to problems for the industry (Ayseli et al., 2020).
China has suspended shrimp import from three Ecuador producers because of the discovery of traces of SARS-CoV-2 on the external packaging of six samples (Godoy et al., 2021). In addition, the epidemiological studies suggested that COVID-19 spread in Qingdao was apparently due to the contamination of cod with SARS-CoV-2 at some point (Liu et al., 2020).
The Effects of COVID-19 on Human Health Concerning the Consumption of Seafood
COVID-19 is known to induce high-grade fever, tiredness, and increased coughing 1 to 14 days after someone becomes infected (Singh et al., 2020). The lockdowns to limit the spread of COVID-19 has hampered the capture and movement of seafood (Ma et al., 2021). Love et al. (2021) have discussed the issues hampering the entire seafood supply chain during this pandemic. Several studies have questioned the spread of COVID-19 through food, especially muscle foods (meat and fish), due to their lowered temperature of processing and storage conditions (Han S. et al., 2021). Han J. et al. (2021) reported the stability of COVID-19 on the skin of muscle foods for 14–21 days, which is a matter of concern. Alternatively, Bondad-Reantaso et al. (2020) ultimately rejected the transmittance of COVID-19 through seafood. Immunity factors have become important by imparting resistance against viral infections. However, dietary supplements and nutraceuticals from aquatic sources have been evaluated for preventing and treating COVID-19 (Das, 2021; Yu et al., 2021).
Furthermore, consuming 26 pounds of seafood per person per annum is recommended to derive the benefits (U.S. Department of Agriculture and U.S. Department of Health and Human Services, n.d.). Seafood is rich in polyunsaturated fatty acids, proteins, bioactive compounds, anti-inflammatory, vitamin-D, B6, and micronutrients. Further, the virus exhibited several variations, imparting them enhanced transmissibility, causing more severe illness, and reduced neutralization of viruses by vaccines impacting treatments (Harvey et al., 2021; Zeyaullah et al., 2021). The negative impact of diet, depression, and obesity, along with the protective effect of seafood consumption on the fatality of COVID-19 patients, was reported (Rajkumar, 2021) based on bivariate analysis of COVID-19 mortality data from 156 countries. Therefore, they are recommending an increase in seafood consumption in COVID-19 patients.
The role of adequate fatty acids and their metabolites on the inactivation of SARS-CoV has been described by Das (2021). Seafood is regarded as one of the most promising sectors with major international trade. Belluzzi et al. (2020) highlighted the immunity improving ability of EPA, which overcomes the immune-suppressing effects of medication. Yu et al. (2021) evaluated the bioactive components derived from tuna proteins degraded to form peptides and showed their antiviral effects when used as a nutritional supplement. It was a strong SARS-CoV-2 inhibitor. These peptides were safe with good solubility. However, only one peptide showed docking with the virus-host with 144 Kcal/mol energy value.
Selenium, an essential micronutrient found in seafood, has been reported to increase immunity, increase vaccine response, and reduce inflammation (Avery and Hoffmann, 2018). All of these factors are associated with the current COVID-19 pandemic. Das (2021) suggested the correct proportion of arachidonic acid, EPA, and docosahexaenoic acid could benefit COVID-19 infections. In addition, zinc is associated with an improved immune system and the inactivation of viral infections (Jayawardena et al., 2020).
The marine secondary metabolite, polyphosphate (polyP 10 μg/ml) from marine bacteria, exhibited a strong antiviral impact on SARS-CoV-2 reduced binding by 42% with cellular receptors by inducing innate immunity and improving mucin barrier. Furthermore, the effect was found to increase with the concentration of polyphosphate (Müller et al., 2020). Inorganic polyphosphate in the form of nanoparticles (magnesium salt of inorganic polyphosphate) with plant metabolite (quercetin) against COVID-19 was evaluated (Neufurth et al., 2021). The combination was said to act as an antioxidant and increased the MUC5AC gene responsible for clearing the lung. Further, polyP could inhibit spike protein by acting on the receptor-binding domain (Geahchan et al., 2021).
Vigorous antimicrobial activity of Abalone viscera against SARS-CoV-2 pseudovirus was exhibited IC50 33 μg/ml (Yim et al., 2021). Gentile et al. (2020) screened (14,064 compounds) and reported 17 potent protease inhibitors against SARS-CoV from marine origin. The selection was confirmed based on structure and ligand-based drug design approach. Cobre et al. (2021) proved the protective (GLM based) effects of seafood, their proteins, and lipids in recovery against COVID. Finally, Sumon et al. (2021) reviewed peptides originating from aquatic organisms for their antiviral potential for application as therapeutics. The review discussed different aquatic antiviral peptides and their ability to induce immune pathways and highlighted their ability to inhibit SARS-CoV-2 through blocking their entry, replication, and release mechanism.
Aatif et al. (2021) reported ACE2 blocking by receptor binding domain using marine seaweed. Dieckol (found in brown alga Ecklonia cava) derivative (DK07) exhibited higher receptor binding ability. The derivative (DK07) showed interaction with crucial amino acids against the mutated United Kingdom variant (VUI-202012/01) of SARS-CoV-2. Yim et al. (2021) reported the inhibition of SARS-CoV-2 by sulfated fucoidan and crude polysaccharides obtained from seaweed species (Undaria pinnatifida sporophyll, Laminaria japonica, Hizikia fusiforme, Sargassum horneri, Codium fragile, Porphyra tenera). Seaweed polysaccharides exhibited high molecular weight (>800 kDa), carbohydrate (62.7%–99.1%), fucose (37.3%–66.2%) content, and antiviral activities against SARS-CoV-2 pseudovirus, except P. tenera. The high antiviral ability was the synergistic effect of bioactive compounds and high molecular weight polysaccharides. The recent review by Fitton et al. (2021) reviewed the role of fucoidan, found in seaweeds against respiratory viral infection (SARS-CoV-2). It clarified the ability of fucoidan to inhibit COVID infection, increase immunity, and enhance inflammatory response. Antiviral activities of purified sulfated polysaccharide and lambda-carrageenan from marine red algae against coronavirus two were reported (Jang et al., 2021). The EC50 value of 0.9 ± 1.1 μg/ml and no toxic effects were observed up to a 300 μg/ml concentration. Further, reduced expression of virus proteins and progeny production were observed in a dose-dependent manner.
Plitidepsin from tunicate (Aplidium albicans) was reported to inhibit SARS-CoV-2 (IC90—1.76 nM), inhibition in human cell line was much effective IC90—0.88 nM, which was over commercially used remdesivir (White K. et al., 2021). Additionally, inhibition of pneumocyte-like cells was also observed IC90—3.14 nM, and replication was also inhibited (White K. et al., 2021).
Aquatic organisms and processed seafood are regarded as potential routes for the transmission of pathogenic microorganisms (Fu et al., 2021). Seafood processing requires special processing facilities and maintenance of the cold chain. However, basic hygienic practices are mostly inadequate in many seafood processing facilities (Cook et al., 2017), although no COVID-19 transmittance has been traced to a seafood purchase (Poelman et al., 2021).
The Effects of Natural Antimicrobials and Functional Foods on the Immune System of Humans With COVID-19
The immune system has a significant role in defending against various infections (Hollman, 2001), including COVID-19 (Benarba and Pandiella, 2020), which became a powerful tool for resisting the COVID-19 disease (Ayivi et al., 2021). Natural antimicrobials from plants and microorganisms (fermented foods, prebiotics, probiotics, and synbiotics) enhance immunity by modulating gut and respiratory systems. They were discussed by Sultan et al. (2014) and Antunes et al. (2020) for their effectiveness against COVID-19. Plant source compounds consisted of immunomodulatory bioactive compounds, while those from microorganisms could be included in therapeutic strategies against COVID-19. Functional foods may improve health and support resistance to several viral infections and nutritionally related diseases (Rastmanesh et al., 2020). Functional foods are foods with one or more specialized functions in the body, usually obtained from naturally occurring components, including improvements in immunity (Han and Hoang, 2020). Galland (2013) suggested that bioactive peptides, fish oils, and fish protein hydrolysates could be functional foods derived from seafood. Mu et al. (2021) have reported that garlic and Rhizome polygonati are available against the SARS-CoV-2 virus. The increased immunity and antimicrobial activity of garlic could be used to prevent COVID-19 infection. Caffeic acid derivative-based functional foods could be used to develop anti-COVID-19 therapies (Adem et al., 2021). Selective and proper functional/bioactive material should be supplemented to enhance immunity (Ayivi et al., 2021).
