Abstract
Objectives
Racial and socioeconomic disparities in the incidence of Legionnaires’ disease have been documented for the past 2 decades; however, the social determinants of health (SDH) that contribute to these disparities are not well studied. The objective of this narrative review was to characterize SDH to inform efforts to reduce disparities in the incidence of Legionnaires’ disease.
Methods
We conducted a narrative review of articles published from January 1979 through October 2019 that focused on disparities in the incidence of Legionnaires’ disease and pneumonia (inclusive of bacterial pneumonia and/or community-acquired pneumonia) among adults and children (excluding articles that were limited to people aged <18 years). We identified 220 articles, of which 19 met our criteria: original research, published in English, and examined Legionnaires’ disease or pneumonia, health disparities, and SDH. We organized findings using the Healthy People 2030 SDH domains: economic stability, education access and quality, social and community context, health care access and quality, and neighborhood and built environment.
Results
Of the 19 articles reviewed, multiple articles examined disparities in incidence of Legionnaires’ disease and pneumonia related to economic stability/income (n = 13) and comorbidities (n = 10), and fewer articles incorporated SDH variables related to education (n = 3), social support (none), health care access (n = 1), and neighborhood and built environment (n = 6) in their analyses.
Conclusions
Neighborhood and built-environment factors such as housing, drinking water infrastructure, and pollutant exposures represent critical partnership and research opportunities. More research that incorporates SDH and multilevel, cross-sector interventions is needed to address disparities in Legionnaires’ disease incidence.
Keywords: Legionnaires’ disease, pneumonia, health disparities, social determinants of health, environmental health
Legionnaires’ disease is a severe respiratory illness resulting from breathing water droplets or aspirating water containing Legionella bacteria. Legionnaires’ disease is a public health challenge, with an estimated annual hospitalization cost of $433 million in the United States. 1 In 2018, 9933 Legionnaires’ disease cases were reported to the Centers for Disease Control and Prevention (CDC) National Notifiable Diseases Surveillance System (NNDSS), representing a nearly 900% increase in Legionnaires’ disease incidence in the United States since 2000. 2 However, Legionnaires’ disease is substantially underdiagnosed; the true number of cases has been estimated to range from 52 000 to 70 000 annually in the United States. 2,3 People at highest risk for Legionnaires’ disease include older adults, males, and people who smoke or who have immunocompromising conditions. National and state surveillance systems have found racial disparities in Legionnaires’ disease incidence. 4 -6
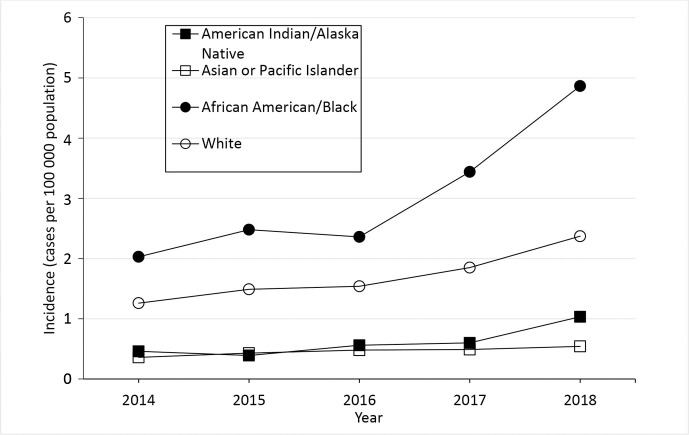
NNDSS data from 2014-2018 demonstrate racial disparities; in 2018, Legionnaires’ disease incidence was almost twice as high among Black people compared with White people (Figure 1). 5 Residents who were non-Hispanic Black and lived in high-poverty census tracts had the highest rates of Legionnaires’ disease in New York City. 6,7 Incidence of and hospitalization with other types of pneumonia among Black people and among people living in the most impoverished census tracts are also disproportionately high. 8 -12 Risk factors for Legionnaires’ disease are often described from an epidemiologic perspective; however, the reasons for racial and socioeconomic disparities in the incidence of Legionnaires’ disease and targeted interventions to address these gaps have not been as widely explored. 13,14 A literature-based analysis grounded in the social determinants of health (SDH) is needed to identify root causes of Legionnaires’ disease inequities and to inform future studies and comprehensive interventions.
Figure 1.
Crude incidence of reported confirmed cases of Legionnaires’ disease, by year and race, United States, District of Columbia, and New York City, 2014-2018. The crude incidence of cases per 100 000 population is the number of confirmed Legionnaires’ disease cases reported that year divided by the postcensal population estimate for that year x 100 000. Cases of disease due to Legionella are reported to the National Notifiable Diseases Surveillance System (NNDSS) as legionellosis, which includes Legionnaires’ disease, Pontiac fever, and disease due to Legionella infection at extrapulmonary sites, but is referred to as Legionnaires’ disease in this figure (because almost all legionellosis cases reported in the United States are due to Legionnaires’ disease). The year is when the case was reported to the Centers for Disease Control and Prevention. Data sources: 2014-2018 data are from NNDSS. 2
Social determinants of health are the conditions in which people are born, grow, live, work, worship, and age that can affect health outcomes and quality of life. 15,16 Healthy People 2030 organizes SDH into 5 domains: economic stability, education access and quality, social and community context, health care access and quality, and neighborhood and built environment.
A sentinel goal of the Healthy People program is “to achieve health equity, eliminate disparities, and improve the health of all groups.” 17 The objective of this topical review was to narratively characterize factors that may contribute to disparities in incidence of Legionnaires’ disease to inform efforts and research needed to prevent Legionnaires’ disease cases among populations disproportionately affected by Legionnaires’ disease.
Methods
We conducted a review of published literature from the PubMed and Scopus databases of journal articles related to health disparities associated with Legionnaires’ disease, bacterial pneumonia, and/or community-acquired pneumonia. Although Legionnaires’ disease differs from pneumonia of other etiologies by route of transmission, many underlying risk factors (eg, older age, chronic lung disease, smoking) for illness among adults are similar. 6 We expanded our review to include articles describing health disparities associated with other types of community-acquired or bacterial pneumonia to increase the breadth of studies available for analysis. Our search included articles published from January 1979 through October 2019. The Medical Subject Headings (MeSH) search terms included “Legionnaires’ disease,” “legionellosis” (which includes less common presentations of illness caused by Legionella bacteria, such as Pontiac fever and extrapulmonary infection), “racial and ethnic minority health,” “health care disparities,” “health disparities,” and “social determinants of health.” We used Endnote X9 software (Clarivate Analytics) to compile the final article records, and we identified and removed 4 duplicate articles.
