Abstract
Spores of Bacillus cereus were heated and recovered in order to investigate the effect of water activity of media on the estimated heat resistance (i.e., the D value) of spores. The water activity (ranging from 0.9 to 1) of the heating medium was first successively controlled with three solutes (glycerol, glucose, and sucrose), while the water activity of the recovery medium was kept near 1. Reciprocally, the water activity of the heating medium was then kept at 1, while the water activity of the recovery medium was controlled from 0.9 to 1 with the same depressors. Lastly, in a third set of experiments, the heating medium and the recovery medium were adjusted to the same activity. As expected, added depressors caused an increase of the heat resistance of spores with a greater efficiency of sucrose with respect to glycerol and glucose. In contrast, when solutes were added to the recovery medium, under an optimal water activity close to 0.98, a decrease of water activity caused a decrease in the estimated D values. This effect was more pronounced when sucrose was used as a depressor instead of glycerol or glucose. When the heating and the recovery media were adjusted to the same water activity, a balancing effect was observed between the protective influence of the solutes during heat treatment and their negative effect during the recovery of injured cells, so that the overall effect of water activity was reduced, with an optimal value near 0.96. The difference between the efficiency of depressors was also less pronounced. It may then be concluded that the overall protective effect of a decrease in water activity is generally overestimated.
It has been recognized that the heat resistance of bacterial spores depends on the medium in which the spores are heated. The maximum thermostability of most microorganisms was found in the range of between 0.2 and 0.4 water activity (1, 3, 27, 28, 29). In typical ranges of water activities which are found in foodstuffs (aw > 0.8), the heat resistance of microorganisms generally increases at decreasing water activities. However, the apparent effect of the water activity of the medium on spores or vegetative cells is complicated by the specific effect of solutes which are used as depressors. It is generally agreed that the occurrence of such solutes in the medium reduces the heat resistance of microorganisms. This antagonism between the protective effect of an increase in water activity and the opposite specific effect of depressors can explain conflicting data from various authors.
The influence of salt on the thermostability of microorganisms is disputed and depends on the heated type of microorganism. Some authors found no effect of the sodium chloride concentration on the heat resistance of bacteria (9, 29, 32, 42). Others observed a reduced heat resistance of microorganisms at increasing salt concentrations (7, 12, 22, 23). On the contrary, a protective effect of salt was found in several studies (6, 14, 26, 35, 38, 39, 40). Corry (14) deduced from his data that sodium chloride had a thermal protective effect on most heat-sensitive bacteria and the opposite effect on most heat-resistant species. Other solutes show the same opposite influence between their common depressor character which protects spores against heat and their specific effect which, on the contrary, reduces their heat resistance. It has been observed (21) that an increase of the thermal resistance of spores was more pronounced when the decrease of the medium water activity was generated by drying instead of an addition of glycerol, sodium chloride, lithium chloride, or glucose. Baird-Parker et al. (5) could not find any correlation between the heat resistance D (values) of salmonellae and the water activity of heating media when sodium chloride or glycerol were used as depressors. However, these researchers observed a clear protective effect of sucrose that was more pronounced for most heat-sensitive strains. It is generally recognized that sucrose is the most protective depressor, while glucose, sodium chloride, and lithium chloride show a clearly lower influence or even an opposite effect. Glycerol shows an intermediate behavior (13, 19, 20, 26, 37). Interactions between the influences of water activity and heating temperature were often observed. An increase of D values generated by a reduced water activity of the heating medium is generally related to an increase of z values. Moreover, several workers have demonstrated that the effect of the water activity of the heating medium depended on the treatment temperature; for example, in Staphylococcus epidermidis (39) or Listeria monocytogenes (37), the protective effect of decreasing water activity is more pronounced at a higher treatment temperature, while the opposite trend was observed for Staphylococcus aureus (38). A few predictive models describing the effect of the water activity of the heating medium on the heat resistance of spores were developed (8, 18, 31).
