ABSTRACT
Background
Higher levels of intra-abdominal adipose tissue (IAAT) comprising visceral adipose tissue (VAT), intermuscular adipose tissue (IMAT), and liver fat are posited drivers of obesity-related chronic disease risk. Fast food is hypothesized to contribute to IAAT patterns.
Objectives
We quantified levels of abdominal subcutaneous adipose tissue (SAT), IAAT, and odds of metabolic-associated fatty liver disease (MAFLD) in middle age according to average fast-food intake over the preceding 25 y.
Methods
We analyzed data from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Participants underwent 6 clinical exams and measurements over 25 y with computed tomography–measured VAT, SAT, and IMAT (n = 3156), plus MAFLD defined by liver attenuation (≤40 Hounsfield units) and 1 metabolic abnormality at year 25 (2010, n = 3001, n cases = 302). We estimated means of VAT, SAT, IMAT, and liver attenuation at the year 25 exam according to categories of average fast-food intake over the previous 25 y adjusted for sociodemographic and lifestyle factors and logistic regression to estimate the odds ratio of MAFLD at year 25.
Results
With higher average fast-food intake over 25 y (categorized as follows: never–1×/mo, >1×–3×/mo, 1–<2×/wk, 2–<3×/wk, ≥3×/wk), there were monotonic higher levels of VAT (98.5, 127.6, 134.5, 142.0, 145.5 cm3), P-trend < 0.0001, which were consistent across anthropometrically classified obesity categories. There was a similar pattern with liver fat. There were higher levels of IMAT and SAT with higher fast-food intake (P-trend = 0.003, 0.0002, respectively), with amounts leveling off at ≥2×/wk. In addition, compared with participants who ate fast food never–1×/mo, there were monotonic higher odds of having MAFLD at year 25 with higher average fast-food intake, with participants who ate fast food ≥3×/wk having an OR of MAFLD = 5.18 (95% CI: 2.87, 9.37).
Conclusions
There were monotonic higher levels of VAT, liver fat, and odds of having MAFLD in middle age according to higher average fast-food intake over the preceding 25 y.
Keywords: fast-food, visceral adipose tissue, MAFLD, obesity, dietary intake
See corresponding editorial on page 11 and article on page 173.
Basic, clinical, and population research support the hypothesis that high levels of visceral adipose tissue (VAT) and liver fat are the adiposity-related etiologic drivers of risk for cardiometabolic diseases, obesity-related cancers, and Alzheimer disease and related dementias, whereas abdominal subcutaneous adipose tissue (SAT) appears to have a lesser role (1–3). Multiple nonmodifiable and modifiable factors likely affect deposition patterns of VAT and other intra-abdominal adipose tissue (IAAT) depots such as intermuscular adipose tissue (IMAT) and liver fat, with dietary intake hypothesized to be among the most influential modifiable factors (3–6). Yet, there are major gaps in the evidence base linking diet and eating behaviors with IAAT deposition patterns (6), thereby limiting diet-related prevention and risk mitigation efforts toward reducing obesity-related chronic diseases.
One of these gaps is a defining aspect of the typical American diet—fast-food intake (7, 8). In a representative sample of US adults, 37% report eating fast food on any given day (9), and young adults aged 20–39 y consume fast food at an even higher frequency (45% on any given day) (9). The typical fast-food meal, regardless of source, is nutrient poor and energy dense (7, 8) and on days when consumed is associated with significantly higher daily energy intake (10). Indeed, the ubiquity of fast food and its poor nutritional profile is a reason why it is often maligned for health, particularly obesity (11).
To inform this evidence gap, the objective of this analysis was to quantify levels of abdominal SAT and IAAT comprising VAT, IMAT, and liver fat, as well as the odds of metabolic-associated fatty liver disease (MAFLD) in middle age according to average intake of fast food assessed repeatedly over the preceding 25 y.
Methods
Study population
The Coronary Artery Risk Development in Young Adults (CARDIA) study is a prospective, multicenter cohort study designed to investigate the development and determinants of cardiovascular disease and its associated risk factors in young adults. Briefly, 5115 black and white men and women (self-identified), aged 18–30 y, were recruited between 1985 and 1986 from 4 US cities: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota, and Oakland, California. Participant enrollment targeted balance among age, race, sex, and educational attainment. The initial examination included standardized measures of known cardiovascular risk factors as well as psychosocial, dietary, and exercise-related characteristics. Reexamination occurred 2, 5, 7, 10, 15, 20, 25, and 30 y after baseline, with retention of 91%, 86%, 81%, 79%, 74%, 72%, 72%, and 71% of the surviving cohort, respectively. The CARDIA study was approved by the institutional review board at each field center, and informed consent was obtained from all participants prior to enrollment (12).
At each clinical examination, participants were asked to fast overnight for 12 h. Tobacco use, strenuous physical activity, and intake of caffeine, food, and alcohol were proscribed. The examinations followed standardized protocols harmonized over time and included measurements of blood pressure, anthropometrics, phlebotomy, and structured questionnaires on sociodemographics, medical and family history, psychosocial characteristics, and diet, among others. During each clinic exam, blood was drawn from an antecubital vein, and after serum separation, aliquots were stored at –70°C until shipped on dry ice to a central laboratory. Details on the collection, storage, quality control, and methodology for clinical specimens are described elsewhere (12–14), and anthropometry (height, weight, waist circumference) also has been previously described (15). BMI was computed as weight in kilograms divided by squared height in meters.
