Abstract
Alcohol-related liver disease (ALD) is the most common indication for liver transplantation (LT) in the United States. The judicious allocation of organs and improvement in outcomes requires identification and monitoring of patients with ALD at high-risk for relapse post-transplantation. The controversial movement toward early LT for severe alcohol-related hepatitis (SAH) has also raised concern for alcohol relapse. While LT cures ALD, treatment of alcohol use disorder (AUD) must be included in the care plan to prevent a return to drinking and subsequent graft ALD. Patients with underlying AUD must be recognized, offered brief interventions and referred for multimodal multidisciplinary treatment that includes medications and psychotherapies along with sober support groups, family engagement, and a new dedication to healthy living in order to help sustain remission. Such comprehensive care will increase LT candidacy in patients with ALD while optimizing clinical outcomes of patients transplanted with AUD.
Keywords: Alcohol, addiction, relapse, liver, cirrhosis, hepatitis, multidisciplinary, transplant
Introduction
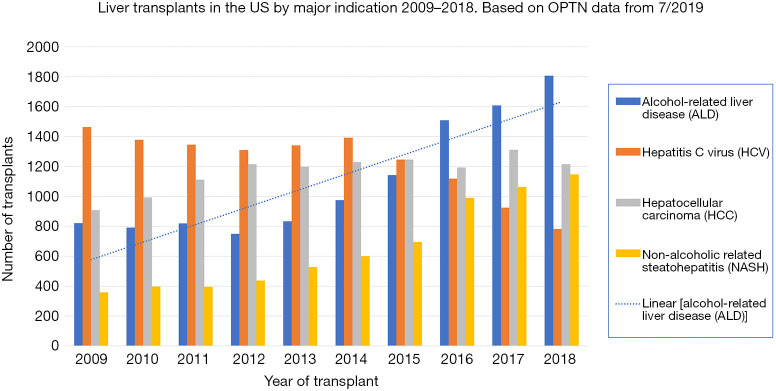
Alcohol-related liver disease (ALD) continues to be a major cause of cirrhosis and a primary indication for liver transplantation (LT); currently, alcohol is responsible for 80% of deaths from liver disease and 50% of deaths from cirrhosis (1). Since the 1990s ALD has risen as a viable indication for LT with similar, if not better, mortality outcomes compared to other conditions (2). As the incidence of Hepatitis C virus (HCV) cirrhosis has declined with effective anti-viral treatment, ALD has become the most common indication for LT in the United States (Figure 1).
Figure 1.
Liver transplants in the US by major indication 2009–2018. Based on OPTN data from 7/2019.
While LT addresses the effects of ALD, patients with ALD often have underlying alcohol use disorder (AUD) and remain at risk for alcohol relapse and subsequent damage to the transplanted organ, which can be mitigated with targeted interventions addressing the underlying AUD. ALD continues to share a strong association with AUD with 80% of patients with ALD presenting for LT with alcohol dependence (3). Sustained or heavy alcohol relapse places the transplanted organ at risk, with one study estimating 1 in 3 patients developing graft cirrhosis due to heavy drinking relapse (4). This risk can be decreased if patients successfully complete an alcohol rehabilitation program prior to LT (2). Such studies suggest the importance of recognizing the underlying AUD as part of the transplant assessment, as well as accurately prognosticating relapse risk post-transplant while attempting to decrease that risk by treating the addiction along with the liver disease.
As liver transplant teams take on more ALD patients, they will need to better evaluate and manage AUD in the context of waitlist management and post-transplantation care. At a liver transplant program in Italy, one approach imbedded intensive addiction treatment within the transplant clinic, which not only reduced relapse rates in comparison to historic controls from 35.1% to 16.4%, but also reduced the 8-year mortality rate from 37.8% to 14.5% (5). Most liver transplant programs do not have such specialized services in their clinics and instead use mental health assessments along with psychosocial scoring systems to stratify relapse risk. A middle ground may be for transplant providers to diagnose the AUD, briefly intervene and co-treat the patient with providers outside the transplant team. Patients identified to be at the highest risk for relapse would merit additional ongoing oversight, including self-monitoring and reporting through mobile technology, routine toxicology at visits, and structured scales to assess relapse. Overall, assessment and subsequent management of AUD varies among transplant centers, and optimal care may occur on a range tailored to the patients AUD severity and relapse risk.
Definition and identification of alcohol addiction, alcohol dependence, and AUD
Studies on AUD in ALD often fail to recognize and define AUD, which is different than quantification of drinks. A clear diagnosis along with staging severity and a review of comorbidities is an essential first step in management. Alcohol addiction, alcohol dependence, and AUD are labels often used interchangeably but they have different definitions. Moreover, the LT literature lacks a standardized approach to assessing AUD, likely driven by uneven expertise distribution, fiscal resources, and different processes of decision-making (3). The identification of the disorder is further impeded by patient factors such as hepatic encephalopathy and alcohol brain damage. Stigma about addiction can also associate with psychological barriers such as shame, guilt, and denial that are often heightened in patients seeking transplantation, impairing accurate assessment of AUD (6). Therefore, a trained psychiatrist or psychologist, one preferably with expertise in addiction, is ultimately best suited to evaluate the candidate’s AUD history, insight into their addiction, and the diagnosis of other psychiatric disorders.
The current model of alcohol addiction has been built and refined by a burgeoning understanding of behavioral neuroscience. Alcohol addiction has been defined as the three repetitive stages of the “addiction cycle” along with their neurobiological correlates outlined by Volkow et al. (7) including (I) binge and intoxication (II) withdrawal and negative affect and (III) preoccupation and anticipation, which correlate with the respective brain regions (I) basal ganglia (II) extended amygdala and (III) prefrontal cortex. Neurotransmitters such as dopamine, opioid peptides, gamma-aminobutyric acid (GABA), and endocannabinoids can mediate positive reinforcement early in the addiction cycle. As the disease progresses negative reinforcement can predominate through glutamate and the down-regulation of GABA. Along with this neurocircuitry, recent data on genetic polymorphisms may help predict addictive alcohol use behavior but are not ready for diagnostic clarity. While the term addiction has been retained for this neuroscientific understanding, the labels of alcohol dependence and AUD define the clinical syndrome and are more useful in practice.
