ABSTRACT
Background
Diabetes is associated with low plasma vitamin C concentrations.
Objectives
We investigated the contribution of dysregulated vitamin C renal physiology, its prevalence, and associated clinical characteristics.
Methods
An essential prerequisite was determination of normal vitamin C renal threshold, the plasma concentration at which vitamin C first appears in urine. Using data from 17 healthy participants who underwent vitamin C depletion–repletion studies with a vitamin C dose range of 15–1250 mg daily, renal threshold was estimated using physiology-based pharmacokinetics modeling. Applying renal threshold 95% CIs, we estimated the minimal elimination threshold, the plasma concentration below which no vitamin C was expected in urine of healthy people. Renal leak was defined as abnormal presence of vitamin C in urine with plasma concentrations below the minimal elimination threshold. Criteria were tested in a cross-sectional cohort study of individuals with diabetes (82) and nondiabetic controls (80) using matched plasma and urine samples.
Results
Vitamin C renal thresholds in healthy men and women were [mean (SD)] 48.5 (5.2) µM and 58.3 (7.5) µM, respectively. Compared with nondiabetic controls, participants with diabetes had significantly higher prevalence of vitamin C renal leak (9% compared with 33%; OR: 5.07; 95% CI: 1.97, 14.83; P < 0.001) and 30% lower mean plasma vitamin C concentrations (53.1 µM compared with 40.9 µM, P < 0.001). Fasting plasma glucose, glycosylated hemoglobin A1c, BMI, micro/macrovascular complications, and protein/creatinine ratio were predictive of vitamin C renal leak.
Conclusions
Increased prevalence of vitamin C renal leak in diabetes is associated with reduced plasma vitamin C concentrations. Glycemic control, microvascular complications, obesity, and proteinuria are predictive of renal leak.
Keywords: diabetes, hyperglycemia, diabetic nephropathy, microvascular complications, vitamin C, nutrition, renal threshold, renal leak
See corresponding editorial on page 3.
Introduction
Vitamin C is a potent water-soluble antioxidant in humans that is required for many reactions, both enzyme dependent and enzyme independent (1, 2). Diabetes and its microvascular and macrovascular complications are associated with increased oxidative stress and decreased antioxidant capacity (3, 4). Compared with healthy populations, vitamin C concentrations are lower in people with diabetes (5–9). In addition, several recent studies have shown a clinical benefit of vitamin C supplementation in diabetes, with varying levels of evidence (10–13). Unfortunately, many studies of vitamin C in diabetes have vitamin C assay limitations, including nonspecificity, interferences, insensitivity, analyte instability, and ambiguous methodology (1, 14–16). Beyond assay uncertainties, reduced vitamin C concentrations in diabetes could be due to limited dietary intake, decreased bioavailability, and increased utilization (1, 2, 17). A major but poorly characterized explanation of reduced vitamin C concentrations in diabetes is abnormal renal loss. To determine whether abnormal renal loss of vitamin C occurs in diabetes, a key prerequisite is knowledge of vitamin C renal physiology in healthy people.
Vitamin C dose–concentration studies indicate that plasma vitamin C concentrations are tightly regulated in healthy individuals (18, 19). These studies are consistent with the existence of a renal threshold, the plasma concentration at which vitamin C first appears in urine. When plasma vitamin C concentrations exceed renal threshold, the amount of filtered vitamin C exceeds renal tubular absorptive capacity, and vitamin C is appropriately excreted into urine. The apical tubular reabsorptive transporter is SVCT1 (20–22). The basolateral efflux transporter has not been identified and may be a transporter other than SVCT1 or SVCT2 (22, 23). Precise sex-specific renal threshold values for women and men are essential for ascertaining when urinary vitamin C excretion is physiologic and appropriate for a given plasma vitamin C concentration or abnormal and potentially indicative of pathology. Prior studies aimed to characterize a normal renal threshold for vitamin C, but measurements suffered from similar methodology limitations as described above for diabetes, with additional concerns of narrow vitamin C dose ranges and minimal accounting for sex differences (24–29).
Our studies had 3 major objectives. First, in healthy men and women, we characterized normal vitamin C renal thresholds using data obtained from vitamin C depletion–repletion and dose–concentration studies (18, 19). Second, we used renal threshold data to determine when urinary vitamin C excretion would be abnormal for a given plasma vitamin C concentration. Abnormal urinary vitamin C excretion is referred to as a renal leak. Third, we used these criteria for renal leak to investigate vitamin C pathophysiology in diabetes. We hypothesized that a renal threshold for vitamin C might be dysregulated in diabetes, and the consequences would be abnormal urinary vitamin C excretion (renal leak). To test our hypothesis, we used a sensitive and specific vitamin C HPLC coulometric electrochemical assay to investigate urinary vitamin C excretion, prevalence of renal leak, and associated clinical characteristics of vitamin C renal leak in a cohort of individuals with diabetes and nondiabetic controls.
Methods
To achieve objectives, 2 component studies were conducted. First was determination of vitamin C renal threshold using data from depletion–repletion studies in healthy men and women. Second was a cross-sectional cohort study of vitamin C renal leak in individuals with diabetes and nondiabetic controls.
Determination of vitamin C renal threshold and renal leak criteria: depletion–repletion studies
Study design, settings
This was a nonrandomized interventional study aimed at determining normal vitamin C renal threshold in healthy men and women using a vitamin C depletion–repletion protocol (92-DK-0033). The data and analyses in this article have not been previously published. The protocol was approved by the Institutional Review Board, National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH.
Participants, interventions
Healthy men and women aged 18–35 y without any acute or chronic medical conditions.
Study interventions
For the whole study, both depletion and repletion phases, participants consumed a vitamin C–restricted diet that contained <5 mg/d. Upon admission, participants were placed on the diet, and all participants achieved nadir plasma vitamin C concentrations <10 μM within 29 d. Participants continued the diet but were then repleted sequentially with 7 escalating doses of oral ascorbic acid, from 30 mg to 2500 mg. Half of each dose was administered twice daily in the fasted state until achievement of steady-state concentration, defined as the mean of 5 or more samples obtained over at least 7 d with <10% SD. Each participant had 5–7 oral and 5–7 intravenous pharmacokinetic studies over a 13-fold or greater dose range. Once steady state was achieved for each dose, 2 sequential bioavailability studies were performed using first oral and then intravenous vitamin C, with collection of urine and plasma samples throughout. Additional information about study design and diet is provided as described (18, 19, 30, 31).
