Summary
Background
Non-alcoholic steatohepatitis (NASH) is the second-leading indication for liver transplantation (LT) worldwide and is projected to become the leading indication. Our study aimed to determine clinical variables that predict post-LT survival in NASH.
Methods
A systematic review and meta-analysis was performed. On June 18, 2020 and April 28, 2022, Ovid MEDLINE ALL, Ovid Embase, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials were searched. No date limits were applied. Inclusion criteria specified the type of study and our study's population/comparison and outcome/timepoints. Pediatric, animal, retransplantation-only, and studies classifying cryptogenic cirrhosis patients with body mass index (BMI) <30 as NASH were excluded. Studies with duplicate cohorts and missing information were excluded from the meta-analysis. Studies were appraised using the Newcastle–Ottawa Scale. This study was preregistered in PROSPERO (CRD42020196915).
Findings
Out of 8583 studies identified, 25 studies were included in the systematic review, while 5 studies were included in the meta-analysis. Our quantitative review suggested that the following variables were predictive of post-LT NASH patient survival: recipient age, functional status, pre-LT hepatoma, model for end-stage liver disease (MELD) score, diabetes mellitus (DM), pre-LT dialysis, hepatic encephalopathy, portal vein thrombosis, hospitalization/ICU at LT, and year of LT. Predictors of graft survival included recipient age, BMI, pre-LT dialysis, and DM. Our pooled meta-analyses included five predictors of patient survival. Increased patient mortality was associated with older recipient age (HR=2·07, 95%CI: 1·71-2·50, I2=0, τ2=0, p=0·40) and pretransplant DM (HR=1·18, 95%CI: 1·08-1·28, I2=0, τ2=0, p=0·76).
Interpretation
Our systematic review and meta-analysis aimed to synthesise predictive variables of mortality in LT NASH patients. Clinically, this might help to identify modifiable risk factors that can be optimized in the post-transplant setting to improve patient outcomes and optimises decision making in the resource-limited LT setting.
Funding
Toronto General and Western Hospital Foundation.
Keywords: NAFLD, NAFL, Fatty liver, NASH, Liver, Steatohepatitis, Transplantation, Transplant, Survival
Research in context.
Evidence before this study
On June 2020 and April 2022, systematic reviews and meta-analyses were sought from the following databases: Ovid MEDLINE ALL, Ovid Embase, and Cochrane. No language restrictions were employed. A broad search strategy was developed for Ovid Medline by combining subject headings Non-alcoholic steatohepatitis (NASH)/ Non-alcoholic fatty liver disease (NAFLD) and “liver transplantation” and “systematic$review” or “meta$analysis”. Only animal studies and grey literature were excluded. Four meta-analyses analyzing post- liver transplantation (LT) outcomes in NASH vs. non-NASH existed. Three of these did not analyze clinical predictors of survival. Six recent systematic reviews/meta-analyses also analyzed the impact of living donor LT (LDLT), COVID-19 infection, bariatric surgery, cardiovascular disease, sarcopenic obesity, and donor BMI on survival in LT recipients, but not in NASH patients specifically.
Added value of this study
The present study employed a broad search and inclusion criteria that was not limited to studies comparing NASH to non-NASH patients, allowing us to identify a larger number of studies. Our quantitative review delineates variables whose hazard ratios significantly predicted post-LT patient survival (recipient age, functional status, pre-LT hepatoma, MELD score, diabetes mellitus (DM), pre-LT dialysis, hepatic encephalopathy, portal vein thrombosis, hospitalization/ICU at LT, year of LT) and graft survival (recipient age, BMI, pre-LT dialysis, DM).
Implications of all the available evidence
To the best of our knowledge, our study is the first to identify several predictive clinical variables with a significant impact on patient and graft survival in post-LT NASH patients. Some of these variables are related to modifiable risk factors that clinicians could use to optimize their patients in the pre- and post-LT setting.
Alt-text: Unlabelled box
Introduction
With the rise in diabetes mellitus (DM) and obesity, non-alcoholic fatty liver disease (NAFLD) has become prevalent worldwide. The estimated prevalence of non-alcoholic steatohepatitis (NASH) is 3-5% globally.1, 2, 3, 4 NASH is expected to affect over 25 million Americans by 2025, and medications are currently limited.5,6 With the curability of hepatitis C virus (HCV) after the advent of direct acting antivirals, NASH has become the second-leading indication for wait-listed adults in the United States, and is expected to become the leading indication for liver transplantation (LT).1,2,7 Currently, it is the leading indication in females without hepatocellular carcinoma (HCC) and older patients.8
NASH often occurs with metabolic co-morbidities including DM, obesity, dyslipidemia, and renal disease. As such, NASH patients are at risk of morbidity and mortality post-LT. Namely, NASH LT recipients have more than double the risk of major cardiovascular events compared to other indications.9 NASH has also been associated with heightened risk of de novo malignancy post-LT.10,11 Before listing NASH patients for transplant, it is critical to accurately determine the risk factors that impact their survival post-LT, as modifiable risks may be more easily mitigated in the pretransplant setting.
Guidelines and recommendations established for the assessment and management of NASH cirrhosis are based on consensus opinion.12 To our knowledge, no systematic reviews or meta-analysis that explore predictors of survival in NASH-only cohorts in the post-LT setting exist. In the current meta-analysis, our aim was to identify predictors of post-transplant survival in NASH to provide modifiable targets and thereby enhance outcomes.
Methods
Search strategy and selection criteria
A search strategy was developed for Ovid Medline using database-specific subject headings and text words that combined the concepts of NASH/NAFLD and liver transplantation. The search strategy was customized for each database. (Appendix I) Searches were performed in the following databases on June 18, 2020 and updated April 28, 2022: Ovid MEDLINE ALL, Ovid Embase, Cochrane Database of Systematic Reviews (Ovid), and Cochrane Central Register of Controlled Trials (Ovid). Animal studies and book/conference materials were excluded. Reference lists of included studies were searched.
Eligibility criteria was based on study design, population, and outcomes. The specific eligibility criteria are defined in Appendix II. This study's original protocol included non-English texts; however, COVID-19 impacted access to library translation services and these were later excluded. We also excluded clinical trials due to the paucity of studies exploring pharmacologic interventions. Our study protocol was pre-registered in PROSPERO (CRD42020196915) and these two changes were updated accordingly.
Abstract and full-text eligibility was determined using separate questionnaires that were optimized through 100-reference pilot screens. Abstract and full-text eligibility was determined by two independent reviewers, who reviewed each source in duplicate (AM/LH and AM/NS, respectively. Discrepancies in rationales for study eligibility were independently reviewed and resolved by a third-party (FQ) using Covidence. As many studies included in the systematic review derived their population from identical transplant registries across overlapping time periods, it was possible that duplicate cohorts existed. As such, to avoid bias from pooling duplicate data in our meta-analysis, studies identified at risk of having overlapping cohorts were excluded from our meta-analysis according to prespecified criteria. (Appendix III) Finally, some studies were included in the meta-analysis; however, a pooled analysis was inappropriate as they employed cut-offs to stratify predictors into value categories. This was the case for three predictors (recipient age, body mass index- BMI, and MELD), and because associated studies employed different categorical cut-offs, results were not compared in a meta-analysis.
Data analysis
Data was collected by two independent reviewers (AM and NS) using a predetermined table. Data collected included the type of study, groups/subgroups, sample size, patient/donor demographic, pre/post-transplant clinical variables, graft variables, and operative variables. To identify predictive variables impacting survival for patients undergoing LT for NASH, an a priori list of demographic and clinical variables was devised using evidence-based and tacit knowledge from Transplant Hepatologists (MB, FQ). Although a predetermined list was drafted a priori, missed variables later identified during data collection were added, which were specified as additions. To quantify the predictive effect of these variables on patient and graft post-LT survival, we extracted hazard ratios obtained from studies’ Cox-proportional hazards regression models. This was collected at the 1-, 3-, 5-, 10-year, and overall timepoints. Hazard ratios were only extracted if the model adjusted for confounding variables relevant to NASH LT patients, which are outlined in the legend of Appendix VI. Furthermore, odds ratios were collected from studies analyzing survival in NASH-only subgroups that were stratified according to predictive variables. These descriptive characteristics were expressed as frequency (%) or mean±SD, or as hazard ratios if provided. As studies expressing data according to the latter format were identified to be at high risk of bias, their effect sizes were not included in the main tables of this systematic review, nor in our meta-analysis. For completeness, basic information pertaining to these high-risk studies were included in the Appendices.