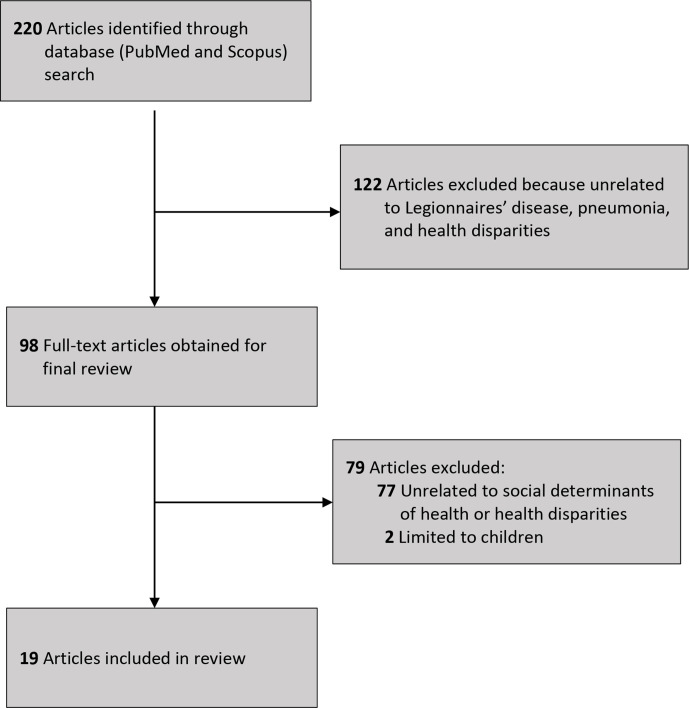
We excluded articles upon review of the title, abstract, and full-text article based on the following criteria: non-original research; non-English publication; and research that did not examine Legionnaires’ disease or pneumonia (eg, bacterial pneumonia), health disparities, and SDH. The initial search resulted in 220 articles. After applying exclusion criteria to the abstracts, we reviewed 98 full-text articles. Of those 98 articles, we excluded 79 articles because they were unrelated to SDH or health disparities and 2 articles because the study population was limited to only people aged <18 years. Nineteen articles met the inclusion criteria and were included in this review (Figure 2, Table). 6 -11,18 -30 The most common reasons for exclusion of full-text articles were that the articles did not explicitly examine disparities in incidence of Legionnaires’ disease or pneumonia or did not characterize aspects of SDH.
Figure 2.
Flow diagram of selection of articles on racial disparities in Legionnaires’ disease incidence and social determinants of health published from January 1979 through October 2019.
Table.
Articles on health disparities in Legionnaires’ disease, by study period (N = 19)
| Study design | Geographic area | Study population/sample size | Outcome measures | Primary results | Primary SDH examined |
|---|---|---|---|---|---|
| Burton et al, 8 2003-2004 | |||||
| Active, population-based surveillance for invasive bacterial disease (ABCs) | Connecticut (entire state), Minnesota (entire state), California (1 county), Colorado (5 counties), Georgia (20 counties), Maryland (6 counties), New York (15 counties), Oregon (3 counties), and Tennessee (11 counties) | 4870 cases of invasive community-acquired bacterial pneumonia among adults aged ≥18 | Annual incidence rate of invasive bacterial pneumonia in each census tract | The RR comparing incidence between Black and White adults was 2.40 (95% CI, 2.24-2.57). The overall incidence rate among Hispanic adults was 2.40 (95% CI, 2.24-2.57). Incidence among Black residents of census tracts with ≥20% of people living in poverty (most impoverished) was 4.4 times the incidence among White residents of census tracts with <5% of people living in poverty (least impoverished). | Economic stability (population employed in working-class occupations, median annual household income, percentage of low-income housing, percentage of high-income housing) |
| Bush et al, 7 2007-2017 | |||||
| Routine surveillance | New York, New York | Cases of Legionnaires’ disease/sociodemographic analysis on 1011 cases from 2015-2017 | Annual incidence rate of Legionnaires’ disease | Legionnaires’ disease incidence (average annual age-adjusted rate per 100 000 residents) was highest among Black residents (6.0) compared with Latino (2.9), White (2.7), and Asian (1.8) residents. Legionnaires’ disease incidence was highest in neighborhoods with the highest poverty levels (5.7) compared with neighborhoods with the lowest poverty levels (3.0). 11% of Legionnaires’ disease patients reported a health care exposure in the 10 days before they developed symptoms. |
|
| Chapman et al, 18 2006-2011 | |||||
| Routine surveillance | Northeast England | 1351 cases of IPD stratified by age (<16, 16-29, 30-44, 45-64, ≥65) | IPD incidence rate | IPD incidence increased linearly with increasing deprivation from 7.0 to 13.6 per 100 000 population. Incidence of IPD was positively correlated with increasing deprivation quintile for income deprivation, employment deprivation, health deprivation and disability, education, skills and training deprivation, crime, and living environment domains. |
|
| Corrado et al, 19 2010-2014 | |||||
| Retrospective analysis | New York, New York | New York City residents aged ≥18; 4 614 108 hospitalizations of adult residents, 283 927 pneumonia-associated | Pneumonia-associated hospitalizations | Neighborhoods with >30% of residents living below the FPL had significantly higher mean annual age-adjusted rates of pneumonia-associated hospitalizations than neighborhoods with <10% of residents living below the FPL (RR = 2.5; 95% CI, 2.5-2.6). |
|
| Farnham et al, 6 2002-2011 | |||||
| Routine surveillance | New York, New York | 1449 legionellosis cases | Incidence of legionellosis | Average incidence per 100 000 population per year for non-Hispanic Black people (2.15 cases) was higher than that for non-Hispanic White people (1.56 cases; P = .13) and significantly higher than that for Hispanic (1.02 cases: P = .003) or Asian/Pacific Islander (0.41 cases; P < .001) people; incidence in the highest poverty areas (3.0 average yearly cases per 100 000 population) was 2.5 times higher than that for the lowest poverty areas (1.2 average yearly cases per 100 000 population); 1278 (88.2%) patients had >1 underlying medical condition that was a known risk factor for legionellosis; current or past smoking (n = 879; 60.7%) and diabetes (n = 506; 34.9%) were the most frequently reported underlying conditions. |
|
| Feemster et al, 10 2005-2008 | |||||
| Population-based surveillance network for pneumococcal disease | 5-county Philadelphia metropolitan region | All hospitalized adult and pediatric patients with pneumococcal bacteremia (stratified by age: <18, 18-39, 40-69, ≥70) | Incidence of pneumococcal bacteremia | Increased disease incidence was associated with higher population density (incidence RR [IRR] = 1.10 [95% CI, 1.00-1.19] per 10 000 people per mile), higher percentage Black population (per 10% increase) (IRR = 1.07; 95% CI, 1.04-1.09), population aged ≤5 years (IRR = 3.49; 95% CI, 1.80-5.18), and population aged ≥65 (IRR = 1.19; 95% CI, 1.00-1.38). | Economic stability (average income, percentage below FPL, average household size) |
| Gleason et al, 20 2003-2013 | |||||
| Geospatial analysis of legionellosis cases | New Jersey | 1634 cases of Legionnaires’ disease | Legionnaires’ disease incidence, cluster incidence | Census tracts with the highest percentages of older age (OR = 0.38; 95% CI, 0.22-0.67); Hispanic population (OR = 0.10; 95% CI 0.05-0.19); poverty (OR = 7.21; 95% CI, 4.04-12.86); and housing units built pre-1950 (OR = 5.69; 95% CI, 2.82-11.50) were positively and significantly associated with legionellosis cluster areas. |