Measures
Fast-food intake
CARDIA assessed fast-food intake with the following question at years 0, 7, 10, 15, 20, and 25: “How many times in a week or month do you eat breakfast, lunch or dinner out in a place such as McDonald's, Burger King, Wendy's, Arby's, Pizza Hut, or Kentucky Fried Chicken?” Questions were open ended, and we calculated a per-week consumption frequency.
Covariates
Dietary intake was assessed at baseline (year 0), year 7, and year 20 using the CARDIA Diet History, an interviewer-administered, validated dietary assessment instrument consisting of a short questionnaire on general dietary practices followed by a comprehensive questionnaire about typical intake of foods using the previous 1 mo as a reference for recall (16). Briefly, interviewers asked study participants open-ended questions about dietary consumption in the past month within 100 food categories, referencing 1609 separate food items in years 0 and 7 and several thousand separate food items in year 20. Follow-up questions for selected foods concerned serving size, frequency of consumption, and common additions to these foods. Provision was made for reporting foods not found in the food-frequency list. Diet history data were coded by the University of Minnesota Nutrition Coordinating Center and categorized into 166 food groups.
Total energy intake (kcal) was calculated from the CARDIA Diet History. We derived a modified alternate Mediterranean (aMed) diet score using methods described elsewhere (17). In brief, the modified aMed score in this analysis assigned 1 point for intake above the cohort-specific median for fruit, vegetables, legumes, nuts, whole-grain products, fish, and MUFA/SFA fat ratio. Individual food group scores were summed for the total aMed score, with a range of 0 to 7. We used the modified aMed diet score because it does not include dietary components widely and often consumed at chain fast-food restaurants, yet provides a metric of dietary quality, and averaged the score and total estimated energy intake from year 0, 7, and 20 data.
Alcohol intake was assessed at each examination with the following question: “Did you drink any alcoholic beverages in the past year?” and, with the use of visual aids to demonstrate a typical drink, were asked 3 follow-up questions to assess the number of drinks of wine, beer, and liquor typically consumed in a week. Assuming that 1 drink of beer, wine, or liquor contains 16.7 mL, 17.0 mL, or 19.2 mL of ethanol on average, respectively, total ethanol consumption in milliliters of ethanol per day was calculated. We estimated average alcohol intake from data at years 0, 7, 10, 15, 20, and 25. Cigarette smoking was evaluated during each in-person CARDIA visit, and we classified participants at year 25 as never, ex, or current smokers for analyses.
Physical activity was assessed at each examination using the CARDIA physical activity questionnaire, a validated interviewer-based self-report of duration and intensity of participation in 13 categories of exercise over the past year (18). Physical activity was reported in exercise units (EU), where 300 EU is approximately equal to 150 min of moderate-intensity physical activity per week or 30 min of moderate-intensity activity 5 d/wk (19). We estimated average physical activity using data from years 0, 7, 10, 15, 20, and 25.
We calculated average household income during the course of the analytic period from assessments at years 5, 7, 10, 15, 20, and 25 that asked for pretax household income for the past 12 mo from all sources in 9 prespecified income brackets ranging from <$5000 to >$100,000. Median values were assigned to each category at each exam and then averaged over 6 exams for use as a covariate. Participants also reported years of education completed at each exam, and we defined education by the highest attained level. We also accounted for employment status, where the participant reported being unemployed, laid off, or looking for work over the course of 25 y. Participants were assigned 1 point for each exam (0, 7, 10, 15, 20, 25), where they reported being unemployed, laid off, or looking for work (0 for not reporting). This variable represents the sum of visits where they reported this status (range: 0–6).
We used study definitions of adjudicated incidence of cardiovascular disease (CVD) (CAD, hospitalization for angina/acute coronary syndrome, heart failure, stroke, transient ischemic attack, peripheral arterial disease) through year 25 to classify participants with a history of CVD at the year 25 exam (20). Diabetes status was assessed at examination years 0, 7, 10, 15, 20, and 25. Prevalence of type 2 diabetes at year 25 was defined as use of diabetes medication (all years, including 2 and 5), a fasting blood glucose concentration of ≥7 mmol/L (126 mg/dL), 2-h postchallenge glucose ≥11.1 mmol/L (200 mg/dL), and/or a glycated hemoglobin (HbA1c) ≥48 mmol/mol (6.5%) at any examination. The 2-h glucose was done at years 10, 20, and 25, whereas HbA1c was done at years 20 and 25.
Outcome
Abdominal adipose tissue depots were measured using computed tomography (CT) scans at year 25. A noncontrast CT scan of the abdomen was performed with multidetector CT scanners [GE 750HD and GE LightSpeed VCT models were used at the Birmingham and Oakland Centers, respectively (GE Healthcare); Siemens Sensation 64 models were used at both Chicago and Minneapolis Centers (Siemens Medical Solutions)]. The images were electronically transmitted to a CT reading center for image analysis and quality control at Wake Forest University School of Medicine (Winston-Salem, NC). CT scans of the abdomen were reconstructed into 5-mm slices with a maximum 50-cm field of view to include the whole abdomen. Adipose tissue depots were measured volumetrically from 2 adjoining 5-mm slices located at the level of the lumbar disk between the fourth and fifth vertebra (VAT and SAT) and L3–L4 for IMAT (to avoid interference with a pelvic bone). Tissues with attenuation between –190 and –30 Hounsfield units (HU) were defined as adipose tissue. Analysts segmented the images based on anatomic boundaries into the entire abdomen, abdominal wall, and intra-abdominal compartments using the Medical Image Processing, Analysis, and Visualization application (http://mipav.cit.nih.gov/index.php). VAT, SAT, and IMAT were quantified in each compartment.