Study outcomes, analyses
The major study outcomes were to determine normal vitamin C renal threshold (the vitamin C plasma concentration in which filtered vitamin C first appears in urine), minimal elimination threshold (MET; the plasma vitamin C concentration 2 SD below the renal threshold), and criteria for abnormal urinary vitamin C excretion (renal leak). Vitamin C renal threshold is defined as the plasma vitamin C concentration during a bioavailability study at which vitamin C first appears in the urine during a repletion phase or when urinary elimination stops during a depletion phase. Data from the NIH depletion study in men and women were used to estimate the vitamin C renal threshold by 2 different methods: physiology-based pharmacokinetics modeling (PBPK) and population curve fitting (PCF) with empirical functions. PBPK used a physiologic-based pharmacokinetics model of vitamin C that incorporated nonlinear absorption, tissue distribution, and renal elimination/reabsorption kinetics with 5 interconnected compartments (absorption, central, tissue, renal tubule, and urine) as well as gut loss and tissue metabolism (more details in Supplemental Methods). Based on data from healthy men, this model facilitated description of pharmacokinetics–time profiles of vitamin C in plasma, urine, and tissues simultaneously (19, 30). Goodness-of-fit analyses support the use of this model to data from healthy women (18, 30). For PCF, plasma and urine values were analyzed using nonlinear mixed-effect modeling. PCF fits empirical functions to data from all participants of each sex to estimate the renal threshold for that population of men and women (details in Supplemental Methods). Using PBPK analyses, we defined the MET as that plasma concentration 2 SD below the mean renal threshold for men and for women based on guidelines for estimating dietary reference intakes (32). When plasma vitamin C concentrations are less than the MET, urinary vitamin C excretion is abnormal, a term referred to as renal leak.
Sample collection and vitamin C assay
Urine and plasma vitamin C were analyzed by HPLC with coulometric electrochemical detection as described, limit of detection 0.01 mg/total urine volume (18, 19, 33, 34) (see Supplemental Methods). Other blood and urine chemistry measurements were performed, using standard laboratory methods, by the Department of Laboratory Medicine Clinical Laboratory Improvement Amendments (CLIA) ID number 21D0665373, Clinical Research Center, NIH.
Cross-sectional cohort study of vitamin C renal leak in individuals with diabetes and nondiabetic controls
Study design
This was a nonrandomized cross-sectional cohort outpatient study aimed at investigating the prevalence and clinical characteristics of vitamin C renal leak in a cohort of individuals with type 1 or type 2 diabetes and nondiabetic controls (04-DK-0021). The protocol was approved by the Institutional Review Boards of the NIDDK, NIH.
Participants and recruitment
The study included a cohort of diabetic and nondiabetic individuals aged 18–65 y, without known acute or chronic medical conditions other than complications of diabetes mellitus or metabolic syndrome. Exclusion criteria included presence of an acute or chronic illness, alcohol abuse or tobacco use, and pregnancy. The exclusion criteria were chosen to avoid conditions that may confound study findings, given reported association of these conditions with vitamin C dysregulation (1, 2, 35). Recruitment was conducted using recruitment flyers and advertisements displayed in the community, on social media, and in online registries.
Screening evaluation
Interested participants were prescreened by telephone and completed a structured questionnaire on their medical history. Eligible participants were then scheduled for an outpatient screening visit at the NIH Clinical Research Center (CRC) for a more detailed clinical history and physical examination by research investigators. Participants who met screening criteria were scheduled for the protocol sampling studies on a rolling basis to accommodate use of the CRC's metabolic unit.
Study interventions
Approximately 2 wk prior to study sampling, participants were instructed to avoid vitamin C supplements. Participants were also instructed to avoid food or drinks (other than water and medications) after midnight, prior to study sampling day. On the morning of study sampling, participants were admitted to the NIH CRC's metabolic unit as outpatients. They were instructed to empty their bladder. One hour later, matched urine and fasting blood samples were obtained for measurement of vitamin C concentrations, chemistry, and related studies. Participants were subsequently provided with a meal and discharged home.
Study groups and comparators for analyses
Two groups of participants who met the inclusion and exclusion criteria were used for data analyses: diabetes cohort and nondiabetic control group. The diabetes cohort comprised men and women with type 1 or type 2 diabetes. The nondiabetic control group was made up of individuals without diabetes and who were not taking any chronic medications.
Study outcomes and endpoints
The primary outcome was prevalence of vitamin C renal leak in the diabetes cohort compared with nondiabetic control. The MET and vitamin C renal leak criteria were derived using data from vitamin C depletion–repletion studies and physiology-based pharmacokinetic modeling (see Methods section on depletion–repletion studies). Using the renal leak criteria, participants were classified as “leakers” or “nonleakers” based on fasting plasma and matched urine vitamin C concentrations. This binary criterion is a qualitative measure indicating the presence or absence of a renal leak in a participant. The prevalence of renal leak was calculated as the proportion of leakers in each population cohort. Secondary outcomes assessed mean differences in plasma vitamin C concentrations between groups. Exploratory outcomes assessed the demographic, clinical, and laboratory covariates associated with vitamin C renal leak and plasma vitamin C concentrations.
Sample collection and vitamin C assay
Urine and plasma vitamin C were analyzed by HPLC with coulometric electrochemical detection as described (18, 19, 34, 36). Other blood and urine chemistry measurements were performed, using standard laboratory methods, by the Department of Laboratory Medicine CLIA ID number 21D0665373, CRC, NIH.
Methodologic approaches used to reduce bias
Exclusion criteria
Conditions associated with vitamin C dysregulation such as smoking and history of chronic diseases (other than diabetes and diabetes-related conditions) were excluded to avoid confounding study findings.
Physiologic variations in vitamin C
Sex-specific criteria for renal leak were used to determine vitamin C renal leak status. In addition, use of 2 SD below mean sex-specific renal threshold was incorporated to control for potential confounding based on potential differences in normal physiologic parameters (such as age) (32).
Dietary considerations
Chronic insufficient dietary intake of vitamin C: Participants on a chronic vitamin C–deficient diet may have low plasma vitamin C concentrations. However, these participants are only considered “leakers” if they also have abnormal urinary excretion of vitamin C, for such low plasma vitamin C concentrations (defined as below the MET). Thus, the sex-specific renal leak criterion avoids the confounding issue of chronic insufficient dietary intake and obviates the need to document intake or its absence.
Postprandial variabilities in vitamin C concentrations: Vitamin C ingestion transiently increases plasma vitamin C concentrations (17–19). This transient postprandial increase will be inconsistent across participants and variable based on the vitamin C content and time of ingestion prior to sampling. We used fasting plasma vitamin C values (no food after midnight the night before) to 1) ensure measured plasma vitamin C accurately reflects participants’ true baseline vitamin C concentration and not the transient postprandial value, 2) avoid erroneous assessment of renal leak status based on transient postprandial changes in plasma vitamin C concentrations, and 3) ensure consistency in how other clinical laboratory covariates are obtained (such as fasting glucose and fasting lipid panel).
Use of vitamin C supplements: Participants were requested to refrain from intake of supplements containing vitamin C 2 wk prior to study to 1) avoid the transient elevations in plasma vitamin C concentrations caused by supplements that are not reflective of their baseline vitamin C status (17–19) and 2) avoid a potential confounding effect associated with variability in ingestion/absorption of supplements, especially in participants with diabetes.