|
| Hayes et al, 9 2001-2014 | |||||
| Retrospective analysis | Nationally representative dataset | 20 361 181 pneumonia-associated hospitalizations | Estimated numbers and rates of pneumonia-associated hospitalizations | Non-Hispanic American Indian/Alaska Native and non-Hispanic Black people had the highest average annual age-adjusted rates of pneumonia-associated hospitalization of all racial/ethnic groups at 439.2 (95% CI, 415.9-462.5) and 438.6 (95% CI, 432.5-444.7) per 100 000 population. The mean age at in-hospital death was lowest in the lowest income quartile (73.1 y [95% CI, 72.9-73.2]) and highest in the highest income quartile (76.4 y [95% CI, 76.2-76.6]). In-hospital death occurred in 10.9% (SE = 0.1%) of pneumonia-associated hospitalizations colisting an immunocompromising condition compared with 6.3% (SE = 0.03%) among those not colisting an immunocompromising condition (P < .001). |
|
| Jeon et al, 21 2006-2008 | |||||
| Retrospective analysis | Referral hospital in upper Manhattan, New York | 52 006 patients | Infections (bloodstream, urinary tract, and pneumonia) present on admission and health care–associated infections | Odds ratio for hospitalization related to community-acquired pneumonia was high for people with low median household income (OR = 1.90; 95% CI, 1.12-3.20), diabetes (OR = 1.09; 95% CI, 0.82-1.44), and Medicaid (OR = 1.90; 95% CI, 1.35-2.67). |
|
| Kempker et al, 22 1999-2005 | |||||
| Longitudinal analysis | Nationally representative dataset | 1523 septicemia deaths | Septicemia, influenza, and pneumonia death rates | Higher HRs related to influenza/pneumonia deaths observed for high poverty (HR = 3.0; 95% CI, 2.1-4.3), diabetes (HR = 1.46; 95% CI, 1.28-1.67), and reported receiving health care >10 times in the past year (HR = 1.87; 95% CI, 1.68-2.08). |
|
| Kim et al, 23 2005 | |||||
| Geospatial analysis | South Korea | 402 979 hospitalizations due to bacterial pneumonia (ages 0 to >65) | Bacterial pneumonia hospitalization rate | Significant β coefficient related to deprivation index (1.993; P < .001). | Economic stability (deprivation index—car ownership, engaged in manual labor) |
| Marzouk et al, 24 2008-2012 | |||||
| Descriptive, cross-sectional | Tunisia | 14 Confirmed Legionnaires’ disease cases | Legionnaires’ disease incidence | 4 of 14 (29%) confirmed cases reported high humidity in their homes. | Neighborhood and built environment (home humidity, living in community) |
| McLaughlin et al, 25 2014-2017 | |||||
| Cross-sectional analysis of medical and prescription claims | United States | 22 223 586 adults aged ≥65 who received PCV13 | PCV13 uptake among adults aged ≥65 | Lower uptake of PCV13 was observed for non-Hispanic Black (36.3%) and Hispanic (30.0%) adults (vs 45.6% for non-Hispanic White adults, P < .01), the poor (30.7% vs 54.2% among lowest vs highest income deciles, P < .01), adults with low educational status (33.0% vs 49.0% among adults with <high school education vs college educated, P < .01). |
|
| Muhammad et al, 26 1998-2009 | |||||
| Active, population-based surveillance of IPD | Nationally representative dataset | 35 925 cases of IPD among adults | IPD prevalence | Diabetes was the third most prevalent comorbidity for IPD among adults aged 18-49 (23%) and ≥65 (19.5%); most common comorbidity for adults aged 50-64 (35%). | Health care access and quality (comorbidities) |
| Nowalk et al, 27 2014 | |||||
| Descriptive, cross-sectional | Nationally representative dataset | 2 193 296 patients hospitalized with pneumococcal disease | Pneumococcal disease hospitalizations | The likelihood of non-immunocompromising pneumococcal high-risk conditions was 12% higher among Black people than among non-Black people. | Health care access and quality (comorbidities) |
| Oggioni et al, 28 2013 | |||||
| Case study | Malta | 1 patient with Legionella coinfection and herpesvirus 3 | Legionella pneumophila and human herpesvirus 3 coinfection | Legionella samples from the patient’s workplace toilet matched clinical samples, demonstrating an epidemiological link between clinical and environmental specimens. | Neighborhood and built environment (environmental samples from home and workplace) |
| Soto et al, 11 1998-1999, 2007-2008 | |||||
| Descriptive, cross-sectional | Connecticut | 5023 cases of IPD | IPD incidence rate and RR of IPD | IPD rates among people living in the highest-poverty census tracts were 2.2 times higher among people living in the poorest census tracts than among people living in the lowest-poverty tracts in 2007-2008; 2.4 times higher in 1998-1999. | Economic stability (census tract high poverty vs low poverty) |
| Storch et al, 29 1976-1979 | |||||
| Case-control study | Nationally representative dataset | 169 cases of Legionnaires’ disease | RR of Legionnaires’ disease | People with Legionnaires’ disease were significantly more likely to have lived near construction sites (RR = 4.4; 95% CI, 1.2-15.7) and have underlying disease (RR = 2.0; 95% CI, 0.9-4.7) than controls. Seven cases were construction workers, and no construction workers were among the controls. |
|
| Wortham et al, 30 1998-2009 | |||||
| Surveillance | Nationally representative dataset | 47 449 cases of IPD (stratified by children aged ≤5 y; children and adults aged >5) in the ABCs/Emerging Infections Program network | IPD rates | IPD rates were 12.6 (95% CI, 12.2-13.1) among White people aged ≥5 years compared with 19.4 (95% CI, 18.0-20.8) among Black people. | Health care (hospitalization status, presence of underlying conditions); these variables were used to predict race where race variable was missing |
Abbreviations: ABCs, Active Bacterial Core surveillance; HR, hazards ratio; ICD-9, International Classification of Diseases, Ninth Revision; IPD, invasive pneumococcal disease; OR, odds ratio; PCV, pneumococcal conjugate vaccine; RR, rate ratio; SDH, social determinant of health.