Liver attenuation, a diagnostic method to assess liver fat, was measured using noncontrast CT images of the upper abdomen while in axial scan mode. Averages of 3 distinct CT slices at the level of the T12–L1 intervertebral space were calculated to provide mean hepatic attenuation in HU. Analysts were trained to avoid measuring regions that included common hepatic lesions and hepatic vasculature. Liver attenuation is inversely related to liver fat content, such that lower levels of liver attenuation indicate higher levels of liver fat (21).
MAFLD was defined as mean liver attenuation ≤40 HU, which is indicative of at least moderate to severe steatosis (22). This status is combined with 1 of 3 other metabolic associated criteria defined in a consensus statement (overweight/obesity, type 2 diabetes, or normal weight defined by BMI) with at least 2 of the following metabolic abnormalities: waist circumference ≥102/88 cm in men and women (or ≥90/80 cm in Asian men and women), blood pressure ≥130/85 mmHg or specific drug treatment, plasma triglycerides ≥150 mg/dL (≥1.70 mmol/L) or specific drug treatment, plasma HDL cholesterol <40 mg/dL (<1.0 mmol/L) for men and <50 mg/dL (<1.3 mmol/L) for women or specific drug treatment, prediabetes [i.e., fasting glucose concentrations 100–125 mg/dL (5.6–6.9 mmol/L), 2-h postload glucose concentrations 140–199 mg/dL (7.8–11.0 mmol), or HbA1c 5.7–6.4% (39–47 mmol/mol)], HOMA-IR score ≥2.5, and plasma high-sensitivity C-reactive protein concentration >2 mg/L (23).
Analysis
We conducted all statistical analyses using SAS, version 9.4 (SAS Institute). Of the original 5115 study members, 3499 participated in the year 25 exam. In total, 3181 had CT/body composition scans, and 3180 had ≥2 assessments of fast-food intake over 25 y, of which 3156 had all data for VAT, SAT, and IMAT. In addition, to address secondary etiology of MAFLD, we excluded participants (from base sample N = 3180) who reported any of the following at the year 25 exam: using any of the following medications (amiodarone, methotrexate, valproate and tamoxifen), a history of hepatitis or cirrhosis, having HIV, or heavy alcohol use. Alcohol-driven etiology is only clear at very high levels of habitual consumption; thus, given the challenges of assessing the absolute level of alcohol intake with accuracy, we applied a threshold (>60 mL/d at year 25) where there is better evidence of alcohol-driven etiology (24). These further exclusions resulted in an analytic sample of N = 3001 for analyses with liver attenuation and MAFLD as an endpoint (Supplemental Figure 1). We calculated average fast-food intake over 25 y and created 5 categories that allowed for cut points with an adequate number of participants and alignment with common population levels of intake. Distributions of dependent variables were examined; all were approximately normal with low skewness and kurtosis. Results of analyses conducted using transformed data did not differ materially from results with transformed data, and thus we present the untransformed data.
We used multivariable general linear regression models to calculate means of VAT, SAT, IMAT, and liver attenuation at the year 25 exam according to categories of average fast-food intake over the previous 25 y. We created 2 statistical models; the first was the unadjusted regressed mean of VAT, SAT, IMAT, and liver attenuation on categories of average 25-y fast-food intake. A second model had adjustments for age, sex, race, study center, education, employment history, household income, smoking, alcohol, diet quality, energy intake, physical activity, and prevalence of type 2 diabetes or history of a CVD event at the year 25 exam. We tested for linear trends in the data by entering fast-food intake as a continuous variable in the model. We carried out stratified analyses by anthropometric obesity definition thresholds using BMI as 30.0 and sex-specific waist circumference (abdominal obesity) thresholds defined by the National Cholesterol Education Program ATP-III (≥40 in. for men and ≥35 in. for women). We also carried out stratified analyses by race (black, white) and sex to check the nature of the estimates across the population subgroups with different lived experiences.
Last, we estimated the odds ratio of having MAFLD at year 25 according to the previous 25-y average of fast-food intake via multivariable logistic regression using the same aforementioned covariates and the reference for comparison being participants who reported the lowest level of average fast-food intake (never–1×/mo). We carried out the following sensitivity analyses for context related to data interpretation: excluding all participants with an adjudicated CVD event, and we compared characteristics of volunteers who participated in year 25 compared with those did not participate to provide insight into any differences and inform interpretation of the results.
Results
The average age of the analytic sample during the year 25 exam with CT-measured body composition was 50 y, 57% were female, and 53% were white. The average fast-food intake frequency per week declined over 25 y: mean (with ∼SD of 2.0) at years 0, 7, 10, 15, 20, and 25 was respectively 2.0, 1.9, 1.8, 1.8, 1.7, and 1.3×/wk. Table 1 presents participant characteristics according to categories of average fast-food intake over 25 y. More than 93% of the participants reported their usual fast-food habits ≥4 times over the 25 y. With higher average fast-food intake, participants were younger at baseline, with lower educational attainment and diet quality, lower household income, lower alcohol intake, lower physical activity, and higher energy intake, and a greater proportion of participants were male and black.
TABLE 1.
