Abstract
Carbapenem-resistant Klebsiella pneumoniae (CRKP), a pathogen that causes severe nosocomial infections and yields a high mortality rate, poses a serious threat to global public health due to its high antimicrobial resistance. Bacteriophages encode polysaccharide-degrading enzymes referred to as depolymerases that cleave the capsular polysaccharide (CPS), one of the main virulence factors of K. pneumoniae. In this study, we identified and characterized a new capsule depolymerase K19-Dpo41 from K. pneumoniae bacteriophage SH-KP156570. Our characterization of K19-Dpo41 demonstrated that this depolymerase showed specific activities against K19-type K. pneumoniae. K19-Dpo41-mediated treatments promoted the sensitivity of a multidrug-resistant K19-type K. pneumoniae strain to the bactericidal effect of human serum and significantly increased the survival rate of Galleria mellonella infected with K19-type K. pneumoniae. Our results provided strong primary evidence that K19-Dpo41 was not only effective in capsular typing of K19-type K. pneumoniae but promising in terms of developing new alternative therapeutic strategies against K19-type CRKP infections in the future.
Keywords: carbapenem-resistant Klebsiella pneumoniae, bacteriophage, depolymerase, capsular typing, anti-infection of CRKP
Introduction
Klebsiella pneumoniae is an opportunistic pathogen that causes a variety of infections, including urinary tract infections, bacteremia, pneumonia, and liver abscesses (Wang et al., 2020). Since the first K. pneumoniae strain containing the enzyme carbapenemase was identified in 1996, carbapenem-resistant K. pneumoniae (CRKP) soon became prevalent, highlighting the issue of lacking available antibiotics and the demand for alternative treatments (Lai et al., 2019). In recent years, the global transmission of CRKP and hypervirulent K. pneumoniae strains significantly amplified the damage caused by this pathogen and brought new challenges for effective pathogen control (Zhang et al., 2015). All these factors pointed out that K. pneumoniae is a major concern to public health (Effah et al., 2020).
Capsular polysaccharide (CPS), also known as K antigen, is the outermost capsule layer around the K. pneumoniae cell, which acts as an essential virulence factor and a defense barrier of K. pneumoniae (Effah et al., 2020). It allows the bacterium to survive inside the host by overcoming the protective mechanisms of the immune system. The capsule hinders the bactericidal action of antimicrobial peptides and blocks complement components, preventing complement-mediated killing (Paczosa and Mecsas, 2016; Li J. et al., 2021). It further impairs phagocytosis and opsonophagocytosis of K. pneumoniae by immune cells (Opoku-Temeng et al., 2019; Assoni et al., 2021). Until now, at least 134 capsular types have been identified (Volozhantsev et al., 2020). Due to the limited source, high cost of antisera, and large number of serological cross-reactions, the traditional serological typing method is not widely used. The new molecular typing method based on a single wzi gene (wzi typing) or, for more accurate typing results, the entire genome of the capsular synthesis region (Kaptive typing), has begun to show its advantages over the serological typing method in epidemiological investigations with its excellent typing performance (Zhou et al., 2016; Lam et al., 2022).
Phage-coded capsular depolymerase is an enzyme that bacteriophages use to degrade the CPS of their host bacteria. Recently, studies on capsular depolymerases have shown that each of them is highly specific to a particular CPS layer, demonstrating that they can be used as a simple and economical method to differentiate capsular types, and act as an antibacterial agent for K. pneumoniae strains of their corresponding capsular types (Pan et al., 2019). Such characteristics of capsular depolymerases have drawn great attention as they have great potential in defeating CRKP. For example, depolymerases can increase K. pneumoniae’s susceptibility to gentamicin at a lower concentration (Bansal et al., 2014); Depolymerase Dpo42 and Dpo43 of K47-type K. pneumoniae make host bacteria fully susceptible to the killing effect of serum complement (Liu et al., 2020; Volozhantsev et al., 2020); Depolymerase KP32gp37 and KP32gp38 of K3-type and K21-type K. pneumoniae, respectively, can efficiently decrease K. pneumoniae resistance to phagocytosis by macrophages (Majkowska-Skrobek et al., 2018). Till now, depolymerases targeting 23 K. pneumonia capsular types, namely, K1, K2, K3, K5, K8, K11, K13, K21, K23, K25, K30, K35, K47, K56, K57, K63, K64, K69, KN1, KN2, KN3, KN4, and KN5, have been identified and reported (Wu et al., 2019; Liu et al., 2020; Squeglia et al., 2020; Volozhantsev et al., 2020; Dunstan et al., 2021; Gorodnichev et al., 2021; Li J. et al., 2021; Pertics et al., 2021).
The number of hospitalized and agriculturally transmitted multi-drug resistant K. pneumoniae strains has shown a rising trend in the past few years in China (Xu et al., 2016; Yang et al., 2021). K19 K. pneumoniae serotype has brought our attention as it was discovered as the second most prevalent serotype in a local hospital CRKP sample population (n = 348) in Shanghai, China (Zhang et al., 2020) and the third most prevalent serotype in a population (n = 592) of local Chinese adults and overseas Chinese in Japan, Malaysia, Singapore, Thailand, and Vietnam (Volozhantsev et al., 2020). However, no report has been published on any depolymerase that specifically targets K19-type K. pneumoniae strains. In this study, we identified a new phage-derived depolymerase targeting specifically K19-type K. pneumoniae strains for the first time. Our results demonstrated that this depolymerase presented great potential in advancing capsular typing and treatments of K19-type CRKP infections.
Materials and Methods
Bacterial Strain Isolation and Identification
Twenty-nine K. pneumoniae strains collected from Renji Hospital, Shanghai Jiao Tong University School of Medicine, and Huashan Hospital, Fudan University (listed in Table 1) were used in this study. All strains were identified by the matrix-assisted laser desorption ionization time-of-flight mass spectrometry (Bruker Diatonic GmbH, Bremen, Germany; D’Andrea et al., 2017). Each K. pneumoniae strain was determined as CRKP with resistance to imipenem or meropenem. The antimicrobial susceptibility was determined using the Kirby-Bauer (K-B) method. The capsular types of these K. pneumoniae strains were determined by wzi gene sequencing according to Brisse et al. (2013). The viable bacterial count was determined using LB agar (1.5% w/v) plates. All strains were cultivated in Luria-Bertani broth (LB, Sangon Biotech, Shanghai, China) at 37°C and stored at –80°C in 50% glycerol. K. pneumoniae strain 6570 was used for the phage isolation, the serum assay and the establishment of the infection model.
TABLE 1.