Based on the results of eligible articles, we aimed to categorize the results into the 5 SDH domains as they related to disparities in incidence of Legionnaires’ disease or pneumonia: economic stability (income and working conditions), education access and quality (education level), social and community context (social capital, stress, discrimination), health care access and quality (comorbidities and health care access), and neighborhood and built environment (housing characteristics, drinking water).
Results
Economic Stability
Several multivariable models indicated that areas with the highest poverty levels have the highest incidence of Legionnaires’ disease. Among community-acquired cases in New York City during 2002-2011, Legionnaires’ disease incidence in the most impoverished areas was 2.5 times higher than in the least impoverished areas. 6 Surveillance data from 2015-2017 in New York City indicated that Legionnaires’ disease incidence in the highest-poverty census tracts was almost double the Legionnaires’ disease incidence in lower-poverty census tracts. 7 This trend was also reflected in the 2015 South Bronx Legionnaires’ disease outbreak, where most Legionnaires’ disease cases were found in impoverished areas. Percentage of the population with income below the federal poverty level was also strongly associated with a census tract being designated as a high-risk legionellosis cluster area in New Jersey (OR = 7.21; 95% CI, 4.04-12.86). 20
Publications on pneumonia also identified population income as being associated with disease incidence. Impoverished areas had the highest incidence of bacteremic pneumonia, invasive pneumococcal disease, and pneumonia deaths. 8,10,11,18,22 Incidence of pneumonia-associated hospitalizations was higher among people living in areas with lower household incomes than among people living in areas with higher household incomes. 9,19,23 In addition, lower accessibility and use of pneumococcal vaccines were associated with people experiencing poverty and racial/ethnic minority communities. 23
An examination of the role of occupation in 1279 cases of community-acquired legionellosis in New York City found most cases were among people employed in hazardous or service industries (ie, transportation, repair, protective services, cleaning, or construction). 6 In 1979, a case-control study of 100 sporadic Legionnaires’ disease cases across the United States also identified a significant occupational difference between cases and controls; 7 Legionnaires’ disease cases were construction workers and no controls were construction workers. 29
Education Access and Quality
Lower education level was associated with a higher census-tract incidence of Legionnaires’ disease in New Jersey, increased incidence of pneumococcal disease in North England, and decreased uptake of pneumococcal vaccine among adults in the United States. 18,20,25
Social and Community Context
No studies measured social and community context factors such as stress, discrimination, or social capital in the context of Legionnaires’ disease.
Health Care Access and Quality
Several studies examined the presence of comorbid illnesses and incidence of Legionnaires’ disease. In our review, surveillance data showed that patients with diabetes had a higher incidence of Legionnaires’ disease and invasive pneumococcal disease than people without diabetes. 6,7,26 In addition, immune-compromising comorbidities were associated with community-acquired pneumonia and pneumonia-related hospitalizations. 9,21,26,27 Two studies that examined invasive pneumococcal disease acknowledged that comorbidities could be a factor to disparities in incidence but did not extensively explore comorbidities. 10,30
Although several articles acknowledged lack of health insurance and medical care as factors contributing to disparities in adverse respiratory-related outcomes, most articles did not explore health care access or type of health insurance in their analyses. One study found that Medicare comprised the largest percentage of primary payer for pneumonia-associated hospitalizations in New York City, followed by Medicaid. 19
Neighborhood and Built Environment
Although residential housing can be a source of exposure to Legionella bacteria, 31,32 few articles examined patient housing characteristics. Gleason et al 20 identified housing characteristics such as percentage of vacant homes, rented homes, and older homes as potential factors associated with legionellosis incidence. Among census tracts in New Jersey, a higher percentage of older (pre-1950) housing was independently associated with increased odds of being a high-risk legionellosis cluster area. 20 Neighborhood density and household crowding were briefly discussed as potential risk factors or mediators for certain types of bacterial pneumonia, but these factors were not directly examined in the articles included in our review. 10,33
Certain housing and facility conditions may create environments conducive to Legionella growth. For example, an examination of Legionella samples in a Legionnaires’ disease patient’s home, workplace, and hotel found that the workplace was the most likely source of exposure. 28 High humidity in the home was listed as a risk factor for Legionnaires’ disease in Tunisia. 24 In England, incidence of invasive pneumococcal disease was correlated with impoverished living conditions. 18 In addition to housing quality, building features and proximity to certain industries were identified as potential risk factors for Legionnaires’ disease. Farnham et al 6 found proximity to cooling towers to be an environmental risk factor for Legionnaires’ disease outbreaks and suggested that continued maintenance of the towers and water systems could be warranted in poor neighborhoods. One study determined that patients with Legionnaires’ disease were more likely than patients without Legionnaires’ disease to reside near construction sites. 29
Only 1 article in our review incorporated drinking water and water infrastructure metrics in its analysis. This study found that drinking water source did not have a significant relationship with legionellosis incidence, although housing age and infrastructure were positively associated risk factors. 20
Discussion
Racial and socioeconomic disparities in Legionnaires’ disease incidence in the United States have been identified in the past 20 years. 5 -7 In addition, the incidence of Legionnaires’ disease has increased since 2000. 2 Numerous factors likely contribute to the increasing incidence of Legionnaires’ disease, including growth in the population aged ≥65; more people with underlying conditions or immunosuppressing medications; proliferation of Legionella in water systems because of warm water temperatures, aging infrastructure, and water conservation measures; and heightened awareness of Legionnaires’ disease among clinicians and public health staff members leading to increased testing and surveillance capacity. Reasons for underdiagnosis of Legionnaires’ disease are likely multifactorial as well. Because Legionnaires’ disease is not clinically distinguishable from pneumonia because of other etiologies, clinicians must order specific testing to confirm the diagnosis. Furthermore, selection of first-line antibiotics to treat community-acquired pneumonia often includes coverage for Legionella. Differences in diagnosis of Legionnaires’ disease by race/ethnicity have not been examined. Studies of racial differences in receipt of guideline-concordant antibiotics have not demonstrated disparities between Black and White patients. 34,35 Through examination of Legionnaires’ disease- and pneumonia-related articles, we identified dimensions of SDH that could contribute to understanding disparities in incidence. Many of these SDH disproportionately affect Black people and may explain some racial disparities. For example, 1 study indicated that Black people have had a higher percentage of household poverty and experienced a lower prevalence of employment, education, and homeownership than non-Hispanic White people. 36