Participant characteristics according to categories of average fast-food intake over 25 y ranging from young adulthood to middle age: Coronary Artery Risk Development in Young Adults study1
| Characteristic | Never–1×/mo (n = 391) | >1×–3× mo (n = 572) | 1–<2×/wk (n = 1113) | 2–<3×/wk (n = 556) | ≥3×/wk (n = 524) |
|---|---|---|---|---|---|
| Age (baseline), y | 27 (24–29) | 26 (23–28) | 25 (22–28) | 25 (22–28) | 24 (21–28) |
| % Female | 72.7 | 66.3 | 56.1 | 50.2 | 42.0 |
| % Black | 18.7 | 36.8 | 51.2 | 59.3 | 61.0 |
| Education | 16 (15–19) | 16 (14–18) | 16 (14–17) | 15 (14–17) | 14.5 (13–16) |
| Employment | 1 (0–2) | 1 (0–3) | 1 (0–3) | 1 (0–3) | 1 (0–3) |
| Income | 66,083 (42,667–90,083) | 58,833 (36,250–84,167) | 52,500 (31,667–78,500) | 48,208 (30,083–70,000) | 49,000 (30,250–67,500) |
| Smoking | 62.4 | 60.4 | 58.6 | 61.8 | 62.7 |
| Alcohol | 7.7 (1.6–15.7) | 6.2 (1.2–14.7) | 5.1 (0.8–14.9) | 4.2 (0.4–13.3) | 4.8 (0.4–14.6) |
| Activity | 394.6 (258.8–553.0) | 320.3 (203.5–478.8) | 302.8 (184.7–456.3) | 279.1 (160.8–435.3) | 296.2 (170.0–470.2) |
| Diet quality | 4 (3.3–5.0) | 3.7 (2.7–4.5) | 3.3 (2.3–4.3) | 3.0 (2.3–4.0) | 3.0 (2.0–4.0) |
| Energy intake | 2170 (1803–2622) | 2203 (1760–2857) | 2399 (1900–3128) | 2620 (2021–3593) | 3010 (2285–4023) |
| Baseline BMI | 22.3 (20.7–24.7) | 23.1(21.0–26.1) | 23.7 (21.4–26.8) | 24.0 (21.7–27.4) | 23.9 (21.4–26.9) |
| Baseline WC | 72.3 (67.0–78.5) | 75.0 (68–82.8) | 77.0 (70.3–84.8) | 77.5 (71.5–85.5) | 78.0 (71.25–85.5) |
| T2D year 25 | 7.3 | 10.9 | 13.7 | 17.9 | 19.0 |
| CVD year 25 | 1.8 | 3.8 | 2.6 | 5.4 | 2.9 |
Values are reported as unadjusted variables as median (25–75th percentile) or proportion of sample. Education: highest level of education attained during study time period. Employment: participants were assigned 1 point for each exam where they reported being unemployed, laid off, or looking for work (0 for not reporting). This variable represents the sum of visits where they reported this status (range: 0–6). Income: mean household pretax income over 6 visits. Alcohol (mL/d): mean alcohol intake over 6 visits. Physical activity: mean exercise units over 6 visits (physical activity score). Diet quality (alternate Mediterranean score, scale 0–7): mean score over 3 visits and mean energy intake (kcal/d) over 3 visits. Smoking: proportion of never smokers at year 25 exam. T2D and CVD at 25 = percentage of cumulative cases of T2D and adjudicated CVD events. CVD, cardiovascular disease; T2D, type 2 diabetes; WC, waist circumference.
We present levels of abdominal adipose tissue depots in middle age (∼50 y) according to categories of average fast-food intake over the preceding 25 y in Table 2. With higher levels of fast-food intake, there were monotonic higher levels of VAT and liver fat. There were higher levels of SAT and IMAT in accordance with higher levels of fast-food intake, but these leveled out at the highest 2 categories of fast-food intake. We observed a similar relation with anthropometric indices (BMI and waist circumference).
TABLE 2.
Abdominal adipose tissue levels and anthropometrics in middle age according to categories of average fast-food intake over preceding 25 y: Coronary Artery Risk Development in Young Adults study1
| Fast-food intake | Never–1×/mo, n = 391 (377) | >1×–3× mo, n = 572 (544) | 1–<2×/wk, n = 1113 (1056) | 2–<3×/wk, n = 556 (526) | ≥3×/wk, n = 524 (498) | P-trend |
|---|---|---|---|---|---|---|
| VAT2 | 98.7 (91.5, 105.8) | 128.1 (122.1, 134.0) | 133.5 (129.2, 137.7) | 142.7 (136.7, 148.7) | 146.1 (139.9, 152.3) | <0.0001 |
| VAT3 | 98.5 (91.2, 105.1) | 127.6 (122.1, 133.0) | 134.5 (130.7, 138.4) | 142.0 (136.5, 147.6) | 145.5 (139.6, 151.4) | <0.0001 |