Host spectrum of phage SH-KP156570 and depolymerase K19-Dpo41 against 29 K. pneumoniae strains.
| Isolate no. | Capsular type | SH-KP156570 |
K19-Dpo41 | |
| Plaques | Halos | |||
| 65701*, 67781, 71241, 73211, 74851, 76961, 77621, 81441, 85761, 18–1992, 18–2012, 18–3712, 19–792, 19–5672, 19–7882, 19–7912, 19–8312, 19–8532, 19–8542, 19–8552 | K19 | + | + | + |
| 50801 | K1 | – | – | – |
| 51461, 51701, 79561 | K2 | – | – | – |
| 63711, 64081 | K20 | – | – | – |
| 80311 | K47 | – | – | – |
| 51691 | K57 | – | – | – |
| 60891 | K64 | – | – | – |
The capsular type of K. pneumoniae strains were determined by wzi genotyping. –, no lysis; +, showed plaques or haloes.
1Isolated from patients at Renji Hospital in 2019 in Shanghai, China.
2Isolated from patients at the Huashan Hospital, affiliated with Fudan University in Shanghai, China.
*Host strain of phage SH-KP156570.
Phage Isolation, One-Step Growth Curve, and Host Spectrum Analysis
Phage SH-KP156570 was isolated from a raw sewage collected at Ruijin Hospital, Shanghai Jiao Tong University School of Medicine by using K. pneumoniae strain 6570 as the host bacterium. After centrifugation, the supernatant was filtered with a 0.22 μm filter (Millex-GP Filter Unit; Millipore, United States) and dripped on a double-layer agar plate covered with K. pneumoniae strain 6570. The lytic phage was then purified according to Pires’s method with minor modifications (Pires et al., 2011). The purified phage named SH-KP156570 was stored in SM buffer (100 mM NaCl, 8 mM MgSO4 7H2O, 50 mM Tris–HCl, pH = 7.5) at 4°C.
The one-step phage growth curve was generated as described previously with some modifications (D’Andrea et al., 2017). Briefly, Phage SH-KP156570 and K. pneumoniae strain 6570 were mixed at an MOI = 0.005. After bathing at 37°C for 5 min, the mixture was centrifuged, resuspended in 3 mL liquid LB medium. Samples were taken for every 3 min and dripped on the double-layer agar plate at a proper dilution. The number of bacteriophage plaque was counted overnight and the titer of bacteriophage was calculated. The number of plaque-forming units (PFU) was then calculated for 1 mL of the concentrated suspension. The latency period was defined as the time between infection and the shortest incubation time, allowing for the production of phages. The burst size was calculated as the ratio of the final count of released phage particles to the initial count of infected bacterial cells during the latent period. Independent experiments were repeated three times.
Host range analysis of phage SH-KP156570 was performed by the spot test using the strains listed in Table 1. In short, 400 μL log-phase bacterial culture was fully mixed with 4 mL 0.5% agar LB and laid on 1.5% agar LB medium. After the upper agar was solidified, the 5 μL purified phage suspension was spotted onto the plate and incubated overnight at 37°C to allow plaques to develop.
Genomic DNA Sequencing and Annotation
The genomic DNA of SH-KP156570 was extracted using the standard protocol as previously described (Guo et al., 2017). In brief, the phage in SM buffer was treated with 1 μg/mL DNaseI and RNaseA (Sigma-Aldrich, United States) at 37°C for 1 h, subsequently 25 mmol/mL ethylenediaminetetraacetic acid. Finally, the concentrated phage DNA was extracted using the UNlQ-10 Column Virus Genomic DNA Isolation Kit (Sangon Biotech, Shanghai, China) and sent to Shanghai Personalbio Biotechnology Co. Ltd for genome sequencing with Illumina high-throughput sequencing platform (Illumina Hiseq 3000). SOAP denovo2 software was used for genome assembly using optimized parameters. Data assembly was proceeding after adapter contamination removing and data filtering by using AdapterRemoval (Lindgreen, 2012) and SOAPec (Luo et al., 2012). The filtered reads were assembled by SPAdes (Bankevich et al., 2012) and A5-miseq (Coil et al., 2015) to constructed scaffolds and contigs. GeneMark was used to predict and analyze the open reading frames (ORFs) of the phage genome. Gene annotation was completed by using the NCBI website1. Function annotation was completed by blast search against different databases, including NR (Non-Redundant Protein Database; Blake and Cohen, 2001), Gene Ontology (Conesa and Götz, 2008), Kyoto Encyclopedia of Gene and Genomes (Moriya et al., 2007), Cluster of Orthologous Groups of proteins (Powell et al., 2014), and Swissprot. CGview (Stothard and Wishart, 2005) was used to give an overview of the genome information. The results of the HHpred database2 were used to locate the gene encoding polysaccharide depolymerase [Uniclust was chosen as the multiple sequence alignment (MSA) generation method, and three was chosen as the maximal number of MSA generation steps].
Cloning, Expression, and Purification of the Recombinant Depolymerase
The ORF41 gene was amplified from the DNA of the purified phage SH-KP156570 using primers 6570-ORF41-F (5′-CAGCAGCAGACGGGAGGATCCATGTCCACGATTACACA ATTC-3′) and 6570-ORF41-R (5′-CTCGAGTGCGGCCG CAAGCTTTTAGTTACTTCTCTCTTCAGC-3′). The 2292-base PCR amplification product was cloned into the N-terminal 6 × His labeled pSUMO3 expression vector (LifeSensors, United States) via BamHI and HindIII sites (New England Biolabs). The recombinant plasmid was verified by DNA sequencing and transformed into E. coli BL21 (DE3). The BL21 cells were cultivated to OD600 = 0.8. Then, the BL21 cells were induced with 0.1 mM isopropyl-β-D-thiogalactopyranoside (IPTG, Sangon Biotech, China). The mixture was centrifuged and the supernatant was discarded. The sediment was resuspended with lysis buffer [20 mM Tris–HCl (pH = 8.0), 0.5M NaCl, 10% glycerol] and phenylmethylsulfonyl fluoride (PMSF, Sangon Biotech, China) to a final concentration of 0.1 mg/mL. After centrifuged, the supernatant was collected. The Ni-NTA column (GE Healthcare, United States) was prerinsed using lysis buffer. After protein binding to Ni-NTA column, it was eluted with a gradient of 20 and 40 mM imidazole, and finally eluted with 300 mM imidazole. Then digested by SUMO protease (LifeSensors, United States). The purified depolymerase was confirmed by SDS-PAGE electrophoresis and named K19-Dpo41.
