Abstract
Introduction
The central nervous system (CNS) is a common site of progression among patients with ROS1-rearranged lung cancer receiving crizotinib. We conducted a phase 2 study to evaluate the intracranial efficacy of lorlatinib in patients with ROS1-rearranged lung cancer who developed CNS-only progression on crizotinib.
Methods
Patients with metastatic ROS1-rearranged lung cancer with CNS-only progression on crizotinib received lorlatinib 100 mg daily. The primary end point was intracranial disease control rate at 12 weeks per modified Response Evaluation Criteria in Solid Tumors version 1.1. Secondary end points included intracranial and extracranial progression-free survival, intracranial objective response rate, and safety/tolerability.
Results
A total of 16 patients were enrolled between November 2016 and January 2019. Nine patients (56%) had received prior CNS radiation, with a median of 10.9 months between radiation and lorlatinib. At 12 weeks, the intracranial disease control rate was 100% and intracranial objective response rate was 87%. While on study, the complee intracranial response rate was 60%. With median follow-up of 22 months, seven patients experienced disease progression, including five patients with CNS relapse. The median intracranial and extracranial progression-free survivals were 38.8 months (95% confidence interval: 16.9–not reported) and 41.1 months (95% confidence interval: 17.6–not reported), respectively. Molecular analysis of plasma or tissue from patients with extracranial progression on lorlatinib revealed ROS1 G2032R (n = 1), ROS1 L2086F (n = 1), and CCDC6-RET fusion plus ROS1 G2032R (n = 1). The safety profile of lorlatinib was consistent with prior studies. There were 11 patients (69%) who required dose reduction, including one patient who discontinued treatment for grade 3 edema. No grade greater than or equal to 4 adverse events were observed.
Conclusions
Lorlatinib induced durable intracranial responses in patients with ROS1-rearranged NSCLC and prior isolated CNS progression on crizotinib.
Keywords: ROS1, Lung cancer, Brain metastasis, Lorlatinib, Crizotinib
Introduction
Approximately one-third of patients with advanced ROS1-rearranged (ROS1+) NSCLC present with central nervous system (CNS) metastases at initial diagnosis.1,2 Despite treatment with ROS1 tyrosine kinase inhibitors (TKIs), such as crizotinib or entrectinib, the CNS remains a site of vulnerability throughout the disease course. Retrospective studies suggest that nearly half of patients will experience CNS-only progression during treatment with crizotinib.2 Owing to the limited ability of crizotinib to cross the blood-brain barrier,3 most patients without baseline brain metastases will develop brain metastases while on treatment with crizotinib.2 Even when treated with entrectinib, a ROS1 TKI that crosses the blood-brain barrier, one-third of patients will experience CNS progression.4 Given the high risk of CNS relapse with the two U.S. Food and Drug Administration (FDA)–approved ROS1 TKIs, therapies that can effectively overcome CNS progression are needed.
Lorlatinib is a brain-penetrant, next-generation ROS1/ALK TKI. In a global phase 1/2 study, lorlatinib induced objective responses in 35% of patients who had experienced extracranial progression on crizotinib, with a median duration of response of 13.8 months.5 Among the subgroup of patients with brain metastases, the intracranial objective response rate (ORR) with lorlatinib was 50%.5 In other molecular subsets of NSCLC (e.g., ALK-rearranged NSCLC), studies suggest that patients with isolated CNS disease may represent a population that is distinct from and more favorable than those with multisite involvement.6 Nevertheless, few studies have prospectively assessed treatment outcomes in patients with this pattern of disease. Notably, in the phase 1/2 study that established the efficacy of lorlatinib in ROS1+ NSCLC, measurable extracranial disease was a prerequisite for enrollment.5
Here, we present the results of an investigator-initiated, single arm, phase 2 study (NCT02927340) designed to assess the intracranial activity of lorlatinib in patients with ROS1+ NSCLC who developed CNS-only progression on crizotinib. On the basis of the hypothesis that isolated CNS progression on crizotinib is primarily driven by limited drug exposure in the CNS, it was anticipated that most CNS-only progression events on crizotinib could be overcome by introducing a more potent ROS1 TKI with proven blood-brain barrier penetration.