| SAT2 | 259.6 (243.1, 276.2) | 329.0 (315.3, 342.7) | 348.2 (338.4, 358.0) | 362.9 (349.0, 376.8) | 346.5 (332.1, 360.8) | <0.0001 |
| SAT3 | 272.0 (256.0, 287.9) | 328.5 (316.0, 341.1) | 345.7 (336.9, 354.4) | 355.6 (343.0, 368.2) | 349.9 (336.4, 363.4) | 0.0002 |
| IMAT2 | 16.5 (15.4, 17.6) | 18.8 (17.9, 19.7) | 18.9 (18.2, 19.5) | 18.7 (17.8, 19.7) | 17.7 (16.8, 18.7) | 0.75 |
| IMAT3 | 15.3 (14.2, 16.4) | 17.8 (16.9, 18.7) | 18.8 (18.2, 19.4) | 19.5 (18.6, 20.3) | 19.0 (18.1, 20.0) | 0.003 |
| Liver2 | 57.9 (56.7, 59.1) | 55.6 (54.6, 56.6) | 55.3 (54.6, 56.0) | 54.6 (53.6, 55.6) | 54.4 (53.3, 55.4) | 0.0005 |
| Liver3 | 58.4 (57.2, 59.6) | 56.4 (55.5, 57.4) | 55.5 (54.8, 56.2) | 53.9 (52.9, 54.9) | 53.4 (52.4, 54.4) | <0.0001 |
| Waist2 | 84.8 (83.3, 86.3) | 92.3 (91.1, 93.6) | 95.5 (94.6, 96.4) | 98.4 (97.2, 99.7) | 98.8 (97.5, 100.1) | <0.0001 |
| Waist3 | 87.7 (86.2, 89.2) | 93.4 (92.2, 94.6) | 95.3 (94.4, 96.1) | 97.1 (95.9, 98.3) | 97.1 (95.9, 98.4) | <0.0001 |
| BMI2 | 26.3 (25.6, 27.0) | 29.5 (28.9, 30.0) | 30.9 (30.5, 31.3) | 31.7 (31.1, 32.2) | 31.4 (30.8, 32.0) | <0.0001 |
| BMI3 | 27.2 (26.5, 27.9) | 29.7 (29.1, 30.2) | 30.8 (30.4, 31.2) | 31.2 (30.7, 31.8) | 31.1 (30.6, 31.7) | <0.0001 |
All values reported as mean (95% CI). n = number of participants with VAT, SAT, and IMAT measures (+ BMI and waist circumference); (n) = number of participants with liver fat. P-trend: linear trend. BMI = kg/m2. VAT, SAT, and IMAT = cm3. Waist = cm. Liver = attenuation measured in Hounsfield units. IMAT, intermuscular adipose tissue; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Model: crude.
Model: adjusted for age, sex, race, study center, education, employment history, household income, smoking, alcohol, diet quality, energy intake, physical activity, prevalence of type 2 diabetes, or history of cardiovascular disease event at year 25 exam.
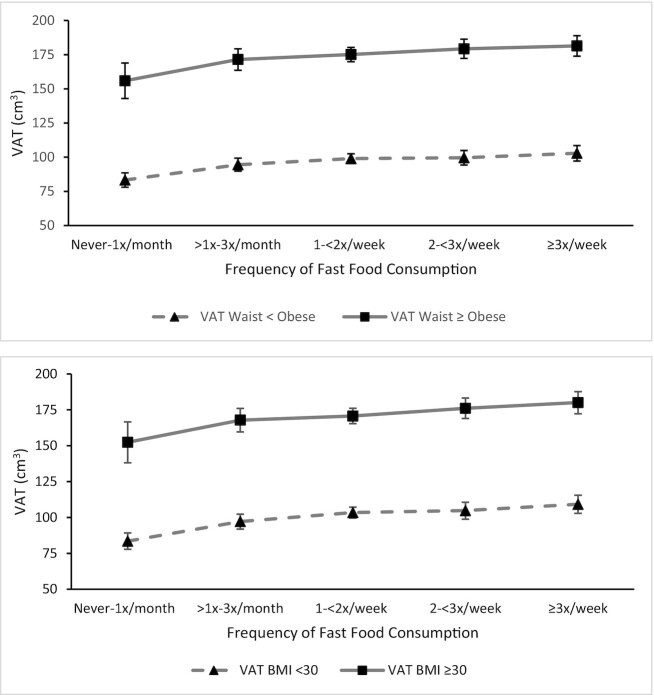
To provide insight into the potential dependence of the abdominal adipose tissue levels on general anthropometric measures, we present levels stratified by anthropometric-defined obesity thresholds in Supplemental Table 1. With higher levels of fast-food intake over 25 y, there were monotonic higher levels of VAT in middle-aged participants with and without abdominal obesity (waist circumference defined) and general obesity (BMI defined) (Figure 1). A similar pattern was observed with liver fat. SAT and IMAT both displayed a threshold-like relation with higher fast-food intake/higher levels across the lower 3 categories and then leveling off.
FIGURE 1.
Visceral adipose tissue levels in middle age according to categories of average fast-food intake over preceding 25 y: stratification by concurrent (year 25) anthropometric-defined obesity thresholds in Coronary Artery Risk Development in Young Adults. All values reported as mean (95% CI). BMI <30, n = 1747; BMI ≥30, n = 1395, P–linear trend = <0.0001 BMI <30, 0.002 BMI ≥30. Waist circumference (WC) < obese, n = 1723; WC obese, n = 1427, P–linear trend = 0.0002 < obese, 0.003 obese. VAT, visceral adipose tissue.
Table 3 reports the odds ratios of MAFLD according to average fast-food intake categories over the previous 25 y. Of the 3001 participants, 302 had MAFLD. Compared with participants who ate at fast-food restaurants never to 1×/mo, there were monotonic higher odds of having MAFLD with each subsequent higher level of average fast-food intake. Participants who ate at fast-food restaurants 3×/wk or more on average over the previous 25 y had a >5-fold higher odds of having MAFLD (OR: 5.18; 95% CI: 2.87, 9.37) after adjustment for demographic, socioeconomic, health status, and lifestyle factors, including diet quality.
TABLE 3.