Han and Hoang (2020) have discussed the role of different functional components/foods that could boost immunity and be used as probable components with the current pandemic. For example, vitamins A, B6, B9, B12, C, D, E, and zinc and iodine help regulate inflammation and protect against viral infections (Calder and Grimble, 2002). Conversely, the deficiency of any of the above nutrients can impair the maintenance of homeostasis (Ayivi et al., 2021).
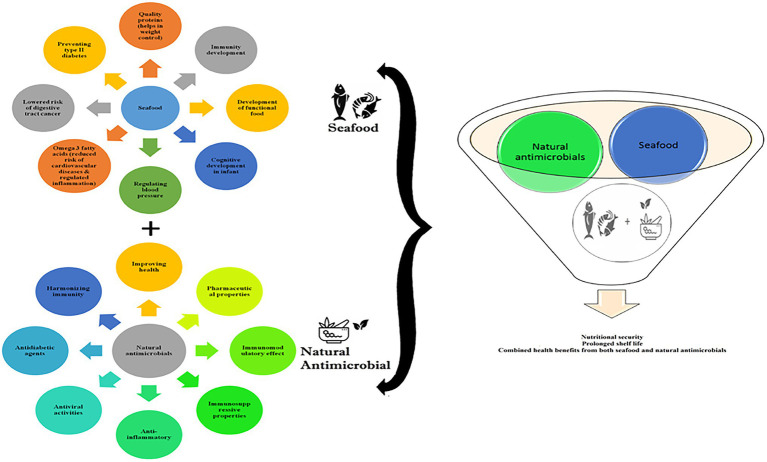
Sultan et al. (2014) have discussed the bioactive components present in garlic, ginger, purple coneflower, black cumin, and liquorice to maintain homeostasis and boost immunity. Phenols, phenolic acids, carotenoids, flavonoids, and other bioactive phytoconstituents in natural plant compounds impart bio-activity and enhance immunity (Chojnacka et al., 2020; Surendran et al., 2020) and have antiviral activity against coronavirus (Nebigil et al., 2020; Rameshkumar et al., 2021). Al Jitan et al. (2018) outlined the different phenolic acids present in plants and their importance in the human diet. Phenolic acids suppress oxidation due to their antioxidant capacity, which is related to increased immunity. Additionally, phenolic content has been shown to suppress inflammation and regulate blood sugar levels (Li et al., 2014). Ding et al. (2018) studied the ability of polyphenols to bind receptors on immune cells and regulate the immune system. They reported improved intestinal immune responses and regulation of allergic diseases. Recently, Surendran et al. (2020) highlighted the plant metabolites that inhibit ACE activity relevant to the current pandemic situation while also lowering the risk of cardiovascular diseases along with some antiviral activity. The health benefits of seafood working with natural antimicrobials are shown in Figure 3. This could provide the effects of seafood discussed in “The Effects of Natural Antimicrobials and Functional Foods on the Immune System of Humans With COVID-19” section and natural compounds discussed in this section. The role of aquatic-based functional foods directly or indirectly associated with boosting immunity, providing resistance against COVID-19, is shown in Table 1.
Figure 3.
Health promoting benefits of seafood and natural antimicrobial.
Table 1.
Impact of aquatic-based functional foods on immune system/COVID-19.
| Functional component | Action | References |
|---|---|---|
| Protein hydrolysate | Immunomodulatory, antioxidant, and anti-hyperglycemic | Roslan et al., 2014; Nasri, 2017; Chakrabarti et al., 2018; Salem et al., 2018; Zamora-Sillero et al., 2018 |
| Peptide | Antihypertensive: prevents activity of angiotensin-I-converting enzyme (ACE), antioxidant, and anti-inflammatory | Roslan et al., 2014; Adnan et al., 2017; Nasri, 2017; Balami et al., 2019; Cong et al., 2020; Durand et al., 2020; Narayanasamy et al., 2020 |
| Lipids | Regulating oxidative stress and anti-inflammatory properties | Suleria et al., 2015; Kundam et al., 2018; Calder et al., 2020 |
| Polysaccharides | Immunomodulatory, anti-inflammatory, antioxidant, and antidiabetic | Zhao et al., 2018; Arasukumar et al., 2019; Lakshmana et al., 2019; Sim et al., 2019 |
Impacts of Natural Antimicrobials on Risk Associated With COVID-19
The emergence and rapid propagation of COVID-19 in such a short time have caused a high number of fatalities. As a result, some efforts have focused on the development of effective antivirals to combat COVID-19. Additionally, the risk of excessive use chemical and synthetic antibiotics may pose a future health threat by increasing the chances of developing antibiotic resistance (Usman et al., 2020). Natural antimicrobials (Plant origin turmeric rhizome, bay leaf, black pepper, oregano, Indian surge tree, red spider lilly, tonka bean, Cinnamomum aromaticum Nees, Vitis vinifera, Japanese apricot, honey, astaxanthin) are rich source of metabolites (essential oils, polyphenolic compounds, triterpenoids, alkaloids, saponins) used to treat several potentially fatal bacterial (Staphylococcus spp., Streptococcus spp., Listeria monocytogenes, Enterococcus) and viral infections (dengue virus, herpes simple virus, human coronaviruses, SARS-CoV, SARS-CoV-2). Additionally, they improve the action of antibiotics by acting as coadjuvant used to treat several potentially fatal viral infections (da Silva Antonio et al., 2020; López-Malo et al., 2020) and are being used against COVID-19 (Li et al., 2005; Chang et al., 2012; Nishide et al., 2019; Rosmalena et al., 2019; da Silva Antonio et al., 2020; Talukdar et al., 2020; Wang et al., 2020; Ali et al., 2021; Rathod et al., 2021a,b; Stan et al., 2021; Zannella et al., 2021). The presence of peptides, polyphenols, hydroxybenzoic acid, hydroxycinnamic acid, lignans, fatty acids, tannins, and alkaloids, have been tested due to their high bioavailability, low toxicity with antiviral abilities without development of resistance (da Silva Antonio et al., 2020; López-Malo et al., 2020; Nebigil et al., 2020). Several studies have focused on screening and application of naturally occurring antiviral compounds exhibiting diverse antiviral activity by restricting entry and replication of virus (Benarba and Pandiella, 2020; da Silva Antonio et al., 2020; Li et al., 2020; Wang et al., 2020).
The inhibitory activity is attributed to phenolics and their antioxidant potential (Sharifi et al., 2013). Natural antimicrobials, including peptides, polyphenols, hydroxybenzoic acid, hydroxycinnamic acid, lignans, fatty acids, tannins, and alkaloids, have been tested due to their high bioavailability and low toxicity (da Silva Antonio et al., 2020; López-Malo et al., 2020). These benefits of antiviral activity without leading to the development of resistance by various microbes in the gut microbiome (Nebigil et al., 2020). In addition, several papers have described the successful screening and applications of naturally occurring antiviral compounds that hamper the entry, attachment, and replication of the virus (Benarba and Pandiella, 2020; da Silva Antonio et al., 2020; Li et al., 2020; Wang et al., 2020).