The current ICD-11 [2018] has simplified the diagnosis of alcohol dependence to three domains which include (I) loss of control over alcohol use leading to (II) social or physical dysfunction and (III) physiologic dependence comprising tolerance and withdrawal (8). In this step-wise syndromic approach, two of the three criteria are required for the diagnosis. A more graded diagnosis of AUD has been developed for the Diagnostic and Statistical Manual 5th edition (DSM-5) for both research and clinical considerations (Figure 2) (9). One of the criteria, craving, defined as a strong subjective desire to drink making it difficult to think of anything else, was added as a neuroadaptive response to prolonged drinking. The AUD is rated mild, moderate, or severe with increasing number of the 11 signs and symptoms (Figure 2).
Figure 2.
DSM-5 criteria for AUD. AUD, alcohol use disorder.
Screening tools to identify and intervene on AUD
Screening tools such as the Alcohol Use Disorder Identification Test (AUDIT) and AUDIT-C may aide the initial identification of AUD. The AUDIT is a 10-item screening tool (scored between 0–40) developed by the World Health Organization (WHO) and validated for clinician-administration and self-report. Patients who score between 8–19 on the AUDIT should be given directed advice on the reduction of hazardous drinking and offered a plan for follow up, while those scoring between 20–40 are referred to an addiction specialist (1). The first three questions rely on the quantification and frequency of alcohol intake, and these questions form the shortened AUDIT-C (Figure 3). In a large systematic review of the AUDIT-C and its longer version, the authors found the shorter version to be equally sensitive and specific for identifying AUD and recommended a cutoff of ≥4 points for men and ≥3 points for women (10).
Figure 3.
AUDIT-C. AUDIT, Alcohol Use Disorder Identification Test.
In addition to these screening tools, laboratory testing suggestive of alcohol use causing ALD should be followed-up with assessment for AUD. Laboratory results of recent drinkers can reveal higher gamma-glutamyl transferase (GGT) values, increased AST/ALT ratio, and increased volume of red blood cells (MCV). While helpful when present, these results may not be present and are not specific for alcohol use causing ALD. Therefore, while laboratory results can be helpful in signaling possible underlying AUD causing ALD, results are nonspecific with limited sensitivity.
In the emergency department and primary care settings the practice of screening, brief intervention, and referral to treatment (SBIRT) identified and reduced drinking in select patients—SBIRT may not be sufficient in patients with heavy alcohol use (11). Nonetheless SBIRT has been trialed in a liver clinic and has been shown to effectively identify AUD and comorbid depression with 82% of enrolled patients finding their quality of life improved and 87% wishing to continue with the behavioral program (12). Brief sessions taking as little as 5 minutes that incorporate motivational interviewing (MI) can be particularly effective in mobilizing patients to seek specialized treatment (1). Therefore, screening for AUD and offering brief interventions as part of usual clinical care, could be an efficient and effective approach to diagnosing and offering initial treatment for AUD.
Additionally, in the hospital, the medical inpatient admission to manage alcohol withdrawal also offers an opportunity to assess motivation for sobriety and make plans for addiction treatment and sober support (13). A consult-liaison psychiatrist could further explore underlying and comorbid mood and anxiety disorders that would be critical to identify as treatment of these conditions can help stabilize the AUD. Blunted affect, hopeless attitude are common emotional and behavioral features of patients who have recently stopped alcohol use (7). In this diagnostic endeavor the primary goal of the mental health professional is to identify the severity of the AUD, its comorbidities and determine the proper intensity of treatment.
Alcohol relapse
Defining alcohol relapse
The identification of alcohol relapse in AUD patients is critically important to optimizing transplant candidate selection and caring for post-transplant patients but is heterogeneously defined and variably assessed (14). Relapse is generally defined as a return to the prior drinking behavior with the word lapse or “slip” signifying a brief use of alcohol without returning to the prior patterns. While relapse is associated with morbidity and mortality, slips may be an opportunity to review risk factors and perform brief intervention to avoid subsequent relapse. Relapse and slips may occur at any time on the waitlist or post-transplant. Combining self-report, structured tools, collateral information, and toxicology should best approximate the true incidence of relapse.
Recognizing relapse rates post-transplant
Studies found waitlisted liver transplant candidates to have relapse rates of 15–25% (6). Post LT meta-analysis identified an annual rate of 5.6% for any alcohol use and 2.5% for heavy alcohol use (15). These annual rates appear to accumulate; by year 5, 28% of recipients with ALD will have relapsed to some alcohol use with 12.5% to severe and problematic use. Additionally, some centers have reported relapse rates after LT as high as 20–25% (4); though this is still lower than relapse rates between 20% and 80% found in the general population after initial treatment for AUD (16). While selection bias for those having undergone transplant may be lowering those rates, the reported rates of up to 1:4 patients post-transplant experiencing harmful relapse are concerning, and this detection is occurring in the setting of non-standardized assessment for alcohol relapse post-LT. A pattern to relapse has emerged with the heaviest drinkers having an early onset as early as 6 weeks with peaking intake at year 3 (17). As transplantation of patients with ALD rising, recognition of relapse rates and standardizing approach to relapse assessment becomes imperative.
Approach to relapse assessment
Relapse assessment involves a multifaceted approach with limitations with use of only a single modality. Self-report may only reveal alcohol relapse in a subset of patients and this may be interviewer dependent. In one prospective study of post LT patients evaluated by their hepatologist and addiction specialist, the hepatologist only identified half of the problematic alcohol use compared to the addiction specialist. In this study the AUDIT also improved detection; identifying 22% more relapse post liver transplant compared to the hepatologist assessment alone (18). Sensitive and specific laboratory tests for alcohol use include the percentage of carboxy-deficient transferrin (%CDT) or Phosphatidylethanol (PEth) in serum and ethyl glucuronide (EtG) in urine. In a sample of LT candidates and recipients, the shorter 3 item AUDIT-C screening tool in combination with the urine EtG had the highest detection rate for alcohol consumption (AuROC =0.98) (19). Blood PEth levels have also been found to correlate with chronic levels of alcohol consumption and their AUDIT score (20). In the post assessment phase, similarly, the most optimal strategy to identify relapse to drinking appears to be a combination of patient interview, caregiver report, and laboratory monitoring.
Overall, AUD is a relapsing/remitting condition with expected relapse and slips. Assessment of relapse should be approached with care and support. The liver transplant team establishes a powerful emotional bond with the recipient. Many patients who have resumed substance use are relieved to learn that the transplant team will not abandon them after a slip or an even more severe relapse. Moreover, it is helpful to not condone or dismiss small amounts of alcohol use in a patient in an abstinence treatment plan (21). What may seem supportive can be distorted by the patient with an addiction and become an excuse to drink more regularly.
