Abstract
The aim was to assess the incremental costs of chimeric antigen receptor (CAR) T-cell therapy (axicabtagene ciloleucel, tisagenlecleucel) compared with standard of care in adult patients with relapsed or refractory diffuse large B-cell lymphoma (r/r DLBCL) from the German third-party payer perspective. A budget impact model was established over a 6-year period. Estimation of the third-line population: partitioned survival model based on outcome data from peer-reviewed literature, a top-down approach based on population forecasts, and age-standardized incidences. Cost data were derived from the controlling department of a tertiary hospital and a German cost-of-illness study. In the scenario analysis, the budget impact of treating second-line DLBCL patients was calculated. One-way deterministic sensitivity analyses were conducted to test the robustness of the model. For the period 2021-2026, 788-867 (minimum population, min) and 1,068-1,177 (maximum population, max) adult third-line r/r DLBCL patients were estimated. The budget impact ranged from €39,419,562; €53,426,514 (min; max) in year 0 to €122,104,097; €165,763,001 (min; max) in year 5. The scenario analysis resulted in a budget impact of €65,987,823; €89,558,611 (min; max) and €204,485,031; €277,567,601 (min; max) for years 0 and 5, respectively. This budget impact analysis showed a significant but reasonable financial burden associated with CAR T-cell therapy for a limited number of patients requiring individualized care. Further, this study presents challenges and future needs in data acquisition associated with cost analysis in personalized medicine. For comprehensive economic discussions, complementary cost-effectiveness analyses are required to determine the value of innovative therapies for r/r DLBCL.
INTRODUCTION
In the last 40 years, there has been an increase in malignant neoplasms of lymphoid hematopoietic and related tissues (C81-C96) among both children and adults in Germany.1 Especially, since 2001, incident cases of non-Hodgkin lymphoma (NHL) have risen by 16.9% and 35.6% for women and men, respectively.2 Among NHL cases, diffuse large B-cell lymphoma (DLBCL) is the most common subtype, with an age-standardized incidence rate of 7 per 100,000 in Europe and the United States.3–5 As a first-line therapy, rituximab-based chemotherapy (R-CHOP), which is curative for 60%–70% of patients, is suggested.6,7 In cases of relapsed or refractory disease (r/r), salvage chemotherapy followed by high-dose (chemo) therapy (HDT) and autologous stem-cell transplantation (ASCT) is recommended.6,8 Transplant-ineligible patients and those who relapse after ASCT have a poor prognosis and limited treatment options.9,10
Since 2018, 2 chimeric antigen receptor (CAR) T-cell therapies, namely tisagenlecleucel (tisa-cel; Kymriah) and axicabtagene Ciloleucel (axi-cel; Yescarta), have been approved. They provide a new therapeutic approach for patients with r/r DLBCL and acute lymphoblastic leukemia (r/r ALL) after ≥2 lines of therapy.11 After extracting and reproducing the patient’s T cells in a laboratory, CARs are integrated into the T-cell membrane and reinfused as CAR T-cells into the patient’s body. Upon binding to the antigen CD19+ at the surface of malignant cells, a specific immune reaction can be induced, activating a cytotoxic mechanism to destroy the CD19+ cancer cells.12 In both pivotal studies, treatment with tisacel (JULIET) and axicel (ZUMA-1) resulted in overall response rates (ORR) of 52% and 83%, respectively, whereas patients treated with conventional therapy (SCHOLAR-1) achieved an ORR of 26%.9,13,14
A 2-year follow-up reported an ORR and CR of 83% and 54% in ZUMA-1 versus 34% and 12% for SOC in SCHOLAR-1. In addition, the 2-year survival rate was 54% for CAR T-cell therapy compared with 20% for SOC indicating an improvement in clinical outcomes for patients treated with CAR T-cells.15
Despite promising clinical outcomes, CAR T-cell therapies are associated with high costs. The reimbursement of axi-cel and tisa-cel for a single infusion amounts to €282,000 and €275,000 in Germany, respectively.16 However, this sum does not include additional costs incurred for other aspects, for example, hospitalization and managing adverse events (AEs). Several cost-effectiveness analyses (CEAs) for treating r/r DLBCL patients with tisa-cel and axi-cel have been conducted to assess their economic value in the United States. Results indicated that CAR T-cell therapies seemed to be mostly cost-effective and did not exceed the willingness-to-pay threshold at a certain probability. However, these findings were highly dependent on the chosen time period and clinical outcomes, such as long-term remission and survival of CAR T patients.17–19
Budget impact analyses (BIA) take on a complementary role to CEAs20 by outlining how the impact on the payer’s budget will change if a new intervention is added to the current mix of treatments and then distributed in routine care.20,21 As personalized therapies such as CAR T-cell therapies are initially associated with significant costs, questions on the future economic impact of innovative therapies for payers are increasingly prominent. A corresponding BIA will provide a clearer idea of the potential economic burden for third-party payers in Germany. Peer-reviewed literature on BIAs is limited, and no analysis on the financial burden of CAR T-cells in German statutory health insurance has been published so far.
The objective of this study was to estimate the incremental costs of treating adult r/r DLBCL patients with axi-cel and tisa-cel in an inpatient setting in comparison to the standard of care (SOC) from the German statutory health insurance perspective. Specifically, the development of the proportion of r/r DLBCL patients treated with CAR T-cells over a 6-year time period was investigated.