Hazard ratios from studies in the systematic review were pooled in the meta-analysis if there were two or more studies without duplicate cohorts that explored a predictive variable. Estimates of log-hazard ratios and standard errors of patient and graft survival at 1, 3, 5, 10-year and overall timepoints were pooled, if it met the above criteria. Pooled outcomes are displayed as Forest plots. For all respective statistical tests, significance was deemed if confidence intervals did not cross the no-difference line.
To assess heterogeneity, the inconsistency index (I² statistic) was used. Substantial heterogeneity was defined as an I2 value >50% or a Q-test yielding a p-value less than 0·1. When trials were heterogeneous, random-effects model was used to calculate the pooled odds ratio and 95% CI. Fixed-effects models were otherwise used. To predict risk of publication bias, counter-enhanced funnel plots were assessed for symmetry. Outliers with significant treatment estimates were excluded in the sensitivity analysis.
The study protocol initially intended to conduct subgroup analyses for various confounding variables to analyze their effects on heterogeneity within the pooled data. However, as all pooled analyses contained two studies, this was not possible. Furthermore, if studies were deemed suspicious for publication bias, we also intended on conducting a sensitivity analysis after excluding outliers; however, the limited number of studies included in the pooled analysis prevented this. All statistical analyses are performed in R (version 3·6·3) by meta and metasens Packages.
As all included references were cohort studies, the Ottawa Newcastle Scale was used for critical appraisal.13 To reduce the effects of study bias on our analysis, studies flagged for high risk of bias were excluded from our synthesis of results.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors in the study had access to the data. Adam Minich, Noor-ul Saba Shaikh, and Fakhar Ali Qazi Arisar verified the data set. All authors were responsible for making the decision to submit this manuscript.
Results
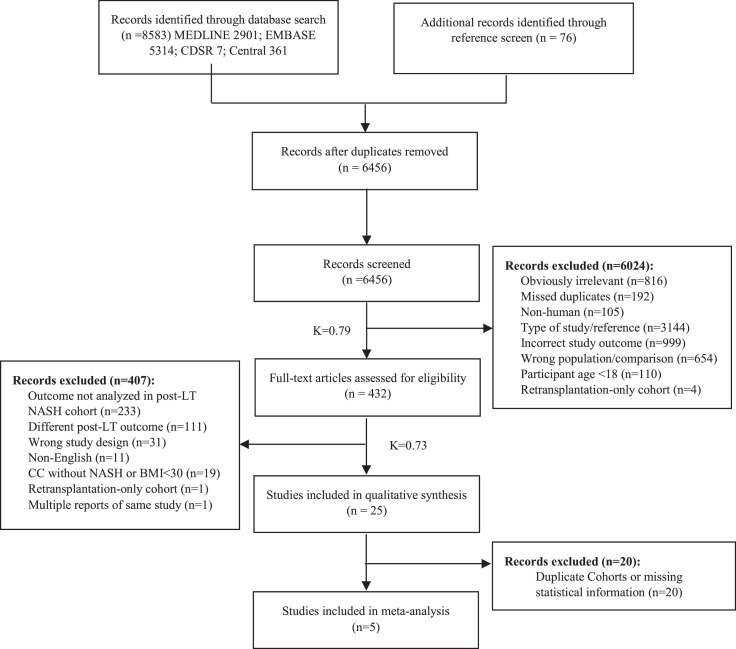
The literature search yielded 8,583 titles and abstracts. 76 additional references were identified by screening 241 reference lists (Figure 1).
Figure 1.
Study selection.
The qualitative synthesis refers to the systematic review, where results from eligible studies were collected and tabulated separately from the meta-analysis stage. Studies were classified as being the “wrong type of study” if they were not observational studies, randomized control trials, systematic reviews, or meta-analyses. “Retransplantation-only” cohorts were solely composed of participants that had previously undergone liver re-transplantation. Inter-rater reliability values were calculated using Cohen's Kappa (Κ).
Twenty-five studies, which explored 27 predictors of patient survival and 11 predictors of graft survival in post-LT NASH patients were included in the systematic review. All studies were cohort studies. Clinically relevant baseline prognostic factors and pertinent study information are displayed in Appendix IV. The definition of NASH in these studies is outlined in Appendix V. Generally, NASH participants had a higher baseline BMI, and a high prevalence of obesity, DM, and hypertension (HTN). Five studies excluded HCC participants.
Bias was limited to studies that did not employ an adjusted Cox-proportional regression model to analyze the effects of various predictive variables. This was often the case for studies conducting survival analyses of NASH subgroups stratified according to various predictors. Studies only using adjusted analyses and were at low risk of bias were tabulated in the results. A breakdown of the risk of bias for each study and their outcome(s) are found in Appendix VI.
25 predictors were associated with statistically significant differences for patient survival, while 11 predictors were significant for graft survival, in at least one study (Tables 1 and 2). The following predictors of patient and/or graft survival were found to be significant and not declared a priori: hospitalization/ventilator support at the time of LT, every 90 days on wait list, retransplantation, era/year of LT, end-stage renal disease, atrial fibrillation, non-calcineurin inhibitor-based immunosuppression, post-LT hypertension, and length of hospital stay. The following variables predicting patient survival had significant HRs with similar directionality across all studies in which they were significant (≥2 studies): recipient age, functional status, HCC, MELD at transplant, DM, dialysis prior to LT, hepatic encephalopathy, era/year of LT, portal vein thrombosis, hospitalization or ICU at LT, and length of hospital stay. The following predictors of graft survival had significant HRs with similar directionality in all studies (≥2 studies): recipient age, dialysis prior to LT, and DM. For both patient and graft survival, hazard ratios of recipient BMI were found to be significant across multiple studies, but different threshold reference BMIs were employed, making it difficult to assess similarities in effect size directionality.
Table 1.
Predictors of patient survival deemed significant or unspecified by included studies.
