Abstract
A number of molybdopterin enzymes, including xanthine oxidoreductase (XOR), aldehyde oxidase (AO), sulfite oxidase (SO), and mitochondrial amidoxime reducing component (mARC), have been identified as nitrate and nitrite reductases. Of these enzymes, XOR has been the most extensively studied and reported to be a substantive source of nitric oxide (NO) under inflammatory/hypoxic conditions that limit the catalytic activity of the canonical NOS pathway. It has also been postulated that XOR nitrite reductase activity extends to red blood cell (RBCs) membranes where it has been immunohistochemically identified. These findings, when combined with countervailing reports of XOR activity in RBCs, incentivized our current study to critically evaluate XOR protein abundance/enzymatic activity in/on RBCs from human, mouse, and rat sources. Using various protein concentrations of RBC homogenates for both human and rodent samples, neither XOR protein nor enzymatic activity (xanthine → uric acid) was detectable. In addition, potential loading of RBC-associated glycosaminoglycans (GAGs) by exposing RBC preparations to purified XO before washing did not solicit detectable enzymatic activity (xanthine → uric acid) or alter NO generation profiles. To ensure these observations extended to absence of XOR-mediated contributions to overall RBC-associated nitrite reduction, we examined the nitrite reductase activity of washed and lysed RBC preparations via enhanced chemiluminescence in the presence or absence of the XOR-specific inhibitor febuxostat (Uloric®). Neither addition of inhibitor nor the presence of the XOR substrate xanthine significantly altered the rates of nitrite reduction to NO by RBC preparations from either human or rodent sources confirming the absence of XO enzymatic activity. Furthermore, examination of the influence of the age (young cells vs. old cells) of human RBCs on XO activity also failed to demonstrate detectable XO protein. Combined, these data suggest: 1) that XO does not contribute to nitrite reduction in/on human and rodent erythrocytes, 2) care should be taken to validate immuno-detectable XO by demonstrating enzymatic activity, and 3) XO does not associate with human erythrocytic glycosaminoglycans or participate in nonspecific binding.
Keywords: Nitrite, Xanthine oxidoreductase, Nitric oxide, Red blood cells, Glycosaminoglycans
1. Introduction
Xanthine oxidoreductase (XOR) catalyzes the final two steps in purine catabolism (hypoxanthine → xanthine → uric acid). XOR is a homodimer with each monomer consisting of four redox centers: a molybdenum cofactor (Mo-co), one FAD site, and two Fe/S clusters. The Mo-co is the site of purine oxidation while NAD+ and O2 reduction occur at the FAD [1–3]. The two Fe/S clusters operate to transfer electrons between the Mo-co and the FAD. The enzyme is translated as xanthine dehydrogenase (XDH) where purine-derived electrons reduce NAD+ to NADH. Under inflammatory conditions post-translational modification of XDH produces xanthine oxidase (XO) where affinity for NAD+ is diminished while affinity for O2 is enhanced resulting in oxidant generation. This capacity to reduce O2 has positioned XO as a critical source of oxidants in a variety of inflammatory processes [4–10].
While elevation in XO levels has long been associated with oxidant production, recent reports have described a salutary function for both XDH and XO whereby their catalytic activity can be utilized to reduce nitrate (NO3−) to nitrite (NO2−) and NO2− to nitric oxide (NO) [11–18]. Several in vitro studies clearly demonstrate the capacity for XO to generate NO from NO2− under inflammatory conditions [19–24]. Importantly, these hypoxic/ischemic conditions limit NO generation from endothelial nitric oxide synthase and have incentivized the supposition that XO- and XDH-mediated NO generation provides compensation [14]. Numerous in vivo reports validate the in vitro findings and demonstrate a clear XOR-dependence on beneficial outcomes associated with nitrite treatment in various inflammatory processes including those associated with the vasculature where vascular-associated XOR has been contributory [11,12,25–29]. However, the details regarding micro-environmental conditions requisite for XOR-mediated NO generation are not completely understood and may involve cooperation with alternative agents such as sulfide to afford maximal benefit [30].
While a detailed accounting of factors describing the biological relevance of XOR-mediated NO formation is ongoing, XOR’s mobility may play a key role in its overall impact as a NO source. For example, XDH can be released from cells (e.g. hepatocytes) to the circulation, rapidly converted to XO by plasma proteases, and bind avidly to the glycocalyx of endothelial cells affording XO capacity to impact vessel homeostasis via alteration of NO levels [31–33]. Recently, immunocytochemical analysis of human RBCs demonstrated the presence of XO on the membrane where it was suggested to participate in NO2− reduction to NO [34,35]. Incentivized by these findings, we examined the extent of XO protein abundance, activity, and nitrite reductase activity in human and rodent RBCs.
2. Materials and methods
Animals, Blood collection and Processing-
Both male and female rodents (Sprague Dawley rats and C57Bl/6 J mice, ages 28–42 weeks) were used for experiments in compliance with WVU IACUC-approved protocols. Blood was collected at time of euthanasia and kept on ice until spun at 1,100×g for 10 min at 4 °C. Plasma was collected and placed in a separate tube while the RBC pellet was washed 3x in a 1:1 ratio with ice-cold potassium phosphate buffer (KPi, 50 mM and pH 7.4) by gentle inversion. RIPA buffer was added and pellets homogenized by needle aspiration. Human blood samples were collected according to the WVU IRB-approved protocol (1303023857). Blood from healthy human subjects (3 men and 3 women, ages 20–50) was collected by venipuncture in tubes coated in potassium EDTA and processed as above. For experiments testing young and old RBCs, blood was collected from healthy volunteers (2 males and 3 females) according to UAB IRB-approved protocol (090504006) by venipuncture and centrifuged (1500×g, 4 °C, 10 min) to pellet RBCs. Blood was collected from 2 male and 3 female volunteers (age range 25–48 years old). Human RBCs were then separated into young and old fractions as previously described [36].
