Abstract
BACKGROUND:
Maternal exposure to adversity during pregnancy has been found to affect infant brain development; however, the specific effect of prenatal crime exposure on neonatal brain connectivity remains unclear. Based on existing research, we hypothesized that living in a high-crime neighborhood during pregnancy would affect neonatal frontolimbic connectivity over and above other individual- and neighborhood-level adversity and that these associations would be mediated by maternal psychosocial stress.
METHODS:
Participants included 399 pregnant women, recruited as part of the eLABE (Early Life Adversity, Biological Embedding, and Risk for Developmental Precursors of Mental Disorders) study. In the neonatal period, 319 healthy, nonsedated infants were scanned using resting-state functional magnetic resonance imaging (repetition time = 800 ms; echo time = 37 ms; voxel size = 2.0 × 2.0 × 2.0 mm3; multiband = 8) on a Prisma 3T scanner and had at least 10 minutes of high-quality data. Crime data at the block group level were obtained from Applied Geographic Solution. Linear regressions and mediation models tested associations between crime, frontolimbic connectivity, and psychosocial stress.
RESULTS:
Living in a neighborhood with high property crime during pregnancy was related to weaker neonatal functional connectivity between the thalamus–anterior default mode network (aDMN) (β = −0.15, 95% CI = −0.25 to −0.04, p = .008). Similarly, high neighborhood violent crime was related to weaker functional connectivity between the thalamus-aDMN (β = −0.16, 95% CI = −0.29 to −0.04, p = .01) and amygdala-hippocampus (β = −0.16, 95% CI = −0.29 to −0.03, p = .02), controlling for other types of adversity. Psychosocial stress partially mediated relationships between the thalamus-aDMN and both violent and property crime.
CONCLUSIONS:
These findings suggest that prenatal exposure to crime is associated with weaker neonatal limbic and frontal functional brain connections, providing another reason for targeted public policy interventions to reduce crime.
Pregnancy is a crucial time for fetal brain development, beginning with neural tube formation in the first 4 weeks after conception and progressing to the establishment of functional brain connectivity (1). Functional networks, albeit in an immature state, have been observed in the second and third trimesters of pregnancy in studies of fetuses and premature neonates (2–4). By the time of birth, the neonatal brain is organized into a collection of resting-state networks that include thalamocortical, interhemispheric, and intrahemispheric connections (5,6). Prior research has demonstrated associations between prenatal stressor exposure and later functional connectivity in offspring (7,8). Specifically, prenatal depression has been shown to alter connectivity between the amygdala and both subcortical and prefrontal areas (9–12). In addition, increases in the number of stressful life events during pregnancy has been associated with reduced amygdala–medial prefrontal cortex connectivity (13). These associations may be driven by stress- and inflammation-related mediators, including cytokines, tryptophan, catecholamines, and cortisol, which can cross the placenta (14). In fact, cortisol concentrations and interleukin 6 levels have been related to neonatal amygdala connectivity and subsequent psychiatric symptoms at age 24 months (15,16). Preclinical models also indicate that the hippocampus, in addition to the amygdala, is affected by prenatal stress and corticotropin-releasing hormone (17–20).
Given the rapid brain development and sensitivity to stress in the prenatal period, neural development in utero may be especially vulnerable to various forms of individual and environmental adversity that increase maternal stress. However, it is unclear whether prenatal exposure to neighborhood crime (i.e., levels of crime in a neighborhood even when an individual has not had a personal victimization experience) is a specific form of adversity associated with brain development over and above other stressors. It is plausible that neighborhood crime relates to brain function over and above direct violence exposure, similar to how neighborhood poverty relates to brain structure and function when controlling for personal income (21–23). Furthermore, neighborhood crime may have distinct effects from neighborhood poverty (or other socioeconomic adversity) based on conceptual models that emphasize the differences between threat and deprivation (24–26) or highlight the importance of a chronic lack of safety (27).
While there are few studies of neighborhood crime, prior literature has demonstrated associations between neighborhood crime, stress, brain development, and mental health. Specifically, neighborhood crime has been related to greater perceived stress in mothers during pregnancy (28) as well as blunted cortisol reactivity to a social stressor in children, even when controlling for directly witnessing violence (29). Survey measures combining direct and neighborhood (i.e., indirect) crime exposure have also been associated with limbic, cingulate, and prefrontal volumes and function in task-based and resting-state functional magnetic resonance imaging (fMRI) paradigms in middle childhood and adolescence (30–33). These brain alterations may have effects on behavior because exposure to neighborhood crime has been related to a variety of physical and mental health problems, including posttraumatic stress disorder and externalizing problems (30,34–36). Overall, living in a high-crime area may be an important form of adversity that is related to greater maternal and child stress, altered brain function, and elevated physical and mental health risk; however, it remains unclear whether neighborhood crime affects brain development in the prenatal period over and above other forms of adversity.
This study aims to fill these gaps in the literature by focusing on the prenatal period, using objective crime data (as opposed to self-reports of community violence exposure), and beginning to examine mechanisms by which neighborhood crime may relate to altered brain function. First, we characterized the relationship between block group–level crime (one of the smallest units of area defined by the U.S. Census), other forms of adversity, and maternal psychosocial stress. Second, we examined the relationship of prenatal exposure to neighborhood crime to functional connectivity within and between limbic and prefrontal regions in neonates. These brain regions were chosen based on prior studies that have found frontolimbic associations with violence exposure in children and adolescents (31,37). Finally, we investigated whether observed functional connectivity differences are mediated by psychosocial stress. We hypothesized that living in an area with higher levels of crime would be associated with greater socioeconomic adversity, but that exposure to neighborhood crime would be associated with decreased functional connectivity between the limbic system and higher-level emotion regulation areas over and above other forms of adversity. In addition, we hypothesized that the relationship between crime and neonatal fMRI would be mediated by psychosocial stress. By investigating these hypotheses, we sought to elucidate whether prenatal crime exposure contributes to intergenerational neurodevelopmental risk.
METHODS AND MATERIALS
Participants
Participants included 399 mother-infant dyads recruited as part of the eLABE (Early Life Adversity, Biological Embedding, and Risk for Developmental Precursors of Mental Disorders) study from two outpatient obstetrics clinics at Washington University in St. Louis. Written informed consent was obtained from mothers prior to participation in the study. All study procedures were approved by the Washington University Institutional Review Board. Survey measures of adversity and psychosocial stress were obtained during each trimester, with exact timing varying based on subject availability at clinical appointments. Neonatal imaging was performed shortly after birth (mean postmenstrual age [PMA] = 41 weeks, range = 37–45 weeks). Inclusion criteria for this study included speaking English, maternal age 18 years or older, and singleton birth. Excluded were women with self-reported alcohol or illicit substance use, other than marijuana, during pregnancy (see the Supplement for analysis excluding marijuana). Anatomical MR images were reviewed by a neuroradiologist (JSS) and pediatric neurologist (CDS). Participants were excluded from the study if they had evidence of brain injury (i.e., any parenchymal abnormality detected on neonatal MRI by a pediatric neuroradiologist and neonatal neurologist). Additional exclusion criteria included maternal congenital infections and known fetal abnormalities, including intrauterine growth restriction.