The nature of the recovery medium in which surviving heated cells are incubated has a great influence on their apparent heat resistance, i.e., their estimated D value (24). It is generally agreed that there is an optimum temperature of incubation for the cell ratio of recovery (16, 36) and the apparent D value (10). Acidification of the recovery medium causes also a reduction in spore recovery and in apparent heat resistance (11, 17, 33, 34, 41). The addition of sodium chloride in the recovery medium causes effects similar to those observed with acidification: a reduction of the viability of cells and a lower apparent D value (7, 12, 22, 30, 32). However, as far as we know, the effect of reducing the water activity of the recovery medium by depressors other than sodium chloride has never been investigated.
The purpose of this work was to investigate and to describe based on a predictive model the influence of the water activity of the recovery medium with glycerol, glucose, and sucrose used as depressors upon the apparent D value of Bacillus cereus spores.
MATERIALS AND METHODS
Microorganism and spore production.
The strain B. cereus CNRZ 110 was obtained from the Institut National de Recherche Agronomique (Paris, France). Spores were kept in distilled water at 4°C. Cells were precultivated at 37°C for 24 h in brain heart infusion (Difco). The preculture was used to inoculate nutritive agar plates (Biokar Diagnostics BK021) with MnSO4 (40 mg liter−1) and CaCl2 (100 mg liter−1) on the surface area. Plates were incubated at 37°C for 5 days. Spores were then collected by scraping the surface of the agar, suspended in sterile distilled water, and washed three times by centrifugation (10,000 × g for 15 min) (Bioblock Scientific model Sigma 3K30). The pellet was then resuspended in 5 ml of distilled water and 5 ml of ethanol. The obtained suspension was kept at 4°C for 12 h to eliminate vegetative nonsporulated bacteria and then washed again three times by centrifugation.
Lastly, the final suspension (ca. 1010 spores ml−1) was distributed into sterile Eppendorf microtubes and kept at 4°C.
Thermal treatment of spore suspension.
D values in citrate-phosphate buffers were determined at 95°C with one replicate at each aw value ranging from 1 to 0.89.
Three solutes (glycerol, glucose, and sucrose) were used to adjust the water activity value. The previous molarities of the different solutes were determined using curves from the model UNIFAC-LARSEN (2). The heating medium was sterilized by filtration, and the aw values were controlled with an aw meter (FA-st1 GBX; France Scientific Instrument).
First, 30 μl of spore suspension was diluted in 3 ml of heating medium. Capillary tubes of 25 μl (Vitrex) were filled with 10 μl of sample and subjected to a thermal treatment in a thermostated oil bath. After being heated, the tubes were cooled in an ice-water bath, washed in a solution of soap, and rinsed with sterile distilled water. Finally, the ends were flamed with ethanol. The capillary tubes were broken at both ends, and their contents poured into a tube containing 9 ml of sterile tryptone salt broth (Biokar Diagnostics) by rinsing with 1 ml of tryptone salt broth contained in a needle-equipped syringe.
Recovery conditions.
Viable spores were counted by duplicate plating at different aw values in nutritive agar (10 g of tryptone, 5 g of meat extract, 5 g of sodium chloride, and 15 g of agar per 1,000 ml) (Biokar Diagnostic) and incubated at 25°C for 6 to 21 days. The aw ranging from 1 to 0.92 was adjusted with glycerol, glucose, or sucrose. To adjust aw values, the previous molarities of the different solutes were determined using curves from the model UNIFAC-LARSEN (2). Nutritive agar was sterilized by autoclaving, and glycerol, glucose, or sucrose solutions were sterilized by filtration to avoid the Maillard reaction. After sterilization, the two solutions were mixed, the pH was adjusted to 7, and the aw value was controlled. The correspondence between the water activity and the concentrations of each depressor is shown in Table 1.
TABLE 1.