Errors from urine and plasma sample collection
To assess for vitamin C renal leak, matched fasting plasma vitamin C measurements and corresponding urine measurements are needed. The fasting vitamin C plasma measurement is used to ensure accurate assessment of baseline vitamin C status. Emptying the bladder and waiting for a fixed period (i.e., 1 h) also avoids erroneous measurement of vitamin C that is confounded by dietary intake from as much as 8–12 h prior (17–19). Waiting 1 h allows for fresh urinary vitamin C measurements that correspond with fasting vitamin C (and all other clinical chemistry measurements such fasting glucose).
Inaccuracies from survey and vitamin C assay
We avoided use of dietary surveys/registries given the strength of the HPLC assay coupled with our study methodology specifically designed to account for not just dietary intake alone (as in surveys) but also urinary vitamin C loss. Dietary intake surveys were also not used to avoid potential errors due to inaccurate reporting of dietary intake (37) resulting in mismatch between reported intake and measured plasma concentrations.
Sample size
At the time of the initial study design, there were no actual, extant data upon which to base a sample size calculation. Sample size calculations were based on scant anecdotal evidence indicating substantial differences in 24-h urinary vitamin C excretion between nondiabetic controls and the diabetes cohort. It was therefore assumed that the magnitude of differences would be between Cohen's d values constituting so-called medium and large differences, corresponding to an effect size (in standard error units for a given statistical measure) between 0.5 and 0.8; the midpoint value of 0.65 was chosen. Using a roughly a 2:1 recruitment goal and standard 2-sample t test, at a 2-sided significance level of 0.05, we projected that 30 individuals without diabetes (nondiabetic controls) and 60 participants with diabetes would yield a statistical power of ∼80% or 81% (38). It was determined that the estimated sample size necessary to determine whether participants with diabetes lose more vitamin C in the urine than normal controls would be approximately 150.
Statistical analyses plan
In the vitamin C depletion–repletion study, the Welch t test was used to assess differences in the estimated vitamin C renal threshold for men compared with women. In the cross-sectional cohort study, descriptive statistics were calculated for baseline characteristics of study participants. Counts and proportions were used for categorical variables, accompanied by the Pearson χ2 test or Fisher exact tests as indicated, while means and standard deviations were used for continuous variables, accompanied by the Welch t test or Wilcoxon–Mann–Whitney tests as indicated. The primary outcome of vitamin C renal leak prevalence in the diabetes cohort compared with control was assessed using group-specific proportions accompanied by an exact binomial confidence interval designed to match a “central” Fisher exact test. This set of estimates is accompanied by an inherently symmetric association measure deemed most informative to follow-on research (studies that may follow individuals at risk for diabetes over time for the development of renal leak) (39): a comparative odds ratio, corresponding 95% CIs, and Fisher exact test P values.
To further explore that the primary study readout—the marginal association of diabetes with renal leak—was not subject to bias due to group differences in baseline covariates, collapsible association measures were employed in place of “adjusting” odds ratios (39): log-binomial regression models (40, 41) and resulting risk ratio estimates (42, 43) were used to assess the persistence of the diabetes–leak association, based on whether 95% CI estimates for the adjusted risk ratio still covered a range of clinically impactful values above 1 (while excluding this null value). For the change-in-effect analyses, we selected nonglycemic baseline variables (Table 1) that were significantly different between the 2 groups [age, BMI, and protein/creatinine ratio (PCR)].
TABLE 1.
Baseline characteristics of participants in the depletion–repletion study and in the cross-sectional study of renal leak in diabetes1
| Cross-sectional cohort studies of vitamin C renal leak | Vitamin C depletion/repletion study (n = 17) | ||
|---|---|---|---|
| Characteristic | Nondiabetic control (n = 80) | Diabetes cohort (n = 82) | |
| Demographics | |||
| Race | |||
| White/Caucasian | 35 (43.8) | 34 (41.5) | 14 (82.4) |
| Black/African American | 36 (45) | 36 (43.9) | 2 (11.8) |
| Other2 | 9 (11.2) | 12 (14.6) | 1 (5.9) |
| Sex | |||
| Male | 30 (37.5) | 40 (48.8) | 7 (41.2) |
| Female | 50 (62.5) | 42 (51.2) | 10 (58.8) |
| Age, mean ± SD, y | 33.5 ± 12.6* | 49.2 ± 12.1* | 22 ± 2 |
| BMI, mean ± SD, kg/m2 | 27.5 ± 5.7* | 32.5 ± 6.7* | 26 ± 11.5 |
| Clinical and laboratory variables | |||
| Renal function | |||
| PCR, mean ± SD, mg/mg | 0.097 ± 0.091* | 0.267 ± 0.557* | NA |
| eGFR,3 mean ± SD, mL/min/1.73 m2 | 112.8 ± 21.1 | 101.6 ± 26.2 | 94 ± 21.2 |
| Glycemic control | |||
| Fasting plasma glucose, mean ± SD, mg/dL | 88.3 ± 8.8* | 147.0 ± 53.3* | 88 ± 4.6 |
| Hemoglobin A1c, mean (SD), % | 5.37 (0.4)* | 7.9 (1.4)* | NA |
| Diabetes history | |||
| Diabetes type | |||
| Type 1 | 21 (25) | ||
| Type 2 | 61 (75) | ||
| Duration of diagnosis, mean ± SD, y | 11.5 ± 11.3 | ||
| Micro- or macrovascular disease | 20 (23.8) | ||
| Medication4 | |||
| Antihypertensives | 45 (53.6) | ||
| Diuretics5 | 15 (17.9) | ||
| ACE/ARB | 41 (48.8) | ||
Values are presented as number (%) unless otherwise indicated. Asterisks indicate that variables are statistically different (P < 0.05, nondiabetic control compared with diabetes cohort, Welch t test). ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; NA, data non available; PCR, protein/creatinine ratio.
The subgroup “Other” is composed of Asian, Hispanic, and Latino and multiracial participants. Racial categories were based on NIH reporting guidelines on racial and ethnic categories (NOT-OD-15-089).
eGFR for all groups was calculated using the Modification of Diet in Renal Disease v4 equation: [186 × (Creatinine / 88.4) – 1.154 × (Age) – 0.203 × (0.742 if female) × (1.210 if black)].
One participant was taking an SGLT2 inhibitor.
Includes loop diuretics, thiazides, and aldosterone antagonists.
To compare group differences (estimated shifts) in mean plasma vitamin C, linear regression analyses with and without adjustments for sex were performed, given the observed sex differences in renal threshold estimates from depletion–repletion studies. We also report diabetes status group-specific mean concentrations of plasma vitamin C and estimated shift in mean concentrations (difference between groups) accompanied by their corresponding (large-sample normal approximation-based) 95% CIs and P values. To assess multiple variables for association with renal leak measures, logistic regression models were employed, yielding estimates of unadjusted (marginal) odds ratios. Given the exploratory intent of these analyses, multiplicity adjustments were not reported. All analyses were conducted using R version 3.6 or higher (The R Project for Statistical Computing).