METHODS
Model design
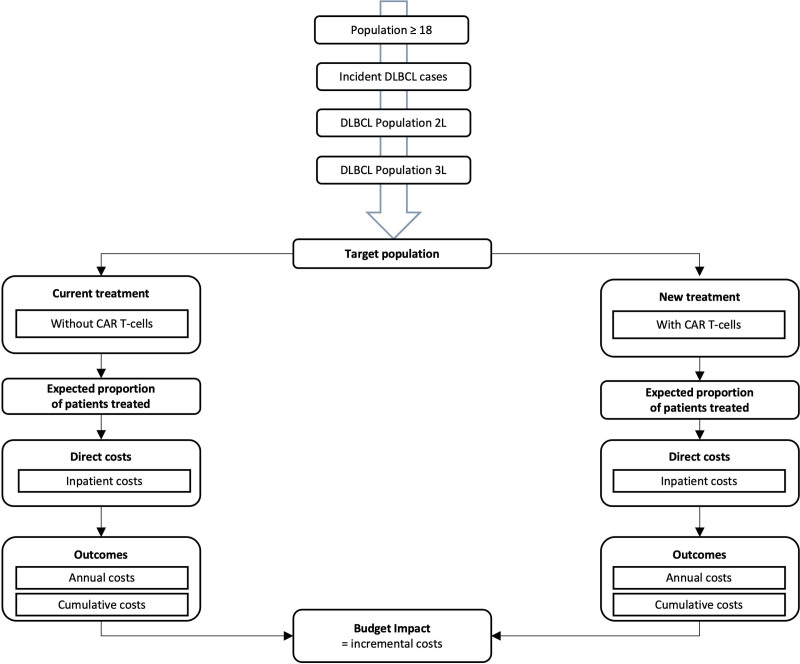
A budget impact model and a 3-state partitioned survival model were used to assess the relevant target population. Inpatient costs of the 2 treatment strategies were compared: standard therapy in the third-line and CAR T-cell treatment. The time period included a baseline year (2021; year 0) and 5 subsequent years (2022–2026; years 1–5) with annual cycles. This BIA was developed according to the International Society for Pharmacoeconomics and Outcomes Research’s (ISPOR) principles of good practices for budget impact analysis.20,22 Additionally, national methodological recommendations provided by the Institute for Quality and Efficiency in Health Care (IQWiG) were considered.21 The resulting model structure is presented in Figure 1. All calculations were performed using Microsoft Excel 2016.
Figure 1.
Budget impact model structure of r/r DLBCL patients treated with CAR T-cells over a 6-y time horizon from the perspective of the German statutory health insurance. 2L = Second-line; 3L = Third-line; cumulative costs = aggregated costs over the 6-y time horizon; CAR = chimeric antigen receptor; r/r DLBCL = relapsed or refractory diffuse large B-cell lymphoma.
Model parameters
Target population
Adult patients (≥18 y) diagnosed with DLBCL (ICD-10: C83.3) who had been treated with at least 2 systemic therapies were included in the model.6 In this analysis, the eligible population was a yearly cohort estimated using an epidemiological top-down approach.
First-line patient population
For the assessment of German incident DLBCL cases per year, the annual German population was identified for the respective time period (6 y) based on general population forecasts by the Federal Statistical Office of Germany (Destatis).23 Therefore, a moderate development of fertility, life expectancy, and migration (G2-L2-W2) was assumed.23 The latest DLBCL age-standardized incidence rate (2017) was extracted from the German Centre for Cancer Registry Data (ZfKD) at the Robert Koch-Institute (RKI) (Table 1). A growing incidence rate per year was assumed due to an increase in German age-standardized incidence rates for DLBCL in recent years.5 Therefore, an average annual growth rate of 2% was calculated based on the reported age-standardized incidence rates from 2009 to 2017 (Table 1).
Table 1.
Model Input
| Parameter | Value | Sources |
|---|---|---|
| Epidemiology | ||
| Standardized incidence rate (per 100,000) | 7.4 | 5 |
| Average annual growth rate (for incidence rate) | 2% | [5] a |
| Statutory insurance coverage | 88% | [24] |
| Treatment and survival | ||
| Cure rate first-line | 60% – 70% | [6] |
| ASCT eligible | 50% | [25] |
| Salvage ORR | 63% | [26] |
| Salvage PFS | 50% | [26] |
| Salvage OS | 71% | [26] |
| ASCT rate | 35% | [27] |
| ASCT ORR | 70.50% | [27] |
| ASCT PFS | 65.50% | [28] |
| ASCT OS | 84% | [28] |
| Salvage ORR (transplant ineligible) | 61% | [10] |
| Salvage PFS (transplant ineligible) | 26% | [10] |
| Salvage OS (transplant ineligible) | 49% | [10] |
| Early mortality conventional chemotherapy | 2% | Based on experts |
| Early mortality ASCT | 5% | [25] b |
| Unit costs (mean values) in € | ||
| Tisagenlecleucel | 345,485 | LMU Hospital controllinga |
| Axicabtagene ciloleucel | 373,324 | LMU Hospital controllinga |
| Standard therapy second-line | 44,750 | [29] |
| Standard therapy third-line | 56,224 | [29] |
| Market share | ||
| Proportion treated with CAR T-cells (baseline) | 16.50% | [30] |
| Average annual growth rate | 23% | [31] c |
aOwn calculation based on data in “Sources.”
bConfirmed by experts.
cEstimation of the growth rate based on German CAR T-cell market projections.
ASCT = autologous stem-cell transplantation; CAR = chimeric antigen receptor; ORR = overall response rate; OS = overall survival; PFS = progression-free survival.