Predicted aw values of glycerol, glucose, and sucrose solutions at 25°C
| Molarity (M) | Glycerol aw | Glucose aw | Sucrose aw |
|---|---|---|---|
| 0.0 | 1.000 | 1.000 | 1.000 |
| 0.5 | 0.991 | 0.991 | 0.991 |
| 1.0 | 0.982 | 0.982 | 0.980 |
| 1.6 | 0.971 | 0.970 | 0.967 |
| 2.0 | 0.964 | 0.963 | 0.958 |
| 2.5 | 0.955 | 0.953 | 0.946 |
| 3.0 | 0.946 | 0.943 | 0.933 |
| 3.5 | 0.937 | 0.933 | 0.920 |
| 4.0 | 0.928 | 0.923 | 0.907 |
| 4.5 | 0.919 | 0.913 | 0.893 |
| 5.0 | 0.910 | 0.903 | 0.879 |
| 5.5 | 0.901 | 0.893 | 0.865 |
Data analysis.
D values were determined on the straight portion of curves obtained when the log number of survivors was plotted against time. The parameters of the models were estimated by simple linear regression carried out with MINITAB software. The goodness of fit of the model was evaluated by using the percent variance R2 value.
RESULTS
Effect of the water activity of the heating medium.
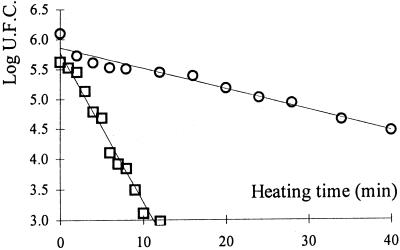
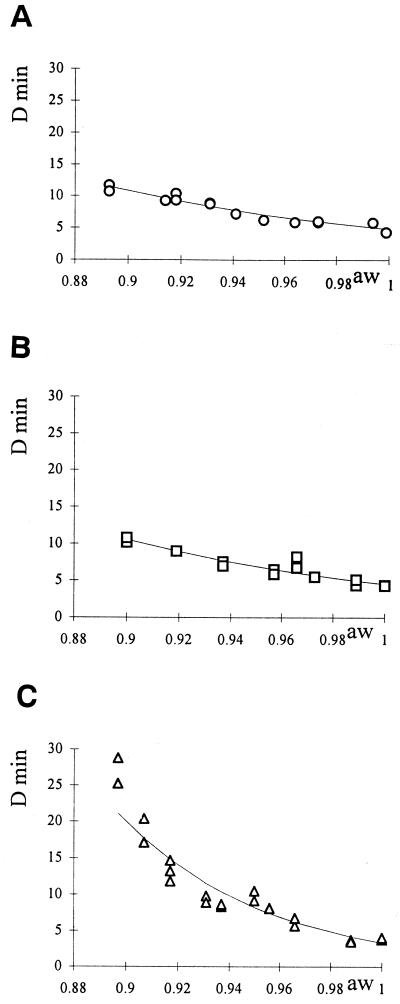
For identical heat treatment, the recovery conditions influence the apparent heat resistance of bacterial spores. A clear protective effect on spores of B. cereus heated at 95°C and at pH 7 was observed when solutes were added to the heating medium for the three types of depressors (Fig. 1 and 2). However, it can be seen that the effect of sucrose is more pronounced than that of glycerol and glucose. While a D95°C of about 4 min at a water activity close to 1 was found, at a water activity of 0.9 the observed D95°C became close to 11.2, 10.5, and 27 min for glycerol, glucose, and sucrose, respectively.
FIG. 1.
Log UFC versus the heating time for B. cereus CNRZ 110 heated at 95°C at pH 7 with an aw of 1 (□) or 0.9 (○) adjusted with sucrose. The aw value of the recovery condition is equal to 1.
FIG. 2.
(A) B. cereus D95°C value versus the aw of the heated medium adjusted with glycerol. Symbols: ○, experimental data; —, calculated values of aw according to equation 1. (B) B. cereus D95°C value versus the aw of the heated medium adjusted with glucose. Symbols: □, experimental data; —, calculated values according to equation 1. (C) B. cereus D95°C value versus the aw of the heated medium adjusted with sucrose. Symbols: ▵, experimental data; —, calculated values according to equation 1.