Risk assessment for relapse
To optimize identifying alcohol relapse, recognizing patients at greatest risk for relapse with subsequent tailoring of testing and intervention will help. Risk assessment includes a survey of risk and protective factors along with a review of the patients’ prior efforts at modifying these factors. Prospective studies of alcohol relapse in liver transplant patients identified a diagnosis of alcohol dependence (severe or moderate AUD), a family history of alcoholism, low social support, and a shorter duration of pre-transplant sobriety to be predictive of relapse post-transplant (22). Additionally, phenotyping AUD into two types to distinguish between a late onset AUD alone (type 1) vs. early onset AUD with other substance abuse (type 2) may help with predicting relapse, with the second type portending a higher risk and a lower benefit from treatment (3). A wealth of data from prospectively studied cohorts, multicenter retrospective cohorts, and studies proposing a scoring tool to assess relapse risk were reviewed to create a comprehensive data collection tool (14,23) which is summarized in Table 1. Using these tools to identify patients at risk for relapse may help tailor relapse assessment and interventions to reduce relapse risk; i.e., patients at higher risk for relapse would undergo more frequent assessment for relapse and be offered more interventions to reduce relapse risk than patients at lower risk for relapse.
Table 1. Variables associated with alcohol relapse in patients with ALD.
| Variables | Notes | Transplant population | Associated alcohol relapse | |||
|---|---|---|---|---|---|---|
| Pre | Post | Slip | Heavy* | |||
| Demographics | ||||||
| Age (24) | Age >50 associated with relapse risk post-LT | + | + | + | ||
| Sex (25) | Women more likely than men to relapse on the waiting list | + | + | |||
| Tobacco use (26,27) | History of smoking more likely to relapse post-LT | + | + | + | ||
| Alcohol use | ||||||
| Continued alcohol use (27) | Continued alcohol use after the formal diagnosis of ALD by a hepatologist associated with relapse post-LT | + | + | |||
| Quantity (24,28,29) | Higher drinks per day and longer years of heavy alcohol consumption associated with increased relapse risk | + | + | + | ||
| Alcohol dependence (17,30) | Diagnosis of pre-transplant alcohol dependence associated with relapse post-LT | + | + | + | ||
| Abstinence (17,24,26,29-33) | Fewer months sober pre-transplant associated with relapse post-LT | + | + | + | + | |
| Rehabilitation (28-30,33,34) | Attendance of a rehabilitation program pre-transplant associated with relapse post-LT | + | + | + | + | |
| Treatment motivation (27,28) | Reluctance to participate in alcohol relapse prevention program associated with increased risk for relapse | + | + | |||
| Consumption consequence (27) | Lack of consumption consequences to employment, income, housing, relationship, or legal matters | + | + | |||
| Self-admission to hospital (28) | Self-admission for treatment of ALD associated with decreased relapse risk | + | + | + | ||
| Legal issues (28,29) | History of alcohol-related legal issues associated with increased risk for relapse | + | + | + | ||
| Family history (17,28,30,34) | Having first degree biologic relatives with AUD associated with relapse post-LT | + | + | + | ||
| Psychiatric factors | ||||||
| Other substance use (17,29,30,34) | Substance use other than alcohol pre-transplant associated with relapse post-LT | + | + | + | ||
| Depression (26,30) | History of depression pre-LT associated with relapse post-LT | + | + | |||
| Perceived stress (17,27) | Reporting perceived stress and poor stress management associated with risk for relapse post-LT | + | + | + | ||
| Confidence (17) | Lack of confidence of receiving another liver if necessary associated with early relapse | + | + | + | ||
| Psychiatric comorbidity (26,28) | Patients with a history of any psychiatric comorbidity were more likely to relapse post-LT | + | + | + | ||
| Personality disorder (35) | Personality disorders associated with relapse | + | + | + | ||
| Medical factors | ||||||
| Noncompliance (35) | Noncompliance was defined as missing a single clinic appointment without contact for rescheduling, missed medication doses, or failure to refill prescription | + | + | + | ||
| Poorer health, fatigue, and bodily pain post LT (17) | Reporting poorer health, fatigue, bodily pain post-LT were more likely to relapse post-LT | + | + | + | ||
| Presence of hepatocellular carcinoma (27) | Absence of hepatocellular carcinoma in patients with ALD associated with increased relapse risk | + | + | |||
| Socioeconomics | ||||||
| Employment (28) | Employment immediately prior to hospitalization for ALD was associated with increased risk for relapse | + | + | + | ||
| Married (24,26,28,30) | Marriage pre-LT associated with decreased risk for relapse post-LT | + | + | + | ||
| Sober sponsor (27,28) | Failure to establish a rehabilitation relationship with another person, i.e., patient identifies a person as their sober sponsor, associates with increased risk for relapse | + | + | |||
| Social support (27,28) | Patient reported lack of social support (none or limited) associates with increased risk for relapse | + | + | |||
| Social events (27) | Continued participation in social events with alcohol present associated with increased risk for alcohol relapse | + | + | |||
| Scores | ||||||
| HRAR (24,36) | Higher HRAR associated with increased relapse risk. The HRAR is a score based on the duration of heavy drinking years, the number of daily drinks, and the number of prior alcoholism treatments | + | + | + | ||
| ARRA (27) | Assigns a point to nine risk factors predictive of alcohol relapse with higher scores more predictive of relapse | + | + | + | ||
| HPSS (28) | Assigns points to the following risk factors to assess alcohol relapse risk, with higher scores suggestive of increased relapse risk | + | + | + | ||
| SALT (29) | Patients with lower scores were at decreased risk for relapse post-LT; points were given for >10 drinks per day, ≥2 failed rehabilitation attempts, history of alcohol-related legal issues, history of non-tetrahydrocannabinol illicit substance use | + | + | + | ||
*, Includes binge and/or sustained relapse outcomes. ALD, alcohol-related liver disease; AUD, alcohol use disorder; HRAR, High-Risk Alcohol Relapse; ARRA, Alcohol Relapse Risk Assessment; HPSS, Hopkins Psychosocial Scoring System; SALT, Sustained Alcohol Use Post-LT; LT, liver transplantation.
Modifiable risk factors such as the severity of symptoms of AUD, comorbid substance abuse, and other psychiatric disorders will be considered later in the treatment section. One critical modifiable risk factor which bears mention here may be social support. Poor social support has been a risk factor associated with post-LT alcohol relapse, while strong social support is believed to mitigate the risk for post-LT alcohol use (15,22). Though it is not known which aspects of this critical factor are essential, this variable may include engaging caregivers facilitating patient adherence to and active participation in addiction counseling as well as creating a supportive environment such as removing alcohol from the home and removal of alcohol from social events. While such modifications can help prevent relapse post-transplantation, other interventions have failed to show efficacy. Some LT teams have patients sign contracts pledging to abstain from alcohol and participate in addiction counseling, yet one study showed no difference in post-LT relapse rates when such written contracts were used (37).