Income was the most common SDH identified in our review, and Black people had the lowest median annual household income compared with other racial groups. 37 Lower income was associated with a higher incidence of Legionnaires’ disease and increased incidence and hospitalization for other respiratory diseases. However, a limitation to this review was that data on Legionnaires’ disease and income were from mostly urban areas and may not reflect data from rural areas. Poverty is a risk factor for increased morbidity and mortality and is correlated with SDH such as lack of medical care access, educational attainment, and inadequate housing conditions that put people at risk for adverse respiratory outcomes. 38 This finding underscores the need for targeted intervention strategies that would increase economic mobility, improve medical access, and further establish the importance of reporting census-tract poverty levels and other socioeconomic indicators in future Legionnaires’ disease research. Our review found that some occupations are associated with increased Legionnaires’ disease risk. Certain occupations have an overrepresentation of racial/ethnic minority groups that experience occupational respiratory exposures and illnesses. Inclusion of job characteristics and working conditions in Legionnaires’ disease research can identify potential occupational and environmental hazards and elucidate how employment status and occupational categories affect Legionnaires’ disease health disparities. 39
Although only a few studies in our review incorporated data on education levels in their analyses, lower levels of education are associated with increased incidence in Legionnaires’ disease, pneumonia, and other respiratory conditions. 20,40 Higher educational attainment is linked to improved employment opportunities and working conditions, higher income, and increased social support. Some racial/ethnic minority populations may have lower levels of educational attainment because of factors such as residential segregation, disinvestment in housing, and underresourced schools. 41,42 Multilevel interventions at the individual, interpersonal, community, and societal levels are most effective to address these educational inequalities. 43
The absence of studies examining community and social contextual factors in our review highlights the need for research to examine the role of these factors in attenuating Legionnaires’ disease health disparities. Two studies, one of which is not included in our review, acknowledge that stress caused by poverty could enhance mechanisms that alter immune function and susceptibility to respiratory illnesses, and these studies did not include variables to measure stress, social support, or social cohesion. 8,44 Social support may protect patients who have chronic obstructive pulmonary disease 44 and other conditions; therefore, exploratory studies could consider social support in future analysis.
Comorbidities of Legionnaires’ disease and other pneumonia-related diseases are frequently described in the literature. 45 -48 Examining the relationship between comorbid illnesses and Legionnaires’ disease may clarify the effect of existing conditions on various racial/ethnic groups. Comorbidity studies showed that Black people have higher rates of influenza and pneumonia morbidity and mortality than White people. Black people are also more likely than White people to have underlying health conditions and experience complications from these infections. 49 To address disparities in the incidence of influenza and pneumococcal disease among Black people, physicians should be proactive in treating comorbid conditions, and more interventions to provide vaccination could be made available. 27,49 In addition, health care access, health care quality, and type of health insurance were not frequently explored in the articles included in our review.
The causes of disparities in health care access and use are multifactorial. Racial/ethnic minority groups have historically encountered barriers to health care access and use. 50 Several studies indicated that the largest contributors to disparities in health care access among racial/ethnic minority groups are socioeconomic disadvantage, lower levels of education, higher rates of occupational hazards, and a lack of health insurance. 51 Exploration of effective interventions to address these disparities in health care access can help to identify potential solutions. For example, community health workers and local community organizations may be able to address challenges associated with health care access and provide additional support in managing comorbidities and respiratory conditions. 52
Neighborhood and built environment factors that may affect Legionnaires’ disease health disparities include housing, drinking water infrastructure, and pollutant exposures. Housing and neighborhood variables include housing vacancies, property ownership status, housing age, housing quality, and proximity to sources of air pollutants. For example, non-White people and poor communities have disproportionate levels of pollution from particulate matter–emitting facilities, which can lead to increased air pollution exposure and exacerbate respiratory conditions. 53 -55
Future studies could examine variables related to poor building infrastructure, building code violations, and air quality. For example, redlining has contributed to residential segregation and resulted in concentrated poverty centers, low-quality housing, decreased access to economic and educational opportunities, and high land-surface temperatures. 56,57 As solutions to address health disparities in neighborhoods and the built environment are explored, the support of community health workers in partnership with environmental health practitioners should also be considered. Their unique ability to identify community needs and provide support through shared experiences, language, and culture has proved to be successful. Community health workers are instrumental in closing gaps in health disparities by assessing community health risks and advocating for housing improvements.
The contribution of household water exposures and community drinking water infrastructure to sporadic, community-acquired Legionnaires’ disease is understudied. Older homes may have impaired premise-plumbing systems (ie, water piping systems in a building that connect the building to the water main line) that can affect water stagnation and quality. A Legionnaires’ disease case investigation in 2018 identified a Wisconsin home built in the 1910s as the most probable source of infection. 31 In addition, older homes may be in communities with overall aging water infrastructure that could contribute to decreased water quality. 20 Previous studies have indicated that potable water sources in the home could be an exposure source, and interventions such as point-of-use filters for homes with immunocompromised people have been recommended. 32,58 Building partnerships with healthy home initiatives to increase awareness about Legionnaires’ disease risks, water system flushing protocols, and drinking water quality may be beneficial.