The three sets of data corresponding to each depressor were fitted according to the Gaillard et al. model (18) which, under isothermal conditions and at a fixed pH of the heating medium, can be reduced to:
 |
1 |
where D(aw,1) is the estimated D value at a water activity of the heating medium aw (which is the controlled variable) and a water activity of the recovery medium of 1. zaw* corresponds to the decrease in water activity of the treatment medium which would cause a 10-fold reduction of the decimal reduction time, with a water activity of the recovery medium of 1. The estimated parameter values are presented in Table 2.
TABLE 2.
Parameter estimates of equation 1
| Depressor | D(1,1) (min) | zaw* (range) | R2 |
|---|---|---|---|
| Glycerol | 4.81 | 0.284 (0.273–0.297) | 0.921 |
| Glucose | 4.54 | 0.274 (0.258–0.293) | 0.872 |
| Sucrose | 3.36 | 0.129 (0.125–0.133) | 0.921 |
Effect of the water activity of the recovery medium.
Whatever the solute used as depressor in the recovery medium, heated spores show the same maximum apparent heat resistance (a D95°C value of ca. 5 min) at an optimum water activity close to 0.98 (see Fig. 3 and 4). Under this optimal value, an increasing concentration of the three depressors causes a decrease in the apparent heat resistance of the spores. Sucrose presents the most pronounced effect, followed in turn by glucose and glycerol. At water activity of 0.92, the estimated D95°C values were 0.9, 1.9, and 2.5 min with sucrose, glucose, and glycerol, respectively.
FIG. 3.
Log UFC versus the heating time for B. cereus CNRZ 110 heated at 95°C at pH 7 with an aw of 1 incubated at 25°C in recovery medium at an aw of 1 (■) or an aw of 0.92 (●) adjusted with sucrose.
FIG. 4.
(A) B. cereus D95°C value versus the aw of the recovery medium adjusted with glycerol. Symbols: ●, experimental data; —, calculated values of aw according to equation 2. (B) B. cereus D95°C value versus the aw of the recovery medium adjusted with glucose. Symbols: ■, experimental data; —, calculated values according to equation 2. (C) B. cereus D95°C value versus the aw of the recovery medium adjusted with sucrose. Symbols: ▴, experimental data; —, calculated values according to equation 2.
We tried to adapt the model describing the influence of the pH of the recovery medium to the apparent thermal resistance of spores, which was tested in our laboratory (15) by substituting pH for water activity, leading to the following equation:
 |
2 |
where D′(1,aw) is the estimated D value at a water activity of the recovery medium a′w (which is the controlled variable) and a water activity of the heating medium of 1. z′aw* corresponds to the decrease of water activity of the recovery medium which would cause a 10-fold reduction of the decimal reduction time, with a water activity of the heating medium of 1. The estimated parameter values are presented in Table 3.
TABLE 3.
Parameter estimates of equation 2
| Depressor | D(1,1) (min) | awopt | zaw′* (range) | R2 |
|---|---|---|---|---|
| Glycerol | 4.75 | 0.978 | 0.103 (0.098–0.108) | 0.893 |
| Glucose | 4.55 | 0.990 | 0.113 (0.106–0.121) | 0.883 |
| Sucrose | 4.52 | 0.978 | 0.068 (0.066–0.070) | 0.941 |
Overall effect of water activity of foods on the apparent heat resistance of spores.
Since foods make up both the heating medium and the recovery medium, a third set of experiments was carried out in which spores were recovered at the same water activity as those of the heating menstruum (Fig. 5). With respect to the second set of data in which the water activity of the heating medium was kept to 1, some noteworthy differences appear. First, the overall influence of water activity becomes relatively slight, while the differences in the curve patterns according to the depressors used are less pronounced than those of Fig. 4. Second, a shift of the optimum water activity from 0.98 toward 0.96 can be observed, with a maximum D95°C value of close to 8 min instead of 5 min. Equations 1 and 2 cannot directly be combined in order to build a model which would take into account the overall effect of the food water activity because they were developed by keeping the water activity of the recovery medium at 1 for equation 1, and keeping the water activity of the heating medium at 1 for equation 2. The accuracy of this third set of data was too poor to allow suitable modeling.