Of the 399 neonates who were recruited for eLABE, 319 were included in these analyses (mean gestational age [GA] = 38 weeks, range = 28–41 weeks) (see the Supplement for full-term infant analysis). Participants were excluded for the following reasons: missing neonatal MRI scans owing to COVID-19 restrictions (n = 14), evidence of brain injury (n = 17), no fMRI data collected (n = 1), no usable T2 for registration (n = 30), did not have ≥10 minutes of usable fMRI data after motion censoring (n = 11), and visible artifacts in functional connectivity data (n = 7). Participants with usable fMRI did not differ significantly from subjects without usable fMRI on GA at birth, adversity levels, or psychosocial stress levels; however, participants with usable fMRI data were significantly (p < .05) younger at the time of MRI scan (PMA = 41.2 weeks) than subjects without usable fMRI data (PMA = 41.8 weeks).
Measures
Geocoding and Crime Rates.
Maternal addresses were collected up to four times during pregnancy, spaced across all three trimesters. The addresses were geocoded using application programming interfaces (APIs) from MapQuest, Open Street Map Contributors, and the censusxy package (38) in R studio. The addresses were then categorized into their respective block groups based on the Block API from the Federal Communications Commission and the censusxy package using the 2010 Decennial Census. Block groups from the birth time point were used because all mothers had addresses collected at birth, with supplemental analyses conducted for mothers who moved during pregnancy (see the Supplement). Of the 319 mothers included in the fMRI analysis, 87% (n = 278) had addresses obtained at multiple time points. Of those mothers, 74% did not move during pregnancy (n = 207), 25% moved once during pregnancy (n = 69), and <1% of mothers moved more than once (n = 2).
Crime data at the block group level were obtained from Applied Geographic Solution’s CrimeRisk Database, which is a commercial dataset that combines data from more than 16,000 law enforcement agencies (39). Applied Geographic Solution crime data is indexed in comparison to the national average, which is set at a value of 100. The two variables of interest in these analyses are crimes against persons (hereafter referred to as violent crime), which combines data on murder, rape, robbery, and aggravated assault, and property crimes, which combines data on burglary, larceny, and motor vehicle theft. Maps of violent crimes and property crimes in the St. Louis area are displayed in Figure 1. The analyzed crime rates do not assess personal exposure to crime. Additional analyses controlled for direct exposure to crime or legal difficulties, as well as physical danger, over the life span using the Stress and Adversity Inventory (see the Supplement for details).
Figure 1.
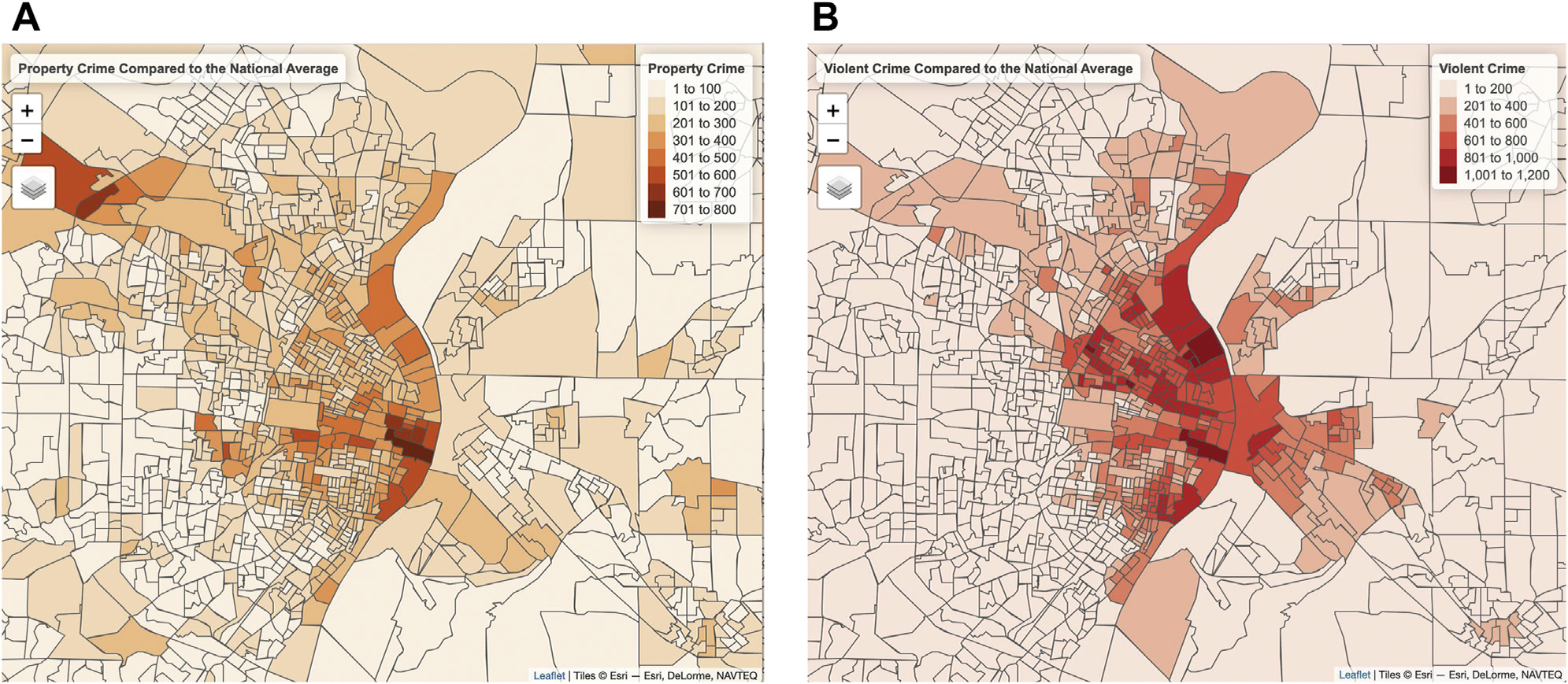
(A) The 2020 property crime rates relative to the national average (set as 100) in St. Louis city and surrounding counties. (B) The 2020 violent crime rates relative to the national average (set as 100) in St. Louis city and surrounding counties. The borders on the maps represent block group boundaries established in the 2010 census.
Functional MRI.
Imaging was performed without sedating medications using a 3T Prisma scanner (Siemens Corp.) and 64-channel head coil. After feeding, the infant was swaddled, placed in a head-stabilizing vacuum fix wrap, and positioned in the scanner. Heart rate and blood oxygenation were measured continuously, and infants were monitored visually via video. Based on visual monitoring, infants slept through scans as indicated by eye closure and minimal movements. A registered nurse was present at all MRI scans in case of emergency. During the scan, T2-weighted images (sagittal, 208 slices, 0.8-mm isotropic resolution, echo time = 563 ms, tissue T2 = 160 ms, repetition time = 3200 or 4500 ms) were collected. Resting-state functional imaging data were collected using a blood oxygen level–dependent gradient-recalled echo-planar multiband sequence (72 slices, 2.0-mm isotropic resolution, echo time = 37 ms, repetition time = 800 ms, multiband factor = 8). FIRMM (40) was used during scanning to monitor realtime participant movement.