Purification of the Capsular Polysaccharide
Klebsiella pneumoniae strain 6570 was cultured in fresh TSB medium at 37°C for 5 days. The purification of CPS was performed according to the method from previous studies with some modifications (Gorodnichev et al., 2021; Li J. et al., 2021). Firstly, 60 μL of formaldehyde solution (36.5%) was added to 10 mL of bacterial culture and incubated 100 rpm at room temperature for 1 h. Subsequently, 1M NaOH was added to the system, with agitation at room temperature for 3 h. Cell suspensions were centrifuged at 16,800 g for 1 h at 4°C (Beckman, JA-25.50, United States). The supernatant was filtered through a 0.22 μm filter and dialyzed overnight in ddH2O with a 12–14 KD MWCO membrane (Thermo Scientific, United States). Then, the cationic detergent cetyltrimethylammonium bromide was added (0.5% w/v) to precipitate polysaccharide. Dissociate with different concentrations of CaCl2 and centrifuge the supernatant. Selective precipitation with 20 and 50% ethanol and washed with NaCl. Next, the sample was subjected to Capto adhere chromatography after ultrafiltration with a 30 KD membrane. The final sample was filtered through a 0.22 μm filter. Finally, the CPS obtained by dialysis was dried and weighed.
Depolymerization Activity Assay of K19-Dpo41 on K. pneumoniae Strain 6570 Capsular Polysaccharide
The activity of K19-Dpo41 was assessed by spot test (Khan Mirzaei and Nilsson, 2015). After K. pneumoniae strain 6570 was poured onto a LB agar, a serial 5 μL polysaccharide depolymerase in different concentration was spotted on the plate and incubated overnight at 37°C. The K19-Dpo41 activity was monitored by observing the formation of translucent halo zones. In addition, the sensitivity of other K. pneumoniae strains to K19-Dpo41 was also determined by single-spot assay (Wu et al., 2019).
Size exclusion chromatography-High performance liquid chromatography (SEC-HPLC) was used to determine the depolymerase activity of K19-Dpo41 on the CPS. The purified CPS was dissolved in 50 mM Na2HPO4 (pH = 7.0) to a final concentration of 0.5 mg/mL and incubated with K19-Dpo41 (10 μg/mL) or ddH2O at 37°C for 30 min. The reactions were terminated by heating at 100°C for 10 min. The mixture was analyzed by a HPLC system (Waters, Milford, MA, United States) equipped with a TSKgel G5000 PWxl column (inner diameter 7.8 mm × 300 mm; Tosoh Corporation Bioscience, Tokyo, Japan) and a refractive index detector (Waters, Milford, MA, United States). The column was run with the mobile phase of the phosphate-buffered saline (PBS, pH = 7.0) at 1 mL/min.
Serum Resistance Assay
The serum resistant assay was performed as previous studies (Liu et al., 2020; Li J. et al., 2021). K. pneumoniae strain 6570 at a concentration of 5 × 104 CFUs were suspended in PBS, treated with either K19-Dpo41 or K64-ORF41 (a depolymerases specific to K. pneumoniae K64 serotype), respectively, to a final concentration of 100 μg/mL. After a pretreatment at 37°C for 1 h, the suspension was mixed at a 1:3 v/v ratio with active or heat-inactivated human serum obtained from healthy volunteers. A control without depolymerase was also set up for significance. The mixture in final volume of 100 μL was incubated for 1 h at 37°C, and then 5 μL aliquots were removed, diluted and cultured on 1.5% LB agar plate for colony enumeration. Each test was performed at least in three independent experiments. Comparisons between any two experimental groups were made by the two-sample Student’s t-test. P < 0.05 was considered statistically significant.
Galleria mellonella Larvae Infection Model
Wax moth larvae (G. mellonella) were obtained from Tianjin Huiyude BioTech, China. The operating procedure was carried out in accordance with the previous studies (Majkowska-Skrobek et al., 2016; Oliveira et al., 2019; Gorodnichev et al., 2021; Shahed-Al-Mahmud et al., 2021). In short, K. pneumoniae strain 6570 from overnight culture were grown in fresh LB at 37°C to exponential phase, harvested (10,000 rpm, 10 min), washed with PBS and suspended in PBS to a concentration of 5 × 106 CFU/mL. Larvae were inoculated with 10 μL bacterial suspension containing 5 × 104 CFUs into the last proleg using 25 μL syringe. The anti-virulence effect of enzyme on K. pneumoniae strain 6570 was estimated by inoculated larvae with either K19-Dpo41 administered at 5 min or 30 min after bacterial infection. Larvae injected with either PBS or 5 × 104 CFUs of K. pneumoniae strain 6570 were included as control groups. After inoculation, caterpillars were kept at 37°C in the dark for 5 days. The results were expressed as the percentage survival rate estimated on the basis of the touch-provoked motility at each day post injection. For each option, at least three independent experiments were performed (10 larvae per trial). The data was presented as the average of three experiments.
Statistics
GraphPad Prism (version 8, GraphPad Software, United States) software was used for the statistical analysis. The data were tabulated as mean ± SD. In the serum resistance analysis, comparisons between any two experimental groups were made by the two-sample Student’s t-test. In the Galleria mellonella larvae infection model, survival curves were plotted using the Kaplan–Meier method, and the survival analysis was performed by using the log-rank Mantel-Cox test. P < 0.05 was considered to be statistically significant.
Ethics Statement
The strain specimens were collected with the written and informed consent of the patients. The conduct and procedures involved in the present work were approved by the Ethics Committee of Shanghai Jiao Tong University School of Medicine.
Results
Antimicrobial Resistance and Capsular Genotypes of K. pneumoniae Strains
All 29 clinical isolates were identified as CRKP and resistant to a variety of antibiotics, including cefuroxime (CXM, 28/29), amikacin (AMK, 15/29), piperacillin-tazobactam (TZP, 28/29), levofloxacin (LVX, 27/29), ciprofloxacin (CIP, 27/29), ceftazidime (CAZ, 29/29), cefoperazone sodium Shubatan (CSL, 27/29), cefepime (FEP, 29/29), Imipenem (IPM, 19/29), meropenem (MEM, 28/29), trimethoprim-sulfamethoxazole (SXT, 17/29), and aztreonam (ATM, 27/29). Twenty-nine K. pneumoniae were genotyped by wzi sequencing. Twenty of them were Type K19, others were K1, K2, K20, K47, K57, and K64 (Table 1).
Bacteriophage SH-KP156570 Is Specific for K19-Type K. Pneumoniae
Klebsiella pneumoniae strain 6570 and the sewage collected from hospital were co-cultured overnight and phage SH-KP156570 was successfully isolated. The phage was spread over K. pneumoniae strain 6570 to form a double-layer agar plate and cleared plaques formations and faint halos were observed. The size of the faint halos increased over time (Figure 1A), which we suspected indicated the presence of a phage-derived depolymerase based on our previous study (Li J. et al., 2021). To understand the relationship between SH-KP156570 and capsular types, we performed a host spectrum analysis of SH-KP156570 on all 29 strains using spot tests. Faint halos and plague were observed over time for all twenty K19-type strains, while absences of plaque or halo were observed for nine non-K19-type strains (Table 1). The results indicated that phage SH-KP156570 targeted K19 CPS exclusively.