FIG. 5.
(A) B. cereus D95°C value versus the aw of both heated and recovery medium adjusted with sucrose. Symbols: ○, experimental data; —, calculated values of aw according to equation 2. (B) B. cereus D95°C value versus the aw of both heated and recovery medium adjusted with glucose. Symbols: □, experimental data; —, calculated values according to equation 2. (C) B. cereus D95°C value versus the aw of both heated and recovery medium adjusted with sucrose. Symbols: ▵, experimental data; —, calculated values according to equation 2.
DISCUSSION
It has been confirmed that an increase in the water activity produces opposite effects on the apparent heat resistance of spores according to whether it concerns the heat treatment or the recovery medium. Inside the investigated water activity range (0.9 to 1), which corresponds to that of most typical foods, a clear protective effect of an increasing water activity of the heating medium can be observed. For a fixed water activity, the degree of protection depends on the type of depressor used; according to our investigations, sucrose showed a more effective protective effect than glycerol and glucose, a finding which is in agreement with observations by other authors (5, 19, 26, 37). As in the case of NaCl, the protective influence of an increase of the water activity could partly be balanced by a specific antagonistic and toxic effect of glycerol and glucose. Moreover, the plasmolyse which is partly responsible for the heat protection of spores is limited by the penetration of glycerol and glucose inside the cells. This limitation does not exist when the depressor is not uptaken inside cells, which is the case with sucrose. Another explanation for the protective effect of a depressor in the heating medium could be an inhibition of spore germination. Anagnastopoulos and Sidhu (4) observed for Bacillus stearothermophilus that the percentage of germination decreases when the water activity decreased and that, at the same water activity, the percentage of germination was lower in a nutrient broth supplemented with sucrose than in a broth supplemented with glycerol. Hydration of spore protoplast is an important condition of spore activation and initiation of germination. Germination is inhibited in the absence of moisture or in a concentrated solution of nonpenetrating solute. The spores which do not germinate are protected during heat treatment and can germinate and grow during recovery. This explanation is in agreement with our results: when the water activity decreases, the heat resistance of spores increases and, at the same water activity, sucrose, a less-penetrating solute in protoplast than glycerol and glucose, shows a greater protective effect than these two solutes. Moreover, according to Anagnastopoulos and Sidhu, the zaw values for glycerol and sucrose (0.28 and 0.13, respectively) correspond to the aw value difference which leads to a 10-fold reduction of the percentage of germination of B. stearothermophilus at 75°C with glycerol and sucrose (0.31 and 0.11, respectively).
It is recognized that the addition of sodium chloride to the recovery medium causes both a reduction of viability of cells and a lower apparent D value of the spores (7, 12, 16, 22, 32). However, as far as we know, the influence of the water activity of the recovery medium and the types of depressors used upon the estimated D values of spores had never been investigated. In our experimental conditions, a maximal apparent heat resistance of spores appeared at an optimal water activity close to 0.98. Below this value, a decrease in the water activity of the recovery medium causes a reduction of the estimated D value of the spores. It is interesting to note regarding this trend that the depressors appear at the same increasing order of effectiveness as was found regarding their protective effect in the heating medium: glycerol, glucose, and sucrose, respectively. This observation is consistent with the behavior of germinated (i.e., nonrefractile) spores, which are rapidly permeable to glycerol, permeable to glucose by active transport, and permeable to sucrose to a lower extent. This order also corresponds to the increasing order of molecular weights and to the decreasing degree of penetration inside the cells. Particularly, the absence of uptake of sucrose by cells keeps a sharp gradient of osmotic pressure between the cell inside and the outside medium, which reduces the viability of surviving cells.