Materials and Methods
Study Design
NCT02927340 is an open-label, investigator-initiated, single-arm phase 2 trial of lorlatinib in patients with ROS1-rearranged NSCLC who developed progressive CNS metastasis on crizotinib without other sites of active, measurable extracranial disease (Supplementary Fig. 1). The study was conducted at the following two institutions: Massachusetts General Hospital and Dana-Farber Cancer Institute. The study was terminated early after enrollment of 16 of 22 planned patients owing to decreasing rate of accrual in the setting of availability of off-label lorlatinib after its approval for ALK-rearranged NSCLC in November 2018. The protocol was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board. Written informed consent was obtained from all patients before screening. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines of the International Conference on Harmonization.
Adult patients (age ≥18 y) with a histologically or cytologically confirmed diagnosis of metastatic (stage IV per American Joint Committee on Cancer version 7.0) NSCLC harboring a ROS1 rearrangement as determined by fluorescence in situ hybridization or tissue-based next-generation sequencing were enrolled. Patients were required to have at least one measurable (≥5 mm) intracranial lesion per modified Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) or evidence of leptomeningeal disease on imaging.7 Cerebrospinal fluid evaluation was not mandated to confirm leptomeningeal disease. Untreated and treated CNS metastases were permitted. To be eligible to enroll after radiation therapy, patients had to have new CNS metastases or irradiated CNS metastases that had unequivocal progression (defined as >20% increase in longest diameter). Patients with symptomatic CNS lesions were eligible. Steroid use was permitted to address neurologic symptoms if the steroid dose was stable or decreasing for at least 1 week before enrollment. Patients with measurable extracranial lesions were excluded, except for patients with extracranial lesions that were not felt to represent active sites of disease based on a prolonged period of stability per investigator assessment. There was no limit on number of prior systemic therapies.
Lorlatinib was administered with a standard starting dose of 100 mg daily taken continuously in a 21-day cycle. Safety assessments were performed at baseline and at subsequent visits. Adverse events (AEs) were graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. Dose reductions and interruptions were allowed as indicated to manage toxicities. All patients underwent baseline tumor assessments, including brain imaging by contrast-enhanced magnetic resonance imaging and computed tomography scans of the chest, abdomen, and pelvis. The study required magnetic resonance imaging slice thickness of 1 mm for brain metastases measuring between 5 and 10 mm. During treatment, imaging was performed every 6 weeks for the initial eight cycles, after which the interval was extended to every 9 weeks. On-treatment computed tomography scans were limited to chest and abdominal imaging unless a patient had evidence of pelvic metastases on baseline imaging.
Response assessment was performed centrally using RECIST v1.1 for extracranial lesions and modified RECIST v1.1 for intracranial lesions.7 Patients with ongoing clinical benefit were permitted to continue treatment beyond progression at the treating investigator’s discretion.
Statistical Design
The primary end point of the study was intracranial disease control (defined as complete response, partial response, or stable disease) at 12 weeks according to modified RECIST v1.1.7 Secondary end points included intracranial progression-free survival (PFS), intracranial duration of response, intracranial ORR, extracranial PFS, and safety and tolerability of lorlatinib. The target rate of effectiveness was defined as an intracranial disease control rate at 12 weeks of 85%. The threshold for ineffectiveness was set at an intracranial disease control rate of 60% or less. The study design had 90% power to detect this difference in intracranial disease control, with a one-side α level of 0.07. All patients who underwent intracranial restaging evaluation at 12 weeks were included in the efficacy analysis. During evaluation of intracranial efficacy end points, patients were not censored at the time of extracranial progression provided they revealed ongoing CNS disease control and continued lorlatinib. The data cutoff for this analysis was February 15, 2021. Analyses were done using SAS version 9.4. The study was originally designed to enroll 22 patients but was terminated for slow accrual after enrolling 16 patients. Thus, the results reported subsequently are descriptive in nature, as formal efficacy analysis could not be performed.