Second-line patient population
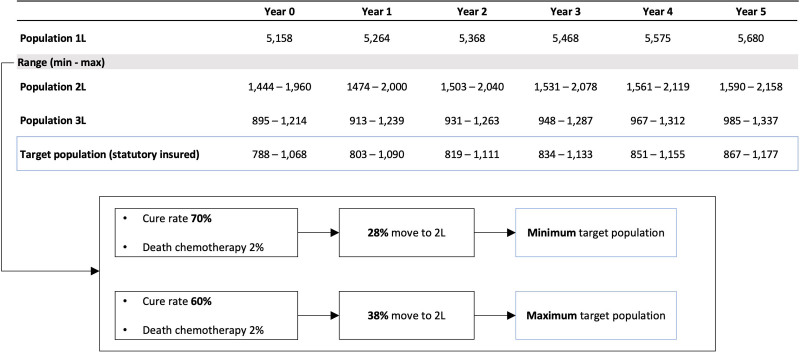
Considering a cure rate of 60%–70% in first-line patients and an early mortality rate of 2% after conventional chemotherapy, 28% (minimum population, min), to 38% (maximum population, max) of incident cases were eligible patients for second-line therapy (Table 1).
Third-line patient population
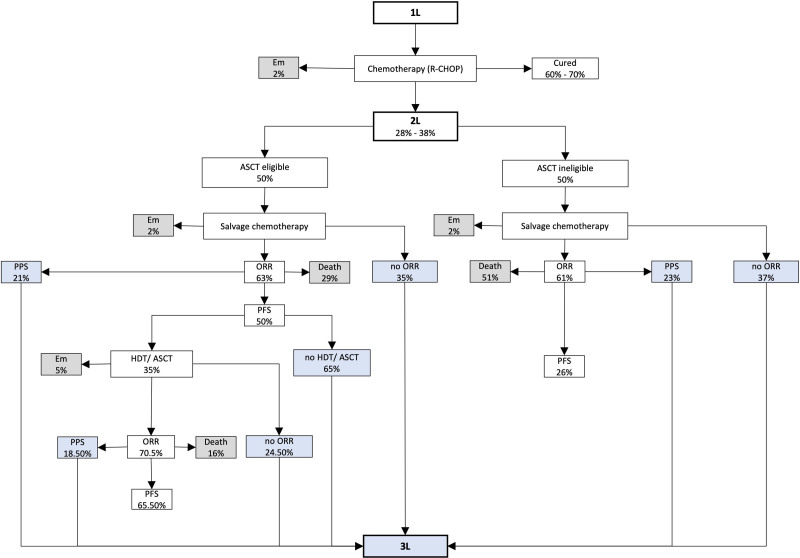
To gather information on clinical outcomes, for example, response rates and survival probabilities in second-line standard therapy, a literature search in PubMed with the following MeSH terms was conducted: “lymphoma, large B-cell, diffuse,” “adult,” “adolescent,” “stem cell transplantation*,” “transplantation, autologous,” “salvage therapy*,” “oxaliplatin,” “overall survival,” and “progression-free survival.” Table 1 presents the clinical parameters that were extracted from the CORAL and ORCHARRD studies, as well as additional references that were considered for transplant-ineligible patients. These input variables were embedded in a simplified therapy algorithm (Figure 2) based on the German DLBCL guideline.6 With each treatment option (chemotherapy and HDT/ASCT, if eligible), the proportion of patients with overall responses was determined. Additionally, the percentage of patients who died after stem-cell transplantation (SCT) (5%) was considered in the calculation (Table 1). To assess the number of third-line patients by means of 1-year survival probabilities, a 3-state partitioned survival model was used. The number of surviving patients with progressive disease or relapse was estimated by post-progression survival (PPS). PPS results from the difference between overall survival (OS) and progression-free survival (PFS). Patients in the death state were estimated using OS probability. Candidates for the third-line were patients with no initial response after treatment, patients with progressive disease, and transplant-eligible patients who did not respond to SCT. According to the chosen cost perspective, the percentage of people covered by statutory health insurance (88%) was applied to the eligible r/r DLBCL population (Table 1).
Figure 2.
Therapy algorithm for the calculation of the third-line DLBCL population. 1L = First-line; 2L = Second-line; 3L = Third-line ASCT = autologous stem-cell transplantation; DLBCL = diffuse large B-cell lymphoma; Em = Early mortality; HDT = high-dose (chemo) therapy ORR = overall response rate; PFS = progression-free survival PPS = postprogression survival; R-CHOP = Rituximab—cyclophosphamide, doxorubicin, vincristine, prednisolone.
Treatment regimens
Standard therapy for third-line patients consisted of chemotherapy, allogeneic SCT, and ASCT. The choice of current treatment patterns was based on a single-center retrospective study of r/r DLBCL patients conducted at the LMU Hospital.29 The new intervention included 2 approved CD19+ CAR T-cell therapies for r/r DLBCL, namely axicabtagene Ciloleucel and tisagenlecleucel.
Market share
CAR T-cell therapy was supposed to replace the current treatment, and the proportion of patients treated with CAR T-cells was assumed to increase over time. Within the CAR T therapy group, the annual distribution of axi-cel and tisa-cel was constant at 50% each, as no specific preferences regarding treatment with 1 of the 2 CAR T-cell therapies were assumed. The proportion of patients treated with CAR T-cells at baseline was assumed to be 16.5% (Table 1). For the following 5 years, an average annual growth rate of 23% was applied (Table 1). The percentages of patients treated with CAR T-cells were 20%, 25%, 31%, 38%, and 47% for the years 2022–2026, respectively.
Costs
In this analysis, only inpatient costs were considered. CAR T-cell therapy is intended for exclusive use in qualified clinical facilities, which is assumed to be in inpatient settings.32,33 Charges for inpatient treatment with CAR T-cells and SOC were derived from codes for diagnosis related groups (DRG), new diagnostic and treatment methods regulation (NUB), and “Zusatzentgelte” (ZE) from the Medical Controlling at the LMU Hospital (aG-DRG billing).