The overall influence of water activity of a single medium which makes up both the heating menstruum and the recovery medium upon the apparent D values of spores had not yet been investigated. Our results show that the protective effect of a decrease in the water activity of the medium during heat treatment is more or less offset by a reduction of the viability of surviving cells during the recovery. Moreover, because depressors, which are most effective in the heat protection of spores, are also responsible for the maximum loss of viability of injured cells, their overall difference in efficiency is greatly reduced. Whereas most authors have determined the optimal water activity of the maximum heat resistance of spores to be between 0.2 and 0.4 because the surviving cells were recovered under optimal conditions when the media of the heat treatment and of the incubation are the same, as for heat-processed foods, the optimal water activity is actually near 0.96.
Investigating separately the influence of water activity of the heating medium on the heat resistance of spores on the one hand and the effect of water activity of the recovery medium on the viability of surviving cells on the other hand obviously provides very interesting and useful data: these two effects must be regarded as two different factors that interact with each other. However, when the heating medium is also the recovery medium, it is worth investigating the overall influence of water activity on the apparent heat resistance of the spores, which reflects both their immediate thermal resistance during heating and their ability to grow in a recovery medium.
In the framework of predictive microbiology, we could develop two separate models for describing the effect of the water activity of the heating menstruum on the one hand and that of water activity of the recovery medium on the other hand upon the apparent heat resistance of the spores. However, further works would be needed in order to develop an overall predictive model adapted for heat-processed food calculations. Such a model would take into account both opposite effects of water activity and would allow us to improve of heat treatment optimization.
REFERENCES
- 1.Ababouch L F, Busta F F. Effect of the thermal treatment in oils on bacterial spores survival. J Appl Bacteriol. 1987;62:491–502. doi: 10.1111/j.1365-2672.1987.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 2.Achard C, Gros J B, Dussap C G. Prédiction de l'activité de l'eau, des températures d'ébullition et de congélation de solutions aqueuses de sucre par un modèle UNIFAC. Ind Aliment Agric. 1992;109:93–101. [Google Scholar]
- 3.Alderton G, Chen J K, Ito K A. Heat resistance of the chemical resistance forms of Clostridium botulinum 62A spores over the water activity range 0 to 0.9. Appl Environ Microbiol. 1980;40:511–515. doi: 10.1128/aem.40.3.511-515.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anagnastopoulos G D, Didhu H S. The effect of water activity and the aw controlling solute on spore germination of Bacillus stearothermophilus. J Appl Bacteriol. 1981;50:335–349. [Google Scholar]
- 5.Baird-Parker A C, Boothroyd M, Jones E. The effect of water activity on the heat resistance of heat sensitive and heat resistant strains of salmonellae. J Appl Bacteriol. 1970;33:515–522. doi: 10.1111/j.1365-2672.1970.tb02228.x. [DOI] [PubMed] [Google Scholar]
- 6.Bean P G, Roberts T A. Effect of sodium chloride and sodium nitrite on the heat resistance of Staphylococcus aureus NCTC 10652 in buffer and meat macerate. J Food Technol. 1975;10:327–332. [Google Scholar]
- 7.Briggs A, Yazdany S. Effect of sodium chloride on the heat and radiation resistance and on the recovery of heated or irradiated spores of the genus Bacillus. J Appl Bacteriol. 1970;33:621–632. doi: 10.1111/j.1365-2672.1970.tb02243.x. [DOI] [PubMed] [Google Scholar]