FIGURE 1.
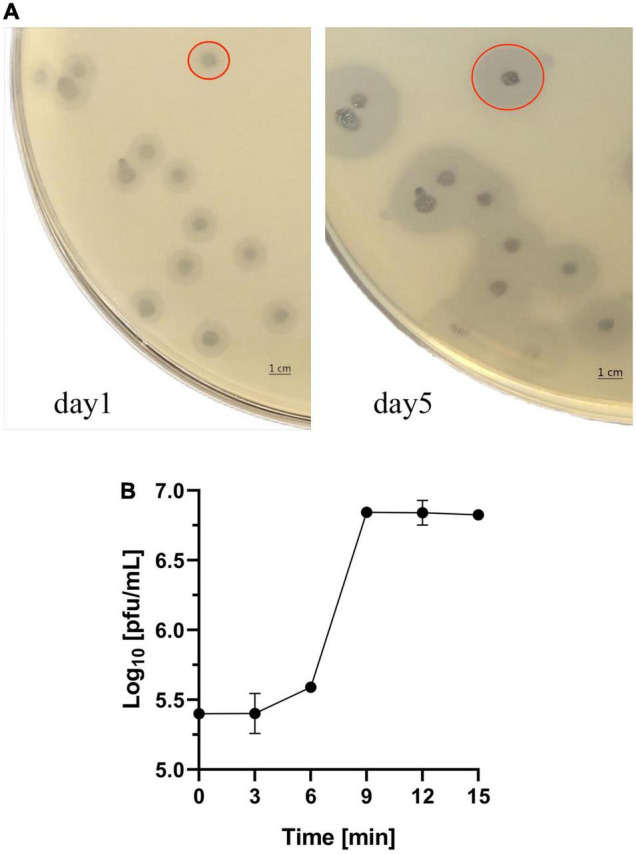
Characterization of Phage SH-KP156570. (A) Interaction between the phage and K. pneumoniae strain 6570. Plagues were formed on the plate. Faint halos could be observed around the plaque on day 1 and the size of the faint halos increased by day 5. The red circle in the picture represented the size of the plaque and halo. (B) One-step growth curve of SH-KP156570. The burst size was calculated as the ratio of the final count of phage particles to the bacterial cells. Phage concentration in PFU/mL as a function of time post-infection was plotted. Error bars represent mean ± SD.
Determination of the Life Cycle of SH-KP156570
The life cycle of SH-KP156570 was revealed with a one-step growth curve. The phage had a short latent period of 6 min followed by a rise period of the phage progeny until a stationary phage was reached at 9 min (Figure 1B). The burst size was about 80 PFU per infected cell.
Genomic DNA Sequencing and Annotation of Phage SH-KP156570
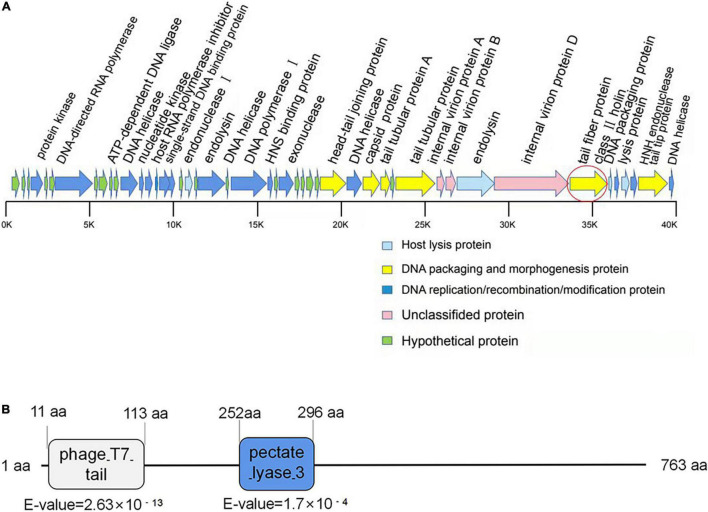
A single contiguous sequence of 38,667 bp was de novo assembled from 8,102,358 raw sequencing reads with more than 30,330 × coverage. Hence, the phage SH-KP156570 had a genome size of 38,667 bp with a GC content of 50.85%. The whole genome sequence of the SH-KP156570 was deposited in National Genomics Data Center3 with the accession number (GWHBGZN00000000). We identified 47 ORFs. Genes encoding putative functional protein were assigned to 29 of the 47 ORFs (61.7%). The average size of these ORFs was 851 bp. Phage-encoded proteins could be divided based on their functions: DNA packaging and morphology-related proteins (6), DNA replication/recombination/modification proteins (16), host lytic proteins (4), and unknown proteins (3). The remaining genes were encoded as hypothetical proteins. Among the 29 functional annotated genes, genes involved in DNA processing and packaging machinery, structural and lytic proteins, DNA replication, DNA recombination, and repair molecular mechanisms were identified. No putative integrase genes, virulence factors, toxins, or antimicrobial resistance genes were found in the genome of SH-KP156570 (Figure 2A).
FIGURE 2.
Bioinformatic analysis of the genome of phage SH-KP156570. (A) Gene map of the phage SH-KP156570. Predicted ORFs were shown. The linear map was based on nucleotide sequences of the whole genome. The red circle denoted ORF41, the gene encoding the putative polysaccharide depolymerase. (B) Bioinformatic analysis of the putative depolymerase of the phage SH-KP156570. The result showed a 763 aa protein with two conserved domains: phage T7 (residue 11–113) and pectate lyase domain (residue 252–296) by using BLASTp and HHpred. ORF41 was determined and identified as protein K19-Dpo41.
Gene ORF41 (2292bp), coding for the tail fiber protein, was predicted to be a depolymerase-encoding gene. The N-terminal conserved domain (11 to 113 aa) of ORF41 protein showed a high similarity with the N-terminal sequence of T7 phage, while the central region (252 to 296 aa) showed a high similarity with the pectate lyase (Figure 2B). This ORF protein contained a β-helical pectin lyase domain, and had a 28% identity to the tail spike protein TSP3 from Escherichia coli phage CBA120 (a lipopolysaccharide lyase of E. coli; Greenfield et al., 2019). These results suggested that the ORF41 protein might be a polysaccharide depolymerase in phage SH-KP156570.