| Predictor Category | Timepoint | Studies which Predictor was Significant | Predictor(s) Significant on AnalysisNo p-value provided, but deemed significant by studyANo p-value provided, nor deemed significant by studyB | Control/Reference Group | HR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Recipient Age at Transplant | Overall | Nagai (2019)14 Dimou (2016)15 Haldar (2019)16 McCabe (2020)17 Agbim (2019)18 Kaswala (2020)19 Henson (2020)20 Rinella (2022)21 Shavelle (2022)22 Yuan (2022)23 |
60–64 years 65–69 years 70+ years 50–64A ≥65a 61–65 >65 50–64 >65 Age at transplant: continuous Age at transplant: continuous Age at transplant: continuousB >65 Age at transplant: continuous Age at transplant: continuous |
Vs. <50 Vs. <50 Vs. <50 Vs. <50 Vs. <50 Vs. ≤45 Vs. ≤45 Vs. <50 Vs. <50 NR NR NR Vs. <55 Continuous Continuous |
1.66 (1.31–2.11) 2.08 (1.63–2.64) 2.66 (1.98–3.57) 1.27 (1.01–1.59) 2.02 (1.58–2.58) 2.07 (1.39–3.08) 1.72 (1.10–2.81) 1.54 (1.29–1.84) 2.14 (1.74–2.64) 1.04 (1.03–1.05) 1.02 (1.01–1.03) NR 1.70 (1.04–2.77) 1.04 (NR) 1.24 (1.17–1.32) |
<0.001 <0.001 <0.001 NR NR <0.001 <0.05 <0.001 <0.001 <0.001 <0.001 NR 0.04 <0.0001 <0.001 |
| 1 year | Nagai (2019)14 | 50–59 60–64 65–69 70+ |
Vs. <50 Vs. <50 Vs. <50 Vs. <50 |
1.61 (1.13–2.3) 1.85 (1.29–2.66) 2.45 (1.7–3.52) 2.95 (1.92–4.54) |
0.008 0.001 <0.001 <0.001 |
|
| 5 years | Henson (2020)20 Karnam (2022)24 |
Age at transplantB: continuous Age at transplant: continuous |
Continuous Continuous |
NR 1.02 (1.01–1.03) |
NR <0.00001 |
|
| Ethnicity | Overall | Nagai (2019)14 Ochoa–Allemant (2020)25 Rinella (2022)21 Yuan (2022)23 |
Ethnicity: Asian Ethnicity: HispanicA Ethnicity: Black Ethnicity: Hispanic/Latino Ethicity: Asian |
Vs. white Vs. non-Hispanic Vs. white Vs. white Vs. white |
0.38 (0.2–0.75) 0.84 (0.71–0.99) 5.25 (2.12–12.96) 0.78 (0.68–0.89) 0.67 (0.46–0.97) |
0.05 NR 0.0003 <0.001 0.03 |
| Functional Status | Overall | Nagai (2019)14 McCabe (2020)17 Shavelle (2022)22 |
Karnosfky score: 10%–30% KPS 3 (40–50%) KPS 4 (10–30%) Karnosfky score: 0–60% |
Vs. 70%–100% Vs. KPS 1 (80–100%) Vs. KPS 1 (80–100%) Vs. 70–100% |
1.7 (1.36–2.13) 1.52 (1.29–1.80) 2.13 (1.8–2.52) 1.57 (NR) |
<0.001 <0.001 <0.001 <0.001 |
| 5 years | Henson (2020)20 Karnam (2022)24 |
Functional status (age ≥65 only subgroup): Some assistanceC Total assistanceD Functional status: Some assistanceC Total assistanceD |
Vs. No assistanceE Vs. No AssistanceE Vs. No assistanceE Vs. No AssistanceE |
1.17 (0.81–1.69) 1.72 (1.18–2.5) 1.16 (1.02–1.32) 1.30 (1.13–1.50) |
0.003 0.003 0.03 0.0003 |
|
| Recipient HCC+ | Overall | Nagai (2019)14 McCabe (2020)17 Kaswala (2020)19 Yuan (2022)23 |
HCC+ HCC+ HCC+ HCC+ |
Vs. no HCC Vs. no HCC Vs. no HCC Vs. no HCC |
1.25 (1.04–1.5) 1.23 (1.08–1.41) 1.37 (1.19–1.58) 1.19 (1.08–1.32) |
0.02 0.002 <0.001 <0.001 |
| Recipient MELD at Transplant | Overall | Dimou (2016)15 Haldar (2019)16 Kaswala (2020)19 Henson (2020)20 Shavelle (2022)22 |
30–39A ≥40a >23 MELD score: overallY MELD score: overallY (age ≥65 only subgroup) 25–40 |
Vs. <20 Vs. <20 Vs. <11 NR NR Vs. 6–10 |
1.86 (1.53–2.23) 2.0 (1.49–2.69) 1.48 (1.04–2.3) 1.01 (1.01–1.02) 1.02 (1.01–1.03) 1.38 (NR) |
NR NR <0.05 <0.001 0.004 0.01 |
| Recipient Diabetes Mellitus pre-LT | Overall | Nagai (2019)14 Dimou (2016)15 Agbim (2019)18 Shavelle (2022)22 Yuan (2022)23 |
DM+ DM+A DM+ DM+ DM+ |
Vs. no DM Vs. no DM NR Vs. no DM Vs. no DM |
1.14 (1.01–1.29) 1.26 (1.09–1.45) 1.13 (0.99–1.29) 1.18 (NR) 1.18 (1.09–1.29) |
0.04 NR <0.001 0.004 <0.001 |
| Recipient BMI | Overall | Nagai (2019)14 Dimou (2016)15 Haldar (2019)16 Agopian (2012)26 Shavelle (2022) Yuan (2022)23 |
30–34.9 35–39.9 25–29a 30–34a 35–39a ≥40a ≤18.5 >18.5, ≤25.0 >40 >35 25–30 ≥30 Continuous |
Vs. 18.5–24.9 Vs. 18.5–24.9 Vs. <25 Vs. <25 Vs. <25 Vs. <25 >25.0,≤30.0 >25.0, ≤30.0 >25.0, ≤30.0 <35 Vs. 18–25 Vs. 18–25 Continuous |
0.73 (0.6–0.89) 0.82 (0.66–1.01) 0.7 (0.56–0.88) 0.7 (0.56–0.88) 0.75 (0.59–0.95) 0.73 (0.55–0.98) 4.29 (1.01–18.21) 2.24 (1.27–3.96) 1.96 (1.16–3.32) 2.3 (NR) 0.7 (NR) 0.69 (NR) 0.99 (0.98–0.99) |
<0.001 0.06 NR NR NR NR <0.05 <0.05 <0.05 0.039 0.01 <0.01 <0.001 |
| 10 years | Satapathy (2020)27 | 25 to <30 ≥30 to <35 ≥35 to <40 ≥40 |
Vs. ≥18 to <25 Vs. ≥18 to <25 Vs. ≥18 to <25 Vs. ≥18 to <25 |
0.62 (0.48–0.79) 0.68 (0.54–0.87) 0.66 (0.51–0.86) 0.64 (0.46–0.89) |
<0.001 <0.001 <0.001 0.007 |
|
| Dialysis Prior to LT | Overall | Agopian (2012)26 Zhang (2019)28 Henson (2020)20 Shavelle (2022)22 Yuan (2022)23 |
Dialysis prior to LT Dialysis prior to LT Dialysis prior to LT (age ≥65 only subgroup) Dialysis 1 week prior to LT Dialysis 1 week prior to LT |
Vs. no dialysis Vs. no dialysis Vs. no dialysis Vs. no dialysis Vs. no dialysis |
2.5 (NR) 1.4 (1.07–1.84) 1.74 (1.24–2.44) 1.86 (NR) 1.53 (1.36–1.71) |
0.029 0.015 0.001 <0.0001 <0.001 |
| 5 years | Karnam (2022)24 | Dialysis 1 week prior to LT | Vs. no dialysis | 2.10 (1.66–2.66) | <0.00001 | |
| Hepatic Encephalopathy | Overall | Nagai (2019)14 Kaswala (2020)19 Shavelle (2022)22 |
Hepatic encephalopathy (grade III & IV) Hepatic encephalopathy: Grade I & II Grade III & IV Hepatic encephalopathy: Grade I & II Grade III & IV |
NR Vs. none Vs. none Vs. none Vs. none |
1.31 (1.11–1.56) 1.16 (1.01–1.34) 1.74 (1.43–2.12) 1.20 (NR) 1.79 (NR) |
0.002 0.02 <0.001 0.009 <0.0001 |
| 5 years | Karnam (2022)24 | Hepatic encephalopathy | Vs. none | 1.15 (1.00–1.31) | 0.04 | |
| Ventilatory support or ICU at LTX | Overall | Zhang (2019)28 Shavelle (2022)22 Shavelle (2022)22 |
Hospitalized not ICU at LT Not hospitalized at LT Hospitalized not ICU at LT Hospitalized and ICU at LT Ventilator at LT |
Vs. ICU at LT Vs. ICU at LT Vs. not hospitalized Vs. not hospitalized Vs. no ventilator |
0.74 (0.57–0.96) 0.6 (0.45–0.78) 1.50 (NR) 2.23 (NR) 2.05 (NR) |