MRI data underwent standard blood oxygen level–dependent preprocessing followed by functional connectivity processing (see the Supplement) done in surface space for cortical areas and volume space for subcortical areas because there is no corresponding surface for deep structures. A minimum of 10 minutes (750 frames) of low-motion (framewise displacement <0.25 mm) neonatal data were required for inclusion. Resting-state functional connectivity measures were computed as the Fisher z-transformed Pearson correlation between time courses from pairs of surface vertices or surface parcels. Values were arranged into a connectivity matrix based upon age-specific resting-state network assignments that were determined and validated using previously published methods (41,42). A total of 11 networks were identified and are displayed in Figure S1. In the neonatal networks, the default mode network (DMN) and frontal parietal network were split into anterior and posterior portions because that was the best fit for the data based on community segregation algorithms (C.M. Sylvester, M.D., Ph.D., et al., unpublished data, 2021). The anterior portions were subsequently examined based on hypotheses derived from prior literature.
Adversity and Psychosocial Stress.
Structural equation modeling was used to create composite measures of socioeconomic advantage and psychosocial stress (43). The model resulted in two factors: one representing socioeconomic advantage and one representing psychosocial stress. The components in the advantage factor included income-to-needs ratio (self-reported family income and household size compared with the federal poverty level), insurance status verified at delivery, mother’s self-reported highest level of education, area deprivation index, and maternal nutrition. The area deprivation index is a geotracking measure that ranks neighborhoods by socioeconomic disadvantage compared with the national average based on census block data, including factors for the domains of income, education, employment, and housing quality (40,44). Maternal nutrition was assessed using the Healthy Eating Index, a validated dietary assessment tool available through the National Institutes of Health used to measure diet quality based on U.S. Dietary Guidelines for Americans (45,46). Dietary information for Healthy Eating Index calculation was obtained using the Diet History Questionnaire (47,48). Higher scores on the advantage factor represent higher levels of advantage, whereas lower scores represent lower levels of advantage (mean = 0, SD = 1). Lead levels were not included in the advantage composite; however, because lead has previously been related to crime (49), additional analyses controlled for census-tract lead levels using data from the Missouri Department of Health and Senior Services (see the Supplement for details).
The components of the psychosocial stress factor included measures of prenatal depression, perceived stress, lifetime stressor exposure, and racial discrimination. Prenatal depression was measured using the Edinburgh Postnatal Depression Scale (50) at each trimester. Perceived stress was assessed at each trimester using the Perceived Stress Scale (51). Lifetime exposure to major life stressors was measured using the total count and severity scores from the Stress and Adversity Inventory (52). Experiences of racial discrimination were assessed using the Everyday Discrimination Scale (53). Higher scores on the psychosocial stress factor represent higher levels of psychosocial stress, whereas lower scores represent lower levels of psychosocial stress (mean = 0, SD = 1). Race itself was not included in the models because it is not a biologically meaningful variable and is instead a proxy for racism and discrimination, which were assessed.
Data Analysis
All analyses were conducted using R and RStudio, specially using the tidyverse, psych, and stats packages (54,55). Distributions of and correlations between crime levels, adversity, and stressors were examined. Initial bivariate correlations between crime rates and hypothesized frontolimbic neonatal brain connections were computed and corrected for multiple comparisons using the Benjamini-Hochberg procedure for false discovery rate in the corr.test function from the psych package (56). Frontolimbic regions were chosen based on prior research (limbic structures: amygdala, hippocampus, thalamus; frontal regions: anterior DMN [aDMN] and anterior frontal parietal network) (Figure 2). Two negative control regions (motor-motor and motor-aDMN) that have not been previously associated with crime exposure were also examined. For a complete bivariate correlation table with corrected p values, see Table S1. Significant bivariate associations were then further related to property and violent crimes in separate linear regression models controlling for GA at birth and PMA at scan (sex was considered but was not related to the independent or dependent variables). Each set of linear regressions (i.e., property and violent crimes) were corrected for multiple comparisons again using the Benjamini-Hochberg procedure for false discovery rate correction (56,57). Next, the adversity composite was added to the models to determine whether neighborhood crime had an effect on neonatal brain function over and above other forms of adversity. Results were once again corrected for multiple comparisons using the false discovery rate procedure (56,57), and the full set of tests are described in the results. Finally, we examined potential mediators of the associations that remained significant after controlling for advantage and correcting for multiple comparisons. Mediation models were then tested for significant connections using the psychosocial stress variable as a mediator and nonparametrically bootstrapped using 1000 simulations with the mediation package in R (58).
Figure 2.
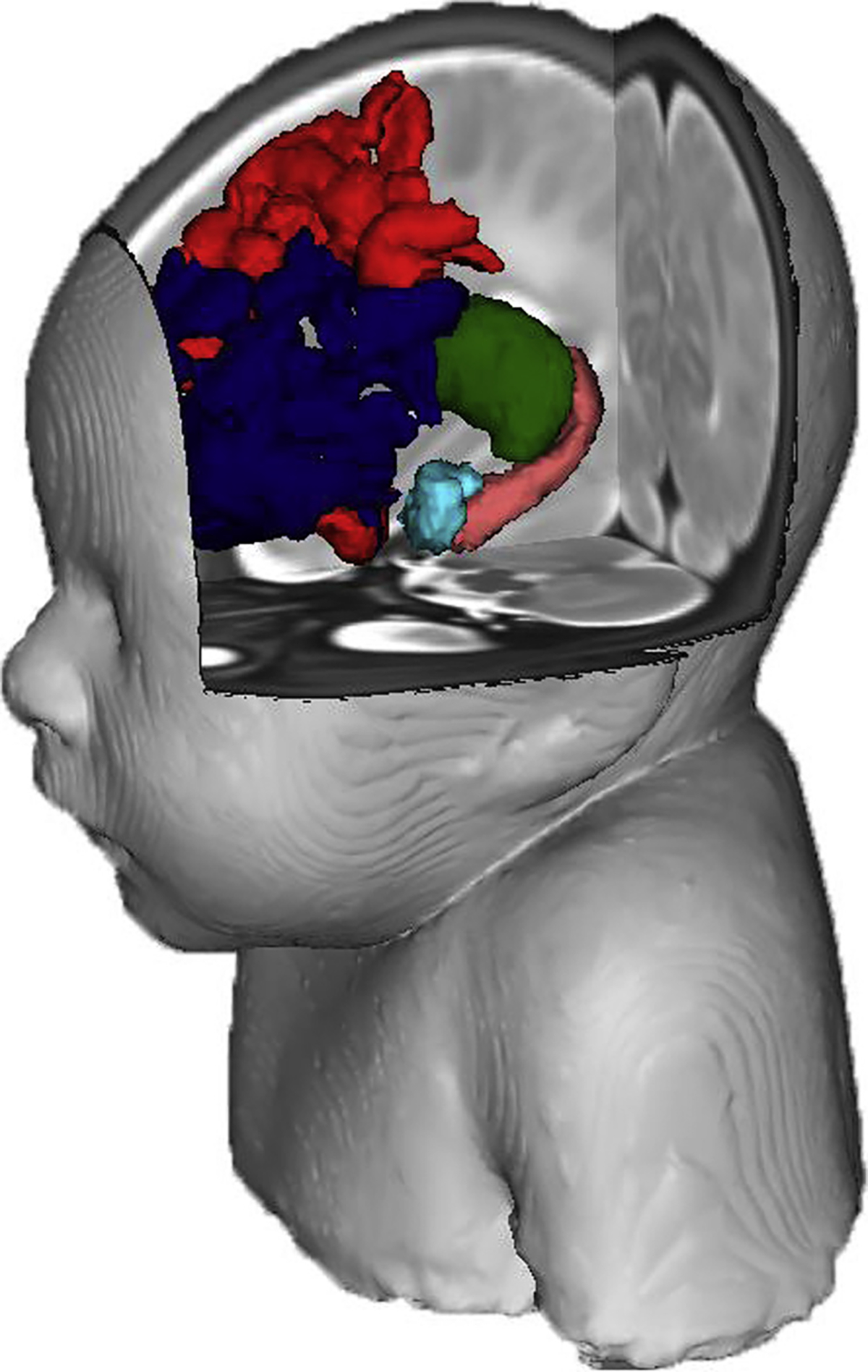
The brain regions of interest (teal = amygdala; pink = hippocampus; green = thalamus; red = anterior default mode network; navy = anterior frontal parietal network) on the neonatal brain. The facial reconstruction was created from an atlas containing multiple neonatal scans.