XOR Activity-
Plasma and RBC homogenates were assessed for XO and/or XDH activity as previously described using reverse phase HPLC coupled to electrochemical (CoulArray) detection of uric acid [37]. One unit of activity (U) is defined as 1 μmole urate formed per min at 37 °C and pH 7.4. The lower limit of detection is 300 nM.
Nitric oxide detection-
Nitric oxide was detected using chemiluminescence (Sievers Nitric Oxide Analyzer). Reaction chamber contained the following at the indicated concentrations using 5 mL KPi, pH 7.0: xanthine (25 μM) and RBC homogenate (5 mg). Reactions were initiated by adding NaNO2− (500 μM). Authentication of NO was accomplished by addition of cPTIO (200 μM). Initial rates of NO generation were used for quantification.
Western Blotting-
Electrophoresis used 4–12% Bis Tris gradient Criterion™ XT Precast Gels (Bio Rad, #3450123). Samples were resolved by SDS-PAGE and transferred to nitrocellulose. Membranes were blocked with Tris-Buffered Saline (TBS)-based LiCOR Intercept blocking buffer (LiCOR, 927–80001). The primary antibody was xanthine oxidase A-3 mouse monoclonal antibody (Santa Cruz, #398548), 1:500 dilution. Secondary antibody was LiCOR, 926–32210. Blots were imaged with the LiCOR Odyssey CLX.
3. Results
XO activity in plasma-
Plasma from human, mouse, and rat was collected as described in the methods. Data shown in Fig. 1A demonstrates robust XO activity in the plasma from rodents as well as XO activity values for human plasma. XDH activity was not detected in any of the plasma samples which was expected due to plasma protease-mediated partial proteolysis resulting in conversion to XO.
Fig. 1.
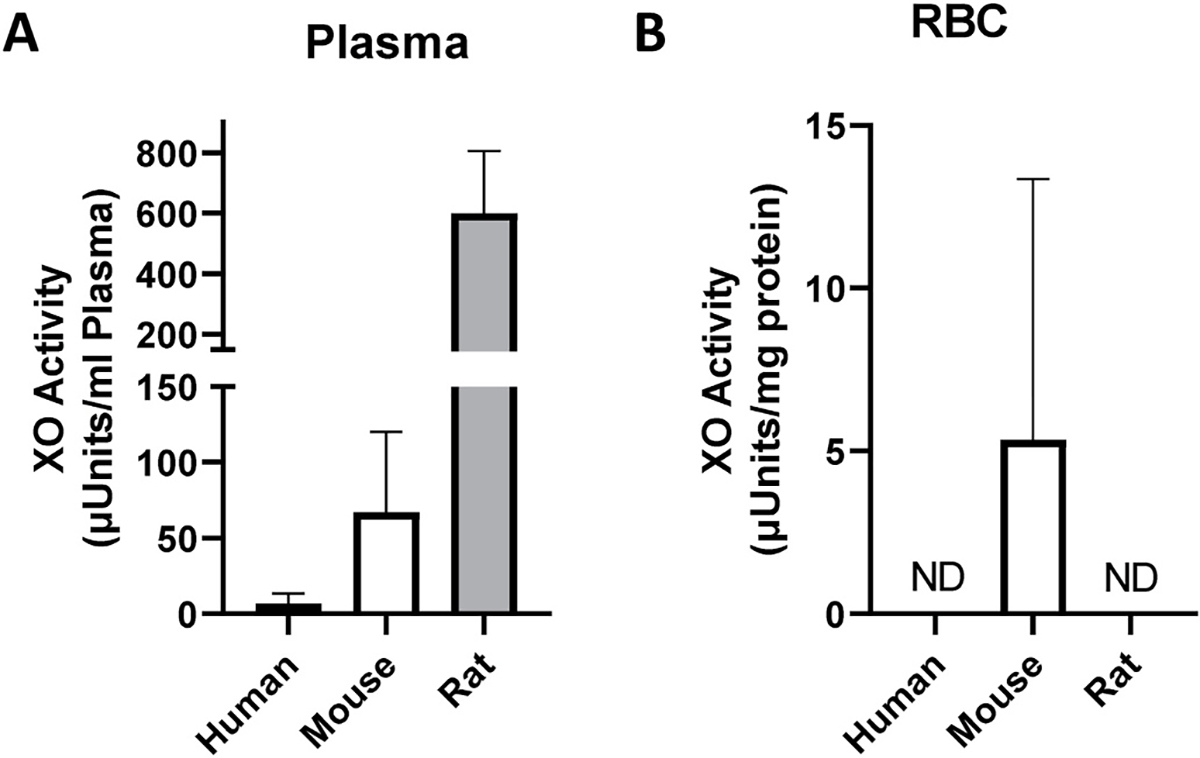
Human, mouse, and rat RBCs do not demonstrate XO activity. Human, mouse, and rat plasma (A) and washed RBC homogenates (B) were analyzed for XO activity as described in the methods. The volume of RBC homogenate used was 16 μL which corresponds to 0.8 mg protein. Data represent the mean ± standard deviation, n = 6 for human, n = 3 mice, and n = 4 for rat samples.
XO activity in RBC homogenates-
RBC homogenates (0.8 mg total protein) were assayed for XO activity as shown in Fig. 1B. Rodent samples contained oxonic acid (100 μM) to eliminate potential contributions of urate oxidase. In the mouse samples, some positive XO activity was indicated whereas in rat and human samples activity was not detected. The presence of XO activity in the 0.2, 0.4 and 1.6 mg of murine RBC homogenate was not detectable suggesting contamination by existing UA to be responsible for the positive value in the 0.8 sample. In all cases, statistical analysis demonstrated no difference between the three groups. Analysis of XDH activity also revealed no detectable enzymatic activity in any of the groups.