- 8.Cerf O, Davey K R, Saoudi A. Thermal inactivation of bacteria: a new predictive model for the combined effects of three environmental factors: temperature, pH and water activity. Food Res Int. 1996;29:219–226. [Google Scholar]
- 9.Chumney R K, Adams D M. Relationship between the increased sensitivity of heat injured Clostridium perfringens spores to surface active antibiotics and to sodium chloride and sodium nitrite. J Appl Bacteriol. 1980;49:55–63. doi: 10.1111/j.1365-2672.1980.tb01043.x. [DOI] [PubMed] [Google Scholar]
- 10.Condon S, Palop A, Raso J, Sala F. Influence of the incubation temperature after heat treatment upon the estimated heat resistance of spores of Bacillus subtilis. Lett Appl Microbiol. 1996;22:149–152. [Google Scholar]
- 11.Cook A M, Brown M R. Relationship between heat activation and percentage colony formation of Bacillus stearothermophilus spores: effect of storage and pH of the recovery medium. J Appl Bacteriol. 1965;28:361–364. doi: 10.1111/j.1365-2672.1965.tb02164.x. [DOI] [PubMed] [Google Scholar]
- 12.Cook A M, Gilbert R J. The effect of sodium chloride on heat resistance of heated spores of Bacillus stearothermophilus. J Appl Bacteriol. 1969;32:96–102. doi: 10.1111/j.1365-2672.1969.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 13.Corry J E. The effect of sugars and polyols on the heat resistance of salmonellae. J Appl Bacteriol. 1974;37:31–43. doi: 10.1111/j.1365-2672.1974.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 14.Corry J E. The effect of water activity on the heat resistance of bacteria. In: Duckworth, editor. Water relations of foods. New York, N.Y: Academic Press, Inc.; 1975. pp. 325–337. [Google Scholar]
- 15.Couvert O, Leguérinel I, Mafart P. Modelling the overall effect of pH on the apparent heat resistance of Bacillus cereus spores. Int J Food Microbiol. 1999;49:57–52. doi: 10.1016/s0168-1605(99)00052-5. [DOI] [PubMed] [Google Scholar]
- 16.Feeherry F E, Munsey B T, Rowley D B. Thermal inactivation and injury of Bacillus stearothermophilus spores. Appl Environ Microbiol. 1987;53:365–370. doi: 10.1128/aem.53.2.365-370.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez P S, Gomez F J, Ocio M J, Sanchez M T, Rodrigo T, Martinez M. Influence of acidification and type of acidulent of the recovery medium on Bacillus cereus spore counts. Lett Appl Microbiol. 1994;19:146–148. [Google Scholar]
- 18.Gaillard S, Leguérinel I, Mafart P. Model for combined effects of temperature, pH and water activity on thermal inactivation of Bacillus cereus spores. J Food Sci. 1998;63:887–889. [Google Scholar]
- 19.Goepfert J M, Iskander I K, Amundson C H. Relation of the heat resistance of salmonellae to the water activity of the environment. Appl Microbiol. 1970;19:429–433. doi: 10.1128/am.19.3.429-433.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Härnulv B J, Johansson M, Snygg B G. Heat resistance of Bacillus stearothermophilus spores at different water activities. J Food Sci. 1977;42:91–93. [Google Scholar]
- 21.Härnulv B J, Snygg B G. Heat resistance of Bacillus subtilis spores at various water activities. J Appl Bacteriol. 1972;35:615–624. doi: 10.1111/j.1365-2672.1972.tb03743.x. [DOI] [PubMed] [Google Scholar]
- 22.Juneja V K, Eblen B S. Influence of sodium chloride on thermal inactivation and recovery of nonproteolytic Clostridium botulinum type B strain KAP B5 spores. J Food Prot. 1995;58:813–816. doi: 10.4315/0362-028X-58.7.813. [DOI] [PubMed] [Google Scholar]
- 23.Lopez M, Mazas M, Gonzalez I, Gonzalez J, Bernardo A. Thermal resistance of Bacillus stearothermophilus spores in different heating systems containing some approved food additives. Lett Appl Microbiol. 1996;23:187–191. doi: 10.1111/j.1472-765x.1996.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 24.Mafart P, Leguérinel I. Modelling the heat stress and the recovery of bacterial spores. Int J Food Microbiol. 1997;37:131–135. doi: 10.1016/s0168-1605(97)00061-5. [DOI] [PubMed] [Google Scholar]