RESULTS
Associations Between Crime, Adversity, and Psychosocial Stress
High levels of block group violent crime were negatively related to advantage, meaning that disadvantaged mothers tended to live in areas with higher crime levels, whereas advantaged participants tended to live in areas with low crime levels (r = −0.52, 95% CI = −0.59 to −0.43, p < .001) (Figure 3A). Disadvantaged mothers (n = 193; advantage z scores < 0) were widely distributed across both safe and dangerous neighborhoods, whereas advantaged mothers (n = 126, advantage z scores > 0) were clustered in safe neighborhoods with low levels of violent crimes (Figure S2). Greater levels of violent crime were also associated with more psychosocial stress in mothers (r = 0.25, 95% CI = 0.14 to 0.35, p < .001) (Figure 3B). Similarly, high levels of property crime were negatively related to advantage because disadvantaged mothers tended to live in areas with higher property crime (r = −0.27, 95% CI = −0.16 to −0.37, p < .001). Higher levels of property crime were also associated with more psychosocial stress among mothers (r = 0.23, 95% CI = 0.13 to 0.33, p < .001).
Figure 3.
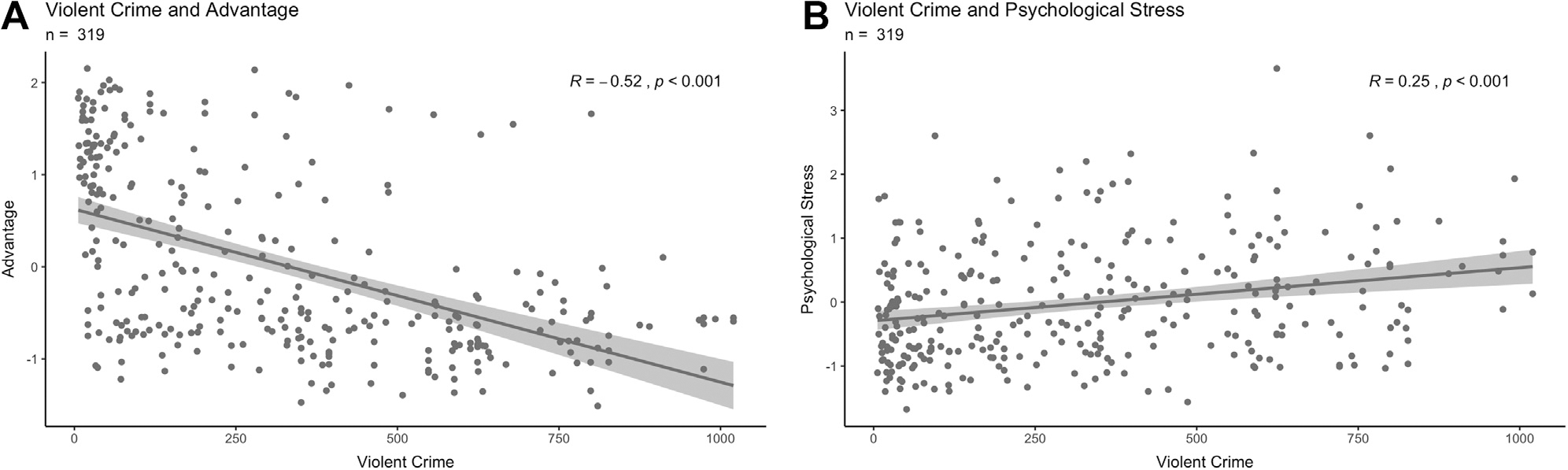
(A) Association between advantage (higher numbers represent increased advantage) and violent crime levels (higher numbers represent more violent crime), scaled to the national average of 100 (n = 319). (B) Association between psychosocial stress (higher numbers represent more psychosocial stress) and violent crime levels, scaled to the national average (n = 319). Each trend line was created from a linear model representing the bivariate correlations. The R and p values of this model are displayed in the upper right-hand corner.
Prenatal Neighborhood Crime Exposure and Neonatal Functional Connectivity
In linear regression models controlling for GA at birth and PMA at scan, functional connectivity between the thalamus-aDMN (β = −0.22, 95% CI = −0.34 to −0.12, p < .001), thalamus–anterior frontal parietal network (β = −0.16, 95% CI = −0.27 to −0.05, p = .005), amygdala-hippocampus (β = −0.12, 95% CI = −0.24 to −0.02, p = .02), and amygdala-aDMN (β = −0.17, 95% CI = −0.29 to −0.07, p = .002) was weaker for infants born to mothers living in areas with high rates of violent crime after correcting for multiple comparisons. Violent crime was also associated with reductions in amygdala-thalamus (β = −0.11, 95% CI = −0.22 to 0.00, p = .059) and amygdala-amygdala (β = −0.11, 95% CI = −0.22 to 0.00, p = .053) connectivity, but these relationships did not meet the threshold for statistical significance. When advantage was added to the significant models, only decreases in the amygdala-hippocampus (β = −0.16, 95% CI = −0.29 to −0.03, p = .02) and thalamus-aDMN (β = −0.16, 95% CI = −0.29 to −0.04, p = .01) connectivity continued to be related to violent crime exposure, after correcting for multiple comparisons (Table 1 and Figure S3). Prenatal exposure to neighborhoods with high rates of property crimes was related to weaker neonatal functional connectivity between the thalamus-aDMN (β = −0.15, 95% CI = −0.25 to −0.04, p = .008) after controlling for advantage (Table 2 and Figure S3). In the negative control analysis, neither motor-motor nor motor-aDMN connectivity was related to violent or property crime (all p values > .2) (Table S2). Results largely remained unchanged when accounting for the percentage of blood tests with elevated lead levels (Table S3) and lifetime exposure to physical danger (Table S4).
Table 1.