Odds ratios of metabolic-associated fatty liver disease (MAFLD) in middle age according to categories of average fast-food intake over preceding 25 y: Coronary Artery Risk Development in Young Adults study1
| Model | Never–1×/mo (n = 18/377) | >1×–3× mo (n = 48/544) | 1–<2×/wk (n = 96/1056) | 2–<3×/wk (n = 63/526) | ≥3×/wk (n = 77/498) | P-trend |
|---|---|---|---|---|---|---|
| Model 1 | 1.00 | 2.11 (1.20, 3.70) | 2.35 (1.38, 4.00) | 3.62 (2.05, 6.37) | 4.99 (2.83, 8.79) | <0.0001 |
| Model 2 | 1.00 | 2.03 (1.15, 3.58) | 2.31 (1.34, 3.98) | 3.50 (1.96, 6.26) | 5.18 (2.87, 9.37) | <0.0001 |
All values reported as OR (95% CI). Model 1: adjusted for age, race, sex, and center. Model 2: model 1 + education, 25-y employment status, 25-y household income, 25-y diet quality, 25-y caloric intake, 25-y physical activity, 25-y alcohol intake, 25-y smoking, and history of CVD. MAFLD was defined as combination of liver attenuation ≤40 Hounsfield units (steatosis) plus 1 of 3 other metabolic-related clinical criteria (overweight/obesity, type 2 diabetes, or normal-weight BMI) and at least 2 metabolic abnormalities: waist circumference ≥102/88 cm in men and women, blood pressure ≥130/85 mmHg or specific drug treatment, plasma triglycerides ≥150 mg/dL (≥1.70 mmol/L) or specific drug treatment, plasma HDL cholesterol <40 mg/dL (<1.0 mmol/L) for men and <50 mg/dL (<1.3 mmol/L) for women or specific drug treatment, prediabetes [i.e., fasting glucose concentrations 100–125 mg/dL (5.6–6.9 mmol/L), 2-h postload glucose concentrations 140–199 mg/dL (7.8–11.0 mmol), or glycated hemoglobin 5.7–6.4% (39–47 mmol/mol)], HOMA-IR score ≥2.5, and plasma high-sensitivity C-reactive protein concentration >2 mg/L. P-trend = Wald test for linear trend.
Results from sensitivity analyses that examined the abdominal adipose tissue metrics by race (Supplemental Tables 2 and 3) were directionally consistent with the main results for VAT and liver fat. However, the results were qualitatively different for SAT and IMAT. There was a more monotonic relation with a threshold between higher fast-food intake and SAT and IMAT accumulation in white participants, whereas there was no apparent association between fast-food and SAT or IMAT in black participants. As well, analyses that categorized participants according to average fast-food intake from 0 to 20 y; by visit years 0, 7, and 10; or by visit years 15, 20, and 25 were also consistent with the main results (Supplemental Tables 4–6). Last, analyses that excluded participants with a history of a CVD event at year 25 were consistent with the main results, and descriptive characteristics of participants who had ≥2 assessments of fast-food intake over 20 y but did not participate in the year 25 exam were consistent with participants in this analysis (data not presented for the latter 2 analyses).
Discussion
Dietary intake is hypothesized to be a primary, consequential driver of IAAT accumulation (25). In this article, we report results from the CARDIA study, where we quantified levels of abdominal SAT, IAAT, and odds of MAFLD in middle age according to a defining aspect of the American diet: fast-food intake habits. We observed that with higher average fast-food intake over the previous 25 y, there were monotonic higher levels of VAT and liver fat, and this was consistent across general obesity categorization (BMI and waist circumference). Furthermore, we observed higher levels of all IAAT metrics with higher average fast-food intake over the first part of adulthood, albeit without the same magnitude as VAT. Last, we observed strong, graded higher odds of MAFLD according to higher fast-food intake.
We are not aware of previous research that has addressed fast-food intake and accumulation of IAAT, but triangulating the minimal evidence base thus far provides important context for these results. First, the amount and type of dietary fat, particularly saturated fatty acids, along with the amount of processed carbohydrates and sugar, appears to lead to greater accumulation of VAT and hepatic adipose tissue (26–29). Second, the average meal from a fast-food restaurant aligns with the aforementioned dietary intake paradigm for greater accumulation of IAAT, as it is higher in calories, saturated fat, cholesterol, and sugar and lowest in overall diet quality compared with meals from other restaurants or prepared at home (7, 30). More broadly, fast-food intake is a predominant factor in overall dietary intake in the United States as ∼37% of adults report eating fast food on any given day, with the highest proportions among young (∼45%) and middle-aged adults (∼38%), with even higher daily intake in non-Hispanic black adults and with increasing family income (9). Of particular note, young and middle-aged adults get ∼15% of their approximate energy intake from fast-food restaurants (10). Finally, there is evidence fast-food intake as a proportion of the usual diet has increased (7, 8). Indeed, this reflects industry trends of an increasing number of fast food restaurants (31). This ubiquity is reflected in the above-noted intake patterns, but the average meal from non-fast-food restaurants is also of low dietary quality (7).
To sum up, although most research examining how dietary intake relates with accumulation of IAAT is limited by temporality considerations, blunt dietary assessment instruments, and few randomized interventions, the results from this study align with what has generally been reported. Specifically, dietary patterns with higher intakes of fruit, vegetables, whole grains, seeds/nuts, and yogurt were associated with lower IAAT, whereas patterns with higher red/processed meats, higher sources of saturated fats, and refined sources of carbohydrates and sugar-sweetened beverages, and ultimately higher energy intake, were associated with higher IAAT accumulation (32–36).