Depolymerization Activity of Recombinant Depolymerase K19-Dpo41
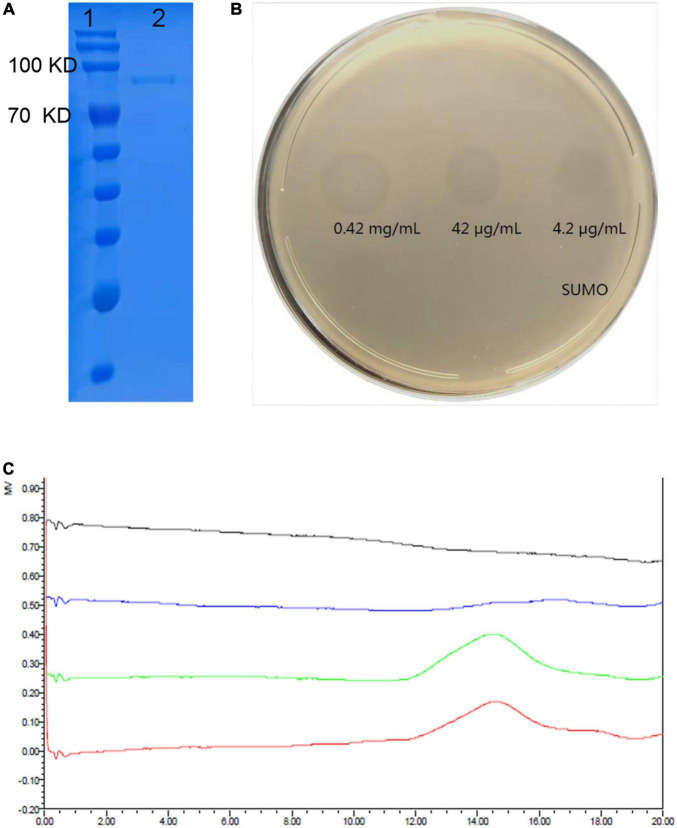
The ORF41 gene of phage SH-KP156570 was cloned into the pSUMO3 expression vector. The recombined K19-ORF41-pSUMO3 was expressed and purified by Ni-NTA column. The purity of the purified K19-Dpo41 (about 84 kDa) was more than 95% confirmed by SDS-PAGE gel analysis (Figure 3A). The purified recombinant depolymerase K19-Dpo41 were diluted to different concentrations (4.2 μg/mL∼0.42 mg/mL) for the spot test to assess the polysaccharide depolymerization activity. Clear halos were observed at different K19-Dpo41 concentrations as low as 4.2 μg/mL (Figure 3B). The SEC-HPLC results confirmed the depolymerization of K19-Dpo41 against the CPS of K. pneumoniae strain 6570. The untreated CPS showed a single peak with a retention time from 12 to 16 min, however, after incubation with K19-Dpo41 at 37°C for 30 min, the peak of CPS disappeared, demonstrating that CPS was degraded by K19-Dpo41 (Figure 3C).
FIGURE 3.
Expression and depolymerization activity of recombinant depolymerase K19-Dpo41. (A) 10% SDS-PAGE gel analysis on the purity of K19-Dpo41. The purity of the purified K19-Dpo41 was more than 95%. Lane 1, protein marker; lane 2, purified K19-Dpo41. (B) Spot test of purified depolymerases K19-Dpo41 on K. pneumoniae strain 6570 lawn. Aliquots of serial dilutions of K19-Dpo41 was spotted onto a plate containing the K. pneumoniae strain 6570. SUMO protein was served as a negative control. (C) Size exclusion chromatography-High performance liquid chromatography (SEC-HPLC) analysis of capsular polysaccharide (CPS) treated with K19-Dpo41. Red line, purified untreated CPS; green line, CPS incubated with 10 μg/mL SUMO at 37°C for 30 min; blue line, CPS treated with 10 μg/mL K19-Dpo41 at 37°C for 30 min; and gray line, K19-Dpo41 incubated at 37°C for 30 min.
The activity of K19-Dpo41 was further tested on all 29 K. pneumoniae strains using the spot assay. On plates incubated with K19-type K. pneumoniae strains, the recombinant protein K19-Dpo41 generated a translucent halo zone (Table 1). Meanwhile, no translucent spots formed on any non-K19-type K. pneumoniae strains as expected. The results showed that depolymerase K19-Dpo41, same as the parental phage SH-KP156570, exhibited specificity to K19-type K. pneumoniae.
K19-Dpo41 Increased the Susceptivity of K. pneumoniae Strain 6570 to Serum Killing
We assessed the bactericidal effect of serum after depolymerase treatment on K. pneumoniae strain 6570. After 1 h of incubation, the survival rate of bacteria treated with K19-Dpo41 decreased by around 70% compared with those of untreated bacteria and bacteria treated with K64-ORF41, a depolymerase specific for K64-type K. pneumoniae, demonstrating that K19-Dpo41 could improve the susceptivity of K19 K. pneumoniae to serum killing (Figure 4A).
FIGURE 4.
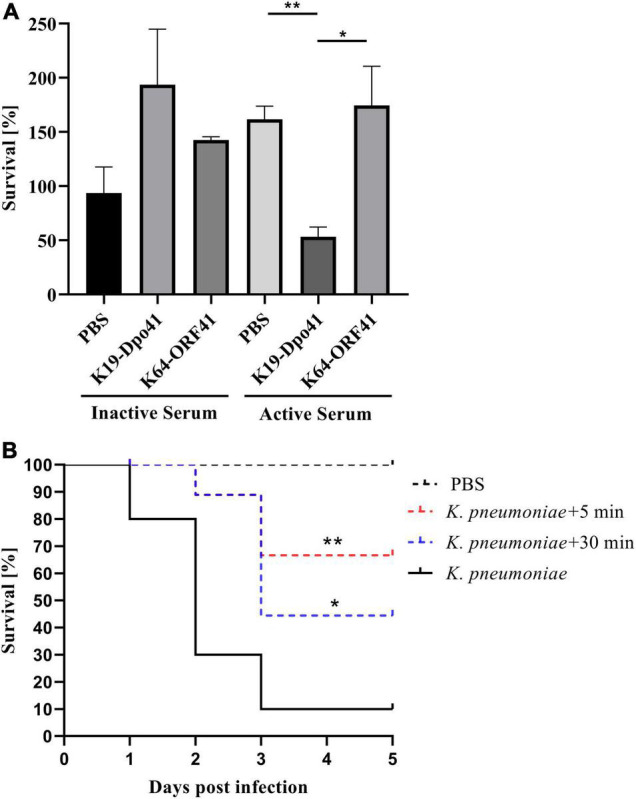
Anti-k. pneumoniae infection effect of K19-Dpo41. (A) Effect of K19-Dpo41 on bacterial susceptibility to serum killing. K. pneumoniae strain 6570 treated with K19-Dpo41 were incubated with active or heat-inactivated human serum obtained from healthy volunteers for 1 h at 37°C. A control group with no K19-Dpo41 was included. A specificity group with K64-ORF41, another depolymerase specifically targeting the CPS of K64-type K. pneumoniae, was also included. The survival rate was defined as the average percent survival of bacteria relative to the initial population. The experiment was repeated three times. Error bars represent mean ± SD. The individual survival rates of each group were compared by Student’s t test. **P < 0.01, *P < 0.05. (B) Effect of K19-Dpo41 on larvae survival rate in a Galleria mellonella larvae infection model. Black solid line, Larvae injected with 5 × 104 CFUs K. pneumoniae strain 6570; red dotted line, K19-Dpo41 (final concentration, 2 μg per larva) administered 5 min after 5 × 104 CFUs K. pneumoniae strain 6570 inoculation; blue dotted line, K19-Dpo41 (final concentration, 2 μg per larva) administered 30 min after 5 × 104 CFUs K. pneumoniae strain 6570 inoculation; black dotted line, Larvae injected with PBS. The mortality were monitored for 5 days (n = 10). Results are the means of three independent experiments. Survival curves were plotted using the Kaplan–Meier method, and differences in survival were calculated by using the log-rank test (GraphPad). **P<0.01 and *p<0.05, mean a significant difference to the K. pneumoniae strain 6570 infected group.