0.022 0.001 <0.0001 <0.0001 <0.0001 |
| 5 years | Karnam (2022)24 | Ventilator at LT | Vs. no ventilator | 1.33 (1.05–1.69) | 0.019 | |
| Era/year of LTX | Overall | Nagai (2019)14 Kaswala (2020)19 Shavelle (2022)22 Yuan (2022)23 |
Era/year of LT: 2014–2015 Year of transplant Year of transplant: continuous Year of LT: 2010–2014 Year of LT: 2015–2019 |
Vs. 2008–2010 NR Continuous 2004–2009 2004–2009 |
0.8 (0.66–0.96) 0.97 (0.96–0.99) 0.97 (NR) 0.79 (0.70–0.87) 0.77 (0.69–0.87) |
0.02 0.02 0.0008 <0.001 <0.001 |
| Re–transplantationX | Overall | Nagai (2019)14 | Retransplantation | First time transplant | 1.75 (1.16–2.65) | 0.01 |
| Serum bilirubin | Overall | Agbim (2019)18 | TSB | NR | 1.02 (1.01–1.02) | <0.001 |
| Portal vein thrombosis | Overall | Agbim (2019)18 Shavelle (2022)22 Yuan (2022)23 |
PVT+ PVT+ PVT+ |
Vs. no PVT Vs. no PVT Vs. no PVT |
1.31 (1.09–1.58) 1.22 (NR) 1.24 (1.12–1.37) |
<0.001 0.01 <0.001 |
| Every 90d on wait listX | Overall | Zhang (2019)28 | Every 90d on waitlist | NR | 1.04 (1.01–1.07) | 0.017 |
| Cold ischemia time | Overall | Henson (2020)20 Yuan (2022)23 |
Cold ischemia time (recipient age ≥65 subgroup) Cold ischemia time (hours)- continuous |
NR Continuous |
1.06 (1.01–1.1) 1.02 (1.01–1.03) |
0.01 0.007 |
| Post-LT biopsy steatosis | 1 year | Malik (2009)29 | Post-LT biopsy steatosis | Vs. Non-steatosis | N/A (% analysis, not regression) | 0.01 |
| Recipient Sex | Overall | McCabe (2020)17 Haldar (2019)16 Rinella (2022)21 |
Male Male Male |
Vs. Female Vs. Female Vs. Female |
1.19 (1.07–1.32) 0.79 (0.63–0.98) 1.27 (0.92–1.75) |
<0.001 <0.05 0.15 |
| Recipient ESRDX | Overall | Rinella (2022)21 | ESRD | Vs. no ESRD | 1.55 (1.04–2.31) | 0.03 |
| Pre-LT Atrial FibrillationX | Overall | Rinella (2022)21 | Atrial fibrillation | Vs. no atrial fibrillation | 1.95 (1.06–3.57) | 0.03 |
| Post-LT HTNX | Overall | Rinella (2022)21 | Post-LT HTN | Vs. no HTN post-LT | 0.55 (0.37–0.79) | 0.002 |
| Immunosuppr- essionX |
Overall | Rinella (2022)21 | Non-calcineurin inhibitor (CNI) regimen | Vs. CNI alone | 2.05 (1.19–3.51) | 0.009 |
| Length of Hospital StayX | Overall | Shavelle (2022)22 Yuan (2022)23 |
11–30 days 31+ days 30 day interval |
Vs. 0–10 days Vs. 0–10 days NR |
1.22 (NR) 2.40 (NR) 1.20 (1.18–1.22) |
0.002 <0.0001 <0.001 |
| DCD Donor | Overall | Nagai (2019)14 | DCD Donor | NR | 1.46 (1.16–1.86) | 0.001 |
| Donor age at death | Overall | Agopian (2012)26 Yuan (2022)23 |
Donor age at death/donation >55y Donor age (10 year interval) |
NR NR |
2.3 (NR) 1.03 (1.00–1.05) |
0.024 0.03 |
| Donor blood group | Overall | Haldar (2019)16 | Donor Blood group: B | Vs. A | 0.37 (0.22=0.63) | <0.001 |
| Donor sex | Overall | Henson (2020)20 Haldar (2019)16 Nagai (2019)14 |
Male (age ≥65) Male Female |
Vs. Female (age ≥65) Vs. Female Vs. Male |
0.8 (0.54–1.0) 0.97 (0.78–1.2) 1.1 (0.97–1.25) |
0.05 NS 0.12 |
Some assistance needed = “some dependance” or KPS 50%–70%.
Full assistance needed = “total dependance” or KPS 10%–40%.
No assistance = “no dependance” or Karnosky Performance Status 80%–100%.
Not an a priori determined variable- added in during data extraction.
At listing.
Table 2.
Predictors of graft survival deemed significant or unspecified by included studies.
| Predictor Category | Timepoint | Studies which Predictor was Significant | Predictor(s) Significant on AnalysisNo p-value provided, but deemed significant by studyANo p-value provided, nor deemed significant by studyB | Control/Reference Group | HR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Recipient age | Overall | Nagai (2019)14 Dimou (2016)15 Agbim (2019)18 |
60–64 65–69 70+ Age at transplantA: ≥65 Age at transplant: overall |
Vs. <50 Vs. <50 Vs. <50 <50 NR |
1.8 (1.2–2.71) 2.11 (1.39–3.21) 2.65 (1.5–4.69) 1.73 (1.38–2.18) 1.03 (1.02–1.04) |
0.004 <0.001 0.02 NR <0.001 |
| 1 year | Nagai (2019)14 | 50–59 60–64 65–69 70+ |
Vs. <50 Vs. <50 Vs. <50 Vs. <50 |
1.42 (1.05–1.92) 1.53 (1.12–2.08) 1.98 (1.45–2.71) 2.19 (1.49–3.22) |
0.02 0.007 <0.001 <0.001 |
|
| Recipient BMI | Overall | Dimou (2016)15 Agopian (2012)26 |
BMIA: 25–29 30–34 35–39 BMI: >35 |
Vs. <25 Vs. <25 Vs. <25 <35 |
0.7 (0.56–0.87) 0.74 (0.6–0.92) 0.79 (0.63–0.99) 2.1 (NR) |
NR NR NR 0.039 |
| 10 years | Satapathy (2020)27 | BMI: 25 to <30 ≥30 to <35 ≥35 to <40 ≥40 |
Vs. ≥18 to <25 Vs. ≥18 to <25 Vs. ≥18 to <25 Vs. ≥18 to <25 |
0.62 (0.48–0.79) 0.68 (0.54–0.87) 0.66 (0.51–0.86) 0.64 (0.46–0.89) |
<0.001 0.004 0.01 0.008 |
|
| Dialysis prior to LT | Overall | Agopian (2012)26 Zhang (2019)28 |
Dialysis prior to LT Dialysis prior to LT |
Vs. no dialysis Vs. no dialysis |
2.2 (NR) 1.4 (1.07–1.84) |
0.037 0.015 |
| Diabetes Mellitus | Overall | Dimou (2016)15 Agbim (2019)18 |
Diabetes mellitusA Diabetes mellitusA |
Vs. no DM Vs. no DM |
1.21 (1.06–1.39) 1.11 (0.98–1.25) |
NR <0.001 |
| Serum bilirubin | Overall | Agbim (2019)18 | TSB | NR | 1.01 (1.01–1.02) | <0.001 |
| Portal Vein Thrombosis | Overall | Agbim (2019)18 | Portal vein thrombosis | NR | 1.37 (1.15–1.63) | <0.001 |
| Ventilatory Support or ICU at LTX | Overall | Zhang (2019)28 | Ventilatory support or ICU at LT: Hospitalized not ICU Not hospitalized |
Vs. ICU Vs. ICUI |
0.72 (0.56–0.94) 0.57 (0.43–0.75) |
0.014 <0.0001 |
| Every 90d on wait listX | Overall | Zhang (2019)28 | Every 90d on waiting list | NS | 1.04 (1.01–1.07) | 0.011 |
| Recipient MELD | Overall | Dimou (2016)15 | MELDA: 30–39 ≥40 |
Vs. <20 Vs. <20 |
1.86 (1.53–2.23) 2.0 (1.49–2.69) |
NR NR |
| Type of Donor | Overall | Dimou (2016)15 | Type of Donor: Extended criteria donorB |
Vs. no ECD |
1.18 (1.03–1.36) |
NR |
| Allograft MacrosteatosisX | 1 year | Altshuler (2022)30 | Macrosteatosis ≥30% | Vs. <30% | 1.44 (1.01–2.06) | 0.05 |
Not an a priori determined variable- added in during data extraction.