The Effects of Antimicrobials on COVID-19
Antimicrobials are substances capable of inhibiting/retarding, and restricting the growth of microorganisms. However, COVID-19 is an enveloped, symmetrical and single-stranded RNA virus (Khan et al., 2020). The entry of SARS-CoV requires an angiotensin-converting enzyme (ACE) 2, followed by its cleavage by the transmembrane serine protease (Hoffmann et al., 2020) as a receptor and papain-like proteinase (Jiang et al., 2020) for replication of polyproteins. To date, chemical pharmaceuticals (host protein targeted, virus targeted, and nucleoside analogs) and traditional Chinese medicines have been administered to protect against COVID-19 by disturbing human host interaction (Li et al., 2020). Therefore, the primary active sites should be the structural and non-structural proteins.
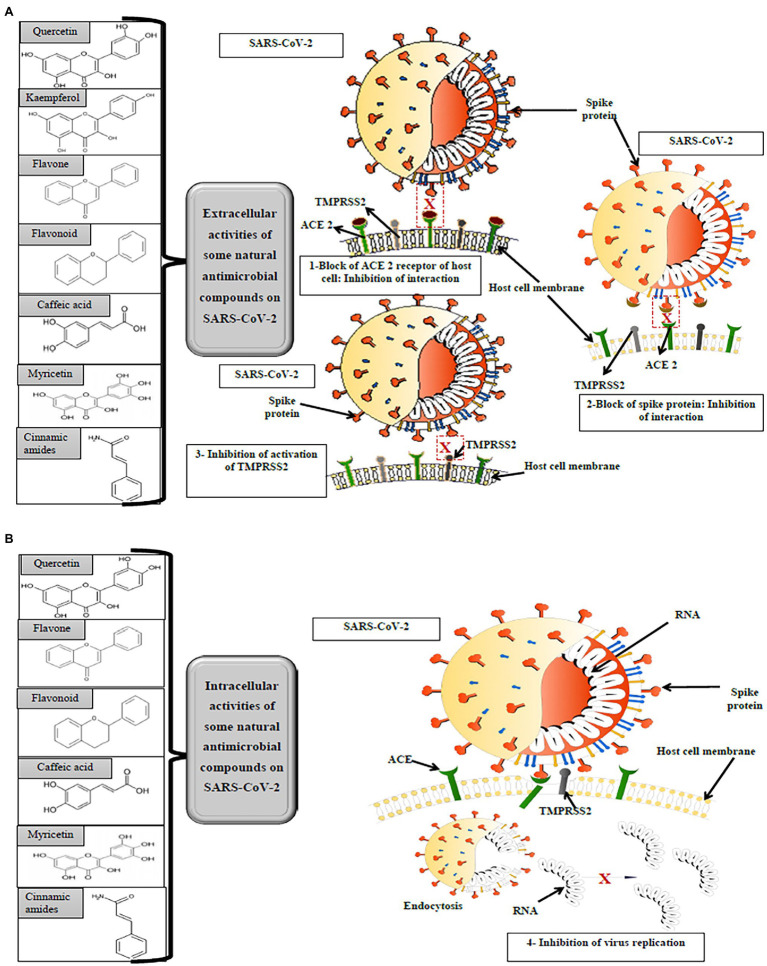
A proposed action of antimicrobials is to block the ACE 2, transmembrane serine protease 2 (TMPRSS2), and target papain-like proteinase (PLPRO). Benarba and Pandiella (2020) discussed and justified the ability of natural products to block ACE 2, thus avoiding the spread and expression of COVID-19. The anti-transmembrane serine protease activity of natural kaempferol (Da et al., 2019) and flavonoids (Rameshkumar et al., 2021) have been reported. In addition, several natural compounds such as cinnamic amides and flavonoids have been shown to inhibit papain and chymotrypsin-like proteinases (Benarba and Pandiella, 2020). Figure 4 shows the action of some natural compounds against SARS CoV2.
Figure 4.
The different antimicrobial activities of some natural compounds against SARS-CoV-2.
Natural Antimicrobials (Plant Source) Against COVID-19
Plants are rich sources of antimicrobial compounds and drugs against several viral infections and diseases (da Silva Antonio et al., 2020; López-Malo et al., 2020). They are rich in natural antioxidants capable of reducing reactive oxygen species and reducing viral spread. Excess use of antimicrobials from synthetic sources may lead to the development of resistance (Usman et al., 2020) which can be avoided by using natural antimicrobials, derivatives, or mimics due to their diverse mechanisms of action (López-Malo et al., 2020). Plant-derived products selectively blocked the ACE 2 receptors (Benarba and Pandiella, 2020).
Specific natural compounds administered as a treatment were reported to show a recovery of 90% in some cases and prevented infections in healthy persons (Benarba and Pandiella, 2020). The Chinese traditional medicines adopted by Chinese health agencies are mixtures of different plants, and their parts consist of Lonicera japonica, Scutellaria baicalensis, and Forsythia suspensa; and Ephedrae herba, Armeniacae semenamarum, Glycyrrhizae radix, and Gypsum fibrosum, respectively and showed anti-SARS-CoV-2 activity (Yang et al., 2020). The plant and parts used were a rich source of metabolites and their mimics that improved health, and in some cases, the infection was wholly eradicated (Wang et al., 2020). Benarba and Pandiella (2020) suggested positive impacts of plant-derived products against SARS-CoV-2. Natural products were reported to inhibit the entry and replication of the virus. Motwalli and Alazmi (2021) suggested the effectiveness of natural zinc compounds (PubChem ID: 95372568 and 1,776,037) to inhibit the activity of SARS-CoV-2. Bioactive compounds originating from plants have been highlighted for their anti-SARS CoV2 activity (Ayivi et al., 2021). Among nucleoside analogs, remdesivir (an adenosine analog; Jorgensen et al., 2020), favipiravir (a guanine analog; Chen et al., 2020), and β-4-N4-hydroxycytidine (a ribonucleoside analog) inhibited SARS-CoV infections and replication by terminating RNA synthesis (Gordon et al., 2020) and activity (Chen et al., 2020).
Other traditional Chinese medicines approved for use include combinations of Chinese herbs. For example, Lianhuaqingwen with 11 herbs and Scutellariae Radix (dried roots of Scutellariae baicalensis Georgi) was found to effectively inhibit replication and activity of SARS-CoV, respectively (Wang et al., 2020). Naturally occurring flavonoid compounds in all 64 samples were evaluated. Among them, herbacetin, pectolinarin, and rhoifolin showed in vitro dose-dependent inhibition of SARS-CoV protease activity (Jo et al., 2020). Rhizoma polygonati, a Chinese herb, was evaluated against COVID-19 by Mu et al. (2021). A total of 10 active chemical compounds were identified, and all of them showed strong binding to the SARS-CoV virus. Two active compounds, diosgenin and (+)-syringaresinol-O-beta-D-glucoside, bound the protein better than ridasivir and lopinavir. Nebigil et al. (2020) evaluated flavaglines (cyclopental [b] benzofurans), which are found in several traditional Chinese medicines. The therapeutic potential of flavaglines was due to the regulation of the eukaryotic initiation factor 4A (eIF4A) and the inhibition of viral signaling. In addition, the flavaglines targeted proteins, helping to avoid issues of mutation and resistance. Computationally evaluated 36 flavonoids (with interaction energy of >−9 kcal/mol) from among 458 compounds against the SARS-CoV-2 main protease (Rameshkumar et al., 2021). Five compounds showed good bindings with all three types of targeted proteins and might be suitable inhibitors of SARS-CoV.
Future Challenges of COVID-19 for Seafood Safety
The recent lockdowns imposed to control the pandemic have hampered aquaculture and fishing activities, causing an increase in demand and seafood prices, even though no study has shown any foodborne transmission of SARS-CoV-2. Some recent publications have highlighted the transmission of the virus through faulty processing conditions and procedures (Zuber and Brüssow, 2020). In addition, contaminated packaging materials have been a challenge for the food industry. However, no actual COVID has been shown to occur even if found on food or food packaging.