Inadequate drinking water quality, access, and infrastructure are critical factors that have been posited to negatively affect environmental health disparities. 59 Water age, water pipe material, water quality, and drinking water source may influence growth of Legionella and other opportunistic pathogens. 39,60 Racial/ethnic minority groups and low-income populations are more likely than non-Hispanic White and high-income populations to be exposed to contaminated drinking water and have poor access to safe drinking water. Including drinking water quality, access, infrastructure information, and environmental justice indicators in research will help to identify possible associations among drinking water violations, socioeconomic factors, and Legionnaires’ disease incidence.
Public Health Implications
The disproportionately higher incidence of Legionnaires’ disease among Black people and people of low socioeconomic status warrants public health action. Our review revealed that income and comorbidities are commonly studied aspects of SDH, and SDH such as job characteristics, education, social support, stress, health care access, health insurance type, and built environment are areas for future study. Knowledge gaps related to built-environment factors (eg, housing, drinking water infrastructure, pollutant exposures) suggest that more research can elucidate the role of cumulative environmental risks in Legionnaires’ disease health disparities. We posit that an SDH perspective can reveal why some communities are more likely than other communities to have vacant buildings with poorly maintained water systems, older housing with deteriorating plumbing, and declining drinking water infrastructure, which could lead to conditions conducive for Legionella growth in pipes.
Future research and programs should incorporate SDH data sources with geocoded addresses from Legionnaires’ disease surveillance data. 61,62 Data sources such as the CDC Environmental Health Tracking Network, CDC/ATSDR Social Vulnerability Index, Robert Wood Johnson Foundation County Health Rankings, and other indices may provide links to environmental and socioeconomic variables that are often not addressed in Legionnaires’ disease research. 63 -65 The findings from our review illustrate the need for increased awareness about the SDH related to Legionnaires’ disease. Outreach to organizations that have an interest in addressing poor housing quality, comorbidities, air pollution, and conditions that disproportionately affect low-income and racial/ethnic minority populations should be prioritized.
In our review, we explored the disproportionate incidence of Legionnaires’ disease and pneumonia among Black people and low-income populations. A present concern is the COVID-19 pandemic and existing comorbidities associated with increased hospitalizations and a higher mortality rate in historically disenfranchised and racial/ethnic minority communities. 66,67 The COVID-19 pandemic is exacerbating the adverse health consequences of racial and socioeconomic disparities in health and health care. 68 Our research reflects a commonality of respiratory conditions and adverse outcomes as a result of longstanding health inequities.
Acknowledgments
The authors thank Jamila Eatman and Na’Taki Osborne Jelks, PhD, MPH, from Spelman College, and Jeffrey Hall, PhD, MSPH, from the Centers for Disease Control and Prevention (CDC) Office of Minority Health and Health Equity for initial conceptual ideas and discussions.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of CDC or the Agency for Toxic Substances and Disease Registry.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Candis M. Hunter, PhD, MSPH https://orcid.org/0000-0001-8063-1758
References
- 1. Collier SA., Stockman LJ., Hicks LA., Garrison LE., Zhou FJ., Beach MJ. Direct healthcare costs of selected diseases primarily or partially transmitted by water. Epidemiol Infect. 2012;140(11):2003-2013. 10.1017/S0950268811002858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . National Notifiable Diseases Surveillance System: national notifiable infectious diseases and conditions, United States: weekly tables. 2020. Accessed March 1, 2020. https://wonder.cdc.gov/nndss/nndss_weekly_tables_menu.asp
- 3. National Academies of Sciences, Engineering, and Medicine . Management of Legionella in Water Systems. National Academies Press; 2020. [PubMed] [Google Scholar]
- 4. Dooling KL., Toews K-A., Hicks LA. et al. Active Bacterial Core surveillance for legionellosis—United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2015;64(42):1190-1193. 10.15585/mmwr.mm6442a2 [DOI] [PubMed] [Google Scholar]
- 5. Barskey A., Lackraj D., Tripathi PS. et al. Legionnaires’ Disease Surveillance Summary Report, United States: 2016-2017. Centers for Disease Control and Prevention; 2020. Accessed March 1, 2020. https://www.cdc.gov/legionella/health-depts/surv-reporting/2016-17-surv-report-508.pdf
- 6. Farnham A., Alleyne L., Cimini D., Balter S. Legionnaires’ disease incidence and risk factors, New York, New York, USA, 2002-2011. Emerg Infect Dis. 2014;20(11):1795-1802. 10.3201/eid2011.131872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bush C., Mensah N., Fitzhenry R. Legionnaires’ disease in New York City, 2007 to 2017. Epi Data Brief. 2018;106:1-17. [Google Scholar]
- 8. Burton DC., Flannery B., Bennett NM. et al. Socioeconomic and racial/ethnic disparities in the incidence of bacteremic pneumonia among US adults. Am J Public Health. 2010;100(10):1904-1911. 10.2105/AJPH.2009.181313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayes BH., Haberling DL., Kennedy JL., Varma JK., Fry AM., Vora NM. Burden of pneumonia-associated hospitalizations: United States, 2001-2014. Chest. 2018;153(2):427-437. 10.1016/j.chest.2017.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feemster KA., Li Y., Localio AR. et al. Risk of invasive pneumococcal disease varies by neighbourhood characteristics: implications for prevention policies. Epidemiol Infect. 2013;141(8):1679-1689. 10.1017/S095026881200235X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soto K., Petit S., Hadler JL. Changing disparities in invasive pneumococcal disease by socioeconomic status and race/ ethnicity in Connecticut, 1998-2008. Public Health Rep. 2011;126(suppl 3):81-88. 10.1177/00333549111260S313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiemken TL., Carrico RM., Furmanek SP. et al. Socioeconomic position and the incidence, severity, and clinical outcomes of hospitalized patients with community-acquired pneumonia. Public Health Rep. 2020;135(3):364-371. 10.1177/0033354920912717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prussin AJ II., Schwake DO., Marr LC. Ten questions concerning the aerosolization and transmission of Legionella in the built environment. Build Environ. 2017;123:684-695. 10.1016/j.buildenv.2017.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Langley G., Schaffner W., Farley MM. et al. Twenty years of Active Bacterial Core surveillance. Emerg Infect Dis. 2015;21(9):1520-1528. 10.3201/eid2109.141333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marmot M, Friel S, Bell R, Houweling TAJ, Taylor S, Commission on Social Determinants of Health . Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372(9650):1661-1669. 10.1016/S0140-6736(08)61690-6 [DOI] [PubMed] [Google Scholar]