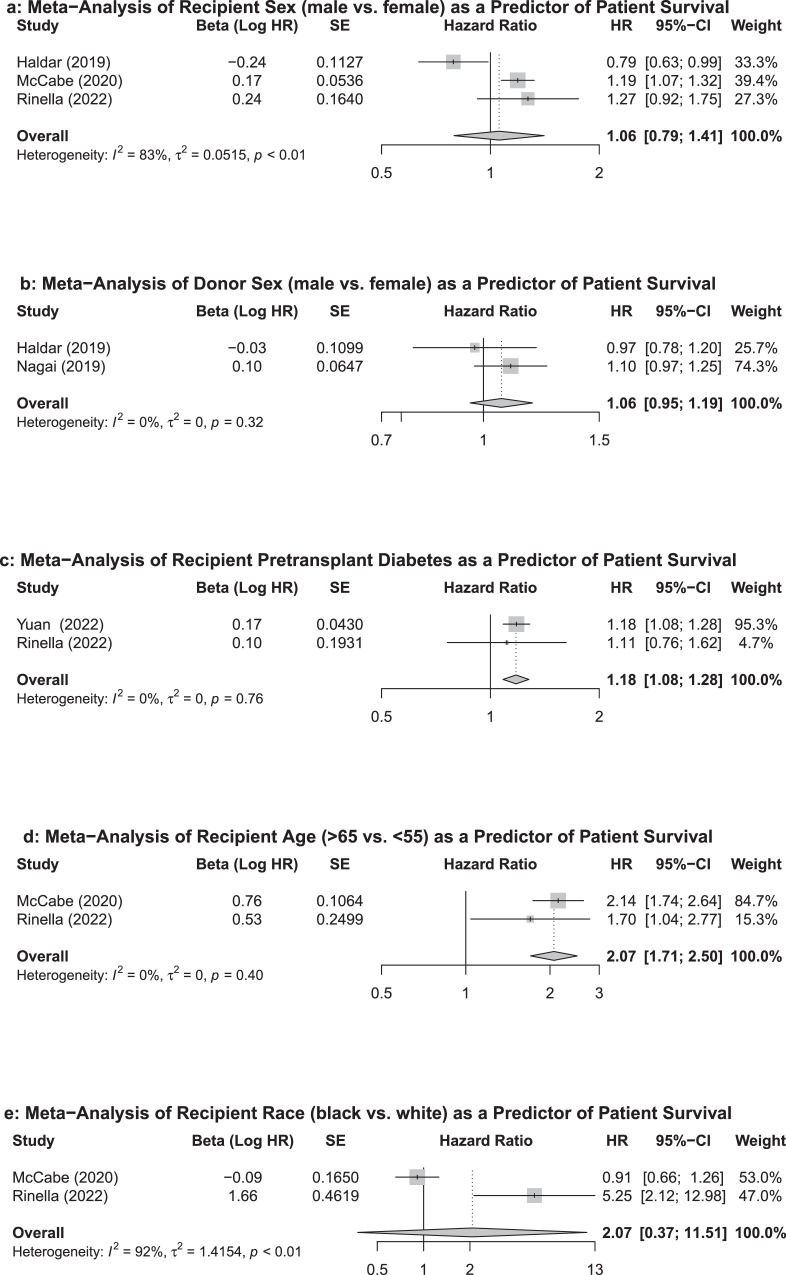
Five studies, which explored five predictors of patient survival, satisfied eligibility criteria for the meta-analysis. The pooled HRs for the three studies exploring recipient sex as a predictor of patient survival showed no association (HR=1·06 95%CI: 0·79-1·41) (Figure 2a). Random effects estimates were used due to observed heterogeneity (I2=83%, τ2=0·0515, p<0·01). The two studies exploring the effects of donor sex on patient survival also did not show association (HR=1·06 95%CI: 0·95-1·19) (Figure 2b). A fixed effects estimate was used (I2=0, τ2=0, p=0·32). Pooled results demonstrate that recipient pretransplant DM significantly decreases post-LT survival (HR=1·18, 95%CI: 1·08-1·28) (Figure 2c). A fixed effects estimate was used (I2=0, τ2=0, p=0·76). Older age (>65 years vs. <50 years) also predicted a poorer patient survival on pooled analysis (HR=2·07, 95%CI: 1·71-2·50) when fixed effects were used (I2=0, τ2=0, p=0·40) (Figure 2d). Finally, black (vs. white) race had no significant predictive effect on patient survival on pooled analysis (HR=2·07, 95%CI: 0·37-11·51) (Figure 2e). Random effects estimates were used due to high heterogeneity (I2=92%, τ2=1.4154, p<0.01). Some studies met eligibility for inclusion in the meta-analysis but their hazard ratios could not be pooled due to incongruent predictor variable cut-off values (Table 3). In general, the two non-duplicate studies that analyzed recipient age demonstrate that, in a dose-dependent manner, age was associated with poorer post-LT patient survival. However, the age cutoffs used as reference in the two studies were different from those employed in the pooled analysis of recipient age, limiting their inclusion in pooled analysis. One of the two non-duplicate studies analyzing recipient BMI demonstrated mild obesity was significantly associated with improved patient survival, while the other demonstrated that morbid (class III) obesity and a lean BMI were significantly associated with poorer survival. Similar to age, BMI data could not be pooled due to different BMI cutoffs used as references between the two studies. Finally, one of two studies analyzing recipient MELD demonstrated that this variable was not significantly associated with post-LT mortality, while the other showed significance at values >23.
Figure 2.
Forest plots and pooled effect estimates of predictors of patient survival.
Results are shown for (A) recipient sex, (B) donor sex, (C) pretransplant DM, (D) recipient age, (E) recipient race. Dotted lines represent pooled summary estimates of hazard ratios and diamonds represent their 95% confidence intervals (CI). Squares represent hazard ratios from individual studies and solid lines portray their 95% confidence intervals. HR: hazard ratio, SE: Standard Error.
Table 3.
Predictor studies eligible for pooled analysis but excluded due to incongruent stratification of predictor.