One controversial technique to mitigate risk for alcohol relapse requires 6 months of pre-LT sobriety. While patients with shorter duration of pre-transplant sobriety appear to be at increased risk for alcohol relapse, optimal pre-transplant sobriety length to mitigate relapse risk remains unknown. In practice, the 6-month rule of sobriety not only assesses the patient’s ability to maintain sustained sobriety but also allows time for possible improvement of liver function without LT. Nonetheless, reaching 6-month of sobriety does not predict a decreased relapse risk, and patients that are low-risk for relapse can present acutely too sick to survive 6-month without a LT. Further study of predictors of relapse-risk are necessary to optimize and personalize pre-transplant abstinence to the candidate being considered to obtain the best clinical outcome.
Instruments evaluating the patient’s risk for relapse may help with creating such objective criteria. Currently proposed instruments include the High-Risk Alcohol Relapse (HRAR) scale and the Alcohol Relapse Risk Assessment (ARRA) (24,27). From the 25 factors tested in the ARRA, nine predicted relapse, with four of them found to be the Vaillant prognostic factors: (I) continued engagement in social activities with alcohol present (II) no rehabilitation relationship, (III) low motivation for alcohol treatment, and (IV) a lack of nonmedical consequences to relapse (3). More recently, two additional scales have been developed to predict relapse risk in patients transplanted with severe alcohol-related hepatitis (SAH) with short pre-transplant sobriety, the Hopkins Psychosocial Scoring System (HPSS) and the Sustained Alcohol Use Post-LT (SALT) (28,29). The simple four item SALT score was derived from a large multi-center retrospective cohort of liver transplant candidates and recipients with alcoholic hepatitis. A low SALT score had a strong negative predictive value for sustained alcohol use and has been posited as an easy tool to identify patients at low risk for relapse. In these studies prior multiple rehabilitation attempts were shown to be a risk factor for relapse. Future should involve the analysis of prospective use of these tools and tools which rely on modifiable risk factors (Table 1).
SAH: diagnosis and risk assessment under pressure
When considering LT in patients with ALD, a difficult scenario involves assessing relapse risk in patients presenting with SAH felt to have high short-term mortality. SAH can have no prior symptomatic or clinical warning of alcohol use causing life-threatening ALD, leaving patients little time to consider the negative role of AUD in their lives. This typically occurs in a younger population thought to have high 3-month mortality. Pilot studies carefully transplanted a subset of these patients with no prior liver decompensation and high motivation for sobriety; these patients were afforded early listing for transplant despite not having the standard 6 months of sobriety (29,38,39). These studies may be limited by the current clinical definitions of SAH and do not address that >95% of SAH occurs in the setting of cirrhosis with <60% having hepatitis on explant pathology (14). Despite mixed liver pathology, these patients are classically feeling ill with liver symptomatology for the first time in their life, and thus many never entered a rehabilitation program nor had significant sobriety. Transplanting this population tests the ethics of beneficence in the setting of a limited resource. An addiction paradigm may be helpful in assessing and helping patients with high mortality risk and low relapse risk. Due to a lack of time to treat active AUD, the liver transplant teams adopting this approach use more restrictive psychosocial selection criteria to mitigate the relapse risk.
One practical paradigm used for this controversial selection is to identify patients with new insight into the AUD and motivation for treatment in the context of a first hospitalization for a severe liver decompensating event. Insight into the underlying alcohol addiction and willingness for treatment in the context of medical hospitalization for the initial SAH episode presents a practical paradigm to clarify in patients ready for this controversial practice. Only considering patients who have sought sobriety after illness may be selecting for patients who have less of an addiction drive and/or more decision-making control. Retrospective study of these highly selected patients reports relapse rates of 17% at 3 years (30), only slightly greater than and within the reported range of relapse of other less selective, transplanted ALD populations, 11.9% (range, 6.5–21%) (40). Therefore, given the potential mortality benefit and the within standard of care relapse risk, selective candidate consideration may be appropriate.
Candidate selection in this scenario can be difficult; in these emergency scenarios the process of conducting a thorough psychiatric evaluation is complicated by many factors. The foundation of the evaluation is the patient interview which necessitates an alert, cooperative and forthright patient to gather accurate information to make the correct diagnosis, such as AUD, and formulate an equitable treatment plan. In many SAH cases, the precipitous medical decline does not allow patients time for lengthy contemplation of their situation or psychological adjustment to the crisis being faced. Often patients feel overwhelmed about their situation and can be in denial of the need for life saving transplantation. The psychiatrist or psychologist should use a non-judgmental approach and identify that they are a part of the transplant team. Nevertheless, issues of shame and guilt can be heightened for the patient during a transplant evaluation and can make taking a comprehensive history challenging. Patients may minimize their alcohol use and AUD problems in an effort to manage impressions for transplant candidacy. Information from collateral sources; medical records, family, medical care providers, essential to supplement and corroborate information from the patient interview, may be difficult to obtain with limited time.
These constraints prevent the majority of candidates from ultimately becoming eligible for liver transplant listing. In the first studies published on this approach, only 25–30% of all potential transplant candidates evaluated psychosocially were deemed acceptable by this method (38,39). While the low conversion of LT evaluations to LT listings in this population may be improved with the previously mentioned interview techniques, programs must remain objective and realistic about relapse risk and its impact on the program. Ultimately, each SAH patient is unique and requires an expert liver transplant team to interview, summarize and consider all of the evaluation data to make a reasoned decision, with future studies investigating techniques to optimize accurate candidate selection.
Staging of illness and treatment of AUD in the setting of LT
AUD is both an illness of the brain and its corresponding behavior with limited study in patients with ALD. Both biological and cognitive neuroscience inform the treatment of addiction; to treating the brain regions responsible for the drive to use alcohol as well as the positive and negative reinforcers of behavior. Hepatic encephalopathy and alcohol-related dementia must also be considered, managed and taken into account in any treatment plan for addiction. Severe AUD is also associated with other neurological syndromes such as spastic paraparesis, seizures, and language and visuospatial dysfunction which require adequate neurological management (40). Addiction treatment planning should then occur in concert with other specialists. In small studies of liver transplant recipients, lower rates of alcohol use were reported in patients who engaged in addiction treatment pre and post-LT in comparison to those who did not engage (40). Such findings suggest future study of AUD interventions may be beneficial to optimizing outcomes in LT recipients. Ideally, the staging of the addiction and its treatment planning is accomplished in the outpatient setting where the patient’s cognition may be less compromised (3).