Seafood safety remains a challenging topic due to their susceptibility to spoilage requiring the maintenance of a chill and cold chain through processing, transportation, and storage. The seafood supply chain has a large number of links leading to many different risks. Several studies have shown that natural antimicrobials in ice and seafood can retard microbial growth, thus preventing deterioration. Natural antimicrobials also preserve the proteins and fats present in seafood. The latest help retain the improvements in immunity and the faster disease recovery associated with this seafood (Baptista et al., 2020) and prove the ability of seafood to build resilient food systems and sustainable healthy diets (Ahern et al., 2021).
Conclusion
The recent COVID-19 pandemic caused by SARS-CoV-2, a highly pathogenic virus, has challenged everyone. Additionally, the pandemic has highlighted the importance of food, nutrition, and health. The seafood chain’s risk of transmitting COVID-19 is high due to the extensive exchange of hands. The available literature suggests high bioactive compounds found in seafood should be further explored for antiviral capacities. As processing and preservation of seafood are carried in cold temperature conditions, special care must be taken to monitor workers’ health and avoid the spread of COVID-19. The natural antimicrobials used as functional materials to preserve and enhance seafood should be evaluated for their ability to mitigate the risk of COVID-19 spread. The literature also discusses the importance of seafood, its authenticity, and its ability to supplement the development of a strong immunity to fight SARS-CoV-2 infections.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work is supported by the PRIMA program under project BioProMedFood (ref. no. 2019-SECTION2-4 Project ID 1467). The PRIMA program is part of the European Union. This research was financially supported by the Scientific and Technological Research Council of Turkey (TUBITAK): N— UPAG 119N492 (PRIMA Programme Section 2). This publication was made possible by grant number NC.X-267-5-12-170-1 from the National Institute of Food and Agriculture (NIFA) and the Department of Family and Consumer Sciences and the Agriculture Research Station at North Carolina Agriculture and Technical State University (Greensboro, NC, United States). The authors extend appreciation to the International Scientific Partnership Program ISPP at King Saud University for funding this research work through ISPP-153.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Abbreviations
- ACE 2
Angiotensin-converting enzyme
- CDC
Centers for Disease Control and Prevention
- COVID-19
Coronavirus disease 2019
- EPA
Eicosapentaenoic
- MAP
Modified atmosphere packaging
- MERS-CoV
Middle east respiratory syndrome coronavirus
- PLPRO
Target papain-like proteinase
- RNA
Ribonucleic acid
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- TMPRSS2
Transmembrane serine protease 2
- WHO
World Health Organization
References
- Aatif M., Muteeb G., Alsultan A., Alshoaibi A., Khelif B. Y. (2021). Dieckol and its derivatives as potential inhibitors of SARS-CoV-2 spike protein (UK strain: VUI 202012/01): a computational study. Mar. Drugs 19:242. doi: 10.3390/md19050242, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdool Karim S. S., de Oliveira T. (2021). New SARS-CoV-2 variants—clinical, public health, and vaccine implications. N. Engl. J. Med. 384, 1866–1868. doi: 10.1056/NEJMc2100362, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adem Ş., Eyupoglu V., Sarfraz I., Rasul A., Zahoor A. F., Ali M., et al. (2021). Caffeic acid derivatives (CAFDs) as inhibitors of SARS-CoV-2: CAFDs-based functional foods as a potential alternative approach to combat COVID-19. Phytomedicine 85:153310. doi: 10.1016/j.phymed.2020.153310, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adnan M., Patel M., Hadi S. (2017). Functional and health promoting inherent attributes of Enterococcus hirae F2 as a novel probiotic isolated from the digestive tract of the freshwater fish Catla catla. PeerJ 5:e3085. doi: 10.7717/peerj.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern M., Thilsted S., Oenema S., Kühnhold H. (2021). The role of aquatic foods in sustainable healthy diets. UN Nutrition Discussion Paper. Available at: https://www.unnutrition.org/wp-content/uploads/FINAL-UN-Nutrition-Aquatic-foods-Paper_EN_.pdf
- Ahmadiara E. (2020). Possibility of Faecal-Oral transmission of novel coronavirus (SARS-CoV-2) via consumption of contaminated foods of animal origin: a hypothesis. J. Food Qual. Hazards Control. 7, 2–3. doi: 10.18502/jfqhc.7.1.2445 [DOI] [Google Scholar]
- Ali S. G., Ansari M. A., Alzohairy M. A., Almatroudi A., Alomary M. N., Alghamdi S., et al. (2021). Natural products and nutrients against different viral diseases: prospects in prevention and treatment of SARS-CoV-2. Medicina 57:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Jitan S., Alkhoori S. A., Yousef L. F. (2018). Phenolic acids from plants: extraction and application to human health. Stud. Nat. Prod. Chem. 58, 389–417. doi: 10.1016/B978-0-444-64056-7.00013-1 [DOI] [Google Scholar]
- Antunes A. E., Vinderola G., Xavier-Santos D., Sivieri K. (2020). Potential contribution of beneficial microbes to face the COVID-19 pandemic. Food Res. Int. 136:109577. doi: 10.1016/j.foodres.2020.109577, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasukumar B., Prabakaran G., Gunalan B., Moovendhan M. (2019). Chemical composition, structural features, surface morphology and bioactivities of chitosan derivatives from lobster (Thenus unimaculatus) shells. Int. J. Biol. Macromol. 135, 1237–1245. doi: 10.1016/j.ijbiomac.2019.06.033 [DOI] [PubMed] [Google Scholar]
- Attwood S., Hajat C. (2020). How will the COVID-19 pandemic shape the future of meat consumption? Public Health Nutr. 23, 3116–3120. doi: 10.1017/S136898002000316X, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery J. C., Hoffmann P. R. (2018). Selenium, selenoproteins, and immunity. Nutrients 10:1203. doi: 10.3390/nu10091203, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayivi R., Ibrahim S. A., Colleran H., Silva R., Williams L., Galanakis C., et al. (2021). COVID-19: human immune response and the influence of food ingredients and active compounds. BCHD 4, 100–148. doi: 10.31989/bchd.v4i6.802 [DOI] [Google Scholar]
- Ayseli Y. I., Aytekin N., Buyukkayhan D., Aslan I., Ayseli M. T. (2020). Food policy, nutrition and nutraceuticals in the prevention and management of COVID-19: advice for healthcare professionals. Trends Food Sci. Technol. 105, 186–199. doi: 10.1016/j.tifs.2020.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balami S., Sharma A., Karn R. (2019). Significance of nutritional value of fish for human health. MJHR 2, 32–34. doi: 10.2478/mjhr-2019-0012 [DOI] [Google Scholar]
- Baptista R. C., Horita C. N., Sant’Ana A. S. (2020). Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: a review. Food Res. Int. 127:108762. doi: 10.1016/j.foodres.2019.108762, PMID: [DOI] [PubMed] [Google Scholar]
- Bassett H. R., Lau J., Giordano C., Suri S. K., Advani S., Sharan S. (2021). Preliminary lessons from COVID-19 disruptions of small-scale fishery supply chains. World Dev. 143:105473. doi: 10.1016/j.worlddev.2021.105473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi A., Scaioli E., Ricciardiello L., Marasco G., Belluzzi C. (2020). Eicosapentaenoic free fatty acid to treat patients with SARS-Cov2 infection. Med. Hypotheses 143:110095. doi: 10.1016/j.mehy.2020.110095, PMID: [DOI] [PubMed] [Google Scholar]