Anti-k. pneumoniae Infection Efficacy of K19-Dpo41 Treated K. pneumoniae in a Galleria mellonella Larvae Infection Model
We also evaluated the anti-k. pneumoniae infection efficacy of K19-Dpo41 using K. pneumoniae infected G. mellonella larvae model. Our results showed that in the K. pneumoniae infection group, 90% of the larvae injected with K. pneumoniae strain 6570 died within 3 day. In contrast, a single dose of K19-Dpo41 given 30 min after bacteria injection increased the survival rate of the larvae to 50%, and the same dose given 5 min reached to 70%. No mortality of larvae was observed in the group injected with PBS.
Discussion
Klebsiella pneumoniae is a clinically significant organism that has proposed a significant amount of threat to public health (Ashurst and Dawson, 2021). The prevalence of CRKP, one of the deadliest strains of K. pneumoniae, is a challenging medical and economical concern that needs to be addressed immediately, especially in countries where infections have become endemic (Karampatakis et al., 2016; Mohammad Ali Tabrizi et al., 2018; Shankar et al., 2018; Effah et al., 2020). Specifically in China, a considerable number of multi-drug resistant K. pneumoniae strains have been isolated and identified from hospitalized patients and local agriculture lands from multiple regions in the past few years (Xu et al., 2016; Yang et al., 2021). Among currently known CRKP strains, K19 serotype was determined as one of the most prevalent K. pneumoniae serotypes in many isolates in previous studies (Volozhantsev et al., 2020; Zhang et al., 2020). It was also found to be strongly associated with the blaKPC–2 antimicrobial resistance gene (Liao et al., 2021). Therefore, the development of a novel, effective therapeutic approach to overcome K19-type K. pneumoniae is urgently needed.
Recently, phage-derived capsular depolymerases that degrade the distinct CPS of K. pneumoniae have been discovered as promising alternatives to treat K. pneumoniae infections. Compared to using phages that directly lyse bacteria, depolymerase-mediated treatments show higher specificity (Pan et al., 2017). They are also found to be safer since phage therapies may result in the development of bacterial resistance to phages and new antimicrobial resistance, which would not be a concern when using depolymerases (Loc-Carrillo and Abedon, 2011; Principi et al., 2019). So far, 34 distinct depolymerases that targeted a range of 22 K. pneumoniae capsular types were discovered (Wu et al., 2019; Liu et al., 2020; Squeglia et al., 2020; Volozhantsev et al., 2020; Dunstan et al., 2021; Gorodnichev et al., 2021; Li J. et al., 2021; Pertics et al., 2021). These depolymerases had great potential for capsular typing of K. pneumoniae, and they were found to be effective in raising the susceptivity of bacteria to serum killing (Pan et al., 2019; Li J. et al., 2021). However, depolymerases distinctly targeting K19-type CRKP, an epidemiologically significant CRKP strain in China, have never been reported. Our study was the first that identified a new phage-derived depolymerase (K19-Dpo41), which specifically degrades the capsule of K19-type K. pneumoniae.
In our previous study, we confirmed that using capsular depolymerase, which directly identified the composition and structure of polysaccharides, could trace the mutations in the CPS synthesis region of the K. pneumoniae genome. It was a more reliable capsular typing method compared to the wzi genotyping (Li J. et al., 2021). The depolymerase K19-Dpo41 we isolated and characterized in this study had high specificity for K19-type K. pneumoniae, which allowed an accurate typing of K19-serotype K. pneumoniae.
The antibacterial effect of K. pneumoniae phage-derived depolymerases made them a potential therapeutic alternative for treating K. pneumoniae infections (Blundell-Hunter et al., 2021; Gorodnichev et al., 2021; Li M. et al., 2021). Unlike phages that directly lysed the host bacteria, depolymerases only cleaved the CPS so that the bacteria became more sensitive to the activities of the surrounding complement and phagocytes, activating the host innate immune responses (Li J. et al., 2021). To assess the anti-virulence effect of K19-Dpo41, we conducted the serum complement-mediated killing assay and used G. Mellonella Larvae infection model to see the depolymerase’s effect on the susceptivity of the K19-type K. pneumoniae strain 6570. The serum-killing assay showed that the survival rate of bacteria pretreated with K19-Dpo41 decreased significantly compared to untreated bacteria or bacteria pretreated with non-specific depolymerase K64-ORF41, a depolymerase that was found not targeting and degrading K19 but instead K64 capsular serotype (Figure 4A). K19-Dpo41 also significantly reduced the mortality of G. mellonella larvae infected with K19-type K. pneumoniae strain 6570 (Figure 4B). These results suggested that K19-Dpo41 could become a promising agent in treating K19-type K. pneumoniae infections.
In conclusion, our study demonstrated that K19-Dpo41, a novel depolymerase derived from phage SH-KP156570, was able to degrade the capsule of K19-type K. pneumoniae, promoted the susceptivity of the bacteria to serum complement lysis, as well as effectively increased the survival rate of G. mellonella larvae in an in vivo infection model. This depolymerase and its enzymatic activity we characterized would surely be found to have beneficial applications relative to the treatment, prevention, and control of severe CRKP infections, and for more accurate, efficient capsular typing.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://ngdc.cncb.ac.cn/gwh, GWHBGZN00000000.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
YH, YW, MG, HL, QL, and PH drafted the manuscript and performed the data analysis. YH, YW, and MG planned and performed experiments. YH, YW, MG, QL, and PH were responsible for experimental design. All authors have read and revised the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant number 81971896) and the Natural Science Foundation of Shanghai Program (grant number 19ZR1428600).