The mean costs for standard therapy were selected from a single-center retrospective cost study assessing the costs of DLBCL patients treated with ≥third-line standard care (Table 1). Costs for CAR T-cell therapy were based on administrative hospital claims data (Table 1). Patients were analyzed from the leukapheresis up to the end of the CAR T associated inpatient stay at the department. The mean inpatient stay was 22 days post CAR T-cell retransfusion. The cutoff points were discharge (eg, relocation to another hospital); getting further DLBCL-treatment unrelated to CAR T-cell therapy. Costs per patient were separately assessed for treatment with axi-cel and tisa-cel. The mean costs per patient for SOC and CAR T-cell therapy were multiplied by the respective calculated share of the patient population. The resulting costs per year were considered to be independent of each other.
Further, the costs of tocilizumab were more precisely assessed for the subsequent sensitivity analysis as tocilizumab served as a proxy for the costs of managing possible AEs of CAR T-cell therapies. In addition, no discounting was applied as this BIA was conducted over a short period of time with no means to determine the net present value of the budget impact.20,22 Table 1 presents the mean costs per patient for axi-cel and tisa-cel.
Model output
Relevant outcomes were the incremental costs of CAR T-cell therapies compared with those of standard treatment. The budget impact was assessed annually and cumulatively (aggregated over a 6-y period).
Analyses
Base case analysis
The budget impact of r/r DLBCL third-line patients treated with CAR T-cells represented the base case.
Scenario analysis
In the scenario analysis, the budget impact of CAR T-cell therapy in second-line patients was assessed. Even though treatment with CAR T-cells is currently approved for ≥third-line, there are ongoing phase III trials testing the efficacy of axi-cel and tisa-cel in patients relapsing after first-line therapy.34,35 The target population consisted of second-line DLBCL patients covered by statutory health insurance. Salvage chemotherapy, HDT, and ASCT are the standard treatments according to the German DLBCL guidelines.6 For the estimation of patients treated with CAR T-cells, the same market share at baseline (16.5%) and average annual growth rate (23%) were applied equal to the base case (Table 1). The mean costs for standard therapy were extracted from the retrospective cost study for second-line DLBCL patients (Table 1). The cost of CAR T-cell treatment remained equal to the base case values.
Sensitivity analysis
A 1-way deterministic sensitivity analysis was conducted to test the robustness of the model by varying the following model parameters: the proportion of patients treated with CAR T-cells at baseline (market share at year 0), the corresponding average annual growth rate for the market share, and costs for both CAR T-cell therapies, standard therapy for third-line patients, and tocilizumab for axi-cel and tisa-cel, respectively. An increase and decrease of values by 20%, according to standard modelling practices, were applied. The variation of population parameters, such as clinical outcomes, was not included, as uncertainty has already been presented by specifying a minimum and maximum population. The impact of the parameter variation was illustrated in the form of a tornado diagram. Relevant outcomes of the sensitivity analyses were the cumulative budget impacts (min, max).
RESULTS
Base case analysis
Target population
In first-line therapy, 5,158–5,680 patients with newly diagnosed DLBCL were treated during the period 2021–2026. The numbers of patients in second-line therapy were estimated to be 1,444–1,590 (min) and 1,960–2,158 (max). The calculated third-line target population amounted to 788–867 (min) and 1,068–1,177 (max). Figure 3 presents a top-down overview of the annual population size from the first to the third-line. For detailed information on the top-down approach and the calculation of the third-line population, see the Suppl. Appendix. During the period 2021–2026, the numbers of patients treated with CAR T-cells increased from 130 to 402 (min) and from 176 to 546 (max), respectively.
Figure 3.
Top-down calculation of the DLBCL target population. 1L = First-line, 2L = Second-line; 3L = Third-line; DLBCL = diffuse large B-cell lymphoma.
Budget impact
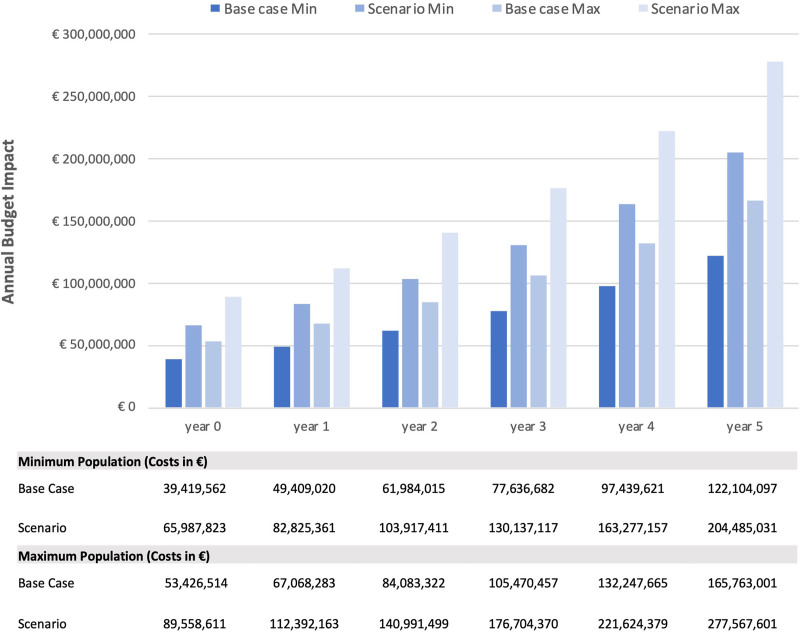
At baseline, the total cost of CAR T-cell therapy was €83,724,074; €113,473,746 (min; max) compared with €44,304,512; €60,047,232 (min; max) when treated with standard therapy. At year 5, the total cost of CAR T-cell therapy was €170,850,035; €231,938,649 (min; max) compared with €48,746,208; €66,175,648 (min; max) without the introduction of CAR T-cells (Suppl. Appendix). The budget impact ranged from €39,419,562, €53,426,514 (min; max) in 2021 to €122,104,097; €165,763,001 (min; max) in 2026 (Figure 4). The cumulative budget impact for CAR T-cell therapy was €447,992,998; €608,059,242 (min; max) over the 6-year time period.