- Benarba B., Pandiella A. (2020). Medicinal plants as sources of active molecules against COVID-19. Front. Pharmacol. 11:1189. doi: 10.3389/fphar.2020.01189, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondad-Reantaso M. G., Mackinnon B., Bin H., Jie H., Tang-Nelson K., Surachetpong W., et al. (2020). Viewpoint: SARS-CoV-2 (The cause of COVID-19 in humans) is not known to infect aquatic food animals nor contaminate their products. Asian Fish. Sci. 33, 74–78. doi: 10.33997/j.afs.2020.33.1.009 [DOI] [Google Scholar]
- Boziaris I. S. (ed.) (2014). “Introduction to seafood processing-assuring quality and safety of seafood,” in Seafood Processing—Technology, Quality, and Safety Chichester, UK: John Wiley & Sons, 1–18. [Google Scholar]
- Calder P. C., Carr A. C., Gombart A. F., Eggersdorfer M. (2020). Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 12:1181. doi: 10.3390/nu12041181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P., Grimble R. (2002). Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 56, S14–S19. doi: 10.1038/sj.ejcn.1601478 [DOI] [PubMed] [Google Scholar]
- Ceniti C., Tilocca B., Britti D., Santoro A., Costanzo N. (2021). Food safety concerns in “COVID-19 era”. Microbiol. Res. 12, 53–68. doi: 10.3390/microbiolres12010006 [DOI] [Google Scholar]
- Centers for Disease Control and Prevention (2021). Protecting Seafood Processing Workers from COVID-19. Available at: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/grc-745768?lang=en
- Chakrabarti S., Guha S., Majumder K. (2018). Food-derived bioactive peptides in human health: challenges and opportunities. Nutrients 10:1738. doi: 10.3390/nu10111738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F. R., Yen C. T., Ei-Shazly M., Lin W. H., Yen M. H., Lin K. H., et al. (2012). Anti-human coronavirus (anti-HCoV) triterpenoids from the leaves of Euphorbia neriifolia. Nat. Prod. Commun. 7, 1415–1417. [PubMed] [Google Scholar]
- Chen C., Zhang Y., Huang J., Yin P., Cheng Z., Wu J., et al. (2020). Favipiravir versus arbidol for COVID-19: a randomized clinical trial. Infectious Diseases (except HIV/AIDS) [Preprint]. doi: 10.1101/2020.03.17.20037432 [DOI]
- Chitrakar B., Zhang M., Bhandari B. (2021). Improvement strategies of food supply chain through novel food processing technologies during COVID-19 pandemic. Food Control 125:108010. doi: 10.1016/j.foodcont.2021.108010, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacka K., Witek-Krowiak A., Skrzypczak D., Mikula K., Młynarz P. (2020). Phytochemicals containing biologically active polyphenols as an effective agent against Covid-19-inducing coronavirus. J. Funct. Foods 73:104146. doi: 10.1016/j.jff.2020.104146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobre A. F., Surek M., Vilhena R. O., Böger B., Fachi M. M., Momade D. R., et al. (2021). Influence of foods and nutrients on COVID-19 recovery: a multivariate analysis of data from 170 countries using a generalized linear model. Clin. Nutr. doi: 10.1016/j.clnu.2021.03.018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L., Bremer P., Kaye-Blake W., Mirosa M. (2020). Chinese consumers’ perceptions of immune health and immune-boosting remedies including functional foods. J. Food Prod. Mark. 26, 55–78. doi: 10.1080/10454446.2020.172088 [DOI] [Google Scholar]
- Cook E. A. J., De Glanville W. A., Thomas L. F., Kariuki S., de Clare Bronsvoort B. M., Fèvre E. M. (2017). Working conditions and public health risks in slaughterhouses in western Kenya. BMC Public Health 17, 1–12. doi: 10.1186/s12889-016-3923-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Antonio A., Wiedemann L. S. M., Veiga-Junior V. F. (2020). Natural products’ role against COVID-19. RSC Adv. 10, 23379–23393. doi: 10.1039/D0RA03774E [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da J., Xu M., Wang Y., Li W., Lu M., Wang Z. (2019). Kaempferol promotes apoptosis while inhibiting cell proliferation via androgen-dependent pathway and suppressing vasculogenic mimicry and invasion in prostate cancer. Anal. Cell. Pathol. 2019, 1–10. doi: 10.1155/2019/1907698, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M., Li H., Yan N., Huang J., Zhao L., Xu S., et al. (2020). Long-term survival of salmon-attached SARS-CoV-2 at 4°C as a potential source of transmission in seafood markets. BioRxiv [Preprint]. doi: 10.1101/2020.09.06.284695 [DOI] [Google Scholar]
- Das U. N. (2021). Essential fatty acids and their metabolites in the pathobiology of (coronavirus disease 2019) COVID-19. Nutrition 82:111052. doi: 10.1016/j.nut.2020.111052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Faria Coelho-Ravagnani C., Corgosinho F. C., Sanches F. L. F. Z., Prado C. M. M., Laviano A., Mota J. F. (2021). Dietary recommendations during the COVID-19 pandemic. Nutr. Rev. 79, 382–393. doi: 10.1093/nutrit/nuaa067, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Jiang H., Fang J. (2018). Regulation of immune function by polyphenols. J. Immunol. Res. 2018, 1–8. doi: 10.1155/2018/1264074, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda-Chodak A., Lukasiewicz M., Zięć G., Florkiewicz A., Filipiak-Florkiewicz A. (2020). Covid-19 pandemic and food: present knowledge, risks, consumers fears and safety. Trends Food Sci. Technol. 105, 145–160. doi: 10.1016/j.tifs.2020.08.020, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand R., Pellerin G., Thibodeau J., Fraboulet E., Marette A., Bazinet L. (2020). Screening for metabolic syndrome application of a herring by-product hydrolysate after its separation by electrodialysis with ultrafiltration membrane and identification of novel anti-inflammatory peptides. Sep. Purif. Technol. 235:116205. [Google Scholar]
- Eftimov T., Popovski G., Petković M., Seljak B. K., Kocev D. (2020). COVID-19 pandemic changes the food consumption patterns. Trends Food Sci. Technol. 104, 268–272. doi: 10.1016/j.tifs.2020.08.017, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitton J. H., Park A. Y., Karpiniec S. S., Stringer D. N. (2021). Fucoidan and lung function: value in viral infection. Mar. Drugs 19:4. doi: 10.3390/md19010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas J., Vaz-Pires P., Câmara J. S. (2020). From aquaculture production to consumption: freshness, safety, traceability and authentication, the four pillars of quality. Aquaculture 518:734857. doi: 10.1016/j.aquaculture.2019.734857 [DOI] [Google Scholar]
- Fu S., Wang W., Wang Q., He F., Hao J., Pang B. (2021). Surveillance of enteric pathogens in imported seafood and environmental surfaces in five seafood markets before the outbreak of COVID-19. Biosaf. Health 3, 183–186. doi: 10.1016/j.bsheal.2021.06.005 [DOI] [Google Scholar]
- Galanakis C. M. (2020). The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods 9:523. doi: 10.3390/foods9040523, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanakis C. M., Rizou M., Aldawoud T. M., Ucak I., Rowan N. J. (2021). Innovations and technology disruptions in the food sector within the COVID-19 pandemic and post-lockdown era. Trends Food Sci. Technol. 110, 193–200. doi: 10.1016/j.tifs.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland L. (2013). “Functional foods: health effects and clinical applications,” in Encyclopedia of Human Nutrition. ed. Caballero B. (United States of America: Elsevier; ), 366–371. [Google Scholar]