References
- Ashurst J. V., Dawson A. (2021). Klebsiella Pneumonia, StatPearls, StatPearls Publishing. Copyright © 2021. Treasure Island, FL: StatPearls Publishing LLC. [Google Scholar]
- Assoni L., Girardello R., Converso T. R., Darrieux M. (2021). Current stage in the development of Klebsiella pneumoniae vaccines. Infect. Dis. Ther. 10 2157–2175. 10.1007/s40121-021-00533-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich A., Nurk S., Antipov D., Gurevich A. A., Dvorkin M., Kulikov A. S., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19 455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal S., Harjai K., Chhibber S. (2014). Depolymerase improves gentamicin efficacy during Klebsiella pneumoniae induced murine infection. BMC Infect. Dis. 14:456. 10.1186/1471-2334-14-456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake J. D., Cohen F. E. (2001). Pairwise sequence alignment below the twilight zone. J. Mol. Biol. 307 721–735. 10.1006/jmbi.2001.4495 [DOI] [PubMed] [Google Scholar]
- Blundell-Hunter G., Enright M. C., Negus D., Dorman M. J., Beecham G. E., Pickard D. J., et al. (2021). Characterisation of bacteriophage-encoded depolymerases selective for key Klebsiella pneumoniae capsular exopolysaccharides. Front. Cell. Infect. Microbiol. 11:686090. 10.3389/fcimb.2021.686090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisse S., Passet V., Haugaard A. B., Babosan A., Kassis-Chikhani N., Struve C., et al. (2013). wzi Gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J. Clin. Microbiol. 51 4073–4078. 10.1128/jcm.01924-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coil D., Jospin G., Darling A. E. (2015). A5-miseq: an updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics 31 587–589. 10.1093/bioinformatics/btu661 [DOI] [PubMed] [Google Scholar]
- Conesa A., Götz S. (2008). Blast2GO: a comprehensive suite for functional analysis in plant genomics. Int. J. Plant Genomics 2008:619832. 10.1155/2008/619832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea M. M., Marmo P., Henrici De Angelis L., Palmieri M., Ciacci N., Di Lallo G., et al. (2017). φBO1E, a newly discovered lytic bacteriophage targeting carbapenemase-producing Klebsiella pneumoniae of the pandemic Clonal Group 258 clade II lineage. Sci. Rep. 7:2614. 10.1038/s41598-017-02788-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan R. A., Bamert R. S., Belousoff M. J., Short F. L., Barlow C. K., Pickard D. J., et al. (2021). Mechanistic insights into the capsule-targeting depolymerase from a Klebsiella pneumoniae bacteriophage. Microbiol. Spectr. 9:e0102321. 10.1128/Spectrum.01023-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effah C. Y., Sun T., Liu S., Wu Y. (2020). Klebsiella pneumoniae: an increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 19:1. 10.1186/s12941-019-0343-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodnichev R. B., Volozhantsev N. V., Krasilnikova V. M., Bodoev I. N., Kornienko M. A., Kuptsov N. S., et al. (2021). Novel Klebsiella pneumoniae K23-specific bacteriophages from different families: similarity of depolymerases and their therapeutic potential. Front. Microbiol. 12:669618. 10.3389/fmicb.2021.669618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield J., Shang X., Luo H., Zhou Y., Heselpoth R. D., Nelson D. C., et al. (2019). Structure and tailspike glycosidase machinery of ORF212 from E. coli O157:H7 phage CBA120 (TSP3). Sci. Rep. 9:7349. 10.1038/s41598-019-43748-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Huang J., Yan G., Lei L., Wang S., Yu L., et al. (2017). Identification and characterization of Dpo42, a novel depolymerase derived from the Escherichia coli phage vB_EcoM_ECOO78. Front. Microbiol. 8:1460. 10.3389/fmicb.2017.01460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karampatakis T., Antachopoulos C., Iosifidis E., Tsakris A., Roilides E. (2016). Molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in Greece. Future Microbiol. 11 809–823. 10.2217/fmb-2016-0042 [DOI] [PubMed] [Google Scholar]
- Khan Mirzaei M., Nilsson A. S. (2015). Isolation of phages for phage therapy: a comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS One 10:e0118557. 10.1371/journal.pone.0118557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y.-C., Lu M.-C., Hsueh P.-R. (2019). Hypervirulence and carbapenem resistance: two distinct evolutionary directions that led high-risk clones to epidemic success. Expert Rev. Mol. Diagn. 19 825–837. 10.1080/14737159.2019.1649145 [DOI] [PubMed] [Google Scholar]
- Lam M. M. C., Wick R. R., Judd L. M., Holt K. E., Wyres K. L. (2022). Kaptive 2.0: updated capsule and lipopolysaccharide locus typing for the Klebsiella pneumoniae species complex. Microb. Genom. 8. 10.1099/mgen.0.000800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Sheng Y., Ma R., Xu M., Liu F., Qin R., et al. (2021). Identification of a depolymerase specific for K64-serotype Klebsiella pneumoniae: potential applications in capsular typing and treatment. Antibiotics 10:144. 10.3390/antibiotics10020144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Li P., Chen L., Guo G., Xiao Y., Chen L., et al. (2021). Identification of a phage-derived depolymerase specific for KL64 capsule of Klebsiella pneumoniae and its anti-biofilm effect. Virus Genes 57 434–442. 10.1007/s11262-021-01847-8 [DOI] [PubMed] [Google Scholar]
- Liao W., Huang N., Zhang Y., Sun Y., Chen T., Zeng W., et al. (2021). Comparison of carbapenem-resistant Klebsiella pneumoniae strains causing intestinal colonization and extraintestinal infections: clinical, virulence, and molecular epidemiological characteristics. Front. Public Health 9:783124. 10.3389/fpubh.2021.783124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgreen S. (2012). AdapterRemoval: easy cleaning of next-generation sequencing reads. BMC Res. Notes 5:337. 10.1186/1756-0500-5-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Leung S. S. Y., Huang Y., Guo Y., Jiang N., Li P., et al. (2020). Identification of two depolymerases from phage IME205 and their antivirulent functions on K47 capsule of Klebsiella pneumoniae. Front. Microbiol. 11:218. 10.3389/fmicb.2020.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loc-Carrillo C., Abedon S. T. (2011). Pros and cons of phage therapy. Bacteriophage 1 111–114. 10.4161/bact.1.2.14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R., Liu B., Xie Y., Li Z., Huang W., Yuan J., et al. (2012). SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience 1:18. 10.1186/2047-217x-1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkowska-Skrobek G., Łątka A., Berisio R., Maciejewska B., Squeglia F., Romano M., et al. (2016). Capsule-targeting depolymerase, derived from Klebsiella KP36 phage, as a tool for the development of anti-virulent strategy. Viruses 8:324. 10.3390/v8120324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkowska-Skrobek G., Latka A., Berisio R., Squeglia F., Maciejewska B., Briers Y., et al. (2018). Phage-borne depolymerases decrease Klebsiella pneumoniae resistance to innate defense mechanisms. Front. Microbiol. 9:2517. 10.3389/fmicb.2018.02517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad Ali Tabrizi A., Badmasti F., Shahcheraghi F., Azizi O. (2018). Outbreak of hypervirulent Klebsiella pneumoniae harbouring bla(VIM-2) among mechanically-ventilated drug-poisoning patients with high mortality rate in Iran. J. Glob. Antimicrob. Resist. 15 93–98. 10.1016/j.jgar.2018.06.020 [DOI] [PubMed] [Google Scholar]