XO binding to RBC GAGs-
In order to fully explore the potential for XO to bind RBC-associated GAGs, purified XO (50 mU) was added to the isolated RBCs (50 mM KPi, pH 7.4) and allowed to incubate with gentle inversion for 15 min. Following this incubation, the RBCs were subjected to gentle washing as described in the methods. In a control experiment, 25 mU of purified XO was added to a suspension of heparin-Sepharose 6B beads (HS6B-XO) and incubated and washed in the same manner as the RBCs. As seen in Fig. 2, RBCs with and without exposure to purified XO did not demonstrate XO activity whereas HS6B-XO showed abundant activity indicative of robust binding to the HS6B. Furthermore, addition of 100 μM allopurinol only partially diminished HS6B-XO activity while febuxostat-mediated inhibition was not affected by XO-HS6B binding, a limitation of allo/oxypurinol-based inhibition of GAG-bound XO that we have previously reported [38,39].
Fig. 2.
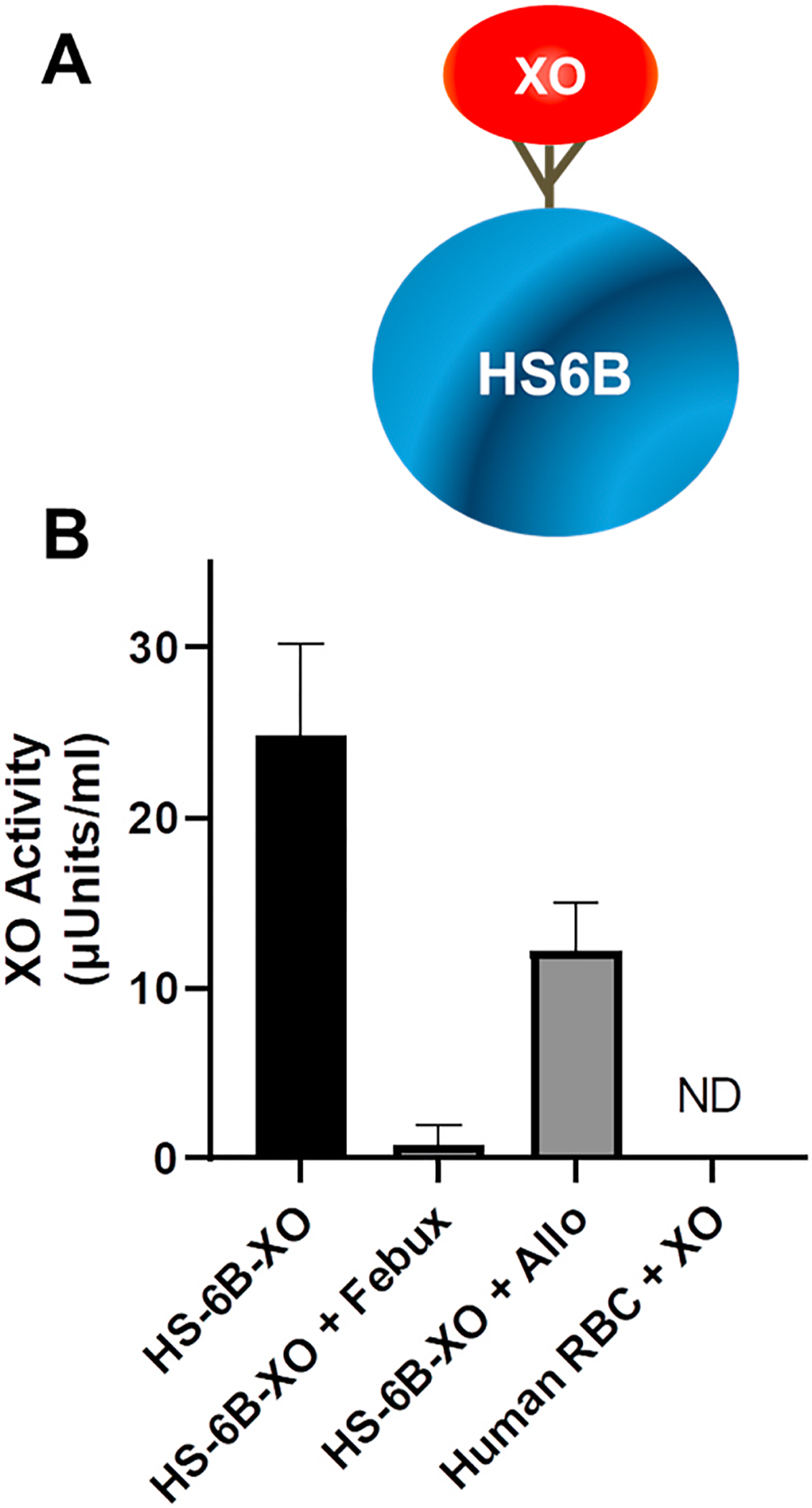
RBCs do not bind XO. A) Cartoon depiction of XO bound to heparin-Sepharose 6B. B) Purified XO (50 mU) was added to human blood for 15 min and then the samples washed under identical conditions as in Fig. 1 and in the methods. Samples were then assessed for XO enzymatic activity as in Fig. 1. Control samples were exposed to either the XO inhibitor allopurinol (Allo) or febuxostat (Febux) as indicated. Data represent the mean ± standard deviation n = 4.