Figure 4.
Annual budget impact in base case and scenario analysis for the minimum and maximum population.
Scenario analysis
The eligible second-line patient population covered by the statutory health insurance was estimated to be 1,271–1,399 (min) and 1,725–1,899 (max) during the period 2021–2026. Considering the resulting market uptake, 210–650 (min) and 284–882 (max) patients were treated with CAR T-cells in the respective time period. The total cost of introducing CAR T- cells ranged from €122,865,073; €166,752,361 (min; max) at baseline to €267,090,281; €362,547,851 (min; max) in year 5 (Suppl. Appendix). The increased population resulted in a higher annual budget impact from €65,987,823; €89,558,611 (min; max) in year 0 to €204,485,031; €277,567,601 (min; max) in year 5 (Figure 4). The scenario analysis reported a 68% higher cumulative budget impact of €750,629,900; €1,018,838,624 (min; max) compared with the base case.
Sensitivity analysis
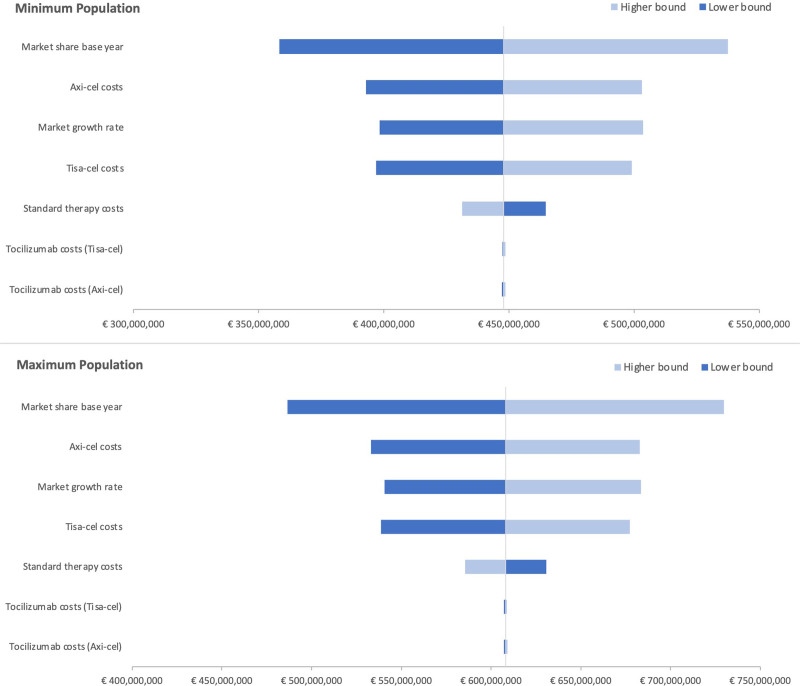
Deterministic sensitivity analyses showed that the cumulative budget impact was most sensitive to the variation in market share at baseline, average growth rate of the market share, and costs of axi-cel and tisa-cel. It remained robust in the case of variation in tocilizumab costs. The variation and outcomes for each parameter are presented in a tornado diagram in Figure 5 for the minimum and maximum target populations.
Figure 5.
Tornado diagram of the minimum and maximum population with cumulative budget impact as outcome parameter.
DISCUSSION
In this study, the budget impact of treating third-line r/r DLBCL patients with CAR T-cells compared with the SOC in an inpatient setting was assessed from the third-party payer perspective. Thus far, no budget impact analysis from the German statutory health insurance perspective has been published based on real-life inpatient cost data of CAR T-cell therapies. The number of DLBCL patients in third-line therapy eligible for CAR T-cell therapy was estimated at 788–867 (min) and 1,068–1,177 (max) in years 0 and 5. The incremental costs for CAR T-cell therapy ranged from €39 to €53 million (min; max) in 2021 and from €122 to €166 million (min; max) in 2026. The cumulative budget impact over the 6-year time period was €448 million; €608 million (min; max).
The annual incremental costs in years 0 and 5 amounted to 0.7%; 1% (min; max) and 2.3%; 3.1% (min; max), respectively, of the German statutory health insurance expenses in 2019 on antineoplastic agents (approximately €5.3 billion).36
Compared with other budget impacts in Germany, treatment of r/r multiple myeloma patients with 3 intravenous (carfilzomib, lenalidomide, dexamethasone [KRd]; elotuzumab, lenalidomide, dexamethasone [ERd]; daratumumab, lenalidomide, dexamethasone [DRd]) and 1 oral therapy (ixazomib, lenalidomide, dexamethasone [IRd]) indicated 1-year budget impacts of €551, €163, €584, and €95 million, respectively. Although therapy costs are not as high as for CAR T-cells, these results clearly exceed our base case budget impact of €39 million for the first year.37
Incremental costs of alternative therapies should be interpreted in the context of the patient population size, the chosen SOC, treatment settings, and lines of therapy. Further options for comparison are limited because only a few BIAs are published for Germany.
From an international point of view, only a limited number of BIAs for CAR T-cell therapies have been published. The existing literature poses a challenge in terms of interpretation as well as the comparability of results owing to the various approaches employed for model structure and methodology across different studies.