| Predictor | Outcome | Studies Eligible for Inclusion in the Meta-Analysis | Sample Size | Stratification Category | HR (95%CI) | P-Value |
|---|---|---|---|---|---|---|
| Recipient MELD | Patient Survival (Overall) | Haldar (2019)16 McCabe (2020)17 |
N=2741 N=11782 |
MELD <11 MELD >11–14 vs. <11 MELD >14–18 vs. <11 MELD >18–23 vs. <11 MELD >23 vs. <11 N/A |
1 1.03 (0.66–1.62) 0.66 (0.44–1.06) 0.71 (0.47–1.15) 1.48 (1.04–2.3) 1 (1–1.01) |
REF NS NS NS <0.05 0.458 |
| Recipient BMI | Patient Survival (Overall) | Haldar (2019)16 Nagai (2019)14 |
N=2741 N=6344 |
BMI ≤18.5 vs. >25.0, ≤30.0 BMI >18.5, ≤25.0 vs. >25.0, ≤30.0 BMI >30, ≤35 vs. >25.0, ≤30.0 BMI >30, ≤35 vs. >25.0, ≤30.0 BMI >40 vs. >25.0, ≤30.0 BMI <18.5 vs. 18.5–24.9 BMI 25–29.9 vs. 18.5–24.9 BMI 30–34.9 vs. 18.5–24.9 BMI 35–39.9 vs. 18.5–24.9 BMI 40+ vs. 18.5–24.9 |
4.29 (1.01–18.21) 2.24 (1.27–3.96) 1.96 (1.16–3.32) 1.38 (0.95–2.01) 1.43 (0.93–2.18) 0.88 (0.38–2) 0.86 (0.7–1.04) 0.73 (0.6–0.89) 0.82 (0.66–1.01) 0.88 (0.69–1.13) |
<0.05 <0.06 NS NS <0.05 0.33 0.12 <0.001 0.06 0.76 |
| Recipient Age | Patient Survival (Overall) | Haldar (2019)16 McCabe (2020)17 |
N=2741 N=11782 |
Age ≤45 Age 46–55 vs. ≤45 Age 56–60 vs. ≤45’ Age 61–65 vs. ≤45 Age >65 vs. ≤45 Age 50–64 vs. <50 |
1 1.31 (0.87–1.98) 1.23 (0.81–1.87) 2.07 (1.39–3.08) 1.72 (1.1–2.71) 1.54 (1.29–1.84) |
REF NS NS <0.001 <0.05 <0.001 |
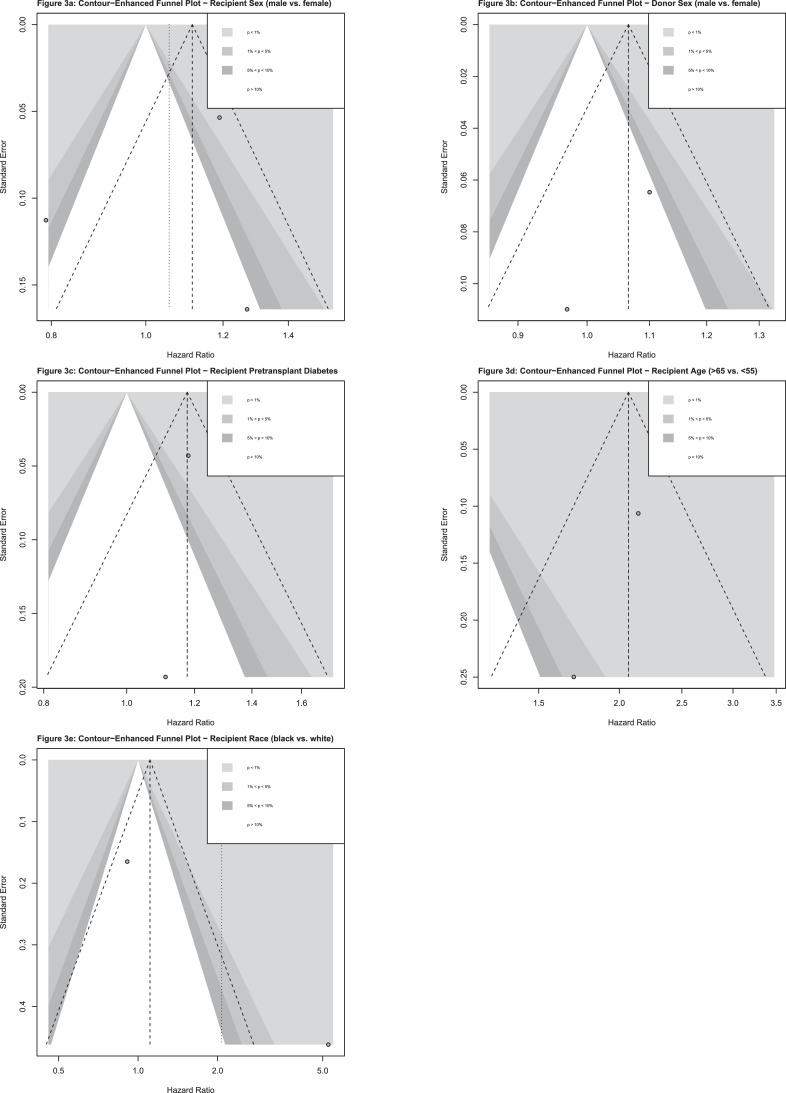
Counter-enhanced funnel plot analysis demonstrated no asymmetry for the predictors of patient survival that were pooled in the meta-analysis, aside from recipient race (Figure 3). As only two studies were included in the meta-analysis of recipient race, no sensitivity analysis was performed. All other timepoints were not likely subject to publication bias.
Figure 3.
Contour-enhanced funnel plots for predictors of patient survival.
Results are shown for (A) recipient sex, (B) donor sex, (C) pretransplant DM, (D) recipient age, (E) recipient race. Each dot represents a result from an individual study. The y-axis portrays the standard error of the effect estimate. The x-axis shows the study's effect estimate, expressed as HR. The superimposed lines represent regions where a test of the effect estimate is statistically significant. Different colors represent varying levels of statistical significance (e.g., <0.01, 0.01–0.05, 0.05–0.1, >0.1).
Discussion
To the best of our knowledge, our systematic review and meta-analysis is the first to provide a comprehensive review of studies analyzing the predictive variables impacting patient and graft survival in NASH-only patients.
Our pooled analysis of predictive variables was limited to five predictors as most studies in the systematic review contained duplicate cohorts. Pooled analyses of the effects of recipient and donor sex, along with recipient black (vs. white) race, suggested that these three variables have no significant effect on patient survival. However, the quality of these results is low due to the few numbers of studies that were included and heterogeneity detected in the pooled analysis for recipient sex and race. Given this poor quality of evidence, we advise clinicians or data scientists to refrain from making survival prognostication estimates using donor sex, recipient sex, and race. Given the large discrepancies in the latter two results, additional studies disseminating the effects of these variables on post-LT survival is warranted.
Increased recipient age was found to significantly elevate mortality risk in post-LT NASH patients in both studies pooled in the meta-analysis, and in the one eligible study that could not be pooled due to difference in categorial age cutoffs employed (Figure 2d, Table 3). Importantly, these studies controlled for confounding variables associated with increased age in their adjusted analysis. Other studies in our review that were excluded from the pooled analysis also confirm the significant predictive effect of age on post-LT survival. Interestingly, in Yong and colleagues’ meta-regression of post-LT survival in NASH vs. non-NASH cohorts, they did not identify age as a predictor.31 This may be explained by major differences between study populations, as the previous review only included NASH vs. non-NASH cohorts to analyze their primary survival outcome and conducted a meta-regression of the NASH subgroup as a secondary outcome. Furthermore, it was unclear whether the meta-regression adjusted for confounding variables that are associated with increased age (e.g., DM, dyslipidemia, chronic kidney disease), which the studies included in our review preformed. Finally, as the studies pooled in our review compared senior and younger-aged cohorts (>65 years vs. <50 years), a different finding may be observed if age was analyzed continuously. Clinically, from a prognostic perspective, we advise that advanced age (>65 years) be incorporated into the process of selecting optimal NASH LT candidates, while considering the limitations from the small number of studies analyzing this variable.
Our meta-analysis also identified recipient pretransplant DM as a significant predictor of reduced overall post-LT survival. This finding is corroborated by other studies that were not included in the pooled analysis due to possibility of duplicate cohorts. To our knowledge, this makes our study the first qualitative synthesis to identify this variable as a predictor of survival in post-LT NASH. Although no heterogeneity was detected, the quality of this finding is limited by the low number of studies in the pooled analysis (n=2). We hypothesize pretransplant DM impacts post-LT survival due to the increased risk of DM-related recurrent NASH and cardiovascular complications in this population. As such, although the quality of evidence is limited, to reduce post-LT mortality, we advocate clinicians mitigate DM burden in NASH patients undergoing LT. We also recommend future studies establish A1c to aid in establishing evidence-based treatment targets.
The two studies eligible for the meta-analysis analyzing recipient MELD demonstrated conflicting results, although their results could not be pooled due to incongruent categorical cutoffs in MELD scores. One demonstrated that increased MELD predicted post-LT mortality in NASH only at values >23, while MELD was insignificant in the comparator study that analyzed MELD continuously.16,17 Importantly, the average MELD score for NASH patients in the latter study was 21.8, which is not representative of the typical NASH LT candidate, and as such this conflicting finding must be interpreted within this context.17 Both hazard ratios were derived from adjusted analyses that was controlled for confounding variables. Other studies included in our review seem to demonstrate with consensus that more representatively-higher MELD scores are significantly predictive of poor survival in post-LT NASH. Therefore, MELD score seems to predict patient survival only at high values that are typically seen in NASH LT candidates. The review published by Yong et al also suggested that the MELD score was predictive of post-LT mortality and hypothesized this may have been related to elevated risk for comorbid systemic disease.31 As comorbid disease was adjusted for in the present review, this instead favors MELD score as being an independent risk factor for post-LT mortality in this population. In conclusion, at scores exceeding 23, clinicians and transplant selection committees should consider recipient MELD scores to have potentially negative, dose-dependent impacts on posttransplant survival in NASH LT recipients, with the caveat that this finding is limited by a small number of supporting studies.