XOR protein analysis of RBC homogenates and impact of cellular age-
To determine if XOR protein was present in/on RBC preparations from human, mouse, and rat samples, Western blot analysis was performed using a commercially-available mouse monoclonal antibody (Santa Cruz) as described in the methods. As seen in Fig. 3A, the dominate band, migrating at ~150 kD and representing a full monomer of the ~300 kD XOR homodimer, is detectable in the positive control (purified XO) lane whereas this band is absent in the lanes containing RBC homogenates. To further validate this monoclonal antibody we probed murine hepatocytes, rat liver homogenates and performed XDH cellular knockdown with siRNA (Suppl. Fig. 1).
Fig. 3.
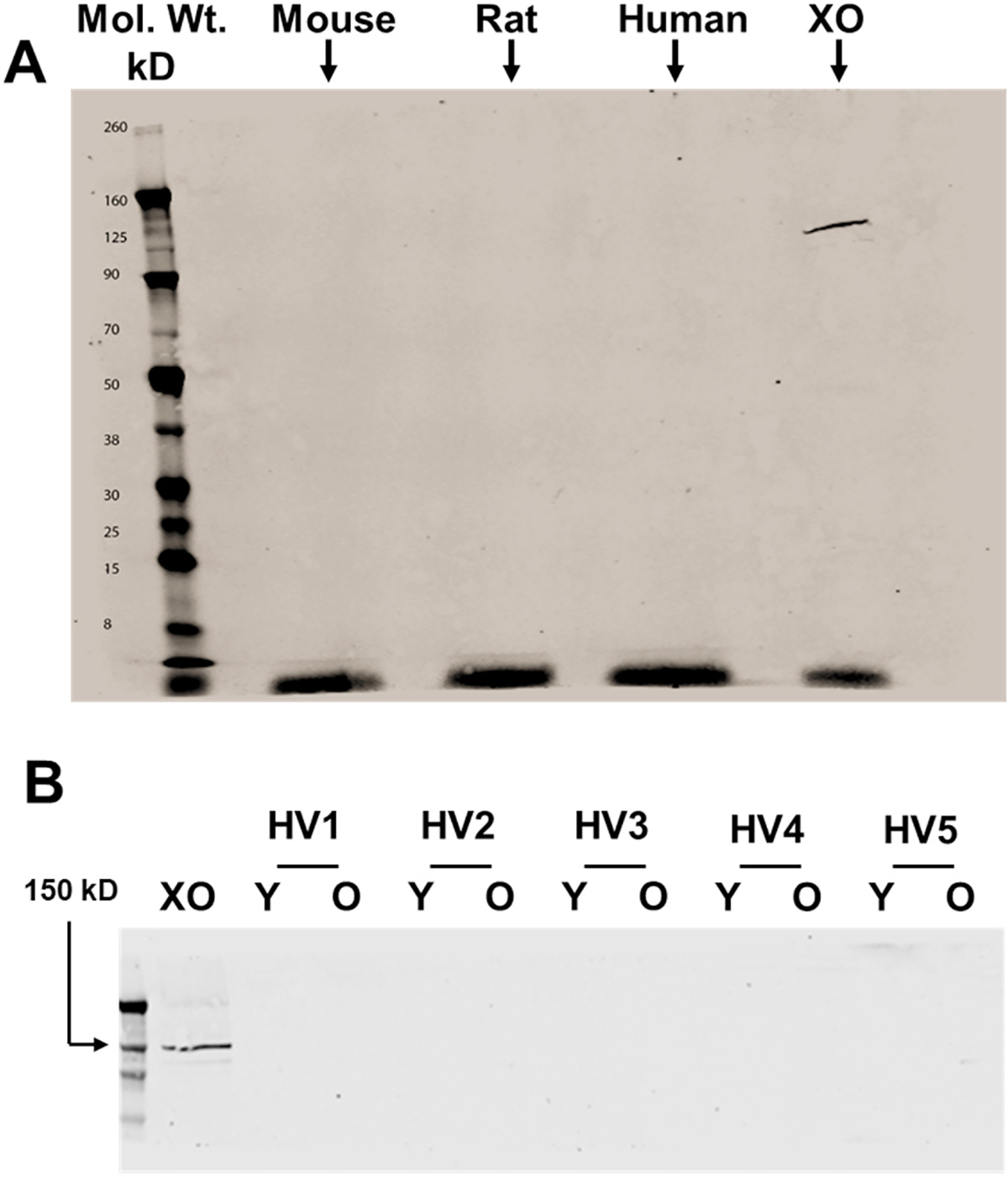
Assessment of RBC-associated XOR protein abundance and impact of cellular age. A) Western blot analysis was performed on human, mouse, and rat RBC homogenates using a monoclonal anti-XOR antibody (Santa Cruz) as described in the methods. Purified XO was used as a positive control and electrophoresed using 0.5 μg total protein. A total of 30 μg of protein was electrophoresed for each homogenate (human, mouse, and rat). Shown is a representative of 3 independent blots representing a total of 3 mice, 3 rats and 3 humans. B) Western blot analysis was performed on five different human RBC homogenates, either young or old, using the same mouse monoclonal anti-XOR antibody as described above. Purified XO was used as a positive control and electrophoresed using 0.5 μg total protein. (HV = healthy volunteer/Y = young/O = old).
Any preparation of human RBCs will comprise cells of different ages spanning the 120d lifespan. Moreover, older RBCs which are destined for clearance typically have altered cell surface characteristics that may allow more or less XO binding. To determine if cellular, age-related alterations impact the content of XO in/on human RBCs, using the same monoclonal antibody, Western blot analysis was conducted on five human samples that were either of young or old cellular age. The positive control band (purified XO) is present at ~150 kD whereas it is not detectable in any other lane indicating the absence of immuno-detectible XO in the patient samples regardless of age, Fig. 3B.