Results
Patient Characteristics
Between November 2016 and January 2019, 16 patients were enrolled. Baseline characteristics of the 16 patients are found in Table 1. The median number of previous lines of therapy was 1 (range: 1–3). All patients had received crizotinib. The median time on crizotinib was 24.7 months (range: 2.9–48.4 mo). No patient had received other ROS1 targeted therapies. Six patients (38%) had received chemotherapy. One patient had received immunotherapy. The CNS was the sole site of progression on crizotinib for 15 (94%) patients. One patient had progression of both CNS and osseous metastases. Most (n = 13, 81%) patients had only parenchymal brain metastases. The remaining three patients had both leptomeningeal and parenchymal CNS disease. Five patients (31%) had symptomatic CNS metastases at the time of enrollment, none of whom required steroids. Nine patients (56%) had irradiated brain metastases, including two patients who had previously completed whole brain radiation. The median interval between completion of the most recent brain radiation and initiation of lorlatinib was 10.9 months (range: 3.5–29.1). Two patients had previously undergone resection of brain metastases
Table 1.
Baseline Characteristics of Study Population
| Characteristics | No. (%) of Patients All Patients (N = 16) |
|---|---|
| Age, y | |
| Median | 54 |
| Range | 33–73 |
| Sex | |
| Male | 3 (19) |
| Female | 13 (81) |
| Race | |
| White | 13 (81) |
| Asian | 2 (13) |
| Unknown | 1 (6) |
| Smoking history | |
| Never | 12 (75) |
| Former | 4 (25) |
| ECOG performance status | |
| 0 | 14 (88) |
| 1 | 1 (6) |
| 2 | 1 (6) |
| Symptomatic brain metastases | |
| Yes | 5 (31) |
| No | 11 (69) |
| Prior brain radiation | |
| Yes | 9 (56) |
| No | 7 (44) |
| Number of prior lines of therapy | |
| 1 | 10 (63) |
| 2 | 5 (31) |
| 3 | 1 (6) |
| Prior chemotherapy | |
| Yes | 6 (37) |
| No | 10 (63) |
ECOG, Eastern Cooperative Oncology Group.
Efficacy
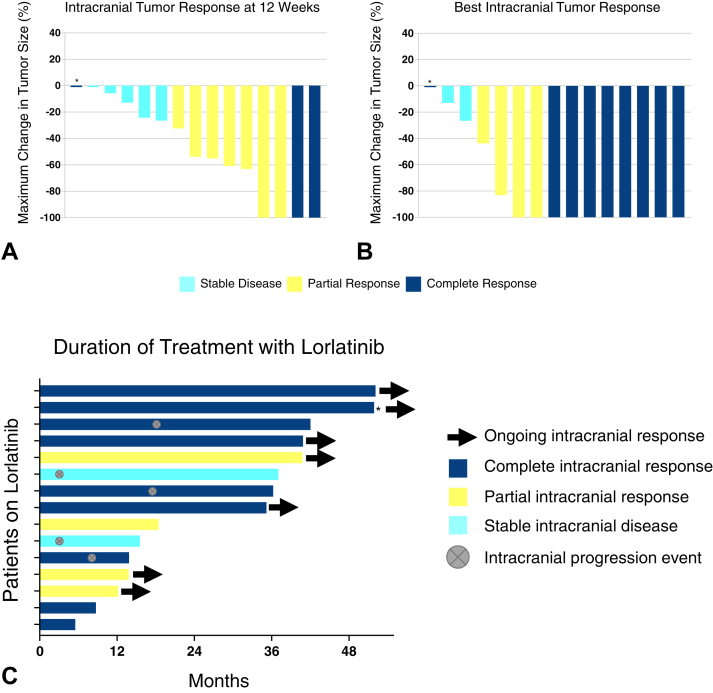
The efficacy analysis included 15 patients. One patient withdrew consent owing to financial hardship after 6 weeks on treatment and was not included in the efficacy analysis. Of the 15 patients who were assessable for intracranial response, one was inadvertently enrolled with nonmeasurable CNS lesions. A total of 10 patients (67%) achieved a confirmed objective intracranial response at 12 weeks, including three patients (20%) with complete intracranial response (Fig. 1A). The patient with nonmeasurable disease achieved complete resolution of CNS lesions by week 12, consistent with complete response. The remaining five patients had stability of intracranial disease. The intracranial disease control rate was 100% at 12 weeks, exceeding the target threshold of 85%. Four patients converted from partial to complete intracranial response during the study. In addition, three patients with initial stable disease achieved intracranial objective response with further follow-up, including two patients with complete responses and one patient with partial response. In total, nine (60%) patients achieved a complete intracranial response, and thirteen (87%) patients achieved an intracranial objective response while on study (Fig. 1B). The median time to intracranial response was 42 days which corresponded to the time of initial response assessment per the clinical trial protocol. Time on therapy for each patient is presented in Figure 1C.