The economic report of the Canadian Agency for Drugs and Technologies in Health (CADTH) reported a 3-year cumulative budget impact of $387 million for introducing tisa-cel into routine care, presenting a similar cost dimension, although our analysis was carried out over a 6-year time period.38 In an economic analysis of CAR T-cell therapies for the treatment of hematological cancers in the former EU-5 (France, Germany, Spain, Italy, and the United Kingdom) and the Netherlands, the eligible patient population for Germany ranged from 996 in 2019 to 1,050 in 2029, which aligns with our calculated target population. Although this analysis attempted to portray the incremental costs of CAR T-cell therapies, no adequate BIA was conducted.39
Although assessing the value of CAR T-cell therapies is not the focus of budget impact analyses, their promising clinical outcomes for r/r DLBCL patients should be taken into consideration when interpreting the calculated incremental costs. Studies on 5-year outcomes for r/r DLBCL patients treated with CAR T-cell therapies have reported an ORR of 58%, with 46% of patients being in CR. In terms of survival, the study documented a PFS of 31% at 5 years.40 A retrospective observational study compared the outcomes of CAR T-cell therapies with alternate treatment methods and showed an ORR of 72% compared with 32% in the control group. Patients previously treated with 2 lines of therapy achieved a median PFS of 6.4 months (mo) when treated with CAR T-cells compared with 2.3 mo for conventional therapy.41
In this BIA, we also performed a scenario analysis to assess the financial burden of CAR T-cell therapies in second-line treatment. Clinical trials evaluating CAR T-cell therapy in second-line for DLBCL or follicular lymphoma (FL) patients showed positive results.34,35,42 For example, the ZUMA-7 study reported an ORR of 83% for CAR T-cell versus 50% for SOC. The CR was 65% versus 32%. In terms of survival, patients treated with CAR T-cell therapy had a longer median EFS (8.3 mo versus 2 mo).35 The target population was estimated to be 1,271; 1,725 (min; max) in 2021 and 1,399; 1,899 (min; max) in 2026. The annual budget impact was calculated at approximately €66 million; €90 million (min; max) in year 0 and €204 million; €278 million (min; max) in year 5. In this case, the budget impact was 1.2% and 1.7% (min; max) and 3.8% and 5.2% (min; max) of the costs for antineoplastic agents from the German statutory health insurance perspective at baseline and in year 5, respectively.36 Due to a greater patient population, the calculated budget impact represents a larger and thus considerable cost indicator. The interpretation depends on the clinical outcomes of CAR T-cell therapies, which must also be taken into consideration to ensure sufficient interpretation.
The calculated budget impact of CAR T-cell therapies seems to represent a manageable cost factor for German third-party payers. Unfortunately, transparency in the budget impact of innovative therapies in Germany is limited to allow a comprehensive comparison. No threshold exists to interpret the severity of the budget impact in the context of German third-party payers’ financial burden.
This budget impact analysis has several limitations. Throughout the process of data collection for suitable input variables, we were confronted with a lack of epidemiological data, comprehensive treatment patterns, and cost data in Germany. The scope of this study was on the inpatient treatment only. Future analyses on outpatient treatment pattern and costs would complement this first budget impact analysis. Additionally, this budget impact only focused on a limited time frame, which is why potential future cost savings, and the development of long-term expenditures could not be analyzed when combined with CEAs. Costs over a longer follow-up period (including long-term toxicity management) are missing and should also be considered in subsequent analyses.
In summary, the results of this BIA provide a first picture of the potential impact of innovative therapies in hematology/oncology. Lately, BIAs have become an increasingly important tool for evaluating the financial impact of new health technologies.22 This kind of model calculation may help to rationalize decisions for budget holders through methodological approaches and provide a foundation for further decisions in terms of resource allocation by decision-makers.20,22 However, due to limited data on peer-reviewed real-world data budget impacts and lack of comparison possibilities within the German healthcare system, it is difficult to assess the financial burden of CAR T-cell therapies. Published budget impact analyses in the context of innovative therapies in hematologic diseases are limited so far. Thus, no further adequate comparisons could be added to the discussion to put our results into perspective. Future efforts in economic analyses, for example, BIAs referring on diagnostic and treatment approaches in haematological malignancies, based on standardized and harmonized methods could support in rational decision making.
This raises the question of what is needed to ensure a comprehensive BIA. Although principles of good practices for BIAs from the ISPOR exist, they only present general recommendations. Moreover, they are not adopted equally due to different needs for data collection, methodological approaches, and reporting methods when conducting BIAs.20 Therefore, clear and structured national recommendations should be developed and applied in the future.22 Further, innovative therapies are on the rise and present new treatment options, for example, for patients with rare disease.43 Besides the much-discussed CAR T-cell therapies, other gene therapies are equally challenging for the respective budget holder.44 For instance, Onasemnogene Abeparvovec for treating spinal muscular atrophy (SMA) has a price of about €2 million per patient. An established budget impact analysis by the Institute for Clinical and Economic Review (ICER) showed that the set threshold of $991 million was not exceeded, reaching 45%, if the entire eligible US patient population of 215 incident SMA type I patients per year. Over a 5-year time period, the per-patient budget impact was about $950,000 compared with the best supportive care Nusinersen.45
Even if innovative therapies cannot be exactly compared in terms of financial burden, they face the same challenges in terms of assessment and reimbursement. New potential payment models and reimbursement policies need to be discussed exclusively for such personalized therapies, as current assessment strategies cannot be applied to therapies with unknown future value (in the long term) and average high costs per patient.
CONCLUSION
We conducted a budget impact analysis for treating r/r DLBCL patients with axi-cel and tisa-cel in Germany from a third-party payer’s perspective.