While the treatment of AUD in ALD is understudied, direction can be sought from the general alcohol addiction literature. Formalized treatment programs have been studied in two large landmark randomized control trials, Matching Alcoholism Treatments to Client Heterogeneity (MATCH) and Combining Medications and Behavioral Interventions (COMBINE). The MATCH trial compared three psychosocial interventions [motivation enhanced therapy (MET), 12-step, and cognitive behavioral therapy (CBT)] and found these interventions to be equally effective with patient’s lack of motivation and psychiatric severity leading to relapse (41). The COMBINE trial found that the combination of medications and behavioral interventions was superior to medication alone (42). Multimodal treatment is the mainstay for other chronic medical conditions such as diabetes where management combines medication, self-monitoring, diet, exercise, overall weight management, and close follow up. Similar approaches are necessary to optimize pre- and post-management of patients with AUD and ALD.
A multimodal AUD treatment plan for AUD in the setting of ALD should include medications, MI, sober group support, family education and engagement, healthy replacement activities, and CBT if indicated. The current model of addiction neuroscience may also help inform multi-modal treatment planning by targeting these four regions of the brain; (I) mesolimbic system and the dopamine reward circuit (II) the orbitofrontal cortex and the decision-making circuit (III) the prefrontal cortex involved in planning and executive function and (IV) the amygdala/hippocampus or emotional memory centers. In this model the patient can be taught the simplistic but apt metaphor of the runaway car with too much gas or acceleration in the mesolimbic reward circuit, poor steering in the decision-making circuit, and the loss of breaks in the pre-frontal cortex. (Figure 4, adapted from NNCI 2019) (43).
Figure 4.
Neurobiology of addiction circuit and the useful metaphor of “driving a car” to help patients understand the neuroscience of AUD along with the corresponding treatment plan (The metaphor of “driving a car” adapted from a video in the National Neuroscience Curriculum Initiative). AUD, alcohol use disorder; AA, Alcoholics Anonymous; SSRIs, Selective serotonin reuptake inhibitors.
While this neuroscience is still evolving, the current knowledge about these four regions may prove useful in organizing treatment goals, and if nothing else, help teach patients how to better address their internal struggles. The following therapies (I) medication-assisted treatment (MAT); (II) motivational enhancement therapy (III) individual therapy and group support and (IV) serotonin reuptake inhibitors (SRIs) have proven efficacy and can be implemented in concert as they work on different regions of the brain that are thought to be dysfunctional in addiction.
MAT
Prescribed medications intended to reduce the reinforcing effects of alcohol have been labeled MAT and include FDA approved AUD medications such as Naltrexone, Acamprosate, and Disulfiram along with others used off-label. These medications may help temper the pleasurable effects of alcohol brought on by the dopaminergic transmission in the mesolimbic reward system. This dopamine reward circuit serves as an accelerator for the initial addiction and a powerful positive reinforcer of behavior. As this circuit is overrun DFosB, a powerful transcription factor that alters gene expression in the nucleus accumbens (NA) mediating the neural adaptation (44). Over time and in states of alcohol withdrawal the circuit will be under-modulated leading to a powerful negative reinforcer of behavior to seek the drug again.
Acute withdrawal or safe detoxication may require inpatient admission using long-acting benzodiazepines and/or anticonvulsant mood stabilizers. In the weeks after acute withdrawal, a post-acute withdrawal syndrome has also been described which responds to short term use of hypnotics such as trazodone and the start of longer-term MAT. MAT is prescribed in the outpatient setting to reduce cravings and help diminish relapse risk. Naltrexone and acamprosate blunt the positive reinforcing effects of alcohol, while disulfiram is useful in creating a negative reinforcer of alcohol use. Disulfiram has been linked to clinical apparent acute liver injury which can be severe and is strongly discouraged in ALD. While naltrexone carries a black-box warning for a risk of liver damage, its connection to clinically apparent liver injury has not been substantiated and its risk may be overvalued (45).
Given the benefit of naltrexone over acamprosate in the COMBINE study, some have advocated for its monitored use after LT (46). Those with a family history of addiction, strong cravings, and a “sweet tooth” may benefit most from naltrexone (47). The long-acting injectable of naltrexone has been found to be more effective than the oral medication likely due to increased adherence and may be safer in liver disease as it avoids first pass metabolism. Routine monitoring of liver function tests may mitigate any further risk of injury. Other off-label anti-craving medications with modest benefit include topiramate, ondansetron, gabapentin, and baclofen. Only baclofen has been studied through randomized controlled trial (RCT) in AUD patients with ALD with 71% achieving sustained abstinence and reduced craving compared to 21% in the placebo group (48). All these medications may ultimately have their effect by reducing cue-induced dopamine release in the NA; they have been summarized in Table 2.
Table 2. Medication-assisted Therapy for AUD in ALD.
| Medication | Dosing | Mechanism | Treatment considerations for AUD in ALD |
|---|---|---|---|
| Naltrexone | 50–150 mg QD; 380 mg IM monthly | Opioid receptor antagonist | FDA approved for AUD; a large number of studies show efficacy; no RCTs in ALD patients. It is heavily metabolized by liver and while hepatotoxicity risk is rare it may occur at high doses |
| Acamprosate | 333–666 mg TID | NMDA receptor antagonist | FDA approved for AUD, a large number of studies show efficacy; no RCTs in ALD patients. No hepatic metabolism but it is contraindicated in severe renal insufficiency |
| Topiramate | 100–400 mg BID | GABA and glutamate modulator | RCTs with a dose escalation design showed efficacy; no RCTs in ALD patients |
| Ondansetron | 1–16 microg/kg BID | 5-HT3 receptor antagonist | One RCT showed reduced drinking in the more severe AUD type 2; no RCTs in ALD patients. Hepatic metabolism but no reports of toxicity |
| Gabapentin | 600–1,800 mg TID | GABA modulator | RCTs with comorbid anxiety disorders and insomnia showed efficacy; no RCTs in ALD patients. Minimal hepatic metabolism and no reports of hepatotoxicity. Dose adjustments for ESRD |
| Baclofen | 10–60 mg TID | GABAB receptor agonist | One RCT evidence for efficacy in advanced ALD Minimal hepatic metabolism and no reports of toxicity |
ALD, alcohol-related liver disease; AUD, alcohol use disorder; QD, once a day; NMDA, N-methyl-D-aspartate; IM, intramuscular; RCT, randomized controlled trial.