Figure 1.
Intracranial antitumor activity of lorlatinib. Waterfall plots depict (A) intracranial tumor response at 12 weeks and (B) best intracranial tumor response while on study, as assessed by modified RECIST version 1.1. (C) Swimmer plot illustrates duration of treatment with lorlatinib. Intracranial response to treatment and brain metastasis status are indicated with symbols (see legend). Asterisk (∗) in A, B, and C indicates a patient with nonmeasurable disease at baseline who achieved a complete intracranial response. RECIST, Response Evaluation Criteria in Solid Tumors.
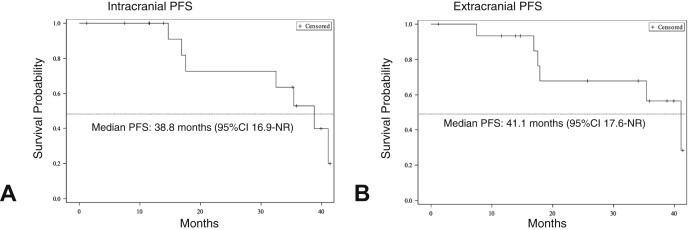
With median follow-up of 22 months from initiation of lorlatinib, seven (46%) of 15 patients experienced disease progression. Two patients progressed exclusively at extracranial sites and discontinued treatment. Five patients (33%) developed progression of brain metastases, including three patients who also experienced progression of extracranial disease. In all three cases where mixed progression was noted, intracranial and extracranial progression events were metachronous. Two of the three patients received focal radiation to progressive sites (n = 1 CNS, n = 1 calvarium) and continued treatment beyond progression, whereas the remaining patient was observed on therapy given slowly enlarging multifocal osseous lesions. The median intracranial PFS was 38.8 months (95% confidence interval [CI]: 16.9–not reported; Fig. 2A). Median intracranial duration of response was not able to be estimated owing to the limited number of progression events. The median extracranial PFS was 41.1 months (95%CI: 17.6–not reported; Fig. 2B).
Figure 2.
PFS on lorlatinib. (A) Kaplan-Meier curve illustrates intracranial PFS on lorlatinib. (B) Kaplan-Meier curve illustrates extracranial PFS on lorlatinib. +, censored; CI, confidence interval; NR, not reached; PFS, progression-free survival.
Safety
All 16 patients were evaluated for safety (Table 2). The most common treatment-related AEs of any grade were hypercholesterolemia (100%), hypertriglyceridemia (75%), peripheral edema (63%), cognitive effects (63%), transaminase elevation (56%), neuropathy (56%), weight gain (38%), and mood effects (31%). Six grade 3 treatment-related AEs were observed, including one patient with hypercholesterolemia, one patient with hypertriglyceridemia, one patient with weight gain, and three patients with lipase elevation. Lipase elevation was not associated with clinical or imaging features of pancreatitis. No patient experienced grade 4 or 5 treatment-related events.
Table 2.
Treatment-Related Adverse Events Occurring in Greater Than or Equal to 10% of Patients
|
Adverse Event |
No. (%) of Patients With Treatment-Related Adverse Event by Gradea |
|||
|---|---|---|---|---|
| All Grades | Grade 1 | Grade 2 | Grade 3 | |
| Hypercholesterolemia | 16 (100) | 8 (50) | 7 (44) | 1 (6) |
| Hypertriglyceridemia | 12 (75) | 7 (44) | 4 (25) | 1 (6) |
| Cognitive effects | 10 (63) | 9 (56) | 1 (6) | 0 (0) |
| Peripheral edema | 10 (63) | 7 (44) | 3 (19) | 0 (0) |
| AST elevation | 9 (56) | 9 (56) | 0 (0) | 0 (0) |
| ALT elevation | 9 (56) | 9 (56) | 0 (0) | 0 (0) |
| Peripheral neuropathy | 9 (56) | 7 (44) | 1 (6) | 0 (0) |
| Weight gain | 6 (38) | 4 (25) | 1 (6) | 1 (6) |
| Mood effects | 5 (31) | 4 (25) | 1 (6) | 0 (0) |
| Polyphagia | 4 (25) | 4 (25) | 0 (0) | 0 (0) |
| Myalgia | 4 (25) | 4 (25) | 0 (0) | 0 (0) |
| Arthralgia | 3 (19) | 3 (19) | 0 (0) | 0 (0) |
| Lipase elevation | 3 (19) | 0 (0) | 0 (0) | 3 (19) |
| Amylase elevation | 2 (13) | 2 (13) | 0 (0) | 0 (0) |
| Median nerve neuropathy | 2 (13) | 1 (6) | 1 (6) | 0 (0) |
| Constipation | 2 (13) | 1 (6) | 1 (6) | 0 (0) |
| Fatigue | 2 (13) | 2 (13) | 0 (0) | 0 (0) |
| Headache | 2 (13) | 2 (13) | 0 (0) | 0 (0) |
ALT, alanine transaminase; AST, aspartate transaminase.