- 16. Office of Disease Prevention and Health Promotion . Healthy People 2020 topics and objectives: social determinants of health. Accessed January 16, 2020. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health
- 17. Office of Disease Prevention and Health Promotion . About Healthy People. Updated June 25, 2020. Accessed June 25, 2020. https://www.healthypeople.gov/2020/About-Healthy-People
- 18. Chapman KE., Wilson D., Gorton R. Invasive pneumococcal disease and socioeconomic deprivation: a population study from the North East of England. J Public Health. 2013;35(4):558-569. 10.1093/pubmed/fdt011 [DOI] [PubMed] [Google Scholar]
- 19. Corrado RE., Lee D., Lucero DE., Varma JK., Vora NM. Burden of adult community-acquired, health-care–associated, hospital-acquired, and ventilator-associated pneumonia: New York City, 2010 to 2014. Chest. 2017;152(5):930-942. 10.1016/j.chest.2017.04.162 [DOI] [PubMed] [Google Scholar]
- 20. Gleason JA., Ross KM., Greeley RD. Analysis of population-level determinants of legionellosis: spatial and geovisual methods for enhancing classification of high-risk areas. Int J Health Geogr. 2017;16(1): 10.1186/s12942-017-0118-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jeon CY., Muennig P., Furuya EY., Cohen B., Nash D., Larson EL. Burden of present-on-admission infections and health care–associated infections, by race and ethnicity. Am J Infect Control. 2014;42(12):1296-1302. 10.1016/j.ajic.2014.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kempker JA., Kramer MR., Waller LA., Martin GS. Risk factors for septicemia deaths and disparities in a longitudinal US cohort. Open Forum Infect Dis. 2018;5(12): 10.1093/ofid/ofy305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim AM., Kang S., Park JH., Yoon TH., Kim Y. A spatial analysis of geographic variation and factors associated with hospitalization for bacterial pneumonia in Korea. BMC Pulm Med. 2019;19(1): 10.1186/s12890-019-0798-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marzouk M., Boukadida A., Chouchene I. et al. Analysis of severe legionellosis hospitalized in intensive care units in Tunisia. Bull Soc Pathol Exot. 2015;108(3):191-196. 10.1007/s13149-014-0414-1 [DOI] [PubMed] [Google Scholar]
- 25. McLaughlin JM., Swerdlow DL., Khan F. et al. Disparities in uptake of 13-valent pneumococcal conjugate vaccine among older adults in the United States. Hum Vaccin Immunother. 2019;15(4):841-849. 10.1080/21645515.2018.1564434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muhammad RD., Oza-Frank R., Zell E. et al. Epidemiology of invasive pneumococcal disease among high-risk adults since the introduction of pneumococcal conjugate vaccine for children. Clin Infect Dis. 2013;56(5):e59-e67. 10.1093/cid/cis971 [DOI] [PubMed] [Google Scholar]
- 27. Nowalk MP., Wateska AR., Lin CJ. et al. Racial disparities in adult pneumococcal vaccination indications and pneumococcal hospitalizations in the U.S. J Natl Med Assoc. 2019;111(5):540-545. 10.1016/j.jnma.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oggioni C., Za A., Auxilia F. et al. Legionnaires’ disease contracted from patient workplace: first report of a severe case of coinfection with varicella-zoster virus. Am J Infect Control. 2016;44(10):1164-1165. 10.1016/j.ajic.2016.03.057 [DOI] [PubMed] [Google Scholar]
- 29. Storch G., Baine WB., Fraser DW. et al. Sporadic community-acquired Legionnaires’ disease in the United States. Ann Intern Med. 1979;90(4):596-600. 10.7326/0003-4819-90-4-596 [DOI] [PubMed] [Google Scholar]
- 30. Wortham JM., Zell ER., Pondo T. et al. Racial disparities in invasive streptococcus pneumoniae infections, 1998-2009. Clin Infect Dis. 2014;58(9):1250-1257. 10.1093/cid/ciu108 [DOI] [PubMed] [Google Scholar]
- 31. Schumacher A., Kocharian A., Koch A., Marx J. Fatal case of Legionnaires’ disease after home exposure to Legionella pneumophila serogroup 3—Wisconsin, 2018. MMWR Morb Mortal Wkly Rep. 2020;69(8):207-211. 10.15585/mmwr.mm6908a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Orkis LT., Harrison LH., Mertz KJ., Brooks MM., Bibby KJ., Stout JE. Environmental sources of community-acquired Legionnaires’ disease: a review. Int J Hyg Environ Health. 2018;221(5):764-774. 10.1016/j.ijheh.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 33. Koch K, Søgaard M, Nørgaard M, Thomsen RW, Schønheyder HC, Danish Collaborative Bacteremia Network . Socioeconomic inequalities in risk of hospitalization for community-acquired bacteremia: a Danish population-based case-control study. Am J Epidemiol. 2014;179(9):1096-1106. 10.1093/aje/kwu032 [DOI] [PubMed] [Google Scholar]
- 34. Frei CR., Mortensen EM., Copeland LA. et al. Disparities of care for African-Americans and Caucasians with community-acquired pneumonia: a retrospective cohort study. BMC Health Serv Res. 2010;10(1):1-11. 10.1186/1472-6963-10-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mortensen EM., Cornell J., Whittle J. Racial variations in processes of care for patients with community-acquired pneumonia. BMC Health Serv Res. 2004;4(1):1-7. 10.1186/1472-6963-4-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cunningham TJ., Croft JB., Liu Y., Lu H., Eke PI., Giles WH. Vital signs: racial disparities in age-specific mortality among Blacks or African Americans—United States, 1999-2015. MMWR Morb Mortal Wkly Rep. 2017;66(17):444-456. 10.15585/mmwr.mm6617e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Semega J., Kollar M., Creamer J., Mohanty A. Income and Poverty in the United States: 2018. Current Population Reports P60-266(RV). US Census Bureau; 2019. [Google Scholar]
- 38. Beckles GL., Truman BI. Education and income—United States, 2005 and 2009. MMWR Suppl. 2011;60(suppl l):13-17. [PubMed] [Google Scholar]