XOR-mediated nitrite reductase activity-
Mouse, rat, and human samples were next analyzed for contributions of XOR to overall NO2− reductase activity, Fig. 4. Samples were prepared in the reaction chamber with 100 μL of RBC homogenate (5 mg total protein) in KPi pH 7.0 with or without xanthine (20 μM) and with or without febuxostat (10 μM). Reactions were initiated by the addition of NaNO2− (500 μM) and authentication of NO was determined by addition of the NO spin trap carboxy-PTIO. As seen in an example of a typical experiment in Fig. 4A, NO formation was detected following addition of NO2− in human samples that was not altered by the presence of febuxostat. Quantitation revealed that neither the presence of xanthine nor febuxostat altered NO generation indicating the absence of XO-catalyzed NO2− reduction, Fig. 4B.
Fig. 4.
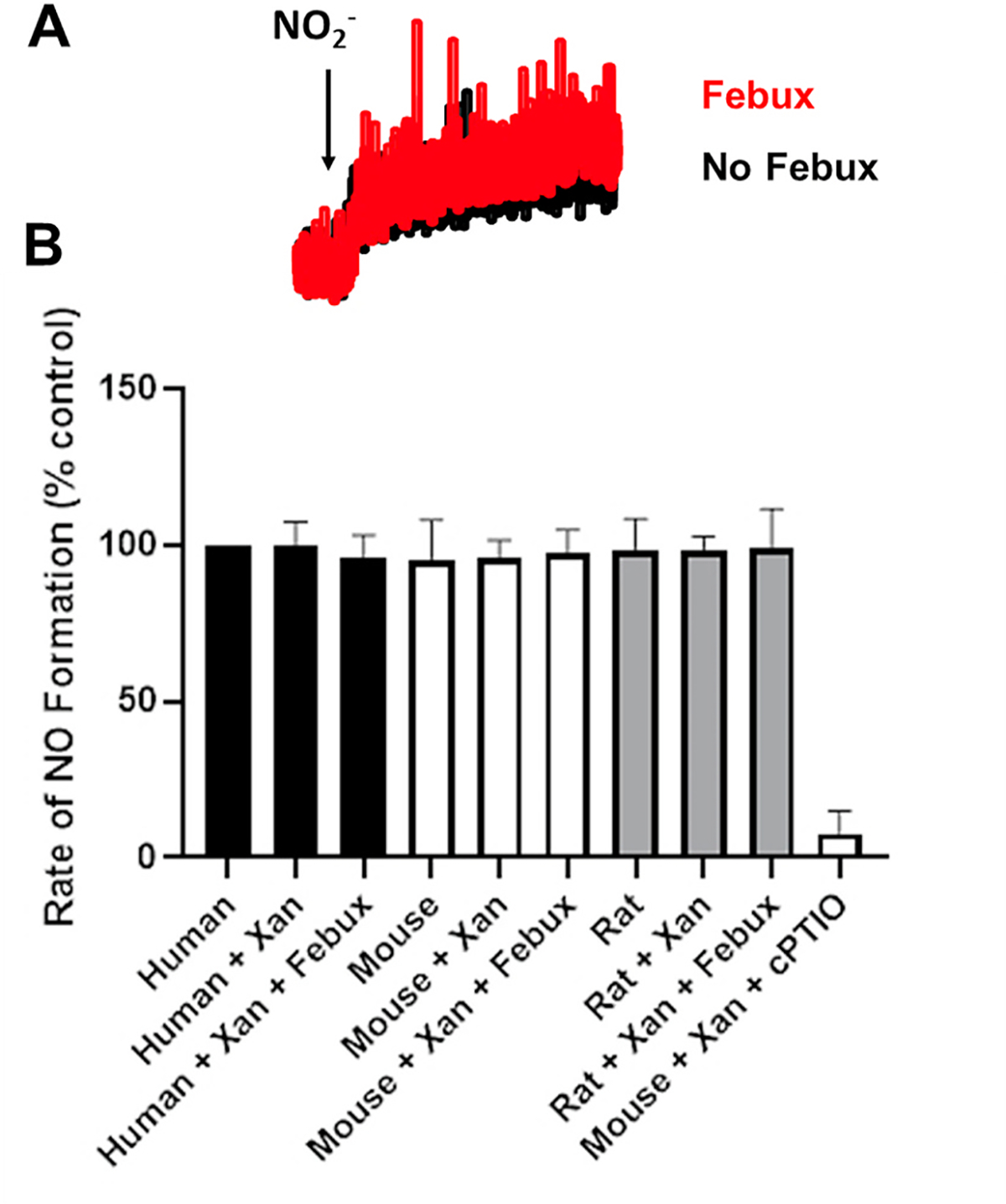
Human and Rodent RBCs do not demonstrate XO-catalyzed nitrite reductase activity. Human, mouse, and rat samples were analyzed for XOR-dependent contributions to overall NO2− reductase activity using the Sievers Nitric Oxide Analyzer (NOA) as detailed in the methods. Samples were prepared in the reaction chamber with 50 μL of RBC homogenate (5 mg total protein) in KPi pH 7.0 with or without xanthine (25 μM) and with or without febuxostat (10 μM). Reactions were initiated by the addition of NaNO2− (500 μM). A) Representative NO generation profiles from human RBC homogenates + xanthine with or without addition of febuxostat (Febux). B) Human, mouse, and rat RBC homogenate values (alone or with xanthine or with xanthine and febuxostat). Authentication of NO was determined by diminution of the signal by the presence of the electron spin resonance NO spin trap carboxy-PTIO. Data represent the mean ± SD of n = 4.