To provide further context for interpretation, we note limitations of the current study. The SAT measurement in the study did not delineate between superficial and deep SAT, which could further inform the topic. Furthermore, CT is considered a valid and clinically appropriate method of measuring liver fat, but magnetic resonance imaging is considered to have measurement advantages over CT (37). The CARDIA study has a high participant retention rate after 25 y, but there is still attrition (and thus potential selection bias). Our comparison of participant characteristics in those who participated at the year 25 exam compared with those who did not demonstrated no material differences, suggesting selection bias does not likely explain the results. In addition, even if validated, self-reported data for dietary intake and other lifestyle-related factors likely have some level of differential and nondifferential measurement error, which has the potential to influence the results in different ways. Last, the adjusted compared with minimally adjusted models for means of SAT and IAAT, as well as in the logistic regression models for estimating the odds of MAFLD, did not produce materially different results, suggesting the results are robust. Due to only 1 measure at year 25, we were not able to examine longitudinal patterns of IAAT and estimate risk for incident MAFLD, although we speculate that most cases of MAFLD at year 25 did not have MAFLD at baseline, given the strong link to general weight gain and obesity.
Fast-food intake is a defining aspect of the typical American diet because it is commonly consumed across demographic and socioeconomic strata (9, 38). Because of this ecologic-level homogeneity, the CARDIA study provides a unique and insightful cohort with longitudinal individual-level data on the topic, so important questions related to individual-level fast-food intake are able to be informed. In addition, the cohort commenced at a critical period for chronic disease development in the life course, namely, in early adulthood, when many habits and influences on health are assorting participants into different trajectories of health status and disease risk (39). The results from this analysis robustly demonstrate that middle-aged adults who ate fast food more frequently over the previous 25 y have significantly higher odds of MAFLD and an IAAT profile, particularly higher VAT, aligned with poorer current and future cardiometabolic health and chronic disease risk.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Linda Van Horn for her role as a CARDIA study reviewer and efforts to review an initial draft of this manuscript.
The authors’ responsibilities were as follows—AOO: conceptualized project, designed research plan, analyzed data, wrote first draft of paper, has primary responsibility for final content; DRJ: provided study oversight, study analytic insight, input and editing of manuscript writing; LBVW: provided analytic insight and input and editing of manuscript writing; MAP: contributed to research plan and input/editing of manuscript writing; and all authors: have read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Partially supported by NIH/National Heart, Lung, and Blood Institute (NHLBI) (R21HL121627) to AOO, DRJ, and MAP and NIH/National Institute on Aging (R01AG055018) to AOO. The NIH had no role in any aspect of this research. The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the NHLBI in collaboration with the University of Alabama at Birmingham (HHSN268201800005I and HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). This article has been reviewed by CARDIA for scientific content.
MAP is a member of the Journal's Editorial Board and played no role in the evaluation of this submission.
Supplemental Figure 1 and Supplemental Tables 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: aMed, alternate Mediterranean; CARDIA, Coronary Artery Risk Development in Young Adults; CT, computed tomography; CVD, cardiovascular disease; EU, exercise units; HbA1c, glycated hemoglobin; HU, Hounsfield units; IAAT, intra-abdominal adipose tissue; IMAT, intermuscular adipose tissue; MAFLD, metabolic-associated fatty liver disease; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Contributor Information
Andrew O Odegaard, Department of Epidemiology and Biostatistics, University of California, Irvine, CA, USA.
David R Jacobs, Jr, Department of Epidemiology and Community Health, University of Minnesota, Minneapolis, MN, USA.
Lisa B Van Wagner, Department of Medicine, Division of Gastroenterology & Hepatology and Department of Preventive Medicine, Division of Epidemiology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Mark A Pereira, Department of Epidemiology and Community Health, University of Minnesota, Minneapolis, MN, USA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 2. Newcombe EA, Camats-Perna J, Silva ML, Valmas N, Huat TJ, Medeiros R. Inflammation: the link between comorbidities, genetics, and Alzheimer's disease. J Neuroinflamm. 2018;15(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai Iet al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–25. [DOI] [PubMed] [Google Scholar]
- 4. Stanhope KL, Goran MI, Bosy-Westphal A, King JC, Schmidt LA, Schwarz JMet al. Pathways and mechanisms linking dietary components to cardiometabolic disease: thinking beyond calories. Obes Rev. 2018;19(9):1205–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8(7):616–27. [DOI] [PubMed] [Google Scholar]
- 6. Fischer K, Pick JA, Moewes D, Nöthlings U. Qualitative aspects of diet affecting visceral and subcutaneous abdominal adipose tissue: a systematic review of observational and controlled intervention studies. Nutr Rev. 2015;73(4):191–215. [DOI] [PubMed] [Google Scholar]
- 7. Liu J, Rehm CD, Micha R, Mozaffarian D. Quality of meals consumed by US adults at full-service and fast-food restaurants, 2003–2016: persistent low quality and widening disparities. J Nutr. 2020;150(4):873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCrory MA, Harbaugh AG, Appeadu S, Roberts SB. Fast-food offerings in the United States in 1986, 1991, and 2016 show large increases in food variety, portion size, dietary energy, and selected micronutrients. J Acad Nutr Diet. 2019;119(6):923–33. [DOI] [PubMed] [Google Scholar]