No grade 4 or 5 events were observed.
Furthermore, 11 patients (69%) required dose reduction to manage AEs, including seven (44%) patients who needed greater than or equal to two dose reductions. Mean lorlatinib dose intensity was 66 mg. The most common reasons for dose reduction were neurocognitive effects which occurred in six patients (38%) and neuropathy which occurred in three cases (19%). Other notable reasons for dose reduction included peripheral edema accompanied by grade 2 left ventricular ejection fraction decrease (n = 1, 6%) and mental status change in the setting of posterior reversible encephalopathy syndrome (PRES, n = 1, 6%). The patient with ejection fraction decrease recovered cardiac function with dose interruption allowing him to maintain treatment at lorlatinib 50 mg for an additional 12 months before it was stopped for progression. The patient with PRES had concomitant use of intraocular bevacizumab which may have been a predisposing factor. Lorlatinib was reduced to 75 mg without recrudescence of PRES symptoms and maintained for 18 months before transitioning to commercial lorlatinib. One patient discontinued lorlatinib owing to toxicity, specifically intolerable edema despite dose reduction to 25 mg.
Patient Disposition
All 16 patients have discontinued treatment on study. One patient discontinued lorlatinib because of treatment toxicity (edema) and seven patients ultimately stopped treatment owing to disease progression. The remaining patients, all of whom had ongoing intracranial disease control, withdrew from the study owing to financial hardship (n = 1) or transition to commercial lorlatinib (n = 7).
Molecular Alterations Identified at Progression on Lorlatinib
Three patients with extracranial progression underwent repeat biopsies (tissue, n = 2; plasma, n = 1) at the time of relapse. ROS1 kinase domain mutations were detected in the two lorlatinib-resistant tissue biopsies: ROS1 G2032R (n = 1, after 8 mo on lorlatinib) and L2086F (n = 1, after 17 mo on lorlatinib), both of which have previously been described in lorlatinib-resistant models.8 Notably, neither patient had undergone a biopsy before initiating lorlatinib, precluding confirmation that the mutations were acquired on lorlatinib. Plasma testing in the third case revealed ROS1 G2032R, including a CCDC6-RET fusion, after 13 months on lorlatinib. CCDC6-RET fusions have been reported as a rare actionable resistance mechanism in EGFR-mutant NSCLC,9 but have not previously been reported in ROS1+ NSCLC. None of the five patients with CNS progression underwent resection of brain metastases.
Discussion
Despite the systemic activity of crizotinib and entrectinib,4,10 CNS relapse is common at disease progression. Indeed, the CNS is among the initial sites of progression in at least one-third of patients treated with either drug, with a subset of patients experiencing progression confined to the brain.2,4 CNS progression is particularly problematic for patients with baseline brain metastases. Indeed, an updated analysis from the pooled studies that led to the FDA approval of entrectinib reported CNS progression in 48.2% of patients with pretreatment brain metastases compared with 2.9% of patients without baseline brain metastases.11 There are currently no FDA-approved targeted therapies for patients that have progressed on crizotinib or entrectinib. Nevertheless, several investigational next-generation ROS1 TKIs (e.g., lorlatinib, taletrectinib, and repotrectinib) have encouraging intracranial activity in small cohorts of crizotinib-pretreated patients with progressive brain metastases.5,12,13 As CNS-only relapse is often purported to reflect drug pharmacokinetics (i.e., limited bioavailability of drug in the CNS) rather than true acquired resistance to therapy,3,14 we launched this study to specifically investigate whether the distinct pathogenesis of CNS-only progression on crizotinib would translate to enhanced sensitivity to subsequent treatment with lorlatinib, a more potent ROS1 TKI with established CNS penetration.