Results
Determination of vitamin C renal threshold in healthy women and men: data from depletion–repletion studies
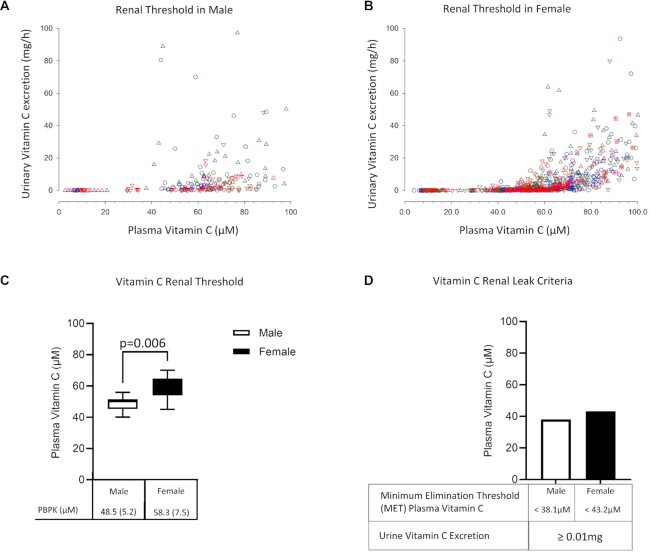
Our first objective was to determine a normal vitamin C renal threshold: that plasma concentration above which vitamin C would be expected to be excreted in urine. Data from 17 healthy volunteers (7 men and 10 women) were included in the final analyses (Figure 1A). Baseline characteristics of participants are shown in Table 1. Measurements of urinary vitamin C excretion as a function of matched plasma vitamin C concentrations are displayed for 7 men (Figure 2A) and 10 women (Figure 2B). Visual inspection of the data indicated a threshold plasma vitamin C concentration, above which vitamin C was detected in urine. We calculated the renal threshold for men and women using 2 analytical models: a PBPK model and a PCF model (see Methods and Supplemental Methods). Using the PBPK approach, the estimated vitamin C renal threshold was [mean (SD)] 48.5 (5.2) µM for healthy men and 58.3 (7.5) µM for healthy women (Figure 2C, Supplemental Figure 1A). The PCF approach revealed similar renal threshold values of 48.3 µM in healthy men and 60.4 µM in healthy women (Supplemental Figure 1). The renal threshold estimates in men and women were significantly different (P = 0.006, Figure 2C).
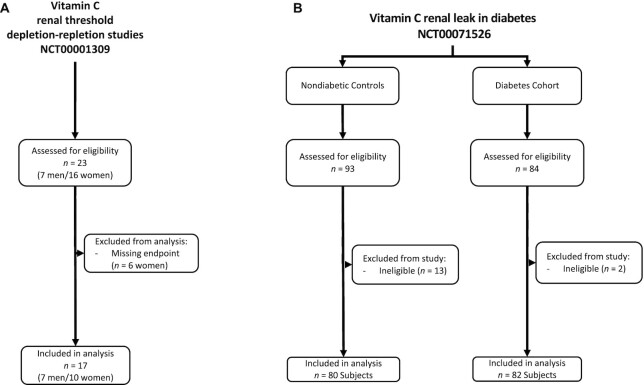
FIGURE 1.
Enrollment and patient flow for vitamin C depletion–repletion study and cross-sectional cohort study of individuals with diabetes and nondiabetic controls.
FIGURE 2.
Vitamin C depletion–repletion study in healthy participants. (A, B) Urinary vitamin C excretion as a function of matched plasma vitamin C concentrations in healthy men (A) and women (B). Pharmacokinetic (PK) studies were performed as 1 component of dose–concentration studies (18, 19, 30) by administering intravenous or oral doses (15–1250 mg) of vitamin C on successive days at steady state. Blood samples were obtained at fixed intervals (18, 19). All urine voided during the test period was collected, and each urine void formed a timed collection. The total amount of vitamin C in each urine sample (mg) is indicated on the y-axis. Each symbol shape coupled to a specific color represents a different participant. Data are shown from 10–14 PK studies (5–7 oral doses; 5–7 intravenous doses) for each participant. Participants were 7 men and 10 women. (C) Estimated vitamin C renal threshold values in healthy men (open bar) and women (filled bar) using physiology-based pharmacokinetic modeling [PBPK, mean (SD)]. (D) Vitamin C renal leak criteria for men (open bar) and women (filled bar). Minimal elimination threshold (MET) was defined as 2 SD below mean renal threshold using PBPK modeling (see Methods section). Vitamin C renal leak was defined as abnormal urinary vitamin C excretion (>0.01 mg/total urine volume, limit of detection) occurring when plasma vitamin C concentrations are below MET values of 38.1 µM for men and 43.2 µM for women.
Next, we determined the MET, the plasma vitamin C concentration below which urinary vitamin C excretion would be abnormal. MET was defined as 2 SD below the renal threshold for each sex (see Methods). Using the PBPK model, the MET was 38.1 µM for healthy men and 43.2 µM for healthy women (Figure 2D). Taken together, when plasma vitamin C concentrations are less than the MET values of 38.1 µM in men or 43.2 µM in women, urinary vitamin C excretion would be abnormal and is termed a vitamin C renal leak (Figure 2D).
Vitamin C renal leak in individuals with diabetes compared with nondiabetic controls
Of the 177 individuals who were assessed for eligibility, 15 participants were excluded due to ineligibility. In total, 162 participants completed the protocol study, 80 participants in the diabetes cohort and 82 participants in the nondiabetic control group. All volunteers who participated in protocol studies were included in final analyses. The demographic, clinical, and laboratory characteristics of individuals in the cohort with diabetes and the nondiabetic control group are shown in Table 1. Both groups had a similar racial makeup. Compared with the nondiabetic controls, the diabetes cohort had a comparatively lower proportion of women (51% compared with 63%), an older mean age (49 y compared with 34 y), and a higher BMI (in kg/m2; 32.5 compared with 27.5) (Table 1). For the baseline clinical variables, the mean estimated glomerular filtration rate (eGFR) was not significantly different in both groups. However, the diabetes cohort had a higher mean PCR, fasting plasma glucose, and hemoglobin A1c.