Violent Crime and Neonatal Frontolimbic Connectivity
| Variable | Amygdala-Hippocampus | Amygdala-aDMN | Thalamus-aDMN | Thalamus-aFPN | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p Value | q Value | β | 95% CI | p Value | q Value | β | 95% CI | p Value | q Value | β | 95% CI | p Value | q Value | |
| Intercept | 0.00 | −0.11 to 0.10 | .90 | >.90 | 0.00 | −0.11 to 0.11 | >.90 | >.90 | 0.00 | −0.10 to 0.11 | >.90 | >.90 | 0.00 | −0.10 to 0.11 | >.90 | >.90 |
| Violent Crime | −0.16 | −0.29 to −0.03 | .02a | .03a | −0.11 | −0.24 to 0.02 | .09 | .13 | −0.16 | −0.29 to −0.04 | .01a | .03a | −0.07 | −0.20 to 0.05 | .25 | .25 |
| PMA at Scan | −0.12 | −0.25 to 0.00 | .05 | .05 | 0.15 | −0.04 to 0.28 | .01a | .02a | 0.20 | 0.08 to 0.32 | .002a | .003a | 0.21 | 0.09 to 0.33 | <.001a | .003a |
| GA at Birth | 0.18 | 0.06 to 0.30 | .003a | .01a | −0.08 | −0.20 to 0.04 | .19 | .26 | −0.07 | −0.18 to 0.05 | .30 | .30 | −0.14 | −0.25 to −0.02 | .02a | .04a |
| Advantage | −0.06 | −0.19 to 0.06 | .33 | .33 | 0.14 | 0.01 to 0.26 | .03a | .06 | 0.12 | 0.00 to 0.25 | .05 | .06 | 0.17 | 0.05 to 0.29 | .007a | .03a |
| No. of Observations | 319 | 319 | 319 | 319 | ||||||||||||
| R2/R2 Adjusted | 0.05/0.04 | 0.08/0.06 | 0.11/0.10 | 0.09/0.08 | ||||||||||||
| Model p Value | <.001a | <.001a | <.001a | <.001a | ||||||||||||
q Values corrected for multiple comparisons using a false discovery rate procedure.
aDMN, anterior default mode network; aFPN, anterior frontal parietal network; GA, gestational age; PMA, postmenstrual age.
Statistically significant at α = 0.05.
Table 2.
Property Crime and Neonatal Frontolimbic Connectivity
| Characteristic | Thalamus-aDMN | |||
|---|---|---|---|---|
| β | 95% CI | p Value | q Value | |
| Intercept | 0.00 | −0.10 to 0.11 | >.90 | >.90 |
| Property Crime | −0.14 | −0.25 to −0.04 | .008a | .008a |
| PMA at Scan | 0.20 | 0.08 to 0.32 | .001a | .001a |
| GA at Birth | −0.05 | −0.17 to 0.06 | .40 | .40 |
| Advantage | 0.16 | 0.05 to 0.27 | .004a | .004a |
| No. of Observations | 319 | |||
| R2/R2 Adjusted | 0.11/0.10 | |||
| Model p Value | <.001a | |||
q Values corrected for multiple comparisons using a false discovery rate procedure.
aDMN, anterior default mode network; GA, gestational age; PMA, postmenstrual age.
Statistically significant at α = 0.05.
Mediations by Maternal Psychosocial Stress
Maternal psychosocial stress partially mediated the direct associations between the thalamus-aDMN and both violent and property crimes (indirect effects; p = .03 and p = .03, respectively). These were partial mediations because the direct effects remained significant after accounting for the mediation pathway (Figure 4). No other associations between crime and functional connectivity that contributed variance over and above adversity were mediated by maternal psychosocial stress.
Figure 4.
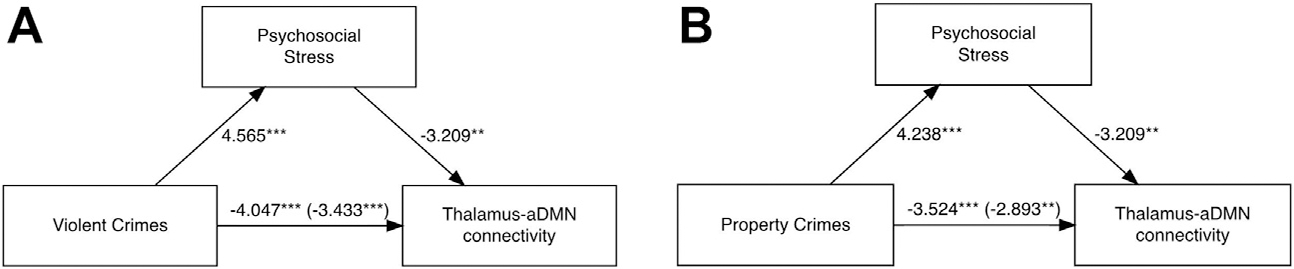
(A) The mediation model containing violent crime (independent variable), psychosocial stress (mediator), and thalamus–anterior default mode network (aDMN) connectivity (dependent variable). (B) The mediation model containing property crime (independent variable), psychosocial stress (mediator), and thalamus-aDMN connectivity (dependent variable). The numbers displayed on each path are the F statistics with the c′ path in parentheses. ***p < .001, **p < .01. In a bootstrapping analysis with 1000 simulations, all paths remained significant. Data from 319 subjects are presented.
DISCUSSION
Consistent with hypotheses, our data show that prenatal exposure to objectively identified violent and property crimes is related to weaker connectivity between the amygdala, hippocampus, thalamus, and aDMN after accounting for other types of adversity. Both violent and property crime exposure during pregnancy were related to weaker thalamus-aDMN connectivity, with violent crime exposure being additionally related to weaker amygdala-hippocampus connectivity. Associations between prenatal crime exposure and thalamus-aDMN connectivity were mediated by self-reported levels of maternal psychosocial stress. These results suggest that living in an unsafe neighborhood may have additional effects on neonatal brain function over and above living in an impoverished area, which is consistent with prior research and conceptual models of adversity (24,25,27), and that maternal psychosocial stress plays a role in these associations.
Frontolimbic Connectivity and the Role of the Thalamus
These results are consistent with prior studies showing that that frontolimbic connectivity is related to survey measures of violent crime exposure in children and adolescents (31,37). In addition, studies using task-based fMRI paradigms of youths exposed to violence showed increased activation in the amygdala in response to angry faces (59) as well as increased amygdala, hippocampal, and ventromedial prefrontal cortex activation in an emotional response inhibition task (37). These findings broadly align with our results, although we also discovered several associations between prenatal crime exposure and neonatal thalamic connectivity.
Despite its role in the limbic system, the thalamus has previously received limited attention, especially in relation to neighborhood crime. The thalamus is classically thought of as a relay station or gatekeeper for sensory information that flows from primary cortices to higher-level association cortices (60,61); yet it is important to highlight that the medial dorsal, anterior, and lateral dorsal nuclei also relay limbic and visceral information (62). The medial dorsal nucleus (commonly called the paraventricular thalamic nucleus) relays signals from the amygdala and other subcortical regions to the anterior cingulate, orbitofrontal, and prefrontal cortices (62). Preclinical models have shown that the paraventricular nucleus determines reactions to threats (63) and controls fear circuits in the amygdala (64). Furthermore, the paraventricular nucleus has been consistently shown to be responsive to both acute and chronic stressors because it is densely innervated by neurotransmitters involved in the stress-response system (65,66). This nucleus also plays a role in habituation to chronic stress and may use a cholecystokinin-mediated pathway to alter hypothalamic-pituitary-adrenal axis function in chronically stressed individuals, as evidenced by studies in rodent models (67,68). Although the development of the paraventricular nucleus and its prenatal function (69) is not well studied, it is possible that the thalamus is responding to chronic maternal stress signals arising from living in a high-crime area, which may in turn affect later regulation of threat and the hypothalamic-pituitary-adrenal axis. Neonatal alterations in thalamic connectivity may also be important for behavior because alterations in the paraventricular nucleus, among other portions of the thalamus, have been associated with a variety of poor psychiatric outcomes (65,70).