MET
Decision making and motivation are associated with orbitofrontal and anterior cingulate connectivity and must be engaged during referral for treatment. Orbitofrontal inputs from the NA and amygdala mediate positive and negative reinforcement for decision making in addiction (49). Complex attention and motivation is further sustained by the anterior cingulate which may be compromised in even mild hepatic encephalopathy (3). Poor decision making can manifest as avoidant coping which predicts AUD relapse in long term follow up (50). Programmatic adoption of tools to improve partnership and acceptance of the patient with AUD will help reduce avoidant behavior and improve motivation.
Insight and motivation can be improved through MET and MI. MET is a therapy designed to help patients advance from contemplation to planning. This type of therapy was originally developed as a manual-guided intervention for the MATCH trial which utilized MI in regular sessions (41). In a small controlled trial of waitlisted patients with ALD, MET was shown to reducing drinking behavior (51). MI is a brief intervention delivered by any clinician to enhance motivation to change behavior. It is a collaborative approach of increasing the patient’s ambivalence about their behavior with the ultimate goal to resolve this ambivalence and promote healthy action. The key principles of this therapy include an exploration of the gap between patient’s present and desired health status without confrontation communication (“change talk”) and supporting their efforts to make changes. MET and brief MI are complementary to MAT treatment.
Individual and group support
The pre-frontal cortex serves as the breaks to the runaway drive of the mesolimbic system. Diminished self-awareness and poor insight into addiction related to deficits in the prefrontal cortex may compromise further preparation and motivated action to change (52). Unharnessed internal and external stimuli can prompt a risk for relapse; whereas executive functions, planning and organization, can negate this impulse to drink again. Early in sobriety this internal “cognitive control” system may still be compromised. Group support such as Alcoholics Anonymous (AA or 12-step) can help give healthy choices for the patient who is having difficulty with cognitive control. The MATCH trial found 12-step to be equivalent to professional help like MET and CBT. When using control groups, abstinent rates are often twice as high in individuals who attended AA compared to those who did not (53). Nonetheless, it has been estimated that 40% of AA members drop out in the first year and this faith-based approach may not be suited for all patients (54). SMART recovery is a similar but non-faith-based program which relies on group support to prevent relapse. While any non-drinking replacement activities may be helpful to prevent relapse, the caring relationships that form in these settings may be a key ingredient and thus attendance of patients with AUD in such programs should be encouraged if not required by LT centers (3).
Beyond group meetings, an individual sponsor can serve as a guide through sobriety and be available to the patient who is struggling with craving or who has had a recent slip. Family therapy is often needed to ensure that the family understands the extent of the addiction so they may also support the sobriety. Even if the patient is not using AA, family and friends may benefit from the emotional support group Al-Anon. While there is no evidence that an “alcohol contract” confirming the transplant candidate’s commitment to abstinence affects alcohol consumption after liver transplant it may help serve as a conversation with the family about their ongoing engagement (37).
Selective serotonin reuptake inhibitors (SSRIs) and psychotherapy
The development of AUD involves elevated anxiety, low mood, and increased sensitivity to stress. This negative affect is mediated by pathological neuroadaptations in the extended amygdala which cause the individual to return to drinking in order to escape the dysphoria (7). Explicit and implicit emotional memories may have further conditioned individuals to drinking behavior. Screening and treatment of co-existing psychiatric disorders has been consistently found to be an essential part of addiction treatment (55). The more common type 1 AUD with late onset drinking may better respond to this dual diagnosis treatment (3). SSRIs and tricyclic antidepressants (TCAs) can stabilize mood and improve abstinence rates in depressed relapsing alcoholic individuals (56) and may be the most appropriate pharmacological interventions for transplant recipients if concurrent mood symptoms are present. Some studies have shown that SSRIs may increase rates of relapse, especially in those with type 2 AUD (57).
Psychotherapy may be particularly helpful in stabilizing reactive anxiety and depression. Emotional and episodic memory of past alcohol use and its setting can long seed risk of relapse in those with unstable anxiety and mood disorders. The stress response, partially modulated by the amygdala, and long remembered by the amygdala/hippocampal complex, manifest as adjustment reactions and less commonly post-traumatic stress disorder (PTSD), upsetting the usual psychological homeostasis, driving the individual to revert to older coping strategies. CBT can particularly help with teaching the patient about their past pairing of environmental cues and rewards through conditioning. High physiologic and social cues to drink (i.e., alcohol expectancies) and confidence to control behavior (i.e., self-efficacy) can be modified by CBT. Through therapy, the patient may also find strategies to engaged natural rewards such as social events and exercise, which will then increase their salience for the future. CBT along with other therapies like dialectical behavioral therapy (DBT) can help reduce reactivity to harmful internal cues to relapse.
Conclusions
The burden of ALD is rising and will increase demand to identify AUD and engage patients early in multimodal treatment. Using a multi-modal (screening interviews, laboratory testing, medications) as well as multi-disciplinary (hepatologist, social worker, psychiatrist) approach, AUD should not present a barrier to listing. In building a therapeutic alliance with patients and their families, confronting feelings of shame and guilt, recruiting the care and support of friends and family, and treating underlying psychiatric co-morbidities, patients can feel comfortable discussing cravings or relapses prior to and post-transplant. As for any other chronic medical illness, a longitudinal multimodal treatment plan for AUD should be established early in the treatment course to work to stabilize the various reversible risks for relapse in concert with LT consideration. Further studies in optimizing this approach, including care coordination, use of medications and behavioral interventions, and relapse monitoring, will be essential to creating a framework that minimizes relapse risk while maximizing transplant candidacy, inevitably improving clinical outcomes.