Renal leak prevalence between groups
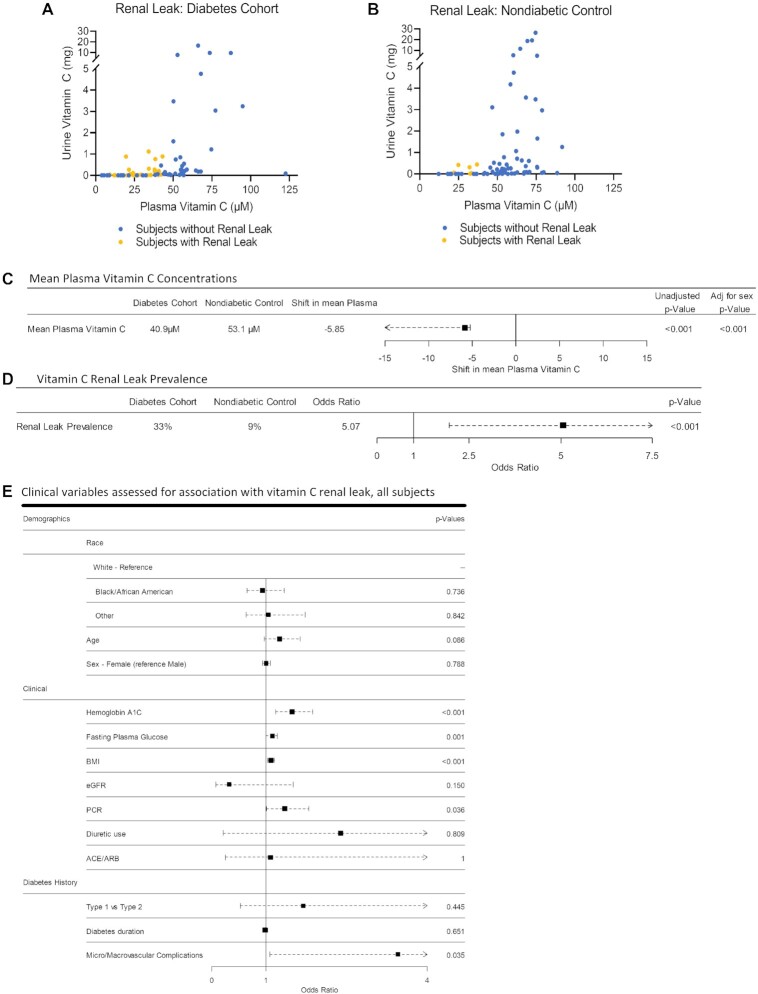
Vitamin C renal leak status for all participants in each group was determined using vitamin C measurements in matched fasting plasma and timed urine samples (Figure 3A, B). The mean plasma vitamin C concentration in the diabetes cohort was significantly lower than the nondiabetic control group with and without adjustment for sex (40.9 µM compared with 53.1 µM, P < 0.001) (Figure 3C, Supplemental Figure2A,B). The prevalence of vitamin C renal leak in the diabetes cohort was significantly higher compared with the nondiabetic control group (33% compared with 9%; OR: 5.07; 95% CI: 1.97, 14.83; P < 0.001) (Figure 3D, Supplemental Figure 2C). Change-of-effect analyses adjusting for group differences in baseline variables did not change the association between diabetes and renal leak (Supplemental Figure 2D).
FIGURE 3.
Cross-sectional cohort study of vitamin C renal leak in a cohort of individuals with diabetes and nondiabetic controls. (A, B) Urinary vitamin C excretion as a function of plasma vitamin C concentrations in the diabetes cohort (n = 82, A) and nondiabetic control group (n = 80, B). In all panels, each circle represents each participant with a renal leak (yellow) or without a renal leak (blue), assessed using matched urine and plasma vitamin C measurements. (C) Plasma vitamin C concentrations in the diabetes cohort compared with the nondiabetic control group. Square indicates estimated shift in mean plasma vitamin C with horizontal points indicating 95% CI. P values are shown for estimated shift, unadjusted and adjusted for sex, n = 162. (D) Vitamin C renal leak prevalence in the diabetes cohort compared with nondiabetic controls. Square indicates odds ratio with horizontal line indicating 95% CI, n = 162. (E) Demographics and clinical variables associated with vitamin C renal leak. All participants were included in the analysis. Squares indicate odds ratio with horizontal points indicating 95% CI, n = 162. Diuretic use includes loop diuretics, thiazides, and aldosterone antagonists. ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration; PCR, protein/creatinine ratio.
Clinical variables associated with vitamin C renal leak in all participants
Among demographic parameters assessed, age, sex, and race were not associated with renal leak (Figure 3E, Supplemental Figure 3). Among the clinical parameters evaluated, BMI, fasting glucose, hemoglobin A1c, and history of micro/macrovascular complications were associated with renal leak. Diabetes type (type 1 compared with type 2), duration, eGFR, use of diuretics, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers were not associated with renal leak (Figure 3E, Supplemental Figure 3).
Discussion
As a prerequisite to understanding vitamin C renal dysregulation in diabetes, we defined normal physiologic parameters associated with renal regulation of vitamin C in healthy men and women using data from depletion–repletion and pharmacokinetics studies coupled with a highly sensitive and specific assay. We provided precise sex-specific estimates of normal vitamin C renal thresholds: the plasma vitamin C concentration in which vitamin C first appears in urine. Renal threshold, in turn, was required to determine the MET, the plasma vitamin C concentrations below which urinary vitamin C excretion would be abnormal in healthy individuals. We integrated these physiologic parameters into simplified criteria for determining when urinary vitamin C excretion would be abnormal at a given plasma vitamin C concentration: a renal leak. There was significantly higher prevalence of vitamin C renal leak in the cohort with diabetes (33%) compared with nondiabetic controls (9%). The diabetes–renal leak association persisted following statistical adjustments for between-group differences in nonglycemic variables (Table 1, Supplemental Figure 2D). Mean fasting plasma vitamin C concentrations were also significantly lower in the diabetes cohort (40.9 µM) compared with nondiabetic controls (53.1 µM). Fasting glucose, hemoglobin A1c, BMI, micro/macrovascular diabetic complications, and PCR were associated with a renal leak. Although glucosuria was not directly measured, we can infer the presence of glucosuria when plasma glucose concentrations exceed the renal threshold of ∼180 mg/dL (44). Renal leak occurred at plasma glucose concentrations below as well as above the 180-mg/mL threshold concentrations, indicating that there is no discernable relation between renal leak and glucosuria (Supplemental Figure 4). Taken together, these findings support the hypothesis that increased prevalence of renal leak is associated with low plasma vitamin C concentrations observed in individuals with diabetes (3, 9, 45). Vitamin C renal leak has 2 interdependent components. First, vitamin C renal leak describes the presence of low plasma vitamin C concentrations below the MET values of 38.1 µM in men and 43.2 µM in women. Second, renal leak describes the incongruous excretion of vitamin C for such low plasma vitamin C concentrations. Together, low plasma vitamin C concentrations below the MET combined with abnormal urinary excretion of the vitamin indicate dysregulated vitamin C renal physiology in diabetes (1, 25).