In this investigator-initiated, single-arm, phase 2 study, lorlatinib was found to have robust intracranial activity in patients with CNS-only progression on crizotinib, with an intracranial ORR and intracranial disease control rate of 87% and 100%, respectively. During the study, a complete intracranial response was observed in 60% of patients. Our findings suggest that introducing the CNS-penetrant, next-generation ROS1 TKI lorlatinib is an effective strategy for overcoming isolated CNS progression on crizotinib. The CNS efficacy outcomes of lorlatinib in our study are more encouraging than the intracranial activity of entrectinib in a similar patient population (ORR 11%, median PFS 4.7 mo).15 In the global phase 1/2 study that initially established the activity of lorlatinib in ROS1+ NSCLC, the intracranial ORR among 24 patients with measurable or nonmeasurable baseline CNS metastases who had progressed on crizotinib was 50%, with an intracranial complete response rate of 38%, which is lower than what we have observed in our study.5 Although our study was not designed to compare outcomes of patients with mixed site progression versus those with CNS-only progression, our findings combined with the global phase 1/2 study findings confirm that lorlatinib is an efficacious therapy in either context, with some suggestion that intracranial activity may be even more pronounced in patients with CNS-only relapse. Although we did not characterize the molecular profile of enlarging or new brain metastases in patients enrolling in our study, the high intracranial ORR (87%) in our study relative to the systemic ORR (35%) among crizotinib-pretreated patients in the global phase 1/2 trial raises the possibility that brain metastases that arise in the setting of isolated CNS relapse on crizotinib may remain ROS1 dependent.
The safety profile of lorlatinib in our study was consistent with previous studies.5,16 Specifically, the most common treatment-related AEs were lipid abnormalities, edema, alanine aminotransferase and aspartate aminotransferase elevation, peripheral neuropathy, cognitive effects, and mood effects. Nevertheless, the overall rate of dose reduction for treatment-related AEs was higher in our study (69%) than the global phase 1/2 study (25%).5 Neurocognitive toxicity and neuropathy were the most common reasons for dose reduction. The median interval between initiating lorlatinib and dose reduction was 42 days (range: 10–566 d). As 57% of patients in the global study had baseline brain metastases,5 it is unlikely that the differences in treatment tolerance were primarily driven by the decision to exclusively enroll patients with CNS involvement. Of note, the global study excluded patients with symptomatic brain metastases whereas approximately one-third of patients in our study had symptomatic CNS disease at study entry. As patients with baseline symptomatic disease may have increased difficulty tolerating additional neurologic side effects and treating providers may have a lower threshold to reduce lorlatinib dose in this context, it is possible that the higher rate of dose reduction reflects differences in baseline functioning of the patients enrolled in each study. It is also possible that different approaches to dose reduction in general (i.e., greater enthusiasm for dose reduction in our study compared with the global studies) may have contributed to this discrepancy. Larger studies conducted at multiple institutions with a variety of investigators are overall better positioned to weather potential imbalances that can arise in smaller studies such as ours.
Our study has several limitations, including its design as a single-arm, phase 2 trial that enrolled a small number of patients. We intentionally targeted a small sample size as we were primarily recruiting a subset of patients belonging to a rare molecular subtype of NSCLC. To overcome this limitation, we sought a dramatic effect size (intracranial ORR = 85%) that could be captured in a smaller study. Unfortunately, the target sample size was not achieved owing to slow accrual in the setting of off-label access to the study drug on its approval for ALK-rearranged NSCLC. Central to the concept of this study was the desire to gain additional insights into the disease biology of CNS-specific relapses on crizotinib. To this end, our study design incorporated optional cerebrospinal fluid sampling before treatment and serially during treatment. Nevertheless, none of the patients elected to undergo this procedure. Thus, the molecular analyses included in this study only pertain to sampling of extracranial lesions. As a result, although the clinical outcomes reported in this study are encouraging, the underlying biology of progressive brain metastases on crizotinib remains poorly elucidated. Finally, patients in our study did not receive entrectinib or investigational next-generation ROS1 TKIs before initiating lorlatinib. As a result, our study design limits drawing conclusions about the intracranial activity of lorlatinib in patients who experience intracranial progression on ROS1 TKIs other than crizotinib.