- 25.Mafart P, Leguérinel I. Modeling combined effects of temperature and pH on heat resistance of spores by a linear-Bigelow equation. J Food Sci. 1998;63:6–8. [Google Scholar]
- 26.Mazas M, Martinez S, Lopez M, Alvarez A B, Martin R. Thermal inactivation of Bacillus cereus spores affected by the solutes used to control water activity of the heating medium. Int J Food Microbiol. 1999;53:61–67. doi: 10.1016/s0168-1605(99)00145-2. [DOI] [PubMed] [Google Scholar]
- 27.Molin G, Snygg B G. Effect of lipid materials on heat resistance of bacterial spores. Appl Bacteriol. 1967;15:1422–1426. doi: 10.1128/am.15.6.1422-1426.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murrell W G, Scott W J. The heat resistance of bacterial spores at various water activities. J Gen Microbiol. 1966;43:411–425. doi: 10.1099/00221287-43-3-411. [DOI] [PubMed] [Google Scholar]
- 29.Pfeiffer J, Kessler H G. Effect of relative humidity of hot air on the heat resistance of Bacillus cereus spores. J Appl Bacteriol. 1994;77:121–128. [Google Scholar]
- 30.Pivnick H, Thacker C. Effect of sodium chloride and pH on inactivation of growth by heat-damaged spores of Clostridium botulinum. J Inst Can Technol Alim. 1970;3:70–75. [Google Scholar]
- 31.Reichart O. Modelling the destruction of Escherichia coli on the base of reaction kinetics. Int J Food Microbiol. 1994;23:449–465. doi: 10.1016/0168-1605(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 32.Roberts T A, Gilbert R J, Ingram M. The effect of sodium chloride on heat resistance and recovery of heated spores of Clostridium sporogenes PA3679. J Appl Bacteriol. 1966;29:549–555. [Google Scholar]
- 33.Sanchez T, Rodrigo M, Ocio M J, Fernandez P S, Martinez A. Growth and heat resistance of Clostridium sporogenes PA3679 spores heated and recovered in acidified media. J Food Prot. 1995;58:656–660. doi: 10.4315/0362-028X-58.6.656. [DOI] [PubMed] [Google Scholar]
- 34.Santos M H, Zarzo J T. Evaluation of citric acid and GDL in the recovery at different pH levels of Bacillus cereus spores subjected to HTST treatment conditions. Int J Food Microbiol. 1996;29:241–254. doi: 10.1016/0168-1605(95)00035-6. [DOI] [PubMed] [Google Scholar]
- 35.Sofos T A. Antimicrobial effects of sodium and other ions in foods: a review. J Food Safety. 1983;6:45–78. [Google Scholar]
- 36.Sugiyama H. Studies on factors affecting the heat resistance of spores of Clostridium botulinum. J Bacteriol. 1951;62:81–96. doi: 10.1128/jb.62.1.81-96.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sumner S S, Sandros T M, Hardon M C, Scott V N, Bernard D T. Heat resistance of Salmonella typhimurium and Listeria monocytogenes in sucrose solutions of various water activities. J Food Sci. 1991;56:1741–1743. [Google Scholar]
- 38.Tuncan E U, Martin S E. Combined effects of salts and temperature on the thermal destruction of Staphylococcus aureus MF-31. J Food Sci. 1990;55:833–836. [Google Scholar]
- 39.Verrips T, Van Rhee R. Effects of egg yolk and salt on Micrococcocea heat resistance. Appl Environ Microbiol. 1983;45:1–5. doi: 10.1128/aem.45.1.1-5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viljoen J A. Heat resistance studies. 2. The protective effect of sodium chloride on bacterial spores heated in pea liquor. J Infect Dis. 1926;39:286–290. [Google Scholar]
- 41.Young K, Foegeding P M. Acetic, lactic and citric acids and pH inhibition of Listeria monocytogenes Scott A and the effect of intracellular pH. J Appl Bacteriol. 1993;47:515–520. [PubMed] [Google Scholar]
- 42.Zaleski S, Sobolewska-Ceronik K, Ceronik E, Daczkowska E, Mazur E, Bogusla T, Zerek W. Effects of Baltic fishes freshness on thermal resistance of bacterial spores. 1. Thermal reduction of Bacillus subtilis spores suspended in heat-denatured extracts from meat of herring and cod of different freshness. Acta Aliment Polo. 1978;5:163–176. [Google Scholar]