An additional benefit of using renal leak to characterize urinary vitamin C loss in those with diabetes is that renal leak accounts for normal differences in plasma vitamin C concentrations based on physiologic variables such as sex and age. This is supported by our study findings that showed sex and age were not associated with renal leak (Figure 3E, Supplemental Figure 3). There are two reasons for this observation: 1) renal leak criteria are sex specific, based on MET values derived from depletion–repletion studies in women and men (17, 19), and 2) MET is defined as 2 SD below mean renal threshold values, sufficiently differentiating normal from abnormal, such that renal leak outcomes are not influenced by differences in age.
In diabetes, it is likely that there is a complex relation between vitamin C renal leak, low plasma vitamin C concentrations, and microvascular complications such as diabetic nephropathy. This complex relation may be further modulated by glycemic control, insulin resistance, and metabolic syndrome. Our study showed that elevated fasting glucose, hemoglobin A1c, PCR, BMI, and history of micro/macrovascular complications were all predictive of a renal leak. Chronic hyperglycemia, insulin resistance, and obesity are associated with increased oxidative stress (4, 46–50). Increased oxidative stress contributes to microvascular complications such as diabetic nephropathy (4). For a disease characterized by increased oxidative stress, the presence of a vitamin C renal leak could exacerbate the already diminished antioxidant capacity with implications for disease pathogenesis (51). This disease-induced increase in nutritional requirement represents a form of relative nutritional deficiency. Recent studies have demonstrated improved albuminuria (52, 53), and glycemic parameters (10–13) following oral vitamin C supplementation. However, larger studies that account for renal leak status might identify individuals or cohorts who could benefit from early and regular vitamin C supplementation.
Several molecular mechanisms may underlie the significant association we observed between ascorbate renal leak and micro/macrovascular disease (Figure 3E). Renal leak in diabetes indicates dysfunctional ascorbate tubular reabsorption. One broad potential mechanism is that diabetes, perhaps via oxidative injury (54), constrains the vitamin C transporters (23) and/or the cells that express these transporters. Another potential mechanism is that diabetes induces local hypoxia in renal tubules, in an intraorgan location especially sensitive to hypoxia (55). Local hypoxia could be driven by impaired red blood cell deformability from diabetes itself (56), compounded by low red blood cell ascorbate concentrations that can alter red blood cell physiology (57, 58). Renal leak may be an early manifestation of oxidative injury and/or microvascular hypoxia and may be a harbinger of downstream micro/macrovascular disease, analogous to proteinuria (59, 60).
Given the cross-sectional study design, the renal leak measurements that we describe here are single snapshots in time. Currently, we do not know how changes in disease status would modulate the onset or progression of vitamin C renal leak and whether the dynamics vary in different patient populations. Longitudinal studies will be necessary to evaluate the diagnostic value and temporal relation between vitamin C renal leak status and features of diabetic nephropathy such as proteinuria or reduced glomerular filtration rate. Similarly, longitudinal studies are needed to unravel mechanisms of renal leak in relation to metabolic syndrome often associated with diabetes, including obesity and hypertension.
Imperatives to obtaining precise renal threshold estimates were use of a study design that incorporated a broad range of plasma vitamin C concentrations for each male and female participant, coupled with a highly sensitive and specific assay measurements. Previous characterizations of vitamin C renal threshold in humans have been limited by insensitive, nonspecific, or poorly described vitamin C measurement techniques further compromised by artifact in human plasma samples, as well as absence of a broad range of vitamin C plasma concentrations in each individual studied (24–29, 61). By addressing these limitations, our study provides a pathway for more translational application of vitamin C renal physiology to disease conditions such as diabetes, in which antioxidants actions may play a role.
Although depletion–repletion design remains the most physiologic way of dynamically estimating the normal renal threshold values of a micronutrient, the complexity and inpatient time commitment necessitated inclusion of a restricted (younger) age bracket. This potentially limits generalizability of renal threshold estimates to older individuals. However, this limitation is not applicable to renal leak outcomes given as the renal leak criteria accounted for potential physiologic variations in vitamin C physiology by use of 2 SD below the mean renal threshold to determine the MET (32). Thus, individuals who met the criteria for renal leak were much more likely to have dysregulated vitamin C physiology regardless of age or sex. This is evidenced by our findings that show age and sex were not predictive of renal leak (Figure 3E).
Renal leak can be tested by a block–time urine collection coupled to a plasma sample, as described for many measurements here. Block–time measures, over 30 or 60 min, for example, are convenient and feasible in outpatient settings, especially when an overnight fast precedes measurement to avoid biased estimates from vitamin C ingestion within the prior 8–12 h. Urine collections for 24 h may be less preferable for several reasons, not just inconvenience. Because they are usually performed at home, there is potential for incomplete collection due to error or inadvertent oxidation due to improper urine handling. There may be confounding data in participants who have plasma concentrations slightly below the threshold but who consume vitamin C–enriched juices or foods during the 24 h of sample collection. Multiple plasma measurements could be helpful but are not feasible in an outpatient setting.
Renal leak status also has novel importance for vitamin C dietary recommendations and guidelines. Currently, there are no guidelines for increased intake in chronic diseases. If renal leak occurs, increasing daily vitamin C intake could increase plasma values to those found in healthy people who ingest the RDA of vitamin C (18, 19, 32). Based on evidence provided here, it is possible that vitamin C renal leak may lead to vitamin C losses that should be replaced by higher recommended intake in people with diabetes. Studies to explore these possibilities are feasible and have clear implications for prevention of complications of diabetes.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the volunteers who participated in these studies; the Intramural Research Programs, NIDDK and National Heart, Lung, and Blood Institute, NIH; and the metabolic unit staff, NIDDK clinical core staff, and clinical center nutrition staff, NIH. Funders had no input into study outcomes.
The authors’ responsibilities were as follows—P-CV, IE, SP, and ML: conceptualization; P-CV, IE, SP, Yaohui Wang, Yu Wang, and ML: methodology; IE, P-CV, HT, Yaohui Wang, Yu Wang, SS, MN, PA, SP, and ML: investigation; ML: resources; P-CV, IE, HS, and ML: data curation; IE, P-CV, and ML: writing—original draft; IE, P-CV, KW, and ML: writing—review and editing; ML: funding acquisition; ML: supervision; P-CV, IE, HS, KW, and ML: formal analysis. The authors report no conflicts of interest.
Notes
Supported by Intramural Research Program, NIDDK, NIH: Z01-DK053211-15 (to ML).
Supplemental Methods and Supplemental Figures 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CLIA, Clinical Laboratory Improvement Amendments; eGFR, estimated glomerular filtration rate; MET, minimum elimination threshold; NIDDK, National Institutes of Diabetes and Digestive and Kidney Diseases; PCF, population curve fitting; PCR, protein/creatinine ratio; PKPB, physiology-based pharmacokinetics modeling.
Contributor Information
Ifechukwude Ebenuwa, Molecular and Clinical Nutrition Section, Digestive Diseases Branch Intramural Research Program, Bethesda, MD, USA.
Pierre-Christian Violet, Molecular and Clinical Nutrition Section, Digestive Diseases Branch Intramural Research Program, Bethesda, MD, USA.