Acknowledgments
The authors are grateful to Peter Shapiro MD and the writing group of the Consultation-Liaison Psychiatry Service at Columbia University for critical feedback during paper preparation.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sanjaya K. Satapathy, David Bernstein, Nitzan Roth) for the series “Liver Transplantation in NASH and ALD” published in Translational Gastroenterology and Hepatology. The article has undergone external peer review.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Connor JP, Haber PS, Hall WD. Alcohol use disorders. Lancet 2016;387:988-98. 10.1016/S0140-6736(15)00122-1 [DOI] [PubMed] [Google Scholar]
- 2.Marroni CA, Fleck AM, Jr, Fernandes SA, et al. Liver transplantation and alcoholic liver disease: History, controversies, and considerations. World J Gastroenterol 2018;24:2785-805. 10.3748/wjg.v24.i26.2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beresford TP, Lucey MR. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol 2018;53:135-44. 10.1093/alcalc/agx104 [DOI] [PubMed] [Google Scholar]
- 4.Dumortier J, Dharancy S, Cannesson A, et al. Recurrent alcoholic cirrhosis in severe alcoholic relapse after liver transplantation: a frequent and serious complication. Am J Gastroenterol 2015;110:1160-6. 10.1038/ajg.2015.204 [DOI] [PubMed] [Google Scholar]
- 5.Addolorato G, Mirijello A, Leggio L, et al. Liver transplantation in alcoholic patients: impact of an alcohol addiction unit within a liver transplant center. Alcohol Clin Exp Res 2013;37:1601-8. 10.1111/acer.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimartini AF, Dew MA. Monitoring alcohol use on the liver transplant wait list: therapeutic and practical issues. Liver Transpl 2012;18:1267-9. 10.1002/lt.23529 [DOI] [PubMed] [Google Scholar]
- 7.Volkow ND, Koob GF, McLellan AT. Neurobiologic advances from the brain disease model of addiction. N Engl J Med 2016;374:363-71. 10.1056/NEJMra1511480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. ICD-11 for Mortality and Morbidity Statistics (ICD-11 MMS), 2018 version. Available online: https://icd.who.int/browse11/l-m/en
- 9.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington DC: American Psychiatric Association, 2013. [Google Scholar]
- 10.Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res 2007;31:185-99. 10.1111/j.1530-0277.2006.00295.x [DOI] [PubMed] [Google Scholar]
- 11.Saitz R. Alcohol screening and brief intervention in primary care: absence of evidence for efficacy in people with dependence or very heavy drinking. Drug Alcohol Rev 2010;29:631-40. 10.1111/j.1465-3362.2010.00217.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma M, Horrow J, Navarro V. A behavioral health program for alcohol use disorder, substance abuse, and depression in chronic liver disease. Hepatol Commun 2019;3:646-55. 10.1002/hep4.1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivest J, Jutras-Aswad D, Shapiro PA. Treating the "unhealthy alcohol user" on medical wards: beyond withdrawal. J Psychiatr Pract 2013;19:213-26. 10.1097/01.pra.0000430505.52391.48 [DOI] [PubMed] [Google Scholar]
- 14.Shen NT, Salajegheh A, Brown RS, Jr. A call to standardize definitions, data collection, and outcome assessment to improve care in alcohol-related liver disease. Hepatology 2019;70:1038-44. 10.1002/hep.30587 [DOI] [PubMed] [Google Scholar]
- 15.Dew MA, DiMartini AF, Steel J, et al. Meta-analysis of risk for relapse to substance use after transplantation of the liver or other solid organs. Liver Transpl 2008;14:159-72. 10.1002/lt.21278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finney JW, Moos RH, Timko C. The course of treated and untreated substance use disorders: remission and resolution, relapse and mortality. In McCrady BS, Epstein EE. editors. Addictions: a comprehensive guidebook. New York: Oxford University Press, 1999:30-49. [Google Scholar]
- 17.DiMartini A, Dew MA, Day N, et al. Trajectories of alcohol consumption following liver transplantation. Am J Transplant 2010;10:2305-12. 10.1111/j.1600-6143.2010.03232.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnadieu-Rigole H, Olive L, Nalpas B, et al. Follow-up of alcohol consumption after liver transplantation: interest of an addiction team? Alcohol Clin Exp Res 2017;41:165-70. 10.1111/acer.13276 [DOI] [PubMed] [Google Scholar]
- 19.Piano S, Marchioro L, Gola E, et al. Assessment of alcohol consumption in liver transplant candidates and recipients: the best combination of the tools available. Liver Transpl 2014;20:815-22. 10.1002/lt.23881 [DOI] [PubMed] [Google Scholar]
- 20.Afshar M, Burnham EL, Joyce C, et al. Cut-point levels of phosphatidylethanol to identify alcohol misuse in a mixed cohort including critically ill patients. Alcohol Clin Exp Res 2017;41:1745-53. 10.1111/acer.13471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiMartini A, Shenoy A, Dew MA. Organ transplantation. In: Levenson JL. editor. The American psychiatric press textbook of psychosomatic medicine: psychiatric care of the medically ill. 3rd ed. Washington DC: American Psychiatric Publishing, 2018:859-906. [Google Scholar]
- 22.Rustad JK, Stern TA, Prabhakar M, et al. Risk factors for alcohol relapse following orthotopic liver transplantation: a systematic review. Psychosomatics 2015;56:21-35. 10.1016/j.psym.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 23.Shen NT, Shenoy A, Brown RS, Jr, et al. Identifying risk of alcohol relapse after liver transplantation: what tools do we need? Liver Transpl 2019;25:1133-5. 10.1002/lt.25579 [DOI] [PubMed] [Google Scholar]
- 24.De Gottardi A, Spahr L, Gelez P, et al. A simple score for predicting alcohol relapse after liver transplantation: results from 387 patients over 15 years. Arch Intern Med 2007;167:1183-8. 10.1001/archinte.167.11.1183 [DOI] [PubMed] [Google Scholar]
- 25.Iasi MS, Vieira A, Anez CI, et al. Recurrence of alcohol ingestion in liver transplantation candidates. Transplant Proc 2003;35:1123-4. 10.1016/S0041-1345(03)00333-6 [DOI] [PubMed] [Google Scholar]
- 26.Chuncharunee L, Yamashiki N, Thakkinstian A, et al. Alcohol relapse and its predictors after liver transplantation for alcoholic liver disease: a systematic review and meta-analysis. BMC Gastroenterol 2019;19:150. 10.1186/s12876-019-1050-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigue JR, Hanto DW, Curry MP. The Alcohol Relapse Risk Assessment: a scoring system to predict the risk of relapse to any alcohol use after liver transplant. Prog Transplant 2013;23:310-8. 10.7182/pit2013604 [DOI] [PubMed] [Google Scholar]