In summary, our phase 2 trial reveals that lorlatinib has substantial and durable intracranial activity in patients with isolated CNS progression on crizotinib. The intracranial disease control rate of 100% and intracranial complete response rate of 60% found with lorlatinib in this context support the notion that isolated CNS progression events on crizotinib can be overcome by introducing a more potent, CNS-penetrant ROS1 TKI.
CRediT Authorship Contribution Statement
Jaime L. Schneider: Data curation, Formal analysis, Visualization, Writing—review and editing.
Alona Muzikansky: Formal analysis, Writing—review and editing.
Jessica J. Lin, Elizabeth A. Krueger, Inga T. Lennes, Joseph O. Jacobson, Michael Cheng, Rebecca S. Heist, Zofia Piotrowska: Data curation, Writing—review and editing.
Justin F. Gainor: Conceptualization, Methodology, Data curation, Writing—review and editing.
Alice T. Shaw: Conceptualization, Methodology, Data curation, Supervision, Writing—review and editing.
Ibiay Dagogo-Jack: Conceptualization, Methodology, Data curation, Supervision, Investigation, Writing—review and editing.
Acknowledgments
The study was funded in full by Pfizer. The authors thank the patients, their families and caregivers, and the co-investigators, study staff, and research coordinators at the two participating centers.
Footnotes
Disclosure: Dr. Dagogo-Jack has received honoraria from Foundation Medicine, Creative Education Concepts, OncLive, ASCO Post, DAVA Oncology, Medscape, Total Health, and American Lung Association; consulting fees from AstraZeneca, Boehringer Ingelheim, Bayer, BostonGene, Catalyst, Genentech, Janssen, Novocure, Pfizer, Sanofi-Genzyme, Syros, and Xcovery; research support from Array, Genentech, Novartis, Pfizer, and Guardant Health; and travel support from Array and Pfizer. Dr. Schneider served as a compensated consultant for Genentech, C4 Therapeutics, Blueprint Medicines, Nuvalent, Turning Point Therapeutics, Bayer, Novartis, and Elevation Oncology; received honorarium and travel support from Pfizer; received institutional research funds from Hengrui Therapeutics, Turning Point Therapeutics, Neon Therapeutics, Relay Therapeutics, Bayer, Elevation Oncology, Roche/Genentech, Pfizer, Linnaeus Therapeutics, Nuvalent, and Novartis; and received CME funding from OncLive, MedStar Health, and Northwell Health. Dr. Piotrowska has received consulting fees from Janssen, Daiichi Sankyo, Takeda, Cullinan, C4 Therapeutics, Jazz, Blueprint, Eli Lilly, Incyte, AstraZeneca, Genentech, Spectrum, and Novartis, and receives institutional research support from Novartis, Takeda, Spectrum, AstraZeneca, Tesaro/GlaxoSmithKline, Cullinan, Daiichi Sankyo, AbbVie, Janssen, and Blueprint. Dr. Heist has received consulting honoraria from Novartis, Daiichi Sankyo, EMD Serono, Boehringer Ingelheim, Tarveda, and Apollomics, and has research funding (to institution, not to self) from Agios, AbbVie, Daiichi Sankyo, Novartis, Eli Lilly, Mirati, Corvus, Genentech Roche, Exelixis, and Turning Point. Dr. Gainor has served as a compensated consultant or received honoraria from Bristol-Myers Squibb, Genentech, Takeda, Loxo/Lilly, Blueprint, Oncorus, Regeneron, Gilead, Moderna, AstraZeneca, Pfizer, Novartis, Merck, iTeos, Karyopharm, Silverback Therapeutics, and GlydeBio; research support from Novartis, Genentech/Roche, and Takeda; institutional research support from Bristol-Myers Squibb, Tesaro, Moderna, Blueprint, Jounce, Array Biopharma, Merck, Adaptimmune, Novartis, and Alexo; and has an immediate family member who is an employee with equity at Ironwood Pharmaceuticals. Dr. Shaw has served as a compensated consultant or received honoraria from Achilles, Archer, ARIAD, Bayer, Blueprint Medicines, Chugai, Daiichi Sankyo, EMD Serono, Foundation Medicine, Genentech/Roche, Guardant, Ignyta, KSQ Therapeutics, LOXO, Natera, Novartis, Pfizer, Servier, Syros, Taiho Pharmaceutical, Takeda, and TP Therapeutics; has received research (institutional) funding from Daiichi Sankyo, Ignyta, Novartis, Pfizer, Roche/Genentech, and TP Therapeutics; has received travel support from Pfizer and Genentech/Roche; has served on the Board of Directors of Syros Pharmaceuticals; and is currently employed by Novartis. The remaining authors declare no conflict of interest.