In the context of expenses for antineoplastic agents and national budget impacts in Germany for cancer therapies, for example, multiple myeloma, our documented budget impact of CAR T-cell therapies seems to be reasonable for a limited number of patients. Internationally viewed, other documented BIAs of CAR T-cell therapies presented comparable cost dimensions. For a better interpretation of the results, updated assessment policies and reimbursement strategies are needed for such innovative therapies. This study may provide general guidance for future reimbursement strategies for personalized medicine. Subsequent collection of epidemiological data, information on treatment patterns, and costs from outpatient and inpatient settings is needed to assure comprehensive budget impact analyses in the future. Additionally, further investigations, for example, CEAs that describe the value of CAR T-cell therapies, need to be taken into consideration including clinical outcomes of innovative therapies.
AUTHOR CONTRIBUTIONS
DS and BM have been involved throughout the process of data collection, data analysis, interpretation of results as well as drafting and writing up the final version of the article. CS, WS, VB, and TW supported as clinicians on the data collection in the clinical department of the LMU. MD supported as clinical expert on lymphoma and has been involved in data collection as well as critical interpretation of results. KB contributed to the development of the conceptional study design, data collection, data analyses, critical interpretation of results, and writing the draft as well as the final version of the article. All authors were involved in critically reviewing the article and gave approval of the version to be published.
DISCLOSURES
MD is an editor for HemaSphere. The remaining authors have no conflicts of interest to disclose.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES
- 1.Barnes B, Kraywinkel K, Nowossadeck E, et al. Bericht zum Krebsgeschehen in Deutschland 2016. 2016. Available at: https://edoc.rki.de/handle/176904/3264. Accessed April 10, 2021.
- 2.Robert-Koch-Institut (RKI). Wie steht es um unsere Gesundheit? 2016. Available at: https://www.rki.de/DE/Content/Gesundheitsmonitoring/Gesundheitsberichterstattung/GBEDownloadsGiD/2015/02_gesundheit_in_deutschland.html. Accessed April 10, 2021.
- 3.Schmitz N, Stelljes M, Bazarbachi A. Diffuse large B-cell lymphoma. In: Carreras E, Dufour C, Mohty M, et al., eds. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Cham (CH): Springer; 2019. [PubMed] [Google Scholar]
- 4.Smith A, Crouch S, Lax S, et al. Lymphoma incidence, survival and prevalence 2004-2014: sub-type analyses from the UK’s Haematological Malignancy Research Network. Br J Cancer. 2015;112:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zentrum für Krebsregisterdaten (ZfKD). Datenbankabfrage—Inzidenz, Altersstandardisierte Rate pro 100.000 Einwohner in Deutschland. Available at: https://www.krebsdaten.de/Krebs/SiteGlobals/Forms/Datenbankabfrage/datenbankabfrage_stufe2_form.html. Accessed April 10, 2021.
- 6.Lenz G, Chapuy B, Glaß B, et al. Diffuses großzelliges B-Zell-Lymphom. 2021. Available at: https://www.onkopedia.com/de/onkopedia/guidelines/diffuses-grosszelliges-b-zell-lymphom/@@guideline/html/index.html. Accessed March 5, 2021.
- 7.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23:4117–4126. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt C, Schneller F, Rudelius M, et al. Diffuses großzelliges B-Zell-Lymphom. In: Dreyling M, ed., Maligne Lymphome: Empfehlungen zur Diagnostik, Therapie und Nachsorge. Tumorzentrum Munich Zuckschwerdt Verlag; 2019, 208-226. [Google Scholar]
- 9.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mounier N, El Gnaoui T, Tilly H, et al. Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy. A phase II Lymphoma Study Association trial. Haematologica. 2013;98:1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsallab M, Levine BL, Wayne AS, et al. CAR T-cell product performance in haematological malignancies before and after marketing authorisation. Lancet Oncol. 2020;21:e104–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shank BR, Do B, Sevin A, et al. Chimeric antigen receptor T cells in hematologic malignancies. Pharmacotherapy. 2017;37:334–345. [DOI] [PubMed] [Google Scholar]
- 13.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 15.Neelapu SS, Locke FL, Bartlett NL, et al. A comparison of two-year outcomes in ZUMA-1 (Axicabtagene Ciloleucel) and SCHOLAR-1 in patients with refractory large B cell lymphoma. Blood. 2019;134: 4095. [Google Scholar]
- 16.Siegmund-Schultze N. Neue Strategien in der Onkologie: CAR-T-Zellen erreichen die klinische Praxis. Deutsches Ärzteblatt. 2019:116. Available at: https://www.aerzteblatt.de/archiv/211164/Neue-Strategien-in-der-Onkologie-CAR-T-Zellen-erreichen-die-klinische-Praxis. Accessed April 11, 2021. [Google Scholar]
- 17.Lin JK, Muffly LS, Spinner MA, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in multiply relapsed or refractory adult large B-cell lymphoma. J Clin Oncol. 2019;37:2105–2119. [DOI] [PubMed] [Google Scholar]
- 18.Roth JA, Sullivan SD, Lin VW, et al. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J Med Econ. 2018;21:1238–1245. [DOI] [PubMed] [Google Scholar]
- 19.Whittington MD, McQueen RB, Ollendorf DA, et al. Long-term survival and cost-effectiveness associated with axicabtagene ciloleucel vs chemotherapy for treatment of B-cell lymphoma. JAMA Netw Open. 2019;2:e190035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices–budget impact analysis. Value Health. 2007;10:336–347. [DOI] [PubMed] [Google Scholar]
- 21.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG). Allgemeine methoden—version 6.0. 2020. Available at: https://www.iqwig.de/methoden/allgemeine-methoden_version-6-0.pdf?rev=180500. Accessed March 1, 2021.