- 28.Lee BP, Chen PH, Haugen C, et al. Three-year results of a pilot program in early liver transplantation for severe alcoholic hepatitis. Ann Surg 2017;265:20-9. 10.1097/SLA.0000000000001831 [DOI] [PubMed] [Google Scholar]
- 29.Lee BP, Vittinghoff E, Hsu C, et al. Predicting Low Risk for Sustained Alcohol Use After Early Liver Transplant for Acute Alcoholic Hepatitis: The Sustained Alcohol Use Post-Liver Transplant Score. Hepatology 2019;69:1477-87. 10.1002/hep.30478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiMartini A, Day N, Dew MA, et al. Alcohol consumption patterns and predictors of use following liver transplantation for alcoholic liver disease. Liver Transpl 2006;12:813-20. 10.1002/lt.20688 [DOI] [PubMed] [Google Scholar]
- 31.Osorio RW, Ascher NL, Avery M, et al. Predicting recidivism after orthotopic liver transplantation for alcoholic liver disease. Hepatology 1994;20:105-10. [DOI] [PubMed] [Google Scholar]
- 32.Hartl J, Scherer MN, Loss M, et al. Strong predictors for alcohol recidivism after liver transplantation: non-acceptance of the alcohol problem and abstinence of <3 months. Scand J Gastroenterol 2011;46:1257-66. 10.3109/00365521.2011.603160 [DOI] [PubMed] [Google Scholar]
- 33.Carbonneau M, Jensen LA, Bain VG, et al. Alcohol use while on the liver transplant waiting list: a single-center experience. Liver Transpl 2010;16:91-7. 10.1002/lt.21957 [DOI] [PubMed] [Google Scholar]
- 34.DiMartini A, Day N, Dew MA, et al. Alcohol use following liver transplantation: a comparison of follow-up methods. Psychosomatics 2001;42:55-62. 10.1176/appi.psy.42.1.55 [DOI] [PubMed] [Google Scholar]
- 35.Gish RG, Lee A, Brooks L, et al. Long-term follow-up of patients diagnosed with alcohol dependence or alcohol abuse who were evaluated for liver transplantation. Liver Transpl 2001;7:581-7. 10.1053/jlts.2001.25455 [DOI] [PubMed] [Google Scholar]
- 36.Yates WR, Booth BM, Reed DA, et al. Descriptive and predictive validity of a high-risk alcoholism relapse model. J Stud Alcohol 1993;54:645-51. 10.15288/jsa.1993.54.645 [DOI] [PubMed] [Google Scholar]
- 37.Masson S, Marrow B, Kendrick S, et al. An 'alcohol contract' has no significant effect on return to drinking after liver transplantation for alcoholic liver disease. Transpl Int 2014;27:475-81. 10.1111/tri.12283 [DOI] [PubMed] [Google Scholar]
- 38.Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011;365:1790-800. 10.1056/NEJMoa1105703 [DOI] [PubMed] [Google Scholar]
- 39.Im GY, Kim-Schluger L, Shenoy A, et al. Early liver transplantation for severe alcoholic hepatitis in the united states--a single-center experience. Am J Transplant 2016;16:841-9. 10.1111/ajt.13586 [DOI] [PubMed] [Google Scholar]
- 40.Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation 2016;100:981-7. 10.1097/TP.0000000000001156 [DOI] [PubMed] [Google Scholar]
- 41.Project MATCH. (Matching Alcoholism Treatment to Client Heterogeneity): rationale and methods for a multisite clinical trial matching patients to alcoholism treatment. Alcohol Clin Exp Res 1993;17:1130-45. 10.1111/j.1530-0277.1993.tb05219.x [DOI] [PubMed] [Google Scholar]
- 42.Anton RF, O'Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 2006;295:2003-17. 10.1001/jama.295.17.2003 [DOI] [PubMed] [Google Scholar]
- 43.Karampahtsis C, Travis M, Arbuckle M. Talking pathways to patients: addiction. 2019. Available online: https://www.nncionline.org/course/talking-pathways-to-patients-addiction
- 44.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci 2011;12:623-37. 10.1038/nrn3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LiverTox. Clinical and research information on drug-induced liver injury. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases, 2012. Available online: https://livertox.nlm.nih.gov/Naltrexone.htm [PubMed]
- 46.Weinrieb RM, Van Horn DH, McLellan AT, et al. Alcoholism treatment after liver transplantation: lessons learned from a clinical trial that failed. Psychosomatics 2001;42:110-6. 10.1176/appi.psy.42.2.110 [DOI] [PubMed] [Google Scholar]
- 47.Garbutt JC, Kampov-Polevoy AB, Kalka-Juhl LS, et al. Association of the sweet-liking phenotype and craving for alcohol with the response to naltrexone treatment in alcohol dependence: a randomized clinical trial. JAMA Psychiatry 2016;73:1056-63. 10.1001/jamapsychiatry.2016.2157 [DOI] [PubMed] [Google Scholar]
- 48.Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet 2007;370:1915-22. 10.1016/S0140-6736(07)61814-5 [DOI] [PubMed] [Google Scholar]
- 49.Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci 2006;29:116-24. 10.1016/j.tins.2005.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moos RH, Moos BS. Treated and untreated alcohol-use disorders: course and predictors of remission and relapse. Eval Rev 2007;31:564-84. 10.1177/0193841X07306749 [DOI] [PubMed] [Google Scholar]
- 51.Weinrieb RM, Van Horn DH, Lynch KG, et al. A randomized, controlled study of treatment for alcohol dependence in patients awaiting liver transplantation. Liver Transpl 2011;17:539-47. 10.1002/lt.22259 [DOI] [PubMed] [Google Scholar]
- 52.Moeller SJ, Hajcak G, Parvaz MA, et al. Psychophysiological prediction of choice: relevance to insight and drug addiction. Brain 2012;135:3481-94. 10.1093/brain/aws252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaskutas LA. Alcoholics anonymous effectiveness: faith meets science. J Addict Dis 2009;28:145-57. 10.1080/10550880902772464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lilienfeld SO, Arkowitz H. Does alcoholics anonymous work? Scientific American. 2011. Available online: https://www.scientificamerican.com/article/does-alcoholics-anonymous-work/
- 55.Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA 2004;291:1887-96. 10.1001/jama.291.15.1887 [DOI] [PubMed] [Google Scholar]
- 56.Cornelius JR, Bukstein O, Salloum I, et al. Alcohol and psychiatric comorbidity. Recent Dev Alcohol 2003;16:361-74. [DOI] [PubMed] [Google Scholar]
- 57.Dundon W, Lynch KG, Pettinati HM, et al. Treatment outcomes in type A and B alcohol dependence 6 months after serotonergic pharmacotherapy. Alcohol Clin Exp Res 2004;28:1065-73. 10.1097/01.ALC.0000130974.50563.04 [DOI] [PMC free article] [PubMed] [Google Scholar]