Type of Crime Exposure
While living in a neighborhood with either high violent or high property crime was related to decreases in thalamus-aDMN connectivity, violent crime exposure was related to amygdala-hippocampus connectivity whereas property crime was not. However, these standardized beta weights were not significantly different from one another using the Z test described in Paternoster et al. (71) (see the Supplement). Furthermore, different areas had high property crime levels as opposed to high violent crime levels, so different mothers in our sample may have been affected by different types of crime (Figure 1). Future studies would be needed to further investigate whether the type of crime exposure, including specific crimes within these categories (e.g., murder), have differential effects on brain function.
Psychosocial and Biological Stress
The results indicate that self-reported maternal psychosocial stress partially mediated the relationship between prenatal crime exposure and neonatal thalamus-DMN connectivity, but not the other associations investigated. One potential explanation for this is that psychosocial and biological stress levels are not always correlated, especially in chronically stressed individuals (72,73). Although acute laboratory-based stressors have been shown to induce biological stress reactivity [e.g., as indexed by cortisol and cytokines], these biological responses are not always strongly related to individuals’ self-reported stress experiences (74,75). In addition, it is possible that perceived psychosocial stress measures more closely index some mechanisms of stress (e.g., cortisol, cytokines, tryptophan, catecholamines) than others (76). As such, biological stress pathways that are not well indexed by psychosocial stress may be mediating crime-brain relationships and not accounted for in our models. It is also possible that multiple biological stress mechanisms may respond to neighborhood crime and, in turn, affect different functional connections in the brain. Finally, mothers may be underreporting their psychosocial symptoms because these were assessed in a survey rather than a clinical interview. Future studies will need to examine which mechanisms of biological stress, if any, mediate the relationships between living in a high-crime area and functional connectivity.
Limitations
While there are several strengths of using objective crime metrics as opposed to survey measures of violence exposure, we were unable to control for direct exposure to crime that occurred during pregnancy because these measures were not included in the study. The crime/legal and physical danger domains of the Stress and Adversity Inventory were examined in supplementary analyses, but these measures represent lifetime stressor exposure that included, but was not limited to, pregnancy. Future work will be necessary to distinguish between the effects of living in a high-crime area and being either a victim or witness of criminal behavior. In addition, this study was not able to assess how long each mother lived in a high-crime neighborhood. It may be the case that chronic exposure to dangerous neighborhoods (or even exposure during a particular period, such as early childhood) shapes mothers’ stress responsivity to crime.
Future Directions
This study demonstrates neonatal alterations in frontolimbic resting-state functional connectivity that are associated with living in high-crime areas during pregnancy; however, the long-term impacts are unclear. In adolescents, studies have found that changes in brain function associated with exposure to community violence may be protective in certain domains, such as cardiac health (77), but maladaptive in other domains, such as externalizing behavior (30). Future studies are needed to determine how the neonatal functional brain alterations associated with prenatal neighborhood crime exposure are related to later behavior. Additional research is also needed to determine whether reductions in neonatal frontolimbic connectivity persist and alter the trajectory of brain development. Finally, future investigations comparing multiple time points of crime exposure throughout development are needed to establish whether pregnancy is a sensitive period for these effects and determine whether there are sensitive periods for resiliency.
Overall, this study provides evidence of intergenerational transmission of an environmental stressor, specifically exposure to neighborhood crime, that may alter brain function at birth. These early alterations in brain development demonstrate perpetuation of systemic injustice and inequity across generations, which could pose particular problems if future studies demonstrate that they persist and negatively affect behavior. Although evidence-based crime prevention and reduction is already a well-established goal of public policy, this study provides further evidence of its potential as a tool to avoid associated intergenerational changes in brain function.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
Research reported in this publication was supported by the National Institutes of Health (Grant No. R01 MH113883 [to JLL, CDS, BBW, DMB, CER] and Grant No. F30 HD104313-01A1 [to RGB]), March of Dimes Prematurity Research Center at Washington University, Intellectual and Developmental Disabilities Research Center at Washington University (Grant No. P50 HD103525), Washington University in St. Louis Center for the Study of Race, Ethnicity, and Equity Small Grant [to RGB, DMB], Children’s Discovery Institute, McDonnell Center for Systems Neuroscience, the Washington University Medical Scientist Training Program [to RGB], and the California Initiative to Advance Precision Medicine (Grant No. OPR21101 [to GMS]).
The views are those of the authors and not necessarily those of the funding organizations.
We thank the Washington University Neonatal Developmental Research Group, eLABE staff, and the families involved with the study.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.biopsych.2022.01.020.
Contributor Information
Rebecca G. Brady, Division of Biology and Biomedical Sciences, Washington University School of Medicine Department of Neurology, Washington University School of Medicine.
Cynthia E. Rogers, Department of Pediatrics, Washington University School of Medicine Department of Psychiatry, Washington University School of Medicine.
Trinidi Prochaska, Department of Psychiatry, Washington University School of Medicine.
Sydney Kaplan, Department of Neurology, Washington University School of Medicine.
Rachel E. Lean, Department of Psychiatry, Washington University School of Medicine
Tara A. Smyser, Department of Psychiatry, Washington University School of Medicine
Joshua S. Shimony, Mallinckrot Institute of Radiology, Washington University School of Medicine
George M. Slavich, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, California
Barbara B. Warner, Department of Pediatrics, Washington University School of Medicine
Deanna M. Barch, Department of Psychiatry, Washington University School of Medicine Mallinckrot Institute of Radiology, Washington University School of Medicine; Department of Psychological and Brain Sciences, Washington University in St. Louis, St. Louis, Missouri.
Joan L. Luby, Department of Pediatrics, Washington University School of Medicine Department of Psychiatry, Washington University School of Medicine.
Christopher D. Smyser, Department of Neurology, Washington University School of Medicine Department of Pediatrics, Washington University School of Medicine; Mallinckrot Institute of Radiology, Washington University School of Medicine.