Sebastian Padayatty, Molecular and Clinical Nutrition Section, Digestive Diseases Branch Intramural Research Program, Bethesda, MD, USA.
Yaohui Wang, Molecular and Clinical Nutrition Section, Digestive Diseases Branch Intramural Research Program, Bethesda, MD, USA.
Yu Wang, Molecular and Clinical Nutrition Section, Digestive Diseases Branch Intramural Research Program, Bethesda, MD, USA.
Henry Sun, Suntech Research Institutes, Rockville, MD, USA.
Preston Adhikari, Molecular and Clinical Nutrition Section, Digestive Diseases Branch Intramural Research Program, Bethesda, MD, USA.
Sheila Smith, Molecular and Clinical Nutrition Section, Digestive Diseases Branch Intramural Research Program, Bethesda, MD, USA.
Hongbin Tu, Molecular and Clinical Nutrition Section, Digestive Diseases Branch Intramural Research Program, Bethesda, MD, USA.
Mahtab Niyyati, Molecular and Clinical Nutrition Section, Digestive Diseases Branch Intramural Research Program, Bethesda, MD, USA.
Kenneth Wilkins, Biostatistics Program, Office of Clinical Research Support, Office of the Director, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA.
Mark Levine, Molecular and Clinical Nutrition Section, Digestive Diseases Branch Intramural Research Program, Bethesda, MD, USA.
Data Availability
Data described in the manuscript will be made available upon request pending application and approval.
References
- 1. Padayatty SJ, Levine M. Vitamin C: the known and the unknown and Goldilocks. Oral Dis. 2016;22(6):463–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281(15):1415–23. [DOI] [PubMed] [Google Scholar]
- 3. Maxwell SR, Thomason H, Sandler D, Leguen C, Baxter MA, Thorpe GH, Jones AF, Barnett AH. Antioxidant status in patients with uncomplicated insulin- dependent and non-insulin-dependent diabetes mellitus. Eur J Clin Invest. 1997;27(6):484–90. [DOI] [PubMed] [Google Scholar]
- 4. Sundaram RK, Bhaskar A, Vijayalingam S, Viswanathan M, Mohan R, Shanmugasundaram KR. Antioxidant status and lipid peroxidation in type II diabetes mellitus with and without complications. Clin Sci (Colch). 1996;90(4):255–60. [DOI] [PubMed] [Google Scholar]
- 5. Hirsch IB, Atchley DH, Tsai E, Labbe RF, Chait A. Ascorbic acid clearance in diabetic nephropathy. J Diabetes Complications. 1998;12(5):259–63. [DOI] [PubMed] [Google Scholar]
- 6. Seghieri G, Martinoli L, Miceli M, Ciuti M, D'Alessandri G, Gironi A, Palmieri L, Anichini R, Bartolomei G, Franconi F. Renal excretion of ascorbic acid in insulin dependent diabetes mellitus. Int J Vitam Nutr Res. 1994;64(2):119–24. [PubMed] [Google Scholar]
- 7. Will JC, Byers T. Does diabetes mellitus increase the requirement for vitamin C?. Nutr Rev. 1996;54(7):193–202. [DOI] [PubMed] [Google Scholar]
- 8. Chen H, Karne RJ, Hall G, Campia U, Panza JA, Cannon RO III, Wang Y, Katz A, Levine M, Quon MJ. High-dose oral vitamin C partially replenishes vitamin C levels in patients with type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am J Physiol Heart Circ Physiol. 2006;290(1):H137–455. [DOI] [PubMed] [Google Scholar]
- 9. Sargeant LA, Wareham NJ, Bingham S, Day NE, Luben RN, Oakes S, Welch A, Khaw KT. Vitamin C and hyperglycemia in the European Prospective Investigation into Cancer—Norfolk (EPIC-Norfolk) study: a population-based study. Diabetes Care. 2000;23(6):726–32. [DOI] [PubMed] [Google Scholar]
- 10. Mason SA, Keske MA, Wadley GD. Effects of vitamin C supplementation on glycemic control and cardiovascular risk factors in people with type 2 diabetes: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2021;44(2):618–30. [DOI] [PubMed] [Google Scholar]
- 11. Ashor AW, Siervo M, van der Velde F, Willis ND, Mathers JC. Systematic review and meta-analysis of randomised controlled trials testing the effects of vitamin C supplementation on blood lipids. Clin Nutr. 2016;35(3):626–37. [DOI] [PubMed] [Google Scholar]
- 12. Tareke AA, Hadgu AA. The effect of vitamin C supplementation on lipid profile of type 2 diabetic patients: a systematic review and meta-analysis of clinical trials. Diabetol Metab Syndr. 2021;13(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El-Aal AA, El-Ghffar EAA, Ghali AA, Zughbur MR, Sirdah MM. The effect of vitamin C and/or E supplementations on type 2 diabetic adult males under metformin treatment: a single-blinded randomized controlled clinical trial. Diabetes Metab Syndr. 2018;12(4):483–9. [DOI] [PubMed] [Google Scholar]
- 14. Washko PW, Hartzell WO, Levine M. Ascorbic acid analysis using high-performance liquid chromatography with coulometric electrochemical detection. Anal Biochem. 1989;181(2):276–82. [DOI] [PubMed] [Google Scholar]
- 15. Washko PW, Welch RW, Dhariwal KR, Wang Y, Levine M. Ascorbic acid and dehydroascorbic acid analyses in biological samples. Anal Biochem. 1992;204(1):1–14. [DOI] [PubMed] [Google Scholar]
- 16. Dhariwal AP, Grosvenor CE, Antunes-Rodrigues J, McCann SM. Studies on the purification of ovine prolcatin-inhibiting factor. Endocrinology. 1968;82(6):1236–41. [DOI] [PubMed] [Google Scholar]
- 17. Levine M, Espey MG, Padayatty SJ. Vitamin C: a concentration function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci U S A. 2001;98(17):9842–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich J, King Jet al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a Recommended Dietary Allowance. Proc Natl Acad Sci U S A. 1996;93(8):3704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399(6731):70–5. [DOI] [PubMed] [Google Scholar]
- 21. Corpe CP, Tu H, Eck P, Wang J, Faulhaber-Walter R, Schnermann J, Margolis S, Padayatty S, Sun H, Wang Yet al. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J Clin Invest. 2010;120(4):1069–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids. 2008;34(3):347–55. [DOI] [PubMed] [Google Scholar]
- 23. Eck P, Kwon O, Chen S, Mian O, Levine M. The human sodium-dependent ascorbic acid transporters SLC23A1 and SLC23A2 do not mediate ascorbic acid release in the proximal renal epithelial cell. Physiol Rep. 2013;1(6):e00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ralli EP, Friedman GJ, Rubin SH. The mechanism of the excretion of vitamin C by the human kidney. J Clin Invest. 1938;17:765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedman AH, Charles NC. Retinal oxalosis in two diabetic patients. Am J Ophthalmol. 1974;78(2):189–95. [DOI] [PubMed] [Google Scholar]