- Geahchan S., Ehrlich H., Rahman M. A. (2021). The anti-viral applications of marine resources for COVID-19 treatment: an overview. Mar. Drugs 19:409. doi: 10.3390/md19080409, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile D., Patamia V., Scala A., Sciortino M. T., Piperno A., Rescifina A. (2020). Putative inhibitors of SARS-CoV-2 main protease from a library of marine natural products: a virtual screening and molecular modeling study. Mar. Drugs 18:225. doi: 10.3390/md18040225, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy M. G., Kibenge M. J., Kibenge F. S. (2021). SARS-CoV-2 transmission via aquatic food animal species or their products: a review. Aquaculture 536:736460. doi: 10.1016/j.aquaculture.2021.736460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C. J., Tchesnokov E. P., Woolner E., Perry J. K., Feng J. Y., Porter D. P., et al. (2020). Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 295, 6785–6797. doi: 10.1074/jbc.RA120.013679, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakovirta M., Hakovirta J. (2020). Transmittance and survival of SARS-CoV-2 in global trade: The role of supply chain and packaging. J. Package. Technol. Res. 4, 261–265. doi: 10.1007/s41783-020-00101-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadani J. D., Hasan M. I., Baldi A. J., Hossain S. J., Shiraji S., Bhuiyan M. S. A., et al. (2020). Immediate impact of stay-at-home orders to control COVID-19 transmission on socioeconomic conditions, food insecurity, mental health, and intimate partner violence in Bangladeshi women and their families: an interrupted time series. Lancet Glob. Health 8, e1380–e1389. doi: 10.1016/S2214-109X(20)30366-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B., Hoang B. X. (2020). Opinions on the current pandemic of COVID-19: use functional food to boost our immune functions. J. Infect. Public Health 13, 1811–1817. doi: 10.1016/j.jiph.2020.08.014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Roy P. K., Hossain I., Byun K.-H., Choi C., Ha S.-D. (2021). COVID-19 pandemic crisis and food safety: implications and inactivation strategies. Trends Food Sci. Technol. 109, 25–36. doi: 10.1016/j.tifs.2021.01.004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Zhang X., He S., Jia P. (2021). Can the coronavirus disease be transmitted from food? A review of evidence, risks, policies and knowledge gaps. Environ. Chem. Lett. 19, 5–16. doi: 10.1007/s10311-020-01101-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W. T., Carabelli A. M., Jackson B., Gupta R. K., Thomson E. C., Harrison E. M., et al. (2021). SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 19, 409–424. doi: 10.1038/s41579-021-00573-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havice E., Marschke M., Vandergeest P. (2020). Industrial seafood systems in the immobilizing COVID-19 moment. Agric. Hum. Values 37, 655–656. doi: 10.1007/s10460-020-10117-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271.e8–280.e8. doi: 10.1016/j.cell.2020.02.052, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofherr J., Martinsohn J., Cawthorn D., Rasco B., Naaum A. M. (2016). “Regulatory frameworks for seafood authenticity and traceability,” in Seafood Authenticity and Traceability. eds. Naaum A. M., Hanner R. H. (United States of America: Elsevier; ), 47–82. [Google Scholar]
- Hollman P. C. H. (2001). Evidence for health benefits of plant phenols: local or systemic effects? J. Sci. Food Agric. 81, 842–852. doi: 10.1002/jsfa.900 [DOI] [Google Scholar]
- Hu L., Gao J., Yao L., Zeng L., Liu Q., Zhou Q., et al. (2021). Evidence of foodborne transmission of the coronavirus (COVID-19) through the animal products food supply chain. Environ. Sci. Technol. 55, 2713–2716. doi: 10.1021/acs.est.0c06822, PMID: [DOI] [PubMed] [Google Scholar]
- Inanli A. G., Tümerkan E. T. A., El Abed N., Regenstein J. M., Özogul F. (2020). The impact of chitosan on seafood quality and human health: a review. Trends Food Sci. Technol. 97, 404–416. doi: 10.1016/j.tifs.2020.01.029 [DOI] [Google Scholar]
- Jang Y., Shin H., Lee M. K., Kwon O. S., Shin J. S., Kim Y.-I., et al. (2021). Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2. Sci. Rep. 11, 1–12. doi: 10.1038/s41598-020-80896-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. (2020). Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab. Syndr. Clin. Res. Rev. 14, 367–382. doi: 10.1016/j.dsx.2020.04.015, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Hillyer C., Du L. (2020). Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 41, 355–359. doi: 10.1016/j.it.2020.03.007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S., Kim S., Shin D. H., Kim M.-S. (2020). Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 35, 145–151. doi: 10.1080/14756366.2019.1690480, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen S. C., Kebriaei R., Dresser L. D. (2020). Remdesivir: review of pharmacology, pre-clinical data, and emerging clinical experience for COVID-19. pharmacotherapy: the journal of human pharmacology and drug. Therapy 40, 659–671. doi: 10.1002/phar.2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S. R., Spratt A. N., Cohen A. R., Naqvi S. H., Chand H. S., Quinn T. P., et al. (2021). Evolutionary analysis of the delta and delta plus variants of the SARS-CoV-2 viruses. J. Autoimmun. 124:102715. doi: 10.1016/j.jaut.2021.102715, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansiime M. K., Tambo J. A., Mugambi I., Bundi M., Kara A., Owuor C. (2021). COVID-19 implications on household income and food security in Kenya and Uganda: findings from a rapid assessment. World Dev. 137:105199. doi: 10.1016/j.worlddev.2020.105199, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Siddique R., Shereen M. A., Ali A., Liu J., Bai Q., et al. (2020). Emergence of a novel coronavirus, severe acute respiratory syndrome coronavirus 2: biology and therapeutic options. J. Clin. Microbiol. 58, e00187–e00220. doi: 10.1128/JCM.00187-20, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber B., Fischer W. M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., et al. (2020). Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 182, 812–827. doi: 10.1101/2020.04.29.069054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulawik P., Rathod N. B., Ozogul Y., Ozogul F., Zhang W. (2022). Recent developments in the use of cold plasma, high hydrostatic pressure, and pulsed electric fields on microorganisms and viruses in seafood. Crit. Rev. Food Sci. Nutr. 1-15. doi: 10.1080/10408398.2022.2077298 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Kundam D. N., Acham I. O., Girgih A. T. (2018). Bioactive compounds in fish and their health benefits. Asian Food Sci. J. 4, 1–14. doi: 10.9734/AFSJ/2018/41803 [DOI] [Google Scholar]
- Lakshmana S. S., Raghu C., Arjun H. A., Anantharaman P. (2019). In vitro and in silico inhibition properties of fucoidan against α-amylase and α-D-glucosidase with relevance to type 2 diabetes mellitus. Carbohydr. Polym. 209, 350–355. [DOI] [PubMed] [Google Scholar]
- Lam S. D., Bordin N., Waman V. P., Scholes H. M., Ashford P., Sen N., et al. (2020). SARS-CoV-2 spike protein predicted to form complexes with host receptor protein orthologues from a broad range of mammals. Sci. Rep. 10, 1–14. doi: 10.1038/s41598-020-71936-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavilla I., Costas-Rodríguez M., Bendicho C. (2013). “Authentication of fishery products,” in Comprehensive Analytical Chemistry, Vol. 60. eds. de la Guardia M., Gonzálvez A. (United States of America: Elsevier; ), 657–717. [Google Scholar]
- Li C., Wang L., Ren L. (2020). Antiviral mechanisms of candidate chemical medicines and traditional Chinese medicines for SARS-CoV-2 infection. Virus Res. 286:198073. doi: 10.1016/j.virusres.2020.198073, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Lee H.-S., Kim S.-H., Moon B., Lee C. (2014). Antioxidant and anti-inflammatory activities of methanol extracts of Tremella fuciformis and its major phenolic acids. J. Food Sci. 79, C460–C468. doi: 10.1111/1750-3841.12393, PMID: [DOI] [PubMed] [Google Scholar]