REFERENCES
- 1.Keunen K, Counsell SJ, Benders MJNL (2017): The emergence of functional architecture during early brain development. Neuroimage 160:2–14. [DOI] [PubMed] [Google Scholar]
- 2.Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ (2010): Longitudinal analysis of neural network development in preterm infants. Cereb Cortex 20:2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomason ME, Scheinost D, Manning JH, Grove LE, Hect J, Marshall N, et al. (2017): Weak functional connectivity in the human fetal brain prior to preterm birth. Sci Rep 7:39286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turk E, van den Heuvel Ml, Benders MJ, de Heus R, Franx A, Manning JH, et al. (2019): Functional connectome of the fetal brain. J Neurosci 39:9716–9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner RG, Wheelock MD, Neil JJ, Smyser CD (2021): Structural and functional connectivity in premature neonates. Semin Perinatol 45:151473. [DOI] [PubMed] [Google Scholar]
- 6.Smyser CD, Snyder AZ, Shimony JS, Mitra A, Inder TE, Neil JJ (2016): Resting-state network complexity and magnitude are reduced in prematurely born infants. Cereb Cortex 26:322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheinost D, Sinha R, Cross SN, Kwon SH, Sze G, Constable RT, Ment LR (2017): Does prenatal stress alter the developing connectome? Pediatr Res 81:214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lautarescu A, Craig MC, Glover V (2020): Prenatal stress: Effects on fetal and child brain development. Int Rev Neurobiol 150:17–40. [DOI] [PubMed] [Google Scholar]
- 9.Scheinost D, Kwon SH, Lacadie C, Sze G, Sinha R, Constable RT, Ment LR (2016): Prenatal stress alters amygdala functional connectivity in preterm neonates. Neuroimage Clin 12:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soe NN, Wen DJ, Poh JS, Chong YS, Broekman BF, Chen H, et al. (2018): Perinatal maternal depressive symptoms alter amygdala functional connectivity in girls. Hum Brain Mapp 39:680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posner J, Cha J, Roy AK, Peterson BS, Bansal R, Gustafsson HC, et al. (2016): Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl Psychiatry 6:e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu A, Anh TT, Li Y, Chen H, Rifkin-Graboi A, Broekman BFP, et al. (2015): Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl Psychiatry 5:e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphreys KL, Camacho MC, Roth MC, Estes EC (2020): Prenatal stress exposure and multimodal assessment of amygdala-medial prefrontal cortex connectivity in infants. Dev Cogn Neurosci 46:100877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakers F, Rupprecht S, Dreiling M, Bergmeier C, Witte OW, Schwab M (2020): Transfer of maternal psychosocial stress to the fetus. Neurosci Biobehav Rev 117:185–197. [DOI] [PubMed] [Google Scholar]
- 15.Graham AM, Rasmussen JM, Entringer S, Ben Ward E, Rudolph MD, Gilmore JH, et al. (2019): Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol Psychiatry 85:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M, et al. (2018): Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol Psychiatry 83:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goelman G, Ilinca R, Zohar I, Weinstock M (2014): Functional connectivity in prenatally stressed rats with and without maternal treatment with ladostigil, a brain-selective monoamine oxidase inhibitor. Eur J Neurosci 40:2734–2743. [DOI] [PubMed] [Google Scholar]
- 18.Negron-Oyarzo I, Neira D, Espinosa N, Fuentealba P, Aboitiz F (2015): Prenatal stress produces persistence of remote memory and disrupts functional connectivity in the hippocampal-prefrontal cortex axis. Cereb Cortex 25:3132–3143. [DOI] [PubMed] [Google Scholar]
- 19.Bock J, Wainstock T, Braun K, Segal M (2015): Stress in utero: Prenatal programming of brain plasticity and cognition. Biol Psychiatry 78:315–326. [DOI] [PubMed] [Google Scholar]
- 20.Curran MM, Sandman CA, Poggi Davis E, Glynn LM, Baram TZ (2017): Abnormal dendritic maturation of developing cortical neurons exposed to corticotropin releasing hormone (CRH): Insights into effects of prenatal adversity? PLoS One 12:e0180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor RL, Cooper SR, Jackson JJ, Barch DM (2020): Assessment of neighborhood poverty, cognitive function, and prefrontal and hippocampal volumes in children. JAMA Netw Open 3:e2023774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomlinson RC, Burt SA, Waller R, Jonides J, Miller AL, Gearhardt AN, et al. (2020): Neighborhood poverty predicts altered neural and behavioral response inhibition. Neuroimage 209:116536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gard AM, Maxwell AM, Shaw DS, Mitchell C, Brooks-Gunn J, McLanahan SS, et al. (2021): Beyond family-level adversities: Exploring the developmental timing of neighborhood disadvantage effects on the brain. Dev Sci 24:e12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaughlin KA, Sheridan MA, Lambert HK (2014): Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev 47:578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaughlin KA, Weissman D, Bitran D (2019): Childhood adversity and neural development: A systematic review. Annu Rev Dev Psychol 1:277–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin KA, Sheridan MA, Humphreys KL, Belsky J, Ellis BJ (2021): The value of dimensional models of early experience: Thinking clearly about concepts and categories. Perspect Psychol Sci 16:1463–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith KE, Poliak SD (2021): Rethinking concepts and categories for understanding the neurodevelopmental effects of childhood adversity. Perspect Psychol Sci 16:67–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shannon MM, Clougherty JE, McCarthy C, Elovitz MA, Nguemeni Tiako MJ, Melly SJ, Burris HH (2020): Neighborhood violent crime and perceived stress in pregnancy. Int J Environ Res Public Health 17:5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theall KP, Shirtcliff EA, Dismukes AR, Wallace M, Drury SS (2017): Association between neighborhood violence and biological stress in children. JAMA Pediatr 171:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weissman DG, Gelardi KL, Conger RD, Robins RW, Hastings PD, Guyer AE (2018): Adolescent externalizing problems: Contributions of community crime exposure and neural function during emotion introspection in Mexican-origin youth. J Res Adolesc 28:551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxbe D, Khoddam H, Piero LD, Stoycos SA, Gimbel SI, Margolin G, Kaplan JT (2018): Community violence exposure in early adolescence: Longitudinal associations with hippocampal and amygdala volume and resting state connectivity. Dev Sci 21: e12686. [DOI] [PubMed] [Google Scholar]
- 32.Butler O, Yang XF, Laube C, Kühn S, Immordino-Yang MH (2018): Community violence exposure correlates with smaller gray matter volume and lower IQ in urban adolescents. Hum Brain Mapp 39:2088–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reda MH, Marusak HA, Ely TD, van Rooij SJH, Stenson AF, Stevens JS, et al. (2021): Community violence exposure is associated with hippocampus-insula resting state functional connectivity in urban youth. Neuroscience 468:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowler PJ, Tompsett CJ, Braciszewski JM, Jacques-Tiura AJ, Baltes BB (2009): Community violence: A meta-analysis on the effect of exposure and mental health outcomes of children and adolescents. Dev Psychopathol 21:227–259. [DOI] [PubMed] [Google Scholar]
- 35.Wright AW, Austin M, Booth C, Kliewer W (2017): Systematic review: Exposure to community violence and physical health outcomes in youth. J Pediatr Psychol 42:364–378. [DOI] [PubMed] [Google Scholar]
- 36.Sams DP, Truscott SD (2004): Empathy, exposure to community violence, and use of violence among urban, at-risk adolescents. Child Youth Care Forum 33:33–50. [Google Scholar]
- 37.van Rooij SJH, Smith RD, Stenson AF, Ely TD, Yang X, Tottenham N, et al. (2020): Increased activation of the fear neurocircuitry in children exposed to violence. Depress Anxiety 37:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prener C, Fox B: Censusxy: Access the U.S. Census Bureau’s geocoding A.P.I. System. Available at: https://CRAN.R-project.org/package=censusxy. Accessed August 19, 2021.