4. Discussion
As sources of NO alternative to the canonical NOS-mediated pathway are beginning to be more clearly understood, a significant role for XOR is becoming apparent. It has been proposed that RBC-associated XOR is contributory to NO2− reduction to NO [34,35]. In these studies, XOR protein was reported to be present in/on the RBC membrane and a portion of the RBC-mediated NO2− reductase activity sensitive to the XOR inhibitor, allopurinol. While these studies did not assess enzymatic activity, they referenced previous publications that did report XOR activity in RBCs [40–42]. However, these reports are problematic in supporting RBC-associated XOR activity as they: 1) did not describe or reference their methods of XO activity assessment [41], 2) utilized approaches that did not account for existing uric acid (absence of controls with an XO inhibitor, [40,42], and 3) report levels of XO activity for human RBCs (mU/mg Hb or U/mL) that are greater than those seen in rat liver [40,42]. A potential explanation for the magnitude of the reported XO activity values in the studies described above is the absence of controls accounting for existing uric acid. Without this control, only variations in existing uric acid are realized unless there is pronounced XO activity. Herein, we accounted for existing uric acid by using sister tubes loaded with allopurinol and report no detectable XO activity in human and rodent RBCs. This is in agreement with a report showing the absence of XO activity in RBCs from humans using radio-labeled xanthine (8- [14]C) as a substrate and radiolabeled uric acid (14C) as the detected product [43]. In addition, we can also conclude that human RBCs do not bind and sequester XO via interaction with GAGs as seen in Fig. 2. While this experiment focused on binding to the artificial GAG, heparin-Sepharose, it also served to confirm the absence of observable non-specific binding to RBCs.
Whereas the absence of XO activity in RBCs seems conclusive, it does not dismiss the presence of XO protein as demonstrated previously [34]. This report clearly shows an abundance of immuno-detectable protein associated with the membrane of human RBCs using a rabbit polyclonal anti-XOR antibody. We used a mouse monoclonal anti-XOR antibody (Santa Cruz) that has demonstrated, in our experience, the greatest specificity among those commercially available; yet, we did not detect XO protein, Fig. 3. Similar, yet inconclusive, results were obtained with an alternative rabbit polyclonal anti-XO antibody (Rockland), a notoriously nonspecific antibody (Suppl. Fig. 2). A plausible answer to why our findings oppose the immunocytochemistry findings reported previously lies in appreciating the literature describing why, with regard to XOR, immunocyto/histochemistry often countervails enzymatic activity results. In 1991, Clare and Lecce described the presence of antibodies (IgG, IgA, and IgM) in commercially-available purified XO preparations and concluded that antisera raised against these preparations would give rise to antibodies recognizing epitopes on these contaminant antibodies and thus could indicate the presence of XO where none existed [44]. This may also explain why we see faint hints of bands migrating near the 145 kD XO control band when using the above-described nonspecific rabbit polyclonal anti-XO antibody, Suppl. Fig. 2.
To further validate our findings, the NO2− reductase capacity of human and rodent RBCs was assessed for XO-mediated contributions. RBC homogenates were analyzed for NO2− reductase activity using xanthine as a reducing substrate for XO as seen in Fig. 4. Whereas the addition of NO2− to the reaction chamber induced some NO formation, the presence of xanthine and/or febuxostat did not alter the rate of generation. These results counter those reported previously that demonstrated a measurable component of RBC-associated, XOR-mediated NO2− reduction to NO [34]. This report differed slightly from our experiment as XOR-mediated contributions were apparent at pH 6.8 and not significant at pH 7.4. We also endeavored to favor acidic conditions similar to those encountered in vivo under hypoxia/inflammation and thus our study was conducted at pH 7.0. This is an important concept as pH plays a critical role in the capacity of XOR to catalyze NO2− reduction [14]. While the difference between 6.8 and 7.0 may be considered small, we must consider this discrepancy as a potential explanation for the difference between the previous report and our observations. However, when combined with the absence of detectable XO protein and enzymatic activity, we conclude that this slight difference in pH does not account for the absence of XO-mediated NO2− reductase activity. In fact, our results are in agreement with a previous report demonstrating the absence of XO-dependent NO2− reduction to NO in RBCs as evidenced by the absence of an effect of the XO inhibitor allopurinol on several readouts including: 1) nitrosyl-hemoglobin formation after addition of NO2− to red cells as measured by electron paramagnetic resonance, 2) NO release from a red cell/nitrite mixtures as measured by chemiluminescence and 3) platelet activation [45]. In addition, this report demonstrated that carbon monoxide (CO), which inhibits heme-mediated NO production from NO2− while not affecting XO activity, abrogated nitrosyl-hemoglobin formation measured by EPR.
In summary, data herein demonstrate the absence of XO protein, enzymatic activity, and NO2− reductase activity in human and rodent RBCs as well as the inability of these RBCs to bind purified XO. In addition, the cellular age of the RBCs does not affect XO content. While these findings affirm the concept that immuno-detectible protein does not equate to enzymatic activity, they are limited to the models tested herein and thus do not rule out all potential for a circumstance whereby XO may be present in or associated with RBCs.
Supplementary Material
Acknowledgements
This work was supported by AHA 19TPA34850089, R01 DK124510-01, R01 HL153532-01A1, P20 GM109098 (EEK), BINP R56 NS117754-01 (PDC), R01 HL 133864, R01 HL 128304, AHA Grant 19EIA34770095 (ACS) RO1 HL153113 (RPP). We also thank our volunteers for their blood donations and Albert and Bill Cree from Cree Farms, Greene Co, Green, PA for donating fresh bovine cream.
Abbreviations:
- GAGs
glycosaminoglycans
- HS6B
heparin-Sepharose 6B
- H2O2
hydrogen peroxide
- NO
nitric oxide
- NOS
nitric oxide synthase
- O2•−
superoxide
- XDH
xanthine dehydrogenase
- XO
xanthine oxidase
- XOR
xanthine oxidoreductase
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.freeradbiomed.2021.07.012.