Two non-duplicate studies analyzed recipient BMI as a predictor for post-LT NASH survival. One study demonstrated mild obesity was significantly associated with reduced mortality post-LT, while no differences in mortality risk were observed for other BMI strata. The other study demonstrated a significant increased risk of mortality in underweight and class III obesity patients, with no significant differences for class I or II.16,14 Both studies adjusted for important confounding factors in their regression analyses. Other studies included in our systematic review but not eligible for the meta-analysis affirmed that underweight and morbidly obese patients experienced higher risk of overall patient mortality, while mildly obese patients had conflicting results. Interestingly, one study analyzed the effects of BMI on long-term (10-year) survival in post-LT NASH, which demonstrated sizeable reductions in the hazard ratios in all higher BMI strata.27 Most studies affirm an elevated risk in patient mortality in underweight and morbidly obese NASH LT patients, likely because these patients have decreased reserve to withstand surgical stress and complications. Studies demonstrating that obese patients are subject to higher rates of complications and multiorgan failure in the early posttransplant period support this hypothesis.32 The discordance in BMI data is important to be clarified in future studies given the growing indication of NASH for LT, while candidates with higher BMI are increasingly turned-down for LT.33 Morbid obesity is also considered a contraindication to LT.34 Guidelines and consensus statements discouraging LT in obese patients are supported by evidence demonstrating increased post-LT mortality with higher BMI; however, these studies are not nuanced to NASH-specific cohorts and may pertain to other non-NASH indications.35 As such, we recommend that future studies analyzing the effects of BMI on post-LT survival in NASH analyze outcomes at short- and long-term follow-up periods. In light of this evidence, we recommend that patients maintain a normal BMI for optimal post-LT survival and cardiovascular protection; however, mild obesity is not a major concern from a survival perspective. This should be interpreted with caution given the low number of studies and the mild inconsistency in results that inform the statement regarding management of patients with mild obesity.
Although the remaining predictive variables analyzed in our review were not eligible for the meta-analyses due to the possibility for duplicate cohorts, a brief discussion of findings is warranted. For predicting patient survival in post-LT NASH, the following variables were also found to be significant across multiple studies: functional status, HCC, dialysis prior to LT, hepatic encephalopathy, era/year of LT (Table 1). All regression analyses of these variables were adjusted for clinically relevant confounding variables. Interestingly, many of these predictors overlap with a tool developed by Karnam and colleagues, which used demographic data from SRTR to identify seven factors impacting 5-year survival in patients undergoing LT for NASH.36 The factors included in the calculator that overlapped with our findings were: age at LT, functional status, hepatic encephalopathy, ventilatory support at LT, dialysis prior to LT.14, 16, 17, 15, 18, 19, 20, 26, 28 The remaining two predictive variables in their study, serum creatinine and presence of transjugular intrahepatic portosystemic shunts, were not analyzed in our eligible studies. Our study also identified novel variables, likely because our review was not restricted to the SRTR database (Table 1). Of note, recipient DM was found to have a small but significant predictive effect on post-LT patient and graft mortality in NASH. We hypothesize this is due to the increased risk of DM-related recurrent NASH and cardiovascular complications in this population. Interestingly, Yong and colleagues’ recent meta-regression reported MELD as being the sole predictor of post-LT mortality in NASH.31 Discrepancies likely resulted as their study excluded NASH-only studies, which informed the bulk of our study results. It was also unclear if their meta-regression of predictors adjusted for pertinent demographic and clinical variables that may have confounded results. Although the quality of evidence is significantly limited from potential duplication of cohorts, the non-modifiable predictors of post-LT mortality identified (HCC, functional status, hepatic encephalopathy, ventilatory support and/or dialysis prior to LT) should be used, from a prognostic perspective, to guide selection of optimal NASH LT candidates. However, this evidence is limited as these findings may be subject to duplicate cohorts.
Our study also identified several predictors of graft survival, which to our knowledge, has not been explored in any review (Table 2). Similar to patient survival, there was study consensus showing that increased recipient age and dialysis prior to LT predicted mortality in post-LT NASH. BMI again had conflicting results, but higher BMI was protective against mortality in the one study analyzing its long-term (10-year) influence.27 Importantly, graft survival results should be interpreted with caution as duplicate cohorts may have been present.
There were limitations to the present study. All studies were non-randomized cohort studies, and the quality of the results should be interpreted within this context. Next, a conservative approach for excluding duplicate cohorts led to the exclusion of many UNOS/OPTN/SRTR database studies. Although studies using data from the same registry with overlapping timeframes may not have duplicate cohorts, there was no feasible method to rule-out this possibility. Furthermore, we employed a BMI cutoff of >30 in our definition of CC as NASH, resulting in 19 potentially eligible studies to be excluded.37 Non-English texts were also excluded from our analysis due to the COVID19 pandemic and difficulty accessing library translation services. Finally, as a large list of predictive variables were analyzed, the probability of false-positive findings increases.
In summary, patient survival in post-LT NASH was influenced by recipient age, MELD scores >23, DM, functional status, HCC, dialysis prior to LT, hepatic encephalopathy, and era/year of LT. Study consistency observed for the latter five variables may have been increased by the prescience of duplicate cohorts, but prior non-meta-analytic syntheses have reported similar findings for all these variables. This evidence may form the foundation for prognostication and resource allocation decisions in patients undergoing LT for NASH. It may also suggest, with a low degree of evidence, that post-LT mortality may be augmented in these patients through control of DM. The predictive effect of recipient BMI was inconsistent among studies, and given the recent evidence suggesting that high BMI may augment risk of mortality in the long-term post-LT period, we suggest that NASH patients should not be rejected for transplant on the basis of BMI alone. Studies that yielded significance for all predictors discussed here employed adjusted Cox regression analyses, which controlled for pertinent confounding variables. All studies were also at low of risk of bias.
Contributors
Development and execution of literature search strategy (AO); Abstract screen (AM, LH, FQ); Full-text review (AM, NS, FQ); Data extraction (AM, NS) and data verification (NS, AM, FQ); Data analysis and determination of study eligibility for meta-analysis (SK); Creation of tables and figures (AM, SK); Manuscript writing (AM, NS, FQ, AA, MB); Manuscript revisions (MB, KP, FQ, AA, AM, SK, AO).
All authors verify that full access to study data was provided and accept responsibility to submit for publication.
Data sharing statement
Data from the cohorts analyzed and pooled by the present study were derived from previously published observational studies. Any query can be directed to the corresponding author.
Declaration of interests
Dr. Bhat reports grants from Novo Nordisk, grants from Ipsen, grants from Paladin, grants from Natera, grants from Oncoustics, grants from MedoAI, grants from Lallemand, personal fees from Novartis, personal fees from Lupin, outside the submitted work. All other authors have nothing to disclose.
Acknowledgments
Toronto General and Western Hospital Foundation.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101534.