- Li S. Y., Chen C., Zhang H. Q., Guo H. Y., Wang H., Wang L., et al. (2005). Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 67, 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Yang M., Zhao X., Guo Y., Wang L., Zhang J., et al. (2020). Cold-chain transportation in the frozen food industry may have caused a recurrence of COVID-19 cases in destination: successful isolation of SARS-CoV-2 virus from the imported frozen cod package surface. Biosaf. Health 2, 199–201. doi: 10.1016/j.bsheal.2020.11.003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Malo A., Alzamora S. M., Paris M. J., Lastra-Vargas L., Coronel M. B., Gómez P. L., et al. (2020). “Naturally occurring compounds–plant sources,” in Antimicrobials in Food. eds. Michael Davidson P., Matthew Taylor T., David J. R. D. (United States of America: ) 527–594. [Google Scholar]
- Love D. C., Allison E. H., Asche F., Belton B., Cottrell R. S., Froehlich H. E., et al. (2021). Emerging COVID-19 impacts, responses, and lessons for building resilience in the seafood system. Global. Food Secur. 28:100494. doi: 10.1016/j.gfs.2021.100494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N. L., Peng W., Soon C. F., Hassim M. F. N., Misbah S., Rahmat Z., et al. (2021). Covid-19 pandemic in the lens of food safety and security. Environ. Res. 193:110405. doi: 10.1016/j.envres.2020.110405, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meharoof M., Gul S., Qureshi N. W. (2020). Indian seafood trade and COVID-19: anticipated impacts and economics. Food Sci. Rep. 1, 54–58. [Google Scholar]
- Motwalli O., Alazmi M. (2021). Analysis of natural compounds against the activity of SARS-CoV-2 NSP15 protein towards an effective treatment against COVID-19: a theoretical and computational biology approach. J. Mol. Model. 27, 1–11. doi: 10.1007/s00894-021-04750-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu C., Sheng Y., Wang Q., Amin A., Li X., Xie Y. (2021). Potential compound from herbal food of Rhizoma Polygonati for treatment of COVID-19 analyzed by network pharmacology: viral and cancer signaling mechanisms. J. Funct. Foods 77:104149. doi: 10.1016/j.jff.2020.104149, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. E., Neufurth M., Wang S., Tan R., Schröder H. C., Wang X. (2020). Morphogenetic (mucin expression) as well as potential anti-corona viral activity of the marine secondary metabolite polyphosphate on A549 cells. Mar. Drugs 18:639. doi: 10.3390/md18120639, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakat Z., Bou-Mitri C. (2021). COVID-19 and the food industry: readiness assessment. Food Control 121:107661. doi: 10.1016/j.foodcont.2020.107661, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanasamy A., Balde A., Raghavender P., Shashanth D., Abraham J., Joshi I., et al. (2020). Isolation of marine crab (Charybdis natator) leg muscle peptide and its anti-inflammatory effects on macrophage cells. Biocatal. Agric. Biotechnol. 25:101577. [Google Scholar]
- Nasri M. (2017). Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. Adv. Food Nutr. Res. 81, 109–159. doi: 10.1016/bs.afnr.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Nebigil C. G., Moog C., Vagner S., Benkirane-Jessel N., Smith D. R., Désaubry L. (2020). Flavaglines as natural products targeting eIF4A and prohibitins: from traditional Chinese medicine to antiviral activity against coronaviruses. Eur. J. Med. Chem. 203:112653. doi: 10.1016/j.ejmech.2020.112653, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufurth M., Wang X., Wang S., Schröder H. C., Müller W. E. (2021). Caged dexamethasone/quercetin nanoparticles, formed of the morphogenetic active inorganic polyphosphate, are strong inducers of MUC5AC. Mar. Drugs 19:64. doi: 10.3390/md19020064, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. T., Haider W., Solgaard H. S., Ravn-Jonsen L., Roth E. (2015). Consumer willingness to pay for quality attributes of fresh seafood: a labeled latent class model. Food Qual. Prefer. 41, 225–236. doi: 10.1016/j.foodqual.2014.12.007 [DOI] [Google Scholar]
- Nishide M., Ikeda K., Mimura H., Yoshida M., Mitani T., Hajime Koyama A. (2019). Antiviral and virucidal activities against herpes simplex viruses of umesu phenolics extracted from Japanese apricot. Microbiol. Immunol. 63, 359–366. doi: 10.1111/1348-0421.12729 [DOI] [PubMed] [Google Scholar]
- Olatunde O. O., Shiekh K. A., Benjakul S. (2021). Pros and cons of cold plasma technology as an alternative non-thermal processing technology in seafood industry. Trends Food Sci. Technol. 111, 617–627. doi: 10.1016/j.tifs.2021.03.026 [DOI] [Google Scholar]
- Plante J. A., Liu Y., Liu J., Xia H., Johnson B. A., Lokugamage K. G., et al. (2021). Spike mutation D614G alters SARS-CoV-2 fitness. Nature 592, 116–121. doi: 10.1038/s41586-020-2895-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelman M. P., Gillebaart M., Schlinkert C., Dijkstra S. C., Derksen E., Mensink F., et al. (2021). Eating behavior and food purchases during the COVID-19 lockdown: a cross-sectional study among adults in the Netherlands. Appetite 157:105002. doi: 10.1016/j.appet.2020.105002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman P., Naidu A. S., Clemens R. (2020). COVID-19 and food safety: risk management and future considerations. Nutr. Today 55, 125–128. doi: 10.1097/NT.0000000000000415 [DOI] [Google Scholar]
- Rajkumar R. P. (2021). Cross-national variations in COVID-19 mortality: the role of diet, obesity and depression. Diseases 9:36. doi: 10.3390/diseases9020036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshkumar M. R., Indu P., Arunagirinathan N., Venkatadri B., El-Serehy H. A., Ahmad A. (2021). Computational selection of flavonoid compounds as inhibitors against SARS-CoV-2 main protease, RNA-dependent RNA polymerase and spike proteins: a molecular docking study. Saudi J. Biol. Sci. 28, 448–458. doi: 10.1016/j.sjbs.2020.10.028, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastmanesh R., Marotta F., Tekin I. (2020). Call for mobilization of functional foods, antioxidants, and herbal antivirals in support of international campaign to control coronavirus. BCHD 3, 90–94. doi: 10.31989/bchd.v3i5.717 [DOI] [Google Scholar]
- Rathod N. B., Kulawik P., Ozogul F., Regenstein J. M., Ozogul Y. (2021a). Biological activity of plant-based carvacrol and thymol and their impact on human health and food quality. Trends Food Sci. Technol. 116, 733–748. doi: 10.1016/j.tifs.2021.08.023 [DOI] [Google Scholar]
- Rathod N. B., Ranveer R. C., Benjakul S., Kim S. K., Pagarkar A. U., Patange S., et al. (2021b). Recent developments of natural antimicrobials and antioxidants on fish and fishery food products. Compr. Rev. Food Sci. Food Saf. 20, 4182–4210. doi: 10.1111/1541-4337.12787 [DOI] [PubMed] [Google Scholar]
- Rizou M., Galanakis I. M., Aldawoud T. M., Galanakis C. M. (2020). Safety of foods, food supply chain and environment within the COVID-19 pandemic. Trends Food Sci. Technol. 102, 293–299. doi: 10.1016/j.tifs.2020.06.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roslan J., Yunos K. F. M., Abdullah N., Kamal S. M. M. (2014). Characterization of fish protein hydrolysate from tilapia (Oreochromis niloticus) by-product. Agric. Agric. Sci. Procedia 2, 312–319. doi: 10.1016/j.aaspro.2014.11.044 [DOI] [Google Scholar]
- Rosmalena R., Elya B., Dewi B. E., Fithriyah F., Desti H., Angelina M., et al. (2019). The antiviral effect of indonesian medicinal plant extracts against dengue virus in vitro and in silico. Pathogens 8:85. doi: 10.3390/pathogens8020085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem R., Ktari N., Bkhairia I., Nasri R., Mora L., Kallel R., et al. (2018). In vitro and in vivo anti-diabetic and anti-hyperlipidemic effects of protein hydrolysates from Octopus vulgaris in alloxanic rats. Food Res. Int. 106, 952–963. doi: 10.1016/j.foodres.2018.01.068 [DOI] [PubMed] [Google Scholar]