References
- [1].Enroth C, Eger BT, Okamoto K, Nishino T, Nishino T, Pai EF, Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: structure-based mechanism of conversion, Proc. Natl. Acad. Sci. U. S. A 97 (2000) 10723–10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nishino T, Okamoto K, The role of the [2Fe-2s] cluster centers in xanthine oxidoreductase, J. Inorg. Biochem 82 (2000) 43–49. [DOI] [PubMed] [Google Scholar]
- [3].Iwasaki T, Okamoto K, Nishino T, Mizushima J, Hori H, Sequence motif-specific assignment of two [2Fe-2S] clusters in rat xanthine oxidoreductase studied by site-directed mutagenesis, J. Biochem 127 (2000) 771–778. [DOI] [PubMed] [Google Scholar]
- [4].Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA, Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease, Proc. Natl. Acad. Sci. U. S. A 98 (2001) 15215–15220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD, Allopurinol improves endothelial dysfunction in chronic heart failure, Circulation 106 (2002) 221–226. [DOI] [PubMed] [Google Scholar]
- [6].Butler R, Morris AD, Belch JJ, Hill A, Struthers AD, Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension, Hypertension 35 (2000) 746–751. [DOI] [PubMed] [Google Scholar]
- [7].Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, Sastre J, Vina J, Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol, Diabetes 51 (2002) 1118–1124. [DOI] [PubMed] [Google Scholar]
- [8].Mink RB, Dutka AJ, Hallenbeck JM, Allopurinol pretreatment improves evoked response recovery following global cerebral ischemia in dogs, Stroke 22 (1991) 660–665. [DOI] [PubMed] [Google Scholar]
- [9].Tan S, Yokoyama Y, Dickens E, Cash TG, Freeman BA, Parks DA, Xanthine oxidase activity in the circulation of rats following hemorrhagic shock, Free Radical Biol. Med 15 (1993) 407–414. [DOI] [PubMed] [Google Scholar]
- [10].Parks DA, Bulkley GB, Granger DN, Hamilton SR, McCord JM, Ischemic injury in the cat small intestine: role of superoxide radicals, Gastroenterology 82 (1982) 9–15. [PubMed] [Google Scholar]
- [11].Alef MJ, Vallabhaneni R, Carchman E, Morris SM Jr., Shiva S, Wang Y, Kelley EE, Tarpey MM, Gladwin MT, Tzeng E, Zuckerbraun BS, Nitrite-generated NO circumvents dysregulated arginine/NOS signaling to protect against intimal hyperplasia in Sprague-Dawley rats, J. Clin. Invest 121 (2011) 1646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Baker JE, Su J, Fu X, Hsu A, Gross GJ, Tweddell JS, Hogg N, Nitrite confers protection against myocardial infarction: role of xanthine oxidoreductase, NADPH oxidase and K(ATP) channels, J. Mol. Cell. Cardiol 43 (2007) 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bueno M, Wang J, Mora AL, Gladwin MT, Nitrite Signaling in Pulmonary Hypertension: Mechanisms of Bioactivation, Signaling, and Therapeutics, Antioxidants & Redox Signaling (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cantu-Medellin N, Kelley EE, Xanthine Oxidoreductase-Catalyzed Reduction of Nitrite to Nitric Oxide : Insights Regarding where, when and How, Nitric Oxide: Biology and Chemistry/official journal of the Nitric Oxide Society (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lu P, Liu F, Yao Z, Wang CY, Chen DD, Tian Y, Zhang JH, Wu YH, Nitrite-derived nitric oxide by xanthine oxidoreductase protects the liver against ischemia-reperfusion injury, Hepatobiliary & pancreatic diseases international : HBPD INT 4 (2005) 350–355. [PubMed] [Google Scholar]
- [16].Sugimoto R, Okamoto T, Nakao A, Zhan J, Wang Y, Kohmoto J, Tokita D, Farver CF, Tarpey MM, Billiar TR, Gladwin MT, McCurry KR, Nitrite reduces acute lung injury and improves survival in a rat lung transplantation model, Am. J. Transplant. : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 12 (2012) 2938–2948. [DOI] [PubMed] [Google Scholar]
- [17].Huang L, Borniquel S, Lundberg JO, Enhanced xanthine oxidoreductase expression and tissue nitrate reduction in germ free mice, Nitric oxide: biology and chemistry/official journal of the Nitric Oxide Society 22 (2010) 191–195. [DOI] [PubMed] [Google Scholar]
- [18].Park JW, Thomas SM, Schechter AN, Piknova B, Control of rat muscle nitrate levels after perturbation of steady state dietary nitrate intake, Nitric Oxide 109–110 (2021) 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL, Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase, J. Biol. Chem 283 (2008) 17855–17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li H, Cui H, Liu X, Zweier JL, Xanthine oxidase catalyzes anaerobic transformation of organic nitrates to nitric oxide and nitrosothiols: characterization of the mechanism and link between organic nitrate and guanylyl cyclase activation, J. Biol. Chem 280 (2005) 16594–16600. [DOI] [PubMed] [Google Scholar]
- [21].Li H, Samouilov A, Liu X, Zweier JL, Characterization of the effects of oxygen on xanthine oxidase-mediated nitric oxide formation, J. Biol. Chem 279 (2004) 16939–16946. [DOI] [PubMed] [Google Scholar]
- [22].Godber BLJ, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R, Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase, J. Biol. Chem 275 (2000) 7757–7763. [DOI] [PubMed] [Google Scholar]
- [23].Li H, Samouilov A, Liu X, Zweier JL, Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of it role in nitric oxide generation in anoxic tissues, J. Biol. Chem 276 (2001) 24482–24489. [DOI] [PubMed] [Google Scholar]