- 39.Nau C, Sidell M, Clift K, Koebnick C, Desai J, Rohm-Young D (2019): A commercially available crime index may be a reliable alternative to actual census-tract crime in an urban area. Prev Med Rep 17:100996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kind AJH, Buckingham WR (2018): Making neighborhood-disadvantage metrics accessible - The neighborhood atlas. N Engl J Med 378:2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eggebrecht AT, Elison JT, Feczko E, Todorov A, Wolff JJ, Kandala S, et al. (2017): Joint attention and brain functional connectivity in infants and toddlers [published correction appears in Cereb Cortex 2020; 30: 3433–3434]. Cereb Cortex 27:1709–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheelock MD, Hect JL, Hernandez-Andrade E, Hassan SS, Romero R, Eggebrecht AT, Thomason ME (2019): Sex differences in functional connectivity during fetal brain development. Dev Cogn Neurosci 36:100632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luby JL, Barch DM, Warner B, Rogers C, Smyser C, Triplett R, et al. (2021): Modeling prenatal adversity/advantage: Effects on birth weight. medRxiv. 10.1101/2021.12.16.21267938. [DOI] [Google Scholar]
- 44.University of Wisconsin School of Medicine and Public Health. 2018 area deprivation index (v.3). Available at: https://www.neighborhoodatlas.medicine.wisc.edu/. Accessed September, 2021.
- 45.National Cancer Institute: The Healthy Eating Index - population ratio method. Available at: https://epi.grants.cancer.gov/hei/population-ratio-method.html. Accessed September, 2021.
- 46.National Cancer Institute: Background on Diet History Questionnaire II (DHQ-II) for U.S. & Canada. Available at: https://epi.grants.cancer.gov/dhq2/about/. Accessed September, 2021.
- 47.Diet*Calc Analysis Program [Computer software]. Version 1.5.0. (2012). Bethesda, MD: National Cancer Institute, Epidemiology and Genomics Research Program. [Google Scholar]
- 48.Diet History Questionnaire, version 2.0 (2010): National Institutes of Health, Epidemiology and Genomics Research Program. National Cancer Institute. [Google Scholar]
- 49.Boutwell BB, Nelson EJ, Emo B, Vaughn MG, Schootman M, Rosenfeld R, Lewis R (2016): The intersection of aggregate-level lead exposure and crime. Environ Res 148:79–85. [DOI] [PubMed] [Google Scholar]
- 50.Cox JL, Holden JM, Sagovsky R (1987): Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 150:782–786. [DOI] [PubMed] [Google Scholar]
- 51.Cohen S, Kamarck T, Mermelstein R (1983): A global measure of perceived stress. J Health Soc Behav 24:385–396. [PubMed] [Google Scholar]
- 52.Slavich GM, Shields GS (2018): Assessing lifetime stress exposure using the Stress and Adversity Inventory for Adults (Adult STRAIN): An overview and initial validation. Psychosom Med 80:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis TT, Yang FM, Jacobs EA, Fitchett G (2012): Racial/ethnic differences in responses to the everyday discrimination scale: A differential item functioning analysis. Am J Epidemiol 175:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, et al. (2019): Welcome to the tidyverse. J Open Source Softw 4:1686. [Google Scholar]
- 55.Revelle W: psych: Procedures for psychological, psychometric, and personality research. Available at: https://CRAN.R-project.org/package=psych. Accessed February 17, 2022.
- 56.Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57:289–300. [Google Scholar]
- 57.Dunn OJ (1961): Multiple comparisons among means. J Am Stat Assoc 56:52–64. [Google Scholar]
- 58.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K (2014): mediation: R package for Causal Mediation Analysis. J Stat Softw 59:1–38.26917999 [Google Scholar]
- 59.White SF, Voss JL, Chiang JJ, Wang L, McLaughlin KA, Miller GE (2019): Exposure to violence and low family income are associated with heightened amygdala responsiveness to threat among adolescents. Dev Cogn Neurosci 40:100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sherman SM, Guillery RW (2002): The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci 357:1695–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steriade M, Llinás RR (1988): The functional states of the thalamus and the associated neuronal interplay. Physiol Rev 68:649–742. [DOI] [PubMed] [Google Scholar]
- 62.Taber KH, Wen C, Khan A, Hurley RA (2004): The limbic thalamus. J Neuropsychiatry Clin Neurosci 16:127–132. [DOI] [PubMed] [Google Scholar]
- 63.Salay LD, Ishiko N, Huberman AD (2018): A midline thalamic circuit determines reactions to visual threat. Nature 557:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, et al. (2015): The paraventricular thalamus controls a central amygdala fear circuit. Nature 519:455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsu DT, Kirouac GJ, Zubieta JK, Bhatnagar S (2014): Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front Behav Neurosci 8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reinelt J, Uhlig M, Müller K, Lauckner ME, Kumral D, Schaare HL, et al. (2019): Acute psychosocial stress alters thalamic network centrality. Neuroimage 199:680–690. [DOI] [PubMed] [Google Scholar]
- 67.Bhatnagar S, Viau V, Chu A, Soriano L, Meijer OC, Dallman MF (2000): A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. J Neurosci 20:5564–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaferi A, Bhatnagar S (2006): Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology 147:4917–4930. [DOI] [PubMed] [Google Scholar]
- 69.Colonnese MT, Phillips MA (2018): Thalamocortical function in developing sensory circuits. Curr Opin Neurobiol 52:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peters SK, Dunlop K, Downar J (2016): Cortico-striatal-thalamic loop circuits of the salience network: A central pathway in psychiatric disease and treatment. Front Syst Neurosci 10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paternoster R, Brame R, Mazerolle P, Piquero A (1998): Using the correct statistical test for the equality of regression coefficients. Criminology 36:859–866. [Google Scholar]
- 72.Dawe K, Montgomery A, McGee H, Panagopoulou E, Morgan K, Hackshaw L, Vedhara K (2016): The effects of perceived stress on biological parameters in healthcare professionals: A systematic review. J Health Psychol 21:607–618. [DOI] [PubMed] [Google Scholar]
- 73.van Eck M, Berkhof H, Nicolson N, Sulon J (1996): The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom Med 58:447–458. [DOI] [PubMed] [Google Scholar]
- 74.Dickerson SS, Kemeny ME (2004): Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull 130:355–391. [DOI] [PubMed] [Google Scholar]
- 75.Herzberg MP, Hunt RH, Thomas KM, Gunnar MR (2020): Differential brain activity as a function of social evaluative stress in early adolescence: Brain function and salivary cortisol. Dev Psychopathol 32:1926–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slavich GM (2020): Psychoneuroimmunology of stress and mental health. In: Harkness KL, Hayden EP, editors. The Oxford Handbook of Stress and Mental Health. Oxford, United Kingdom: Oxford University Press, 519–546. [Google Scholar]
- 77.Miller GE, Chen E, Armstrong CC, Carroll AL, Ozturk S, Rydland KJ, et al. (2018): Functional connectivity in central executive network protects youth against cardiometabolic risks linked with neighborhood violence. Proc Natl Acad Sci U S A 115:12063–12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