- Moriya Y., Itoh M., Okuda S., Yoshizawa A. C., Kanehisa M. (2007). KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 35 W182–W185. 10.1093/nar/gkm321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira H., Mendes A., Fraga A. G., Ferreira A., Pimenta A. I., Mil-Homens D., et al. (2019). K2 capsule depolymerase is highly stable, is refractory to resistance, and protects larvae and mice from Acinetobacter baumannii sepsis. Appl. Environ. Microbiol. 85:e00934-19. 10.1128/aem.00934-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opoku-Temeng C., Kobayashi S. D., DeLeo F. R. (2019). Klebsiella pneumoniae capsule polysaccharide as a target for therapeutics and vaccines. Comput. Struct. Biotechnol. J. 17 1360–1366. 10.1016/j.csbj.2019.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paczosa M. K., Mecsas J. (2016). Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. 80 629–661. 10.1128/mmbr.00078-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. J., Lin T. L., Chen C. C., Tsai Y. T., Cheng Y. H., Chen Y. Y., et al. (2017). Klebsiella phage PhiK64-1 encodes multiple depolymerases for multiple host capsular types. J. Virol. 91:e02457-16. 10.1128/JVI.02457-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. J., Lin T. L., Chen Y. Y., Lai P. H., Tsai Y. T., Hsu C. R., et al. (2019). Identification of three podoviruses infecting Klebsiella encoding capsule depolymerases that digest specific capsular types. Microb. Biotechnol. 12 472–486. 10.1111/1751-7915.13370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertics B. Z., Cox A., Nyúl A., Szamek N., Kovács T., Schneider G. (2021). Isolation and characterization of a novel lytic bacteriophage against the K2 capsule-expressing hypervirulent Klebsiella pneumoniae strain 52145, and identification of its functional depolymerase. Microorganisms 9:650. 10.3390/microorganisms9030650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires D., Sillankorva S., Faustino A., Azeredo J. (2011). Use of newly isolated phages for control of Pseudomonas aeruginosa PAO1 and ATCC 10145 biofilms. Res. Microbiol. 162 798–806. 10.1016/j.resmic.2011.06.010 [DOI] [PubMed] [Google Scholar]
- Powell S., Forslund K., Szklarczyk D., Trachana K., Roth A., Huerta-Cepas J., et al. (2014). eggNOG v4.0: nested orthology inference across 3686 organisms. Nucleic Acids Res. 42 D231–D239. 10.1093/nar/gkt1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Principi N., Silvestri E., Esposito S. (2019). Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front. Pharmacol. 10:513. 10.3389/fphar.2019.00513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahed-Al-Mahmud M., Roy R., Sugiokto F. G., Islam M. N., Lin M. D., Lin L. C., et al. (2021). Phage φAB6-borne depolymerase combats Acinetobacter baumannii Biofilm Formation and Infection. Antibiotics 10:279. 10.3390/antibiotics10030279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar C., Nabarro L. E., Anandan S., Ravi R., Babu P., Munusamy E., et al. (2018). Extremely high mortality rates in patients with carbapenem-resistant, hypermucoviscous Klebsiella pneumoniae blood stream infections. J. Assoc. Physicians India 66 13–16. [PubMed] [Google Scholar]
- Squeglia F., Maciejewska B., Łątka A., Ruggiero A., Briers Y., Drulis-Kawa Z., et al. (2020). Structural and functional studies of a Klebsiella phage capsule depolymerase tailspike: mechanistic insights into capsular degradation. Structure 28 613–624.e4. 10.1016/j.str.2020.04.015 [DOI] [PubMed] [Google Scholar]
- Stothard P., Wishart D. S. (2005). Circular genome visualization and exploration using CGView. Bioinformatics 21 537–539. 10.1093/bioinformatics/bti054 [DOI] [PubMed] [Google Scholar]
- Volozhantsev V. N., Shpirt M. A., Borzilov I. A., Komisarova V. E., Krasilnikova M. V., Shashkov S. A., et al. (2020). Characterization and therapeutic potential of bacteriophage-encoded polysaccharide depolymerases with β galactosidase activity against Klebsiella pneumoniae K57 capsular type. Antibiotics 9:732. 10.3390/antibiotics9110732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Zhao G., Chao X., Xie L., Wang H. (2020). The characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. Int. J. Environ. Res. Public Health 17:6278. 10.3390/ijerph17176278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Wang R., Xu M., Liu Y., Zhu X., Qiu J., et al. (2019). A novel polysaccharide depolymerase encoded by the phage SH-KP152226 confers specific activity against multidrug-resistant Klebsiella pneumoniae via biofilm degradation. Front. Microbiol. 10:2768. 10.3389/fmicb.2019.02768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Zhou Y., Zhai X., Du Z., Wu H., Han Y., et al. (2016). Emergence and characterization of tigecycline resistance in multidrug-resistant Klebsiella pneumoniae isolates from blood samples of patients in intensive care units in northern China. J. Med. Microbiol. 65 751–759. 10.1099/jmm.0.000299 [DOI] [PubMed] [Google Scholar]
- Yang Y., Peng Y., Jiang J., Gong Z., Zhu H., Wang K., et al. (2021). Isolation and characterization of multidrug-resistant Klebsiella pneumoniae from raw cow milk in Jiangsu and Shandong provinces, China. Transbound. Emerg. Dis. 68 1033–1039. 10.1111/tbed.13787 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Sun J., Mi C., Li W., Zhao S., Wang Q., et al. (2015). First report of two rapid-onset fatal infections caused by a newly emerging hypervirulent K. pneumonia ST86 strain of serotype K2 in China. Front. Microbiol. 6:721. 10.3389/fmicb.2015.00721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. Y., Qin R., Lu Y. H., Shen J., Zhang S. Y., Wang C. Y., et al. (2020). Capsular polysaccharide and lipopolysaccharide O type analysis of Klebsiella pneumoniae isolates by genotype in China. Epidemiol. Infect. 148:e191. 10.1017/s0950268820001788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K., Lokate M., Deurenberg R. H., Tepper M., Arends J. P., Raangs E. G. C., et al. (2016). Use of whole-genome sequencing to trace, control and characterize the regional expansion of extended-spectrum β-lactamase producing ST15 Klebsiella pneumoniae. Sci. Rep. 6:20840. 10.1038/srep20840 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://ngdc.cncb.ac.cn/gwh, GWHBGZN00000000.