- 39. Ahonen EQ., Fujishiro K., Cunningham T., Flynn M. Work as an inclusive part of population health inequities research and prevention. Am J Public Health. 2018;108(3):306-311. 10.2105/AJPH.2017.304214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flory JH., Joffe M., Fishman NO., Edelstein PH., Metlay JP. Socioeconomic risk factors for bacteraemic pneumococcal pneumonia in adults. Epidemiol Infect. 2009;137(5):717-726. 10.1017/S0950268808001489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Braveman P., Egerter S., Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381-398. 10.1146/annurev-publhealth-031210-101218 [DOI] [PubMed] [Google Scholar]
- 42. Owens A. Income segregation between school districts and inequality in students’ achievement. Sociol Educ. 2018;91(1):1-27. 10.1177/0038040717741180 [DOI] [Google Scholar]
- 43. Alvidrez J., Castille D., Laude-Sharp M., Rosario A., Tabor D. The National Institute on Minority Health and Health Disparities research framework. Am J Public Health. 2019;109(suppl 1):S16-S20. 10.2105/AJPH.2018.304883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beck AF., Florin TA., Campanella S., Shah SS. Geographic variation in hospitalization for lower respiratory tract infections across one county. JAMA Pediatr. 2015;169(9):846-854. 10.1001/jamapediatrics.2015.1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Torres A., Blasi F., Dartois N., Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015;70(10):984-989. 10.1136/thoraxjnl-2015-206780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu AT., Kamali A., Vugia DJ. Legionella epidemiologic and environmental risks. Curr Epidemiol Rep. 2019;6(3):310-320. 10.1007/s40471-019-00207-3 [DOI] [Google Scholar]
- 47. Wickramasekaran RN., Sorvillo F., Kuo T. Legionnaires’ disease and associated comorbid conditions as causes of death in the U.S., 2000-2010. Public Health Rep. 2015;130(3):222-229. 10.1177/003335491513000309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fields BS., Benson RF., Besser RE. Legionella and Legionnaires’ disease: 25 years of investigation. Clin Microbiol Rev. 2002;15(3):506-526. 10.1128/CMR.15.3.506-526.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Churchwell AL., Schaffner W. Facing down the triple threat of influenza and pneumococcal disease in African Americans: focusing on prevention. J Natl Med Assoc. 2011;103(3):278-280. 10.1016/S0027-9684(15)30289-3 [DOI] [PubMed] [Google Scholar]
- 50. Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care . Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. [PubMed] [Google Scholar]
- 51. Krieger N., Chen JT., Waterman PD., Rehkopf DH., Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the Public Health Disparities Geocoding Project. Am J Public Health. 2003;93(10):1655-1671. 10.2105/AJPH.93.10.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Woods ER., Bhaumik U., Sommer SJ. et al. Community asthma initiative to improve health outcomes and reduce disparities among children with asthma. MMWR Suppl. 2016;65(1):11-20. 10.15585/mmwr.su6501a4 [DOI] [PubMed] [Google Scholar]
- 53. Mikati I., Benson AF., Luben TJ., Sacks JD., Richmond-Bryant J. Disparities in distribution of particulate matter emission sources by race and poverty status. Am J Public Health. 2018;108(4):480-485. 10.2105/AJPH.2017.304297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tessum CW., Apte JS., Goodkind AL. et al. Inequity in consumption of goods and services adds to racial-ethnic disparities in air pollution exposure. Proc Natl Acad Sci U S A. 2019;116(13):6001-6006. 10.1073/pnas.1818859116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ho S-C., Chuang K-J., Lee K-Y. et al. Chronic obstructive pulmonary disease patients have a higher risk of occurrence of pneumonia by air pollution. Sci Total Environ. 2019;677:524-529. 10.1016/j.scitotenv.2019.04.358 [DOI] [PubMed] [Google Scholar]
- 56. Williams DR., Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404-416. 10.1016/S0033-3549(04)50068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hoffman JS., Shandas V., Pendleton N. The effects of historical housing policies on resident exposure to intra-urban heat: a study of 108 US urban areas. Climate. 2020;8(1):12. 10.3390/cli8010012 [DOI] [Google Scholar]
- 58. Pedro-Botet ML., Stout JE., Yu VL. Legionnaires’ disease contracted from patient homes: the coming of the third plague? Eur J Clin Microbiol Infect Dis. 2002;21(10):699-705. 10.1007/s10096-002-0813-2 [DOI] [PubMed] [Google Scholar]
- 59. VanDerslice J. Drinking water infrastructure and environmental disparities: evidence and methodological considerations. Am J Public Health. 2011;101(suppl 1):S109-S114. 10.2105/AJPH.2011.300189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang H., Masters S., Hong Y. et al. Effect of disinfectant, water age, and pipe material on occurrence and persistence of Legionella, mycobacteria, Pseudomonas aeruginosa, and two amoebas. Environ Sci Technol. 2012;46(21):11566-11574. 10.1021/es303212a [DOI] [PubMed] [Google Scholar]
- 61. Hadler JL., Vugia DJ., Bennett NM., Moore MR. Emerging infections program efforts to address health equity. Emerg Infect Dis. 2015;21(9):1589-1594. 10.3201/eid2109.150275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Krieger N., Chen JT., Waterman PD., Rehkopf DH., Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312-323. 10.2105/AJPH.2003.032482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Centers for Disease Control and Prevention . National Environmental Public Health Tracking Network. Accessed May 6, 2021. https://ephtracking.cdc.gov/
- 64. Agency for Toxic Substances and Disease Registry . CDC/ATSDR Social Vulnerability Index. Accessed March 1, 2021. https://www.atsdr.cdc.gov/placeandhealth/svi/
- 65. Robert Wood Johnson Foundation . County health rankings & roadmaps. Accessed March 1, 2021. https://www.countyhealthrankings.org
- 66. Hooper MW., Nápoles AM., Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466-2467. 10.1001/jama.2020.8598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tai DBG., Shah A., Doubeni CA., Sia IG., Wieland ML. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72(4):703-706. 10.1093/cid/ciaa815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shippee TP., Akosionu O., Ng W. et al. COVID-19 pandemic: exacerbating racial/ethnic disparities in long-term services and supports. J Aging Soc Policy. 2020;32(4-5):323-333. 10.1080/08959420.2020.1772004 [DOI] [PMC free article] [PubMed] [Google Scholar]