Cite this article as: Schneider JL, Muzikansky A, Lin JJ, et al. A phase 2 study of lorlatinib in patients with ROS1-rearranged lung cancer with brain-only progression on crizotinib. JTO Clin Res Rep. 2022;3:100347.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100347.
Supplementary Data
References
- 1.Gainor J.F., Tseng D., Yoda S., et al. Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non-small-cell lung cancer. JCO Precis Oncol. 2017;2017 doi: 10.1200/PO.17.00063. PO.17.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patil T., Smith D.E., Bunn P.A., et al. The incidence of brain metastases in stage IV ROS1-rearranged non-small cell lung cancer and rate of central nervous system progression on crizotinib. J Thorac Oncol 11. 2018;13:1717–1726. doi: 10.1016/j.jtho.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa D.B., Kobayashi S., Pandya S.S., et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29:e443–e445. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 4.Drilon A., Siena S., Dziadziuszko R., et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21:261–270. doi: 10.1016/S1470-2045(19)30690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw A.T., Solomon B.J., Chiari R., et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2019;20:1691–1701. doi: 10.1016/S1470-2045(19)30655-2. [DOI] [PubMed] [Google Scholar]
- 6.Johung K.L., Yeh N., Desai N.B., et al. Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis. J Clin Oncol. 2016;34:123–129. doi: 10.1200/JCO.2015.62.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long G.V., Trefzer U., Davies M.A., et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 8.Lin J.J., Choudhury N.J., Yoda S., et al. Spectrum of mechanisms of resistance to crizotinib and lorlatinib in. Clin Cancer Res. 2021;27:2899–2909. doi: 10.1158/1078-0432.CCR-21-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piotrowska Z., Isozaki H., Lennerz J.K., et al. Landscape of acquired resistance to osimertinib in. Cancer Discov. 2018;8:1529–1539. doi: 10.1158/2159-8290.CD-18-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw A.T., Ou S.H., Bang Y.J., et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dziadziuszko R., Krebs M.G., De Braud F., et al. Updated integrated analysis of the efficacy and safety of entrectinib in locally advanced or metastatic. J Clin Oncol. 2021;39:1253–1263. doi: 10.1200/JCO.20.03025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drilon A., Ou S.I., Cho B.C., et al. Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent- front mutations. Cancer Discov. 2018;8:1227–1236. doi: 10.1158/2159-8290.CD-18-0484. [DOI] [PubMed] [Google Scholar]
- 13.Ou S.I., Fujiwara Y., Shaw A.T., et al. Efficacy of taletrectinib (AB-106/DS-6051b) JTO Clin Res Rep. 2021;2 doi: 10.1016/j.jtocrr.2020.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metro G., Lunardi G., Floridi P., et al. CSF concentration of crizotinib in two ALK-positive non-small-cell lung cancer patients with CNS metastases deriving clinical benefit from treatment. J Thorac Oncol. 2015;10:e26–e27. doi: 10.1097/JTO.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 15.Drilon A., Chiu C., Fan Y., et al. Long-term efficacy and safety of entrectinib in ROS1 fusion-positive nonsmall cell lung cancer. JTO Clin Res Reports. 2020;21:261–270. doi: 10.1016/j.jtocrr.2022.100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girard N., Galland-Girodet S., Avrillon V., et al. Lorlatinib for advanced ROS1+ non-small-cell lung cancer: results of the IFCT-1803 LORLATU study. ESMO Open. 2022;7 doi: 10.1016/j.esmoop.2022.100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.