- [24].Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR, Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions, FEBS (Fed. Eur. Biochem. Soc.) Lett 427 (1998) 225–228. [DOI] [PubMed] [Google Scholar]
- [25].Carlstrom M, Larsen FJ, Nystrom T, Hezel M, Borniquel S, Weitzberg E, Lundberg JO, Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 17716–17720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lundberg JO, Carlstrom M, Larsen FJ, Weitzberg E, Roles of dietary inorganic nitrate in cardiovascular health and disease, Cardiovasc. Res 89 (2011) 525–532. [DOI] [PubMed] [Google Scholar]
- [27].Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ, Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver, J. Clin. Invest 115 (2005) 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, Bauer PM, Choi JJ, Curtis E, Choi AM, Gladwin MT, Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation, Circulation 121 (2010) 98–109. [DOI] [PubMed] [Google Scholar]
- [29].Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, Kevil CG, Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis, J Am Heart Assoc 1 (2012), e004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pardue S, Kolluru GK, Shen X, Lewis SE, Saffle CB, Kelley EE, Kevil CG, Hydrogen sulfide stimulates xanthine oxidoreductase conversion to nitrite reductase and formation of NO, Redox Biol (2020) 101447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Adachi T, Fukushima T, Usami Y, Hirano K, Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial-cell surface, Biochem. J 289 (1993) 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fukushima T, Adachi T, Hirano K, The heparin-binding site of human xanthine oxidase, Biol. Pharm. Bull 18 (1995) 156–158. [DOI] [PubMed] [Google Scholar]
- [33].Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks DA, Freeman BA, Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling, J. Biol. Chem 274 (1999) 4985–4994. [DOI] [PubMed] [Google Scholar]
- [34].Webb AJ, Milsom AB, Rathod KS, Chu WL, Qureshi S, Lovell MJ, Lecomte FM, Perrett D, Raimondo C, Khoshbin E, Ahmed Z, Uppal R, Benjamin N, Hobbs AJ, Ahluwalia A, Mechanisms underlying erythrocyte and endothelial nitrite reduction to nitric oxide in hypoxia: role for xanthine oxidoreductase and endothelial nitric oxide synthase, Circ. Res 103 (2008) 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ghosh SM, Kapil V, Fuentes-Calvo I, Bubb KJ, Pearl V, Milsom AB, Khambata R, Maleki-Toyserkani S, Yousuf M, Benjamin N, Webb AJ, Caulfield MJ, Hobbs AJ, Ahluwalia A, Enhanced vasodilator activity of nitrite in hypertension: critical role for erythrocytic xanthine oxidoreductase and translational potential, Hypertension 61 (2013) 1091–1102. [DOI] [PubMed] [Google Scholar]
- [36].Owusu BY, Stapley R, Honavar J, Patel RP, Effects of erythrocyte aging on nitric oxide and nitrite metabolism, Antioxidants Redox Signal. 19 (2013) 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Harmon DB, Mandler WK, Sipula IJ, Dedousis N, Lewis SE, Eckels JT, Du J, Wang Y, Huckestein BR, Pagano PJ, Cifuentes-Pagano E, Homanics GE, Van’t Erve TJ, Stefanovic-Racic M, Jurczak MJ, O’Doherty RM, Kelley EE, Hepatocyte-specific ablation or whole-body inhibition of xanthine oxidoreductase in mice corrects obesity-induced systemic hyperuricemia without improving metabolic abnormalities, Diabetes 68 (2019) 1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kelley EE, Trostchansky A, Rubbo H, Freeman BA, Radi R, Tarpey MM, Binding of xanthine oxidase to glycosaminoglycans limits inhibition by oxypurinol, J. Biol. Chem 279 (2004) 37231–37234. [DOI] [PubMed] [Google Scholar]
- [39].Malik UZ, Hundley NJ, Romero G, Radi R, Freeman BA, Tarpey MM, Kelley EE, Febuxostat inhibition of endothelial-bound XO: implications for targeting vascular ROS production, Free Radic. Biol. Med 51 (2011) 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zoroglu SS, Armutcu F, Ozen S, Gurel A, Sivasli E, Yetkin O, Meram I, Increased oxidative stress and altered activities of erythrocyte free radical scavenging enzymes in autism, Eur. Arch. Psychiatr. Clin. Neurosci 254 (2004) 143–147. [DOI] [PubMed] [Google Scholar]
- [41].Kaynar H, Meral M, Turhan H, Keles M, Celik G, Akcay F, Glutathione peroxidase, glutathione-S-transferase, catalase, xanthine oxidase, Cu-Zn superoxide dismutase activities, total glutathione, nitric oxide, and malondialdehyde levels in erythrocytes of patients with small cell and non-small cell lung cancer, Canc. Lett 227 (2005) 133–139. [DOI] [PubMed] [Google Scholar]
- [42].Avci A, Atli T, Erguder IB, Varli M, Devrim E, Aras S, Durak I, Effects of garlic consumption on plasma and erythrocyte antioxidant parameters in elderly subjects, Gerontology 54 (2008) 173–176. [DOI] [PubMed] [Google Scholar]
- [43].Al-Khalidi UA, Chaglassian TH, The species distribution of xanthine oxidase, Biochem. J 97 (1965) 318–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Clare DA, Lecce JG, Copurification of bovine milk xanthine oxidase and immunoglobulin, Arch. Biochem. Biophys 286 (1991) 233–237. [DOI] [PubMed] [Google Scholar]
- [45].Liu C, Wajih N, Liu X, Basu S, Janes J, Marvel M, Keggi C, Helms CC, Lee AN, Belanger AM, Diz DI, Laurienti PJ, Caudell DL, Wang J, Gladwin MT, Kim-Shapiro DB, Mechanisms of human erythrocytic bioactivation of nitrite, J. Biol. Chem 290 (2015) 1281–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