- 22.Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17:5–14. [DOI] [PubMed] [Google Scholar]
- 23.Statistisches Bundesamt (destatis). Bevölkerungsvorausberechnung bis 2060 in Deutschland. 2020. Available at: https://www-genesis.destatis.de/genesis/online?sequenz=tabelleErgebnis&selectionname=12421-0001#abreadcrumb. Accessed April 4, 2021.
- 24.Bundesministerium für Gesundheit. Mitglieder und Versicherte der Gesetzlichen Krankenversicherung (GKV). 2020. Available at: https://www.bundesgesundheitsministerium.de/themen/krankenversicherung/zahlen-und-fakten-zur-krankenversicherung/mitglieder-und-versicherte.html. Accessed July 15, 2021.
- 25.Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011:498–505. [DOI] [PubMed] [Google Scholar]
- 26.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Imhoff GW, McMillan A, Matasar MJ, et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD study. J Clin Oncol. 2017;35:544–551. [DOI] [PubMed] [Google Scholar]
- 28.Assouline S, Li S, Gisselbrecht C, et al. The conditional survival analysis of relapsed DLBCL after autologous transplant: a subgroup analysis of LY.12 and CORAL. Blood Adv. 2020;4:2011–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moertl B, Dreyling M, Schmidt C, et al. Inpatient treatment of relapsed/refractory diffuse large B-cell lymphoma (r/r DLBCL): A health economic perspective. Clinical Lymphoma Myeloma and Leukemia. 2021. [DOI] [PubMed] [Google Scholar]
- 30.Xie J, Wu A, Liao L, et al. Characteristics and treatment patterns of patients with relapsed/refractory diffuse large B-cell lymphoma who received ≥3 lines of therapies. Blood. 2020;136:4–5..32614961 [Google Scholar]
- 31.Grand View Research. T-cell therapy market size, share & trends analysis report by modality, by therapy (CAR T-cell, tumor-infiltrating lymphocytes), by indication (hematologic malignancies, solid tumors), by region, and segment forecasts, 2021–2028. 2021. Available at: https://www.grandviewresearch.com/industry-analysis/t-cell-therapy-market. Accessed May 27, 2021.
- 32.Novartis Pharma. Fachinformation (Zusammenfassung der Merkmale des Arzneimittels/SmPC), Kymriah 1,2 × 106 bis 6 × 108 Zellen Infusionsdispersion. 2021. Available at: https://www.fachinfo.de/pdf/022124. Accessed May 10, 2021.
- 33.Kite Pharma. Fachinformation (Zusammenfassung der Merkmale des Arzneimittels)—Yescarta. 2020. Available at: https://www.cart-zelltherapie.de/fachkreise/wp-content/uploads/sites/2/2020/10/Fachinformation_YESCARTA_Stand_Juni_2020.pdf. Accessed May 10, 2021.
- 34.Bishop MR, Dickinson M, Purtill D, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386:629–639. [DOI] [PubMed] [Google Scholar]
- 35.Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–654. [DOI] [PubMed] [Google Scholar]
- 36.IGES Institut. Arzneimittel-Atlas. Ausgaben der GKV für Krebsmedikamente. 2020. Available at: https://www.arzneimittel-atlas.de/arzneimittel/l01-antineoplastische-mittel/ausgaben/. Accessed July 5, 2021.
- 37.Basic E, Kappel M, Misra A, et al. Budget impact analysis of the use of oral and intravenous therapy regimens for the treatment of relapsed or refractory multiple myeloma in Germany. Eur J Health Econ. 2020;21:1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canadian Agency for Drugs and Technologies in Health (CADTH). CADTH optimal use report. Tisagenlecleucel for diffuse large B-cell lymphoma: economic review report. 2019. Available at: https://cadth.ca/sites/default/files/pdf/car-t/op0538-tisagenlecleucel-economic-report-DLBCL-jan2019.pdf. Accessed June 22, 2021. [PubMed]
- 39.Heine R, Thielen FW, Koopmanschap M, et al. Health economic aspects of chimeric antigen receptor T-cell therapies for hematological cancers: present and future. HemaSphere. 2021;5:e524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chong EA, Ruella M, Schuster SJ. Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N Engl J Med. 2021;384:673–674. [DOI] [PubMed] [Google Scholar]
- 41.Sermer D, Batlevi C, Palomba ML, et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020;4:4669–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamdar M, Solomon SR, Arnason JE, et al. 91 lisocabtagene maraleucel (liso-cel), a CD19-directed chimeric antigen receptor (CAR) T cell therapy, versus standard of care (SOC) with salvage chemotherapy (CT) followed by autologous stem cell transplantation (ASCT) as second-line (2L) treatment in patients (Pts) with relapsed or refractory (R/R) large B-cell lymphoma (LBCL): results from the randomized phase 3 transform study. 2021. Available at: https://ash.confex.com/ash/2021/webprogram/Paper147913.html. Accessed February 14, 2022.
- 43.Elverum K, Whitman M. Delivering cellular and gene therapies to patients: solutions for realizing the potential of the next generation of medicine. Gene Ther. 2020;27:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gene therapies should be for all. Nat Med. 2021;27:1311. [DOI] [PubMed] [Google Scholar]
- 45.Institute for Clinical and Economic Review (ICER). Spinraza and Zolgensma for spinal muscular atrophy: effectiveness and value. final evidence report. 2019. Available at: https://icer.org/wp-content/uploads/2020/10/ICER_SMA_Final_Evidence_Report_110220.pdf. Accessed August 18, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.