- 26. Lewis JS, Storvick CA, Hauck HM, Patterson I, Higano S, Hawthorne B. Renal threshold for ascorbic acid in twelve normal adults: with a note on the state of tissue reserves of subjects on an intake of ascorbic acid approximating the suggested daily allowance: one figure. J Nutr. 1943;25(2):185–96. [Google Scholar]
- 27. Kallner A, Hartmann D, Hornig D. Steady-state turnover and body pool of ascorbic acid in man. Am J Clin Nutr. 1979;32(3):530–9. [DOI] [PubMed] [Google Scholar]
- 28. Oreopoulos DG, Lindeman RD, VanderJagt DJ, Tzamaloukas AH, Bhagavan HN, Garry PJ. Renal excretion of ascorbic acid: effect of age and sex. J Am Coll Nutr. 1993;12(5):537–42. [DOI] [PubMed] [Google Scholar]
- 29. Klosterman AM, Haines JE, Hauck HM, Kline AB. The renal threshold for ascorbic acid; a modified method for its estimation with results for 12 adult subjects. J Nutr. 1947;33(5):505–9. [DOI] [PubMed] [Google Scholar]
- 30. Graumlich JF, Ludden TM, Conry-Cantilena C, Cantilena LR Jr, Wang Y, Levine M. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm Res. 1997;14(9):1133–9. [DOI] [PubMed] [Google Scholar]
- 31. King J, Wang Y, Welch RW, Dhariwal KR, Conry-Cantilena C, Levine M. Use of a new vitamin C–deficient diet in a depletion/repletion clinical trial. Am J Clin Nutr. 1997;65(5):1434–40. [DOI] [PubMed] [Google Scholar]
- 32. Institute of Medicine (US), Panel on Dietary Antioxidants and Related Compounds . Dietary Reference Intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington (DC): National Academy Press; 2000: 1–20., 383–400. [PubMed] [Google Scholar]
- 33. Dhariwal KR, Hartzell WO, Levine M. Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am J Clin Nutr. 1991;54(4):712–6. [DOI] [PubMed] [Google Scholar]
- 34. Li H, Tu H, Wang Y, Levine M. Vitamin C in mouse and human red blood cells: an HPLC assay. Anal Biochem. 2012;426(2):109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140(7):533–7. [DOI] [PubMed] [Google Scholar]
- 36. Dhariwal KR, Washko P, Hartzell WO, Levine M. Ascorbic acid within chromaffin granules: in situ kinetics of norepinephrine biosynthesis. J Biol Chem. 1989;264(26):15404–9. [PubMed] [Google Scholar]
- 37. Macdiarmid J, Blundell J. Assessing dietary intake: who, what and why of under-reporting. Nutr Res Rev. 1998;11(2):231–53. [DOI] [PubMed] [Google Scholar]
- 38. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York (NY): Routledge; 1988. [Google Scholar]
- 39. Hall GH, Round AP. Bayes theorem for the clinician. West Engl Med J. 1990;105(4):106–9. [PMC free article] [PubMed] [Google Scholar]
- 40. Marschner IC, Gillett AC. Relative risk regression: reliable and flexible methods for log-binomial models. Biostatistics. 2012;13(1):179–92. [DOI] [PubMed] [Google Scholar]
- 41. Donoghoe MW, Marschner IC. logbin: an R package for relative risk regression using the log-binomial model. J Stat Softw. 2018;86(9):1–22. [Google Scholar]
- 42. Cummings P. The relative merits of risk ratios and odds ratios. Arch Pediatr Adolesc Med. 2009;163(5):438–45. [DOI] [PubMed] [Google Scholar]
- 43. Hauck WW, Neuhaus JM, Kalbfleisch JD, Anderson S. A consequence of omitted covariates when estimating odds ratios. J Clin Epidemiol. 1991;44(1):77–81. [DOI] [PubMed] [Google Scholar]
- 44. Marsenic O. Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis. 2009;53(5):875–83. [DOI] [PubMed] [Google Scholar]
- 45. Will JC, Ford ES, Bowman BA. Serum vitamin C concentrations and diabetes: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 1999;70(1):49–52. [DOI] [PubMed] [Google Scholar]
- 46. Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48(1):1–9. [DOI] [PubMed] [Google Scholar]
- 47. Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7(5):e330–41. [DOI] [PubMed] [Google Scholar]
- 48. Keaney JF Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJet al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23(3):434–9. [DOI] [PubMed] [Google Scholar]
- 49. Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19(3):257–67. [DOI] [PubMed] [Google Scholar]
- 50. Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci. 2000;97(22):12222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–20. [DOI] [PubMed] [Google Scholar]
- 52. Bolignano D, Cernaro V, Gembillo G, Baggetta R, Buemi M, D'Arrigo G. Antioxidant agents for delaying diabetic kidney disease progression: a systematic review and meta-analysis. PLoS One. 2017;12(6):e0178699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Farvid MS, Jalali M, Siassi F, Hosseini M. Comparison of the effects of vitamins and/or mineral supplementation on glomerular and tubular dysfunction in type 2 diabetes. Diabetes Care. 2005;28(10):2458–64. [DOI] [PubMed] [Google Scholar]
- 54. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kribben A, Wieder ED, Wetzels JF, Yu L, Gengaro PE, Burke TJ, Schrier RW. Evidence for role of cytosolic free calcium in hypoxia-induced proximal tubule injury. J Clin Invest. 1994;93(5):1922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shin S, Ku YH, Ho JX, Kim YK, Suh JS, Singh M. Progressive impairment of erythrocyte deformability as indicator of microangiopathy in type 2 diabetes mellitus. Clin Hemorheol Microcirc. 2007;36(3):253–61. [PubMed] [Google Scholar]
- 57. Tu H, Li H, Wang Y, Niyyati M, Wang Y, Leshin J, Levine M. Low red blood cell vitamin C concentrations induce red blood cell fragility: a link to diabetes via glucose, glucose transporters, and dehydroascorbic acid. EBioMedicine. 2015;2(11):1735–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tu H, Wang Y, Li H, Brinster LR, Levine M. Chemical transport knockout for oxidized vitamin C, dehydroascorbic acid, reveals its functions in vivo. EBioMedicine. 2017;23:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Borch-Johnsen K, Kreiner S. Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. BMJ. 1987;294(6588):1651–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yudkin JS, Forrest RD, Jackson CA. Microalbuminuria as predictor of vascular disease in non-diabetic subjects. Islington Diabetes Survey. Lancet North Am Ed. 1988;332(8610):530–3. [DOI] [PubMed] [Google Scholar]
- 61. Hodges RE. What's new about scurvy?. Am J Clin Nutr. 1971;24(4):383–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript will be made available upon request pending application and approval.