Appendix. Supplementary materials
References
- 1.Younossi ZM. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatol Baltim Md. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 5.Burke A, Lucey M. Non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and orthotopic liver transplantation. Am J Transplant. 2004;4(5):686–693. doi: 10.1111/j.1600-6143.2004.00432.x. [DOI] [PubMed] [Google Scholar]
- 6.Dyson JK, Anstee QM, McPherson S. Republished: non-alcoholic fatty liver disease: a practical approach to treatment. Postgrad Med J. 2015;91(1072):92–101. doi: 10.1136/postgradmedj-2013-100404rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 8.Younossi ZM, Stepanova M, Ong J, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2021;19(3):580–589.e5. doi: 10.1016/j.cgh.2020.05.064. [DOI] [PubMed] [Google Scholar]
- 9.Narayanan P, Mara K, Izzy M, et al. Recurrent or de novo allograft steatosis and long-term outcomes after liver transplantation. Transplantation. 2019;103(1):e14–e21. doi: 10.1097/TP.0000000000002317. [DOI] [PubMed] [Google Scholar]
- 10.Bhat M, Mara K, Dierkhising R, Gender Watt KD. Race and disease etiology predict de novo malignancy risk after liver transplantation: insights for future individualized cancer screening guidance. Transplantation. 2019;103(1):91–100. doi: 10.1097/TP.0000000000002113. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Hu Z, Zhang Q, et al. Spectrum of de novo cancers and predictors in liver transplantation: analysis of the scientific registry of transplant recipients database. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newsome PN, Allison ME, Andrews PA, et al. Guidelines for liver transplantation for patients with non-alcoholic steatohepatitis. Gut. 2012;61(4):484–500. doi: 10.1136/gutjnl-2011-300886. [DOI] [PubMed] [Google Scholar]
- 13.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa CA: Ottawa Hospital Research Institute; 2013 [cited 2021 Mar 7]. Available from: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp
- 14.Nagai S, Collins K, Chau LC, et al. Increased risk of death in first year after liver transplantation among patients with nonalcoholic steatohepatitis vs liver disease of other etiologies. Clin Gastroenterol Hepatol. 2019;17(13):2759–2768.e5. doi: 10.1016/j.cgh.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Dimou FM, Mehta HB, Adhikari D, Harland RC, Riall TS, Kuo Y-F. The role of extended criteria donors in liver transplantation for nonalcoholic steatohepatitis. Surgery. 2016;160(6):1533–1543. doi: 10.1016/j.surg.2016.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haldar D, Kern B, Hodson J, et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European Liver Transplant Registry study. J Hepatol. 2019;71(2):313–322. doi: 10.1016/j.jhep.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCabe P, Galoosian A, Wong RJ. Patients with alcoholic liver disease have worse functional status at time of liver transplant registration and greater waitlist and post-transplant mortality which is compounded by older age. Dig Dis Sci. 2020;65(5):1501–1511. doi: 10.1007/s10620-019-05891-1. [DOI] [PubMed] [Google Scholar]
- 18.Agbim U, Jiang Y, Kedia SK, et al. Impact of nonmalignant portal vein thrombosis in transplant recipients with nonalcoholic steatohepatitis. Liver Transpl. 2019;25(1):68–78. doi: 10.1002/lt.25322. [DOI] [PubMed] [Google Scholar]
- 19.Kaswala DH, Zhang J, Liu A, et al. A comprehensive analysis of liver transplantation outcomes among ethnic minorities in the United States. J Clin Gastroenterol. 2020;54(3):263–270. doi: 10.1097/MCG.0000000000001236. [DOI] [PubMed] [Google Scholar]
- 20.Henson JB, Wilder JM, Kappus MR, et al. Transplant outcomes in older patients with nonalcoholic steatohepatitis compared to alcohol-related liver disease and hepatitis C. Transplantation. 2020;104(6):e164–e173. doi: 10.1097/TP.0000000000003219. [DOI] [PubMed] [Google Scholar]
- 21.Rinella ME, Satapathy SK, Brandman D, et al. Factors impacting survival in those transplanted for nash cirrhosis: data from the NailNASH consortium. Clin Gastroenterol Hepatol. 2022;S1542-3565(22) doi: 10.1016/j.cgh.2022.02.028. In press. [DOI] [PubMed] [Google Scholar]
- 22.Shavelle RM, Saur RC, Kwak JH, Brooks JC, Hameed B. Life expectancy after liver transplantation for NASH. Prog Transpl. 2022;32(2):102–111. doi: 10.1177/15269248221087441. 152692482210874. [DOI] [PubMed] [Google Scholar]
- 23.Yuan L, Hanlon CL, Terrault N, et al. Portrait of regional trends in liver transplantation for nonalcoholic steatohepatitis in the United States. Am J Gastroenterol. 2022;117(3):433–444. doi: 10.14309/ajg.0000000000001591. [DOI] [PubMed] [Google Scholar]
- 24.Karnam RS, Mitsakakis N, Saracino G, Lilly L, Asrani SK, Bhat M. Predicting long-term survival after liver transplantation in patients with NASH cirrhosis. Clin Gastroenterol Hepatol. 2022;20(3):704–705. doi: 10.1016/j.cgh.2021.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Ochoa-Allemant P, Ezaz G, Trivedi HD, Sanchez-Fernandez Lady, Bonder A. Long-term outcomes after liver transplantation in the Hispanic population. Liver Int. 2020;40(2):437–446. doi: 10.1111/liv.14248. [DOI] [PubMed] [Google Scholar]
- 26.Agopian VG, Kaldas FM, Hong JC, et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg. 2012;256(4):624–633. doi: 10.1097/SLA.0b013e31826b4b7e. [DOI] [PubMed] [Google Scholar]
- 27.Satapathy SK, Jiang Y, Agbim U, et al. Posttransplant outcome of lean compared with obese nonalcoholic steatohepatitis in the United States: the obesity paradox. Liver Transpl. 2020;26(1):68–79. doi: 10.1002/lt.25672. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Boktour MR. Effects of share 35 policy on liver transplantation outcomes for patients with nonalcoholic steatohepatitis. Prog Transplant. 2019;29(3):248–253. doi: 10.1177/1526924819854481. [DOI] [PubMed] [Google Scholar]
- 29.Malik SM, deVera ME, Fontes P, Shaikh O, Sasatomi E, Ahmad J. Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis: recurrent nash following it for nash cirrhosis. Liver Transpl. 2009;15(12):1843–1851. doi: 10.1002/lt.21943. [DOI] [PubMed] [Google Scholar]
- 30.Altshuler PJ, Dang H, Frank AM, et al. Evaluating outcomes related to donor and recipient metabolic environment: macrosteatotic allografts and nonalcoholic steatohepatitis. Liver Transplantation. 2022;28(4):623–635. doi: 10.1002/lt.26313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yong JN, Lim WH, Ng CH, et al. Outcomes of non-alcoholic steatohepatitis following liver transplantation. An updated meta-analysis and systematic review. Clin Gastroenterol Hepatol. 2021 doi: 10.1016/j.cgh.2021.11.014. S1542-3565(21)01226-X. In press. [DOI] [PubMed] [Google Scholar]
- 32.Sawyer RG, Pelletier SJ, Pruett TL. Increased early morbidity and mortality with acceptable long-term function in severely obese patients undergoing liver transplantation: liver transplantation in obese patients. Clin Transplant. 1999;13(1pt2):126–130. doi: 10.1034/j.1399-0012.1999.130111.x. [DOI] [PubMed] [Google Scholar]
- 33.Segev DL, Thompson RE, Locke JE, et al. Prolonged waiting times for liver transplantation in obese patients. Ann Surg. 2008;248(5):863–870. doi: 10.1097/SLA.0b013e31818a01ef. [DOI] [PubMed] [Google Scholar]
- 34.Pais R, Barritt AS, Calmus Y, et al. NAFLD and liver transplantation: current burden and expected challenges. J Hepatol. 2016;65(6):1245–1257. doi: 10.1016/j.jhep.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States: obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35(1):105–109. doi: 10.1053/jhep.2002.30318. [DOI] [PubMed] [Google Scholar]
- 36.Karnam RS, Mitsakakis N, Saracino G, Lilly L, Asrani SK, Bhat M. Predicting long-term survival after liver transplantation in patients With NASH cirrhosis. Clin Gastroenterol Hepatol. 2021;20(3):704–705. doi: 10.1016/j.cgh.2021.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Li J, Riaz DR, Shi G, Liu C, Dai Y. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2014;12(3):394–402.e1. doi: 10.1016/j.cgh.2013.09.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.