- Shahbaz M., Bilal M., Moiz A., Zubair S., Iqbal H. M. (2020). Food safety and COVID-19: precautionary measures to limit the spread of coronavirus at food service and retail sector. J. Pure Appl. Microbiol. 14(Suppl 1), 749–756. doi: 10.22207/JPAM.14.SPL1.12 [DOI] [Google Scholar]
- Sharifi N., Souri E., Ziai S. A., Amin G., Amanlou M. (2013). Discovery of new angiotensin converting enzyme (ACE) inhibitors from medicinal plants to treat hypertension using an in vitro assay. DARU J. Pharm. Sci. 21:74. doi: 10.1186/2008-2231-21-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S. Y., Shin Y. E., Kim H. K. (2019). Fucoidan from Undaria pinnatifida has anti-diabetic effects by stimulation of glucose uptake and reduction of basal lipolysis in 3T3-L1 adipocytes. Nutr. Res. 65, 54–62. doi: 10.1016/j.nutres.2019.02.002 [DOI] [PubMed] [Google Scholar]
- Singh P., Tripathi M. K., Yasir M., Khare R., Tripathi M. K., Shrivastava R. (2020). Potential inhibitors for SARS-CoV-2 and functional food components as nutritional supplement for COVID-19: a review. Plant Foods Hum. Nutr. 75, 458–466. doi: 10.1007/s11130-020-00861-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan D., Enciu A. M., Mateescu A. L., Ion A. C., Brezeanu A. C., Stan D., et al. (2021). Natural compounds with antimicrobial and antiviral effect and nanocarriers used for their transportation. Front. Pharmacol. 6:723233. doi: 10.3389/fphar.2021.723233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll J. S., Harrison H. L., De Sousa E., Callaway D., Collier M., Harrell K., et al. (2021). Alternative seafood networks during COVID-19: implications for resilience and sustainability. Front. Sustain. Food Syst. 5:614368. doi: 10.3389/fsufs.2021.614368 [DOI] [Google Scholar]
- Suleria H. A. R., Osborne S., Masci P., Gobe G. (2015). Marine-based nutraceuticals: an innovative trend in the food and supplement industries. Mar. Drugs 13, 6336–6351. doi: 10.3390/md13106336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan M. T., Buttxs M. S., Qayyum M. M. N., Suleria H. A. R. (2014). Immunity: plants as effective mediators. Crit. Rev. Food Sci. Nutr. 54, 1298–1308. doi: 10.1080/10408398.2011.633249, PMID: [DOI] [PubMed] [Google Scholar]
- Sumon T. A., Hussain M. A., Hasan M., Rashid A., Abualreesh M. H., Jang W. J., et al. (2021). Antiviral peptides from aquatic organisms: functionality and potential inhibitory effect on SARS-CoV-2. Aquaculture 541:736783. doi: 10.1016/j.aquaculture.2021.736783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran O. N., Haridas M., Szakacs G., Sabu A. (2020). “Modified plant metabolites as nutraceuticals,” in Plant Metabolites: Methods, Applications and Prospects. eds. Sukumaran S. T., Sugathan S., Abdulhameed S. (Singapore: Springer; ), 167–180. [Google Scholar]
- Talukdar J., Dasgupta S., Nagle V., Bhadra B. (2020). COVID-19: potential of microalgae derived natural astaxanthin as adjunctive supplement in alleviating cytokine storm. [DOI] [PMC free article] [PubMed]
- Tian H., Liu Y., Li Y., Wu C.-H., Chen B., Kraemer M. U., et al. (2020). An investigation of transmission control measures during the first 50 days of the COVID-19 epidemic in China. Science 368, 638–642. doi: 10.1126/science.abb6105, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokur B. T., Korkmaz K. (2021). The evaluation of avian influenza and coronavirus as human pathogenic enveloped viruses for possible health risk in seafood: a review. J. Anatol. Environ. Animal Sci. 6, 31–42. doi: 10.35229/jaes.796262 [DOI] [Google Scholar]
- Torrinhas R. S., Calder P. C., Lemos G. O., Waitzberg D. L. (2021). Parenteral fish oil: an adjuvant pharmacotherapy for coronavirus disease 2019? Nutrition 81:110900. doi: 10.1016/j.nut.2020.110900, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services (n.d.). Dietary Guidelines for Americans, 2020–2025. 9th Edition. December 2020. Available at: DietaryGuidelines.gov
- Usman M., Farooq M., Hanna K. (2020). Environmental side effects of the injudicious use of antimicrobials in the era of COVID-19. Sci. Total Environ. 745:141053. doi: 10.1016/j.scitotenv.2020.141053, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Huang J., Yeung A. W. K., Tzvetkov N. T., Horbańczuk J. O., Willschke H., et al. (2020). The significance of natural product derivatives and traditional medicine for COVID-19. PRO 8:937. doi: 10.3390/pr8080937 [DOI] [Google Scholar]
- White E. R., Froehlich H. E., Gephart J. A., Cottrell R. S., Branch T. A., Agrawal Bejarano R., et al. (2021). Early effects of COVID-19 on US fisheries and seafood consumption. Fish Fish. 22, 232–239. doi: 10.1111/faf.12525, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K. M., Rosales R., Yildiz S., Kehrer T., Miorin L., Moreno E., et al. (2021). Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 371, 926–931. doi: 10.1126/science.abf4058, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2019). Coronavirus Disease 2019 COVID-19: Situation Report. WHO. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200221-sitrep-32-covid-19.pdf (Accessed May 31, 2022).
- World Health Organization (2020). COVID-19 and Food Safety: Guidance for Food Businesses: Interim Guidance, 07 April 2020. World Health Organization.
- Yang Y., Islam M. S., Wang J., Li Y., Chen X. (2020). Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 16, 1708–1717. doi: 10.7150/ijbs.45538, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim S.-K., Kim K., Kim I., Chun S., Oh T., Kim J.-U., et al. (2021). Inhibition of SARS-CoV-2 virus entry by the crude polysaccharides of seaweeds and abalone viscera in vitro. Mar. Drugs 19:219. doi: 10.3390/md19040219, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Kan R., Ji H., Wu S., Zhao W., Shuian D., et al. (2021). Identification of tuna protein-derived peptides as potent SARS-CoV-2 inhibitors via molecular docking and molecular dynamic simulation. Food Chem. 342:128366. doi: 10.1016/j.foodchem.2020.128366, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahran E. M., Albohy A., Khalil A., Ibrahim A. H., Ahmed H. A., El-Hossary E. M., et al. (2020). Bioactivity potential of marine natural products from scleractinia-associated microbes and in silico anti-SARS-CoV-2 evaluation. Mar. Drugs 18:645. doi: 10.3390/md18120645, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora-Sillero J., Gharsallaoui A., Prentice C. (2018). Peptides from fish by-product protein hydrolysates and its functional properties: an overview. Mar. Biotechnol. 20, 118–130. doi: 10.1007/s10126-018-9799-3 [DOI] [PubMed] [Google Scholar]
- Zannella C., Giugliano R., Chianese A., Buonocore C., Vitale G. A., Sanna G., et al. (2021). Antiviral activity of Vitis vinifera leaf extract against SARS-CoV-2 and HSV-1. Viruses 13:1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyaullah M., AlShahrani A. M., Muzammil K., Ahmad I., Alam S., Khan W. H., et al. (2021). COVID-19 and SARS-CoV-2 variants: current challenges and health concern. Front. Genet. 12:693916. doi: 10.3389/fgene.2021.693916, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Ji Z., Yue Y., Liu H., Wang J. (2021). Infection risk assessment of COVID-19 through aerosol transmission: a case study of South China seafood market. Environ. Sci. Technol. 55, 4123–4133. doi: 10.1021/acs.est.0c02895, PMID: [DOI] [PubMed] [Google Scholar]
- Zhao C., Yang C., Liu B., Lin L., Sarker S. D., Nahar L., et al. (2018). Bioactive compounds from marine macroalgae and their hypoglycemic benefits. Trends Food Sci. Technol. 72, 1–12. doi: 10.1016/j.tifs.2017.12.001 [DOI] [Google Scholar]
- Zuber S., Brüssow H. (2020). COVID 19: challenges for virologists in the food industry. Microb. Biotechnol. 13, 1689–1701. doi: 10.1111/1751-7915.13638, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]