- 9. Fryar CD, Hughes JP, Herrick KA, Ahluwalia N. Fast food consumption among adults in the United States, 2013–2016. NCHS Data Brief. 2018;(322):1–8. [PubMed] [Google Scholar]
- 10. Fryer CD, Ervin RB. Caloric intake from fast food among adults: United States, 2007–2010. NCHS Data Brief. 2013;(114):1–8. [PubMed] [Google Scholar]
- 11. Brown WV, Carson JA, Johnson RK, Kris-Etherton P. JCL roundtable: fast food and the American diet. J Clin Lipidol. 2015;9(1):3–10. [DOI] [PubMed] [Google Scholar]
- 12. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jret al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–16. [DOI] [PubMed] [Google Scholar]
- 13. Bild DE, Jacobs DR, Liu K, Williams OD, Hilner JE, Perkins LLet al. Seven-year trends in plasma low-density-lipoprotein cholesterol in young adults: the CARDIA study. Ann Epidemiol. 1996;6(3):235–45. [DOI] [PubMed] [Google Scholar]
- 14. Park K, Gross M, Lee DH, Holvoet P, Himes JH, Shikany JM, Jacobs DR Jr.. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. 2009;32(7):1302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE. Longitudinal changes in adiposity associated with pregnancy. The CARDIA study. Coronary Artery Risk Development in Young Adults Study. JAMA. 1994;271(22):1747–51. [PubMed] [Google Scholar]
- 16. McDonald A, Van Horn L, Slattery M, Hilner J, Bragg C, Caan Bet al. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc. 1991;91(9):1104–12. [PubMed] [Google Scholar]
- 17. Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai Net al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82(1):163–73. [DOI] [PubMed] [Google Scholar]
- 18. Jacobs DRJ, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of a short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil. 1989;9(11):448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parker ED, Schmitz KH, Jacobs DR Jr, Dengel DR, Schreiner PJ. Physical activity in young adults and incident hypertension over 15 years of follow-up: the CARDIA study. Am J Public Health. 2007;97(4):703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reis JP, Allen N, Gunderson EP, Lee JM, Lewis CE, Loria CMet al. Excess body mass index- and waist circumference-years and incident cardiovascular disease: the CARDIA study. Obesity. 2015;23(4):879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, Sirlin CB. Fatty liver: imaging patterns and pitfalls. Radiographics. 2006;26(6):1637–53. [DOI] [PubMed] [Google Scholar]
- 22. Li Q, Dhyani M, Grajo JR, Sirlin C, Samir AE. Current status of imaging in nonalcoholic fatty liver disease. World J Hepatol. 2018;10(8):530–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. [DOI] [PubMed] [Google Scholar]
- 24. Hashimoto E, Tokushige K, Ludwig J. Diagnosis and classification of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: current concepts and remaining challenges. Hepatol Res. 2015;45(1):20–8. [DOI] [PubMed] [Google Scholar]
- 25. Zamboni M, Rossi AP, Fantin F, Budui SL, Zoico E, Zamboni GA, Mazzali G. Predictors of ectopic fat in humans. Curr Obes Rep. 2014;3(4):404–13. [DOI] [PubMed] [Google Scholar]
- 26. Henkin Y, Kovsan J, Gepner Y, Shai I. Diets and morbid tissues—history counts, present counts. Br J Nutr. 2015;113(Suppl 2):S11–8. [DOI] [PubMed] [Google Scholar]
- 27. Parry SA, Hodson L. Influence of dietary macronutrients on liver fat accumulation and metabolism. J Investig Med. 2017;65(8):1102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luukkonen PK, Sädevirta S, Zhou Y, Kayser B, Ali A, Ahonen Let al. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care. 2018;41(8):1732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meisinger C, Rospleszcz S, Wintermeyer E, Lorbeer R, Thorand B, Bamberg Fet al. Isocaloric substitution of dietary carbohydrate intake with fat intake and MRI-determined total volumes of visceral, subcutaneous and hepatic fat content in middle-aged adults. Nutrients. 2019;11(5):1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolfson JA, Leung CW, Richardson CR. More frequent cooking at home is associated with higher Healthy Eating Index–2015 score. Public Health Nutr. 2020;23(13):2384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Le T. Fast Food Restaurants in the US: INDUSTRY REPORT 72221A. IBISWorld. 2021 [Internet]. Available from: https://www.ibisworld.com.
- 32. Odegaard AO, Choh AC, Czerwinski SA, Towne B, Demerath EW. Sugar-sweetened and diet beverages in relation to visceral adipose tissue. Obesity. 2012;20(3):689–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maskarinec G, Lim U, Jacobs S, Monroe KR, Ernst T, Buchthal SDet al. Diet quality in midadulthood predicts visceral adiposity and liver fatness in older ages: the Multiethnic Cohort Study. Obesity. 2017;25(8):1442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shah RV, Murthy VL, Allison MA, Ding J, Budoff M, Frazier-Wood ACet al. Diet and adipose tissue distributions: the Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 2016;26(3):185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gepner Y, Shelef I, Schwarzfuchs D, Zelicha H, Tene L, Yaskolka Meir Aet al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: CENTRAL magnetic resonance imaging randomized controlled trial. Circulation. 2018;137(11):1143–57. [DOI] [PubMed] [Google Scholar]
- 36. Ma J, Hennein R, Liu C, Long MT, Hoffmann U, Jacques PFet al. Improved diet quality associates with reduction in liver fat, particularly in individuals with high genetic risk scores for nonalcoholic fatty liver disease. Gastroenterology. 2018;155(1):107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang YN, Fowler KJ, Hamilton G, Cui JY, Sy EZ, Balanay Met al. Liver fat imaging—a clinical overview of ultrasound, CT, and MR imaging. Br J Radiol. 2018;91(1089):20170959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zagorsky JL, Smith PK. The association between socioeconomic status and adult fast-food consumption in the U.S. Econ Hum Biol. 2017;27:12–25. [DOI] [PubMed] [Google Scholar]
- 39. Lawrence EM, Mollborn S, Hummer RA. Health lifestyles across the transition to adulthood: implications for health. Soc Sci Med. 2017;193:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.